Abstract
Peptidyl-prolyl isomerases (PPIs) catalyse the cis–trans isomerisation of peptide bonds N-terminal to proline residues in polypeptide chains. They have roles in the folding of newly synthesised proteins and in the function of the immune system. In addition, members of the parvulin-like family of PPIs have been implicated in cell cycle control. Their activity is directed by the prior phosphorylation of target proteins in both yeast and mammalian cells. More recent data have illustrated that they may also influence other nuclear events. This review examines PPI activity in the context of eukaryotic transcriptional regulation. The findings are consistent with a two-step model of conformational control, in which the outcome depends on the transcription factor involved.
Introduction
Peptidyl-prolyl isomerases (PPIs) are ubiquitous proteins expressed in prokaryotic and eukaryotic cells alike. Their primary catalytic function is to facilitate the cis–trans isomerisation of peptide bonds N-terminal to proline (Pro) residues within polypeptide chains (see Figure 1). Hence a role for PPIs in the folding of newly synthesised proteins was inferred and indeed chaperone-like activity has been associated with several PPIs (Kruse et al., 1995; Schmid, 1995). The earlier findings that immunosuppressant drugs bind to PPIs and that inhibition of PPI activity is essential for immune suppression also implicated the enzymes in immune function (Schreiber, 1991), although it is now recognised that they act by sequestering calcineurin, a calcium/calmodulin-dependent protein phosphatase, rather than having an immune system-specific function.
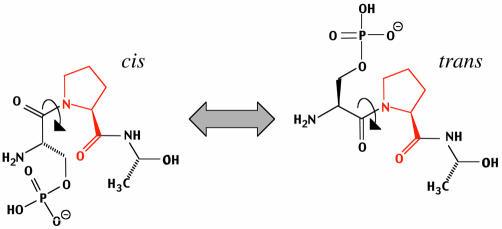
Fig. 1. Peptidyl-prolyl isomerisation. Cis/trans isomerisation of the peptide bond (arrow) preceding proline (in red) in the tri-peptide pSer-Pro-Ala. Specificity for the phosphorylated substrate is a property of the Pin1/Ess1 subfamily of parvulin-like PPIs, although some cyclophilins may be able to isomerise pSer-Pro/pThr-Pro bonds. The cis and trans forms of peptidyl-proline bonds exist in equilibrium. Analysis of protein structure databases indicates that ∼5% of Ser-Pro motifs in proteins exist as cis prolyl bonds (Reimer et al., 1998). While phosphorylation of Ser or Thr preceding a Pro has only a minor effect on the cis/trans equilibrium in short polypeptides (G. Fischer, personal communication), major effects may result in proteins due to vicinal charged side-chains. For example, a significant change in the cis/trans ratio of a Thr-Pro motif in the cytoplasmic tail of the amyloid precursor protein (APP) upon phosphorylation has been reported (Ramelot and Nicholson, 2001).
Based on drug specificity, PPIs have been divided into three distinct classes: the Cyclosporin A (CsA)-binding cyclophilins; the FK506-binding proteins (FKBPs); and the Parvulin-like PPIs, which do not bind immunosuppressants. These three PPI classes are also structurally distinct. Cyclophilins are characterised by an eight-stranded β-barrel that forms a hydrophobic pocket in which CsA binds (Ke et al., 1991; Mikol et al., 1993). FKBPs, in contrast, consist of an amphipathic, five-stranded β-sheet that wraps around a single, short α-helix (Michnick et al., 1991; Van Duyne et al., 1991). Members of the parvulin family, which include the eponymous Escherichia coli protein parvulin, the Drosophila dodo gene product, the Arabidopsis protein PIN1At and the human proteins Pin1 and hPar14 (Rahfeld et al., 1994; Maleszka et al., 1997; Uchida et al., 1999; Landrieu et al., 2000), sport a PPI domain consisting of a half β-barrel, its four antiparallel strands surrounded by four α-helices (Ranganathan et al., 1997).
Unlike cyclophilins and FKBPs, eukaryotic parvulin-like PPIs have been shown to be important for cell cycle progression (Hanes et al., 1989; Lu et al., 1996; Rippmann et al., 2000). Compelling evidence also links parvulin-like PPIs to several aspects of gene regulation. In both instances they appear to work in conjunction with protein kinases to control the activity or stability of key regulatory components. This paper reviews these recent findings, in particular data linking PPI function to the action of RNA polymerase II and several transcription factors.
Ess1 and Pin1 target phosphorylated substrates
The yeast parvulin-like PPI Ess1/Ptf1 (hereafter termed Ess1) and its human orthologue Pin1 both consist of two domains: a short, N-terminal WW domain (with two conserved tryptophans) and a C-terminal catalytic domain. In general, WW domains behave rather like Src homology 3 (SH3) domains insofar as they recognise Pro-rich sequences. Given that the WW domains of Ess1 and Pin1 are indispensable for function, it was inferred that WW domains serve to direct them to target proteins (Ranganathan et al., 1997). Several groups have now shown that the critical recognition motif of the Pin1 WW domain is either Ser-Pro or Thr-Pro. Importantly, these motifs are only recognised when Ser or Thr is phosphorylated (i.e. pSer-Pro or pThr-Pro) (Lu et al., 1999; Verdecia et al., 2000; Landrieu et al., 2001). This finding immediately implied that the substrates of proline-directed protein kinases such as cyclin-dependent kinases (CDKs) and mitogen-activated protein kinases (MAPKs) were likely to be Ess1 and Pin1 targets. As such sites are frequently recognised by the mitotic-phosphoprotein-monoclonal antibody MPM-2 (Davis et al., 1983), a reagent was already available to test this notion.
The first evidence that this is the case was obtained for Cdc25, a Cdc2-directed phosphatase, and its upstream regulator, polo-like kinase (Plk1). Cdc25 is multiply phosphorylated and activated, in part by Cdc2/cyclin B in a positive feedback loop, during the G2/M transition of the cell cycle. Pin1 was found to interact with Cdc25 in mitotic, but not interphase, Xenopus egg extracts. Similarly, Pin1 interacted with Plk1 from HeLa cells arrested in mitosis but not from cells arrested in S phase (Crenshaw et al., 1998). Moreover, interactions between Pin1 and several mitotic regulators—including Cdc25 and Plk1—were shown to be controlled by phosphorylation (Crenshaw et al., 1998; Shen et al., 1998). Further work has indicated that Pin1 acts on Cdc25 in a catalytic manner to promote a conformational change that facilitates its dephosphorylation by PP2A (Zhou et al., 2000; Stukenberg and Kirschner, 2001). It is also worth noting that although pin1 and ess1 deletions characteristically disrupt mitosis—for example, they cause premature mitotic entry in Xenopus oocytes (Winkler et al., 2000) and late mitotic arrest in Candida albicans (Devasahayam et al., 2002)—in many organisms they do not result in a lethal phenotype. Intriguingly, two recent studies have shown that yeast cells lacking ess1 display an increased sensitivity to CsA, implying that cyclophilins may perform functions normally carried out by parvulin-like PPIs (Fujimori et al., 2001; Huang et al., 2001). This apparent functional redundancy also suggests that some cyclophilins may even be able to isomerise pSer-Pro/pThr-Pro bonds.
Roles for PPIs in transcription and gene silencing
The first data to link PPI function to transcriptional control were those implicating a cyclophilin in the regulation of the cMyb protein. Intramolecular interactions between the N-terminal DNA-binding and C-terminal regulatory domains of cMyb suppress its DNA binding. In vitro studies of this inhibition of DNA-binding showed it to require the cyclophilin Cyp-40, and suggested that it involves PPI activity, as inhibition could be blocked by CsA. Provocatively, the oncogenic vMyb protein, which harbours point mutations in its DNA-binding domain, was not bound by Cyp-40 and escaped inhibition, lending circumstantial support to the idea that cyclophilins can influence transcriptional events by causing changes in protein conformation (Leverson and Ness, 1998). At least one FKBP has also been linked to transcriptional events. Association of interferon regulatory factor 4 (IRF-4) with FKBP52 was found to block DNA-binding and transcriptional activation by the PU.1–IRF-4 complex, in a manner dependent on PPI activity (Mamane et al., 2000).
A considerable body of data now implicates parvulin-like PPIs in gene expression. The first evidence derived from a yeast genetic screen for factors involved in pre-mRNA processing. Two temperature-sensitive mutants defective in 3′-end formation were rescued by expression of Ess1. Both mutants were found to harbour substitutions of conserved residues in the PPI domain of Ess1, which reduced the protein’s catalytic activity (Hani et al., 1999). However, the precise role of Ess1 in mRNA 3′-end formation is as yet unclear.
The most compelling evidence linking Pin1 function specifically to transcription also derives from yeast genetic experiments. A screen for suppressors of conditional ess1 mutants yielded five genes, all of which express proteins with known or suspected transcriptional activity. These include components of the polymerase II holoenzyme, a subunit of the Sin3A–Rpd3 histone deacetylase complex (HDAC), a protein with sequence similarities to both human TFIIS and Polycomb/Bithorax family members and a Zn-finger transcription factor (Wu et al., 2000). In line with earlier reports (Albert et al., 1999; Morris et al., 1999), Ess1 was shown to interact physically and genetically with the C-terminal domain (CTD) of polymerase II. While these results indicate a functional association between Ess1 and the general transcription machinery, analysis of gene expression levels suggested that the effects of ess1 mutations on gene transcription are nonetheless selective. This selectivity may be linked to the identity of a sixth gene found to suppress the conditional ess1 phenotype; the fact that this gene, CPR1, encodes the yeast homologue of cyclophilin A (Arevalo-Rodriguez et al., 2000) raised the interesting possibility that the other suppressors act by elevating CPR1 expression. However, no evidence of this was found. Instead, both cyclophilin A and Ess1 were shown to be capable of interacting with the Sin3A–Rpd3 HDAC in vitro and of modulating HDAC-dependent gene silencing in vivo, although paradoxically, with opposite effects. These results provide a further indication that cyclophilin A and Pin1 may have partially overlapping functions.
Little evidence is available to implicate Pin1 directly in the process of transcription in vertebrate cells. Indeed, in a study involving in vitro transcription and in vivo reporter assays, the only evidence of a role for Pin1 was the inhibition of pre-initiation complex formation by Juglone, a naphthoquinone with dubious specificity for parvulin-like PPIs (Hennig et al., 1998; Chao et al., 2001).
Transcription factors as targets for modulation by Ess1 and Pin1
In the last year, Pin1 has been linked to the regulation of several transcription factors (see Table I). Among these is β-catenin, whose regulation involves intracellular localisation and protein stability (Dale, 1998; Hecht and Kemler, 2000). Activation of the Wnt signalling pathway inhibits the phosphorylation of β-catenin by glycogen synthase kinase 3β (GSK-3β), resulting in its stabilisation and nuclear accumulation. Target genes for β-catenin include c-myc, fibronectin and notably cyclin D1 (He et al., 1998; Gradl et al., 1999; Tetsu and McCormick, 1999). In the absence of Wnt signals, the APC protein, which is expressed by the tumour suppressor gene mutated in familial adenomatous polyposis coli (APC), exports β-catenin from the nucleus and recruits it into a cytoplasmic complex with GSK-3β, thus promoting β-catenin degradation and downregulating its target genes (Rubinfeld et al., 1996). Pin1 has been identified as a positive regulator of β-catenin signalling and is thought to function by antagonising the interaction between β-catenin and APC. Overexpression of Pin1 reduces the level of β-catenin associating with APC in HeLa cells and increases nuclear β-catenin, effects that require both the WW domain and PPI function. Pin1 binds to β-catenin at a pSer-Pro motif (Ser246) within the armadillo repeats, at a position close to the APC interaction interface, and a β-catenin protein mutated at Ser246 is no longer influenced by Pin1 (Ryo et al., 2001). These findings are particularly noteworthy in the context of oncogenesis, in which β-catenin is implicated, as Pin1 was found to be overexpressed several fold in invasive breast tumours.
Table I. Transcription factors potentially regulated by Ess1 and Pin1 identified to date.
| Transcription factor | Protein kinase | Consequence | Reference |
|---|---|---|---|
| β-catenin |
GSK-3β |
Dissociation from APC, nuclear accumulation |
Ryo et al. (2001) |
| c-Jun |
JNK/SAPKs |
Transcriptional activation |
Wulf et al. (2001) |
| CF2 |
dMAPK |
Ubiquitination, degradation |
Hsu et al. (2001) |
| NFAT |
NFAT kinases |
Transcriptional inhibition |
Liu et al. (2001) |
| CTH1 |
ND |
ND |
Wu et al. (2000) |
| YKL005C | ND | ND | Wu et al. (2000) |
ND, not determined.
A second mechanism by which Pin1 may promote elevated cyclin D1 expression, which is detected in 50% of breast cancers, involves cooperation with Ras to activate the transcription factor c-Jun. Activated c-Jun N-terminal kinases/stress activated protein kinases (JNK/SAPKs) translocate to the nucleus, dock with the δ-domain of c-Jun and phosphorylate two Ser-Pro motifs (Ser63 and Ser73), thereby activating c-Jun for transcription (Chang and Karin, 2001). These events are stimulated by oncogenic Ras. Co-immunoprecipitation and in vitro interaction experiments indicate that at least one of the motifs is recognised by Pin1. Moreover, Pin1 and Ha-Ras signalling are able to activate cyclin D1 reporter expression cooperatively through an AP-1 binding site (Wulf et al., 2001). The implication of these findings is that Pin1 acts as a MAPK-sensitive relay switch by binding to phosphorylated c-Jun and altering the conformation of its N-terminal transactivation domain. It is noteworthy that although mice lacking Pin1 were initially reported to develop normally, further studies have shown that they display cell-proliferation phenotypes common to cyclin D1-deficient mice (Fujimori et al., 1999; Liou et al., 2002). In this context Pin1 was also shown to interact with cyclin D1 via pThr286, enhancing both its nuclear localisation and stability.
Pin1 has also been found to function downstream of a MAPK during oogenesis in Drosophila. Dodo, the Drosophila Pin1 homologue, acts in the epidermal growth factor receptor (EGFR) pathway that initiates dorsoventral patterning of somatic follicle cells within the developing egg. As a result of EGFR signalling, phosphorylation of the transcription factor CF2 at a single site by dMAPK targets the protein for proteasomal degradation. These events are blocked by proteasomal inhibitors, mutation of the phosphorylation site in CF2 (Thr40) or a Dodo deficiency (Hsu et al., 2001). Thus, in this scenario, Dodo may interact with CF2 to induce a conformation that is more amenable to recognition by a SCF-family E3 ligase and subsequent ubiquitylation.
Pin1 also appears to act in the T cell activation pathway targeted by the immunosuppressant CsA. In quiescent cells, the T cell-specific NFAT transcription is phosphorylated and resides within the cytoplasm. However, following T cell activation, calcium signalling leads to NFAT dephosphorylation by calcineurin, and to its subsequent translocation to the nucleus (Graef et al., 2001). Overexpression of Pin1 in Jurkat T cells has been reported to prevent NFAT activation by saturating pSer-Pro motifs and thereby blocking their dephosphorylation by calcineurin (Liu et al., 2001). It is still unclear, however, how Pin1 acts at physiological levels. It may even promote the dephosphorylation of NFAT by calcineurin, and thereby exert a positive effect on transcription.
Pin1 is also regulated by WW domain phosphorylation
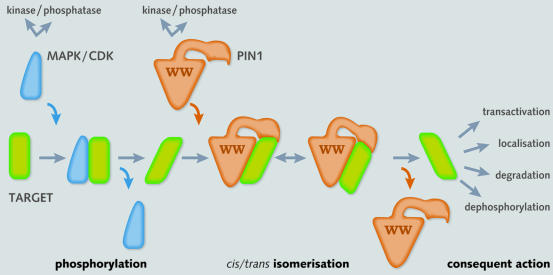
The underlying theme of the preceding examples is that Pin1 and its yeast homologue act in concert with protein kinases in a two-step ‘tag and twist’ mechanism to reconfigure target protein structures with context-dependent outcomes (see Figure 2). This concept has now been complicated by other recent findings. First, Pin1 is itself a protein kinase substrate (Lu et al., 2002). Phosphorylation at Ser16 in the WW domain appears to be regulated in a cell cycle-dependent manner, whereby cells in G2/M contain unphosphorylated Pin1. As phosphorylated Pin1, or a Pin1 mutant in which Ser16 is substituted by glutamate, fail to bind mitotic phosphoproteins, phosphorylation may serve to constrain the actions of Pin1 exclusively to nuclear proteins phosphorylated during the later phase of the cell cycle. The Pin1 kinase and phosphatase have yet to be identified unequivocally.
Fig. 2. Two-step tag and twist mechanism involving a protein kinase and PPI. Phosphorylation of a target protein (green) by a proline-directed serine/threonine kinase (blue) creates a docking site for the WW domain of Ess1/Pin1 (brown). Subsequent isomerisation by the catalytic domain changes the conformation of the target protein to regulate transactivation, intracellular localisation, dephosphorylation or ubiquitylation through modulation of interactions with accessory transcription factors, phosphatases, E3 ligases or other proteins.
Secondly, the conformation of the polypeptide backbone can determine the initial phosphorylation rate of a Ser-Pro motif. For example, ERK2 shows a clear preference in vitro for the cis form of the Ser-Pro peptide bond in a short peptide substrate (Weiwad et al., 2000). This finding suggests that in a protein with multiple Ser-Pro/Thr-Pro motifs, e.g. Cdc25, an initial phosphorylation event may trigger a succession of alternating phosphorylation–isomerisation steps, each one revealing a further target for modification, to prescribe the overall conformational change. By extension, peptidyl-prolyl bond conformation may also affect the specificity with which other proteins, including other WW-domain proteins (Sudol et al., 2001) or protein phosphatases such as PP2A interact with pSer-Pro/pThr-Pro motifs, thereby influencing the half-lives of target phosphoproteins.
Perspectives
To date the role of protein conformational change, which is recognised to be of fundamental importance in other areas, notably prion biology, has received limited attention in the context of transcriptional regulation. However, the body of evidence now available firmly implicates PPI activity both in the regulatory mechanisms governing several disparate transcription factors and in the function of RNA polymerase II itself. They may act on their target proteins either at catalytic concentrations or stoichiometrically; data supporting both modes of action have been described. A range of biochemical and biophysical approaches will now be required to unravel the intricacies of substrate recognition, peptidyl-prolyl bond equilibria and other determinants of conformational change in transcriptionally active proteins. PPIs may be set to turn heads.

Peter E. Shaw
Acknowledgments
Acknowledgements
I would like to thank Dr Gunter Fischer for discussions, Drs Cornelia de Moor, Roger Patient, Tahir Pillay and the anonymous referees for their comments on the manuscript and Wendy Solis for help with its preparation. Thanks also to Dr Charlie Laughton for help with Figure 1. Work in the author’s laboratory is funded by grants from the Wellcome Trust, the Association of International Cancer Research (AICR) and the Institut de Recherches Servier.
REFERENCES
- Albert A., Lavoie, S. and Vincent, M. (1999) A hyperphosphorylated form of RNA polymerase II is the major interphase antigen of the phosphoprotein antibody MPM-2 and interacts with the peptidyl-prolyl isomerase Pin1. J. Cell Sci., 112, 2493–2500. [DOI] [PubMed] [Google Scholar]
- Arevalo-Rodriguez M., Cardenas, M.E., Wu, X., Hanes, S.D. and Heitman, J. (2000) Cyclophilin A and Ess1 interact with and regulate silencing by the Sin3-Rpd3 histone deacetylase. EMBO J., 19, 3739–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. and Karin, M. (2001) Mammalian MAP kinase signalling cascades. Nature, 410, 37–40. [DOI] [PubMed] [Google Scholar]
- Chao S.-H., Greenleaf, A. and Price, D.L. (2001) Juglone, an inhibitor of the peptidyl-prolyl isomerase Pin1, also directly blocks transcription. Nucleic Acids Res., 29, 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenshaw D.G., Yang, J., Means, A.R. and Kornbluth, S. (1998) The mitotic peptidyl-prolyl isomerase, Pin1, interacts with Cdc25 and Plx1. EMBO J., 17, 1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale T.C. (1998) Signal transduction by the Wnt family of ligands. Biochem. J., 329, 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F.M., Tsao, T.Y., Fowler, S.K. and Rao, P.N. (1983) Monoclonal antibodies to mitotic cells. Genes Dev., 80, 2926–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devasahayam G., Chaturvedi, V. and Hanes, S.D. (2002) The Ess1 prolyl isomerase is required for growth and morphogenetic switching in Candida albicans. Genetics, 160, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori F., Takahashi, K., Uchida, C. and Uchida, T. (1999) Mice lacking Pin1 develop normally, but are defective in entering cell cycle from G0 arrest. Biochem. Biophys. Res. Commun., 265, 658–663. [DOI] [PubMed] [Google Scholar]
- Fujimori F. et al. (2001) Crosstalk of prolyl isomerases, Pin1/Ess1, and cyclophilin A. Biochem. Biophys. Res. Commun., 289, 181–190. [DOI] [PubMed] [Google Scholar]
- Gradl D., Kuhl, M. and Wedlich, D. (1999) The Wnt/Wg signal transducer β-catenin controls fibronectin expression. Mol. Cell. Biol., 19, 5576–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef I.A., Chen, F. and Crabtree, G.R. (2001) NFAT signalling in vertebrate development. Curr. Opin. Genet. Dev., 11, 505–512. [DOI] [PubMed] [Google Scholar]
- Hanes S.D., Shank, P.R. and Bostian, K.A. (1989) Sequence and mutational analysis of ESSI, a gene essential for growth in Saccharomyces cerevisiae. Yeast, 5, 55–72. [DOI] [PubMed] [Google Scholar]
- Hani J., Schelber, B., Bernhardt, A., Domdey, H., Fischer, G., Wiebauer, K. and Rahfeld, J. (1999) Mutations in a peptidyl-cis/trans-isomerase gene lead to a defect in 3′-end formation of a pre-mRNA in Saccharomyces cerevisiae. J. Biol. Chem., 274, 108–116. [DOI] [PubMed] [Google Scholar]
- He T.C., Sparks, A.B., Rago, C., Hermeking, H., Zawel, L., da Costa, L.T., Morin, P.J., Vogelstein, B. and Kinzler, K.W. (1998) Identification of c-Myc as a target of the APC pathway. Science, 281, 1509–1512. [DOI] [PubMed] [Google Scholar]
- Hecht A. and Kemler, R. (2000) Curbing the nuclear activities of β-catenin. EMBO rep., 1, 24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig L., Christner, C., Kipping, M., Schelbert, B., Rücknagel, K.P., Grabley, S., Küllertz, G. and Fischer, G. (1998) Selective inactivation of parvulin-like peptidyl-prolyl cis/trans isomerases by Juglone. Biochemistry, 37, 5953–5960. [DOI] [PubMed] [Google Scholar]
- Hsu T., McRackan, D., Vincent, T.S. and Gert De Couet, H. (2001) Drosophila Pin1 prolyl isomerase Dodo is a MAP kinase signal responder during oogenesis. Nat. Cell. Biol., 3, 538–543. [DOI] [PubMed] [Google Scholar]
- Huang H., Forsburg, S.L., John, U.P., O’Connell, M.J. and Hunter, T. (2001) Isolation and characterization of the Pin1/Ess1p homologue in Schizosaccharomyces pombe. J. Cell Sci., 114, 3779–3788. [DOI] [PubMed] [Google Scholar]
- Ke H.M., Zydowskz, L.D., Liu, J. and Walsh, C.T. (1991) Crystal structure of recombinant human T-cell cyclophilin A at 2.5 Å resolution. Proc. Natl Acad. Sci. USA, 88, 9483–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse M., Brunke, M., Escher, A., Szalay, A.A., Tropschug, M. and Zimmermann, R. (1995) Enzyme assembly after de novo synthesis in rabbit reticulocyte lysate involves molecular chaperones and immunophilins. J. Biol. Chem., 270, 2588–2594. [DOI] [PubMed] [Google Scholar]
- Landrieu I., Vezlder, D.L., Fruchart, J., Odaert, B., Casteels, P., Portetelle, D., Van Montague, M., Inze, D. and Lippens, G. (2000) The Arabidopsis thaliana Pin1 At gene encodes a single-domain phosphorylation-dependent peptidyl prolyl cis/trans isomerase. J. Biol. Chem., 275, 10577–10581. [DOI] [PubMed] [Google Scholar]
- Landrieu I., Odaert, B., Wieruszeski, J., Drobecq, H., Rousselot-Pailley, P., Inze, D. and Lippens, G. (2001) p13SUC1 and the WW domain of PIN1 bind to the same phosphothreonine-proline epitope. J. Biol. Chem., 276, 1434–1438. [DOI] [PubMed] [Google Scholar]
- Leverson J.D. and Ness, S.A. (1998) Point mutations in v-Myb disrupt a cyclophilin-catalyzed negative regulatory mechanism. Mol. Cell, 1, 203–211. [DOI] [PubMed] [Google Scholar]
- Liou Y., Ryo, A., Huang, H., Lu, P., Bronson, R., Fujimori, F., Uchida, T., Hunter, T. and Lu, K.P. (2002) Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc. Natl Acad. Sci. USA, 99, 1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Youn, H., Zhou, X.Z., Lu, K.P. and Liu, J.O. (2001) Binding and regulation of the transcription factor NFAT by the peptidyl prolyl cis-trans isomerase Pin1. FEBS Lett., 496, 105–108. [DOI] [PubMed] [Google Scholar]
- Lu K.P., Hanes, S.D. and Hunter, T. (1996) A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature, 380, 544–547. [DOI] [PubMed] [Google Scholar]
- Lu P.-J., Wulf, G., Zhou, X.Z., Davies, P. and Lu, K.P. (1999) The prolyl-isomerase Pin1 restores the function of Alzheimer-associated phos-phorylated tau protein. Nature, 399, 784–788. [DOI] [PubMed] [Google Scholar]
- Lu P.-J., Zhou, X.Z., Liou, Y.-C., Noel, J.P. and Lu, K.P. (2002) Critical role of WW domain phosphorylation in regulating phosphoserine binding activity and Pin1 function. J. Biol. Chem., 277, 2381–2384. [DOI] [PubMed] [Google Scholar]
- Maleszka R., Lupas, A., Hanes, S.D. and Miklos, G.L. (1997) The dodo gene family encodes a novel protein involved in signal transduction and protein folding. Gene, 203, 89–93. [DOI] [PubMed] [Google Scholar]
- Mamane Y., Sharma, S., Petropoulos, L., Lin, R. and Hiscott, J. (2000) Posttranslational regulation of IRF-4 activity by the immunophilin FKBP52. Immunity, 12, 129–140. [DOI] [PubMed] [Google Scholar]
- Michnick S.W., Rosen, M.K., Wandless, T.J., Karplus, M. and Schreiber, S.L. (1991) Solution structure of FKBP, a rotamase enzyme and receptor for FK506 and rapamycin. Science, 252, 836–839. [DOI] [PubMed] [Google Scholar]
- Mikol V., Kallen, J., Pflugl, G. and Walkinshaw, M.D. (1993) X-ray structure of a monomeric cyclophilin A-cyclosporin A crystal complex at 2.1 Å resolution. J. Mol. Biol., 234, 1119–1130. [DOI] [PubMed] [Google Scholar]
- Morris D.P., Phatnani, H.P. and Greenleaf, A.L. (1999) Phosphocarboxyl-terminal domain binding and the role of prolyl isomerase in pre-mRNA 3′-end formation. J. Biol. Chem., 274, 31583–31587. [DOI] [PubMed] [Google Scholar]
- Rahfeld J.U., Rucknagel, K.P., Schelbert, B., Ludwig, B., Hacker, J., Mann, K. and Fischer, G. (1994) Confirmation of the existence of a third family among peptidyl-prolyl cis/trans isomerases. Amino acid sequence and recombinant production of parvulin. FEBS Lett., 352, 180–184. [DOI] [PubMed] [Google Scholar]
- Ramelot T.A. and Nicholson, L.K. (2001) Phosphorylation-induced structural changes in the amyloid precursor protein cytoplasmic tail detected by NMR. J. Mol. Biol., 307, 871–884. [DOI] [PubMed] [Google Scholar]
- Ranganathan R., Lu, K.P., Hunter, T. and Noel, J.P. (1997) Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell, 89, 875–886. [DOI] [PubMed] [Google Scholar]
- Reimer U., Scherer, G., Drewello, M., Kruber, S., Schutkowski, M. and Fischer, G. (1998) Side-chain effects on peptidyl-prolyl cis/trans isomerases. J. Mol. Biol., 279, 449–460. [DOI] [PubMed] [Google Scholar]
- Rippmann J.F. et al. (2000) Phosphorylation-dependent proline isomerization catalyzed by Pin1 is essential for tumor cell survival and entry into mitosis. Cell Growth Differ., 11, 409–416. [PubMed] [Google Scholar]
- Rubinfeld B., Albert, I., Porfiri, E., Fiol, C., Munemitsu, S. and Polakis, P. (1996) Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science, 272, 1023–1026. [DOI] [PubMed] [Google Scholar]
- Ryo A., Nakamura, M., Wulf, G., Liou, Y. and Lu, K.P. (2001) Pin1 regulates turnover and subcellular localization of β-catenin by inhibiting its interaction with APC. Nat. Cell Biol., 3, 793–801. [DOI] [PubMed] [Google Scholar]
- Schmid F.X. (1995) Prolyl isomerases join the fold. Curr. Biol., 5, 993–994. [DOI] [PubMed] [Google Scholar]
- Schreiber S.L. (1991) Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science, 251, 283–287. [DOI] [PubMed] [Google Scholar]
- Shen M., Stukenberg, P.T., Kirschner, M.W. and Lu, K.P. (1998) The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev., 12, 706–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenberg P.T. and Kirschner, M.W. (2001) Pin1 acts catalytically to promote a conformational change in Cdc25. Mol. Cell, 7, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Sudol M., Sliwa, K. and Russo, T. (2001) Functions of WW domains in the nucleus. FEBS Lett., 490, 190–195. [DOI] [PubMed] [Google Scholar]
- Tetsu O. and McCormick, F. (1999) β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature, 398, 422–426. [DOI] [PubMed] [Google Scholar]
- Uchida T., Fujimori, F., Tradler, T., Fischer, G. and Rahfeld, J.U. (1999) Identification and characterisation of a 14 kDa human protein as a novel parvulin-like peptidyl-prolyl cis/trans isomerase. FEBS Lett., 446, 278–282. [DOI] [PubMed] [Google Scholar]
- Van Duyne S.D., Standaert, R.F., Karplus, P.A., Schreiber, S.L. and Clardy, J. (1991) Atomic structure of FKBP-FK506, an immunophilin–immuno-suppressant complex. Science, 252, 839–842. [DOI] [PubMed] [Google Scholar]
- Verdecia M.A., Bowman, M.E., Lu, K.P., Hunter, T. and Noel, J.P. (2000) Structural basis for phosphoserine-proline recognition by group IV WW domains. Nat. Struct. Biol., 7, 639–643. [DOI] [PubMed] [Google Scholar]
- Weiwad M., Küllertz, G., Schutkowski, M. and Fischer, G. (2000) Evidence that the substrate backbone conformation is critical to phosphorylation by p42 MAP kinase. FEBS Lett., 478, 39–42. [DOI] [PubMed] [Google Scholar]
- Winkler K.E., Swenson, K.L., Kornbluth, S. and Means, A.R. (2000) Requirement of the prolyl isomerase Pin1 for the replication checkpoint. Science, 287, 1644–1647. [DOI] [PubMed] [Google Scholar]
- Wu X., Wilcox, C.B., Devasahayam, G., Hackett, R.L., Arevalo-Rodriguez, M., Cardenas, M.E., Heitman, J. and Hanes, S.D. (2000) The Ess1 prolyl isomerase is linked to chromatin remodeling complexes and the general transcription machinery. EMBO J., 19, 3727–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf G.M., Ryo, A., Wulf, G.G., Lee, S.W., Niu, T., Petkova, V. and Lu, K.P. (2001) Pin1 is overexpressed in breast cancer and cooperates with Ras signalling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J., 20, 3459–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.Z., Kops, O., Werner, A., Lu, P.J., Shen, M., Stoller, G., Kullertz, G. and Lu, K.P. (2000) Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and Tau proteins. Mol. Cell, 6, 873–883. [DOI] [PubMed] [Google Scholar]