Abstract
The secreted phospholipases A2 (sPLA2s) comprise a family of small secreted proteins with the ability to catalyze the generation of bioactive lipids through glycophospholipid hydrolysis. Recently, a large number of receptor proteins and extracellular binding partners for the sPLA2s have been identified, suggesting that these secreted factors might exert a subset of their broad spectrum of biological activities independently of their enzymatic activity. Here, we describe an activity for the sPLA2 group XII (sPLA2-gXII) gene during Xenopus laevis early development. In the ectoderm, sPLA2-gXII acts as a neural inducer by blocking bone morphogenetic protein (BMP) signaling. Gain of function in embryos leads to ectopic neurogenesis and to the specification of ectopic olfactory sensory structures, including olfactory bulb and sensory epithelia. This activity is conserved in the Drosophila melanogaster, Xenopus, and mammalian orthologs and appears to be independent of the lipid hydrolytic activity. Because of its effect on olfactory neurogenesis, we have renamed this gene Rossy, in homage to the Spanish actress Rossy de Palma. We present evidence that Rossy/sPLA2-gXII can inhibit the transcriptional activation of BMP direct-target gene reporters in Xenopus and mouse P19 embryonic carcinoma cells through the loss of DNA-binding activity of activated Smad1/4 complexes. Collectively, these data represent the first evidence for signaling cross talk between a secreted phospholipase A2 and the BMP/transforming growth factor β pathways and identify Rossy/sPLA2-gXII as the only factor thus far described which is sufficient to induce anterior sensory neural structures during vertebrate development.
The patterning of embryonic germ layers involves the interplay of bone morphogenetic protein (BMP) and transforming growth factor β (TGFβ) signaling pathways, which act to specify distinct cellular fates prior to and during gastrulation (17, 37, 41). BMP and TGFβ ligands signal through the activation of the receptor-associated Smad proteins (37). BMP signaling differentially promotes Smad1/5/8 phosphorylation, and TGFβ ligands mainly activate Smad2 and Smad3. Receptor-activated Smads bind to Smad4 and exert their activities through an interaction with cell- and tissue-specific transcription factors that act to direct the specificity of target genes (37). During gastrulation, signaling mediated by BMPs is critical in dorsoventral fate specification in all germ layers (44). In the ectoderm, the output of BMP signaling modulates the choice between epidermal and neural fates, and BMP inhibition constitutes the hallmark of neural fate acquisition (41, 63). Many distinct direct neural inducing molecules have been identified in Xenopus laevis, and they all share the common property of inhibiting BMP signaling (41). Among the neural inducers, many act intracellularly to inhibit Smad activation (such as Smad7 [6, 20, 47]), compete for binding to Smad4 (such as Smad6 [18]), degrade active Smad complexes via the proteosome (such as the Smurfs [12, 27]), and inactivate Smad complexes through direct binding (such as Ski [66]) or through recruitment of transcriptional corepressors (such as Ski, SnoN, and TGIF [49, 55, 60, 64, 66]). The activity of such inhibitors is modulated through the influence of various signaling pathways that fine-tune the responsiveness of a cell to BMP and TGFβ signaling (37). During development, the interpretation and translation of the BMP morphogen gradient into distinct cellular fates are poorly understood, although it is likely that signaling integration through the modulation of such negatively acting factors plays a critical role during cell fate specification (44).
Phospholipases A2 (PLA2s) are enzymes that catalyze the hydrolysis of glycerophospholipids to yield fatty acids and lysophospholipids and are considered key enzymes in the generation of biologically active lipids during inflammation (46, 54, 58). Within the PLA2 superfamily, two main classes of enzymes have been identified based on their subcellular distribution (54, 58): the cytosolic PLA2s (46) and a large number of molecules that are released into the extracellular space and are therefore termed the secreted PLA2s (sPLA2s) (8, 9, 14, 56, 58). These secreted molecules are Ca2+-dependent phospholipases, disulfide-rich proteins of 14 to 18 kDa. Current evidence suggests that the biology of the sPLA2s is complex, both in the types of responses they elicit as well as in their mechanism of action. It has been postulated that the biological activity of this class of secreted proteins might involve receptor-activation and signaling in a fashion independent of their enzymatic activity (14). Two biochemically distinct classes of receptors (M and N types [2, 9, 48]) and a variety of extracellular binding proteins have been identified (58), suggesting that the sPLA2s might signal through receptor activation. However, there is no conclusive evidence that the bioactivities of the sPLA2s might arise from two distinct mechanisms, one mediated through lipid hydrolysis and one mediated through direct activation of a receptor.
Recently, a novel sPLA2 has been characterized as an independent class within the sPLA2s (14). This molecule, termed sPLA2-gXII (for group XII), is a highly conserved protein that retains the active-site histidine and the histidine and aspartic acid catalytic dyad found in other sPLA2s (see Fig. 2) (45). Although this protein retains weak lipid hydrolyase activity (14), both the pattern of cysteines outside the active site and the Ca2+-binding loop are quite distinct from other sPLA2s. Furthermore, the unique distribution of human sPLA2-gXII suggests that it might fulfill specific functions (14). As part of a gain-of-function screen aimed at identifying factors with embryological activities during Xenopus laevis development, we discovered that sPLA2-gXII modulates germ layer specification. Overexpression of mouse, Drosophila melanogaster, and Xenopus orthologs of sPLA2-gXII in the prospective neural territory leads to ectopic neurogenesis and to the induction of ectopic olfactory sensory structures, including olfactory bulb and sensory epithelium. Because of the effects of sPLA2-gXII on ectopic olfactory generation, we renamed sPLA2-gXII “Rossy” (after Pedro Almodovar's actress Rossy de Palma, most likely the “most famous nose in Spain”).
FIG. 2.
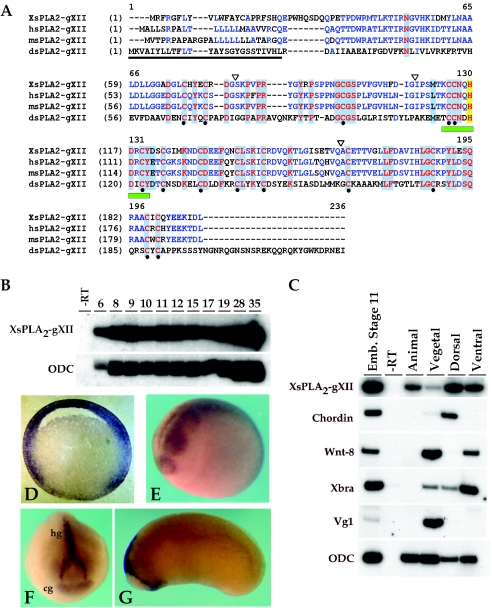
Sequence alignment of Rossy orthologs and expression of Xenopus Rossy mRNA. (A) Alignment of Xenopus, human, mouse, and Drosophila cDNAs. Identical residues are highlighted in red. Notice conservation in the 14 cysteines (black dots) and the phospholipase active site (green box) (24). Signal sequence is underlined. Exon-intron junctions are marked by inverted triangles. (B) Temporal expression of xRossy by RT-PCR. Numbers are embryonic stages. Stage 6.5 corresponds to maternal transcripts. (C) RT-PCR analysis of dissected embryos at stage 10.5. Chordin, dorsal mesoderm; Wnt-8, ventrolateral mesoderm; Xbra, pan-mesoderm; Vg-1, endoderm; ODC (ornithine decarboxylase), loading control. (D to G) Expression of xRossy by in situ hybridization at (D) stage 10.5, (E) stage 19 to 20, and (F and G) tadpole stages. Notice expression in the animal pole in panel D, neural tissue in panel E, and hatching (hg) and cement glands (cg) in panels F and G. The section in panel D is 50 μm.
In this report, we describe the characterization of Rossy's bioactivities in the ectoderm. Expression of Rossy in animal cap explants promotes neural marker expression. Molecular analysis suggests that signaling mediated by Rossy in Xenopus embryos and explants arises from the modulation of BMP and TGFβ pathways. Rossy leads to a specific inhibition of the BMP pathway in Xenopus embryos and in mouse P19 embryonic carcinoma cells. This inhibition occurs at the nuclear level by interfering with the DNA-binding activity of Smad1/Smad4 complexes, as judged by binding to the BMP-responsive element (BRE) in P19 cells. The selective inhibition of Smad1/4 activity and the neurogenic effects constitute the first demonstrated developmental role for a secreted PLA2.
MATERIALS AND METHODS
Cloning of Xenopus Rossy (xRossy).
Degenerate primers to amplify amino acids 108 to 190 were as follows: 5′-TAGACGAATTCTGYTGYAAYCARCAYGA-3′ (residues CCNQHD) and 5′-TAGACCTCGAGRTCNGTYTTYTCYTCRTA-3′ (encoding residues YEEKTD). PCRs were performed as follows: 95°C for 3 min; 5 cycles of 95°C for 1 min, 45°C for 2 min, and 72°C for 3 min; 30 cycles of 95°C for 1 min, 56°C for 1 min, and 72°C for 1 min; and a final extension step for 10 min at 72°C. The degenerate PCR product was used to obtain full-length clones from a gastrula stage cDNA library (a gift of R. Harland).
Embryonic expression of xRossy.
Expression of xRossy by in situ hybridization with xRossy digoxigenin riboprobes was performed as described previously (16). Reverse transcription (RT)-PCR analysis of xRossy was performed with 5′-GACTGTGATGAGGAGTTTCAG-3′ (forward) and 5′-CTCCAGGTAAGGTTTACATCC-3′ (reverse).
Embryo manipulations and RNA injections.
Capped mRNAs were microinjected with (per embryo) xRossy (250 pg to 1 ng), bmp-4 (1 ng), Smad-1 (1 ng), Smad-4a (1 ng), and OAZ (100 pg).
Plasmids and constructs.
Full-length Xenopus sPLA2-gXII open reading frame was amplified by PCR and subcloned into the pCS2++ vector. Drosophila sPLA2-gXII was subcloned into pCS2++ by PCR. The point mutant H113E version of mouse sPLA2-gXII was engineered by PCR with a QuickchangeM kit (Stratagene) following the manufacturer's instructions. C-terminal hemagglutinin (HA)-tagged mouse sPLA2-gXII was performed by conventional PCR.
Expression analysis of human Rossy in embryonic stem cells.
Human H1 embryonic stem (ES) cells (WiCell, Wis.) were grown in feeder-free conditions as described previously (51). Primers for human Oct3/4 and glyceraldehyde-3 phosphate dehydrogenase were as described previously (52). Primers for human Rossy were (sense) GACGGATCTAAGCCTTTCCC and (antisense) CCACTGTTGTTTCACATGCC.
Luciferase reporter experiments.
In animal cap experiments, embryos were injected with 25 pg to 50 pg of reporters together with various RNAs in one blastomere at the four-cell stage (BRE4-luciferase) or the dorsal marginal zone (ARE3-luciferase, TOP-FLASH, Goosecoid-luciferase, or Brachyury-luciferase). Assays were measured in triplicate, and embryos were lysed at stage 11 using the Promega luciferase reporter assay kit. In P19 cells, cells were transfected in 12 wells with reporters (150 ng/well) and renilla luciferase (3.5 ng/well), together with various cDNAs using Invitrogen Lipofectamine 2000 reagent. Cells were exposed to 10 ng/ml of recombinant BMP4 (rhBMP4) or activin proteins (R&D Systems) 18 h prior to luciferase measurements using the Promega dual luciferase kit.
Smad1 activity and oligonucleotide pulldown experiments.
Smad1 phosphorylation in P19 cells was measured 30 min following exposure to rhBMP4 with a polyclonal antibody against phospho-Smad1 (Upstate Biotechnology). Anti-Smad antibodies were from Upstate Biotechnology. For the oligonucleotide pulldown experiments, we made 5′-biotinylated BRE oligonucleotides (from IT-DNA) encoding the wild type or a 3′ box mutant BRE (19). Oligonucleotides were annealed to form double strands and incubated with the extracts as described previously (19).
Microarray analysis.
Microarrays with 5,000 cDNAs from a gastrula stage library were performed as described previously (43). One nanogram of Rossy RNA was injected into the animal pole at the two-cell stage, and RNAs were harvested at stage 10.5. Results represent data obtained from four data points (two microarray experiments with inverse dye hybridizations). Primers used for RT-PCR are as follows (5′ to 3′): clone 8-G4/Akt-like, (S) GTTTTTACAGAGGACAGAGCACG and (AS) CAAATAACCTCTCATGGTCTTGG; clone 22-A2/xSall1, (S) CAAACTCAATACTTCCCTCAACG and (AS) CTGGAAGAAGAGAATGATTTCCC; clone 29-E12/calreticulin, (S) AGTAAAAGCGATAAGTAACCGGC and (AS) AACAGGCACCCATTTATAGATCC; clone 34-A8/Bcl-7b, (S) TGGATTTATATTTCGGGCTAAGG and (AS) 5′-AAAGTTCTGCATGGCAGTATAGC.
RESULTS
Biological activity of Rossy during early Xenopus development.
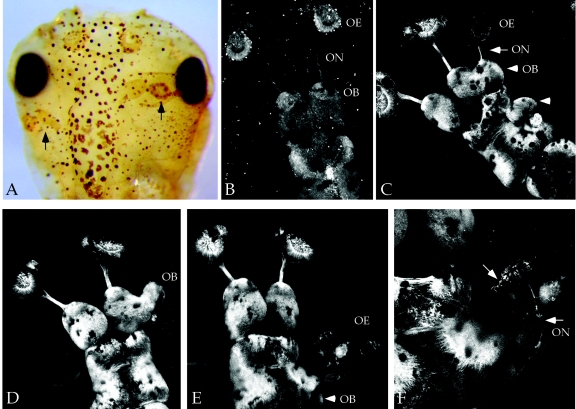
Secreted molecules play key instructive and inhibitory roles during development, modulating the extent of the signaling events that regulate early germ layer specification and patterning. In an effort to identify novel factors that affect early fate decisions in Xenopus, we overexpressed a collection of mouse RNAs encoding secreted factors at the four-cell stage. One clone, sPLA2-gXII, consistently led to patterning defects. Embryos injected at the eight-cell stage in one dorsal animal blastomere (prospective neural ectoderm) showed ectopic neural tissue, as judged by immunostaining with neural-specific antibodies (Fig. 1A). Remarkably, the ectopic neural tissue resembled well-organized sensory structures, although ectopic neurons were also observed away from the central nervous system. In order to verify these effects, we injected Rossy RNA into transgenic frogs expressing tau-green fluorescent protein under the control of the neuronal β-tubulin promoter (36). In these experiments, injections in one dorsal animal blastomere at the eight-cell stage led to ectopic telencephalic structures (Fig. 1C), which included well-differentiated olfactory bulbs and sensory epithelia in roughly 10% of injected embryos (Fig. 1C to F). The frequency of duplicated sensory epithelia was less than that of olfactory bulbs, and we never observed sensory-like structures in the absence of a morphologically distinct bulb. However, in several cases, we detected an evagination of ectopic olfactory bulbs in the absence of ectopic olfactory nerves (Fig. 1D). Interestingly, ectopic olfactory structures were observed arising from the midbrain, suggesting that Rossy's activity transformed the fate of emerging neural tissue (Fig. 1E to G).
FIG. 1.
Expression of Rossy in prospective neural ectoderm promotes neurogenesis and olfactory sensory development in Xenopus. Phenotypes of Rossy RNA injections (500 pg) in a dorsal animal blastomere at the eight-cell stage are shown. (A) Neural tissue staining with 6F11 antibody (Developmental Hybridoma, Iowa) reveals ectopic neuronal structures (arrows). This embryo received injections in both dorsal animal blastomeres. (B to F) Composite Z-stack confocal images of transgenic green fluorescent protein-β-tubulin embryos (27). (B) Uninjected embryo. (C to F) Embryos injected into dorso-animal blastomere. (C) Arrowheads point to ectopic olfactory bulbs. The arrow points to ectopic olfactory sensory epithelia and nerve innervating the secondary bulb on the injected side. (D) Notice ectopic bulb evagination. (E and F) Ectopic structures in the midbrain, including sensory epithelia (arrowhead). Arrows in panel F point to neurons in ectopic epithelia and ectopic nerve. ON, olfactory nerve; OB, olfactory bulb; OE, olfactory epithelium.
To test whether Rossy's neurogenic effects required catalytic activity, we eliminated the active-site histidine, a residue required for lipid hydrolysis (H130E in mouse Rossy [mRossy] [14, 45]). We find that all the embryonic activities are maintained in this point mutant (see below). The effects of this mutant in olfactory generation did not differ from those of the wild-type construct, either in the frequency or in morphological appearance (not shown). This evidence strongly suggests that the neurogenic effects of Rossy are independent of its lipid hydrolytic activity.
Embryonic expression of Xenopus Rossy.
In order to assess the embryonic expression and function of Rossy, we cloned Xenopus Rossy. This gene is conserved phylogenetically, and the open reading frame is composed of four exons, as identified in genomic database searches. The conservation between the frog and Drosophila cDNAs is considerably lower (26% identity), the homologies stretching to the active site and Ca2+-binding pocket (14) (Fig. 2A). Interestingly, the 14 cysteines are conserved evolutionarily, suggesting that structural conservation, rather than primary sequence, is critical for its function. Indeed, the Drosophila ortholog displays similar activities in all assays tested.
We determined Rossy's mRNA distribution by RT-PCR and whole-mount in situ hybridization. Transcripts are detected maternally and throughout early development (Fig. 2B). In the gastrulating embryo, xRossy is expressed in the ectoderm and animal pole, dorsal and ventral marginal zone mesendoderm, and, more weakly, the vegetal pole (Fig. 2C). This expression is corroborated by in situ hybridization during gastrula stages (Fig. 2D). xRossy is also expressed at neurula stages in the anterior neural plate (Fig. 2E to G), and it becomes restricted to cement and hatching glands, tissues known to be sensitive to BMP signaling (13, 22).
Neuralizing activity of Rossy in ectodermal explants.
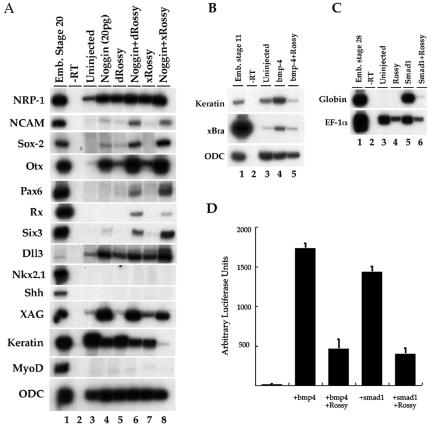
Although complex, the Rossy gain-of-function phenotypes can be associated with genes that modulate the BMP pathway. Because of the ectopic anterior neural structures, we investigated whether there is a molecular interaction between the activity of Rossy and this pathway. We analyzed cell fate changes in ectodermal explants injected with xRossy RNA. Indeed, xRossy induced, albeit weakly, Otx1/2 (a telencephalic marker), XAG (cement gland), and NRP-1 (pan-neural) and decreased epidermal keratin (not shown) without inducing mesendodermal genes. By these criteria, Rossy directly neuralizes the ectoderm, suggesting an inhibitory interaction between Rossy and BMP signaling. Next, and because Rossy acts as a weak neural inducer, we tested whether it could potentiate the effect of low doses of noggin (20 pg) in animal caps. Indeed, both the Drosophila and Xenopus orthologs can synergize with noggin, as judged by the expression of Sox-2, NCAM, and NRP-1 and of the anterior neural markers Otx1/2, Pax6, Six3, and Rx (Fig. 3A). Interestingly, we never detected expression of ventral markers such as Nkx2.1 or Shh, suggesting that the type of neural tissue induced by Rossy is dorsal. On their own, xRossy and Drosophila Rossy weakly induced xDll-3 (mDlx-5 ortholog), a gene required for the generation of olfactory structures (35). Overall, the effects of Rossy in the explants and in vivo suggested that its effects could be due to an interference with BMP signaling.
FIG. 3.
Rossy directly neuralizes animal cap explants and synergizes with noggin in anterior neural fate specification. (A) Injection of Drosophila and Xenopus Rossy RNA (500 pg to 1 ng) induces expression of NRP-1 (pan-neural), XAG (cement gland), and Otx1/2 (anterior neural). Both clones synergize with low doses of noggin (20 pg) in the induction of NCAM and Sox-2 and of Pax6, Rx, and Six3 (anterior neural). MyoD, muscle. (B and C) Interaction of Rossy with BMP signaling. Rossy (500 pg) was coinjected in the animal pole with bmp-4 (1 ng; B) or Smad-1 (1 ng; C). RT-PCR analysis was performed at stage 11 (B) or stage 28 (C). Rossy expression blocked Bra (mesoderm) and globin (a marker of blood) induction in the animal caps following BMP signaling. Ornithine decarboxylase (ODC) and EF-1α are loading controls. (D) Rossy blocks activation of a BRE (BRE4) luciferase reporter, even when coinjected with Smad1 (D).
To test this hypothesis, we microinjected RNAs for Rossy and bmp4 or Smad-1 (3) in the animal pole at the two-cell stage (Fig. 3B and C). Activation of the BMP pathway in animal caps leads to ventral mesoderm formation during gastrulation and blood development during tadpole stages (33) (Fig. 3B and C). Coinjection of Rossy abolished the induction of brachyury and the upregulation of epidermal keratin by bmp4 in gastrula stage explants (Fig. 3B), and it inhibited the induction of globin by Smad-1 in tadpoles (Fig. 3C), suggesting that Rossy can inhibit the BMP pathway downstream of receptor activation. In order to confirm these results, we tested whether injection of Rossy could affect the activation of a BMP-responsive promoter, the BRE (19). When injected into the animal cap, the activity of a BRE4-luciferase reporter can be stimulated by coinjection with bmp4 or Smad1 RNAs (Fig. 3D). Coexpression of Rossy leads to a marked decrease in reporter activity, even in the presence of Smad1, suggesting that its effects suppressed BMP signaling intracellularly. The inhibitory effects of Rossy were selective for the BRE, since the activity of a Bra-luciferase reporter was unchanged, as were other reporters such as the Smad-dependent goosecoid-luciferase promoter and the Wnt-sensitive reporter, TOP-FLASH (not shown; 7, 32). Interestingly, the activity of the TGFβ-sensitive activin response element, ARE3-luciferase (34), increased severalfold when Rossy was injected in the dorsal marginal zone at the four-cell stage (not shown), consistent with a selective effect of Rossy on BMP/TGFβ pathways. The effect of Rossy on the ARE3 reporter in Xenopus could be due to a modulation of TGFβ target genes or to the inhibition of the BMP pathway through competition for the available Smad4 pool (37, 42).
Induction of ectopic in vivo anterior neural marker expression by Rossy.
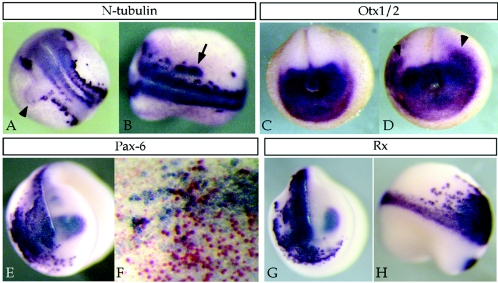
The effects of Rossy on animal caps and in olfactory generation reflected a strong activity of Rossy on emerging neural tissue. Therefore, we monitored the expression of various neural markers in embryos injected with Rossy. The effects were more pronounced on dorsal anterior markers, where Rossy led to ectopic or expanded expression of Pax6, Rx, Six3, and Otx1/2 (Fig. 4 and not shown). The effects of Rossy led to a mediolateral expansion of anterior neural markers and N-tubulin (Fig. 4) as well as posterior expansion of the anterior markers Otx1/2 and Rx (Fig. 4C, D, G, and H). This occurred at the expense of cranial crest or placodal gene expression (not shown), consistent with the BMP inhibition model, whereby neural fates are promoted at the expense of peripheral fates (37, 42). The gain of Pax6 expression (a dorsal marker [53]) and loss of Xash1 (a ventral marker [not shown]) also confirm the specification of dorsal neural tissue formed by Rossy in the explants.
FIG. 4.
Effects of Rossy on neural marker expression in vivo. Albino embryos were injected into one dorsoanterior blastomere at the four-cell stage, and embryos were processed for in situ hybridization. (A and B) Mediolateral expansion (arrowhead in panel A) and ectopic expression of N-tubulin (arrow in panel B). (C) Otx1/2 expression in a control embryo and in an embryo injected on both sides with Rossy RNA (D). Arrowheads point to mediolateral and posterior expansion of Otx expression. (E) Upregulation of Pax6 in injected side. The embryo in panel F was coinjected with xRossy and β-galactosidase, stained for LacZ activity (red), and processed for Pax6 in situ expression. Most injected cells (expressing LacZ) do not express Pax6, suggesting a non-cell-autonomous effect on Pax6 expression. (G and H) Expansion of Rx expression.
Epistatic analysis of Rossy signaling.
In order to dissect the biochemical mechanism of Rossy signaling and its specificity, we addressed its ability to modify various components of the BMP/TGF-β pathways and basic fibroblast growth factor (bFGF)/Ras, Wnt, or Stat3 signaling in animal cap assays. We evaluated the responsiveness of the animal caps to bFGF or TGFβ activation, in the case of mesoderm induction (3, 29, 62), and Wnt signaling. Ectodermal explants were dissected at blastula (stage 9) stages and incubated with recombinant bFGF or activin, and mesendodermal markers were monitored. Rossy mildly enhances the effects of bFGF in the explants, as judged by the expression of Bra and Wnt-8 (Fig. 5). In activin-induced mesoderm, Rossy markedly changed the responsiveness of the explants (Fig. 5). Similar results were observed in explants injected with Xnr-1, another TGFβ ligand (not shown). Rossy decreased ventrolateral mesodermal expression upon activin exposure, as judged by Bra (pan-mesodermal), Wnt-8 (ventral), and Hb-9 (lateral). Interestingly, expression of both cardiac actin (muscle) and collagen type II (notochord) were downregulated, whereas Hex and Cerberus (anterior endoderm) were upregulated. By contrast, expression of chordin was unchanged, suggesting a selective effect on mesodermal gene expression. These experiments suggest that Rossy alters the responsiveness of ectodermal cells to TGFβs and supports that Rossy acts to modulate BMP/TGFβ pathways. In contrast, coinjection of Rossy with Xwnt-8 did not affect the induction of direct Wnt target genes Xnr-3 and Sia (not shown) or the levels of activated Ras or Stat3 signaling (not shown), suggesting that the effects of Rossy are specific for BMP/TGFβ pathways.
FIG. 5.

Specificity of Rossy activity in the ectoderm and change in the responsiveness of the animal cap cells to bFGF and TGFβs. Embryos (Emb.) were injected at the two-cell stage, and explants were cultured with bFGF (100 ng/ml) or activin oocyte-conditioned medium. RT-PCR analysis of animal caps treated with bFGF (lanes 5 to 6) or activin (lanes 7 to 8) is shown. bFGF exposure leads to ventral mesodermal fates, as judged by Brachyury and Wnt8 expression. Rossy weakly enhanced these markers (lane 6). In activin-treated caps, notice the decrease in cardiac actin (muscle), brachyury, and collagen (Coll.) type II (notochord) and the increase in Hex and Cerberus (anterior endoderm) and Otx 1/2 (anterior neural). CG, cement gland; Slug, neural crest; Hb9, lateral mesoderm; ODC, ornithine decarboxylase.
Rossy inhibits BMP signaling in P19 mouse embryonic carcinoma cells.
In order to address whether Rossy modulates BMP/TGFβ signaling in other contexts, we tested Rossy's activities in mammalian cells. Interestingly, we found that the mammalian genes are strongly expressed in human, primate, and mouse undifferentiated ES cells (51), as judged by RT-PCR analysis (Fig. 6A) and in the transcriptional profile of human ES cells (51). Similarly, mRossy is expressed in P19 mouse embryonic carcinoma cells. P19 cells were transfected with Rossy cDNA, and the activity of several BMP-sensitive reporters was assayed. As in Xenopus, mRossy inhibited the effects of Bmp4 on Vent-1 and BRE4-luciferase reporters, even in cells cotransfected with Smad1 and Smad4 and the transcriptional coactivator OAZ (19) (Fig. 6B and C). In these assays, Drosophila, Xenopus, and murine Rossy genes showed similar effects (Fig. 6 and not shown). Furthermore, the point mutant lacking the active-site histidine retained complete reporter inhibitory activity (Fig. 6C). By contrast, Rossy did not inhibit the TGFβ-responsive reporter 3TP-lux (5) (Fig. 6D) in the presence of activin, suggesting that the effect of Rossy is specific for BMP-responsive promoters.
FIG. 6.
Transfection of Rossy in P19 mouse embryonic carcinoma cells inhibits a variety of BMP-responsive promoters. (A) Expression of human Rossy in H1 pluripotent embryonic stem cells (WiCell) as measured by RT-PCR. Oct3/4, pluripotent ES cell marker; glyceraldehyde-3-phosphate dehydrogenase (3GPD), loading control. (B to E) Luciferase reporter assays were performed in P19 cells 48 h after transfection. Rossy transfection blocked the activation of xVent-1-luciferase (B) and BRE4-luciferase (C) even in the presence of Smad1, Smad4, and OAZ. In C, the active-site mutant mRossy(H-E) retains complete BMP-inhibitory activity. (D) Rossy expression did not inhibit the TGFβ-responsive promoter 3TP-lux. (F) The mTlx2 promoter does not require OAZ for activation, and it can also be inhibited by Rossy. (E) Lack of effect of Rossy on the pGL-P reporter.
Activation of the BRE element requires the activity of OAZ (19). However, OAZ is required only for a subset of BMP target genes (19). For instance, regulation of the mouse Tlx2 (mTlx2) gene is not mediated through OAZ (19, 57). Therefore, we tested the effects of Rossy on an mTlx2 reporter. mRossy also blocked BMP4 stimulation of the Tlx2 promoter (Fig. 6E). Additionally, the endogenous activity of this reporter was lowered (Fig. 6E). In this experiment, OAZ reduced the activation of the Tlx2 reporter because it competes for binding of the Smads and is used as a control for the responsiveness of the reporter (Fig. 6E). Indeed, OAZ and Rossy cooperated in inhibiting the responsiveness of the reporter upon BMP stimulation. This result suggested a more general effect mediated by Rossy in the inhibitory modulation of BMP signaling and ruled out an inhibitory mechanism dependent on OAZ regulation. Additionally, we also tested whether Rossy could affect pGL-P, a Smad-independent, OAZ-dependent promoter (19), which can be activated by OAZ/Olf complexes (19). Olf is a transcription factor implicated in olfactory neurogenesis (59). In these experiments, Rossy did not enhance activation of this promoter, suggesting that recruitment of OAZ to non-Smad-dependent promoters via Olf is unlikely to be the mechanism by which Rossy inhibits the BMP pathway (Fig. 6F).
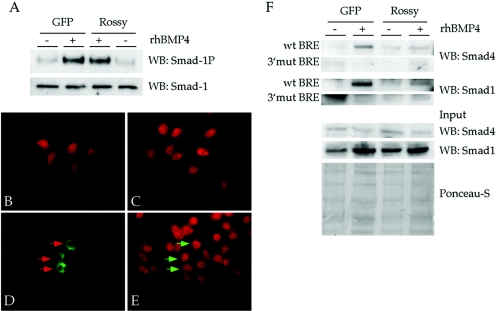
The effects of Rossy on the BMP promoters can arise from a change in the specificity of Smad1/4 complexes towards various binding partners and the choice of promoters and not necessarily on a direct transcriptional repression. In order to address at which step of the pathway Rossy acts, we monitored Smad1 phosphorylation, nuclear translocation, and DNA-binding activity in P19 cells (Fig. 7). We find that transfection of mRossy DNA does not affect Smad1 activation or the nuclear translocation of phosphorylated Smad1 (Fig. 7A to E). In order to monitor the binding of Smad complexes to DNA, we tested whether endogenous Smads bound a single BRE in oligonucleotide pulldown experiments (Fig. 7F) (19). In these experiments, BMP4 leads to binding of Smad1 and Smad4 to the BRE (Fig. 7F). Binding is abolished when extracts are incubated with a point mutant BRE (3′ mut) (19). In the presence of Rossy, binding to the BRE is inhibited (Fig. 7F), as detected by the lack of interaction of Smad1 and Smad4 with the BRE. This suggests that the mechanism of inhibition of Rossy is due to an inability of activated Smad1/4 complexes to bind their cognate sites.
FIG. 7.
Mechanism of BMP inhibition mediated by Rossy signaling in P19 cells. (A) Smad1 is phosphorylated in P19 cells expressing mRossy. GFP, green fluorescent protein; WB, Western blot. (B to E) Nuclear translocation of phosphorylated Smad1 following BMP4 stimulation is unaltered in P19 cells expressing an HA-tagged mRossy (C). (B) Unstimulated cells. (C) Cells stimulated with rhBMP4 protein for 30 min. (D and E) Expression of Rossy-HA in P19 cells stimulated with rhBMP4 (green signal) and of phospho-Smad1 (red signal). Arrows point to normal nuclear translocation of Smad1 in cells expressing Rossy (E). (F) Rossy expression inhibits the ability of activated Smad1/4 complex to bind the BRE element in an oligonucleotide pulldown assay (32). Notice the large decrease in immunoreactivity of Smad1 and Smad4 in the bound material. wt, wild type.
Identification of potential target genes of Rossy activity in the ectoderm.
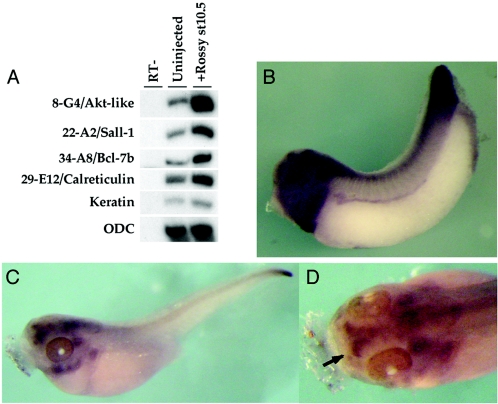
In an effort to identify potential downstream target genes of Rossy, we profiled animal caps injected with Rossy RNA. Here, we report four of the genes upregulated during early gastrulation by Rossy (Fig. 8). These genes are the XSall-1 transcription factor (clone 22-A2 [26]), a novel Akt homolog (clone 8-G4), a homolog of the Bcl-7b gene (clone 29-E12), and calreticulin (clone 34-A8). The Sall-1 gene is a direct target of dpp in Drosophila (10) and has been implicated in neuronal fate acquisition and sensory development (10, 11). Furthermore, it is expressed in the telencephalon and olfactory structures in Xenopus (Fig. 8B and C) (26) and mice (4, 50), suggesting that Sall-1 activity might be regulated by Rossy in olfactory specification. The upregulation of xSall1 by Rossy, together with the ectopic expression of xPax6, is likely to contribute to the specific effect on olfactory structures compared to eye fates. Further experiments are required to determine whether the genes identified in our array are direct targets of Rossy activity and their functional involvement in olfactory bulb generation.
FIG. 8.
Identification of potential target genes of Rossy in the ectoderm. Animal caps were injected with Rossy RNA, and cDNAs from uninjected and injected gastrula-stage animal caps were competitively hybridized to a microarray containing 5,000 gastrula cDNAs (5). Several genes were selected based on their differential expression in four hybridizations. The differential expression of these genes was confirmed by RT-PCRs using gene-specific primers (A). (B to D) mRNA expression analysis of xSalI in the anterior neural plate and olfactory bulbs in Xenopus embryos. The arrow in D points to the olfactory bulb expression of xSalI in tadpoles. At neurula stages, expression was restricted to sensory placodes and anterior neural plate (not shown). st10.5, stage 10.5; ODC, ornithine decarboxylase.
DISCUSSION
Rossy signaling and olfactory specification.
The emergence of a large number of secreted phospholipases A2 and binding partners, together with the unclear correlation between their enzymatic properties and biological responses (56), has prompted the speculation that the sPLA2s signal through an unknown mechanism, independent of their enzymatic properties. Our work has shown that expression of Rossy leads to defects that can be phenotypically and molecularly associated with the modulation of BMP/TGFβ pathways. However, the specific effect on anterior olfactory structures suggests that this activity results in the regulation of a specific program. Thus far, Rossy is the only factor endowed with such an activity. The functional conservation in the Drosophila and mammalian genes suggests that the fly homolog might play similar roles in this species. In light of our discovery that the xSall1 is regulated by Rossy, this gene might be associated with olfactory field generation in vertebrates. Indeed, Sall1 homologs have been widely associated with nervous system development (4, 26, 50) and in the specification of sensory structures in the Drosophila thorax (50). In all species tested, family members show expression in developing neural tissue, and frog Sall-1 and mouse Sall-1 are expressed in the olfactory bulbs (our work and reference 4). SallI has been postulated to act as a transcriptional repressor (28) and is itself a target of BMP signaling in Drosophila (10).
In our hands, Pax6 and Rx expression was upregulated in embryo and in neuralized explants by Rossy. The expression of Rossy in the hatching gland marks the dorsal-most region of anterior neural tissue and flanks the formation of the neurogenic placodes and telencephalon. Because of its effects on Pax6 and olfactory structures, it is likely that Rossy modulates the position and fate of the placodes and dorsoanterior regions of the neural tube. Although both Rx and Pax6 are required for eye formation (38), mice lacking these genes also show loss of the forebrain in Rx−/− mice (39) and of olfactory structures in Pax6-deficient mice (24, 25). Although the molecules implicated in olfactory bulb specification are largely unknown, FGF signaling is required for bulb morphogenesis (21, 40). In addition, retinoic acid signaling from the frontonasal mesenchyme promotes olfactory bulb formation through Pax6 expression (1, 30). Therefore, the effects of Rossy could result from Pax6 upregulation, an effect on FGF signaling, or both.
In addition, the arrival of axonal projections from the olfactory epithelium has been postulated to play a key role in olfactory bulb evagination (15) through an effect on telencephalic proliferation. However, this has been recently challenged in mice deficient for fgfr1 in the telencephalon (21) and in Dlx-5-deficient mice (35). We observed the duplicated olfactory bulbs in the absence of duplicated sensory epithelia, suggesting that the initial evagination is independent of the olfactory nerve. In several embryos, we found ectopic olfactory sensory epithelial structures whose axons innervated the ectopic bulbs, even within the midbrain region. Given the modulatory role of Rossy on the BMP pathway, and the recent report that a BMP-related member, GDF-11, acts to inhibit olfactory neuron generation (65), it will be of interest to determine whether Rossy acts to regulate olfactory neurogenesis in mice through a negative effect on GDF-11. Although the effects of Rossy on olfactory generation cannot be explained exclusively through a negative regulation of the BMP pathway, the effects on xSall-1 in animal caps suggest that Pax6/Sall-1 overexpression might direct telencephalic cell fates towards an olfactory identity.
Modulation of BMP/TGFβ pathways by Rossy.
Overall, Rossy activity inhibits several BMP target genes. The finding that an extracellular factor can inhibit the intracellular signal transducers Smad1 and Smad4 suggests that Rossy likely activates a cascade whose input acts to block a subset of Smad-dependent responses. Our experiments in P19 cells have shown that this effect is likely mediated through the regulation of the DNA-binding activity of activated Smad1/4 complexes. In that regard, we postulate that the observed inhibition of known BMP target genes arises from a shift in target gene preference in Smad1/4 complexes, rather than in an inhibition of the BMP pathway per se.
Overall, our results suggest that Rossy might act in an instructive manner through the activation of receptors and transduction of a signal with the ability to modulate Smad signaling. Although two classes of receptors have been described, only the M type has been cloned. The N-type receptor consists of at least two subunits and is expressed in the nervous system (9, 56). Whether Rossy can bind to this receptor is presently unknown, although expression in P19 cells of the soluble version of the sPLA2 180-kDa M-type receptor (56) does not interfere with the activity of Rossy (not shown), suggesting that they do not bind in this assay. A previous report has suggested that mitogen-activated protein kinase activation results from the exposure of astrocytic cells to sPLA2-gIIA (23). However, we were unable to inhibit the activity of Rossy in animal caps with amounts of dominant-negative Ras that can effectively block mesoderm induction by bFGF (61). Interestingly, two of the genes identified in our microarrays, an Akt homolog and calreticulin, are implicated in calcium homeostasis. Therefore, a topic of current interest in our lab is whether Rossy signaling involves Akt activation and calcium mobilization.
The functional conservation in mutants lacking the active-site histidine suggests that Rossy can elicit biological responses independently of a catalytic activity. However, both Rossy and other sPLA2s are capable of lipid hydrolysis (14, 31, 58), and there is evidence for a phospholipase A2 enzymatic activity in early zebra fish development. Therefore, the range of biological effects of the sPLA2s might be dependent on both modes of signaling. In support of a lipid hydrolysis-independent activity of sPLA2s, both the Rossy's zebra fish homolog (14) and the group XIII sPLA2 (GenBank accession number Q99P27) lack the active-site histidine.
Overall, we have demonstrated that Rossy signaling can act to modulate BMP/TGFβ pathways and promote neurogenesis. The activity of this factor has been remarkably conserved, suggesting an ancestral function. The maternal expression of the Drosophila and Xenopus Rossy, and the expression of the mammalian orthologs in pluripotent ES cells, suggests that it might also modulate these pathways in embryonic stem cell differentiation.
Acknowledgments
We thank Dan Stetler and Alison North for help with the confocal images, N. Marsh-Armstrong for the transgenic embryos, A. Hata for the Tlx2 reporter, and Dan Besser, Alin Vonica, and Joan Seoane for comments on the manuscript.
This work is funded by NIH grant HD32105 to A.H.B. and a Helen Hay Whitney Foundation fellowship to I.M.-S.
REFERENCES
- 1.Anchan, R. M., D. P. Drake, C. F. Haines, E. A. Gerwe, and A. S. LaMantia. 1997. Disruption of local retinoid-mediated gene expression accompanies abnormal development in the mammalian olfactory pathway. J. Comp. Neurol. 379:171-184. [PubMed] [Google Scholar]
- 2.Ancian, P., G. Lambeau, M. G. Mattei, and M. Lazdunski. 1995. The human 180-kDa receptor for secretory phospholipases A2. Molecular cloning, identification of a secreted soluble form, expression, and chromosomal localization. J. Biol. Chem. 270:8963-8970. [DOI] [PubMed] [Google Scholar]
- 3.Baker, J. C., and R. M. Harland. 1996. A novel mesoderm inducer, Madr2, functions in the activin signal transduction pathway. Genes Dev. 10:1880-1889. [DOI] [PubMed] [Google Scholar]
- 4.Buck, A., A. Kispert, and J. Kohlhase. 2001. Embryonic expression of the murine homologue of SALL1, the gene mutated in Townes-Brocks syndrome. Mech. Dev. 104:143-146. [DOI] [PubMed] [Google Scholar]
- 5.Carcamo, J., A. Zentella, and J. Massague. 1995. Disruption of transforming growth factor beta signaling by a mutation that prevents transphosphorylation within the receptor complex. Mol. Cell. Biol. 15:1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casellas, R., and A. H. Brivanlou. 1998. Xenopus Smad7 inhibits both the activin and BMP pathways and acts as a neural inducer. Dev. Biol. 198:1-12. [DOI] [PubMed] [Google Scholar]
- 7.Cho, K. W., B. Blumberg, H. Steinbeisser, and E. M. De Robertis. 1991. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell 67:1111-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cupillard, L., K. Koumanov, M. G. Mattei, M. Lazdunski, and G. Lambeau. 1997. Cloning, chromosomal mapping, and expression of a novel human secretory phospholipase A2. J. Biol. Chem. 272:15745-15752. [DOI] [PubMed] [Google Scholar]
- 9.Cupillard, L., R. Mulherkar, N. Gomez, S. Kadam, E. Valentin, M. Lazdunski, and G. Lambeau. 1999. Both group IB and group IIA secreted phospholipases A2 are natural ligands of the mouse 180-kDa M-type receptor. J. Biol. Chem. 274:7043-7051. [DOI] [PubMed] [Google Scholar]
- 10.de Celis, J. F., R. Barrio, and F. C. Kafatos. 1996. A gene complex acting downstream of dpp in Drosophila wing morphogenesis. Nature 381:421-424. [DOI] [PubMed] [Google Scholar]
- 11.de Celis, J. F., R. Barrio, and F. C. Kafatos. 1999. Regulation of the spalt/spalt-related gene complex and its function during sensory organ development in the Drosophila thorax. Development 126:2653-2662. [DOI] [PubMed] [Google Scholar]
- 12.Ebisawa, T., M. Fukuchi, G. Murakami, T. Chiba, K. Tanaka, T. Imamura, and K. Miyazono. 2001. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J. Biol. Chem. 276:12477-12480. [DOI] [PubMed] [Google Scholar]
- 13.Gammill, L. S., and H. Sive. 2000. Coincidence of otx2 and BMP4 signaling correlates with Xenopus cement gland formation. Mech. Dev. 92:217-226. [DOI] [PubMed] [Google Scholar]
- 14.Gelb, M. H., E. Valentin, F. Ghomashchi, M. Lazdunski, and G. Lambeau. 2000. Cloning and recombinant expression of a structurally novel human secreted phospholipase A2. J. Biol. Chem. 275:39823-39826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong, Q., and M. T. Shipley. 1995. Evidence that pioneer olfactory axons regulate telencephalon cell cycle kinetics to induce the formation of the olfactory bulb. Neuron 14:91-101. [DOI] [PubMed] [Google Scholar]
- 16.Harland, R. 1991. In situ hybridization: an improved wholemount method for Xenopus embryos. Methods Cell Biol. 36:675-678. [DOI] [PubMed] [Google Scholar]
- 17.Harland, R., and J. Gerhart. 1997. Formation and function of Spemann's organizer. Annu. Rev. Cell Dev. Biol. 13:611-667. [DOI] [PubMed] [Google Scholar]
- 18.Hata, A., G. Lagna, J. Massague, and A. Hemmati-Brivanlou. 1998. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 12:186-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hata, A., J. Seoane, G. Lagna, E. Montalvo, A. Hemmati-Brivanlou, and J. Massague. 2000. OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell 100:229-240. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi, H., S. Abdollah, Y. Qiu, J. Cai, Y. Y. Xu, B. W. Grinnell, M. A. Richardson, J. N. Topper, M. A. Gimbrone, Jr., J. L. Wrana, and D. Falb. 1997. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell 89:1165-1173. [DOI] [PubMed] [Google Scholar]
- 21.Hebert, J. M., M. Lin, J. Partanen, J. Rossant, and S. K. McConnell. 2003. FGF signaling through FGFR1 is required for olfactory bulb morphogenesis. Development 130:1101-1111. [DOI] [PubMed] [Google Scholar]
- 22.Hemmati-Brivanlou, A., and D. A. Melton. 1994. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell 77:273-281. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez, M., S. L. Burillo, M. S. Crespo, and M. L. Nieto. 1998. Secretory phospholipase A2 activates the cascade of mitogen-activated protein kinases and cytosolic phospholipase A2 in the human astrocytoma cell line 1321N1. J. Biol. Chem. 273:606-612. [DOI] [PubMed] [Google Scholar]
- 24.Hill, R. E., J. Favor, B. L. Hogan, C. C. Ton, G. F. Saunders, I. M. Hanson, J. Prosser, T. Jordan, N. D. Hastie, and V. van Heyningen. 1991. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 354:522-525. [DOI] [PubMed] [Google Scholar]
- 25.Hogan, B. L., E. M. Hirst, G. Horsburgh, and C. M. Hetherington. 1988. Small eye (Sey): a mouse model for the genetic analysis of craniofacial abnormalities. Development 103(Suppl.):115-119. [DOI] [PubMed] [Google Scholar]
- 26.Hollemann, T., R. Schuh, T. Pieler, and R. Stick. 1996. Xenopus Xsal-1, a vertebrate homolog of the region specific homeotic gene spalt of Drosophila. Mech. Dev. 5:19-32. [DOI] [PubMed] [Google Scholar]
- 27.Kavsak, P., R. K. Rasmussen, C. G. Causing, S. Bonni, H. Zhu, G. H. Thomsen, and J. L. Wrana. 2000. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol. Cell 6:1365-1375. [DOI] [PubMed] [Google Scholar]
- 28.Kiefer, S. M., B. W. McDill, J. Yang, and M. Rauchman. 2002. Murine Sall1 represses transcription by recruiting a histone deacetylase complex. J. Biol. Chem. 277:14869-14876. [DOI] [PubMed] [Google Scholar]
- 29.Lagna, G., A. Hata, A. Hemmati-Brivanlou, and J. Massague. 1996. Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature 383:832-836. [DOI] [PubMed] [Google Scholar]
- 30.LaMantia, A. S., M. C. Colbert, and E. Linney. 1993. Retinoic acid induction and regional differentiation prefigure olfactory pathway formation in the mammalian forebrain. Neuron 10:1035-1048. [DOI] [PubMed] [Google Scholar]
- 31.Lambeau, G., L. Cupillard, and M. Lazdunski. 1997. Membrane receptors for venom phospholipase A2, p. 389-412. In R. M. Kini (ed.), Venom phospholipase A2 enzymes: structure, function and mechanism. John Wiley & Sons, Chichester, United Kingdom.
- 32.Latinkic, B. V., M. Umbhauer, K. A. Neal, W. Lerchner, J. C. Smith, and V. Cunliffe. 1997. The Xenopus Brachyury promoter is activated by FGF and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes Dev. 11:3265-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, F., A. Hata, J. C. Baker, J. Doody, J. Carcamo, R. M. Harland, and J. Massague. 1996. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature 381:620-623. [DOI] [PubMed] [Google Scholar]
- 34.Liu, F., C. Pouponnot, and J. Massague. 1997. Dual role of the Smad4/DPC4 tumor suppressor in TGFbeta-inducible transcriptional complexes. Genes Dev. 11:3157-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long, J. E., S. Garel, M. J. Depew, S. Tobet, and J. L. R. Rubenstein. 2003. DLX5 regulates development of peripheral and central components of the olfactory system. J. Neurosci. 23:568-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh-Armstrong, N., H. Huang, D. L. Berry, and D. D. Brown. 1999. Germ-line transmission of transgenes in Xenopus laevis. Proc. Natl. Acad. Sci. USA 96:14389-14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massague, J., and Y. Chen. 2000. Controlling TGF-β signaling. Genes Dev. 14:627-644. [PubMed] [Google Scholar]
- 38.Mathers, P. H., and M. Jamrich. 2000. Regulation of eye formation by the Rx and Pax6 homeobox genes. Cell. Mol. Life Sci. 57:186-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathers, P. H., A. Grinberg, K. A. Mahon, and M. Jamrich. 1997. The Rx homeobox gene is essential for vertebrate eye development. Nature 387:603-607. [DOI] [PubMed] [Google Scholar]
- 40.Meyers, E. N., M. Lewandoski, and G. R. Martin. 1998. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 18:136-141. [DOI] [PubMed] [Google Scholar]
- 41.Muñoz-Sanjuán, I. and A. H. Brivanlou. 2001. Early posterior/ventral fate specification in the vertebrate embryo. Dev. Biol. 237:1-17. [DOI] [PubMed] [Google Scholar]
- 42.Muñoz-Sanjuán, I., and A. H. Brivanlou. 2002. Neural induction, the default model and embryonic stem cells. Nat. Rev. Neurosci. 3:271-280. [DOI] [PubMed] [Google Scholar]
- 43.Muñoz-Sanjuán, I., E. Bell, C. R. Altmann, A. Vonica, and A. H. Brivanlou. 2002. Gene profiling during neural induction in Xenopus laevis: regulation of BMP signaling by post-transcriptional mechanisms and TAB3, a novel TAK1-binding protein. Development 129:5529-5540. [DOI] [PubMed] [Google Scholar]
- 44.Muñoz-Sanjuán, I. and A. H. Brivanlou. Modulation of BMP signaling during vertebrate gastrulation. In C. Stern (ed.), Vertebrate gastrulation, in press. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Murakami, M., S. Shimbara, T. Kambe, H. Kuwata, M. V. Winstead, J. A. Tischfield, and I. Kudo. 1998. The functions of five distinct mammalian phospholipase A2s in regulating arachidonic acid release. J. Biol. Chem. 273:14411-14423. [DOI] [PubMed] [Google Scholar]
- 46.Murakami, M., Y. Nakatani, G. Atsumi, K. Inoue, and I. Kudo. 1997. Regulatory functions of phospholipase A2. Crit. Rev. Immunol. 17:225-283. [DOI] [PubMed] [Google Scholar]
- 47.Nakao, A., M. Afrakhte, A. Moren, T. Nakayama, J. L. Christian, R. Heuchel, S. Itoh, M. Kawabata, N. E. Heldin, C. H. Heldin, and P. ten Dijke. 1997. Identification of Smad7, a TGF beta-inducible antagonist of TGF-beta signalling. Nature 389:631-635. [DOI] [PubMed] [Google Scholar]
- 48.Nicolas, J. P., Y. Lin, G. Lambeau, F. Ghomashchi, M. Lazdunski, and M. H. Gelb. 1997. Localization of structural elements of bee venom phospholipase A2 involved in N-type receptor binding and neurotoxicity. J. Biol. Chem. 272:7173-7181. [DOI] [PubMed] [Google Scholar]
- 49.Nomura, T., M. M. Khan, S. C. Kaul, H. D. Dong, R. Wadhwa, C. Colmenares, I. Kohno, and S. Ishii. 1999. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev. 13:412-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ott, T., K. H. Kaestner, A. P. Monaghan, and G. Schutz. 2001. The mouse homolog of the region specific homeotic gene spalt of Drosophila is expressed in the developing nervous system and in mesoderm-derived structures. Mech. Dev. 56:117-128. [DOI] [PubMed] [Google Scholar]
- 51.Sato, N., I. M. Sanjuan, M. Heke, M. Uchida, F. Naef, and A. H. Brivanlou. 2003. Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev. Biol. 260:404-413. [DOI] [PubMed] [Google Scholar]
- 52.Schuldiner, M., O. Yanuka, J. Itskovitz-Eldor, D. A. Melton, and N. Benvenisty. 2000. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 97:11307-11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson, T. I., and D. J. Price. 2002. Pax6: a pleiotropic player in development. Bioessays 24:1041-1051. [DOI] [PubMed] [Google Scholar]
- 54.Six, D. A., and E. A. Dennis. 2000. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim. Biophys. Acta 1488:1-19. [DOI] [PubMed] [Google Scholar]
- 55.Stroschein, S. L., W. Wang, S. Zhou, Q. Zhou, and K. Luo. 1999. Negative feedback regulation of TGF-beta signaling by the SnoN oncoprotein. Science 286:771-774. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki, N., J. Ishizaki, Y. Yokota, K. Higashino, T. Ono, M. Ikeda, N. Fujii, K. Kawamoto, and K. Hanasaki. 2000. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase. J. Biol. Chem. 275:5785-5793. [DOI] [PubMed] [Google Scholar]
- 57.Tang, S. J., P. A. Hoodless, Z. Lu, M. L. Breitman, R. R. McInnes, J. L. Wrana, and M. Buchwald. 1998. The Tlx-2 homeobox gene is a downstream target of BMP signalling and is required for mouse mesoderm development. Development 125:1877-1887. [DOI] [PubMed] [Google Scholar]
- 58.Valentin, E., and G. Lambeau. 2000. Increasing molecular diversity of secreted phospholipases A(2) and their receptors and binding proteins. Biochim. Biophys. Acta 1488:59-70. [DOI] [PubMed] [Google Scholar]
- 59.Wang, S. S., R. Y. Tsai, and R. R. Reed. 1997. The characterization of the Olf-1/EBF-like HLH transcription factor family: implications in olfactory gene regulation and neuronal development. J. Neurosci. 17:4149-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, W., F. V. Mariani, R. M. Harland, and K. Luo. 2000. Ski represses bone morphogenetic protein signaling in Xenopus and mammalian cells. Proc. Natl. Acad. Sci. USA 97:14394-14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinstein, D. C., J. Marden, F. Carnevali, and A. Hemmati-Brivanlou. 1998. FGF-mediated mesoderm induction involves the Src-family kinase Laloo. Nature 394:904-908. [DOI] [PubMed] [Google Scholar]
- 62.Whitman, M., and D. A. Melton. 1992. Involvement of p21ras in Xenopus mesoderm induction. Nature 357:252-254. [DOI] [PubMed] [Google Scholar]
- 63.Wilson, P. A., and A. Hemmati-Brivanlou. 1995. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376:331-333. [DOI] [PubMed] [Google Scholar]
- 64.Wotton, D., R. S. Lo, S. Lee, and J. Massague. 1999. A smad transcriptional corepressor. Cell 97:29-39. [DOI] [PubMed] [Google Scholar]
- 65.Wu, H.-H., S. Ivkovic, R. C. Murray, S. Jaramillo, K. Lyons, J. E. Johnson, and A. L. Calof. 2003. Autoregulation of neurogenesis by GDF11. Neuron 37:197-207. [DOI] [PubMed] [Google Scholar]
- 66.Xu, W., K. Angelis, D. Danielpour, M. M. Haddad, O. Bischof, J. Campisi, E. Stavnezer, and E. E. Medrano. 2000. Ski acts as a corepressor with Smad2 and Smad3 to regulate the response to type beta transforming growth factor. Proc. Natl. Acad. Sci. USA 97:5924-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]