Abstract
Naringin is a natural flavonoid with therapeutic properties found in citrus fruits and an active natural product from herbal plants. Naringin has become a focus of attention in recent years because of its ability to actively participate in the body's immune response and maintain the integrity of the immune barrier. This review aims to elucidate the mechanism of action and therapeutic efficacy of naringin in various inflammatory diseases and to provide a valuable reference for further research in this field. The review provided the chemical structure, bioavailability, pharmacological properties, and pharmacokinetics of naringin and found that naringin has good therapeutic potential for inflammatory diseases, exerting anti-inflammatory, anti-apoptotic, anti-oxidative stress, anti-ulcerative and detoxifying effects in the disease. Moreover, we found that the great advantage of naringin treatment is that it is safe and can even alleviate the toxic side effects associated with some of the other drugs, which may become a highlight of naringin research. Naringin, an active natural product, plays a significant role in systemic diseases' anti-inflammatory and antioxidant regulation through various signaling pathways and molecular mechanisms.
Keywords: Naringin, Inflammatory disease, Anti-Inflammatory, Antioxidant
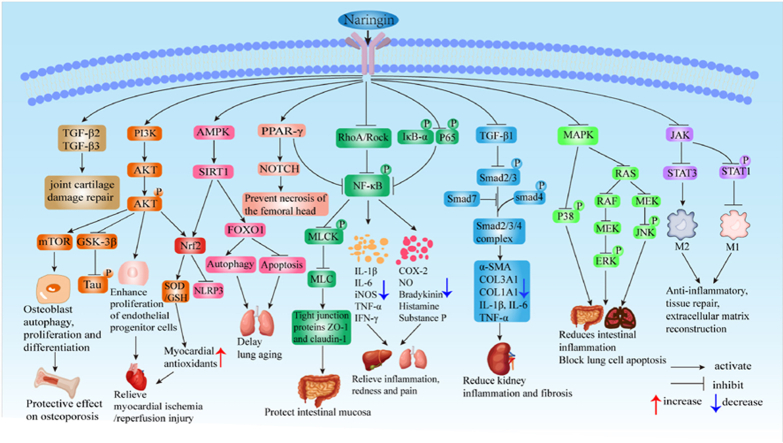
Graphical abstract
1. Introduction
Naringin is a natural flavonoid in grapes, citrus fruits, and Chinese herbal medicine. It is a flavanone glycoside formed by the flavanone naringenin and the disaccharide neohesperidose, mainly derived from yellowish dihydro flavonoids extracted from the dried peel of the plant Rutaceae and grapefruit [1,2]. Isolation of naringin from plants or fruits involves four steps: extraction, identification, isolation, and purification. It is based on microwave-assisted extraction (MAE), high-performance liquid chromatography-photodiode array detector-mass spectrometry (HPLC-DAD-MS/MS), and high-speed countercurrent chromatography (HSCCC) highly effective strategies [3]. Determination of naringin is most commonly performed by high-performance liquid chromatography (HPLC) and ultra-performance liquid chromatography (UHPLC) combined with a mass spectrometer (MS) or photodiode array (PAD) detectors [4]. Studies have found that naringin plays an essential role in promoting anti-inflammatory [5], anti-apoptotic [6], anti-tumor [7,8], anti-ulcer [9], oxidative stress [10], antiviral [11] and promoting skeletal muscle fiber remodeling [12,13]. Naringin has been approved as a potential antitussive and expectorant agent in clinical trials [14]. The powerful anti-inflammatory effect of naringin can treat and relieve a variety of inflammatory diseases in multiple organs of the body and airway inflammation [15], exhibit renal and neuroprotective effects [16,17], and therapeutic effects in the prevention and treatment of cardiovascular disease [18] and metabolic syndrome [19].
2. Materials and methodology
A search strategy was performed to extract the available literature from the PubMed database. The search terms “naringin," “bioavailability," “signaling pathways," and “Pharmacokinetics" combined with terms like inflammatory diseases such as neuroinflammatory diseases, cardiovascular inflammatory diseases, inflammatory bowel diseases, renal inflammation, skin inflammation, and joint inflammation were searched. Original research, including prospective and retrospective studies and review papers, was included and cross-referenced.
3. Results and discussion
3.1. Naringin chemical structure and bioavailability
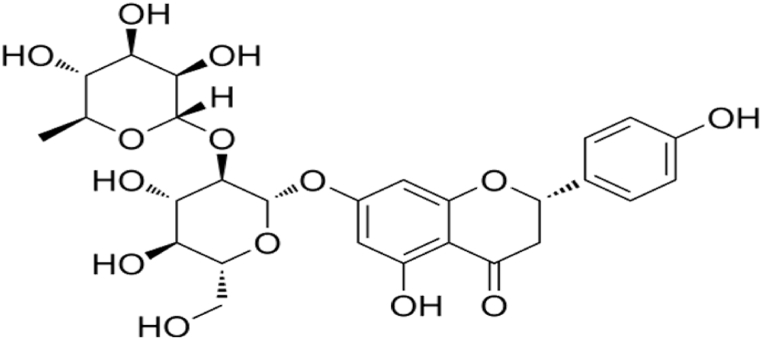
Naringin, chemically 4′,5,7-trihydroxyflavanone-7-rhamnoglucoside (Fig. 1), is weakly alkaline, pale yellow or white powder with the bitter taste of grapefruit juice [20]. Naringenin is the aglycone of naringin, and various biological enzymes can help eliminate the glycosides of naringin to produce naringenin, which is then converted to other metabolites [21,22]. Both naringenin and naringin have anti-inflammatory effects [23]. Naringin is a naringenin, an aglycone and neohesperidose flavanone glycoside bound to the –OH group at the carbon C-7, and it has a bitter taste [20].
Fig. 1.
Chemical structure of naringin.
Naringin is a natural product with abundant sources, a wide range of pharmacological effects, low toxicity, and few systemic adverse reactions when used as a therapeutic agent [24,25]. The oral bioavailability of naringin in humans has been reported to be only 5–9% [26]. The lower bioavailability may be closely related to the extensive metabolism of naringin, first-pass elimination, and the exocytosis of metabolites [27]. Naringin metabolism is a complex process catalyzed by various enzymes [21,22,28,29]. Phase II enzymes metabolize naringin in the intestine, and the resulting hydrophilic phase II metabolites are transported out of the cell into the intestinal lumen or bile by efflux transporter proteins. Some naringin can be transferred to the liver via enterohepatic circulation and conjugated into glucuronidated and sulfated metabolites that can be absorbed by organs and tissues [27]. There is substantial evidence that naringin has powerful biological activities, and relative safety does benefit human health. Therefore, to improve the bioavailability and pharmacological properties of naringin, it is currently proposed to encapsulate naringin within its structure and thus release it in a controlled manner by using various modern nanocarriers or liposomes such as preparations, including nanogels, phytocomplexes, lipid nanoparticles, polymers, inorganic nanoparticles, and demonstrated that naringin esterification could improve the physical and chemical properties of naringin and thus enhance its bioavailability [[30], [31], [32], [33]]. In vitro and in vivo toxicological evaluation of naringin-loaded nanocapsules and nanocapsules showed no signs of toxicity [34], and the carrier binding to naringin may exert a synergistic effect. The metal-organic framework (MOF) has been shown to encapsulate naringin as a nontoxic loading carrier that can improve and modulate immune system function using the synergy between naringin and MOF [24]. In addition, the naringin-loaded proposomal gel (PPG) improved the permeability of naringin and showed better wound closure [35]. Although naringin has low solubility in lipophilic systems, esterification can overcome it. Lee J et al. have demonstrated that naringenin esterification can improve the physical and chemical properties of naringin and thus enhance its bioavailability [36]. Naringin acetate includes an enzymatic reaction between naringin and an acyl donor, and studies in recent years have confirmed that naringin acetate can promote esterification reactions, resulting in high conversion rates in a short period [37].
3.2. Spectral properties of naringin
Citrus flavonoids are polyphenolic flavonoids that exist mainly as glycosides in citrus fruits, with naringin and hesperidin being the most abundant [38]. They can be converted to their glycosidic ligands, naringenin, and hesperidin. To date, there are only scattered fragments of spectroscopic literature on flavonoids. The main reason is the difficulty in obtaining and isolating individual flavonoids and their wide variety of species. Flavonoids are categorized into six groups: isoflavones, flavanones, flavonols, flavonoids, flavanols, and anthocyanins [39]. Naringin, naringenin, hesperetin, and hesperidin are included in the flavanones.
Flavonoids have a similar backbone, with a core structure consisting of an oxygen-containing heterocyclic ring of 15 carbon atoms in a C6–C3–C6 skeleton, represented by rings A, C, and B, respectively [39]. The various flavonoids differ mainly in the different substitution positions of the three groups (hydroxyl, methoxy, and glycosyl). Spectroscopic techniques are an excellent way to identify and compare the structures of various flavonoids.
Surface-enhanced Raman spectroscopy (SERS) has the characteristics of fast analysis, high sensitivity, and non-destructiveness. SERS showed that most citrus flavonoids displayed similar spectra below 1000 cm−1 [40]. The characteristic peaks were mainly at 1100-1500 cm−1 (assigned to different O–H bond) and 1550-1650 cm−1 (assigned to C O bond) [40]. Among them, the band at 1650-1700 cm−1 (assigned to C(H)–C(H) bond) can distinguish flavanones from other analogs [40]. For flavanone analogs, the band at 1221 cm−1 belonging to v (C–H) of CH3 can be used to distinguish the hesperetin analogs (hesperidin, hesperetin) from the naringenin analogs (naringin, naringenin) [40]. However, it is challenging to determine naringin from naringenin by using Sers alone, and SERS is now primarily used in combination with thin-layer liquid chromatography for further differentiation.
In addition, naringin and naringenin have slightly different UV absorption properties. The absorption spectra of flavonoids usually consist of two main bands, band I (300–380 nm) and band II (240–295 nm). The band II (283 nm) of naringin was shifted downward by 5 nm compared to naringenin (288 nm), while the band I of naringin and naringenin were relatively unchanged and almost identical [41].
In addition, the typical bands for naringenin, such as carbonyl stretching at around 1660 cm−1 for both IR and Raman spectra or vibration of C–C in aromatic ring around 1618 cm−1 and 1019 cm−1 for IR and at about 1613 cm−1 and 1104 cm−1 for Raman spectra are present [39]. Thus, the different positions of the three substituents on flavonoids cause differences in spectral features, and joint evaluation of multiple spectroscopic techniques can help to distinguish flavonoids better.
3.3. Pharmacokinetics of naringin
Interestingly, there are significant sex-related and species-specific differences in the pharmacokinetics of naringin and naringenin [42]. In trials combining non-clinical and clinical data, the pharmacokinetics and metabolism of naringin after oral and intravenous administration in rats, dogs, and humans were evaluated. It was found that the pharmacokinetic process of naringin was prolonged in humans after oral administration of naringin compared to those in rats and dogs [43], a difference that is thought to be closely related to biochemical processes, blood flow rate, and volume or surface area of the species [43]. After a single oral administration of naringin in a constructed rat animal model, naringin was widely distributed in multiple organs, and the concentration of naringin in tissues was much higher than in plasma, particularly in the trachea and lungs [ [44], [45]]. In clinical studies, plasma naringin concentrations increased to a maximum at approximately 2 h (Tmax, 2.09 ± 1.15 h) and decreased to 50 % of Cmax at about 3 h (t 1/2, 2.69 ± 1.77 h) [43].
3.4. Molecular mechanism of naringin reducing drug toxicity
Naringin has potential hepatoprotective effects on acetaminophen (APAP)-induced injury. The major cellular processes of APAP-induced hepatocytotoxicity include increased oxidative stress and mitochondrial dysfunction [46,47]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor in oxidative stress that regulates cellular defenses against toxicity and oxidative damage by expressing genes for oxidative stress and drug detoxification, and Nrf2 can also participate in metabolic reprogramming, immune response, autophagy regulation, protein balance, inflammation regulation, and mitochondrial physiology regulation [[48], [49], [50]]. In an in vitro model established by primary rat hepatocytes and HepG2 cells, naringin could activate the AMPK/Nrf2 pathway, reducing in vitro oxidative stress by promoting AMPK phosphorylation and upregulating Nrf2 expression [51]. At the same time, this process can promote mitochondrial fusion by increasing the expression of fusion proteins (i.e., Mfn1, Opa1), thereby reducing APAP-induced hepatocellular and mitochondrial damage [51,52]. Similarly, in APAP-induced mouse models of acute hepatotoxicity, naringin mediates Nrf2 activation by upregulating cation transport regulator-like protein 2 (CHAC2), alleviating acetaminophen-induced acute liver injury [53]. In most cases, Nrf2 activation plays a protective role in maintaining human health, and naringin can activate Nrf2 signaling to protect human health. Therefore, naringin plays a role in treating APAP-induced liver injury in mice, reducing the expression of liver injury markers in a dose-dependent manner, with potential mechanisms including antioxidant, rescue glucose metabolism, amino acid regulation, and purine metabolism [54].
Cyclophosphamide (CYCP) is a synthetic alkylated anti-tumor drug that destroys cancer and non-cancer cells, leading to cancer regression and multi-organ toxicity. Naringin can prevent cyclophosphamide-induced hepatotoxicity in rats by attenuating oxidative stress, fibrosis, and inflammation by the molecular mechanism that naringin can attenuate CYCP-induced upregulation of hepatic chemokine ligand 2 (CCL2), interferon (IFN) α1, interleukin (IL) -1β, and transforming growth factor (TGF) β1, reversing CYCP-induced reduction in hepatic glutathione (GSH) levels and catalase (CAT) activity [55]. Naringin exerts its anti-inflammatory, antioxidant, and apoptotic activities by increasing tumor necrosis factor (TNF)-α and IL-17 levels and reducing hepatic p53 and caspase-3 expression, and also has a specific mitigating effect on diclofenac-induced hepatotoxicity in male Wistar rats [56].
Methotrexate (MTX) is a commonly used chemotherapy drug that exerts adverse toxic effects on both kidney and germ cells. Exposure to MTX increases nitric oxide (NO) production, depleting CAT, superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GPx), and reduced GSH in testicular tissue [57]. Naringin can significantly counteract the above effects of MTX, suggesting that naringin may be a potential candidate for the toxic effects of MTX on reproduction. Similarly, naringin has a nephroprotective effect in methotrexate-induced nephrotoxicity in male rats, which involves significant increases in antioxidant enzymes and GSH levels. However, the nephroprotective molecular mechanism of naringin still needs to be further studied [58].
Cisplatin (CP) and aminoglycosides are effective anti-tumors or bactericidal drugs, and their application may cause hair cell (HCs) death, so there is a high probability of ototoxicity. In zebrafish animal models, naringin can play antioxidant, anti-apoptotic, proliferative, and HCs regeneration roles by downregulating the expression of genes related to apoptosis (P53 or Bcl-2, Bax, and Caspase-3) and pyroptosis (caspb and caspa or Caspase-1 and NLRP3), thereby achieving anti-ototoxic potential in vivo and in vitro [59].
Doxorubicin (DOX) is an anti-tumor antibiotic, and toxic side effects on the heart can lead to cardiomyocyte damage and irreversible heart failure, limiting its clinical application. In a constructed DOX-treated mouse model, naringin could inhibit the effects of DOX on apoptosis, inflammation, and oxidative stress in vitro experiments by promoting the expression of ECHS1, which is an essential reason for protecting mouse hearts from damage [60].
Oxaliplatin (OXL), a chemotherapy drug used for metastatic and other types of cancer, causes peripheral neuropathy with dose-limiting side effects. A study has revealed that naringin can reduce oxidative stress, inflammation, and apoptosis in OXL-induced sciatic nerve tissue. It significantly attenuates peripheral neuropathy and can be a novel protective agent in preventing OXL-induced peripheral neuropathy [61].
Naringin reduces the toxic effects of various clinical drugs mainly by reducing oxidative stress, inflammation, apoptosis, and pyroptosis, promoting cell proliferation and regeneration, and balancing the metabolism of nutrients (glucose and proteins), suggesting that naringin combined with other drugs may be a better clinical approach in treating diseases.
3.5. Inflammation and naringin
Inflammation is a protective response to environmental exposure, infection, tissue damage, and autoimmune induction, mainly manifested as redness, swelling, heat, pain, immune cell infiltration, and dysfunction [62]. However, severe and uncontrolled persistent inflammation is a pathological process with local or systemic tissue involvement that leads to inflammatory disease [63]. Inflammatory disorders share common physiologic and pathological features and are caused by excessive, abnormal, and sustained immune responses in immune cells and inflammatory responses in stromal cells [64,65]. Thus, early control of the development of abnormal immune reactions is a promising strategy for treating inflammatory diseases.
Peripherally induced inflammation promotes transient activation of TNF-α-mediated primer neural stem cells (NSCs), which can enhance the systemic inflammation alarm of adult NSCs through TNF-α receptor signaling and drive inflammation [66]. Accurate regulation of homeostasis in the inflammatory process is significant for treating inflammation-related diseases. Naringin is a natural anti-inflammatory antioxidant that actively participates in the body's immune response and maintains the integrity of the immune barrier. It can enhance autophagy flux by activating the AMPK/SIRT1 pathway, protecting human nuclear myelocytes from TNF-α-induced inflammation, oxidative stress, and loss of cellular homeostasis [67]. It is worth noting that inflammation and oxidative stress are two-way processes. Thus, limiting oxidative stress is also an effective way to control inflammatory diseases.
3.6. Naringin against neuroinflammatory diseases
Neuroinflammation is primarily caused by an immune response and long-term activation of the brain's glial cells (astrocytes and microglia). Usually, it is a crucial process in the pathogenesis of neurodegenerative diseases (NDDs) [64,68]. NDDs mainly include cerebral ischemia, brain injury, Alzheimer's disease (AD), and Parkinson's disease (PD). The pathophysiology of neurodegeneration is complex, and effective treatments for this disease are lacking. Thus, naringin extracted from medicinal plants or fruits shows promise in targeting multiple signaling pathways to treat different types of neuroinflammatory disorders.
3.6.1. Naringin and Alzheimer's disease
Alzheimer's disease (AD) is the most common neurodegenerative disease, with the clinical manifestation of an individual's cognitive dysfunction and progression to dementia. The leading cause of AD is the accumulation of hyperphosphorylated tau protein, Amyloid-β (Aβ) plaques, and neurofibrillary tangles (NFTs) [69]. So far, studies have shown that estrogen and mediated cell signaling pathways have some neuroprotective effects on AD in the absence of effective and safe cures and drugs [70]. Unfortunately, long-term estrogen therapy can cause unpredictable side effects such as uterine cancer.
As a phytoestrogen, naringin has few harmful side effects, and it can bind to estrogen receptors (ERs) to initiate estrogen-like biological effects [71]. Studies have found that naringin stimulates the expression of ER β, activates the PI3K/AKT signaling pathway, and inhibits the activity of glycogen synthase kinase (GSK)-3β and phosphorylation of Tau protein [72]. As an alternative to long-term estrogen therapy, the application of naringin is a valuable research direction for anti-AD treatment. Naringin has a strong iron chelating ability, which can reduce the formation of amyloid plaques. Therefore, it can be used for neuroprotection and prevention of AD [73]. Meanwhile, naringin can also improve memory deficits and cognitive dysfunction by regulating multiple metabolic pathways and exerting neuroprotective effects in animal models of AD [74]. As an example, expression of the brain-derived neurotrophic factor (BDNF)/cAMP response element binding protein (CREB)/tropomyosin receptor kinase B (TrkB) signaling pathway plays a crucial role in improving learning and memory [75]. Aβ inhibits the expression of TrkB and CREB in the hippocampus. Still, naringin can enhance the presentation of BDNF, TrkB, and CREB in the long term and attenuate the activated cyclooxygenase-2 under inflammatory conditions, thereby compensating for the learning and memory deficits in the rat model of AD-like behavior [76]. In addition, naringin exerts therapeutic benefits in Aβ-induced rat animal models through the renin-angiotensin system. The mechanism of action is that naringin improves not only Aβ-induced cholinergic dysfunction but also reverses Aβ-induced apoptosis, maintains increased levels of mitochondrial calcium uniporter, and decreases the level of hemeoxygenase-1 in all brain regions, thereby countering amyloid β-induced brain cognitive deficits and mitochondrial toxicity in rats [77].
Furthermore, naringin can also reduce the expression of phosphorylated p-P38/P38 [78], one of the main proteins in the mitogen-activated protein kinase (MAPK) family, p38 MAPK, together with c-Jun N-terminal kinase (JNK), is the main pathway by which MAPK regulates cellular physiological responses [79]. When p38 MAPK is activated, it forms a stress MAPK signaling pathway, often triggered by environmental stress and cytokines to induce inflammation and accelerate apoptosis [80]. However, whether naringin exerts neuroprotective effects via the P38 MAPK pathway needs further verification.
At present, naringin has been shown to reduce the inflammatory response, oxidative stress, and endoplasmic reticulum stress by inhibiting the TLR4/NF-κB signaling pathway, thereby improving cognitive dysfunction and hippocampal histopathological injury in rats [81]. In summary, naringin can exert neuroprotective effects through a variety of mechanisms, including the BDNF/CREB)/TrkB signaling pathway, TLR4/NF-κB signaling pathway, renin-angiotensin system, amyloid β metabolism, Tau protein hyperphosphorylation, acetylcholinergic system, oxidative stress, and apoptosis. Of note, naringin primarily enhances long-term memory in animal models of AD by regulating autophagy, oxidative stress, and tau expression [82].
Due to the bitter taste of naringin, it is necessary to find alternative derivatives while retaining their functional advantages. Naringin dihydrochalcone (NDC), a natural naringin derivative widely used in food, has a sweet taste and a powerful antioxidant effect. The study has found that NDC can weaken Aβ deposition in the brain of AD mice and reduce the activated microglia and astrocytes around the plaque, inhibiting neuroinflammation [83].
3.6.2. Naringin and Parkinson's disease
Parkinson's disease (PD) is also a chronic neurodegenerative disorder with degenerative death of dopaminergic neurons in the substantia nigra of the midbrain, followed by a decrease in dopamine levels in the striatum [84], characterized by bradykinesia, resting tremor, and muscle rigidity and postural gait disturbance. Despite extensive research, treatment options for the disease are limited and can only relieve symptoms rather than stop disease progression. Naringin is a flavonoid with neuroprotective activity, which can promote the production of glia-derived neurotrophic factor (GDNF) by dopaminergic neurons in the substantia nigra striatum, reduce the level of TNF-α in microglia, and then protect dopaminergic projection in the substantia nigra striatum in PD, playing an anti-inflammatory and preventive therapeutic role in brain dopaminergic neurons [85,86]. In line with this, Kim HD et al. further demonstrated that naringin does prevent dopaminergic neuronal degeneration but may not be sufficient to restore the substantia nigra striatal dopaminergic neuronal projection in a mouse model of PD [87]. In addition, there is growing evidence that the Nrf2-centric signaling pathway may be a critical pharmacological target for treating NDDs [88]. In animal models of rotenone-induced PD, naringin can exert neuroprotective activity on rotenone-induced toxicity in animals via the Nrf2-mediated pathway [89]. Therefore, it is speculated that naringin may be another option for future PD treatment management.
3.6.3. Naringin and cerebral ischemia-reperfusion injury
Recently, in an in vitro cell experiment, naringin was found to prevent PC12 cell damage in an oxygen-glucose deprivation/reoxygenation (OGD/R) model by targeting NF-κB1 and regulating the HIF-1α/AKT/mTOR signaling pathway, providing new ideas for the treatment of cerebral ischemia-reperfusion injury (IRI) [90]. In addition, naringin can also attenuate cerebral IRI injury by inhibiting peroxynitrite-mediated mitochondrial autophagy [91]. It is worth noting that prolonged total cerebral ischemia or severe ischemia can cause cerebral infarction, while naringin can protect against cerebral infarction by inhibiting neuronal apoptosis and inflammation. For example, naringin could reduce apoptosis of rat hippocampal nerve cells and secretion of inflammatory factors such as TNF-α and IL-6 in rat animal models with cerebral infarction and further promote the expression of p-AKT protein in a concentration-dependent manner, thereby activating the PI3K/AKT pathway in neurons [92].
3.6.4. Naringin and depression
In addition, naringin can mediate neurogenesis in adults by activating CREB signaling to combat depression [93]. In line with this, studies have found that flavonoid extract naringin can be recovered from agricultural residues with anxiolytic and antidepressant-like effects [94]. Therefore, naringin may have an excellent therapeutic potential for anxiety and depression.
It has been found that the leptin-Janus kinase (JAK)/signal transducer and activator of transcription (STAT) 3 signaling pathway is activated in the microglia of sleep-deprived patients. Naringin can promote microglial polarization to M2-type macrophages by inhibiting phosphorylation of the JAK/STAT3 signaling pathway, thereby promoting anti-inflammatory, tissue repair, extracellular matrix reconstruction, and exerting neuroprotective effects [95].
3.7. Naringin against cardiovascular inflammatory diseases
Cardiovascular disease (CVD) is a class of diseases of the heart or blood vessels that persist for a long time and harm human health. Hyperglycemia, hyperlipidemia, hypertension, obesity, heavy alcohol consumption, lack of exercise, smoking, inflammation, and clonal hematopoiesis are important risk factors for CVD [96,97]. These risk factors help initiate mechanisms such as arterial wall inflammation and oxidation, leading to the formation of fatty fibrous lesions over time [96]. Therefore, atherosclerosis is the pathological basis for the development of CVD.
3.7.1. Naringin and atherosclerosis
Atherosclerosis (AS) is a chronic inflammatory vascular disease driven by risk factors, lipid metabolism disorders as the pathological basis of AS, early arterial lesions starting from the intima, initial deposition of lipoproteins in the vascular wall, endothelium activation and expression of chemokines and adhesion molecules leading to leukocyte recruitment and infiltration into the subendothelium, and subsequent accumulation of cholesterol and low-density lipoprotein (LDL) into macrophages in the arterial wall [98,99], which eventually leads to thickening and hardening of the artery wall, narrowing of the lumen of the vessel, and even blockage. Naringin has been shown to lower cholesterol and triglyceride (TG), promote lipolysis, and regulate fatty acid β oxidation in a dose-dependent manner [[100], [101], [102], [103], [104]]. Various studies involved in vitro and in vivo models have shown that naringin has a positive effect on inhibiting AS progression. Hypercholesterolemia is one of the risk factors for AS, and naringin reverses vascular dysfunction and oxidative stress in hypercholesterolemia rats by reducing iNOS, LOX-1, and NADPH oxidase subunit expression. This process also involves a decrease in ROS production, increasing NO bioavailability and improving rat aortic endothelial function [105]. In addition, studies have revealed that the main potential pathway for naringin to alleviate is the gut microbiota-liver-cholesterol axis, which promotes the synthesis of bile acids in cholesterol by inhibiting the FXR/FGF15 pathway and upregulating CYP7A1 [98]. Moreover, the results also show that naringin promotes reverse cholesterol transport by downregulating PCSK9/IDOL [106]. In addition to the mechanisms described above, the potential protective effects of naringin against AS can reverse apoptosis and inflammation triggered by oxidative LDL (ox-LDL) by inhibiting the YAP pathway [6]. In human umbilical vein endothelial cells (HUVECs), naringin restores endothelial barrier integrity by preventing VE-cadherin disassembly and F-actin remodeling, as well as down-regulation of pro-inflammatory factors such as IL-1β, IL-6, and IL-18 [6]. In conclusion, naringin has an apparent regulatory effect on AS pathological lipid metabolism and inflammatory state, which may attenuate the onset of AS to a certain extent.
3.7.2. Naringin and diabetic cardiomyopathy
Diabetic cardiomyopathy (DCM) is the primary disease of people with diabetes and is characterized by diastolic dysfunction leading to heart failure and death. Unfortunately, even strict blood sugar control is not practical in preventing it. Naringin limits the increase in ROS production, reduces calpain activity, TNF-α, and IL-6 levels, and decreases NF-κB expression, thereby avoiding cardiomyopathy in type 2 diabetes (T2DM) mice [107].
3.7.3. Naringin and diabetic cardiac autonomic neuropathy
Diabetic cardiac autonomic neuropathy (DCAN) is one of the most common complications associated with diabetes. In cases of diabetes, P2Y14 is significantly overexpressed, releasing inflammatory factors (IL-1β) in large quantities, forming gap connections between nerve cells, which promote DCAN, which can lead to cardiac damage [108]. Naringin reduces the expression of P2Y14 receptors and inflammatory factors and restores the function of the antioxidant GPX4/NRF2 pathway, thereby effectively alleviating DCAN [108].
3.7.4. Naringin and cardiac hypertrophy
Cardiomyocytes exit the cell cycle and undergo terminal differentiation shortly after birth, so adult cardiac hypertrophy increases in the size of a single cardiomyocyte [109]. Hypertrophy is initially an adaptive response of cardiomyocytes to stimuli to maintain cardiac output during harmful stimuli, a process known as physiological hypertrophy. However, sustained stimulation in the later stages can cause myocardial decompensation. That is, pathological hypertrophy may lead to heart failure. Myocardial hypertrophy involves different signaling pathways related to regulation. ERK, a signaling molecule downstream of the MAPK pathway (also known as RAS-RAF-MEK-ERK), is a key player in the pathophysiology of cardiac hypertrophy and appears to play a beneficial role by preventing cell death and hypertrophy during chronic stress overload [110]. In addition, naringin improves hyper fructose-induced cardiac hypertrophy by regulating the AMPK-mTOR signaling axis to reduce cardiomyocyte hypertrophy [111].
Leptin is a product of the obesity gene and plays a role in the pro-inflammatory immune response, angiogenesis, cardiomyocyte hypertrophy, and lipolysis [112,113]. Chen J et al. demonstrated that in cardioblasts (H9c2 cells), leptin induces rat cardiomyocyte hypertrophy through p38/MAPK activation. In contrast, naringin protects heart cells from high glucose-induced damage by inhibiting the leptin-induced p38/MAPK pathway [114]. In subsequent studies, naringin can attenuate high glucose-induced leptin expression and mitigate high glucose-induced cardiomyocyte damage and inflammatory responses by inhibiting the JAK2/STAT3 pathway in H9c2 cardiomyocytes [115].
3.7.5. Naringin and myocardial ischemia-reperfusion injury
Ischemia-reperfusion injury is one of the significant risk factors associated with cardiovascular morbidity and mortality. Naringin can be used as an emerging therapeutic drug with the potential to treat ischemic diseases. Endothelial progenitor cells (EPCs) can be involved in neovascularization in vivo. Activation of the CXCL12/CXCR4 axis by the PI3K/AKT pathway enhances endothelial progenitor cell proliferation [116], exhibiting a positive role in treating myocardial IRI. Naringin can significantly promote the phosphorylation of Akt, so naringin alleviates myocardial IRI in rats by promoting apoptosis, oxidative stress, and autophagy inhibition mediated by the PI3K/Akt pathway [117]. In addition, naringin can also inhibit cardiomyocyte apoptosis, inflammatory response, and oxidative stress by reducing the expression of lysis caspase 3 protein, IL-23, IL-6, and TNF-α, SOD, which in turn has a particular alleviating effect on myocardial IRI in rats [118]. In a recent study, naringin can also significantly upregulate miR-126 expression, and miR-126 can bind to GSK-3β and downregulate its expression, thereby increasing β-catenin activity in cardiomyocytes to reduce myocardial IRI [119].
3.8. Naringin against inflammatory bowel diseases
Several recent studies have demonstrated that naringin can significantly improve inflammatory bowel diseases, including ulcerative colitis (UC) and sepsis-induced intestinal damage, by inhibiting inflammatory responses and regulating intestinal flora homeostasis [[120], [121], [122], [123]]. Among them, naringin inhibits the intestinal inflammatory response involving several pathways and signaling molecules.
3.8.1. Naringin and ulcerative colitis
Peroxisome proliferator-activated receptor γ (PPARγ) is an essential member of the nuclear receptor superfamily, which can inhibit the expression of pro-inflammatory genes and is considered a necessary regulator of inflammation [124]. Therefore, modulating PPARγ and PPARγ-related pathways has great promise for treating UC. As an example, in dextran sodium sulfate (DSS)-induced mouse UC animal models, naringin significantly alleviated DSS-induced disease activity index (DAI), colon length shortening, and colonic pathological damage, and correspondingly reduced tissue and serum secretion of inflammatory cytokines (TNF-α, IL-6, and IL-1β) [125]. On the one hand, the expression of PPAR-γ in DSS-induced colitis is significantly reduced, which may lead to an increase in the expression of the nuclear transcription factor NF-κB. However, naringin can activate DSS-induced PPARγ and subsequently inhibit NF-κB activation, reduce inflammatory cytokines, and inhibit inflammation [126]. Furthermore, the symptomatic process of naringin in colitis relief can be counteracted by the PPAR-γ inhibitor BADGE, suggesting that PPAR-γ may be the target of the therapeutic effect of naringin-induced colitis [126]. On the other hand, the MAPK pathway in DSS-induced mice is also significantly activated, and naringin significantly inhibits p38, ERK, and JNK phosphorylation levels, thereby inhibiting the MAPK signaling pathway and reducing NLRP3 inflammasome activation [9]. Therefore, naringin can inhibit NF-κB and MAPK signaling pathways, reduce the production of pro-inflammatory factors, and have a specific remission effect on DSS-induced UC in mice. Similarly, in a recent study, naringin was shown to mitigate TNBS-induced colitis in rats by reducing pro-inflammatory cytokines and improving antioxidant status [127]. In addition, naringin may lessen the severity of colitis by inhibiting pro-inflammatory mediators (GM-CSF/M-CSF, IL-6, and TNF-α) and the NF-κB/IL-6/STAT3 signaling pathway in colorectal tumor pathogenesis [128]. In conclusion, NG may be a compelling candidate for treating UC patients.
3.8.2. Naringin and sepsis-induced intestinal injury
Macrophages are an essential component of the innate immune system and contribute significantly to the inflammatory response. Once activated, they can polarize into two different phenotypes, classically activated macrophages (M1) and alternatively activated macrophages (M2), and the nuclear receptor PPARγ can drive macrophage phenotypic changes [129]. In animal models of intestinal injury in sepsis, naringin therapy significantly inhibited M1 macrophage polarization and stimulated M2 macrophage polarization by activating the PPARγ/miR-21 axis to inhibit STAT1 signaling [130], alleviating sepsis-induced intestinal injury. In addition, naringin can improve impaired intestinal permeability and inhibit the release of pro-inflammatory factors (TNF-α and IL-6), attenuate MLC phosphorylation and NF-κB activation through the RhoA/ROCK pathway in vivo and in vitro, increase the expression of tight junction proteins ZO-1 and claudin-1, protecting against sepsis-induced intestinal injury [131].
3.9. Naringin against inflammatory lung disease
The anti-inflammatory effect of naringin under lung injury is mediated by inhibition of NF-κB signaling, and decreased levels of anti-inflammatory cytokines (IL-6, TNF-α, IL-1 β) can be detected [124]. At the same time, naringin treatment also significantly reduced the expression levels of p-p38 and p-JNK proteins, inhibited the MAPK signaling pathway, and blocked apoptosis of lung cells [132]. In mouse animal models of acute lung injury, naringin can reduce the protein expression of NF-κB, STAT3, and COX-2 and reduce the expression level of pro-inflammatory mediators, upregulating anti-inflammatory mediator levels [133]. Naringin has been found to reduce airway inflammation and lung permeability by upregulating Aquaporin1 (AQP1) expression in mouse animal models of chronic obstructive pulmonary disease (COPD) [15]. Nrf2 is an important mediator to protect cells from oxidative stress and can downregulate the expression of the NLRP3 inflammasome for anti-inflammatory effects [134]. During lung aging, naringin can induce SIRT1 to activate the Nrf2/NQO1 pathway to inhibit oxidative stress and promote the sirt1-FOXO1 pathway to induce autophagy [135]. Thus, delaying cellular aging can prolong the life span of experimental animals.
Naringin may act as an antiasthmatic drug to relax tracheal smooth muscle in rats, mainly by opening Ca2+-activated K+ channels to relax tracheal smooth muscle, mediating plasma membrane hyperpolarization and reducing Ca2+ influx [136]. At the same time, naringin is a non-toxic bitter substance of plant origin that promotes the proliferation of cultured human airway epithelial cells and activates bitter receptors (TAS2Rs), thereby initiating the relaxation of airway smooth muscle cells (ASMCs) [137]. Therefore, as a novel bronchodilator, naringin has great potential in treating asthma.
3.10. Naringin against liver inflammation
3.10.1. Naringin and nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is a disorder of lipid metabolism caused by various factors other than alcohol and other definite liver damage, and excessive fructose intake leads to hepatic lipid accumulation through increased TG synthesis [138]. Risk factors for nonalcoholic liver disease include obesity, diabetes mellitus, insulin resistance, hyperlipidemia, nutrition, and genetic factors [139]. In addition, there is emerging evidence that gut bacteria are strongly associated with the development of NAFLD [140]. Currently, clinical guidelines recommend a weight loss target of 7–10% to improve the character of NAFLD [141]. The lipid-lowering effect of naringin can effectively reduce NALFD induced by a high-fat diet, attenuate body weight, and decrease liver lipid accumulation in mice [142]. Naringin can improve intestinal bacterial dysbiosis and liver resistance to oxidative stress and inflammation [143]. In a novel tissue engineering fatty liver model, naringin can downregulate CD36, acetyl-CoA carboxylase, and fatty acid synthetase, increase PPAR-α, thereby reducing fatty acid uptake and new fat production, and increase fatty acid oxidation to improve lipid metabolism disorders in the liver [144]. Qu Zhi Ke (the peel of Changshan pomelo containing naringin) significantly inhibits systemic and intrahepatic inflammation by inhibiting IL-1β, IL-6, IL-12, TNF-α, and IFN-γ, thereby inhibiting NF-κB signaling pathways in the liver driven by IL-1, TNF-α, and other inflammatory factors [145]. In addition to NF-κB signaling, Qu Zhi Ke can also inhibit MAPK signaling by inhibiting the phosphorylation of ERK and p38 [145]. Among them, P38 is a member of the MAPK subfamily, which can induce the secretion of pro-inflammatory factors (CXCL2, IL-1β, CXCL10, and IL-6) by M1 macrophages to promote the development of steatohepatitis [146]. Therefore, hepatoprotective and anti-inflammatory effects of naringin in NAFLD lie in inhibiting NF-κB and MAPK signaling. In line with this, naringin has been shown to reverse the upregulation of NF-κB and TNF-α expression in rat liver in fructose-induced rat NAFLD animal models and target activation of antioxidant mediator Nrf2/HO-1 pathways to block fructose-induced NAFLD progression in rats [147]. As NAFLD progresses, it may become a common chronic liver disease, and liver fibrosis develops. Liver fibrosis is mainly the activation of hepatic stellate cells, which in turn occurs excessive accumulation of extracellular matrix and collagen deposits, which can lead to the development of cirrhosis and even liver cancer [148,149]. Naringin has anti-fibrotic properties with few side effects and is also a promising source in treating liver fibrosis.
3.10.2. Naringin and diabetes-induced hepatitis
Naringin improves T2DM-induced steatohepatitis by inhibiting RAGE/NF-κB-mediated mitochondrial apoptosis [150]. However, in streptozotocin-induced rat models of type I diabetes, apoptosis of two pathways increases in diabetic states: protein expression of Fas/FasL/caspase-3 and an increase in Bax/Bcl-2 ratio and increased oxidative stress in the liver in diabetic rats shows increased levels of NF-κB, Cox-2, and IL-6. At the same time, NF-κB promotes iNOS gene expression and subsequent NO formation, increasing nitrosylation of proteins [151]. However, naringin can significantly eliminate these effects, highlighting its antioxidant, anti-nitrifying, and anti-inflammatory properties.
3.11. Naringin against renal inflammation
3.11.1. Naringin and renal interstitial fibrosis
Renal interstitial fibrosis is typical of all progressive kidney diseases. The presence of profibrotic factors and inflammation often accompanies the fibrotic process. TGF-β1 is considered a key mediator of tissue fibrosis, and inhibiting TGF-β subtype TGF-β1 or its downstream signaling pathway can significantly limit renal fibrosis [152]. Smad2 and Smad3 are the two major downstream regulators that promote TGF-β1-mediated tissue fibrosis, which can bind to Smad4 to form complexes that trigger the expression of fibrotic genes. In contrast, Smad7, as a negative feedback regulator of the TGF-β1/Smad pathway, prevents TGF-β1-mediated fibrosis [153]. In rat models and fibrotic cell models of renal interstitial fibrosis, naringin can significantly reduce gene expression levels of α-smooth muscle actin (α-SMA), collagen 1 (COL1A1), collagen 3 (COL3A1), IL-1β, IL-6, and TNF-α, thereby reducing renal inflammation and fibrosis [154]. Notably, naringin reduces the expression of Smad2/3 and Smad4 phosphorylation and antagonizes renal interstitial fibrosis by inhibiting the TGF-β/Smad pathway.
3.11.2. Naringin and diabetic nephropathy
In rat in vitro cultured mesangial cells, naringin can regulate the NLRP3-caspase-1-IL-1β/IL-18 signaling pathway to inhibit NLRP3 inflammasomes, reduce the expression of inflammatory factors and then improve diabetic nephropathy by exerting anti-inflammatory effects [155]. In addition, studies have found that naringin can prevent diabetic nephropathy in rats by blocking oxidative stress and mitochondrial dysfunction [156].
3.11.3. Naringin and renal ischemia-reperfusion injury
In one study, naringin was found to benefit renal protection from renal IRI in rats by downregulating microRNA-10a, caspase-3, and Bax and upregulating the expression of Bcl-2 in renal tissue [157]. In addition, in rat animal models of renal IRI, naringin can improve the expression of SOD and Nrf-2 after renal IRI injury, enhance antioxidant activity, and thus have a clear protective role against renal and distal myocardial injury [158].
3.12. Naringin against skin inflammation
The skin is the body's biological barrier against external environmental stimuli and stress, and inflammation or wounds occur when its physiological structure is destroyed. Skin wound injuries are common and can lead to severe complications if not treated properly. Naringin can accelerate wound healing by upregulating the expression of growth factors (VEGF-A, B, C, and VEGF-R3) [159]. In addition, inhibiting NF-κB and COX-2 by topical application of naringin hydrogels enhances the repair and regeneration of deep dermal wounds [160]. Naringin has potent anti-inflammatory and antioxidant activities, not only able to downregulate gene expression of the inflammatory mediator (TNF-α) and apoptosis (Bax) but also to increase levels of SOD and GSH [161]. The combination of naringin/sericin can downregulate pro-inflammatory cytokines (including IL-6, IL-12p40, IL-23, and TNF-α) to help human peripheral blood mononuclear cells (hPBMCs) in patients with psoriasis exert anti-inflammatory effects [162]. Chronic inflammation of skin disorders to a later stage may develop into skin fibrosis. Naringin may play a protective role against fibrosis in skin tissue in vivo by reducing collagen production and lowering levels of fibrosis-related genes [163]. Similarly, naringin inhibits the phosphorylation of Akt and Akt downstream proteins in fibroblasts, thereby inhibiting the development of proliferative scars [164].
3.13. Naringin and joint inflammation
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by persistent synovial hyperplasia and progressive erosion of articular cartilage. Potential mechanisms for the therapeutic effects of naringin in RA are mainly through the PI3K/Akt and MAPK/ERK signaling pathways that inhibit the production of inflammatory cytokines and matrix metalloproteinases (MMP), promoting apoptosis in RA fibroblast-like synoviocytes [165]. Similarly, naringin prevents cartilage destruction in osteoarthritis by inhibiting the NF-κB signaling pathway [166]. Furthermore, naringin can prevent steroid-induced avascular necrosis of the femoral head by upregulating PPARγ and activating the Notch signaling pathway [167]. In addition, naringin increases the expression of TGF-β2, TGF-β3, and Sox-9 in cartilage defects through the TGF-β/ALK5/Smad2/3 signaling pathway, thereby achieving good results in joint cartilage quality repair at the defect site [168], so naringin has good application potential in osteochondral tissue engineering.
3.14. Safety assessment of naringin
Safety assessment plays a vital role in researching and developing natural products. Wang Y et al. evaluated the potential toxicological effects of naringin on the reproductive system of SD rats, and the results showed that naringin gavage had minimal or no effect on clinical signs, reproductive performance, estrous cycle, spermatogenesis evaluation, gross pathology, and histopathology in rats [169]. Naringin's no-observed adverse effect level (NOAEL) on fertility and early embryonic development in rats was at least 1250 mg/kg/d [170]. In addition, Li P et al. compared the liver and kidney serological indices and toxicological changes in tissues and organs of rats given naringin with those of normal rats and found that naringin did not induce significant hepatotoxicity and nephrotoxicity [170,171].
Subsequently, the team further observed the morbidity and mortality of Beagles after continuous oral administration of naringin for 3 and 6 months, resulting in no toxicology-related events [172]. In vitro experiments also verified that naringin was cytotoxic to cancer cells at low concentrations but not healthy cells [173]. At the same time, naringin did not cause significant DNA damage at non-cytotoxic concentrations but was protective against DNA damage in healthy cells. These in vivo and in vitro results indicate that naringin has a good safety profile.
However, Ranawat's team found that naringin was able to reduce the number of apoptotic germ cells as well as sperm count and viability in mice, thereby causing damaging effects on testicular tissue [174]. This is mainly because that although naringin is a potent antioxidant, it may also be a pro-oxidant [174]. Limited toxicokinetic data are available for naringin. The toxicity of 22 polyphenol-rich compounds was assessed using a high-content screening analysis. The early and late toxicity of naringin on cells was further observed by observing the effects of naringin on the mitochondrial membrane potential, cell membrane integrity, and nuclear size of the respective cells of five cell lines (HepG2, Caco-2, A549, HMEC-1, and 3T3), which confirmed that naringin is the least toxic phenolic compound [175]. Meanwhile, the total drug concentration detected 24 h after oral naringin administration in rats was significantly reduced, suggesting that naringin does not accumulate excessively in the body after a few days of administration [176,177]. This provides a reference for further research on naringin in human clinical studies.
Currently, studies on the adverse effects of naringin have focused more on animal studies, and few studies have documented adverse effects in humans from naringin-derived foods. However, Ortiz-Andrade et al. demonstrated through in vitro and in vivo experiments and computerized methods that naringin is relatively safe for human use, virtually non-toxic, and valuable for drug development [178]. In addition, studies have demonstrated that no alterations in serum hepatic or renal parameters and no significant adverse events (pruritus, vomiting, diarrhea, etc.) have been observed in healthy adults who ingested doses of 150–900 mg of citrus extract (28 % naringenin and 8.5 % naringin) [179]. At these doses, naringin metabolites were in circulation and cleared within 24 h. Meanwhile, naringenin (at 8 μM in blood and the appropriate dose) increased the thermogenic genes of primary human adipocytes, so it is suggested that a twice-daily intake of 300 mg of citrus extract would benefit humans [179]. However, the main component of citrus extract in this trial was naringenin rather than naringin, so more clinical trials may be needed to confirm naringin's safety in its pure form in humans.
3.15. Naringin clinical trial data
Naringin has been approved by the China Food and Drug Administration for clinical trials as a new drug because of its cough suppressant, phlegm, and low toxicity characteristics [180]. Current clinical trials of naringin/naringenin have focused on significant hypoglycemic, hypolipidemic, hypotensive [181], anti-inflammatory, and anti-oxidative stress efficacy, as well as the potential to affect the bioavailability of other medications in clinical applications (Table 1). However, these clinical trial models have focused more on the therapeutic effects of naringin in patients with different types of dyslipidemia. In addition, It is important to note that naringin should be avoided with honey, as honey significantly reduces the absorption of naringin/naringenin in humans [182]. Therefore, future clinical validation of naringin in other disease models must be supplemented.
Table 1.
Clinical trials on the efficacy of naringin, naringenin, and its enriched food sources.
| Model | Dose | Mechanism | Key findings | Ref. |
|---|---|---|---|---|
| Hypercholesterolemic subjects | Naringin, 400mg/capsule/day | Decreased plasma total cholesterol (TC), LDL, and Apo B; increased erythrocyte SOD and CAT activity | Lowering blood lipid, antioxidant | [183] |
| Patients with moderate hypercholesterolemia | Naringin, 500mg/capsule/day |
TC and LDL cholesterol (LDL-C) concentrations are not reduced | Naringin does not lower blood lipids in patients with moderately high cholesterol | [184] |
| Adult patients with dyslipidemia | Naringin. 450 mg/day |
TC, LDL, and TG decreased; lipocalin increased slightly | Reducing weight in patients with dyslipidemia | [102] |
| Prediabetes patients | Citrus Flavonoid Supplement (4 % Naringin). 200, 400, or 800 mg/day for 12 weeks |
Decreased fasting glucose, HOMA-IR, HbA1c, glucagon, C-peptide, hsCRP, IL-6, and TNF-α; increased GLP-1, lipocalin in the blood. | Anti-inflammatory, anti-hyperglycemic and antioxidant | [185] |
| Patients with T2DM and mixed hyperlipidemia | 650 mg of the BPF powder (BPF, main ingredient naringin) | LDL particles (LDL-P), TC, LDL-C, TG, blood glucose reduction | Lowering of blood glucose and blood lipids, complementary treatment of cardiometabolic disorders | [186,187] |
| T2DM | Mediterranean Diet (naringenin, naringin) | Decreased IL-6, oxidative stress marker 8-hydroxy-2′-deoxyguanosine (8-OHdG) | Reducing Inflammation in T2DM | [188] |
| Participants with abdominal obesity/dyslipidemia | 1240 mg/day total polyphenols (naringin, naringenin) | Increases fatty acid oxidation in the liver, reduces inflammation by inhibiting nuclear factor κ-light chain enhancers in activated B cells, increases lipocalin | Blood pressure lowering, diet program to treat NAFLD. | [189] |
| older adults experiencing subjective cognitive decline (SCD) | 400 mg Citrus Peel Extract (naringenin 3 mg) | IL-10, IL1-Ra, soluble TNF receptor I, soluble TNF receptor II increased; CXCL8/IL-8, IL-1β, IL-6, IL-17, TNF-α, IP10, MIP-1α, CCL2/MCP-1, CCL5/RANTES, IL-18, NO, thiobarbituric acid reactive substances, SOD decreased | Improves immediate memory, visuospatial/structural, language, attention, and delayed memory; reduces oxidative stress and neuronal damage and anti-inflammatory effects | [190] |
| Healthy volunteers | Felodipine with 250 mL grapefruit juice | Significantly inhibited CYP3A4 activity | Peak concentrations were higher, but half-life was unchanged. Grapefruit juice significantly increased the bioavailability of felodipine | [[191], [192], [193]] |
| Healthy volunteers | aliskiren with 300 mL grapefruit juice | Naringin inhibits human intestinal organic anion transporting polypeptide (OATP) 1A2 | Reducing exposure to aliskiren | [194] |
| Healthy volunteers | Fexofenadine with 300 ml grapefruit juice | Naringin inhibits the OATP1A2 part of the intestine | Reduces the bioavailability of oral fexofenadine | [195] |
| Healthy volunteers | talinolol and 1050 mg of naringin | The high dose may inhibit the efflux transporter protein P-glycoprotein (P-gp), counteracting uptake inhibition. | Short-term high-dose supplementation with naringin does not affect the pharmacokinetics of talinolol. | [196] |
| Healthy volunteers | Nisoldipine coated tablets with 250 ml water, 250 ml grapefruit juice | Increasing the maximum concentration of nisoldipine and decreasing the time to reach the maximum concentration | [197] |
3.16. Future prospects
Naringin has a low cost, comprehensive efficacy, and a long consumption history. It is widely used in basic and applied research on various inflammatory damages, showing significant anti-inflammatory and antioxidant effects [5,10]. The latest study also demonstrated the protective effect of naringin on various system cells (Cardiac cells, Endothelial progenitor stem cells, Human amniotic fluid-derived stem cells, Human periodontal ligament stem cells, Bone marrow stem cells, neuronal cells) [198], highlighting the critical role of the Nrf2 pathway. In this review, we systematically explored the molecular mechanism of naringin in improving inflammatory diseases in nervous, cardiovascular, bone, and other tissues and found that naringin mainly plays an essential role in regulating the immune microenvironment and the recovery of metabolic disorders. However, the analysis of the pathway mechanism of naringin on the therapeutic effect of inflammatory diseases has yet to form a systematic and comprehensive theory. Hence, an in-depth exploration of its pharmacological effects and molecular mechanisms is still the focus and hotspot of future research. It is worth noting that the metabolic, toxicity, and safety evaluation data of naringin clinical trials still need to be completed and further supplemented.
4. Conclusion
Naringin is an active pharmaceutical component rich in flavonoids in citrus plants, which has good anti-inflammatory, antioxidant, anti-apoptotic, anti-tissue fibrosis, and hypolipidemic effects. Given the poor bioavailability and low water solubility of naringin, a new drug carrier encapsulation of naringin can improve its pharmacological properties and pharmacokinetics, thereby improving the utilization rate of naringin in vivo. In in vitro and in vitro experiments, naringin can exert powerful anti-inflammatory and antioxidant properties through various molecular mechanisms and signaling pathways, which have good alleviating effects on various inflammatory diseases. In addition, naringin treatment has few adverse effects and can reduce the toxicity of other drug treatments at the relevant doses, thereby reducing the toxic side effects of drugs. Therefore, after further research, naringin may be a promising candidate for the future treatment of novel and safe inflammatory diseases. However, the current bioactive role of naringin in inflammatory diseases relies heavily on animal models and in vitro experiments, and more clinical trials are needed to validate its beneficial role in human health further.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81873163), the Key Project of Natural Science Foundation of Shandong Province (ZR2020KC024), and the Natural Science Foundation of Shandong Province (ZR2017LH042).
National Natural Science Foundation of China, China; Key Project of Natural Science Foundation of Shandong Province, China; Natural Science Foundation of Shandong Province, China?
Ethics declarations
Review and/or approval by an ethics committee was not needed for this study as this is a review.
Informed consent was not required for this study as this is a review.
Data availability statement
No data was used for the research described in the article.
CRediT authorship contribution statement
Yuan Peng: Conceptualization, Writing – original draft. Ruyi Qu: Investigation, Methodology. Shuqin Xu: Visualization. Hongsheng Bi: Resources, Supervision. Dadong Guo: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chen R., Qi Q.L., Wang M.T., Li Q.Y. Therapeutic potential of naringin: an overview. Pharm. Biol. 2016 Dec;54(12):3203–3210. doi: 10.1080/13880209.2016.1216131. Epub 2016 Aug 26. PMID: 27564838. [DOI] [PubMed] [Google Scholar]
- 2.Joshi R., Kulkarni Y.A., Wairkar S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: an update. Life Sci. 2018 Dec 15;215:43–56. doi: 10.1016/j.lfs.2018.10.066. Epub 2018 Nov 1. PMID: 30391464. [DOI] [PubMed] [Google Scholar]
- 3.Wang C., Pan Y., Fan G., Chai Y., Wu Y. Application of an efficient strategy based on MAE, HPLC-DAD-MS/MS and HSCCC for the rapid extraction, identification, separation and purification of flavonoids from Fructus Aurantii Immaturus. Biomed. Chromatogr. 2010 Mar;24(3):235–244. doi: 10.1002/bmc.1278. PMID: 19591241. [DOI] [PubMed] [Google Scholar]
- 4.Csuti A., Sik B., Ajtony Z. Measurement of naringin from citrus fruits by high-performance liquid chromatography - a review. Crit. Rev. Anal. Chem. 2022 Jun 6:1–14. doi: 10.1080/10408347.2022.2082241. Epub ahead of print. PMID: 35658668. [DOI] [PubMed] [Google Scholar]
- 5.Yuan S., Li Y., Li J., Xue J.C., Wang Q., Hou X.T., Meng H., Nan J.X., Zhang Q.G. Traditional Chinese medicine and natural products: potential Approaches for inflammatory bowel disease. Front. Pharmacol. 2022 Jul 7;13 doi: 10.3389/fphar.2022.892790. PMID: 35873579; PMCID: PMC9301246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao H., Liu M., Liu H., Suo R., Lu C. Naringin protects endothelial cells from apoptosis and inflammation by regulating the Hippo-YAP Pathway. Biosci. Rep. 2020 Mar 27;40(3) doi: 10.1042/BSR20193431. 10.1042/BSR20193431. Erratum in: Biosci Rep. 2020 Jul 31;40(7): PMID: 32091090; PMCID: PMC7056449. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Memariani Z., Abbas S.Q., Ul Hassan S.S., Ahmadi A., Chabra A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021 Sep;171 doi: 10.1016/j.phrs.2020.105264. Epub 2020 Nov 6. PMID: 33166734. [DOI] [PubMed] [Google Scholar]
- 8.Ansari B., Aschner M., Hussain Y., Efferth T., Khan H. Suppression of colorectal carcinogenesis by naringin. Phytomedicine. 2022 Feb;96 doi: 10.1016/j.phymed.2021.153897. Epub 2021 Dec 20. PMID: 35026507. [DOI] [PubMed] [Google Scholar]
- 9.Cao H., Liu J., Shen P., Cai J., Han Y., Zhu K., Fu Y., Zhang N., Zhang Z., Cao Y. Protective effect of naringin on DSS-induced ulcerative colitis in mice. J. Agric. Food Chem. 2018 Dec 19;66(50):13133–13140. doi: 10.1021/acs.jafc.8b03942. Epub 2018 Dec 4. PMID: 30472831. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y., Liu S. Bioactivity of naringin and related mechanisms. Pharmazie. 2021 Aug 1;76(8):359–363. doi: 10.1691/ph.2021.1504. 10.1691/ph.2021.1504. PMID: 34412734. [DOI] [PubMed] [Google Scholar]
- 11.Ali A.M., Kunugi H. Propolis, Bee honey, and their components protect against Coronavirus disease 2019 (COVID-19): a review of in Silico, in vitro, and clinical studies. Molecules. 2021 Feb 25;26(5):1232. doi: 10.3390/molecules26051232. PMID: 33669054; PMCID: PMC7956496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge X., Zhou G. Protective effects of naringin on glucocorticoid-induced osteoporosis through regulating the PI3K/Akt/mTOR signaling pathway. Am J Transl Res. 2021 Jun 15;13(6):6330–6341. PMID: 34306372; PMCID: PMC8290725. [PMC free article] [PubMed] [Google Scholar]
- 13.Li P., Zhang S., Song H., Traore S.S., Li J., Raubenheimer D., Cui Z., Kou G. Naringin promotes skeletal muscle fiber remodeling by the AdipoR1-APPL1-AMPK signaling pathway. J. Agric. Food Chem. 2021 Oct 13;69(40):11890–11899. doi: 10.1021/acs.jafc.1c04481. Epub 2021 Sep 29. PMID: 34586803. [DOI] [PubMed] [Google Scholar]
- 14.Zeng X., Su W., Liu B., Chai L., Shi R., Yao H. A review on the pharmacokinetic properties of naringin and its therapeutic efficacies in respiratory diseases. Mini Rev. Med. Chem. 2020;20(4):286–293. doi: 10.2174/1389557519666191009162641. PMID: 32134369. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H.H., Zhou X.J., Zhong Y.S., Ji L.T., Yu W.Y., Fang J., Ying H.Z., Li C.Y. Naringin suppressed airway inflammation and ameliorated pulmonary endothelial hyperpermeability by upregulating Aquaporin1 in lipopolysaccharide/cigarette smoke-induced mice. Biomed. Pharmacother. 2022 Jun;150 doi: 10.1016/j.biopha.2022.113035. Epub 2022 Apr 28. PMID: 35658207. [DOI] [PubMed] [Google Scholar]
- 16.Amini N., Maleki M., Badavi M. Nephroprotective activity of naringin against chemical-induced toxicity and renal ischemia/reperfusion injury: a review. Avicenna J Phytomed. 2022 Jul-Aug;12(4):357–370. doi: 10.22038/AJP.2022.19620. PMID: 35782769; PMCID: PMC9121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed S., Khan H., Aschner M., Hasan M.M., Hassan S.T.S. Therapeutic potential of naringin in neurological disorders. Food Chem. Toxicol. 2019 Oct;132 doi: 10.1016/j.fct.2019.110646. Epub 2019 Jun 26. PMID: 31252025. [DOI] [PubMed] [Google Scholar]
- 18.Heidary Moghaddam R., Samimi Z., Moradi S.Z., Little P.J., Xu S., Farzaei M.H. Naringenin and naringin in cardiovascular disease prevention: a preclinical review. Eur. J. Pharmacol. 2020 Nov 15;887 doi: 10.1016/j.ejphar.2020.173535. Epub 2020 Sep 8. PMID: 32910944. [DOI] [PubMed] [Google Scholar]
- 19.Raja Kumar S., Mohd Ramli E.S., Abdul Nasir N.A., Ismail N.H.M., Mohd Fahami N.A. Preventive effect of naringin on metabolic syndrome and its mechanism of action: a systematic review. Evid Based Complement Alternat Med. 2019 Feb 3;2019 doi: 10.1155/2019/9752826. PMID: 30854019; PMCID: PMC6377991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam M., Ahsan F., Mahmood T., Shamim A., Parveen S., Shariq M., Ansari V.A. Meticulous parade on naringin respecting its pharmacological activities and novel formulations. Avicenna J Phytomed. 2022 Sep-Oct;12(5):457–474. doi: 10.22038/AJP.2022.20001. PMID: 36249455; PMCID: PMC9516400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng X., Zheng Y., He Y., Zhang J., Peng W., Su W. Microbial metabolism of naringin and the impact on antioxidant capacity. Nutrients. 2022;14(18) doi: 10.3390/nu14183765. 3765. Published 2022 Sep. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shilpa V.S., Shams R., Dash K.K., et al. Phytochemical properties, extraction, and pharmacological benefits of naringin: a review. Molecules. 2023;28(15):5623. doi: 10.3390/molecules28155623. Published 2023 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miles E.A., Calder P.C. Effects of citrus fruit juices and their bioactive components on inflammation and immunity: a narrative review. Front. Immunol. 2021 Jun 24;12 doi: 10.3389/fimmu.2021.712608. PMID: 34249019; PMCID: PMC8264544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge X., Jiang F., Wang M., Chen M., Li Y., Phipps J., Cai J., Xie J., Ong J., Dubovoy V., Masters J.G., Pan L., Ma S. Naringin@Metal-Organic framework as a multifunctional bioplatform. ACS Appl. Mater. Interfaces. 2022 Dec 23 doi: 10.1021/acsami.2c19904. Epub ahead of print. PMID: 36562661. [DOI] [PubMed] [Google Scholar]
- 25.Li P., Wang S., Guan X., Liu B., Wang Y., Xu K., Peng W., Su W., Zhang K. Acute and 13 weeks subchronic toxicological evaluation of naringin in Sprague-Dawley rats. Food Chem. Toxicol. 2013 Oct;60:1–9. doi: 10.1016/j.fct.2013.07.019. Epub 2013 Jul 18. PMID: 23871784. [DOI] [PubMed] [Google Scholar]
- 26.Felgines C., Texier O., Morand C., Manach C., Scalbert A., Régerat F., Rémésy C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000 Dec;279(6):G1148–G1154. doi: 10.1152/ajpgi.2000.279.6.G1148. PMID: 11093936. [DOI] [PubMed] [Google Scholar]
- 27.Guo X., Cao X., Fang X., Guo A., Li E. Involvement of phase II enzymes and efflux transporters in the metabolism and absorption of naringin, hesperidin and their aglycones in rats. Int. J. Food Sci. Nutr. 2022;73(4):480–490. doi: 10.1080/09637486.2021.2012562. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M., Zhu S., Yang W., Huang Q., Ho C.T. The biological fate and bioefficacy of citrus flavonoids: bioavailability, biotransformation, and delivery systems. Food Funct. 2021;12(8):3307–3323. doi: 10.1039/d0fo03403g. [DOI] [PubMed] [Google Scholar]
- 29.Najmanová I., Vopršalová M., Saso L., Mladěnka P. The pharmacokinetics of flavanones. Crit. Rev. Food Sci. Nutr. 2020;60(18):3155–3171. doi: 10.1080/10408398.2019.1679085. [DOI] [PubMed] [Google Scholar]
- 30.Bhia M., Motallebi M., Abadi B., Zarepour A., Pereira-Silva M., Saremnejad F., Santos A.C., Zarrabi A., Melero A., Jafari S.M., Shakibaei M. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics. 2021 Feb 23;13(2):291. doi: 10.3390/pharmaceutics13020291. PMID: 33672366; PMCID: PMC7926828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Zou Z., Liu S., Miao S., Liu H. Nanogels as novel nanocarrier systems for efficient delivery of CNS therapeutics. Front. Bioeng. Biotechnol. 2022 Jul 19;10 doi: 10.3389/fbioe.2022.954470. PMID: 35928954; PMCID: PMC9343834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmad A., Prakash R., Khan M.S., Altwaijry N., Asghar M.N., Raza S.S., Khan R. Enhanced antioxidant effects of naringenin nanoparticles synthesized using the high-energy ball milling method. ACS Omega. 2022 Sep 19;7(38):34476–34484. doi: 10.1021/acsomega.2c04148. PMID: 36188293; PMCID: PMC9521026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zverev Y.F., Rykunova A.Y. Modern nanocarriers as a factor in increasing the bioavailability and pharmacological activity of flavonoids. Appl. Biochem. Microbiol. 2022;58(9):1002–1020. doi: 10.1134/S0003683822090149. Epub 2022 Dec 16. PMID: 36540406; PMCID: PMC9756931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budel R.G., da Silva D.A., Moreira M.P., Dalcin A.J.F., da Silva A.F., Nazario L.R., Majolo J.H., Lopes L.Q.S., Santos R.C.V., Antunes Soares F.A., da Silva R.S., Gomes P., Boeck C.R. Toxicological evaluation of naringin-loaded nanocapsules in vitro and in vivo. Colloids Surf. B Biointerfaces. 2020 Apr;188 doi: 10.1016/j.colsurfb.2019.110754. Epub 2019 Dec 23. PMID: 31887647. [DOI] [PubMed] [Google Scholar]
- 35.Kumari S.D., Chevala N.T., Jitta S.R., Kumar L., Verma R., Jose J. Design and development of naringin-loaded proposomal gel for wound healing. J. Cosmet. Dermatol. 2022 Oct;21(10):5187–5202. doi: 10.1111/jocd.15029. Epub 2022 May 16. PMID: 35486446. [DOI] [PubMed] [Google Scholar]
- 36.Lee J., Kim K., Son J., Lee H., Song J.H., Lee T., Jeon H., Kim H.S., Park S.J., Yoo H.Y., Park C. Improved productivity of naringin oleate with flavonoid and fatty acid by efficient enzymatic esterification. Antioxidants. 2022 Jan 27;11(2):242. doi: 10.3390/antiox11020242. PMID: 35204125; PMCID: PMC8868195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baek Y., Lee S., Son J., Lee T., Oh J.M., Lee S.H., Kim H.U., Seo S.W., Park S.J., Yoo H.Y., Park C. Efficient production of naringin acetate with different acyl donors via enzymatic transesterification by lipases. Int. J. Environ. Res. Publ. Health. 2022 Mar 3;19(5):2972. doi: 10.3390/ijerph19052972. PMID: 35270665; PMCID: PMC8910296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh B., Singh J.P., Kaur A., Singh N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020;132 doi: 10.1016/j.foodres.2020.109114. [DOI] [PubMed] [Google Scholar]
- 39.Krysa M., Szymańska-Chargot M., Zdunek A. FT-IR and FT-Raman fingerprints of flavonoids - a review. Food Chem. 2022;393 doi: 10.1016/j.foodchem.2022.133430. [DOI] [PubMed] [Google Scholar]
- 40.Li Y., Zhao C., Lu C., et al. Simultaneous determination of 14 bioactive citrus flavonoids using thin-layer chromatography combined with surface enhanced Raman spectroscopy. Food Chem. 2021;338 doi: 10.1016/j.foodchem.2020.128115. [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi M., LaRocca C.A., Bernat J.D., Lindsey J.S. Digital database of absorption spectra of diverse flavonoids enables structural comparisons and quantitative evaluations. J. Nat. Prod. 2023;86(4):1087–1119. doi: 10.1021/acs.jnatprod.2c00720. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y., Trevethan M., Wang S., Zhao L. Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: an update on bioavailability, pharmacokinetics, and mechanisms. J. Nutr. Biochem. 2022 Jun;104 doi: 10.1016/j.jnutbio.2022.108967. Epub 2022 Feb 18. PMID: 35189328; PMCID: PMC9058202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai Y., Peng W., Yang C., Zou W., Liu M., Wu H., Fan L., Li P., Zeng X., Su W. Pharmacokinetics and metabolism of naringin and active metabolite naringenin in rats, dogs, humans, and the differences between species. Front. Pharmacol. 2020 Mar 27;11:364. doi: 10.3389/fphar.2020.00364. PMID: 32292344; PMCID: PMC7118210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng X., Yao H., Zheng Y., He Y., He Y., Rao H., Li P., Su W. Tissue distribution of naringin and derived metabolites in rats after a single oral administration. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2020 Jan 1;1136 doi: 10.1016/j.jchromb.2019.121846. Epub 2019 Oct 31. PMID: 31821965. [DOI] [PubMed] [Google Scholar]
- 45.Najmanová I., Vopršalová M., Saso L., Mladěnka P. The pharmacokinetics of flavanones. Crit. Rev. Food Sci. Nutr. 2020;60(18):3155–3171. doi: 10.1080/10408398.2019.1679085. [DOI] [PubMed] [Google Scholar]
- 46.McGill M.R., Hinson J.A. The development and hepatotoxicity of acetaminophen: reviewing over a century of progress. Drug Metab. Rev. 2020 Nov;52(4):472–500. doi: 10.1080/03602532.2020.1832112. Epub 2020 Oct 14. PMID: 33103516; PMCID: PMC8427730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramachandran A., Visschers R.G.J., Duan L., Akakpo J.Y., Jaeschke H. Mitochondrial dysfunction as a mechanism of drug-induced hepatotoxicity: current understanding and future perspectives. J Clin Transl Res. 2018 May 28;4(1):75–100. doi: 10.18053/jctres.04.201801.005. PMID: 30873497; PMCID: PMC6261533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional regulation by Nrf2. Antioxidants Redox Signal. 2018 Dec 10;29(17):1727–1745. doi: 10.1089/ars.2017.7342. Epub 2017 Oct 20. PMID: 28899199; PMCID: PMC6208165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He F., Ru X., Wen T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020 Jul 6;21(13):4777. doi: 10.3390/ijms21134777. PMID: 32640524; PMCID: PMC7369905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baird L., Yamamoto M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell Biol. 2020 Jun 15;40(13) doi: 10.1128/MCB.00099-20. PMID: 32284348; PMCID: PMC7296212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Q., Yu P., Bi Y., Li Z., Guo W., Chen Y., Duan Z. Naringin regulates mitochondrial dynamics to protect against acetaminophen-induced hepatotoxicity by activating the AMPK/Nrf2 signaling pathway in vitro. Braz. J. Med. Biol. Res. 2022 Oct 17;55 doi: 10.1590/1414-431X2022e12040. PMID: 36259797; PMCID: PMC9578698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang S.W., Haydar G., Taniane C., Farrell G., Arias I.M., Lippincott-Schwartz J., Fu D. AMPK activation prevents and reverses drug-induced mitochondrial and hepatocyte injury by promoting mitochondrial fusion and function. PLoS One. 2016 Oct 28;11(10) doi: 10.1371/journal.pone.0165638. PMID: 27792760; PMCID: PMC5085033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhai X., Dai T., Chi Z., Zhao Z., Wu G., Yang S., Dong D. Naringin alleviates acetaminophen-induced acute liver injury by activating Nrf2 via CHAC2 upregulation. Environ. Toxicol. 2022 Jun;37(6):1332–1342. doi: 10.1002/tox.23487. Epub 2022 Feb 18. PMID: 35179299. [DOI] [PubMed] [Google Scholar]
- 54.Lin Z., Wang G., Gu W., Zhao S., Shen Z., Liu W., Zheng G., Chen B., Cai Y., Li M., Wan C.C., Yan T. Exploration of the protective mechanism of naringin in the acetaminophen-induced hepatic injury by metabolomics. Oxid Med Cell Longev. 2022 Sep 16. 2022 doi: 10.1155/2022/7138194. PMID: 36160708; PMCID: PMC9507767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akamo A.J., Rotimi S.O., Akinloye D.I., Ugbaja R.N., Adeleye O.O., Dosumu O.A., Eteng O.E., Amah G., Obijeku A., Cole O.E. Naringin prevents cyclophosphamide-induced hepatotoxicity in rats by attenuating oxidative stress, fibrosis, and inflammation. Food Chem. Toxicol. 2021 Jul;153 doi: 10.1016/j.fct.2021.112266. Epub 2021 May 14. PMID: 33992719. [DOI] [PubMed] [Google Scholar]
- 56.Hassan R.A., Hozayen W.G., Abo Sree H.T., Al-Muzafar H.M., Amin K.A., Ahmed O.M. Naringin and hesperidin counteract diclofenac-induced hepatotoxicity in male wistar rats via their antioxidant, anti-inflammatory, and antiapoptotic activities. Oxid Med Cell Longev. 2021 Aug 11. 2021 doi: 10.1155/2021/9990091. PMID: 34422219; PMCID: PMC8376442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elsawy H., Alzahrani A.M., Alfwuaires M., Abdel-Moneim A.M., Khalil M. Beneficial role of naringin against methotrexate-induced injury to rat testes: biochemical and ultrastructural analyses. Redox Rep. 2022 Dec;27(1):158–166. doi: 10.1080/13510002.2022.2101832. PMID: 35861275; PMCID: PMC9310850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elsawy H., Alzahrani A.M., Alfwuaires M., Abdel-Moneim A.M., Khalil M. Nephroprotective effect of naringin in methotrexate induced renal toxicity in male rats. Biomed. Pharmacother. 2021 Nov;143 doi: 10.1016/j.biopha.2021.112180. Epub 2021 Sep 15. PMID: 34536756. [DOI] [PubMed] [Google Scholar]
- 59.Li M., Liu J., Liu D., Duan X., Zhang Q., Wang D., Zheng Q., Bai X., Lu Z. Naringin attenuates cisplatin- and aminoglycoside-induced hair cell injury in the zebrafish lateral line via multiple pathways. J. Cell Mol. Med. 2021 Jan;25(2):975–989. doi: 10.1111/jcmm.16158. Epub 2020 Dec 3. PMID: 33274582; PMCID: PMC7812295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Z., Yang S., Deng Y., Wang L., Zhang Y., Feng Z., Li H., Chi Z., Xie Y., Dong D. Naringin interferes doxorubicin-induced myocardial injury by promoting the expression of ECHS1. Front. Pharmacol. 2022 Apr 12;13 doi: 10.3389/fphar.2022.859755. PMID: 35496302; PMCID: PMC9039159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Semis H.S., Kandemir F.M., Caglayan C., Kaynar O., Genc A., Arıkan S.M. Protective effect of naringin against oxaliplatin-induced peripheral neuropathy in rats: a behavioral and molecular study. J. Biochem. Mol. Toxicol. 2022 Sep;36(9) doi: 10.1002/jbt.23121. Epub 2022 Jun 7. PMID: 35670529. [DOI] [PubMed] [Google Scholar]
- 62.Müller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr. Bull. 2018 Aug 20;44(5):973–982. doi: 10.1093/schbul/sby024. PMID: 29648618; PMCID: PMC6101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porritt R.A., Binek A., Paschold L., Rivas M.N., McArdle A., Yonker L.M., Alter G., Chandnani H.K., Lopez M., Fasano A., Van Eyk J.E., Binder M., Arditi M. The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children. J. Clin. Invest. 2021 Oct 15;131(20) doi: 10.1172/JCI151520. PMID: 34437303; PMCID: PMC8516454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan J., Zhou L., Zhang C., Xu Q., Sun Y. Targeting protein phosphatases for the treatment of inflammation-related diseases: from signaling to therapy. Signal Transduct. Targeted Ther. 2022 Jun 4;7(1):177. doi: 10.1038/s41392-022-01038-3. PMID: 35665742; PMCID: PMC9166240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi Y., Wang Y., Li Q., Liu K., Hou J., Shao C., Wang Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018 Aug;14(8):493–507. doi: 10.1038/s41581-018-0023-5. PMID: 29895977. [DOI] [PubMed] [Google Scholar]
- 66.Belenguer G., Duart-Abadia P., Jordán-Pla A., Domingo-Muelas A., Blasco-Chamarro L., Ferrón S.R., Morante-Redolat J.M., Fariñas I. Adult neural stem cells are alerted by systemic inflammation through TNF-α receptor signaling. Cell Stem Cell. 2021 Feb 4;28(2):285–299.e9. doi: 10.1016/j.stem.2020.10.016. Epub 2020 Nov 17. PMID: 33207218. [DOI] [PubMed] [Google Scholar]
- 67.Chen R., Gao S., Guan H., Zhang X., Gao Y., Su Y., Song Y., Jiang Y., Li N. Naringin protects human nucleus pulposus cells against TNF-α-induced inflammation, oxidative stress, and loss of cellular homeostasis by enhancing autophagic flux via AMPK/SIRT1 activation. Oxid. Med. Cell. Longev. 2022 Feb 27:2022. doi: 10.1155/2022/7655142. 7655142. PMID: 35265264; PMCID: PMC8898769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madore C., Yin Z., Leibowitz J., Butovsky O. Microglia, lifestyle stress, and neurodegeneration. Immunity. 2020 Feb 18;52(2):222–240. doi: 10.1016/j.immuni.2019.12.003. Epub 2020 Jan 7. PMID: 31924476; PMCID: PMC7234821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leng F., Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 2021 Mar;17(3):157–172. doi: 10.1038/s41582-020-00435-y. Epub 2020 Dec 14. PMID: 33318676. [DOI] [PubMed] [Google Scholar]
- 70.Uddin M.S., Rahman M.M., Jakaria M., et al. Estrogen signaling in Alzheimer's disease: molecular insights and therapeutic targets for Alzheimer's dementia. Mol. Neurobiol. 2020;57(6):2654–2670. doi: 10.1007/s12035-020-01911-8. [DOI] [PubMed] [Google Scholar]
- 71.Petrine Jéssica C.P., Bruno Del Bianco-Borges The influence of phytoestrogens on different physiological and pathological processes: an overview. Phytother Res. : PT. 2021;35(1):180–197. doi: 10.1002/ptr.6816. [DOI] [PubMed] [Google Scholar]
- 72.Qiu Q., Lei X., Wang Y., et al. Naringin protects against tau hyperphosphorylation in aβ25-35-injured PC12 cells through modulation of ER, PI3K/AKT, and GSK-3β signaling pathways. Behav. Neurol. 2023:2023. doi: 10.1155/2023/1857330. 1857330. Published 2023 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jahanshahi M., Khalili M., Margedari A. Naringin chelates excessive iron and prevents the formation of amyloid-beta plaques in the Hippocampus of iron-overloaded mice. Front. Pharmacol. 2021 Jul 2;12 doi: 10.3389/fphar.2021.651156. PMID: 34276359; PMCID: PMC8283124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goyal A., Verma A., Dubey N., Raghav J., Agrawal A. Naringenin: a prospective therapeutic agent for Alzheimer's and Parkinson's disease. J. Food Biochem. 2022 Dec;46(12) doi: 10.1111/jfbc.14415. Epub 2022 Sep 15. PMID: 36106706. [DOI] [PubMed] [Google Scholar]
- 75.Baek S.Y., Li F.Y., Kim D.H., Kim S.J., Kim M.R. Enteromorpha prolifera extract improves memory in scopolamine-treated mice via downregulating amyloid-β expression and upregulating BDNF/TrkB pathway. Antioxidants. 2020 Jul 15;9(7):620. doi: 10.3390/antiox9070620. PMID: 32679768; PMCID: PMC7402154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi G.Y., Kim H.B., Hwang E.S., Park H.S., Cho J.M., Young-Ki H., Kim J.H., Mun M.K., Maeng S., Park J.H. Naringin enhances long-term potentiation and recovers learning and memory deficits of amyloid-beta induced Alzheimer's disease-like behavioral rat model. Neurotoxicology. 2022 Dec 19;S0161–813X(22) doi: 10.1016/j.neuro.2022.12.007. 00200-00205. Epub ahead of print. PMID: 36549596. [DOI] [PubMed] [Google Scholar]
- 77.Varshney Vibhav, Garabadu Debapriya. Naringin exhibits mas receptor-mediated neuroprotection against amyloid beta-induced cognitive deficits and mitochondrial toxicity in rat brain. Neurotox. Res. 2021;39(4):1023–1043. doi: 10.1007/s12640-021-00336-y. [DOI] [PubMed] [Google Scholar]
- 78.Meng X., Fu M., Wang S., Chen W., Wang J., Zhang N. Naringin ameliorates memory deficits and exerts neuroprotective effects in a mouse model of Alzheimer's disease by regulating multiple metabolic pathways. Mol. Med. Rep. 2021 May;23(5):332. doi: 10.3892/mmr.2021.11971. Epub 2021 Mar 24. PMID: 33760152; PMCID: PMC7974313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coulthard L.R., White D.E., Jones D.L., McDermott M.F., Burchill S.A. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009 Aug;15(8):369–379. doi: 10.1016/j.molmed.2009.06.005. Epub 2009 Aug 6. PMID: 19665431; PMCID: PMC3016890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sui X., Kong N., Ye L., Han W., Zhou J., Zhang Q., He C., Pan H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014 Mar 28;344(2):174–179. doi: 10.1016/j.canlet.2013.11.019. Epub 2013 Dec 11. PMID: 24333738. [DOI] [PubMed] [Google Scholar]
- 81.Dai X.J., Jia Y., Cao R., Zhou M.N. Naringin prevents cognitive dysfunction in aging rats by inhibiting toll-like receptor 4 (TLR4)/NF-κB pathway and endoplasmic reticulum stress. Evid Based Complement Alternat Med. 2023;2023 doi: 10.1155/2023/2919811. Published 2023 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hassan H.M., Elnagar M.R., Abdelrazik E., Mahdi M.R., Hamza E., Elattar E.M., ElNashar E.M., Alghamdi M.A., Al-Qahtani Z., Al-Khater K.M., Aldahhan R.A., Eldesoqui M. Neuroprotective effect of naringin against cerebellar changes in Alzheimer's disease through modulation of autophagy, oxidative stress and tau expression: an experimental study. Front. Neuroanat. 2022 Oct 14;16 doi: 10.3389/fnana.2022.1012422. PMID: 36312298; PMCID: PMC9615142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang W., Zhou K., Zhou Y., An Y., Hu T., Lu J., Huang S., Pei G. Naringin dihydrochalcone ameliorates cognitive deficits and neuropathology in APP/PS1 transgenic mice. Front. Aging Neurosci. 2018 Jun 5;10:169. doi: 10.3389/fnagi.2018.00169. PMID: 29922152; PMCID: PMC5996202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tolosa E., Garrido A., Scholz S.W., Poewe W. Challenges in the diagnosis of Parkinson's disease. Lancet Neurol. 2021 May;20(5):385–397. doi: 10.1016/S1474-4422(21)00030-2. PMID: 33894193; PMCID: PMC8185633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh A., Tripathi P., Yadawa A.K., Singh S. Promising polyphenols in Parkinson's disease therapeutics. Neurochem. Res. 2020 Aug;45(8):1731–1745. doi: 10.1007/s11064-020-03058-3. Epub 2020 May 27. PMID: 32462543. [DOI] [PubMed] [Google Scholar]
- 86.Leem E., Nam J.H., Jeon M.T., Shin W.H., Won S.Y., Park S.J., Choi M.S., Jin B.K., Jung U.J., Kim S.R. Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of Parkinson's disease. J. Nutr. Biochem. 2014 Jul;25(7):801–806. doi: 10.1016/j.jnutbio.2014.03.006. Epub 2014 Apr 1. PMID: 24797334. [DOI] [PubMed] [Google Scholar]
- 87.Kim H.D., Jeong K.H., Jung U.J., Kim S.R. Naringin treatment induces neuroprotective effects in a mouse model of Parkinson's disease in vivo, but not enough to restore the lesioned dopaminergic system. J. Nutr. Biochem. 2016 Feb;28:140–146. doi: 10.1016/j.jnutbio.2015.10.013. Epub 2015 Nov 4. PMID: 26878791. [DOI] [PubMed] [Google Scholar]
- 88.Bai X., Bian Z., Zhang M. Targeting the Nrf2 signaling pathway using phytochemical ingredients: a novel therapeutic road map to combat neurodegenerative diseases. Phytomedicine. 2022 Nov 30;109 doi: 10.1016/j.phymed.2022.154582. Epub ahead of print. PMID: 36610130. [DOI] [PubMed] [Google Scholar]
- 89.Garabadu D., Agrawal N. Naringin exhibits neuroprotection against rotenone-induced neurotoxicity in experimental rodents. NeuroMolecular Med. 2020 Jun;22(2):314–330. doi: 10.1007/s12017-019-08590-2. Epub 2020 Jan 8. PMID: 31916219. [DOI] [PubMed] [Google Scholar]
- 90.Cao W., Feng S.J., Kan M.C. Naringin targets NFKB1 to alleviate oxygen-glucose deprivation/reoxygenation-induced injury in PC12 cells via modulating HIF-1α/AKT/mTOR-Signaling pathway. J. Mol. Neurosci. 2021 Jan;71(1):101–111. doi: 10.1007/s12031-020-01630-8. Epub 2020 Jun 18. PMID: 32557145. [DOI] [PubMed] [Google Scholar]
- 91.Feng J., Chen X., Lu S., Li W., Yang D., Su W., Wang X., Shen J. Naringin attenuates cerebral ischemia-reperfusion injury through inhibiting peroxynitrite-mediated mitophagy activation. Mol. Neurobiol. 2018 Dec;55(12):9029–9042. doi: 10.1007/s12035-018-1027-7. Epub 2018 Apr 7. PMID: 29627876. [DOI] [PubMed] [Google Scholar]
- 92.Yang J., Yuan L., Wen Y., Zhou H., Jiang W., Xu D., Wang M. Protective effects of naringin in cerebral infarction and its molecular mechanism. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020 Jan 4;26 doi: 10.12659/MSM.918772. PMID: 31901198; PMCID: PMC6977645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao C., Wu M., Du Q., Deng J., Shen J. Naringin mediates adult hippocampal neurogenesis for antidepression via activating CREB signaling. Front. Cell Dev. Biol. 2022 Apr 7;10 doi: 10.3389/fcell.2022.731831. PMID: 35478969; PMCID: PMC9037031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hernández-Vázquez L., Cassani J., Heyerdahl-Viau I., Martínez-Casares R.M., Luna H., Dorantes-Barrón A.M., Arrieta-Báez D., Estrada-Reyes R. Recovery of naringin-rich flavonoid extracts from agroresidues with anxiolytic- and antidepressant-like effects in mice. Molecules. 2022 Dec 3;27(23):8507. doi: 10.3390/molecules27238507. PMID: 36500599; PMCID: PMC9740236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li L., Liu R., He J., et al. Naringin regulates microglia BV-2 activation and inflammation via the JAK/STAT3 pathway. Evid Based Complement Alternat Med. 2022;2022 doi: 10.1155/2022/3492058. Published 2022 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S., Tokgözoğlu L., Lewis E.F. Atherosclerosis. Nat Rev Dis Primers. 2019 Aug 16;5(1):56. doi: 10.1038/s41572-019-0106-z. PMID: 31420554. [DOI] [PubMed] [Google Scholar]
- 97.Ma S.R., Tong Q., Lin Y., Pan L.B., Fu J., Peng R., Zhang X.F., Zhao Z.X., Li Y., Yu J.B., Cong L., Han P., Zhang Z.W., Yu H., Wang Y., Jiang J.D. Berberine treats atherosclerosis via a vitamine-like effect down-regulating Choline-TMA-TMAO production pathway in gut microbiota. Signal Transduct. Targeted Ther. 2022 Jul 7;7(1):207. doi: 10.1038/s41392-022-01027-6. PMID: 35794102; PMCID: PMC9259588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tedgui A., Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 2006 Apr;86(2):515–581. doi: 10.1152/physrev.00024.2005. PMID: 16601268. [DOI] [PubMed] [Google Scholar]
- 99.Doran A.C. Inflammation resolution: implications for atherosclerosis. Circ. Res. 2022 Jan 7;130(1):130–148. doi: 10.1161/CIRCRESAHA.121.319822. Epub 2022 Jan 7. PMID: 34995137; PMCID: PMC8842990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.George M.Y., Menze E.T., Esmat A., Tadros M.G., El-Demerdash E. Naringin treatment improved main clozapine-induced adverse effects in rats; emphasis on weight gain, metabolic abnormalities, and agranulocytosis. Drug Dev. Res. 2021 Nov;82(7):980–989. doi: 10.1002/ddr.21800. Epub 2021 Feb 3. PMID: 33537987. [DOI] [PubMed] [Google Scholar]
- 101.Hager-Theodorides A.L., Massouras T., Simitzis P.E., Moschou K., Zoidis E., Sfakianaki E., Politi K., Charismiadou M., Goliomytis M., Deligeorgis S. Hesperidin and naringin improve broiler meat fatty acid profile and modulate the expression of genes involved in fatty acid β-oxidation and antioxidant defense in a dose dependent manner. Foods. 2021 Mar 31;10(4):739. doi: 10.3390/foods10040739. PMID: 33807218; PMCID: PMC8065613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barajas-Vega J.L., Raffoul-Orozco A.K., Hernandez-Molina D., Ávila-González A.E., García-Cobian T.A., Rubio-Arellano E.D., Ramirez-Lizardo E.J. Naringin reduces body weight, plasma lipids and increases adiponectin levels in patients with dyslipidemia. Int. J. Vitam. Nutr. Res. 2022 Jul;92(3–4):292–298. doi: 10.1024/0300-9831/a000658. Epub 2020 Jun 9. PMID: 32513069. [DOI] [PubMed] [Google Scholar]
- 103.Yu X., Meng X., Yan Y., Wang H., Zhang L. Extraction of naringin from pomelo and its therapeutic potentials against hyperlipidemia. Molecules. 2022 Dec 18;27(24):9033. doi: 10.3390/molecules27249033. PMID: 36558166; PMCID: PMC9783781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J., Wang Q., Zhu S., Huang J., Liu Z., Qi R. Naringin reduces fat deposition by promoting the expression of lipolysis and β-oxidation related genes. Obes. Res. Clin. Pract. 2022 Dec 6 doi: 10.1016/j.orcp.2022.11.004. S1871-403X(22)00129-6. Epub ahead of print. PMID: 36494293. [DOI] [PubMed] [Google Scholar]
- 105.Pengnet S., Prommaouan S., Sumarithum P., Malakul W. Naringin reverses high-cholesterol diet-induced vascular dysfunction and oxidative stress in rats via regulating LOX-1 and NADPH oxidase subunit expression. BioMed Res. Int. 2019 Oct 24;2019 doi: 10.1155/2019/3708497. PMID: 31781614; PMCID: PMC6855071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang F., Zhao C., Tian G., Wei X., Ma Z., Cui J., Wei R., Bao Y., Kong W., Zheng J. Naringin alleviates atherosclerosis in ApoE-/- mice by regulating cholesterol metabolism involved in gut microbiota remodeling. J. Agric. Food Chem. 2020 Nov 11;68(45):12651–12660. doi: 10.1021/acs.jafc.0c05800. Epub 2020 Oct 27. PMID: 33107729. [DOI] [PubMed] [Google Scholar]
- 107.Uryash A., Mijares A., Flores V., Adams J.A., Lopez J.R. Effects of naringin on cardiomyocytes from a rodent model of type 2 diabetes. Front. Pharmacol. 2021 Aug 23;12 doi: 10.3389/fphar.2021.719268. PMID: 34497520; PMCID: PMC8419284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tang G., Pi L., Guo H., Hu Z., Zhou C., Hu Q., Peng H., Xiao Z., Zhang Z., Wang M., Peng T., Huang J., Liang S., Li G. Naringin relieves diabetic cardiac autonomic neuropathy mediated by P2Y14 receptor in superior cervical ganglion. Front. Pharmacol. 2022 Apr 21;13 doi: 10.3389/fphar.2022.873090. PMID: 35529431; PMCID: PMC9068893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakamura M., Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018 Jul;15(7):387–407. doi: 10.1038/s41569-018-0007-y. PMID: 29674714. [DOI] [PubMed] [Google Scholar]
- 110.Gallo S., Vitacolonna A., Bonzano A., Comoglio P., Crepaldi T. ERK: a key player in the pathophysiology of cardiac hypertrophy. Int. J. Mol. Sci. 2019 May 1;20(9):2164. doi: 10.3390/ijms20092164. PMID: 31052420; PMCID: PMC6539093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Park J.H., Ku H.J., Kim J.K., Park J.W., Lee J.H. Amelioration of high fructose-induced cardiac hypertrophy by naringin. Sci. Rep. 2018 Jun 21;8(1):9464. doi: 10.1038/s41598-018-27788-1. PMID: 29930336; PMCID: PMC6013481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Majumdar P., Chen S., George B., Sen S., Karmazyn M., Chakrabarti S. Leptin and endothelin-1 mediated increased extracellular matrix protein production and cardiomyocyte hypertrophy in diabetic heart disease. Diabetes Metab Res Rev. 2009 Jul;25(5):452–463. doi: 10.1002/dmrr.964. PMID: 19391127. [DOI] [PubMed] [Google Scholar]
- 113.Obradovic M., Sudar-Milovanovic E., Soskic S., Essack M., Arya S., Stewart A.J., Gojobori T., Isenovic E.R. Leptin and obesity: role and clinical implication. Front. Endocrinol. 2021 May 18;12 doi: 10.3389/fendo.2021.585887. PMID: 34084149; PMCID: PMC8167040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen J., Mo H., Guo R., You Q., Huang R., Wu K. Inhibition of the leptin-induced activation of the p38 MAPK pathway contributes to the protective effects of naringin against high glucose-induced injury in H9c2 cardiac cells. Int. J. Mol. Med. 2014 Mar;33(3):605–612. doi: 10.3892/ijmm.2014.1614. Epub 2014 Jan 7. PMID: 24399619. [DOI] [PubMed] [Google Scholar]
- 115.Luo C., Ke X., Xiong S., Sun Y., Xu Q., Zhang W., Lei Y., Ding Y., Zhen Y., Feng J., Cheng F., Chen J. Naringin attenuates high glucose-induced injuries and inflammation by modulating the leptin-JAK2/STAT3 pathway in H9c2 cardiac cells. Arch. Med. Sci. 2019 May 7;17(5):1145–1157. doi: 10.5114/aoms.2019.84854. PMID: 34522243; PMCID: PMC8425238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao Z., Ma X., Ma J., Sun X., Li F., Lv J. Naringin enhances endothelial progenitor cell (EPC) proliferation and tube formation capacity through the CXCL12/CXCR4/PI3K/Akt signaling pathway. Chem. Biol. Interact. 2018 Apr 25;286:45–51. doi: 10.1016/j.cbi.2018.03.002. Epub 2018 Mar 13. PMID: 29510123. [DOI] [PubMed] [Google Scholar]
- 117.Li F., Zhan Z., Qian J., Cao C., Yao W., Wang N. Naringin attenuates rat myocardial ischemia/reperfusion injury via PI3K/Akt pathway-mediated inhibition of apoptosis, oxidative stress and autophagy. Exp. Ther. Med. 2021 Aug;22(2):811. doi: 10.3892/etm.2021.10243. Epub 2021 May 28. PMID: 34131434; PMCID: PMC8193209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu W., Cheng L., Li X., Zhao L., Hu X., Ma Z. Short-term pretreatment of naringin isolated from Citrus wilsonii Tanaka attenuates rat myocardial ischemia/reperfusion injury. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022 Sep;395(9):1047–1059. doi: 10.1007/s00210-022-02255-x. Epub 2022 Jun 6. PMID: 35666279. [DOI] [PubMed] [Google Scholar]
- 119.Guo X., Ji Q., Wu M., Ma W. Naringin attenuates acute myocardial ischemia-reperfusion injury via miR- 126/GSK-3β/β-catenin signaling pathway. Acta Cir. Bras. 2022 Apr 8;37(1) doi: 10.1590/acb370102. PMID: 35416858; PMCID: PMC9000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li P., Yao X., Zhou Q., Meng X., Zhou T., Gu Q. Citrus peel flavonoid extracts: health-beneficial bioactivities and regulation of intestinal microecology in vitro. Front. Nutr. 2022 May 24;9 doi: 10.3389/fnut.2022.888745. PMID: 35685878; PMCID: PMC9171401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sost M.M., Ahles S., Verhoeven J., Verbruggen S., Stevens Y., Venema K. A citrus fruit extract high in polyphenols beneficially modulates the gut microbiota of healthy human volunteers in a validated in vitro model of the colon. Nutrients. 2021 Nov 1;13(11):3915. doi: 10.3390/nu13113915. PMID: 34836169; PMCID: PMC8619629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen S.Y., Zhou Q.Y., Chen L., Liao X., Li R., Xie T. The Aurantii Fructus Immaturus flavonoid extract alleviates inflammation and modulate gut microbiota in DSS-induced colitis mice. Front. Nutr. 2022 Oct 5;9 doi: 10.3389/fnut.2022.1013899. PMID: 36276817; PMCID: PMC9581122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fidélix M., Milenkovic D., Sivieri K., Cesar T. Microbiota modulation and effects on metabolic biomarkers by orange juice: a controlled clinical trial. Food Funct. 2020 Feb 26;11(2):1599–1610. doi: 10.1039/c9fo02623a. PMID: 32016250. [DOI] [PubMed] [Google Scholar]
- 124.von Knethen A., Soller M., Brüne B. Peroxisome proliferator-activated receptor gamma (PPAR gamma) and sepsis. Arch. Immunol. Ther. Exp. 2007 Jan-Feb;55(1):19–25. doi: 10.1007/s00005-007-0005-y. PMID: 17277894. [DOI] [PubMed] [Google Scholar]
- 125.Cao R., Wu X., Guo H., Pan X., Huang R., Wang G., Liu J. Naringin exhibited therapeutic effects against DSS-induced mice ulcerative colitis in intestinal barrier-dependent manner. Molecules. 2021 Oct 31;26(21):6604. doi: 10.3390/molecules26216604. PMID: 34771012; PMCID: PMC8588024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dong J., Chen Y., Yang F., Zhang W., Wei K., Xiong Y., Wang L., Zhou Z., Li C., Wang J., Chen D. Naringin exerts therapeutic effects on mice colitis: a study based on transcriptomics combined with functional experiments. Front. Pharmacol. 2021 Aug 24;12 doi: 10.3389/fphar.2021.729414. PMID: 34504431; PMCID: PMC8421552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hambardikar V.R., Mandlik D.S. Protective effect of naringin ameliorates TNBS-induced colitis in rats via improving antioxidant status and pro-inflammatory cytokines. Immunopharmacol. Immunotoxicol. 2022 Jun;44(3):373–386. doi: 10.1080/08923973.2022.2049813. Epub 2022 Mar 10. PMID: 35254187. [DOI] [PubMed] [Google Scholar]
- 128.Zhang Y.S., Wang F., Cui S.X., Qu X.J. Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol. Ther. 2018 Aug 3;19(8):735–744. doi: 10.1080/15384047.2018.1453971. Epub 2018 Apr 25. PMID: 29580144; PMCID: PMC6067845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Daniel B., Nagy G., Czimmerer Z., Horvath A., Hammers D.W., Cuaranta-Monroy I., Poliska S., Tzerpos P., Kolostyak Z., Hays T.T., Patsalos A., Houtman R., Sauer S., Francois-Deleuze J., Rastinejad F., Balint B.L., Sweeney H.L., Nagy L. The nuclear receptor PPARγ controls progressive macrophage polarization as a ligand-insensitive epigenomic ratchet of transcriptional memory. Immunity. 2018 Oct 16;49(4):615–626.e6. doi: 10.1016/j.immuni.2018.09.005. PMID: 30332629; PMCID: PMC6197058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Z.L., Yang B.C., Gao M., Xiao X.F., Zhao S.P., Liu Z.L. Naringin improves sepsis-induced intestinal injury by modulating macrophage polarization via PPARγ/miR-21 axis. Mol. Ther. Nucleic Acids. 2021 Jul 16;25:502–514. doi: 10.1016/j.omtn.2021.07.005. PMID: 34589273; PMCID: PMC8463290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Z., Gao M., Yang B., Zhang H., Wang K., Liu Z., Xiao X., Yang M. Naringin attenuates MLC phosphorylation and NF-κB activation to protect sepsis-induced intestinal injury via RhoA/ROCK pathway. Biomed. Pharmacother. 2018 Jul;103:50–58. doi: 10.1016/j.biopha.2018.03.163. Epub 2018 Apr 7. PMID: 29635128. [DOI] [PubMed] [Google Scholar]
- 132.Kim J.K., Park J.H., Ku H.J., Kim S.H., Lim Y.J., Park J.W., Lee J.H. Naringin protects acrolein-induced pulmonary injuries through modulating apoptotic signaling and inflammation signaling pathways in mice. J. Nutr. Biochem. 2018 Sep;59:10–16. doi: 10.1016/j.jnutbio.2018.05.012. Epub 2018 Jun 6. PMID: 29957300. [DOI] [PubMed] [Google Scholar]
- 133.Ahmad S.F., Attia S.M., Bakheet S.A., Zoheir K.M., Ansari M.A., Korashy H.M., Abdel-Hamied H.E., Ashour A.E., Abd-Allah A.R. Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of NF-κb, STAT3 and pro-inflammatory mediators and enhancement of IκBα and anti-inflammatory cytokines. Inflammation. 2015 Apr;38(2):846–857. doi: 10.1007/s10753-014-9994-y. PMID: 25117567. [DOI] [PubMed] [Google Scholar]
- 134.Matsumaru Daisuke, Motohashi Hozumi. vol. 10. Antioxidants; Basel, Switzerland: 1929. p. 12. (The KEAP1-NRF2 System in Healthy Aging and Longevity). 30 Nov. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Salama A.A.A., Yassen N.N., Mansour H.M. Naringin protects mice from D-galactose-induced lung aging and mitochondrial dysfunction: implication of SIRT1 pathways. Life Sci. 2023;324 doi: 10.1016/j.lfs.2023.121471. [DOI] [PubMed] [Google Scholar]
- 136.Shi R., Xu J.W., Xiao Z.T., Chen R.F., Zhang Y.L., Lin J.B., Cheng K.L., Wei G.Y., Li P.B., Zhou W.L., Su W.W. Naringin and naringenin relax rat tracheal smooth by regulating BKCa activation. J. Med. Food. 2019 Sep;22(9):963–970. doi: 10.1089/jmf.2018.4364. Epub 2019 Jun 28. PMID: 31259654. [DOI] [PubMed] [Google Scholar]
- 137.Ni K., Guo J., Bu B., Pan Y., Li J., Liu L., Luo M., Deng L. Naringin as a plant-derived bitter tastant promotes proliferation of cultured human airway epithelial cells via activation of TAS2R signaling. Phytomedicine. 2021 Apr;84 doi: 10.1016/j.phymed.2021.153491. Epub 2021 Feb 5. PMID: 33601237. [DOI] [PubMed] [Google Scholar]
- 138.Rada P., González-Rodríguez Á., García-Monzón C., Valverde Á.M. Understanding lipotoxicity in NAFLD pathogenesis: is CD36 a key driver? Cell Death Dis. 2020 Sep 25;11(9):802. doi: 10.1038/s41419-020-03003-w. PMID: 32978374; PMCID: PMC7519685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Juanola O., Martínez-López S., Francés R., Gómez-Hurtado I. Non-Alcoholic fatty liver disease: metabolic, genetic, epigenetic and environmental risk factors. Int. J. Environ. Res. Publ. Health. 2021 May 14;18(10):5227. doi: 10.3390/ijerph18105227. PMID: 34069012; PMCID: PMC8155932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang W., Kong D. The intestinal microbiota as a therapeutic target in the treatment of NAFLD and ALD. Biomed. Pharmacother. 2021 Mar;135 doi: 10.1016/j.biopha.2021.111235. Epub 2021 Feb 6. PMID: 33561650. [DOI] [PubMed] [Google Scholar]
- 141.Aron-Wisnewsky J., Warmbrunn M.V., Nieuwdorp M., Clément K. Nonalcoholic fatty liver disease: modulating gut microbiota to improve severity? Gastroenterology. 2020 May;158(7):1881–1898. doi: 10.1053/j.gastro.2020.01.049. Epub 2020 Feb 8. PMID: 32044317. [DOI] [PubMed] [Google Scholar]
- 142.Zhou C., Lai Y., Huang P., Xie L., Lin H., Zhou Z., Mo C., Deng G., Yan W., Gao Z., Huang S., Chen Y., Sun X., Lv Z., Gao L. Naringin attenuates alcoholic liver injury by reducing lipid accumulation and oxidative stress. Life Sci. 2019 Jan 1;216:305–312. doi: 10.1016/j.lfs.2018.07.031. Epub 2018 Jul 18. PMID: 30031061. [DOI] [PubMed] [Google Scholar]
- 143.Mu H., Zhou Q., Yang R., Zeng J., Li X., Zhang R., Tang W., Li H., Wang S., Shen T., Huang X., Dou L., Dong J. Naringin attenuates high fat diet induced non-alcoholic fatty liver disease and gut bacterial dysbiosis in mice. Front. Microbiol. 2020 Nov 13;11 doi: 10.3389/fmicb.2020.585066. PMID: 33281780; PMCID: PMC7691324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang X., Zhang Y., Gao W., Guo Z., Wang K., Liu S., Duan Z., Chen Y. Naringin improves lipid metabolism in a tissue-engineered liver model of NAFLD and the underlying mechanisms. Life Sci. 2021 Jul 15;277 doi: 10.1016/j.lfs.2021.119487. Epub 2021 Apr 20. PMID: 33862107. [DOI] [PubMed] [Google Scholar]
- 145.Jiang J., Yan L., Shi Z., Wang L., Shan L., Efferth T. Hepatoprotective and anti-inflammatory effects of total flavonoids of Qu Zhi Ke (peel of Citrus changshan-huyou) on non-alcoholic fatty liver disease in rats via modulation of NF-κB and MAPKs. Phytomedicine. 2019 Nov;64 doi: 10.1016/j.phymed.2019.153082. Epub 2019 Sep 3. PMID: 31541796. [DOI] [PubMed] [Google Scholar]
- 146.Zhang X., Fan L., Wu J., Xu H., Leung W.Y., Fu K., Wu J., Liu K., Man K., Yang X., Han J., Ren J., Yu J. Macrophage p38α promotes nutritional steatohepatitis through M1 polarization. J. Hepatol. 2019 Jul;71(1):163–174. doi: 10.1016/j.jhep.2019.03.014. Epub 2019 Mar 23. Erratum in: J Hepatol. 2020 Sep;73(3):742-743. PMID: 30914267. [DOI] [PubMed] [Google Scholar]
- 147.Pengnet S., Sumarithum P., Phongnu N., Prommaouan S., Kantip N., Phoungpetchara I., Malakul W. Naringin attenuates fructose-induced NAFLD progression in rats through reducing endogenous triglyceride synthesis and activating the Nrf2/HO-1 pathway. Front. Pharmacol. 2022 Dec 15;13 doi: 10.3389/fphar.2022.1049818. PMID: 36588703; PMCID: PMC9797507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ribera J., Vilches C., Sanz V., de Miguel I., Portolés I., Córdoba-Jover B., Prat E., Nunes V., Jiménez W., Quidant R., Morales-Ruiz M. Treatment of hepatic fibrosis in mice based on targeted plasmonic hyperthermia. ACS Nano. 2021 Apr 27;15(4):7547–7562. doi: 10.1021/acsnano.1c00988. Epub 2021 Mar 15. PMID: 33720693. [DOI] [PubMed] [Google Scholar]
- 149.Xu W., Wang L., Niu Y., Mao L., Du X., Zhang P., Li Z., Li H., Li N. A review of edible plant-derived natural compounds for the therapy of liver fibrosis. Eur. J. Gastroenterol. Hepatol. 2023 Feb 1;35(2):133–152. doi: 10.1097/MEG.0000000000002483. Epub 2022 Dec 8. PMID: 36574304. [DOI] [PubMed] [Google Scholar]
- 150.Syed A.A., Reza M.I., Shafiq M., Kumariya S., Singh P., Husain A., Hanif K., Gayen J.R. Naringin ameliorates type 2 diabetes mellitus-induced steatohepatitis by inhibiting RAGE/NF-κB mediated mitochondrial apoptosis. Life Sci. 2020 Sep 15;257 doi: 10.1016/j.lfs.2020.118118. Epub 2020 Jul 20. PMID: 32702445. [DOI] [PubMed] [Google Scholar]
- 151.Rodríguez V., Plavnik L., Tolosa de Talamoni N. Naringin attenuates liver damage in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018 Sep;105:95–102. doi: 10.1016/j.biopha.2018.05.120. Epub 2018 May 28. PMID: 29852394. [DOI] [PubMed] [Google Scholar]
- 152.Meng X.M., Nikolic-Paterson D.J., Lan H.Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 2016 Jun;12(6):325–338. doi: 10.1038/nrneph.2016.48. Epub 2016 Apr 25. PMID: 27108839. [DOI] [PubMed] [Google Scholar]
- 153.Hu H.H., Chen D.Q., Wang Y.N., Feng Y.L., Cao G., Vaziri N.D., Zhao Y.Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018 Aug 25;292:76–83. doi: 10.1016/j.cbi.2018.07.008. Epub 2018 Jul 11. PMID: 30017632. [DOI] [PubMed] [Google Scholar]
- 154.Wang R., Wu G., Dai T., Lang Y., Chi Z., Yang S., Dong D. Naringin attenuates renal interstitial fibrosis by regulating the TGF-β/Smad signaling pathway and inflammation. Exp. Ther. Med. 2021 Jan;21(1):66. doi: 10.3892/etm.2020.9498. Epub 2020 Nov 23. PMID: 33365066; PMCID: PMC7716641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen F., Wei G., Xu J., Ma X., Wang Q. Naringin ameliorates the high glucose-induced rat mesangial cell inflammatory reaction by modulating the NLRP3 Inflammasome. BMC Compl. Alternative Med. 2018 Jun 22;18(1):192. doi: 10.1186/s12906-018-2257-y. PMID: 29929501; PMCID: PMC6014005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pérez A., Mukdsi J., Valdez L., et al. Naringin prevents diabetic nephropathy in rats through blockage of oxidative stress and attenuation of the mitochondrial dysfunction. Can. J. Physiol. Pharmacol. 2023;101(7):349–360. doi: 10.1139/cjpp-2022-0449. [DOI] [PubMed] [Google Scholar]
- 157.Amini N., Sarkaki A., Dianat M., Mard S.A., Ahangarpour A., Badavi M. The renoprotective effects of naringin and trimetazidine on renal ischemia/reperfusion injury in rats through inhibition of apoptosis and downregulation of micoRNA-10a. Biomed. Pharmacother. 2019 Apr;112 doi: 10.1016/j.biopha.2019.01.029. Epub 2019 Feb 16. PMID: 30780111. [DOI] [PubMed] [Google Scholar]
- 158.Amini N., Sarkaki A., Dianat M., Mard S.A., Ahangarpour A., Badavi M. Protective effects of naringin and trimetazidine on remote effect of acute renal injury on oxidative stress and myocardial injury through Nrf-2 regulation. Pharmacol. Rep. 2019 Dec;71(6):1059–1066. doi: 10.1016/j.pharep.2019.06.007. Epub 2019 Jun 14. PMID: 31604166. [DOI] [PubMed] [Google Scholar]
- 159.Yen J.H., Chio W.T., Chuang C.J., Yang H.L., Huang S.T. Improved wound healing by naringin associated with MMP and the VEGF pathway. Molecules. 2022 Mar 4;27(5):1695. doi: 10.3390/molecules27051695. PMID: 35268795; PMCID: PMC8911856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vabeiryureilai M., Lalrinzuali K., Jagetia G.C. NF-κB and COX-2 repression with topical application of hesperidin and naringin hydrogels augments repair and regeneration of deep dermal wounds. Burns. 2022 Feb;48(1):132–145. doi: 10.1016/j.burns.2021.04.016. Epub 2021 Apr 21. PMID: 33972147. [DOI] [PubMed] [Google Scholar]
- 161.Alsakhawy M.A., Abdelmonsif D.A., Haroun M., Sabra S.A. Naringin-loaded Arabic gum/pectin hydrogel as a potential wound healing material. Int. J. Biol. Macromol. 2022 Dec 1;222(Pt A):701–714. doi: 10.1016/j.ijbiomac.2022.09.200. Epub 2022 Sep 25. PMID: 36170930. [DOI] [PubMed] [Google Scholar]
- 162.Deenonpoe R., Prayong P., Thippamom N., Meephansan J., Na-Bangchang K. Anti-inflammatory effect of naringin and sericin combination on human peripheral blood mononuclear cells (hPBMCs) from patient with psoriasis. BMC Compl. Alternative Med. 2019 Jul 10;19(1):168. doi: 10.1186/s12906-019-2535-3. PMID: 31291937; PMCID: PMC6617890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mohammadi M., Kohan L., Saeidi M., Saghaeian Jazi M., Mohammadi S. The antifibrotic effects of naringin in a hypochlorous acid (HOCl)-induced mouse model of skin fibrosis. Immunopharmacol. Immunotoxicol. 2022 Oct;44(5):704–711. doi: 10.1080/08923973.2022.2077217. Epub 2022 May 18. PMID: 35583493. [DOI] [PubMed] [Google Scholar]
- 164.Song Y., Guo B., Ma S., Chang P., Tao K. Naringin suppresses the growth and motility of hypertrophic scar fibroblasts by inhibiting the kinase activity of Akt. Biomed. Pharmacother. 2018 Sep;105:1291–1298. doi: 10.1016/j.biopha.2018.06.103. Epub 2018 Jun 23. PMID: 30021366. [DOI] [PubMed] [Google Scholar]
- 165.Aihaiti Y., Song Cai Y., Tuerhong X., Ni Yang Y., Ma Y., Shi Zheng H., Xu K., Xu P. Therapeutic effects of naringin in rheumatoid arthritis: network pharmacology and experimental validation. Front. Pharmacol. 2021 May 14;12 doi: 10.3389/fphar.2021.672054. PMID: 34054546; PMCID: PMC8160516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhao Y., Li Z., Wang W., Zhang H., Chen J., Su P., Liu L., Li W. Naringin protects against cartilage destruction in osteoarthritis through repression of NF-κB signaling pathway. Inflammation. 2016 Feb;39(1):385–392. doi: 10.1007/s10753-015-0260-8. PMID: 26438631. [DOI] [PubMed] [Google Scholar]
- 167.Huang D., Li Z., Chen B., Fang G., Sun X., Li F., Xu H., Chen Y., Ding W. Naringin protects against steroid-induced avascular necrosis of the femoral head through upregulation of PPARγ and activation of the Notch signaling pathway. Mol. Med. Rep. 2018 Feb;17(2):3328–3335. doi: 10.3892/mmr.2017.8247. Epub 2017 Dec 11. PMID: 29257271. [DOI] [PubMed] [Google Scholar]
- 168.Ye C., Chen J., Qu Y., Qi H., Wang Q., Yang Z., Wu A., Wang F., Li P. Naringin in the repair of knee cartilage injury via the TGF-β/ALK5/Smad2/3 signal transduction pathway combined with an acellular dermal matrix. J Orthop Translat. 2021 Aug 6;32:1–11. doi: 10.1016/j.jot.2021.06.004. PMID: 35591936; PMCID: PMC9072805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang Y., Wu H., Chen P., Su W., Peng W., Li P. Fertility and early embryonic development toxicity assessment of naringin in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2021;123 doi: 10.1016/j.yrtph.2021.104938. [DOI] [PubMed] [Google Scholar]
- 170.Li P., Wang S., Guan X., et al. Acute and 13 weeks subchronic toxicological evaluation of naringin in Sprague-Dawley rats. Food Chem. Toxicol. 2013;60:1–9. doi: 10.1016/j.fct.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 171.Li P., Wang S., Guan X., et al. Six months chronic toxicological evaluation of naringin in Sprague-Dawley rats. Food Chem. Toxicol. 2014;66:65–75. doi: 10.1016/j.fct.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 172.Li P., Wu H., Wang Y., Peng W., Su W. Toxicological evaluation of naringin: acute, subchronic, and chronic toxicity in Beagle dogs. Regul. Toxicol. Pharmacol. 2020;111 doi: 10.1016/j.yrtph.2020.104580. [DOI] [PubMed] [Google Scholar]
- 173.Secerli J., Adatepe Ş., Altuntas S., Topal G.R., Erdem O., Bacanlı M. In vitro toxicity of naringin and berberine alone, and encapsulated within PMMA nanoparticles. Toxicol. Vitro. 2023;89 doi: 10.1016/j.tiv.2023.105580. [DOI] [PubMed] [Google Scholar]
- 174.Ranawat P., Bakshi N. Naringenin; a bioflavonoid, impairs the reproductive potential of male mice. Toxicol. Mech. Methods. 2017;27(6):417–427. doi: 10.1080/15376516.2017.1296048. [DOI] [PubMed] [Google Scholar]
- 175.Boncler M., Golanski J., Lukasiak M., Redzynia M., Dastych J., Watala C. A new approach for the assessment of the toxicity of polyphenol-rich compounds with the use of high content screening analysis. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0180022. Published 2017 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu M., Yang C., Zou W., et al. Toxicokinetics of naringin, a putative antitussive, after 184-day repeated oral administration in rats. Environ. Toxicol. Pharmacol. 2011;31(3):485–489. doi: 10.1016/j.etap.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 177.Yang C.P., Liu M.H., Zou W., Guan X.L., Lai L., Su W.W. Toxicokinetics of naringin and its metabolite naringenin after 180-day repeated oral administration in beagle dogs assayed by a rapid resolution liquid chromatography/tandem mass spectrometric method. J. Asian Nat. Prod. Res. 2012;14(1):68–75. doi: 10.1080/10286020.2011.632369. [DOI] [PubMed] [Google Scholar]
- 178.Ortiz-Andrade R., Araujo-León J.A., Sánchez-Recillas A., et al. Toxicological screening of four bioactive citroflavonoids: in vitro, in vivo, and in silico approaches. Molecules. 2020;25(24):5959. doi: 10.3390/molecules25245959. Published 2020 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rebello C.J., Beyl R.A., Lertora J.J.L., et al. Safety and pharmacokinetics of naringenin: a randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metabol. 2020;22(1):91–98. doi: 10.1111/dom.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bai Y., Peng W., Yang C., et al. Pharmacokinetics and metabolism of naringin and active metabolite naringenin in rats, dogs, humans, and the differences between species. Front. Pharmacol. 2020;11:364. doi: 10.3389/fphar.2020.00364. Published 2020 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Naeini F., Namkhah Z., Ostadrahimi A., Tutunchi H., Hosseinzadeh-Attar M.J. A comprehensive systematic review of the effects of naringenin, a citrus-derived flavonoid, on risk factors for nonalcoholic fatty liver disease. Adv. Nutr. 2021;12(2):413–428. doi: 10.1093/advances/nmaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hou Y.C., Hsiu S.L., Yen H.F., Chen C.C., Chao P.D. Effect of honey on naringin absorption from a decoction of the pericarps of Citrus grandis. Planta Med. 2000;66(5):439–443. doi: 10.1055/s-2000-8582. [DOI] [PubMed] [Google Scholar]
- 183.Jung U.J., Kim H.J., Lee J.S., et al. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin. Nutr. 2003;22(6):561–568. doi: 10.1016/s0261-5614(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 184.Demonty I., Lin Y., Zebregs Y.E., et al. The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J. Nutr. 2010;140(9):1615–1620. doi: 10.3945/jn.110.124735. [DOI] [PubMed] [Google Scholar]
- 185.Ribeiro C.B., Ramos F.M., Manthey J.A., Cesar T.B. Effectiveness of Eriomin® in managing hyperglycemia and reversal of prediabetes condition: a double-blind, randomized, controlled study. Phytother Res. 2019;33(7):1921–1933. doi: 10.1002/ptr.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mollace V., Scicchitano M., Paone S., et al. Hypoglycemic and hypolipemic effects of a new lecithin formulation of bergamot polyphenolic fraction: a double blind, randomized, placebo- controlled study. Endocr., Metab. Immune Disord.: Drug Targets. 2019;19(2):136–143. doi: 10.2174/1871530319666181203151513. [DOI] [PubMed] [Google Scholar]
- 187.Fuhr U., Kummert A.L. The fate of naringin in humans: a key to grapefruit juice-drug interactions? Clin. Pharmacol. Ther. 1995;58(4):365–373. doi: 10.1016/0009-9236(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 188.Al-Aubaidy H.A., Dayan A., Deseo M.A., et al. Twelve-Week mediterranean diet intervention increases citrus bioflavonoid levels and reduces inflammation in people with type 2 diabetes mellitus. Nutrients. 2021;13(4):1133. doi: 10.3390/nu13041133. Published 2021 Mar 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yaskolka Meir A., Rinott E., Tsaban G., et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut. 2021;70(11):2085–2095. doi: 10.1136/gutjnl-2020-323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Galluzzi S., Zanardini R., Ferrari C., et al. Cognitive and biological effects of citrus phytochemicals in subjective cognitive decline: a 36-week, randomized, placebo-controlled trial. Nutr. J. 2022;21(1):64. doi: 10.1186/s12937-022-00817-6. Published 2022 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bailey D.G., Dresser G.K., Kreeft J.H., Munoz C., Freeman D.J., Bend J.R. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin. Pharmacol. Ther. 2000;68(5):468–477. doi: 10.1067/mcp.2000.110774. [DOI] [PubMed] [Google Scholar]
- 192.Bailey D.G., Arnold J.M., Munoz C., Spence J.D. Grapefruit juice--felodipine interaction: mechanism, predictability, and effect of naringin. Clin. Pharmacol. Ther. 1993;53(6):637–642. doi: 10.1038/clpt.1993.84. [DOI] [PubMed] [Google Scholar]
- 193.Bailey D.G., Kreeft J.H., Munoz C., Freeman D.J., Bend J.R. Grapefruit juice-felodipine interaction: effect of naringin and 6',7'-dihydroxybergamottin in humans. Clin. Pharmacol. Ther. 1998;64(3):248–256. doi: 10.1016/S0009-9236(98)90173-4. [DOI] [PubMed] [Google Scholar]
- 194.Rebello S., Zhao S., Hariry S., et al. Intestinal OATP1A2 inhibition as a potential mechanism for the effect of grapefruit juice on aliskiren pharmacokinetics in healthy subjects. Eur. J. Clin. Pharmacol. 2012;68(5):697–708. doi: 10.1007/s00228-011-1167-4. [DOI] [PubMed] [Google Scholar]
- 195.Bailey D.G., Dresser G.K., Leake B.F., Kim R.B. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin. Pharmacol. Ther. 2007;81(4):495–502. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- 196.Nguyen M.A., Staubach P., Tamai I., Langguth P. High-dose short-term administration of naringin did not alter talinolol pharmacokinetics in humans. Eur. J. Pharmaceut. Sci. 2015;68:36–42. doi: 10.1016/j.ejps.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 197.Bailey D.G., Arnold J.M., Strong H.A., Munoz C., Spence J.D. Effect of grapefruit juice and naringin on nisoldipine pharmacokinetics. Clin. Pharmacol. Ther. 1993;54(6):589–594. doi: 10.1038/clpt.1993.195. [DOI] [PubMed] [Google Scholar]
- 198.Calabrese E.J., Pressman P., Hayes A.W., et al. Naringin commonly acts via hormesis. Sci. Total Environ. 2023;896 doi: 10.1016/j.scitotenv.2023.164728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.