Abstract
Adherence of parasite-infected erythrocytes (IEs) to the microvascular endothelium of various organs, a process known as sequestration, is a feature of Plasmodium falciparum malaria. This event is mediated by specific adhesive interactions between parasite proteins, expressed on the surface of IEs, and host molecules. P. falciparum IEs can bind to purified chondroitin sulfate A (CS-A), to the proteoglycan thrombomodulin through CS-A side chains, and to CS-A present on the surface of brain and lung endothelial cells and placental syncytiotrophoblasts. In order to identify structural characteristics of CS-A important for binding, oligosaccharide fragments ranging in size from 2 to 20 monosaccharide units were isolated from CS-A and CS-C, following controlled chondroitin lyase digestion, and used as competitive inhibitors of IE binding to immobilized ligands. Inhibition of binding to CS-A was highly dependent on molecular size: a CS-A tetradecasaccharide fraction was the minimum length able to almost completely inhibit binding. The effect was dose dependent and similar to that of the parent polysaccharide, and the same degree of inhibition was not found with the CS-C oligosaccharides. There was no effect on binding of IEs to other ligands, e.g., CD36 and intercellular adhesion molecule 1. Hexadeca- and octadecasaccharide fractions of CS-A were required for maximum inhibition of binding to thrombomodulin. Analyses of oligosaccharide fractions and polysaccharides by electrospray mass spectrometry and high-performance liquid chromatography suggest that the differences between the activities of CS-A and CS-C oligosaccharides can be attributed to differences in sulfate content and sulfation pattern and that iduronic acid is not involved in IE binding.
The adherence of purified infected erythrocytes (IEs) to the microvascular endothelium of various organs is a key feature of Plasmodium falciparum infection and contributes to the survival of parasites by aiding replication and evasion of splenic clearance. This process can lead to postcapillary venules becoming packed with adherent IEs, an important pathological characteristic of cerebral malaria (1, 44), and to the accumulation of IEs in the intervillous spaces of the placenta (8). A range of cell adhesion molecules has been reported for P. falciparum, including thrombospondin (33), CD36 (2, 29), intercellular adhesion molecule 1 (ICAM-1) (5), vascular cell adhesion molecule 1, E-selectin (30), platelet/endothelial cell adhesion molecule/CD31 (41), P-selectin (42), and heparan sulfate (14).
Previously, cell-associated or purified chondroitin sulfate A (CS-A or chondroitin-4-sulfate) has been identified as a cell adhesion molecule for IEs in static and flow-based assays (13, 16, 32, 35). IEs can also bind to the proteoglycan thrombomodulin (TM), via CS-A side chains, making it a candidate for anchoring CS-A ligands at the cell surface (20, 36). Interest in CS-A as a cell surface carbohydrate ligand has grown following reports that IEs can adhere to Saimiri brain and human lung endothelial cells (32) and placental syncytiotrophoblasts (18, 27) in a CS-A-dependent manner. Also, binding to CS-A may be involved in the placental sequestration of IEs and may partially explain the increased susceptibility to malaria during pregnancy (18).
Chondroitin sulfate (CS) chains comprise repeating disaccharide units of hexuronic acid (HexA) β1-3 linked to N-acetylgalactosamine (GalNAc), i.e., -(4HexAβ1-3GalNAc1)n-. However, CS chains show heterogeneity in sulfation patterns and uronic acid compositions (glucuronic acid-iduronic acid [GlcA-IdoA]), depending on the polysaccharide source (22), due to different sulfate substitutions and differing degrees of isomerization of GlcA to IdoA. Typically, GalNAc is mono-O sulfated at either the 4- or the 6-O position and this differentiates the principal CS-A and CS-C disaccharide units, respectively. CS-B (or dermatan sulfate) is similar in sulfation to CS-A but the uronic acid is predominantly IdoA.
In order to investigate specific structural characteristics of CS-A that are involved in binding parasitized erythrocytes, various sizes of CS-A and CS-C oligosaccharide fragments obtained by lyase digestion have been used to study inhibition of IE binding to immobilized CS-A and TM, together with an analysis of sulfation and uronic acid composition, allowing derivation of the minimum size oligosaccharide containing the putative inhibitory motif.
MATERIALS AND METHODS
Chemicals.
CS-A (sodium salt from bovine trachea) and -C (sodium salt from shark cartilage), chondroitin lyase ABC (EC 4.2.2.4, from Proteus vulgaris) and disaccharide standards ΔUA-GalNAc (0-S), ΔUA-GalNAc(4SO3H) (4-S), ΔUA-GalNAc(6SO3H) (6-S), ΔUA(2SO3H)-GalNAc(6SO3H) (diS-D), and ΔUA-GalNAc(4SO3H,6SO3H) (diS-E) were purchased from Sigma Chemical Co. (Poole, Dorset, United Kingdom).
Preparation and characterization of CS oligosaccharide fragments.
CS-A and -C were partially depolymerized by controlled digestion with chondroitin lyase ABC as described previously (9). Briefly, CS-A and CS-C (200 mg each) were incubated with 0.5 U of chondroitin lyase ABC in 4 ml of sodium phosphate buffer (50 mM, pH 7.0) containing 0.2 M NaCl at 30°C. Aliquots of reaction solutions were diluted and monitored by UV absorbance at 232 nm at timed intervals. The reaction was stopped by heating the solution at 100°C for 1 min when digestion was 60% complete.
After being desalted on a short column of Sephadex G-10 (1.6 by 36 cm), the digests were fractionated on a Bio-Gel P-4 column (1.6 by 90 cm) and eluted with 0.2 M ammonium acetate at a flow rate of 15 ml/h. The eluate was monitored on-line by UV at 232 nm and also by refractive index. Di- to dodecasaccharide fractions together with the larger oligomer pool were collected and freeze-dried. The larger oligomers were rechromatographed on a Bio-Gel P-10 column under the same conditions, and tetradeca- to eicosasaccharide fractions were isolated. Ammonium acetate was removed by repeated coevaporation with water by lyophilization.
Each oligosaccharide fraction was quantified by UV absorption at 232 nm with CS disaccharide 6-S as a standard. The UV activity of oligosaccharide fragments results from 4,5-unsaturated uronic acid residues (ΔUA) that are created by lyase cleavage at the GalNAc1-4HexA bond. UV absorption for ΔUA-containing CS-A and CS-C oligosaccharides was assumed to be equivalent, and the calculated quantities were checked against the dry weights of the samples. Dried oligosaccharides were dissolved in phosphate-buffered saline (1 mg/ml) before use in binding assays.
The major components in Bio-Gel P-4 fractions were detected by electrospray mass spectrometry (ES-MS) (11, 12). Compositions of oligosaccharides in terms of GalNAc, HexA, and sulfate were derived from the measured molecular masses.
Disaccharide composition analysis.
Analysis was essentially as described previously (10). Oligosaccharides were freeze-dried, dissolved in 28 μl of 5 mM sodium phosphate (pH 7.0) containing 0.2 M NaCl, and digested exhaustively at 37°C overnight with 5 mU of chondroitin ABC lyase in 2 μl of the same phosphate buffer. Disaccharides (0.5 to 1 nmol) were separated by strong anion exchange high-performance liquid chromatography on an S5-SAX column (4.6 by 250 mm; Phase Separation Ltd) with a titanium-lined Gilson liquid chromatographic system. Disaccharides were eluted at a flow rate of 1 ml/min with UV detection at 232 nm by using increasing concentrations of NaCl (pH 3.5) from 0 to 0.075 M for 15 min followed by 0.075 to 0.9 M for 25 min.
Parasitized erythrocytes.
Brazilian P. falciparum isolate ItG2F6 was cloned to give FAF6 (7). This was selected for binding to endothelial cells to give FAF-EA8 (6) and was subsequently selected for binding to Chinese hamster ovary cells and immobilized CS-A to give the isolate CS2 (35). In cytoadherence assays, FAF6 binds to CD36 and FAF-EA8 binds to CD36, ICAM-1, and (weakly) CS-A. CS2 binds at high levels to CS-A but does not bind to ICAM-1 or CD36. HCS3 was derived from a patient isolate by selection for binding to immobilized CS-A. Selection was performed three times. Parasites were cultured as previously described (38) and synchronized every 1 to 2 weeks by sorbitol lysis (23).
Purified ligands.
CS-A from porcine rib cartilage (Sigma Chemical Co., Sydney, Australia) was covalently linked to dipalmitoylphosphatidylethanolamine as previously described (39) to facilitate immobilization on plastic and was used at a concentration of 50 μg/ml. Recombinant soluble human TM containing CS chains (a gift of B. Grinnell and B. Gerlitz, Lilly Corporate Center, Indianapolis, Ind.) was used at a concentration of 1 μg/ml. Platelet-derived CD36 was a gift of M. Berndt, Baker Institute, Melbourne, Australia, and was used at a 1-μg/ml concentration. Recombinant soluble ICAM-1 (A. Boyd, Walter and Eliza Hall Institute of Medical Research, Parkville, Australia) was used at a 10-μg/ml concentration.
Cytoadherence assays.
Assays were performed using trophozoite-infected erythrocytes at 4 to 5% parasitemia and 2% hematocrit. Similar to that of a previously described method (21), holes were punched in a layer of plastic sealing film (Nescofilm; Nippon Shoji Kaisha Ltd, Osaka, Japan) that was then pressed firmly into place in a polystyrene petri dish (150 by 15 mm) (Falcon 1058; Becton Dickinson, Lincoln Park, N.J.), creating shallow 6-mm-diameter wells. Up to 30 wells could be created with a single piece of plastic film. Purified ligands were spotted (10 μl) into each well and were incubated at 4°C overnight in a humid box. Prior to performing the assay, ligand spots were aspirated and each well was blocked with 1% bovine serum albumin for 30 to 60 min at room temperature and subsequently washed three times with RPMI-HEPES medium. A 45-μl suspension of IEs in RPMI-HEPES medium containing 10% pooled human serum, pH 6.8, was added to each well, and the plate was incubated for 45 min at 37°C. After the plastic film was carefully removed, plates were washed five times with RPMI-HEPES medium (pH 6.8, 37°C) to remove unbound cells by slowly adding 25 ml of medium to the dish, gently agitating, and then aspirating the medium from the side by using suction. Bound cells were fixed with 2% glutaraldehyde for 2 h at 4°C, stained with 10% Giemsa, and counted microscopically. This approach enabled the testing of many samples together in one dish, under the same conditions, favoring a more reliable comparison of the inhibitory effects of different CS fractions. CS oligo- and polysaccharides were tested for inhibition of binding by adding them to the parasite suspension at a final concentration of 90 μg/ml (unless otherwise stated) 5 min prior to performing the assay. Phosphate-buffered saline was used as a control and samples were randomized and coded in all experiments.
RESULTS
Inhibition of binding to immobilized CS-A by oligosaccharides.
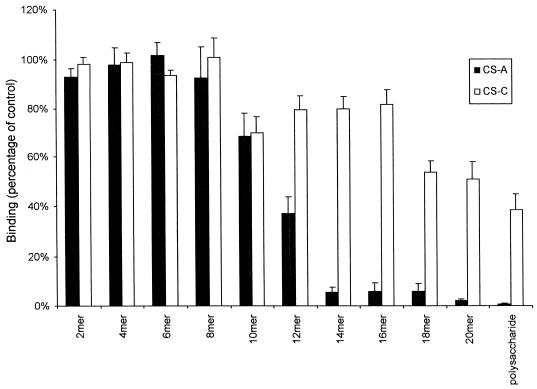
Ten oligosaccharide fractions of 2 to 20 monosaccharide residues in length derived from either CS-A or CS-C were tested, at a fixed concentration of 90 μg/ml, for inhibition of binding of IEs to immobilized CS-A by using the parasite line CS2. Figure 1 shows that the inhibitory effects of the CS-A oligosaccharides are highly length specific. Nearly complete inhibition of binding was found only with the CS-A-derived tetradecamer or larger oligosaccharides and polysaccharide. Significant inhibition was seen with the CS-A deca- and dodecasaccharide fractions, averaging 31 and 63% inhibition, respectively, but not with shorter oligosaccharides. The same degree of inhibition was absent using the CS-C-derived oligosaccharides, and only the CS-C polysaccharide gave an average inhibition of more than 50%, which was never complete in any experiment at the maximum concentration tested.
FIG. 1.
Inhibition of binding of CS2 parasites to immobilized CS-A by oligosaccharides (di- to eicosamers) and polysaccharides from CS-A and CS-C at a concentration of 90 μg/ml. Values are means (± standard errors) for quadruplicate experiments.
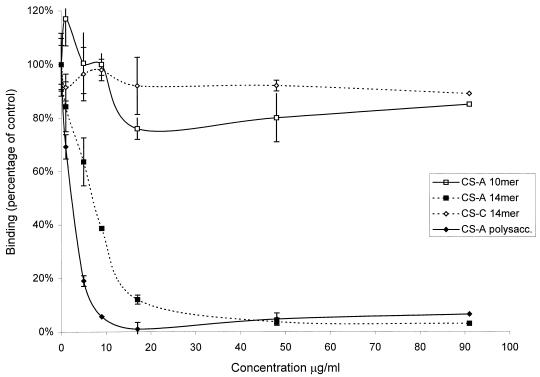
The inhibitory effect of the CS-A tetradecasaccharide fraction on IE binding to immobilized CS-A was further tested over a range of concentrations and compared to that of CS-A polysaccharide by using the CS-A decamer and CS-C tetradecamer fractions as negative controls. The resulting concentration versus binding plot for the CS-A tetradecasaccharides paralleled that for the CS-A polysaccharide, consistent with specific competitive inhibition of receptor-ligand binding (Fig. 2). The 50% inhibitory concentrations (IC50) derived for the CS-A tetradecasaccharide and polysaccharide were similar and were at least 10-fold lower than those of the CS-C tetradecamer and CS-A decamer fractions (Table 1).
FIG. 2.
Inhibition of binding of CS2 parasites to immobilized CS-A at increasing concentrations of oligosaccharides and polysaccharide. Values are means ± standard errors for triplicate experiments.
TABLE 1.
IC50 for CS-A decasaccharide, tetradecasaccharide, and polysaccharide and CS-C tetradecasaccharide
| Saccharide fraction | IC50 (μg/ml)a |
|---|---|
| CS-A decamer | >100 |
| CS-A tetradecamer | 7.5 |
| CS-A polysaccharide | 2.5 |
| CS-C tetradecamer | >100 |
Values were determined by linear regression of the x intercept using data points from the inhibition curve (Fig. 2).
Erythrocytes infected by P. falciparum HCS3, selected for high binding to immobilized CS-A, could be inhibited in a dose-dependent manner by free CS-A but not by CS-B or CS-C, and there was no binding seen to immobilized CS-B used as a control (data not shown). As with erythrocytes infected by CS2, the inhibitory effects of the CS-A oligosaccharides showed the same significant increases for tetradecamers and larger oligosaccharides. For the CS-A fractions, binding as a percentage of that for the control averaged 75% for the decamer, 45% for the dodecamer, 21% for the tetradecamer, and <10% for each of the hexadeca-, octadeca-, and eicosamer and for the polysaccharide fractions. Binding averaged 60% or higher with each of the CS-C fractions from deca- to eicosamer and the polysaccharide.
Inhibition of binding to TM, CD36, and ICAM-1.
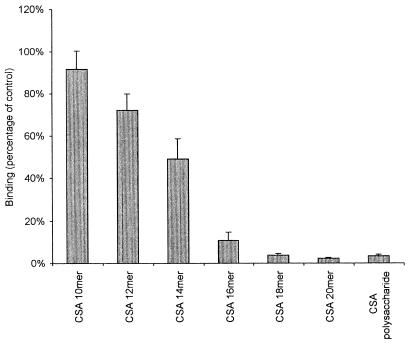
The effects of CS-A oligosaccharides on binding of CS2 IEs to immobilized TM are shown in Fig. 3. The inhibitory effect of the CS-A tetradecasaccharide fraction was about 50% but this effect was significantly increased when the hexadecamer and higher fractions were used under the same experimental conditions as those used for binding to CS-A. Little or no inhibition was achieved with the CS-C oligosaccharide fractions at the same concentration (data not shown). In preliminary experiments, increasing the concentration of TM above 1 μg/ml when coating plates for binding assays significantly reduced the degree of inhibition by CS-A oligosaccharides.
FIG. 3.
Inhibition of binding of CS2 to immobilized recombinant human TM by CS-A oligosaccharides and polysaccharide at a concentration of 90 μg/ml. Values are means ± standard errors for duplicate experiments.
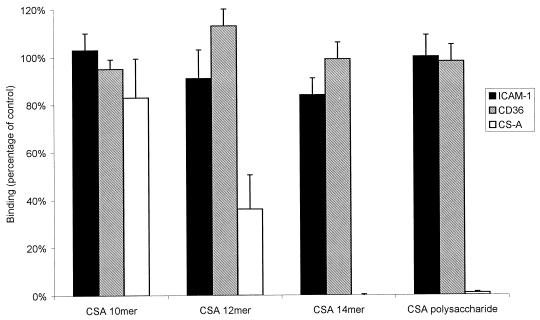
The oligosaccharides and polysaccharide had little or no effect on the binding of FAF6-infected IEs to CD36 or of FAF-EA8-infected IEs to ICAM-1 (Fig. 4). Specifically, the CS-A tetradecasaccharide fraction had no effect on binding to CD36 and ICAM-1 compared to that of the same length CS-C oligosaccharides. This is consistent with a specific interaction between CS-A and a receptor present only on parasite lines CS2 and HCS3.
FIG. 4.
Effect on binding of parasite lines FAF-EA8 and FAF6 to ICAM-1 and CD36, respectively, and of CS2 to CS-A by CS-A oligosaccharides and polysaccharide at a concentration of 90 μg/ml. Values are means ± standard errors for duplicate experiments.
Characterization of CS oligosaccharides.
The molecular sizes and compositions of the major components in oligosaccharide fractions from gel filtration chromatography of CS polysaccharide digests were determined by ES-MS analysis. The principal ions in spectra of deca- and tetradecasaccharide fractions from both CS-A and CS-C are given in Table 2. In CS-A fractions, the dominant mass ions were consistent with ΔUA-[GalNAc(SO3H)-HexA]n-1-GalNAc(SO3H), where n equals the number of disaccharide repeats. The relative abundance of mono-undersulfated species (−SO3) in the CS-A fractions was 25%. In CS-C fractions, the major ΔUA-[GalNAc(SO3H)-HexA]n-1-GalNAc(SO3H) components were accompanied by mono-oversulfated (+SO3) and mono-undersulfated species with relative abundances of 35 and 15%, respectively. This indicates that CS-C has an overall higher sulfate content than CS-A.
TABLE 2.
ES-MS of CS deca- and tetradecasaccharide fractions
| Saccharide fraction | Observed mass peaka
|
||
|---|---|---|---|
| Major | −SO3 | +SO3 | |
| CS-A decasaccharide | 2,296 (100) | 2,216 (23) | —b |
| CS-A tetradecasaccharide | 3,215 (100) | 3,135 (27) | — |
| CS-C decasaccharide | 2,296 (100) | 2,216 (17) | 2,376 (34) |
| CS-C tetradecasaccharide | 3,215 (100) | 3,135 (10) | 3,296 (35) |
Percentages of relative abundance are in parentheses.
—, not detected.
Disaccharide composition analyses of the tetradecasaccharide fractions and polysaccharides (Table 3) were in agreement with the compositions derived from ES-MS of intact oligosaccharides. The CS-A tetradecasaccharide fraction produced more nonsulfated disaccharide (0-S) (11%) than that of CS-C (2%), whereas CS-C gave rise to more disulfated disaccharide (diS-D) (7%) than CS-A (1%). As expected, there was a higher proportion of mono-4-sulfated disaccharides in the CS-A fraction (44%) than in the CS-C fraction (15%). A trace amount of diS-E was also present in both tetradecasaccharide fractions. GlcA-IdoA composition analysis by high-resolution 1H nuclear magnetic resonance (NMR) (40) of CS-A and CS-C polysaccharide fractions gave no detectable signals from IdoA residues (data not shown).
TABLE 3.
Disaccharide composition analysis of CS tetradeca- and polysaccharides
| Saccharide fraction | Composition (%)
|
||||
|---|---|---|---|---|---|
| 0-S | 6-S | 4-S | diS-D | diS-E | |
| CS-A tetradecasaccharide | 11 | 46 | 44 | <1 | <1 |
| CS-C tetradecasaccharide | 2 | 77 | 15 | 7 | <1 |
| CS-A polysaccharide | 6 | 40 | 55 | <1 | <1 |
| CS-C polysaccharide | 1 | 74 | 17 | 7 | 1 |
DISCUSSION
Using competitive inhibition assays with oligosaccharide fragments of defined molecular size, this study has shown that a CS-A tetradecasaccharide is the minimum-length fraction to cause complete inhibition of binding of parasitized erythrocytes to immobilized CS-A polysaccharides. The inhibition of binding by oligosaccharides was highly dependent on chain length and CS type, consistent with the presence of a unique sequence(s), comprising the CS-A binding epitope of IEs, found only in the tetradecasaccharide and higher oligosaccharide fractions. This effect was demonstrated with IEs of two different P. falciparum strains, CS2 and HCS3. Specificity was further confirmed by results indicating that inhibition with the tetradecasaccharide fraction was dose dependent and saturable, with a derived IC50 similar to that of the parent polysaccharide and more than 10-fold lower than that of the corresponding CS-C tetradecamer or CS-A decamer. Additionally, there was little or no effect on binding of IEs to CD36 or ICAM-1, reflecting a specific interaction between CS-A and parasite strains CS2 and HCS3. Finally, the response was restricted to the CS-A oligosaccharides. The CS-C used in this study contains up to 15% CS-A, which may explain the modest inhibition of binding seen with the larger CS-C oligosaccharide fractions and polysaccharide.
In the present study, we used CS-A from bovine trachea rather than from porcine rib cartilage, as was used in a previous study (35), because it contains predominantly GlcA with little or no IdoA, allowing a more direct comparison with CS-C. The major difference between the inhibitory activities of CS-A and CS-C tetradecasaccharides can, therefore, be attributed to differences in sulfate content and sulfation pattern. ES-MS and high-performance liquid chromatography disaccharide composition analyses showed fewer sulfates and a higher proportion of 4-O- than 6-O-sulfated GalNAc in the components of the CS-A tetradecasaccharide fraction than in those of the CS-C tetradecasaccharide fraction, and by high-resolution NMR analysis undetectable levels of IdoA were found in both the CS-A and CS-C polysaccharides used. The extra sulfate in the oversulfated region of CS-C was at the 2-O- position of GlcA residues as in the disaccharide ΔUA(2SO3H)-GalNAc(6SO3H) and as previously identified in the unique trisaccharide motif -GalNAc(4SO3H)-GlcA(2SO3H)-GalNAc(6SO3H)- (10). Hence, 2-O- sulfation appears not to be required for IE binding. CS-A from porcine rib cartilage, containing approximately equal amounts of IdoA and GlcA, has been shown to directly bind IEs and to inhibit binding of IEs to cell-associated CS-A, whereas CS-B, which has a sulfation pattern similar to that of to CS-A but contains mostly IdoA, does not (35). Taken together with the results from the present study, this suggests that IdoA is not involved in binding IEs; rather IE binding is dependent on an oligosaccharide sequence(s) with a backbone of -4GlcAβ1-3GalNAc1- and a unique sulfation pattern. Fractionation and detailed analysis of individual oligosaccharides in the tetradecamer and adjacent fractions, together with evaluation of their inhibitory activities, may further define structural features of CS-A binding motifs.
TM contains one or two CS-A chains (17, 19), to which parasites may bind, and its distribution in brain (45) and placenta (37), where parasite sequestration is known to occur, makes TM a likely target for IE adhesion. The CS chains of human TM are predominantly constituted of 4-O-sulfated GalNAc with GlcA (28). Inhibition of binding of IEs to immobilized human TM by oligosaccharides in the present study was dependent on size and CS type, being strongly inhibited by CS-A hexadecasaccharide and higher oligosaccharide fractions, similar to results obtained with CS-A as the binding ligand. Previous work demonstrated that CS-A polysaccharides of differing GlcA-IdoA contents had different inhibitory effects on binding of IEs to TM (36). However, the sulfate contents and sulfation patterns of these CS-As were unknown and may have accounted for inhibitory differences. TM may be the principal cell surface proteoglycan containing CS-A; however, glycosylation of TM can be variable (25), which may affect the pattern of sequestration in vivo. Additionally, the possible heterogeneity of CS chains present on cell surfaces in the human population may affect susceptibility to infection and/or severe disease.
Further studies are needed to clarify the role of adhesion of IEs to CS-A in parasite sequestration in organs such as the placenta and the brain. If specific structural motifs in CS-A chains are implicated, defined oligosaccharides may provide reagents to target sequestered parasites or to prevent sequestration of circulating IEs. In Saimiri monkeys, intramuscular administration of CS-A polysaccharides was found to inhibit and reverse parasite sequestration following infection with a CS-A-binding P. falciparum strain (31). CSs have also been safely administered to humans, both orally and parenterally, achieving levels in serum equivalent to those that inhibit binding of IEs in vitro (15). CS is normally present in human serum at a concentration of >1 μg/ml and is made up of approximately 60% nonsulfated disaccharides and 40% mono-4-sulfated disaccharides, with GlcA being the principal uronic acid (43). It may be useful to examine changes in serum CS levels that occur with malaria infection and in pregnancy.
The receptor on the surface of IEs that binds to CS-A remains to be identified but is likely to be the P. falciparum erythrocyte membrane protein 1 (PfEMP1) (34). As a strain-specific variant protein (6), PfEMP1 is expressed on the surface of IEs (24, 26) and binds to CD36, thrombospondin, ICAM-1 (3, 4), heparan sulfate (14), and perhaps other host molecules. The possibility that the CS-A tetradecasaccharides are inhibitory by specifically binding to PfEMP1 on the surface of IEs will be the focus of further investigation. Identification of a protein receptor for defined CS-A oligosaccharides will enable elucidation of the cytoadherence receptor-ligand interaction and should lead to a better understanding of cytoadherence in relation to the pathogenesis of disease.
ACKNOWLEDGMENTS
We thank Kathy Davern and John Reeder for technical assistance and helpful discussions provided during this study and J. Luo (MRC Toxicology Unit, Leicester, United Kingdom) and H. Kogelberg (The Glycosciences Laboratory) for performing the ES-MS and NMR analyses, respectively. Human erythrocytes and serum were kindly provided by the Red Cross Blood Bank of Victoria, Australia.
The work of the Division of Infection and Immunity is supported by grants from the National Health and Medical Research Council (NHMRC) of Australia, and The Glycosciences Laboratory is supported by a program grant (E400/6221) from the U.K. Medical Research Council. J.G.B. is a recipient of an NHMRC Medical Postgraduate Scholarship. S.J.R. is supported by a Wellcome Trust Career Development Fellowship in Clinical Tropical Medicine.
REFERENCES
- 1.Aikawa M, Iseki M, Barnwell J W, Taylor D, Oo M M, Howard R J. The pathology of human cerebral malaria. Am J Trop Med Hyg. 1990;43:30–37. doi: 10.4269/ajtmh.1990.43.30. [DOI] [PubMed] [Google Scholar]
- 2.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baruch D I, Gormley J A, Ma C, Howard R J, Pasloske B L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 5.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion molecule for Plasmodium falciparum. Nature. 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 6.Biggs B-A, Anders R F, Dillon H E, Davern K M, Martin M, Petersen C, Brown G V. Adherence of infected erythrocytes to venular endothelium selects for antigenic variants of Plasmodium falciparum. J Immunol. 1992;149:2047–2054. [PubMed] [Google Scholar]
- 7.Biggs B-A, Goozé L, Wycherley K, Wollish W, Southwell B, Leech J H, Brown G V. Antigenic variation in Plasmodium falciparum. Proc Natl Acad Sci USA. 1991;88:9171–9174. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulmer J N, Rasheed F N, Francis N, Morrison L, Greenwood B M. Placental malaria. I. Pathological classification. Histopathology. 1993;22:211–218. doi: 10.1111/j.1365-2559.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 9.Chai W, Kogelberg H, Lawson A M. Generation and structural characterization of a range of unmodified chondroitin sulfate oligosaccharide fragments. Anal Biochem. 1996;237:88–102. doi: 10.1006/abio.1996.0205. [DOI] [PubMed] [Google Scholar]
- 10.Chai W, Lawson A M, Gradwell M J, Kogelberg H. Structural characterization of two hexasaccharides and an octasaccharide from chondroitin sulfate C containing the unusual sequence (4-sulpho)-N-acetylgalactosamine-1-4-(2-sulpho)-glucuronic acid-1-3-(6-sulpho)-N-acetylgalactosamine. Eur J Biochem. 1998;251:114–121. doi: 10.1046/j.1432-1327.1998.2510114.x. [DOI] [PubMed] [Google Scholar]
- 11.Chai W, Luo J, Lim C K, Lawson A M. Characterization of heparin oligosaccharide mixtures as ammonium salts using electrospray mass spectrometry. Anal Biochem. 1998;70:2060–2066. doi: 10.1021/ac9712761. [DOI] [PubMed] [Google Scholar]
- 12.Chai W, Luo J, Lim C K, Lawson A M. Proceedings of the 45th ASMS Conference on Mass Spectrometry and Allied Topics, Palm Springs, Calif. 1997. Mixture analysis of glycosaminoglycan oligosaccharide fragments by electrospray mass spectrometry; p. 1019. [Google Scholar]
- 13.Chaiyaroj S C, Angkasekwinai P, Buranakiti A, Looareesuwan S, Rogerson S J, Brown G V. Cytoadherence characteristics of Plasmodium falciparum isolates from Thailand: evidence for chondroitin sulfate A as a cytoadherence receptor. Am J Trop Med Hyg. 1996;55:76–80. doi: 10.4269/ajtmh.1996.55.76. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Barragan A, Fernandez V, Sundstrom A, Schlichtherle M, Sahlen A, Carlson J, Datta S, Wahlgren M. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J Exp Med. 1998;187:15–23. doi: 10.1084/jem.187.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conte A, de Bernardi M, Palmieri L, Lualdi P, Mautone G, Ronca G. Metabolic fate of exogenous chondroitin sulfate in man. Arzneimittelforschung. 1991;41:768–772. [PubMed] [Google Scholar]
- 16.Cooke B M, Rogerson S J, Brown G V, Coppel R L. Adhesion of malaria-infected red blood cells to chondroitin sulfate A under flow conditions. Blood. 1996;88:4040–4044. [PubMed] [Google Scholar]
- 17.Dittman W A, Majerus P W. Structure and function of thrombomodulin: a natural anticoagulant. Blood. 1990;75:329–336. [PubMed] [Google Scholar]
- 18.Fried M, Duffy P E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 19.Gerlitz B, Hassell T, Vlahos C J, Parkinson J F, Bang N U, Grinnell B W. Identification of the predominant glycosaminoglycan-attachment site in soluble recombinant human thrombomodulin: potential regulation of functionality by glycosyltransferase competition for serine474. Biochem J. 1993;295:131–140. doi: 10.1042/bj2950131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gysin J, Pouvelle B, Le Tonqueze M, Edelman L, Boffa M-C. Chondroitin sulfate of thrombomodulin is an adhesion receptor for Plasmodium falciparum-infected erythrocytes. Mol Biochem Parasitol. 1997;88:267–271. doi: 10.1016/s0166-6851(97)00082-0. [DOI] [PubMed] [Google Scholar]
- 21.Hasler T, Albrecht G R, van Schravendijk M R, Aguiar J C, Morehead K E, Pasloske B L, Ma C, Barnwell J W, Greenwood B, Howard R J. An improved microassay for Plasmodium falciparum cytoadherence using stable transformants of Chinese hamster ovary cells expressing CD36 or intercellular adhesion molecule-1. Am J Trop Med Hyg. 1993;48:332–347. doi: 10.4269/ajtmh.1993.48.332. [DOI] [PubMed] [Google Scholar]
- 22.Karamanos N K, Syrokou A, Vanky P, Nurminen M, Hjerpe A. Determination of 24 variously sulfated galactosaminoglycan- and hyaluronan-derived disaccharides by high-performance liquid chromatography. Anal Biochem. 1994;221:189–199. doi: 10.1006/abio.1994.1396. [DOI] [PubMed] [Google Scholar]
- 23.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 24.Leech J H, Barnwell J W, Miller L H, Howard R J. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984;159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin J-H, McLean K, Morser J, Young T A, Wydro R M, Andrews W H, Light D R. Modulation of glycosaminoglycan addition in naturally expressed and recombinant human thrombomodulin. J Biol Chem. 1994;269:25021–25030. [PubMed] [Google Scholar]
- 26.Magowan C, Wollish W, Anderson L, Leech J H. Cytoadherence by Plasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J Exp Med. 1988;168:1307–1320. doi: 10.1084/jem.168.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maubert B, Guilbert L J, Deloron P. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect Immun. 1997;65:1251–1257. doi: 10.1128/iai.65.4.1251-1257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nawa K, Sakano K, Fujiwara H, Sato Y, Sugiyama N, Teruuchi T, Iwamoto M, Marumoto Y. Presence and function of chondroitin-4-sulfate on recombinant on recombinant human soluble thrombomodulin. Biochem Biophys Res Commun. 1990;171:729–737. doi: 10.1016/0006-291x(90)91207-9. [DOI] [PubMed] [Google Scholar]
- 29.Ockenhouse C F, Tandon N N, Magowan C, Jamieson G A, Chulay J D. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989;243:1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- 30.Ockenhouse C F, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan K E, Thway Y, Win K, Aikawa M, Lobb R R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pouvelle B, Meyer P, Robert C, Bardel L, Gysin J. Chondroitin-4-sulfate impairs in vitro and in vivo cytoadherence of Plasmodium falciparum infected erythrocytes. Mol Med. 1997;3:508–518. [PMC free article] [PubMed] [Google Scholar]
- 32.Robert C, Pouvelle B, Meyer P, Muanza K, Fukioka H, Aikawa M, Scherf A, Gysin J. Chondroitin-4-sulphate (proteoglycan), a receptor for Plasmodium falciparum-infected erythrocyte adherence on brain microvascular endothelial cells. Res Immunol. 1995;146:383–393. doi: 10.1016/0923-2494(96)81042-x. [DOI] [PubMed] [Google Scholar]
- 33.Roberts D D, Sherwood J A, Spitalnik S L, Panton L J, Howard R J, Dixit V M, Frazier W A, Miller L H, Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985;318:64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- 34.Rogerson S J, Brown G V. Chondroitin sulfate A as an adherence receptor for Plasmodium falciparum-infected erythrocytes. Parasitol Today. 1997;13:70–75. doi: 10.1016/s0169-4758(96)10081-8. [DOI] [PubMed] [Google Scholar]
- 35.Rogerson S J, Chaiyaroj S C, Ng K, Reeder J C, Brown G V. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogerson S J, Novakovic S, Cooke B M, Brown G V. Plasmodium falciparum-infected erythrocytes adhere to the proteoglycan thrombomodulin in static and flow-based systems. Exp Parasitol. 1997;86:8–18. doi: 10.1006/expr.1996.4142. [DOI] [PubMed] [Google Scholar]
- 37.Salem H H, Maruyama I, Ishii H, Majerus P W. Isolation and characterization of thrombomodulin from human placenta. J Biol Chem. 1984;259:12246–12251. [PubMed] [Google Scholar]
- 38.Southwell B, Brown G V, Forsyth K P, Smith T, Philip G, Anders R F. Field applications of agglutination and cytoadherence assays with Plasmodium falciparum from Papua New Guinea. Trans R Soc Trop Med Hyg. 1989;83:464–469. doi: 10.1016/0035-9203(89)90248-4. [DOI] [PubMed] [Google Scholar]
- 39.Sugiura N, Sakurai K, Hori Y, Karasawa K, Suzuki S, Kimata K. Preparation of lipid-derivatized glycosaminoglycans to probe a regulatory function of the carbohydrate moieties of proteoglycans in cell-matrix interaction. J Biol Chem. 1993;268:15779–15787. [PubMed] [Google Scholar]
- 40.Toida T, Qiu G, Matsunaga T, Sagehashi Y, Imanari T. Gas chromatography-mass spectrometric determination of iduronic and glucuronic acid in glycosaminoglycans after reduction of carboxylic acid groups using sodium borodeuteride. Anal Sci. 1992;8:799–804. [Google Scholar]
- 41.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med. 1997;2:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 42.Udomsangpetch R, Reinhardt P H, Schollaardt T, Elliott J F, Kubes P, Ho M. Promiscuity of clinical Plasmodium falciparum isolates for multiple adhesion molecules under flow conditions. J Immunol. 1997;158:4358–4364. [PubMed] [Google Scholar]
- 43.Volpi N, Cusmano M, Venturelli T. Qualitative and quantitative studies of heparin and chondroitin sulfates in normal human plasma. Biochim Biophys Acta. 1995;1243:49–58. doi: 10.1016/0304-4165(94)00123-f. [DOI] [PubMed] [Google Scholar]
- 44.Warrell D A, Molyneux M E, Beales P F. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84:1–65. [PubMed] [Google Scholar]
- 45.Wong V L Y, Hofman F M, Ishii H, Fisher M. Regional distribution of thrombomodulin in human brain. Brain Res. 1991;556:1–5. doi: 10.1016/0006-8993(91)90540-c. [DOI] [PubMed] [Google Scholar]