Abstract
Individuals diagnosed with autism often display alterations in visual spatial attention toward visual stimuli, but the underlying cause of these differences remains unclear. Recent evidence has demonstrated that covert spatial attention, rather than remaining constant at a cued location, samples stimuli rhythmically at a frequency of 4–8 Hz (theta). Here we tested whether rhythmic sampling of attention is altered in autism. Participants were asked to monitor three locations to detect a brief target presented 300–1200 ms after a spatial cue. Visual attention was oriented to the cue and modified visual processing at the cued location, consistent with previous studies. We measured detection performance at different cue-target intervals when the target occurred at the cued location. Significant oscillations in detection performance were identified using both a traditional time-shuffled approach and a new autoregressive surrogate method developed by Brookshire in 2022. We found that attention enhances behavioral performance rhythmically at the same frequency in both autism and control group at the cued location. However, rhythmic temporal structure was not observed in a subgroup of autistic individuals with co-occurring attention-deficit/hyperactivity disorder (ADHD). Our results imply that intrinsic brain rhythms which organize neural activity into alternating attentional states is functional in autistic individuals, but may be altered in autistic participants who have a concurrent ADHD diagnosis.
Keywords: attention, rhythmic attention, spatial attention, shifting attention, autism
Lay Summary
Many people with autism struggle with shifting their attention from one task or object to another, which can make it challenging for them to switch between different topics or activities. However, our study showed that attention switches between objects in the same way in adults with and without autism. These results imply that a fundamental temporal property of spatial attention is not changed in autistic adults.
Introduction
Individuals diagnosed with Autism Spectrum Disorder display changes in a wide range of attention-related functions, including selective, sustained, spatial, and shifting attention operations (e.g., Casey et al., 1993; Allen & Courchesne, 2001; Remington et al., 2009; Fan et al., 2012). One prominent hypothesis is a general difficulty disengaging attention from one stimulus and shifting to another, which results in a sort of pause or delay in orienting visual attention (Mo et al., 2019; Renner et al., 2006; Wainwright-Sharp & Bryson, 1993; Casey et al., 1993). This difference in attentional modulation may contribute to some features of autism such as insistence on sameness. Consistent with this view, slower shifting of visual attention has been observed in children, adolescents and adults with autism compared to individuals without autism (Keehn et al., 2010; Townsend et al., 1996). However, on some measures, for example, when given adequate time, autistic individuals do not show impaired performance in shifting attention (Richard & Lajiness-Oneill, 2015; Pascualvaca et al., 1998). As the results are mixed, Pascualvaca et al. proposed that autism involves altered coordination and modulation of attentional resources, rather than deficits in attention shifting per se (Pascualvaca et al., 1998). But there is little evidence for the altered coordination of attentional resources in autism.
In non-autistic individuals, recent evidence has demonstrated that, rather than remaining constant at the presently attended location, covert attention samples the environment rhythmically at a frequency of 4–8 Hz (theta) (Fiebelkorn et al., 2013; Landau & Fries, 2012). Thus, even under conditions that maintain attention at a cued location, there are alternating periods of either attention-related sensory sampling or attention shifting (Fiebelkorn & Kastner, 2019). This rhythmic reweighting of attentional prioritization is thought to prevent overfocused attention on any given location and promote a more effective sampling of the environment. Recent reports of slower binocular rivalry in autism suggest altered intrinsic neural dynamics of cortical processing in the autistic brain (Spiegel et al., 2019; Robertson et al., 2013). Although they affect perception differently, binocular rivalry and attention are both involved in the dynamic selection of visual stimuli (Mitchell et al., 2004). Thus, we hypothesized that rhythmic sampling of attention may operate differently in autism.
In the present study, we investigated the coordination of attentional resources in autism by examining the rhythmic sampling of attention. We used an established cue-target paradigm (Fiebelkorn et al., 2013) with variable cue-target-interval to examine the time course of covert spatial attention. Our results show that covert spatial attention operates rhythmically in both the autistic and non-autistic groups at the cued location, at the same sampling frequency. Additionally, in secondary exploratory analyses, rhythmic temporal structure was not observed in a subgroup of autistic individuals with co-occurring attention-deficit/hyperactivity disorder (ADHD). These results imply that intrinsic brain rhythms which organize neural activity into alternating attentional states is functional in autistic individuals, but may be altered in autistic participants who have a concurrent ADHD diagnosis.
Method
Participants
Participants included 29 participants (Age: 20–30 years) diagnosed with autism and 29 non-autistic subjects that served as a comparison group (Age: 20–30 years), matched on age, sex-assigned-at-birth and IQ (Table 1). All participants had normal or corrected-to-normal vision. Diagnosis of ASD was confirmed based on a telehealth adaptation of the Autism Diagnostic Observation Schedule, second edition (ADOS-2,Lord et al., 2012), the Childhood Autism Rating Scales- High Functioning (CARS-HF) (Schopler et al., 2010), and a DSM-5 checklist, conducted by research reliable clinicians (American Psychiatric Association, 2013). More information about the remote autism diagnostic confirmation assessment via Telehealth are available by contacting authors. Scores on the Autism Quotient-28 (AQ-28) were also used to confirm a diagnosis of ASD, autistic participants had to score >65, while non-autistic participants had to score <65 (Baron-Cohen et al., 2001). ADOS-2 Overall Comparison Score was calculated based on Hus’s paper (Hus et al., 2014; Hus and Lord 2014). Autistic and non-autistic participants with a diagnosis of epilepsy or other neurological disorders, a past serious head injury, motor impairments and sensory impairments were not recruited. To be included in the study, all participants were required to be stable on their medication for at least 3 months. Eight participants with ASD had a self-reported diagnosis of ADHD. All non-autistic participants had no ADHD diagnosis history. All participants gave informed written consent to participate in the study, which was approved by the Institutional Review Board of the University of Washington.
Table 1.
Participant Characteristics
| autistic | non-autistic | p value | t | |
|---|---|---|---|---|
| Sex ratio (males: females) |
11:18 | 11:18 | ||
| Age in years (mean ± SD) |
23.6± 3.3 | 23.8±3.0 | 0.8081 | 0.2522 |
| IQ Full Score-4 | 117.9±13.0 | 115.3±10.0 | 0.3983 | 0.8514 |
| ADOS-2 Overall Comparison Score (mean ± SD) |
6.8 ± 1.7 |
Experimental design
The experimental design was adapted from the task used by Fiebelkorn and colleagues (2013). Participants were asked to perform a detection task. In each trial, two bars were presented either horizontally or vertically around the central fixation point. After 400 – 800 ms, a spatial cue (100 ms) randomly appeared at one of the four ends of the two bars. Following a variable cue-target interval (37 cue-target intervals, in steps of 25 ms from 300–1200 ms, randomly selected in each trial), a near-threshold target Gabor patch was presented for 100 ms. Targets appeared at the cued location, the uncued location at the same bar or an uncued location at the different bar in 63%, 16% and 16% of all trials, respectively. Total number of trials was 350, split into 5 runs, and each participant completed 6 trials for each cue-target interval condition where the target appeared in the cued location. There was no target on 5% trials (catch trials) and these trials were excluded from data analysis. Participants were asked to maintain central fixation and press the button as soon as they detected the target. The contrast of the target grating was adjusted for each participant before the experiment using a staircase procedure to ensure that average performance at the cued location was approximately 75%. The false alarm rates were 34.96% for the autistic group and 22.55% for the non-autistic group. While these values are higher than in typical signal detection tasks, they are within an acceptable range and consistent with our goal of maintaining task difficulty at a level that yields ~75% accuracy. When misses and false alarm occurred, there was an auditory beep as feedback. The main analysis in the manuscript consists of only trials in which the target appeared at the cued locations. Analyses of the trials in which the target appeared in uncued locations (in the same or different bar) are regarded as preliminary due to the low number of trials in each of these conditions, and are only briefly reported.
Data analysis
All data were analyzed with MATLAB (R2019b) and Python 3. First, we calculated the detection accuracy within 75 ms bins of each cue-target interval in steps of 25 ms. Second, the time course of target detection was detrended by removing a polynomial trend with degree 2. Third, the detrended time course was converted into the frequency domain using Fast Fourier Transform (FFT). The absolute value of the complex FFT output provided spectral amplitude measurements. Fourth, we averaged the spectral amplitude across participants for each group (autistic and non-autistic) and condition (cued location, uncued-same object location and uncued-different object location). Finally, the peak frequency was defined as frequency with the largest spectral amplitude within 0.1–20 Hz. Group differences were examined using between-subjects t-tests. Additional Bayesian analysis was used to assess the relative evidence for the absence of groups differences (quantify support for the null hypothesis) and was carried out using the bayesFactor Toolbox in Matlab by Bart Krekelberg (https://klabhub.github.io/bayesFactor/#1).
Two methods were applied to test the significance of oscillation. First, we followed the widely used shuffling-in-time procedure (Fiebelkorn et al., 2013; Landau & Fries, 2012): we shuffled the time course of target detection in time and then calculated the spectrum by the same procedure as described for the empirical data. After 1000 randomizations, p-value at each frequency was computed as the proportion that the empirical amplitude is smaller than the amplitude of the shuffled data. Multiple comparisons across frequencies were corrected by Bonferroni correction.
In a second analysis, we adopted a recent approach proposed by Brookshire (Brookshire, 2022), based on generating a surrogate distribution with an autoregressive (AR) model. The reason we adopted this method was because the shuffling-in-time procedure does not separate rhythmic from arrhythmic structure, which might lead to the misidentification of significant behavioral oscillations. For each participant, an AR model with a single positive coefficient (AR(1)) was fit to the binned time courses of target detection performance using exact maximum likelihood with the Kalman filter. This AR(1) model captures the lag-1 autocorrelated aperiodic structure of the original time courses. Then the surrogate time courses were generated from the AR(1) model 10000 times. We processed the surrogate data as described above for real data (detrend and FFT) and data obtained with the surrogate distribution. The averaged power spectrum (across participants) of the real data was subsequently compared to the surrogate distribution of averaged power spectra (across participants), frequency by frequency. Frequencies where the real power spectrum exceeded the 95th percentile of the surrogate distribution were considered significant. Multiple comparisons across frequencies were corrected by Bonferroni correction.
Results
Attention samples stimuli rhythmically in autistic and non-autistic individuals
To study the rhythmic sampling of attention, we used an established behavioral approach (Fiebelkorn et al., 2013) (Figure 1) to examine the temporal dynamics of behavioral performance at the attended location. Participants were asked to maintain central fixation and report the appearance of a brief target after a spatial cue which indicates target location in 63% of all trials. The cue-target interval varied from 300 to 1200 ms. Thus, we can measure participants’ detection performance at different cue-target intervals while they covertly monitored the cued location. For each participant, the contrast of the target grating was adjusted before the main experiment to ensure that average performance at the cued location was approximately 75%. For autistic participants, the average detection accuracy was 73% ± 2.7% (mean ± SEM) and 64% ± 2.7% (mean ± SEM) when the target appeared at cued locations and uncued locations (same object and different object) respectively. For non-autistic participants, the average detection accuracy was 77% ± 2% (mean ± SEM) and 66% ± 2.6% (mean ± SEM) when the target appeared at cued locations and uncued locations (same object and different object) respectively. Both groups benefited from valid cueing (cued vs uncued location paired t test for each group: p<0.001). Fluctuations of detection performance at the cued location was observed in both the autistic and non-autistic individuals (Figure 2A). Since there are differences in the phase of individual oscillatory time courses, we converted the behavioral time courses to the frequency domain individually and identified the significant oscillation frequency using a nonparametric test that shuffles the data in time (Fiebelkorn et al., 2013). Our results showed that behavioral performance fluctuated periodically at theta-band in both autistic and non-autistic group (Figure 2B, p < 0.05, corrected for multiple comparisons). There was no significant difference in the peak frequency between groups (Figure 2C, autistic: 3.05 ± 0.23; non-autistic: 3.10 ± 0.24, t56 = 0.1568, p = 0.876). Additionally, the observed Bayes Factor (Morey & Wagenmakers, 2014) of 4.2463 (BF01) also indicates evidence in support of the null hypothesis (no group difference in peak frequency). These findings suggest that attention samples stimuli rhythmically at the same frequency in autistic and non-autistic individuals.
Figure 1. Experiment design.
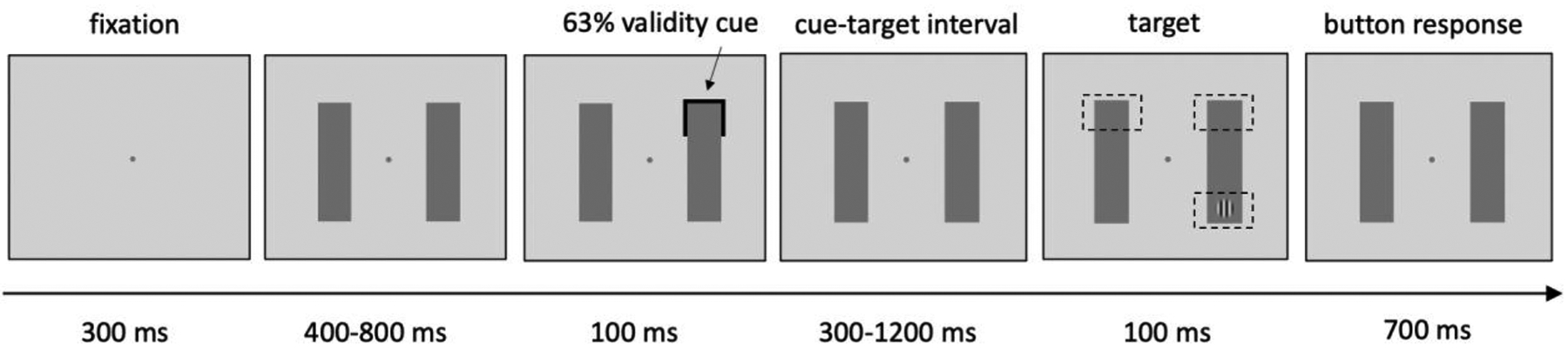
Two vertical or horizontal bars were shown on the screen. After a 100 ms cue and a variable cue-target interval, a near threshold target grating was either presented at the cued location, uncued location or absent. The contrast of grating is increased in this figure for the reader’s convenience. Participants were asked to keep fixation to the central dot and press a button as soon as they detect the target.
Figure 2.
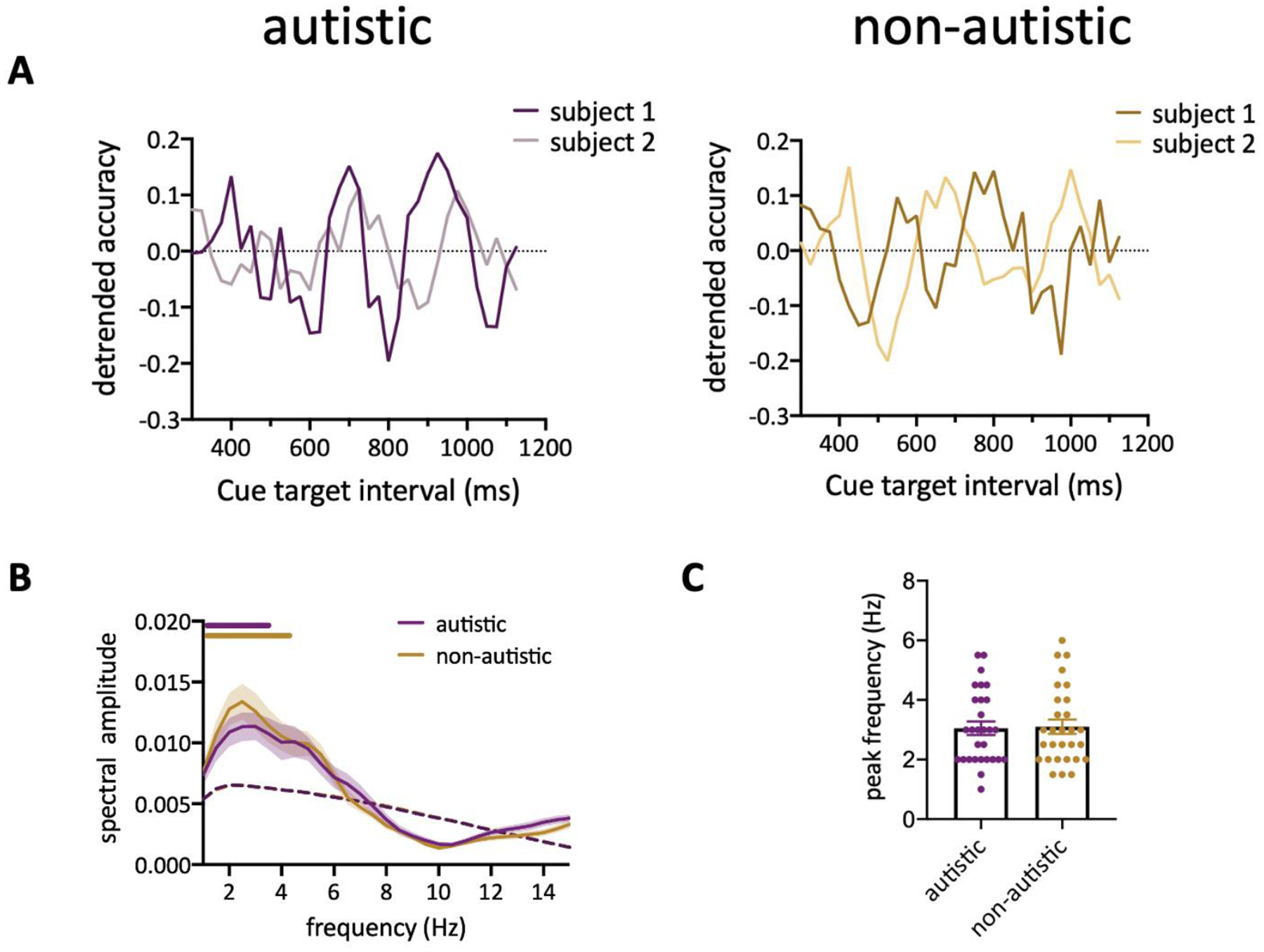
(A) Behavioral time course from two example subjects from each group. (B) Group-averaged amplitude spectra of the behavioral time course. The purple and yellow horizontal bar indicate the significant frequencies (p<0.05, corrected for multiple comparison) for autistic and non-autistic group, respectively. Shaded areas indicate SEM across participants. The dashed lines represent significant threshold defined by the 95th percentile of the surrogate data. (C) Averaged peak frequency across participants.
Oscillations are not explained by aperiodic temporal structure
A recent study by Brookshire proposed that rhythmic oscillations of attention could be a false positive finding, resulting from aperiodic temporal structure (Brookshire, 2022). The standard analysis technique of the shuffling-in-time procedure used to test for the null hypothesis eliminates aperiodic temporal structure, which can lead to the misidentification of significant behavioral oscillations. To rule out the confounding effect of aperiodic temporal structure, an alternative analysis method proposed by Brookshire was used to test the significance of the result. In the new method, a surrogate distribution was created using an autoregressive (AR) model which captures aperiodic structures. We found that the behavioral performance exhibits a significant oscillation at about 5 Hz in both the autistic (Figure 3 Left; p < 0.05 for 3.5–5.5 Hz) and non-autistic group (Figure 3 Right; p < 0.05 for 4–6 Hz). Additionally, although with limited number of trials, significant periodicity at the uncued same-object location and uncued different-object location were also observed in both ASD and NT participants (Figure S1). Thus, the result of the new analysis method is in-line with the results of the traditional analysis, confirming that rhythmic sampling of attention appears to be the same in autistic and non-autistic individuals.
Figure 3.
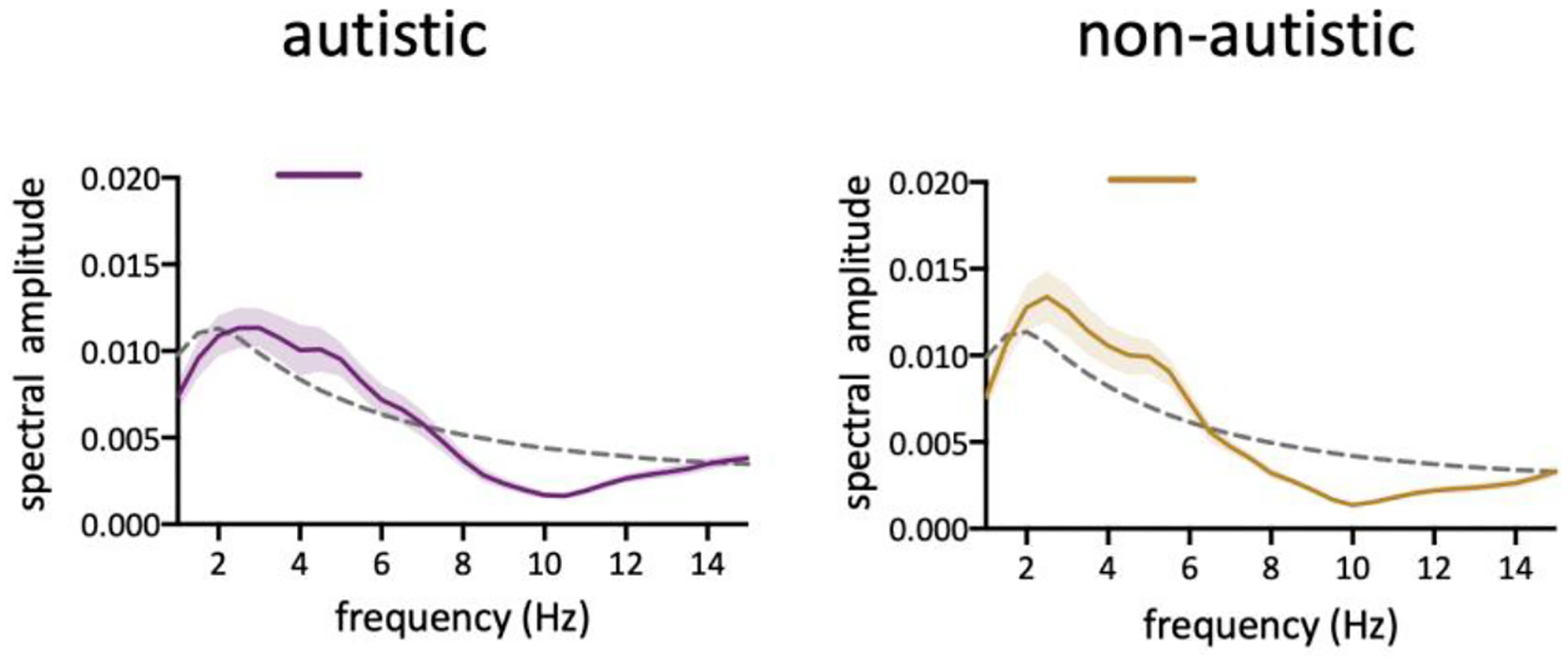
The autoregressive surrogate method uncovered oscillations in behavioral time course. Horizontal bars above the curve indicate the significant frequencies (p<0.05, corrected for multiple comparison). The dashed line represents significant threshold defined by the 95th percentile of the surrogate data.
Attentional rhythm is atypical in autistic individuals with co-occurring ADHD
Symptoms of inattention, hyperactivity and impulsivity are common in ASD and estimated to occur in up to 50% of individuals (Murray 2010). In the current sample, 8 autistic participants (out of 29) had a self-reported diagnosis of ADHD. As ADHD is obviously related to attention, and could impact the current findings, we used the AR surrogate method to analyze the cued location data separating the autistic group according to their ADHD status. Behavioral performance in the autistic-nonADHD group fluctuated periodically in the same theta-band compared to the non-autistic group (Figure 4A Left; p < 0.05 for 4.5–6 Hz). In the autistic+ADHD group, no significant oscillation was observed (Figure 4A Right), and the peak frequency of behavioral performance was significantly lower in these participants (Figure 4B, t27 = 2.563, p = 0.016). To exclude the possibility that the absence of significant oscillation in autistic+ADHD was due to the small number of participants, we randomly sampled 8 participants from the autistic-nonADHD group and found significant rhythmic temporal structure (Figure 4C, p < 0.05 for 4.5 Hz). These results suggest that attentional oscillations could be altered in ADHD, or in co-occurring ASD+ADHD. However, this is an exploratory result with only 8 participants, hence future research is needed to elucidate this issue.
Figure 4.
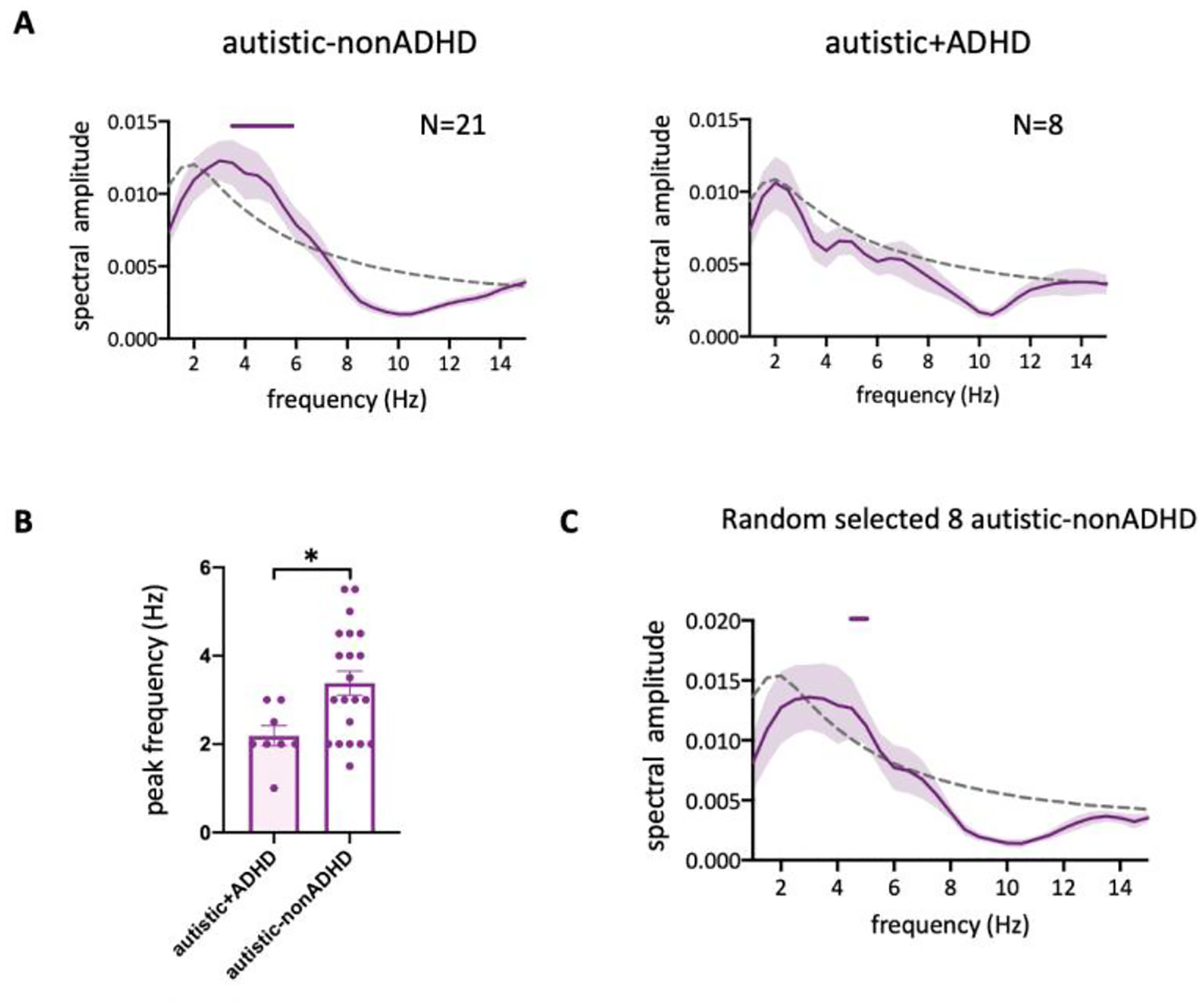
(A) and (C) Group-averaged amplitude spectra of the behavioral time course. Horizontal bars above the curve indicate the significant frequencies (p<0.05, corrected for multiple comparison). The dashed line represents significance threshold defined by the 95th percentile of the surrogate data. N is the number of subjects. All data are analyzed using AR method. (B) Averaged peak frequency across participants. Nautistic+ADHD=8, Nautistic-nonADHD =21.
Discussion
The present study was designed to test whether rhythmic attentional sampling is altered in autistic individuals. For this purpose, we used a target detection task with variable cue-target intervals to uncover the temporal dynamics of attention. First, our results replicated the rhythmic attentional switching observed in prior studies (Fiebelkorn et al., 2013; Landau & Fries, 2012) in non-autistic individuals, even after accounting for aperiodic temporal structure. The results further show that spatial attention operates rhythmically at theta band in both autistic and non-autistic groups, with no difference in peak frequency between groups. However, an exploratory analysis suggests that attention might switch differently in autistic participants who have a concurrent ADHD diagnosis.
Interestingly, in 2022, Brookshire found no evidence for rhythmic temporal structure after reanalyzing published datasets in a new approach that can account for non-oscillatory temporal structure. We applied Brookshire’s method on our data, but generated surrogate data from fitted AR model based on behavioral time courses of each participant instead of the averaged time course for all participants. Because of the oscillatory phase difference in individual participants (Figure S3; Fiebelkorn et al., 2013; Re et al., 2019; Chen et al., 2017), averaging the behavioral performance across participants may generate different results. A recent study, which employed the analysis pipeline recommended by Brookshire, also highlighted the importance of fitting autoregressive models to individual time series rather than averaged time courses. This is because the behavioral rhythms of individuals may vary in phase, and averaging them could cause the behavioral rhythms to be lost (Chota et al, 2022). Therefore, it is likely that attentional oscillation was not observed in Brookshire’s study due to fitting autoregressive models to averaged time courses, as described in his code. However, his proposed AR surrogate analysis is highly valuable in distinguishing between rhythmic and arrhythmic structures.
Fiebelkorn and colleagues (2013) have found that the detection performance showed two peaks at 4 Hz and 8 Hz for targets at the cued location or an uncued location within the same object, and one peak at 4 Hz for targets at an uncued location in a different object. In the current study we found rhythms at 4 Hz for the detection rates at the cued location, but not at 8 Hz. Similar to our findings, Helfrich and colleagues in 2018 also detected a 4 Hz rhythmic pattern at the cued location using a similar paradigm (Helfrich et al., 2018). We hypothesize that the differences between these studies may stem from distinct task requirements. In the Fiebelkorn et al., 2013 study, the cue validity was 75% and the performance requirement was only 65%. They suggested that sustained attention at the cued location interfered with the full 4Hz attentional switching between two objects, hence allowing an 8Hz rhythmic pattern within objects to emerge. However, in our study, as well as Helfrich’s 2018 study, the task accuracy demands were elevated - cue validity was 72% and 75%, respectively, while performance requirements were 75% and 80%. Consequently, subjects needed to exert more effort on attentional switching between objects to meet these higher performance standards. This increased attentional shifting may have downplayed the preferential processing at the cued location, thus emphasizing the 4Hz periodicity between objects. Consistent with this explanation, a recent study investigated how the sequential sampling mechanism adapts to the need of attending to from one to four locations and their results suggested that that the generally stable rhythmic attention mechanism could flexibly adjusts its sampling rate to accommodate increased attentional demands (Jiang et al., In press).
It is possible that alterations in attention early in development may influence the development of social communication abilities and contribute to various features of autism. Consistent with this idea, differences in orienting of attention, such as disengaging, shifting, and reengaging attention, have been widely reported in autism. Numerous studies have found evidence of impaired attention disengagement in both autistic adults (Mo et al., 2019; Wainwright-Sharp & Bryson,1993; Casey et al., 1993; Sacrey et al., 2014; Kawakubo et al., 2007) and infants at high risk for developing autism (Elsabbagh et al., 2009; Zwaigenbaum et al., 2005; Landry & Bryson, 2004). Additionally, slower shifting of visual attention has been observed (Keehn et al., 2010; Townsend et al., 1996). These findings have led to the hypothesis that attention alterations in autism represent a general reduction in the orienting response, which is not specific to social stimuli (Renner et al., 2006).
Although there is an extensive body of literature demonstrating differences in attentional processes between individuals with and without autism, other studies have provided contrasting evidence and indicate that attentional abilities are largely intact in autism (Grubb et al., 2013). When given adequate time, autistic individuals do not show impaired performance in shifting attention (Richard & Lajiness-Oneill, 2015; Pascualvaca et al., 1998). In addition, impaired attentional disengagement was not observed in autistic children (Fischer et al., 2014) and toddlers newly diagnosed with ASD (Fischer et al., 2016). These results do not support the notion that attentional disengagement is impaired at an early stage, leading to an altered developmental trajectory. One explanation for the conflicting findings could be that differences in attention orientation stem from heterogeneous comorbid conditions in the autistic group. For example, individuals with autism may have an abnormally narrow or an abnormally broad focus of attention, depend upon the presence or absence of reductions in parietal lobe volume (Townsend & Courchesne,1994). Another possibility is that attentional differences are more closely related to changes in higher-order functions, such as semantic and social information processing during the perception of scenes, objects, faces, and voices. In fact, many autistic individuals tend to focus on a single aspect of an object or environment while ignoring others. Some studies have also shown that autistic children have more difficulty switching their attention between different sensory modalities (Reed & McCarthy, 2012), and autistic individuals seem to have less preference for looking at scene regions with rich semantic-level saliency (Wang et al., 2015). According to Weak Central Coherence Theory, individuals with ASD tend to process local-level information at the expense of global and semantic information (Happe and Frith, 2006). Thus, autistic individuals not only have differences in the distribution of attention across spatial locations (Townsend & Courchesne,1994; Allen & Courchesne, 2001), but also across different levels of image attributes (Remington et al., 2012). Although we didn’t observe any altered attentional oscillation in individuals with autism, it is important to note that attention is a complex, multi-faceted cognitive process, and our study specifically focused on rhythmic sampling of attention, which is just one aspect of it. Our findings add to the nuanced understanding of attentional processes in autism, suggesting that while some aspects of attention might differ between individuals with and without autism, others like rhythmic attentional sampling do not.
A recent Electrocorticography (ECoG) study was designed to investigate the structural basis for rhythmic sampling of attention and demonstrated that rhythmic behavioral performance is linked to the neural oscillations in the frontoparietal attention network (Helfrich et al., 2018). Although the study of altered theta brain oscillations and their link to sensory and perceptual processing in autism remains limited, disruptions of neural oscillation in autism have been observed in multiple frequency bands (Simon & Wallace 2016; Milne et al., 2009; Snijders et al., 2013; Sun et al., 2012). In some studies, the atypical orienting of visual attention in autism were related to structural abnormalities in parietal lobe (Townsend & Courchesne 1994; Townsend et al., 1996). But our results implied a typical intrinsic theta-band oscillations in the frontoparietal attention network.
ADHD is a common disorder characterized by inattention, hyperactivity and impulsivity. It affects both children and adults, with inattention symptoms being more prominent in adults (Faraone et al., 2006; Simon et al., 2009). It is estimated that about 30% - 50% individuals with ASD meet diagnostic criteria for ADHD, although it is not fully understood why both conditions often co-occur (Rong et al.,2021; Antshel et al., 2013; Joshi et al., 2017; Matson et al., 2013). Interestingly, attention seems to switch differently in autistic individuals who have ADHD. The results suggest that attention may switch at a slower rate, or even not follow a rhythmic pattern, in people with co-occurring ASD and ADHD. This finding is consistent with previous research showing reduced rhythmic modulation of behavior and performance in participants with ADHD (Dankner et al., 2017; Yordanova et al., 2011). However, no ADHD evaluation was included in the current study, and no dimensional assessment of ADHD traits was made. Thus, this exploratory analysis is limited to relatively few participants who, as part of our assessment process, reported a previous ADHD diagnosis. Moreover, future research including participants with ADHD without autism is warranted to examine whether atypical attentional oscillation is a characteristic of ADHD, or is unique to people with co-occurring ASD and ADHD.
Binocular rivalry is a phenomenon that occurs when two different images are presented to each eye simultaneously. Instead of perceiving a combined image, perception alternates between the two images. Converging experimental evidence has shown that attention plays an important role in binocular rivalry (Zhang et al., 2011; Ooi & He, 1999), and samples competing percepts periodically (Davidson et al., 2018), but the exact relationship between rhythmic sampling of attention and binocular rivalry is not fully understood. Dynamics in binocular rivalry are correlated with structure and function of parietal and frontal cortex (Lumer et al., 1998). Interestingly, rhythmic perceptual sampling is also an inherent feature of the frontoparietal network (Fiebelkorn & Kastner, 2019; Helfrich et al., 2018). A computational model proposed that rivalry relies on both attentional modulation and mutual inhibition, which explained some phenomena reported in rivalry (Li et al., 2017). Similarly, it has been hypothesized that the perceptual alternations in binocular rivalry are, at least in part, the result of modulation by right-sided fronto-parietal brain regions in attention network (Tong et al., 2006; Lumer et al., 1998). In autism, it has been consistently found that the rate of transitions in binocular rivalry is slower (Robertson et al., 2013; Spiegel et al., 2019; Choi et al., 2023; Freyberg et al., 2015), however our current results show that spatial attention sampling rate is not changed. Future work is needed to clarify the relationship between attentional oscillations and binocular rivalry and their underlying cortical dynamics, and how these mechanisms may differ and interact in autism.
In conclusion, our findings indicate that attention oscillates rhythmically in both non-autistic and autistic individuals, in the absence of coexisting ADHD. This suggests that a fundamental property of spatial attention is preserved in autistic adults. However, attention may process visual information differently in autistic individuals who also have ADHD. One limitation of the present study is the exclusive inclusion of autistic adults with average or above IQ, which raises questions about the generalizability of the findings to the broader autistic community and the developmental trajectory of attentional systems. Further research is necessary to explore these aspects and provide a more comprehensive understanding of potential attention differences in autism.
Supplementary Material
Acknowledgments
This research was supported by NIH R01MH11847 to SOM and SJW.
Footnotes
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Behavioral time course from autistic and non-autistic group are available on Open Science Framework (https://osf.io/byd9h/).
References
- Allen G, & Courchesne E (2001). Attention function and dysfunction in autism. Frontiers in Bioscience : A Journal and Virtual Library, 6(2), D105–19. 10.2741/allen [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Antshel KM, Zhang-James Y, & Faraone SV (2013). The comorbidity of ADHD and autism spectrum disorder. Expert Review of Neurotherapeutics, 13(10), 1117–1128. 10.1586/14737175.2013.840417 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, & Clubley E (2001). The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31(1), 5–17. 10.1023/a:1005653411471 [DOI] [PubMed] [Google Scholar]
- Brookshire G (2022). Putative rhythms in attentional switching can be explained by aperiodic temporal structure. Nature Human Behaviour. 10.1038/s41562-022-01364-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Gordon CT, Mannheim GB, & Rumsey JM (1993). Dysfunctional attention in autistic savants. Journal of Clinical and Experimental Neuropsychology, 15(6), 933–946. 10.1080/01688639308402609 [DOI] [PubMed] [Google Scholar]
- Chen A, Wang A, Wang T, Tang X, & Zhang M (2017). Behavioral oscillations in visual attention modulated by Task difficulty. Frontiers in Psychology, 8(SEP), 1–9. 10.3389/fpsyg.2017.01630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YB, Mentch J, Haskins AJ, Van Wicklin C, & Robertson CE (2023). Visual processing in genetic conditions linked to autism: A behavioral study of binocular rivalry in individuals with 16p11.2 deletions and age-matched controls. Autism Research, (September 2022), 1–10. 10.1002/aur.2901 [DOI] [PubMed] [Google Scholar]
- Chota S, Leto C, van Zantwijk L, & Van der Stigchel S (2022). Attention rhythmically samples multi-feature objects in working memory. Scientific Reports, 12(1), 1–12. 10.1038/s41598-022-18819-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankner Y, Shalev L, Carrasco M, & Yuval-Greenberg S (2017). Prestimulus Inhibition of Saccades in Adults With and Without Attention-Deficit/Hyperactivity Disorder as an Index of Temporal Expectations. Psychological Science, 28(7), 835–850. 10.1177/0956797617694863 [DOI] [PubMed] [Google Scholar]
- Davidson MJ, Alais D, van Boxtel JJA, & Tsuchiya N (2018). Attention periodically samples competing stimuli during binocular rivalry. ELife, 7, 1–25. 10.7554/eLife.40868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Holmboe K, Tucker L, Csibra G, Baron-Cohen S, … Johnson MH (2009). Visual orienting in the early broader autism phenotype: disengagement and facilitation. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 50(5), 637–642. 10.1111/j.1469-7610.2008.02051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Bernardi S, Dam NT, Anagnostou E, Gu X, Martin L, … Hof PR (2012). Functional deficits of the attentional networks in autism. Brain and Behavior, 2(5), 647–660. 10.1002/brb3.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, & Mick E (2006). The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychological Medicine, 36(2), 159–165. 10.1017/S003329170500471X [DOI] [PubMed] [Google Scholar]
- Fiebelkorn IC, & Kastner S (2019). A Rhythmic Theory of Attention. Trends in Cognitive Sciences, 23(2), 87–101. 10.1016/j.tics.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Pinsk MA, & Kastner S (2018). A Dynamic Interplay within the Frontoparietal Network Underlies Rhythmic Spatial Attention. Neuron, 99(4), 842–853.e8. 10.1016/j.neuron.2018.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Saalmann YB, & Kastner S (2013). Rhythmic sampling within and between objects despite sustained attention at a cued location. Current Biology, 23(24), 2553–2558. 10.1016/j.cub.2013.10.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Koldewyn K, Jiang YV, & Kanwisher N (2014). Unimpaired attentional disengagement and social orienting in children with autism. Clinical Psychological Science, 2(2), 214–223. 10.1177/2167702613496242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Smith H, Martinez-Pedraza F, Carter AS, Kanwisher N, & Kaldy Z (2016). Unimpaired attentional disengagement in toddlers with autism spectrum disorder. Developmental Science, 19(6), 1095–1103. 10.1111/desc.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg J, Robertson CE, & Baron-Cohen S (2015). Reduced perceptual exclusivity during object and grating rivalry in autism. Journal of Vision, 15(13), 1–12. 10.1167/15.13.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MA, Behrmann M, Egan R, Minshew NJ, Carrasco M, & Heeger DJ (2013). Endogenous Spatial Attention: Evidence for Intact Functioning in Adults With Autism. Autism Research, 6(2), 108–118. 10.1002/aur.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, & Frith U (2006). The weak coherence account: detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders, 36(1), 5–25. 10.1007/s10803-005-0039-0 [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Fiebelkorn IC, Szczepanski SM, Lin JJ, Parvizi J, Knight RT, & Kastner S (2018). Neural Mechanisms of Sustained Attention Are Rhythmic. Neuron, 99(4), 854–865.e5. 10.1016/j.neuron.2018.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, & Lord C (2014). The autism diagnostic observation schedule, module 4: Revised algorithm and standardized severity scores. Journal of Autism and Developmental Disorders, 44(8), 1996–2012. 10.1007/s10803-014-2080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Gotham K, & Lord C (2014). Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders, 44(10), 2400–2412. 10.1007/s10803-012-1719-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, He S, & Zhang J. The adaptive flexibility of rhythmic attentional sampling in attending to multiple targets. Journal of Experimental Psychology: General. In press [DOI] [PubMed] [Google Scholar]
- Joshi G, Faraone SV, Wozniak J, Tarko L, Fried R, Galdo M, … Biederman J (2017). Symptom Profile of ADHD in Youth With High-Functioning Autism Spectrum Disorder: A Comparative Study in Psychiatrically Referred Populations. Journal of Attention Disorders, 21(10), 846–855. 10.1177/1087054714543368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo Y, Kasai K, Okazaki S, Hosokawa-Kakurai M, Watanabe K-I, Kuwabara H, … Maekawa H (2007). Electrophysiological abnormalities of spatial attention in adults with autism during the gap overlap task. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 118(7), 1464–1471. 10.1016/j.clinph.2007.04.015 [DOI] [PubMed] [Google Scholar]
- Keehn B, Lincoln AJ, Müller RA, & Townsend J (2010). Attentional networks in children and adolescents with autism spectrum disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 51(11), 1251–1259. 10.1111/j.1469-7610.2010.02257.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau AN, & Fries P (2012). Attention samples stimuli rhythmically. Current Biology, 22(11), 1000–1004. 10.1016/j.cub.2012.03.054 [DOI] [PubMed] [Google Scholar]
- Landry R, & Bryson SE (2004). Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry and Allied Disciplines, 45(6), 1115–1122. 10.1111/j.1469-7610.2004.00304.x [DOI] [PubMed] [Google Scholar]
- Li HH, Rankin J, Rinzel J, Carrasco M, & Heeger DJ (2017). Attention model of binocular rivalry. Proceedings of the National Academy of Sciences of the United States of America, 114(30), E6192–E6201. 10.1073/pnas.1620475114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S (2012) Autism diagnostic observation schedule: ADOS. Los Angeles: Western Psychological Services. [Google Scholar]
- Lumer ED, Friston KJ, & Rees G (1998). Neural correlates of perceptual rivalry in the human brain. Science (New York, N.Y.), 280(5371), 1930–1934. 10.1126/science.280.5371.1930 [DOI] [PubMed] [Google Scholar]
- Matson JL, Rieske RD, & Williams LW (2013). The relationship between autism spectrum disorders and attention-deficit/hyperactivity disorder: An overview. Research in Developmental Disabilities, 34(9), 2475–2484. 10.1016/j.ridd.2013.05.021 [DOI] [PubMed] [Google Scholar]
- Milne E, Scope A, Pascalis O, Buckley D, & Makeig S (2009). Independent component analysis reveals atypical electroencephalographic activity during visual perception in individuals with autism. Biological Psychiatry, 65(1), 22–30. 10.1016/j.biopsych.2008.07.017 [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Stoner GR, & Reynolds JH (2004). Object-based attention determines dominance in binocular rivalry. Nature, 429(6990), 410–413. 10.1038/nature02584 [DOI] [PubMed] [Google Scholar]
- Mo S, Liang L, Bardikoff N, & Sabbagh MA (2019). Shifting visual attention to social and non-social stimuli in Autism Spectrum Disorders. Research in Autism Spectrum Disorders, 65(152), 56–64. 10.1016/j.rasd.2019.05.006 [DOI] [Google Scholar]
- Morey RD, & Wagenmakers E-J (2014). Simple relation between Bayesian order-restricted and point-null hypothesis tests. Statistics & Probability Letters, 92, 121–124. 10.1016/j.spl.2014.05.010 [DOI] [Google Scholar]
- Murray MJ (2010). Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Current Psychiatry Reports, 12(5), 382–388. 10.1007/s11920-010-0145-3 [DOI] [PubMed] [Google Scholar]
- Ooi TL, & He ZJ (1999). Binocular rivalry and visual awareness: The role of attention. Perception, 28(5), 551–574. 10.1068/p2923 [DOI] [PubMed] [Google Scholar]
- Pascualvaca DM, Fantie BD, Papageorgiou M, & Mirsky AF (1998). Attentional capacities in children with autism: is there a general deficit in shifting focus? Journal of Autism and Developmental Disorders, 28(6), 467–478. 10.1023/a:1026091809650 [DOI] [PubMed] [Google Scholar]
- Re D, Inbar M, Richter CG, & Landau AN (2019). Feature-Based Attention Samples Stimuli Rhythmically. Current Biology, 29(4), 693–699.e4. 10.1016/j.cub.2019.01.010 [DOI] [PubMed] [Google Scholar]
- Reed P, & McCarthy J (2012). Cross-modal attention-switching is impaired in autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(6), 947–953. 10.1007/s10803-011-1324-8 [DOI] [PubMed] [Google Scholar]
- Remington A, Campbell R, & Swettenham J (2012). Attentional status of faces for people with autism spectrum disorder. Autism : The International Journal of Research and Practice, 16(1), 59–73. 10.1177/1362361311409257 [DOI] [PubMed] [Google Scholar]
- Remington A, Swettenham J, Campbell R, & Coleman M (2009). Selective attention and perceptual load in autism spectrum disorder. Psychological Science, 20(11), 1388–1393. 10.1111/j.1467-9280.2009.02454.x [DOI] [PubMed] [Google Scholar]
- Renner P, Grofer Klinger L, & Klinger MR (2006). Exogenous and Endogenous Attention Orienting in Autism Spectrum Disorders. Child Neuropsychology, 12(4–5), 361–382. 10.1080/09297040600770753 [DOI] [PubMed] [Google Scholar]
- Richard AE, & Lajiness-Oneill R (2015). Visual attention shifting in autism spectrum disorders. Journal of Clinical and Experimental Neuropsychology, 37(7), 671–687. 10.1080/13803395.2015.1042838 [DOI] [PubMed] [Google Scholar]
- Robertson CE, Kravitz DJ, Freyberg J, Baron-Cohen S, & Baker CI (2013). Slower Rate of binocular rivalry in autism. Journal of Neuroscience, 33(43), 16983–16991. 10.1523/JNEUROSCI.0448-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Yang CJ, Jin Y, & Wang Y (2021). Prevalence of attention-deficit/hyperactivity disorder in individuals with autism spectrum disorder: A meta-analysis. Research in Autism Spectrum Disorders, 83(February), 101759. 10.1016/j.rasd.2021.101759 [DOI] [Google Scholar]
- Sacrey LAR, Armstrong VL, Bryson SE, & Zwaigenbaum L (2014). Impairments to visual disengagement in autism spectrum disorder: A review of experimental studies from infancy to adulthood. Neuroscience and Biobehavioral Reviews, 47, 559–577. 10.1016/j.neubiorev.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Schopler E, Van Bourgondien M, Wellman G, & Love S (2010). Childhood autism rating scale-second edition (CARS2): Manual. Los Angeles: Western Psychological Services. [Google Scholar]
- Simon DM, & Wallace MT (2016). Dysfunction of sensory oscillations in Autism Spectrum Disorder. Neuroscience and Biobehavioral Reviews, 68, 848–861. 10.1016/j.neubiorev.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V, Czobor P, Bálint S, Mészáros A, & Bitter I (2009). Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. The British Journal of Psychiatry : The Journal of Mental Science, 194(3), 204–211. 10.1192/bjp.bp.107.048827 [DOI] [PubMed] [Google Scholar]
- Snijders TM, Milivojevic B, & Kemner C (2013). Atypical excitation-inhibition balance in autism captured by the gamma response to contextual modulation. NeuroImage. Clinical, 3, 65–72. 10.1016/j.nicl.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A, Mentch J, Haskins AJ, Robertson CE (2019). Slower Binocular Rivalry in the Autistic Brain. Current Biology, 29(17), 2948–2953.e3. 10.1016/j.cub.2019.07.026 [DOI] [PubMed] [Google Scholar]
- Sun L, Grützner C, Bölte S, Wibral M, Tozman T, Schlitt S, … Uhlhaas PJ (2012). Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal-posterior cortices. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32(28), 9563–9573. 10.1523/JNEUROSCI.1073-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F, Meng M, & Blake R (2006). Neural bases of binocular rivalry. Trends in Cognitive Sciences, 10(11), 502–511. 10.1016/j.tics.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Townsend J, & Courchesne E (1994). Parietal damage and narrow “spotlight” spatial attention. Journal of Cognitive Neuroscience, 6(3), 220–232. 10.1162/jocn.1994.6.3.220 [DOI] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, & Egaas B (1996). Slowed orienting of covert visual-spatial attention in autism: Specific deficits associated with cerebellar and parietal abnormality. Development and Psychopathology, 8(3), 563–584. 10.1017/S0954579400007276 [DOI] [Google Scholar]
- Townsend J, Harris NS, & Courchesne E (1996). Visual attention abnormalities in autism: Delayed orienting to location. Journal of the International Neuropsychological Society, 2(6), 541–550. 10.1017/S1355617700001715 [DOI] [PubMed] [Google Scholar]
- Wainwright-Sharp JA, & Bryson SE (1993). Visual orienting deficits in high-functioning people with autism. Journal of Autism and Developmental Disorders, 23(1), 1–13. 10.1007/BF01066415 [DOI] [PubMed] [Google Scholar]
- Wang S, Jiang M, Duchesne XM, Laugeson EA, Kennedy DP, Adolphs R, & Zhao Q (2015). Atypical Visual Saliency in Autism Spectrum Disorder Quantified through Model-Based Eye Tracking. Neuron, 88(3), 604–616. 10.1016/j.neuron.2015.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordanova J, Albrecht B, Uebel H, Kirov R, Banaschewski T, Rothenberger A, & Kolev V (2011). Independent oscillatory patterns determine performance fluctuations in children with attention deficit/hyperactivity disorder. Brain : A Journal of Neurology, 134(Pt 6), 1740–1750. 10.1093/brain/awr107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Jamison K, Engel S, He B, & He S (2011). Binocular rivalry requires visual attention. Neuron, 71(2), 362–369. 10.1016/j.neuron.2011.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, & Szatmari P (2005). Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience : The Official Journal of the International Society for Developmental Neuroscience, 23(2–3), 143–152. 10.1016/j.ijdevneu.2004.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Behavioral time course from autistic and non-autistic group are available on Open Science Framework (https://osf.io/byd9h/).


