Abstract
Although eukaryotic G-protein coupled receptor (GPCR) systems are well known for their ability to detect and mediate rapid responses to extracellular signals, the full range of stimuli to which they respond may not yet have been identified. Activation of GPCRs by hormones, pheromones, odorants, neurotransmitters, light and different taste compounds is well established. However, the recent discovery of a glucose-sensing GPCR system in Saccharomyces cerevisiae has unexpectedly added common nutrients to this list of stimuli. This GPCR system mediates glucose activation of adenylate cyclase during the switch from respirative/gluconeogenic metabolism to fermentation. The GPCR system involved in pheromone signalling in S. cerevisiae has already served as an important model and tool for the study of GPCR systems in higher eukaryotic cell types. Here, we highlight the similarities and differences between these two signalling systems. We also indicate how the new glucose-sensing system can serve as a model for GPCR function and as a tool with which to screen for heterologous components of signalling pathways as well as for novel ligands in high-throughput assays.
Yeast GPCRs for pheromone and glucose sensing
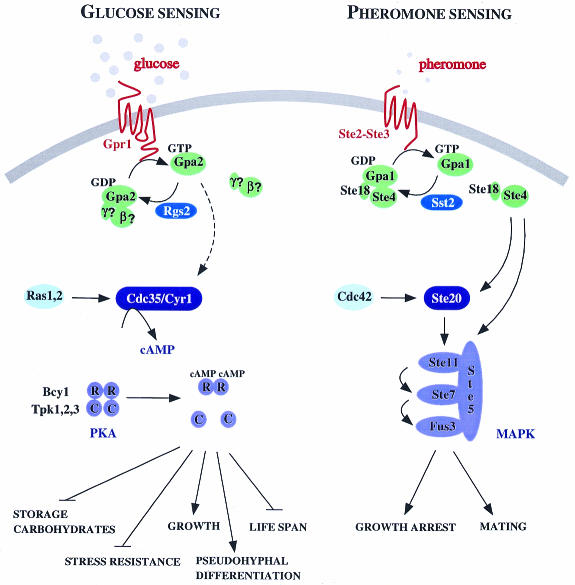
The human genome encodes five to six hundred G-protein coupled receptors (GPCRs), 14 Gα proteins, at least five Gβ proteins, seven Gγ proteins and 31 regulators of heterotrimeric G-protein signalling (RGS proteins). In yeast only two GPCR systems have been identified. Due to space constraints, we will focus in this review on the GPCR systems rather than on the downstream components of the signalling pathways. The pheromone response pathway of the genetically tractable organism Saccharomyces cerevisiae has served as a model system and as a powerful tool for the in vivo study of GPCR systems (Dohlman et al., 1991; Pausch, 1997). All major sensing and signalling components and targets of this pathway seem to have been identified and most of them are related in sequence and function to at least a subset of mammalian homologues. Both haploid yeast cell types (α and a) express mating-type specific gene products such as the a-factor pheromone and the α-factor receptor (Ste2) in a-cells, and the α-factor and the a-factor receptor (Ste3) in α-cells. Pheromone binding to either receptor stimulates the exchange of GDP for GTP on the Gα protein Gpa1, which in turn dissociates from the βγ dimer, consisting of Ste4 and Ste18 (Whiteway et al., 1989). The Ste4–Ste18 dimer transmits the signal to Ste20, the first member of the p21-activated protein kinase (PAK) family, which activates a MAP-kinase cascade (Leberer et al., 1992; Figure 1). This activation results in cell cycle arrest, formation of a ‘shmoo’ cell shape and fusion with a cell of the opposite mating type. An interesting aspect of this response is desensitization to the continued presence of pheromone, a process that is similar to hormone desensitization in mammalian cells. At the level of the receptor, Ste2 and Ste3 are downregulated via hyperphosphorylation of several C-terminal residues, followed by ubiquitylation, internalization and degradation. At the level of the Gα protein, Gpa1, desensitization depends on the GTPase stimulating protein, Sst2, the founding member of the RGS-protein family.
Fig. 1. Overview of pheromone and glucose signalling in S. cerevisiae. The putative glucose receptor Gpr1 activates the Gα protein Gpa2, for which no β or γ subunits have been identified yet. Rgs2 stimulates the GTPase activity of Gpa2 and thus inhibits glucose-induced cAMP signalling. Gpa2 is thought to activate adenylate cyclase (Cdc35/Cyr1) but direct biochemical evidence for this is still lacking. The basal activity of adenylate cyclase also depends on the Ras1 and Ras2 proteins of which the signalling function, if any, remains unclear. Activation of PKA by cAMP results in stimulation of growth and pseudohyphal differentiation, loss of stress resistance, mobilization of trehalose and glycogen and in reduced life-span. Pheromone sensing depends on the Ste2 and Ste3 receptors that respectively bind α- and a-factor and that transmit the signal to the heterotrimeric G-protein consisting of the Gα protein Gpa1 and the βγ subunit Ste4 and Ste18. The RGS protein Sst2 is able to diminish signalling by stimulating the GTPase activity of Gpa1. Ste4 recruits both the scaffolding protein Ste5 and Ste20 (which can also be stimulated by Cdc42) to the membrane resulting in activation of the mating MAP kinase cascade (Ste11, Ste7 and Fus3). Activation of the MAP kinase pathway results in growth arrest and conjugation.
Yeast cells prefer glucose as a carbon source because of its rapid fermentation to ethanol, which inhibits the growth of competing micro-organisms. During growth on glucose, yeast cells exhibit many characteristics different from those of cells growing on a non-fermentable carbon source using respiration and gluconeogenesis. Fermenting yeast cells display active glycolysis, grow more rapidly than non-fermenting cells, are sensitive to environmental stress (heat, freezing, high-osmolarity, salinity, etc.), and accumulate low levels of the carbohydrates trehalose and glycogen. Glucose triggers the switch to a fermentative life-style by transiently activating cAMP synthesis and thereby activating cAMP-dependent protein kinase (PKA), which controls a broad range of targets (Figure 1). Activation of this cascade is dependent on a GPCR system consisting of the putative glucose receptor Gpr1 and the cognate Gα protein Gpa2 (Colombo et al., 1998; Kraakman et al., 1999). The RGS protein Rgs2 downregulates glucose-induced cAMP signalling via stimulation of the intrinsic GTPase activity of Gpa2 (Versele et al., 1999). A remarkable complication is that glucose-induced cAMP signalling is also strictly dependent on glucose phosphorylation (Beullens et al., 1988). Ras1 and Ras2 are required for basal adenylate cyclase activity and, hence, for cell viability, but they are apparently not involved in glucose activation of cAMP synthesis (Colombo et al., 1998). In addition to its role during the transition to fermentation, the Gpr1–Gpa2 system is involved in the induction of pseudohyphal differentiation (growth as chain-like multicellular filaments) in response to nitrogen starvation in the presence of glucose (Lorenz and Heitman, 1997; Xue et al., 1998). However, the mechanism for sensing nitrogen limitation remains unclear: ammonium transporters could be involved or the strong transcriptional induction of GPR1 under nitrogen starvation conditions might be important in this respect.
G-protein coupled receptors Ste2, Ste3 and Gpr1
Three GPCRs are known in S. cerevisiae: Ste2, Ste3 and Gpr1. Although Ste2 and Ste3 are both coupled to Gpa1 and activate the mating pathway, the sequence similarity between them is limited. Except for pheromone receptors in Schizosaccharomyces pombe and Kluyveromyces lactis, Ste2 and Ste3 are largely unrelated in sequence to other GPCRs. Nevertheless, the yeast pheromone receptors can be functionally replaced by several mammalian GPCRs to the extent that the pheromone pathway can be activated by the corresponding mammalian agonists (Dohlman et al., 1991; Pausch, 1997; Brown et al., 2000).
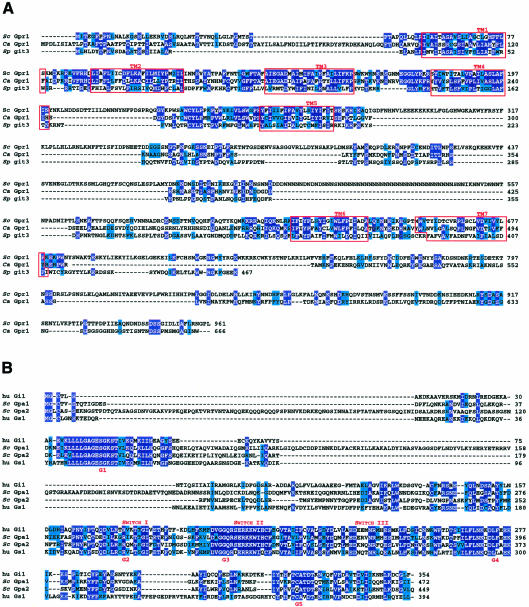
Gpr1 was identified both by two-hybrid screening using Gpa2 as bait (Xue et al., 1998; Kraakman et al., 1999) and by screening for mutants deficient in glucose-induced loss of heat resistance (Kraakman et al., 1999). As shown in Figure 2A, a Candida albicans gene called Gpr1 and the S. pombe gene git3 are related in sequence to S. cerevisiae Gpr1 gene. The similarity is not only apparent in the transmembrane (TM) domains but also in the connecting loops, most prominently in the second intracellular and extracellular loops.
Fig. 2. (A) Alignment of putative glucose-sensing G-protein coupled receptors: S. cerevisiae Gpr1, C. albicans Gpr1 and S pombe git3. Transmembrane regions (TM1–7) are indicated in red, residues identical for at least two aligned sequences are in white letters on a dark blue background, residues similar in at least two sequences are shaded in light blue. (B) Alignment of human Gi1 and Gs1, and S. cerevisiae Gpa1 and Gpa2. Identity between Gpa1 and Gi1 is 37%, between Gpa1 and Gs1 29%; identity between Gpa2 and Gi1 is 44% compared to 34% between Gpa2 and Gs1. Residues identical in at least three aligned sequences are in white letters on a dark blue background and similar residues in at least three sequences are shaded in light blue.
Schizosaccharomyces pombe contains a glucose-sensing GPCR system for activation of the cAMP pathway that is similar to that of S. cerevisiae. In S. pombe, glucose-induced cAMP signalling inhibits gluconeogenesis by triggering the repression of the gene encoding fructose-1,6-bisphosphatase (fbp1). This requires the Gα protein gpa2 and the putative glucose receptor git3 (Welton and Hoffman, 2000). In C. albicans the cAMP pathway is involved in the switch from yeast to hyphal growth, similar to the induction of pseudohyphal differentiation in S. cerevisiae (Whiteway, 2000). Although no experimental data yet link C. albicans Gpr1 to glucose sensing or to cAMP signalling, the high similarity of the region between TM1 and TM5 of these three GPCRs could be indicative of a new GPCR family involved in glucose sensing.
In contrast to the unique function of pheromones in activating the mating pathway in yeast, glucose has multiple functions. In addition to its role as a carbon and energy source, it exerts many regulatory effects and apparently serves as a primary signalling molecule in several signal transduction pathways, of which the glucose repression pathway, the Snf3–Rgt2 glucose signalling pathway and the cAMP pathway are the best characterized. Moreover, control of the cAMP pathway occurs at more than one level. In addition to activation of the Gpr1–Gpa2 GPCR system, glucose activation of the cAMP pathway also requires internalization and phosphorylation of the glucose (Beullens et al., 1988). It appears as if the latter is required to render adenylate cyclase responsive to GPCR stimulation, but it is still unclear at what point along the pathway it acts. Recently, it was shown that extracellular glucose could still trigger a cAMP signal in a yeast strain lacking detectable uptake of glucose (hxt1-7 hexose transporter deletion strain) provided an internal source of the sugar was available to sustain a low level of glucose phosphorylation (Rolland et al., 2000). This was achieved by pre-addition of a low level of maltose, which is taken up by the cells by a specific maltose transporter and is unable to activate the cAMP pathway itself. This ‘extracellular glucose-induced cAMP signal’ was strictly dependent on the presence of Gpr1. Although this does not formally exclude indirect sensing of extracellular glucose via other transmembrane proteins or extracellular proteins transmitting the signal to Gpr1, these experiments point strongly to Gpr1 as a receptor for extracellular glucose. Using the same experimental system, it was shown that Gpr1-dependent cAMP signalling is specific for d-glucose and sucrose; no other sugar or sugar analogue tested could trigger activation.
The apparent affinity of Gpr1 for its putative ligand, glucose, is very low: 20–30 mM glucose are necessary for half-maximal activation of Gpr1-dependent cAMP signalling in vivo. However, this low apparent affinity fits with the physiological context in which Gpr1-dependent glucose sensing is functional: yeast cells switch their metabolism completely from respiration to fermentation only at concentrations of at least 20 mM (Thevelein, 1991). Whereas other glucose-sensing mechanisms in yeast detect glucose concentrations in the µM range, Gpr1 has a specific role in detecting a high concentration of glucose. Recent data on the effect of cAMP signalling in extending life-span in yeast (defined as the period between its birth as a bud from a mother cell and the production of its own last daughter bud) are consistent with the very low affinity of Gpr1 for glucose. Life-span can be extended by limiting glucose availability; i.e. cells grown in the presence of 25 mM live longer than cells grown on 100 mM glucose. Likewise, deletion of GPR1 prolongs life-span in the presence of 100 mM glucose (Lin et al., 2000).
The heterotrimeric G-proteins Gpa1, Ste4, Ste18 and Gpa2
The GPA1 and GPA2 genes hybridize to probes that are based on the sequence of mammalian Gα proteins (Nakafuku et al., 1987). As illustrated in Figure 2B, both Gpa1 and Gpa2 share significant sequence similarity with mammalian Gi as well as Gs proteins, particularly in the regions involved in GTP hydrolysis. Although Gpa1 functions as an inhibitory Gα protein in the pheromone pathway, while all available evidence points to a stimulatory function for Gpa2 in cAMP signalling, both Gpa1 and Gpa2 are more closely related to mammalian Gi than to mammalian Gs. Both Gpa1 and Gpa2 have striking, unique sequence extensions: Gpa1 contains an internal extension (amino acids 100 to 190) and Gpa2 has an N-terminal non-homologous section (amino acids 15 to 95). The roles of these regions in the function or the regulation of Gpa1 or Gpa2 are not known.
The identification of Gpa2 as a new Gα protein raised the question as to which βγ dimer associates with it. The structural requirements for a Gβ subunit are seven WD-40 repeats, which form a propeller-like structure, preceded by a helical N-terminus. Smith et al. (1999) have classified WD proteins with similar binding partners based on the homology of predicted surface residues. In this study, only one genuine Gβ-like protein was identified in yeast, namely Ste4.
We have tested the possibility that, in addition to its role in pheromone signalling, Ste4 functions as the β subunit of Gpa2. However, Ste4 and Gpa2 do not interact with each other in a two-hybrid assay, neither deletion nor overexpression of STE4 affect cAMP signalling or PKA controlled phenotypes and, also, simultaneous overexpression of STE4 and STE18 does not have any effect on the cAMP pathway (L. Buainain, M. Versele and J.M. Thevelein, unpublished results). Lorenz and Heitman (1997) also could not find any evidence for the involvement of Ste4 or Ste18 in cAMP-dependent pseudohyphal growth. Moreover, STE4 and STE18 are haploid-specific genes, i.e. they are not expressed in diploid cells (Whiteway et al., 1989). Nevertheless, Gpa2-dependent glucose-induced cAMP signalling is similar in haploids and diploids, and pseudohyphal growth is confined to diploid cells.
Given the considerations above, if Gpa2 has a cognate β subunit, it is likely to be divergent with respect to the sequence and/or structure of the typical Gβ protein. Lorenz and Heitman (1997) have deleted eight genes encoding candidate β subunits and three genes coding for putative γ proteins, but none of these mutants were affected in pseudohyphal growth. Several residues in the Switch regions that have been shown to contact β subunits in other Gα proteins are conserved in Gpa2 (Wall et al., 1998; see Figure 2B). In analogy to the G203A Gi or G226A Gs mutants that are catalytically active but defective in dissociation from the βγ subunit (Berghuis et al., 1996), we and others have mutated Gpa2 Gly229 to Ala. The gpa2gly299ala mutant is defective both in glucose-induced cAMP signalling and in pseudohyphal development, which may either reflect a catalytically inactive protein (unlike any other analogous mutant Gα protein tested) or which may be caused by a cognate βγ subunit that constitutively binds Gpa2gly299ala (L. Buainain, M. Versele and J.M. Thevelein, unpublished results; Lorenz and Heitman, 1997).
On the other hand, the N-terminus of Gα proteins also interacts with the β subunit (Wall et al., 1998). The N-terminal sequence extension in Gpa2 perhaps indicates that the putative Gβ protein for Gpa2 might be different from the classical Gβ protein. Also, for Cryptococcus neoformans GPA1—a Gα protein involved in regulating virulence via cAMP signalling and sharing the N-terminal extension with Gpa2—no Gβ subunit has been reported yet. Gα proteins in other organisms may also lack any, or at least a classical, Gβ subunit. In S. pombe, there is also only one Gβ protein (that lacks the typical helical N-terminus but which is otherwise closely related to Gβ proteins) and one Gγ protein while there are two Gα proteins. While the git5 Gβ protein interacts with the gpa2 Gα protein for glucose activation of the cAMP pathway in S. pombe, the gpa1 Gα protein acts in the pheromone pathway and possibly lacks a Gβ protein, as does Gpa2 in S. cerevisiae (Landry and Hoffman, 2001). The Caenorhabditis elegans genome encodes only two Gβ proteins while there are 20 Gα proteins. Perhaps in this case the Gγ subunits provide additional selectivity, but there are only two Gγ homologues (and two RGS proteins containing a Gγ-like GGL domain) in the C. elegans genome. Even with all possible βγ combinations, this yields only eight different dimers, leaving another 12 Gα proteins without a unique, conventional βγ.
Several other Gpa2-binding proteins have been isolated in two-hybrid screens but none of these exhibits any sequence similarity to classical Gβ proteins (Lorenz et al., 2000; L. Kraakman and J.M. Thevelein, unpublished results). One of these, Ime2, is a protein kinase that is essential for meiosis. Interestingly, in the presence of glucose or nitrogen, Gpa2 inhibits entry into meiosis through direct inhibition of Ime2, independent of cAMP (Donzeau and Bandlow, 1999). The roles of two other Gpa2-binding proteins (encoded by YAL056 and YGL121) have not been established yet. It remains to be investigated whether these novel genes actually encode alternative Gβ subunits with a role in the control of cAMP signalling or whether they encode Gpa2 effectors.
The RGS proteins Sst2 and Rgs2
The GTP-hydrolysis rate of Gα proteins can be accelerated by RGS proteins. RGS protein family members share the so-called RGS domain, which is sufficient for in vitro GTPase stimulating activity, but have divergent N- and C-termini. The Sst2 and Rgs2 RGS proteins are specific for Gpa1 and Gpa2, respectively, and neither can complement the deficiency of the other (Versele et al., 1999). Sst2 seems to exert its effect as a negative regulator of Gpa1 by participating in a feedback-inhibition system involving the downstream MAP-kinases Fus3 and Kss1. Pheromone-induced MAP-kinase dependent phosphorylation of Sst2 stabilizes the protein, thereby stimulating the GTPase activity of Gpa1 and downregulating the pheromone pathway (Garrison et al., 1999). In addition SST2 is transcriptionally induced upon pheromone stimulation, which results in increased Sst2 protein levels (Dohlman et al., 1996).
In contrast to Sst2, the precise physiological role of Rgs2 is not well understood. Overexpression of RGS2 attenuates glucose-induced cAMP signalling and deletion of RGS2 results in a higher glucose-induced cAMP signal and in high-PKA phenotypes (Versele et al., 1999). The effects of Rgs2 are more prominent in stationary phase and in low-PKA mutants, suggesting that Rgs2-downregulation of Gpa2 is not part of the negative feedback exerted by PKA on cAMP signalling, but instead confers an additional level of regulation, possibly only under specific conditions.
The yeast GPCR systems as screening tools
The importance of GPCR systems is illustrated by the fact that 30% of all clinically prescribed drugs function as GPCR agonists or antagonists (Stadel et al., 1997). Five to six hundred genes encoding GPCRs are present in the human genome but for many of these no ligand has been found so far. These so-called orphan receptors are among the most promising targets for the development of novel drugs.
The similarity between pheromone signalling in yeast and GPCR-mediated signalling in humans has allowed the development of both chemical high-throughput screening to isolate GPCR agonists or antagonists (Pausch, 1997) and genetic screens to isolate new mammalian gene products involved in GPCR functioning (Cismowski et al., 1999). For these purposes, the yeast pheromone signalling pathway has been coupled to a mammalian receptor (e.g. via a hybrid Gpa1–Gα protein) and the downstream MAP kinase cascade has been linked to a reporter system that allows high-throughput screening. A chemical drug library or a human cDNA library can then be screened for inhibitors or activators of this reporter system.
The recent identification and characterization of Gpr1, Gpa2 and Rgs2 as a new yeast GPCR module opens the possibility to use this system in such applications as well. The different components of the glucose-sensing GPCR system may allow the isolation of different heterologous regulators of GPCR signalling, as illustrated by the differential complementation of sst2 and rgs2 mutants by mammalian RGS proteins. Mammalian RGS4 and RGS16 complement both mutants, whereas RGS5 only complements the rgs2 mutant which made possible the first demonstration of in vivo RGS activity for RGS5 (Versele et al., 1999).
Furthermore, the intrinsic phenotypic effects of glucose-induced cAMP signalling can be used instead of a reporter system for high-throughput screens. The rapid loss of stress resistance upon glucose-activation of the Gpr1/Gpa2 system has already been exploited successfully as a selection method in yeast: mutants in CYR1 (encoding adenylate cyclase) and GPR1 were isolated by heat shock treatment shortly after the initiation of fermentation, and RGS2 was isolated as a high dosage-suppressor of the loss of heat resistance after glucose-addition (Kraakman et al., 1999; Versele et al., 1999; Van Dijck et al., 2000). In a similar approach, mammalian cDNA libraries can be screened for GPCR components or regulators of GPCR systems, and drug libraries can be screened for agonists or antagonists of a heterologous receptor that is coupled via a hybrid Gpa2–Gα protein to the downstream cascade. Another possibility is to use the synthetic lethality of the inactivation of Gpa2 or Gpr1 and either Ras2 or the Sch9 protein kinase as a basis for selection (Xue et al., 1998; Kraakman et al., 1999).
Perspectives
Glucose and pheromone are possibly the two single molecules with the most dramatic impact on yeast proliferation and physiology. Saccharomyces cerevisiae uses two distinct GPCR systems for their detection. As opposed to pheromone sensing, detection of a common nutrient such as glucose by a GPCR system is rather unexpected. One of the most typical features of GPCRs, the exceptionally high ligand-affinity, is entirely absent. Gpr1 is apparently activated only by the metabolizable sugars glucose and sucrose and, at least in the case of glucose, only at the high concentrations that are capable of sustaining fermentation. Hence, the receptor appears to be sensing the presence of compounds with a specific nutritive value. This sets it apart from gustatory and olfactory GPCRs, which do not serve to detect the mere presence of nutrients (and other compounds) but rather their taste and flavour properties.
The identification in other yeasts of components homologous to the glucose-sensing system, points to the probable existence of a novel family of GPCR systems involved in nutrient sensing. This could be a singularity of yeasts, but research on the yeast pheromone pathway has already amply demonstrated that so-called yeast peculiarities often turn out to be of more general significance. If nutrient sensing GPCR systems are a wide-spread phenomenon in eukaryotes, in particular if the GPCRs also act on pivotal effectors like adenylate cyclase, it might have a dramatic impact on the field of signal transduction in that it would add a novel layer of regulation.
An intriguing aspect of the glucose-sensing system is the apparent lack of a classical Gβ subunit to interact with Gpa2. This might lead to the identification of novel types of Gβ subunits or possibly even to the discovery that members of a subgroup of Gα proteins function as monomers. In addition, the new GPCR system offers novel possibilities for the development of genetic and chemical high-throughput screens in yeast.

Mathias Versele, Katleen Lemaire & Johan M. Thevelein


Acknowledgments
Acknowledgements
Original research in our laboratory has been supported by a fellowship from the Institute for Scientific and Technological Research (IWT) to M.V. and by grants from the Flemish Interuniversity Institute of Biotechnology (VIB/PRJ2), the Fund for Scientific Research-Flanders, the Research Fund of the Katholieke Universiteit Leuven (Concerted Research Actions) and Interuniversity Attraction Poles Network P4/30.
References
- Berghuis A.M., Lee, E., Raw, A.S., Gilman, A.G. and Sprang, S.R. (1996) Structure of the GDP-Pi complex of Gly203→Ala giα1: a mimic of the ternary product complex of gα-catalyzed GTP hydrolysis. Structure, 4, 1277–1290. [DOI] [PubMed] [Google Scholar]
- Beullens M., Mbonyi, K., Geerts, L., Gladines, D., Detremerie, K., Jans, A.W.H. and Thevelein, J.M. (1988) Studies on the mechanism of the glucose-induced cAMP signal in glycolysis and glucose repression mutants of the yeast Saccharomyces cerevisiae. Eur. J. Biochem., 172, 227–231. [DOI] [PubMed] [Google Scholar]
- Brown A.J. et al. (2000) Functional coupling of mammalian receptors to the yeast mating pathway using novel yeast/mammalian G protein α-subunit chimeras. Yeast, 16, 11–22. [DOI] [PubMed] [Google Scholar]
- Cismowski M.J., Takesono, A., Ma, C., Lizano, J.S., Xie, X., Fuernkranz, H., Lanier, S.M. and Duzic, E. (1999) Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nature Biotechnol., 17, 878–883. [DOI] [PubMed] [Google Scholar]
- Colombo S. et al. (1998) Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J., 17, 3326–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H.G., Thorner, J., Caron, M.G. and Lefkowitz, R.J. (1991) Model systems for the study of seven-transmembrane-segment receptors. Annu. Rev. Biochem., 60, 653–688. [DOI] [PubMed] [Google Scholar]
- Dohlman H.G., Song, J.P., Ma, D.R., Courchesne, W.E. and Thorner, J. (1996) Sst2, a negative regulator of pheromone signaling in the yeast Saccharomyces cerevisiae: Expression, localization, and genetic interaction and physical association with Gpa1 (the G-protein α subunit). Mol. Cell. Biol., 16, 5194–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzeau M. and Bandlow, W. (1999) The yeast trimeric guanine nucleotide-binding protein α subunit, Gpa2p, controls the meiosis-specific kinase Ime2p activity in response to nutrients. Mol. Cell. Biol., 19, 6110–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison T.R., Zhang, Y., Pausch, M., Apanovitch, D., Aebersold, R. and Dohlman, H.G. (1999) Feedback phosphorylation of an RGS protein by MAP kinase in yeast. J. Biol. Chem., 274, 36387–36391. [DOI] [PubMed] [Google Scholar]
- Kraakman L., Lemaire, K., Ma, P., Teunissen, A.W.R.H., Donaton, M.C.V., Van Dijck, P., Winderickx, J., de Winde, J.H. and Thevelein, J.M. (1999) A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol. Microbiol., 32, 1002–1012. [DOI] [PubMed] [Google Scholar]
- Landry S. and Hoffman, C.S. (2001) The git5 Gβ and git11 Gγ form an atypical dimer acting in the fission yeast glucose/cAMP pathway. Genetics, 157, 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E., Dignard, D., Harcus, D., Thomas, D.Y. and Whiteway, M. (1992) The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein βγ subunits to downstream signalling components. EMBO J., 11, 4815–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.J., Defossez, P.A. and Guarente, L. (2000) Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science, 289, 2126–2128. [DOI] [PubMed] [Google Scholar]
- Lorenz M.C. and Heitman, J. (1997) Yeast pseudohyphal growth is regulated by GPA2, a G protein α homolog. EMBO J., 16, 7008–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M.C., Pan, X., Harashima, T., Cardenas, M.E., Xue, Y., Hirsch, J.P. and Heitman, J. (2000) The G protein-coupled receptor gpr1 is a nutrient sensor that regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Genetics, 154, 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakafuku M., Itoh, H., Nakamura, S. and Kaziro, Y. (1987) Occurrence in Saccharomyces cerevisiae of a gene homologous to the cDNA coding for the α subunit of mammalian G proteins. Proc. Natl Acad. Sci. USA, 84, 2140–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pausch M.H. (1997) G-protein-coupled receptors in Saccharomyces cerevisiae: high-throughput screening assays for drug discovery. Trends Biotechnol., 15, 487–494. [DOI] [PubMed] [Google Scholar]
- Rolland F., de Winde, J.H., Lemaire, K., Ma, P., Boles, E., Thevelein, J.M. and Winderickx, J. (2000) Glucose-induced cAMP signalling in yeast requires both a G-protein coupled receptor system for extracellular glucose detection and a separable hexose kinase dependent sensing process. Mol. Microbiol., 38, 348–358. [DOI] [PubMed] [Google Scholar]
- Smith T.F., Gaitatzes, C., Saxena, K. and Neer, E.J. (1999) The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci., 24, 181–185. [DOI] [PubMed] [Google Scholar]
- Stadel J.M., Wilson, S. and Bergsma, D.J. (1997) Orphan G protein-coupled receptors: a neglected opportunity for pioneer drug discovery. Trends Pharmacol. Sci., 18, 430–437. [DOI] [PubMed] [Google Scholar]
- Thevelein J.M. (1991) Fermentable sugars and intracellular acidification as specific activators of the Ras-adenylate cyclase signalling pathway in yeast: the relationship to nutrient-induced cell cycle control. Mol. Microbiol., 5, 1301–1307. [DOI] [PubMed] [Google Scholar]
- Van Dijck P., Ma, P., Versele, M., Gorwa, M.F., Colombo, S., Lemaire, K., Bossi, D., Loiez, A. and Thevelein, J.M. (2000) A baker’s yeast mutant (fil1) with a specific, partially inactivating mutation in adenylate cyclase maintains a high stress resistance during active fermentation and growth. J. Mol. Microbiol. Biotechnol., 2, 521–530. [PubMed] [Google Scholar]
- Versele M., de Winde, J.H. and Thevelein, J.M. (1999) A novel regulator of G-protein signalling in yeast, Rgs2, downregulates glucose-activation of the cAMP pathway through direct inhibition of Gpa2. EMBO J., 18, 5577–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M.A., Posner, B.A. and Sprang, S.R. (1998) Structural basis of activity and subunit recognition in G protein heterotrimers. Structure, 6, 1169–1183. [DOI] [PubMed] [Google Scholar]
- Welton R.M. and Hoffman, C.S. (2000) Glucose monitoring in fission yeast via the gpa2 Gα, the git5 Gβ and the git3 putative glucose receptor. Genetics, 156, 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteway M. (2000) Transcriptional control of cell type and morphogenesis in Candida albicans. Curr. Opin. Microbiol., 3, 582–588. [DOI] [PubMed] [Google Scholar]
- Whiteway M., Hougan, L., Dignard, D., Thomas, D.Y., Bell, L., Saari, G.C., Grant, F.J., O’Hara, P. and MacKay, V.L. (1989) The STE4 and STE18 genes of yeast encode potential β and γ subunits of the mating factor receptor-coupled G protein. Cell, 56, 467–477. [DOI] [PubMed] [Google Scholar]
- Xue Y., Batlle, M. and Hirsch, J.P. (1998) GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p G subunit and functions in a Ras-independent pathway. EMBO J., 17, 1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]