Abstract
Small globular proteins contain internal cavities and packing defects that reduce thermodynamic stability but seem to play a role in controlling function by defining pathways for the diffusion of the ligand/substrate to the active site. In the case of myoglobin (Mb), a prototype for structure–function relationship studies, the photosensitivity of the adduct of the reduced protein with CO, O2 and NO allows events related to the migration of the ligand through the matrix to be followed. The crystal structures of intermediate states of wild-type (wt) and mutant Mbs show the photolysed CO to be located either in the distal heme pocket (primary docking site) or in one of two alternative cavities (secondary docking sites) corresponding to packing defects accessible to an atom of xenon. These results convey the general picture that pre-existing internal cavities are involved in controlling the dynamics and reactivity of the reactions of Mb with O2 and other ligands, including NO.
Introduction
As the first protein whose three-dimensional structure was deciphered at atomic resolution (Kendrew et al., 1960), myoglobin (Mb) has enjoyed a central position in protein science, so much so that it has sometimes been called the ‘hydrogen atom of molecular biology’. This small monomeric hemeprotein has a rich optical spectrum (Antonini and Brunori, 1971), which has made possible detailed ligand binding studies and helped in the characterization of site-directed mutants (Varadarajan et al., 1985; Springer and Sligar, 1987). Mb’s function in the heart and skeletal muscle is O2 storage and delivery (Stryer, 1975). In the 1960s, it was discovered that Mb facilitates O2 diffusion from the periphery of the cell to the mitochondria, for respiration (see Wittenberg, 1970). Ferrous Mb is known to bind several other small molecules; among these, carbon monoxide (CO) and nitric oxide (NO) have been frequently studied and their equilibrium and kinetic properties compared with those of O2 (Springer et al., 1994). Very recently, it was proposed (Brunori, 2001) that oxymyoglobin (MbO2) may also have the role of scavenging NO in the red muscle; since NO is known to inhibit mitochondrial respiration (Cleeter et al., 1994), this reaction of MbO2 may protect the energy-producing machinery and thus prevent muscle failure.
Taking advantage of the photosensitivity of the heme Fe–ligand bond (Haldane and Lorrain-Smith, 1897; Gibson and Ainsworth, 1957), Gibson (1959) performed experiments on the ligand-rebinding kinetics of Mb and hemoglobin (Hb) after photodissociation induced by a microsecond pulse of white light. These were among the first applications of flash technology, an approach that paved the way for a dynamic view of structural biology that became prolific after the introduction of fast laser technology. In 1975, the concept that geminate rebinding and conformational substates play a central role in controlling the reactivity of Mb with ligands was introduced (Austin et al., 1975). Recently, crystallographic experiments on reaction intermediates of Mb (including an X-ray diffraction experiment with nanosecond time resolution; Srajer et al., 1996) provided the structural basis for an interpretation of the extensive kinetic data obtained by transient spectroscopy in solution (Brunori, 2000; Schlichting and Chu, 2000; Scott et al., 2001; and references therein).
This paper presents our view of the structural dynamics of a protein as may be deduced from studies on the reactions of Mb with O2 and other ligands using different experimental approaches. In particular, we address the question concerning the role of cavities and packing defects in the interior of globular proteins, in controlling reactivity.
Cavities and packing defects
Small globular proteins have an internal structure that reveals a number of packing defects which were identified on inspection of their three-dimensional high resolution models (Richards, 1977). These cavities, with volumes ranging from 30 to 100 Å3, are an integral part of the protein matrix; those found in Mb are coated by hydrophobic residues and therefore do not contain bound water molecules. Packing defects imply an energetic cost and reduce the overall thermodynamic stability of a protein. In fact, filling these voids using mutagenesis strategies increases the melting temperature of globular proteins (such as T4 lysozyme and cytochrome c552; Anderson et al., 1993; Hasegawa et al., 1999), mimicking the behavior of thermophilic proteins, which are generally more stable and less flexible than their mesophilic counterparts (Jaenike, 1999).
Over and above the general notion that conformational mobility is essential for efficient catalysis, one may wonder why internal packing defects were retained during evolution, given that stability is crucial in maintaining the native state of a protein. In this review, we offer a structural interpretation for the functional advantages that may be conferred on a protein by the retention of internal cavities, the conclusions being largely based on analysis of laser-activated kinetic experiments in solution and on recent crystallographic data for reaction intermediates of Mb. Thus, the general theme addressed below is whether there is a functional significance to maintaining cavities and packing defects in the interior of a globular protein during evolution, and how they may contribute to control and optimal fitness.
The discovery of the geminate states
The structure of Mb shows that the heme is deeply buried in the protein, and there is no obvious channel through which O2 could diffuse towards the metal for binding. It was, therefore, postulated that fluctuations of the backbone and the side-chains are essential for O2 transport over a range of time that is compatible with mammalian physiology. More than 30 years ago, Perutz and Matthews (1966) proposed that ligand migration from the heme pocket to the solvent, and vice versa, involves rotation of the side-chain of the distal His64(E7), which is at H-bonding distance from bound O2 (Perutz, 1989). The His-gate hypothesis was supported by crystallographic work on different myoglobins (Bolognesi et al., 1982), and is nowadays on a firm basis due to extensive kinetic experiments carried out on site-directed mutants (see Scott et al., 2001). Ligand binding to Mb has been described by a four-state scheme that, although oversimplified, provides a useful framework for this discussion:
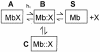 1
1
As outlined above, all the liganded states of ferrous Mb (including MbO2, MbCO and MbNO) are photosensitive (Gibson and Ainsworth, 1957); thus, hitting ligand-bound Mb (state A) with an intense and short light pulse leads to breakage of the bond and relaxation of the protein (Henry et al., 1983; Gibson, 1989). If the photodissociated ligand diffuses into the solvent (state S), rebinding to Mb will follow bimolecular kinetics. However, a fraction of the (photo)dissociated ligand remains momentarily ‘trapped’ within the protein matrix; this quota may recombine by intramolecular diffusion and collision with the iron, in a process called geminate rebinding (Austin et al., 1975). Thus, migration of the photodissociated ligand can be exploited to probe the internal structure of the protein and its fluctuations on a time-scale ranging from picoseconds to microseconds. Scheme 1 shows two geminate states (B and C) because geminate rebinding can be biphasic or multiphasic. The conundrum is how to correlate geminate states B and C and their relative populations to the internal structure of the protein. The binding of xenon to Mb suggests that there may be a link between internal cavities and dynamics states.
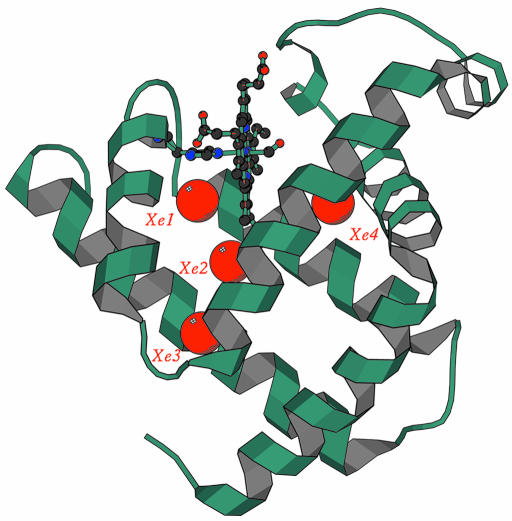
In 1965, Kendrew and his collaborators (Shoenborn et al., 1965) discovered that xenon binds to Mb. Twenty years later, Tilton et al. (1984) solved the structure of Met-Mb equilibrated with xenon gas. As shown in Figure 1, the crystallographic data revealed four xenon atoms (bound to sites called Xe1 to Xe4), which occupy pre-existing cavities (with radii >5Å) lined with hydrophobic side-chains. This finding has acquired significance because of crystallographic experiments on reaction intermediates that bridge the gap between xenon binding cavities and transient docking sites for the dissociated ligand trapped in the matrix.
Fig. 1. The xenon binding sites of myoglobin. Crystal structure, at 1.9 Å resolution, of sperm whale met-Mb under Xe gas at 7 atm. pressure. The four xenon atoms bound are shown as red spheres, and the protein backbone as a ribbon; the heme is seen edge-on, with the proximal His92 (F8) on the left. Xe1 and Xe2 are relatively close to each other on the proximal side of the heme; Xe3 resides in a cavity near the surface, far (13.5 Å) from the iron; Xe4 is on the distal side of the heme, ∼8 Å from the iron (modified from Tilton et al., 1984).
Crystallography of reaction intermediates
Srajer et al. (1996) followed the X-ray diffraction of Mb single crystals with nanosecond time resolution at room temperature, and showed the immediate disappearance of CO density adjacent to the iron and its motion into the heme plane. The resolution in this technically demanding experiment was not sufficient to define pathways followed by the photodissociated ligand. The structures of geminate states at low or ultra-low temperatures have been obtained by several groups during the last seven years (Schlichting et al., 1994; Teng et al., 1994, 1997; Hartman et al., 1996; Brunori et al., 2000; Chu et al., 2000; Ostermann et al., 2000); these high resolution results showed the location of CO following photodissociation.
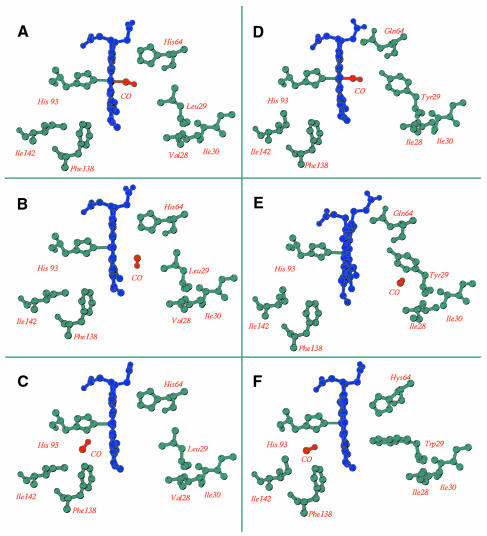
The picture emerging from these studies is summarized in Figure 2, which presents a compilation of data obtained from experiments carried out in two different space groups with two Mbs (horse and sperm whale) and two site-directed mutants. In a nutshell, the crystallographic data show that the photolysed CO can be seen at more than one docking site inside the protein. The primary docking site is close to the Fe2+ in the distal pocket, the CO lying parallel to the heme plane, ∼3.6 Å from the metal (Figure 2B), as shown by ultra-low temperature (20–40 K) experiments on wild-type (wt) Mb. This position, which is occupied a few picoseconds after photodissociation (Lim et al., 1997), is presumed to be associated with the faster geminate rebinding phase (state B).
Fig. 2. Three-dimensional structure of myoglobin and photochemical intermediates. Structure of the active site of horse heart (A–C) and sperm whale (D–F) Mb. The heme is seen edge-on with the proximal His92(F8) on the left coordinated to the heme iron, and the distal heme pocket on the right. The CO is colored red and indicated explicitly in all panels. In (A), the CO is bound to the heme iron of wt Mb, but moves to the primary docking site in the distal pocket after photolysis at 22 K (B). However, if photodissociation is initiated at 160 K, the CO migrates to the proximal side of the heme into a secondary docking site, as shown in (C). When the experiment is carried out with Mb mutants containing an aromatic residue (Tyr or Trp) at postion 29, the CO initially bound to the heme in the dark (D) migrates to the secondary docking site distal to the heme (E) upon photolysis at 20 K, but moves to that on the proximal side (F) if photolysis is performed at 180 K [modified from Chu et al. (2000) for (A–C); from Brunori et al. (2000) for (D and E); and from Ostermann et al. (2000) for (F)].
Photodissociated CO has also been observed at two secondary docking sites at greater distance from the Fe2+. These overlap with two of the xenon binding sites (namely Xe1 and Xe4). High occupancy of the Xe4 site (Figure 2E) has been observed at low temperatures in two mutants of sperm whale Mb, both involving substitution of Leu29(B10) with an aromatic amino acid (Tyr or Trp) residue (Brunori et al., 2000; Ostermann et al., 2000). At higher temperatures (∼180 K), the CO migrates to the proximal side of the heme in wt horse Mb (Figure 2C; Chu et al., 2000), as well as in the mutant Leu29(B10)→Trp (Figure 2F; Ostermann et al., 2000). The latter study was also informative because it provided a coherent correlation between results obtained by crystallography and infrared spectroscopy, which together show that kinetic changes occur after extended illumination because ligands migrate to the Xe1 cavity. Whether the photodissociated ligand can diffuse out of these cavities directly to the solvent (as suggested by molecular dynamics simulations, see Elber and Karplus, 1990) is uncertain, but the majority (∼80%) of the ligand escapes from the His-gate (see above), as extensively discussed by Scott et al. (2001). At any rate, the photolysed ligand has been observed only in two secondary docking sites (Figure 2C, E and F), which overlap with two of the four xenon binding sites identified by Tilton et al. (1984).
Structural dynamics
The time-course and the yield of the geminate phases are both sensitive to the immediate environment of the heme, and therefore to the conformation of the protein (Frauenfelder et al., 1988; Ansari et al., 1994). It has been proposed that the biphasic (or multiphasic) time-course of the geminate phase (shown in Figure 3) reflects rebinding of the ligand from distinct docking sites at various distances from the heme. By reference to Scheme 1, it was assumed that rebinding from the B state (Mb:X) is faster than from the C state (Mb::X), because in the former the photodissociated ligand is closer to the metal. This interpretation was championed by Gibson et al. (1992), based on the geminate kinetics of a number of mutants and chemically modified Mbs, e.g. an Mb containing cobalt instead of iron (Ikeda-Saito et al., 1993). Although by no means universally accepted (see, for example, Friedman, 1985), this proposal provides a convenient model to relate the speed of geminate rebinding to the physical location of the photodissociated ligand with respect to the heme-iron and, in this respect, differs from the view extensively elaborated by Frauenfelder et al. (1988).
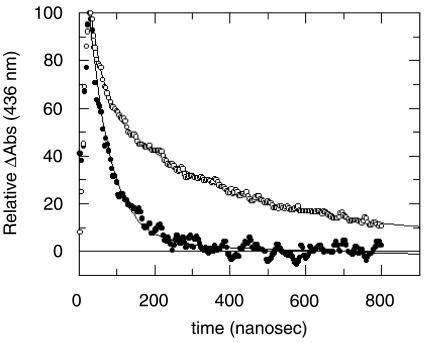
Fig. 3. Geminate rebinding of NO to myoglobin. The figure depicts the time-course of geminate rebinding of NO to the Mb mutant Mb-YQR (L29Y; H64Q; T67R; modified from Brunori et al., 1999b). The upward deflection at shorter times indicates the laser-induced photodissociation obtained with a 9 ns laser pulse of green light. The geminate recombination of dissociated NO yields a biphasic time-course in the absence of xenon (open circles); however, under xenon at high pressure (filled circles), the geminate recombination becomes faster and more homogeneous because the slow kinetic component essentially disappears (and the total amplitude of the excursion decreases to one half; not shown in this representation). This result is consistent with the hypothesis that the slower component of the geminate rebinding reflects the ligand trapped in secondary docking sites, which in the presence of xenon are not available to host the ligand.
A paper by Scott and Gibson (1997) provided convincing evidence in support of this hypothesis. By following the geminate rebinding of O2 in the absence and in the presence of xenon (at pressures up to 12 atm.), these authors observed that the amplitude of the slower geminate component often decreased or disappeared in the presence of xenon; this suggested that when the secondary docking sites are occupied by this gas, they are not available to host the photolysed ligand. This effect was also observed with several site-directed mutants. The data in Figure 3 suggested (Brunori et al., 1999b) that the unusually slow geminate recombination of NO observed with a triple-mutant of sperm whale Mb was due to substantial migration of the photolysed ligand into a secondary docking site, and to hindrance by Tyr29 at position B10 (see Figure 2E). These experimental observations are generally consistent with earlier molecular dynamics simulations carried out by Elber and Karplus (1990), who suggested three major trajectories, with the ligand trapped in the xenon binding cavities and making rare and rapid transitions between them.
Concluding remarks and biological implications
We have discussed the hypothesis that internal cavities and packing defects in Mb are the docking sites transiently occupied by the ligand (O2, CO, NO) during its trajectory through the protein, and define a pathway for migration to and from the heme, which is deeply buried. The kinetic behavior of geminate rebinding depends on the ligand being trapped momentarily inside the protein, and a correlation between geminate rates and location of the ligand at a limited number of specific docking sites seems consistent with the results of recent X-ray diffraction studies of intermediate states. Low temperature crystallographic experiments on the photolytic intermediates of Mb (Figure 2) identified a primary docking site (which hosts the photolysed ligand fairly close to the iron in the distal heme pocket) and two secondary docking sites at greater distance. The latter coincide with two of the voids occupied by xenon (at high pressure), as depicted in Figure 1. The effect of xenon on the geminate recombination time-course (Scott and Gibson, 1997; Brunori et al., 1999b) provided a link between kinetics of rebinding and location of the photolysed ligand in the matrix, supporting the view that rebinding is slower when the ligand is trapped momentarily at greater distance from the active site. Since overall rates of O2 binding and dissociation dictate efficient delivery to tissues and thus physiology, we previously concluded that the dynamics of the internal architecture of a protein has evolutionary value (Brunori et al., 1999a).
This view of the internal packing defects may also provide a clue for understanding the control of reactivity. A cavity containing a molecule may participate in and, in some sense, catalyze a chemical process, as illustrated by the mechanism of the reaction of NO with oxyhemoglobin or oxymyoglobin (Doyle and Hoekstra, 1981; Eich et al., 1996). In the skeletal and heart muscle the latter process may protect cytochrome c oxidase from NO inhibition (Cleeter et al., 1994), acquiring physiopathological significance as suggested by Brunori (2001). The reaction of NO with O2 in solution involves a ter-molecular process (Ford et al., 1993), 2NO + O2 → 2NO2, which at the concentrations of NO prevailing in the cell (<1 µM) is very slow. This is why even in well oxygenated tissues, the lifetime of biologically synthesized NO is quite long (many seconds to minutes), which explains how this messenger can diffuse away from the site of synthesis to meet its targets. In contrast, oxidation of NO to nitrate via the reaction MbO2 + NO → Met-Mb+ + NO3–, is very rapid (Eich et al., 1996), with half-times that may be in the millisecond time range in the red muscle. This huge enhancement in reactivity depends on the superoxide character acquired by O2 once bound to the heme iron (Weiss, 1964); transient trapping of NO in the internal protein cavities, as discussed in this review, enhances the probability of encounter of NO with ‘activated’ metal bound O2– and, thereby, the in situ formation of peroxynitrite (Herold, 1999). Thus, cavities acquire the status of catalytically active components of a macromolecular reactor.
In conclusion, we have put forward the general concept that cavities have a functional role, and recognize a potential selective advantage in building a protein with packing defects, over and above the common view that they simply enhance flexibility, and thereby effective catalysis, at the optimal temperature for the organism. In essence, there seems to be some merit in retaining internal packing defects during evolution.

Maurizio Brunori & Quentin H. Gibson

Acknowledgments
Acknowledgements
The authors are grateful to Dr W.A. Eaton (NIH, Bethesda, MD) for carefully reading and commenting on the manuscript, and to Drs L. Savino and B. Vallone (Rome, Italy) for preparing the figures. Financial support by the MURST of Italy (PRIN 1999, ‘Dinamica strutturale di emoproteine’), and by the NIH, Bethesda (GM 14276) is acknowledged.
References
- Anderson D.E., Hurley, J.H., Nicholson, H., Basse, W.A. and Matthews, B.W. (1993) Hydrophobic core repacking and aromatic–aromatic interaction in the thermostable mutant of T4 lysozyme Ser117→Phe. Protein Sci., 2, 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Jones, C.M., Henry, E.R., Hofrichter, J. and Eaton, W.A. (1994) Conformational relaxation and ligand binding in myoglobin. Biochemistry, 33, 5128–5145. [DOI] [PubMed] [Google Scholar]
- Antonini E. and Brunori, M. (1971) Hemoglobin and myoglobin in their reactions with ligands. North Holland Publishing Co., Amsterdam, The Netherlands.
- Austin R.H., Beeson, K.W., Eisenstein, L., Frauenfelder, H. and Gunsalus, I.C. (1975) Dynamics of ligand binding to myoglobin. Biochemistry, 14, 5355–5373. [DOI] [PubMed] [Google Scholar]
- Bolognesi M., Cannillo, E., Ascenzi, P., Giacometti, G.M., Merli, A. and Brunori, M. (1982) Reactivity of ferric Aplysia and sperm whale myoglobins towards imidazole. J. Mol. Biol., 158, 305–315. [DOI] [PubMed] [Google Scholar]
- Brunori M. (2000) The structural dynamics of myoglobin. Biophys. Chem., 86, 221–230. [DOI] [PubMed] [Google Scholar]
- Brunori M. (2001) Nitric oxide, cytochrome-c-oxidase and myoglobin. Trends Biochem. Sci., 26, 21–23. [DOI] [PubMed] [Google Scholar]
- Brunori M., Cutruzzolà, F., Savino, C., Travaglini-Allocatelli, C., Vallone, B. and Gibson, Q.H. (1999a) Does picosecond protein dynamics have survival value? Trends Biochem. Sci., 24, 223–255. [DOI] [PubMed] [Google Scholar]
- Brunori M., Cutruzzolà, F., Savino, C., Travaglini-Allocatelli, C., Vallone, B. and Gibson, Q.H. (1999b) Structural dynamics of ligand diffusion in the protein matrix: a study on a new myoglobin mutant Y(B10) Q(E7) R(E10). Biophys. J., 76, 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M., Vallone, B., Cutruzzolà, F., Travaglini-Allocatelli, C., Berendzen, J., Chu, K., Sweet, R.M. and Schlichting, I. (2000) The role of cavities in protein dynamics: crystal structure of a photolytic intermediate of a mutant myoglobin. Proc. Natl Acad. Sci. USA, 97, 2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K., Vojtchovsky, J., McMahon, B.H., Sweet, R.M., Berendsen, J. and Schlichting, I. (2000) Structure of a ligand-binding intermediate in wild-type carbonmonoxy myoglobin. Nature, 403, 921–923. [DOI] [PubMed] [Google Scholar]
- Cleeter M.W.J., Cooper, J.M., Darley-Usmar, V.M., Moncada, S. and Schapira, A.H.V. (1994) Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. FEBS Lett., 345, 50–54. [DOI] [PubMed] [Google Scholar]
- Doyle M.P. and Hoekstra, J.W. (1981) Oxidation of nitrogen oxides by bound dioxygen in hemeproteins. J. Inorg. Biochem., 14, 351–358. [DOI] [PubMed] [Google Scholar]
- Eich R.F. et al. (1996) Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry, 35, 6976–6983. [DOI] [PubMed] [Google Scholar]
- Elber R. and Karplus, M. (1990) Enhanced sampling in molecular dynamics: use of the time-dependent Hartree approximation for a simulation of carbon monoxide diffusion through myoglobin. J. Am. Chem. Soc., 112, 9161–9175. [Google Scholar]
- Ford P.C., Wink, D.A. and Stanbury, D.M. (1993) Autoxidation kinetics of aqueous nitric oxide. FEBS Lett., 326, 1–3. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak, F. and Young, R.D. (1988) Conformational substates in proteins. Annu. Rev. Biophys. Biophys. Chem., 17, 451–479. [DOI] [PubMed] [Google Scholar]
- Friedman J.M. (1985) Structure, dynamics and reactivity of hemoglobin. Science, 228, 1273–1284. [DOI] [PubMed] [Google Scholar]
- Gibson Q.H. (1959) The photochemical formation of a quickly reacting form of hemoglobin. Biochem. J., 71, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q.H. (1989) Hemoproteins, ligand and quanta. J. Biol. Chem., 264, 20155–20158. [PubMed] [Google Scholar]
- Gibson Q.H. and Ainsworth, S. (1957) Photosensitivity of haem compounds. Nature, 180, 1416–1417. [DOI] [PubMed] [Google Scholar]
- Gibson Q.H., Regan, R., Elber, R.R., Olson, J.S. and Carver, T.E. (1992) Distal pocket residues affect picosecond ligand recombination in myoglobin: an experimental and molecular dynamics study of position 29 mutants. J. Biol. Chem., 267, 22022–22034. [PubMed] [Google Scholar]
- Haldane J. and Lorrain-Smith, T. (1897) The oxygen tension of arterial blood. J. Physiol., 20, 497–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman H., Zinser, S., Komnions, P., Schneider, R.T., Nienhaus, G.U. and Parak, F. (1996) X-ray structure determination of a metastable state of carbonmonoxymyoglobin after photodissociation. Proc. Natl Acad. Sci. USA, 93, 7013–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa J. et al. (1999) Stabilization of Pseudomonas aeruginosa cytochrome c551 by systematic aminoacid substitutions based on the structure of thermophilic Hydrogenobacter thermophilus cytochrome c552. J. Biol. Chem., 274, 37533–37537. [DOI] [PubMed] [Google Scholar]
- Henry E.R., Sommer, J.H., Hofrichter, J. and Eaton, W.A. (1983) Geminate recombination of carbon-monoxide to myoglobin. J. Mol. Biol. 166, 443–451. [DOI] [PubMed] [Google Scholar]
- Herold S. (1999) Kinetic and spectroscopic characterization of an intermediate peroxynitrite complex in the nitrogen monoxide induced oxidation of oxyhemoglobin. FEBS Lett., 443, 81–84. [DOI] [PubMed] [Google Scholar]
- Ikeda-Saito M., Dou, Y., Yonetani, T., Olson, J.S., Li, T., Regan, R. and Gibson, Q.H. (1993) Ligand diffusion in the distal heme pocket of myoglobin. J. Biol. Chem., 268, 6855–6857. [PubMed] [Google Scholar]
- Jaenike R. (1999) Stability and folding of domain proteins. Progr. Biophys. Mol. Biol., 71, 155–241. [DOI] [PubMed] [Google Scholar]
- Kendrew J.C., Dickerson, R.E., Strandberg, B.E., Hart, R.G., Davis, D.D., Phillips, D.C. and Shore, V.C. (1960) The three dimensional structure of myoglobin. Nature, 185, 422–427. [DOI] [PubMed] [Google Scholar]
- Lim M., Jackson, T.A. and Anfirud, P.A. (1997) Ultrafast rotation and trapping of carbon monoxide dissociated from myoglobin. Nature Struct. Biol., 4, 209–214. [DOI] [PubMed] [Google Scholar]
- Ostermann A., Waschipky, R., Parak, F.G. and Nienhaus, G.U. (2000) Ligand binding and conformational motions in myoglobin. Nature, 404, 205–208. [DOI] [PubMed] [Google Scholar]
- Perutz M.F. (1989) Myoglobin and haemoglobin: role of distal residues in reactions with haem ligands. Trends Biochem. Sci., 14, 42–44. [DOI] [PubMed] [Google Scholar]
- Perutz M.F. and Matthews, F.S. (1966) An X-ray study of azide methaemoglobin. J. Mol. Biol., 21, 199–207. [DOI] [PubMed] [Google Scholar]
- Richards F.M. (1977) Areas, volumes, packing and protein structure. Annu. Rev. Biophys. Bioeng., 6, 151–176. [DOI] [PubMed] [Google Scholar]
- Schlichting I. and Chu, K. (2000) Trapping intermediates in the crystal: ligand binding to myoglobin. Curr. Opin. Struct. Biol., 10, 744–752. [DOI] [PubMed] [Google Scholar]
- Schlichting I., Berendzen, J., Phillips, G.N. and Sweet, R.M. (1994) Crystal structure of photolysed carbonmonoxy-myoglobin. Nature, 371, 808–812. [DOI] [PubMed] [Google Scholar]
- Scott E.E. and Gibson, Q.H. (1997) Ligand migration in sperm whale myoglobin. Biochemistry, 36, 11909–11917. [DOI] [PubMed] [Google Scholar]
- Scott E.E., Gibson, Q.H. and Olson, J.S. (2001) Mapping the pathways for O2 entry into and exit from myoglobin. J. Biol. Chem., 276, 5177–5188. [DOI] [PubMed] [Google Scholar]
- Shoenborn B.P., Watson, H.C. and Kendrew, J.C. (1965) Binding of xenon to sperm whale myoglobin. Nature, 207, 28–30. [DOI] [PubMed] [Google Scholar]
- Springer B.A. and Sligar, S.G. (1987) High-level expression of sperm whale myoglobin in Escherichia coli. Proc. Natl Acad. Sci. USA, 84, 8961–8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B.A., Sligar, S.G., Olson, J.S. and Phillips, G.N. (1994) Mechanisms of ligand recognition in myoglobin. Chem. Rev., 94, 699–714. [Google Scholar]
- Srajer V. et al. (1996) Photolysis of the carbon monoxide complex of myoglobin: nanosecond time-resolved crystallography. Science, 274, 1726–1729. [DOI] [PubMed] [Google Scholar]
- Stryer L. (1975) Biochemistry. W.H. Freeman and Co., San Francisco, CA, p. 43. [Google Scholar]
- Teng T.Y., Srajer, V. and Moffat, K. (1994) Photolysis-induced structural changes in single crystals of carbonmonoxy myoglobin at 40 K. Nature Struct. Biol., 1, 701–705. [DOI] [PubMed] [Google Scholar]
- Teng T.Y., Srajer, V. and Moffat, K. (1997) Initial trajectory of carbon monoxide after photodissociation from myoglobin at cryogenic temperatures. Biochemistry, 36, 12087–12100. [DOI] [PubMed] [Google Scholar]
- Tilton R.F., Kuntz, I.D. and Petsko, G.A. (1984) Cavities in proteins: structure of a metmyoglobin–xenon complex solved to 1.9 Å. Biochemistry, 23, 2849–2857. [DOI] [PubMed] [Google Scholar]
- Varadarajan R., Szabo, A. and Boxer S.G. (1985) Cloning, expression in Escherichia coli, and reconstitution of human myoglobin. Proc. Natl Acad. Sci. USA, 82, 5681–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J.J. (1964) Nature of the iron–oxygen bond in oxyhemoglobin. Nature, 203, 182. [PubMed] [Google Scholar]
- Wittenberg J. (1970) Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol. Rev., 50, 559–636. [DOI] [PubMed] [Google Scholar]