Abstract
Ferroptosis is a newly discovered non-apoptotic and iron-dependent type of cell death. Ferroptosis mainly takes place owing to the imbalance of anti-oxidation and oxidation in the body. It is regulated via a number of factors and pathways both inside and outside the cell. Ferroptosis is closely linked with brain and various neurological disorders (NDs). In the human body, the brain contains the highest levels of polyunsaturated fatty acids, which are known as lipid peroxide precursors. In addition, there is also a connection of glutathione depletion and lipid peroxidation with NDs. There is growing evidence regarding the possible link between neuroinflammation and multiple NDs, such as Alzheimer's disease, amyotrophic lateral sclerosis, Parkinson's disease, Huntington's disease, and stroke. Recent studies have demonstrated that disruptions of lipid reactive oxygen species (ROS), glutamate excitatory toxicity, iron homeostasis, and various other manifestations linked with ferroptosis can be identified in various neuroinflammation-mediated NDs. It has also been reported that damage-associated molecular pattern molecules including ROS are generated during the events of ferroptosis and can cause glial activation via activating neuroimmune pathways, which subsequently leads to the generation of various inflammatory factors that play a role in various NDs. This review summarizes the regulation pathways of ferroptosis, the link between ferroptosis as well as inflammation in NDs, and the potential of a range of therapeutic agents that can be used to target ferroptosis and inflammation in the treatment of neurological disorders.
Keywords: Ferroptosis, Neuroinflammation, Apoptosis, Alzheimer's disease, Phytochemicals
1. Introduction
Neurological disorders (NDs) are responsible for causing substantial morbidity and mortality worldwide [1]. The number of deaths and disabilities is also increasing because of aging and population overgrowth, which is indicating that the management and prevention of primary neurological conditions are largely unsuccessful. Neuroinflammation is a common etiopathogenic factor in various NDs and neurodegenerative diseases, including Alzheimer's disease (AD), Huntington's disease (HD), Parkinson's disease (PD), stroke, and amyotrophic lateral sclerosis (ALS) [[2], [3], [4], [5]]. Neuroinflammation is a well-orchestrated and complex mechanism involved with the interactions of peripheral immune cells and glial cells in the central nervous system (CNS) [6]. It has been observed that activations of glia and peripheral lymphocytes trigger the release of various inflammatory factors that can further injure the nervous system. Various studies have revealed that disruption of iron homeostasis, iron-dependent lipid reactive oxygen species (ROS) accumulation, and multiple other manifestations are present in some neuroinflammation-caused NDs, which further indicates that ferroptosis has a significant contribution in the incidence and development of several NDs including AD, stroke, PD, ALS, and HD [7].
In recent times, ferroptosis has been identified as a type of regulated cell death [8]. Ferroptosis is an iron-dependent, non-apoptotic, and oxidative form of cell death [9]. Ferroptosis differs from autophagy, necrosis, and apoptosis in terms of heredity, biochemistry, and morphology [10]. Ferroptosis is primarily characterized by lipid peroxidation (LPO) and glutathione (GSH) consumption, which also includes specific oxidation of phosphatidylethanolamine (PE) comprising adrenal acid and arachidonic acid (AA) [11]. It has been reported that various unique gene set is essential for erastin-mediated ferroptosis including tetratricopeptide repeat domain 35, citrate synthase, F0–F1-ATPase subunit C3, iron response element-binding protein 2, ribosomal protein L8, and acetyl-CoA synthetase family member [12]. Indeed, ferroptosis has a significant contribution in the brain and NDs [13]. In the human body, the highest level of polyunsaturated fatty acids (PUFAs) is found in the brain [14]. There is a close link between LPO and GSH depletion with NDs, such as neurodegeneration, stroke, and neurotrauma [15]. An elevated level of ferroptosis PE marker has been observed following traumatic brain injury [16]. Furthermore, cellular ferroptosis has been detected in dopaminergic neurons in the case of PD and various other neurodegenerative disorders [17]. In mouse models of ischemic stroke, several ferroptosis inhibitors including liproxstatin-1 (Lip-1) and ferrostatin-1 (Fer-1) markedly decreased functional deficits. This further suggests that ferroptosis is linked with ischemia-reperfusion injury and stroke [18]. Multiple in vitro and in vivo studies have detected GSH peroxidase 4 (GPX4) genetic defects in neuronal death [[19], [20], [21]].
GPX4 is an important ferroptosis regulator. GPX4 is a GSH-dependent enzyme that is responsible for reducing lipid hydroperoxides (L-OOH) to lipid alcohols (L-OH), which further averts the iron (Fe2+)-dependent accumulation of toxic lipid ROS. In addition, GSH plays a role as a GPX4 cofactor and also plays a role in preserving GPX4 levels via the exchange of cysteine and glutamate through cystine/glutamate antiporter system Xc−. Cysteine is a reduced form of cysteine, which serves as a GSH synthesis precursor. Therefore, the suppression of GSH synthesis or system Xc− and direct GPX4 inactivation can cause significant GPX4 inactivation and GSH depletion, which can eventually result in the buildup of lipid peroxides (LPs) and ferroptosis cell death. As the iron-dependent buildup of oxidatively damaged phospholipids is a hallmark feature of ferroptosis, both LPO and iron metabolism are also two important mechanisms apart from GPX4. Indeed, iron is crucial for the execution of ferroptosis and the buildup of LPs. The impairment of iron metabolism, such as storing iron (ferritin), iron export (ferroportin), iron uptake (transferrin receptor), and ferritinophagy induced by nuclear receptor coactivator 4 (NCOA4) and lysosome, can further trigger iron overload and subsequently can catalyze the generation of toxic lipid ROS via Fenton reaction [22]. PUFAs are usually prone to LPO and are required for the execution of ferroptosis [23]. The substrates of the synthetic lipid signaling mediums are free PUFAs, however they are required to be oxidized into signals of ferroptotic and esterified into membrane phospholipids [24]. Therefore, the localization and abundance of PUFAs regulate the extent of ferroptosis and the extent of LPO [25].
A range of ferroptosis inhibitors with different targets have already been identified, such as GPX4, Fer-1, antioxidants, iron chelators, and multiple phytochemicals [9,12,[26], [27], [28], [29]]. This review summarizes ferroptosis and its regulatory mechanisms as well as the relationship between ferroptosis and neuroinflammation. Moreover, the role of ferroptosis and inflammation in neurological disorders and the potential of a range of therapeutic agents that can be used to target ferroptosis and inflammation in the treatment of neurological disorders.
2. Methods
The search strategy for this review aimed at identifying all original research, books, clinical trials, and review articles that discussed the role of ferroptosis pathways in neuroinflammation and neurological disorders as well as their treatment strategies. In this regard, a range of databases including ScienceDirect, Scopus, Web of Science, PubMed, and Google Scholar were searched from 2012 to April 2023. The terms or combinations of terms that were used while searching the databases include ferroptosis, ferroptosis pathways, neuroinflammation, neurological disorders, ferroptosis regulation, targeting ferroptosis to treat neurological disorders, Alzheimer's disease, Parkinson's disease, Amyotrophic lateral sclerosis, Huntington's disease, stroke, iron chelators, deferiprone, quercetin, ferrostatin-1, vitamin E, liproxstatin-1, baicalein, coenzyme Q10, alpha-lipoic acid, puerarin, and phytochemicals.
3. Ferroptosis
Ferroptosis is a new type of oxidative cell death that is mediated by the buildup of iron-mediated LPO [9,30,31]. Immunological and morphological features of ferroptosis are different from autophagy, apoptosis, and necroptosis [32]. The characteristics of cell ferroptosis include the ruptured mitochondrial outer membrane, elevated mitochondrial membrane density, significantly reduced or even disappeared mitochondrial cristae, considerably smaller mitochondria, normal size of the nucleus, and no chromatin agglutination [32,33]. Moreover, cell ferroptosis occurs along with the presence of ROS, iron, and PUFA-containing phospholipids. Phospholipid peroxidation leads to the destruction of cell membrane integrity, which eventually can result in ferroptosis [30,34]. Ferroptosis is induced by iron-dependent peroxidation of phospholipids, which is controlled via several cellular metabolic pathways, such as redox homeostasis, sugars, iron handling, lipids, mitochondrial function, and metabolism of amino acids, along with several signaling mechanisms associated with diseases. Ferroptosis also induces many degenerative pathologies and organ injuries. Interestingly, cancer cells resistant to therapies, specifically those prone to metastasis and in the mesenchymal state are highly susceptible to ferroptosis. Therefore, pharmacological ferroptosis modulation is highly promising for the treatment of various degenerative disorders, ischaemic organ injuries, and drug-resistant cancers associated with extensive levels of lipid peroxidation [30].
3.1. Regulatory mechanisms of ferroptosis
3.1.1. Iron metabolism
Iron is the most abundant essential trace element found in the human body [35]. Iron is an important metal that is associated with numerous physiological processes including DNA synthesis, cellular respiration, oxygen transport, and biosynthesis of neurotransmitters in the nervous system [36]. Homeostasis of iron is vital for the development and survival of normal cells [37]. On the other hand, since iron plays a role in mediating ROS generation and enzyme function in LPO, therefore ferroptosis is strictly regulated via iron metabolism regulators including iron utilization, storage, uptake, and efflux. Moreover, transcriptional and translational regulation of iron homeostasis provides an assimilated network to estimate ferroptosis sensitivity. It has been observed that impaired ferroptosis is associated with a range of iron-linked pathological diseases or conditions, including neurodegenerative diseases, cancer, and ischemia-reperfusion injury [38].
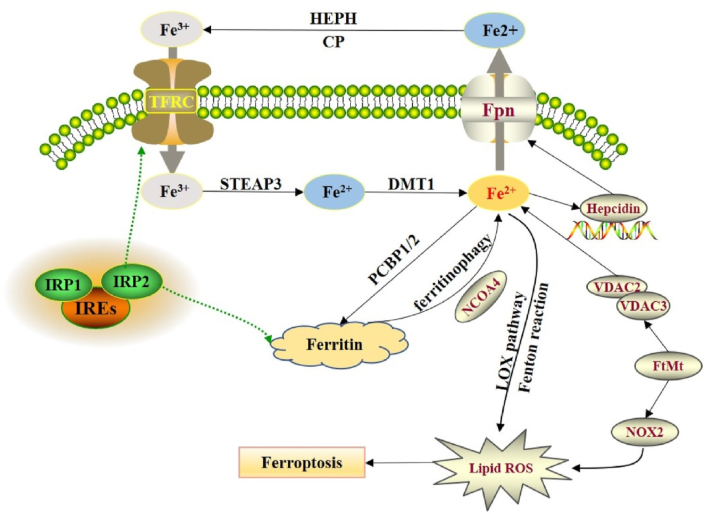
Posttranscriptional regulation of the iron regulatory proteins (IRP1 and IRP2) regulates cellular iron metabolism [39]. Under normal physiological conditions, IRP1 and IRP2 can control several iron metabolism genes including ferritin heavy chain 1 (FTH1) and TFRC to retain the stability of unstable iron pools [40]. Moreover, iron can exist both in ferrous (Fe2+) and ferric (Fe3+) forms, whereas circulating iron can exist in ferric form (Fe3+) by binding with transferrin (TF). It has been reported that free Fe3+ can enter into the cell via the membrane protein TF receptor 1 (TFR1) and subsequently can be stored in the nucleosome, while Fe3+ is converted to Fe2+ via six transmembrane epithelial antigen of the prostate 3 (STEAP3) (Fig. 1) [41,42]. Subsequently, divalent metal transporter 1 (DMT1) transports Fe2+ from the endosome to the cytoplasm [43].
Fig. 1.
Regulation of iron homeostasis in ferroptosis. Reproduced with permission from Elsevier, Reference [42]. Abbreviations: FPN, ferroportin; DMT1, divalent metal transporter 1; FtMt, ferritin mitochondria; HEPH, hephaestin; PCBP, poly-(rC)-binding protein; NCOA4, nuclear receptor coactivator 4; NOX2, NADPH oxidase 2; ROS, reactive oxygen species; STEAP3, six transmembrane epithelial antigen of the prostate 3; VDAC, voltage-dependent anion channels.
Fe2+ is typically stored in the ferritocyte stock protein complex (comprised of ferritin light chain and FTH1) to avert ROS generation and keep the balance of unstable iron pools [44]. The membrane protein ferritin 1 mediates the export of Fe2+ to the extracellular space [45]. Interestingly, if the utilization, storage, transport, and uptake of intracellular iron fails, then an excess level of free Fe2+ deposits in the cell, and the Fenton reaction is initiated, which leads to the generation of ROS and hydroxyl radicals [46]. ROS then alters and interferes with DNA, lipids, and proteins, and an array of peroxidation reactions take place with PUFAs on the cell membrane, which further produces LPs and results in obliteration of the cell membrane structures, which eventually results in cell ferroptosis [47]. Accumulation of iron primarily takes place because of barriers including DMT1, TFR1, and ferroportin (FPN), which further lead to loss of iron transport regulation (Fig. 1) [40]. On the other hand, NCOA4-mediated degradation of the ferritin phagocytosis cascade leads to an elevated level of iron storage, then Fenton reaction/mitochondrial injury/lipoxygenases (LOXs) activity results in elevated iron level in the active iron pool [48]. Subsequently, an increased level of ROS results in ferroptosis. Furthermore, Fe2+ also plays a role as a cofactor of various metabolic enzymes, mediates the ROS generation, and increases the function of several metabolic enzymes including PDH1 and LOXs [39,40].
3.1.2. Amino acid metabolism
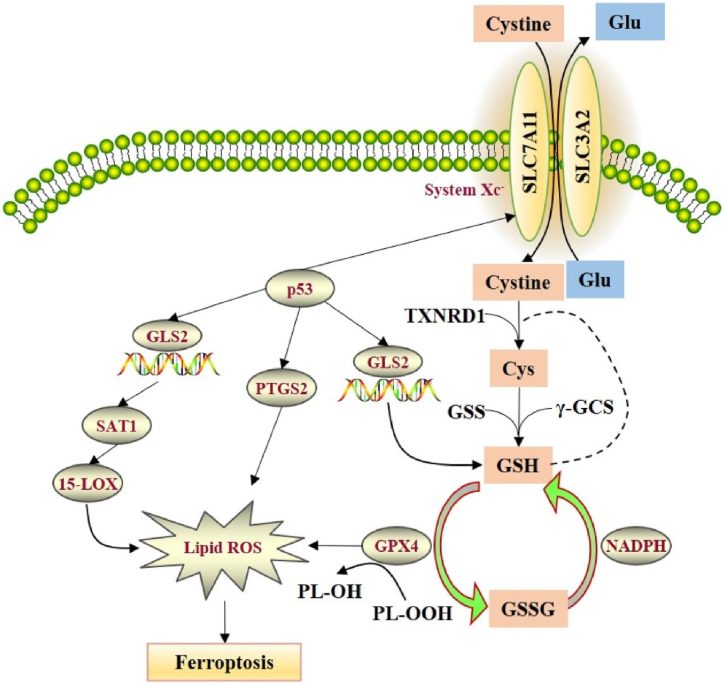
It has already been revealed that cystine/glutamic acid metabolism has a significant contribution in ferroptosis [49]. System Xc− is an amino acid antiporter that contains heavy-chain SLC3A2 and light-chain solute carrier family 7 member 11 (SLC7A11) connected by disulfide bonds, which transports cystine inward and glutamate outward in a 1:1 ratio [50]. Interestingly, cystine is moved to cells and reduced to cysteine, which then binds with glycine and glutamic acid to generate GSH. GSH is a vital antioxidant that provides protection to cells against oxidative stress (OS) and is the substrate for the lipid repair activity of GPX4. GPX4 is a strong antioxidant enzyme. Along with GSH as the reducing agent, it catalyzes the conversion of peroxide bond into a hydroxyl group and converts the peroxide into a lipid alcohol, which induces the loss of its oxidative function, therefore suppresses ferroptosis. Ras selective lethal small molecule 3 (RSL3) and erastin play roles as activators of ferroptosis [40]. Erastin can also suppress its function via binding with SLC7A11, which affects cystine transfer and reduces the generation of GSH, which further leads to the failure of cells to eliminate LPs in time. These events further result in injury of cell membranes and eventually induce ferroptosis (Fig. 2) [51]. It has been observed that RSL3 can cause GPX4 inactivation via covalently binding with GPX4 and disturb the cellular redox homeostasis, which results in an elevated level of LPO and ultimately ferroptosis [52]. Furthermore, transcription factors can induce the SLC7A11 expression to control ferroptosis, where several tumor suppressor genes including BECN1, BAP1, and TP53 play role as negative regulators of SLC7A11 and nuclear transcription factor (NRF2) play a role as a positive regulator to increase SLC7A11 expression [[53], [54], [55]].
Fig. 2.
Regulation mechanisms of System Xc-in ferroptosis. Reproduced with permission from Elsevier, Reference [42]. Abbreviations: GLS2, glutaminase 2; GSH, glutathione; GPX4, glutathione peroxidase 4; ROS, reactive oxygen species; γ-GCS, cysteine synthase; GSSG, oxidized GSH; SAT1, spermidine/spermine N1-acetyltransferase 1; PTGS2, prostaglandin endoperoxide synthase 2; SLC7A11, solute carrier family 7 member 11; SLC3A2, recombinant solute carrier family 3 member 2; TXNRD1, thioredoxin reductase 1.
3.1.3. Lipid peroxidation
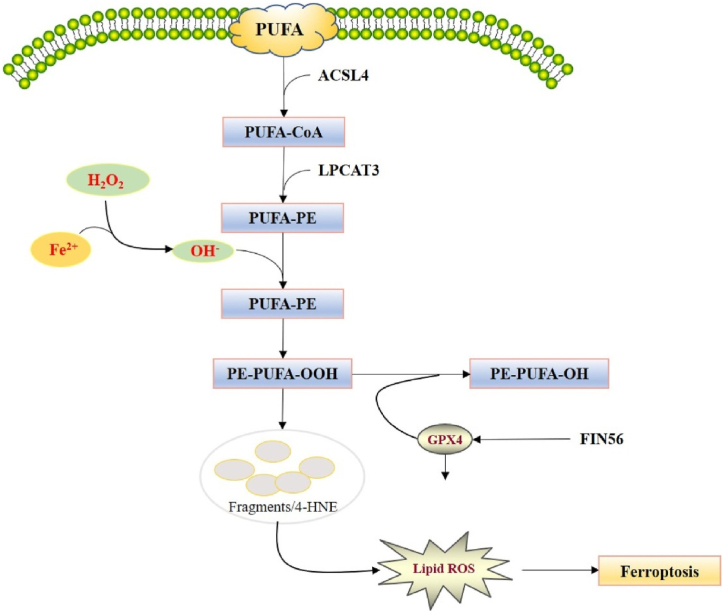
ROS are byproducts of cellular metabolism. ROS are associated with cell signaling and maintaining stability under normal metabolic conditions. On the other hand, excessive buildup of intracellular ROS can result in cell death under pathological conditions [40]. LPs function as an important mediator of numerous pathological conditions, such as neurodegenerative diseases, cancer, and inflammation [56]. OH• can induce LPO to produce lipid peroxyl radicals and lipid radicals, which can further react with PUFAs to produce LPs and ultimately result in ferroptosis (Fig. 3) [57]. There are three pathways through which iron can participate in the accumulation of ROS [58]. ROS can react with PUFAs in the lipid membrane to trigger LPO, which further mediates intracellular ferroptosis [27,[59], [60], [61]]. It has been detected that PUFAs possess dyalenyl hydrogen atoms; therefore they react easily with ROS to cause LPO [62]. PEs consist of AA or adrenic acid that mediate cellular ferroptosis. Thus, ferroptosis could be elevated by providing AA or various other PUFAs and via suppressing the functions of lysophosphatidylcholine acyltransferase 3 (LPCAT3) and acyl-CoA synthetase long-chain family member 4 (ACSL4) [40,63]. Thus, ferroptosis could be elevated via supplementing AA or other PUFAs and via suppressing the functions of LPCAT3 and ACSL4 [40,63].
Fig. 3.
Regulation of lipid peroxidation in ferroptosis. Reproduced with permission from Elsevier, Reference [42]. Abbreviations: ACSL4, acyl-CoAsynthetase long-chain family member 4; LPCAT3, lysophosphatidylcholine acyltransferase 3; GPX4, glutathione peroxidase 4; PUFA, polyunsaturated fatty acid.
4. The link between ferroptosis and neuroinflammation
Inflammation usually takes place in response to tissue injury or pathogen, which is usually stated as a complex biological response [64]. Besides recognition of pathogens, the immune system has the capacity to respond to cellular injuries, such as inflammatory diseases, systemic autoimmune diseases, and acute organ rejection. Following stress-induced injury, endogenous danger signals have the ability to sense harmful stimuli via activating the immune response. Danger-associated molecular patterns (DAMPs) can induce such type of inflammation in response to cell death and stress [65]. It has been confirmed that ferroptosis contributes in the pathological mechanisms of various NDs (Table 1). Therefore studies have been carried out to elucidate the events of ferroptosis that play a role in neuroinflammation to neurological conditions [7]. DAMP molecules are generated during the events of ferroptosis, which then cause glial activation via activating the neuroimmune system. In addition, glial activation leads to the generation of several inflammatory factors which then results in neuronal injury and various NDs [7].
Table 1.
Ferroptosis is associated with the pathogenesis of various neurological disorders.
| Neurological disorders | Characteristics of ferroptosis | References |
|---|---|---|
| Alzheimer's disease | Reduced expression of ferroportin; reduced levels of glutathione peroxidase 4 (GPX4), glutathione (GSH), and solute carrier family 7 member 11 (SLC7A11); iron overload; elevated level of nuclear receptor coactivator 4 | [[66], [67], [68], [69], [70], [71], [72]] |
| Parkinson's disease | Reduced level of coenzyme Q10 (CoQ10); iron overload; ferritinophagy; reduced level of GSH and SLC7A11; elevated expression of divalent metal transporter 1 | [66,[73], [74], [75], [76], [77]] |
| Amyotrophic lateral sclerosis | Lipid peroxidation; aberrant metabolism of GSH; iron dysregulation; iron overload | [78,79] |
| Huntington's disease | Reduced level of GSH; iron overload | [66,80] |
| Strokes | Increased level of reactive oxygen species; reduced level of CoQ10; iron overload; reduced levels of GPX4, GSH, and SLC7A11 | [66,[81], [82], [83], [84], [85], [86], [87], [88]] |
4.1. Reactive oxygen species and nuclear factor kappa B (NF-κB) signaling
GPX4 deficiency occurs during the events of ferroptosis, which can cause lipid ROS accumulation and result in ferroptosis [52,89]. GPX4 uses GSH as a cofactor in order to eliminate LPO from the membrane. Interestingly, GPX4 reduces the complex hydroperoxide, such as cholesterol hydroperoxide and phosphatidyl hydroperoxide, to the corresponding alcohol to avoid detrimental OS and keep the redox balance. OS and ROS are common features linked with inflammation [90]. ROS causes activation of the nuclear factor kappa B (NF-κB) pathway. Key components of the NF-κB pathway include the transcription factor NF-κB, the IκB kinase (IKK) complex, and the inhibitory IκB protein. It has been observed that external stimuli can induce IKK to cause phosphorylation, ubiquitination, and eventually degradation of IκB protein. Several posttranslational modifications can cause activation of the released dimer of NF-κB, which is then transported to the nucleus to bind with certain DNA sequences, which then can mediate the transcription of target genes. In some cell types, ROS (particularly H2O2) can cause the activation of IKK. ROS induces IKKγ/NEMO dimerization, which is the IKK subunit, via mediating the generation of disulfide bonds between Cys347 and Cys54, which further leads to IKK activation and also initiates the NF-κB signaling pathway [91].
Moreover, ROS control of the upstream NF-κB activation pathways is also reflected in the case of selective phosphorylation of IκBα. Typically, IκBα is phosphorylated at Ser36 and Ser32, however H2O2 can cause IκBα ubiquitination and degradation by affecting the IκBα phosphorylation at Tyr42 or various other tyrosine residues. It has been observed that the released dimer of NF-κB can migrate to the nucleus, where it can bind with the κB site on the DNA sequence and can mediate the transcription of various target genes. GPX4 has a significant contribution in eliminating peroxides by suppressing the NF-κB activation and decreasing ROS accumulation. NF-κB is closely linked with inflammation, which has been found to be expressed in nearly all cell tissues including astrocytes, neurons, and microglia [92]. In addition, it controls several functional target genes and plays roles in several biological mechanisms including tumorigenesis, apoptosis, inflammation, and immune response. Microglia are the primary immune cells of the brain and they have a significant contribution in normal brain development and neuroinflammatory disorders. Chronic NF-κB activation triggers the transcription of several neuroinflammatory genes and mediates the release of various inflammatory cytokines including neurotoxins, adhesion molecules, nitric oxide, interleukin-1 beta (IL-1β), interleukin (IL)-6, and tumor necrosis factor alpha (TNF-α), which further induces neuronal apoptosis [93]. In the brain, ROS accumulation during the events of ferroptosis can result in NF-κB activation in the glia and also can lead to neuroinflammation [7].
4.2. Lipid peroxidation and neuroinflammation
LPO is an important hallmark of ferroptosis. It has been revealed that lipids form LPs in order to interfere with the membrane structures as well as activities of membrane proteins and to trigger intracellular signal transduction and induce various other cascades that result in cell death [9,94]. It has been demonstrated that lipids might have a significant contribution in metabolism, immunity, inflammation, and several biofilms. Excessive levels of reactive nitrogen species (RNS) including ONOO− and NO·, as well as ROS including hydroxyl radical (·OH) and H2O2 can result in OS [95] and oxidize various biological macromolecules including proteins, DNA, or lipids. ·OH is the most detrimental and dynamic reactive oxygen radical, which is generated via H2O2 in the presence of metal ions. It is well established that hydroxyl radicals have a significant contribution in LPO, which causes the oxidization of lipids with carbon-carbon double bonds, particularly PUFAs. The C C units are separated by a single-bonded C atom in the case of PUFAs, where hydrogen atoms (diallyl hydrogens) are attached to these C atoms (bis-allylic hydrogens) and are susceptible to hydroxyl radical. Thus, PUFAs including AA are known as extremely oxidized fatty acids. PUFA peroxides and their final products including reactive aldehydes such as 4-hydroxynonaldehyde (4-HNE) can result in carbonylation of proteins, which is linked with neurodegenerative diseases, atherosclerosis, and various other diseases [96].
PUFAs can go through enzymatic or nonenzymatic oxidation. LOXs are ferric enzymes that can catalyze the oxygenation of PUFA to generate fatty acid hydroperoxides. AA cascade includes enzymes that are linked with LPO metabolism. AA leads to the generation of bioactive eicosanoids (including epoxy-fatty acids, leukotrienes, and prostaglandins) by utilizing lipid peroxidase-related enzymes including cytochrome P450 enzymes, LOXs, and cyclooxygenase (COX). COX-2 controls synaptic and cerebrovascular plasticity in the nervous system under physiological conditions. In contrast, under pathological conditions, AA metabolism induced via COX resulted in an increased level of prostaglandin in a rat model of focal cerebral ischemia [7,97]. Prostaglandins play an important role in excitotoxic and ischemic nerve injury. During LPO, COX-2 overexpression induces neurotoxicity via prostaglandin E2 (PGE2). Moreover, if the toxic stimulation continues, it can further result in secondary neuroinflammation injury of the CNS. It has been demonstrated that the use of COX-2 inhibitors decreased the concentration of various proinflammatory cytokines including TNF-α and IL-1β. Gene knockout or drug-mediated inhibition of 15-LOX decreased the generation of TNF-α and IL-1 [7,98,99].
4.3. Damage-associated molecular patterns (DAMPs) in neuroinflammation
Iron disruption is a common characteristic of ferroptosis. Excessive levels of iron from the periphery and hemoglobin can be transferred by various transporters and ion channels, such as FPN, TFR1, and DMT1. Fe2+ can also catalyze lipid ROS production via the Fenton reaction, which can further lead to the continuous buildup of intracellular lipid ROS and eventually ferroptosis. In addition, excess levels of Fe2+ can be transferred from the microglia, which can lead to iron metabolism disorder [100]. In addition to the role of an immune cell, Glia in the CNS constantly keep track of the dynamic balance of the internal environment, which helps in resisting exogenous and endogenous pathogenic stimuli [101]. Pattern recognition receptors (PRRs) help immune cells in recognizing pathogens, which include DAMPs and pathogen-associated molecular patterns. The family of toll-like receptor (TLR) is the main form of PRRs, which is an abundantly present immune cell and is widely expressed in CNS glia. DAMPs are endogenous danger signal molecules encoded directly by endogenous genes of the host. Interestingly, it can be secreted directly under specific conditions of tissue injury, devoid of the necessity for protein synthesis. DAMPs that are derived from proinflammatory cytokines and ferroptosis cells give prefeedback signals that can further strengthen the programmed death of more cells [102]. Along with the spread of inflammation throughout the body, extreme death cycles can cause barrier disturbance, which might ultimately result in severe multiple-organ failure. Iron, erythrocyte, hemoglobin, and DAMPs that are generated during the events of ferroptosis can be detected via PRRs including TLRs in glia [103].
Activated astrocytes and microglia release chemokines, ROS, and various inflammatory cytokines including IL-18, TNF-α, IL-10, IL-12, IL-6, IFN-β, IL-1β, and IFN-α, which can further play roles in infection-caused neuroinflammation in the nervous system [104]. It has been observed that the TLR3 signal stimulates a highly strong proinflammatory polarization response and the characteristic of the response includes an increased concentration of IFN-β, IL-10, C-X-C motif chemokine ligand 10, IL-6, TNF-α, and IL-12. Activated TLR signals in glia are associated with neuronal damage under the function of exogenous and endogenous pathogens or the secretion of products from cell injury in case of noninfectious or infectious CNS diseases owing to ferroptosis of nerve cells. After primary neuritis, high mobility group box-1 (HMGB1), S100 protein, and amyloid are secondary important factors in the case of AD, which can worsen the generation of various proinflammatory cytokines. HMGB1 is a member of Ia DAMP class that can be detected via PRRs on multiple cells. It has been demonstrated that class Ia DAMPs signal can harm adjacent PRR-containing cells including phagocytes by inducing sterile inflammation [7,105].
4.4. P53 gene, ferroptosis and neuroinflammation
The P53 gene is a tumor suppressor gene that induces apoptosis, senescence, and suppression of the cell cycle, which is important in development and tumorigenesis. It has been revealed that acetylated defective P53 mutants can mediate ferroptosis [106]. In a study, Jiang et al. [107] revealed that the functions of H1299 cells containing the silenced P53 gene did not alter when these cells were exposed to ROS [107]. Nonetheless, 90% of the H1299 cells died due to ROS exposure following activation of the P53 gene, which is suggesting that P53 activation decreased the antioxidant property of these cells. The P53 gene regulates GSH synthesis via suppressing Xc− [108]. P53 can also mediate ferroptosis via increasing spermine N1-acetyltransferase 1 (SAT1) expression, which is a crucial rate-limiting enzyme in polyamine catabolism and can cause acetylation of spermine. Aberrant expression of SAT1 and polyamine metabolism is linked with numerous disorders including cancer and NDs [109]. SAT1 function can also elevate various stress reactions, such as inflammatory stimuli and OS. SAT1 activation might result in ROS-mediated LPO and ferroptosis, which further leads to neuroinflammatory injury. SAT1 overexpression results in a fast reduction of spermine in cells, which eventually leads to substantial mitochondrial apoptosis and growth inhibition [110]. SAT1 stimulation was found to be linked with 15-LOX expression, while PD146176 (a 15-LOX inhibitor)-mediated suppression of 15-LOX reduced SAT1-induced ferroptosis [111]. Along with COX-2, 15-LOX is associated with AA metabolism, generating ROS, peroxides, prostaglandins, and other constituents to trigger inflammatory damage of the nervous system [7,112].
5. The role of ferroptosis-mediated inflammation in neurological disorders
5.1. Alzheimer's disease
Alzheimer's disease (AD) is the most prevalent form of dementia and one of the important features of AD pathogenesis is neuroinflammation [113]. Key characteristics of AD pathogenesis include extracellular senile plaques that are generated by the deposition of amyloid beta (Aβ) and hyperphosphorylation of tau, loss of neurons, and proliferation of glial cells. Toxic Aβ accumulation is one of the major contributors in AD pathogenesis [114]. In addition, inflammation, weakened glial activity, mitochondrial dysfunction, autophagy, OS, and altered metal homeostasis are also linked with AD pathogenesis [115,116]. Deposition of iron is one of the first reported brain chemical alterations in AD individuals [66]. In the case of AD, pathological iron accumulation in neurons in the brain can lead to the generation of a higher level of free radicals via the Fenton reaction, which is a key factor that results in OS [66]. OS, LPO, and ferroptosis are observed in some individuals with AD, following disturbance of iron homeostasis mediated via increased levels of ferritin in the absence of elevated iron [117]. The level of ferritin in AD brains was found to differ from physiological ferritin, whereas its catalytic site could be utilized in the Fenton reaction to induce OS. A comparatively higher level of iron might alter Aβ precursor protein (APP) processing via iron-dependent ferroptosis, which can induce the generation of neurofibrillary tangles and plaques as well as can contribute in AD pathogenesis [118]. Brain atrophy and FPN downregulation in the hippocampus and neocortex were detected in the hippocampus of mouse models and brains of AD individuals, therefore FPN downregulation is extensively associated with progressive atrophy of AD brains via inducing ferroptosis [67]. Aβ accumulation is also observed in neurons of AD brains. This accumulation can take place in several subcellular areas, which further causes synaptic disturbance and suppresses the ubiquitin-proteasome system, and mediates toxic effects including proinflammatory responses and mitochondrial dysfunction [119].
Ferroptotic cell death was found to induce Aβ cytotoxicity and the slow Aβ accumulation in neurons might result in sustained cellular ferroptosis, which can further lead to additional toxic effects [120]. Interestingly, supplementation with γ-glutamylcysteine and tetrahydroxy stilbene glycoside can cause restoration of the GSH-GPX4 antioxidant system and decrease ROS levels in individuals with AD, thus can decrease Aβ-mediated brain damage [68,121]. Deferoxamine (DFO), a metal ion chelator, can decrease the iron accumulation in brain areas of experimental animals, which can suppress plaque formation or Aβ lamellae and also dissolve the already generated lamellae [69]. In AD, clinical treatment with DFO slowed down the dementia-associated symptoms [66]. Both iron chelating and antioxidant properties are exhibited by alpha-lipoic acid. Alpha-lipoic acid treatment markedly blocked tau-mediated LPO and iron overload by decreasing the loss of neurons in mouse models of AD [70]. Nrf2 pathway activation can decrease ferroptosis, neuroinflammation, and OS, which ultimately improve cognitive impairments [71]. Moreover, it was observed in elderly AD individuals with cardiac insufficiency that mitochondrial aldehyde dehydrogenase (ALDH2) can eradicate the manifestations of elevated NCOA4, reduced GPX4, and SLC7A11 in APP/PS1 mouse models via suppressing ACSL4-dependent ferroptosis, and ameliorate their cardiac insufficiency as well as cognitive impairments [72]. Indeed, targeting ferroptosis pathways including the GPX4 antioxidant system, lipid metabolism, and iron metabolism can be an effective strategy in developing AD therapies [66].
5.2. Parkinson's disease
Parkinson's disease (PD) is a chronic neurodegenerative disorder primarily caused by the deficiency of dopamine in the brain [122,123]. Major features of PD pathogenesis include death and degeneration of dopamine in the midbrain, along with a marked dopamine loss in the substantia nigra pars compacta as well as the occurrence of Lewy Body in the cytoplasm of remaining neurons in the substantia nigra [124]. The actual cause of this pathological mechanism is not clear, however OS, aging, environmental factors, genetic factors, and various other factors might be associated with this pathological mechanism [125]. Moreover, neuroinflammation is closely associated with PD pathogenesis [126]. It has been observed that ferroptosis is also associated with PD pathogenesis. Increased levels of LPO, depletion of GSH, and iron deposits have been observed in the substantia nigra pars compacta [[127], [128], [129]]. Furthermore, ferroptosis is associated with the development of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mediated PD in mice. Fer-1 treatment decreased the injury of dopaminergic neurons in models of rotenone- and MPTP-induced PD [128]. Suppression of TFR1 decreased α-synuclein aggregation in MPP+-treated neurofibroma cells. An increased level of TFR1 has also been observed in ferroptotic cells. Deferiprone (DFP) treatment decreased OS and increased dopamine function to reduce existing motor symptoms and slow the advancement of motor impairments [130] in individuals with early PD. Fer-1 treatment decreased the level of OS, accumulation of excessive levels of ROS, and α-synuclein deposition in the striatum and substantia nigra in a rotenone-induced cell injury model and MPTP-induced PD model. Nonetheless, erastin (a ferroptosis inducer) administration reduced the number of dopaminergic neurons, elevated the aberrant α-synuclein accumulation in mouse models, and induced ferroptotic cell death [[131], [132], [133]].
5.3. Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease. ALS characteristics include progressive muscular weakness and atrophy resulting from the loss of motor neurons in the brainstem, spinal cord, primary motor cortex, and corticospinal tracts. There is growing evidence that suggests that neuroinflammation has a significant contribution in ALS pathogenesis. In the case of ALS, neuroinflammation is characterized via the activation of reactive astrocytes and microglia, association of complement, and infiltration of macrophages and lymphocytes [134]. Various characteristics of ferroptosis including LPO, aberrant metabolism of GSH, and iron dysregulation have already been detected in ALS. Accumulation of iron has already been observed in the spinal cord and motor cortex SOD1G93A mouse models and individuals with ALS, which has been associated with abnormal OS and redox chemistry. Depletion of GSH was observed in the affected CNS tissues of SOD1G93A mouse models and ALS individuals, which was associated with system Xc− dysregulation in mutant SOD1 mouse models [135,136]. Mutant SOD1 mouse models and ALS individuals are characterized by protuberant LPO in affected CNS tissues [137,138]. Neuron-specific GPX4 deletion in mouse models led to motor neuron degeneration, paralysis, and muscle atrophy, which is suggesting the expression of GPX4 in cell susceptibility in the case of ALS [139]. Collectively, these findings indicate that ferroptosis has a significant contribution in the death of motor neurons in ALS. GPX4 depletion has extensively been observed in motor neurons and spinal cords of ALS mouse models and ALS individuals. In addition, overexpression of GPX4 in SOD1G93A mouse models markedly alleviated symptoms and loss of motor neurons in novel human GPX4 transgenic mouse models. These observations were found to be in line with the observation of a recent study, where crossed SOD1G93A mouse models were crossed with independent GPX4 transgenic mouse models [140,141]. In that study, it was confirmed that ferroptosis has a significant contribution in inducing the death of motor neurons in ALS [141].
5.4. Huntington's disease
Huntington's disease (HD) is an inherited neurodegenerative disorder that takes place owing to the amplification of CAG repeat in the HTT gene. HD clinical manifestations include mood disorders, dementia, and dance-like movements [142]. Neuroinflammation is a hallmark of HD, which is characterized by the presence of reactive microglia, inflammatory factors, and astrocytes [143,144]. Ferroptosis is linked with glutamate excitotoxicity and GSH-induced redox reactions in the case of HD. Aberrant concentrations of iron ions, GSH, glutamate, and buildup of intracellular lipid ROS have also been identified in HD individuals [145,146]. Various features of ferroptosis including reduced GPX functions and blocked synthesis of GSH have been observed in animal models of HD [147,148].
5.5. Stroke
Globally, stroke is the leading cause of disability and the second leading cause of death, along with a growing incidence rate. A stroke or cerebrovascular accident can occur due to elevated cerebral vascular sclerosis, inadequate blood supply to the brain, or elevated levels of cerebrovascular blood pressure, along with enormous neuronal death [149]. It has already been demonstrated that ferroptosis can take place in neuronal cells during the onset of stroke, where ferroptosis can induce the pathophysiological mechanism of stroke. On the other hand, ferroptosis suppression has a protective function on stroke and can result in ameliorated stroke prognosis [150]. OS, iron buildup, excitatory toxicity, and inflammation are also observed during the onset of stroke [151]. Free radical scavengers, antioxidants, and iron-chelating agents were found to decrease cerebrovascular damage following stroke [152,153]. Features of ferroptosis have been detected in reperfusion in vivo, mouse cortical nerve cells following ischemia, and hippocampal neurons in vitro stroke models, while treatment with ferroptosis inhibitors markedly reversed cell death [154,155]. Indeed, ferroptosis is closely linked with stroke and suppression of ferroptosis can reverse the stroke-mediated neuronal injury. Therefore, therapeutically targeting ferroptosis can be effective in treating stroke [40,156].
6. Targeting ferroptosis and inflammation in the treatment of neurological disorders
6.1. Deferiprone (DFP) and deferoxamine (DFO)
Iron chelators inhibit ferroptosis via targeting free iron in labile iron pools and avert iron from providing electrons to oxygen to form ROS [157]. Two major groups of iron chelators include membrane-impermeable and membrane-permeable (lipophilic iron chelators) [158]. Several lipophilic iron chelators including 2,2-bipyridyl and ciclopirox have the ability to target areas of iron accumulation, penetrate the blood-brain barrier and cell membrane, and eliminate chelatable iron from labile iron pools or transport it to various other proteins. In order to suppress ferroptosis, lipophilic iron chelators avert lipid ROS formation and PUFA fragment oxidation [159]. Furthermore, lipophilic iron chelators can also cause direct inactivation of ferrous enzymes that mediate membrane lipid oxidation and hypoxia-inducible factor-prolyl hydroxylases [158]. The aforesaid activity provides neuroprotective functions via causing the activation of hypoxia-adaptive genetic programs, stabilizing HIF-1α, and inducing hypoxia [160]. On the other hand, membrane-impermeable iron chelators including DFP and DFO can accumulate in lysosomes via endocytosis from where they capture extra free iron [161].
DFP is a metal chelator that is utilized to address the overload of iron and it has great potential in the treatment of disorders that are linked with iron accumulation. DFP can penetrate the blood-brain barrier and can bind with iron at several intracellular regions. Moreover, DFP has anti-inflammatory properties. Ferroptosis that takes place in chondrocytes is mediated via IL-1β, which mediates inflammation and destruction of chondrocytes. In a study, Guo et al. confirmed that DFO showed protective properties on chondrocytes and slowed the progression of osteoarthritis by suppressing chondrocyte ferroptosis [162]. Interestingly, DFP pretreatment ameliorated ketamine or sevoflurane-mediated cognitive deficit and improved behavioral testing ability in both aged and adolescent rat models [163]. Indeed, the cognitive deficit is strongly linked with neuroinflammation and iron overload, and divalent metal transporter 1 (DMT1) is linked as a key link between immunity and iron signaling [164]. It has been revealed by several in vitro and in vivo studies that iron chelators can have a significant contribution in PD treatment [165].
DFP decreased OS, improved existing motor symptoms, increased utilization of cellular dopamine, and averted worsening in MPTP-treated mice [166]. Treatment with iron chelators elevated GSH peroxide functions and decreased dyskinesia in PD patients in the cerebrospinal fluid within six months in a phase III clinical trial [167,168]. Normal systemic iron homeostasis was observed during the use of iron chelators in most individuals with the neurodegenerative disorder [169]. Overuse of iron chelation therapy was found to be unsuitable for PD patients and might cause anemia and iatrogenic iron depletion. Thus, iron chelation therapy ought to be used at a low dose to provide neuroprotective properties with minimum side effects [170]. After treatment with DFP at the dose of 30 mg/10 g/day, dyskinesia was markedly decreased at 12 months, clinical benefits were seen at 6 months, and a normal iron level was restored at 18–24 months. Moreover, it was suggested that a low dose of DFP can be safe along with a risk for reversible neutropenia of 1–3% [166]. In a study, Zhang et al. [171] observed that ferroptosis took place at a comparatively low ferric citrate level, whereas apoptosis took place along with an increased level of iron. Collectively, this result suggests the importance of early death in the case of PD and suggests that ferroptosis can lead to iron overload mediated by apoptosis [172].
6.2. Ferrostatin-1 (Fer-1)
Fer-1 is a synthetic antioxidant. It has demonstrated that Fer-1 is a selective and strong suppressor of ferroptosis. Fer-1 has been discovered through high-throughput screening of small molecule libraries via utilizing cell assays, where ferroptosis was mediated via either pharmacological suppression of system Xc− [173] or deletion of the gene encoding the GPx4 [174]. It was observed that Fer-1 suppressed ROS accumulation [175]. It has been observed that angiotensin II (Ang II) can cause activation of astrocytes, which can lead to the substantial generation of various inflammatory factors that play roles in damage, which ultimately results in cell death in astrocytes [176]. Increased angiotensin II type 1 receptor (AT1R) activity in neurons can cause inflammation, cell death, and cognitive deficit. AT1R activation can mediate antioxidant as well as anti-inflammatory properties, cell survival, and cognition in the CNS [177]. Alleviation of Fer-1 on Ang II resulted in the activation of AT1R, which indicates its protective function in astrocytes. Fer-1 can also mitigate Ang II-mediated ferroptosis and inflammation via suppressing ROS concentrations and causing activation of the Keap1/Nrf2/HO-1 signaling. Indeed, Fer-1 suppressed iron-induced apoptosis and cell death in the early PD stages [171]. Fer-1 showed protective properties in animal models of AD. It has been observed that Fer-1 improved memory loss and neuronal death mediated via Aβ aggregation in vivo and in vitro [67].
6.3. Liproxstatin-1 (Lip-1)
Ischemia-reperfusion (I/R) injury can arise from an initially restricted blood supply to a tissue or organ followed by the perfusion or restoration, which can result in mortality and morbidity in several pathologies. Furthermore, I/R injury can take place in multiple organs such as the lung, liver, kidney, brain, and heart, and its processes including microvascular dysfunction, mitochondrial dysfunction, calcium overload, inflammation, oxidative stress, and the activation of cell death signaling [178]. Lip-1 deceased serum inflammation mediators and ferroptosis in I/R injury [179]. Cao et al. [180] confirmed that Lip-1 restrained microglial activation and decreased the in vivo rise of IL-1β, TNF-α, and IL-6 in subarachnoid hemorrhage. Various inflammatory mediators including IL-1β are regarded as the main matrix metallopeptidase 9 activators [180]. Furthermore, Lip-1 improved the Aβ aggregation-mediated memory loss and neuronal death in vitro and in vivo [67].
6.4. Alpha-lipoic acid (α-LA)
Alpha-lipoic acid (α-LA) is a naturally occurring iron chelator and an antioxidant, which is an attractive drug candidate for the development of anti-neuroinflammatory therapy [181]. In a study, Kim et al. [181] indicated that α-LA can decrease mitogen-activated protein kinase signaling, NF-κB, activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome, and secretion of various pro-inflammatory cytokines in lipopolysaccharide (LPS)-mediated BV-2 microglial cells and regulates microglial cells M1/M2 polarization. They have also suggested that α-LA be an effective drug candidate for the treatment of chronic neurodegenerative and inflammatory [181]. In addition, α-LA markedly rescued cognitive deficit and tauopathy in P301S Tau transgenic mouse models. α-LA blocked tau-mediated LPO and iron overload linked with ferroptosis. α-LA also improved these irregularities via decreasing the level of ROS and elevating the GPX4 expressions [70].
6.5. Quercetin
Quercetin is a natural bioflavonoid widely present in vegetables and fruits including capers, onions, and berries [182]. Quercetin shows anti-inflammatory and antioxidant properties against numerous neurodegenerative, metabolic and inflammatory diseases [[182], [183], [184]]. Quercetin provides protection against neuroinflammation via suppressing the generation of nitric oxide in microglia, which can further result in the suppression of NF-κB signaling and prevention of inflammation-associated neuronal damage [185,186]. In addition, quercetin decreased iron bioavailability, decreased serum iron levels, hindered intestinal iron absorption, and upregulated hepcidin (the main regulator of systemic iron homeostasis) expression. Quercetin is also a powerful natural iron chelator that can decrease iron overload-induced tissue injury via keeping steady iron levels. Moreover, quercetin eliminated ROS as well as various oxidizing substances and played a role as a natural ferroptotic cell death inhibitor [[187], [188], [189], [190]].
6.6. Baicalein
Baicalein is a flavonoid derived from the roots of Scutellaria baicalensis. Baicalein is widely used in many countries because of its anti-inflammatory, antiviral, and antibacterial properties [191]. Baicalein decreased the generation of various pro-inflammatory cytokines including IL-6, TNF-α, and IL-1β in MPTP-induced PD in mice [191,192]. Baicalein also exerted neuroprotective properties in MPTP-treated mouse models [191,192]. Moreover, baicalein can act as a natural ferroptosis inhibitor and can provide protection against OS-induced damage. It has been demonstrated that baicalein exerted a more significant function against ferroptotic cell death as compared to various classic inhibitors including deferoxamine mesylate, β-mercaptoethanol, Fer-1, and Lip-1 [133,193]. In a study, Xie et al. [194] observed that baicalein might suppress erastin-mediated ferroptosis of pancreatic cancer cells via suppressing GSH depletion, LPO, and degradation of GPX4. Baicalein also activates the Nrf2 pathway, which further averts erastin-mediated degradation of Nrf2 and suppression of OS. Moreover, baicalein decreased the PE oxidation in ferroptosis and improved the recovery and prognosis of cerebral cortical impact [195].
6.7. Puerarin
Puerarin is an isoflavone glycoside isolated from Pueraria lobata (Willd.) Ohwi, which is widely used in traditional Chinese medicine [196]. It has been revealed that puerarin can act as a natural ferroptosis inhibitor and provide protection to the neurons by suppressing ROS production. Puerarin also suppressed iron overload, regulated iron homeostasis, and decreased ROS levels, which ultimately resulted in the suppression of ferroptosis [197,198]. Puerarin inhibited the overload of iron in the cerebral cortex and ameliorated memory disorders and spatial learning in AD mouse models, however the exact mechanisms are yet to be revealed [133,199]. Furthermore, puerarin alleviated neuroinflammation decreased Aβ deposition, reduced hyperphosphorylated tau protein levels, and prevented neuronal loss [200]. Puerarin also ameliorated impaired synaptic plasticity via controlling the p38 MAPK-CREB pathway in AD [201].
6.8. Vitamin E
Vitamin E shows several important properties including cholesterol-lowering, anti-inflammatory, and antioxidant properties along with anti-ferroptosis activity. It has also been indicated that vitamin E supplementation can be effective in the treatment of neurodegenerative diseases including PD and AD [202]. Antioxidant properties of vitamin E are closely associated with their anti-inflammatory effects, as OS is a part of inflammation [203]. Nonetheless, there are certain activities that are not dependent on these antioxidant properties. Vitamin E has the ability to interfere with inflammation at different levels, such as the synthesis of signaling molecules, signaling cascades, and transcription factors [202,204]. A vitamin E-deficient diet may lead to worsened behavioral dysfunction and hippocampal neurodegeneration, where Lip-1 ameliorated neurodegeneration and cognitive function in mouse models [205]. In addition, high-dose vitamin E supplementation terminated LPO, neutralized peroxidative free radicals, and decreased the risk of cognitive deficit in AD individuals [206]. Vitamin E also showed clinical benefits in individuals with mild-moderate AD by reducing caregiver burden and slowing functional decline, in a large randomized clinical trial [207,208].
6.9. Coenzyme Q10
Coenzyme Q10 (CoQ10) is an antioxidant that offers protection to lipoproteins and cellular membranes. CoQ10 is an important part of the mitochondrial electron transport chain [209]. It has been observed that a nanomicellar, water-soluble formulation of CoQ10 significantly suppressed the formation of Aβ plaques and ameliorated long-term memory [210]. CoQ10 also prevents the propagation and initiation of LPO [211,212]. Therefore, CoQ10 might serve as an effective ferroptosis inhibitor. CoQ10 also reduced LPO and upregulated GSH in mouse models. Various studies have also confirmed the efficacy and safety of high-dose CoQ10 supplementation in multiple neuropsychiatric disorders [213,214]. In a study, Li et al. [215] reported that CoQ10 averted Aβ25–35–mediated IκBα degradation and p65 nuclear translocation by reducing PGE2 generation and COX-2 expressions. Thus, CoQ10-mediated suppression of NF–κB signaling in PC12 cells may lead to the downregulation of several pro-inflammatory mediators, which can lead to an anti-inflammatory effect [215]. The therapeutic agents that can be used to target ferroptosis and inflammatory pathways in neuroinflammation and neurological disorders have been summarized in Table 2.
Table 2.
Summary of therapeutic agents that can be used to target ferroptosis and inflammatory pathways in neuroinflammation and neurological disorders.
| Therapeutic agents | Characteristics | Anti-inflammatory and anti-ferroptosis mechanisms | References |
|---|---|---|---|
| Deferiprone | Antioxidant, iron chelator | Decreases oxidative stress (OS); reduces iron overload | [166,170,171] |
| Ferrostatin-1 | Antioxidant, iron chelator | Inhibits reactive oxygen species (ROS) accumulation; activates angiotensin II type 1 receptor; suppresses ROS; activates the Keap1/Nrf2/HO-1 signaling | [67,171,175] |
| Liproxstatin-1 | Antioxidant, iron chelator | Reduces serum inflammation mediators; decreases the in vivo rise of interleukin (IL)-1β, tumor necrosis factor alpha (TNF-α), and IL-6; inhibits lipid peroxidation | [[178], [179], [180]] |
| Alpha-lipoic acid | Antioxidant, iron chelator | Decreases the level of ROS; elevates the GPX4 expressions; decreases mitogen-activated protein kinase and nuclear factor kappa B (NF-κB) signaling; activates NLRP3 inflammasome; secretes pro-inflammatory cytokines, reduces iron overload | [70,181] |
| Quercetin | Antioxidant | Inhibits the generation of nitric oxide, suppresses NF-κB signaling, eliminates ROS and several oxidizing substances; decreases iron overload | [[185], [186], [187], [188], [189], [190]] |
| Baicalein | Antioxidant | Decreases various inflammatory cytokines such as IL-6, TNF-α, and IL-1β; provides protection against OS-induced damage, suppresses OS; suppresses glutathione (GSH) depletion, lipid peroxidation (LPO), and phosphatidylethanolamine oxidation | [191,192,194] |
| Puerarin | Antioxidant | Inhibits ROS production; regulates p38 MAPK-CREB pathway; suppresses iron overload; regulates iron homeostasis | [197,198] |
| Vitamin E | Antioxidant | Decreases synthesis of signaling molecules, signaling cascades, and transcription factors; terminates LPO; neutralizes peroxidative free radicals | [202,204,206] |
| Coenzyme Q10 | Antioxidant | Degrades IκBα and nuclear translocation of p65 by decreasing prostaglandin E2 generation and cyclooxygenase-2 expression; suppresses NF–κB signaling; downregulates several pro-inflammatory mediators; decreases propagation and initiation of LPO; upregulates GSH | [211,212,215] |
7. Conclusion and perspectives
Characteristics of ferroptosis include iron metabolism disorder, suppression of glutamate transport, and accumulation of lipid ROS, which can further induce OS as well as neuroinflammation and subsequently exacerbate CNS injury. Neuroinflammation involves the overgeneration of inflammatory mediators, invading T cells, reactive astrocytes, and activated microglia. Neuroinflammation is not essential during the primary stage of various NDs, however prolonged inflammation can lead to the exacerbation of diseases. Numerous studies have already demonstrated that ferroptosis is closely linked with stroke, HD, PD, AD, and various other NDs. Various studies have revealed the link between ferroptosis and inflammation in NDs, however differences and similarities in the molecular mechanisms of ferroptosis in terms of different NDs. Ferroptosis inhibitors have already demonstrated their efficacy in multiple experimental animal models in improving the severity of NDs, nonetheless clinical efficacy of these inhibitors in treating chronic neuroinflammation is yet to be verified. Furthermore, more studies on inflammation and ferroptosis will enhance the understanding of the role of ferroptosis and associated inflammation in neurological disorders and advance the development of novel therapeutic agents which will be effective in treating and preventing inflammation and ferroptosis in NDs.
CRediT authorship contribution statement
Syam Mohan: Writing – original draft, Formal analysis, Data curation, Conceptualization. Hassan A. Alhazmi: Formal analysis, Data curation. Rym Hassani: Methodology, Formal analysis. Gulrana Khuwaja: Writing – original draft, Formal analysis, Data curation. V.P.Maheshkumar: Writing – review & editing, Data curation. Afaf Aldahish: Writing – original draft, Formal analysis, Data curation. Kumarappan Chidambaram: Writing – original draft, Data curation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number ISP23-81.
References
- 1.Feigin V.L., Vos T., Nichols E., Owolabi M.O., Carroll W.M., Dichgans M., Deuschl G., Parmar P., Brainin M., Murray C. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol. 2020;19:255–265. doi: 10.1016/S1474-4422(19)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilhus N.E., Deuschl G. Neuroinflammation — a common thread in neurological disorders. Nat. Rev. Neurol. 2019;15:429–430. doi: 10.1038/s41582-019-0227-8. [DOI] [PubMed] [Google Scholar]
- 3.Brambilla R. Neuroinflammation, the thread connecting neurological disease: cluster: “neuroinflammatory mechanisms in neurodegenerative disorders.”. Acta Neuropathol. 2019;137:689–691. doi: 10.1007/s00401-019-02009-9. [DOI] [PubMed] [Google Scholar]
- 4.Mishra A., Bandopadhyay R., Singh P.K., Mishra P.S., Sharma N., Khurana N. Neuroinflammation in neurological disorders: pharmacotherapeutic targets from bench to bedside. Metab. Brain Dis. 2021;36:1591–1626. doi: 10.1007/s11011-021-00806-4. [DOI] [PubMed] [Google Scholar]
- 5.Rehman M.U., Sehar N., Dar N.J., Khan A., Arafah A., Rashid S., Rashid S.M., Ganaie M.A. Mitochondrial dysfunctions, oxidative stress and neuroinflammation as therapeutic targets for neurodegenerative diseases: an update on current advances and impediments. Neurosci. Biobehav. Rev. 2023;144 doi: 10.1016/J.NEUBIOREV.2022.104961. [DOI] [PubMed] [Google Scholar]
- 6.Kumar D., Jahan S., Khan A., Siddiqui A.J., Redhu N.S., Wahajuddin, Khan J., Banwas S., Alshehri B., Alaidarous M. Neurological manifestation of SARS-CoV-2 induced inflammation and possible therapeutic strategies against COVID-19. Mol. Neurobiol. 2021;58:3417–3434. doi: 10.1007/S12035-021-02318-9/TABLES/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y., Song Y., Chen H., Li Q., Gao Y., Lu G., Luo C. Ferroptosis mediated by lipid reactive oxygen species: a possible causal link of neuroinflammation to neurological disorders. Oxid. Med. Cell. Longev. 2021:2021. doi: 10.1155/2021/5005136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon S.J. Ferroptosis. Bug or feature? Immunol. Rev. 2017;277:150–157. doi: 10.1111/imr.12533. [DOI] [PubMed] [Google Scholar]
- 9.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Herde K., Krysko D.V. Ferroptosis: oxidized PEs trigger death. Nat. Chem. Biol. 2017;13:4–5. doi: 10.1038/nchembio.2261. [DOI] [PubMed] [Google Scholar]
- 12.Speer R.E., Karuppagounder S.S., Basso M., Sleiman S.F., Kumar A., Brand D., Smirnova N., Gazaryan I., Khim S.J., Ratan R.R. Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by “antioxidant” metal chelators: from ferroptosis to stroke. Free Radic. Biol. Med. 2013;62:26–36. doi: 10.1016/j.freeradbiomed.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenny E.M., Fidan E., Yang Q., Anthonymuthu T.S., New L.A., Meyer E.A., Wang H., Kochanek P.M., Dixon C.E., Kagan V.E., et al. Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit. Care Med. 2019;47:410–418. doi: 10.1097/CCM.0000000000003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazinet R.P., Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 15.Weiland A., Wang Y., Wu W., Lan X., Han X., Li Q., Wang J. Ferroptosis and its role in diverse brain diseases. Mol. Neurobiol. 2019;56:4880–4893. doi: 10.1007/s12035-018-1403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y., Song J., Wang Y., Wang X., Culmsee C., Zhu C. The potential role of ferroptosis in neonatal brain injury. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Do Van B., Gouel F., Jonneaux A., Timmerman K., Gelé P., Pétrault M., Bastide M., Laloux C., Moreau C., Bordet R., et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol. Dis. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Tuo Q.Z., Lei P., Jackman K.A., Li X.L., Xiong H., Li X.L., Liuyang Z.Y., Roisman L., Zhang S.T., Ayton S., et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatr. 2017;22:1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- 19.Yoo S.E., Chen L., Na R., Liu Y., Rios C., Van Remmen H., Richardson A., Ran Q. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic. Biol. Med. 2012;52:1820–1827. doi: 10.1016/j.freeradbiomed.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L., Hambright W.S., Na R., Ran Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J. Biol. Chem. 2015;290:28097–28106. doi: 10.1074/jbc.M115.680090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren J.X., Sun X., Yan X.L., Guo Z.N., Yang Y. Ferroptosis in neurological diseases. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang W.S., Kim K.J., Gaschler M.M., Patel M., Shchepinov M.S., Stockwell B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagan V.E., Mao G., Qu F., Angeli J.P.F., Doll S., Croix C.S., Dar H.H., Liu B., Tyurin V.A., Ritov V.B., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao M.Y., Liu T., Zhang L., Wang M.J., Yang Y., Gao J. Role of ferroptosis in neurological diseases. Neurosci. Lett. 2021;747 doi: 10.1016/j.neulet.2020.135614. [DOI] [PubMed] [Google Scholar]
- 26.Ward R.J., Zucca F.A., Duyn J.H., Crichton R.R., Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skouta R., Dixon S.J., Wang J., Dunn D.E., Orman M., Shimada K., Rosenberg P.A., Lo D.C., Weinberg J.M., Linkermann A., et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang W.S., Sriramaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen C.R., Hawkins B.E., Prough D.S. Finding the hidden (statistical) platform. Crit. Care Med. 2019;47:480–483. doi: 10.1097/CCM.0000000000003611. [DOI] [PubMed] [Google Scholar]
- 30.Jiang X., Stockwell B.R., Conrad M. Ferroptosis. Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockwell B.R., Jiang X., Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30:478–490. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., Kang R., Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S., Gao X., Zhou S. New target for prevention and treatment of neuroinflammation: microglia iron accumulation and ferroptosis. ASN Neuro. 2022:14. doi: 10.1177/17590914221133236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louandre C., Ezzoukhry Z., Godin C., Barbare J.-C., Mazière J.-C., Chauffert B., Galmiche A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer. 2013;133:1732–1742. doi: 10.1002/ijc.28159. [DOI] [PubMed] [Google Scholar]
- 36.DeGregorio-Rocasolano N., Martí-Sistac O., Gasull T. Deciphering the iron side of stroke: neurodegeneration at the crossroads between iron dyshomeostasis, excitotoxicity, and ferroptosis. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torti S.V., Torti F.M. Iron and cancer: more ore to Be mined. Nat. Rev. Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X., Yu C., Kang R., Tang D. Iron metabolism in ferroptosis. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsarou A., Pantopoulos K. Basics and principles of cellular and systemic iron homeostasis. Mol. Aspect. Med. 2020;75 doi: 10.1016/j.mam.2020.100866. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Tang B., Zhu J., Yu J., Hui J., Xia S., Ji J. Emerging mechanisms and targeted therapy of ferroptosis in neurological diseases and neuro-oncology. Int. J. Biol. Sci. 2022;18:4260–4274. doi: 10.7150/ijbs.72251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan N., Zhang J.J. The emerging roles of ferroptosis in vascular cognitive impairment. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ou M., Jiang Y., Ji Y., Zhou Q., Du Z., Zhu H., Zhou Z. Role and mechanism of ferroptosis in neurological diseases. Mol. Metabol. 2022;61 doi: 10.1016/j.molmet.2022.101502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., Kang R., Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao H., Zhao Y., Li H., Lei L. Ferroptosis as an emerging target in inflammatory diseases. Prog. Biophys. Mol. Biol. 2020;155:20–28. doi: 10.1016/j.pbiomolbio.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Masaldan S., Bush A.I., Devos D., Rolland A.S., Moreau C. Striking while the iron is hot: iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2019;133:221–233. doi: 10.1016/j.freeradbiomed.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y.Y., Wan W.P., Zhao S., Ma Z.G. L-type calcium channels are involved in iron-induced neurotoxicity in primary cultured ventral mesencephalon neurons of rats. Neurosci. Bull. 2020;36:165–173. doi: 10.1007/s12264-019-00424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu H., Yang C., Jian L., Guo S., Chen R., Li K., Qu F., Tao K., Fu Y., Luo F., et al. Sulfasalazine-induced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol. Rep. 2019;42:826–838. doi: 10.3892/or.2019.7189. [DOI] [PubMed] [Google Scholar]
- 48.Fujimaki M., Furuya N., Saiki S., Amo T., Imamichi Y., Hattori N. Iron supply via NCOA4-mediated ferritin degradation maintains mitochondrial functions. Mol. Cell Biol. 2019;39 doi: 10.1128/mcb.00010-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayano M., Yang W.S., Corn C.K., Pagano N.C., Stockwell B.R. Loss of cysteinyl-TRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dixon S.J., Patel D., Welsch M., Skouta R., Lee E., Hayano M., Thomas A.G., Gleason C., Tatonetti N., Slusher B.S., et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014 doi: 10.7554/eLife.02523. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L., Li X., Liu L., Yu B., Xue Y., Liu Y. Erastin sensitizes glioblastoma cells to temozolomide by restraining XCT and cystathionine-γ-lyase function. Oncol. Rep. 2015;33:1465–1474. doi: 10.3892/or.2015.3712. [DOI] [PubMed] [Google Scholar]
- 52.Yang W.S., Sriramaratnam R., Welsch M.E., Shimada K., Skouta R., Viswanathan V.S., Cheah J.H., Clemons P.A., Shamji A.F., Clish C.B., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie Y., Zhu S., Song X., Sun X., Fan Y., Liu J., Zhong M., Yuan H., Zhang L., Billiar T.R., et al. The tumor suppressor P53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20:1692–1704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y., Koppula P., Gan B. Regulation of H2A ubiquitination and SLC7A11 expression by BAP1 and PRC1. Cell Cycle. 2019;18:773–783. doi: 10.1080/15384101.2019.1597506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen D., Tavana O., Chu B., Erber L., Chen Y., Baer R., Gu W. NRF2 is a major target of ARF in P53-independent tumor suppression. Mol. Cell. 2017;68:224–232.e4. doi: 10.1016/j.molcel.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaschler M.M., Stockwell B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017;482:419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shah R., Shchepinov M.S., Pratt D.A. Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent. Sci. 2018;4:387–396. doi: 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 60.Doll S., Conrad M. Iron and ferroptosis: a still ill‐defined liaison. IUBMB Life. 2017;69:423–434. doi: 10.1002/iub.1616. [DOI] [PubMed] [Google Scholar]
- 61.Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang W.S., Kim K.J., Gaschler M.M., Patel M., Shchepinov M.S., Stockwell B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng H., Stockwell B.R. Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2006203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wallach D., Kang T.B., Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat. Rev. Immunol. 2014;14:51–59. doi: 10.1038/nri3561. [DOI] [PubMed] [Google Scholar]
- 65.Li J.Y., Yao Y.M., Tian Y.P. Ferroptosis: a trigger of proinflammatory state progression to immunogenicity in necroinflammatory disease. Front. Immunol. 2021;12:3336. doi: 10.3389/fimmu.2021.701163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Y., Lin W., Rao T., Zheng J., Zhang T., Zhang M., Lin Z. Ferroptosis and its potential role in the nervous system diseases. J. Inflamm. Res. 2022;15:1555–1574. doi: 10.2147/JIR.S351799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao W.-D., Pang P., Zhou X.-T., Hu F., Xiong W., Chen K., Wang J., Wang F., Xie D., Hu Y.-Z., et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer's disease. Cell Death Differ. 2021;28:1548–1562. doi: 10.1038/s41418-020-00685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y., Chen Z., Li B., Yao H., Zarka M., Welch J., Sachdev P., Bridge W., Braidy N. Supplementation with γ-glutamylcysteine (γ-GC) lessens oxidative stress, brain inflammation and amyloid pathology and improves spatial memory in a murine model of AD. Neurochem. Int. 2021;144 doi: 10.1016/j.neuint.2020.104931. [DOI] [PubMed] [Google Scholar]
- 69.Agrawal M., Saraf S., Saraf S., Antimisiaris S.G., Chougule M.B., Shoyele S.A., Alexander A. Nose-to-Brain drug delivery: an update on clinical challenges and progress towards approval of anti-alzheimer drugs. J. Contr. Release. 2018;281:139–177. doi: 10.1016/j.jconrel.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y.H., Wang D.W., Xu S.F., Zhang S., Fan Y.G., Yang Y.Y., Guo S.Q., Wang S., Guo T., Wang Z.Y., et al. α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S tau transgenic mice. Redox Biol. 2018;14:535–548. doi: 10.1016/j.redox.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shao L., Dong C., Geng D., He Q., Shi Y. Ginkgolide B protects against cognitive impairment in senescence-accelerated P8 mice by mitigating oxidative stress, inflammation and ferroptosis. Biochem. Biophys. Res. Commun. 2021;572:7–14. doi: 10.1016/j.bbrc.2021.07.081. [DOI] [PubMed] [Google Scholar]
- 72.Zhu Z. yun, Liu Y. dong, Gong Y., Jin W., Topchiy E., Turdi S., Gao Y. feng, Culver B., Wang S. yi, Ge W., et al. Mitochondrial aldehyde dehydrogenase (ALDH2) rescues cardiac contractile dysfunction in an APP/PS1 murine model of Alzheimer's disease via inhibition of ACSL4-dependent ferroptosis. Acta Pharmacol. Sin. 2022;43:39–49. doi: 10.1038/s41401-021-00635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian Y., Lu J., Hao X., Li H., Zhang G., Liu X., Li X., Zhao C., Kuang W., Chen D., et al. FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson's disease. Neurotherapeutics. 2020;17:1796–1812. doi: 10.1007/s13311-020-00929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Devos D., Moreau C., Devedjian J.C., Kluza J., Petrault M., Laloux C., Jonneaux A., Ryckewaert G., Garçon G., Rouaix N., et al. Targeting chelatable iron as a therapeutic modality in Parkinson's disease. Antioxidants Redox Signal. 2014;21:195–210. doi: 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asanuma M., Miyazaki I. Glutathione and related molecules in parkinsonism. Int. J. Mol. Sci. 2021:22. doi: 10.3390/ijms22168689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mischley L.K., Allen J., Bradley R. Coenzyme Q10 deficiency in patients with Parkinson's disease. J. Neurol. Sci. 2012;318:72–75. doi: 10.1016/j.jns.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi L., Huang C., Luo Q., Xia Y., Liu W., Zeng W., Cheng A., Shi R., Zhengli C. Clioquinol improves motor and non-motor deficits in MPTP-induced monkey model of Parkinson's disease through AKT/MTOR pathway. Aging. 2020;12:9515–9533. doi: 10.18632/aging.103225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang D., Liang W., Huo D., Wang H., Wang Y., Cong C., Zhang C., Yan S., Gao M., Su X., et al. SPY1 inhibits neuronal ferroptosis in amyotrophic lateral sclerosis by reducing lipid peroxidation through regulation of GCH1 and TFR1. Cell Death Differ. 2023;30:369–382. doi: 10.1038/s41418-022-01089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bertrand R.L. Iron accumulation, glutathione depletion, and lipid peroxidation must occur simultaneously during ferroptosis and are mutually amplifying events. Med. Hypotheses. 2017;101:69–74. doi: 10.1016/j.mehy.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 80.Skouta R., Dixon S.J., Wang J., Dunn D.E., Orman M., Shimada K., Rosenberg P.A., Lo D.C., Weinberg J.M., Linkermann A., et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tuo Q.Z., Lei P., Jackman K.A., Li X.L., Xiong H., Li X.L., Liuyang Z.Y., Roisman L., Zhang S.T., Ayton S., et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatr. 2017;22:1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- 82.Nasoohi S., Simani L., Khodagholi F., Nikseresht S., Faizi M., Naderi N. Coenzyme Q10 supplementation improves acute outcomes of stroke in rats pretreated with atorvastatin. Nutr. Neurosci. 2019;22:264–272. doi: 10.1080/1028415X.2017.1376928. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y., Min J.W., Feng S., Subedi K., Qiao F., Mammenga E., Callegari E., Wang H. Therapeutic role of a cysteine precursor, OTC, in ischemic stroke is mediated by improved proteostasis in mice. Transl Stroke Res. 2020;11:147–160. doi: 10.1007/s12975-019-00707-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fang Y., Gao S., Wang X., Cao Y., Lu J., Chen S., Lenahan C., Zhang J.H., Shao A., Zhang J. Programmed cell deaths and potential crosstalk with blood–brain barrier dysfunction after hemorrhagic stroke. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Q., Han X., Lan X., Gao Y., Wan J., Durham F., Cheng T., Yang J., Wang Z., Jiang C., et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2 doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y., Wang J., Chen S., Wu P., Xu S., Wang C., Shi H., Bihl J. MiR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SH-SY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res. Ther. 2020;11 doi: 10.1186/s13287-020-01836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karuppagounder S.S., Alin L., Chen Y., Brand D., Bourassa M.W., Dietrich K., Wilkinson C.M., Nadeau C.A., Kumar A., Perry S., et al. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E2 to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann. Neurol. 2018;84:854–872. doi: 10.1002/ana.25356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang H., Wen M., Chen J., Yao C., Lin X., Lin Z., Ru J., Zhuge Q., Yang S. Pyridoxal isonicotinoyl hydrazone improves neurological recovery by attenuating ferroptosis and inflammation in cerebral hemorrhagic mice. BioMed Res. Int. 2021:2021. doi: 10.1155/2021/9916328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu H., Santo A., Jia Z., Li Y. GPx4 in bacterial infection and polymicrobial sepsis: involvement of ferroptosis and pyroptosis. Reactive Oxygen Species. 2019;7:154. doi: 10.20455/ros.2019.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mao H., Zhao Y., Li H., Lei L. Ferroptosis as an emerging target in inflammatory diseases. Prog. Biophys. Mol. Biol. 2020;155:20–28. doi: 10.1016/j.pbiomolbio.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 91.Zhou L., Yeo A.T., Ballarano C., Weber U., Allen K.N., Gilmore T.D., Whitty A. Disulfide-mediated stabilization of the IκB kinase binding domain of NF-κb essential modulator (NEMO) Biochemistry. 2014;53:7929–7944. doi: 10.1021/bi500920n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh S. Sen, Rai S.N., Birla H., Zahra W., Rathore A.S., Singh S.P. NF-ΚB-Mediated neuroinflammation in Parkinson's disease and potential therapeutic effect of polyphenols. Neurotox. Res. 2020;37:491–507. doi: 10.1007/s12640-019-00147-2. [DOI] [PubMed] [Google Scholar]
- 93.Wang C., Yuan W., Hu A., Lin J., Xia Z., Yang C.F., Li Y., Zhang Z. Dexmedetomidine alleviated sepsis-induced myocardial ferroptosis and septic heart injury. Mol. Med. Rep. 2020;22:175–184. doi: 10.3892/mmr.2020.11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang W.S., Kim K.J., Gaschler M.M., Patel M., Shchepinov M.S., Stockwell B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ito F., Sono Y., Ito T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants. 2019;8 doi: 10.3390/antiox8030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fritz K.S., Petersen D.R. An overview of the chemistry and biology of reactive aldehydes. Free Radic. Biol. Med. 2013;59:85–91. doi: 10.1016/j.freeradbiomed.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Font-Nieves M., Sans-Fons M.G., Gorina R., Bonfill-Teixidor E., Salas-Peŕdomo A., Maŕquez-Kisinousky L., Santalucia T., Planas A.M. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E 2 production in astrocytes. J. Biol. Chem. 2012;287:6454–6468. doi: 10.1074/jbc.M111.327874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu M.-Y., Lin T.-H., Chiu Y.-C., Liou H.-C., Yang R.-S., Fu W.-M. Involvement of 15-lipoxygenase in the inflammatory arthritis. J. Cell. Biochem. 2012;113:2279–2289. doi: 10.1002/jcb.24098. [DOI] [PubMed] [Google Scholar]
- 99.Abrial C., Grassin-Delyle S., Salvator H., Brollo M., Naline E., Devillier P. 15-Lipoxygenases regulate the production of chemokines in human lung macrophages. Br. J. Pharmacol. 2015;172:4319–4330. doi: 10.1111/bph.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bogdan A.R., Miyazawa M., Hashimoto K., Tsuji Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem. Sci. 2016;41:274–286. doi: 10.1016/j.tibs.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Q., Zhou J. Neuroinflammation in the central nervous system: symphony of glial cells. Glia. 2019;67:1017–1035. doi: 10.1002/glia.23571. [DOI] [PubMed] [Google Scholar]
- 102.Kim E.H., Wong S.W., Martinez J. Programmed necrosis and disease:we interrupt your regular programming to bring you necroinflammation. Cell Death Differ. 2019;26:25–40. doi: 10.1038/s41418-018-0179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Proneth B., Conrad M. Ferroptosis and necroinflammation, a yet poorly explored link. Cell Death Differ. 2019;26:14–24. doi: 10.1038/s41418-018-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Minkiewicz J., de Rivero Vaccari J.P., Keane R.W. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013;61:1113–1121. doi: 10.1002/glia.22499. [DOI] [PubMed] [Google Scholar]
- 105.Sarhan M., Land W.G., Tonnus W., Hugo C.P., Linkermann A. Origin and consequences of necroinflammation. Physiol. Rev. 2018;98:727–780. doi: 10.1152/physrev.00041.2016. [DOI] [PubMed] [Google Scholar]
- 106.Li J., Cao F., Yin H. liang, Huang Z. jian, Lin Z., tao, Mao N., Sun B., Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11 doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang L., Hickman J.H., Wang S.J., Gu W. Dynamic roles of P53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle. 2015;14:2881–2885. doi: 10.1080/15384101.2015.1068479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lane D.J.R., Merlot A.M., Huang M.L.H., Bae D.H., Jansson P.J., Sahni S., Kalinowski D.S., Richardson D.R. Cellular iron uptake, trafficking and metabolism: key molecules and mechanisms and their roles in disease. Biochim. Biophys. Acta Mol. Cell Res. 2015;1853:1130–1144. doi: 10.1016/j.bbamcr.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 109.Kang R., Kroemer G., Tang D. The tumor suppressor protein P53 and the ferroptosis network. Free Radic. Biol. Med. 2019;133:162–168. doi: 10.1016/j.freeradbiomed.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mandal S., Mandal A., Park M.H. Depletion of the polyamines spermidine and spermine by overexpression of spermidine/spermine N1-acetyltransferase 1 (SAT1) leads to mitochondria-mediated apoptosis in mammalian cells. Biochem. J. 2015;468:435–447. doi: 10.1042/BJ20150168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ou Y., Wang S.J., Li D., Chu B., Gu W. Activation of SAT1 engages polyamine metabolism with P53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E6806–E6812. doi: 10.1073/pnas.1607152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Macías-Rodríguez R.U., Inzaugarat M.E., Ruiz-margáin A., Nelson L.J., Trautwein C., Cubero F.J. Reclassifying hepatic cell death during liver damage: ferroptosis—a novel form of non-apoptotic cell death? Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21051651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Winblad B., Amouyel P., Andrieu S., Ballard C., Brayne C., Brodaty H., Cedazo-Minguez A., Dubois B., Edvardsson D., Feldman H., et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 114.Morris G.P., Clark I.A., Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer's disease. Acta Neuropathol Commun. 2014;2:1–21. doi: 10.1186/s40478-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Belaidi A.A., Bush A.I. Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. J. Neurochem. 2016;139:179–197. doi: 10.1111/jnc.13425. [DOI] [PubMed] [Google Scholar]
- 116.Nixon R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 117.Ashraf A., Jeandriens J., Parkes H.G., So P.W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer's disease: evidence of ferroptosis. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ayton S., Portbury S., Kalinowski P., Agarwal P., Diouf I., Schneider J.A., Morris M.C., Bush A.I. Regional brain iron associated with deterioration in Alzheimer's disease: a large cohort study and theoretical significance. Alzheimer's Dementia. 2021;17:1244–1256. doi: 10.1002/alz.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takahashi R.H., Nagao T., Gouras G.K. Plaque Formation and the intraneuronal accumulation of β-amyloid in Alzheimer's disease. Pathol. Int. 2017;67:185–193. doi: 10.1111/pin.12520. [DOI] [PubMed] [Google Scholar]
- 120.Huang L., McClatchy D.B., Maher P., Liang Z., Diedrich J.K., Soriano-Castell D., Goldberg J., Shokhirev M., Yates J.R., Schubert D., et al. Intracellular amyloid toxicity induces oxytosis/ferroptosis regulated cell death. Cell Death Dis. 2020;11:828. doi: 10.1038/s41419-020-03020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gao Y., Li J., Wu Q., Wang S., Yang S., Li X., Chen N., Li L., Zhang L. Tetrahydroxy stilbene glycoside ameliorates Alzheimer's disease in APP/PS1 mice via glutathione peroxidase related ferroptosis. Int. Immunopharm. 2021:99. doi: 10.1016/j.intimp.2021.108002. [DOI] [PubMed] [Google Scholar]
- 122.Emamzadeh F.N., Surguchov A. Parkinson's disease: biomarkers, treatment, and risk factors. Front. Neurosci. 2018;12:612. doi: 10.3389/fnins.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bhalla S., Mehan S., Khan A., Rehman M.U. Protective role of IGF-1 and GLP-1 signaling activation in neurological dysfunctions. Neurosci. Biobehav. Rev. 2022;142 doi: 10.1016/J.NEUBIOREV.2022.104896. [DOI] [PubMed] [Google Scholar]
- 124.Mhyre T.R., Boyd J.T., Hamill R.W., Maguire-Zeiss K.A. Parkinson's disease. Subcell. Biochem. 2012;65:389–455. doi: 10.1007/978-94-007-5416-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu Y., Zhao J., Zhao Y., Zhou L., Qiao H., Xu Q., Liu Y. The role of ferroptosis in neurodegenerative diseases. Mol. Biol. Rep. 2022:1. doi: 10.1007/s11033-022-08048-y. –7. [DOI] [PubMed] [Google Scholar]
- 126.Troncoso-Escudero P., Parra A., Nassif M., Vidal R.L. Outside in: unraveling the role of neuroinflammation in the progression of Parkinson's disease. Front. Neurol. 2018;9:860. doi: 10.3389/fneur.2018.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lei P., Ayton S., Finkelstein D.I., Spoerri L., Ciccotosto G.D., Wright D.K., Wong B.X.W., Adlard P.A., Cherny R.A., Lam L.Q., et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- 128.Do Van B., Gouel F., Jonneaux A., Timmerman K., Gelé P., Pétrault M., Bastide M., Laloux C., Moreau C., Bordet R., et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol. Dis. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 129.Guiney S.J., Adlard P.A., Bush A.I., Finkelstein D.I., Ayton S. Ferroptosis and cell death mechanisms in Parkinson's disease. Neurochem. Int. 2017;104:34–48. doi: 10.1016/j.neuint.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 130.Abeyawardhane D.L., Fernández R.D., Murgas C.J., Heitger D.R., Forney A.K., Crozier M.K., Lucas H.R. Iron redox chemistry promotes antiparallel oligomerization of α-synuclein. J. Am. Chem. Soc. 2018;140:5028–5032. doi: 10.1021/jacs.8b02013. [DOI] [PubMed] [Google Scholar]
- 131.Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ito K., Eguchi Y., Imagawa Y., Akai S., Mochizuki H., Tsujimoto Y. MPP+ induces necrostatin-1-and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Dis. 2017;3 doi: 10.1038/cddiscovery.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu L., Liu M., Liang J., Li N., Yang D., Cai J., Zhang Y., He Y., Chen Z., Ma T. Ferroptosis as a new mechanism in Parkinson's disease therapy using traditional Chinese medicine. Front. Pharmacol. 2021;12:1270. doi: 10.3389/fphar.2021.659584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu J., Wang F. Role of neuroinflammation in amyotrophic lateral sclerosis: cellular mechanisms and therapeutic implications. Front. Immunol. 2017;8:1005. doi: 10.3389/fimmu.2017.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Choi I.Y., Lee P., Statland J., McVey A., Dimachkie M., Brooks W., Barohn R. Reduction in cerebral antioxidant, glutathione (GSH), in patients with ALS: a preliminary study (P6.105) Neurology. 2015:84. [Google Scholar]
- 136.Mesci P., Zaïdi S., Lobsiger C.S., Millecamps S., Escartin C., Seilhean D., Sato H., Mallat M., Boillée S. System XC- is a mediator of microglial function and its deletion slows symptoms in amyotrophic lateral sclerosis mice. Brain. 2015;138:53–68. doi: 10.1093/brain/awu312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saccon R.A., Bunton-Stasyshyn R.K.A., Fisher E.M.C., Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136:2342–2358. doi: 10.1093/brain/awt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Philips T., Rothstein J.D. Rodent models of amyotrophic lateral sclerosis. Curr. Protoc. Pharmacol. 2015;2015 doi: 10.1002/0471141755.ph0567s69. 5.67.1-5.67.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen L., Hambright W.S., Na R., Ran Q. Ablation of the ferroptosis inhibitor glutathione peroxidase 4 in neurons results in rapid motor neuron degeneration and paralysis. J. Biol. Chem. 2015;290:28097–28106. doi: 10.1074/jbc.M115.680090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen L., Na R., Danae McLane K., Thompson C.S., Gao J., Wang X., Ran Q. Overexpression of ferroptosis defense enzyme Gpx4 retards motor neuron disease of SOD1G93A mice. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-021-92369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang T., Tomas D., Perera N.D., Cuic B., Luikinga S., Viden A., Barton S.K., McLean C.A., Samson A.L., Southon A., et al. Ferroptosis mediates selective motor neuron death in amyotrophic lateral sclerosis. Cell Death Differ. 2022;29:1187–1198. doi: 10.1038/s41418-021-00910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wyant K.J., Ridder A.J., Dayalu P. Huntington's disease—update on treatments. Curr. Neurol. Neurosci. Rep. 2017;17:1–11. doi: 10.1007/s11910-017-0739-9. [DOI] [PubMed] [Google Scholar]
- 143.Saba J., Couselo F.L., Bruno J., Carniglia L., Durand D., Lasaga M., Caruso C. Neuroinflammation in Huntington's disease: a starring role for astrocyte and microglia. Curr. Neuropharmacol. 2021;20:1116–1143. doi: 10.2174/1570159x19666211201094608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lois C., González I., Izquierdo-García D., Zürcher N.R., Wilkens P., Loggia M.L., Hooker J.M., Rosas H.D. Neuroinflammation in Huntington's disease: new insights with 11C-PBR28 PET/MRI. ACS Chem. Neurosci. 2018;9:2563–2571. doi: 10.1021/acschemneuro.8b00072. [DOI] [PubMed] [Google Scholar]
- 145.Dubinsky J.M. Towards an understanding of energy impairment in Huntington's disease brain. J. Huntingtons Dis. 2017;6:267–302. doi: 10.3233/JHD-170264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kumar A., Kumar V., Singh K., Kumar S., Kim Y.S., Lee Y.M., Kim J.J. Therapeutic advances for Huntington's disease. Brain Sci. 2020;10 doi: 10.3390/brainsci10010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ribeiro M., Rosenstock T.R., Cunha-Oliveira T., Ferreira I.L., Oliveira C.R., Rego A.C. Glutathione redox cycle dysregulation in Huntington's disease knock-in striatal cells. Free Radic. Biol. Med. 2012;53:1857–1867. doi: 10.1016/j.freeradbiomed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 148.Mason R.P., Casu M., Butler N., Breda C., Campesan S., Clapp J., Green E.W., Dhulkhed D., Kyriacou C.P., Giorgini F. Glutathione peroxidase activity is neuroprotective in models of Huntington's disease. Nat. Genet. 2013;45:1249–1254. doi: 10.1038/ng.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Krishnamurthi R.V., Feigin V.L., Forouzanfar M.H., Mensah G.A., Connor M., Bennett D.A., Moran A.E., Sacco R.L., Anderson L.M., Truelsen T., et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet Global Health. 2013;1:e259. doi: 10.1016/S2214-109X(13)70089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Y., Fang Y., Zhang Z., Luo Y., Zhang A., Lenahan C., Chen S. Ferroptosis: an emerging therapeutic target in stroke. J. Neurochem. 2022;160:64–73. doi: 10.1111/jnc.15351. [DOI] [PubMed] [Google Scholar]
- 151.Tan Q., Fang Y., Gu Q. Mechanisms of modulation of ferroptosis and its role in central nervous system diseases. Front. Pharmacol. 2021:12. doi: 10.3389/fphar.2021.657033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou Y., Zhang S., Fan X. Role of polyphenols as antioxidant supplementation in ischemic stroke. Oxid. Med. Cell. Longev. 2021:2021. doi: 10.1155/2021/5471347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choi K.-H., Park M.-S., Kim J.-T., Nam T.-S., Choi S.-M., Kim B.-C., Kim M.-K., Cho K.-H. The serum ferritin level is an important predictor of hemorrhagic transformation in acute ischaemic stroke. Eur. J. Neurol. 2012;19:570–577. doi: 10.1111/j.1468-1331.2011.03564.x. [DOI] [PubMed] [Google Scholar]
- 154.Tuo Q.Z., Lei P., Jackman K.A., Li X.L., Xiong H., Li X.L., Liuyang Z.Y., Roisman L., Zhang S.T., Ayton S., et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatr. 2017;22:1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- 155.Zille M., Karuppagounder S.S., Chen Y., Gough P.J., Bertin J., Finger J., Milner T.A., Jonas E.A., Ratan R.R. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke. 2017;48:1033–1043. doi: 10.1161/STROKEAHA.116.015609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weiland A., Wang Y., Wu W., Lan X., Han X., Li Q., Wang J. Ferroptosis and its role in diverse brain diseases. Mol. Neurobiol. 2019;56:4880–4893. doi: 10.1007/s12035-018-1403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 158.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gao M., Monian P., Quadri N., Ramasamy R., Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Karuppagounder S.S., Ratan R.R. Hypoxia-inducible factor prolyl hydroxylase inhibition: robust new target or another big bust for stroke therapeutics. J. Cerebr. Blood Flow Metabol. 2012;32:1347–1361. doi: 10.1038/jcbfm.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ren J.X., Sun X., Yan X.L., Guo Z.N., Yang Y. Ferroptosis in neurological diseases. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo Z., Lin J., Sun K., Guo J., Yao X., Wang G., Hou L., Xu J., Guo J., Guo F. Deferoxamine alleviates osteoarthritis by inhibiting chondrocyte ferroptosis and activating the Nrf2 pathway. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.791376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu J., Yang J.J., Cao Y., Li H., Zhao H., Yang S., Li K. Iron overload contributes to general anaesthesia-induced neurotoxicity and cognitive deficits. J. Neuroinflammation. 2020;17 doi: 10.1186/s12974-020-01777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hansen J.B., Tonnesen M.F., Madsen A.N., Hagedorn P.H., Friberg J., Grunnet L.G., Heller R.S., Nielsen A.O., Storling J., Baeyens L., et al. Divalent metal transporter 1 regulates iron-mediated ros and pancreatic β cell fate in response to cytokines. Cell Metabol. 2012;16:449–461. doi: 10.1016/j.cmet.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 165.Do Van B., Gouel F., Jonneaux A., Timmerman K., Gelé P., Pétrault M., Bastide M., Laloux C., Moreau C., Bordet R., et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol. Dis. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 166.Devos D., Moreau C., Devedjian J.C., Kluza J., Petrault M., Laloux C., Jonneaux A., Ryckewaert G., Garçon G., Rouaix N., et al. Targeting chelatable iron as a therapeutic modality in Parkinson's disease. Antioxidants Redox Signal. 2014;21:195–210. doi: 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guiney S.J., Adlard P.A., Bush A.I., Finkelstein D.I., Ayton S. Ferroptosis and cell death mechanisms in Parkinson's disease. Neurochem. Int. 2017;104:34–48. doi: 10.1016/j.neuint.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 168.Martin-Bastida A., Ward R.J., Newbould R., Piccini P., Sharp D., Kabba C., Patel M.C., Spino M., Connelly J., Tricta F., et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson's disease. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ward R.J., Zucca F.A., Duyn J.H., Crichton R.R., Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moreau C., Duce J.A., Rascol O., Devedjian J.-C., Berg D., Dexter D., Cabantchik Z.I., Bush A.I., Devos D. Iron as a therapeutic target for Parkinson's disease. Mov. Disord. 2018;33:568–574. doi: 10.1002/mds.27275. [DOI] [PubMed] [Google Scholar]
- 171.Zhang P., Chen L., Zhao Q., Du X., Bi M., Li Y., Jiao Q., Jiang H. Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson's disease. Free Radic. Biol. Med. 2020;152:227–234. doi: 10.1016/j.freeradbiomed.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 172.Ren J.X., Sun X., Yan X.L., Guo Z.N., Yang Y. Ferroptosis in neurological diseases. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., Herbach N., Aichler M., Walch A., Eggenhofer E., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Skouta R., Dixon S.J., Wang J., Dunn D.E., Orman M., Shimada K., Rosenberg P.A., Lo D.C., Weinberg J.M., Linkermann A., et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gowrisankar Y.V., Clark M.A. Angiotensin II induces interleukin-6 expression in astrocytes: role of reactive oxygen species and NF-ΚB. Mol. Cell. Endocrinol. 2016;437:130–141. doi: 10.1016/j.mce.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 177.Chen Y., Zhang P., Chen W., Chen G. Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol. Lett. 2020;225:9–15. doi: 10.1016/j.imlet.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 178.Chen Y., Fan H., Wang S., Tang G., Zhai C., Shen L. Ferroptosis: a novel therapeutic target for ischemia-reperfusion injury. Front. Cell Dev. Biol. 2021;9:2203. doi: 10.3389/fcell.2021.688605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li Y., Feng D., Wang Z., Zhao Y., Sun R., Tian D., Liu D., Zhang F., Ning S., Yao J., et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–2299. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cao Y., Li Y., He C., Yan F., Li J.-R., Xu H.-Z., Zhuang J.-F., Zhou H., Peng Y.-C., Fu X.-J., et al. Selective ferroptosis inhibitor liproxstatin-1 attenuates neurological deficits and neuroinflammation after subarachnoid hemorrhage. Neurosci. Bull. 2021;37:535–549. doi: 10.1007/s12264-020-00620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim S.M., Ha J.S., Han A.R., Cho S.W., Yang S.J. Effects of α-lipoic acid on LPS-induced neuroinflammation and NLRP3 inflammasome activation through the regulation of BV-2 microglial cells activation. BMB Rep. 2019;52:613–618. doi: 10.5483/BMBRep.2019.52.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Benameur T., Soleti R., Porro C. The potential neuroprotective role of free and encapsulated quercetin mediated by mirna against neurological diseases. Nutrients. 2021;13 doi: 10.3390/nu13041318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li Y., Yao J., Han C., Yang J., Chaudhry M.T., Wang S., Liu H., Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8 doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Andres S., Pevny S., Ziegenhagen R., Bakhiya N., Schäfer B., Hirsch-Ernst K.I., Lampen A. Safety aspects of the use of quercetin as a dietary supplement. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700447. [DOI] [PubMed] [Google Scholar]
- 185.Chen W.W., Zhang X., Huang W.J. Role of neuroinflammation in neurodegenerative diseases (Review) Mol Med Rep. 2016;13:3391–3396. doi: 10.3892/mmr.2016.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liao Y.R., Lin J.Y. Quercetin intraperitoneal administration ameliorates lipopolysaccharide-induced systemic inflammation in mice. Life Sci. 2015;137:89–97. doi: 10.1016/j.lfs.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 187.Sangkhae V., Nemeth E. Regulation of the iron homeostatic hormone hepcidin. Adv. Nutr. 2017;8:126–136. doi: 10.3945/an.116.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Guo X., Chen M., Zeng H., Liu P., Zhu X., Zhou F., Liu J., Zhang J., Dong Z., Tang Y., et al. Quercetin attenuates ethanol-induced iron uptake and myocardial injury by regulating the angiotensin II-L-Type calcium channel. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700772. [DOI] [PubMed] [Google Scholar]
- 189.Mazhar M., Kabir N., Simjee S.U. Quercetin modulates iron homeostasis and INOS expression of splenic macrophages in a rat model of iron deficiency anemia. Chin. J. Nat. Med. 2018;16:580–589. doi: 10.1016/S1875-5364(18)30095-5. [DOI] [PubMed] [Google Scholar]
- 190.Xiao L., Luo G., Tang Y., Yao P. Quercetin and iron metabolism: what we know and what we need to know. Food Chem. Toxicol. 2018;114:190–203. doi: 10.1016/j.fct.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 191.Rui W., Li S., Xiao H., Xiao M., Shi J. Baicalein attenuates neuroinflammation by inhibiting NLRP3/caspase-1/GSDMD pathway in MPTP-induced mice model of Parkinson's disease. Int. J. Neuropsychopharmacol. 2020;23:762–773. doi: 10.1093/ijnp/pyaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zheng Z.V., Cheung C.Y., Lyu H., Chan H.Y., Li Y., Bian Z.X., Wang K.K.W., Poon W.S. Baicalein enhances the effect of low dose levodopa on the gait deficits and protects dopaminergic neurons in experimental parkinsonism. J. Clin. Neurosci. 2019;64:242–251. doi: 10.1016/j.jocn.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 193.Xie Y., Song X., Sun X., Huang J., Zhong M., Lotze M.T., Zeh H.J., Kang R., Tang D. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 2016;473:775–780. doi: 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 194.Xie Y., Song X., Sun X., Huang J., Zhong M., Lotze M.T., Zeh H.J., Kang R., Tang D. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 2016;473:775–780. doi: 10.1016/j.bbrc.2016.03.052. [DOI] [PubMed] [Google Scholar]
- 195.Kenny E.M., Fidan E., Yang Q., Anthonymuthu T.S., New L.A., Meyer E.A., Wang H., Kochanek P.M., Dixon C.E., Kagan V.E., et al. Ferroptosis contributes to neuronal death and functional outcome after traumatic brain injury. Crit. Care Med. 2019;47:410–418. doi: 10.1097/CCM.0000000000003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhou Y.-X., Zhang H., Peng C. Puerarin: a review of pharmacological effects. Phytother Res. 2014;28:961–975. doi: 10.1002/ptr.5083. [DOI] [PubMed] [Google Scholar]
- 197.Roh J.L., Kim E.H., Park J.Y., Kim J.W., Kwon M., Lee B.H. Piperlongumine selectively kills cancer cells and increases cisplatin antitumor activity in head and neck cancer. Oncotarget. 2014;5:9227–9238. doi: 10.18632/oncotarget.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chen Y., Liu J.M., Xiong X.X., Qiu X.Y., Pan F., Liu D., Lan S.J., Jin S., Yu S. Bin, Chen X.Q. Piperlongumine selectively kills hepatocellular carcinoma cells and preferentially inhibits their invasion via ROS-ER-MAPKs-CHOP. Oncotarget. 2015;6:6406–6421. doi: 10.18632/oncotarget.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang Y., Kong W.N., Chai X.Q. Compound of icariin, Astragalus, and puerarin mitigates iron overload in the cerebral cortex of Alzheimer's disease mice. Neural Regen Res. 2018;13:731–736. doi: 10.4103/1673-5374.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang H.J., Huang C.Y., Lee M., Lin J.Y., Hsieh-Li H.M. Puerariae radix prevents anxiety and cognitive deficits in mice under oligomeric Aβ-induced stress. Am. J. Chin. Med. 2019;47:1459–1481. doi: 10.1142/S0192415X19500757. [DOI] [PubMed] [Google Scholar]
- 201.Liu C., Zhang J., Lun X., Li L. LncRNA PVT1 promotes hypoxia-induced cardiomyocyte injury by inhibiting MiR-214-3p. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/4604883. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 202.Regner-Nelke L., Nelke C., Schroeter C.B., Dziewas R., Warnecke T., Ruck T., Meuth S.G. Enjoy Carefully: the Multifaceted Role of Vitamin e in Neuro-Nutrition. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221810087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Simpson D.S.A., Oliver P.L. Ros generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants. 2020;9:1–27. doi: 10.3390/antiox9080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lewis E.D., Meydani S.N., Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019;71:487–494. doi: 10.1002/iub.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hambright W.S., Fonseca R.S., Chen L., Na R., Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8–17. doi: 10.1016/j.redox.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Basambombo L.L., Carmichael P.H., Côté S., Laurin D. Use of vitamin E and C supplements for the prevention of cognitive decline. Ann. Pharmacother. 2017;51:118–124. doi: 10.1177/1060028016673072. [DOI] [PubMed] [Google Scholar]
- 207.Dysken M.W., Sano M., Asthana S., Vertrees J.E., Pallaki M., Llorente M., Love S., Schellenberg G.D., McCarten J.R., Malphurs J., et al. Effect of vitamin E and memantine on functional decline in alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA. 2014;311:33–44. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dysken M.W., Guarino P.D., Vertrees J.E., Asthana S., Sano M., Llorente M., Pallaki M., Love S., Schellenberg G.D., McCarten J.R., et al. Vitamin E and memantine in Alzheimer's disease: clinical trial methods and baseline Data. Alzheimer's Dementia. 2014;10:36–44. doi: 10.1016/j.jalz.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Aaseth J., Alexander J., Alehagen U. Coenzyme Q10 supplementation – in ageing and disease. Mech. Ageing Dev. 2021;197 doi: 10.1016/j.mad.2021.111521. [DOI] [PubMed] [Google Scholar]
- 210.Muthukumaran K., Kanwar A., Vegh C., Marginean A., Elliott A., Guilbeault N., Badour A., Sikorska M., Cohen J., Pandey S. Ubisol-Q10 (a nanomicellar water-soluble formulation of CoQ10) treatment inhibits alzheimer-type behavioral and pathological symptoms in a double transgenic mouse (TgAPEswe, PSEN1dE9) model of Alzheimer's disease. J. Alzheim. Dis. 2018;61:221–236. doi: 10.3233/JAD-170275. [DOI] [PubMed] [Google Scholar]
- 211.Varela-López A., Giampieri F., Battino M., Quiles J.L. Coenzyme Q and its role in the dietary therapy against aging. Molecules. 2016;21 doi: 10.3390/molecules21030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Morris G., Anderson G., Berk M., Maes M. Coenzyme Q10 depletion in medical and neuropsychiatric disorders: potential repercussions and therapeutic implications. Mol. Neurobiol. 2013;48:883–903. doi: 10.1007/s12035-013-8477-8. [DOI] [PubMed] [Google Scholar]
- 213.Gerwyn M., Maes M. Mechanisms explaining muscle fatigue and muscle pain in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a review of recent findings. Curr. Rheumatol. Rep. 2017;19:1–10. doi: 10.1007/s11926-017-0628-x. [DOI] [PubMed] [Google Scholar]
- 214.Morris G., Berk M., Galecki P., Walder K., Maes M. The neuro-immune pathophysiology of central and peripheral fatigue in systemic immune-inflammatory and neuro-immune diseases. Mol. Neurobiol. 2016;53:1195–1219. doi: 10.1007/s12035-015-9090-9. [DOI] [PubMed] [Google Scholar]
- 215.Li L., Xu D., Lin J., Zhang D., Wang G., Sui L., Ding H., Du J. Coenzyme Q10 attenuated β-amyloid25–35–induced inflammatory responses in PC12 cells through regulation of the NF–ΚB signaling pathway. Brain Res. Bull. 2017;131:192–198. doi: 10.1016/j.brainresbull.2017.04.014. [DOI] [PubMed] [Google Scholar]