Abstract
In response to changes in the extracellular environment, cells coordinate intracellular activities to maximize their probability of survival and proliferation. Eukaryotic cells, from yeast to mammals, transduce diverse extracellular stimuli through the cell by multiple mitogen-activated protein kinase (MAPK) cascades. Exposure of cells to increases in extracellular osmolarity results in rapid activation of a highly conserved family of MAPKs, known as stress-activated MAPKs (SAPKs). Activation of SAPKs is essential for the induction of adaptive responses required for cell survival upon osmostress. Recent studies have begun to shed light on the broad effects of SAPK activation in the modulation of several aspects of cell physiology, ranging from the control of gene expression to the regulation of cell division.
Introduction
Most cells are sensitive to osmotic imbalances and, when subjected to increases in extracellular osmolarity, lose water and shrink. To adapt to such high osmotic conditions, cells generally accumulate small organic molecules (e.g. glycerol), which allow them to balance their osmotic pressure with that of the external environment. However, osmostress not only induces the accumulation of osmolytes, but also has a great impact on cellular physiology. In yeast, for instance, it causes cytoskeletal reorganization, changes in cell-wall dynamics, alteration of ion homeostasis, metabolic adjustments and cell-cycle arrest, as well as having a huge impact on gene expression (reviewed by Hohmann, 2002). Our current knowledge confirms that many principles of osmoadaptation are conserved across eukaryotes, and therefore the use of yeasts as basic models has been of great value in elucidating the signal transduction mechanisms underlying the response to high osmolarity.
In Saccharomyces cerevisiae, changes in the osmolarity of the medium have been reported to affect different signalling pathways. The best-characterized signalling system by far involves the mitogen-activated protein (MAP) kinase Hog1, a relative of the p38 and c-Jun N-terminal kinase (JNK) families of stress-activated protein kinases (SAPKs). In addition to the HOG pathway, a second signal transduction pathway, mediated by cAMP-activated protein kinase A (PKA), has been shown to broadly modulate osmostress-induced gene expression in yeast. More detailed reviews of PKA involvement in osmostress signalling have been published over the years (e.g. Hohmann, 2002). We therefore focus on the role of SAPKs in osmostress, in both yeast and mammals.
Signal transduction by SAPKs
MAP kinase (MAPK) cascades are common signalling modules found in both higher and lower eukaryotic cells and are composed of three consecutively activated tiers of kinases: MAPKKK, MAPKK and MAPK. Eukaryotic organisms contain multiple MAPK families organized into discrete cascades, and two major pathways that are activated by environmental and genotoxic stress have been identified in mammals. The SAPKs, including the JNKs and the p38 MAPKs, are important components of these pathways. A prototype of the SAPK family is the yeast Hog1 MAPK, which specifically responds to increased extracellular osmolarity and is required for cell survival under these conditions. Conservation of the stress MAPK cascades between yeast and humans is indicated by the fact that individual kinases in the yeast pathway can be replaced by the corresponding human enzymes.
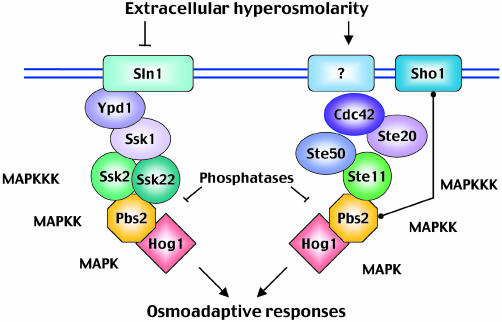
Osmostress sensors. It was proposed that, in mammalian cells, osmotic shock causes activation of JNK by non-specific clustering and internalization of cell surface receptors such as the epidermal growth factor (EGF) receptor (Rosette and Karin, 1996). However, in yeast, specific osmosensing devices seem to be responsible for detecting changes in osmolarity. The HOG pathway is activated predominantly by two independent mechanisms that lead to the activation of either the Ssk2 and Ssk22 or the Ste11 MAPKKKs, respectively (Figure 1). The first mechanism involves a ‘two-component’ osmosensor, composed of the Sln1-Ypd1-Ssk1 proteins. The Sln1 transmembrane protein has intrinsic histidine kinase activity and is a homologue of bacterial two-component signal transducers. Using a phospho-relay mechanism involving the Ypd1 and Ssk1 proteins, Sln1 is able to control the activity of Ssk1, which in turn interacts with and regulates the Ssk2 and Ssk22 MAPKKKs and subsequent Pbs2 activation (Posas et al., 1998). Pbs2 activation can also be achieved by a second, independent mechanism (Figure 1) that involves the transmembrane protein Sho1, the MAPKKK Ste11, the Ste11-binding protein Ste50, the Ste20 p21-activated kinase (PAK) and the small GTPase Cdc42 (Posas et al., 1998; Hohmann, 2002). Activation of Pbs2 by Ste11 requires the interaction of Pbs2 with Sho1 and, although this interaction is thought to be regulated (Reiser et al., 2000), the basic activation mechanism for this remains unclear (Raitt et al., 2000). Other, less well-characterized osmosensing mechanisms could also be feeding signals into the HOG pathway (Van Wuytswinkel et al., 2000). Since mammalian cells do not seem to have specific stress sensors similar to Sln1, determination of the sensor mechanism coupled to Sho1 could help to decipher the molecular identity of mammalian osmosensors.
Fig. 1. Schematic diagram of the yeast HOG pathway. Pbs2 integrates signals from two major independent upstream osmosensing mechanisms, which leads to the activation of specific MAPKKKs. Under osmostress, activated Pbs2 activates the Hog1 MAPK, which induces a set of osmoadaptive responses.
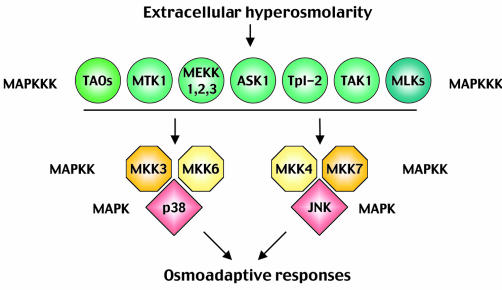
SAPK pathway components and organization. The central core of the yeast HOG pathway comprises a layer of MAPKKKs (Ssk2, Ssk22 and Ste11) that are responsible for the activation of the MAPKK Pbs2. Once activated, Pbs2 phosphorylates and activates the Hog1 MAPK (Figure 1). Mammalian cells activate three different MAPKs in response to osmostress: p38, JNK and ERK5. Since the role of ERK5 is still unclear, we will concentrate on the p38 and JNK MAPK pathways (Figure 2). Both p38 and JNK are encoded by several genes, which display differential tissue distribution, substrate specificity and sensitivity to chemical inhibitors (Kültz and Burg, 1998; Kyriakis and Avruch, 2001). Moreover, activation of these MAPKs can be achieved by several MAPKKs that have overlapping activities but differential specificities. MKK3 and MKK6 activate p38 MAPKs, whereas MKK4 and MKK7 are mainly responsible for the activation of JNK; all four MAPKKs can be activated by osmostress. Three different families of MAPKKKs (MEKKs, MLKs and TAOs) regulate the activation of MAPKKs and integrate the signals from upstream components into the cascades. However, the complexity of the regulation of these enzymes makes it difficult to formulate a clear picture of the stimuli that drive their activation. One of the MAPKKKs that mediates osmostress signalling is MTK1/MEKK4. MTK1 is activated by osmostress, and the overexpression of a dominant negative MTK1 can partially block the subsequent activation of p38 (Takekawa et al., 1997).
Fig. 2. Mammalian SAPK pathways. The MAPKKKs upstream of JNK and p38 belong to three broad protein kinase families differentiated by colour in the figure (TAOs, MEKKs and MLKs). There are four MAPKKs with overlapping activities but with different specificity.
The actual mechanisms of MAPKKK activation by osmostress in mammalian cells remain largely uncharacterized, but they could be similar to the mechanisms that function in yeast. For instance, Ssk2 activation by Ssk1 involves binding to the N-terminal non-catalytic domain of Ssk2. Interestingly, binding of GADD45 proteins to the human homologue of Ssk2, MTK1, results in activation of the MAPKKK (Takekawa and Saito, 1998). Moreover, in yeast, Ste11 activation is controlled in part by Ste20 and the small G-protein Cdc42; and, in mammals, Ste20-like kinases and small G-proteins have been shown to regulate MAPKKKs (Kyriakis and Avruch, 2001).
The organization of all these components into MAPK modules has been proposed to be directed by protein–protein interactions between kinases and by scaffolding proteins. Many proteins with a scaffolding function have already been reported to interact with SAPKs (e.g. JIP1/JSAP1 for JNK, and JIP2 for p38) and with signalling components that possess intrinsic scaffolding properties (i.e. MKK4 and Pbs2). It has been proposed that scaffolding proteins could contribute to signal specificity by insulating different MAPK modules. This seems to be the case for the yeast HOG pathway, since binding of Ste11 to Pbs2 MAPKK restricts Ste11 to activating only Pbs2, and not other MAPKKs (Harris et al., 2001). Thus, it is possible that specific scaffolding proteins could direct osmostress stimuli to restricted SAPK cascades. Although scaffolds could play a role in signalling specificity, MAPK substrate specificity and negative feedback loops involving, for instance, protein phosphatases may also be important (Chang and Karin, 2001).
Spatial organization of SAPK components. The upstream effectors of MAPK cascades seem to have specific sites of action in the cell (e.g. Cdc42), and membrane recruitment of MAPKKKs can be very important for cascade activation, as is the case for the recruitment of the MAPKKK Raf1 by the small G-protein Ras1. MAPKKs are actively excluded from the nucleus by mechanisms that involve a conserved nuclear export signal and the nucleo-cytoplasmic shuttling of MAPKs. Stimuli-dependent translocation of the MAPK ERK1 into the nucleus is mediated by its dissociation from its MAPKK and by its subsequent dimerization. Osmostress-induced phosphorylation of Hog1, rather than its kinase activity, is also essential for its nuclear accumulation (Ferrigno et al., 1998; Reiser et al., 1999); and, similarly to ERK1, Hog1 contains a dimerization domain, although the importance of this in vivo has not yet been demonstrated. However, since the concentration of Hog1 vastly exceeds that of the MAPKK, other mechanisms for controlling Hog1 localization may also come into play. For example, interactions with specific transport carriers or with nuclear retention factors may affect the nuclear accumulation of Hog1, as well as of other SAPKs (Ferrigno and Silver, 1999).
Downregulation of stress signalling. The magnitude and duration of signalling through SAPKs are critical determinants of their biological effect. Activation of Hog1 in response to mild osmolarity occurs within a minute and is extremely transient (Maeda et al., 1995). This suggests that the MAPK functions as a biological switch that must be actively downregulated, both under basal conditions and during adaptation. In yeast, two major families of phosphatases interact with and inactivate Hog1: the Ser/Thr protein phosphatases type 2C (PP2C) and the protein tyrosine phosphatases (PTP). The importance of Hog1 downregulation is illustrated by the fact that simultaneous deletion of the PTC1 and PTP2 phosphatase genes results in inhibition of the cell proliferation that is caused by constitutive activation of the HOG pathway (Maeda et al., 1993). A similar scheme is thought to operate in mammalian cells, and both PP2C and PTP have recently been shown to regulate p38 (Takekawa et al., 1998; Saxena et al., 1999). In addition, another family of dual-specificity phosphatases must play a key role in the regulation of MAPKs, since, for instance, the M3/6 and MKP-7 phosphatases have been shown to regulate JNK1 and p38 SAPKs (Keyse, 2000).
Adaptive responses
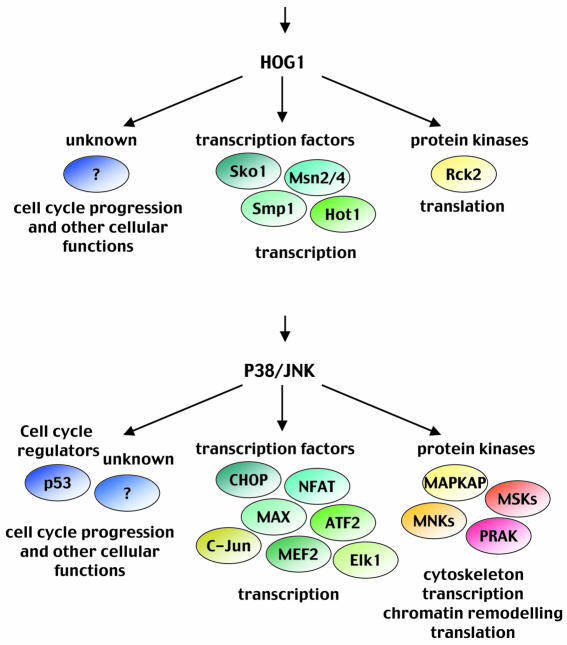
Downstream targets for SAPKs. Many SAPK targets have been described, both in the cytoplasm and the nucleus, which indicates that multiple cellular functions are under their control. SAPKs are proline-directed kinases. However, substrate specificity is not only defined by the targeted amino acid but also by specific docking domains present on the substrate protein and by specific substrate binding motifs in MAPKs. This interesting combination can account for the very high selectivity among MAPK subgroups (Tanoue et al., 2000). The substrates that mediate the adaptive response induced by SAPKs can be classified mainly into two groups: effector kinases and transcription factors (Figure 3).
Fig. 3. Activation of SAPKs by osmostress has a great impact on cell physiology. In response to stress, the yeast Hog1 (upper panel) or the mammalian p38 and JNK (lower panel) induce diverse osmoadaptive responses.
Transcriptional regulation. One of the main functions of MAPKs in response to osmostress is the regulation of gene expression. In mammalian cells, p38 controls the expression of >100 genes (Ono and Han, 2000). In budding yeast, genome-wide transcription studies revealed that a large number of genes (∼7%) show significant but transient changes in their expression levels after a mild osmotic shock and that the Hog1 MAPK plays a key role in much of this global gene regulation. These osmostress-regulated genes are implicated mainly in carbohydrate metabolism, general stress protection, protein production and signal transduction. This global change in transcription could account, at least in part, for the metabolic adjustments required for osmostress adaptation (Hohmann, 2002).
There is no unifying mechanism by which SAPKs and MAPKs modulate gene expression. JNK and p38 target several transcription factors directly, enhancing their ability to activate transcription (e.g. MEF2A/C, Elk1/Sap1a). Moreover, phosphorylation of c-Jun and AFT-2 by p38 and JNK kinases yields the formation of activation protein 1 (AP-1) complexes. JNK also inhibits the nuclear translocation, and therefore the function, of the activator NFAT by phosphorylation. JNK and p38 MAPKs also modulate gene expression through remodelling the structure of chromatin. p38 controls the phosphorylation of histone H3 and the high mobility group protein 14 (HMG-14) through the activation of the mitogen- and stress-activated protein kinase 1 (MSK-1) (Kyriakis and Avruch, 2001). On the other hand, acetylation of histones H2B and H4 by ATF-2 is stimulated by JNK phosphorylation (Kawasaki et al., 2000). Additionally, p38 has been reported to phosphorylate the TATA-binding protein (TBP), a prerequisite for its binding to the TATA box (Carter et al., 1999). Whether any of these MAPK functions control gene expression during osmostress signalling remains to be determined.
In yeast, five transcription factors have been proposed to be controlled by the Hog1 MAPK. Hot1, Smp1, Msn2 and Msn4 activate, whereas Sko1 represses or activates, different subsets of osmotic-inducible and Hog1-regulated genes (Rep et al., 2000; Proft et al., 2001; E. de Nadal and F. Posas, unpublished results). Sko1 is an ATF/CREB factor whose repressive activity via the Cyc8–Tup1 complex is inhibited by Hog1 in response to osmostress (Proft et al., 2001). Msn2 and Msn4 are generic stress factors controlled by PKA and Hog1 by an unknown mechanism. Hot1 physically interacts with Hog1, and its binding to DNA and subsequent transactivation activity are regulated by its phosphorylation by the kinase (Rep et al., 1999; Alepuz et al., 2001).
In addition to the conventional MAPK role in regulating transcription factor activity by direct phosphorylation, the finding that Hog1 can associate with chromatin at promoter regions of target genes adds a new dimension to gene regulation by signalling kinases. Activated Hog1 is recruited to osmotic-inducible promoters through interaction with specific transcription factors (Alepuz et al., 2001). Furthermore, recent results support a model in which promoter-localized Hog1 stimulates transcription by phosphorylation of specific components of the RNA pol II holoenzyme (E. de Nadal, P.M. Alepuz, M. Zapater, G. Ammerer and F. Posas, unpublished results). Additionally, Hog1 has been reported to bind to Sko1-dependent promoters through its interaction with Sko1, and both proteins are required for the recruitment of the SAGA histone acetylase and SWI/SNF nucleosome-remodelling complexes in response to osmostress (Proft and Struhl, 2002). These results suggest that MAPK-mediated modification of the transcription machinery at gene promoters could be a common feature of transcriptional regulation.
Post-transcriptional control of gene expression. Recent studies have highlighted roles for the MAPKs in the post-transcriptional and translational control of gene expression. Both p38 and JNK kinase pathways have been shown to contribute to cytokine/stress-induced gene expression by stabilizing mRNAs through an ARE (AU-rich element, present in 3′-UTR transcripts) targeted mechanism. AREs regulate mRNA turnover by modulating poly(A)-shortening rates and the subsequent decay of mRNA. In the yeast system, inhibition of the Hog1 pathway also leads to destabilization of the ARE-bearing transcript (Vasudevan and Peltz, 2001). In addition to ARE-mediated mRNA decay, unstable mRNAs can be targeted for rapid decay by other pathways. For instance, JNK is involved in IL-2 mRNA stabilization in activated T cells (Chen et al., 2000). Although this is a very interesting mechanism by which SAPKs can regulate gene expression, its role in osmostress still needs to be characterized.
Regulation of protein synthesis. In response to increases in external osmolarity, there is a transient decrease in protein synthesis (Norbeck and Blomberg, 1998; Uesono and Toh, 2002) caused by a decrease in amino-acid uptake, repression of ribosomal protein genes and a decrease in translation efficiency. The HOG pathway appears not to be involved in the initial inhibition of translation, but rather in the reactivation of translation under stress as an adaptation mechanism (Uesono and Toh, 2002). The yeast Rck2 kinase, which resembles the mammalian CaM kinases, is regulated by Hog1 (Bilsland-Marchesan et al., 2000; Teige et al., 2001). Reduction of protein synthesis upon stress was similar in hog1 and rck2 cells, which suggests that the effects of Hog1 on translation are mediated by Rck2 kinase (Teige et al., 2001). Rck2 could affect translation by directly regulating the elongation factor EF-2, but an effect on initiation factors cannot be excluded. In mammalian cells, stress-induced regulation of protein synthesis is mediated by phosphorylation of the eukaryotic initiation factor eIF4E by Mnk1 or of EF-2 by the eEF-2 kinase. Both kinases have been reported to be regulated by p38 in response to stress (Waskiewicz et al., 1999; Knebel et al., 2001). Thus, adaptive responses to restore protein translation could be mediated by similar mechanisms in yeast and mammals.
Cell-cycle control by SAPKs. Progression through the cell cycle is critically dependent on the presence of nutrients and stress stimuli. In response to osmostress, cells transiently modulate cell-cycle progression to allow adaptation. The role of SAPKs in cell-cycle control was first proposed in Schizosaccharomyces pombe for the Spc1/Sty1 MAPK (Shiozaki and Russell, 1995). The Spc1/Sty1 MAPK pathway displays strong structural similarities to the HOG pathway. However, it is activated not only by osmostress but also in response to a whole range of environmental conditions (e.g. heat shock, oxidative stress and UV light). It was initially identified as having a role in cell-cycle control, since alteration of its components, resulting in either hyperactivation or signal abrogation, resulted in cell defects, and only later was this pathway related to environmental responses (Wilkinson and Millar, 2000). More recently, a role for p38 and JNK MAPK pathways in cell-cycle progression has been reported in several organisms (reviewed by Ambrosino and Nebreda, 2001; Pearce and Humphrey, 2001). Although in mammals the role of JNK in cell-cycle control has not been clearly defined, it has been proposed that depletion of JNK1 or JNK2 suppresses cell growth under non-stress basal conditions. Moreover, the proliferation of Jnk –/– cells is reduced due to G1–S defects caused by a decrease in AP-1 transcription. p38 has been shown to regulate G0, G1–S and G2–M transitions. Depending on the cell type, p38 can induce either progression or inhibition at the G1–S transition by differential regulation of specific cyclin levels (cyclin A or D1) as well as by pRb and p53 phosphorylation (Wilkinson and Millar, 2000; Ambrosino and Nebreda, 2001). Less is known about the involvement of SAPKs in the regulation of the cell cycle upon osmostress. Mammalian cells regularly exposed to osmotic imbalances, such as inner medullary epithelial cells (IMEs), respond to high osmolarity by arresting in G1–S, G2 and mitosis. To protect cells from hypertonicity, p53 inhibits DNA replication and transition from G1 to S (Dmitrieva et al., 2001), and p53 is also known to be phosphorylated in response to stress by p38 MAPK. G2–S and M delays seem to be p53-independent. For instance, hypertonicity causes a rapid activation of the G2–M checkpoint through activation of p38, which causes a drop in Cdc2 kinase activity.
In yeast, a similar scenario is being unravelled. Increases in external osmolarity induce a transient growth arrest that results in accumulation of cells in G1 and G2–M phases and a concomitant drop of cells in S phase (Alexander et al., 2001). It is not clear whether the Hog1 MAPK is acting in the G1–S delay, but it has been reported that Hog1, together with the kinase Swe1, regulates a G2 delay by affecting yeast Cdc2 kinase activity (Alexander et al., 2001; J. Clotet, X. Escoté, E. Garí, M. Aldea and F. Posas, unpublished results). It is worth noting that mutations that inactivate the SLN1 osmosensor or inactivate both the PTC1 and PTP2 phosphatases block cell growth because of inappropriate hyperactivation of the Hog1 MAPK. This could be caused by improper activation of several cell-cycle checkpoints.
Perspectives
In recent years, we have learned that stress-activated MAPK pathways play a pivotal role in osmostress signal transduction, in both yeast and mammals. Moreover, osmostress responses, which were initially thought to be limited to the modification of the expression of a small number of genes, have now been shown to be involved in many aspects of cell biology. Still, some key questions regarding osmostress signalling remain to be clarified. A major issue is the identification of the mechanisms required to detect changes in osmolarity in mammalian cells. Much is known about the components of the central core of SAPK cascades; however, the mechanisms of MAPKKK activation and how signal specificity is achieved in response to osmostress remain unclear. Control of gene expression is a major outcome of SAPK activation, and the precise regulatory mechanisms have only started to be elucidated. Future identification of SAPK targets that are specifically modified in response to osmostress will result in a better understanding of the molecular mechanisms required to survive environmental changes.

Paula M. Alepuz, Francesc Posas & Eulàlia de Nadal. Francesc Posas is the recipient of an EMBO Young Investigator Award
Acknowledgments
Acknowledgements
Work in our laboratory is supported by grants from Ministerio de Ciencia y Tecnología PM99-0028, DURSI (Generalitat de Catalunya) and the EMBO Young Investigator Programme (to F.P.).
References
- Alepuz P.M., Jovanovic, A., Reiser, V. and Ammerer, G. (2001) Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol. Cell, 7, 767–777. [DOI] [PubMed] [Google Scholar]
- Alexander M.R., Tyers, M., Perret, M., Craig, B.M., Fang, K.S. and Gustin, M.C. (2001) Regulation of cell cycle progression by Swe1p and Hog1p following hypertonic stress. Mol. Biol. Cell, 12, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino C. and Nebreda, A.R. (2001) Cell cycle regulation by p38 MAP kinases. Biol. Cell, 93, 47–51. [DOI] [PubMed] [Google Scholar]
- Bilsland-Marchesan E., Arino, J., Saito, H., Sunnerhagen, P. and Posas, F. (2000) Rck2 kinase is a substrate for the osmotic stress-activated mitogen-activated protein kinase Hog1. Mol. Cell. Biol., 20, 3887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A.B., Knudtson, K.L., Monick, M.M. and Hunninghake, G.W. (1999) The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression. The role of TATA-binding protein (TBP). J. Biol. Chem., 274, 30858–30863. [DOI] [PubMed] [Google Scholar]
- Chang L. and Karin, M. (2001) Mammalian MAP kinase signalling cascades. Nature, 410, 37–40. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Gherzi, R., Andersen, J.S., Gaietta, G., Jurchott, K., Royer, H.D., Mann, M. and Karin, M. (2000) Nucleolin and YB-1 are required for JNK-mediated interleukin-2 mRNA stabilization during T-cell activation. Genes Dev., 14, 1236–1248. [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva N., Michea, L. and Burg, M. (2001) p53 protects renal inner medullary cells from hypertonic stress by restricting DNA replication. Am. J. Physiol. Renal Physiol., 281, F522–F530. [DOI] [PubMed] [Google Scholar]
- Ferrigno P. and Silver, P.A. (1999) Regulated nuclear localization of stress-responsive factors: how the nuclear trafficking of protein kinases and transcription factors contributes to cell survival. Oncogene, 18, 6129–6134. [DOI] [PubMed] [Google Scholar]
- Ferrigno P., Posas, F., Koepp, D., Saito, H. and Silver, P.A. (1998) Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin β homologs NMD5 and XPO1. EMBO J., 17, 5606–5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K., Lamson, R.E., Nelson, B., Hughes, T.R., Marton, M.J., Roberts, C.J., Boone, C. and Pryciak, P.M. (2001) Role of scaffolds in MAP kinase pathway specificity revealed by custom design of pathway-dedicated signaling proteins. Curr. Biol., 11, 1815–1824. [PubMed] [Google Scholar]
- Hohmann S. (2002) Osmotic stress signaling and osmoadaptation in yeast. Microbiol. Mol. Biol. Rev., 66, 300–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Schiltz, L., Chiu, R., Itakura, K., Taira, K., Nakatani, Y. and Yokoyama, K.K. (2000) ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature, 405, 195–200. [DOI] [PubMed] [Google Scholar]
- Keyse S.M. (2000) Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr. Opin. Cell Biol., 12, 186–192. [DOI] [PubMed] [Google Scholar]
- Knebel A., Morrice, N. and Cohen, P. (2001) A novel method to identify protein kinase substrates: eEF2 kinase is phosphorylated and inhibited by SAPK4/p38δ. EMBO J., 20, 4360–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kültz D. and Burg, M. (1998) Evolution of osmotic stress signaling via MAP kinase cascades. J. Exp. Biol., 201, 3015–3021. [DOI] [PubMed] [Google Scholar]
- Kyriakis J.M. and Avruch, J. (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev., 81, 807–869. [DOI] [PubMed] [Google Scholar]
- Maeda T., Tsai, A.Y. and Saito, H. (1993) Mutations in a protein tyrosine phosphatase gene (PTP2) and a protein serine/threonine phosphatase gene (PTC1) cause a synthetic growth defect in Saccharomyces cerevisiae. Mol. Cell. Biol., 13, 5408–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Takekawa, M. and Saito, H. (1995) Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science, 269, 554–558. [DOI] [PubMed] [Google Scholar]
- Norbeck J. and Blomberg, A. (1998) Amino acid uptake is strongly affected during exponential growth of Saccharomyces cerevisiae in 0.7 M NaCl medium. FEMS Microbiol. Lett., 158, 121–126. [DOI] [PubMed] [Google Scholar]
- Ono K. and Han, J. (2000) The p38 signal transduction pathway: activation and function. Cell. Signal., 12, 1–13. [DOI] [PubMed] [Google Scholar]
- Pearce A.K. and Humphrey, T.C. (2001) Integrating stress-response and cell-cycle checkpoint pathways. Trends Cell Biol., 11, 426–433. [DOI] [PubMed] [Google Scholar]
- Posas F., Takekawa, M. and Saito, H. (1998) Signal transduction by MAP kinase cascades in budding yeast. Curr. Opin. Microbiol., 1, 175–182. [DOI] [PubMed] [Google Scholar]
- Proft M. and Struhl, K. (2002) Hog1 kinase converts the Sko1–Cyc8–Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol. Cell, 9, 1307–1317. [DOI] [PubMed] [Google Scholar]
- Proft M., Pascual-Ahuir, A., de Nadal, E., Arino, J., Serrano, R. and Posas, F. (2001) Regulation of the Sko1 transcriptional repressor by the Hog1 MAP kinase in response to osmotic stress. EMBO J., 20, 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitt D.C., Posas, F. and Saito, H. (2000) Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J., 19, 4623–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V., Ruis, H. and Ammerer, G. (1999) Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol. Biol. Cell, 10, 1147–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser V., Salah, S.M. and Ammerer, G. (2000) Polarized localization of yeast Pbs2 depends on osmostress, the membrane protein Sho1 and Cdc42. Nat. Cell Biol., 2, 620–627. [DOI] [PubMed] [Google Scholar]
- Rep M., Reiser, V., Gartner, U., Thevelein, J.M., Hohmann, S., Ammerer, G. and Ruis, H. (1999) Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell. Biol., 19, 5474–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rep M., Krantz, M., Thevelein, J.M. and Hohmann, S. (2000) The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem., 275, 8290–8300. [DOI] [PubMed] [Google Scholar]
- Rosette C. and Karin, M. (1996) Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science, 274, 1194–1197. [DOI] [PubMed] [Google Scholar]
- Saxena M., Williams, S., Brockdorff, J., Gilman, J. and Mustelin, T. (1999) Inhibition of T cell signaling by mitogen-activated protein kinase-targeted hematopoietic tyrosine phosphatase (HePTP). J. Biol. Chem., 274, 11693–11700. [DOI] [PubMed] [Google Scholar]
- Shiozaki K. and Russell, P. (1995) Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature, 378, 739–743. [DOI] [PubMed] [Google Scholar]
- Takekawa M. and Saito, H. (1998) A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell, 95, 521–530. [DOI] [PubMed] [Google Scholar]
- Takekawa M., Posas, F. and Saito, H. (1997) A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J., 16, 4973–4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa M., Maeda, T. and Saito, H. (1998) Protein phosphatase 2Cα inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J., 17, 4744–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T., Adachi, M., Moriguchi, T. and Nishida, E. (2000) A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat. Cell Biol., 2, 110–116. [DOI] [PubMed] [Google Scholar]
- Teige M., Scheikl, E., Reiser, V., Ruis, H. and Ammerer, G. (2001) Rck2, a member of the calmodulin-protein kinase family, links protein synthesis to high osmolarity MAP kinase signaling in budding yeast. Proc. Natl Acad. Sci. USA, 98, 5625–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesono Y. and Toh, E. (2002) Transient inhibition of translation initiation by osmotic stress. J. Biol. Chem., 277, 13848–13855. [DOI] [PubMed] [Google Scholar]
- Van Wuytswinkel O., Reiser, V., Siderius, M., Kelders, M.C., Ammerer, G., Ruis, H. and Mager, W.H. (2000) Response of Saccharomyces cerevisiae to severe osmotic stress: evidence for a novel activation mechanism of the HOG MAP kinase pathway. Mol. Microbiol., 37, 382–397. [DOI] [PubMed] [Google Scholar]
- Vasudevan S. and Peltz, S.W. (2001) Regulated ARE-mediated mRNA decay in Saccharomyces cerevisiae. Mol. Cell, 7, 1191–1200. [DOI] [PubMed] [Google Scholar]
- Waskiewicz A.J., Johnson, J.C., Penn, B., Mahalingam, M., Kimball, S.R. and Cooper, J.A. (1999) Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol. Cell. Biol., 19, 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson M.G. and Millar, J.B. (2000) Control of the eukaryotic cell cycle by MAP kinase signaling pathways. FASEB J., 14, 2147–2157. [DOI] [PubMed] [Google Scholar]