Abstract
When the intracellular pathogen Listeria monocytogenes infects cultured human mucosecreting polarized HT29-MTX cells apically, it induces the stimulation of mucus exocytosis without cell entry. Using a set of isogenic mutants and purified listeriolysin O (LLO), we identified the L. monocytogenes thiol-activated exotoxin LLO as the agonist of mucus secretion. We demonstrated that the LLO-induced mucus exocytosis did not result from the LLO membrane-damaging activity. We found that LLO-induced mucus exocytosis is an event requiring the binding of LLO to a brush border-associated receptor and membrane oligomerization of the exotoxin. By a pharmacological approach, we demonstrated that no regulatory system or intracellular transducing signal known to be involved in control of mucin exocytosis was activated by LLO. Based on the present data, the stimulatory action of LLO on mucin exocytosis could be accounted for either by an unknown signaling system which remains to be determined or by direct action of LLO with the membrane vesicle components involved in the intracellular vesicular transport of mucins.
The existence of an intestinal stage in listeriosis is consistent with recent outbreaks in which gastroenteritis and fever were important features of Listeria infection (11, 63). However, the mechanisms whereby Listeria monocytogenes is established locally are largely unknown. The mucus layer is the first obstacle encountered by enteric pathogens (78). Indeed, the human small intestinal mucosa has a mucus coating at the surface. In vivo, for some pathogenic bacteria the mucus gel could serve at least two functions (24). First, it might be a source of nutrients for bacterial growth, thus positively influencing intestinal colonization by adhering bacteria which have the ability to survive in and multiply in the outer areas of the mucus layer. Second, the mucus coat overlying the microvillous surface contributes to host defenses by preventing bacterial adhesion or invasion and toxin binding to the mucosal surface. In a recent study involving a rat ligated ileal loop system, the inoculation of Listeria cells into the loops was associated with the massive release of mucus by goblet cells (62). Consistent with this observation, we report here that L. monocytogenes infection in the human polarized mucosecreting cells induces the stimulation of mucus exocytosis. L. monocytogenes is a gram-positive facultative intracellular pathogen that causes severe food-borne infections in humans and many animals. It is capable of infecting both macrophages and nonprofessional phagocytes, including epithelial cells, fibroblastic cells, and hepatocytes (28, 29). Recent sophisticated studies have finely dissected the successive stages of the L. monocytogenes cell infection first proposed by Tilney and Portnoy (73). The intracellular lifestyle of this bacterium is characterized by rapid phagolysosomal lysis, escape into the eukaryotic cytoplasm, and actin-based propulsion. To develop this cycle, L. monocytogenes uses several molecular determinants of pathogenicity (for reviews, see references 16 and 61). Contiguous genes required for L. monocytogenes pathogenesis are organized on the chromosome. A majority of the genes are located on a single 10-kb chromosomal locus in the order prfA, plcA, hly, mpl, actA, and plcB; inlA and inlB are located in an independent operon. These genes are regulated by the pleiotropic activator prfA (9, 13, 15, 44).
To identify the L. monocytogenes virulence factor acting as an agonist of mucus secretion, we used a set of L. monocytogenes EGD site-directed prfA insertion mutants (9), an isogenic actA1 mutant (59), isogenic chromosomal deletion mutants with mutations in the inlA locus (14), and isogenic chromosomal deletion mutants with deletions at hly-2 and plcB2 (9). We describe a novel cellular function for the L. monocytogenes thiol-activated exotoxin listeriolysin O (LLO). To examine the mechanism by which LLO induces mucus exocytosis, we used attenuated mutants of L. monocytogenes LO28 (54) and neutralizing monoclonal antibodies (MAbs) against LLO (57). Also, we used a pharmacological approach to attempt to identify the cellular transducing signal activated by LLO in the production of mucus exocytosis.
MATERIALS AND METHODS
Bacteria.
EGD (26, 46) and LO28 (54) wild-type and mutant strains (9, 14, 54, 59) were used (Table 1). EGD-SmR is a streptomycin-resistant derivative of strain EGD. Listeria strains were routinely grown for 18 h at 37°C in tryptic soy broth (TSB) or brain heart infusion (BHI) broth with streptomycin (60 μg/ml) for EGD-SmR, or plated on sheep blood or tryptic soy agar (TSA) plates. For LLO expression, Listeria EGD-SmR was grown in BHI–1% glucose–1% charcoal for 6 h at 37°C.
TABLE 1.
Mucin exocytosis induced by L. monocytogenes mutants apically infecting HT29-MTX cells
| Strain | Genotype | [3H]mucin exocytosis (cpm/106 cells)a |
|---|---|---|
| None (control cells) | 564 ± 14 | |
| EGD-SmR | L. monocytogenes serovar 1/2a, streptomycin resistant | 1,511 ± 157** |
| EGD | L. monocytogenes serovar 1/2a | 1,443 ± 194** |
| BUG 949 | EGD ΔinlAB | 1,364 ± 103** |
| BUG 947 | EGD ΔinlA | 999 ± 141** |
| BUG 1047 | EGD ΔinlB | 1,361 ± 162** |
| ΔplcB2 | EGD ΔplcB | 1,479 ± 206** |
| ΔprfA1 | EGD ΔprfA | 688 ± 43* |
| Δhly2 | EGD Δhly | 489 ± 97* |
| EGD-SmR hly mutant | EGD-SmR Δhly | 478 ± 37* |
Statistical analysis between control and mutants was performed by Student’s t test: *, no significant difference; **, significant difference (P < 0.01).
S. typhimurium SL1344 (19) was a gift from B. A. D. Stocker (Stanford University, Stanford, California).
Reagents and drugs.
d-[6-3H]glucosamine hydrochloride (specific activity, 20 to 40 Ci/mmol) was from ICN, Orsay, France. N5-[Methylamidino]-l ornithine or NG-methyl-d-arginine (NMMA) (Sigma, St. Louis, Mo.), EGTA (Sigma), staurosporine (Sigma), genistein (4′,5,7-trihydroxyisoflavone) (Sigma), U 73122 (1-[6-[[17 beta-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione) (Biomol), dantrolene (1-[(5-[p-nitrophenyl]furfurylidene)-amino] hydantoin) (Sigma), bapta/AM (1,2-bis[2-aminophenoxy]ethane-N,N,N′,N′-tetraacetic acid tetrakis [acetoxymethyl] ester) (Sigma), H7 [1-(5-isoquinolinylsulfonyl)-2-methylpiperazine] (Sigma), quinine hydrocloride (Sigma), indomethacin (1-[-chlorobenzoyl]-5-methoxy-2-methylindole-3-acetic acid) (Sigma), pertussis toxin from Bordetella pertussis (Sigma), 2-chloroadenosine (Sigma), crude PKI peptide from bovine heart (Sigma), and Dulbecco’s modified Eagle’s medium (DMEM) (1.8 mM Ca2+) and S-DMEM (0.1 μM Ca2+) (Gibco BRL) were also used.
Cell culture.
We used the mucus-secreting HT29-MTX cell subpopulation (43) selected from the mainly undifferentiated parental HT-29 cell line (20) by growth adaptation to methotrexate (10−6 M). This subpopulation remains stable when it is subcultured under standard conditions, i.e., in standard glucose-containing medium. Cells were routinely grown in DMEM (25 mM glucose) (Gibco BRL), supplemented with 10% inactivated (30 min for 56°C) fetal bovine serum (Gibco BRL). Monolayers of cells were prepared on glass coverslips, which were placed in six-well tissue culture plates (Corning Glass Works, Corning, N.Y.). For determination of mucin exocytosis, HT29-MTX cells were grown in filters mounted in a 12-chamber culture apparatus (Costar culture plate inserts; pore size, 3 μm; 1.2 × 106 cells per chamber), which delineates an apical (luminal) and a basolateral (serosal) reservoir. For maintenance purposes, the cells were passaged weekly by using 0.02% trypsin in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 3 mM EDTA. Experiments and cell maintenance were carried out at 37°C under a 10% CO2–90% air atmosphere. The culture medium was changed daily. Cultures were used at late postconfluence, i.e., after 20 days in culture.
Infection of HT29-MTX cells.
Before infection, the HT29-MTX monolayers were washed twice with PBS. Infecting bacteria were suspended in the culture medium, and a total of 1 ml (108 CFU/ml) of this suspension was added to each well of the culture plate insert in the apical or basolateral reservoir. The plates were incubated at 37°C under 10% CO2–90% air and were subsequently washed three times with sterile PBS.
To determine the cell-associated bacteria (extracellular plus intracellular bacteria), the infected-cell monolayers were osmotically lysed with sterile H2O. Appropriate dilutions were plated on TSA to determine the number of viable cell-associated bacteria by bacterial colony counts.
Quantitative determination of bacterial internalization was performed by the method established by Isberg and Leong (31) with the aminoglycoside antibiotic gentamicin. After incubation, monolayers were washed twice with sterile PBS and then incubated for 60 min in a medium containing gentamicin (50 μg/ml). Bacteria adherent to the HT29-MTX brush border membranes were rapidly killed, whereas those located within HT29-MTX cells were not. The monolayers were washed with PBS and osmotically lysed with sterile H2O. Appropriate dilutions were plated on TSA to determine the number of viable cell-associated bacteria by bacterial colony counts.
Measurement of cell integrity.
In each experiment, the integrity of the confluent polarized monolayers was checked by measuring transepithelial membrane resistance with a volt-ohmmeter (Millicel ERS; Millipore). Moreover, cell integrity in several experiments was determined by measuring the amount of lactate dehydrogenase in the culture medium postinfection (Enzyline LDH kit; Biomérieux, Dardilly, France).
Measurement of secretory mucins.
Secretory mucins were quantified by the previously described specific and sensitive electrophoretic method (2). Briefly, the HT29-MTX cells were metabolically labelled with 10 μCi of d-[6-3H]glucosamine hydrochloride per ml in DMEM for 24 h. The labeled cells were washed three times with serum-free DMEM. After bacterial infection or treatment of the cells with the bacterial spent culture supernatant, mucin secretion in the apical compartment was measured. The apical medium was removed, and the monolayer was rinsed with additional DMEM to remove adherent mucins. The mucin-containing medium was dialyzed against several changes of deionized water at 4°C for 36 to 48 h and subsequently freeze-dried. The secretory glycoproteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on 3% polyacrylamide gels. The band at the stacking-gel/running-gel interface, which contains the mucins, was cut out, placed in a borosilicate vial, and incubated overnight at 40°C in the presence of 0.5 ml of Soluene 350 (Packard). Radioactivity was determined by liquid-scintillation counting. The results were expressed as counts per minute (cpm) per 106 cells.
IL-1 enzyme-linked immunosorbent assay.
The level of interleukin-1 (IL-1) was measured in cell lysate plus culture medium (intracellular plus extracellular) by a sensitive and specific non-cross-reacting enzyme-linked immunosorbent assay ELISA (R&D Systems; standard limit of detection, 0.2 pg ml−1). Cell lysate plus culture medium was prepared in the presence of leupeptin and aprotinin (2 μg/ml). The results were expressed as picograms per 106 cells.
Purification of LLO.
LLO was purified from L. monocytogenes EGD-SmR by an unpublished method. Briefly, 500 μl of an overnight bacterial culture in BHI broth was grown for 8 h at 37°C with shaking in 10 ml of P3 broth (0.5% [wt/vol] Protease Peptone no. 3 [Difco], 0.5% [wt/vol] yeast extract, 0.5 mM Na2HPO4, 0.5 mM KH2PO4, 0.1% [wt/vol] sterile charcoal, and sterile H2O, complemented with 1% [wt/vol] glucose). A 10-ml volume of P3 culture was used to inoculate 1 liter of the same medium supplemented with charcoal (0.1%, wt/vol). After 12 h of incubation at 37°C without shaking, the bacteria were removed by centrifugation at 5,000 × g for 20 min at 4°C. The cell-free supernatant was centrifugated at 10,000 × g for 20 min at 4°C and filtered through a 0.45-μm-pore-size Stericup HV5 filter unit (Millipore Corp., Bedford, Mass.). EGTA (1 mM) and phenylmethylsulfonyl fluoride (1 mM) were added to the supernatant to block proteases. Ammonium sulfate was added to give a final concentration of 40%. After 30 min of stirring at 4°C, the precipitate was collected by centrifugation at 10,000 × g at 4°C. The precipitate was suspended in deionized H2O and dialyzed for 2 h at 4°C against H2O containing sodium azide (0.02%, wt/vol) and then overnight at 4°C against H2O containing sodium azide (0.02%, wt/vol). The concentrated crude supernatant was then applied to a Q Sepharose column (Pharmacia, Uppsala, Sweden) and eluted with 50 mM Tris-HCl (pH 6.7, the pKi of LLO). The hemolytic titers of the fractionated samples were determined by the microplate method with sheep erythrocytes as previously described (54). Fractions showing high levels of hemolytic activity were pooled, concentrated 20-fold with a centrifuge ultrafilter (Ultrafree-15 centrifugal filter; Millipore), and stored at −80°C. To determine the purity of LLO, SDS-polyacrylamide gel electrophoresis was performed. Purified LLO migrated as a 58-kDa band in Coomassie blue-stained SDS-polyacrylamide gels and was judged to be more than 95% pure.
Scanning electron microscopy.
After the bacterial adhesion assay, cells were fixed with 2.5% (vol/vol) glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.4) for 1 h at room temperature, washed with sodium phosphate buffer, postfixed for 30 min with 2% (wt/vol) OsO4 in the same buffer, washed three times with the same buffer, and dehydrated in a graded series (30 to 100%) of ethanol. The cells were dried in a critical-point dryer (Balzers CPD030) and coated with gold. The specimens were then examined under a JEOL electron microscope.
Statistics.
Data are expressed as mean ± standard error of the mean of several experiments, with at least three monolayers from three successive passages of HT29-MTX cells per experiment. The statistical significance was assessed by a Student t test.
RESULTS
Infection of the mucosecreting HT29-MTX cell subpopulation by L. monocytogenes is followed by stimulation of mucus exocytosis.
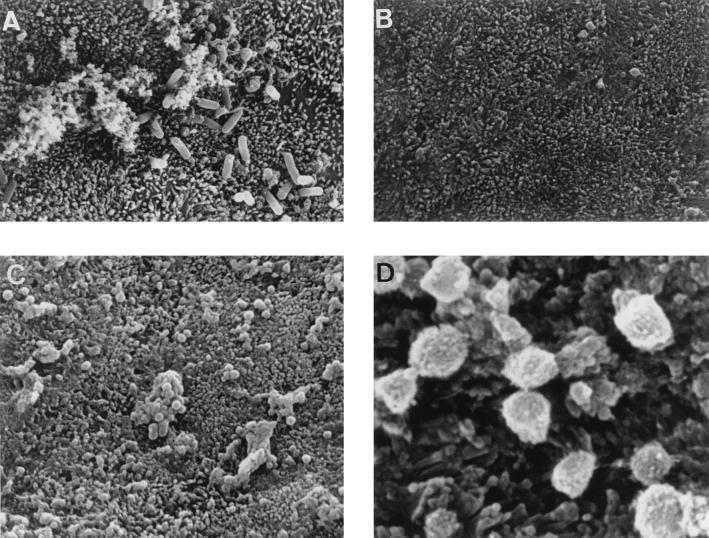
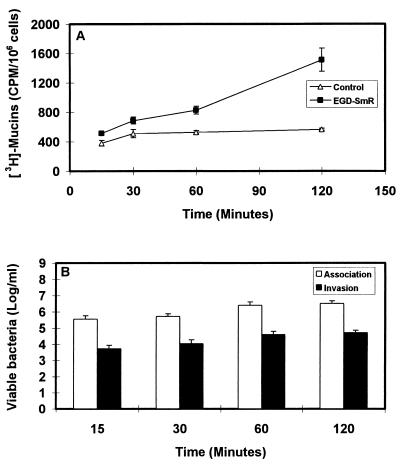
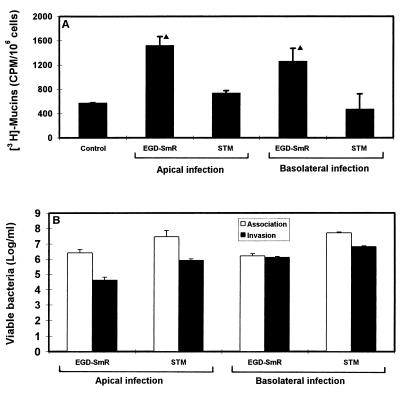
Cultured polarized human mucosecreting HT29-MTX cells were apically infected by L. monocytogenes. As shown in Fig. 1A, the L. monocytogenes bacteria are associated with the brush border of the cells and mucus secretion occurs at the vicinity of the adhering bacteria. Quantification of the mucin exocytosis was conducted in cells in which the mucins had previously been metabolically radiolabeled. As shown in Fig. 2A, stimulation of the mucin exocytosis developed postinfection as a function of time. At 2 h postinfection, a highly significant fourfold increase in mucin exocytosis was observed. The cell integrity of the L. monocytogenes-infected cells was examined by determination of the release of the lactate dehydrogenase into the culture medium and measurement of the transepithelial resistance. The infected cells did not release more intracellular enzyme than the noninfected cells did (control, 16 ± 5 U/ml; L. monocytogenes-infected cells, 17 ± 4 U/ml). The transepithelial resistance measured showed no change between the infected and noninfected cells (control, 207 ± 12 Ω; L. monocytogenes-infected cells, 210 ± 15 Ω). These results demonstrate that the cell integrity of the HT29-MTX cells is not altered upon L. monocytogenes infection. The cell association and cell entry of L. monocytogenes were quantified. As shown in Fig. 2B, L. monocytogenes rapidly associated with the cells and the level of cell-associated bacteria increased as a function of time. In contrast, a low level of bacteria located intracellularly was observed, although a slight but nonsignificant increase in bacterial cell entry developed as a function of time. When examining the basolateral route of infection for L. monocytogenes, we observed that efficient cell invasion did not modify the level of stimulation of mucin exocytosis compared with the apical route of infection (Fig. 3).
FIG. 1.
Scanning electron micrographs of cultured human mucosecreting HT29-MTX cells infected apically with L. monocytogenes EGD-SmR or treated with L. monocytogenes EGD-SmR-SCS. (A) Infected cells showing secreted mucus at the vicinity of the infecting bacteria. (B) Untreated cells showing a regular brush border. (C and D) Low and high magnifications of cells subjected to L. monocytogenes EGD-SmR-SCS, showing a great increase in mucus secretion. LLO-induced mucin exocytosis stimulation was observed after immunolabeling of secreted mucins at the HT29-MTX cell surface with the anti-mucin M1 MAb (not shown).
FIG. 2.
Mucin exocytosis (A) and bacterial cell association or cell entry (B) in HT29-MTX cells apically infected with 108 CFU of L. monocytogenes EGD-SmR per ml as a function of the time postinfection.
FIG. 3.
Mucin exocytosis (A) and bacterial cell association or cell entry (B) in HT29-MTX cells infected apically or basolaterally with 108 CFU of L. monocytogenes EGD-SmR and S. typhimurium SL1344 per ml. STM, S. typhimurium. Statistical analysis between control and infected cells was performed by Student’s t test: ▴, significant difference (P < 0.01).
To examine if mucin exocytosis results from the cell entry of an enteroinvasive pathogen, we further examined if Salmonella typhimurium is able to induce mucin exocytosis in HT29-MTX cells. As shown in Fig. 3, S. typhimurium efficiently invaded the cells after both apical and basal infection but it failed to induce stimulation of the basal mucin exocytosis.
Taken together, these results demonstrate that L. monocytogenes is able to promote an efficient stimulation of mucus exocytosis after infection by the apical and basal routes in the polarized, fully differentiated, human mucosecreting HT29-MTX cell subpopulation. The lack of mucin exocytosis stimulation by S. typhimurium infection indicates that the mucin exocytosis stimulation did not result from a single-cell entry of an enteroinvasive pathogen.
LLO is the L. monocytogenes virulence factor acting as the agonist of mucus exocytosis in the infected HT29-MTX cell subpopulation.
To identify the L. monocytogenes virulence factor involved in stimulation of mucus exocytosis, we examined a set of L. monocytogenes EGD site-directed insertion mutants (9), the isogenic actA1 mutant (59), isogenic chromosomal deletion mutants with mutations in the inlA locus (14), and isogenic chromosomal deletion mutants with deletions at hly2 and plcB2 (9) (Table 1).
Entry into undifferentiated Caco-2 cells (27, 28) requires the region encoding mRNA for internalin A (inlA) and internalin B (inlB) (26) and the cell surface protein E-cadherin as a receptor (51). The inlAB locus is required for entry of L. monocytogenes into cultured hepatocytes (29). Dramsi et al. (14) recently demonstrated that inlB is required for invasion of cultured hepatocytes but not Caco-2 cells. We found here that the mutants BUG 947 (EGD ΔinlA), BUG 1047 (EGD ΔinlB), and BUG 949 (EGD ΔinlAB) conserved the ability to induce mucus exocytosis, demonstrating that the L. monocytogenes gene inlA, inlB, or both were not involved.
After cell entry, L. monocytogenes is propelled through the cytoplasm and spreads from cell to cell by inducing the formation of a pseudopod-like structure containing bacteria, which invades the neighboring host cells. Propulsion of L. monocytogenes through the cytoplasm requires polymerization of the host actin; this requires the bacterial surface protein product of the actA gene (37). Since L. monocytogenes induces mucin exocytosis through the basal route of infection and cell entry, we have examined if the product of the actA gene was involved. Using the actA2 mutant, we found that this gene was not involved, since the mutant conserved the ability to stimulate mucin exocytosis.
L. monocytogenes secretes virulence factors such as the broad-range PLC encoded by plcB (40, 41, 47, 49, 68, 76) and LLO (10, 30, 50, 52). We have examined if these secreted virulence factors participate in the L. monocytogenes-induced stimulation of mucus exocytosis. The mutant EGD ΔplcB conserved the ability to induce mucus exocytosis. It was interesting that the EGD ΔprfA mutant had only 50% of the activity of EGD, in agreement with the fact that the prfA gene is a pleiotropic regulator that activates transcription of the LLO gene. We observed that the EGD-SmR hly and EGD Δhly mutants failed to induce mucus exocytosis, demonstrating that the LLO encoded by the hly gene acts as an agonist of mucin exocytosis in the human cultured HT29-MTX cell subpopulation.
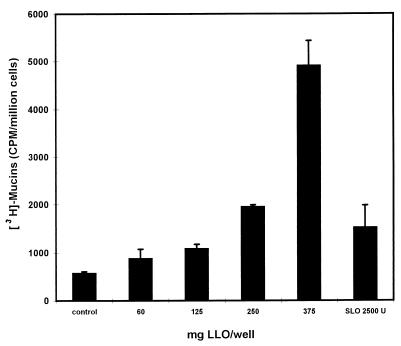
Since LLO is secreted by the bacteria, we have examined the activity of 6-h EGD-SmR and EGD-SmR hly spent culture supernatants (EGD-SmR-SCS and EGD-SmR hly-SCS, respectively) (Table 2). A high level of mucus exocytosis stimulation was obtained with EGD-SmR-SCS (10-fold increase), in comparison with the level of stimulation obtained during the cell contact of the L. monocytogenes bacteria (fourfold increase). As observed by scanning electron microscopy, the EGD-SmR-SCS-treated HT29-MTX cells showed an increase in budding of mucus secreted by the goblet cells compared with nonstimulated cells (Fig. 1B to D). Like the EGD-SmR hly bacteria, the EGD-SmR hly-SCS failed to induce mucus exocytosis. The difference in the level of stimulation between the L. monocytogenes infection and EGD-SmR-SCS treatment could be explained by the level of LLO produced during the 2 h of bacterium-cell contact in the cell culture medium and the 6-h bacterial culture. It has been reported that culture conditions influence the production of LLO (12). When examining this point (Table 2), we observed an increase in the mucus exocytosis stimulation when L. monocytogenes was cultured in TSB or BHI broth supplemented with glucose. Moreover, mucus exocytosis stimulation was highly increased when the bacteria were cultured in BHI containing glucose supplemented with charcoal. These results are consistent with the increase in LLO production in the L. monocytogenes culture medium during the glucose supplementation, which was revealed by an increase in the hemolytic titer in the spent culture supernatant. To show that LLO is the L. monocytogenes virulence factor inducing mucin exocytosis in human polarized epithelial mucosecreting cells, we have purified LLO from the EGD-SmR-SCS. As shown in Fig. 4, purified LLO applied at the apical domain of the HT29-MTX cells dose-dependently induces mucin exocytosis. A low level of activity was found for streptolysin O, another thiol-activated toxin.
TABLE 2.
Mucin exocytosis induced by L. monocytogenes EGD-SmR-SCS and EGD-SmR cultured under different conditions
| Conditions | 3[H]mucin exocytosis (cpm/106 cells)a | Hemolytic titerb |
|---|---|---|
| Control | 565 ± 57 | |
| EGD-SmR-SCS | 4,217 ± 831** | 2,256 |
| EGD-SmR hly-SCS | 371 ± 138* | 4 |
| TSBc | 1,570 ± 18** | 128 |
| TSBc + glucose | 2,888 ± 242** | 256 |
| BHIc | 1,739 ± 281** | 128 |
| BHI + glucosec | 3,293 ± 77** | 256 |
| BHI + glucose + charcoalc | 5,155 ± 613** | 2,256 |
Statistical analysis between control and assay conditions was performed by Student’s t test: *, no significant difference; **, significant difference (P < 0.01).
Determined as described by Michel et al. (54).
L. monocytogenes EGD-SmR culture conditions.
FIG. 4.
Dose-dependent induction of mucin exocytosis in HT29-MTX cells subjected apically to purified LLO.
LLO-induced stimulation of mucus exocytosis is not related to LLO membrane-damaging activity.
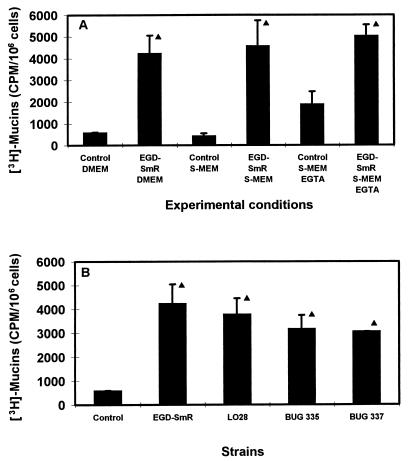
LLO is a thiol-activated toxin (for a review, see reference 1) that induces damage in the phagolysosomal membrane, allowing release of the bacterium into the cytosol of the host cell before the bacterium is propelled through the cytoplasm. LLO, in the same fashion as streptolysin O (3, 36), exerts its cytolytic activity by a mechanism that is not entirely defined but involves pore formation in the cell membrane. We have previously indicated that the cell integrity was conserved in the L. monocytogenes-infected cells, considering the lack of release of the lactate dehydrogenase into the culture medium. However, LLO could induce micropore formation in the membrane, conserving cell integrity but allowing the entry into the cell of small molecules present in the cell culture medium that act as agonists of mucus exocytosis, such as Ca2+ (for reviews, see references 23 and 38). To examine this point in HT29-MTX cells treated with EGD-SmR-SCS, we have conducted experiments in which a cell culture medium with a low Ca2+ was used. As shown in Fig. 5A, the levels of LLO-induced mucus exocytosis were identical when a normal Ca2+ concentration (1.8 mM) or a low Ca2+ concentration (0.1 μm) was used. Moreover, chelating the extracellular Ca2+ with EGTA (500 μm) for 30 min did not antagonize the LLO-induced stimulation of mucus exocytosis. The lack of relationship between LLO-induced stimulation of mucus exocytosis and the LLO membrane-damaging activity was confirmed by the use of two mutant strains: BUG 335 (LO28 Trp-491 to Ala) and BUG 337 (LO28 Trp-492 to Ala), for which a decrease of 95 and 99.9%, respectively, in the hemolytic activity in culture supernatants occurred (54). As shown in Fig. 5B, the two mutants conserved the LLO-induced stimulation of mucus exocytosis compared with the activity of the EGD-SmR and LO28 control strains. Taken together, these results suggest that the LLO-induced mucus exocytosis activity is not related to a micropore-forming activity.
FIG. 5.
Role of the LLO membrane-damaging activity in L. monocytogenes EGD-SmR-SCS-induced stimulation of mucus exocytosis in HT29-MTX cells. (A) Mucus exocytosis promoted by EGD-SmR-SCS applied apically to HT29-MTX cells in the presence of a low-Ca2+ cell culture medium (S-DMEM) and after chelation of the extracellular Ca2+ by EGTA. (B) Mucus exocytosis promoted by the spent culture supernatant of the EGD-SmR, LO28, and mutant strains: BUG 335 (LO28 Trp-491 to Ala) and BUG 337 (LO28 Trp-492 to Ala). ▴, significant difference (P < 0.01) from control.
LLO-induced stimulation of mucus exocytosis is an event occurring after the LLO interaction with a brush border-associated receptor in HT29-MTX cells.
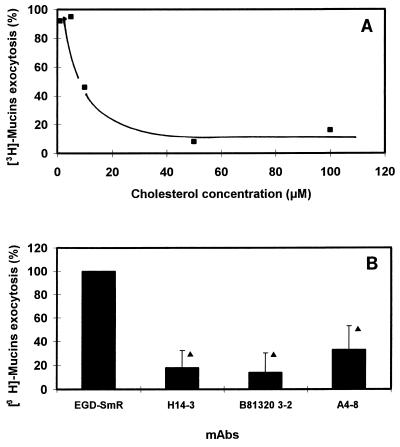
For the thiol-activated membrane-damaging toxins, cholesterol is assumed to be the toxin binding site on the surface of eucaryotic cells, since membranes lacking this component are insensitive to the action of toxins (for a review, see reference 1). Moreover, inhibition of hemolytic activity by cholesterol is a characteristic of the thiol-activated toxins. Since LLO is a thiol-activated toxin, it was of interest to examine whether the LLO-induced stimulation of mucus exocytosis was affected by cholesterol. EGD-SmR-SCS was incubated for 30 min at 37°C with increasing concentrations of cholesterol before being added to the HT29-MTX cells. As shown in Fig. 6A, the LLO-induced stimulation of mucus exocytosis was dose-dependently inhibited by cholesterol.
FIG. 6.
L. monocytogenes EGD-SmR-SCS-induced mucus exocytosis occurs after LLO interaction with an HT29-MTX brush border-associated receptor. (A) Stimulation of mucus exocytosis promoted by EGD-SmR-SCS applied apically to the HT29-MTX cells in the presence of increasing concentrations of cholesterol. (B) Stimulation of mucus exocytosis promoted by EGD-SmR-SCS applied apically to the HT29-MTX cells in the presence of neutralizing MAbs against LLO. MAbs H14-3 (5 μg/ml) and B8B20-3-2 (5 μg/ml) allow LLO binding and prevent cell lysis. A4-8 (10 μg/ml) inhibits both LLO binding and cell lysis. ▴, significant difference (P < 0.01) from EGD-SmR.
It has been suggested that after binding to the membrane, oligomerization of thiol-activated toxins is a prerequisite for their activity (for a review, see reference 1). Nato et al. (57) have produced neutralizing MAbs against LLO. We found that that MAbs H14-3 and B8B20-3-2, which recognize identical or overlapping epitopes, allowing binding but preventing lysis, blocked the LLO-induced stimulation of mucus exocytosis (Fig. 6B). Using the MAb A4-8, which recognizes a distinct epitope inhibiting binding and preventing lysis, we observed an inhibition of the LLO-induced stimulation of mucus exocytosis (Fig. 6B).
Is the LLO-activated MAP kinase and LLO-induced IL-1 production involved in the LLO-induced stimulation of mucus exocytosis?
Considering that the mitogen-activated protein kinase (MAP kinase) is activated by LLO (71, 72) we have examined the possible role of MAP kinase in the LLO-induced stimulation of mucus exocytosis in EGD-SmR-SCS-treated HT29-MTX cells. The MAP kinase blocker genistein (150 μM for 30 min), which is able to block LLO-induced MAP kinase activation (72), failed to inhibit LLO-induced mucus exocytosis (control, 564 ± 145 cpm/106 cells; L. monocytogenes-infected cells, 4,784 ± 145 cpm/106 cells; L. monocytogenes-infected cells plus genistein, 4,266 ± 344 cpm/106 cells).
IL-1 production was increased by LLO (80). IL-1 is known to be an agonist of mucin exocytosis in HT29-Cl.16E cells (34, 35) via nitric oxide (NO)-dependent and -independent mechanisms (74, 75). We have determined the intracellular plus extracellular IL-1 levels in control and L. monocytogenes EGD-SmR-SCS-treated HT29-MTX cells. We found no change in the intracellular plus extracellular IL-1 contents after L. monocytogenes EGD-SmR-SCS treatment (control, 2 ± 0.1 pg/106 cells; L. monocytogenes EGD-SmR-SCS-treated cells, 2 ± 0.2 pg/106 cells).
Is the LLO-induced mucus exocytosis promoted by a host cell transducing signal pathway activated after LLO binding to an apical membrane-associated receptor?
Mucin exocytosis in the human intestinal mucosecreting cells responds to stimuli such as neuroendocrine receptor and coupled signaling systems, inflammatory agents, and immune system agents, and it appears to be associated with the electrolyte secretory pathways (for reviews, see references 23 and 38). On the basis of our knowledge of mucin exocytosis regulation, we have investigated which mucin exocytosis regulatory pathway(s) could be involved in the LLO-induced stimulation of mucus exocytosis in HT29-MTX cells. For this purpose, we have developed a strategy of using inhibitors (Table 3).
TABLE 3.
Mucin exocytosis induced by the L. monocytogenes EGD-SmR-SCS applied apically to HT29-MTX cells in the presence of transducing signal molecule inhibitors
| Inhibitor | 3[H]mucin exocytosis (cpm/106 cells)a | Specificity | Reference(s)b |
|---|---|---|---|
| None (control) | 565 ± 145 | ||
| EGD-SmR-SCS | 4,784 ± 339 | ||
| U73122 | 6,946 ± 1,124* | PLC | 70 |
| NMMA | 4,551 ± 101* | NO synthase | 75 |
| PKI | 5,876 ± 296* | PKA | 33 |
| Pertussis toxin | 4,364 ± 662* | G proteins | 23, 69 |
| Bapta/AM | 7,282 ± 1,489* | Intracellular calcium | 6, 69 |
| Dantrolene | 4,768 ± 846* | Calcium release | 6 |
| Staurosporine | 7,165 ± 1,039* | PKC | 22, 70 |
| H7 | 5,845 ± 1,106* | PKC | 22, 70 |
| Indomethacin | 5,125 ± 996* | Prostaglandin E2 | 56, 70 |
| Quinine | 5,110 ± 126* | K+ channels | 53 |
| 2-Chloroadenosine | 6,395 ± 69* | cGMP | 60 |
Statistical analysis between EGD-SmR-SCS and inhibitors was performed by Student’s t test: *, no significant difference.
The references are to studies of the inhibition of stimulated mucin exocytosis in intestinal cells by the blockers used here.
We found that quinine (1 mM for 30 min) was unable to block LLO-induced mucus exocytosis, showing that LLO-associated mucin exocytosis is purinergic P2 receptor independent (53). We show that LLO-induced mucus exocytosis is not inhibited by pertussis toxin treatment (0.6 μg/ml for 20 h), suggesting that it is G protein independent (5) or that it results from pertussis toxin-resistant G proteins (18). The protein kinase A (PKA) inhibitor peptide (PKI peptide) (10 μg/ml for 3 h) failed to block the LLO-induced stimulation of mucus exocytosis, indicating that cyclic AMP (cAMP)-dependent mucin exocytosis through activation of PKA (4, 17, 32, 33, 39, 42, 64) is not required. Moreover, we found that the prostaglandin E2 blocker indomethacin (5 μM for 8 h) is inactive against LLO-induced mucus exocytosis, confirming the above results indicating that it is cAMP independent. The observation that the PLC inhibitor U73122 (10 μM for 30 min) failed to inhibit LLO-induced mucus exocytosis demonstrates that Ca2+-dependent mucin exocytosis (2, 6, 22, 65, 70) resulting from PLC activation, releasing Ca2+ from intracellular stores, does not occur. Moreover, using the intracellular Ca2+ chelator Bapta/AM (50 μM for 30 min) and the Ca2+ release blocker dantrolene (150 μM for 30 min), we confirmed that the LLO-induced stimulation of mucus exocytosis is Ca2+ independent. We found that LLO-induced stimulation of mucus exocytosis is independent of PKC activation (21, 22), since two PKC blockers, staurosporine (0.1 μg/ml for 60 min) and H7 (25 μM for 60 min), were inactive. The NO synthase blocker NMMA (500 μM for 60 min) was unable to inhibit LLO-induced mucus exocytosis, indicating that this phenomenon is NO independent (8, 74). By using the nucleotide analog 2-chloroadenosine (2ClAdo) (1 mM for 20 h), we observed that the LLO-induced mucus exocytosis appears independent of cGMP production (23, 60).
In conclusion, for this set of experiments, in which we examined each known agonist of mucin secretion and the described regulatory pathways controlling mucin exocytosis, the data presented seems to indicate that none of the well-established regulatory pathways could be implicated in LLO-induced mucus exocytosis.
DISCUSSION
Two major cell types, i.e., enterocytes and goblet cells, are represented in the intestinal mucosa. The human small intestinal mucosa has a mucus coating at the surface (58). For some endogenous and pathogenic bacteria, the mucus gel could serve at least two functions. First, it might be a source of nutrients for bacterial growth, thus positively influencing intestinal colonization by the adhering bacteria which have the ability to survive in and multiply in the outer areas of the mucus layer. Second, the mucus coat overlying the microvillous surface contributes to host defense by preventing bacterial adhesion or invasion and toxin binding to the mucosal surface. Several pathogens and bacterial toxins promote (25, 67) or inhibit (7, 55) mucus exocytosis. In this paper, we present evidence that L. monocytogenes stimulates mucin secretion in polarized cultured human mucosecreting HT29-MTX cells by the action of its exotoxin LLO. LLO is a secreted protein of 58 kDa that is essential for L. monocytogenes survival in the infected host (for reviews, see references 16 and 61). The unique cellular function of LLO currently described concerns its role in the escape of the bacterium from the single-membrane phagosome during the cell cycle of L. monocytogenes infection. Disruption in the phagosomal membrane by LLO could result from pore formation within lipid membrane bilayers, a common mechanism for the SH-activated toxins (for a review, see reference 1). To determine which mechanism LLO uses to induce mucus exocytosis, we have investigated whether LLO uses the successive stages of the mechanism of action of the pore-forming SH-activated toxins. It is well established that the SH-activated toxins bind to a lipidic membranous receptor before being oligomerized into higher-order structures on the membrane. We found that the mechanism by which LLO induces mucin exocytosis in mucosecreting cells involves both the receptor binding and the oligomerization stages. Indeed, we observed that cholesterol dose-dependently inhibits LLO-induced mucus exocytosis, suggesting that receptor recognition is required. Using two MAbs blocking LLO oligomerization after binding to receptor, we observed a strong inhibition of the LLO-induced mucin exocytosis. Moreover, a MAb blocking both the receptor binding and the oligomerization stages inhibited LLO-induced mucus exocytosis. In contrast, we clearly demonstrated that the LLO-induced mucin exocytosis was cytolysis independent. This was expected because of the lack of cell alteration and lysis, indicating that the effect did not result from LLO-induced pore formation. Moreover, this result was confirmed by manipulating the concentration of extracellular Ca2+, an agonist of mucin secretion, which could enter the cells upon LLO-induced micropore formation. Indeed, we found that the LLO-induced mucin exocytosis was not blocked when the extracellular Ca2+ was chelated or was absent from the culture medium.
It was previously reported that several cytotonic toxins use the endocytic pathway to enter the cell, partly or totally to interact with the regulatory systems which control the production of second messengers triggering cellular disorders (for a review, see reference 67). We believe that LLO acts intracellularly with a system controlling the apical vectorized vesicular mucin transport. Indeed, when the mucosecreting cells were infected through the apical domain, the stimulation of mucin exocytosis occurred without significant bacterial entry. Stimulation of mucin exocytosis was even found when the spent culture supernatant containing LLO was applied at the apical domain of the cells. When the L. monocytogenes bacteria infected the cells via the basolateral domain, allowing efficient cell entry of the bacteria, an efficient stimulation of mucin exocytosis occurred. In this situation, L. monocytogenes uses intracellular LLO with or without other enzymes to escape from the phagosomes. It was recently described that LLO is delivered in the cytoplasm of the cells (77). In consequence, LLO could interact with and stimulate a system which controls the vesicular mucin transport. Taken together, these results suggest that the LLO could cross the apical membrane and enter the cell to stimulate mucin exocytosis. However, it remains to be determined if LLO uses the endocytic pathway to enter the cell.
In the present work, we have investigated whether LLO-induced stimulation of mucin exocytosis results from the activation of a known regulatory system producing one of the intracellular second messengers identified as an agonist of mucin exocytosis. Our results demonstrate that none of these second messengers was involved in LLO activity. This raises a question about the secretion pathways which are targeted by the LLO; i.e., are the unregulated and/or the regulated secretory pathways involved? The intracellular processing of mucins in mucus-secreting cells involves synthesis, oligomerization into the endoplasmic reticulum, glycosylation into the cis- and trans-Golgi network, and storage in granules (for a review, see reference 23). Granules containing mucin are guided to the cell surface via microtubules. The viscous mucus contained in granules is extruded after fusion of granule and plasma membranes and formation of a fusion pore. This process requires an expulsionary force. There are two secretory pathways in cells that secrete proteins. One is a steady vesicular constitutive pathway in which no storage occurs, since the vesicles are transported directly to the plasma membrane and undergo immediate exocytosis of the proteins inside. This pathway does not require a signal to release the vesicle contents. The second pathway involves packaging and storage of mucins into granules, from which the release of the mucins enclosed is regulated by specific stimuli involving transducing signal molecules. A future challenge will be to determine the mechanism by which LLO increases mucin exocytosis. A hypothesis is that LLO could act directly in the granules containing mucins. Two granule populations have been observed in mucosecreting cells. Granules involved in single-granule exocytosis are located at the periphery of the granule mass and participate in the unstimulated secretion. In contrast, the regulated secretion mobilizes a population of inert granules centrally in the cell, which requires compound exocytosis. With this in mind, we are attempting to determine the population of vesicles which is mobilized upon LLO-induced stimulation. In other secretory systems, the rab GTPases, members of the Ras-related GTPase superfamily, are involved in the processes by which the transport vesicles identify and fuse with their cognate target membranes (for a review, see reference 45). Recently, rab GTPases have been identified in polarized human intestinal cells. Indeed, rab3B (79) and rab13 (81) have been localized at the apical pole very near the junctional complexes, which may provide the machinery required for docking and fusion of some apical vesicles. We believe that LLO could intracellularly target protein members of the rab family or their regulators to induce the stimulation of mucin exocytosis observed in the human intestinal mucus-secreting cells. Considering that there is currently no information about the involvement of the rab GTPases in regulation of the traffic of the vesicles containing mucins in the intestinal mucin-secreting cells, LLO could provide a useful tool for future examinations of the regulation of this secretory system.
ACKNOWLEDGMENTS
We thank T. Chakraborty for generously providing mutants. We are grateful to P. Cossart for the generous gift of mutants and anti-LLO MAbs.
REFERENCES
- 1.Alouf J E, Geoffroy C. Structure activity relationships in sulphydryl-activated toxins. In: Allouf J E, Fehrenbach F J, Freer J H, Jeljaszewicz J, editors. Bacterial protein toxins. London, United Kingdom: Academic Press, Ltd.; 1984. pp. 165–171. [Google Scholar]
- 2.Augeron C, Voisin T, Maoret J J, Berthon B, Laburthe M, Laboisse C. Neurotensin and neuromedin N stimulate mucin ouput in the human goblet cell clone Cl.16E: a neurotensin receptor-mediated event. Am J Physiol. 1992;262:G470–G476. doi: 10.1152/ajpgi.1992.262.3.G470. [DOI] [PubMed] [Google Scholar]
- 3.Bahkdi S, Tranum-Jensen J, Sziegoleit A. Mechanism of membrane damage by streptolysin O. Infect Immun. 1985;47:52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beubler E, Kollar G, Saria A, Bukhave K, Rask-Madsen J. Involvement of 5-hydroxytryptamine, prostaglandin-E2, and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology. 1989;96:368–376. doi: 10.1016/0016-5085(89)91560-6. [DOI] [PubMed] [Google Scholar]
- 5.Böhm S K, Grady E F, Bunnett N W. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bou-Hanna C, Berthon B, Combettes L, Claret M, Laboisse C. Role of calcium in carbachol- and neurotensin-induced mucin exocytosis in a human colonic goblet cell line and cross-talk with the cyclic AMP pathway. Biochem J. 1994;299:579–585. doi: 10.1042/bj2990579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branka J E, Vallette G, Jarry A, Bou-Hanna C, Lemarre P, Nguyen Van P, Laboisse C L. Early functional effects of Clostridium difficile toxin A on human colonocytes. Gastroenterology. 1997;112:1887–1894. doi: 10.1053/gast.1997.v112.pm9178681. [DOI] [PubMed] [Google Scholar]
- 8.Branka J E, Vallette G, Jarry A, Laboisse C L. Stimulation of mucin exocytosis from human epithelial cells by nitric oxide: evidence for a cGMP-dependent and a cGMP-independent pathway. Biochem J. 1997;323:521–524. doi: 10.1042/bj3230521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty T, Leimester-Wächter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cossart P, Vicente M F, Mengaud J, Baquero F, Perez-Diaz J C, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton C B, Austin C C, Sobel S, Hayes P S, Bibb W F, Graves L M, Swaminathan B, Proctor M E, Griffin P M. An outbreack of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med. 1997;336:100–105. doi: 10.1056/NEJM199701093360204. [DOI] [PubMed] [Google Scholar]
- 12.Datta A R, Kothary M H. Effects of glucose, growth temperature, and pH on listeriolysin O production in Listeria monocytogenes. Appl Environ Microbiol. 1993;59:3495–3497. doi: 10.1128/aem.59.10.3495-3497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domann E, Zechel S, Lingnau A, Hain T, Darji A, Nichterlein T, Wehland J, Chakraborty T. Identification and characterization of a novel prfA-regulated gene in Listeria monocytogenes, whose product, IrpA, is highly homologous to internalin proteins, which contain leucine-rich repeats. Infect Immun. 1997;65:101–109. doi: 10.1128/iai.65.1.101-109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of InlB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 15.Dramsi S, Kocks C, Forestier C, Cossart P. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature and the pleiotropic activator prfA. Mol Microbiol. 1993;9:931–941. doi: 10.1111/j.1365-2958.1993.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 16.Dramsi S, Lebrun M, Cossart P. Molecular and genetic determinants involved in invasion of mammalian cells by Listeria monocytogenes. Curr Top Microbiol Immunol. 1996;209:61–78. doi: 10.1007/978-3-642-85216-9_4. [DOI] [PubMed] [Google Scholar]
- 17.Enss M L, Wagner S, Schmidt-Wittig U, Heim H K, Bell W, Hedrich H J. Effect of PGE2 on ammount and composition of high molecular weight glycoproteins released by human mucous cells in primary culture. Prostaglandins Leukotrienes Essent Fatty Acids. 1997;56:93–98. doi: 10.1016/s0952-3278(97)90503-2. [DOI] [PubMed] [Google Scholar]
- 18.Fields T A, Casey P J. Signalling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem J. 1997;321:561–571. doi: 10.1042/bj3210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlay B B, Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 20.Fogh J, Fogh J M, Orfeo T. One hundred and twenty seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 21.Forstner G, Zhang Y, McCool D, Forstner J. Mucin secretion by T84 cells. Stimulation by PKC, Ca2+, and a protein kinase activated by Ca2+ ionophore. Am J Physiol. 1993;264:G1096–G1102. doi: 10.1152/ajpgi.1993.264.6.G1096. [DOI] [PubMed] [Google Scholar]
- 22.Forstner G, Zhang Y, McCool D, Forstner J. Regulation of mucin secretion in T84 adenocarcinoma cells by forskolin: relationship to Ca2+ and PKC. Am J Physiol. 1994;266:G606–G612. doi: 10.1152/ajpgi.1994.266.4.G606. [DOI] [PubMed] [Google Scholar]
- 23.Forstner G G. Signal transduction, packaging and secretion of mucins. Annu Rev Physiol. 1995;57:585–605. doi: 10.1146/annurev.ph.57.030195.003101. [DOI] [PubMed] [Google Scholar]
- 24.Forstner J F. Intestinal mucins in health and disease. Digestion. 1978;17:234–263. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- 25.Forstner J F, Roomi N W, Fahim E E F, Forstner G G. Cholera toxin stimulates secretion of immunoreactive intestinal mucin. Am J Physiol. 1981;240:G10–G15. doi: 10.1152/ajpgi.1981.240.1.G10. [DOI] [PubMed] [Google Scholar]
- 26.Gaillard J L, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from Gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 27.Gaillard J L, Berche P, Mounier J, Richard S, Sansonetti P J. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaillard J L, Finlay B B. Effect of cell polarization and differentiation on entry of Listeria monocytogenes into the enterocyte-like Caco-2 cell line. Infect Immun. 1996;64:1299–1308. doi: 10.1128/iai.64.4.1299-1308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaillard J L, Jaubert F, Berche P. The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J Exp Med. 1996;183:359–369. doi: 10.1084/jem.183.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geoffroy C, Gaillard J L, Alouf J E, Berche P. Purification, characterization, and toxicity of the sulfhydryl-actived hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987;55:1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isberg R R, Leong J M. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 32.Jarry A, Merlin D, Hopfer U, Laboisse C L. Cyclic AMP-induced mucin exocytosis is dependent of Cl− movements in human colonic epithelial cells (HT29-Cl.16E) Biochem J. 1994;304:675–678. doi: 10.1042/bj3040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarry A, Merlin D, Velcich A, Hopfer U, Augenlicht L H, Laboisse C L. Interferon-γ modulates cAMP-induced mucin exocytosis without affecting mucin gene expression in a human colonic goblet cell line. Eur J Pharmacol. 1994;267:95–103. doi: 10.1016/0922-4106(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 34.Jarry A, Muzeau F, Laboisse C. Cytokine effect in a human colonic goblet cell line. Dig Dis Sci. 1992;37:1170–1178. doi: 10.1007/BF01296556. [DOI] [PubMed] [Google Scholar]
- 35.Jarry A, Vallette G, Branka J E, Laboisse C. Direct secretory effect of interleukin-1 via type I receptors in human colonic mucous epithelial cells (HT29-Cl.16E) Gut. 1996;38:240–242. doi: 10.1136/gut.38.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kehoe M A, Miller L, Walker J A, Boulnois G. Nucleotide sequence of the SLO (SLO) gene: structural homologies between SLO and other membrane damaging, thiol-actived toxins. Infect Immun. 1987;55:3228–3232. doi: 10.1128/iai.55.12.3228-3232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 38.Laboisse C, Jarry A, Branka J E, Merlin D, Bou-Hanna C, Vallette G. Regulation of mucin exocytosis from intestinal goblet cells. Biochem Soc Trans. 1995;23:810–813. doi: 10.1042/bst0230810. [DOI] [PubMed] [Google Scholar]
- 39.Laburthe M, Augeron C, Rouyer-Fessard C, Roumagnac I, Maoret J J, Grasset E, Laboisse C. Functional VIP receptors in the human mucus-secreting colonic epithelial cell line CL.16E. Am J Physiol. 1989;256:G440–G450. doi: 10.1152/ajpgi.1989.256.3.G443. [DOI] [PubMed] [Google Scholar]
- 40.Leighton I, Threfall D R, Dakley C L. Phospholipase C activity in culture filtrates from Listeria monocytogenes. In: Woodbine M, editor. Problems of listeriosis. Leicester, England: Leicester University Press; 1975. pp. 239–241. [Google Scholar]
- 41.Leimester-Wächter M, Domann E, Chakraborty T. Detection of a gene encoding phosphatidylinositol specific phospholipase C that is co-ordinately expressed with listeriolysin in Listeria monocytogenes. Mol Microbiol. 1991;5:361–366. doi: 10.1111/j.1365-2958.1991.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 42.Lencer W I, Delp C, Neutra M R, Madara J L. Mechanism of cholera toxin action on a polarized human epithelial cell line: role of vesicular trafic. J Cell Biol. 1992;117:1197–1209. doi: 10.1083/jcb.117.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990;50:6334–6343. [PubMed] [Google Scholar]
- 44.Lingnau A, Domann E, Hudel M, Bock M, Nichterlein T, Wehland J, Chakraborty T. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by prfA-dependent and -independent mechanisms. Infect Immun. 1995;63:3896–3903. doi: 10.1128/iai.63.10.3896-3903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macara I G, Lounsbury K M, Richards S A, McKiernan C, Bar-Sagi D. The ras superfamily of GTPases. FASEB J. 1995;10:625–630. doi: 10.1096/fasebj.10.5.8621061. [DOI] [PubMed] [Google Scholar]
- 46.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 47.Marquis H, Goldfine H, Portnoy D A. Proteolytic pathways of activation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J Cell Biol. 1997;137:1381–1392. doi: 10.1083/jcb.137.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mellman I. Endocytis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 49.Mengaud J, Braun-Breton C, Cossart P. Identification of phosphatidylinositol-specific-phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor. Mol Microbiol. 1991;5:367–372. doi: 10.1111/j.1365-2958.1991.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 50.Mengaud J, Chenevert J, Geoffroy C, Gaillard J L, Cossart P. Identification of the structural gene encoding the SH-activated hemolysin of Listeria monocytogenes: listeriolysin O is homologous to streptolysin O and pneumolysin. Infect Immun. 1987;55:3225–3227. doi: 10.1128/iai.55.12.3225-3227.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mengaud J, Ohayon H, Gounon P, Mège R M, Cossart P. E-cadherin is the receptor for internalin, a surfase protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 52.Mengaud J, Vicente M F, Chenevert J, Pereira J M, Geoffroy C, Gicquel-Sanzey B, Baquero F, Perez-Diaz J C, Cossart P. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect Immun. 1988;56:766–772. doi: 10.1128/iai.56.4.766-772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merlin D, Augeron C, Tien X Y, Guo X, Laboisse C L, Hopfer U. ATP-stimulated electrolyte and mucin secretion in the human intestinal goblet cell line HT29-Cl.16E. J Membr Biol. 1994;137:137–149. doi: 10.1007/BF00233483. [DOI] [PubMed] [Google Scholar]
- 54.Michel E, Reich K A, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990;12:2167–2178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 55.Micots I, Augeron C, Laboisse C L, Muzeau F, Mégraud F. Mucin exocytosis: a major target for Helicobacter pylori. J Clin Pathol. 1993;46:241–245. doi: 10.1136/jcp.46.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore B A, Sharkey K A, Mantle M. Role of 5-HT in cholera toxin-induced mucin secretion in the rat small intestine. Am J Physiol. 1996;270:G1001–G1009. doi: 10.1152/ajpgi.1996.270.6.G1001. [DOI] [PubMed] [Google Scholar]
- 57.Nato F, Reich K, Lhopital S, Rouyre S, Geoffroy C, Mazie J C, Cossart P. Production and characterization of neutralizing and nonneutralizing monoclonal antibodies against listeriolysin O. Infect Immun. 1991;59:4641–4646. doi: 10.1128/iai.59.12.4641-4646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neutra M R, Forstner J F. Gastrointestinal mucus synthesis, secretion and function. In: Johnson L R, editor. Physiology of the gastrointestinal tract. 2nd ed. New York, N.Y: Raven Press; 1987. pp. 975–1009. [Google Scholar]
- 59.Niebuhr K, Chakraborty T, Rohde M, Gazlig T, Jansen B, Köllner P, Wehland J. Localization of the ActA polypeptide of Listeria monocytogenes in infected tissue culture cell lines: ActA is not associated with actin “comets.”. Infect Immun. 1993;61:2793–2802. doi: 10.1128/iai.61.7.2793-2802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parkinson S J, Alekseev A E, Gomez L A, Wagner F, Terzic A, Waldman S A. Interruption of Escherichia coli heat-stable enterotoxin-induced guanylyl cyclase signaling and associate chloride current in human intestinal cells by 2-chloroadenosine. J Biol Chem. 1997;272:754–758. doi: 10.1074/jbc.272.2.754. [DOI] [PubMed] [Google Scholar]
- 61.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pron B, Boumaila C, Jaubert F, Sarnaki S, Monnet J P, Berche P, Gaillard J L. Comprehensive study of the intestinal stage of listeriosis in a rat ligated loop system. Infect Immun. 1997;66:747–755. doi: 10.1128/iai.66.2.747-755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rieto F X, Pinner R W, Tosca M L, Cartter M L, Graves L M, Reeves M W, Weaver R E, Plikaytis B D, Broome C V. A point-source foodborne listeriosis outbreak: documented incubation period and possible mild illness. J Infect Dis. 1994;170:693–696. doi: 10.1093/infdis/170.3.693. [DOI] [PubMed] [Google Scholar]
- 64.Roomi N, Laburthe M, Fleming N, Crowther R, Forstner J. Cholera-induced mucin secretion of rat intestine: lack of effect of cyclic AMP, cycloheximide, VIP, and colchicine. Am J Physiol. 1984;247:G140–G148. doi: 10.1152/ajpgi.1984.247.2.G140. [DOI] [PubMed] [Google Scholar]
- 65.Roumagnac I, Laboisse C L. A mucus-secreting human colonic epithelial cell line responsive to cholinergic stimulation. Biol Cell. 1987;61:65–68. doi: 10.1111/j.1768-322x.1987.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 66.Roumagnac I, Laboisse C L. A simple immunofiltration assay for mucins secreted by a human colonic epithelial cell line. J Immunol Methods. 1989;122:265–271. doi: 10.1016/0022-1759(89)90273-1. [DOI] [PubMed] [Google Scholar]
- 67.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steel D M, Hanrahan J W. Muscarinic-induced mucin secretion and intracellular signaling by hamster tracheal goblet cells. Am J Physiol. 1997;272:L230–L237. doi: 10.1152/ajplung.1997.272.2.L230. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi S, Okabe S. Stimulatory effects of sucralfate on secretion and synthesis of mucus by rabbit gastric mucosal cells. Dig Dis Sci. 1996;41:498–504. doi: 10.1007/BF02282325. [DOI] [PubMed] [Google Scholar]
- 71.Tang P, Rosenshine I, Cossart P, Finlay B B. Listeriolysin O activates mitogen-activated protein kinase in eucaryotic cells. Infect Immun. 1996;64:2359–2361. doi: 10.1128/iai.64.6.2359-2361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang P, Rosenshine I, Finlay B B. Listeria monocytogenes, an invasive bacterium, stimulates MAP kinase upon attachment to epithelial cells. Mol Biol Cell. 1994;5:455–464. doi: 10.1091/mbc.5.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vallette G, Jarry A, Branka J E, Laboisse C L. A redox-based mechanism for induction of interleukin-1 production by nitric oxide in a human colonic epithelial cell line (HT29-Cl.16E) J Biochem. 1996;313:35–38. doi: 10.1042/bj3130035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vallette G, Jarry A, Lemarre P, Branka J E, Laboisse C L. NO-dependent and NO-indépendant IL-1 production by a human colonic epithelial cell line under inflammatory stress. Br J Pharmacol. 1997;121:187–192. doi: 10.1038/sj.bjp.0701118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vasquez-Boland J A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Villanueva M S, Sijts A J A M, Pamer E G. Listeriolysin is processed efficiently into an MHC class I-associated epitope in Listeria monocytogenes-infected cells. J Immunol. 1995;155:5227–5233. [PubMed] [Google Scholar]
- 78.Walker W A. Role of the mucosal barrier in toxin/microbial attachment to the gastrointestinal tract. Ciba Found Symp. 1985;112:34–47. doi: 10.1002/9780470720936.ch3. [DOI] [PubMed] [Google Scholar]
- 79.Weber E, Berta G, Tousson A, St. John P, Green M W, Gopalokrishnan U, Jilling T, Sorscher E J, Elton T S, Abrahamson D R, Kirk K L. Expression and polarized targeting of Rab3 isoform in epithelial cells. J Cell Biol. 1994;125:583–594. doi: 10.1083/jcb.125.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshikawa H, Kawamura I, Fujita M, Tsukada H, Arakawa M, Mitsuyama M. Membrane damage and interleukin-1 production in murine macrophages exposed to listeriolysin O. Infect Immun. 1993;61:1334–1339. doi: 10.1128/iai.61.4.1334-1339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zahraoui A, Joberty G, Arpin M, Fontaine J J, Hellio R, Tavitian A, Louvard D. A small rab GTPase is distributed in cytoplasmic vesicles in nonpolarized cells but colocalizes with the tight junction marker ZO-1 in polarized epithelial cells. J Cell Biol. 1994;124:101–115. doi: 10.1083/jcb.124.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]