Abstract
Platelets have important hemostatic functions in repairing blood vessels upon tissue injury. Cytokines, growth factors, and metabolites stored in platelet α-granules and dense granules are released upon platelet activation and clotting. Emerging evidence indicates that such platelet-derived signaling factors are instrumental in guiding tissue regeneration. Here, we discuss the important roles of platelet-secreted signaling factors in skeletal muscle regeneration. Chemokines secreted by platelets in the early phase after injury are needed to recruit neutrophils to injured muscles, and impeding this early step of muscle regeneration exacerbates inflammation at later stages, compromises neo-angiogenesis and the growth of newly formed myofibers, and reduces post-injury muscle force production. Platelets also contribute to the recruitment of pro-regenerative stromal cells from the adipose tissue, and the platelet releasate may also regulate the metabolism and proliferation of muscle satellite cells, which sustain myogenesis. Therefore, harnessing the signaling functions of platelets and the platelet secretome may provide new avenues for promoting skeletal muscle regeneration in health and disease.
Keywords: skeletal muscle regeneration, platelets, platelet secretome, neutrophils, angiogenesis, muscle repair, platelet-rich plasma
Graphical Abstract
Skeletal muscles have the remarkable capacity to regenerate in response to damage, and this requires the infiltration of neutrophils and, subsequently, of other immune cells to the injured muscle. How neutrophils are recruited to the site of muscle damage was, however, largely unknown. This minireview reports the emerging evidence that signaling factors secreted by platelets are key for the recruitment of neutrophils to injured muscles and for the subsequent regeneration and re-establishment of muscle homeostasis.
Introduction
Platelets have important roles in wound healing because of their capacity to repair blood vessels. In addition to having a role in hemostasis, platelets are emerging as important signaling centers that instruct the subsequent steps of tissue regeneration that follow blood clotting [1]. Specifically, growth factors, cytokines, and metabolites are stored in platelet α-granules and dense granules that are released by platelets upon activation [2,3]. On this basis, several studies have tested the impact of administering the platelet releasate (PR) and the platelet-rich plasma (PRP), which is enriched for platelets and platelet-secreted factors [4]. Both the platelet releasate and the platelet-rich plasma have been proposed to boost wound healing and regeneration in several tissues including the skeletal muscle [4,5].
Previous studies have described a remarkable capacity for skeletal muscle to regenerate in response to xenobiotics, trauma, and strenuous exercise [6,7] and that this regenerative capacity declines with aging [7-10] and is altered in several diseases [11,12], including cancer [13]. Upon the death of muscle cells (myofibers), different waves of immune populations (e.g. neutrophils, monocytes, and macrophages) invade the injured muscles [14-16] and set the stage for the formation of new myofibers from satellite (~stem) cells in the process of myogenesis [6,7,17,18], which is also accompanied by the formation of new blood vessels (neo-angiogenesis) [19-22].
In this minireview, we will discuss recent developments in understanding how platelets guide skeletal muscle regeneration via the secretion of growth factors and cytokines (Figure 1). From the results of these studies, platelets emerge as important signaling regulators that initiate muscle regeneration.
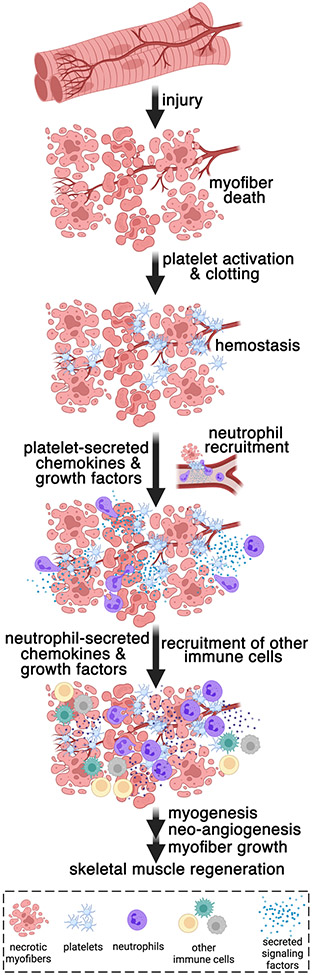
FIGURE 1.
Skeletal damage occurs during myopathies and in response to xenobiotics and exercise. Upon injury, circulating platelets are activated and clot to ensure the repair and integrity of the blood vessels adjacent to the necrotic myofibers. In addition to their role in hemostasis, platelets have signaling functions in muscle regeneration. Platelet-secreted chemokines are necessary for the recruitment of neutrophils to the damaged muscle [25], and this initial step of regeneration is followed by the subsequent infiltration of other immune cell populations into the injured muscle. In addition, platelet- and neutrophil-secreted growth factors (e.g., VEGF) contribute to other aspects of muscle regeneration, such as neo-angiogenesis and myofiber growth. Platelet-secreted factors collectively initiate muscle regeneration by recruiting neutrophils to the injured muscle, the growth of new myofibers and blood vessels (myogenesis and angiogenesis), and the optimal recovery of muscle force production post injury [25]. The scheme was drawn with BioRender.
Platelet-secreted chemokines promote muscle regeneration by recruiting neutrophils to injured muscles
Platelets have emerged as important signaling centers of inter-cellular communication in many physiological and disease contexts [1,23,24]. We have recently found that platelets are the first responders that mount the regenerative response of skeletal muscle [25]. By virtue of their hemostatic functions, platelet clusters are found within the muscle blood vessels at the site of injury [25]. Although capillaries may not be directly damaged by myotoxic agents, it was previously reported that the blood vessel network is indirectly disrupted because of myofiber necrosis [26]. Interestingly, we found that antibody-based platelet depletion from 2 hours before the time of muscle injury impairs subsequent regeneration [25]. Myogenesis does not seem to be affected by platelet depletion because there is a similar number of newly formed myofibers that are positive for embryonic myosin heavy chain (eMHC) in the platelet-depleted mice as in the IgG mock-treated mice. However, platelet depletion exacerbates inflammation at later stages of regeneration, and this impairs myofiber growth and leads to ultrastructural defects in post-injury muscles [25]. Because platelets are recruited immediately after injury to the damaged muscle, they may influence any of the following steps of muscle regeneration, which entails the sequential recruitment of waves of immune cell populations, the clearance of the debris of necrotic myofibers, and the activation, proliferation, and fusion of muscle satellite (~stem) cells to form new myofibers (myogenesis).
Neutrophils are recruited to injured muscles within the first 24 hours after injury and gradually transition back to circulation or undergo apoptosis at the site of injury [15]. Neutrophils are tasked with removing the debris resulting from the necrosis of myofibers and with secreting the signaling factors that promote regeneration and that recruit subsequent immune cell populations [15]. Despite this pivotal role in the early phase of muscle regeneration, it was largely unknown how neutrophils are timely recruited to the damaged muscle upon injury. Our study now indicates that platelets are necessary to recruit neutrophils to injured muscles and that this occurs via the secretion of platelet-specific chemokines with potent neutrophil chemoattractant activities [25]. In particular, we found that neutrophil infiltration, which occurs by day 1 after injury, is significantly reduced by platelet depletion. Cytokine arrays indicate that the intramuscular protein levels of the neutrophil chemoattractants CXCL5 and CXCL7/PPBP, which are chemokines specifically expressed by platelets [27], are high at day 1 after injury but that their levels are significantly reduced by platelet depletion [25]. Because CXCL7/PPBP is 1000x more abundant than other platelet-specific chemokines with neutrophil chemoattractant activities [25], we next examined its role and found that it is necessary for optimal muscle regeneration: in the absence of CXCL7, the infiltration of neutrophils into injured muscles was reduced, resulting in reduced myofiber size and muscle force production at 14 days post-injury [25]. Further confirming the important role of neutrophils in muscle regeneration downstream of platelet-induced chemokine signaling, we found that antibody-based depletion of neutrophils similarly reduces myofiber size and muscle force production post-injury [25]. Altogether, this study identifies a key role of platelets in regenerating muscle by guiding the infiltration of neutrophils into injured muscles (Figure 1).
In addition to recruiting neutrophils to injured muscles, platelets may also play a role in the intramuscular infiltration of other cell types needed for optimal regeneration. It was recently found that the subcutaneous adipose tissue is the source of mesenchymal/adipose stromal cells (ASCs) which are similar to the fibro-adipogenic progenitor cells (FAPs) that reside in skeletal muscles and promote regeneration. Interestingly, ASCs express high levels of podoplanin, a ligand for the C-type lectin-like receptor 2 (CLEC-2) which activates platelets. Platelets interact with ASCs via CLEC-2/podoplanin and promote the mobilization of ASCs from the adipose tissue and their infiltration into the injured muscle. Consequently, podoplanin knockdown or platelet depletion reduces the number of ASCs/FAPs present in the muscle on day 1 after injury, and this impairs muscle regeneration [28].
Altogether, these studies indicate a key role of platelets in initiating regeneration via the recruitment of neutrophils and other pro-regenerative cell populations to the injured muscle.
Platelet-secreted factors can promote myoblast proliferation and metabolic fitness
Application of the platelet releasate (PR) was found to promote the proliferation of cultured C2C12 myoblasts and muscle satellite cells in vitro [29-31]. These effects were ascribed to growth factors secreted by platelets, including VEGF and PDGF: pharmacologic inhibitors of VEGF and PDGF receptor signaling impeded PR-induced myoblast proliferation [29-32]. Continuous PR administration maintained the myoblasts in a proliferative phase, indicating that the PR application needs to be transient to promote myogenesis. However, PR administration after myoblast fusion promoted the differentiation of myotubes [29-31], indicating that the platelet secretome can aid different steps of myogenesis [4]. In addition, it was found that a platelet extract promotes the chemotaxis of muscle satellite cells in vitro, and that this can be largely prevented by anti-TGF-β neutralizing antibodies, indicating that these effects are due to platelet-secreted TGF-β [33].
It was also found that the PR improves the mitochondrial respiratory capacity of muscle satellite cells, suggesting that platelet-secreted factors sustain myoblast proliferation and myogenesis by boosting metabolism [29-31]. Although these effects can be mediated by platelet-secreted signaling factors, mitochondria can also be released by platelets and uptaken by nearby cells and that such mitochondrial transfer improves the metabolic and regenerative capacity of the recipient cells [34]. These studies suggest that platelet-secreted factors can influence muscle satellite cell proliferation and migration, and this may occur at least in part by boosting their metabolic capacity.
While these findings are certainly interesting, they are primarily based on in vitro systems; therefore much remains to be learned about how the PR regulates myogenesis in vivo, and whether this is a therapeutic effect of exogenous PR application, or it is also a normal outcome of platelet-induced signaling during regeneration. However, as discussed above, platelets are recruited to injured muscles immediately after damage, several days before the onset of myogenesis [25]. Because platelets typically release their granules upon clotting and activation, any ensuing effect on myogenesis in vivo may be the indirect result of the effects of platelets on earlier steps of regeneration (such as neutrophil recruitment, inflammation, and neo-angiogenesis, as discussed in the paragraphs above and below). Although no effect of platelet depletion was found on myogenesis in vivo, the assessment of eMHC [25] indicates that exogenous (therapeutic) application of the PR may indeed boost several aspects of myogenesis, as observed in cell culture systems [29-31,33].
In agreement with this possibility, an interesting study found that intraperitoneal injection of the PR from wild-type platelets can rescue the muscle regeneration deficits of ApoE knockout mice, at least in part by promoting myoD and myogenin expression in differentiating myoblasts, and by promoting the growth of newly formed myofibers [35]. The decreased regenerative capacity of ApoE knockout mice arises from hyperlipidemia and metabolic stress, suggesting that the systemic delivery of the PR from wild-type mice may help normalize metabolism in ApoE knockout mice, possibly via ApoE released by platelets [2,3]. Further studies should determine whether platelets and the PR influence myogenesis in distinct disease contexts and whether this response also occurs in vivo in physiological conditions (i.e. in wild-type mice).
Platelet-secreted factors promote myofiber growth and neo-angiogenesis during regeneration
Muscle regeneration requires the assembly of blood vessels around the newly formed myofibers [19,21,26,36]. Consistently, a key pro-angiogenic factor, VEGF, promotes skeletal muscle regeneration and is normally expressed by myofibers in uninjured muscles [37]. At day 1 after injury (a time point at which the myofibers are necrotic), we found that platelet depletion reduces the intramuscular levels of VEGF [25]. Because myofibers are necrotic on day 1 after injury and because both platelets and neutrophils are highly present in injured muscles at that stage [25] and are both known sources of VEGF [38-41], neutrophils and platelets appear to be the major source of VEGF at this early stage of regeneration [25]. Moreover, the high levels of MMP9 and other metalloproteases expressed by neutrophils release additional VEGF from the extracellular matrix, further increasing VEGF bioavailability [42,43]. Consistent with the secretion of VEGF and other pro-angiogenic factors by platelets and by neutrophils, we found that platelet depletion reduces capillary density in regenerating skeletal muscles [25]. Such a decline in regenerative angiogenesis may contribute to the reduced growth of newly formed myofibers in the skeletal muscles of mice treated with platelet-depleting antibodies [25]. However, paradoxically, platelets also secrete signaling factors that reduce angiogenesis. This is the case for CXCL4/PF4, which is secreted by platelets in response to signaling via the complement 5a receptor C5aR1 [44]. Consequently, it was found that platelet-specific deletion of C5aR1 promotes neo-angiogenesis in regenerating muscles by reducing CXCL4/PF4 secretion [44].
Collectively, these findings indicate that platelets secrete several signaling factors that regulate angiogenesis: although platelets generally have pro-angiogenic functions [45], the secretion of CXCL4/PF4 and of other anti-angiogenic factors such as endostatin [46] may constitute a rheostat that impedes an excessive induction of neo-angiogenesis by platelets during muscle regeneration. In other contexts, it was proposed that pro- and anti-angiogenic signaling factors are stored in separate platelet granules and that their release occurs differently depending on the specific stimulus that activates platelets [47]. Whether this holds true during skeletal muscle regeneration and how it is regulated is unknown but certainly of interest.
Altogether, these findings suggest that the formation of novel blood vessels is promoted by platelets and platelet-secreted pro-angiogenic signaling factors during muscle regeneration: however, this occurs under the tight control of anti-angiogenic factors secreted by platelets that may constitute a negative feedback loop to limit or shut down the pro-angiogenic program induced by platelet-secreted VEGF.
Conclusion
The studies discussed above highlight platelets' important signaling roles in skeletal muscle regeneration. Several aspects of muscle regeneration are promoted by platelets, including infiltration of immune cells, neo-angiogenesis, growth of newly formed myofibers, and possibly also satellite cell function and myogenesis. Future studies should dissect the specific components of platelet-initiated signaling that are necessary for muscle regeneration in different contexts characterized by muscle damage and/or impaired regenerative capacity, such as myopathies, cancer, and aging. Moreover, because of their capacity to be selectively recruited to the site of injury and release the content of their secretory granules, platelets could be engineered to deliver specific therapeutics to the site of injury to further improve or modify the characteristics of the regenerating muscle. In addition, recombinant versions of platelet-secreted factors could be delivered to the muscle to augment platelet-induced muscle regeneration. In summary, platelets and their secretome offer promising therapeutic avenues for promoting skeletal muscle regeneration during aging and disease.
ACKNOWLEDGMENTS
M.L. is supported by the National Cancer Institute (R01CA245301). Research at St. Jude Children’s Research Hospital is supported by ALSAC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interests.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Smyth SS, McEver RP, Weyrich AS, Morrell CN, et al. 2009. Platelet functions beyond hemostasis. J Thromb Haemost 7: 1759–66. [DOI] [PubMed] [Google Scholar]
- 2.Parsons MEM, Szklanna PB, Guerrero JA, Wynne K, et al. 2018. Platelet Releasate Proteome Profiling Reveals a Core Set of Proteins with Low Variance between Healthy Adults. Proteomics 18: e1800219. [DOI] [PubMed] [Google Scholar]
- 3.Piersma SR, Broxterman HJ, Kapci M, de Haas RR, et al. 2009. Proteomics of the TRAP-induced platelet releasate. J Proteomics 72: 91–109. [DOI] [PubMed] [Google Scholar]
- 4.Chellini F, Tani A, Zecchi-Orlandini S, Sassoli C. 2019. Influence of Platelet-Rich and Platelet-Poor Plasma on Endogenous Mechanisms of Skeletal Muscle Repair/Regeneration. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimauro I, Grasso L, Fittipaldi S, Fantini C, et al. 2014. Platelet-rich plasma and skeletal muscle healing: a molecular analysis of the early phases of the regeneration process in an experimental animal model. PLoS One 9: e102993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charge SB, Rudnicki MA. 2004. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–38. [DOI] [PubMed] [Google Scholar]
- 7.Wagers AJ, Conboy IM. 2005. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell 122: 659–67. [DOI] [PubMed] [Google Scholar]
- 8.Brack AS, Munoz-Canoves P. 2016. The ins and outs of muscle stem cell aging. Skelet Muscle 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz-Canoves P, Neves J, Sousa-Victor P. 2020. Understanding muscle regenerative decline with aging: new approaches to bring back youthfulness to aged stem cells. FEBS J 287: 406–16. [DOI] [PubMed] [Google Scholar]
- 10.Blanc RS, Kallenbach JG, Bachman JF, Mitchell A, et al. 2020. Inhibition of inflammatory CCR2 signaling promotes aged muscle regeneration and strength recovery after injury. Nat Commun 11: 4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouche M, Munoz-Canoves P, Rossi F, Coletti D. 2014. Inflammation in muscle repair, aging, and myopathies. Biomed Res Int 2014: 821950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Paepe B, De Bleecker JL. 2013. Cytokines and chemokines as regulators of skeletal muscle inflammation: presenting the case of Duchenne muscular dystrophy. Mediators Inflamm 2013: 540370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwata Y, Suzuki N, Ohtake H, Kamauchi S, et al. 2016. Cancer cachexia causes skeletal muscle damage via transient receptor potential vanilloid 2-independent mechanisms, unlike muscular dystrophy. J Cachexia Sarcopenia Muscle 7: 366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tidball JG. 2005. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288: R345–53. [DOI] [PubMed] [Google Scholar]
- 15.Tidball JG. 2017. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol 17: 165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panci G, Chazaud B. 2021. Inflammation during post-injury skeletal muscle regeneration. Semin Cell Dev Biol 119: 32–8. [DOI] [PubMed] [Google Scholar]
- 17.Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, et al. 2010. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest 120: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt LC, Xu B, Finkelstein D, Fan Y, et al. 2015. The glucose-sensing transcription factor MLX promotes myogenesis via myokine signaling. Genes Dev 29: 2475–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latroche C, Weiss-Gayet M, Muller L, Gitiaux C, et al. 2017. Coupling between Myogenesis and Angiogenesis during Skeletal Muscle Regeneration Is Stimulated by Restorative Macrophages. Stem Cell Reports 9: 2018–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, et al. 2010. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A 107: 3287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floriano JF, Emanueli C, Vega S, Barbosa AMP, et al. 2022. Pro-angiogenic approach for skeletal muscle regeneration. Biochim Biophys Acta Gen Subj 1866: 130059. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen NL, Morton AB, Segal SS. 2023. Angiogenesis precedes myogenesis during regeneration following biopsy injury of skeletal muscle. Skelet Muscle 13: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labelle M, Begum S, Hynes RO. 2011. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20: 576–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labelle M, Begum S, Hynes RO. 2014. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A 111: E3053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graca FA, Stephan A, Minden-Birkenmaier BA, Shirinifard A, et al. 2023. Platelet-derived chemokines promote skeletal muscle regeneration by guiding neutrophil recruitment to injured muscles. Nat Commun 14: 2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen NL, Norton CE, Shaw RL, Cornelison DDW, et al. 2022. Myofibre injury induces capillary disruption and regeneration of disorganized microvascular networks. J Physiol 600: 41–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandt E, Ludwig A, Petersen F, Flad HD. 2000. Platelet-derived CXC chemokines: old players in new games. Immunol Rev 177: 204–16. [DOI] [PubMed] [Google Scholar]
- 28.Sastourne-Arrey Q, Mathieu M, Contreras X, Monferran S, et al. 2023. Adipose tissue is a source of regenerative cells that augment the repair of skeletal muscle after injury. Nat Commun 14: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scully D, Naseem KM, Matsakas A. 2018. Platelet biology in regenerative medicine of skeletal muscle. Acta Physiol (Oxf) 223: e13071. [DOI] [PubMed] [Google Scholar]
- 30.Scully D, Sfyri P, Verpoorten S, Papadopoulos P, et al. 2019. Platelet releasate promotes skeletal myogenesis by increasing muscle stem cell commitment to differentiation and accelerates muscle regeneration following acute injury. Acta Physiol (Oxf) 225: e13207. [DOI] [PubMed] [Google Scholar]
- 31.Scully D, Sfyri P, Wilkinson HN, Acebes-Huerta A, et al. 2020. Optimising platelet secretomes to deliver robust tissue-specific regeneration. J Tissue Eng Regen Med 14: 82–98. [DOI] [PubMed] [Google Scholar]
- 32.Pinol-Jurado P, Gallardo E, de Luna N, Suarez-Calvet X, et al. 2017. Platelet-Derived Growth Factor BB Influences Muscle Regeneration in Duchenne Muscle Dystrophy. Am J Pathol 187: 1814–27. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff R 1997. Chemotaxis of skeletal muscle satellite cells. Dev Dyn 208: 505–15. [DOI] [PubMed] [Google Scholar]
- 34.Levoux J, Prola A, Lafuste P, Gervais M, et al. 2021. Platelets Facilitate the Wound-Healing Capability of Mesenchymal Stem Cells by Mitochondrial Transfer and Metabolic Reprogramming. Cell Metab 33: 283–99 e9. [DOI] [PubMed] [Google Scholar]
- 35.Barlow J, Sfyri PP, Mitchell R, Verpoorten S, et al. 2021. Platelet releasate normalises the compromised muscle regeneration in a mouse model of hyperlipidaemia. Exp Physiol 106: 700–13. [DOI] [PubMed] [Google Scholar]
- 36.Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, et al. 2007. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol 293: R651–61. [DOI] [PubMed] [Google Scholar]
- 37.Wagner PD. 2011. The critical role of VEGF in skeletal muscle angiogenesis and blood flow. Biochem Soc Trans 39: 1556–9. [DOI] [PubMed] [Google Scholar]
- 38.Webb NJ, Bottomley MJ, Watson CJ, Brenchley PE. 1998. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci (Lond) 94: 395–404. [DOI] [PubMed] [Google Scholar]
- 39.Salgado R, Benoy I, Bogers J, Weytjens R, et al. 2001. Platelets and vascular endothelial growth factor (VEGF): a morphological and functional study. Angiogenesis 4: 37–43. [DOI] [PubMed] [Google Scholar]
- 40.Gaudry M, Bregerie O, Andrieu V, El Benna J, et al. 1997. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood 90: 4153–61. [PubMed] [Google Scholar]
- 41.Gong Y, Koh DR. 2010. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res 339: 437–48. [DOI] [PubMed] [Google Scholar]
- 42.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. 2007. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A 104: 20262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J 2018. Neutrophils in tissue injury and repair. Cell Tissue Res 371: 531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nording H, Baron L, Haberthur D, Emschermann F, et al. 2021. The C5a/C5a receptor 1 axis controls tissue neovascularization through CXCL4 release from platelets. Nat Commun 12: 3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kisucka J, Butterfield CE, Duda DG, Eichenberger SC, et al. 2006. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci U S A 103: 855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klement GL, Yip TT, Cassiola F, Kikuchi L, et al. 2009. Platelets actively sequester angiogenesis regulators. Blood 113: 2835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Battinelli EM, Markens BA, Italiano JE Jr. 2011. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood 118: 1359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.