Abstract
Purpose
The objective of this study was to investigate the risk of cardiovascular toxicities related to PD-1/PD-L1 inhibitors in solid tumors.
Methods
A literature search was performed following the participants, interventions, comparisons, outcomes, and study design (PICOS) principles, and the study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Data analysis was conducted using Review Manager version 5.4.
Results
This meta-analysis included 69 randomized controlled trials (RCTs) divided into five groups based on the treatment regimens: PD-1/PD-L1 + chemotherapy versus chemotherapy, PD-1/PD-L1 versus chemotherapy, PD-1/PD-L1 versus placebo, PD-1/PD-L1 + CTLA-4 versus PD-1/PD-L1 and PD-1/PD-L1 + CTLA-4 versus chemotherapy. Compared to chemotherapy treatment alone, PD-1/PD-L1 +chemotherapy significantly increased the risk of hypertension [all-grade (OR = 1.27, 95% CI [1.05, 1.53], p = 0.01); grade 3–5 (OR = 1.36, 95% CI [1.04, 1.79], p = 0.03)], hypotension [all-grade (OR = 2.03, 95% CI [1.19, 3.45], p = 0.009); grade 3–5 (OR = 3.60, 95% CI [1.22, 10.60], p = 0.02)], arrhythmia [all-grade (OR = 1.53, 95% CI [1.02, 2.30], p = 0.04); grade 3–5 (OR = 2.91, 95% CI [1.33, 6.39], p = 0.008)] and myocarditis [all-grade (OR = 2.42, 95% CI [1.06, 5.54], p = 0.04)]. The risk of all-grade hypotension (OR = 2.87, 95% CI [1.26, 6.55], p = 0.01) and all-grade arrhythmia (OR = 2.03, 95% CI [1.13, 3.64], p = 0.02) significantly increased when treated with PD-1/PD-L1 inhibitors compared to the placebo. The risks of cardiovascular toxicities are significantly higher with PD-1+CTLA-4 compared to PD-1 alone (OR = 2.02, 95% CI [1.12, 3.66], p = 0.02).
Conclusion
PD-1/PD-L1 inhibitor leads to an increased risk of cardiovascular toxicities, especially hypertension, hypotension, arrhythmia, and myocarditis.
Keywords: PD-1/PD-L1 inhibitors, solid tumors, cardiotoxicity, vascular toxicity, meta-analysis
Introduction
In recent years, the programmed cell death 1/programmed cell death 1 ligand 1 (PD-1/PD-L1) inhibitor has been used as an immunotherapy and has led to substantial advancements in the prognosis of diverse cancer types (1). It can enhance the immune response by blocking the inhibitory signal of the T cell response and exerting anti-tumor effects (2). However, the enhanced destructive effect of T cells can also damage normal cells and tissues. Clinicians are becoming aware of its adverse effects on almost all organ types (3). Adverse effects often include immune-related pneumonitis, liver damage, endocrine organ abnormalities, and adverse skin reactions (4). Although cardiovascular toxicities, such as myocarditis, arrhythmia, blood pressure abnormalities, and heart failure, are uncommon, their prognoses are poor (5, 6). Therefore, additional attention should be paid to cardiovascular toxicity.
PD-1/PD-L1 inhibitors are currently recommended in various therapeutic combinations. Previous reviews and meta-analyses have summarized cardiovascular toxicities associated with different treatment regimens (7, 8). The completion of more clinical trials may have affected the original analysis results. The original topic that could not be analyzed because of insufficient data may have to be reoperated and completed. Therefore, given that cardiovascular toxicities are now considered major determinants of prognosis (9), it is necessary to conduct a new meta-analysis for this study. This will further guide the antitumor treatment of patients with solid tumors.
Materials and methods
Search strategy and selection criteria
This study was consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (10). Randomized controlled trials (RCTs) on solid tumors with cardiovascular toxicities published between July 2013 and May 2023 were searched based on the principle of PICOS (participants, interventions, comparisons, outcomes, and study design). The following medical subject heading (MeSH) terms were used: nivolumab, pembrolizumab, atezolizumab, tislelizumab, penpulimab, avelumab, durvalumab, camrelizumab, Opdivo, Bavencio, Keytruda, Imfinzi, AK105, MPDL3280A, Tecentriq, MK-3475, and BMS 963558. RCTs mentioned in the relevant reviews and references were also searched to avoid missing data. Five individuals were selected for literature search and data extraction. All conflicts were jointly discussed and resolved by the corresponding author.
The following selection criteria were used: 1) RCTs published between July 2013 and May 2023; 2) participants diagnosed with solid tumors treated with at least one PD-1 or PD-L1 inhibitor; 3) clinical trials reporting all-grade or grade 3–5 adverse effects; 4) research published in English. The exclusion criteria were as follows: 1) no treatment with PD-1/PD-L1; 2) non-RCT studies; 3) RCTs not involving cardiovascular toxicities; 4) single-arm studies without a control group.
Data extraction
Five individuals independently obtained the following baseline information from the included studies: year of publication, name of the first author, name of the study, national clinical trial (NCT) number, treatment lines, names of tumors, names of drugs, treatment arms, and the total number of people included in each study.
Publication bias and quality assessments
The Cochrane Collaboration tool was used to evaluate the risk of bias in the RCTs and funnel plots were used to evaluate publication bias (11). Seven sources of bias were evaluated in each RCT: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). Each domain was independently assigned a ‘high’, ‘low’, or ‘unclear’ risk of bias by all authors, with disagreements adjudicated by the corresponding author.
Heterogeneity assessment and statistical analysis
Review Manager (RevMan) version 5.4. was used to analyze the relevant data using the Mantel–Haenszel method (12). I2 values were applied to estimate heterogeneity among the included clinical trials, which were classified into three grades: low, moderate, and high (I2 values <25%, 25%–50%, and >50%, respectively) (13). When I2 was greater than 50%, significant heterogeneity was considered, and the source of heterogeneity was determined by subgroup analysis. Owing to the inherent heterogeneity among the included trials, the random effect (RE) was applied to analyze the odds ratio (OR) and corresponding 95% confidence interval (CI) (14). Funnel plots derived from the fixed effect (FE) model were used to evaluate publication bias. All reported P values were two-sided, and P < 0.05 was deemed to be statistically significant.
Results
Literature search results
We retrieved 638 relevant records from the PubMed database. The RCTs screening process was shown in Figure 1 , and the baseline characteristics are presented in Table 1 . Bias assessments of the included trials were completed and were presented in Figure 2 . After thoroughly reviewing the complete texts of all trials included in this meta-analysis, a total of 10 prevalent cardiovascular toxicities were incorporated, comprising hypertension (n = 36) (22, 24, 25, 29–32, 34–37, 39, 40, 42–48, 51, 52, 54, 56, 62, 63, 65, 68, 69, 71, 72, 75, 77, 78, 81, 83, 84), hypotension (n = 14) (25, 29–32, 36, 40, 42, 52, 62, 68, 71, 75, 76, 78, 83, 84), arrhythmia (n = 32) (21–24, 29, 30, 32, 36, 37, 41, 42, 45–47, 57, 58, 61, 62, 65–69, 71, 72, 75, 76, 78, 83, 84), myocarditis (n = 31) (17, 21–25, 28, 30, 31, 33, 37, 38, 49, 50, 52, 53, 56, 59, 62, 63, 67, 68, 70, 72–74, 78–81, 84, 91), heart failure (n = 17) (20, 22, 25, 30–32, 34, 37, 45–47, 49, 62, 65, 67, 68, 78), myocardial infarction (n = 22) (15, 16, 23, 27, 30, 34, 36, 37, 39, 40, 46, 47, 52, 55, 62, 63, 65, 68, 70, 72, 78, 83, 84), pericardial diseases (n = 4) (32, 68, 76, 78), thrombosis (n = 18) (15, 25–27, 30, 34, 36, 40, 47, 52, 55, 62, 67, 68, 71, 76, 78, 83), embolism (n = 21) (15, 20, 22, 27, 30, 36, 38, 40–42, 45–48, 55, 62, 66–68, 83, 84), and vasculitis (n = 13) (19, 25, 27, 32, 51, 62, 64, 67, 68, 72, 80–84).
Figure 1.
The flow diagram of the included randomized controlled trials (RCTs).
Table 1.
The baseline characteristics of the RCTs included in this meta-analysis (Total of 69 clinical trials).
| NO | First author and year | Study | Treatment lines | Tumor type | Drug | PD-1/ PD-L1 | Treatment regimen | Enrollment |
|---|---|---|---|---|---|---|---|---|
| PD-1/PD-L1 + chemotherapy VS chemotherapy | ||||||||
| 1 | Forde PM, 2022 (15) | CheckMate 816 (NCT 02998528) | first line | NSCLC | Nivolumab | PD-1 | Nivolumab + platinum-based chemotherapy VS platinum-based chemotherapy | 352 |
| 2 | Langer CJ, 2016 (16) | KEYNOTE-021 (NCT 02039674) | first line | NSCLC | Pembrolizumab | PD-1 | Pembrolizumab + carboplatin VS carboplatin + pemetrexed | 121 |
| 3 | Rodríguez-Abreu D, 2021 (17) | KEYNOTE-189 (NCT 02578680) | first line | NSCLC | Pembrolizumab | PD-1 | Pembrolizumab + pemetrexed-platinum VS pemetrexed-platinum | 607 |
| Garassino MC, 2023 (18) | ||||||||
| 4 | Novello S, 2023 (19) | KEYNOTE-407 (NCT 02775435) | first line | NSCLC | Pembrolizumab | PD-1 | Pembrolizumab + carboplatin+paclitaxel/nab-paclitaxel VS carboplatin + paclitaxel/nab-paclitaxel | 558 |
| 5 | Zhou C, 2021 (20) | CameL (NCT 03134872) | first line | NSCLC | Camrelizumab | PD-1 | Camrelizumab + carboplatin + pemetrexed VS carboplatin + pemetrexed | 412 |
| 6 | Wang Z, 2023 (21) | CHOICE-01 (NCT 03856411) | first line | NSCLC | Toripalimab | PD-1 | Toripalimab + nab-paclitaxel + carboplatin VS nab-paclitaxel + carboplatin | 464 |
| 7 | Lu Z, 2022 (22) | ORIENT-15(NCT 03748134) | first line | ESCC | Sintilimab | PD-1 | Sintilimab + cisplatin + paclitaxel VS cisplatin + paclitaxel | 659 |
| 8 | Luo H, 2021 (23) | ESCORT-1st (NCT 03691090) | first line | ESCC | Camrelizumab | PD-1 | Camrelizumab + paclitaxel + cisplatin VS paclitaxel + cisplatin | 595 |
| 9 | Wang ZX, 2022 (24) | JUPITER-06 (NCT 03829969) | first line | ESCC | Toripalimab | PD-1 | Toripalimab + paclitaxel + cisplatin VS paclitaxel + cisplatin | 514 |
| 10 | Xu J, 2023 (25) | RATIONALE-306 (NCT 03783442) | first line | ESCC | Tislelizumab | PD-1 | Tislelizumab + platinum agent and fluoropyrimidine/capecitabine/paclitaxel VS platinum agent and fluoropyrimidine/capecitabine/paclitaxel | 645 |
| 11 | Janjigian YY, 2021 (26) | CheckMate 649 (NCT 02872116) | first line | GJC | Nivolumab | PD-1 | Nivolumab + capecitabine+oxaliplatin / leucovorin+fluorouracil+oxaliplatin VS capecitabine+oxaliplatin / leucovorin+fluorouracil+oxaliplatin | 1549 |
| 12 | Kang YK, 2022 (27) | CheckMate 649 (NCT 02872116) | first line | GC/GJC | Nivolumab | PD-1 | Nivolumb + oxaliplatin + capecitabine VS oxaliplatin + capecitabin | 717 |
| 13 | Tolaney SM, 2020 (28) | NCT 03051659 | second or others | BRCA | Pembrolizumab | PD-1 | Pembrolizumab + eribulin VS eribulin | 88 |
| 14 | Schmid P, 2022 (29) | KEYNOTE-522 (NCT 03036488) | first line | TNBC | Pembrolizumab | PD-1 | Pembrolizumab + paclitaxel + carboplatin VS paclitaxel + carboplatin | 1172 |
| 15 | Powles T, 2021 (30) | KEYNOTE-361 (NCT 02853305) | first line | UC | Pembrolizumab | PD-1 | Pembrolizumab + gemcitabine+cisplatin/carboplatin VS gemcitabine+cisplatin/carboplatin | 691 |
| 16 | Mai HQ, 2021 (31) | JUPITER-02 (NCT 03581786) | first line | NPC | Toripalimab | PD-1 | Toripalimab +gemcitabine-cisplatin VS gemcitabine-cisplatin | 289 |
| 17 | Yang Y, 2021 (32) | CAPTAIN-1st(NCT 03707509) | first line | NPC | Camrelizumab | PD-1 | Camrelizumab + gemcitabine + cisplatin VS gemcitabine + cisplatin | 263 |
| 18 | Nishio M, 2021 (33) | IMpower132 (NCT 02657434) | first line | NSCLC | Atezolizumab | PD-L1 | Atezolizumab + carboplatin / cisplatin and pemetrexed VS carboplatin / cisplatin and pemetrexed | 565 |
| 19 | Socinski MA, 2018 (34) | IMpower150 (NCT 02366143) | first line | NSCLC | Atezolizumab | PD-L1 | Atezolizumab + bevacizumab + carboplatin + paclitaxel VS bevacizumab + carboplatin + paclitaxel | 787 |
| Reck M, 2020 (35) | ||||||||
| 20 | West H, 2019 (36) | IMpower130 (NCT 02367781) | first line | NSCLC | Atezolizumab | PD-L1 | Atezolizumab + carboplatin + nab-paclitaxel VS carboplatin + nab-paclitaxel | 705 |
| 21 | Zhou C, 2022 (37) | GEMSTONE-302 (NCT 03789604) | first line | NSCLC | Sugemalimab | PD-L1 | Sugemalimab + platinum-based chemotherapy VS platinum-based chemotherapy | 479 |
| 22 | Johnson ML, 2023 (38) | POSEIDON (NCT 03164616) | first line | NSCLC | Durvalumab | PD-L1 | Durvalumab + platinum-based chemotherapy VS platinum-based chemotherapy | 667 |
| 23 | Paz-Ares L, 2019 (39) | CASPIAN(NCT 03043872) | first line | SCLC | Durvalumab | PD-L1 | Durvalumab + platinum–etoposide VS platinum–etoposide | 531 |
| Goldman JW, 2021 (40) | ||||||||
| 24 | Wang J, 2022 (41) | CAPSTONE-1(NCT 03711305) | first line | SCLC | Adebrelimab | PD-L1 | Adebrelimab + carboplatin + etoposide VS carboplatin + etoposide | 462 |
| 25 | Pusztai L, 2021 (42) | I-SPY2 (NCT 01042379) | first line | BRCA | Durvalumab | PD-L1 | Durvalumab + olaparib + paclitaxel VS paclitaxel | 372 |
| 26 | Emens LA, 2021 (43) | IMpassion130 (NCT 02425891) | first line | TNBC | Atezolizumab | PD-L1 | Atezolizumab + nab-paclitaxel VS nab-paclitaxel | 890 |
| 27 | Mittendorf EA, 2020 (44) | IMpassion031 (NCT 03197935) | first line | TNBC | Atezolizumab | PD-L1 | Atezolizumab + nab-paclitaxel + doxorubicin + cyclophosphamide VS nab-paclitaxel + doxorubicin + cyclophosphamide | 331 |
| 28 | Pujade-Lauraine E, 2021 (45) | JAVELIN Ovarian 200(NCT 02580058) | first line | Multiple cancers | Avelumab | PD-L1 | Avelumab + pegylated liposomal doxorubicin VS pegylated liposomal doxorubicin | 359 |
| 29 | Lee NY, 2021 (46) | JAVELIN Head and Neck 100(NCT 02952586) | first line | HNSCC | Avelumab | PD-L1 | Avelumab+chemoradiotherapy VS chemoradiotherapy | 692 |
| 30 | Monk BJ, 2021 (47) | JAVELIN Ovarian 100(NCT 02718417) | first line | EOC | Avelumab | PD-L1 | Avelumab + carboplatin + paclitaxel VS carboplatin + paclitaxel + observation | 662 |
| 31 | Moore KN, 2021 (48) | IMagyn050/GOG 3015/ENGOT-OV39(NCT 03038100) | first line | OC | Atezolizumab | PD-L1 | Atezolizumab + bevacizumab + carboplatin + paclitaxel VS bevacizumab + carboplatin + paclitaxel | 1286 |
| 32 | Powles T, 2022 (49) | IMbassador 250 (NCT 03016312) | second or others | PCA | Atezolizumab | PD-L1 | Atezolizumab + enzalutamide VS enzalutamide | 750 |
| 33 | Mettu NB, 2022 (50) | BACCI (NCT 02873195) | second or others | CRC | Atezolizumab | PD-L1 | Atezolizumab + capecitabine + bevacizumab VS capecitabine + bevacizumab | 132 |
| 34 | Galsky MD, 2020 (51) | IMvigor130 (NCT 02807636) | first line | UC | Atezolizumab | PD-L1 | Atezolizumab+platinum-based chemotherapy VS platinum-based chemotherapy | 843 |
| PD-1/PD-L1 VS chemotherapy | ||||||||
| 1 | Huang J, 2020 (52) | ESCORT (NCT 03099382) | second or others | ESCC | Camrelizumab | PD-1 | Camrelizumab VS docetaxel/irinotecan | 448 |
| 2 | Kojima T, 2020 (53) | KEYNOTE-181 (NCT 02564263) | second or others | ESCC | Pembrolizumab | PD-1 | Pembrolizumab VS paclitaxel/docetaxel/irinotecan | 610 |
| 3 | Chan ATC, 2023 (54) | KEYNOTE-122 (NCT 02611960) | second or others | NPC | Pembrolizumab | PD-1 | Pembrolizumab VS capecitabine/gemcitabine/docetaxel | 228 |
| 4 | Diaz LA Jr, 2022 (55) | KEYNOTE-177 (NCT 02563002) | first line | CRC | Pembrolizumab | PD-1 | Pembrolizumab VS 5-fluorouracil–based therapy | 296 |
| André T, 2020 (56) | ||||||||
| 5 | Powles T, 2021 (30) | KEYNOTE-361 (NCT 02853305) | first line | UC | Pembrolizumab | PD-1 | Pembrolizumab VS gemcitabine+cisplatin/carboplatin | 644 |
| 6 | Winer EP, 2021 (57) | KEYNOTE-119 (NCT 02555657) | second or others | TNBC | Pembrolizumab | PD-1 | Pembrolizumab VS capecitabine/eribulin/gemcitabine/vinorelbine | 601 |
| 7 | Herbst RS, 2016 (58) | KEYNOTE-010 (NCT 01905657) | second or others | NSCLC | Pembrolizumab | PD-1 | Pembrolizumab VS docetaxel | 652 |
| 8 | Mok TSK, 2019 (59) | KEYNOTE-042 (NCT 02220894) | first line | NSCLC | Pembrolizumab | PD-1 | Pembrolizumab VS platinum-based chemotherapy | 1251 |
| de Castro G Jr, 2023 (60) | ||||||||
| 9 | Borghaei H, 2015 (61) | CheckMate 057 (NCT 01673867) | second or others | NSCLC | Nivolumab | PD-1 | Nivolumab VS docetaxel | 555 |
| 10 | Sezer A, 2021 (62) | EMPOWER-Lung 1 (NCT 03088540) | first line | NSCLC | Cemiplimab | PD-1 | Cemiplimab VS platinum-doublet chemotherapy | 697 |
| 11 | Barlesi F, 2018 (63) | JAVELIN Lung 200 (NCT 02395172) | second or others | NSCLC | Avelumab | PD-L1 | Avelumab VS docetaxel | 758 |
| 12 | Jassem J, 2021 (64) | IMpower110 (NCT 02409342) | first line | NSCLC | Atezolizumab | PD-L1 | Atezolizumab VS platinum-based chemotherapy | 549 |
| Herbst RS, 2020 (65) | ||||||||
| 13 | Galsky MD, 2020 (51) | IMvigor130 (NCT 02807636) | first line | UC | Atezolizumab | PD-L1 | Atezolizumab VS platinum-based chemotherapy | 744 |
| 14 | van der Heijden MS, 2021 (66) | IMvigor211 (NCT 02302807) | second or others | UC | Atezolizumab | PD-L1 | Atezolizumab VS vinflunine/paclitaxel/docetaxel | 902 |
| 15 | Powles T, 2020 (67) | DANUBE (NCT 02516241) | first line | UC | Durvalumab | PD-L1 | Durvalumab VS gemcitabine+cisplatin/carboplatin | 658 |
| 16 | Pujade-Lauraine E, 2021 (45) | JAVELIN Ovarian 200(NCT 02580058) | first line | Multiple cancers | Avelumab | PD-L1 | Avelumab VS pegylated liposomal doxorubicin | 364 |
| PD-1/PD-L1 VS placebo | ||||||||
| 1 | Choueiri TK, 2021 (68) | KEYNOTE-564 (NCT 03142334) | second or others | RCC | Pembrolizumab | PD-1 | Pembrolizumab VS placebo | 984 |
| Powles T, 2022 (69) | ||||||||
| 2 | Janjigian YY, 2021 (70) | KEYNOTE-811 (NCT 03615326) | second or others | GC | Pembrolizumab | PD-1 | Pembrolizumab VS Placebo | 433 |
| 3 | Cohen EEW, 2019 (71) | KEYNOTE-040 (NCT 02252042) | second or others | HNSCC | Pembrolizumab | PD-1 | Pembrolizumab VS Standard-of-Care | 480 |
| 4 | Colombo N, 2021 (72) | KEYNOTE-826 (NCT 03635567) | first line | CCA | Pembrolizumab | PD-1 | Pembrolizumab VS Placebo | 616 |
| 5 | Eggermont AMM, 2020 (73) | KEYNOTE-054(NCT 02362594) | second or others | melanoma | Pembrolizumab | PD-1 | Pembrolizumab VS Placebo | 1011 |
| 6 | Long GV, 2022 (74) | KEYNOTE-716 (NCT 03553836) | second or others | melanoma | Pembrolizumab | PD-1 | Pembrolizumab VS Placebo | 969 |
| 7 | Zimmer L, 2020 (75) | IMMUNED (NCT 02523313) | second or others | melanoma | Nivolumab | PD-1 | Nivolumab VS Placebo | 107 |
| 8 | Fennell DA, 2021 (76) | CONFIRM (NCT 03063450) | second or others | mesothelioma | Nivolumab | PD-1 | Nivolumab VS placebo | 332 |
| 9 | Sugawara S, 2021 (77) | TASUKI-52 (NCT 03117049) | first line | NSCLC | Nivolumab | PD-1 | Nivolumab VS Placebo | 548 |
| 10 | Antonia SJ, 2017 (78) | PACIFIC (NCT 02125461) | second or others | NSCLC | Durvalumab | PD-L1 | Durvalumab VS Placebo | 709 |
| 11 | Zhou Q, 2022 (79) | GEMSTONE-301 (NCT 03728556) | second or others | NSCLC | Sugemalimab | PD-L1 | Sugemalimab VS placebo | 381 |
| 12 | Felip E, 2021 (80) | IMpower010 (NCT 02486718) | second or others | NSCLC | Atezolizumab | PD-L1 | Atezolizumab VS placebo | 990 |
| Kenmotsu H, 2022 (81) | ||||||||
| 13 | Horn L, 2018 (82) | IMpower133 (NCT 02763579) | first line | SCLC | Atezolizumab | PD-L1 | Atezolizumab VS Placebo | 394 |
| 14 | Bellmunt J, 2021 (83) | IMvigor010 (NCT 02450331) | first line | UC | Atezolizumab | PD-L1 | Atezolizumab VS Observation | 787 |
| 15 | Pal SK, 2022 (84) | IMmotion010(NCT 03024996) | second or others | RCC | Atezolizumab | PD-L1 | Atezolizumab VS placebo | 773 |
| PD-1/PD-L1 + CTLA-4 VS PD-1/PD-L1 | ||||||||
| 1 | Antonia SJ, 2016 (85) | CheckMate 032 (NCT 01928394) | second or others | SCLC | Nivolumab | PD-1 | Nivolumab + ipilimumab VS nivolumab | 159 |
| 2 | Boyer M, 2021 (86) | KEYNOTE-598 (NCT 03302234) | first line | NSCLC | Pembrolizumab | PD-1 | Pembrolizumab+ipilimumab VS pembrolizumab | 563 |
| 3 | Gettinger SN, 2021 (87) | Lung-MAP S1400I(NCT 02785952) | second or others | SCLC | Nivolumab | PD-1 | Nivolumab + ipilimumab VS nivolumab | 247 |
| 4 | Hodi FS, 2018 (88) | CheckMate 067 (NCT 01844505) | first line | melanoma | Nivolumab | PD-1 | Nivolumab + ipilimumab VS Nivolumab | 626 |
| 5 | Powles T, 2020 (67) | DANUBE (NCT 02516241) | first line | UC | Durvalumab | PD-L1 | Durvalumab + tremelimumab VS Durvalumab | 685 |
| PD-1/PD-L1 + CTLA-4 VS chemotherapy | ||||||||
| 1 | Baas P, 2021 (89) | CheckMate 743 (NCT 02899299) | first line | pleural mesothelioma | Nivolumab | PD-1 | Nivolumab + ipilimumab VS chemotherapy | 584 |
| 2 | Paz-Ares L, 2021 (90) | CheckMate 9LA (NCT 03215706) | first line | NSCLC | Nivolumab | PD-1 | Nivolumab + ipilimumab VS chemotherapy | 707 |
| 3 | Powles T, 2020 (67) | DANUBE (NCT 02516241) | first line | UC | Durvalumab | PD-L1 | Durvalumab + tremelimumab VS Chemotherapy | 653 |
PD-1, Programmed cell death 1; PD-L1, Programmed cell death 1 ligand 1; CTLA-4, anti-cytotoxic T-lymphocyte antigen-4; HR, Hazard Ratios; OR, Odds Ratio; CI, Confidence Interval; RE, Random Effect; FE, Fixed Effect; NSCLC, Non-Small-Cell Lung Cancer; SCLC, Small-Cell Lung Cancer; BRCA, Breast Cancer; UC, Urothelial Carcinoma; HNSCC, Head and Neck Squamous Cell Carcinoma; CCA, Cervical Cancer; TNBC, Triple-Negative Breast Cancer; GC, Gastric Cancer; GC/GJC, Gastric or gastro-oesophageal junction cancer; ESCC, Oesophagea/Esophagea Squamous Cell Carcinoma; NPC, Nasopharyngeal Cancer; CRC, Colorectal Cancer; EOC, Epithelial Ovarian Cancer; OC, Ovarian Cancer; GEC, Gastroesophageal adenocarcinoma; RCC, Renal Cell Carcinoma; PCA, Prostate Cancer; HCC, Hepatocellular Carcinoma; EC, Esophageal Cancer; MPM, Malignant Pleural Mesothelioma.
Figure 2.
Forest plots depicting the risk of hypertension in PD-1/PD-L1 + chemotherapy versus chemotherapy. (A1) The risk of hypertension of all-grade: subgroup analyses were conducted according to PD-1/PD-L1. (A2) The risk of hypertension of grade 3-5: subgroup analyses were performed based on PD-1/PD-L1. (A3) The risk of hypertension of grade 3-5: subgroup analyses were performed based on types of tumors. Forest plot depicting the risk of hypertension in PD-1/PD-L1 versus chemotherapy. (B) The risk of hypertension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Forest plot depicting the risk of hypertension in PD-1/PD-L1 versus placebo. (C) The risk of hypertension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1.
Characteristics of identified trials
We first divided the 63 clinical trials into five groups according to treatment regimen. The specific grouping methods are as follows.
Group 1: PD-1/PD-L1 + chemotherapy versus chemotherapy; n = 34 (15, 16, 19–51, 91). Seventeen clinical trials included PD-1 (15–17, 19–32) and seventeen clinical trials included PD-L1 (33–51).
Group 2: PD-1/PD-L1 versus chemotherapy; n = 16 (30, 45, 51–67). Ten clinical trials included PD-1 (30, 52–62) and six included PD-L1 (45, 51, 63–67).
Group 3: PD-1/PD-L1 versus placebo; n = 15 (17, 27, 68–82, 84). Nine clinical trials included PD-1 (68–77) and six included PD-L1 (78–84).
Group 4: PD-1/PD-L1 + CTLA-4 versus PD-1/PD-L1; n = 5 (67, 85–88). Four clinical trials included PD-1 (85–88) and one included PD-L1 (67).
Group 5: PD-1/PD-L1 + CTLA-4 versus chemotherapy; n = 3 (67, 89, 90). Two clinical trials included PD-1 (89, 90) and one included PD-L1 (67).
Risk of hypertension
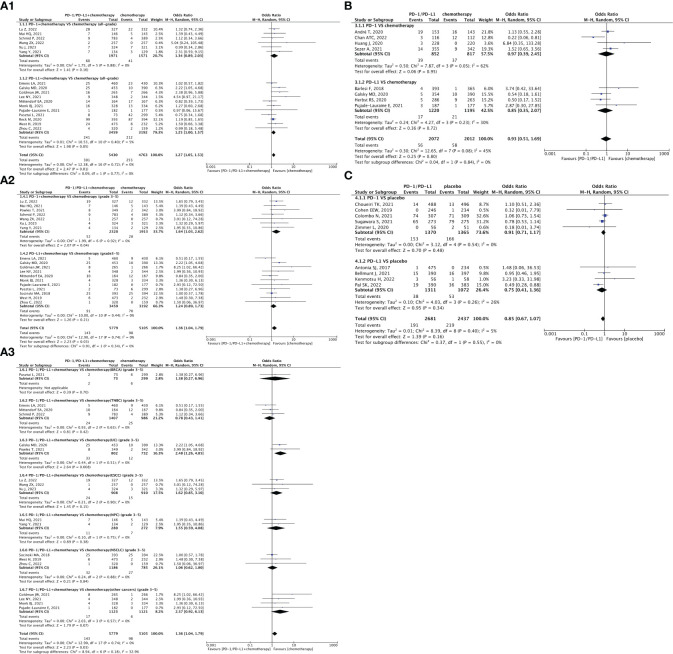
Thirty-six clinical trials reported hypertension (22, 24, 25, 29–32, 34–37, 39, 40, 42–48, 51, 52, 54, 56, 62, 63, 65, 68, 69, 71, 72, 75, 77, 78, 81, 83, 84). In comparison to chemotherapy, PD-1/PD-L1 + chemotherapy resulted in a significantly increased risk of all-grade hypertension (OR = 1.27, 95% CI [1.05, 1.53], p = 0.01, I2 = 0%; Figure 2A1 ), especially for the subgroup of first-line treatment (OR = 1.27, 95% CI [1.05, 1.53], p = 0.01, I2 = 0%; Figure 2A1 ) (22, 24, 25, 29, 31, 32, 35–37, 40, 42–47, 51). Similar trend were also be found in grade 3–5 hypertension (OR = 1.36, 95% CI [1.04, 1.79], p = 0.03, I2 = 0%; Figure 2A2 ). Among them, the PD-1 subgroup (OR = 1.64, 95% CI [1.03, 2.62], p = 0.04, I2 = 0%; Figure 2A2 ), first-line treatment (OR = 1.36, 95% CI [1.04. 1.79], p = 0.03, I2 = 0%; Figure 2A2 ), or urothelial carcinoma (UC) (OR = 2.48, 95% CI [1.26, 4.85], p = 0.008, I2 = 0%; Figure 2A3 ) were more likely to cause grade 3–5 hypertension (22, 24, 25, 29–32, 34, 36, 37, 40, 42–47, 51). No heterogeneity was observed among the studies.
Compared with chemotherapy alone ( Figure 2B ) (45, 51, 52, 54, 56, 62, 63, 65) or the placebo ( Figure 2C ) (68, 71, 72, 75, 77), the effects of PD-1/PD-L1 inhibitors on hypertension, indicated by non-significant statistical analysis results, were weaker than those of the control groups. The corresponding funnel plots are shown in the Supplementary Data ( Supplementary Figure 2 ).
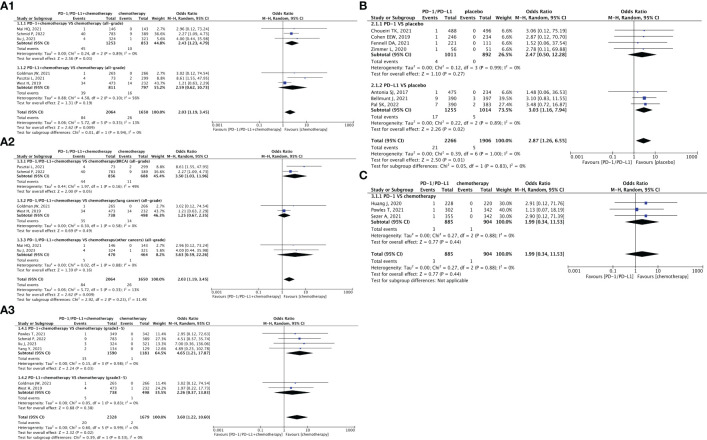
Risk of hypotension
There were fourteen clinical trials reporting hypotension (25, 29–32, 36, 40, 42, 52, 62, 68, 71, 75, 76, 78, 83, 84). The risk of all-grade hypotension (OR = 2.03, 95% CI [1.19, 3.45], p = 0.009, I2 = 13%; Figure 3A1 ) and grade 3–5 hypotension (OR = 3.60, 95% CI [1.22, 10.60], p = 0.02, I2 = 0%; Figure 3A3 ) associated with chemotherapy were significantly lower than those associated with PD-1/PD-L1 + chemotherapy. This difference was particularly notable in the PD-1 subgroup [(all-grade (OR = 2.43, 95% CI [1.23, 4.79], p = 0.01, I2 = 0%; Figure 3A1 ); grade 3–5 (OR = 4.65, 95% CI [1.21, 17.87], p = 0.03, I2 = 0%; Figure 3A3 ], and first-line treatment subgroup [all-grade (OR = 2.03, 95% CI [1.19. 3.45], p = 0.009, I2 = 13%; Figure 3A1 ); grade 3–5 (OR = 3.60, 95% CI [1.22, 10.60], p = 0.02, I2 = 0%; Figure 3A3 )] (25, 29, 31, 36, 40, 42). Furthermore, in the subgroup of breast cancer (BRCA), PD-1/PD-L1 + chemotherapy exhibited a tendency toward a higher risk of all-grade hypotension (OR = 3.50, 95% CI [1.03, 11.96], p = 0.05, I2 = 49%; Figure 3A2 ).
Figure 3.
Forest plots depicting the risk of hypotension in PD-1/PD-L1 + chemotherapy versus chemotherapy. (A1) The risk of hypotension of all-grade: subgroup analyses were conducted according to PD-1/PD-L1. (A2) The risk of hypotension of all-grade: subgroup analyses were conducted according to types of tumors. (A3) The risk of hypotension of grade 3-5: subgroup analysis was conducted according to PD-1/PD-L1. Forest plot depicting the risk of hypotension in PD-1/PD-L1 versus placebo. (B) The risk of hypotension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Forest plot depicting the risk of hypotension in PD-1/PD-L1 versus chemotherapy. (C) The risk of hypotension of grade 3-5: subgroup analysis was conducted according to PD-1.
Compared to placebo, PD-1/PD-L1 substantially increased the risk of all-grade hypotension (OR = 2.87, 95% CI [1.26, 6.55], p = 0.01, I2 = 0%; Figure 3B ), especially PD-L1 (OR = 3.03, 95% CI [1.16, 7.94], p = 0.02, I2 = 0%; Figure 3B ) (68, 71, 75, 76, 78, 83, 84). No significant heterogeneity was observed in the aforementioned results. PD-1/PD-L1 did not demonstrate a higher risk of grade 3–5 hypotension when compared to chemotherapy alone ( Figure 3C ) (30, 52, 62). The corresponding funnel plots are shown in Supplementary Data ( Supplementary Figure 3 ).
Risk of arrhythmia
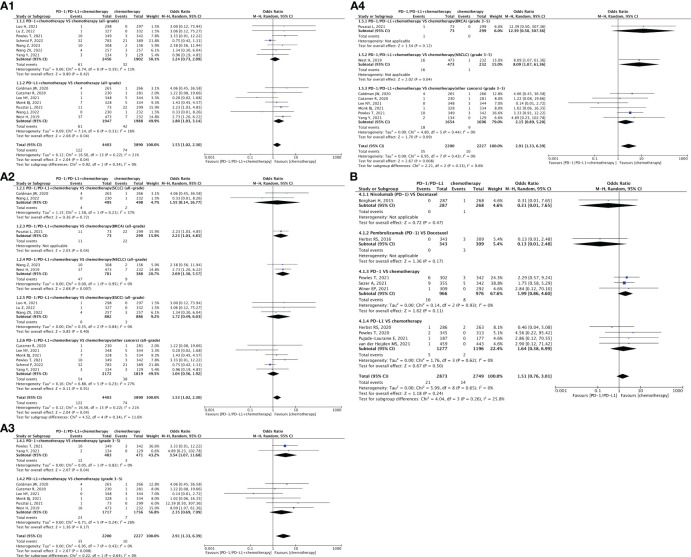
Thirty-two clinical trials reported arrhythmia (21–24, 29, 30, 32, 36, 37, 41, 42, 45–47, 57, 58, 61, 62, 65–69, 71, 72, 75, 76, 78, 83, 84). Compared with chemotherapy, the combination of PD-1/PD-L1 inhibitors with chemotherapy exhibited a significantly higher risk of all-grade arrhythmia (OR = 1.53, 95% CI [1.02, 2.30], p = 0.04, I2 = 21%; Figure 4A1 ) and grade 3–5 arrhythmia (OR = 2.91, 95% CI [1.33, 6.39], p = 0.008, I2 = 0%; Figure 4A3 ). This effect was particularly prominent in the subgroups of first-line treatment [all-grade (OR = 1.53, 95% CI [1.02, 2.30], p = 0.04, I2 = 21%; Figure 4A1 ); grade 3–5 (OR = 2.91, 95% CI [1.33, 6.39], p = 0.008, I2 = 0%; Figure 4A3 )], and non-small cell lung cancer (NSCLC) [all-grade (OR = 2.69, 95% CI [1.30, 5.57], p = 0.007, I2 = 0%; Figure 4A2 ); grade 3–5 (OR = 8.09, 95% CI [1.07, 61.36], p = 0.04; Figure 4A4 )] (21–24, 29, 30, 32, 36, 40–42, 46, 47). Specifically, the combination of PD-L1 and chemotherapy demonstrated a higher risk of causing all-grade arrhythmias (OR = 1.80, 95% CI [1.03, 3.14], p = 0.04, I2 = 16%; Figure 4A1 ), whereas PD-1 combined with chemotherapy was more prone to inducing grade 3–5 arrhythmia (OR = 3.54, 95% CI [1.07, 11.68], p = 0.04, I2 = 0%; Figure 4A3 ). Additionally, among BRCA patients, there was an increased risk of developing all-grade arrhythmia with PD-1/PD-L1 + chemotherapy (OR = 2.23, 95% CI [1.03, 4.85], p = 0.04; Figure 4A2 ). Notably, no significant heterogeneity was observed among the findings.
Figure 4.
Forest plots depicting the risk of arrhythmia in PD-1/PD-L1 + chemotherapy versus chemotherapy. (A1) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to PD-1/PD-L1. (A2) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to types of tumors. (A3) The risk of arrhythmia of grade 3-5: subgroup analyses were conducted according to PD-1/PD-L1. (A4) The risk of arrhythmia of grade 3-5: subgroup analyses were conducted according to types of tumors. Forest plot depicting the risk of arrhythmia in PD-1/PD-L1 versus chemotherapy. (B) The risk of arrhythmia of all-grade: subgroup analysis was conducted according to PD-1/PD-L1.
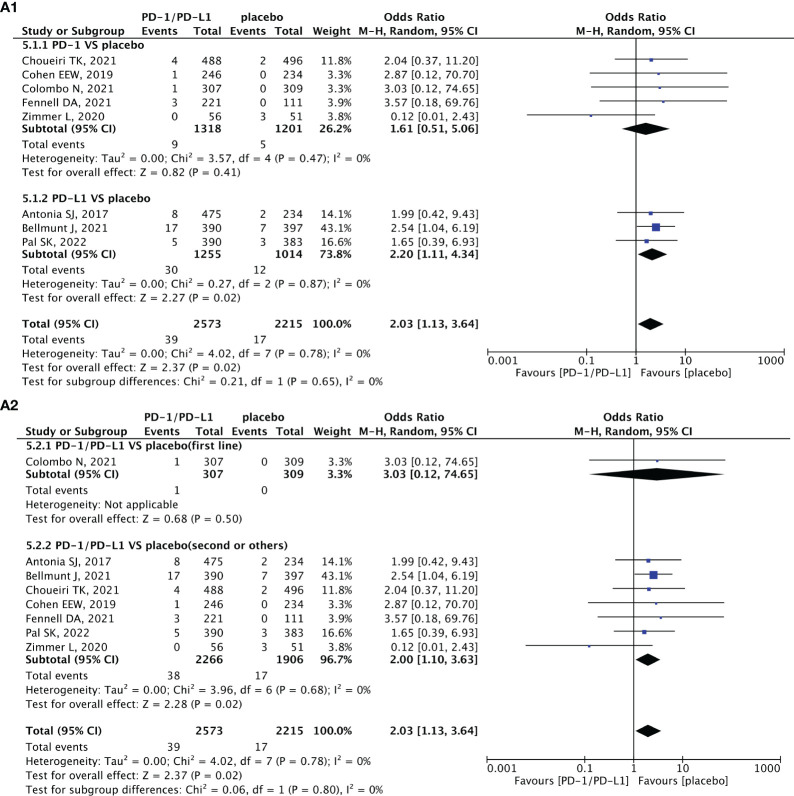
When comparing PD-1/PD-L1 inhibitors (nivolumab and pembrolizumab) with chemotherapy (specifically docetaxel), it was observed that nivolumab and pembrolizumab carried a lower risk of inducing hypotension; however, the difference was not statistically significant ( Figure 4B ) (30, 45, 57, 58, 61, 62, 65–67). Compared to placebo, PD-1/PD-L1 inhibitors showed a tendency toward a higher risk of all-grade arrhythmia (OR = 2.03, 95% CI [1.13, 3.64], p = 0.02, I2 = 0%; Figure 5A ), particularly within the PD-L1 subgroup (OR = 2.20, 95% CI [1.11, 4.34], p = 0.02, I2 = 0%; Figure 5A1 ) and second-line treatment subgroup (OR = 2.00, 95% CI [1.10, 3.63], p = 0.02, I2 = 0%; Figure 5A2 ) (68, 71, 72, 75, 76, 78, 83, 84). No heterogeneity was observed in the aforementioned results. The corresponding funnel plots are presented in Supplementary Data ( Supplementary Figures 4 , 5 ).
Figure 5.
Forest plots depicting the risk of arrhythmia in PD-1/PD-L1 versus placebo. (A1) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to PD-1/PD-L1. (A2) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to treatment lines.
Risk of myocarditis
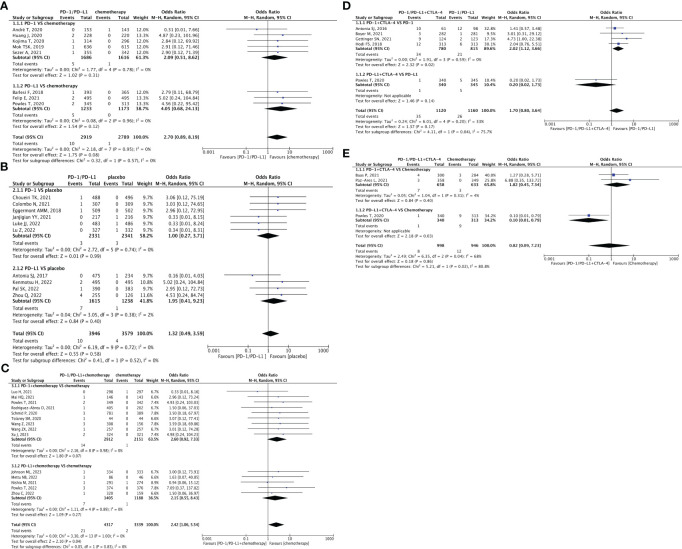
The adverse effects of myocarditis were reported in thirty-one clinical trials (17, 21–25, 28, 30, 31, 33, 37, 38, 49, 50, 52, 53, 56, 59, 62, 63, 67, 68, 70, 72–74, 78–81, 84, 91). No significant difference was observed in the risk of myocarditis between PD-1/PD-L1 monotherapy and chemotherapy ( Figure 6A ) (52, 53, 56, 59, 62, 63, 67, 80) or between PD-1/PD-L1 monotherapy and placebo ( Figure 6B ) (22, 68, 70, 72–74). However, the risk of all-grade myocarditis associated with chemotherapy was significantly lower than that associated with PD-1/PD-L1 + chemotherapy (OR = 2.42, 95% CI [1.06, 5.54], p = 0.04, I2 = 0%; Figure 6C ) (17, 21–25, 28, 30, 31, 33, 37, 38, 50, 69, 91). No heterogeneity was found in the above result. The corresponding funnel plots are provided in the Supplementary Data ( Supplementary Figures 6A–C ).
Figure 6.
Forest plot depicting the risk of myocarditis in PD-1/PD-L1 versus chemotherapy. (A) The risk of myocarditis of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Forest plot depicting the risk of myocarditis in PD-1/PD-L1 versus placebo. (B) The risk of myocarditis of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Forest plot depicting the risk of myocarditis in PD-1/PD-L1 + chemotherapy versus chemotherapy. (C) The risk of myocarditis of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Forest plot depicting the risk of cardiovascular toxicities in PD-1/PD-L1 + CTLA-4 versus PD-1/PD-L1. (D) The risk of cardiovascular toxicities of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Forest plot depicting the risk of cardiovascular toxicities in PD-1/PD-L1 + CTLA-4 versus chemotherapy. (E) The risk of cardiovascular toxicities of all-grade: subgroup analysis was conducted according to PD-1/PD-L1.
Risk of cardiovascular toxicity associated with CTLA-4
Five clinical trials compared PD-1/PD-L1 + CTLA-4 with PD-1/PD-L1 (67, 85–88). Among them, four RCTs included PD-1, and the results suggested a significantly higher risk following combination therapy than following PD-1 monotherapy (OR = 2.02, 95% CI [1.12, 3.66], p = 0.02, I2 = 0%; Figure 6D ). Three clinical trials compared PD-1/PD-L1 + CTLA-4 versus chemotherapy (67, 89, 90). Only one of these studies involved PD-L1 combined with CTLA-4, and the results indicated a lower risk of cardiovascular toxicity for this treatment than chemotherapy (OR = 0.10, 95% CI [0.01, 0.79], p = 0.03; Figure 6E ). The corresponding funnel plots are provided in the Supplementary Data ( Supplementary Figure 6D, E ).
Risk of myocardial infarction, heart failure, and pericardial diseases
There were twenty-two clinical trials reporting on myocardial infarction (15, 16, 23, 27, 30, 34, 36, 37, 39, 40, 46, 47, 52, 55, 62, 63, 65, 68, 70, 72, 78, 83, 84). Heart failure was reported in seventeen clinical trials (20, 22, 25, 30–32, 34, 37, 45–47, 49, 62, 65, 67, 68, 78). Only four clinical trials reported pericardial diseases (32, 68, 76, 78). No statistically significant differences were observed in the risk of all-grade heart failure between the PD-1/PD-L1 versus chemotherapy or PD-1/PD-L1 + chemotherapy versus chemotherapy groups (20, 22, 25, 31, 32, 34, 37, 45–47, 49, 62, 63, 65, 67), myocardial infarction (15, 16, 23, 27, 30, 34, 36, 37, 39, 40, 46, 47, 52, 55, 62, 63, 65), or pericardial diseases (32, 68, 76, 78). Additionally, no statistically significant difference was observed in the risk of all-grade heart failure (78, 84) or myocardial infarction (68, 70, 72, 78, 83, 84) with PD-1/PD-L1 or placebo. The specific statistical data is presented in Tables 2 , 3 .
Table 2.
The risk of all-grade myocardial infarction, heart failure, pericardial diseases, embolism, thrombosis and vasculis: subgroup analyses were carried out based on PD-1/PD-L1.
| Treatment regimen | PD-1/PD-L1+chemotherapy VS chemotherapy | PD-1/PD-L1 VS chemotherapy | PD-1/PD-L1 VS placebo | |
|---|---|---|---|---|
| myocardial infraction | PD-1 | OR=0.69, 95% CI [0.11, 4.40], p=0.70 | OR=0.80, 95% CI [0.20, 3.29], p=0.76 | OR=2.16, 95% CI [0.46, 10.09], p=0.33 |
| PD-L1 | OR=0.86, 95% CI [0.32, 2.32], p=0.77 | OR=0.92, 95% CI [0.10, 8.91], p=0.95 | OR=1.91, 95% CI [0.32, 11.36], p=0.48 | |
| heart failure | PD-1 |
OR=1.43, 95% CI [0.33, 6.26], p=0.64 |
OR=0.72, 95% CI [0.16, 3.24], p=0.67 |
OR=2.04, 95% CI [0.18, 22.54], p=0.56 |
| PD-L1 | OR=1.17, 95% CI [0.52, 2.63], p=0.70 | OR=0.56, 95% CI [0.13, 2.30], p=0.42 | OR=3.22, 95% CI [0.37, 28.43], p=0.29 | |
| pericardial diseases | PD-1 |
OR=0.96, 95% CI [0.06, 15.55], p=0.98 |
N/A |
OR=3.82, 95% CI [0.44, 33.23], p=0.22 |
| PD-L1 | OR=2.42, 95% CI [0.46, 12.82], p=0.03 | N/A | OR=2.48, 95% CI [0.12, 51.79], p=0.56 | |
| emobolism | PD-1 |
OR=1.17, 95% CI [0.33, 4.13], p=0.81 |
OR=1.28, 95% CI [0.15, 10.61], p=0.82 |
OR=1.37, 95% CI [0.09, 19.88], p=0.82 |
| PD-L1 | OR=1.05, 95% CI [0.66, 1.66], p=0.85 | OR=1.49, 95% CI [0.18, 12.17], p=0.71 | OR=1.03, 95% CI [0.26, 4.01], p=0.97 | |
| thrombosis | PD-1 |
OR=0.67, 95% CI [0.15, 2.98], p=0.60 |
OR=0.96, 95% CI [0.29, 3.15], p=0.95 |
OR=0.54, 95% CI [0.09, 3.47], p=0.52 |
| PD-L1 | OR=1.74, 95% CI [0.79, 3.84], p=0.17 | OR=0.18, 95% CI [0.01, 3.77], p=0.27 | OR=0.58, 95% CI [0.12, 2.73], p=0.49 | |
| vasculitis | PD-1 |
OR=0.80, 95% CI [0.20, 3.29], p=0.76 |
OR=0.32, 95% CI [0.01, 7.89], p=0.49 |
OR=5.07, 95% CI [0.24, 105.95], p=0.30 |
| PD-L1 | OR=0.80, 95% CI [0.20, 3.29], p=0.76 | OR=0.83, 95% CI [0.17, 4.01], p=0.81 | OR=1.02, 95% CI [0.24, 4.43], p=0.98 |
PD-1, Programmed cell death 1; PD-L1, Programmed cell death 1 ligand 1; OR, Odds Ratio; CI, Confidence Interval; N/A, not available.
Table 3.
The risk of all-grade myocardial infarction, heart failure, pericardial diseases, embolism, thrombosis and vasculis: subgroup analyses were carried out based on treatment lines.
| Treatment regimen | PD-1/PD-L1+chemotherapy VS chemotherapy | PD-1/PD-L1 VS chemotherapy | PD-1/PD-L1 VS placebo | |
|---|---|---|---|---|
| myocardial infraction | first line | OR=0.82, 95% CI [0.34, 1.96], p=0.65 | OR=1.22, 95% CI [0.30, 4.98], p=0.78 | OR=1.62, 95% CI [0.20, 13.22], p=0.65 |
| second or others | N/A | OR=0.31, 95% CI [0.03, 3.03], p=0.32 | OR=2.28, 95% CI [0.56, 9.25], p=0.25 | |
| heart failure | first line |
OR=1.08, 95% CI [0.52, 2.25], p=0.84 |
OR=0.48, 95% CI [0.16, 1.45], p=0.19 |
N/A |
| second or others | OR=4.05, 95% CI [0.45, 36.44], p=0.21 | OR=4.67, 95% CI [0.22, 97.56], p=0.32 | OR=2.62, 95% CI [0.52, 13.16], p=0.24 | |
| pericardial diseases | first line |
OR=1.90, 95% CI [0.45, 7.93], p=0.38 |
N/A | N/A |
| second or others | N/A | N/A | OR=3.30, 95% CI [0.57, 19.25], p=0.18 | |
| emobolism | first line |
OR=1.06, 95% CI [0.69, 1.64], p=0.79 |
OR=1.21, 95% CI [0.26, 5.65], p=0.81 |
OR=0.33, 95% CI [0.01, 8.24], p=0.50 |
| second or others | N/A | OR=2.90, 95% CI [0.12, 71.42], p=0.51 | OR=1.34, 95% CI [0.39, 4.65], p=0.64 | |
| thrombosis | first line | OR=1.41, 95% CI [0.70, 2.83], p=0.34 |
OR=0.64, 95% CI [0.20, 2.09], p=0.46 |
OR=0.54, 95% CI [0.09, 3.47], p=0.52 |
| second or others | N/A | OR=2.91, 95% CI [0.12, 71.76], p=0.51 | OR=0.58, 95% CI [0.12, 2.73], p=0.49 | |
| vasculitis | first line |
OR=1.51, 95% CI [0.86, 2.65], p=0.15 |
OR=0.82, 95% CI [0.17, 3.97], p=0.80 |
OR=1.35, 95% CI [0.09, 19.84], p=0.82 |
| second or others | N/A | OR=0.33, 95% CI [0.01, 8.19], p=0.50 | OR=1.38, 95% CI [0.27, 7.19], p=0.70 |
PD-1: Programmed cell death 1; PD-L1: Programmed cell death 1 ligand 1; OR: Odds Ratio; CI: Confidence Interval; N/A, not available.
Risk of embolism, thrombosis, and vasculitis
Twenty-one clinical trials reported embolism (15, 20, 22, 27, 30, 36, 38, 40–42, 45–48, 55, 62, 66–68, 83, 84), eighteen reported thrombosis (15, 25–27, 30, 34, 36, 40, 47, 52, 55, 62, 67, 68, 71, 76, 78, 83) and thirteen reported vasculitis (19, 25, 27, 32, 51, 62, 64, 67, 68, 72, 80–84). No significant differences were observed in the risk of all-grade embolism between the PD-1/PD-L1 versus chemotherapy/placebo group and the PD-1/PD-L1 + chemotherapy versus chemotherapy group (15, 20, 22, 27, 30, 36, 38, 40–42, 45–48, 55, 62, 66–68, 83, 84), thrombosis (15, 25–27, 30, 34, 36, 40, 47, 52, 55, 62, 67, 68, 71, 76, 78, 83), or vasculitis (19, 25, 27, 32, 51, 62, 64, 67, 68, 72, 80–84). The specific statistical data is presented in Tables 2 , 3 .
Discussion
This meta-analysis included recently completed RCTs and provided updated information on the cardiotoxicity of PD-1/PD-L1 inhibitors. With a larger sample size and more detailed subgroups, this study provided several novel findings, indicating that the combination of PD-1/PD-L1 inhibitors with chemotherapy carries a considerably higher risk of myocarditis and hypotension than conventional chemotherapy alone. An increasing number of people are now paying attention to the cardiovascular toxicities of PD-1/PD-L1, and this study provides strong supporting evidence for these concerns. Additionally, it assists doctors in making preliminary assessments of the potential causes of these side effects when they detect cardiovascular issues in patients. This, in turn, allows for a more significant improvement in patient prognosis without compromising their anti-tumor treatment. Additionally, this study supports previous meta-analyses (7, 8) and preclinical evidence (9) (92, 93), highlighting the substantial increase in cardiovascular toxicities associated with PD-1/PD-L1 inhibitors. Flow cytometry and metabolomic assays revealed that PD-1/PD-L1 treatment in mice resulted in an increase in the overall lymphocyte count and changes in lipid metabolism within the cardiac tissue. These findings provide evidence that PD-1/PD-L1 disrupts immune homeostasis and energy production in the heart (9). Furthermore, single-cell sequencing revealed that endothelial cells constituted the majority of cells in the cardiac interstitium. Notably, these endothelial cells, along with cardiomyocytes and vascular endothelial cells, exhibit high levels of PD-L1 expression on their surfaces (92, 93). The use of PD-1/PD-L1 inhibitors can enable T cells to nonselectively target normal cells in the heart. Consequently, these factors increase the risk of cardiovascular toxicity.
This study demonstrated a notable increase in the risk of hypertension with the use of PD-1/PD-L1 inhibitors in combination with chemotherapy (22, 24, 25, 29, 31, 32, 35–37, 40, 42–47, 51). This trend was specifically observed in the subgroups of PD-1 inhibitors, first-line treatment, and urothelial carcinoma (UC), which has not been reported in previous meta-analyses. This phenomenon may be attributed to the immune-enhancing effects of PD-1/PD-L1 inhibitors. Owing to the high expression of PD-L1 on vascular endothelial cells (94), medications that enhance non-specific attack by T cells can also cause damage to vascular endothelial cells. This weakens the ability of cells to regulate blood pressure, leading to blood pressure fluctuations (95). However, the exact mechanism requires further investigation. In addition, while PD-1/PD-L1 did not exhibit statistically significant outcomes compared with chemotherapy or placebo, it can be inferred that PD-1/PD-L1 carries a reduced risk of inducing hypertension compared with the placebo group. This novel fact should be applied in clinical settings; when hypertension occurs after using PD-1/PD-L1, initial focus should be on identifying factors unrelated to this medication, such as potential drug interactions, unhealthy lifestyle choices, underlying health conditions, age, or gender.
Despite the lack of significant differences in the risk of heart failure among the treatment regimens in this study (20, 22, 25, 31, 32, 34, 37, 45–47, 49, 62, 63, 65, 67, 78, 84), the potential detrimental effects of PD-1/PD-L1 on cardiac function should not be overlooked. Michel et al. (9) observed that six of seven patients with stage IV progressive melanoma treated with PD-1 had decreased left ventricular ejection fraction (LVEF) and exhibited no significant signs of myocarditis four weeks after the first treatment. In addition, this study also concluded that PD-1/PD-L1 alone (68, 71, 75, 76, 78, 83, 84) or in combination with chemotherapy (25, 29, 31, 36, 40, 42) leads to an appreciably higher risk of hypotension, which was first reported in a meta-analysis, and could not be ruled out as a manifestation of reduced ejection following a decrease in cardiac function due to PD-1/PD-L1. This trend was particularly evident in the PD-1 + chemotherapy, PD-L1 alone, first-line treatment, or breast cancer subgroups. In addition to diminished cardiac pumping, hypotension cannot exclude the less common drug-induced hypersensitivity syndrome (DIHS), which results from excessive activation of T-cell function by immune checkpoint inhibitors (ICIs) (96). Vasodilation and increased permeability of the vessel wall lead to plasma extravasation, which reduces the intravascular blood volume and vasogenic hypotension. However, the exact mechanisms remain to be further elucidated.
In a comparison of PD-1/PD-L1 + chemotherapy versus chemotherapy (21–24, 29, 30, 32, 36, 40–42, 46, 47) and PD-1/PD-L1 versus placebo (68, 71, 72, 75, 76, 78, 83, 84), the use of PD-1/PD-L1-related therapy was associated with a considerably increased risk of arrhythmias. Particularly in the NSCLC subgroup, the combination of PD-1/PD-L1 inhibitors with chemotherapy led to a notably higher occurrence of all-grade or grade 3–5 arrhythmia (21, 36). This is broadly consistent with the results of previous meta-analyses or reviews by Herrmann and Liu et al. (7, 97). In addition, although there was no statistically significant difference in the risk of arrhythmia between PD-1/PD-L1 inhibitors and chemotherapy, the two PD-1 inhibitors, nivolumab and pembrolizumab, exhibited a lower risk of arrhythmia than docetaxel. Thus, more important with docetaxel is the prevention of several serious complications, such as myocardial ischemia due to abnormal heart rhythms. Additionally, positive results may be obtained concerning the apparent subjective discomfort experienced by the patients. Currently, physicians can easily ascertain abnormal heart rhythms and collect these data using Holter (24h dynamic electrocardiogram) or other devices. However, additional fundamental research is required to investigate the mechanisms by which PD-1/PD-L1 affects the cardiac conduction system.
Clinical evidence has indicated that immunotherapy can cause myocarditis, which should be taken seriously. The severity of immune-associated myocarditis varies from mild cases without apparent inflammation to severe cases that may be associated with heart failure, cardiogenic shock, and a high mortality rate in the case of rapidly progressing fulminant myocarditis (98, 99). Hu et al. concluded that immunotherapy drastically increased the risk of myocardial disease compared with conventional antitumor therapy (100). This is the first study to provide evidence that the combination of PD-1/PD-L1 inhibitors and chemotherapy is associated with an elevated risk of myocarditis (17, 21–25, 28, 30, 31, 33, 37, 38, 50, 69, 91). However, no positive results were obtained in the subgroup analysis, which should be conducted in additional RCTs. The exact mechanism of immune-associated myocarditis remains unclear, but some preclinical studies have made some conjectures, such as inflammation due to T-cell activation (101). Given the poor prognosis of this disease, more clinical data and basic research are required.
The combination of PD-1/PD-L1 and CTLA-4 blockade substantially enhances the immune responses and survival rates in certain cancers (102). However, it also increases the risk of adverse effects. This study found that the risk of cardiovascular toxicity following PD-1 combined with CTLA-4 treatment was noticeably higher than following PD-1 treatment alone, and these results were consistent with prior findings. Preclinical trials have revealed that when PD-1 on the surface of myocardial cells binds to PD-L1 on the surface of T lymphocytes, it prevents T lymphocytes from attacking the myocardium. CTLA-4, on the other hand, prevents lymphocyte proliferation and spread. Therefore, the simultaneous inhibition of both pathways inevitably leads to indiscriminate T lymphocyte attacks on myocardial tissue, resulting in an increased risk of cardiovascular toxicity with the combined use of ICIs (103). Further research is required to decrease the occurrence of adverse event while maintaining the efficacy of the combination.
Cardiovascular toxicities associated with ICIs can be indicated by several biomarkers, including inflammatory markers such as C-reactive protein, erythrocyte sedimentation rate, and white blood cell count, as well as cardiac injury markers like troponin I, creatine kinase-MB, and brain natriuretic peptide. The development of ICI adverse effects is attributed to excessive enhancement of immune function, leading to inadvertent harm to normal cells. In response, we initially administered symptomatic treatments involving a variety of immunosuppressive agents, including corticosteroids, cytotoxic drugs, calcineurin inhibitors, and biologics. Secondly, the severity of the adverse effects needs to be assessed to determine whether temporary or permanent discontinuation of the medication is warranted. In addition, screening specific patients before initiating treatment can help prevent adverse effects. For instance, it is not recommended for individuals with autoimmune diseases, organ transplant recipients, patients with active hepatitis, or elderly patients to use ICIs. Furthermore, patients with pre-existing cardiovascular disorders should be monitored (104).
This meta-analysis further refined the cardiovascular toxicity of PD-1/PD-L1 through a comprehensive analysis of 69 RCTs. Moreover, there was no heterogeneity or insignificant heterogeneity among the RCTs included in this meta-analysis; thus, the results were reliable. However, this study had some limitations. Only 11% of the original studies searched reported the above cardiovascular toxicity events. In an initial comparison of morbidity data, PD-1/PD-L1 treatment resulted in a higher number of cardiovascular adverse events than conventional treatment. However, the final meta-analysis did not yield positive results. First, it can be inferred that PD-1/PD-L1 therapy is safe. However, it should also be noted that cardiovascular adverse events may not have received sufficient attention from doctors and patients, resulting in patients not seeking medical treatment promptly or first consulting physicians not collecting data on time. Therefore, due to the lack of sufficient sample size, this study was unable to collect baseline information for subgroup analyses of additional possible risk factors or to shed light on the specifics of chemotherapy. Furthermore, this meta-analysis exclusively included RCTs; most of these only reported a greater than certain percentage of cardiovascular toxicities, which may lead to the underreporting of some rare diseases with low incidence.
Conclusion
The combination of PD-1/PD-L1 with chemotherapy increases the risk of hypertension, hypotension, arrhythmia, and myocarditis. The incidence of hypotension or arrhythmia associated with PD-1/PD-L1 inhibitors was substantially higher than that associated with placebo. When hypertension is observed in patients receiving PD-1/PD-L1 inhibitors, factors other than ICIs should be considered as potential contributors in the first instance.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author contributions
CZ: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft. FW: Writing – original draft. WM: Writing – original draft. JZ: Investigation, Supervision, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Shandong Province (ZR2021MH245).
Abbreviations
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, Randomized Controlled Trial; ICI, Immune Checkpoint Inhibitor; PD-1, Programmed cell death 1; PD-L1, Programmed cell death 1 ligand 1; CTLA-4, anti-cytotoxic T-lymphocyte antigen-4; HR, Hazard Ratios; OR, Odds Ratio; CI, Confidence Interval; RE, Random Effect; FE, Fixed Effect; NSCLC, Non-Small-Cell Lung Cancer; SCLC, Small-Cell Lung Cancer; BRCA, Breast Cancer; UC, Urothelial Carcinoma; HNSCC, Head and Neck Squamous Cell Carcinoma; CCA, Cervical Cancer; TNBC, Triple-Negative Breast Cancer; GC, Gastric Cancer; GC/GJC, Gastric or gastro-oesophageal junction cancer; ESCC, Oesophagea/Esophagea Squamous Cell Carcinoma; NPC, Nasopharyngeal Cancer; CRC, Colorectal Cancer; EOC, Epithelial Ovarian Cancer; OC, Ovarian Cancer; GEC, Gastroesophageal adenocarcinoma; RCC, Renal Cell Carcinoma; PCA, Prostate Cancer; HCC, Hepatocellular Carcinoma; EC, Esophageal Cancer; MPM, Malignant Pleural Mesothelioma; N/A, not available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1255825/full#supplementary-material
The assessment of bias risk in the studies included in this meta-analysis.
Funnel plots depicting the risk of hypertension in PD-1/PD-L1 + chemotherapy versus chemotherapy. (A1) The risk of hypertension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. (A2) The risk of hypertension of grade 3-5: subgroup analyses were conducted according to PD-1/PD-L1. (A3) The risk of hypertension of grade 3-5: subgroup analyses were conducted according to types of tumors. Funnel plot depicting the risk of hypertension in PD-1/PD-L1 versus chemotherapy. (B) The risk of hypertension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of hypertension in PD-1/PD-L1 versus placebo. (C) The risk of hypertension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1.
Funnel plots depicting the risk of hypotension in PD-1/PD-L1 + chemotherapy versus chemotherapy. (A1) The risk of hypotension of all-grade: subgroup analyses were conducted according to PD-1/PD-L1. (A2) The risk of hypotension of all-grade: subgroup analyses were conducted according to types of tumors. (A3) The risk of hypotension of grade 3-5: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of hypotension in PD-1/PD-L1 versus placebo. (B) The risk of hypotension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of hypotension in PD-1/PD-L1 versus chemotherapy. (C) The risk of hypotension of grade 3-5: subgroup analysis was conducted according to PD-1.
Funnel plots depicting the risk of arrhythmia in PD-1/PD-L1 + chemotherapy versus chemotherapy. (A1) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to PD-1/PD-L1. (A2) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to types of tumors. (A3) The risk of arrhythmia of grade 3-5: subgroup analyses were conducted according to PD-1/PD-L1. (A4) The risk of arrhythmia of grade 3-5: subgroup analyses were conducted according to types of tumors. Funnel plot depicting the risk of arrhythmia in PD-1/PD-L1 versus chemotherapy. (B) The risk of arrhythmia of all-grade: subgroup analysis was conducted according to PD-1/PD-L1.
Funnel plot depicting the risk of arrhythmia in PD-1/PD-L1 versus placebo. (A1) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to PD-1/PD-L1. (A2) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to treatment lines.
Funnel plot depicting the risk of myocarditis in PD-1/PD-L1 versus chemotherapy. (A) The risk of myocarditis of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of myocarditis in PD-1/PD-L1 versus placebo. (B) The risk of myocarditis of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of myocarditis in PD-1/PD-L1 + chemotherapy versus chemotherapy. (C) The risk of myocarditis of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of cardiovascular toxicities in PD-1/PD-L1 + CTLA-4 versus PD-1/PD-L1. (D) The risk of cardiovascular toxicities of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of cardiovascular toxicities in PD-1/PD-L1 + CTLA-4 versus chemotherapy. (E) The risk of cardiovascular toxicities of all-grade: subgroup analysis was conducted according to PD-1/PD-L1.
References
- 1. Kraehenbuehl L, Weng C-H, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol (2022) 19(1):37–50. doi: 10.1038/s41571-021-00552-7 [DOI] [PubMed] [Google Scholar]
- 2. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med (2016) 375(18):1767–78. doi: 10.1056/NEJMra1514296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph A, Simonaggio A, Stoclin A, Vieillard-Baron A, Geri G, Oudard S, et al. Immune-related adverse events: a retrospective look into the future of oncology in the intensive care unit. Ann Intensive Care (2020) 10(1):143. doi: 10.1186/s13613-020-00761-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J For Immunotherapy Cancer (2021) 9(6). doi: 10.1136/jitc-2021-002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thuny F, Naidoo J, Neilan TG. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J (2022) 43(42):4458–68. doi: 10.1093/eurheartj/ehac456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salem J-E, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol (2018) 19(12):1579–89. doi: 10.1016/S1470-2045(18)30608-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu S, Gao W, Ning Y, Zou X, Zhang W, Zeng L, et al. Cardiovascular toxicity with PD-1/PD-L1 inhibitors in cancer patients: A systematic review and meta-analysis. Front Immunol (2022) 13:908173. doi: 10.3389/fimmu.2022.908173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin (2020) 70(2):86–104. doi: 10.3322/caac.21596 [DOI] [PubMed] [Google Scholar]
- 9. Michel L, Helfrich I, Hendgen-Cotta UB, Mincu R-I, Korste S, Mrotzek SM, et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J (2022) 43(4):316–29. doi: 10.1093/eurheartj/ehab430 [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delgado-Rodríguez M, Sillero-Arenas M. Systematic review and meta-analysis. Med Intensiva (Engl Ed) (2018) 42(7):444–53. doi: 10.1016/j.medin.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 13. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Norton EC, Dowd BE, Maciejewski ML. Odds ratios-current best practice and use. JAMA. (2018) 320(1):84–5. doi: 10.1001/jama.2018.6971 [DOI] [PubMed] [Google Scholar]
- 15. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med (2022) 386(21):1973–85. doi: 10.1056/NEJMoa2202170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol (2016) 17(11):1497–508. doi: 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol (2021) 32(7):881–95. doi: 10.1016/j.annonc.2021.04.008 [DOI] [PubMed] [Google Scholar]
- 18. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol (2023) 41(11):1992–8. doi: 10.1200/jco.22.01989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol (2023) 41(11):1999–2006. doi: 10.1200/JCO.22.01990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med (2021) 9(3):305–14. doi: 10.1016/S2213-2600(20)30365-9 [DOI] [PubMed] [Google Scholar]
- 21. Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-small-cell lung cancer: A multicenter randomized phase III trial (CHOICE-01). J Clin Oncol (2023) 41(3):651–63. doi: 10.1200/JCO.22.00727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. (2022) 377:e068714. doi: 10.1136/bmj-2021-068714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. (2021) 326(10):916–25. doi: 10.1001/jama.2021.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Z-X, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell (2022) 40(3):277–88.e3. doi: 10.1016/j.ccell.2022.02.007 [DOI] [PubMed] [Google Scholar]
- 25. Xu J, Kato K, Raymond E, Hubner RA, Shu Y, Pan Y, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol (2023) 24(5):483–95. doi: 10.1016/S1470-2045(23)00108-0 [DOI] [PubMed] [Google Scholar]
- 26. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang Y-K, Chen L-T, Ryu M-H, Oh D-Y, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23(2):234–47. doi: 10.1016/S1470-2045(21)00692-6 [DOI] [PubMed] [Google Scholar]
- 28. Tolaney SM, Barroso-Sousa R, Keenan T, Li T, Trippa L, Vaz-Luis I, et al. Effect of eribulin with or without pembrolizumab on progression-free survival for patients with hormone receptor-positive, ERBB2-negative metastatic breast cancer: A randomized clinical trial. JAMA Oncol (2020) 6(10):1598–605. doi: 10.1001/jamaoncol.2020.3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med (2022) 386(6):556–67. doi: 10.1056/NEJMoa2112651 [DOI] [PubMed] [Google Scholar]
- 30. Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SYS, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(7):931–45. doi: 10.1016/S1470-2045(21)00152-2 [DOI] [PubMed] [Google Scholar]
- 31. Mai H-Q, Chen Q-Y, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med (2021) 27(9):1536–43. doi: 10.1038/s41591-021-01444-0 [DOI] [PubMed] [Google Scholar]
- 32. Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol (2021) 22(8):1162–74. doi: 10.1016/S1470-2045(21)00302-8 [DOI] [PubMed] [Google Scholar]
- 33. Nishio M, Saito H, Goto K, Watanabe S, Sueoka-Aragane N, Okuma Y, et al. IMpower132: Atezolizumab plus platinum-based chemotherapy vs chemotherapy for advanced NSCLC in Japanese patients. Cancer Sci (2021) 112(4):1534–44. doi: 10.1111/cas.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med (2018) 378(24):2288–301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 35. Reck M, Wehler T, Orlandi F, Nogami N, Barone C, Moro-Sibilot D, et al. Safety and patient-reported outcomes of atezolizumab plus chemotherapy with or without bevacizumab versus bevacizumab plus chemotherapy in non-small-cell lung cancer. J Clin Oncol (2020) 38(22):2530–42. doi: 10.1200/JCO.19.03158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(7):924–37. doi: 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 37. Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol (2022) 23(2):220–33. doi: 10.1016/S1470-2045(21)00650-1 [DOI] [PubMed] [Google Scholar]
- 38. Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol (2023) 41(6):1213–27. doi: 10.1200/JCO.22.00975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. (2019) 394(10212):1929–39. doi: 10.1016/S0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 40. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2021) 22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8 [DOI] [PubMed] [Google Scholar]
- 41. Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23(6):739–47. doi: 10.1016/S1470-2045(22)00224-8 [DOI] [PubMed] [Google Scholar]
- 42. Pusztai L, Yau C, Wolf DM, Han HS, Du L, Wallace AM, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell (2021) 39(7):989–98.e5. doi: 10.1016/j.ccell.2021.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Emens LA, Adams S, Barrios CH, Diéras V, Iwata H, Loi S, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol (2021) 32(8):983–93. doi: 10.1016/j.annonc.2021.05.355 [DOI] [PubMed] [Google Scholar]
- 44. Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet. (2020) 396(10257):1090–100. doi: 10.1016/S0140-6736(20)31953-X [DOI] [PubMed] [Google Scholar]
- 45. Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard I-L, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol (2021) 22(7):1034–46. doi: 10.1016/S1470-2045(21)00216-3 [DOI] [PubMed] [Google Scholar]
- 46. Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol (2021) 22(4):450–62. doi: 10.1016/S1470-2045(20)30737-3 [DOI] [PubMed] [Google Scholar]
- 47. Monk BJ, Colombo N, Oza AM, Fujiwara K, Birrer MJ, Randall L, et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(9):1275–89. doi: 10.1016/S1470-2045(21)00342-9 [DOI] [PubMed] [Google Scholar]
- 48. Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol (2021) 39(17):1842–55. doi: 10.1200/JCO.21.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Powles T, Yuen KC, Gillessen S, Kadel EE, Rathkopf D, Matsubara N, et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med (2022) 28(1):144–53. doi: 10.1038/s41591-021-01600-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mettu NB, Ou F-S, Zemla TJ, Halfdanarson TR, Lenz H-J, Breakstone RA, et al. Assessment of capecitabine and bevacizumab with or without atezolizumab for the treatment of refractory metastatic colorectal cancer: A randomized clinical trial. JAMA Netw Open (2022) 5(2):e2149040. doi: 10.1001/jamanetworkopen.2021.49040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. (2020) 395(10236):1547–57. doi: 10.1016/S0140-6736(20)30230-0 [DOI] [PubMed] [Google Scholar]
- 52. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol (2020) 21(6):832–42. doi: 10.1016/S1470-2045(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 53. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu C-H, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888 [DOI] [PubMed] [Google Scholar]
- 54. Chan ATC, Lee VHF, Hong RL, Ahn MJ, Chong WQ, Kim SB, et al. Pembrolizumab monotherapy versus chemotherapy in platinum-pretreated, recurrent or metastatic nasopharyngeal cancer (KEYNOTE-122): an open-label, randomized, phase III trial. Ann Oncol (2023) 34(3):251–61. doi: 10.1016/j.annonc.2022.12.007 [DOI] [PubMed] [Google Scholar]
- 55. Diaz LA, Shiu K-K, Kim T-W, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol (2022) 23(5):659–70. doi: 10.1016/S1470-2045(22)00197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. André T, Shiu K-K, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med (2020) 383(23):2207–18. doi: 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 57. Winer EP, Lipatov O, Im S-A, Goncalves A, Muñoz-Couselo E, Lee KS, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(4):499–511. doi: 10.1016/S1470-2045(20)30754-3 [DOI] [PubMed] [Google Scholar]
- 58. Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 59. Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 60. de Castro G, Kudaba I, Wu Y-L, Lopes G, Kowalski DM, Turna HZ, et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non-small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥ 1% in the KEYNOTE-042 study. J Clin Oncol (2023) 41(11):1986–91. doi: 10.1200/JCO.21.02885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. (2021) 397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2 [DOI] [PubMed] [Google Scholar]
- 63. Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol (2018) 19(11):1468–79. doi: 10.1016/S1470-2045(18)30673-9 [DOI] [PubMed] [Google Scholar]
- 64. Jassem J, de Marinis F, Giaccone G, Vergnenegre A, Barrios CH, Morise M, et al. Updated overall survival analysis from IMpower110: atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1-selected NSCLC. J Thorac Oncol Off Publ Int Assoc For Study Lung Canc (2021) 16(11):1872–82. doi: 10.1016/j.jtho.2021.06.019 [DOI] [PubMed] [Google Scholar]
- 65. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med (2020) 383(14):1328–39. doi: 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- 66. van der Heijden MS, Loriot Y, Durán I, Ravaud A, Retz M, Vogelzang NJ, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma: A long-term overall survival and safety update from the phase 3 IMvigor211 clinical trial. Eur Urol (2021) 80(1):7–11. doi: 10.1016/j.eururo.2021.03.024 [DOI] [PubMed] [Google Scholar]
- 67. Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol (2020) 21(12):1574–88. doi: 10.1016/S1470-2045(20)30541-6 [DOI] [PubMed] [Google Scholar]
- 68. Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang Y-H, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med (2021) 385(8):683–94. doi: 10.1056/NEJMoa2106391 [DOI] [PubMed] [Google Scholar]
- 69. Powles T, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23(9):1133–44. doi: 10.1016/S1470-2045(22)00487-9 [DOI] [PubMed] [Google Scholar]
- 70. Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature (2021) 600(7890):727–30. doi: 10.1038/s41586-021-04161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M-J, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. (2019) 393(10167):156–67. doi: 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 72. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med (2021) 385(20):1856–67. doi: 10.1056/NEJMoa2112435 [DOI] [PubMed] [Google Scholar]
- 73. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III melanoma: updated results from the EORTC 1325-MG/KEYNOTE-054 trial. J Clin Oncol (2020) 38(33):3925–36. doi: 10.1200/JCO.20.02110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Long GV, Luke JJ, Khattak MA, de la Cruz Merino L, Del Vecchio M, Rutkowski P, et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol (2022) 23(11):1378–88. doi: 10.1016/S1470-2045(22)00559-9 [DOI] [PubMed] [Google Scholar]
- 75. Zimmer L, Livingstone E, Hassel JC, Fluck M, Eigentler T, Loquai C, et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. (2020) 395(10236):1558–68. doi: 10.1016/S0140-6736(20)30417-7 [DOI] [PubMed] [Google Scholar]
- 76. Fennell DA, Ewings S, Ottensmeier C, Califano R, Hanna GG, Hill K, et al. Nivolumab versus placebo in patients with relapsed Malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol (2021) 22(11):1530–40. doi: 10.1016/S1470-2045(21)00471-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sugawara S, Lee JS, Kang JH, Kim HR, Inui N, Hida T, et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann Oncol (2021) 32(9):1137–47. doi: 10.1016/j.annonc.2021.06.004 [DOI] [PubMed] [Google Scholar]
- 78. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 79. Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin Q, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol (2022) 23(2):209–19. doi: 10.1016/S1470-2045(21)00630-6 [DOI] [PubMed] [Google Scholar]
- 80. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. (2021) 398(10308):1344–57. doi: 10.1016/S0140-6736(21)02098-5 [DOI] [PubMed] [Google Scholar]
- 81. Kenmotsu H, Sugawara S, Watanabe Y, Saito H, Okada M, Chen-Yoshikawa TF, et al. Adjuvant atezolizumab in Japanese patients with resected stage IB-IIIA non-small cell lung cancer (IMpower010). Cancer Sci (2022) 113(12):4327–38. doi: 10.1111/cas.15564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 83. Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(4):525–37. doi: 10.1016/S1470-2045(21)00004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pal SK, Uzzo R, Karam JA, Master VA, Donskov F, Suarez C, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet. (2022) 400(10358):1103–16. doi: 10.1016/S0140-6736(22)01658-0 [DOI] [PubMed] [Google Scholar]
- 85. Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol (2016) 17(7):883–95. doi: 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 86. Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab plus ipilimumab or placebo for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%: randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol (2021) 39(21):2327–38. doi: 10.1200/JCO.20.03579 [DOI] [PubMed] [Google Scholar]
- 87. Gettinger SN, Redman MW, Bazhenova L, Hirsch FR, Mack PC, Schwartz LH, et al. Nivolumab plus ipilimumab vs nivolumab for previously treated patients with stage IV squamous cell lung cancer: the lung-MAP S1400I phase 3 randomized clinical trial. JAMA Oncol (2021) 7(9):1368–77. doi: 10.1001/jamaoncol.2021.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol (2018) 19(11):1480–92. doi: 10.1016/S1470-2045(18)30700-9 [DOI] [PubMed] [Google Scholar]
- 89. Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable Malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. (2021) 397(10272):375–86. doi: 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 90. Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0 [DOI] [PubMed] [Google Scholar]
- 91. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med (2020) 382(9):810–21. doi: 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 92. Ren Z, Yu P, Li D, Li Z, Liao Y, Wang Y, et al. Single-cell reconstruction of progression trajectory reveals intervention principles in pathological cardiac hypertrophy. Circulation. (2020) 141(21):1704–19. doi: 10.1161/CIRCULATIONAHA.119.043053 [DOI] [PubMed] [Google Scholar]
- 93. Wang L, Yu P, Zhou B, Song J, Li Z, Zhang M, et al. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat Cell Biol (2020) 22(1):108–19. doi: 10.1038/s41556-019-0446-7 [DOI] [PubMed] [Google Scholar]
- 94. Liu S, Qin T, Liu Z, Wang J, Jia Y, Feng Y, et al. anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis (2020) 11(5):309. doi: 10.1038/s41419-020-2511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Magder S. The meaning of blood pressure. Crit Care (2018) 22(1):257. doi: 10.1186/s13054-018-2171-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mirza S, Hill E, Ludlow SP, Nanjappa S. Checkpoint inhibitor-associated drug reaction with eosinophilia and systemic symptom syndrome. Melanoma Res (2017) 27(3):271–3. doi: 10.1097/CMR.0000000000000326 [DOI] [PubMed] [Google Scholar]
- 97. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol (2020) 17(8):474–502. doi: 10.1038/s41569-020-0348-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Andres MS, Ramalingam S, Rosen SD, Baksi J, Khattar R, Kirichenko Y, et al. The spectrum of cardiovascular complications related to immune-checkpoint inhibitor treatment : Including myocarditis and the new entity of non inflammatory left ventricular dysfunction. Cardiooncology. (2022) 8(1):21. doi: 10.1186/s40959-022-00147-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dong H, Qi Y, Kong X, Wang Z, Fang Y, Wang J. PD-1/PD-L1 inhibitor-associated myocarditis: epidemiology, characteristics, diagnosis, treatment, and potential mechanism. Front Pharmacol (2022) 13:835510. doi: 10.3389/fphar.2022.835510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hu J, Tian R, Ma Y, Zhen H, Ma X, Su Q, et al. Risk of cardiac adverse events in patients treated with immune checkpoint inhibitor regimens: A systematic review and meta-analysis. Front Oncol (2021) 11:645245. doi: 10.3389/fonc.2021.645245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lichtman AH. The heart of the matter: protection of the myocardium from T cells. J Autoimmun (2013) 45:90–6. doi: 10.1016/j.jaut.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res (2019) 38(1):255. doi: 10.1186/s13046-019-1259-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Love VA, Grabie N, Duramad P, Stavrakis G, Sharpe A, Lichtman A. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res (2007) 101(3):248–57. doi: 10.1161/CIRCRESAHA.106.147124 [DOI] [PubMed] [Google Scholar]
- 104. Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J For Immunotherapy Canc (2016) 4:50. doi: 10.1186/s40425-016-0152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The assessment of bias risk in the studies included in this meta-analysis.
Funnel plots depicting the risk of hypertension in PD-1/PD-L1 + chemotherapy versus chemotherapy. (A1) The risk of hypertension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. (A2) The risk of hypertension of grade 3-5: subgroup analyses were conducted according to PD-1/PD-L1. (A3) The risk of hypertension of grade 3-5: subgroup analyses were conducted according to types of tumors. Funnel plot depicting the risk of hypertension in PD-1/PD-L1 versus chemotherapy. (B) The risk of hypertension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of hypertension in PD-1/PD-L1 versus placebo. (C) The risk of hypertension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1.
Funnel plots depicting the risk of hypotension in PD-1/PD-L1 + chemotherapy versus chemotherapy. (A1) The risk of hypotension of all-grade: subgroup analyses were conducted according to PD-1/PD-L1. (A2) The risk of hypotension of all-grade: subgroup analyses were conducted according to types of tumors. (A3) The risk of hypotension of grade 3-5: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of hypotension in PD-1/PD-L1 versus placebo. (B) The risk of hypotension of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of hypotension in PD-1/PD-L1 versus chemotherapy. (C) The risk of hypotension of grade 3-5: subgroup analysis was conducted according to PD-1.
Funnel plots depicting the risk of arrhythmia in PD-1/PD-L1 + chemotherapy versus chemotherapy. (A1) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to PD-1/PD-L1. (A2) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to types of tumors. (A3) The risk of arrhythmia of grade 3-5: subgroup analyses were conducted according to PD-1/PD-L1. (A4) The risk of arrhythmia of grade 3-5: subgroup analyses were conducted according to types of tumors. Funnel plot depicting the risk of arrhythmia in PD-1/PD-L1 versus chemotherapy. (B) The risk of arrhythmia of all-grade: subgroup analysis was conducted according to PD-1/PD-L1.
Funnel plot depicting the risk of arrhythmia in PD-1/PD-L1 versus placebo. (A1) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to PD-1/PD-L1. (A2) The risk of arrhythmia of all-grade: subgroup analyses were conducted according to treatment lines.
Funnel plot depicting the risk of myocarditis in PD-1/PD-L1 versus chemotherapy. (A) The risk of myocarditis of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of myocarditis in PD-1/PD-L1 versus placebo. (B) The risk of myocarditis of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of myocarditis in PD-1/PD-L1 + chemotherapy versus chemotherapy. (C) The risk of myocarditis of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of cardiovascular toxicities in PD-1/PD-L1 + CTLA-4 versus PD-1/PD-L1. (D) The risk of cardiovascular toxicities of all-grade: subgroup analysis was conducted according to PD-1/PD-L1. Funnel plot depicting the risk of cardiovascular toxicities in PD-1/PD-L1 + CTLA-4 versus chemotherapy. (E) The risk of cardiovascular toxicities of all-grade: subgroup analysis was conducted according to PD-1/PD-L1.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.