Abstract
Purpose
This review article provides the readers with an in-depth insight in understanding and interpreting various research literatures on the masseter vestibular evoked myogenic potentials (mVEMP). The article also reviews the contemporary researches involving the clinical applications of the mVEMP.
Conclusions
Masseter VEMP is an evolving yet clinically promising neuro-otology test tool that has recently gained more research interest and is considered an additional tool to diagnose various vestibular disorders. Masseter VEMP assesses the functional integrity of the acoustic-masseteric and vestibulo-masseteric reflex pathways. The mVEMP could be used as a complementary test to evaluate the same peripheral generator as the cervical VEMP but a different central pathway i.e., vestibulo-trigeminal pathway. Various research studies that have experimented on parameters such as the effect of different electrode montages (zygomatic vs mandibular configurations), stimulation rates, filter settings and stimuli used to evoke mVEMP have been discussed in this article that could assist in the optimization of a comprehensive clinical protocol. The latency and the amplitude of mVEMP waveforms serve as significant parameters in differentiating normals from those of the clinical populations. Along with the cVEMPs and oVEMPs, mVEMP might help diagnose brainstem lesions in REM Sleep behaviour disorders, Multiple Sclerosis and Parkinson's disease. However, further studies are required to probe in this area of research.
Keywords: Masseter vestibular evoked myogenic potentials (mVEMP), Vestibulo-masseteric pathway, Acoustic-masseteric pathway
1. Introduction
Vestibular evoked myogenic potential (VEMP) is clinically a promising neurophysiological test tool that assesses the functional integrity of the saculocollic and otolith ocular reflex pathways. VEMPs are obtained preferentially from the otoliths and not from the semicircular canals, making it an efficient clinical tool to assess the otoliths and their respective pathways. VEMP is a short latency electromyogram (EMG) potential that can be evoked by loud air conduction sounds (Colebatch et al., 1994), bone conduction vibrations applied to the skull (Todd et al., 2007), and/or galvanic stimulations (Watson et al., 1998) provided to the vestibular end organs. Clinically, air conduction clicks/tone burst stimuli are used to record the VEMP.
The vestibular system is typically insensitive to the acoustic stimulus. However, a high-intensity acoustic stimulus can stimulate the vestibular system. It has been found that individuals with profound hearing loss have the presence of VEMPs, whereas it is absent for individuals with both cochlear and vestibular loss (Bickford et al., 1964; Colebatch and Halmagyi, 1992). Bickford and colleagues discovered a response recorded at inion to loud clicks, which were present solely during the contraction of the sternocleidomastoid muscle of the neck (Cody et al., 1964, 1969). This suggests that the responses are myogenic in origin. Researchers have obtained the VEMP responses from various muscles such as neck extensor muscles, masseter muscle, soleus muscle, gastrocnemius muscle, triceps muscle and the inferior oblique muscle (Wu et al., 1999; Deriu et al., 2007; Todd et al., 2007; Ruddisil & Hain, 2008; Cherchi et al., 2009; Cunha et al., 2014).
Originally, VEMP was recorded from the sternocleidomastoid (SCM) muscle, which is known as the cervical vestibular evoked myogenic potentials(cVEMP) (Colebatch and Halmagyi, 1992; Colebatch et al., 1994). Later, another test method was adopted where the myogenic response potentials were recorded by placing the electrodes at the inferior oblique muscle. This is known as ocular vestibular evoked myogenic potentials(oVEMP) (Rosengren et al., 2005). Since then, the cVEMP and the oVEMP have been the widely practised vestibular tests employed to evaluate the integrity of the sacculo-collic and the utriculo-ocular pathways, respectively. Recently, a new electromyogenic test known as masseter VEMP (mVEMP) has gained more research and clinical interest. mVEMP assesses the integrity of the vestibulo-trigeminal pathway (Deriu et al., 2005). During the mVEMP recording, the surface electrodes are applied to the tonically contracted masseter muscles to record the myogenic responses obtained bilaterally.
2. Masseter VEMP (mVEMP)
Research studies have demonstrated that tonically activated masseter muscles in healthy individuals manifest a short-latency inhibitory EMG response bilaterally in response to galvanic/acoustic stimulations (Meier-Ewert et al., 1974; Deriu et al., 2003, 2005). These short-latency electromyogram potentials are recorded from the masseter muscles and are thus denoted as masseter vestibular-evoked myogenic potentials (Masseter VEMP or mVEMP). The electrode is positioned on the lower third of the superficial layer of the masseter muscle (Meier-Ewert et al., 1974; Deriu et al., 2003, 2005, 2007). The typical electrode placement for recording mVEMP is provided in fig. 4.
Fig. 4.
Electrode montages used to record mVEMP. (A) Zygomatic electrode montage (B) Mandibular Electrode montage.
A preliminary study showed that the acoustic jaw reflex was present only in those with normal hearing and intact auditory nerves (Meier-Ewert et al., 1974). Hearing-impaired patients with intact vestibular systems did not manifest any sort of jaw reflex to intense acoustic stimulations around 90–100 dB. Thus, the authors presumed that the acoustic-jaw reflex originates from the cochlear receptors, not the vestibular end organs. They also stated that it could be a sign of local protection or the startle reflex towards loud sounds (Meier-Ewert et al., 1974).
In contrast, a study by Deriu et al. (2003) reported the presence of an inhibitory reflex recorded from the masseter muscle on unilateral and bilateral galvanic vestibular stimulations. They found a biphasic potential at p11/n15 latencies. They demonstrated An interesting method where the inhibitory responses were recorded by tilting the subjects on both sides to 30°. During the head tilt procedure, it was found that the p11/n15 responses were asymmetrically modulated. This proved that the responses are vestibular and not from the auditory system. Fig. 1 shows the typical mVEMP recorded from a young, healthy subject.
Fig. 1.
Masseter VEMP waveform consisting of P1 and N1 peak.
Fig. 1 can be inserted here.
Deriu et al. (2005) showed that the p11 peak was present when loud clicks were provided whereas, p16 was observed when the sound was given at lower stimulus level. The intensity levels for elicitation of p16 peak is around 98–113 dBSPL and the intensity levels for elicitation of p11 peak ia around 128–138 dBSPL(De Natale et al., 2018). It was reported that the p11 responses were asymmetrically modulated when the subjects were asked to tilt their heads on both sides. Interestingly, this asymmetrical modulation was not observed with the p16 peak, showing that this response is of pure cochlear origin. The authors concluded that, on providing loud acoustic stimuli such as 100dbnHL clicks, we can observe two partially overlapping reflexes originating from both the hearing and the vestibular end organs. The predominantly visible peaks during such loud stimulation are p11 and n21 which is basically the overlap of p11/n15 (vestibular origin) and p16/n21 (cochlear origin) responses. At the threshold level of the stimulus, only p16/n21 is present which is predominantly cochlear in origin (Deriu et al., 2005).
The p11/n15 responses recorded from the masseter muscle are similar to the p13/n23 responses obtained in cVEMP from the SCM muscle (Colebatch et al., 1994). Some of the observed similarities include (a) both are elicited at higher threshold levels (90-100dBnHL); (b) both are inhibitory reflex responses (Colebatch and Rothwell 1993); (c) Elicitation of both reflexes requires tonic contraction of the corresponding muscles; (d) In both the cases, we can observe a positive correlation between the background level of tonic EMG activity and the amplitude of the responses (Deriu et al., 2005); (e) Both p11/n15 response of mVEMP and p13/n23 responses of cVEMP were observed to be absent in subjects with vestibular pathologies (Colebatch et al., 1994; Deriu et al., 2007).
Although the peripheral generators of mVEMP and cVEMP are same, there are a few noticeable differences between the two potentials. (a) Responses obtained from the masseter muscle are always bilateral; conversely, those obtained from the SCM muscle are ipsilateral (Colebatch et al., 1994; Deriu et al., 2003, 2005). This could be due to the nature of the function of those respective muscles. The masseter muscles work together, assisting in the appropriate positioning of the mandible. Thus, it is possible to contract both the masseters simultaneously, and the inhibitory responses can be recorded bilaterally. Vinayagar and Sinha (2023) reported that the amplitude of the mVEMP to bilateral sound stimulations is larger compared to the ipsilateral and contralateral sound stimulations.Wang and Young (2003) reported that the cVEMP amplitude to a binaural stimulus is not different compared to a monoaural stimulus indicating that the pathway of cVEMP is unilateral. Also, the corrected amplitude of cVEMP (p13) is approximately 30% larger than the mVEMP's amplitude (Deriu et al., 2005). The larger amplitude for cVEMP could be due to stronger vestibular projections to the sternocleidomastoid muscle than the masseter muscles(Deriu et al., 2005). The amplitude of cVEMP could also be due to more muscle thickness of the sternocliode mastoid muscle compared to the masseter muscle. Larger cVEMP amplitude compress to oVEMP has also been reported due to muscle thickness(Bansal et al., 2013).The neck muscles play a major role in the postural control compared to the masseter musckle; hence, the vestibular projections to the neck muscles could be comparatively larger(Deriu et al., 2005).
3. Physiology of the vestibulomassetric reflex
The masseter muscles help in the mastication process by elevating the mandible and assisting in the occlusion of the teeth, thus maintaining the jaw against gravity. These muscles support speaking, chewing, and jaw positioning in static and dynamic situations. (Lund and Olsson, 1983; Manns et al., 1987). Both auditory and vestibular stimulations are said to provoke responses from the masseters (Meier-Ewert et al., 1974; Deriu et al., 2003, 2005). Studies have also provided quantitative shreds of evidence suggesting that there is an effect on the tonic masseter muscles during both angular dynamics and tilting of the head (Hickenbottom et al., 1985; Deriu et al., 2000). Deriu et al. (2000) reported that the head tilt was found to modulate the masseter muscle activity, which was macular in origin.
The principal characteristic function of the vestibulo-masseteric reflex is to respond to an unanticipated sudden head tilt (Deriu et al., 2005). For example, the masseter muscles are inhibited when the head is suddenly dropped down whereas excitation occurs when there is a sudden pitch up of the head. During such sudden movements of the head, the vestibular system provides fine-tuned input to the masseters to maintain the head's orientation in space. The macular inputs from the vestibular system are asymmetrical with respect to the orientation of the jaw during head tilts and this provides evidence that the vestibulo-masseteric polysynaptic pathway assists in keeping the jaw in equilibrium with respect to the movement of the head in space (Tolu and Pugliatti, 1993). Studies also report that the vestibular system modulates rhythmic jaw motions (opening and closing phases alternatively) required during the mastication process (Hattori et al., 2010).
4. Vestibulo-masseteric and acoustic-masseteric reflex pathways
4.1. Vestibulo-masseteric reflex pathway
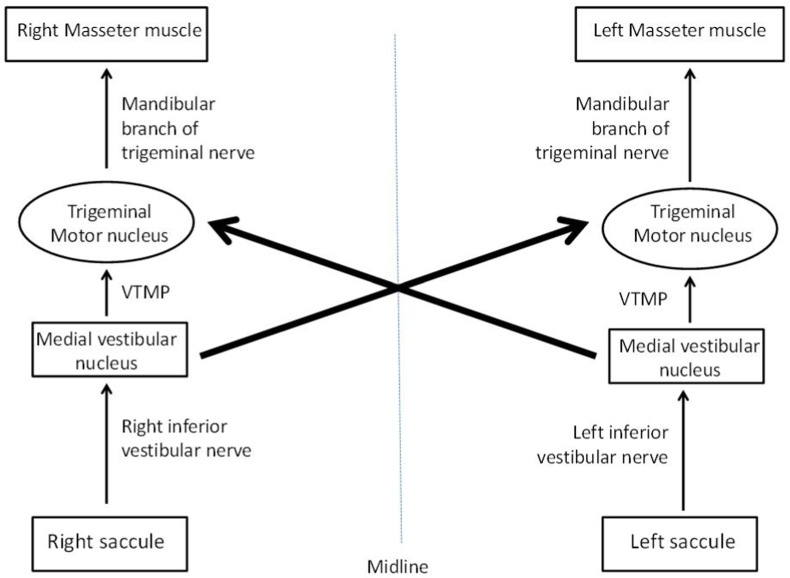
Fig. 2 can be inserted here.
Fig. 2.
Schematic diagram of vestibular-masseteric reflex pathway Darker arrows indicate stronger pathway
VTMP- Vestibulo-trigeminal monosynaptic pathway.
The vestibular-masseteric reflex pathway originates from the saccule located in the inner ear. mVEMP and cervical VEMP have been reported to share a common origin: the saccule and inferior vestibular nerve (Deriu et al., 2005, 2007). When the sensory hair cells of the saccular macula get stimulated, the information is passed on to the Scarpa's ganglion which is then carried along the inferior vestibular branch of the vestibulocochlear nerve to the brainstem. From the vestibular nerve, the information is carried out to the ipsilateral medial vestibular nucleus. From there, the nerve takes a decussation path and ends at the ipsilateral and contralateral trigeminal motor nuclei via the vestibulo-trigeminal monosynaptic pathway (VTMP). The decussated VTMP fibers are the majority in number than the ipsilateral ones (Tolu and Pugliatti, 1993; Giaconi et al., 2006). From the trigeminal nucleus, the signals are carried to the masseter muscles via the mandibular branch of the trigeminal motor nerve. The lower third of the masseter muscle is said to receive comparatively heavy innervation, and thus, electrodes placed on that specific site yield responses of larger magnitude (Giaconi et al., 2006). Fig. 2 represents the block diagram of the vestibular-masseteric reflex pathway. The vestibulomassetric reflex pathways have been confirmed in animal studies (Tolu and Pugliatti, 1993; Giaconi et al., 2006; Cuccurazzu et al., 2007).
4.2. Acoustic-masseteric reflex pathway
Fig. 3 can be inserted here.
Fig. 3.
Schematic diagram of acoustic-masseteric reflex pathway
ATMP- Auditory-trigeminal monosynaptic pathway.
Research studies report that stimulation of the auditory pathway can also bring about changes in the masseter muscles' potentials, which can be recorded as inhibitory reflex responses (Meier-Ewert et al., 1974; Deriu et al., 2005). When the sound stimulates the cochlea, the auditory nerve fibres carry the auditory information to the ipsilateral dorsal cochlear nucleus (DCN). From this site, the ipsilateral and contralateral pathway carries the information to the trigeminal motor nucleus bilaterally. Stimulation of one cochlea is carried to both the ipsi- and contralateral trigeminal nucleus, from which the information is carried on to the masseter muscles via the mandibular branch of the trigeminal nerve. Thus, the responses can be recorded bilaterally even on stimulating a single ear. The acoustic-jaw reflex could be a sign of local protective or the startle reflex towards loud sounds (Meier-Ewert et al., 1974).
The acoustic-jaw inhibitory reflex pathway could be hypothesized to be similar to the pathway to the tensor tympani muscle which is also supplied by the trigeminal motor nerve. The similarity between the two pathways (The acoustic-masseteric reflex pathway and the tensor tympani reflex pathway) is both get stimulated as a startle reflex when there is a loud sound. The tensor tympani reflex pathway also gets stimulated to non-auditory stimuli and self-generated sounds such as chewing and swallowing (Mukerji et al., 2010; Edmonson et al., 2022). Fig. 3 represents the acoustic-masseteric reflex pathway.
5. Electrode montage to record mVEMP
Two distinct belly tendon montages can be employed to record the mVEMP. The first montage method involves the inverting electrode placed on the mandibular angle, termed as mandibular reference. In the second method, the inverting electrode is positioned on the medial point of the zygomatic arch and is referred as the zygomatic reference (Deriu et al., 2005). The active or the non-inverting electrode is placed on the lower third of the masseter muscle and the ground electrode is placed on the lower forehead. The majority of the researchers have used the zygomatic reference to record the mVEMP (de Natale et al., 2019; Vignesh et al., 2021; Romero et al., 2022).
When compared between both belly tendon montage methods, the corrected amplitude, reliability, and the response rate have been reported to be higher for the zygomatic electrode placement than for the mandibular electrode placement method (de Natale et al., 2019; Loi et al., 2020). The test-retest reliability of the zygomatic reference montage has been reported to be superior compared to that of the mandibular reference method (Loi et al., 2020). This could be due to the reduction in the "reference contamination" when the zygomatic configuration montage method is employed (Loi et al., 2020; Piker et al., 2018). However, Thirusangu and Sinha (2022) reported no significant differences in the latency and amplitude of mVEMP between the two electrode montage configurations using tone burst stimulus. Also, de Natale et al. (2019), reported the presence of p11 peak in only one of the two reference configuration methods. Loi et al. (2020) have suggested employing both montage configurations to obtain the highest detection rates in mVEMP (Loi et al., 2020). Fig. 4 shows the two electrode montages used for recording mVEMP.
Fig. 4 can be inserted here.
6. Test-retest reliability of masseter VEMP
Loi et al. (2020) studied the reliability measure across zygomatic and mandibular montage configurations using click stimuli and reported that the zygomatic montage has excellent test-retest reliability compared to the mandibular montage method. The test-retest reliability of mVEMP recorded using 500 Hz tone-burst stimuli in young healthy individuals has been reported to be excellent (Vignesh et al., 2021). However, there are some variability in latency and amplitude parameters of mVEMP for a few individuals only. The latency is slightly prolonged or earlier and the amplitude of mVEMP is either reduced or enlarged in the second session of test-retest reliability measures (Vignesh et al., 2021). This could be due to disparity in the placement of electrodes between the sessions and could be due to minuscule variations in the extent of masseter muscle contraction (Vignesh et al., 2021).
7. Stimulus parameters
7.1. Types of stimuli
mVEMP can be recorded using both electrical and sound stimulations (Deriu et al., 2003, 2005). Electrical vestibular stimulations evoke masseter responses on both sides (Deriu et al., 2003). Current pulses of 5 mA and a frequency of 3Hz are employed to obtain the reflex responses. The amplitude and the latency of the p11 wave are indistinguishable with both electrical and acoustic stimuli; whereas n15 are barely noticeable on sound stimulation but it was clear when electrical stimuli were provided(Deriu et al., 2005). This could be due to a better neural synchrony with electrical stimulations than acoustic stimulations.
Clicks, Chirp and tone-burst stimuli have been primarily used to record mVEMP (Deriu et al., 2005, 2007; Ginatempo et al., 2013; Vignesh et al., 2021; Romero et al., 2022; Sangu Srinivasan et al., 2022; Thirusangu and Sinha, 2022; Neupane et al., 2023; Vinayagar and Sinha, 2023). Vignesh et al. (2021) compared the mVEMP responses obtained using tone bursts with that of those obtained with clicks in a study by de Natale et al. (2015b). It was outlined that the tone burst stimuli produced more robust p11/n21 peaks than click stimuli; the latencies of the peaks were more prolonged, and the amplitude was larger when tone burst was used to elicit mVEMP. The peak-to-peak amplitude of mVEMP recorded with tone-burst is higher compared to click stimulus(Vinayagar and Sinha, 2023). The mVEMP has been also recorded with ipsilateral, contralateral and binaural stimulations using the 500 Hz tone burst stimuli(Vinayagar and Sinha, 2023). The only similarity reported in the mVEMP responses obtained using clicks and tone bursts was that the corrected amplitude asymmetry ratio of the ipsi- and contralateral responses were indistinguishable (Vignesh et al., 2021).
Neupane et al. (2023) reported that the latencies of p11-n21 are shorter when elicited using clicks and 500Hz NB CE-Chirps than compared to those obtained with 500Hz tonebursts. 500Hz NB CE-Chirps tend to have earlier stimulus onset time compared to tonebursts and this could be the reason for the presence of early latencies when chirps were procured (Çoban et al., 2021; Karaçaylı et al., 2020; Mat et al., 2021). The duration of 500 Hz tone burst stimulus is relatively longer than that of clicks and chirps stimuli; saccule and the vestibular nerves are hypothesized to respond effectively when stimulated using a long-duration stimulus (Cheng et al., 2003; Wu et al., 2007; Singh and Apeksha, 2014). Research studies generally use a 500 Hz tone burst with a duration of 10 msec, whereas click is generally 0.1 msec(Singh and Apeksha, 2014).
8. Repetition rate of the stimuli
Earlier studies have employed repetition rate of 3Hz to elicit mVEMP (Deriu et al., 2005, 2007), whereas recent researchers have studied mVEMP responses using 5Hz stimulation rates irrespective of the stimuli used i.e. clicks, chirps or tonebursts (Kilinc et al., 2023; Neupane et al., 2023; Vinayagar and Sinha, 2023; Xie et al., 2022). 5Hz repetition rates have been reported to produce comparatively better morphology and replicability of the waveforms in cervical VEMPs since there is a noticeable intersubject difference in the responses to using higher repetition rates (Wu et al., 1999).
9. Number of stimuli averages
Deriu et al. (2005) recorded mVEMP using both electrical and acoustical stimuli. They used 300 to 500 stimuli to average for acoustically evoked mVEMP and 800 averages for electrically evoked mVEMP. Further, all the studies using auditory stimuli such as tonebursts and clicks adopted 300 to 500 numbers averages (de Natale et al., 2015a, 2019; Magnano et al., 2014; Deriu et al., 2007; Vignesh et al., 2021). Although Deriu et al. (2007) reported the requirement of a higher number of stimulus averages to record mVEMP, none of the studies has compared the impact of various stimulus averages on mVEMP amplitude and latency.
10. Intensity
Deriu et al. (2005) used the different levels of intensity ranging from 80 to 100 dB nHL click stimulus to record mVEMP and reported the mVEMP threshold to be 80 dBnHL. The amplitude of the mVEMP varies at different intensities. The response of mVEMP at 90 dBnHL is 40 % lesser than 100 dB of click stimuli(Deriu et al., 2005). de Natale et al. (2019) reported that the p11 and n21 components of the vestibulo masseteric reflex can be detected at 128–138 dB SPL.Vignesh et al. (2021) reported clear, consistent, and replicable p11 and n21 waveforms in all the forty-four subjects at 125 dB pe SPL.
11. Amplifier gain
Amplification of masseter VEMP by 5000 times reveals a clear, replicable p11 and n21 peaks (de Natale et al., 2015b, 2019; Magnano et al., 2014; Deriu et al., 2005; Loi et al., 2020; Vignesh et al., 2021). de Natale et al. (2018) used amplification of the recorded response by 3000 times for all three VEMPs. No significant reason was mentioned for the amplification. To date, none of the studies have compared the effect of different amounts of gain/amplification on recorded mVEMP.
12. Band pass filter
Earlier studies conducted on mVEMP using clicks were carried out with bandpass filters ranging from 0.3 to 2000 Hz (Deriu et al., 2005, 2007), whereas the subsequent studies used 5–5000 Hz (de Natale et al., 2015a, 2018, 2019; Magnano et al., 2014; Loi et al., 2020). Studies that have used click stimuli employed the filter setting of 5–5000 Hz (Magnano et al., 2014, 2016; de Natale et al., 2015a, 2015b, 2018, 2019; Puligheddu et al., 2019; Loi et al., 2020). For the waveforms obtained using toneburst stimuli, studies have used filter settings ranging from 0.3 to 2000Hz (Vignesh et al., 2021; Sangu Srinivasan et al., 2022); 5 to 1500 (Romero et al., 2022); 0.1 to 3000 (Thirusangu and Sinha, 2022; Vinayagar and Sinha, 2023); 10–1000 Hz (Kilinc et al., 2023). There is no uniformity in the filter settings used across studies mainly for the toneburst stimuli which needs to be looked on in the upcoming research studies. Table 1shows the summary of stimulus and acquisition parameters of mVEMP.
Table-1.
Stimulus and acquisition parameters used across studies on Masseter VEMP.
|
S.No |
Stimulus parameters |
Acquisition parameters |
|||||
|---|---|---|---|---|---|---|---|
| Authors | Type of stimuli used | Repetition rate | Number of stimuli averages | Stimulus intensity | Amplifier gain | Bandpass filter | |
| 1 | Deriu et al. (2005) | clicks | 3Hz | 300 | 70–100 dB nHL | 5000 | 0.3–2000Hz |
| 2 | Deriu et al. (2007) | clicks | 3Hz | 300–500 | 70–100 dB nHL | – | 0.3–2000Hz |
| 3 | Ginatempo et al. (2013) | clicks | 5Hz | – | 143-108 dB SPL | – | – |
| 4 | Magnano et al. (2014) | clicks | 5Hz | 300–500 | 143-108 dB SPL | 5000 | 5–5000Hz |
| 5 | de Natale et al. (2015a) | clicks | 5Hz | 300–500 | 140 dB SPL | 5000 | 5–5000Hz |
| 6 | de Natale et al. (2015b) | clicks | 5Hz | 300–500 | 140dBSPL | 5000 | 5–5000Hz |
| 7 | Magnano et al. (2016) | clicks | 5Hz | 300–500 | 143-108 dB SPL | 5000 | 5–5000Hz |
| 8 | de Natale et al. (2018) | clicks | 5Hz | 300–500 | 138 dB SPL | 3000 | 5–5000Hz |
| 9 | de Natale et al. (2019) | clicks | 5Hz | 300–500 | 138 dB SPL & 108 dB SPL | 5000 | 5–5000Hz |
| 10 | Puligheddu et al. (2019) | clicks | 5Hz | 250–400 | 138 dB SPL | 5000 | 5–5000Hz |
| 11 | Loi et al. (2020) | clicks | 5Hz | 300–500 | 138 dB SPL & 108 dB SPL | 5000 | 5–5000Hz |
| 12 | Vignesh et al. (2021) | Tonebursts (500Hz) | 5.1Hz | 300 | 125 dB peSPL | 5000 | 0.3–2000Hz |
| 13 | Vignesh et al. (2021) | Tonebursts (500Hz) | 5.1Hz | 300 | 125 dB peSPL | 5000 | 0.3–2000Hz |
| 14 | Romero et al. (2022) | Tonebursts (500Hz) | 5.4Hz | 128 (minimum) | 125 dB peSPL | 2000 | 5–1500Hz |
| 15 | Vinayagar and Sinha (2023) | Tonebursts (500Hz) | 5.1Hz | 300 | 125 dB SPL | 5000 | 0.1–3000Hz |
| 16 | Xie et al. (2022) | Clicks | 5Hz | 200–300 | 125 dB SPL | – | – |
| 17 | Vinayagar and Sinha (2023) | Tonebursts (500Hz) | 5.1Hz | 300 | 125dBSPL | 5000 | 0.1–3000Hz |
| 18 | Neupane et al. (2023) | CE-Chirps, Clicks, Tonebursts (500Hz) | 5.1Hz | 200 | 95dBnHL | 5000 | 0.3–1500Hz |
| 19 | Kilinc et al. (2023) | Tonebursts (500Hz) | 5.1Hz | 200 | 75-100dBnHL | – | 10–1000Hz |
Table 1 can be inserted here.
13. Masseter VEMP in a normal population
13.1. Latency and amplitude
Deriu et al. (2005) reported the presence of two short latency responses evoked by click stimuli at 100 dB that varied in terms of latency, amplitude, and threshold. At 100 dB, the p11 wave was noticed at an average latency of 7.0–9.2ms; often, the n15 wave was detected as just a minute deflection from the p11/n21 biphasic waveform. Both the latency and amplitude of the waveform were reported to be equal on ipsi- and contralateral stimulations. The amplitude of p11-n21 peak complex of mVEMP to a binaural acoustic stimulation is comparatively larger than the ipsi- and the contralateral (Deriu et al., 2005, 2007; Vinayagar and Sinha, 2023). On varying the EMG magnitude level, as EMG levels increased, amplitude increased while latency remained unchanged (Deriu et al., 2005; Romero et al., 2022). The latency and amplitude values obtained in different studies are summarized below in Table 2.
Table 2.
Latency and amplitude value of mVEMP across various studies.
| Sl. No |
Authors | Type of stimulus | P11 latency(msec) |
N21 latency(msec) |
P11–N21 amplitude complex(μv) |
|||
|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Mean | Standard deviation | |||
| 1. | de Natale et al. (2019) | Clicks | 11.37 | 0.91 | 19.75 | 1.61 | 0.68 | 0.33 |
| 2. | Loi et al. (2020),* | Clicks | 12.0 | 1.0 | 20.4 | 1.3 | 0.45 | 0.16 |
| 3. | Loi et al. (2020)** | Clicks | 12.1 | 1.1 | 20.4 | 1.4 | 0.66 | 0.32 |
| 4. | Vignesh et al. (2021) | 500 Hz tone burst | 12.85 | 1.54 | 21.25 | 1.36 | 1.11 | 0.46 |
| 5. | Kilinc et al.(2023) | 500 Hz tone burst | 15.90 | 1.68 | 25.86 | 1.48 | 0.13 | 0.07 |
*Zygomatic electrode montage.
**Mandibular electrode montage.
Insert Table 2here.
There is no significant difference in latency or amplitude between zygomatic and mandibular configurations (de Natale et al., 2019). The test-retest reliability of mVEMP for both the mandibular and zygomatic electrode montages are excellent (Loi et al., 2020). Vignesh et al. (2021) also reported no significant effect of ear and gender on latency and amplitude of mVEMP. The authors reported a very good test-retest reliability of the mVEMP in healthy subjects. However, Kilinc et al. (2023) reported shorter latencies of p11 and n21 for females compared to males, which could be due to shorter reflex pathways in females compared to the males. The p11-n21 amplitude is larger for the contralateral than the ipsilateral acoustic stimulation (Vinayagar and Sinha, 2023). This could be due to the difference in the ipsi- and contralateral vestibulo-masseteric reflex pathways. The larger amplitude on stimulation to the contralateral side could be due to the presence of stronger contralateral vestibulo-trigeminal motor pathways than the ipsilateral ones (Deriu et al., 1999). However, Vignesh et al. (2021) reported no significant differences in amplitude parameters between ipsilateral and contralateral recording in healthy individuals.
14. Masseter VEMP in the clinical population
14.1. Parkinson's disease
Parkinson's disease involves degeneration of the brainstem nuclei (Grinberg et al., 2010). One of the symptoms of this condition is manifested as the mismodulation of neurophysiological responses such as VEMP. mVEMP, cVEMP, and the oVEMP are reported to be abnormal in patients with Parkinson's disease (de Natale et al., 2015a). The amplitude of mVEMP is significantly smaller in patients with Parkinson's disease than in the healthy group. There was no significant deviation in the latency parameters in these populations. The authors concluded that further studies are required to test the usefulness of VEMPs in different stages of Parkinson's disease (de Natale et al., 2015a). The study also reported that the rate of abnormality was higher in mVEMP (66.7%) than cVEMP (41.7%) and oVEMP (45.8%), thus proving the efficiency and sensitivity of masseteric VEMP in identifying brainstem pathological conditions such as PD. The abnormality of mVEMP increases with the severity of Parkinson's disease(de Natale et al., 2015b). The abnormality rate is higher for mVEMP than the cVEMP and oVEMP in both the early and advanced stages of Parkinso's disease(de Natale et al., 2015b).
14.1.1. Multiple sclerosis
Magnano et al. (2014) studied mVEMP in sixty patients with multiple sclerosis. The authors also correlated the results of the mVEMP with the MRI data in individuals with multiple sclerosis. In mVEMP the peak latency of p11 and N21 peak are significantly prolonged in individuals with multiple sclerosis compared to the normal individuals. The authors reported that the mVEMP was abnormal in 62% of the individuals with multiple sclerosis, whereas the MRI was abnormal in 70% of the patients with multiple sclerosis. The MRI results suggested monolateral/or bilateral supratemporal/cerebellar lesions and in the brainstem in 98.3% and 71.7% of individuals with multiple sclerosis. Within the brainstem, midbrain was affected in 38.3%, pons was affected in 68.3% and medulla in 43.3% of the patients. A good correlation was observed between the MRI and mVEMP results in multiple sclerosis. The authors suggested that mVEMP can complement the standard MRI tests in the early detection of Multiple sclerosis.
Magnano et al. (2016) studied forty-five individuals with multiple sclerosis using masseter mVEMP, ABR, clinical and MRI data. MRI results suggested affected midbrain in 45%, affected pons in 73.3% and affected medulla in 48.9% of the patients with multiple sclerosis. The authors reported a significant delay in latency and a significant reduction in amplitude of the mVEMP in multiple sclerosis individuals. The follow-up examination after 15 months for the individuals with mVEMP showed significant alterations in clinical and mVEMP with no change in clinical and MRI tests. The proportion of altered vestibulomassetric reflex at baseline was 57.8 %, and it was significantly changed to 71.1 % during the follow-up. The authors concluded that the combined evoked potentials and brainstem reflex testing help to track the pathophysiology of brainstem dysfunction.
Sangu Srinivasan et al. (2022) studied cVEMP, oVEMP and mVEMP on forty-five patients with multiple sclerosis. They reported that mVEMP had a higher detection rate (82.22%) of the abnormality than the other two VEMPs. The authors also observed that mVEMP could potentially identify those with multiple sclerosis even during the absence of any clinical and radiological brainstem dysfunctions in them.
14.1.2. REM sleep disorders
de Natale et al. (2018) recorded cVEMP, oVEMP, and mVEMP in twenty participants with idiopathic REM sleep behaviour disorders and twenty-two healthy participants. The rate of abnormality of cVEMP was 45% for cVEMP, 50% for oVEMP, and 65% for masseter VEMP in participants with idiopathic REM sleep behaviour disorder. The amplitude for all three VEMPS was reduced in these individuals. The authors concluded that there is a consistent and extensive brainstem abnormality in idiopathic REM sleep behaviour disorder patients. However, the authors also warranted testing the potential of VEMPs in detecting the prognosis of idiopathic REM sleep behaviour disorders over time.
Puligheddu et al. (2019) studied cVEMP, oVEMP, and mVEMP in seventeen patients with idiopathic random eye movement sleep behaviour disorder and twenty-two normal individuals. The authors reported intact VEMP response in only 25 % of patients with an idiopathic random eye movement disorder. The authors also reported an abnormal amplitude asymmetry ratio in individuals with idiopathic random eye movement sleep behaviour disorder. They concluded that the VEMP battery with other neurological tests could improve the early detection of idiopathic random eye movement sleep behaviour disorder.
15. Future clinical applications of masseter VEMP
Vestibular evoked myogenic potentials have been used widely in the vestibular clinical setup to assess the otolithic organs. The cervical VEMP assesses the sacullocoliic pathways, whereas, the ocular VEMP assesses the otolith-ocular pathways. The peripheral generators for cVEMP and oVEMP are the saccule and the utricle, respectively. The literature on mVEMP has started to emerge recently. However, the much wider clinical applications of mVEMP need to be explored further. The peripheral generator of the mVEMP is saccule, the same as that of the cervical VEMP. However, the two potentials have distinctive neural pathways; hence the cVEMP and mVEMP provide neurophysiological information regarding the vestibulo-collic and vestibulo-masseteric pathways, respectively. Thus, the mVEMP could be employed as a supplementary tool to evaluate the same peripheral generator but a different central pathway.
It is also evident from various studies that mVEMP is superior to cVEMP and oVEMP in identifying neural lesions in numerous pathologies. de Natale et al. (2018) reported abnormal cVEMP in 45% of patients, abnormal oVEMP in 50%, and abnormal mVEMP in 65% of patients with idiopathic REM sleep behaviour disorder. Sangu Srinivasan et al. (2022) reported that mVEMP had a higher detection rate (82.22%) of brainstem dysfunction in multiple sclerosis patients. The detection rate of brainstem abnormality in multiple sclerosis was much higher for mVEMP compared to the cVEMP and oVEMP. Magnano et al. (2016) also reported that mVEMP can identify brainstem lesions in multiple sclerosis patients even without any clinical symptoms and with normal MRI scan results. The lesions of the medulla also result in abnormal vestibulomassetric reflexes (Magnano et al., 2014). de Natale et al. (2015a) reported that the rate of abnormality was higher for mVEMP (66.7%) than cVEMP (41.7%) and oVEMP (45.8%) in patients with Parkinson's disease. Even for the advanced stage of Parkinson's disease, the detection rate of abnormal neural pathways is much higher for mVEMP compared to the cVEMP and oVEMP(de Natale et al., 2015b). No studies have assessed the vestibulomassetric reflex pathway in various peripheral vestibular pathologies such as labyrinthitis, vestibular neuritis, Meniere's disease, etc. Also, the mVEMP assessing the neural pathways in various central pathologies is sparse. Hence, further studies are required to understand the vestibulomassetric reflex pathways and the effect of different peripheral and central vestibular pathologies.
16. Conclusion
mVEMP is a new tool to assess the vestibulo-massetric reflex pathway. Few published studies have explored the clinical application of mVEMP in a few neural disorders. However, the wide clinical application of mVEMP needs to be explored thoroughly. The mVEMP can be employed as a supplementary tool in clinical vestibular practice. mVEMP is much easier to administer, does not cause any discomfort to the subjects, and can be recorded with any equipment used to record the cervical VEMP.
Declarations
The authors declare no conflict of interest.
Funding
There are no funding source for this paper.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Bansal S., Sahni S., Sinha S. Cervical and ocular vestibular evoked myogenic potentials in individuals with severe to profound hearing loss. J Hear Science. 2013;3(4):56–63. doi: 10.17430/889971. [DOI] [Google Scholar]
- Bickford R.G., Jacobson J.L., Cody D., Thane R. Nature of average evoked potentials to sound and other stimuli in man. Ann. N. Y. Acad. Sci. 1964;8(112):204–223. doi: 10.1111/j.1749-6632.1964.tb26749.x. [DOI] [PubMed] [Google Scholar]
- Cheng P.W., Huang T.W., Young Y.H. The influence of clicks versus short tone bursts on the vestibular evoked myogenic potentials. Ear Hear. 2003;24(3):195–197. doi: 10.1097/01.aud.0000069225.80220.cb. [DOI] [PubMed] [Google Scholar]
- Cherchi M., Bellinaso N.P., Card K., Covington A., Krumpe A., Pfeifer M.S., et al. Sound evoked triceps myogenic potentials. Otol. Neurotol. 2009;30(4):545–550. doi: 10.1097/mao.0b013e31819d89eb. [DOI] [PubMed] [Google Scholar]
- Çoban V.K., Öçal F.C.A., Karaçaylı C., Satar B. Differences in bone conduction ocular vestibular evoked myogenic potentials to 500 Hz narrow band chirp stimulus and 500 Hz tone burst. Auris Nasus Larynx. 2021;48(4):590–593. doi: 10.1016/j.anl.2020.11.008. [DOI] [PubMed] [Google Scholar]
- Cody D.T.R., Bickford R.G., Klass D.W. Averaged evoked myogenic responses in normal man Laryngoscope. 1969;8(2–3):391–397. doi: 10.1288/00005537-196903000-00007. [DOI] [PubMed] [Google Scholar]
- Cody D.T.R., Jacobson J.L., Walker J.C., Bickford R.G. LXIV averaged evoked myogenic and cortical potentials to sound in man. Ann. Otol. Rhinol. Laryngol. 1964;73(3):763–777. doi: 10.1177/000348946407300315. [DOI] [PubMed] [Google Scholar]
- Colebatch J.G., Rothwell J.C. Vestibular-evoked EMG responses in human neck muscles. J Physiol. 1993;473:18. [Google Scholar]
- Colebatch J.G., Halmagyi G.M. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992;42(8):1635. doi: 10.1212/wnl.42.8.1635. 1635. [DOI] [PubMed] [Google Scholar]
- Colebatch J.G., Halmagyi G.M., Skuse N. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J. Neurol. Neurosurg. Psychiatry. 1994;57(2):190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccurazzu B., Deriu F., Tolu E., Yates B.J., Billig I. A monosynaptic pathway links the vestibular nuclei and masseter muscle motoneurons in rats. Exp. Brain Res. 2007;176(4):665–671. doi: 10.1007/s00221-006-0834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha L.C.M., Labanca L., Tavares M.C., Gonçalves D.U. Vestibular evoked myogenic potential (VEMP) with galvanic stimulation in normal subjects. Braz J Otorhinolaryngol. 2014;80:48–53. doi: 10.5935/1808-8694.20140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Natale E.R., Ginatempo F., Laccu I., Figorilli M., Manca A., Mercante B., et al. Vestibular evoked myogenic potentials are abnormal in idiopathic REM sleep behavior disorder. Front. Neurol. 2018;9:911. doi: 10.3389/fneur.2018.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Natale E.R., Ginatempo F., Mercante B., Manca A., Magnano I., Ortu E., et al. Vestibulo masseteric reflex and acoustic masseteric Reflex. Normative data and effects of age and gender. Clin. Neurophysiol. 2019;130(9):1511–1519. doi: 10.1016/j.clinph.2019.05.021. [DOI] [PubMed] [Google Scholar]
- de Natale E.R., Ginatempo F., Paulus K.S., Manca A., Mercante B., Pes G.M., et al. Paired neurophysiological and clinical study of the brainstem at different stages of Parkinson's Disease. Clin. Neurophysiol. 2015;126(10):1871–1878. doi: 10.1016/j.clinph.2014.12.017. [DOI] [PubMed] [Google Scholar]
- de Natale E.R., Ginatempo F., Paulus K.S., Pes G.M., Manca A., Tolu E., et al. Abnormalities of vestibular-evoked myogenic potentials in idiopathic Parkinson's disease are associated with clinical evidence of brainstem involvement. Neurol. Sci. 2015;36:995–1001. doi: 10.1007/s10072-014-2054-4. [DOI] [PubMed] [Google Scholar]
- Deriu F., Ortu E., Capobianco S., Giaconi E., Melis F., Aiello E., et al. Origin of sound‐evoked EMG responses in human masseter muscles. J Physiol. 2007;580(1):195–209. doi: 10.1113/jphysiol.2006.123240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu F., Podda M.V., Chessa G., Tolu E. Trigeminal integration of vestibular and forelimb nerve inputs. Arch. Ital. Biol. 1999;137(1):63–73. [PubMed] [Google Scholar]
- Deriu F., Podda M.V., Milia M., Chessa G., Sau G., Pastorino M., et al. Masseter muscle activity during vestibular stimulation in man. Arch. Ital. Biol. 2000;138(3):205–215. [PubMed] [Google Scholar]
- Deriu F., Tolu E., Rothwell C. A short latency vestibulomasseteric reflex evoked by electrical stimulation over the mastoid in healthy humans. J Physiol. 2003;553(1):267–279. doi: 10.1113/jphysiol.2003.047274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu F., Tolu E., Rothwell J.C. A sound-evoked vestibulomasseteric reflex in healthy humans. J. Neurophysiol. 2005;93(5):2739–2751. doi: 10.1152/jn.01005.2004. [DOI] [PubMed] [Google Scholar]
- Edmonson A., Iwanaga J., Olewnik Ł., Dumont A.S., Tubbs R.S. The function of the tensor tympani muscle: a comprehensive review of the literature. Anat Cell Biol. 2022;55(2):113–117. doi: 10.5115/acb.21.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaconi E., Deriu F., Tolu E., Cuccurazzu B., Yates B.J., Billig I. Transneuronal tracing of vestibulo-trigeminal pathways innervating the masseter muscle in the rat. Exp. Brain Res. 2006;171:330–339. doi: 10.1007/s00221-005-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginatempo F., Ortu E., Pilurzi G., Tolu E., Deriu F. 121. Vestibulo-masseteric reflex (VMR) and acoustic-masseteric reflex (AMR): Normative values. Clin. Neurophysiol. 2013;124(11):e216. doi: 10.1016/j.clinph.2019.05.021. [DOI] [PubMed] [Google Scholar]
- Grinberg L.T., Rueb U., Alho A.T., Heinsen H. Brainstem pathology and non-motor symptoms in PD. J. Neurol. Sci. 2010;289(1–2):81–88. doi: 10.1016/j.jns.2009.08.021. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Shimizu Y., Satoh C., Watanabe M. Masticatory motion is controlled in humans by a limited set of muscle synergies. Tohoku J. Exp. Med. 2010;220(3):217–222. doi: 10.1620/tjem.220.217. [DOI] [PubMed] [Google Scholar]
- Hickenbottom R.S., Bishop B., Moriarty T.M. Effects of whole-body rotation on masseteric motoneuron excitability. Exp. Neurol. 1985;89(2):442–453. doi: 10.1016/0014-4886(85)90103-7. [DOI] [PubMed] [Google Scholar]
- Karaçaylı C., Akın Öçal F.C., Çoban V.K., Satar B. Normative data of ocular vestibular evoked myogenic potentials in response to chirp stimulus. J Int Adv Otol. 2020;16(3):378–381. doi: 10.5152/iao.2020.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kılınç E., Gençtürk E., Taşcı B., Şerbetçioğlu M.B. Normalızatıon of masseter VEMP and comparıson wıth cervıcal VEMP ın normal ındıvıduals Egypt. J. Otolaryngol. 2023;39(1):1–7. doi: 10.1186/s43163-023-00416-0. [DOI] [Google Scholar]
- Loi N., Manca A., Ginatempo F., Deriu F. The vestibulo-masseteric reflex and the acoustic-masseteric reflex: a reliability and responsiveness study in healthy subjects. Exp. Brain Res. 2020;238:1769–1779. doi: 10.1007/s00221-020-05804-z. [DOI] [PubMed] [Google Scholar]
- Lund J.P., Olsson K.A. The importance of reflexes and their control during jaw movement. Trends Neurosci. 1983;6:458–463. doi: 10.1016/0166-2236(83)90219-9. [DOI] [Google Scholar]
- Magnano I., Pes G.M., Cabboi M.P., Pilurzi G., Ginatempo F., Achene A., et al. Comparison of brainstem reflex recordings and evoked potentials with clinical and MRI data to assess brainstem dysfunction in multiple sclerosis: a short-term follow-up. Neurol. Sci. 2016;37(9):1457–1465. doi: 10.1007/s10072-016-2604-z. [DOI] [PubMed] [Google Scholar]
- Magnano I., Pes G.M., Pilurzi G., Cabboi M.P., Ginatempo F., Giaconi E., et al. Exploring brainstem function in multiple sclerosis by combining brainstem reflexes, evoked potentials, clinical and MRI investigations. Clin. Neurophysiol. 2014;125(11):2286–2296. doi: 10.1016/j.clinph.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Manns A., Chan C., Miralles R. Influence of group function and canine guidance on electromyographic activity of elevator muscles. J. Prosthet. Dent. 1987;57(4):494–501. doi: 10.1016/0022-3913(87)90024-2. [DOI] [PubMed] [Google Scholar]
- Mat Q., Duterme J.P., Tainmont S., Lelubre C., Manto M. Optimizing ocular vestibular evoked myogenic potentials with narrow band CE-chirps. Ear Hear. 2021;42(5):1373–1380. doi: 10.1097/aud.0000000000001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ewert K., Gleitsmann K., Reiter F. Acoustic jaw reflex in man: its relationship to other brainstem and microreflexes. Electroencephalogr. Clin. Neurophysiol. 1974;36:629–637. doi: 10.1016/0013-4694(74)90229-6. [DOI] [PubMed] [Google Scholar]
- Mukerji S., Windsor A.M., Lee D.J. Auditory brainstem Circuits that mediate the Middle ear muscle reflex. Trends Amplif. 2010;14(3):170–191. doi: 10.1177/1084713810381771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupane A.K., Bhagat H., Bheda K. Comparison of chirp versus tone burst–and click-evoked masseteric vestibular evoked myogenic potentials in normal-hearing adults. Am. J. Audiol. 2023;1–11 doi: 10.1044/2022_aja-22-00155. [DOI] [PubMed] [Google Scholar]
- Piker E.G., Jacobson G.P., Makowiec K.F., Atabek P.M., Krolewicz S. The medial canthus reference electrode is not electrically indifferent to the ocular vestibular evoked myogenic potential. Otol. Neurotol. 2018;39(10):e1069–e1077. doi: 10.1097/mao.0000000000001978. [DOI] [PubMed] [Google Scholar]
- Puligheddu M., Figorilli M., Serra A., Laccu I., Congiu P., Tamburrino L., et al. REM Sleep without atonia correlates with abnormal vestibular-evoked myogenic potentials in isolated REM sleep behavior disorder. Sleep. 2019;42(9):zsz128. doi: 10.1093/sleep/zsz128. [DOI] [PubMed] [Google Scholar]
- Romero D.J., Jacobson G.P., Roberts R.A. The effect of EMG magnitude on the masseter vestibular evoked myogenic potential (mVEMP) J Otol. 2022;17(4):203–210. doi: 10.1016/j.joto.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren S.M., Todd N.M., Colebatch J.G. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin. Neurophysiol. 2005;116(8):1938–1948. doi: 10.1016/j.clinph.2005.03.019. HYPERLINK. [DOI] [PubMed] [Google Scholar]
- Rudisill H.E., Hain T.C. Lower extremity myogenic potentials evoked by acoustic stimuli in healthy adults. Otol. Neurotol. 2008;29(5):688–692. doi: 10.1097/MAO.0b013e3181730377. [DOI] [PubMed] [Google Scholar]
- Sangu Srinivasan V., Rangappan Munirathinam B., Singh N.K., Rajalakshmi K. Usefulness of masseter vestibular evoked myogenic potentials in identifying brainstem dysfunction among individuals with multiple sclerosis. Int. J. Audiol. 2022;1–9 doi: 10.1080/14992027.2022.2065548. [DOI] [PubMed] [Google Scholar]
- Singh N.K., Apeksha K. The effect of rise/fall time of 500 Hz short tone bursts on cervical vestibular evoked myogenic potential. J. Vestib. Res. 2014;24(1):25–31. doi: 10.3233/ves-130503. [DOI] [PubMed] [Google Scholar]
- Thirusangu V.P., Sinha S.K. Effect of electrode montage on 500-hz tone burst evoked masseter vestibular evoked myogenic potential. Am. J. Audiol. 2022;31(2):403–410. doi: 10.1044/2022_aja-22-00016. HYPERLINK. [DOI] [PubMed] [Google Scholar]
- Todd N.P., Rosengren S.M., Aw S.T., Colebatch J.G. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin. Neurophysiol. 2007;118(2):381–390. doi: 10.1016/j.clinph.2006.09.025. HYPERLINK. [DOI] [PubMed] [Google Scholar]
- Tolu E., Pugliatti M. The vestibular system modulates masseter muscle activity. J. Vestib. Res. 1993;3(2):163–171. [PubMed] [Google Scholar]
- Vignesh S.S., Singh N.K., Rajalakshmi K. Tone burst masseter vestibular evoked myogenic potentials: Normative values and test–retest reliability. Journal J Am Acad Audiol. 2021;32(5):308–314. doi: 10.1055/s-0041-1728718. [DOI] [PubMed] [Google Scholar]
- Vinayagar P.T., Sinha S.K. Characteristics of ipsilateral, contralateral and bilateral masseter vestibular-evoked myogenic potentials in healthy adults. J. Laryngol. Otol. 2023;1–24 doi: 10.1017/s0022215123000051. HYPERLINK. [DOI] [PubMed] [Google Scholar]
- Wang S.J., Young Y.H. Vestibular evoked myogenic potentials using simultaneous binaural acoustic stimulation. Hear. Res. 2003;185(1–2):43–48. doi: 10.1016/s0378-5955(03)00256-9. [DOI] [PubMed] [Google Scholar]
- Watson S.R., Fagan P., Colebatch J.G. Galvanic stimulation evokes short-latency EMG responses in sternocleidomastoid which are abolished by selective vestibular nerve section. Electroencephalogr. Clin. Neurophysiol. 1998;109(6):471–474. doi: 10.1016/s0924-980x(98)00033-2. HYPERLINK. [DOI] [PubMed] [Google Scholar]
- Wu C.H., Young Y.H., Murofushi T. Tone burst-evoked myogenic potentials in human neck flexor and extensor. Acta Otolaryngol. 1999;119(7):741–744. doi: 10.1080/00016489950180351. [DOI] [PubMed] [Google Scholar]
- Wu H.J., Shiao A.S., Yang Y.L., Lee G.S. Comparison of short tone burst-evoked and click-evoked vestibular myogenic potentials in healthy individuals. J. Chin. Med. Assoc. 2007;70(4):159–163. doi: 10.1016/s1726-4901(09)70350-8. [DOI] [PubMed] [Google Scholar]
- Xie W.Y., Shen Y., Chen Y., Zhuang S., Wang Y.L., Jin H., et al. REM sleep without atonia and vestibular-evoked myogenic potentials: clinical brainstem dysfunction in early-stage Parkinson's disease and isolated REM sleep behavior disorder. Sleep Med. 2022;89:122–129. doi: 10.1016/j.sleep.2021.12.004. [DOI] [PubMed] [Google Scholar]