Abstract
Intermittent theta burst stimulation (iTBS), a time-saving and cost-effective repetitive transcranial magnetic stimulation regime, has been shown to improve cognition in patients with Alzheimer’s disease (AD). However, the specific mechanism underlying iTBS-induced cognitive enhancement remains unknown. Previous studies suggested that mitochondrial functions are modulated by magnetic stimulation. Here, we showed that iTBS upregulates the expression of iron-sulfur cluster assembly 1 (ISCA1, an essential regulatory factor for mitochondrial respiration) in the brain of APP/PS1 mice. In vivo and in vitro studies revealed that iTBS modulates mitochondrial iron-sulfur cluster assembly to facilitate mitochondrial respiration and function, which is required for ISCA1. Moreover, iTBS rescues cognitive decline and attenuates AD-type pathologies in APP/PS1 mice. The present study uncovers a novel mechanism by which iTBS modulates mitochondrial respiration and function via ISCA1-mediated iron-sulfur cluster assembly to alleviate cognitive impairments and pathologies in AD. We provide the mechanistic target of iTBS that warrants its therapeutic potential for AD patients.
Keywords: Intermittent theta burst stimulation, Alzheimer’s disease, Iron-sulfur cluster assembly 1, Mitochondrial dysfunction, Neurodegeneration
Introduction
Alzheimer’s disease (AD), the most common form of dementia, is characterized by a progressive cognitive decline that seriously impairs individuals’ independence, resulting in a reduced quality of life and a heavy social burden [1]. However, therapeutic strategies targeting the major pathological hallmarks of AD, including senile plaques and neurofibrillary tangles, have always been frustrated [2, 3]. It is imperative to explore alternative approaches to halt the early neurodegeneration of AD prior to the breakthrough of disease-modifying medications.
A growing body of evidence suggests that mitochondrial dysfunction may play a fundamental role in AD pathogenesis [4–6]. Specifically, inherited variance and neurotoxin mediated mitochondrial DNA disturbance promotes the loss of mitochondrial structural and functional integrity, including mitochondrial fragmentations, energy metabolism impairment, and mitophagy dysfunction, resulting in neuronal damage and apoptosis in AD [4, 7]. Thus, targeting mitochondrial dysfunction is a promising strategy for the therapy of AD. Interestingly, several important physiological processes associated with mitochondria, including ATP synthesis, reactive oxide species generation, Ca2+ homeostasis, and apoptosis have been shown to be modulated by static or time-varying magnetic fields [8–11], suggesting that modulation of mitochondrial function through magnetic fields has therapeutic potential for AD.
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive method of brain stimulation based on pulsed magnetic fields [12] and has moved towards clinical applications in multiple fields, including major depressive disorder, neuropathic pain and limb motor rehabilitation at the post-acute stage [13]. Intermittent theta burst stimulation (iTBS), an emerging rTMS paradigm based on the endogenous theta oscillatory rhythm found in the hippocampus, displays a better efficiency than conventional rTMS in neural circuit repair [14]. Previous studies have revealed that iTBS facilitates multiple cognitive domains such as memory, language, visuospatial, attention and executive functions in AD patients [15–17]. Moreover, high-frequency rTMS alleviates oxidative stress through the PI3K/Akt/GLT-1 signaling pathway in a 3xTg-AD mouse model, suggesting that rTMS may modulate mitochondrial function in AD [10].
Iron-sulfur cluster assembly 1 (ISCA1) belongs to the evolutionarily-conserved A-type protein family and is involved in the mitochondrial iron-sulfur cluster (ISC) assembly machinery which is essential for the optimal mitochondrial function [18–20]. Pathogenic mutation of ISCA1 leads to instability of ISC in the mitochondrial [4Fe-4S] proteins in patients with multiple mitochondrial dysfunction syndromes [21, 22]. ISCA1 knockdown experiments suggest that it behaves as an ISC carrier to facilitate the maturity of ISC-containing proteins and thus enhances mitochondrial respiration during the late biogenesis of mitochondrial [4Fe-4S] proteins [20]. Moreover, emerging evidence has shown that ISCA1 might be modulated by magnetic fields, thereby affecting neural activity via certain downstream pathways [23, 24], suggesting that ISCA1 is likely a vital target for mitochondrial modulation via external magnetic stimulation, such as iTBS.
Here, we examined the therapeutic effects of iTBS on behavioral deficits and pathologies in APP/PS1 mice. Moreover, we determined whether iTBS modulates brain mitochondrial dysfunction such as glucose uptake, oxidative stress, and mitochondrial dynamics in APP/PS1 mice. Finally, we investigated the molecular mechanisms underlying the effects of mitochondrial modulation by iTBS via ISCA1 knockdown experiments in the SH-SY5Y-APP695 cell line.
Materials and Methods
Animals
All operations complied with the care and use of laboratory animal guidelines and were approved by the Laboratory Animal Welfare and Ethics Committee of the Army Medical University. All animal experiments were conducted in accordance with the care and use of laboratory animal guidelines issued by the National Institute of Health Guide. The 6-month-old female APP/PS1 mice were transferred to C57BL/6 background and age- and sex-matched wild-type mice were purchased from Jackson Laboratory. The mice were maintained under a 12-h light/dark cycle with free access to food in the Experimental Animal Centre of Daping Hospital (Chongqing, China) and randomly split into three groups: (1) wild-type mice with sham stimulation (Wt sham); (2) APP/PS1 mice with sham stimulation (Tg sham); and (3) APP/PS1 mice with iTBS treatment (Tg iTBS).
Cell Culture
SH-SY5Y cells transfected with the human APP695 gene carrying the Swedish mutant (SH-SY5Y-APP695) were used to conduct cell experiments. The cells were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM, HyClone, Logan, USA) supplemented with 1% penicillin-streptomycin (HyClone, Logan, USA) and 10% fetal bovine serum (FBS; Gibco, Carlsbad, USA) at 37 °C with 5% CO2. Geneticin™ selective antibiotic (Invitrogen, Carlsbad, USA) was used to screen the cells which stably expressed the human APP695 mutant gene. SH-SY5Y-APP695 cells were transfected with siRNA-ISCA1 (siISCA1) or siRNA-NC (siNC) using Lipofectamine 2000 transfection reagent (Thermofisher, Waltham, USA). The siRNA sequences (5′–3′) were as follows: siISCA1: GGAGAUUCUGAUGAAGAAGUUTT, siNC: UUCUCCGAACGUGUCACGUTT.
iTBS Treatment
For animal experiments, the magnetic stimulation was applied by a CCY-I stimulator (Yiruide, Wuhan, China) with an experimental coil (3 cm diameter). The mice were held by hand to ensure the coil was centered tangentially to the exposed head via a restraining device [25]. The mice were subjected to this device for 10 min per day for 3 consecutive days to eliminate anxiety. The protocol of one iTBS session contained 40 trains of 10 bursts at a frequency of 5 Hz, 3 pulses at 50 Hz in each burst, in a total of 1200 stimuli. The iTBS treatment was administered in one session per day for 21 consecutive days. The stimulation intensity was 80%–100% of the resting motor threshold detected by a preliminary experiment as described previously [26]. Mice in the sham stimulation group were fixed in the same apparatus and only heard the noise generated by the stimulator but were not stimulated.
For in vitro iTBS, the cells were seeded on 6-well plates (3 × 105 cells per well) and cultured in a medium containing 5% FBS 24 h before and during treatment processing. The same stimulator and protocol were used in the cell stimulation experiment at the maximum output intensity of the device. To determine the dose-effect relationship between iTBS and the expression of ISCA1, the cells were subjected to sham stimulation or various pulses of stimulation (150, 300, or 600) for 5 days. In the interfering experiments, iTBS was administered 24 h after transfection with 600 stimuli per day for 5 consecutive days.
Behavioral Tests
The Morris water maze (MWM) test, open field test (OFT), and Y-maze test were applied to measure the behavioral performance of Wt sham, Tg sham and Tg iTBS mice as previously described [27]. Briefly, we administered the MWM test to examine spatial learning and memory. The mice were trained for 5 consecutive days in the platform trial. The spatial learning of each mouse was assessed by escape latency. In the probe trial, memory consolidation was assessed by the percentage of time spent in the target quadrant and the number of platform crossings. The OFT was used to assess locomotor activity and anxiety-like behaviors. The mice were placed in the center of an open field apparatus and allowed to explore freely for 5 min. The total distance traveled, and the number of rearing were recorded to determine the locomotor activity and anxiety-like behaviors, respectively. Recognition memory was assessed by the Y-maze test. The mice were placed in the center zone of the Y-maze apparatus and allowed to explore freely for 5 min with the novel arm closed. After a 30-min interval, the mice were allowed to explore all three arms freely for 5 min. The percentage of novel arm entries and time spent in the novel arm were recorded to evaluate spatial recognition memory.
18F-FDG Positron Emission Tomography (PET)
Brain glucose uptake was measured using a small-animal PET scanner (PINGSENG, Kunshan, China). Before the experiment, the mice were fasted for 12 h with free access to water. The mice were anesthetized with 1%–2% isoflurane and injected with 300 µCi 18F-FDG. 1 h after injection, dynamic 3-dimensional imaging was performed for 10 min. The regions of interest (ROIs) were manually drawn over a sagittal brain slice using PMOD software (PMOD, Zurich, Switzerland). The standard uptake value (SUV) of 18F-FDG calculated as (mean ROI activity)/(injected dose/body weight) was used to reflect the rate of glucose uptake.
Electrophysiology
The electrophysiology tests were used to assess the excitability of cortical neuron in the Wt sham, Tg sham and Tg iTBS mice as we previously reported [28]. Briefly, the brain was quickly removed and impregnated in sucrose solution (containing, in mmol/L, 200 sucrose, 1.5 KCl, 0.5 CaCl2, 4.0 MgCl2, 1.0 KH2PO4, 25 NaHCO3, 10 Na-ascorbate and 20 dextrose) aerated with 95% O2 and 5% CO2. 300 µm coronal slices were sectioned using a vibratome (VT1000; Leica, Wetzlar, USA) and immediately transferred to artificial cerebrospinal fluid (containing, in mmol/L, 130 NaCl, 2.5 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.25 NaH2PO4, 25 NaHCO3, and 10 dextrose). Pyramidal neurons in superficial layers of the cortex were identified for whole-cell recordings. Neurons were stabilized for at least 5 min prior to data collection and were discarded if the series resistance increased by > 20%. All recordings were conducted at 30 °C–33 °C using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, USA). The mean action potential (AP) numbers and AP threshold were analyzed with pClamp 9.2 software (Molecular Devices, Sunnyvale, USA). Whole-cell recordings and data analysis were performed by another investigator blinded to the slice grouping.
Brain Tissue Preparation
Under anesthesia with 0.3% pentobarbital sodium (50 mg/kg, i.p.), the 7-month-old mice were intracardially perfused with normal saline. After rapidly removing the brain, one hemisphere was fixed in 4% paraformaldehyde and then sectioned to 30 μm coronal brain sections were cut for histological analysis. The remaining hemisphere was stored at − 80 °C for biochemical analysis. For Golgi staining, the mice were decapitated under anesthesia without perfusion, and brains were prepared for use.
Histology and Quantification
Immunohistochemistry and Immunofluorescence
The free-floating method was used for the immunohistochemical and immunofluorescent staining [29]. Briefly, the sections were incubated in the penetrative buffer (0.5% Triton X-100 in 3% H2O2) for 30 min and blocked in 3% bovine serum albumin for 1 h at room temperature, followed by incubation with primary antibodies overnight at 4 °C. The primary antibodies were as follows: 6E10 (BioLegend, San Diego, USA), anti-NeuN (Abcam, Cambridge, UK), anti-MAP2 (Abcam, Cambridge, UK), anti-GFAP (Abcam, Cambridge, UK), anti-CD68 (Abcam, Cambridge, UK), anti-3-NT (Abcam, Cambridge, UK) and anti-c-fos (CST, Danvers, USA). The sections were incubated in secondary antibody for 1 h at 37 °C. Images were acquired under the same conditions at the same time using an optical microscope (Zeiss, Oberkochen, Germany). The area fraction or integrated fluorescence intensity of positive staining was quantified with ImageJ software by another investigator blinded to the grouping.
Congo Red Staining
For Congo red staining, the sections were mounted on glass slides and impregnated in the prepared working saturated sodium chloride solution at room temperature for 20 min. Then the sections were immediately transferred to working Congo red solution and left for 2 h, followed by 10 s dehydration in absolute alcohol three times.
TUNEL Staining
Apoptosis was detected using the In Situ Death Detection Kit (Roche, Basel, USA), following the manufacturer's specification with modifications. In brief, the sections were treated with permeabilization solution on ice for 2 min and then incubated in blocking solution at room temperature for 20 min to remove endogenous peroxidase, followed by incubation in TUNEL reaction mixture at 37 °C for 1 h in the dark. The apoptotic cells were visualized by DAB substrate after incubation in Converter-POD for 30 min at 37 °C.
Golgi Staining
The neuronal dendrites and dendritic spines were measured by Golgi staining using the Golgi-Cox OptimStain Kit (Hitobiotec, Kingsport, USA) as we previously reported [30]. Briefly, 150 µm coronal sections were cut on a freezing microtome after the preparation of samples via sequential impregnation in various solutions provided by the manufacturer. The sections were mounted on a gelatin-coated slide and dried in a lucifugal fume hood overnight, followed by immersion in staining solution. 100× Z-stack images of hippocampal dendrites were collected for quantitative analysis.
Western Blot
For western blot analysis, equal amounts of protein (~ 40 mg per sample) were run on 4%–20% SDS-PAGE gels (Genscript, Nanjing, China) and then transferred to nitrocellulose membranes. The primary antibodies used to probe the specific bands were as follows: ISCA1 (Invitrogen, Carlsbad, USA); PSD93, PSD95, SNAP25, VAMP1, and IBA57 from Abcam, UK; DRP1 (Phospho-S616) and β-actin from CST, USA; ISCA2, NDUFS3, NDUFS5, SDHB, ACO2, COX IV, OPA1, MFN2, DRP1, MFF and FIS1 from Proteintech, China. The bands were visualized with IRDye 800 CW secondary antibodies (Li-COR, Lincoln, USA). All target protein results are presented as intensity relative to β-actin.
Elisa and Oxidative Stress Analysis
Human Aβ40 and Aβ42 (Invitrogen, Carlsbad, USA) levels in Tris buffer solution (TBS), 2% sodium dodecyl sulfonate (SDS) and 70% formic acid (FA) extracts of APP/PS1 mouse brain tissue, or in SH-SY5Y-APP695 cell lysates, and pro-inflammatory factors IL-1β, IL-6, TNF-α and IFN-γ (Raybio, Atlanta, USA) levels in TBS extracts of APP/PS1 mouse brain tissue were quantified using the corresponding Elisa kits according to the specifications provided by the manufacturers. For oxidative stress analysis, the lipid peroxidation product malonaldehyde (MDA), superoxide dismutase (SOD), reduced glutathione (GSH), and total antioxidant capacity (T-AOC) were measured with corresponding assay kits (Jiancheng Institute, Nanjing, China), respectively.
Seahorse Analysis
The mitochondrial respiration was assessed by Seahorse analysis using the XF Cell Mito Stress Test Kit (Agilent, Santa Clara, USA), following the manufacturer's instructions. In brief, the treated cells were seeded in the specialized microplates and cultured with XF DMEM supplemented with 1 mmol/L pyruvate, 2 mmol/L glutamine, and 10 mmol/L glucose for 24 h, followed sequentially by 1.5 µmol/L oligomycin, 2.0 µmol/L FCCP and 0.5 µmol/L of rotenone and antimycin A treatment in each well. The curves of oxygen consumption rate (OCR) were drawn, and the ATP production, basal respiration, and spare respiratory capacity were calculated automatically to evaluate mitochondrial respiration by Agilent Seahorse analysis software.
Flow Cytometry
Mitochondrial membrane potential (Δψ) was assessed by flow cytometry using a JC-1 assay kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Briefly, 3 × 105 cells were harvested in a centrifuge tube after 5 consecutive days of treatment and then resuspended in 0.5 mL fresh medium. An identical volume of working JC-1 solution was added to each sample and incubated at 37 °C for 20 min in the dark. JC-1 monomers (green) and aggregates (red) were measured via the FITC and PE channels, respectively. The JC-1 monomers ratio, indicative of a depolarized Δψ, was calculated as monomers/(monomers + aggregates) to assess the level of mitochondrial dysfunction.
Mitochondrial Staining
To observe mitochondrial fusion and fission, we measured the mitochondrial morphology of the SH-SY5Y-APP695 cells using a Mitochondrial staining kit (Abcam, Cambridge, UK). The cells were seeded (3 × 104/well) and cultured on the coverslips. After 5 consecutive days iTBS treatment, the cells were incubated in the dye working solution at 37 °C for 2 h, followed by fixation with 4% paraformaldehyde solution for 20 min at room temperature. Images were acquired using a VS200 slide scanning system (Olympus, Tokyo, Japan). For the mitochondrial dynamics assay, mitochondria were divided into three classes: interconnected network (category I), large dot in the cytosol (category II) and fragments surrounding the nucleus (category III) as previously described [31]. Category I and category II + III represented mitochondrial fusion and fission, respectively. At least 500 cells were counted per sample and three biological replicates were assigned in each group.
Quantitative Real-Time PCR
Total RNA from cultured cells was extracted with TRIzol reagent (Ambion, Austin, USA), and then reverse-transcribed into cDNA using the PimeScript RT reagent kit (Takara, Otsu, Japan). Quantitative real-time PCR (qRT-PCR) was performed on a CFX96TM Real-Time System (BIO-RAD, Hercules, USA). The gene-specific primers were obtained from the literature or NCBI. The primer sequences (5′–3′) of the genes were as follows: ISCA1: forward: CTGAGCATGTGGGTCTGAAA, reverse: TTCCCTTGATGTTGGGGTTA; ACTB: forward: GACGGCCAGGTCATCACTATTG, reverse: AGGAAGGCTGGAAAAGAGCC.
Statistics
All data processing and statistical analysis were performed with SPSS 22.0 and GraphPad Prism 8.0. Statistically significant differences between groups were determined using unpaired two-tailed t test, one-way ANOVA followed by Tukey's post hoc test for multiple comparisons, or two-way ANOVA for multiple timepoints comparisons. P < 0.05 indicates a statistically significant difference.
Results
iTBS Treatment Rescues Behavioral Deficits in APP/PS1 Mice
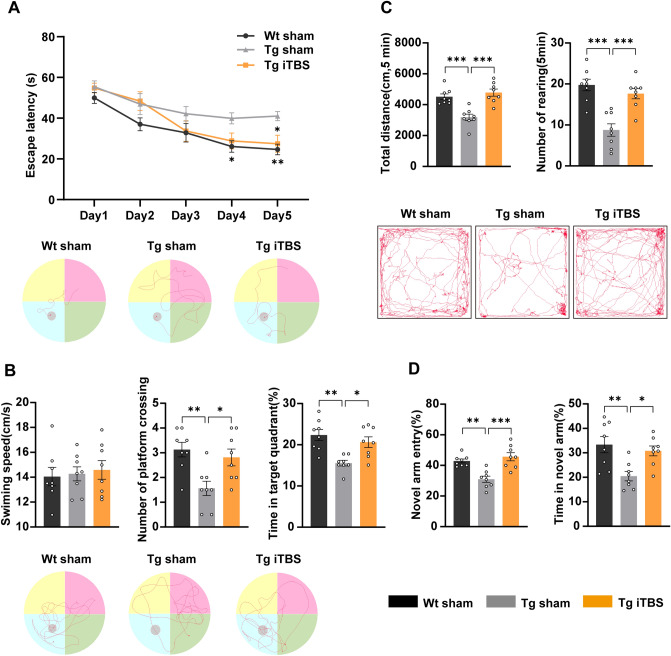
To evaluate the therapeutic effects of iTBS on cognitive decline in APP/PS1 mice, we applied the MWM test, OFT, and Y-maze test. The mice were treated with iTBS or sham stimulation at 6 months of age when behavioral phenotypes appeared. The APP/PS1 mice with sham stimulation revealed a significant cognitive impairment compared with Wt mice as expected (Fig. 1). In the MWM test, the mice treated with iTBS performed better, as substantiated by a significant reduction in escape latency in the platform trials (Fig. 1A), more times to cross-platform and a longer target quadrant time in the probe trials (Fig. 1B). The swimming speed during probe trials showed no significant difference between groups (Fig. 1B). The data suggested that iTBS significantly improves spatial learning and memory consolidation in APP/PS1 mice. In the OFT, the total distance traveled and the number of rearings were significantly increased in the Tg iTBS group compared to the Tg sham (Fig. 1C), indicating better locomotor activity and enhanced anxiety-like behavior. The Y-maze test results demonstrated that both the entry numbers and the residence time in the new arm were higher in the iTBS group (Fig. 1D), suggesting better performance of spatial recognition memory.
Fig. 1.
iTBS preserves the memory and behavior of APP/PS1 mice. A Latency to escape and representative trajectories (Day5) in the platform trials of the MWM test. B Swimming speed, number of platform crossing, percentage of time in the target quadrant and representative trajectories (the last day) in the probe trials of the MWM test. C Total distance traveled, number of rearing and representative trajectories in OFT. D Percentage of number and time of novel arm entries in the Y-maze test. Data are expressed as the mean ± SEM. n = 8 per group, two-way ANOVA is used for multiple timepoint comparisons, one-way ANOVA followed by Turkey’s post hoc test is used for multiple comparisons, *P < 0.05, **P < 0.01, ***P < 0.001. Wt: wild-type mice, Tg: APP/PS1 mice.
iTBS Treatment Reduces Aβ Load in the Brain of APP/PS1 Mice
Total Aβ plaques and compact Aβ plaques were identified by 6E10 immunohistochemical staining and Congo red staining, respectively, to assess the effect of iTBS treatment on Aβ deposition in the brain of APP/PS1 mice. The ratio of plaque area and plaque numbers in the hippocampus and neocortex were significantly reduced by iTBS treatment compared with the Tg sham group (Fig. 2A, B). Consistent with the histochemical results, ELISA assays also revealed a significant reduction in the Aβ40, Aβ42, and total Aβ levels of the TBS, SDS and FA brain extracts in the iTBS treatment group (Fig. 2C).
Fig. 2.
iTBS reduces the Aβ load in the brain of APP/PS1 mice. A Representative images of total and compact Aβ plaques detected by the 6E10 antibody and Congo red, respectively (scale bars, 500 μm). Insets are magnified images of positive staining. B Comparison of area fraction and plaque density of total plaques or compact plaques in the hippocampus and neocortex between groups. C Quantification of Aβ40, Aβ42, and total Aβ in brain TBS, SDS and FA extracts using Elisa. Values are performed as mean ± SEM. n = 8 per group, *P < 0.05, **P < 0.01, ***P < 0.001, unpaired two-tailed t test. Tg: APP/PS1 mice.
iTBS Treatment Attenuates Neurodegeneration and Neuroinflammation in APP/PS1 Mice
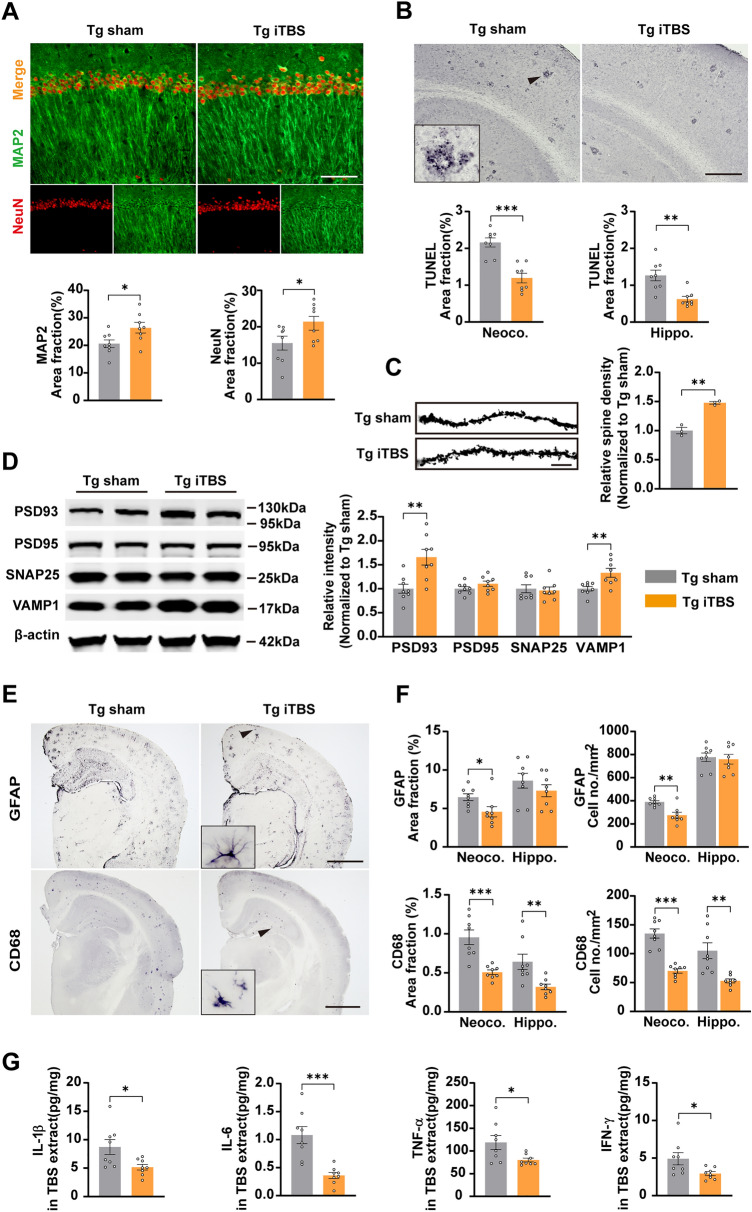
We explored whether iTBS treatment affected neurodegeneration in APP/PS1 mice, including neurite degeneration, neuronal apoptosis, and synaptic plasticity impairment. The area fractions of NeuN- and MAP2-positive staining in the hippocampus increased in the iTBS treatment group compared to those of the sham stimulation mice (Fig. 3A). We also found that TUNEL-positive staining for apoptotic cells in the hippocampus was significantly reduced by iTBS (Fig. 3B). These data suggested that iTBS promotes the survival and integrity of hippocampal neurons in APP/PS1 mice. Moreover, the density of dendritic spines measured with Golgi staining in hippocampal neurons (Fig. 3C) and the levels of the synaptic proteins PSD93 and VAMP1 (Fig. 3D) were significantly increased by iTBS. Although no significant difference in PSD95 or SNAP25 was found between groups (Fig. 3D), these data indicated that iTBS improves the synaptic plasticity impairment in APP/PS1 mice. For neuroinflammation, we showed that astrocytosis detected by GFAP staining in the neocortex and microgliosis detected by CD68 staining in the neocortex and hippocampus were significantly reduced in the Tg iTBS group, while activated astrocyte in the hippocampus showed no significant differences (Fig. 3E, F). We also found that the levels of IL-1β, IL-6, TNF-α, and IFN-γ in the TBS brain tissue extracts decreased in iTBS-treated mice (Fig. 3G), suggesting the anti-neuroinflammatory effects of iTBS in AD.
Fig. 3.
iTBS attenuates other AD-type pathologies in the brain of APP/PS1 mice. A Representative images of immunofluorescent co-staining of NeuN and MAP2 and quantification of area fractions of positive staining in the CA1 region of the hippocampus. (scale bar, 20 μm). B Representative images and quantification of neuronal apoptosis in the neocortex and hippocampus visualized by TUNEL staining (scale bar, 200 μm). Inset is the magnified image of positive staining. C Representative images of Golgi-stained neuronal dendrites in the hippocampal CA1 region (scale bar, 10 μm) and comparison of spine intensity between the two groups. (n = 3). D Representative western blotting bands and quantification of synaptic proteins in each group. E Representative images of activated astrocyte (GFAP) and microglia (CD68) immunohistochemical staining in the brain of APP/PS1 (scale bar, 500 μm) mice. Insets are the magnified morphology of positive staining. F Area fraction and cell density of GFAP- or CD68-positive plaques in the neocortex and hippocampus in each group. G Elisa assays of IL-1β, IL-6, TNF-α, and IFN-γ levels in the brain TBS extracts. Values are presented as the mean ± SEM. n = 8 per group, *P < 0.05, **P < 0.01, ***P < 0.001, unpaired two-tailed t test. Tg: APP/PS1 mice.
iTBS Treatment Prevents the Decline of Neuronal Activity in APP/PS1 Mice
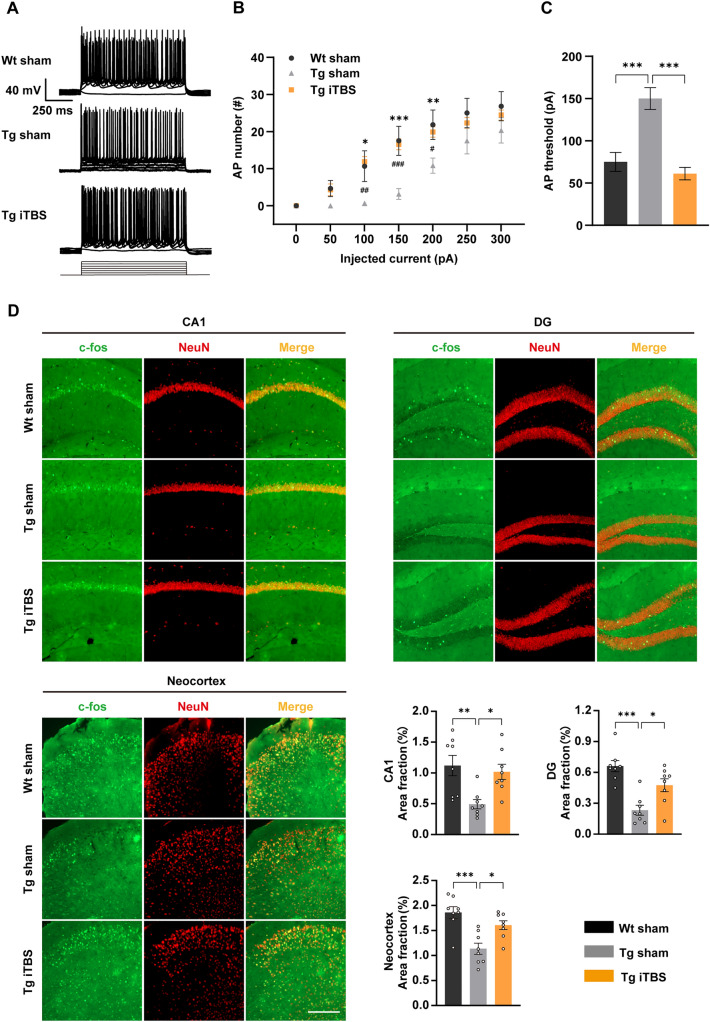
In the present study, we found that iTBS improved cognition and pathologies in AD mice. We wondered if neuronal excitability, an essential component for maintaining normal cognition, is regulated by iTBS. Thus, we investigated the excitability of pyramidal neurons in the cortex by characterizing the AP firing patterns. A series of depolarizing currents (0 pA–300 pA in 50 pA steps) 1000 ms in duration was applied to elicit APs. We found that the neuronal excitability was impaired in 7-month-old APP/PS1 mice, as reflected by a lower mean number of APs in the Tg sham group compared to that in the Wt group (Fig. 4A, B). In contrast, the neuronal excitability was significantly elevated by iTBS treatment (Fig. 4A, B). This phenomenon mainly occurred at a relatively low stimulus intensity, which may more closely mimic living conditions. Moreover, the AP threshold was significantly higher in the Tg sham group than in the Wt group, and this was also attenuated by iTBS (Fig. 4C). We further conducted NeuN and c-fos immunofluorescent co-staining to reflect neuronal activity. Consistent with the electrophysiological test results, the area fractions of c-fos-positive neurons were lower in the hippocampus (CA1 and DG regions) and cortex of APP/PS1 mice than in Wt mice, while iTBS significantly increased the area fractions of c-fos-positive neurons in APP/PS1 mice (Fig. 4D). Collectively, these results suggested that iTBS reverses the neuronal hypoactivity in the brain of AD.
Fig. 4.
iTBS improves neuronal hypoactivity in the brain of APP/PS1 mice. A Representative traces showing the firing properties of cortical neurons in the Wt sham, Tg sham, and Tg iTBS group (0 pA–300 pA current injection 1000 ms in duration, in 50 pA steps). B Mean AP numbers at different stimulus intensities among groups (n = 6 for Wt sham, n = 6 for Tg sham, n = 9 for Tg iTBS, *P < 0.05, **P < 0.01, ***P < 0.001 for Wt sham versus Tg sham, #P < 0.05, ##P < 0.01, ###P < 0.001 for Tg iTBS versus Tg sham two-way ANOVA). C Comparison of the AP thresholds of cortical neurons among groups (n = 6 for Wt sham, n = 6 for Tg sham, n = 9 for Tg iTBS, one-way ANOVA followed by Turkey’s post hoc test). D Representative images of immunofluorescent co-staining of NeuN and c-fos (scale bar, 100 μm) and quantification of area fractions of positive staining in the hippocampus (CA1 and DG regions) and neocortex. (n = 8 per group, *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Turkey’s post hoc test). Data are presented as the mean ± SEM. Wt: wild-type mice, Tg: APP/PS1 mice.
iTBS Treatment Improves Glucose Uptake Impairment, Alleviates Oxidative Stress, and Preserves Mitochondrial Dynamics in the Brain of APP/PS1 Mice
Mitochondria affects neuronal excitability through energy metabolism, which has been reported to be modulated by magnetic stimulation [8]. To explore whether iTBS regulates mitochondrial function in AD, we measured the glucose uptake, oxidative stress and mitochondrial dynamics in the brain of APP/PS1 mice. 18F-FDG PET was applied to assess the effect of iTBS on brain glucose uptake by measuring the SUV of 18F-FDG in mice. Compared to Wt mice, the 7-month-old APP/PS1 mice displayed a significant decrease in SUV in multiple brain regions, including the cortex, hippocampus, thalamus, and cerebellum (Fig. 5A, B). Importantly, the SUV in the entire brain of APP/PS1 mice was significantly restored by iTBS (Fig. 5A, B), indicating that iTBS can preserve brain glucose uptake in AD.
Fig. 5.
iTBS improves mitochondrial dysfunction in the brain of APP/PS1 mice. A Representative PET images of 18F-FDG uptake in the brain of Wt sham, Tg sham, and Tg iTBS mice. B Comparison of standard uptake value (SUV) of 18F-FDG in multiple brain regions among groups. (n = 4, one-way ANOVA followed by Turkey’s post hoc test). C Representative images of 3-NT immunohistochemical staining and quantitative analysis of area fractions of positive staining in the hippocampal CA1 region, CA3 region, and neocortex (scale bar, 20 μm). D Analysis of lipid peroxide (MDA), antioxidase (SOD), non-enzymatic antioxidant (GSH), and total antioxidant ability (T-AOC) using the corresponding biochemical assay kit. E Representative western blotting bands and quantification of mitochondrial fusion and fission proteins between groups. Data are the mean ± SEM. n = 8 per group, *P < 0.05, **P < 0.01, ***P < 0.001, unpaired two-tailed t test. Str, striatum; CG, central gray; SC, superior colliculi; Tha, thalamus; HpTh, hypothalamus; BF, basal forebrain; OB, Olfactory Bulb; Cer, cerebellum; BS, brain stem; Amy, amygdala; Hip, hippocampus; Cor, Cortex. Wt: wild-type mice, Tg: APP/PS1 mice.
In oxidative stress analysis, the area fraction of positive staining for the production of protein peroxidation (3-NT) was significantly lower in the iTBS group than in the sham stimulation group (Fig. 5C). The product of lipid peroxidation (MDA) in the fraction of brain homogenates was also significantly reduced by iTBS (Fig. 5D). Moreover, the antioxidase (SOD), non-enzymatic antioxidant (GSH) and total antioxidant activity (T-AOC) were preserved by iTBS (Fig. 5D). These data suggested that iTBS attenuates oxidative damage in the brain of APP/PS1 mice.
Next, we evaluated the alterations of mitochondrial dynamics via western blot analysis. The results demonstrated that the levels of mitochondrial fusion proteins, including MFN2 and OPA1, were significantly increased and phosphorylated mitochondrial fission protein DRP1 (Ser616) was significantly decreased by iTBS compared to the sham stimulation group, while the DRP1, MFF, and FIS1 levels showed no significant differences between groups (Fig. 5E), suggesting that iTBS preserves mitochondrial fusion and suppresses mitochondrial fission to prevent mitochondrial fragmentation in AD.
iTBS Upregulates the Expression of ISCA1 and Modulates the Mitochondrial ISC Assembly Pathway
ISCA1 plays a crucial role in the maintenance of optimal mitochondrial function. We first found that the expression of ISCA1 decreased in the brain of 7-month-old APP/PS1 mice compared to Wt mice (Fig. 6A). To determine whether the levels of ISCA1 changed under a pulsed magnetic field, we treated SH-SY5Y-APP695 cells with or without different pulses of iTBS through a repetitive magnetic stimulation method in vitro as previously described [32]. We found that iTBS treatment increased the expression of ISCA1 in cell lysates in a dose-dependent manner (Fig. 6B). Moreover, iTBS treatment also significantly increased the levels of ISCA1 in the brain of APP/PS1 mice (Fig. 6C).
Fig. 6.
iTBS increases the expression of ISCA1 and facilitates mitochondrial ISC assembly. A Western blotting bands and quantification of ISCA1 in the brain of 7-month-old C57BL/J Wt mice and APP/PS1 Tg mice (n = 6 for Wt mice, n = 7 for Tg mice). B Representative western blotting bands and quantification of ISCA1 in the lysates of SH-SY5Y-APP695 cells treated with or without various pulses of iTBS for 5 days. (n = 3, *P < 0.05, **P + 0.01 vs 0 pulse, one-way ANOVA followed by Turkey’s post hoc test). C Representative western blotting bands and quantification of ISCA1 and ISC assembly-related proteins in brain homogenates of Tg sham and Tg iTBS mice. D Representative western blotting bands and quantification of mitochondrial ISC-containing enzyme. Values are expressed as mean ± SEM. n = 8 per group, *P < 0.05, **P < 0.01, ***P < 0.001, unpaired two-tailed t test. Wt: wild-type mice, Tg: APP/PS1 mice.
Furthermore, we investigated the effects of iTBS on the mitochondrial ISC assembly pathway in APP/PS1 mice. We found that iTBS significantly increased ISC assembly-related proteins, including ISCA2 and IBA57 relative to the Tg sham group (Fig. 6C). As terminal products of the ISC assembly pathway, a series of mitochondria-related ISC-containing enzymes were detected. The results demonstrated that the subunits of mitochondrial complex I (NDUFS3 and NDUFS5) were upregulated by iTBS, whereas the levels of ACO2, SDHB, and COX IV displayed no significant differences between groups (Fig. 6D). Taken together, these data suggested that ISCA1 and ISCA1-mediated mitochondrial ISC assembly pathway are promoted by iTBS, which may enhance mitochondrial respiration in AD.
ISCA1 is Required for the Modulation of Mitochondrial Function and Anti-Aβ by iTBS In Vitro
To verify the role of ISCA1 in the iTBS-induced improvement of mitochondrial function, we performed siRNA-mediated knockdown experiments in SH-SY5Y-APP695 cells, which were treated without or with 600 pulses of iTBS. Firstly, we found that Cy3-coupled siRNA was transfected into the cells reflected by the distinct red fluorescence on Day 2 after transfection compared to control (Fig. 7A). Moreover, the Cy3 fluorescence was observed in the cells on Day 7 after transfection, suggesting that the siRNA remained in the cells for at least 7 days (Fig. 7A). The efficiency of knockdown was further assessed by western blot and qRT-PCR, and the results revealed that the expression of ISCA1 was significantly knocked down in the SH-SY5Y-APP695 cells compared to the siNC group (Fig. 7B, C), suggesting that the siRNA against ISCA1 used in our project was sufficient to interfere with the expression of ISCA1. Moreover, we further found that the expression of ISCA1 was significantly reduced in ISCA1-deficient cells with or without iTBS (Fig. 7D, E), suggesting that the knockdown effect of siRNA-ISCA1 continued until completing iTBS.
Fig. 7.
ISCA1 knockdown abolishes the modulation of mitochondrial ISC assembly by iTBS in SH-SY5Y-APP695 cells. A The transfection effect of Cy3-coupled siRNA in the control, Day 2, and Day 7 post-transfection group. Scale bar, 100 μm. B Representative western blotting bands and quantitative analysis of ISCA1 in the cell lysates at Day 2 post-transfection. C Analysis of ISCA1 mRNA levels using qRT-PCR in the SH-SY5Y-APP695 cells at Day 2 post-transfection. D, E Representative western blotting bands and quantitative analysis of ISCA1 and ISC assembly-related proteins in the cell lysates. F, G Representative western blotting bands and quantitative analysis of mitochondrial ISC-containing proteins in the cell lysates. Data are presented as the mean ± SEM. n = 3 per group, *P < 0.05, **P < 0.01, ***P < 0.001, ns, no significant difference, the unpaired two-tailed t test is used for comparison between two groups, one-way ANOVA followed by Turkey’s post hoc test is used for multiple comparisons. BF: bright field. siNC: siRNA-NC, siISCA1: siRNA-ISCA1.
Consistent with the in vivo data, we found that the levels of ISC assembly-related proteins (ISCA2 and IBA57) (Fig. 7D, E) and mitochondrial complex I (NDUFS3 and NDUFS5) (Fig. 7F, G) were significantly increased by iTBS in the SH-SY5Y-APP695 cells. Importantly, ISCA1 knockdown abolished the beneficial effects of iTBS (Fig. 7D–G). However, there were no significant differences in the levels of ACO2 and SDHB among groups (Fig. 7D–G). These data indicated that ISCA1 is indispensable for ISC assembly modulated by iTBS.
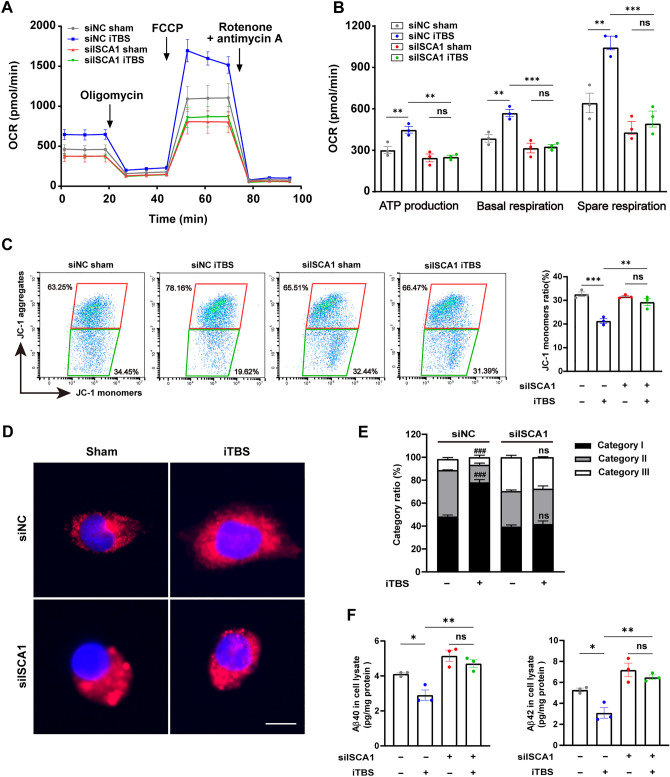
The impact of ISCA1 depletion on the effect of iTBS on mitochondrial respiration was investigated in SH-SY5Y-APP695 cells using Seahorse analysis. We found that 5 consecutive days of iTBS significantly enhanced the mitochondrial respiratory function as reflected by an increase in ATP production, basal respiration, and spare respiratory capacity of non-knockdown cells, whereas no significant difference was detected in ISCA1-deficient cells when the same measures were applied (Fig. 8A, B). Next, we applied flow cytometry to measure mitochondrial membrane potential (Δψ) using the JC-1 assay kit. The iTBS-treated cells displayed a significant reduction in the JC-1 monomer ratio compared with the control group (Fig. 8C), suggesting that iTBS preserves the Δψ. Notably, ISCA1 knockdown suppressed the effect of iTBS on the Δψ in the ISCA1-deficient cells (Fig. 8C). For mitochondrial fusion and fission, we applied mitochondrial staining to detect mitochondrial morphology in SH-SY5Y-APP695 cells. Mitochondria were divided into three categories as described in the materials and methods subsection. We found that iTBS significantly increased the ratio of category I in the mitochondria of the SH-SY5Y-APP695 cells (Fig. 8D, E), suggesting that iTBS facilitated mitochondrial fusion. Moreover, ISCA1 depletion abolished the iTBS-induced mitochondrial fusion (Fig. 8D, E). Collectively, these results demonstrated the specific role of ISCA1 in iTBS modulating the maturation of mitochondrial iron-sulfur enzyme, which is essential for mitochondrial respiration and function.
Fig. 8.
ISCA1 knockdown abolishes the effects of iTBS on mitochondria and Aβ in SH-SY5Y-APP695 cells. A, B Seahorse analysis of oxygen consumption rate (OCR) at baseline and in response to oligomycin, FCCP, and rotenone with antimycin A and quantification of ATP production, basal respiration, and spare respiratory capacity among groups. C Representative flow cytometry images and quantitative analysis of mitochondrial membrane potential using the JC-1 assay kit. D Representative images of mitochondrial morphology among groups (scale bar, 20 μm). E Comparison of the ratio of category I and category II + III among groups. ###P < 0.001 for siNC iTBS versus siNC sham. F Elisa assay for Aβ40 and Aβ42 levels in the cell lysates. Bars express as the mean ± SEM. n = 3 in each group, *P < 0.05, **P < 0.01, ***P < 0.001, ns, no significant difference, one-way ANOVA followed by Turkey’s post hoc test for multiple comparisons. siNC: siRNA-NC, siISCA1: siRNA-ISCA1.
To further assess the role of ISCA1 in the inhibitory effects of iTBS on Aβ, we measured Aβ levels using Elisa assay in SH-SY5Y-APP695 cells. The levels of Aβ40 and Aβ42 were significantly reduced by iTBS in the siRNA-NC treated cells (Fig. 8F). Moreover, this anti-Aβ toxic effect disappeared in the ISCA1 deficient cells (Fig. 8F). These data suggested that ISCA1 is required in the process by which iTBS reduces the levels of Aβ.
Discussion
Currently, the enormous burdens of AD on the home caregivers and society drive an urgent demand for disease-modifying therapies, but unfortunately none are actually available. Nevertheless, novel therapeutic strategies for AD are still being actively developed, and non-invasive neuromodulation techniques such as rTMS demonstrate a promising prospect [15, 33–35]. In the current study, we found that early intervention by iTBS, a time-saving and cost-effective rTMS regime, effectively rescued the impaired learning and memory and attenuated AD-type pathologies, including Aβ deposition, neurodegeneration, and neuroinflammation in APP/PS1 mice. Importantly, these alterations might be mediated by the ISCA1-induced ISC assembly pathway that attenuates mitochondrial dysfunction. Based on our findings, we uncovered a novel mechanism underlying iTBS treatment that modulates the expression of ISCA1 and ISCA1-induced mitochondrial ISC assembly, facilitating mitochondrial respiration and function, resulting in the improvement of cognitive impairment and pathologies in AD.
iTBS Modulates Mitochondrial Dysfunction in AD
Targeting mitochondria is an important therapeutic strategy for AD, but as yet no targeted medication has been successfully developed. Non-drug approaches for mitochondrial modulation show novel hope; for example, in an optogenetic study, light has been found to regulate mitochondrial Δψ and ATP biosynthesis to extend the lifespan of Caenorhabditis elegans [36, 37]. Moreover, previous studies have also revealed that the mitochondrion can be modulated by static or time-varying magnetic fields [8–10]. In the present study, we further confirmed that iTBS, a specific magnetic stimulus, can regulate mitochondrial functions such as respiration, energy metabolism, oxidative stress, Δψ, and dynamics in animal and cellular models of AD.
The brain utilizes glucose primarily for bioenergetics, the basic and central function of mitochondria, whereas glucose metabolism declines in amnestic mild cognitive impairment and AD patients [38, 39]. Impaired glucose uptake leads to a decreased ATP production, resulting in synaptic dysfunction and eventual neuronal death [40, 41]. Although previous studies revealed many inconsistent results about glucose uptake in the brain of APP/PS1 mice aged 6 months–7 months [42, 43], we found that the glucose uptake was decreased in multiple brain regions of 7-month-old APP/PS1 mice, and iTBS attenuated glucose uptake disorder, as reflected by a higher SUV of 18F-FDG in the iTBS-treated mice. This suggests that iTBS increases the substrate supply for ATP synthesis by accelerating the uptake and utilization of glucose. Moreover, iTBS upregulated the levels of mitochondrial complex I subunits and elevated the basic and spare respiratory capacity, suggesting that iTBS promotes oxidative phosphorylation through the electron transport chain which is the main location of ATP biosynthesis in the mitochondrion [44]. Collectively, we propose that iTBS facilitates several key points of ATP production, thereby improving mitochondrial dysfunction in AD.
Previous studies have suggested that iTBS induces excitatory effects in the neurons [45]. However, currently, the iTBS-induced activity improvement has been attributed to the enhancement of long-term potentiation (LTP) -like synaptic plasticity [45, 46] with little attention given to alterations in intrinsic membrane properties. Aβ toxicity-induced neuronal hyperexcitability is well-studied in the brain of AD. Additionally, hypoactive neurons (termed silent neurons) also exist in the brain of AD [47], and the number of hypoactive and hyperactive neurons both increase with progressive pathologies [48]. In the present study, we found that the neuronal excitability was inhibited in the brain of APP/PS1 mice compared to that of wild-type mice, which may be explained why these hypoactive cells observed in the brain of APP/PS1 mice are most likely the silent neurons. The emergence of silent neurons is deemed as the consequence of compensatory remodeling of inhibitory networks in AD mice [49]. Both the aberrant hyperexcitability and the compensatory inhibition may induce synaptic plasticity impairment and contribute to cognitive decline in AD [49]. Intriguingly, we found that iTBS elevated the excitability of these silent neurons to a level similar to that of WT mice and attenuated cognitive deficits in AD mice. Given the crucial role of mitochondria-related cellular energy metabolism in maintaining proper neuronal activity [50], we postulate that iTBS modulates mitochondrial respiration to facilitate neuronal energy metabolism, providing the energetic base for various physiological activities of neurons, thus recovering the excitability of hypoactive neurons in AD. It is noteworthy that the role of iTBS in neuronal hyperexcitability in AD remains unclear. It is necessary to further investigate the alterations of neuronal excitability after iTBS using in vivo two-photon calcium imaging in the future.
ISCA1 Participates in the Therapeutic Effects of iTBS
The mitochondrial iron-sulfur cluster assembly pathway is crucial for the synthesis of cellular iron-sulfur protein [51]. ISC-containing proteins are involved in various biological processes from cellular energy biogenesis to DNA synthesis and repair [52]. The mitochondrion is the main subcellular location for the biosynthesis of ISC-containing proteins. Previous studies have suggested that the ISC machinery can be divided into two main steps: in the early stage, an ISC is transiently assembled on the scaffold protein ISCU [53]; in the late stage, the ISC is transferred to recipient apo-proteins by coordination with specific amino-acid residues to formulate mature [4Fe-4S] proteins [19, 20]. Emerging evidence has shown that ISCA1, interacting with ISCA2 and IBA57, is required for [4Fe-4S] protein biogenesis via the late-acting ISC machinery [20]. In the current study, we found that iTBS elevated the expression of late ISC assembly-related proteins (ISCA1, ISCA2, and IBA57) and ISC-containing proteins (NDUFS3 and NDUFS5), suggesting that iTBS promotes the mitochondrial ISC assembly pathway in AD mice. Furthermore, we found that siRNA-mediated knockdown of ISCA1 in SH-SY5Y-APP695 cells abolished the effects of iTBS on the ISC assembly pathway and mitochondrial function. Collectively, these results suggest that mitochondrial ISC assembly is effectively modulated by targeting ISCA1 using iTBS in AD.
A previous study has revealed that disturbed mitochondrial dynamics correlate with complex I abnormalities and reduced cellular respiration [54]. A recent study suggests that miR-128 inhibits mitochondrial complex I and respiration to disturb mitochondrial dynamics by directly targeting NDUFS4 [55]. In the current study, we found that iTBS preserved mitochondrial fusion and suppressed mitochondrial fission to prevent mitochondrial fragmentation in AD mice. Moreover, our in vitro study showed that ISCA1 was involved in the iTBS-induced regulation of mitochondrial dynamics, suggesting that ISCA1 may affect mitochondrial dynamics via oxidative respiration. There is evidence showing that the transfer of iron-sulfur cluster affects the dynamics of mitochondrial structure and morphology [56]. Since ISCA1 is a central regulatory factor in iron-sulfur cluster transfer, there may be some undiscovered mechanisms in which ISCA1 directly regulates mitochondrial dynamics through iron-sulfur cluster assembly.
The Role of iTBS-Induced Mitochondrial Modulation in AD Pathologies
It is well known that the overproduction and clearance dysfunction of Aβ plays an essential role in the pathogenesis of AD. Previous studies revealed that rTMS treatment alleviates brain Aβ burden through two-fold effects: on one hand, rTMS suppresses Aβ production via suppressing BACE1 expression; on the other hand, it enhances Aβ clearance via facilitating the brain drainage system [57, 58]. Consistently, we found that iTBS, a biomimetic rTMS program, effectively reduced the brain Aβ load in APP/PS1 mice, and the knockdown of ISCA1 abolished the effect of iTBS on Aβ in vitro. Thus, we propose a potential mechanism underlying the anti-Aβ effect of iTBS that it promotes mitochondrial respiration through ISCA1 to inhibit the amyloidogenic processing of amyloid precursor protein (APP). Indeed, previous studies have shown that altered mitochondrial energy metabolism drives changes in APP and Aβ homeostasis [59]. A mitochondrial respiratory chain inhibitor promotes APP amyloidogenic processing to ultimately induce the overproduction of Aβ [60]. Conversely, methylene blue, a widely studied drug with potentially beneficial effects on mitochondrial respiration and energy metabolism, is shown to inhibit the expression the BACE1 and Aβ production in AD mice.
Etiologically, the Aβ hypothesis suggests that the neuroinflammation caused by astrocytosis and microgliosis occurs secondary to Aβ pathologies [61]. Therefore, the inhibitory effect of iTBS on glial activation may be due to the reduction of Aβ burden in the brain of APP/PS1 mice. However, previous study reveals that repetitive transcranial magnetic stimulation (rTMS) facilitates neuroprotective polarization of cultured primary astrocytes exposed to oxygen-glucose deprivation and reoxygenation [62]. In addition, iTBS also alleviates neuroinflammation via inhibiting the TLR4/NFκB/NLRP3 pathway in cerebral ischemic mice, which is abolished by the microglia inhibitor (PLX3397) [63]. These studies collectively suggest that iTBS can directly act on glial cells to exert a neuroprotective effect. Further exploration of the glial modulation of iTBS in AD animal or cell models is significant for uncovering the cellular mechanism of iTBS-induced cognitive improvement in AD.
In the present study, we found that iTBS increased dendritic spine density and upregulated the levels of synaptic-related proteins PSD93 and VAMP1 in the brain of AD mice, while there was no significant difference in the SNAP25 and PSD95. There are several explanations for this discrepancy. Firstly, a previous study shows that the level of PSD95 remains unaltered under consecutive 14 sessions of high-frequency rTMS treatment [64], suggesting that the duration of iTBS treatment may not reach the threshold to induce alterations in SNAP25 and PSD95 levels. Moreover, the levels of SNAP25 and PSD95 in the brain of 7-month-old APP/PS1 mice may not have decreased yet, and iTBS improves synaptic plasticity without affecting these synaptic markers [65]. Lastly, PSD93 and VAMP1 may be more involved in the regulation of structural plasticity by iTBS, which is required to be verified in the future.
It is noteworthy that there are some limitations to our study. We preliminarily discover that ISCA1 is required for iTBS-induced mitochondrial modulation in siRNA-mediated knockdown experiments in SH-SY5Y-APP695 cells, and an animal model of conditional knockout is needed to further verify the role of ISCA1 in the process of magnetic therapy in the future. Moreover, we suggest that activated ISCA1 may facilitate the mitochondrial ISC assembly and thus modulates mitochondrial function. However, it is unknown how the magnetically activated ISCA1 further motivates ISC assembly. To address this question and identify mechanisms underlying magnetoreception, future multidisciplinary approaches including quantum physics, computer simulation, biochemistry, molecular biology and neurobiology, will be required.
In conclusion, our findings uncover that the emerging and promising iTBS pattern effectively preserves cognition and alleviates pathologies in APP/PS1 mice. Moreover, we provide the first direct evidence that mammalian ISCA1 is necessary for iTBS-induced mitochondrial modulation in AD. The present study provides the mechanistic target of iTBS that warrants future therapeutic potential for patients with AD.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81901142) and funds for key support objects of Third Military Medical University.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflict of interest
The authors declare that there are no conflict of interest.
Footnotes
Yang Zhu and Hao Huang contributed equally to this work.
Contributor Information
Shi-Hao Gao, Email: f.gll@163.com.
Yan-Jiang Wang, Email: wayaja@163.com.
Chang-Yue Gao, Email: gaochangyue@163.com.
References
- 1.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long JM, Holtzman DM. Alzheimer disease: An update on pathobiology and treatment strategies. Cell. 2019;179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings J, Lee G, Nahed P, Kambar MEZN, Zhong K, Fonseca J, et al. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement (N Y) 2022;8:e12295. doi: 10.1002/trc2.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Zhao F, Ma X, Perry G, Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol Neurodegener. 2020;15:30. doi: 10.1186/s13024-020-00376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma C, Kim S, Nam Y, Jung UJ, Kim SR. Mitochondrial dysfunction as a driver of cognitive impairment in Alzheimer’s disease. Int J Mol Sci. 2021;22:4850. doi: 10.3390/ijms22094850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashleigh T, Swerdlow RH, Beal MF. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimer's Dement. 2022;19:333–342. doi: 10.1002/alz.12683. [DOI] [PubMed] [Google Scholar]
- 7.Miller B, Kim SJ, Mehta HH, Cao K, Kumagai H, Thumaty N, et al. Mitochondrial DNA variation in Alzheimer’s disease reveals a unique microprotein called SHMOOSE. Mol Psychiatry. 2023;28:1813–1826. doi: 10.1038/s41380-022-01769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Wang Z, Zhang L, Li Z, Tian X, Fang J, et al. Cellular ATP levels are affected by moderate and strong static magnetic fields. Bioelectromagnetics. 2018;39:352–360. doi: 10.1002/bem.22122. [DOI] [PubMed] [Google Scholar]
- 9.Zhu X, Liu Y, Cao X, Liu H, Sun A, Shen H, et al. Moderate static magnetic fields enhance antitumor CD8+ T cell function by promoting mitochondrial respiration. Sci Rep. 2020;10:14519. doi: 10.1038/s41598-020-71566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao H, Zuo C, Gu Z, Huang Y, Yang Y, Zhu L, et al. High frequency repetitive transcranial magnetic stimulation alleviates cognitive deficits in 3xTg-AD mice by modulating the PI3K/Akt/GLT-1 axis. Redox Biol. 2022;54:102354. doi: 10.1016/j.redox.2022.102354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenz M, Galanis C, Müller-Dahlhaus F, Opitz A, Wierenga CJ, Szabó G, et al. Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat Commun. 2016;7:10020. doi: 10.1038/ncomms10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pell GS, Roth Y, Zangen A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol. 2011;93:59–98. doi: 10.1016/j.pneurobio.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018) Clin Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Dufor T, Grehl S, Tang AD, Doulazmi M, Traoré M, Debray N, et al. Neural circuit repair by low-intensity magnetic stimulation requires cellular magnetoreceptors and specific stimulation patterns. Sci Adv. 2019;5:eaav9847. doi: 10.1126/sciadv.aav9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Ji GJ, Geng Z, Wang L, Yan Y, Wu Y, et al. Accelerated intermittent theta-burst stimulation broadly ameliorates symptoms and cognition in Alzheimer’s disease: A randomized controlled trial. Brain Stimul. 2022;15:35–45. doi: 10.1016/j.brs.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Golaszewski S, Kunz A, Schwenker K, Sebastianelli L, Versace V, Ferrazzoli D, et al. Effects of intermittent Theta burst stimulation on the clock drawing test performances in patients with alzheimer’s disease. Brain Topogr. 2021;34:461–466. doi: 10.1007/s10548-021-00836-2. [DOI] [PubMed] [Google Scholar]
- 17.Leblhuber F, Geisler S, Ehrlich D, Steiner K, Kurz K, Fuchs D. High frequency repetitive transcranial magnetic stimulation improves cognitive performance parameters in patients with Alzheimer’s disease - an exploratory pilot study. Curr Alzheimer Res 2022: 681–688. [DOI] [PubMed]
- 18.Suraci D, Saudino G, Nasta V, Ciofi-Baffoni S, Banci L. ISCA1 orchestrates ISCA2 and NFU1 in the maturation of human mitochondrial[4Fe-4S]proteins. J Mol Biol. 2021;433:166924. doi: 10.1016/j.jmb.2021.166924. [DOI] [PubMed] [Google Scholar]
- 19.Sheftel AD, Wilbrecht C, Stehling O, Niggemeyer B, Elsässer HP, Mühlenhoff U, et al. The human mitochondrial ISCA1, ISCA2, and IBA57 proteins are required for[4Fe-4S]protein maturation. Mol Biol Cell. 2012;23:1157–1166. doi: 10.1091/mbc.e11-09-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beilschmidt LK, Ollagnier de Choudens S, Fournier M, Sanakis I, Hograindleur MA, Clémancey M, et al. ISCA1 is essential for mitochondrial Fe4S4 biogenesis in vivo. Nat Commun. 2017;8:15124. doi: 10.1038/ncomms15124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukla A, Hebbar M, Srivastava A, Kadavigere R, Upadhyai P, Kanthi A, et al. Homozygous p.(Glu87Lys) variant in ISCA1 is associated with a multiple mitochondrial dysfunctions syndrome. J Hum Genet. 2017;62:723–727. doi: 10.1038/jhg.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebigot E, Hully M, Amazit L, Gaignard P, Michel T, Rio M, et al. Expanding the phenotype of mitochondrial disease: Novel pathogenic variant in ISCA1 leading to instability of the iron-sulfur cluster in the protein. Mitochondrion. 2020;52:75–82. doi: 10.1016/j.mito.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Qin S, Yin H, Yang C, Dou Y, Liu Z, Zhang P, et al. A magnetic protein biocompass. Nat Mater. 2016;15:217–226. doi: 10.1038/nmat4484. [DOI] [PubMed] [Google Scholar]
- 24.Long X, Ye J, Zhao D, Zhang SJ. Magnetogenetics: Remote non-invasive magnetic activation of neuronal activity with a magnetoreceptor. Sci Bull. 2015;60:2107–2119. doi: 10.1007/s11434-015-0902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C, Lu R, Wang L, Yun W, Zhou X. Restraint devices for repetitive transcranial magnetic stimulation in mice and rats. Brain Behav. 2019;9:e01305. doi: 10.1002/brb3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gersner R, Kravetz E, Feil J, Pell G, Zangen A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. J Neurosci. 2011;31:7521–7526. doi: 10.1523/JNEUROSCI.6751-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiao SS, Yao XQ, Liu YH, Wang QH, Zeng F, Lu JJ, et al. Edaravone alleviates Alzheimer’s disease-type pathologies and cognitive deficits. Proc Natl Acad Sci U S A. 2015;112:5225–5230. doi: 10.1073/pnas.1422998112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao SH, Tao Y, Zhu Y, Huang H, Shen LL, Gao CY. Activation of dopamine D2 receptors alleviates neuronal hyperexcitability in the lateral entorhinal cortex via inhibition of HCN current in a rat model of chronic inflammatory pain. Neurosci Bull. 2022;38:1041–1056. doi: 10.1007/s12264-022-00892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YJ, Pollard A, Zhong JH, Dong XY, Wu XB, Zhou HD, et al. Intramuscular delivery of a single chain antibody gene reduces brain Abeta burden in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2009;30:364–376. doi: 10.1016/j.neurobiolaging.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Gao M, Huang H, Gao SH, Liao LY, Tao Y, et al. p75NTR ectodomain ameliorates cognitive deficits and pathologies in a rapid eye movement sleep deprivation mice model. Neuroscience. 2022;496:27–37. doi: 10.1016/j.neuroscience.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Neitemeier S, Jelinek A, Laino V, Hoffmann L, Eisenbach I, Eying R, et al. BID links ferroptosis to mitochondrial cell death pathways. Redox Biol. 2017;12:558–570. doi: 10.1016/j.redox.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao CG, Qin J, Sun W, Ju F, Zhao YL, Wang R, et al. rTMS regulates the balance between proliferation and apoptosis of spinal cord derived neural stem/progenitor cells. Front Cell Neurosci. 2020;13:584. doi: 10.3389/fncel.2019.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grover S, Wen W, Viswanathan V, Gill CT, Reinhart RMG. Long-lasting, dissociable improvements in working memory and long-term memory in older adults with repetitive neuromodulation. Nat Neurosci. 2022;25:1237–1246. doi: 10.1038/s41593-022-01132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Qi G, Yu C, Lian G, Zheng H, Wu S, et al. Cortical plasticity is correlated with cognitive improvement in Alzheimer’s disease patients after rTMS treatment. Brain Stimul. 2021;14:503–510. doi: 10.1016/j.brs.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Yao Q, Tang F, Wang Y, Yan Y, Dong L, Wang T, et al. Effect of cerebellum stimulation on cognitive recovery in patients with Alzheimer disease: A randomized clinical trial. Brain Stimul. 2022;15:910–920. doi: 10.1016/j.brs.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Berry BJ, Trewin AJ, Milliken AS, Baldzizhar A, Amitrano AM, Lim Y, et al. Optogenetic control of mitochondrial protonmotive force to impact cellular stress resistance. EMBO Rep. 2020;21:e49113. doi: 10.15252/embr.201949113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry BJ, Vodičková A, Müller-Eigner A, Meng C, Ludwig C, Kaeberlein M, et al. Optogenetic rejuvenation of mitochondrial membrane potential extends C. elegans lifespan. Nat Aging. 2023;3:157–161. doi: 10.1038/s43587-022-00340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 39.An Y, Varma VR, Varma S, Casanova R, Dammer E, Pletnikova O, et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement. 2018;14:318–329. doi: 10.1016/j.jalz.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen P, Shen Z, Wang Q, Zhang B, Zhuang Z, Lin J, et al. Reduced cerebral glucose uptake in an alzheimer’s rat model with glucose-weighted chemical exchange saturation transfer imaging. Front Aging Neurosci. 2021;13:618690. doi: 10.3389/fnagi.2021.618690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shivamurthy VKN, Tahari AK, Marcus C, Subramaniam RM. Brain FDG PET and the diagnosis of dementia. AJR Am J Roentgenol. 2015;204:W76–W85. doi: 10.2214/AJR.13.12363. [DOI] [PubMed] [Google Scholar]
- 42.He C, Li Q, Cui Y, Gao P, Shu W, Zhou Q, et al. Recurrent moderate hypoglycemia accelerates the progression of Alzheimer’s disease through impairment of the TRPC6/GLUT3 pathway. JCI Insight. 2022;7:e154595. doi: 10.1172/jci.insight.154595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu A, Tang Y, Zeng Q, Wang X, Tian H, Zhou Y, et al. Electroacupuncture enhances cognition by promoting brain glucose metabolism and inhibiting inflammation in the APP/PS1 mouse model of alzheimer’s disease: A pilot study. J Alzheimers Dis. 2020;77:387–400. doi: 10.3233/JAD-200242. [DOI] [PubMed] [Google Scholar]
- 44.Nolfi-Donegan D, Braganza A, Shiva S. Nolfi-Donegan, Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. doi: 10.1016/j.redox.2020.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 46.Koch G, Bonnì S, Casula EP, Iosa M, Paolucci S, Pellicciari MC, et al. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke: A randomized clinical trial. JAMA Neurol. 2019;76:170–178. doi: 10.1001/jamaneurol.2018.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 48.Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, et al. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2012;109:8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kann O, Kovács R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 51.Lill R, Freibert SA. Mechanisms of mitochondrial iron-sulfur protein biogenesis. Annu Rev Biochem. 2020;89:471–499. doi: 10.1146/annurev-biochem-013118-111540. [DOI] [PubMed] [Google Scholar]
- 52.Lill R. Function and biogenesis of iron-sulphur proteins. Nature. 2009;460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- 53.Webert H, Freibert SA, Gallo A, Heidenreich T, Linne U, Amlacher S, et al. Functional reconstitution of mitochondrial Fe/S cluster synthesis on Isu1 reveals the involvement of ferredoxin. Nat Commun. 2014;5:5013. doi: 10.1038/ncomms6013. [DOI] [PubMed] [Google Scholar]
- 54.Rosenfeld M, Brenner-Lavie H, Ari SGB, Kavushansky A, Ben-Shachar D. Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiatry. 2011;69:980–988. doi: 10.1016/j.biopsych.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Sharma K, Chandra A, Hasija Y, Saini N. MicroRNA-128 inhibits mitochondrial biogenesis and function via targeting PGC1α and NDUFS4. Mitochondrion. 2021;60:160–169. doi: 10.1016/j.mito.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Karmi O, Marjault HB, Bai F, Roy S, Sohn YS, Darash Yahana M, et al. A VDAC1-mediated NEET protein chain transfers[2Fe-2S]clusters between the mitochondria and the cytosol and impacts mitochondrial dynamics. Proc Natl Acad Sci U S A. 2022;119:e2121491119. doi: 10.1073/pnas.2121491119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang Z, Tan T, Du Y, Chen L, Fu M, Yu Y, et al. Low-frequency repetitive transcranial magnetic stimulation ameliorates cognitive function and synaptic plasticity in APP23/PS45 mouse model of alzheimer’s disease. Front Aging Neurosci. 2017;9:292. doi: 10.3389/fnagi.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Y, Jin J, Lv R, Luo Y, Dai W, Li W, et al. Repetitive transcranial magnetic stimulation increases the brain’s drainage efficiency in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun. 2021;9:102. doi: 10.1186/s40478-021-01198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swerdlow RH. Mitochondria and mitochondrial cascades in Alzheimer’s disease. J Alzheimers Dis. 2018;62:1403–1416. doi: 10.3233/JAD-170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabuzda D, Busciglio J, Chen LB, Matsudaira P, Yankner BA. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J Biol Chem. 1994;269:13623–13628. doi: 10.1016/S0021-9258(17)36875-8. [DOI] [PubMed] [Google Scholar]
- 61.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong Y, Liu Q, Peng M, Bai M, Li J, Sun R, et al. High-frequency repetitive transcranial magnetic stimulation improves functional recovery by inhibiting neurotoxic polarization of astrocytes in ischemic rats. J Neuroinflammation. 2020;17:150. doi: 10.1186/s12974-020-01747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo L, Liu M, Fan Y, Zhang J, Liu L, Li Y, et al. Intermittent theta-burst stimulation improves motor function by inhibiting neuronal pyroptosis and regulating microglial polarization via TLR4/NFκB/NLRP3 signaling pathway in cerebral ischemic mice. J Neuroinflammation. 2022;19:141. doi: 10.1186/s12974-022-02501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Dong GY, Wang LX. High-frequency transcranial magnetic stimulation protects APP/PS1 mice against Alzheimer’s disease progress by reducing APOE and enhancing autophagy. Brain Behav. 2020;10:e01740. doi: 10.1002/brb3.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li K, Wang X, Jiang Y, Zhang X, Liu Z, Yin T, et al. Early intervention attenuates synaptic plasticity impairment and neuroinflammation in 5xFAD mice. J Psychiatr Res. 2021;136:204–216. doi: 10.1016/j.jpsychires.2021.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.