Abstract
Objectives
The gut microbiota can mediate both pro and anti-inflammatory responses. In patients with psoriatic arthritis (PsA), we investigated the impact of faecal microbiota transplantation (FMT), relative to sham transplantation, on 92 inflammation-associated plasma proteins.
Methods
This study relates to the FLORA trial cohort, where 31 patients with moderate-to-high peripheral PsA disease activity, despite at least 3 months of methotrexate treatment, were included in a 26-week, double-blind, randomised, sham-controlled trial. Participants were allocated to receive either one gastroscopic-guided healthy donor FMT (n=15) or sham (n=16). Patient plasma samples were collected at baseline, week 4, 12 and 26 while samples from 31 age-matched and sex-matched healthy controls (HC) were collected at baseline. Samples were analysed using proximity extension assay technology (Olink Target-96 Inflammation panel).
Results
Levels of 26 proteins differed significantly between PsA and HC pre-FMT (adjusted p<0.05), of which 10 proteins were elevated in PsA: IL-6, CCL20, CCL19, CDCP1, FGF-21, HGF, interferon-γ (IFN-γ), IL-18R1, monocyte chemotactic protein 3, and IL-2. In the FMT group, levels of 12 proteins changed significantly across all timepoints (tumour necrosis factor (TNF), CDCP1, IFN-γ, TWEAK, signalling lymphocytic activation molecule (SLAMF1), CD8A, CD5, Flt3L, CCL25, FGF-23, CD6, caspase-8). Significant differences in protein levels between FMT and sham-treated patients were observed for TNF (p=0.002), IFN-γ (p=0.011), stem cell factor (p=0.024), matrix metalloproteinase-1 (p=0.038), and SLAMF1 (p=0.042). FMT had the largest positive effect on IFN-γ, Axin-1 and CCL25 and the largest negative effect on CCL19 and IL-6.
Conclusions
Patients with active PsA have a distinct immunological plasma protein signature compared with HC pre-FMT. FMT affects several of these disease markers, including sustained elevation of IFN-γ.
Trial registration number
Keywords: Arthritis, Psoriatic; Cytokines; Inflammation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Psoriatic arthritis (PsA) is a chronic, systemic, immune-mediated disease associated with disturbances of the gut microbial environment. The disease is driven by inflammation-associated plasma proteins such as interleukin (IL)-23, IL-17, tumour necrosis factor (TNF), interferon-γ (IFN-γ), IL-1, IL-6, IL-12, and IL-15.
Clinical response to faecal microbiota transplantation (FMT) has been coupled to changes in inflammation-associated plasma protein levels, notably TNF, IL-6, and IL-10, in patients with inflammatory bowel disease.
WHAT THIS STUDY ADDS
This report is the first to assess, in a randomised controlled setting, the systemic immunological response to FMT, relative to sham transplantation, in immune-mediated arthritis.
We identified 26 inflammation-associated plasma proteins that differed significantly between patients with active peripheral PsA and age-matched and sex-matched healthy controls pre-FMT. Levels of 12 proteins changed significantly in the FMT-treated PsA patients, of which three proteins (TNF, IFN-γ and SLAMF1) differed significantly between patients receiving FMT versus sham transplantation.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
We observed long-lasting, increased levels of IFN-γ in response to FMT in patients with active peripheral PsA irrespectively of treatment outcome. The clinical implications of our findings have yet to be determined.
Introduction
Psoriatic arthritis (PsA) is a chronic, systemic, seronegative, immune-mediated disease associated with changes in the gut microbial environment.1 2 The immunopathogenesis of PsA is still incompletely understood, but the systemic activation of the interleukin (IL)−23/IL-17 cytokine axis and other pro-inflammatory mediators including tumour necrosis factor (TNF), interferon-γ (IFN-γ), IL-1, IL-6, IL-12 and IL-15 are recognised pro-inflammatory proteins implicated in the self-perpetuating inflammatory response driving the disease.3 Inducer, enhancer and effector cytokines, such as IL-23, IL-17 and TNF, respectively, orchestrate the inflammatory disease process in PsA4 and are currently the main therapeutic targets of cytokine inhibition.5 Despite expansion of the pharmacological toolbox within the last 20 years, obtaining sustained remission remains a considerable clinical challenge in PsA.5 6
The accumulating evidence implicating the intestinal milieu as a potential disease initiator and/or modifier of PsA inflammation has motivated investigation into the immunomodulatory potential of interventions targeting the complex interaction between host and gut microbes.7 One such therapy is faecal microbiota transplantation (FMT), where stool containing viable microbes and luminal content from healthy screened donors is used to modify the intestinal environment of the recipient.8 Although donor selection, sample administration and microbial engraftment are considered key factors of FMT success,9 10 a recent study encompassing metagenomic data from more than 316 FMT courses suggests that neither colonisation by donor strains, displacement of recipient species, nor the reinstatement of specific function seems to be crucial determinants for a beneficial outcome across various treatment indications.11 From this study, the effects of FMT may likely be mediated in concert with other important factors such as host immune modulation.12 This suggested FMT mode-of-action may be especially true for immune-mediated diseases.13
Fever is a well-known self-limiting side effect occurring in some recipients within hours of FMT.13 Even in healthy adults with a presumed intact intestinal barrier, FMT may induce blood leukocytosis and neutrophilia, along with a transient decrease in total lymphocyte count.14 However, the systemic immunological effects following FMT have never been investigated in immune-mediated arthritis and only scarcely reported in other conditions.15–25 In ulcerative colitis, decreased levels of TNF and IL-6 have been shown to be associated with remission following FMT, while decrease of IL-10 levels was associated with non-remission.15 A recent observational study reported reduced levels of IL-6 and CD4+T cell memory/naïve ratio in the peripheral blood of FMT-treated patients with systemic lupus erythematosus.23 These findings motivate more research into the systemic immunological effects following FMT to improve treatment outcome in immune-related conditions.26
Novel antibody-based measurement techniques using proximity extension assay (PEA) enable quantification of a broad panel of plasma proteins, which can be used to identify new proteins of interest not previously linked to the pathogenesis of PsA.27 28 In this exploratory study based on data collected from the FLORA trial,29 we aimed to investigate the immunological profile of steady-state dose methotrexate (MTX)-treated patients with PsA with moderate to high peripheral disease activity by comparing levels of 92 inflammation-associated plasma proteins (cytokines, chemokines, enzymes and shed surface receptors) between PsA patients and age-matched and sex-matched healthy controls (HC), and subsequently explore the impact of FMT on these plasma proteins relative to sham transplantation.
Methods
Study design
In the present exploratory study, we combined data on inflammation-associated plasma protein levels collected prospectively at four time points (baseline (BL), week 4, week 12 and week 26) from 31 Danish patients with PsA enrolled in the FLORA trial (15 treated with one gastroscopic-guided FMT and 16 treated with one gastroscopic-guided sham transplantation) with BL data from 4 FMT donors and 31 age-matched and sex-matched HC (FLORA trial).29 For all patients, we collected patient-reported outcomes at all four time points. Additional clinical parameters evaluated by a rheumatologist were collected at BL, week 12 and week 26. The primary trial endpoint (treatment failure) was evaluated continuously throughout the trial. If a patient was deemed treatment failure, they were offered treatment escalation, which in all but one case was initiation of TNF inhibitors (TNFi). Hence, some patients had treatment change between time points, which we took into account when performing the statistical analyses. However, we did not start any new treatments with local or systemic immunosuppressants until after the 4-week visit.
Participants
The main patient inclusion criteria were age 18–75; a diagnosis of PsA in accordance with the Classification for Psoriatic Arthritis (CASPAR)30; and active peripheral arthritis, defined as at least three swollen joints, despite ongoing steady-state dose treatment with MTX at the maximal tolerable dose (15–25 mg/week) for at least 3 months prior to study inclusion. The complete list of study eligibility criteria and the extensive programme for donor selection and screening have been reported previously.29 We recruited HC (n=31) at the local blood bank facility who matched the patient demography regarding to sex and age (within a 5-year interval).
Collection of blood samples
Plasma samples from venous blood of FMT donors and HC were collected one time (BL) during two different periods: from April to July 2017 and from February to March 2021, respectively. For patients included in the FLORA trial, samples were collected at four time points (BL, week 4, week 12 and week 26) from May 2017 to June 2020. Patients were fasting at BL and week 26, but not at weeks 4 and 12. Plasma samples were collected and treated with EDTA, separated (centrifuged at 2000G for 10 min at 20°C) and frozen within 3.5 hours after collection and stored at −80°C until analyses.
Analysis of 92 inflammation-associated plasma proteins
Frozen plasma aliquots were shipped on dry ice to the Olink facility (BioXpedia A/S laboratory, Aarhus, Denmark) without prior thawing in March 2021.31 The analyses were performed using the Olink inflammation panel encompassing 92 inflammation-associated plasma proteins (online supplemental table S1).31 During the first run, four samples did not pass the Olink internal quality control. These four samples, however, passed the quality control following a second run and data were included in the final data sheet. The Olink high-throughput proteomic platform employs a proximity extension assay technology, in which a pair of oligonucleotide-labelled antibody probes bind to the targeted protein, and if the two probes are in close proximity, a PCR target sequence is formed by a proximity-dependent DNA polymerisation event and the resulting sequence is subsequently detected and quantified using standard real-time PCR. Data are then normalised and transformed using internal extension controls and inter-plate controls, to adjust for intrarun and interrun variation. The final assay read-out is given in Normalised Protein eXpression (NPX),32 which is an arbitrary unit on log2-scale, where a high value corresponds to a higher protein concentration. Each protein has specific lower limit of quantification and an upper limit of quantification between which a 1-unit increase in NPX correspond to a twofold increase in protein concentration. Internal controls specify a run-specific lower limit of detection as three times the SD over background.
rmdopen-2023-003750supp001.pdf (171.3KB, pdf)
Patient and public involvement
Patients were involved in the planning, conduct and reporting of the clinical part of the randomised trial, but they were not directly involved in the planning or reporting of the laboratory analyses presented in this study.
Statistics
The statistical analysis of proteomic data was performed on protein data received from BioXpedia without further normalisation. All analyses were done in R V.4.3.0 according to the prespecified statistical analysis plan (SAP), which we closed and signed in 21 June 2022 before looking at the protein data in August 2022. The full version of the SAP is available as a online supplemental file A. In the primary analyses, we included actual NPX levels (data as observed) regardless of the lower limit of detection. To determine whether the protein NPXs were normally distributed, we used density plots, quantile–quantile plots and the Shapiro-Wilk test. From the latter normality test, it became clear that the data were not normally distributed. However, after further investigation of the density plots and quantile–quantile plots, we decided that repeated measures ANOVA and mixed ANOVA could be used (as specified in the SAP).
rmdopen-2023-003750supp002.pdf (12.2MB, pdf)
First, seeking to identify inflammation-associated proteins of special interest in PsA patients with active disease, we used the Wilcoxon rank-sum test to compare BL levels of each inflammation-associated plasma protein between HC versus patient, HC versus FMT donors, and patients allocated to FMT versus sham. Due to the multiple testing applied in this study, we used the Benjamini-Hochberg sequential procedure to control the false discovery rate. Using a non-supervised cluster analysis, we grouped samples and visualised the results in a dendrogram to explore if patient samples or HC and FMT donors would cluster. In the patient group, we further performed an in-group hierarchical clustering analysis to explore whether we could detect a cluster structure based on protein levels at BL among patients.
Second, to investigate potential relations between inflammation-associated plasma proteins and the four preselected disease activity measures (swollen joint count, Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis count, tender point count and Health Assessment Questionnaire Disability Index (HAQ-DI)), random forest regression, which is a supervised machine learning algorithm, was applied using the package randomForest V.4.7.1 with default settings, except importance=true. This function implements Breiman’s random forest algorithm and provides percentage of variance explained as the output of the model. For each of the models, we plotted the variable importance (VImp) and variable interaction (VInt) of the random forest regression based on the Gini index. Skewed variables were log-transformed before building the model. For each of the models, we visualised the VImp values and VInt values of the proteins by creating a heatmap using the leaf-sorting algorithm.33 We also applied random forest classification to predict treatment outcome (failure vs success) of patients at all timepoints. This prediction was done using all inflammation-associated proteins and only using TNF protein separately, to determine not only if TNFi therapy would have an impact on the prediction of treatment status but also if levels of all proteins correlated with treatment status.
Third, to compare changes in protein levels between FMT and sham-treated patients, we used the mixed ANOVA to explore in-group factors (time points) combined with between-group factors (FMT vs sham) across time points (BL, week 4, week 12, week 26) for each of the inflammation-associated proteins. Finally, we investigated the effect sizes of FMT and add-on treatment with TNFi on protein levels from BL to week 26 using a mixed effect model to investigate whether some of the effects observed in the FMT group could be explained by the initiation of TNFi in more patients allocated to FMT compared with patients allocated to sham.
Results
Patients, FMT donors and HC
BL demographic and disease activity measures for PsA patients, FMT donors and HC are presented in table 1. Additional information are found in the FLORA trial publication on the clinical outcomes.29
Table 1.
Baseline demographics and disease characteristics of healthy controls (HC), FMT donors, and patients with psoriatic arthritis (PsA)
| Characteristic | HC (n=31) | FMT donors (n=4) | PsA patients (n=31) |
| Female sex, number (%) | 20 (65%) | 2 (50%) | 20 (65%) |
| Age, year | * | 37.8 (10.0) | 50.7 (13.6) |
| Body mass index (kg/m2) | – | 23.8 (1.4) | 31.4 (7.2) |
| Time since diagnosis, year† | – | – | 3.7 (0.5 to 8.3) |
| Rheumatoid factor IgM negative, number (%)‡ | – | – | 28 (93%) |
| Anti-citrullinated peptide antibody negative, number (%)‡ | – | – | 30 (100%) |
| HLA-B27 negative, number (%) | – | 4 (100%) | 28 (90%) |
| HAQ-DI§ | – | – | 0.83 (0.50) |
| Swollen joint 66 count | – | – | 7.1 (2.8) |
| SPARCC enthesitis index¶ | – | – | |
| Score≥1, number (%) | – | – | 28 (90%) |
| Score in patients with a score≥1 | – | – | 7.6 (3.8) |
| Tender point count | – | – | 6.8 (4.6) |
| C-reactive protein, mg/L | – | 5.27 (6.43) | |
| Methotrexate | |||
| Oral administration route, number (%) | – | – | 6 (19%) |
| Oral dose, mg/week | – | – | 18.3 (4.1) |
| Subcutaneous administration route, number (%) | – | – | 25 (81%) |
| Subcutaneous dose, mg/week | – | – | 20.0 (3.8) |
Data are mean (SD) or n (%) unless otherwise stated.
*Age of each HC corresponds to a patient matched within a 5-year age intervals: 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, and 65–69. Only one (71-year old) patient was matched with a HC in a lower age category (interval 65–69), since blood donors are retired when they turn 70 years. The lower age limit for HC would be 20–24 years while the upper range limit would be 64–69 years.
†Time since diagnosis of PsA is presented as median and IQR.
‡Presence of rheumatoid factor (IgM) and anti-citrullinated peptide antibody was not accessed in one patient from the FMT group.
§Scores on the Health Assessment Questionnaire Disability Index (HAQ-DI) range from 0 to 3, with higher scores indicating greater disability.
¶SpondyloArthritis Research Consortium of Canada (SPARCC) Enthesitis Index range from 0 to 16, with higher scores indicating more severe disease.
DMARD, disease-modifying anti-rheumatic drug; FMT, faecal microbiota transplantation.
Levels of inflammation-associated plasma proteins in PsA differ markedly from those in HC
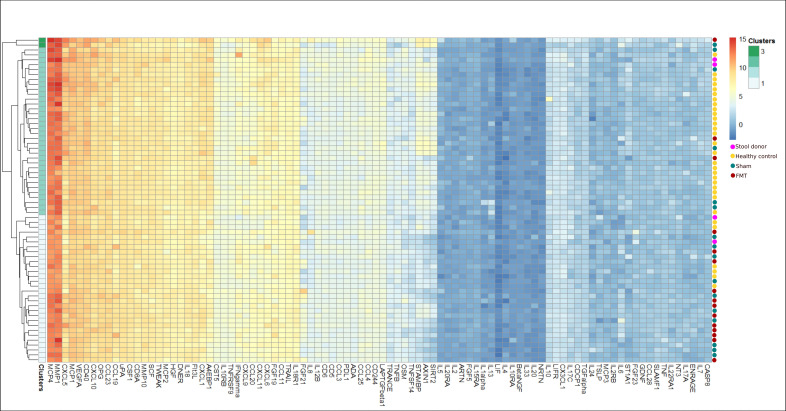
We first performed hierarchical clustering to determine the similarity of the protein profiles of the two PsA groups (sham and FMT) and the two control groups (HC and donors) at BL. The resulting dendrogram and heatmap (online supplemental results, online supplemental figures S1 and S2) showed that the majority of the study population (PsA, HC, donors) was separated into two clusters, one containing mostly patients with 22 out of 31 (71%) and one containing mostly HCs with 27 out of 35 (77%). Two FMT donors were located in the HC dominated cluster while the other two were located in the cluster dominated by PsA patients. Next, we compared protein levels between PsA and HC using Wilcoxon rank sum test. After adjusting for multiple testing, we identified 26 proteins that differed significantly between PsA and HC (table 2). As expected, we did not identify any differences in BL protein levels between PsA patients allocated to FMT and sham (p>0.49). This finding was also confirmed by the heatmap of protein levels in patients with PsA showing an equal BL distribution of patients from each of the treatment arms (figure 1).
Table 2.
Baseline levels of inflammation-associated plasma proteins that significantly differ between patients with PsA and healthy controls (HC)
| Protein | PsA (n=31) |
FMT donors (n=4) |
HC (n=31) |
Difference PsA vs HC (95% CI) |
P values PsA vs HC | Adjusted P values PsA vs HC |
| Increased NPX | ||||||
| IL-6 | 1.44 (0.832, 2.16) | 0.011 (−0.368, 0.479) | 0.336 (0.005, 0.732) | −1.13 (−1.60 to 0.743) | <0.0001 | <0.0001 |
| CCL20 | 6.24 (5.81, 6.92) | 5.84 (5.20, 6.50) | 5.54 (5.20, 5.85) | −0.702 (−1.00 to 0.385) | <0.0001 | 0.0001 |
| CCL19 | 8.08 (7.54, 8.67) | 7.64 (7.02, 8.34) | 7.42 (7.21, 7.77) | −0.625 (−1.07 to 0.280) | <0.001 | 0.0013 |
| CDCP1 | 1.67 (1.42, 1.92) | 0.865 (0.398, 1.26) | 1.23 (0.939, 1.60) | −0.380 (−0.613 to 0.094) | <0.001 | 0.0025 |
| FGF-21 | 4.34 (3.50, 5.08) | 3.26 (2.29, 4.06) | 3.51 (2.81, 4.21) | −0.862 (−1.48 to 0.222) | <0.001 | 0.0027 |
| HGF | 7.15 (6.92, 7.47) | 6.80 (6.38, 7.21) | 6.89 (6.69, 7.12) | −0.259 (−0.465 to 0.067) | <0.001 | 0.0038 |
| IFN-γ | 6.34 (5.88, 7.10) | 6.27 (5.56, 6.29) | 5.82 (5.47, 6.39) | −0.533 (−0.860 to 0.0425) | 0.0027 | 0.013 |
| IL-18R1 | 6.83 (6.56, 7.26) | 6.47 (6.18, 6.78) | 6.65 (6.25, 6.96) | −0.292 (−0.540 to 0.060) | 0.0031 | 0.014 |
| MCP-3 | 0.473 (0.199, 0.808) | 0.165 (−0.183, 0.395) | 0.160 (−0.181, 0.530) | −0.276 (−0.514 to 0.033) | 0.0054 | 0.021 |
| IL-2 | −0.425 (−0.673,–0.209) | −0.367 (−0.593,–0.232) | −0.634 (−0.827,–0.457) | −0.190 (−0.236 to 0.071) | 0.0057 | 0.020 |
| Decreased NPX | ||||||
| 4E-BP1 | 6.58 (6.03, 7.25) | 7.11 (6.06, 8.50) | 8.41 (7.98, 8.70) | 1.67 (0.878 to 1.81) | <0.0001 | <0.0001 |
| STAMBP | 2.92 (2.40, 3.64) | 4.00 (2.86, 5.04) | 4.67 (4.10, 5.17) | 1.65 (0.871 to1.91) | <0.0001 | <0.0001 |
| SIRT2 | 1.72 (1.14, 2.73) | 3.06 (1.73, 4.13) | 3.56 (3.13, 4.50) | 1.81 (0.895 to 2.14) | <0.0001 | <0.0001 |
| Axin-1 | 2.27 (1.61, 3.47) | 2.79 (1.62, 4.11) | 4.31 (3.46, 4.87) | 1.77 (0.833 to 2.21) | <0.0001 | <0.0001 |
| ST1A1 | 0.171 (−0.272, 0.844) | −0.167 (−0.604, 0.772) | 1.33 (0.793, 1.68) | 1.02 (0.237 to 1.19) | <0.0001 | <0.0001 |
| Caspase-8 | 0.494 (0.327, 0.884) | 0.975 (0.165, 1.73) | 0.929 (0.717, 1.14) | 0.381 (−0.017 to 0.406) | <0.0001 | <0.001 |
| TWEAK | 7.61 (7.44, 7.82) | 7.93 (7.54, 8.47) | 7.88 (7.72, 8.12) | 0.279 (0.158 to 0.456) | <0.0001 | <0.001 |
| CD244 | 4.66 (4.49, 4.81) | 4.90 (4.55, 5.24) | 4.96 (4.77, 5.09) | 0.270 (0.087 to 0.377) | <0.0001 | <0.001 |
| Stem cell factor | 7.97 (7.68, 8.14) | 8.14 (8.09, 8.29) | 8.19 (8.00, 8.40) | 0.265 (0.080 to 0.432) | <0.001 | 0.001 |
| CX3CL1 | 2.51 (2.27, 2.78) | 2.72 (2.54, 3.06) | 2.78 (2.59, 3.05) | 0.299 (0.107 to 0.534) | <0.001 | 0.0025 |
| Cystatin D | 5.12 (4.78, 5.36) | 5.33 (5.04, 5.65) | 5.42 (5.04, 5.72) | 0.315 (0.074 to 0.551) | <0.001 | 0.0035 |
| CD40L receptor | 9.53 (9.32, 9.75) | 9.54 (9.35, 9.82) | 9.81 (9.52, 1.02) | 0.291 (−0.009 to 0.450) | 0.0011 | 0.0060 |
| DNER | 7.10 (6.98, 7.23) | 7.33 (7.19, 7.56) | 7.22 (7.10, 7.39) | 0.128 (0.009 to 0.244) | 0.0034 | 0.015 |
| ADA | 3.91 (3.64, 4.18) | 3.98 (3.65, 4.37) | 4.18 (3.92, 4.29) | 0.229 (−0.032 to 0.368) | 0.0037 | 0.015 |
| LAP TGF-beta-1 | 4.53 (4.29, 4.87) | 4.67 (4.23, 5.17) | 4.82 (4.57, 5.04) | 0.262 (−0.006 to 0.460) | 0.0041 | 0.016 |
| MCP-1 | 9.86 (9.56, 10.3) | 9.78 (9.48, 1.05) | 1.01 (9.88, 1.03) | 0.245 (−0.016 to 0.393) | 0.0073 | 0.026 |
P values were adjusted using Benjamini-Hochberg correction. Normalised Protein eXpression (NPX) is an arbitrary value with no units, where a high value corresponds to a higher protein concentration.
ADA, adenosine deaminase; CCL-20, C-C motif chemokine 20; CDCP1, Cub domain-containing protein 1; CX3CL1, Fractalkine; DNER, delta and notch-like epidermal growth factor-related receptor; 4E-BP1, eukaryotic translation initiation factor 4E-binding protein 1; FGF-21, fibroblast growth factor 21; HGF, hepatocyte growth factor; IFN-γ, interferon-γ; IL-6, interleukin 6; IL-18R1, Interleukin-18 receptor 1; LAP TGF-beta-1, Latency-associated peptide transforming growth factor beta 1; MCP-3, monocyte chemotactic protein 3; SIRT2, SIR2-like protein 2; ST1A1, sulfotransferase 1A1; STAMPB, STAM-binding protein; TWEAK, tumour necrosis factor (Ligand) superfamily, member 12.
Figure 1.
Heatmap showing profiles of inflammation-associated plasma protein levels of each PsA patient at baseline using hierarchical clustering. Dendrogram (left) shows four clusters of which two clusters consist of only one patient each. The two remaining clusters contain 7 and 22 patients. Red and blue dots (right) show which treatment group (FMT or sham) each patient was allocated to. Colour gradients show the expression level (Normalised Protein eXpression (NPX)). The equal distribution of samples from each allocation group highlights the baseline homogeneity of inflammation-associated plasma protein profiles between patients receiving FMT (n=15) and sham transplantation (n=16). FMT, faecal microbiota transplantation.
rmdopen-2023-003750supp003.pdf (4.2MB, pdf)
FMT affects inflammation-associated plasma protein levels
In the FMT group, levels of 12 inflammation-associated plasma proteins changed significantly across all timepoints (BL, week 4, week 12, week 26), of which all, but one, showed an overall increase: TNF (p<0.001), Cub domain-containing protein 1 (CDCP1) (p=0.003), TNF superfamily, member 12 (p=0.003), IFN-γ (p=0.004), T-cell surface glycoprotein CD8 alpha chain (p=0.012), T-cell surface glycoprotein CD5 (p=0.018), Fms-related tyrosine kinase 3 ligand (p=0.044), C-C motif chemokine 25 (CCL-25) (p=0.044), fibroblast growth factor 23 (FGF-23) (p=0.045), T-cell surface glycoprotein CD6 (p=0.049) and signalling lymphocytic activation molecule (SLAMF1) (p=0.011) (online supplemental figure S3). Only caspase-8 showed sustained reduced levels following FMT (p=0.045). In the sham group, levels of stem cell factor (SCF) (p=0.006), CCL-3 (p=0.012), CCL-25 (p=0.018), IL-5 (p=0.022), monocyte chemotactic protein 4 (MCP-4) (p=0.029) and C-X-C motif chemokine 5 (CXCL-5) (p=0.043) changed significantly across all time points, all showing an initial decreasing trend from BL to week 12 followed by an increase from week 12 to week 26 (online supplemental results, online supplemental figure S4).
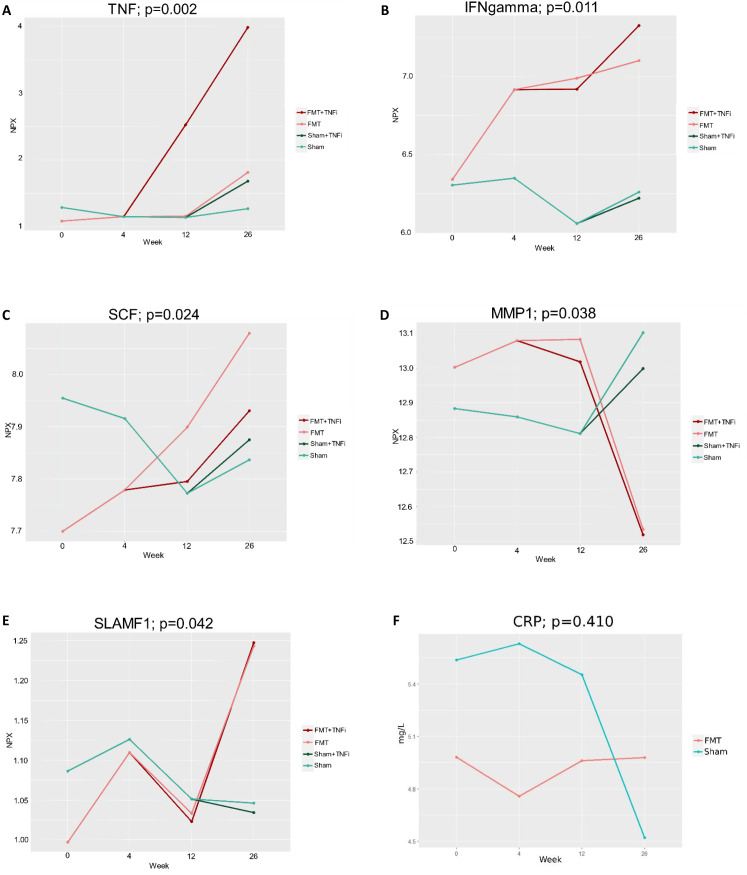
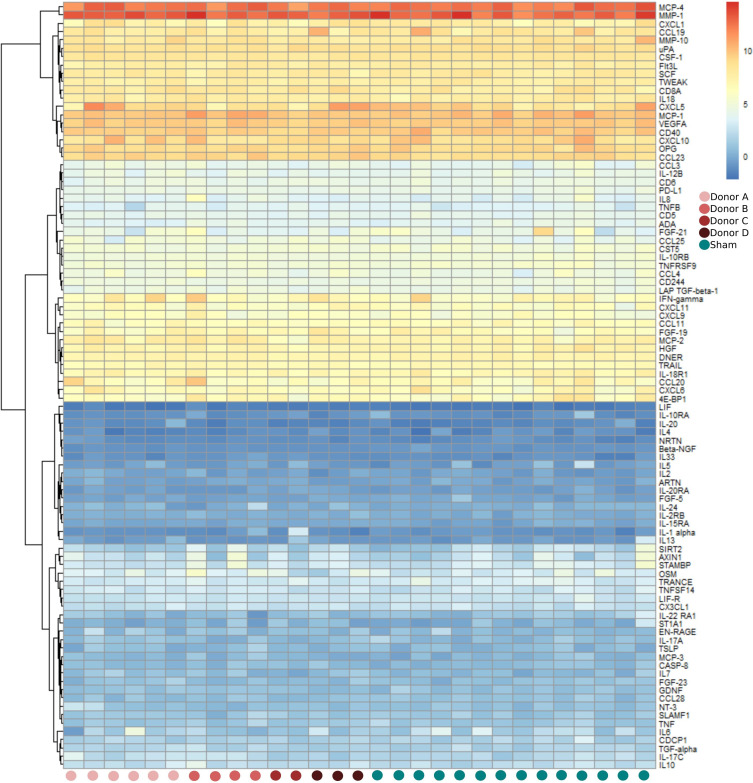
Five proteins differed significantly between FMT and sham-treated patients: TNF (p=0.002), IFN-γ (p=0.011), SCF (p=0.024), matrix metalloproteinase-1 (p=0.038) and SLAMF1 (p=0.042), figure 2 and online supplemental figure S5. To investigate whether some of the effects observed in the FMT group could be explained by treatment with TNFi, we identified the top-five proteins with the largest increase (positive effect size) or decrease (negative effect size) in NPX value as a result of FMT (table 3). Confirming the results from the mixed ANOVA, FMT treatment had the largest positive effect on IFN-γ, signified by sustained, increased levels of this protein following FMT. Figure 3 shows the relation between a patient’s protein profile at week 4 and the corresponding donor, who provided faeces for the patient’s FMT. Based on this heatmap, we observed no clear signals of any (major) systemic immunological donor-specific effects 4 weeks after FMT.
Figure 2.
Post-intervention changes in levels of inflammation-associated proteins and C-reactive protein (CRP) between faecal microbiota transplantation (FMT) and sham-treated patients. To visualise the influence of TNF inhibitor (TNFi) treatment given to the majority of treatment failures, we have divided the groups accordingly, when the first patient received TNFi treatment. P values were obtained from mixed ANOVA (sham vs FMT). Week 12: sham (n=16); sham+TNFi (n=0); FMT (n=10); FMT+TNFi (n=5). Week 26: sham (n=15); sham+TNFi (n=1); FMT (n=9); FMT+TNFi (n=6). NPX, Normalised Protein eXpression.
Table 3.
Effect sizes of faecal microbiota transplantation (FMT) and treatment with TNF inhibitor (TNFi), respectively, on inflammation-associated plasma protein levels (Normalised Protein eXpression (NPX))
| Protein | FMT | TNF |
| Increased NPX | ||
| IFN gamma | 0.523 | 0.642 |
| AXIN1 | 0.452 | −1.075 |
| CCL25 | 0.403 | 0.217 |
| OSM | 0.390 | 0.074 |
| CCL4 | 0.376 | −0.609 |
| Decreased NPX | ||
| CCL19 | −0.443 | −0.274 |
| IL6 | −0.418 | −0.467 |
| FGF21 | −0.358 | −0.174 |
| IL22RA1 | −0.338 | 0.247 |
| TRANCE | −0.326 | 0.003 |
Only top-five proteins with the largest increase (positive effect size) or decrease (negative effect size) in NPX value as a result of FMT are shown in this table.
CCL-25, C-C motif chemokine 25; FGF-21, fibroblast growth factor 21; IFN-γ, interferon-γ; IL-6, interleukin 6; IL-22 RA1, interleukin 22 receptor subunit alpha-1; TRANCE, TNF-related activation-induced cytokine.
Figure 3.
Heatmap showing profiles of inflammation-associated plasma protein levels of each patient at week 4. Faecal microbiota transplantation (FMT)-treated patients are grouped according to their respective FMT donor: FMT donor A, FMT donor B, FMT donor C, or FMT donor D. Red dots represent patients treated with FMT (n=15) while blue dots represent patients treated with sham transplantation (n=14). Samples from two sham-treated patients were not collected (=missing) at this time point.
Relations between clinical disease activity measures and inflammation-associated plasma proteins
Using random forest regression, we investigated relations between BL inflammation-associated plasma proteins and four preselected clinical disease activity measures. The model calculated the pseudo R2 value/variance explained for each disease activity measurement at BL and results showed that for swollen joint count, the pseudo R2 value was −9.94%, for enthesitis score −0.34%, for tender points −4.88% and for HAQ-DI score −6.33%. This means that the variance of the model was not able to predict the sample better than the grand mean. Consequently, we found no significant relation between protein levels and any of the four preselected clinical disease activity measures at BL. Heatmaps showing both variable importance (VImp) and variable interaction (VInt) of all measured proteins for all four models are presented in online supplemental results (online supplemental figure S6).
Discussion
The gut microbiota is an essential modulator of the host immune system. This microbial virtual organ is not only vital for the mucosal immune system’s development, maintenance and operation but also seems to be a crucial environmental component in various immune-mediated pathologies.34 The relative plasticity of the human intestinal milieu further strengthens its potential as an upcoming treatment target being readily modifiable by life-style changes, dietary intervention, surgery, antibiotics and microbiota-based therapies.35 This report is the first to assess, in a randomised controlled setting, the systemic immunological response to FMT in an immune-mediated rheumatic condition. Levels of 12 proteins changed significantly in the FMT-treated patients with PsA. Three of these proteins (TNF, IFN-γ and SLAMF1) as well as SCF and MMP1 differed significantly between FMT and sham-treated patients across all timepoints. The observed increase in TNF was most likely due to the initiation of TNF inhibition in significantly more FMT-treated than sham-treated patients following week 4, as visualised in figure 2A. However, TNFi therapy could not explain the increases observed in IFN-γ and SLAMF1. From the mixed effect model, we further observed that the proteins with the largest negative effect sizes of FMT were CCL19 and IL-6 (ie, lower protein levels relative to sham) while FMT had the largest positive effect (ie, higher protein levels relative to sham) on IFN-γ (effect size=0.523), Axin-1, and CCL25. Interestingly, initiation of TNFi had a similar, positive effect on IFN-γ levels (effect size=0.642) resulting in an additional increase in IFN-γ levels in the FMT group following week 12.
In agreement with previous reports, we observed that IFN-γ levels were higher in patients with PsA as compared with HCs. The pathogenic role of IFN-γ in psoriatic disease has been reconsidered within recent years, now placing it in the early steps of the psoriatic cascade, where it acts as an upstream cytokine in the IL-23/IL-17 pathway.36 Still, the complex role of IFN-γ in PsA has yet to be fully understood. On the one hand, IFN-γ is known to directly inhibit RANKL-mediated osteoclastogenesis. On the other hand, this cytokine may also promote osteoclastogenic factors such as TNF production by macrophages. Moreover, high IFN-γ BL levels have been shown to improve clinical response after Janus kinase inhibition treatment in patients with skin psoriasis.37 Hence, IFN-γ signalling seems to control both activation and inhibition of immune responses that leads to different functional outcomes.38 That IFN-γ may be associated with both successful and unsuccessful clinical outcome in line with our observations, showing elevated IFN-γ levels in both FMT responders and FMT failures.
T lymphocytes and natural killer cells are the principal sources of IFN-γ.39 IFN-γ secreted by CD8+ T lymphocytes located in the lamina propria of the gut can stimulate phagocytic cells to effectively kill microorganisms that have breached the mucosal barrier.40 41 Hence, increased levels of IFN-γ following FMT could be the result of immune-activating microorganisms transferred from the donor to the intestinal environment of the recipient. However, since levels of C reactive protein (figure 2F) and IL-6 (online supplemental results, online supplemental figure S7), which also play a central role in the integrated immune defence network against infections,42 remained largely unchanged 4 weeks after the intervention while IFN-γ levels continued to increase throughout the trial in FMT-treated patients, other mechanisms mediated by changes in microbial metabolites and/or related to the abnormal immune activation in PsA may likely be involved.7 43 The latter hypothesis could explain why previous studies, in contrast to our observations, have reported no changes or decreasing trends in IFN-γ levels in response to FMT in patients with Clostridioides difficile infections24 and ulcerative colitis.15
SLAMF1 was the other protein that markedly increased following FMT. This protein is a co-stimulatory receptor that co-localises with CD3 on T cell activation and affects downstream signalling including secretion of IFN-γ. SLAMF1 has previously been identified in synovial fluid from patients with PsA.44 Although this protein has been proposed to be a key biomarker in the development and progression of rheumatoid arthritis (RA),45 its function in PsA pathogenesis has yet to be determined. To further complicate the interpretation of the overall cytokine/protein profile associated with FMT, we also found that FMT had the largest negative effect (ie, lower protein levels relative to sham) on IL-6 and CCL19 and no significant change in TNF levels from BL to week 4. This is not indicative of an initial net pro-inflammatory effect of FMT in PsA. Based on these observations and the early clinical findings (including a slight reduction in HAQ-DI in the FMT group within the first weeks of FMT),29 we could hypothesise that if one FMT induces (early) anti-inflammatory effects in active PsA, they do not seem to be long-lasting in the majority of patients treated.
FMT may have immune modulating capacities in immune-mediated rheumatic conditions, which has recently been demonstrated in an observational study of 20 patients with systemic lupus erythematosus treated with capsule FMT. In this study, levels of IL-6 and CD4+memory/naïve T ratio in the peripheral blood decreased significantly at week 12.23 Similar anti-inflammatory effects have been reported in a prospective cohort study of 62 patients with ulcerative colitis,15 where levels of TNF and IL-6 decreased significantly in the responder group after FMT. Findings from three other small FMT studies of patients with ulcerative colitis revealed mixed results.18 22 46 Based on these studies, immunological response to FMT may vary due to patient and disease-specific characteristics in addition to donor variability and differences in FMT product types, localisation of administration, dosage, therapy frequency, application of pre-FMT antibiotics and engraftment success.26 Notably, we observed that of the four FMT donors participating in the trial, two of them were located within the HC-dominated cluster, whereas the other two were located in the PsA-dominated cluster at BL. The small study size, however, limits our ability to explore the potential clinical impact of this observation.
Another unexpected finding of this study was that six inflammation-associated plasma proteins (SCF, CCL-3, CCL-25, IL-5, MCP-4 and CXCL-5) changed significantly across all time points following the sham treatment, all showing an initial decreasing trend from BL to week 12 followed by an increase from week 12 to week 26. One explanation for these changes could be that since patients had moderate to high disease activity at time of trial inclusion, these patients would experience some degree of regression towards the mean (clinical improvement) because of the fluctuating nature of PsA. Still, this phenomenon is unlikely to account for all successful cases (13 out of 16) among the sham-treated patients. Consequently, the unexpected clinical improvement observed in the sham group combined with the documented systemic changes following the sham procedure makes us speculate, if the sham transplant—which only consisted of 250 mL brown-coloured saline—or the delivery procedure to the third part of duodenum using gastroscopic guidance could unintentionally have evoked biological (anti-inflammatory) effects. Potentially, the rinsing of the small intestine could have induced changed to the small intestinal microbial environment. Yet, this theory is purely speculative.
Next, in our PsA cohort consisting of MTX-treated PsA patients with moderate to high peripheral disease activity, we identified increased levels of 10 proteins and decreased levels of 16 proteins as compared with age-matched and sex-matched HC pre-FMT. IL-6 was significantly elevated in our patient cohort as compared with HC. This finding is interesting since blocking of this multi-functional interleukin is not currently recommended for PsA.47 A phase IIb RCT has previously demonstrated significantly improved musculoskeletal manifestations (but only minimal changes in skin disease) following IL-6 blockade in active PsA, but without clear evidence of a dose response.48 Increased levels of IL-6 have also been linked to low-grade inflammation in obesity.49 Although data on body mass index (BMI) were not available for the age-matched and sex-matched HCs in the present study, a previous report found that only 10% of healthy Danish blood donors are obese.50 Therefore, differences in BMI between PsA patients and HCs might have contributed to the observed increased IL-6 levels in PsA.
A study comparing serum proteomic signatures between patients with PsA, skin psoriasis, axial spondyloarthritis and HC using 11 Olink panels encompassing 951 proteins (which also included the 92 proteins measured in the current report) found 68 differentially expressed proteins in PsA compared with HCs.27 However, only two of these proteins were assessed by the Olink Inflammation panel (IL-6 and IL-17A). In our study, the higher levels of IL-17A in PsA (unadjusted p=0.042) were no longer significant after adjustment for multiple testing. The main difference to our study cohort was that patients were recruited early after PsA disease onset, typically within 1 year, they had on average fewer swollen joints and lower enthesitis count, and only two were treated with disease-modifying anti-rheumatic drugs.27 Consequently, the elevated levels of IFN-γ, IL-2, CCL20, CCL19, MCP-3 (also known as CCL7), CDCP1, FGF21, hepatocyte growth factor and IL-18R1, which characterised our patient cohort may reflect higher inflammatory activity despite ongoing treatment with MTX and/or longer disease duration. Increased levels of IFN-γ, IL-6, CCL20 and MCP-3 have also been observed in synovial fluid from patients with PsA, further indicating a key role for these proteins in the disease process of PsA.44 Eight of the 16 lowly expressed proteins have previously been found to be low in patients with knee osteoarthritis compared with HCs.51 A reduction of these proteins in both osteoarthritis and PsA could, therefore, indicate shared (downstream) pathogenic mechanism such as hampered bone formation (caspase-8) and activated peripheral and central pain pathways (SIRT2 and SCF).
The main limitation of this study was the small sample size, which limited our ability to perform multiple linear regression and conduct subgroup analysis of responders versus failures. Hence, even though the study demonstrates a number of differentially circulating cytokines between the FMT and sham groups across the time points, larger studies will be needed to explore if these systemic immunological effects can be beneficial to patients with PsA. Furthermore, PEA is a discovery-oriented technology, which allowed us to assess multiple well known and exploratory proteins in parallel. However, the downside of running this predefined panel was that we missed measurements of other potential relevant proteins previously coupled to PsA pathogenesis such as IFN-α, IL-9, IL-23, CCL18 and ICAM-1. Nor was this assay designed to differentiate between inhibited and active TNF following initiation of TNFi therapy.
Furthermore, statistical analysis of 92 proteins entails a considerable issue with multiple testing and comparisons. We used Benjamini-Hochberg correction to reduce type I errors, but by doing so, we might have neglected potential clinically relevant true positive findings. Finally, the comparison of biomarkers between patients with PsA and age and sex-matched HCs could have been affected by other factors than their disease status, including BMI. In addition, patients, but not HC, were fasting at BL. Consequently, we might have underestimated levels of pro-inflammatory cytokines, including IL-6, in the patient cohort as compared with HC.52 Fasting may also have affected levels of FGF21, which could be one explanation for the higher levels of this protein in patient samples as compared with HC.
The gut microbiota is a major factor in maintaining the balance of pro and anti-inflammatory immune responses in hosts. In this study, we identified 26 well-known as well as novel inflammation-associated plasma proteins, which differed significantly between HC and MTX-treated patients with PsA with moderate to high peripheral disease activity. One single-donor FMT applied to duodenum seemed to affect several of these proteins including IFN-γ, SLAMF1, Axin-1, CCL25, CCL19 and IL-6, which highlights that FMT likely induces systemic immune responses in PsA. The long-lasting elevation of IFN-γ was most striking. Whether or not FMT can potentiate the effect of pharmacological immunosuppressants or may induce clinical beneficial effects by targeting microbial drivers of immune-mediated arthritis will be highly relevant to scrutinise in the years to come.
rmdopen-2023-003750supp004.pdf (153.7KB, pdf)
Acknowledgments
We thank all patients, FMT donors, and healthy controls for their important contribution.
Footnotes
MSK and JRBJ contributed equally.
Contributors: MSK, RR and TE conceived and designed the study. MSK and TE were responsible for funding. MSK and ACN were responsible for recruitment of FMT donors and healthy controls. MSK and JK were responsible for the FMT procedure. MSK, TE, HCH, HM and JP were responsible for patient recruitment. TE, HCH and HM acquired the clinical data. BioXpedia A/S laboratory (Aarhus, Denmark) was responsible for laboratory handling and analysis of samples (MK). MSK, TE and RR wrote the statistical analysis plan. JRBJ and RR performed the statistical analyses with input from MSK and TE. MSK, RR, TE, JK, JRBJ, JRM, BHM and KK interpreted the results. MSK drafted the report with input from JRBJ, TE, RR, JRM and BHM. All authors critically reviewed the report and approved the final version. RR and TE are guarantors. The corresponding author (MSK) attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was supported by Sygeforsikringen 'danmark' (2022-0026), Fabrikant Vilhelm Pedersen’s Mindelegat (on recommendation by the Novo Nordisk Foundation), Medicin Fund of the Danish Regions (Regionernes Medicin- og behandlingspulje), University of Southern Denmark Research Fund, the Danish Rheumatism Association, the Danish Psoriasis Research Foundation, and Research Fund of Odense University Hospital. JRM, BHM, and the Division of Digestive Diseases at Imperial College London receive financial and infrastructure support from the NIHR Imperial Biomedical Research Centre (BRC) based at Imperial College Healthcare NHS Trust and Imperial College London. BHM is the recipient of an NIHR Academic Clinical Lectureship (CL-2019-21-002).
Competing interests: BHM has received consultancy fees from Finch Therapeutics Group, outside of the submitted work. JRM has received consultancy fees from Cultech Ltd., and Enterobiotix Ltd., outside of the submitted work.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request. Data will be shared after review and approval by the ethical and scientific board of the study. Terms of collaboration will be reached together with a signed data access agreement. Data access and subsequent data handling must be in agreement with the European General Data Protection Regulation (GDPR).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Regional Committees on Health Research Ethics for Southern Denmark (DK-S-20150080) and the Danish Data Protection Agency (15/41684). Participants gave informed consent to participate in the study before taking part.
References
- 1.Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol 2015;67:128–39. 10.1002/art.38892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson KN, Bonham KS, Ilott NE, et al. Alterations in the gut microbiome implicate key taxa and metabolic pathways across inflammatory arthritis phenotypes. Sci Transl Med 2023;15:eabn4722. 10.1126/scitranslmed.abn4722 [DOI] [PubMed] [Google Scholar]
- 3.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet 2018;391:2273–84. 10.1016/S0140-6736(18)30830-4 [DOI] [PubMed] [Google Scholar]
- 4.Schett G, Rahman P, Ritchlin C, et al. Psoriatic arthritis from a mechanistic perspective. Nat Rev Rheumatol 2022;18:311–25. 10.1038/s41584-022-00776-6 [DOI] [PubMed] [Google Scholar]
- 5.Leung Y-Y, Korotaeva TV, Candia L, et al. Management of peripheral arthritis in patients with psoriatic arthritis: an updated literature review informing the 2021 GRAPPA treatment recommendations. J Rheumatol 2023;50:119–30. 10.3899/jrheum.220315 [DOI] [PubMed] [Google Scholar]
- 6.Kerschbaumer A, Smolen JS, Dougados M, et al. Pharmacological treatment of psoriatic arthritis: a systematic literature research for the 2019 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2020;79:778–86. 10.1136/annrheumdis-2020-217163 [DOI] [PubMed] [Google Scholar]
- 7.Kragsnaes MS, Miguens Blanco J, Mullish BH, et al. Small intestinal permeability and metabolomic profiles in feces and plasma associate with clinical response in patients with active psoriatic arthritis participating in a fecal Microbiota transplantation trial: exploratory findings from the FLORA trial. ACR Open Rheumatol 2023;5:583–93. 10.1002/acr2.11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kragsnaes MS, Nilsson AC, Kjeldsen J, et al. How do I establish a stool bank for fecal microbiota transplantation within the blood- and tissue transplant service Transfusion 2020;60:1135–41. 10.1111/trf.15816 [DOI] [PubMed] [Google Scholar]
- 9.Danne C, Rolhion N, Sokol H. Recipient factors in faecal microbiota transplantation: one stool does not fit all. Nat Rev Gastroenterol Hepatol 2021;18:503–13. 10.1038/s41575-021-00441-5 [DOI] [PubMed] [Google Scholar]
- 10.Ianiro G, Punčochář M, Karcher N, et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat Med 2022;28:1913–23. 10.1038/s41591-022-01964-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt TSB, Li SS, Maistrenko OM, et al. Drivers and determinants of strain dynamics following fecal microbiota transplantation. Nat Med 2022;28:1902–12. 10.1038/s41591-022-01913-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisbee AL, Petri WA. Considering the immune system during fecal microbiota transplantation for clostridioides difficile infection. Trends Mol Med 2020;26:496–507. 10.1016/j.molmed.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng L, Deng Y, Yang K, et al. Safety and efficacy of fecal microbiota transplantation for autoimmune diseases and autoinflammatory diseases: a systematic review and meta-analysis. Front Immunol 2022;13:944387. 10.3389/fimmu.2022.944387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goloshchapov OV, Olekhnovich EI, Sidorenko SV, et al. Long-term impact of fecal transplantation in healthy volunteers. BMC Microbiol 2019;19:312. 10.1186/s12866-019-1689-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Huang Z, Ding L, et al. Fecal Microbiota transplantation versus glucocorticoids for the induction of remission in mild to moderate ulcerative colitis. J Transl Med 2022;20:354. 10.1186/s12967-022-03569-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al KF, Craven LJ, Gibbons S, et al. Fecal Microbiota transplantation is safe and tolerable in patients with multiple sclerosis: a pilot randomized controlled trial. Mult Scler J Exp Transl Clin 2022;8:20552173221086662. 10.1177/20552173221086662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A, Roy A, Premkumar M, et al. Fecal Microbiota transplantation in alcohol-associated acute-on-chronic liver failure: an open-label clinical trial. Hepatol Int 2022;16:433–46. 10.1007/s12072-022-10312-z [DOI] [PubMed] [Google Scholar]
- 18.Crothers JW, Chu ND, Nguyen LTT, et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study. BMC Gastroenterol 2021;21:281. 10.1186/s12876-021-01856-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Lu Y, Yan Y, et al. Promising treatment for type 2 diabetes: fecal microbiota transplantation reverses insulin resistance and impaired islets. Front Cell Infect Microbiol 2019;9:455. 10.3389/fcimb.2019.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groot P, Scheithauer T, Bakker GJ, et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut 2020;69:502–12. 10.1136/gutjnl-2019-318320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konturek PC, Koziel J, Dieterich W, et al. Successful therapy of clostridium difficile infection with fecal microbiota transplantation. J Physiol Pharmacol 2016;67:859–66. [PubMed] [Google Scholar]
- 22.Zhang T, Cui B, Li P, et al. Short-term surveillance of cytokines and C-reactive protein cannot predict efficacy of fecal microbiota transplantation for ulcerative colitis. PLoS ONE 2016;11:e0158227. 10.1371/journal.pone.0158227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Yi P, Zhu M, et al. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: an EXPLORER trial. J Autoimmun 2022;130:102844. 10.1016/j.jaut.2022.102844 [DOI] [PubMed] [Google Scholar]
- 24.Monaghan T, Mullish BH, Patterson J, et al. Effective fecal microbiota transplantation for recurrent clostridioides difficile infection in humans is associated with increased signalling in the bile acid-Farnesoid X receptor-fibroblast growth factor pathway. Gut Microbes 2019;10:142–8. 10.1080/19490976.2018.1506667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monaghan TM, Duggal NA, Rosati E, et al. A multi-factorial observational study on sequential fecal microbiota transplant in patients with medically refractory clostridioides difficile infection. Cells 2021;10:3234. 10.3390/cells10113234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alam MZ, Maslanka JR, Abt MC. Immunological consequences of microbiome-based therapeutics. Front Immunol 2022;13:1046472. 10.3389/fimmu.2022.1046472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leijten E, Tao W, Pouw J, et al. Broad proteomic screen reveals shared serum proteomic signature in patients with psoriatic arthritis and psoriasis without arthritis. Rheumatology (Oxford) 2021;60:751–61. 10.1093/rheumatology/keaa405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandran V, Cook RJ, Edwin J, et al. Soluble biomarkers differentiate patients with psoriatic arthritis from those with psoriasis without arthritis. Rheumatology (Oxford) 2010;49:1399–405. 10.1093/rheumatology/keq105 [DOI] [PubMed] [Google Scholar]
- 29.Kragsnaes MS, Kjeldsen J, Horn HC, et al. Safety and efficacy of faecal microbiota transplantation for active peripheral psoriatic arthritis: an exploratory randomised placebo-controlled trial. Ann Rheum Dis 2021;80:1158–67. 10.1136/annrheumdis-2020-219511 [DOI] [PubMed] [Google Scholar]
- 30.Coates LC, Conaghan PG, Emery P, et al. Sensitivity and specificity of the classification of psoriatic arthritis criteria in early psoriatic arthritis. Arthritis Rheum 2012;64:3150–5. 10.1002/art.34536 [DOI] [PubMed] [Google Scholar]
- 31.Bioxpedia . Innovative biomarker discovery and validation. Available: https://www.bioxpedia.com/olink-proteomics/ [Accessed 27 Sep 2022].
- 32.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-Plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One 2014;9:e95192. 10.1371/journal.pone.0095192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inglis A, Parnell A, Hurley CB. Visualizing variable importance and variable interaction effects in machine learning models. Journal of Computational and Graphical Statistics 2022;31:766–78. 10.1080/10618600.2021.2007935 [DOI] [Google Scholar]
- 34.Ruff WE, Greiling TM, Kriegel MA. Host-Microbiota interactions in immune-mediated diseases. Nat Rev Microbiol 2020;18:521–38. 10.1038/s41579-020-0367-2 [DOI] [PubMed] [Google Scholar]
- 35.Belvoncikova P, Maronek M, Gardlik R. Gut dysbiosis and fecal microbiota transplantation in autoimmune diseases. Int J Mol Sci 2022;23:10729. 10.3390/ijms231810729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiricozzi A, Romanelli P, Volpe E, et al. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci 2018;19:179. 10.3390/ijms19010179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincken NLA, Welsing PMJ, Silva-Cardoso SC, et al. Suppression of IL-12/IL-23 P40 subunit in the skin and blood of psoriasis patients by tofacitinib is dependent on active interferon-Γ signaling in dendritic cells: implications for the treatment of psoriasis and interferon-driven diseases. Exp Dermatol 2022;31:962–9. 10.1111/exd.14566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skougaard M, Ditlev SB, Søndergaard MF, et al. Cytokine signatures in psoriatic arthritis patients indicate different phenotypic traits comparing responders and non-responders of IL-17A and TNFα inhibitors. Int J Mol Sci 2023;24:6343. 10.3390/ijms24076343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai H, Adamopoulos IE. Psoriatic arthritis under the influence of IFNγ. Clin Immunol 2020;218:108513. 10.1016/j.clim.2020.108513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasetti MF, Simon JK, Sztein MB, et al. Immunology of gut mucosal vaccines. Immunol Rev 2011;239:125–48. 10.1111/j.1600-065X.2010.00970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gogokhia L, Buhrke K, Bell R, et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 2019;25:285–99. 10.1016/j.chom.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol 2017;13:399–409. 10.1038/nrrheum.2017.83 [DOI] [PubMed] [Google Scholar]
- 43.Zhao H, Wang D, Zhang Z, et al. Effect of gut microbiota-derived metabolites on immune checkpoint inhibitor therapy: enemy or friend Molecules 2022;27:4799. 10.3390/molecules27154799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbarroja N, López-Montilla MD, Cuesta-López L, et al. Characterization of the inflammatory proteome of synovial fluid from patients with psoriatic arthritis: potential treatment targets. Front Immunol 2023;14:1133435. 10.3389/fimmu.2023.1133435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li A, Zhang Z, Ru X, et al. Identification of SLAMF1 as an immune-related key gene associated with rheumatoid arthritis and verified in mice collagen-induced arthritis model. Front Immunol 2022;13:961129. 10.3389/fimmu.2022.961129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponce-Alonso M, García-Hoz C, Halperin A, et al. An immunologic compatibility testing was not useful for donor selection in fecal microbiota transplantation for ulcerative colitis. Front Immunol 2021;12:683387. 10.3389/fimmu.2021.683387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kastrati K, Aletaha D, Burmester GR, et al. A systematic literature review informing the consensus statement on efficacy and safety of pharmacological treatment with Interleukin-6 pathway inhibition with biological Dmards in immune-mediated inflammatory diseases. RMD Open 2022;8:e002359. 10.1136/rmdopen-2022-002359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mease PJ, Gottlieb AB, Berman A, et al. The efficacy and safety of clazakizumab, an anti-Interleukin-6 monoclonal antibody, in a phase IIb study of adults with active Psoriatic arthritis. Arthritis Rheumatol 2016;68:2163–73. 10.1002/art.39700 [DOI] [PubMed] [Google Scholar]
- 49.Kumthekar A, Ogdie A. Obesity and psoriatic arthritis: a narrative review. Rheumatol Ther 2020;7:447–56. 10.1007/s40744-020-00215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaspersen KA, Pedersen OB, Petersen MS, et al. Obesity and risk of infection: results from the Danish blood donor study. Epidemiology 2015;26:580–9. 10.1097/EDE.0000000000000301 [DOI] [PubMed] [Google Scholar]
- 51.Giordano R, Petersen KK, Andersen HH, et al. Serum inflammatory markers in patients with knee osteoarthritis: a proteomic approach. Clin J Pain 2020;36:229–37. 10.1097/AJP.0000000000000804 [DOI] [PubMed] [Google Scholar]
- 52.Almeneessier AS, BaHammam AA, Alzoghaibi M, et al. The effects of diurnal intermittent fasting on proinflammatory cytokine levels while controlling for sleep/wake pattern, meal composition and energy expenditure. PLoS One 2019;14:e0226034. 10.1371/journal.pone.0226034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003750supp001.pdf (171.3KB, pdf)
rmdopen-2023-003750supp002.pdf (12.2MB, pdf)
rmdopen-2023-003750supp003.pdf (4.2MB, pdf)
rmdopen-2023-003750supp004.pdf (153.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available upon reasonable request. Data will be shared after review and approval by the ethical and scientific board of the study. Terms of collaboration will be reached together with a signed data access agreement. Data access and subsequent data handling must be in agreement with the European General Data Protection Regulation (GDPR).