Abstract
Autoantibodies to chromatin and double-stranded DNA are a hallmark of systemic lupus erythematosus (SLE). In a mouse model of monogenic human SLE caused by DNASE1L3 deficiency, anti-DNA response is dependent on endosomal nucleic acid-sensing Toll-like receptors TLR7 and TLR9. Here we report that this response also required TLR2, a surface receptor for microbial products that is primarily expressed on myeloid cells. Cell transfers into lymphopenic DNASE1L3-deficient mice showed that TLR2 was required for anti-DNA antibody production by lymphocytes. TLR2 was detectably expressed on B cells and facilitated the production of IL-6 by B cells activated in the presence of microbial products. Accordingly, treatment with broad-spectrum antibiotics or antibody-mediated blockade of IL-6 delayed anti-DNA response in DNASE1L3-deficient mice. These studies reveal an unexpected B cell-intrinsic role of TLR2 in systemic autoreactivity to DNA, and suggest that microbial products may synergize with self-DNA in the activation of autoreactive B cells in SLE.
Introduction
The hallmark of systemic lupus erythematosus (SLE) is the production of antibodies (Abs) to nuclear antigens such as ribonucleoproteins, DNA and DNA-histone complexes. High-affinity IgG Abs to double-stranded DNA (dsDNA) are thought to be particularly pathogenic and associated with the severity of LN (1). Anti-dsDNA Ab production is supported by and in turn activates innate sensing pathways that induce pathogenic type I interferon (IFN-I) and other cytokines (2, 3). However, the identity and precise role of these innate sensing pathways remain poorly understood. For example, the cGAS-STING pathway of intracellular DNA sensing appears paradoxically dispensable or protective in murine models of SLE (4). Similarly, the endosomal Toll-like receptor for unmethylated DNA, TLR9, is protective in certain SLE models, whereas the receptor for single-stranded RNA, TLR7, is uniformly pathogenic (5). In addition, innate receptors for non-nucleic acid products such as TLR2 and/or TLR4 were found to facilitate autoreactivity and inflammation elicited by chemical induction (6, 7) or lymphoproliferation (8). However, the role of these TLRs in relevant genetic models of human anti-DNA reactivity has not been examined, and the mechanism whereby they may contribute to autoreactivity remains unclear.
DNASE1L3 (D1L3) is a unique secreted DNase that can digest native chromatin and thus reduces the levels of microparticle-associated DNA in circulation (9). Null mutations of DNASE1L3 in humans cause monogenic SLE with early onset, anti-dsDNA autoAbs and nephritis (10). Moreover, up to 50% of patients with non-genetic lupus nephritis develop blocking autoAbs to D1L3 (9), emphasizing the role of this enzyme in the sporadic SLE. Accordingly, D1L3 knockout (D1L3KO) mice develop anti-nucleosome (anti-Nuc) and anti-DNA IgG, followed by immune cell activation and immune complex deposition (11). This anti-DNA response results from extrafollicular (ExFo) differentiation of autoreactive B cells, which is supported by the ExFo helper T cells (ExFo-TH). Furthermore, it is facilitated by IFN-I produced by plasmacytoid dendritic cells (pDCs) and requires the combined activity of TLR9 and TLR7 (12). Collectively, these mechanistic features resemble the emerging pathogenesis of human anti-DNA responses (3, 13, 14), and facilitate the analysis of anti-DNA reactivity in the absence of lymphoproliferation, chemical induction or other confounding features. Here we utilized this model to analyze the role of surface TLRs in anti-DNA Ab response.
Materials and Methods
Animals.
All experiments were performed in accordance with the animal protocol approved by the Institutional Animal Care and use committee of NYU Grossman School of Medicine. All the mouse strains were on a C57BL/6 (B6) background and C57BL/6J (Stock 000664) mice bred in the same facility were used as wild-type controls. Mice with the null Dnase1L3LacZ allele on the B6 background have been described (11). Mice deficient for Tlr9, Tlr7, Tlr2, Tlr4, Myd88, Unc93b1 and Rag1KO were purchased from the Jackson Laboratory or MMMRC and bred to generate homozygous single- or double-deficient mice. For adoptive transfers, 4×106 total splenocytes were injected i.v. into Rag1-deficient recipients.
Autoantibody measurements and flow cytometry.
All procedures were done as described in detail previously (12).
Kidney histopathology.
One half of each kidney was fixed and embedded, and 5 μm sections were stained with Hematoxylin and Eosin and captured using the PerkinElmer Vectra multispectral imaging system at 40x magnification. For the analysis of glomerular size, the AI DenseNet module of Halo software (Indica Labs) was trained to identify and measure glomeruli.
In vitro B cell and BMDC stimulation and IL-6 analysis.
Naïve B cells from WT or Tlr2-deficient mice were MACS-purified by negative selection from splenocytes using CD43 microbeads. Purified B cells were suspended in complete RPMI + 10% FBS and plated in round-bottom 96 well plates at 0.5×106 cells/well in 200 μl media. Cells were either activated with 10 μg/ml anti-IgM + 2 μg/ml anti-CD40 (both ultra LEAF-purified, BioLegend) in the presence or absence of 5 μg/ml PGN-EK (InvivoGen); 5 μg/ml Pam3CSK (InvivoGen); 5 μg/ml zymosan or 1 μg/ml LPS (LPS-EK Ultrapure, InvivoGen). Culture supernatants were collected after 60 hr and IL-6 concentration was determined using mouse IL-6 quantikine ELISA kit (R&D Systems).
In vivo IL-6R blockade.
One-year-old Dnase1l3KO mice were bled to ascertain the serum autoantibody titers and randomly assigned to be injected i.p. twice every week for 10 weeks with 100 μg of Isotype control or anti-mouse IL-6R antibody (BioXcell).
Antibiotic treatment.
Dnase1l3KO female mice were randomly assigned to be given sterile water containing 1% sucrose with or without the antibiotic (Abx) cocktail (Vancomycin 0.5 g/L; Ampicillin 1 g/L; Neomycin 1 g/L, and Metronidazole 1 g/L) for 5 months. After every 30 days mice were bled to collect serum while feces were collected to extract DNA using the DNeasy PowerLyzer powerSoil kit (Qiagen), and used to determine the microbial burden as described (15).
Statistical analysis.
Comparisons between multiple groups were performed by one-way ANOVA followed by multiple comparisons analysis (Tukey’s test). Comparisons between two groups were performed by the Mann-Whitney test. All analysis was done using Prism software (GraphPad).
Results and Discussion
TLR2 is required for autoreactivity in DNASE1L3-deficient mice
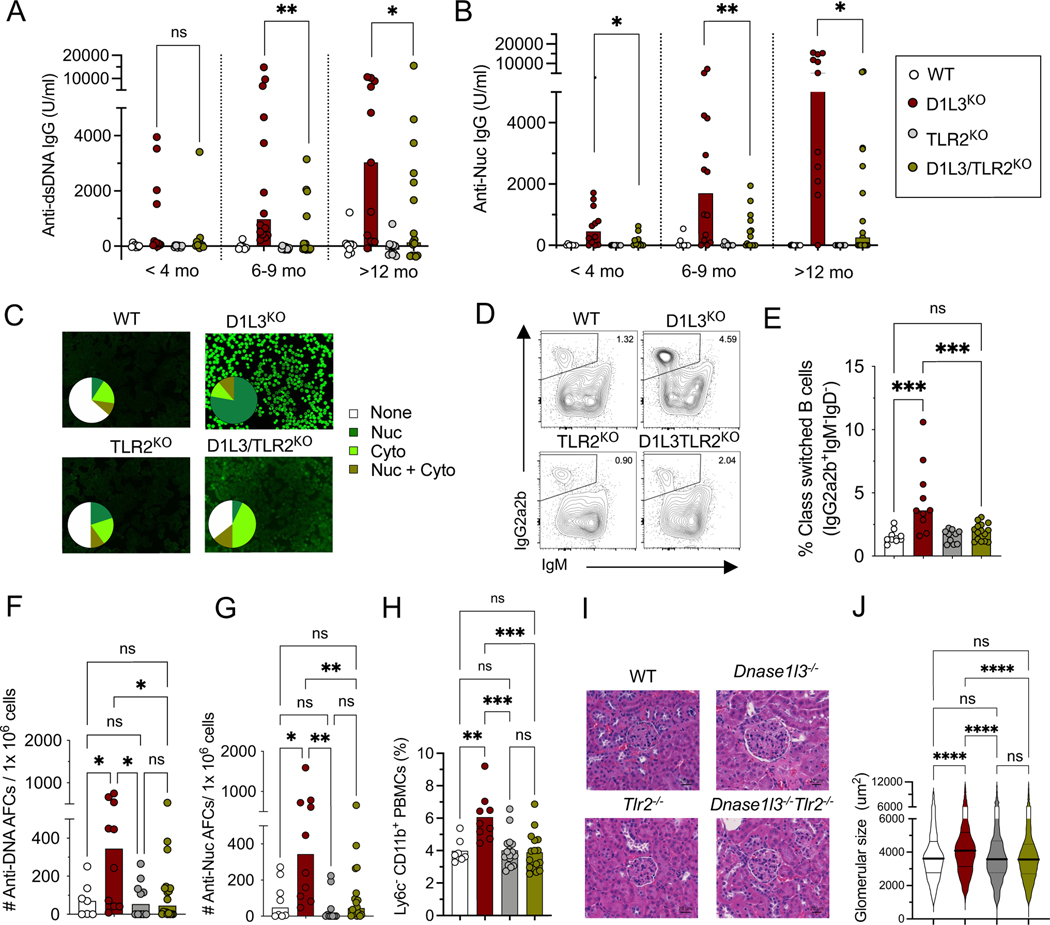
To test the role of surface TLRs in autoreactivity to self-DNA, we generated D1L3KO mice that were also deficient for either TLR4 or TLR2. The deletion of TLR4 did not reduce anti-dsDNA, anti-Nuc or anti-nuclear antibodies (ANA) (Supplemental Fig. 1A-E), suggesting that TLR4 is dispensable for the anti-DNA response. In contrast, the deletion of TLR2 led to significantly reduced anti-dsDNA and anti-Nuc IgG autoAb titers (Fig. 1A, B). The majority of TLR2KO D1L3KO mice lost ANA reactivity and instead showed weak reactivity to cytoplasmic antigens (Fig. 1C). Accordingly, total splenic class-switched B cells (Fig. 1 D, E) and anti-dsDNA and anti-Nuc IgG-secreting splenic antibody-forming cells (AFC) were significantly reduced (Fig. 1F, G). A trend toward lower frequencies of short-lived ExFo B cells (Supplemental Fig. 1F), and a significant decrease in germinal center (GC) B cells (Supplemental Fig. 1G) were also noted. Consistent with lower autoAbs, TLR2 deficiency significantly reduced overall immune cell activation, including reduced expansion of CD11b+ Ly6c− monocytes in the blood (Fig. 1H and S1H); reduced frequency of lymphocytes expressing the interferon-inducible marker Sca-1 (Supplemental Fig. 1I-J); increased frequency of naïve T cells (Supplemental Fig. 1K), and a significant reduction in ExFo-TH and TFH cells (Supplemental Fig. 1L,M). Old D1L3KO mice show increased size of kidney glomeruli, which reflects the deposition of immune complexes (12); this glomerular enlargement was completely rescued by TLR2 deficiency (Fig. 1I, J).
Figure 1. Deletion of TLR2 ameliorates autoimmunity in D1L3KO mice.
Mice deficient for DNASE1L3 (D1L3KO, red symbols), TLR2 (TLR2KO, grey symbols) or both (D1L3/TLR2KO, green symbols) or wild-type controls (WT, open symbols) were analyzed at 1 year of age unless indicated otherwise.
(A-B) Serum anti-dsDNA IgG titers (A) and anti-Nuc titers (B), at the indicated ages as measured by ELISA.
(C) Serum ANA reactivity, showing representative staining images and the distribution of ANA reactivity pattern (9–14 mice per genotype).
(D-E) Frequency of IgG2a2b+ IgM− class-switched B cells in the spleen.
(F-G) Frequency of anti-dsDNA (F), and anti-nucleosome (G) antibody-forming cells (AFCs) per 106 splenocytes as determined by ELISpot.
(H) Fraction of CD11c+ CD11b+ Ly6c− population among total PBMCs as determined by flow cytometry.
(I) Histology images of glomeruli from kidney sections stained with hematoxylin and eosin (scale bars, 20 μm). Representative of 5 female mice per group.
(J) The size of glomeruli in the kidneys of mice of indicated genotypes. Shown are violin plots with median (thick lines) and quartiles (thin lines). All the glomeruli from a single kidney section from 5 mice per genotype were measured.
In all bar graphs, symbols represent individual mice and bars indicate median. Statistical significance: ns, not significant; * p ≤ 0.05; ** p ≤ 0.01; ***p ≤ 0.001; **** p ≤ 0.0001.
To test whether TLR2 is generally required for T cell-dependent antibody responses, we used immunization with the hapten 4-Hydroxy-3-nitrophenylacetyl (NP) conjugated to the protein keyhole limpet hemocyanin (KLH). While the titers of both high-affinity (anti-NP2) and low-affinity (anti-NP30) IgM were reduced in TLR2KO D1L3KO mice compared to D1L3KO mice (Supplemental Fig. 2A), the titers of anti-NP IgG or NP-specific AFC were not different (Supplemental Fig. 2B-D). These results suggest that TLR2 is dispensable for common immunization-induced Ab responses, but is required for autoreactive Ab responses towards self-DNA.
TLR2 promotes autoantibody response within lymphocytes
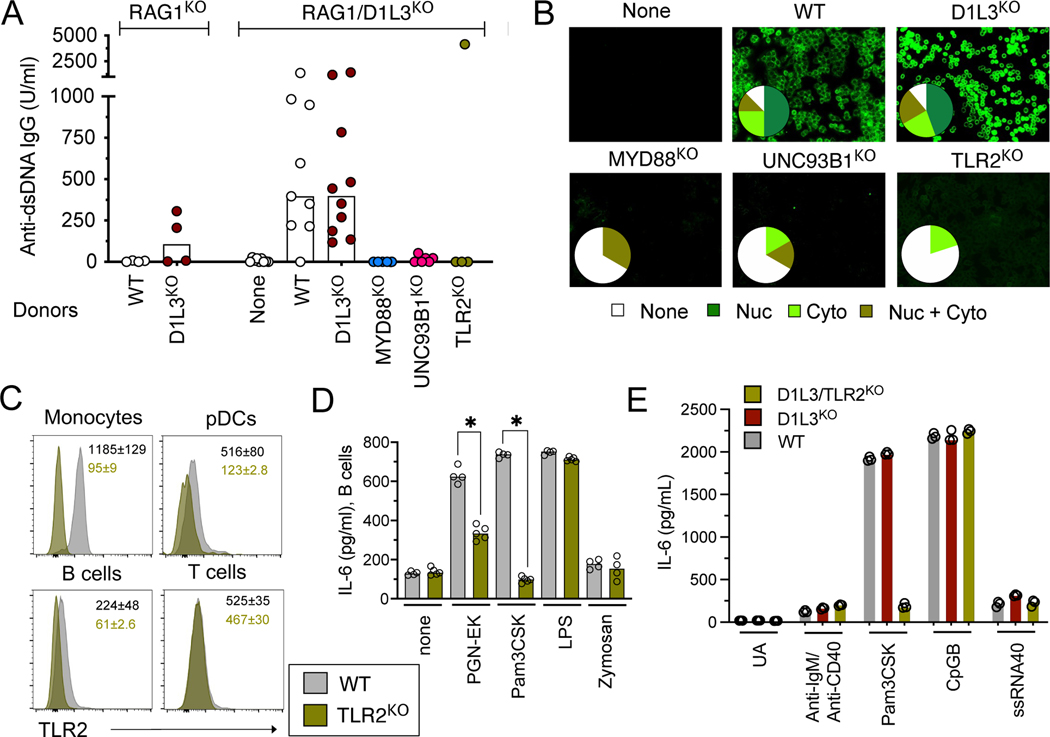
TLR2 is primarily expressed by myeloid cells, in which it can elicit the production of IFN-I in response to bacterial DNA complexed with amyloid protein (7). Moreover, the role of TLR2 in autoimmunity was proposed to reflect its binding to the endogenous alarmin protein HMGB1, which forms complexes with self-DNA (16). However, we found that IFN-I response to CpG-containing immunogenic self-DNA (iDNA) was not dependent on HMGB1 or TLR2 in dendritic cells, and was absent from primary splenocytes (data not shown). To test whether TLR2 may act directly within the antibody-producing cells, we first generated D1L3KO mice on Rag1-deficient background to abolish their lymphocyte compartment. D1L3 is expressed primarily by DCs and macrophages (11), thus the transfer of wild-type (WT) lymphocytes into lymphopenic Rag1KO D1L3KO mice should expose lymphocytes to D1L3-deficient environment and elicit an anti-DNA response. We transferred total splenocytes (comprising primarily B and T cells) from WT or D1L3KO donors into lymphopenic recipients, yielding efficient reconstitution of B and T cell compartments (data not shown). The transfer of WT donor lymphocytes into Rag1KO recipients induced no anti-DNA Abs, as expected (Fig. 2A). In contrast, the transfer of WT donor lymphocytes into Rag1KO D1L3KO recipients induced high anti-dsDNA Ab titers and prominent ANA, similar to the transfer of D1L3KO donor lymphocytes into the same recipients (Fig. 2A,B). These results formally prove that D1L3 acts in a cell-extrinsic manner, and establish a system to test the lymphocyte-intrinsic role of individual genes in this model.
Figure 2. B cell-intrinsic TLR2 promotes autoreactivity and facilitates IL-6 production.
(A) Anti-dsDNA IgG titers as measured by ELISA in the serum of recipient mice after lymphocyte transfer from the indicated donor mice. Each symbol represents an individual recipient mouse, bars show median.
(B) Serum ANA reactivity, showing representative staining images and the distribution of ANA reactivity pattern for groups of D1L3KO RAG1KO recipients reconstituted with lymphocytes of indicated genotypes (WT, n=8; D1L3KO; n=9; MYD88KO; n=3; UNC93B1KO; n=6; TLR2KO; n=5).
(C) The expression of TLR2 on immune cell types by flow cytometry. Shown are representative TLR2 staining histograms in the indicated cell types with the average mean fluorescence intensity (MFI) ±SD from 8 WT and 2 TLR2KO mice.
(D) Concentration of IL-6 as measured by ELISA in the supernatants of B cells from WT and TLR2KO mice stimulated with anti-IgM/ anti-CD40 in the absence or presence of the indicated microbial products. Each symbol represents an individual mouse and bars indicate median.
(E) Concentration of IL-6 as measured by ELISA in the supernatants of B cells from WT, D1L3KO and D1L3/TLR2KO mice stimulated for 60 hr with anti-IgM/anti-CD40 or with the indicated TLR ligands.
We first tested the role of TLR signaling in lymphocytes by using splenocytes from MYD88KO or UNC93B1KO donor mice, in which autoAb response is abrogated ((11) and data not shown). Accordingly, the transfer of MYD88KO or UNC93B1KO splenocytes into Rag1KO D1L3KO recipients did not result in any anti-dsDNA Abs or ANA formation (Fig. 2A, B). Thus, endosomal TLR signaling in lymphocytes is essential for autoreactivity, likely reflecting the well-established functional roles of TLR7 and TLR9 in B cells (17). Notably, the transfer of TLR2KO splenocytes also did not induce any anti-dsDNA Abs or ANA (high anti-dsDNA titers in a single recipient did not correlate with ANA and likely reflect aberrant cross-reactivity) (Fig. 2A,B). Thus, TLR2 acts at least partially in lymphocytes to facilitate their response to self-DNA.
TLR2 promotes IL-6 production by activated B cells
We examined the expression of TLR2 protein on the surface of immune cells, using TLR2KO mice as a genetic control. In addition to the expected strong expression on monocytes, we observed clearly detectable expression on pDCs and B cells (Fig. 2C). No TLR2 could be detected on T cells, suggesting that within the lymphoid compartment, B cells represent the primary TLR2-expressing cell type. We then tested the effect on B cells of microbial TLR ligands such as peptidoglycan from E.coli K12 (PGN-EK), bacterial lipopolysaccharide (LPS) or the fungal glucan zymosan, or their synthetic mimics such as the triacylated lipopeptide Pam3CSK. As expected, these products induced IL-6 production by dendritic cells in a manner that was TLR2-independent for LPS, but was partially or completely TLR2-dependent for PGN-EK and Pam3CSK, respectively (data not shown). Accordingly, PGN-EK and Pam3CSK augmented IL-6 production by B cells activated through the BCR and CD40, and this effect was TLR2-dependent (Fig. 2D). In contrast, zymosan had no effect on activated B cells. To analyze the relative contribution of BCR vs TLR stimulation to IL-6 production, we stimulated B cells from WT, D1L3KO and D1L3/TLR2KO mice either with anti-IgM/anti-CD40 (BCR stimulation) or with the ligands of TLR2 (Pam3CSK), TLR7 (ssRNA40) or TLR9 (CpG-B). BCR stimulation alone induced weak IL-6 production across all genotypes (Fig. 2E), suggesting that i) activated B cells from D1L3KO mice do not spontaneously produce increased IL-6; ii) TLR2 does not contribute to BCR-induced IL-6 production in the absence of microbial ligands. CpG-B induced high IL-6 levels comparable to Pam3CSK, albeit in a TLR2-independent manner as expected (Fig. 2E). Furthermore, the immunogenic CpG-containing DNA from the mouse genome (18) elicted a weak TLR2-independent IL-6 response in D1L3KO B cells (data not shown). Collectively, these data suggest that TLR2, along with endosomal TLRs, may facilitate IL-6 production by B cells and thus promote autoreactivity.
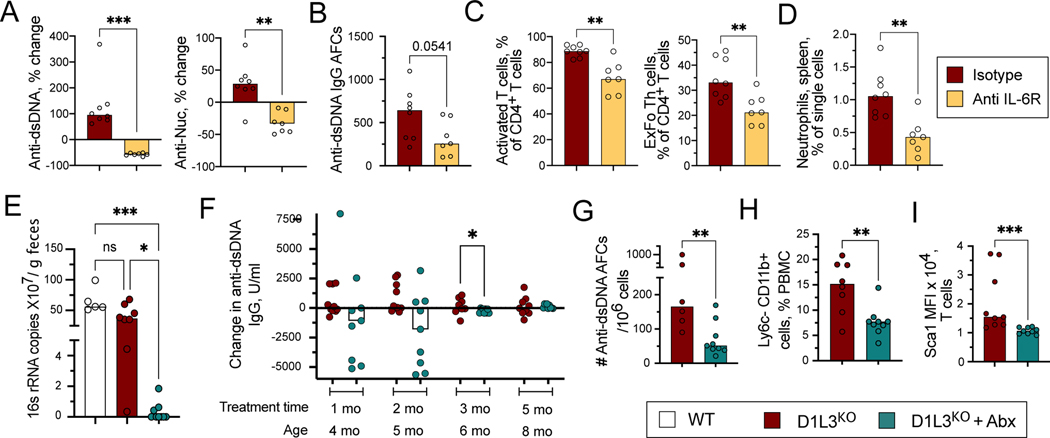
To confirm the role of IL-6 in anti-DNA response, we treated old (11–12 months of age) D1L3KO mice with anti-IL-6R blocking antibody or an isotype control for two months. Isotype control-treated mice showed overall increase in the titers of anti-dsDNA and anti-Nuc, which was abolished by anti-IL-6R blockade (Fig. 3A). The frequency of AFC specific for dsDNA was also reduced after anti-IL-6R treatment, albeit with a marginal significance (Fig. 3B). Furthermore, IL-6R blockade significantly reduced total T cell activation and the frequency of pathogenic ExFo-TH (Fig. 3C). Finally, we observed a decrease in neutrophils (Fig. 3D), an expected effect of IL-6 blockade. These data confirm the pathogenic role of IL-6 in autoAb development in D1L3KO mice, potentially by promoting expansion of pathogenic CD4+ TH cells.
Figure 3. IL-6 and microbiome facilitate autoreactivity in D1L3KO mice.
(A-D) Autoreactivity in 1-year-old D1L3KO mice treated with anti-IL-6R antibody or an isotype control for 10 weeks.
(A) Percent change in the titers of serum anti-dsDNA IgG and anti-Nuc Abs.
(B) Frequency of anti-dsDNA antibody-forming cells (AFCs) per 106 splenocytes as determined by ELISpot.
(C) Frequencies of T cell subsets including activated CD62L− CD44+ CD4+ T cells and CD62L− CD44+ PSGL1lo CD4+ ExFoTh cells in the spleen.
(D) Frequencies of Ly6C+ Ly6G+ neutrophils in the spleen.
(E-I) Three-month-old D1L3KO mice were pre-bled, kept on drinking water with or without antibiotics (Abx) for 5 months and sacrificed.
(E) Levels of bacterial 16s rRNA copies per gram feces after 5 months of treatment.
(F) Percent change in the titers of serum anti-dsDNA IgG at the indicated time points after Abx treatment compared with untreated mice.
(H) Frequency of anti-dsDNA AFCs per 106 splenocytes as determined by ELISpot.
(I) Fraction of CD11c+ CD11b+ Ly6c− population among total PBMCs.
(J) The expression levels of Sca-1 on CD4+ T cells.
In all panels, symbols represent individual mice and bars indicate median. Statistical significance: ns, not significant; * p ≤ 0.05; ** p ≤ 0.01; ***p ≤ 0.001.
Finally, we tested whether products from commensal bacteria might contribute to autoreactivity by treating 3-month-old D1L3KO mice with a combination of antibiotics (Abx) in the drinking water. The mice were monitored monthly for microbial burden and titers of autoAbs and analyzed at the 5-month endpoint. Abx-treated D1L3KO mice showed a greatly reduced bacterial load in the gut compared to control D1L3KO or WT mice kept without Abx, as determined by 16S rRNA analysis from feces (Fig. 3E). Notably, the levels of endotoxin in the plasma were not affected by Abx treatment (data not shown), as reported previously for germ-free rodents (19). Consistent with their relatively young age, control D1L3KO mice showed stable anti-dsDNA titers over the course of the experiment (Fig. 3F). However, 5 out of 9 Abx-treated mice showed a strong reduction of the titers after 1–2 months of treatment, and the overall difference became significant at 3 months of treatment (Fig. 3F). Furthermore, Abx-treated mice at the endpoint harbored significantly fewer anti-dsDNA-forming splenic AFCs (Fig. 3G), fewer inflammatory CD11b+ Ly6C− myeloid cells in the blood (Fig. 3H), and lower Sca-1 expression on T cells (Fig. 3I). Overall, Abx treatment of young D1L3KO mice delayed the anti-DNA response, supporting the role of microbial stimulation in this process.
We report that TLR2, but not TLR4, is required for anti-DNA reactivity in a model of human monogenic SLE that is also dependent on TLR7 and TLR9. We found that both TLR2 and UNC93B1-dependent endosomal TLRs were required in the lymphocyte compartment, and further showed that B cells express functional TLR2. Thus, surface and endosomal TLRs exert their pathogenic function in SLE at least in part through B cells, although their contribution through other cell types such as pDCs is also likely. One potential source of TLR2 signals may be represented by self-DNA, which is increased in the microparticles of D1L3KO mice (9) along with endogenous TLR2 ligands such as HMGB1 (16, 20). While this possibility remains to be investigated in the future, our current data point to the role of canonical TLR2 ligands, i.e. microbial products. The contribution of microbiome to SLE has been recently appreciated, with certain bacterial species found to exacerbate SLE by enhancing the TLR7-induced IFN-I production (21). It was also observed that particular strains of Ruminococcus gnavus that are expanded in patients with severe SLE carry TLR2-stimulating activity (22). Furthermore, pneumococcal TLR agonists and Pam3CSK but not LPS induced anti-dsDNA IgA production in C4-deficient mice (23). Our studies support these observations and suggest that bacteria-derived TLR2 ligands may act directly on autoreactive B cells.
The production of IL-6 by B cells was shown to promote TFH expansion in systemic inflammation models in a TLR7-dependent way (24). Indeed, TLR7 and especially TLR9 ligands elicited IL-6 production in B cells (Fig. 2E), consistent with the collective requirement for these two TLRs in the D1L3KO model (12) and suggesting that they may act in part through IL-6, in parallel to IFN-I. Our data further suggest that IL-6 may be driven by microbial signaling via TLR2 in B cells. It is likely that IL-6 production is limited to autoreactive clones and/or operates at an autocrine/paracrine level, given the normal IL-6 production by polyclonally activated B cells (Fig. 2E) and undetectable IL-6 in the sera of D1L3KO mice (data not shown). Nevertheless, elevated production of IL-6 was described in SLE patients (25). Indeed, the IL-6R inhibitor Tocilizumab reduced anti-DNA Ab levels and disease symptoms in SLE patients, although the resulting neutropenia limited its broader application (26). Our results not only emphasize the important role of IL-6 in the disease, but also highlight its TLR2-dependent production as a likely mechanism of pathogenesis. As such, they warrant the exploration of TLR2 inhibitors as potential tools in the combined therapeutic approaches to SLE.
Supplementary Material
Highlights.
anti-DNA autoantibody response in DNASE1L3-deficient mice requires TLR2
microbial products signal via TLR2 to elicit IL-6 secretion by B cells
Acknowledgments
This work was supported by the NIH grants AR071703 and AR070591 (to B.R.) and AI100853, AR069515 and GM136573 (to L.S.), the Lupus Research Alliance and the Colton Center for Autoimmunity (B.R.), the Uehara Memorial Foundation (S.M.), and the German Research Foundation (DFG) grant EI1185/1-1 (A.E.).
References
- 1.Pisetsky DS, and Lipsky PE 2020. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol 16: 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matta B, and Barnes BJ 2020. Coordination between innate immune cells, type I IFNs and IRF5 drives SLE pathogenesis. Cytokine 132: 154731. [DOI] [PubMed] [Google Scholar]
- 3.Caielli S, Wan Z, and Pascual V. 2023. Systemic Lupus Erythematosus Pathogenesis: Interferon and Beyond. Annu Rev Immunol 41: 533–560. [DOI] [PubMed] [Google Scholar]
- 4.Motwani M, McGowan J, Antonovitch J, Gao KM, Jiang Z, Sharma S, Baltus GA, Nickerson KM, Marshak-Rothstein A, and Fitzgerald KA 2021. cGAS-STING Pathway Does Not Promote Autoimmunity in Murine Models of SLE. Front Immunol 12: 605930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nündel K, and Marshak-Rothstein A. 2019. The role of nucleic acid sensors and type I IFNs in patient populations and animal models of autoinflammation. Current Opinion in Immunology 61: 74–79. [DOI] [PubMed] [Google Scholar]
- 6.Urbonaviciute V, Starke C, Pirschel W, Pohle S, Frey S, Daniel C, Amann K, Schett G, Herrmann M, and Voll RE 2013. Toll-like receptor 2 is required for autoantibody production and development of renal disease in pristane-induced lupus. Arthritis Rheum 65: 1612–23. [DOI] [PubMed] [Google Scholar]
- 7.Tursi SA, Lee EY, Medeiros NJ, Lee MH, Nicastro LK, Buttaro B, Gallucci S, Wilson RP, Wong GCL, and Tukel C. 2017. Bacterial amyloid curli acts as a carrier for DNA to elicit an autoimmune response via TLR2 and TLR9. PLoS Pathog 13: e1006315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lartigue A, Colliou N, Calbo S, Francois A, Jacquot S, Arnoult C, Tron F, Gilbert D, and Musette P. 2009. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J Immunol 183: 6207–16. [DOI] [PubMed] [Google Scholar]
- 9.Hartl J, Serpas L, Wang Y, Rashidfarrokhi A, Perez OA, Sally B, Sisirak V, Soni C, Khodadadi-Jamayran A, Tsirigos A, Caiello I, Bracaglia C, Volpi S, Ghiggeri GM, Chida AS, Sanz I, Kim MY, Belmont HM, Silverman GJ, Clancy RM, Izmirly PM, Buyon JP, and Reizis B. 2021. Autoantibody-mediated impairment of DNASE1L3 activity in sporadic systemic lupus erythematosus. J Exp Med 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tusseau M, Lovsin E, Samaille C, Pescarmona R, Mathieu AL, Maggio MC, Selmanovic V, Debeljak M, Dachy A, Novljan G, Janin A, Januel L, Gibier JB, Chopin E, Rouvet I, Goncalves D, Fabien N, Rice GI, Lesca G, Labalme A, Romagnani P, Walzer T, Viel S, Perret M, Crow YJ, Avcin T, Cimaz R, and Belot A. 2022. DNASE1L3 deficiency, new phenotypes, and evidence for a transient type I IFN signaling. J Clin Immunol 42: 1310–1320. [DOI] [PubMed] [Google Scholar]
- 11.Sisirak V, Sally B, D’Agati V, Martinez-Ortiz W, Ozcakar ZB, David J, Rashidfarrokhi A, Yeste A, Panea C, Chida AS, Bogunovic M, Ivanov II, Quintana FJ, Sanz I, Elkon KB, Tekin M, Yalcinkaya F, Cardozo TJ, Clancy RM, Buyon JP, and Reizis B. 2016. Digestion of Chromatin in Apoptotic Cell Microparticles Prevents Autoimmunity. Cell 166: 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soni C, Perez OA, Voss WN, Pucella JN, Serpas L, Mehl J, Ching KL, Goike J, Georgiou G, Ippolito GC, Sisirak V, and Reizis B. 2020. Plasmacytoid Dendritic Cells and Type I Interferon Promote Extrafollicular B Cell Responses to Extracellular Self-DNA. Immunity 52: 1022–1038.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenks SA, Cashman KS, Woodruff MC, Lee FE, and Sanz I. 2019. Extrafollicular responses in humans and SLE. Immunol Rev 288: 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atisha-Fregoso Y, Toz B, and Diamond B. 2021. Meant to B: B cells as a therapeutic target in systemic lupus erythematosus. The Journal of clinical investigation 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jimeno R, Brailey PM, and Barral P. 2018. Quantitative Polymerase Chain Reaction-based Analyses of Murine Intestinal Microbiota After Oral Antibiotic Treatment. J Vis Exp doi: 10.3791/58481. [DOI] [PubMed]
- 16.Urbonaviciute V, and Voll RE 2011. High-mobility group box 1 represents a potential marker of disease activity and novel therapeutic target in systemic lupus erythematosus. J Intern Med 270: 309–18. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Fitzgerald KA, Cancro MP, and Marshak-Rothstein A. 2015. Nucleic Acid-Sensing Receptors: Rheostats of Autoimmunity and Autoinflammation. J Immunol 195: 3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uccellini MB, Busconi L, Green NM, Busto P, Christensen SR, Shlomchik MJ, Marshak-Rothstein A, and Viglianti GA 2008. Autoreactive B cells discriminate CpG-rich and CpG-poor DNA and this response is modulated by IFN-alpha. J Immunol 181: 5875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama Y, Sugiyama F, Yagami K, Miyaji S, and Kurosawa T. 1993. Comparison of plasma endotoxin levels in germ-free, SPF and conventional laboratory animals (mice and rats). Jikken Dobutsu 42: 89–92. [DOI] [PubMed] [Google Scholar]
- 20.Pisetsky DS 2014. The expression of HMGB1 on microparticles released during cell activation and cell death in vitro and in vivo. Mol Med 20: 158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, Costa FRC, Tiniakou E, Greiling T, Ruff W, Barbieri A, Kriegel C, Mehta SS, Knight JR, Jain D, Goodman AL, and Kriegel MA 2018. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359: 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azzouz D, Omarbekova A, Heguy A, Schwudke D, Gisch N, Rovin BH, Caricchio R, Buyon JP, Alekseyenko AV, and Silverman GJ 2019. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann Rheum Dis 78: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yammani RD, Leyva MA, Jennings RN, and Haas KM 2014. C4 Deficiency is a predisposing factor for Streptococcus pneumoniae-induced autoantibody production. J Immunol 193: 5434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arkatkar T, Du SW, Jacobs HM, Dam EM, Hou B, Buckner JH, Rawlings DJ, and Jackson SW 2017. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med 214: 3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattanaik SS, Panda AK, Pati A, Padhi S, Tripathy R, Tripathy SR, Parida MK, and Das BK 2022. Role of interleukin-6 and interferon-α in systemic lupus erythematosus: A case–control study and meta-analysis. Lupus 31: 1094–1103. [DOI] [PubMed] [Google Scholar]
- 26.Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, Fleisher T, Balow JE, and Lipsky PE 2010. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum 62: 542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.