Abstract
In eukaryotes, the initiation of DNA replication involves the ordered assembly on chromatin of pre-replicative complexes (pre-RCs), including the origin recognition complex (ORC), Cdc6, Cdt1 and the minichromosome maintenance proteins (MCMs). In light of its indispensable role in the formation of pre-RCs, Cdc6 binding to chromatin represents a key step in the regulation of DNA replication and cell proliferation. Here, we study the human Cdc6 (HuCdc6) protein during programmed cell death (apoptosis). We find that HuCdc6, but not HuOrc2 (a member of the ORC) or HuMcm5 (one of the MCMs), is specifically cleaved in several human cell lines induced to undergo apoptosis by a variety of stimuli. Expression of caspase-uncleavable mutant HuCdc6 attenuates apoptosis, delaying cell death. Therefore, an important function for cleavage of HuCdc6 is to prevent a wounded cell from replicating and to facilitate death.
INTRODUCTION
The control of programmed cell death (apoptosis) is crucial in all higher eukaryotes for several biological processes, including morphogenesis, tissue turnover and elimination of potentially tumorigenic cells. Apoptosis can be initiated by a variety of stimuli and leads to suicide of the cell, a process characterized by plasma membrane blebbing, chromatin condensation, fragmentation of the nucleus and DNA degradation. A central component of the apoptotic machinery is a family of cysteine proteases (caspases), which cleave a select set of proteins at aspartic acid (Asp, D) residues, generally at only one or a few specific sites. Fourteen mammalian caspases have been identified so far, three of which, caspases 3, 6 and 7, act as the effector caspases that are largely responsible for the morphological and biochemical changes that occur during apoptosis. A large number of substrates for caspases have been identified and include structural proteins such as nuclear lamins, but also proteins involved in DNA repair, DNA damage signalling and genomic stability, such as polyADP-ribose polymerase (PARP). The cleavage of these proteins ensures the efficient death of the cell (for a comprehensive review, see Earnshaw et al., 1999).
The identification of substrates for caspases is of fundamental importance to understand the downstream events that occur during apoptosis. In the light of this, we have studied the effects of apoptosis on DNA replication proteins. Specifically, we have asked whether Cdc6 was a target of selective cleavage during apoptosis in human cells. Cdc6 is essential for DNA replication and is required for pre-replicative complex (pre-RC) formation. Cdc6 binding to chromatin is dependent on the origin recognition complex (ORC), and in turn it is necessary to recruit the minichromosome maintenance proteins (MCMs), which license the DNA for replication (reviewed in Kelly and Brown, 2000). Recent studies have characterized the role of Cdc6 in the regulation of DNA replication during cell proliferation, and it has become clear that Cdc6 function must be carefully controlled both in yeast and animal cells to prevent inappropriate replication of the genome (Jallepalli et al., 1997; Pelizon et al., 2000). We therefore reasoned that inactivation of an essential replication protein such as Cdc6 would ensure that cells attempting to apoptose cannot replicate and this would facilitate cell death.
Here, we show that human Cdc6 (HuCdc6) is cleaved when human cells undergo apoptosis induced by a variety of different stimuli. We also indicate the consequence of HuCdc6 cleavage on the ordered execution of apoptosis.
RESULTS AND DISCUSSION
HuCdc6 is cleaved during apoptosis
To address whether HuCdc6 protein is cleaved during apoptosis, we used a well-characterized model system in which HL60 cells are induced to apoptose by treatment with the topoisomerase II inhibitor etoposide. Nuclear extracts were prepared from cells treated with this drug over a time course of 8 h, and proteins were analysed by western blotting. We used either a polyclonal antibody raised against the C-terminal fragment of HuCdc6 (amino acid residues 364–547) or a commercially available monoclonal antibody. By using constructs expressing different portions of HuCdc6, we found that the monoclonal antibody detected an epitope at the N-terminus of the protein (between amino acid residues 1 and 271) on western blots (data not shown).
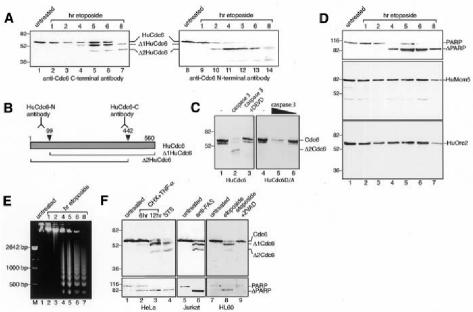
As shown in Figure 1A, cleavage of HuCdc6 is first detected 2–4 h after the addition of etoposide and is complete after 8 h. During apoptosis, the 62 kDa full-length HuCdc6 undergoes cleavage and, by using an anti-C-terminal HuCdc6 antibody, we could detect two smaller products, Δ1Cdc6 and Δ2Cdc6, which migrate with apparent molecular weights of ∼52 and 45 kDa, respectively (lanes 1–7). Similarly, we could demonstrate the cleavage of HuCdc6 by use of an anti-N-terminal HuCdc6 antibody (lanes 8–14). This antibody is able to detect the Δ2Cdc6 fragment and, occasionally, a further smaller fragment with an apparent molecular weight of ∼36 kDa. Either Asp286 or Asp289 are potential cleavage sites able to generate a 36 kDa fragment. However, since we were able to detect this band only at very late stages of apoptosis and after long exposure of the film, we believe this cleavage product derives from a less efficiently recognized site (although in the context of a DXXD caspase-consensus site) and we cannot be certain of its identity. Taken together, these results are compatible with the presence of at least two apoptotic cleavage sites in HuCdc6, one at each end of HuCdc6 (Figure 1B). By considering the size of the cleaved fragments and the sequence requirement for caspases (in several cases, the cleavage occurs after an Asp residue in any sequence context), cleavage of HuCdc6 is likely to occur at amino acid residues Asp99 (producing Δ1Cdc6) and Asp442 (producing Δ2Cdc6). To ascertain whether the sequences 96LVRD99 and 439SEVD442 are indeed the caspase recognition and cleavage sites, we mutated Asp99 and Asp442 to alanines (A), generating the mutant HuCdc6D/A. Wild-type HuCdc6 and mutant HuCdc6D/A proteins were translated in vitro and then incubated with caspase 3. Wild-type HuCdc6 was cleaved by caspase 3, whereas HuCdc6D/A was resistant to cleavage (Figure 1C). This suggests that caspase 3 cleaves HuCdc6 at Asp99 and Asp442 during apoptosis.
Fig. 1. HuCdc6 is cleaved in cells induced to undergo apoptosis. (A) Western blotting of nuclear extracts from HL60 cells treated with etoposide for the times indicated. An anti-C-terminal HuCdc6 antibody (lanes 1–7) and an anti-N-terminal HuCdc6 antibody (lanes 8–14) were used. (B) Diagram showing HuCdc6 and HuCdc6 fragments generated during apoptosis and the regions of HuCdc6 recognized by the antibodies used in this study. (C) Wild-type HuCdc6 and the caspase-resistant mutant HuCdc6D/A were transcribed and translated in vitro and incubated with caspase 3 (400 ng, left panel; 400 and 100 ng, right panel) or without (–). (D) PARP, HuMcm5 and HuOrc2 detected by western blotting of the same nuclear extracts as described above. Since HuMcm5 remains intact and its protein levels unchanged during apoptosis (Schwab et al., 1998), it is also used as a loading control for experiments in (A) and (D). (E) Analysis of genomic DNA fragmentation in HL60 cells by gel electrophoresis. (F) Western blotting of nuclear extracts from HeLa cells (induced to undergo apoptosis by either TNF-α plus CHX, lanes 2 and 3, or by STS, lane 4) and Jurkat cells (treated with anti-Fas antibody, lane 6). In vivo inhibition of caspases by the caspase-family inhibitor ZVAD–FMK is in lane 9. HuCdc6 was detected by using an anti-C-terminal HuCdc6 antibody. Bottom panels show cleavage of PARP.
Importantly, the cleavage of HuCdc6 is an early event during apoptosis and occurs with a similar time course to the cleavage of PARP (Figure 1D, top panel) and to the appearance of DNA laddering (Figure 1E), two distinct features of cells undergoing apoptosis.
By contrast, neither HuMcm5 nor HuOrc2 is cleaved during apoptosis, as shown by their unchanged mobility (Figure 1D). Previously, HuMcm3, another member of the MCM complex, was shown to undergo cleavage during apoptosis (Schwab et al., 1998). Therefore, it seems that components of the pre-RCs are not indiscriminately cleaved during apoptosis in human cells but rather that some components, including HuCdc6, are specifically cleaved.
To investigate whether HuCdc6 cleavage is a general feature of human cells undergoing apoptosis, we used different human cell lines and a variety of well-characterized inducers of apoptosis (Figure 1F). HeLa cells were treated with either TNF-α plus cycloheximide (CHX; lanes 2 and 3) or with staurosporine (STS; lane 4) and Jurkat cells with anti-Fas antibody (lane 6). In all cases, the treatment resulted in effective induction of apoptosis (see cleavage of PARP, bottom panels) and led to the cleavage of HuCdc6 into the same two fragments, Δ1Cdc6 and Δ2Cdc6. These experiments allow us to conclude that HuCdc6 protein is a target for selective cleavage during apoptosis irrespective of the inducing agent and the cell line under investigation, suggesting that HuCdc6 cleavage is an important and universal step in the apoptotic program of human cells. Importantly, the caspase inhibitor ZVAD–FMK effectively inhibits HuCdc6 cleavage in HL60 cells induced into apoptosis by etoposide (Figure 1F, lane 9), which strongly suggests that HuCdc6 breakdown is due to an apoptotic cysteine protease.
Caspase 3 is responsible for cleavage of HuCdc6 in vitro and in vivo
Caspases 3, 6 and 7 are the executioners of apoptosis. Among them, caspase 3 is the one involved in the cleavage of the majority of the substrates examined (Slee et al., 2001). We asked whether HuCdc6 could be cleaved by recombinant caspases in vitro and, if so, whether the pattern of in vitro cleavage corresponds to the one observed in vivo.
HeLa nuclear extract was incubated with activated caspases 3 or 6 in vitro and analysed on western blots. Figure 2A shows that caspase 3 efficiently cleaves HuCdc6 and PARP, while caspase 6 does not cleave HuCdc6 under any condition tested, despite actively cleaving lamin A (Figure 2B). Neither caspases 7 or 8 are able to cleave HuCdc6 in vitro (data not shown). Specific inhibitors Ac–DEVD–CHO and Z–VEID–FMK block cleavage by caspases 3 and 6, respectively (Figure 2A and B, lane 5). Recombinant caspase 3 is also able to specifically cleave HuCdc6 when HeLa nuclei are treated in vitro (Figure 2C). Notably, the pattern of HuCdc6 cleavage in vitro by recombinant caspase 3 is identical to that observed in vivo, resulting in the Δ1Cdc6 and Δ2Cdc6 fragments. The second fragment is detectable after extensive cleavage of HuCdc6 at the highest concentration of caspase 3 (Figure 2C, lane 4). Because of the similarity between the in vitro and in vivo HuCdc6 cleavage pattern, we suggest that caspase 3 is very likely responsible for HuCdc6 cleavage in vivo. Furthermore, HuCdc6 is not cleaved in vivo in caspase-3- deficient MCF-7 cells treated with STS to undergo apoptosis, whereas lamin A is cleaved (Figure 2D).
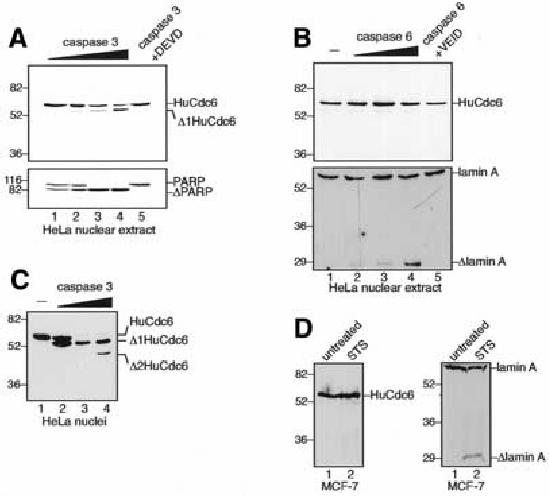
Fig. 2. Caspase 3, but not caspase 6, cleaves HuCdc6 in vitro. (A) HeLa nuclear extract was incubated with caspase 3 (20, 50, 100 and 400 ng) and analysed on western blots using an anti-C-terminal HuCdc6 antibody. The cleavage of PARP by caspase 3 is in the lower panel. Caspase 3 (400 ng) was specifically inhibited by Ac–DEVD–CHO, lane 5. (B) HeLa nuclear extract was incubated with no caspase (–) or caspase 6 (20, 50 and 400 ng) and analysed as in (A). The cleavage of lamin A by caspase 6 is in the lower panel. Caspase 6 (400 ng) was specifically inhibited by Z–VEID–FMK (lane 5). (C) Western blotting of HeLa nuclei incubated with caspase 3 (0.2, 1, and 2 µg). HuCdc6 was detected with an anti-C-terminal antibody. (D) Western blotting of nuclear extracts from the caspase-3-deficient MCF-7 cell line induced to apoptose by STS (left panel). The anti-HuCdc6 antibody is the same as in (A–C). Cleavage of lamin A in the apoptotic MCF-7 cells is in the right panel.
Resistance to apoptosis of cells expressing caspase-uncleavable mutant HuCdc6
To test the impact of HuCdc6 cleavage on cell death, we expressed the uncleavable form of HuCdc6 in HeLa cells by microinjecting a plasmid expressing the HuCdc6D/A gene under the control of the CMV promoter. Cells were then treated with N-methyl-N ′-nitro-N-nitrosoguanidine (MNNG), a DNA-damaging agent, as described in Halappanavar et al. (1999), and the apoptotic response of these cells was compared with that of cells injected with either a plasmid expressing the wild-type HuCdc6 or the vector alone during the following 25 h. In response to MNNG, the cells expressing the uncleavable HuCdc6 protein are more resistant to induction of apoptosis at any time point we analysed, in at least three independent experiments. Significantly, expression of the uncleavable HuCdc6 protein results in a delay of cell death and higher survival of the damaged cells (Figure 3A and B). Although almost the entire population of cells injected with the vector alone or wild-type HuCdc6 are dead (we obtained similar results for these two controls and overlapping curves in the graph), ∼50% of the cells expressing the uncleavable HuCdc6 survive at 12 h. Even 25 h after induction of apoptosis, cells expressing the uncleavable HuCdc6 continue to show more resistance. Attenuation of apoptosis due to prevention of HuCdc6 cleavage is comparable to results obtained for uncleavable PARP (Halappanavar et al., 1999) and lamins (Rao et al., 1996) in terms of delay of apoptosis and percentage of surviving cells.
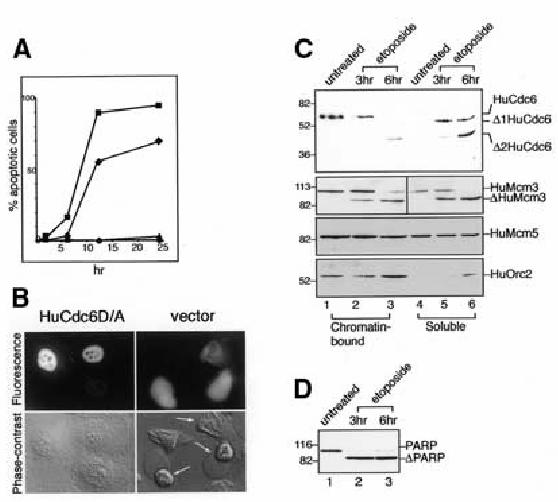
Fig. 3. Cells expressing uncleavable HuCdc6 are resistant to apoptosis. (A) Uncleavable HuCdc6D/A (filled diamonds) and pCDNA3 vector or wild-type HuCdc6 (filled squares) were injected into HeLa cells. Cells were then treated with 200 µM MNNG for 2 h and washed, and apoptotic cells were counted during the following 25 h. HuCdc6D/A (filled triangles) and pCDNA3 vector or wild-type HuCdc6 (filled circles) were injected in control cells. (B) Representative images of cells injected with uncleavable HuCdc 6D/A (left panels) and pCDNA3 vector (right panels). Cells were coinjected with Texas Red as a marker (top panels). Bottom panels show phase-contrast images with the apoptotic cells indicated by arrows (6 h after MNNG treatment). (C) Western blotting of chromatin-bound and soluble protein fractions from untreated and apoptotic cells by using an anti-C-terminal HuCdc6 (top panel), anti-HuMcm3 and anti-HuMcm5 (second and third panel, respectively) antibodies. HuOrc2 was analysed to assess the biochemical fractionation. HuMcm5 was also used as an internal loading control. (D) Western blotting of proliferating and apoptotic HL60 extracts by PARP antibody.
To test whether activities of HuCdc6, namely binding to chromatin and recruitment of the MCMs (Donovan et al., 1997), are impaired as consequence of caspase 3 cleavage, we analysed the distribution of HuCdc6 and MCMs in the chromatin-bound and soluble fractions prepared from untreated HL60 cells and from cells undergoing apoptosis.
Figure 3C (top panel) shows that, in untreated HL60 cells, nuclear HuCdc6 is chromatin-bound, as already shown in Mendez and Stillman (2000). However, upon induction of apoptosis (monitored by cleavage of PARP; Figure 3D), the full-length HuCdc6 band is no longer detectable and the cleaved HuCdc6 fragments are preferentially recovered in the soluble protein fraction. On the basis of these results, we suggest that cleavage of HuCdc6 impairs its binding to chromatin and results in HuCdc6 release from chromatin during progression of apoptosis. In contrast, the binding of the MCMs (Figure 3C; HuMcm3 and HuMcm5 are shown) is not greatly affected during cell death, as shown in a previous study for HuMcm3 (Schwab et al., 1998). The partitioning of HuOrc2 protein (Figure 3C, bottom panel) indicates a good biochemical fractionation (the extensive chromatin degradation occurring as a mid-to-late event results in some HuOrc2 being recovered in the soluble fraction, 6 h etoposide, lane 6).
Interestingly, cleavage of HuCdc6 at amino acid residue Asp442 separates the C-terminus of HuCdc6 from the rest of the protein. Structural data based on the Pyrobaculum aerophilum Cdc6 protein and sequence comparisons (Liu et al., 2000) have identified a wing-helix domain in the C-terminal region of Cdc6 proteins (amino acid residues 452–560 of HuCdc6) whose possible function, also supported by this study, might be mediation of protein–protein and protein–chromatin interactions. In agreement with this, a deletion mutant of Xenopus Cdc6 (XCdc6) lacking the C-terminal portion of the protein (amino acid residues 350–553) is unable to support DNA replication in Cdc6-depleted Xenopus extract (C. Pelizon, unpublished results).
In summary, we show that activation of the apoptotic program leads to cleavage of HuCdc6 and impairs its binding function to chromatin. By releasing HuCdc6 from potential replication sites, damaged cells become unable to replicate DNA. Therefore, we suggest that apoptotic cleavage of HuCdc6 is an important event during cell death to prevent HuCdc6 function and facilitate cell death. In agreement with this, we show that expression of uncleavable HuCdc6 delays cell death and results in a higher survival. Cleavage of HuCdc6 would make not only energetic sense for the dying cell but could provide an important defence against cancer, ensuring that any cells that attempt apoptosis, yet survive in a damage state, are still unable to replicate.
METHODS
Cell culture, extraction and analysis of apoptotic DNA. HL60 cells and Jurkat cells were grown in RPMI 1640 medium. HeLa cells and MCF-7 cells were grown in DMEM. Medium was supplemented with 10% FCS, 100 U/ml penicillin, 10 U/ml streptomycin and 2 mM glutamine. Cell culture reagents were from Gibco-BRL. For induction of apoptosis, HL60 cells were treated with 68 µM etoposide (Sigma) for various times or with 5 µM STS (Alexis Corp.) for 12 h. Apoptosis in HeLa cells was induced with TNF-α (40 ng/ml; Peprotech EC Ltd) and CHX (40 µg/ml; Sigma) for 6 or 12 h or with 5 µM STS for 12 h. Jurkat cells were incubated with 100 ng/ml anti-Fas antibody (MBL International Corp.) for 12 h. The caspase-3-deficient MCF-7 cell line was treated with STS for 23 h. The caspase-family inhibitor ZVAD–FMK (Alexis Corp.) was added to the cell culture medium 30 min before induction of apoptosis. Caspase 3 inhibitor Ac–DEVD–CHO was from PharMingen and caspase 6 inhibitor Z–VEID–FMK was from BioVision. Inhibitors were used according to the manufacturers’ instructions.
DNA was extracted from cells undergoing apoptosis by using DNAzol (Gibco-BRL) and then analysed on a 1.5% agarose gel.
Antibodies. Anti-HuCdc6 polyclonal antibody was raised against the C-terminal fragment of HuCdc6 (amino acid residues 364–547). Anti-HuMcm5 monoclonal antibody was raised against a fragment of HuMcm5 including amino acid residues 367–582. Anti-HuOrc2 polyclonal antibody was described in Stoeber et al. (1998). Monoclonal anti-HuCdc6, anti-HuMcm3 (both from Santa Cruz Biotechnology), anti-PARP (PharMingen) and anti-lamin-A antibodies (Cell Signaling) were used according to manufacturers’ instructions.
Nuclear extract preparation and chromatin isolation. Control cells or those induced to undergo apoptosis were harvested, washed in PBS and disrupted with a Dounce homogenizer in buffer A (10 mM HEPES–KOH pH 7.6, 1.5 mM MgCl2, 10 mM KCl and 1mM DTT). Nuclei were collected by centrifugation and nuclear proteins were extracted with 10 mM HEPES–KOH pH 7.6, 5 mM MgCl2, 300 mM NaCl, 1 mM DTT and 0.2 mM EDTA. Alternatively, nuclei were directly lysed in SDS loading buffer. Chromatin-bound and soluble nuclear proteins were separated as described in Mendez and Stillman (2000).
In vitro cleavage of HuCdc6 protein. HeLa nuclear extract (Computer Cell Culture, Mons, Belgium) was incubated with various amounts of recombinant caspase 3 or 6 in a final buffer composition of 20 mM HEPES pH 7.6, 0.1% CHAPS, 20 mM DTT and 5 mM EDTA, at 37°C. Reactions were terminated by the addition of SDS loading buffer to the reaction mixture. All the caspases were purchased from PharMingen.
Generation of caspase-resistant mutant HuCdc6 and in vitro translation. Plasmid HuCdc6D/A, bearing substitution of Asp99 and Asp442 with alanines, was obtained by site-directed mutagenesis (Stratagene). Briefly, the HuCdc6 gene was cloned into the pCDNA3 vector and was used as a template in PCR reactions with primers containing the desired mutations. In vitro translation was performed using the couple TNT reticulocyte lysate system (Promega). The translation products were incubated with caspase 3 and subjected to western blotting.
Expression of caspase-uncleavable HuCdc6. Plasmid pCDNA3 and the same plasmid expressing either HuCdc6 or HuCdc6D/A were injected into HeLa cells together with Texas Red to identify injected cells at the microscope. Three hours after injection (when proteins were efficiently expressed), cells were exposed to MNNG (Fluka) and the apoptotic responses of these cells were compared. Injected cells were followed by time-lapse microscopy taking florescence and phase-contrast images for 25 h until cells were detached from the dish with a terminal apoptotic phenotype. We counted as apoptotic only those cells showing distinctive morphology features such nuclear shrinkage, membrane blebbing and chromosome condensation (after staining with Hoechst dye). Routinely, 50–100 cells were injected and analysed.
Acknowledgments
ACKNOWLEDGEMENTS
We thank R. Freire for reagents and advice, D. Santamaria, L. Ko Ferrigno and J. Bradbury for comments on the manuscript and S. Dilworth for anti-HuMcm5 antibody.
REFERENCES
- Donovan S., Harwood, J., Drury, L.S. and Diffley, J.F. (1997) Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc. Natl Acad. Sci. USA, 94, 5611–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W.C., Martins, L.M. and Kaufmann, S.H. (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem., 68, 383–424. [DOI] [PubMed] [Google Scholar]
- Halappanavar S.S., Rhun, Y.L., Mounir, S., Martins, L.M., Huot, J., Earnshaw, W.C. and Shah, G.M. (1999) Survival and proliferation of cells expressing caspase-uncleavable Poly(ADP-ribose) polymerase in response to death-inducing DNA damage by an alkylating agent. J. Biol. Chem., 274, 37097–37104. [DOI] [PubMed] [Google Scholar]
- Jallepalli P.V., Brown, G.W., Muzi-Falconi, M., Tien, D. and Kelly, T.J. (1997) Regulation of the replication initiator protein p65cdc18 by CDK phosphorylation. Genes Dev., 11, 2767–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T.J. and Brown, G.W. (2000) Regulation of chromosome replication. Annu. Rev. Biochem., 69, 829–880. [DOI] [PubMed] [Google Scholar]
- Liu J., Smith, C.L., DeRyckere, D., DeAngelis, K., Martin, G.S. and Berger, J.M. (2000) Structure and function of Cdc6/Cdc18: implications for origin recognition and checkpoint control. Mol. Cell, 6, 637–648. [DOI] [PubMed] [Google Scholar]
- Mendez J. and Stillman, B. (2000) Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell. Biol., 20, 8602–8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelizon C., Madine, M.A., Romanowski, P. and Laskey, R.A. (2000) Unphosphorylatable mutants of Cdc6 disrupt its nuclear export but still support DNA replication once per cell cycle. Genes Dev., 14, 2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L., Perez, D. and White, E. (1996) Lamin proteolysis facilitates nuclear events during apoptosis. J. Cell Biol., 135, 1441–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab B.L., Leist, M., Knippers, R. and Nicotera, P. (1998) Selective proteolysis of the nuclear replication factor MCM3 in apoptosis. Exp. Cell Res., 238, 415–421. [DOI] [PubMed] [Google Scholar]
- Slee E.A., Adrain, C. and Martin, S.J. (2001) Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem., 276, 7320–7326. [DOI] [PubMed] [Google Scholar]
- Stoeber K., Mills, A.D., Kubota, Y., Krude, T., Romanowski, P., Marheineke, K., Laskey, R.A. and Williams, G.H. (1998) Cdc6 protein causes premature entry into S phase in a mammalian cell-free system. EMBO J., 17, 7219–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]