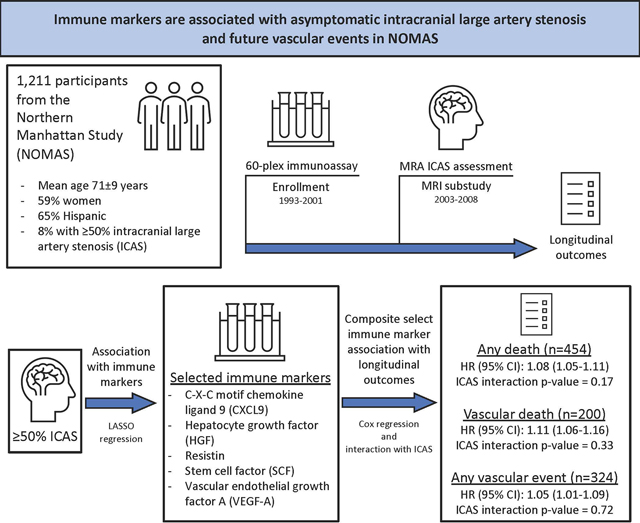
Abstract
Background
Inflammation contributes to atherosclerosis but is incompletely characterized in intracranial large artery stenosis (ICAS). We hypothesized immune markers would be associated with ICAS and modify the risk ICAS confers on future vascular events.
Methods
This study included a subsample of stroke-free participants in the prospective Northern Manhattan Study who had blood samples analyzed with a 60-plex immunoassay (collected 1993–2001) and ICAS assessment with time-of-flight magnetic resonance angiography (obtained 2003–2008). We dichotomized ICAS as either ≥50% stenosis or not (including no ICAS). We ascertained post-MRI vascular events. We used least absolute shrinkage and selection operator (LASSO) procedures to select immune markers independently associated with ICAS. Then, we grouped selected immune markers into a derived composite z-score. Using proportional odds regression, we quantified the association of the composite immune marker z-score, ICAS, and risk of vascular events.
Results
Among 1,211 participants (mean age 71±9 years, 59% women, 65% Hispanic participants), 8% had ≥50% ICAS. Using LASSO regression, we identified C-X-C motif chemokine ligand 9, hepatocyte growth factor, resistin, stem cell factor, and vascular endothelial growth factor A to have the strongest positive relationships with ≥50% ICAS in fully adjusted models. Selected markers were used to derive a composite immune marker z-score. Over an average follow-up of 12 years, we found each unit increase in immune marker z-scores was associated with an 8% (95% confidence interval (CI): 1.05–1.11), 11% (95% CI: 1.06–1.16), and 5% (95% CI: 1.01–1.09) increased hazard of death, vascular death, and any vascular event, respectively, in adjusted models. We did not find a significant interaction between immune marker z-scores and ICAS in their relationship with any longitudinal outcome.
Conclusions
Among a diverse stroke-free population, select serum immune markers were associated with ICAS and future vascular events. Further study is needed to better understand their role in the pathogenesis of ICAS and as a potential therapeutic target in stroke prevention.
Keywords: intracranial large artery stenosis, inflammation, biomarker, stroke
Graphical Abstract
Introduction
Intracranial large artery stenosis (ICAS) is common among community-dwelling older adults and increases risk for future vascular events.1 Further, the risk of recurrent stroke in symptomatic ICAS can exceed 20% at one year in certain populations, despite best medical therapy of antiplatelets and vascular risk factor modification.2 Therefore, new therapeutic approaches to ICAS are needed.
ICAS is often presumed to be caused by underlying atherosclerosis, which is a chronic inflammatory disease of the arterial wall mediated by cholesterol among other factors.3,4 Inflammation is also thought to play a role in vascular aging that may involve vessel wall remodeling to cause non-atherosclerotic stenosis.5 Therefore, inflammation represents a potentially attractive target for therapeutics in ICAS; however, data on inflammation and ICAS are limited to a few non-specific biomarkers. We sought to evaluate the association between ICAS and a panel of immune biomarkers among a diverse stroke-free population. We hypothesized immune markers would be associated with ICAS and modify the risk ICAS confers on future vascular events.
Methods
Study Population
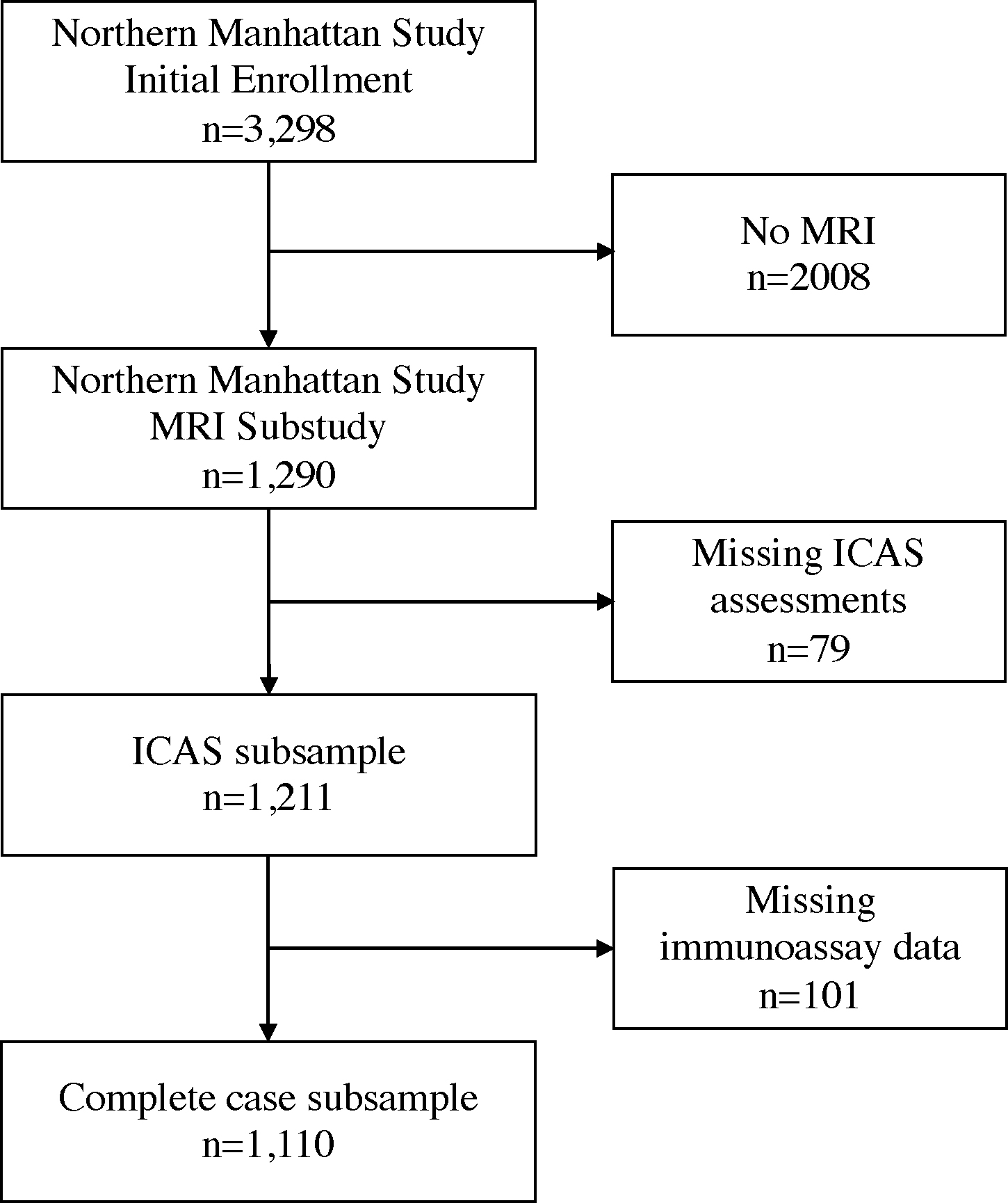
This study included stroke-free participants enrolled in the Northern Manhattan Study (NOMAS), a diverse prospective cohort of stroke risk factors. Details for study recruitment and eligibility were previously described.6 From 1993–2001, NOMAS investigators recruited a total of 3,298 stroke-free participants. In 2003–2008, NOMAS researchers introduced standardized brain magnetic resonance imaging (MRI) protocols and 1091 surviving stroke-free participants aged 50 years or older and 199 household members were invited to participate and screened for eligibility in the MRI substudy, for a total of 1,290 participants. Compared to the overall NOMAS cohort, the MRI subsample is younger and had a lower proportion of vascular risk factors.7 In this study, we included 1,211 participants who had ICAS assessments, of whom 1,110 participants had complete covariate information (Figure 1). The University of Miami and Columbia University institutional review boards approved this study. All participants provided informed consent. The study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (Supplemental Material).8
Figure 1. Study subsample flow diagram.
Immunoassay Assessment
At enrollment, trained phlebotomists drew blood samples from participants, which were processed and stored at −80 degrees Celsius. Laboratory personnel blinded to participant characteristics analyzed the samples using a 60-plex immunoassay. We used a customized, magnetic bead-based immunoassay similar to prior studies.9 The assay included immune biomarkers related to activation of Th1 (pro-inflammatory), Th2 (counter-regulatory-autoimmunity-promoting), Th17 (innate mucosal anti-pathogen defenses, including in gut), T regulatory, B (antibody-producing) and NK cells, and more generally, markers of innate and adaptive immunity, chemokines, growth factors (including those vital to vascular remodeling), neurotrophic factors, cell adhesion markers, and adipokines.10
We coded, randomized and assayed plasma samples in duplicate. Flow- and fluorescence-based Luminex 200™detection platform (Luminex Corporation, Austin, TX) determined the median fluorescence intensities (MFI) of each analyte-specific immunoassay bead set.11 Data were processed using a quality control (QC) algorithm that calibrates performance of an expanded set of serial standard curves and an in-house plasma control run on every assay plate, monitoring intra- and inter-plate covariance (CV) and bead counts. Only plates with mean intra-assay %CV <15% were accepted. Samples that did not meet QC criteria were reanalyzed when feasible. MFI values exceeding machine limits of reliable detection (>25,000) were excluded. Because interpolated concentrations can introduce bias for samples with very low or high values in relation to the serial standard curves, averaged MFI values for all 60 cytokines were used in the final statistical analyses rather than absolute concentrations.12,13 The final data set had a mean intra-assay %CV of 4.6% (standard deviation [sd] 2.2%, range: 1.9–7.9%) and 0.5% of all possible intra-assay %CV values were >25%, across all cytokines. Among the 1,211 participants with ICAS assessments, 110 (8%) individuals had missing immunoassay data.
ICAS Assessment
The NOMAS MRI substudy collected 3-dimensional, time-of-flight magnetic resonance angiography (MRA). Following a standardized protocol, this MRI substudy used a 1.5-T MRI system (Philips Medical Systems, Best, Netherlands) at the Columbia University Medical Center.1 All 11 major intracranial large arteries were visually inspected in the MRA datasets. If arterial stenosis was identified, the narrowest lumen area was measured to define stenosis, referencing the immediately preceding segment with normal lumen (or the next normal appearing lumen if the stenosis was at the arterial origin). Stenoses were categorized as no stenosis, <50% (or luminal irregularities), 50–69%, ≥70%, and flow gap. Stenoses were graded in blinded fashion by a trained neurologist and vascular neurologist independently. Interrater reliability was high (kappa=0.93) for identifying ≥50% stenosis and the interclass correlation coefficient for the ordinal stenosis scale was >0.90 for single and average measures.1 We represented ICAS dichotomously as significant (≥50%) stenosis or not (including no stenosis).
Post-MRI event adjudication
Study participants were screened annually with standardized telephone interviews and/or in-person visits for pre-defined outcomes.14 The outcomes included in the present analysis were any death, vascular death, and any vascular event. We considered vascular death to be death caused by myocardial infarction, stroke, heart failure, pulmonary embolism, or cardiac arrhythmia. We defined vascular events to include vascular death, any stroke or myocardial infarction. NOMAS investigators adjudicated death and pre-defined outcomes by medical record review from New York-Presbyterian Hospital/Columbia University Medical Center (where a majority of participants obtained medical care) or from outside institutions, including foreign hospitals if needed. Two study vascular neurologists, blinded to clinical information, adjudicated stroke independently. A study cardiologist adjudicated myocardial infarction using criteria from the Cardiac Arrythmia Suppression Trial and the Lipid Research Clinics Coronary Primary Prevention Trial.15,16 In the present analysis, we included follow-up data collected until July 2020. Among the MRI substudy, only 3 (0.4%) participants were lost to follow-up and 11 (1.4%) withdrew from active participation.
Covariates
All NOMAS participants underwent a structured questionnaire at time of enrollment. Demographics and smoking history were ascertained by participant self-report. Vascular risk factors were recorded at time of MRI study. Hypertension, diabetes mellitus, and hyperlipidemia were defined by either self-reported diagnosis, medication used to treat each condition, or blood pressure and/or laboratory evidence of these vascular risks, as previously reported.17 Smoking was dichotomized as current smoking or not current smoking at time of MRI.
Statistical Analyses
We first sought to evaluate the association between immune markers (logarithm base 2-transformed and standardized) and ICAS, and to identify a subset of key immune markers among the 60 in the immunoassay that relate to ≥50% ICAS. Second, using selected immune markers, we focused on their relationship with post-MRI vascular events. We used complete case analysis. All two-tailed p-values <0.05 were considered statistically significant.
To assess the association between immune markers and ICAS, we used regularized logistic regression with a Least Absolute Shrinkage and Selection Operator (LASSO) approach to estimate the association between immune markers and ≥50% ICAS with 3 models: 1) panel of immune markers; 2) additionally adjusted for age, sex, race, and ethnicity; and 3) additionally adjusted for hypertension, hyperlipidemia, diabetes mellitus, and smoking history. Immune markers with the 5 greatest positive coefficient estimates were then selected to derive a composite immune marker z-score, which is a summation of the individual z-scores for each selected biomarker. We conducted all LASSO logistic regression using R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) with the glmnet package.18 We were unable to perform random forests and eXtreme Gradient Boosting trees procedures given an imbalance of outcomes (8% of sample) between training and testing sets. Using Pearson’s correlation test, we evaluated the relationship of immune markers selected by LASSO regression with high-sensitivity C-reactive protein (hs-CRP), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-alpha), which were also measured at enrollment.
Next, we evaluated the association between immune marker z-scores and post-MRI vascular events. To calculate hazard ratios (HR) and their 95% confidence intervals (CI) with robust sandwich error variance,19 we conducted Cox proportional hazards regression using 3 models: 1) includes immune marker z-scores and ICAS groups (≥50% stenosis vs reference not ≥50% stenosis); 2) additionally included age, sex, race, and ethnicity; and 3) additionally included hypertension, hyperlipidemia, diabetes mellitus, and smoking history. To evaluate the survival bias because of any death, we modelled the adjusted risk of events using Fine & Gray regression using the same covariates. We also assessed for interaction between the immune marker z-score and ICAS in the relationship with events using a multiplicative interaction term in the fully adjusted model. We used SAS version 9.4 (SAS Institute Inc., Cary, NC) to conduct survival analyses.
Lastly, we created a protein interaction network using the String database (StringDB v11.5) of known and predicted protein-protein interactions and inferring protein associations from co-expression data, according to the standard network instructions provided with a medium stringency threshold of association (0.4).20
Results
Baseline characteristics
Among 1,211 participants (mean age 71±9 years, 59% women, 65% Hispanic participants), 96 (8%) had ≥50% ICAS. A high proportion of participants had comorbid vascular risk factors (78% and 81% of participants with hypertension and hyperlipidemia, respectively). Those with ≥50% ICAS were older and had higher prevalence of hypertension and diabetes mellitus (Table 1).
Table 1.
Study population characteristics by intracranial large artery stenosis (ICAS) groups
| Characteristic | ≥50% ICAS (n=96) | Not ≥50% ICAS (n=1,115) |
|---|---|---|
| Age, mean years (sd) | 74.5 (9.4) | 70.2 (8.8) |
| Women, % | 54 | 59 |
| Race and Ethnicity, % | ||
| Non-Hispanic White | 15 | 18 |
| Non-Hispanic Black | 25 | 17 |
| Hispanic | 60 | 65 |
| Comorbidities, % | ||
| Hypertension | 92 | 77 |
| Hyperlipidemia | 84 | 81 |
| Diabetes Mellitus | 39 | 23 |
| Current Smoking | 7 | 12 |
| Longitudinal Outcomes, % | ||
| Any death | 57 | 36 |
| Vascular death | 33 | 15 |
| Any vascular event (vascular death, myocardial infarction, or any stroke) | 43 | 25 |
| Any ischemic stroke | 2 | 1 |
Immune marker selection
Using LASSO regression with stepwise increasing adjustment for confounders, we identified several immune markers associated with ≥50% ICAS (Table 2). In the fully adjusted model, C-C motif chemokine ligand 2 (CCL2), C-X-C motif chemokine ligand 9 (CXCL9), epidermal growth factor (EGF), hepatocyte growth factor (HGF), interleukin 1 receptor antagonist (IL1RA), resistin, stem cell factor (SCF), soluble intercellular adhesion molecule-1 (sICAM1), and vascular endothelial growth factor A (VEGF-A) were selected predictors for ≥50% ICAS. Of these, CXCL9, HGF, resistin, SCF, and VEGF-A had the strongest positive relationships with ≥50% ICAS (respective β-estimates = 0.032, 0.045, 0.069, 0.088, 0.024), which we used to derive an ICAS-derived composite immune marker z-score. HGF, SCF, and VEGF-A had moderate positive correlations (r range from 0.28 to 0.62) with serum IL-6 and TNF-alpha levels (Supplemental Table). Figure 2 shows a protein interaction network of the selected immune markers.
Table 2.
Regularized logistic regression feature selection and beta estimates in predicting ≥50% intracranial large artery stenosis
| Feature* | Model 1 β-estimate per unit |
Model 2 β-estimate per unit |
Model 3 β-estimate per unit |
|---|---|---|---|
| bNGF | 0.019 | — | — |
| CCL2 | 0.055 | 0.003 | 0.001 |
| CCL5 | −0.065 | — | — |
| CCL11 | 0.031 | 0.020 | — |
| CXCL9 | 0.170 | 0.013 | 0.032† |
| CXCL12a | −0.083 | — | — |
| EGF | −0.329 | −0.174 | −0.157 |
| HGF | 0.134 | 0.047 | 0.045† |
| IFNb | −0.022 | — | — |
| IL1RA | 0.052 | 0.026 | 0.009 |
| Leptin | 0.042 | 0.030 | — |
| PDGFBB | 0.121 | — | — |
| Resistin | 0.153 | 0.060 | 0.069† |
| SCF | 0.132 | 0.092 | 0.088† |
| sFasL | −0.059 | — | — |
| sICAM1 | −0.122 | — | −0.017 |
| VEGF-A | 0.084 | 0.050 | 0.024† |
| VEGF-D | −0.044 | — | — |
| Age (years) | n/a | n/a | 0.042 |
| Hypertension | n/a | n/a | 0.495 |
| Diabetes mellitus | n/a | n/a | 0.348 |
bNGF = beta nerve growth factor; CCL = C-C motif chemokine ligand; CXCL = C-X-C motif chemokine ligand; EGF = epidermal growth factor; HGF = hepatocyte growth factor; IL1RA = interleukin 1 receptor antagonist; PDGFBB = platelet derived growth factor-BB; SCF = stem cell factor; sFasL = soluble Fas ligand; sICAM1 = soluble intercellular adhesion molecule-1; VEGF = vascular endothelial growth factor
Immune markers were log 2 transformed and standardized.
Features constrained from regression model are denoted by “—”.
Features included in z-score derivation are denoted by “†”.
Model 1 includes only immune markers.
Model 2 additionally includes age, sex, race, ethnicity.
Model 3 additionally includes hypertension, hyperlipidemia, diabetes mellitus, and smoking history.
Figure 2. Protein interaction network of selected immune marker features.
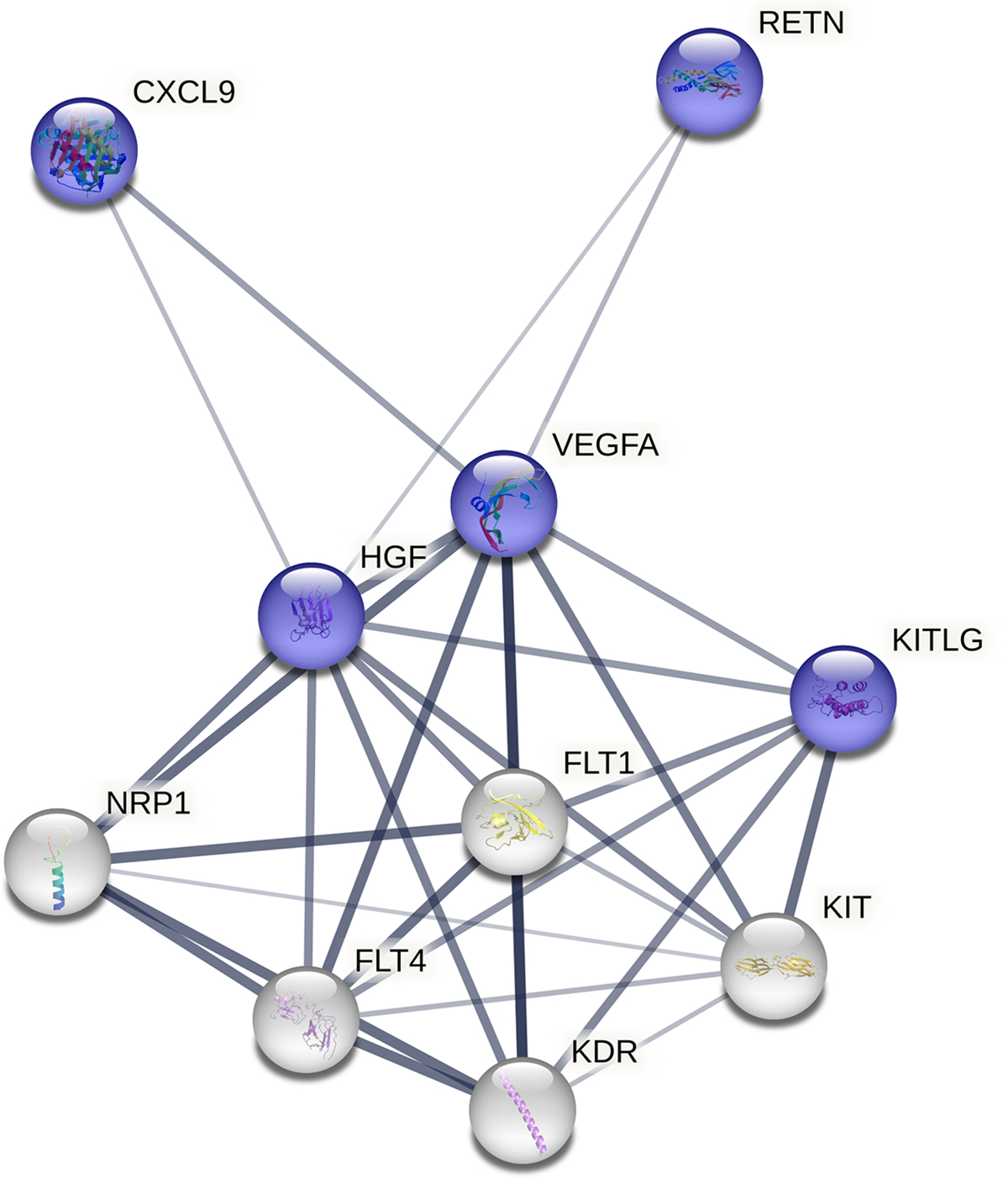
Blue colored nodes represent immune markers selected by regularized regression and white colored nodes represent predicted functional partners. Thickness of node-node line qualifies strength of association. CXCL9 = C-X-C motif chemokine 9; FLT1 = vascular endothelial growth factor 1; FLT4 = vascular endothelial growth factor receptor 3; HGF = hepatocyte growth factor; KDR = vascular endothelial growth factor receptor 2; KIT = mast/stem cell growth factor receptor kit; KITLG = kit ligand (stem cell factor); NRP1 = neuropilin-1; RETN = resistin; VEGFA = vascular endothelial growth factor A
Association of immune marker z-scores and ICAS with longitudinal outcomes
Over an average follow-up of 12 years (range 0.1–17 years), 324 (27%) participants suffered a vascular event and 454 (37%) individuals died. A total of 200 participants (17%) were classified with vascular death. Only 1.4% of included participants had an ischemic stroke during follow-up. Those with ≥50% ICAS experienced greater mortality, vascular death, and vascular events than those without ≥50% ICAS (Table 1).
Evaluating the association between the immune marker z-scores and ICAS with longitudinal outcomes, we found increased immune marker z-scores to be significantly associated with increased hazard of all three outcomes (Table 3). The relationship between immune marker z-scores and all longitudinal outcomes remained similar after adjustment for demographics and vascular risk factors. In fully adjusted models, each unit increase in immune marker z-scores was associated with an 8% (95% confidence interval (CI): 1.05–1.11), 11% (95% CI: 1.06–1.16), and 5% (95% CI: 1.01–1.09) increased hazard of death, vascular death, and any vascular event, respectively. When comparing ≥50% ICAS to not ≥50% stenosis, we found that stepwise inclusion of confounders attenuated the associations with longitudinal outcomes, such that ≥50% ICAS was significantly associated with increased hazard of death (hazard ratio (HR) [95% confidence interval, CI]: 1.47 [1.08–2.01]) and vascular death (HR [95% CI]: 1.94 [1.27–2.95]), but not vascular events (HR [95% CI]: 1.33 [0.91–1.93]) when compared to not ≥50% stenosis.
Table 3.
Cox regression models of selected immune marker z-score, intracranial large artery stenosis, and longitudinal outcomes
| Outcome | Characteristic | Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
Interaction term p-value |
|---|---|---|---|---|---|
| Any death (n=454) | Immune marker z-score | 1.09 (1.06–1.12) | 1.08 (1.05–1.12) | 1.08 (1.05–1.11) | 0.17 |
| ≥50% ICAS* | 2.10 (1.54–2.85) | 1.52 (1.12–2.07) | 1.47 (1.08–2.01) | ||
| Vascular death (n=200) | Immune marker z-score | 1.11 (1.07–1.15) | 1.12 (1.07–1.16) | 1.11 (1.06–1.16) | 0.33 |
| ≥50% ICAS* | 2.95 (1.95–4.45) | 2.06 (1.36–3.13) | 1.94 (1.27–2.95) | ||
| Any vascular event (n=324) | Immune marker z-score | 1.07 (1.04–1.11) | 1.06 (1.02–1.10) | 1.05 (1.01–1.09) | 0.72 |
| ≥50% ICAS* | 1.86 (1.29–2.69) | 1.39 (0.96–2.02) | 1.33 (0.91–1.93) |
HR = hazard ratio; CI = confidence interval; ICAS = intracranial larger artery stenosis
Reference not ≥50% ICAS.
Model 1 includes only immune marker z-score and ICAS.
Model 2 additionally includes age, sex, race, ethnicity.
Model 3 additionally includes hypertension, hyperlipidemia, diabetes mellitus, and smoking history.
We did not find a significant interaction between immune marker z-scores and ≥50% ICAS in their relationship with death (p=0.17), vascular death (p=0.33), or any vascular event (p=0.72).
Discussion
In this diverse stroke-free population, we identify several immune biomarkers associated with ICAS. The biomarkers associated with ICAS have known roles in chemotaxis and atherosclerosis-related angiogenesis.21–23 Notably, these immune markers are independently associated with future mortality and vascular events as a group. However, despite themselves being derived from ICAS, selected immune markers do not appear to have a relationship with ICAS on longitudinal outcomes, suggesting the pathways involving inflammation, ICAS, and vascular events may be more complex than modeled in the present analysis.
The results suggest that plasma immune biomarkers are associated with ICAS after adjustment for demographics and vascular risk factors. Several of the selected immune markers correlated well with previously studied inflammatory biomarkers. Though less specific, prior studies have similarly reported an independent relationship of plasma hs-CRP and uric acid to ICAS among stroke-free individuals.24–26 In symptomatic ICAS, hs-CRP, matrix metalloproteinases (MMPs), and IL-6 were linked to progression of intracranial stenosis after stroke,27,28 however this may represent a different population than the present cohort with asymptomatic ICAS.
Among the panel, individual markers associated with ICAS included CXCL9, HGF, resistin, SCF, and VEGF-A. CXCL9 is a member of the C-X-C chemokine subfamily, many of which are induced by interferon gamma (IFNg), and involved in Th1-type immune responses and T-cell tissue infiltration.29 Symptomatic extracranial carotid atherosclerosis may be characterized by increased CXCL9 expression within the plaque, in addition to other IFNg regulated chemokines.30 Similarly, resistin is an adipocytokine that contributes to atherosclerosis by chemoattracting macrophages into adipose tissue and intracellular activation of nuclear transcription factor-kappa B to cause secretion of various cytokines.21,31 Several longitudinal studies have demonstrated resistin to be associated with atherosclerotic cardiovascular diseases.32,33
HGF is a pleiotropic mesenchymal cytokine involved in the development of epithelial and endothelial cells, but has been linked to the progression of atherosclerosis, coronary heart disease, and stroke.22,34 Both SCF, also called kit ligand, and VEGF-A are growth factors involved in normal and pathologic angiogenesis, including atherosclerosis-related vascular remodeling.23 Injured tissue, such as ischemic brain, may stimulate VEGF signaling to activate MMP-9 in bone marrow and release SCF. Kit ligand then promotes recruitment and mobilization of progenitor hematopoietic and endothelial cells into circulation.23 Together, HGF, SCF, and VEGFA are involved in a network of molecules that contribute to angiogenesis, including vascular endothelial growth factors and neuropilin-1 (Figure 2). Selected chemokines, CXCL9 and resistin, appear loosely related to the network through HGF and VEGF. Jointly, VEGF and related proteomes may be important in the pathogenesis of ICAS.35,36
The composite of selected immune markers was independently associated with longitudinal vascular events. Interestingly, the modest associations with outcomes were not attenuated after controlling for demographics and vascular risk factors. Other studies, including several in the NOMAS cohort, have similarly shown associations between inflammatory markers and certain stroke outcomes had minimal attenuation in fully adjusted models.37–39 Our results reinforce a body of literature that inflammation may represent a source of stroke risk that is not completely explained by traditional vascular risk factors.40 The cause of inflammation, however, can be multifactorial and difficult to disentangle.
Indeed, though these immune markers are derived from asymptomatic ICAS and independently associated with longitudinal vascular events, they did not modify the ICAS associated mortality and event risk. There may be several plausible explanations for this finding. First, the selected biomarkers may represent an epiphenomenon of ICAS in the present analysis if they reflect systemic inflammatory burden and atherosclerosis, as previously discussed. Second, given the statistical power needed to detect interaction effects, our sample size may have been insufficient. Additionally, ischemic stroke constituted a small proportion of this subsample. Therefore, we may not have captured an interaction effect more directly related to ICAS. Third, ICAS by stenosis severity alone may not be sufficient in characterizing stenosis at “high risk” for events. Previous work in NOMAS and other community-based cohorts,1,41 asymptomatic ICAS of 50% stenosis and greater is associated with future vascular events, but not specifically ischemic stroke suggesting other features such as plaque characteristics, downstream hemodynamics, and collateral vessel status may be needed to predict stroke risk in subclinical ICAS.42–45 There is also the possibility that ICAS in our sample may represent non-atherosclerotic causes such as infectious arteriopathies, but atherosclerosis likely dominates as the underlying pathology given the study population’s older age, vascular comorbidities, and subclinical nature of ICAS.
Our results reinforce the potential importance of inflammation as a future therapeutic target in ICAS. Multiple lines of observational evidence have implicated inflammation in the pathogenesis of atherosclerosis, all causes of stroke, and cognitive function.4,10 Though no randomized controlled trial has been yet completed in stroke, colchicine, which has anti-inflammatory properties by inhibiting beta-tubulin polymerization, has been shown to reduce recurrent vascular events among those with myocardial infarction.46 In a meta-analysis of trials designed for the study of coronary artery disease, colchicine-treated participants had half the risk of stroke as control arms.47 The ongoing CONVINCE (Colchicine for Prevention of Vascular Inflammation in Noncardioembolic Stroke, NCT02898610)48 and CASPER (Colchicine After Stroke to Prevent Event Recurrence, ACTRN1261001408875) trials may add more conclusive safety and efficacy data for anti-inflammatory therapies in stroke.
Our study has limitations. First, both the immunoassay and MRA assessment of ICAS were determined at a single point in time, limiting our ability to make causal conclusions. An individual’s inflammatory profile changes over time due to complex interactions with numerous factors such as aging and development of intercurrent illness. Similarly, ICAS may also change over time in terms of stenosis severity. Though a study on the natural history of ICAS-related stroke found 70–80% of concomitant asymptomatic ICAS in the same individuals had static stenosis severity after 5 years,49 the risk for misclassification error in our study remains. Second, immune markers were not assessed in cerebrospinal fluid or cerebrovascular tissue, which may be more specific to intracranial arterial disease. Third, though the immunoassay panel included 60 markers, many other immune and inflammatory markers were not included. Fourth, we did not characterize other possible relevant features of ICAS beyond luminal stenosis. Future study may seek to incorporate multiple ICAS-related elements in addition to inflammatory markers to better predict high risk ICAS. Additionally, a small proportion of this NOMAS subsample had ischemic stroke events, limiting our longitudinal analyses. Lastly, the MRI subsample may not be completely generalizable to a community-dwelling population of older adults. Our study’s strengths include the multiethnic community-based population, the longitudinal design to detect vascular events with low rate of loss to follow-up, and the rigorous ascertainment of vascular outcomes.
In conclusion, we find serum immune markers associated with ICAS among a racially and ethnically diverse stroke-free population. Specifically, the selected cytokines are involved in chemotaxis and atherosclerosis-related angiogenesis. Importantly, these ICAS-derived markers are associated with future vascular events, and therefore, may represent potential therapeutic targets. However, future study is needed to better understand their exact role in pathogenesis of ICAS and vascular outcomes.
Supplementary Material
Sources of Funding
NOMAS was funded by the National Institutes of Health (NIH R01s NS029993, AG057709 and AG066162). DY is supported by NIH/NINDS (T32 NS007153).
Nonstandard Abbreviations and Acronyms
- ICAS
intracranial large artery stenosis
- NOMAS
Northern Manhattan Study
- LASSO
Least Absolute Shrinkage and Selection Operator
- CCL
C motif chemokine ligand
- CXCL
C-X-C motif chemokine ligand
- EGF
epidermal growth factor
- HGF
hepatocyte growth factor
- IL1RA
interleukin 1 receptor antagonist
- SCF
stem cell factor
- sICAM-1
soluble intercellular adhesion molecule-1
- VEGF
vascular endothelial growth factor
- PDGFBB
platelet derived growth factor-BB
- sFasL
soluble Fas ligand
- hs-CRP
high sensitivity C-reactive protein
- MMP
matrix metalloproteinases
- IFNg
interferon gamma
Footnotes
Disclosures
TR reported employment by the University of Miami and grant funding from NIH/NINDS outside of the submitted work. VDB reported grant funding from NIH outside of the submitted work. Outside of the present work, MSE reported receipt of royalties for chapters on stroke published in UpToDate and is an employee of the American Heart Association. JG reported employment by Columbia University, receiving grant funding from the Foundation for the NIH and NIH/National Institute of Aging (NIA), compensation from Trine Law Firm LLC for consultant services, compensation from John Astuno, Jr, LLC and Hanna, Campbell & Powell, LLP for expert witness services, receipt of royalties for chapters on intracranial atherosclerosis published in UpToDate.
References
- 1.Gutierrez J, Khasiyev F, Liu M, DeRosa JT, Tom SE, Rundek T, Cheung K, Wright CB, Sacco RL, Elkind MSV. Determinants and Outcomes of Asymptomatic Intracranial Atherosclerotic Stenosis. J Am Coll Cardiol. 2021;78:562–571. doi: 10.1016/j.jacc.2021.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, Waters MF, Lutsep H, Lopez-Cancio E, Turan TN, et al. Hemodynamic Markers in the Anterior Circulation as Predictors of Recurrent Stroke in Patients With Intracranial Stenosis. Stroke. 2018:STROKEAHA118020840. doi: 10.1161/STROKEAHA.118.020840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Ley K. Lymphocyte migration into atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2015;35:40–49. doi: 10.1161/ATVBAHA.114.303227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly PJ, Lemmens R, Tsivgoulis G. Inflammation and Stroke Risk: A New Target for Prevention. Stroke. 2021;52:2697–2706. doi: 10.1161/STROKEAHA.121.034388 [DOI] [PubMed] [Google Scholar]
- 5.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of Vascular Aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, Paik MC, Shea S. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.str.32.8.1725 [DOI] [PubMed] [Google Scholar]
- 7.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, Sacco RL. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70:425–30. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 9.Hornig M, Montoya JG, Klimas NG, Levine S, Felsenstein D, Bateman L, Peterson DL, Gottschalk CG, Schultz AF, Che X, et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv. 2015;1. doi: 10.1126/sciadv.1400121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkind MSV, Moon M, Rundek T, Wright CB, Cheung K, Sacco RL, Hornig M. Immune markers are associated with cognitive performance in a multiethnic cohort: The Northern Manhattan Study. Brain Behav Immun. 2021;97:186–192. doi: 10.1016/j.bbi.2021.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–255. doi: 10.1016/s0022-1759(00)00238-6 [DOI] [PubMed] [Google Scholar]
- 12.Breen EJ, Polaskova V, Khan A. Bead-based multiplex immuno-assays for cytokines, chemokines, growth factors and other analytes: median fluorescence intensities versus their derived absolute concentration values for statistical analysis. Cytokine. 2015;71:188–198. doi: 10.1016/j.cyto.2014.10.030 [DOI] [PubMed] [Google Scholar]
- 13.Breen EJ, Tan W, Khan A. The Statistical Value of Raw Fluorescence Signal in Luminex xMAP Based Multiplex Immunoassays. Sci Rep. 2016;6:26996. doi: 10.1038/srep26996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boden-Albala B, Roberts ET, Bazil C, Moon Y, Elkind MS, Rundek T, Paik MC, Sacco RL. Daytime sleepiness and risk of stroke and vascular disease: findings from the Northern Manhattan Study (NOMAS). Circ Cardiovasc Qual Outcomes. 2012;5:500–507. doi: 10.1161/CIRCOUTCOMES.111.963801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaefer EJ, Lamon-Fava S, Jenner JL, McNamara JR, Ordovas JM, Davis CE, Abolafia JM, Lippel K, Levy RI. Lipoprotein(a) levels and risk of coronary heart disease in men. The lipid Research Clinics Coronary Primary Prevention Trial. JAMA. 1994;271:999–1003. doi: 10.1001/jama.1994.03510370051031 [DOI] [PubMed] [Google Scholar]
- 16.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201 [DOI] [PubMed] [Google Scholar]
- 17.Yang D, Liu M, Willey JZ, Khasiyev F, Tom SE, Rundek T, Cheung YK, Wright CB, Sacco RL, Elkind MSV, Gutierrez J. Physical Activity Is Inversely Associated With Severe Intracranial Stenosis in Stroke-Free Participants of NOMAS. Stroke. 2023;54:159–166. doi: 10.1161/STROKEAHA.122.039660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 19.So Y, Lin G, Johnston G. Using the PHREG procedure to analyze competing-risks data. Paper/Poster presented at: SAS Global Forum; 2014; [Google Scholar]
- 20.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Askin L, Abus S, Tanriverdi O. Resistin and Cardiovascular Disease: A Review of the Current Literature Regarding Clinical and Pathological Relationships. Curr Cardiol Rev. 2022;18:e290721195114. doi: 10.2174/1573403X17666210729101120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, Fiaccavento R, Carotenuto F, De Vito P, Baldini PM, et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176 [DOI] [PubMed] [Google Scholar]
- 23.Heissig B, Werb Z, Rafii S, Hattori K. Role of c-kit/Kit ligand signaling in regulating vasculogenesis. Thromb Haemost. 2003;90:570–576. doi: 10.1160/TH03-03-0188 [DOI] [PubMed] [Google Scholar]
- 24.Zhang N, Xiang Y, Zhao Y, Ji X, Sang S, Shao S, Ma X, Wang G, Lv M, Xue F, et al. Association of triglyceride-glucose index and high-sensitivity C-reactive protein with asymptomatic intracranial arterial stenosis: A cross-sectional study. Nutr Metab Cardiovasc Dis. 2021;31:3103–3110. doi: 10.1016/j.numecd.2021.07.009 [DOI] [PubMed] [Google Scholar]
- 25.Su BJ, Dong Y, Tan CC, Hou XH, Xu W, Sun FR, Cui M, Dong Q, Tan L, Yu JT. Elevated Hs-CRP Levels Are Associated with Higher Risk of Intracranial Arterial Stenosis. Neurotox Res 2020;37:425–432. doi: 10.1007/s12640-019-00108-9 [DOI] [PubMed] [Google Scholar]
- 26.Ahn JK, Hwang J, Hwang JH, Yoon WT, Chung PW, Ryu S. The association between serum uric acid and asymptomatic intracranial arterial stenosis in middle-aged Koreans. Nutr Metab Cardiovasc Dis. 2018;28:14–22. doi: 10.1016/j.numecd.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 27.Shimizu K, Shimomura K, Tokuyama Y, Sakurai K, Isahaya K, Takaishi S, Kato B, Usuki N, Shimizu T, Yamada K, et al. Association between inflammatory biomarkers and progression of intracranial large artery stenosis after ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22:211–217. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 28.Arenillas JF, Alvarez-Sabin J, Molina CA, Chacon P, Fernandez-Cadenas I, Ribo M, Delgado P, Rubiera M, Penalba A, Rovira A, et al. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke. 2008;39:1456–1463. doi: 10.1161/STROKEAHA.107.498600 [DOI] [PubMed] [Google Scholar]
- 29.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shalhoub J, Viiri LE, Cross AJ, Gregan SM, Allin DM, Astola N, Franklin IJ, Davies AH, Monaco C. Multi-analyte profiling in human carotid atherosclerosis uncovers pro-inflammatory macrophage programming in plaques. Thromb Haemost. 2016;115:1064–1072. doi: 10.1160/TH15-08-0650 [DOI] [PubMed] [Google Scholar]
- 31.Henning RJ. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: a review of the pathophysiology and treatment of obesity. Am J Cardiovasc Dis. 2021;11:504–529. [PMC free article] [PubMed] [Google Scholar]
- 32.Cai X, Allison MA, Ambale-Venkatesh B, Jorgensen NW, Lima JAC, Muse ED, McClelland RL, Shea S, Lebeche D. Resistin and risks of incident heart failure subtypes and cardiac fibrosis: the Multi-Ethnic Study of Atherosclerosis. ESC Heart Fail. 2022;9:3452–3460. doi: 10.1002/ehf2.14064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frankel DS, Vasan RS, D’Agostino RB, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell EJ, Larson NB, Decker PA, Pankow JS, Tsai MY, Hanson NQ, Wassel CL, Longstreth WT Jr., Bielinski SJ. Hepatocyte Growth Factor Is Positively Associated With Risk of Stroke: The MESA (Multi-Ethnic Study of Atherosclerosis). Stroke. 2016;47:2689–2694. doi: 10.1161/STROKEAHA.116.014172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arenillas JF, Alvarez-Sabin J, Montaner J, Rosell A, Molina CA, Rovira A, Ribo M, Sanchez E, Quintana M. Angiogenesis in symptomatic intracranial atherosclerosis: predominance of the inhibitor endostatin is related to a greater extent and risk of recurrence. Stroke. 2005;36:92–97. doi: 10.1161/01.STR.0000149617.65372.5d [DOI] [PubMed] [Google Scholar]
- 36.Yu F, Lu J, Li Z, Zhou X, Zeng S, Zhan Q, Yuan M, Yang Q, Xia J. Correlation of Plasma Vascular Endothelial Growth Factor and Endostatin Levels with Symptomatic Intra- and Extracranial Atherosclerotic Stenosis in a Chinese Han Population. J Stroke Cerebrovasc Dis. 2017;26:1061–1070. doi: 10.1016/j.jstrokecerebrovasdis.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 37.Elkind MSV. Inflammation, atherosclerosis, and stroke. Neurologist. 2006;12:140–148. 10.1097/01.nrl.0000215789.70804.b0 [DOI] [PubMed] [Google Scholar]
- 38.Elkind MSV, Sciacca RR, Boden-Albala B, Rundek T, Paik MC, Sacco RL. Relative elevation in baseline leukocyte count predicts first cerebral infarction. Neurology. 2005;64:2121–2125. 10.1212/01.wnl.0000165989.12122.49 [DOI] [PubMed] [Google Scholar]
- 39.Folsom AR, Gottesman RF, Appiah D, Shahar E, Mosley TH. Plasma d-dimer and incident ischemic stroke and coronary heart disease: the Atherosclerosis Risk in Communities Study. Stroke. 2016;47:18–23. 10.1161/strokeaha.115.011035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grau AJ, Urbanek C, Palm F. Common infections and the risk of stroke. Nat Rev Neurol. 2010;12:681–694. 10.1038/nrneurol.2010.163 [DOI] [PubMed] [Google Scholar]
- 41.Hurford R, Wolters FJ, Li L, Lau KK, Kuker W, Rothwell PM, Oxford Vascular Study Phenotyped C. Prevalence, predictors, and prognosis of symptomatic intracranial stenosis in patients with transient ischaemic attack or minor stroke: a population-based cohort study. Lancet Neurol. 2020;19:413–421. doi: 10.1016/S1474-4422(20)30079-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G, Frankel M, Chimowitz MI, Group WS. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69:2063–2068. doi: 10.1212/01.wnl.0000279338.18776.26 [DOI] [PubMed] [Google Scholar]
- 43.Ryoo S, Lee MJ, Cha J, Jeon P, Bang OY. Differential Vascular Pathophysiologic Types of Intracranial Atherosclerotic Stroke: A High-Resolution Wall Magnetic Resonance Imaging Study. Stroke. 2015;46:2815–2821. doi: 10.1161/STROKEAHA.115.010894 [DOI] [PubMed] [Google Scholar]
- 44.Amin-Hanjani S, Turan TN, Du X, Pandey DK, Rose-Finnell L, Richardson D, Elkind MS, Zipfel GJ, Liebeskind DS, Silver FL, et al. Higher Stroke Risk with Lower Blood Pressure in Hemodynamic Vertebrobasilar Disease: Analysis from the VERiTAS Study. J Stroke Cerebrovasc Dis. 2017;26:403–410. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, Chimowitz MI, Warfarin-Aspirin Symptomatic Intracranial Disease I. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–974. doi: 10.1002/ana.22354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 47.Fiolet ATL, Opstal TSJ, Mosterd A, Eikelboom JW, Jolly SS, Keech AC, Kelly P, Tong DC, Layland J, Nidorf SM, et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2021;42:2765–2775. doi: 10.1093/eurheartj/ehab115 [DOI] [PubMed] [Google Scholar]
- 48.Kelly P, Weimar C, Lemmens R, Murphy S, Purroy F, Arsovska A, Bornstein NM, Czlonkowska A, Fischer U, Fonseca AC, et al. Colchicine for prevention of vascular inflammation in Non-CardioEmbolic stroke (CONVINCE) - study protocol for a randomised controlled trial. Eur Stroke J. 2021;6:222–228. doi: 10.1177/2396987320972566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu WS, Park SS, Kim YS, Lee SH, Kang K, Kim C, Sohn CH, Lee SH, Yoon BW. Long-term natural history of intracranial arterial stenosis: an MRA follow-up study. Cerebrovasc Dis. 2014;38:290–296. doi: 10.1159/000367587 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


