Abstract
Ticks are important vectors of zoonotic diseases and play a major role in the circulation and transmission of many rickettsial species. The aim of this study was to investigate the carriage of Candidatus Rickettsia tarasevichiae (CRT) in a total of 1168 ticks collected in Inner Mongolia to elucidate the potential public health risk of this pathogen, provide a basis for infectious disease prevention, control and prediction and contribute diagnostic ideas for clinical diseases that present with fever in populations exposed to ticks. A total of four tick species, Haemaphysalis concinna (n = 21), Dermacentor nuttalli (n = 122), Hyalomma marginatum (n = 148), and Ixodes persulcatus (n = 877), were collected at nine sampling sites in Inner Mongolia, China, and identified by morphological and molecular biological methods. Reverse transcription PCR targeting the 16S ribosomal RNA (rrs), gltA, groEL, ompB and Sca4 genes was used to detect CRT DNA. Sequencing was used for pathogen species confirmation. The molecular epidemiological analysis showed that three species of ticks were infected with CRT, and the overall positive rate was as high as 42%. The positive rate of I. persulcatus collected in Hinggan League city was up to 96%, and that of I. persulcatus collected in Hulun Buir city was 50%. The pool positive rates of D. nuttalli and H. marginatum collected in Bayan Nur city and H. concinna collected in Hulun Buir city were 0%, 28% and 40%, respectively. This study revealed the high prevalence of CRT infection in ticks from Inner Mongolia and the first confirmation of CRT detected in H. marginatum in China. The wide host range and high infection rate in Inner Mongolia may dramatically increase the exposure of CRT to humans and other vertebrates. The role of H. marginatum in the transmission of rickettsiosis and its potential risk to public health should be further considered.
Introduction
Rickettsia species are a group of important, obligate intracellular, vector-borne pathogens that cause rickettsioses through arthropod vectors such as fleas, ticks, mites or lice [1–3]. Rickettsial diseases constitute a significant public health concern and impose a substantial economic burden on a global scale [3]. Candidatus Rickettsia tarasevichiae (CRT), an emerging tick-borne human pathogen, was initially detected in Ixodes persulcatus collected from various regions of Russia, including western Siberia, eastern Siberia, and the southern Urals in 2003 [4]. Since then, it has been detected in I. persulcatus, Haemaphysalis japonica douglasi, Dermacentor silvarum, Ixodes pavlovskyi, Ixodes trianguliceps and Haemaphysalis concinna within the territory of Russia [5–9]. The presence of CRT has been further documented in I. persulcatus in Estonia [10], Japan [11, 12], Mongolia [13], Korea [14] and the Chinese-Russian border [6]. It has also been detected in rodents and ticks in the northeastern region of China [15–18]. Few cases have been reported in Inner Mongolia, probably due to misdiagnosis resulting from a lack of understanding of CRT. This means that there is a wide range of invertebrate hosts infected with CRT, which threatens human health in the context of increased human activity in wildlife habitats. Despite the limited reports on CRT in Inner Mongolia [19, 20], this region is of great importance due to its vast land area, ecological and environmental diversity, and rich tick species reserve. Inner Mongolia encompasses three distinct ecological zones, including forest, grassland, and Gobi and semi desertification steppe areas from east to west, which makes it a unique and complex environment for tick-borne diseases. Furthermore, the region spans three climatic zones, from east to west, including temperate semi humid, semi arid, and arid zones, which also contribute to the diversity of tick species and their distribution. Therefore, further research is needed to understand the distribution and prevalence of CRT in Inner Mongolia, which could provide valuable insights into the epidemiology and control of tick-borne diseases in China.
However, recent studies have shown that CRT is pathogenic to humans and can even cause death in humans [21–23]. Human infection with CRT was first reported in northeastern China in 2012 [24]. In 2014, eight patients in eastern central China who were infected with CRT and presented with symptoms resembling severe fever with thrombocytopenia syndrome (SFTS) died as a result of misdiagnosis [22]. In May 2017, a fatal case of tick-borne rickettsiosis caused by mixed Rickettsia sibirica subsp. sibirica and CRT infection was reported in Russia [23].
Ticks are considered second to mosquitoes as vectors of human diseases in the world and can carry and transmit hundreds of pathogens, including viruses, bacteria, protozoa and helminths [25, 26]. The prevalence and biodiversity of Rickettsia spp. in I. persulcatus, H. concinna, H. douglasi, Dermacentor nuttalli and D. silvarum ticks from different regions in Inner Mongolia were examined in our previous study [19, 27, 28]. However, there is a lack of research data on CRT, and it is necessary to investigate the status of CRT infection in Inner Mongolia. To this end, we conducted screening for CRT infection in ticks collected from regions where there have been previous reports of tick-borne pathogens (TBPs) [29].
Materials and methods
Ethics statement
The collection of ticks from the body surface of cattle, sheep and goats in this study was verbally approved by the animals’ owners and performed in strict accordance with the National Guidelines for Experimental Animal Welfare of China (2006–398). In addition, this study was reviewed and approved by the Medical Ethics Committee of Inner Mongolia Medical University (No. YKD202302084).
Sample collection and tick species identification
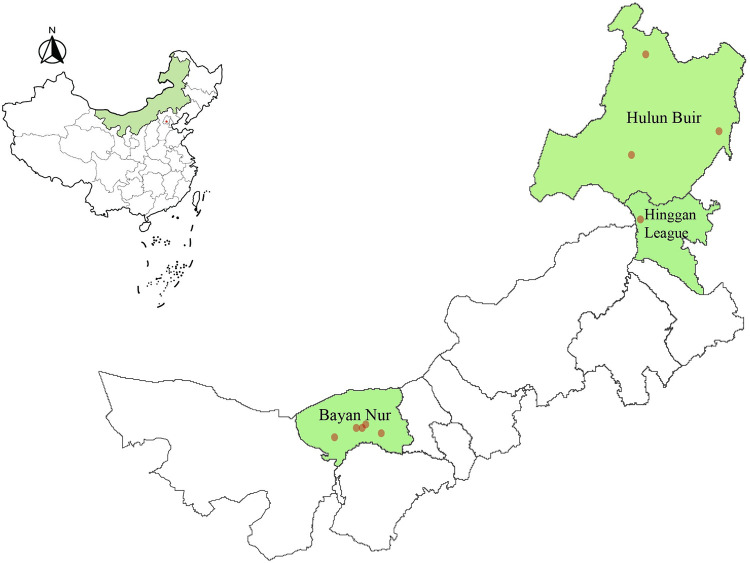
From May 2021 to May 2023, ticks were collected at nine sampling sites with relatively high tick population densities in three central cities, Hulun Buir, Hinggan League, and Bayan Nur, in Inner Mongolia, China (Fig 1 and Table 1). Ticks were collected directly from domestic animals (cattle, sheep and goats) and grasslands and then brought back to the laboratory alive. The tick species were initially identified based on their morphological characteristics, such as coxae, scutum, genital opening, anal aperture and spiracular plates, using stereomicroscopes [30–34]. The classification was further confirmed through gene sequencing of cytochrome c oxidase subunit 1 (COI) [35] or small subunit 16 S ribosomal RNA (rrs) [36]. Following morphological identification, ticks were stored at −80°C until nucleic acid extraction.
Fig 1. Map of tick collection sites in Inner Mongolia, China.
Map is created using the Software program ArcGIS. Map source: Xinliang Xu. Multi-year administrative division boundary data of Chinese cities and municipalities. Resource and Environmental Science Data Registration and Publication System. (http://www.resdc.cn/DOI), 2023. DOI:10.12078/2023010102.
Table 1. Tick sampling information.
| Species of ticks | Group | Animal/Geomorphy | Region | Tick (n) | Coordinate | |
|---|---|---|---|---|---|---|
| Longitude | Latitude | |||||
| Ixodes persulcatus | D | Forest shrub | HingganLeague | 617 | 120°6’ | 47°3’ |
| Dermacentor nuttalli | B | Goat/Sheep | BayanNur | 118 | 109°36’ | 40°55’ |
| 3 | 107°43’ | 41°7’ | ||||
| 1 | 107°59’ | 41°7’ | ||||
| Hyalomma marginatum | E | Goat | BayanNur | 136 | 109°36’ | 40°55’ |
| 3 | 107°43’ | 41°7’ | ||||
| 2 | 107°59’ | 41°7’ | ||||
| 1 | 106°24’ | 40°33’ | ||||
| 6 | 108°32’ | 41°19’ | ||||
| Ixodes persulcatus | K | Forest shrub | HulunBuir | 176 | 121°51’ | 51°41’ |
| 84 | 120°42’ | 49°17’ | ||||
| Haemaphysalis concinna | G | Cattle/Dog | HulunBuir | 21 | 124°35’ | 49°45’ |
| Total | 1168 | |||||
Nucleic acid extraction
To eliminate potential impurities and pathogens from the surface of ticks, each tick was immersed in 75% ethanol for three minutes, washed three times with phosphate-buffered saline (PBS), and finally air-dried on sterile filter paper. After being divided into 130 tick pools of 1–50 ticks depending on feeding status and collection location, the ticks were crushed in a mortar with a sterile pestle at a low temperature maintained by liquid nitrogen and placed in a 1.5 ml centrifuge tube. Total RNA was extracted from each tick pool sample using the TransZol Up Plus RNA Kit (TransGen Biotech, China), and cDNA was then obtained using TransScript® II One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech, China) according to the instructions provided by the manufacturer.
Detection of pathogens
cDNA extracted from ticks was examined using polymerase chain reaction (PCR) targeting the rrs gene, and the amplified gene fragments were sequenced to detect and identify Rickettsiales bacteria in the ticks. For further identification of CRT, the rrs-positive tick samples were further amplified by PCR targeting the gltA, groEL, ompB and Sca4 genes and sequenced. DNA samples were considered CRT-positive when at least two of these four genes were positive.
DNA was amplified using a system of 20 μl, including 11 μL PCR Master Mix (TianGen, China), 1 μl of DNA from each sample, and 0.5 μl each of reverse and forward primer, and filled to volume with double-distilled water. Negative and positive controls were included in each PCR. The amplified PCR products were separated on a 1.5% agarose gel and purified using the Gel DNA Recovery Kit (TianGen, China) according to the manufacturer’s instructions for sequencing. Oligo7 (https://www.oligo.net/downloads.html) was used to design specific primers. The synthesis of primers and sequencing were performed by Sangon Biotech (Shanghai, China). The primers used for amplification are listed in Table 2.
Table 2. Primer sequence list for Candidatus Rickettsia tarasevichiae detection.
| Gene | Name | Primer sequence | Annealing temperature(°C) | Amplicon size(bp) |
|---|---|---|---|---|
| gltA | F | GTCGGTTCTCTTTCAGCATT | 57 | 311 |
| R | CCGGCAATTCTTACTGTTGA | 57 | ||
| ompB | F | GCAACAACTACAGGAACCAC | 56 | 254 |
| R | AACTGGCTACTTCCGATAGC | 56 | ||
| 16S rRNA | F | TGATCCAGCAATACCGAGT | 56 | 382 |
| R | TGATCCAGCAATACCGAGT | 55 | ||
| groEL | F | AGGTCCAAAAGGAAGAAACG | 56 | 354 |
| R | GTACCGACCTGTGCTATTTC | 56 | ||
| sca4 | F | CACTGCTCACTACGAAGAAG | 56 | 358 |
| R | TGAAGATTCAGCTTGTTGCA | 56 |
Phylogenetic analysis
The BLAST nucleotide collection database (nr/nt) was utilized to identify homologies in our gene sequences. The acquired nucleotide sequences from Rickettsiae target genes were edited and assembled using MEGA software version 7.0, while MAFFT v.7.266 was used for multiple nucleotide sequence alignment [37, 38]. Phylogenetic trees were constructed using the maximum likelihood (ML) approach of version 7.0 of the MEGA program, and bootstrap analysis with 1000 replicates was performed to evaluate branch reliability. Branches with values greater than 70% support were deemed significantly different for presentation. The phylogenetic trees were modified and visualized using FigTree v.1.4.3.
Statistical analysis
The gathered data were statistically analyzed using the Statistical Package for Social Sciences Version 21.0 software (SPSS, Chicago, IL, USA). To determine the differences in the positive rate of rickettsiae, the p value was calculated using the Chi-square test or Fisher’s exact test. The statistical significance level was set at p < 0.05.
Results
Species and distribution of the collected ticks
In Inner Mongolia, 1,168 adult ticks were collected from the nine sampling regions of Hulun Buir, Hinggan League, and Bayan Nur. The ticks were all classified as one of four species belonging to four different genera based on their morphological characteristics and gene sequencing data, including H. concinna 2% (21/1168), D. nuttalli 10% (122/1168), H. marginatum 13% (148/1168), and I. persulcatus 75% (877/1168) (Fig 1 and Table 1). Based on the sample point locations, only I. persulcatus 53% (617/1168) were found in Hinggan League. D. nuttalli (10%, 122/1168) and H. marginatum (13%, 148/1168) were discovered in Bayan Nur. I. persulcatus 22% (260/1168) and H. concinna 2% (21/1168) were collected in Hulun Buir (Fig 1 and Table 1). A total of 130 tick pools were created based on region, species, and feeding status for all ticks included in the study. Sequences were entered in GenBank with accession numbers OR272248-OR272253, OR294053-OR294057, OQ852069 and OQ852073.
The phylogenetic trees shown in Fig 2A and 2B are based on the sequences of the rrs and COI genes. The sequences acquired in the present study were clustered with their respective homologs, corresponding to the four species (Fig 2).
Fig 2. Identification of the ticks based on phylogenetic analysis with the rrs gene and COI gene.
The red font indicates the representative sequences from the ticks in the different sampling regions.
Rickettsia bacteria detected in ticks
rrs gene-positive samples were utilized for the identification of CRT-specific segments of the gltA, ompB, gloEL, and sca4 genes. The RT‒PCR results revealed a total positivity rate of 42% (55/130), with the highest pool positivity rate observed in the entire I. persulcatus tick population collected from Hinggan League at 96% (25/26). Additionally, the pool positivity rates for H. marginatum ticks collected from Bayan Nur and for both I. persulcatus and H. concinna ticks collected from Hulun Buir were 28% (14/50), 50% (7/14) and 40% (4/10), respectively. However, no simultaneous detection of two or more CRT-specific genes was observed in any pool of D. nuttalli ticks collected in Bayan Nur (Table 3).
Table 3. Prevalence of Candidatus Rickettsia tarasevichiae detected in tick samples collected in Inner Mongolia, China.
| Parameters | NO.tested (n/ %) | The pool positivity rate (n/ %) | X2-value | P |
|---|---|---|---|---|
| Region | ||||
| HingganLeague | 26/ 20% | 25/ 96% | 51.965 | <0.001 |
| BayanNur | 80/ 62% | 14/ 18% | ||
| HulunBuir | 24/ 18% | 11/46% | ||
| Species of ticks | ||||
| Dermacentor nuttalli | 30/ 23% | 0/ 0% | 50.232 | <0.001 |
| Hyalomma marginatum | 50/ 38% | 14/ 28% | ||
| Ixodes persulcatus | 40/ 31% | 32/ 80% | ||
| Haemaphysalis concinna | 10/ 8% | 4/ 40% | ||
| Species / Region | ||||
| Ixodes persulcatus/HingganLeague | 26/ 20% | 25/ 96% | 58.422 | <0.001 |
| Dermacentor nuttalli/BayanNur | 30/ 23% | 0/ 0% | ||
| Hyalomma marginatum/BayanNur | 50/ 38% | 14/ 28% | ||
| Ixodes persulcatus/HulunBuir | 14/ 11% | 7/ 50% | ||
| Haemaphysalis/concinnaHulunBuir | 10/ 8% | 4/ 40% | ||
| Total(pools) | 130 | 55/ 42% | ||
Analyzing the regional distribution of positive samples, there were differences in the pool positivity rates among the sampling sites. The highest positive rate was found in Hinggan League (25/26, 96%), followed by Hulun Buir (11/24, 46%) and Bayan Nur (14/80, 18%). The analysis results of the collected tick species showed that there were also differences in infection rates among the four different tick species from the genus Ixodes. CRT was the most widely infected and had the highest pool positivity rate among the I. persulcatus ticks (32/40, 80%), followed by H. concinna ticks (4/10, 40%) and H. marginatum ticks (14/50, 28%), and was not detected among the D. nuttalli ticks collected in the present study.
Genetic and phylogenetic analysis
All CRT gene sequences obtained in this study have been deposited into GenBank under accessions OR454091-OR454142, OR449962-OR449989 and OR712487-OR712557. Sequence BLAST analysis showed that the nucleotide similarity between the gltA, groEL, ompB and sca4 genes that we obtained and the corresponding sequences of CRT available in GenBank were 99.55%-100%, 100%, 100%, and 100%, respectively. The nucleotide similarity of all sequences obtained in this investigation to the published sequences on NCBI exceeds 99%.
The ompB gene sequences in this study exhibited the highest similarity with Pr-7477 (OP722685.1) from I. persulcatus in the Far East, Primorsky Krai, Russia [39], while the gltA and groEL gene sequences showed the greatest resemblance to Bayan-68 (MN450397.2, MN450404.2) from I. persulcatus in Harbin, China [40]. Furthermore, the sca4 gene sequence of the Om-111 (OQ540735.1) isolate of I. persulcatus discovered in Western Siberia’s Omsk Province shared a high degree of similarity with the sca4 gene sequences identified in this study.
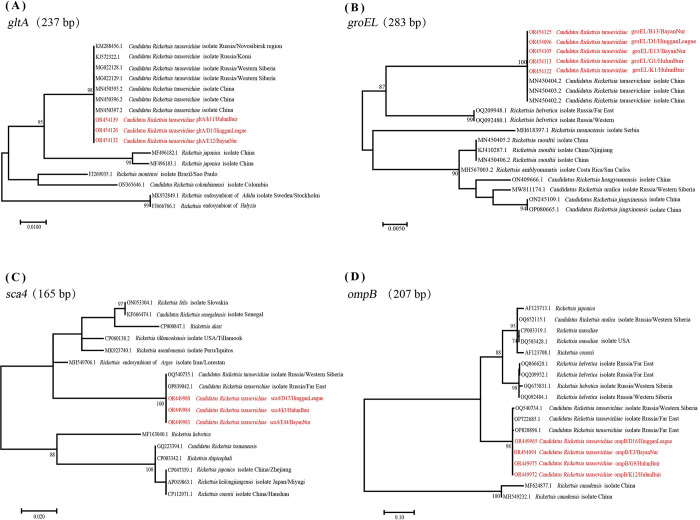
In the phylogenetic trees constructed in MEGA7 using maximum likelihood (ML) based on the gltA, groEL, ompB, and sca4 genes, the sequences representative of this study were clustered with the corresponding published CRT sequences obtained in NCBI (Fig 3).
Fig 3. Phylogeneic tree based on the gltA, groEL, ompB and sca4 genes of CRT.
The genetic identity among different Rickettsia species was inferred by the maximum-likelihood method implemented in MEGA7 and rooted by the midpoint method. The red font indicates the nucleotide sequences of the gltA, groEL, ompB and sca4 gene segments from CRT in the different sampling regions.
Discussion
In this study, the distribution and prevalence of CRT among four tick species collected from nine sampling sites in three leagues in Inner Mongolia were investigated. A total of 1168 ticks, including D. nuttalli, I. persulcatus, H. concinna, and H. marginatum, were collected for this purpose in northern China. Inner Mongolia covers an area of 1,183,000 square kilometers, accounting for 12.3% of China’s land area. The suitable climatic conditions and geographical advantages provide advantages for the development of animal husbandry as well as a good living environment for ticks. Furthermore, the borders of Inner Mongolia with Mongolia and Russia facilitate cross-regional transmission of tick-borne pathogens. Since CRT was first identified in I. persulcatus collected in Russia [4] and subsequently found to be widely distributed in this species, many I. persulcatus ticks were collected for this study. D. nuttalli, a dominant tick species in Inner Mongolia, is known to have a wide distribution and can carry various species of Rickettsia [28, 29]. In a survey of microbial diversity carried by D. nuttalli ticks collected in Hulun Buir, CRT was reported to be present [20, 27]. However, there is limited research on CRT in H. concinna and H. marginatum. CRT infection has been reported in H. concinna [41], but there have been no reports of CRT carriage and transmission by H. marginatum. The Sca family is one of the largest protein families in Rickettsia. Certain members of the family, such as ompB and sca4, are known to be antigenic determinants of Spotted Fever Group (SFG) or Typhus Group (TG) rickettsiae [42]. The gltA and groEL genes have also been proven to be useful for the molecular identification and characterization of Rickettsia [43, 44]. In this study, specific primers were used to screen the gltA, ompB, groEL, and sca4 genes of CRT in the samples. The presence of CRT infection was demonstrated in H. marginatum ticks collected from Bayan Nur in Inner Mongolia, marking the first detection of CRT in H. marginatum. This finding may have important implications for the prevention and control of tick-borne rickettsioses caused by CRT infection in the local population.
Different species of ticks and different sampling locations showed variations in the prevalence of CRT. Among the four tick species collected in this study, I. persulcatus had the highest infection rate of CRT (80%). Pathogens were detected in I. persulcatus samples collected from Hinggan League (96%) and Hulun Buir (46%) cities. The next highest infection rate was observed in H. concinna ticks collected from Hulun Buir (40%), followed by H. marginatum ticks collected from the Bayan Nur region (28%). No CRT infection was detected in D. nuttalli ticks collected from the same region. Previous reports have identified CRT in various tick species, including I. persulcatus, H. japonica douglasi, D. silvarum, I. pavlovskyi, I. trianguliceps, and H. concinna, within the territory of Russia [5–9]. In neighboring Mongolia, the infection rate of CRT in I. persulcatus ranged from 19.5% to 46.6% [13], which is consistent with our research findings. It has also been reported that CRT was detected in 5.42% of D. nuttalli ticks in Hulun Buir, Inner Mongolia [20]. However, we did not detect CRT infection in D. nuttalli ticks, possibly due to differences in climate and environment between the sampling sites. The Hulun Buir sampling site is located in the northwest, adjacent to Russia and Mongolia, while the southeastern region is adjacent to Heilongjiang Province. These areas share similar natural environments and habitats, which may facilitate the cross-regional transmission of the pathogen. The differences in CRT infection rates observed in H. marginatum ticks collected from the Bayan Nur region may be attributed to specific collection locations and differences in the host animals they inhabit. These results provide epidemiological data to support the prevention and control of ticks and tick-borne diseases in Inner Mongolia, China, and contribute to our understanding of the epidemiology of CRT.
The gltA and groEL genes obtained in this study showed the highest similarity to the gltA and groEL genes isolated from Bayan-68 of I. persulcatus in Harbin, China. Similarly, the ompB and sca4 gene sequences were most similar to the ompB and sca4 genes of CRT isolated from Russia. This similarity may be due to the proximity of Inner Mongolia to northeastern China and the Far East/Siberia region of Russia, which share similar natural environments and habitats. Additionally, there are limited genetic data available for the ompB and sca4 genes of CRT in northeastern China.
CRT has been confirmed as the pathogen causing human rickettsioses and belongs to spotted fever group rickettsiae (SFGR) [45]. The first five cases of human infection with CRT were reported in northeastern China in 2012 [21]. The patients were hospitalized with symptoms such as fever, asthenia, anorexia, nausea, headache, eschar, and lymphadenopathy. Initially, their conditions were misdiagnosed because none of the patients presented with the typical rash associated with spotted fever group rickettsiae infections in China. The results of this study demonstrate the exposure of cattle, goats, and humans in Inner Mongolia to CRT. Therefore, investigating the distribution of CRT may contribute to establishing an etiological diagnosis, facilitating appropriate treatment, and implementing public health measures. This study unveils the high prevalence of CRT infection in ticks from Inner Mongolia, and it offers valuable molecular epidemiological data regarding CRT infection not only in Inner Mongolia but also in certain regions of North China, Northwest China, and Northeast China. The detection of CRT infection in H. marginatum for the first time suggests a possible etiological diagnosis for tick-borne rickettsioses caused by H. marginatum bites and contributes to the surveillance of human rickettsioses in China.
Supporting information
(ZIP)
Acknowledgments
We are very grateful to the Inner Mongolia Center for Disease Control and Prevention for providing tick samples. We are very grateful to the Molecular Biology Research Center of Inner Mongolia Medical University for providing experimental facilities and conditions.
Data Availability
All relevant sequences were entered in GenBank with accession numbers OR272248-OR272253, OR294053-OR294057, OQ852069, OQ852073, OR454091-OR454142, OR449962-OR449989 and OR712487-OR712557.
Funding Statement
This work received financial support from the Scientific Research Project of the Mongolian Medicine Collaborative Innovation Center of the Inner Mongolia Autonomous Region (No. MYYXTPY202206 to Jing-Feng Yu). Zhiyuan talent project of Inner Mongolia Medical University (No. ZY0201027 to Jing-Feng Yu). Health Science and Technology Plan of Inner Mongolia Autonomous Region in 2022 (No. 202201213 to Jing-Feng Yu). Natural Science Foundation of Inner Mongolia Autonomous Region (No. 2022LHMS08004 to Jing-Feng Yu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Salje J. Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle. Nat Rev Microbiol. 2021;19(6):375–90. Epub 2021/02/11. doi: 10.1038/s41579-020-00507-2 . [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Geng J, Du J, Wang Y, Qian W, Zheng A, et al. Molecular Identification of Rickettsia Species in Haemaphysalis Ticks Collected from Southwest China. Vector Borne Zoonotic Dis. 2018;18(12):663–8. Epub 2018/08/22. doi: 10.1089/vbz.2017.2231 . [DOI] [PubMed] [Google Scholar]
- 3.Adem PV. Emerging and re-emerging rickettsial infections. Semin Diagn Pathol. 2019;36(3):146–51. Epub 2019/05/19. doi: 10.1053/j.semdp.2019.04.005 . [DOI] [PubMed] [Google Scholar]
- 4.Shpynov S, Fournier PE, Rudakov N, Raoult D. "Candidatus Rickettsia tarasevichiae" in Ixodes persulcatus ticks collected in Russia. Ann N Y Acad Sci. 2003;990:162–72. Epub 2003/07/16. doi: 10.1111/j.1749-6632.2003.tb07358.x . [DOI] [PubMed] [Google Scholar]
- 5.Igolkina Y, Rar V, Vysochina N, Ivanov L, Tikunov A, Pukhovskaya N, et al. Genetic variability of Rickettsia spp. in Dermacentor and Haemaphysalis ticks from the Russian Far East. Ticks Tick Borne Dis. 2018;9(6):1594–603. Epub 2018/08/20. doi: 10.1016/j.ttbdis.2018.07.015 . [DOI] [PubMed] [Google Scholar]
- 6.Yi S, Hongrong J, Wuchun C, Weiming F, Wendong J, Xin W. Prevalence of Candidatus Rickettsia tarasevichiae-Like Bacteria in Ixodid Ticks at 13 Sites on the Chinese-Russian Border. J Med Entomol. 2014;51(6):1304–7. Epub 2015/08/27. doi: 10.1603/ME13189 . [DOI] [PubMed] [Google Scholar]
- 7.Rar V, Livanova N, Tkachev S, Kaverina G, Tikunov A, Sabitova Y, et al. Detection and genetic characterization of a wide range of infectious agents in Ixodes pavlovskyi ticks in Western Siberia, Russia. Parasit Vectors. 2017;10(1):258. Epub 2017/05/27. doi: 10.1186/s13071-017-2186-5 ; PubMed Central PMCID: PMC5445278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igolkina Y, Bondarenko E, Rar V, Epikhina T, Vysochina N, Pukhovskaya N, et al. Genetic variability of Rickettsia spp. in Ixodes persulcatus ticks from continental and island areas of the Russian Far East. Ticks Tick Borne Dis. 2016;7(6):1284–9. Epub 2016/07/18. doi: 10.1016/j.ttbdis.2016.06.005 . [DOI] [PubMed] [Google Scholar]
- 9.Igolkina Y, Yakimenko V, Tikunov A, Epikhina T, Tancev A, Tikunova N, et al. Novel Genetic Lineages of Rickettsia helvetica Associated with Ixodes apronophorus and Ixodes trianguliceps Ticks. Microorganisms. 2023;11(5). Epub 2023/06/15. doi: 10.3390/microorganisms11051215 ; PubMed Central PMCID: PMC10223337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katargina O, Geller J, Ivanova A, Värv K, Tefanova V, Vene S, et al. Detection and identification of Rickettsia species in Ixodes tick populations from Estonia. Ticks Tick Borne Dis. 2015;6(6):689–94. Epub 2015/06/23. doi: 10.1016/j.ttbdis.2015.06.001 . [DOI] [PubMed] [Google Scholar]
- 11.Inokuma H, Ohashi M, Jilintai, Tanabe S, Miyahara K. Prevalence of tick-borne Rickettsia and Ehrlichia in Ixodes persulcatus and Ixodes ovatus in Tokachi district, Eastern Hokkaido, Japan. J Vet Med Sci. 2007;69(6):661–4. Epub 2007/07/06. doi: 10.1292/jvms.69.661 . [DOI] [PubMed] [Google Scholar]
- 12.Hiraoka H, Shimada Y, Sakata Y, Watanabe M, Itamoto K, Okuda M, et al. Detection of rickettsial DNA in ixodid ticks recovered from dogs and cats in Japan. J Vet Med Sci. 2005;67(12):1217–22. Epub 2006/01/07. doi: 10.1292/jvms.67.1217 . [DOI] [PubMed] [Google Scholar]
- 13.Boldbaatar B, Jiang RR, von Fricken ME, Lkhagvatseren S, Nymadawa P, Baigalmaa B, et al. Distribution and molecular characteristics of rickettsiae found in ticks across Central Mongolia. Parasit Vectors. 2017;10(1):61. Epub 2017/02/06. doi: 10.1186/s13071-017-1981-3 ; PubMed Central PMCID: PMC5289011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo JY, Kim YJ, Kim SY, Lee HI. Molecular Detection of Anaplasma, Ehrlichia and Rickettsia Pathogens in Ticks Collected from Humans in the Republic of Korea, 2021. Pathogens. 2023;12(6). Epub 2023/06/28. doi: 10.3390/pathogens12060802 ; PubMed Central PMCID: PMC10301364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan TT, Ma L, Jiang BG, Fu WM, Sun Y, Jia N, et al. First Confirmed Infection of Candidatus Rickettsia Tarasevichiae in Rodents Collected from Northeastern China. Vector Borne Zoonotic Dis. 2020;20(2):88–92. Epub 2019/08/28. doi: 10.1089/vbz.2019.2443 . [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Li Q, Zhang X, Li Z, Wang Z, Song M, et al. Characterization of rickettsiae in ticks in northeastern China. Parasit Vectors. 2016;9(1):498. Epub 2016/09/15. doi: 10.1186/s13071-016-1764-2 ; PubMed Central PMCID: PMC5022169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Zhang S, Liang W, Zhao S, Wang Z, Li H, et al. Survey of tick species and molecular detection of selected tick-borne pathogens in Yanbian, China. Parasite. 2022;29:38. Epub 2022/07/22. doi: 10.1051/parasite/2022039 ; PubMed Central PMCID: PMC9302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao JW, Yao XY, Song XD, Li WJ, Huang HL, Huang SJ, et al. Molecular detection and genetic diversity of Rickettsia spp. in pet dogs and their infesting ticks in Harbin, northeastern China. BMC Vet Res. 2021;17(1):113. Epub 2021/03/09. doi: 10.1186/s12917-021-02823-y ; PubMed Central PMCID: PMC7938463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D, Wulantuya, Fan H, Li X, Li F, Gao T, et al. Co-infection of tick-borne bacterial pathogens in ticks in Inner Mongolia, China. PLoS Negl Trop Dis. 2023;17(3):e0011121. Epub 2023/03/10. doi: 10.1371/journal.pntd.0011121 ; PubMed Central PMCID: PMC10030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao J, Lu Z, Yu Y, Ou Y, Fu M, Zhao Y, et al. Identification of tick-borne pathogens by metagenomic next-generation sequencing in Dermacentor nuttalli and Ixodes persulcatus in Inner Mongolia, China. Parasites & vectors. 2021;14(1):287. Epub 2021/05/29. doi: 10.1186/s13071-021-04740-3 ; PubMed Central PMCID: PMC8161991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia N, Zheng YC, Jiang JF, Ma L, Cao WC. Human infection with Candidatus Rickettsia tarasevichiae. The New England journal of medicine. 2013;369(12):1178–80. Epub 2013/09/21. doi: 10.1056/NEJMc1303004 . [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Li H, Lu QB, Cui N, Yang ZD, Hu JG, et al. Candidatus Rickettsia tarasevichiae Infection in Eastern Central China: A Case Series. Ann Intern Med. 2016;164(10):641–8. Epub 2016/03/29. doi: 10.7326/M15-2572 . [DOI] [PubMed] [Google Scholar]
- 23.Rudakov N, Samoylenko I, Shtrek S, Igolkina Y, Rar V, Zhirakovskaia E, et al. A fatal case of tick-borne rickettsiosis caused by mixed Rickettsia sibirica subsp. sibirica and "Candidatus Rickettsia tarasevichiae" infection in Russia. Ticks Tick Borne Dis. 2019;10(6):101278. Epub 2019/09/04. doi: 10.1016/j.ttbdis.2019.101278 . [DOI] [PubMed] [Google Scholar]
- 24.Jia N, Zheng YC, Jiang JF, Ma L, Cao WC. Human infection with Candidatus Rickettsia tarasevichiae. N Engl J Med. 2013;369(12):1178–80. Epub 2013/09/21. doi: 10.1056/NEJMc1303004 . [DOI] [PubMed] [Google Scholar]
- 25.Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32(6):897–928. Epub 2001/03/15. doi: 10.1086/319347 . [DOI] [PubMed] [Google Scholar]
- 26.Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129 Suppl:S3–14. Epub 2005/06/09. doi: 10.1017/s0031182004005967 . [DOI] [PubMed] [Google Scholar]
- 27.Jiao J, Lu Z, Yu Y, Ou Y, Fu M, Zhao Y, et al. Identification of tick-borne pathogens by metagenomic next-generation sequencing in Dermacentor nuttalli and Ixodes persulcatus in Inner Mongolia, China. Parasites & vectors. 2021;14(1):287. Epub 2021/05/29. doi: 10.1186/s13071-021-04740-3 ; PubMed Central PMCID: PMC8161991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gui Z, Cai H, Qi DD, Zhang S, Fu SY, Yu JF, et al. Identification and genetic diversity analysis of Rickettsia in Dermacentor nuttalli within inner Mongolia, China. Parasit Vectors. 2022;15(1):286. Epub 2022/08/08. doi: 10.1186/s13071-022-05387-4 ; PubMed Central PMCID: PMC9358909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao GP, Wang YX, Fan ZW, Ji Y, Liu MJ, Zhang WH, et al. Mapping ticks and tick-borne pathogens in China. Nature communications. 2021;12(1):1075. Epub 2021/02/19. doi: 10.1038/s41467-021-21375-1 ; PubMed Central PMCID: PMC7889899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valcárcel F, González J, González MG, Sánchez M, Tercero JM, Elhachimi L, et al. Comparative Ecology of Hyalomma lusitanicum and Hyalomma marginatum Koch, 1844 (Acarina: Ixodidae). Insects. 2020;11(5). Epub 2020/05/18. doi: 10.3390/insects11050303 ; PubMed Central PMCID: PMC7290797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L, Ma YM, Yang B, Han WX, Zhao WH, Chai HL, et al. Comparative analysis of microbial communities in different growth stages of Dermacentor nuttalli. Front Vet Sci. 2022;9:1021426. Epub 2022/11/01. doi: 10.3389/fvets.2022.1021426 ; PubMed Central PMCID: PMC9614212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paulauskas A, Sakalauskas P, Kaminskienė E, Šimkevičius K, Kibiša A, Radzijevskaja J. First record of Haemaphysalis concinna (Acari: Ixodidae) in Lithuania. Ticks Tick Borne Dis. 2020;11(5):101460. Epub 2020/05/18. doi: 10.1016/j.ttbdis.2020.101460 . [DOI] [PubMed] [Google Scholar]
- 33.Bugmyrin SV, Belova OA, Bespyatova LA, Ieshko EP, Karganova GG. Morphological features of Ixodes persulcatus and I. ricinus hybrids: nymphs and adults. Exp Appl Acarol. 2016;69(3):359–69. Epub 2016/03/18. doi: 10.1007/s10493-016-0036-3 . [DOI] [PubMed] [Google Scholar]
- 34.Estrada-Peña A, Pfäffle M, Baneth G, Kleinerman G, Petney TN. Ixodoidea of the Western Palaearctic: A review of available literature for identification of species. Ticks Tick Borne Dis. 2017;8(4):512–25. Epub 2017/03/14. doi: 10.1016/j.ttbdis.2017.02.013 . [DOI] [PubMed] [Google Scholar]
- 35.Hebert PD, Ratnasingham S, deWaard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc Biol Sci. 2003;270 Suppl 1(Suppl 1):S96–9. Epub 2003/09/04. doi: 10.1098/rsbl.2003.0025 ; PubMed Central PMCID: PMC1698023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black WCt, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci U S A. 1994;91(21):10034–8. Epub 1994/10/11. doi: 10.1073/pnas.91.21.10034 ; PubMed Central PMCID: PMC44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33(7):1870–4. Epub 2016/03/24. doi: 10.1093/molbev/msw054 ; PubMed Central PMCID: PMC8210823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80. Epub 2013/01/19. doi: 10.1093/molbev/mst010 ; PubMed Central PMCID: PMC3603318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igolkina Y, Nikitin A, Verzhutskaya Y, Gordeyko N, Tikunov A, Epikhina T, et al. Multilocus genetic analysis indicates taxonomic status of "Candidatus Rickettsia mendelii" as a separate basal group. Ticks Tick Borne Dis. 2023;14(2):102104. Epub 2022/12/12. doi: 10.1016/j.ttbdis.2022.102104 . [DOI] [PubMed] [Google Scholar]
- 40.Shao JW, Zhang XL, Li WJ, Huang HL, Yan J. Distribution and molecular characterization of rickettsiae in ticks in Harbin area of Northeastern China. PLoS Negl Trop Dis. 2020;14(6):e0008342. Epub 2020/06/05. doi: 10.1371/journal.pntd.0008342 ; PubMed Central PMCID: PMC7272007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng C, Fu W, Ju W, Yang L, Xu N, Wang YM, et al. Diversity of spotted fever group Rickettsia infection in hard ticks from Suifenhe, Chinese-Russian border. Ticks Tick Borne Dis. 2016;7(5):715–9. Epub 2016/03/16. doi: 10.1016/j.ttbdis.2016.02.023 . [DOI] [PubMed] [Google Scholar]
- 42.Ogata H, La Scola B, Audic S, Renesto P, Blanc G, Robert C, et al. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS genetics. 2006;2(5):e76. Epub 2006/05/17. doi: 10.1371/journal.pgen.0020076 ; PubMed Central PMCID: PMC1458961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo WP, Wang YH, Lu Q, Xu G, Luo Y, Ni X, et al. Molecular detection of spotted fever group rickettsiae in hard ticks, northern China. Transbound Emerg Dis. 2019;66(4):1587–96. Epub 2019/03/29. doi: 10.1111/tbed.13184 . [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, Park HS, Jang WJ, Koh SE, Kim JM, Shim SK, et al. Differentiation of rickettsiae by groEL gene analysis. J Clin Microbiol. 2003;41(7):2952–60. Epub 2003/07/05. doi: 10.1128/JCM.41.7.2952-2960.2003 ; PubMed Central PMCID: PMC165385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker DH, Ismail N. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat Rev Microbiol. 2008;6(5):375–86. Epub 2008/04/17. doi: 10.1038/nrmicro1866 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
Data Availability Statement
All relevant sequences were entered in GenBank with accession numbers OR272248-OR272253, OR294053-OR294057, OQ852069, OQ852073, OR454091-OR454142, OR449962-OR449989 and OR712487-OR712557.