Abstract
Membrane lipidomes are dynamic and their changes generate lipid mediators affecting various biological processes. Phosphatidic acid (PA) has emerged as an important class of lipid mediators involved in a wide range of cellular and physiological responses in plants, animals, and microbes. The regulatory functions of PA have been studied primarily outside the nuclei, but an increasing number of recent studies indicates that some of the PA effects result from its action in nuclei. PA levels in nuclei are dynamic in response to stimuli. Changes in nuclear PA levels can result from activities of enzymes associated with nuclei and/or from movements of PA generated extranuclearly. PA has also been found to interact with proteins involved in nuclear functions, such as transcription factors and proteins undergoing nuclear translocation in response to stimuli. The nuclear action of PA affects various aspects of plant growth, development, and response to stress and environmental changes.
Keywords: Phosphatidic acid, phospholipases, DAG kinases, transcriptional regulation, nuclear signaling, diacylglycerol, stress signaling
1. Introduction
Membrane lipids are not only structural backbones for cellular and intracellular compartmentalization, but also rich sources for generating cellular mediators in response to various stimuli. Phosphatidic acid (PA), which is a central intermediate in glycerolipid metabolism (Fig. 1), has emerged as an important class of lipid mediators in various cellular and physiological processes (Wang et al., 2006; Hong et al., 2010; Shin and Loewen, 2011; Jang et al., 2012; Peng and Frohman, 2012; Kolesnikov et al., 2022). PA affects plant growth, development, reproduction, and responses to abiotic and biotic challenges (reviewed in Wang et al., 2006; Testerink and Munnik, 2011; Hong et al., 2016; Kim and Wang, 2020; Ali et al. 2022; Kolesnikov et al., 2022). In animal systems, PA is involved in multiple pathophysiological processes, such as inflammation, malignant transformation, neurodegenerative disorders, and infection (reviewed in Nelson and Frohman, 2015; Bullen and Soldati-Favre, 2016; Brown et al., 2017). In microbes, PA regulates various processes, such as mediating lipid metabolism and cellular response to nutrient availability (Young et al., 2010; Carman and Han, 2019; Kwiatek et al., 2022).
Fig. 1. Multiple enzymes that produce and remove PA.

Green and red arrows indicate production and removal of PA, respectively. Numbers in parenthesis indicate the number of genes in Arabidopsis. Please refer to the text for abbreviations of lipids and enzymes.
The regulatory functions of PA and its mode of action have been studied primarily outside the nuclei. However, recent studies indicate that some of the PA effects are mediated through its action in nuclei (Kim et al., 2019, 2022, 2023; Cai et al., 2020; Cao et al., 2021; Zhou et al., 2022; Fan et al., 2023). Here, we will review evidence on the production and detection of nuclear PA, PA interaction with proteins that function in nuclei, and cellular and physiological processes affected by nuclear PA, as well as current knowledge gaps in the rapidly progressing research field.
2. Production of PA associated with nuclei
PA is a minor component of membrane lipids, with the simplest head group, phosphate without any modification (Fig. 1). The cellular level of PA is highly dynamic, changing rapidly and transiently in plant response to stress, such as wounding, stress hormones, dehydration, salt, cold/freezing, and pathogen attack (Welti et al., 2002; Zhang et al., 2004; Hong et al., 2009; Zhang et al., 2009; Vu et al., 2012, 2014). The production and removal of PA are mediated by multiple enzymes (Fig. 1), some of which are associated with nuclei. In addition, PA produced by extranuclear enzymes can move to nuclei (Kim et al., 2022).
2.1. Cellular production of PA associated with nuclei
Phospholipase D (PLD) and diacylglycerol (DAG) kinase (DGK) are two major families of enzymes that produce signaling PA. PLD hydrolyzes membrane phospholipids, such as phosphatidylcholine (PC), to PA (Fig. 1). DGK produces PA by phosphorylating DAG that can be produced by phosphoinositide-specific phospholipase C (PI-PLC) or non-specific PLC (NPC) (Fig. 1). PA can be removed by PA phosphohydrolase (PAH), lipid phosphate phosphatase (LPP), phospholipase A (PLA), or PA kinase (PAK) (Fig. 1). Each of the PA-metabolizing enzyme families has multiple members and many of them within the same family have different biochemical and regulatory properties, temporal and spatial expression, and/or subcellular associations (Hong et al., 2016; Ali et al. 2022). For example, the differences, such as Ca2+ requirements, substrate preferences, and stimulus-induced activation of different PLDs (Table 1), can lead to specific temporal and spatial patterns of PA production, as well as molecular species of PA produced, underlying a basis for diverse cellular effects of PA.
Table 1.
Signature Properties and functions of Arabidopsis PLDs
| Group | Ca2+ | PIP2 | Oleate | Substrate Preference | Subcellular Association | Mutant Phenotype* | Reference* |
|---|---|---|---|---|---|---|---|
| Stimulation | |||||||
| PLDa1 | mM | No | No | PC>PE | Cyto-Mic | water loss | Mishra et al, 2006 |
| PLDa3 | mM | No | No | PC<PE | Mic | salt, drought | Hong et al, 2008 |
| PLDb1 | μM | Yes | No | PC= PE | Mic | pathogens | Zhao et al, 2013 |
| PLDg1 | μM | Yes | No | PC<PE | Mic, Nuc | Al | Zhao et al, 2011 |
| PLDd | μM-mM | Yes | Yes | PC<PE | PM | salt, temp | Li et al, 2004; Kim et al, 2022 |
| PLDe | μM-mM | Yes | No | PC<PE | PM, Mic | N deficit | Hong et al., 2009 |
| PLDz1 | No | Yes | No | PC | PM, Mic | Pi deficit | Li et al., 2006a |
| PLDz2 | No | Yes | No | PC | Vacuole | Pi deficit | Li et al., 2006b; Su et al, 2018 |
Selected phenotypes and references only. Mic, microsome; Nuc, nucleus; PM, plasma membrane; Temp, temperature; PC, phosphatidylcholine; PE, phosphatidylethanolamine
Some of the PA-producing and removal enzymes are associated with nuclei. Early studies in animal systems indicate that PA-metabolizing enzymes are associated with the nucleus and isolated nuclei can synthesize PA (Smith and Wells, 1983; Cocco et al., 1987; Siniossoglou, 2013). PAH is translocated between the endoplasmic reticulum (ER) and nucleus in yeast (Ren et al., 2010). Among 12 PLDs in Arabidopsis, PLDγ was associated with isolated nuclei, revealed by an antibody raised against PLDγ (Fan et al., 1999). Arabidopsis has three PLDγs with high sequence similarity, and whether all PLDγs are associated with nuclei remains to be determined. Arabidopsis has seven DGKs that are grouped into cluster I (DGK1 and 2), II (DGK3, 4 and 7), and III (DGK5 and 6). A portion of cluster III DGK5 has been found to be associated with nuclei (Kalachova et al., 2022; Li et al., 2023a). Moreover, disruption of DGK5 decreased, whereas overexpressing it increased nuclear PA levels (Li et al., 2023a).
Since DGK in nuclei phosphorylates DAG to PA, the DAG-producing enzymes, including PI-PLC and NPC, can indirectly affect nuclear PA levels. PI-PLC3 in Arabidopsis is associated with both the nucleus and plasm membrane (Ren et al., 2017), and is involved in lateral root initiation and thermotolerance (Gao et al., 2014; Zhang et al., 2018). The total cellular PA was not changed in plc3, but the effect of PLC3 on nuclear PA levels remains to be determined (Zhang et al., 2018). Three of the six NPCs in Arabidopsis (NPC1, NPC2, and NPC5) are localized to ER (reviewed in Nakamura and Ngo, 2020). Since the outer nuclear membrane is continuous with the ER membrane, the ER-associated PLCs could affect potentially the DAG levels in the outer nuclear membrane, but their effect on nuclear PA levels is not tested.
2.2. PA movement to nuclei
In addition to PA-metabolizing enzymes associated with nuclei, extranuclear PA has been reported to move into nuclei. In animal cells, PA outside the nucleus enters the nucleus via PA trafficking while the precise mechanism remains to be elucidated (Henkels et al., 2016). In plants, PLDδ is involved in heat-induced PA accumulation associated with nuclei (Kim et al., 2022). PLDδ is associated with the plasma membrane (Wang and Wang, 2001). The heat-induced PA elevation in nuclei was diminished by knockout of PLDδ and by the vesicle trafficking inhibitor brefeldin A (BFA), as shown using mass spectrometry (MS)-based analysis of nuclear PA and by live-cell imaging (Kim et al., 2022). The heat-induced nuclear PA elevation that was blocked by BFA was also shown using a PA biosensor (Li et al., 2023b). The results suggest the possibility that PA moves to the nucleus via vesicle trafficking under heat stress (Fig. 2). We propose a topologically possible model for the PA-GAPC co-movement through the outer and inner nuclear membranes without flipping of any lipids or proteins (Fig. 2), and such nuclear envelope trafficking has been proposed in other systems (Burns & Wente, 2012; Bhosle et al., 2019; Corbeil et al., 2020; Arii, 2021). However, it remains unclear whether the transport is direct or via other subcellular compartments such as Golgi complex or endoplasmic reticulum, and hence the specific route by which PA and GAPC move into the nucleus requires further elucidation. In addition, other possibilities exist as described below.
Fig. 2. A proposed model for PLDδ derived PA interacting with GAPC and PA-GAPC co-movement into nuclei under heat.
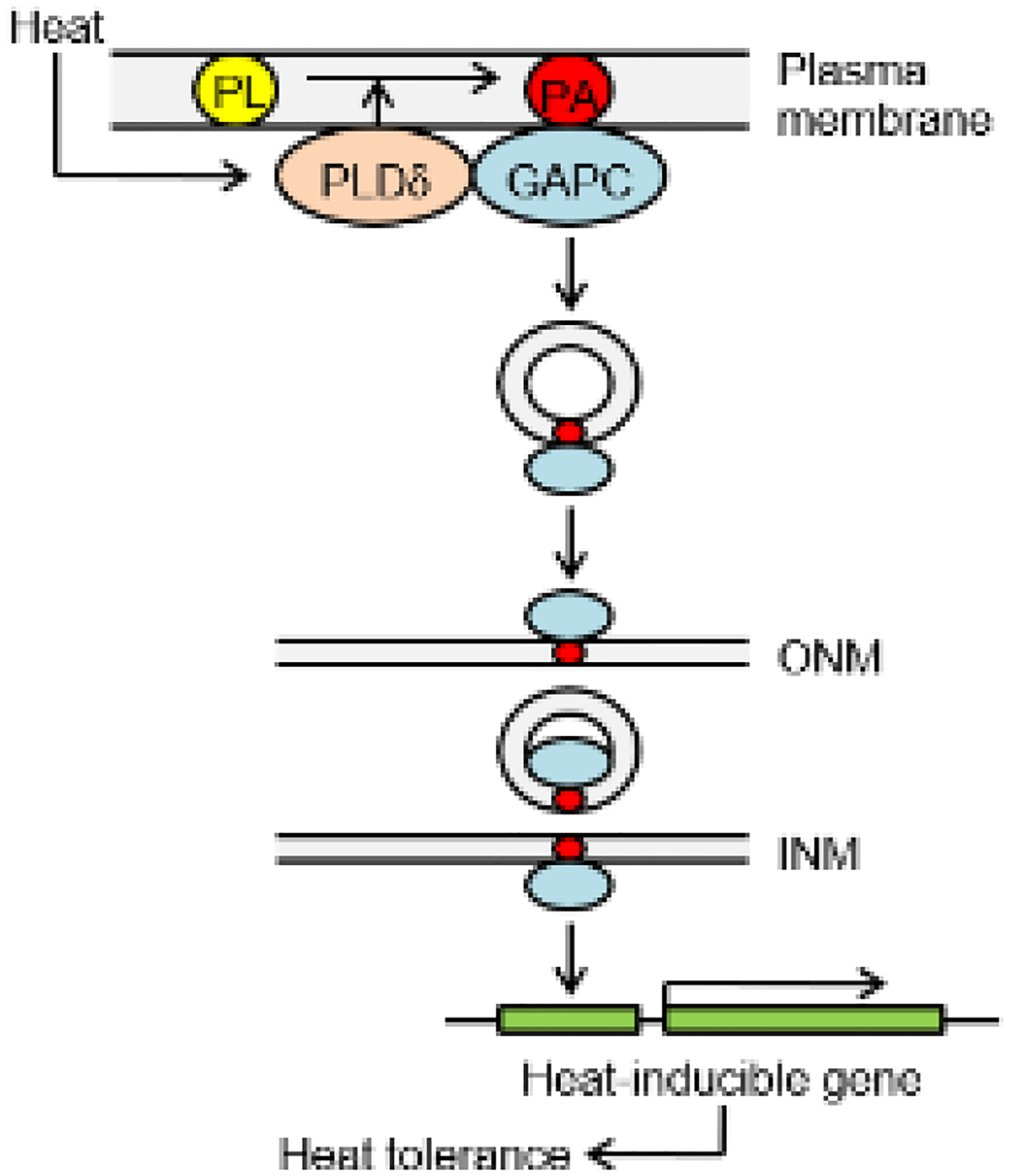
In response to heat stress, PA produced by PLDδ activity promotes GAPC translocation into the nucleus via vesicle trafficking, where GAPC increases the expression of heat-inducible genes, rendering Arabidopsis tolerant to heat stress. For simplicity, membranes are depicted as monolayer and PA acyl chains in vesicle omitted, and outer nuclear membrane (ONM) undervalued. PL, phospholipid; INM, inner nuclear membrane.
The geometrical shape of PA renders it a high propensity to distort membranes. PA in membranes exists in a cone-like shape because it contains two bulky fatty acids and a relatively small head group (Harlos and Eibl, 1981). This structure of PA decreases the packing stability of the lipid bilayer and induces negative (concave) curvature on the membrane, which could be involved in membrane budding and vesicle formation (Kooijman and Burger, 2009). PA has been shown to interact with proteins in vesicle fission/fusion (Homan and Pownall, 1988; Contreras et al., 2010; Tanguy et al., 2019; Zhukovsky et al., 2019). In addition, PA can transverse membrane lipid bilayer spontaneously or assisted by proteins. PA has been found extracellularly in phloem associated with proteins as mobile signals in long-distance signaling (Barbaglia et al., 2016). Furthermore, the nuclear envelope is comprised of double membranes that are physically continuous with ER. ER is connected directly to the outer nuclear membrane (ONM), which is contiguous with the inner nuclear membrane (INM) via membranes surrounding nuclear pore complexes (Barger et al., 2022). Thus, extranuclear PA can be associated with nuclei potentially via direct movement through continuous membrane connections, vesicular trafficking, and/or membrane contact sites. The complex PA behaviors may account for its diverse effects on vesicular trafficking and membrane fusion/fission depending on cell types and conditions (Nakanishi et al., 2004; Valente et al., 2013; Pagliuso et al., 2016; Starr et al., 2016). Thus, the specific route by which PA moves into nuclei could be specific to a given cue, which requires further elucidation.
2.3. Enzymes degrading PA
PA can be removed by various enzymes, such as dephosphorylation by PAHs and LPPs and deacylation by acyl-hydrolases and PLA. The PAH homologs in mammals and invertebrates have been found to be associated with ER and nuclear membranes and regulate gene expression (Zhang and Reue, 2017). Arabidopsis has two PAHs, and both are localized into the cytosol (Nakamura et al., 2009; Eastmond et al., 2010). The double knockout of PAH1 and PAH2 caused overexpansion of the ER membrane (Eastmond et al., 2010). One of nine LPPs in Arabidopsis, LPPα2, is localized to the ER membrane and regulates ER phospholipid biosynthesis (Nguyen and Nakamura, 2023). PLA hydrolyzes the acyl group of phospholipids to produce lysophospholipid and free fatty acids, The secretory PLA2-α was found to move into the nucleus, but its activity toward PA is very low (Mansfeld and Ulbrich-Hofmann, 2007; Froidure et al., 2010). Several members of Arabidopsis PLAs can use PA as substrate and have also been reported to be localized to ER (reviewed in Chen et al., 2013). Since the outer nuclear membrane is continuous with the ER membrane, these ER-localized PA-hydrolyzing enzymes could contribute potentially to the regulation of PA levels in nuclei.
3. Detection of PA associated with nuclei
The ability to detect and quantify PA in nuclei is crucial to determine its function in nuclei. Different approaches have been used to analyze PA, such as MS-based lipidomic profiling and PA biosensors, as well as traditional lipid separation and quantification. PA associated with nuclei has been measured using MS-based lipidomic profiling with the ability to quantify molecular species of PA and PA biosensors for live-cell monitoring of PA dynamics. In addition, fluorescently labeled lipids, including PA, have been applied to detecting lipid distribution and dynamics in nuclei.
3.1. MS-based lipid profiling of nuclear fraction
MS-based lipid profiling has been used to quantify PA from isolated nuclei without apparent cytoplasmic contamination (Cai et al., 2020). Lipids are extracted from the nuclear fraction and analyzed using electrospray ionization tandem MS (ESI-MS/MS, Fig. 3A). For example, a recent study measured PA levels in nuclei isolated from heat-treated WT, pldδ, and BFA-treated WT to determine whether heat stress induced nuclear PA accumulation (Kim et al., 2022). Upon heat stress, total cellular PA levels increased in all the plants, with most PA molecular species being increased. However, heat-induced PA increases were only detected in WT, but not in pldδ or BFA-treated WT.
Fig. 3. Quantification of nuclear PA.

A. A workflow for nuclear isolation and lipid analysis. Isolated nuclei were verified by immunoblotting Histone H3 as a nuclear marker and phosphoenolpyruvate carboxylase (PEPC) as a cytosolic marker. Lipids from nuclei were analyzed using ESI-MS/MS. B. Suppression of PA formation by EGTA during nuclear isolation. Nuclei were isolated from 10-day-old Arabidopsis seedlings by Percoll gradient centrifugation with or without 50 mM EGTA. PA was quantified by ESI-MS/MS and shown as per total nuclear proteins.
One precaution in nuclear fractionation is to prevent lipolytic activity during mechanical disruption of the cells. Major lipolytic activities can be inhibited by including in all buffers used for extraction and fractionation millimolar levels of Ca2+-chelating EGTA that inhibit key lipolytic enzymes, such as PLD (Cai et al., 2020). To test whether the lipolytic activity is inhibited, we compared the PA levels of nuclear fractions between WT and pldα1pldδ treated without and with EGTA (Fig. 3B). PLDα1 and PLDδ are the two most active PLDs in Arabidopsis. The results indicate that including EGTA is required to minimize PA production during nuclear isolation (Fig. 3B).
3.2. PA biosensors detecting PA associated with nuclei in-cell
To detect PA changes at subcellular levels, PA biosensors have been used, including membrane translocation-based single fluorescent PA biosensors and Förster resonance energy transfer (FRET)-based PA sensors. The translocation PA biosensors feature a PA-binding domain (PABD) attached to a fluorescent protein, such as GFP (Nakanishi et al., 2004; Corrotte et al., 2006; Du and Frohman, 2009; Bohdanowicz et al., 2013; Potocký et al., 2014; Li et al., 2023b). The GFP-tagged PABD from the N-terminal region of Arabidopsis respiratory burst oxidase homolog D (GFP-N160RbohD) successfully detected salt-induced PA increase at the plasma membrane (PM) of root cells, while the PA binding-abolished mutant GFP-N160MRbohD failed to detect the PA increase at PM (Li et al., 2023b). Moreover, heat stress induced the translocation of GFP-N160MRbohD from PM to nuclei, which was impaired by suppressing PLD- and DGK-mediated PA production (Li et al., 2023b).
In FRET-based PA probes, the emission spectrum of a donor fluorophore overlaps with the excitation spectrum of an acceptor fluorophore (Nishioka et al., 2010; Ferraz-Nogueira et al., 2014). When the donor gets close to the acceptor, the emission of the acceptor can be detected under the excitation wavelength of the donor. FRET detection is based on ratio imaging, thus having advantages over single-intensity probes because the effects of probe concentration or photobleaching cancel out when two images are divided to yield a ratio. FRET-based PA biosensors have been used to monitor PA changes in plant cells. The construct features the PABD from NADPH oxidase that was fused between cyan (CFP) and yellow (YFP) fluorescent proteins through rigid α-helical linkers consisting of repeated EAAAR sequences (Li et al., 2019). Also, FRET sensors can be targeted to specific subcellular membranes to measure local changes in PA, such as using the non-raft plasma membrane-targeted domain of K-Ras4B (Nishioka et al., 2010). The FRET PA biosensor has detected in real-time PA increases in the PM induced by salt and abscisic acid (ABA) (Li et al., 2019).
3.3. Detection of nuclear PA using fluorescent lipids and FRET
The application of fluorescent lipids has greatly facilitated the study of lipid distribution and dynamics, including PA in nuclei (Klymchenko and Kreder, 2014; Henkels et al., 2016; Kim et al., 2022). When nitrobenzoxadiazole (NBD)-labeled lipids were infiltrated into Arabidopsis seedlings, NBD-PA bound to proteins, which were pulled down by immunoprecipitation, and this helped to verify PA interactions with transcription factors (Kim et al., 2019). To provide spatial information about the interactions, FRET between a CFP-tagged protein and an NBD-labeled lipid has been used to detect lipid-protein interaction in vivo at a subcellular level, including nuclei, as the excitation spectrum of NBD (488 nm) overlaps with the emission spectrum of CFP (Kim et al., 2022; Fig. 4A).
Fig. 4. Spectra of CFP, NBD, and TopFluor TMR fluorophores and FRET between CFP-GAPC and TopFluor TMR-PA in Arabidopsis roots.

A. Spectra in the regions of interest for CFP (dashed line), NBD (dotted line), and TopFluor TMR (solid line). B. Images of WT root cells with 10 μM TopFluor TMR-PA and CFP-GAPC2-expressing root cells stained without or with 10 μM TopFluor TMR-PA. Scale bar = 10 μm. The regions of interest, such as the boxed or elsewhere, could be used to calculated FRET efficiency as described in Yao and Wang (2023).
In addition, the fluorophore BODIPY (TopFluor)-labeled lipids offer some advantages over the NBD-lipids because TopFluor (λMax ex/λMax em = 505 nm/513 nm) is more photostable and hydrophobic (Boldyrev et al., 2007; Kay et al., 2012). TopFluor has low excitation at 405 nm, so it could be a better FRET acceptor than NBD to be paired with CFP (Fig. 4A). The emission spectrum of TopFluor tetramethylrhodamine (TMR)-PA excited at 561 nm shows less overlap with the emission of CFP excited at 406 nm compared with that of NBD excited at 488 nm (Yao and Wang, 2023). TopFluor TMR-PA and CFP-tagged cytosolic glyceraldehyde-3-phosphate dehydrogenase (GAPC) have been used to document PA-GAPC interaction in planta (Fig. 4B; Yao and Wang, 2023). Without stress GAPC mainly interacts with PA in the cytosol (Fig. 4B), whereas some GAPC-PA co-move into nuclei under heat stress (Kim et al., 2022). In addition, recent developments in using click-chemistry to control and detect PA production in mammalian cells (Tai and Baskin, 2022) are promising to be adapted to visualize nuclear PA and investigate the nuclear PA functions. It is worth noting some limitations of the above methods. The fluorophores on PA may hinder the binding of PA to its target protein. The FRET-based PA probes may not detect PA that would be partially embedded in a protein. Hence, a lack of FRET signals does not necessarily mean an absence of PA-protein interaction.
4. PA interactions with proteins that function in nuclei
PA binding to proteins is one of the modes of PA’s cellular actions, and the identification of PA-interacting proteins and subsequent analyses have shed light on how PA acts as cellular mediators (Fig. 5; Kim and Wang, 2020). Physiochemical properties of PA, such as pH-dependent dual deprotonation and the ability to act as an electrostatic/hydrogen-bond switch, make PA a unique class of lipids in interacting with proteins (Kooijman et al., 2007). The study of PA-interacting proteins has led to the identification of several PA-binding proteins in nuclei (Kim et al., 2019; Cai et al., 2020).
Fig. 5. Interaction of PA with proteins that act in nuclei.

Lower, PA has pH-dependent dual protonations and hydrogen bonding to effector proteins that distinguish it from other lipids. Upper, Examples of PA-binding proteins with nuclear functions.
4.1. PA binding to transcription regulators
An earlier study identified WEREWOLF (WER) as PA binding protein that functions in nuclei. WER is a MYB transcription factor that regulates cell differentiation, such as root hair patterning, and the PA-WER interaction affects the WER’s nuclear localization and root hair development (Yao et al., 2013). To explore lipid interactions with transcription factors, an Arabidopsis transcription factor library was screened for lipid binding (Kim et al., 2019). This led to the finding that PA binds to two closely related MYB transcription factors, CIRCADIAN CLOCK ASSOCIATED (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), which are core regulators of the circadian clock in Arabidopsis (Kim et al., 2019). In addition, PA was found to bind the AT-hook motif-containing nuclear localized (AHL) protein, AHL4. Further analysis revealed that the PA-AHL4 interaction modulates transcriptional regulation of triacylglycerol (TAG) degradation and seed germination (Cai et al., 2020).
The PA interactions with LHY/CCA1 and AHL4 were shown with filter binding, liposomal binding, surface plasmon resonance (SPR), and co-immunoprecipitation (Kim et al., 2019; Cai et al., 2020). The binding specificity has been tested against other phospholipids such as PC, phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylserine (PS), and moreover, different PA molecular species. For example, LHY binds to PA-containing, but not PC-only, liposomes, and it binds to specific PA species (e.g. di16:0-PA) but not di18:1PA (Fig. 6). In addition, LHY and PA were co-immunoprecipitated from plant extracts. Furthermore, fluorescence-labelled PA in plants was co-immunoprecipitated with PA-binding LHY/CCA1, but not with a non-PA binding transcription factor (Kim et al., 2019).
Fig. 6. Protein binding with different PA species.

Lipid immunoblotting of different PA species with various PA-binding proteins.
4.2. PA binding to proteins that translocate into nuclei in response to stimuli
PA was shown to interact with GAPCs in Arabidopsis, a cytosolic glycolytic enzyme (Kim et al., 2013; McLoughlin et al., 2013). A portion of GAPC molecules undergoes nuclear translocation from the cytoplasm in response to various stressors, including heat (Vescovi et al., 2013; Schneider et al., 2018; Kim et al., 2020). A recent study showed that PA is involved in the heat-induced nuclear localization of GAPC (Kim et al., 2022). The loss of PLDδ activity in Arabidopsis abolished nuclear PA accumulation and nuclear translocation of GAPC both induced by heat, suggesting that PLDδ mediates the heat-induced PA accumulation in nuclei and the nuclear localization of GAPC (Kim et al., 2022).
Several other proteins that interact with PA also undergo nuclear translocation. GIBBERELLIN (GA)-INSENSITIVE DWARF1 (GID1) is a soluble GA receptor that moves into nuclei in response to GA. PA binds to GID1 in rice, and the mutations of arginines 79 and 82 of GID1 that are required for PA binding abolish its nuclear localization (Cao et al., 2021). A recent study shows that PA binds ABA DEFICIENT 2 (ABA2) and suppresses its enzymatic activity. ABA2 was detected in and outside nuclei, and the loss of the nuclear DGK5 decreased the nuclear association of ABA2. Those results indicate that DGK5 and PA interact with ABA2 and suppress ABA production (Li et al., 2023a). In addition, the scaffolding A1 subunit of protein phosphatase 2A (PP2AA1) mediates the dephosphorylation of auxin transporter PIN-FORMED 1 (PIN1) and regulates the distribution of auxin. PA binds to PP2AA1 in Arabidopsis, and exogenous PA induces the accumulation of PP2AA1 on the membrane. The inhibition of PLD-mediated PA production by 1-butanol causes the perinuclear aggregation of PP2AA1 (Gao et al., 2013).
A recent study indicates that PA promotes the nuclear translocation of the calcium-dependent protein kinase (CDPKs /CPK) CPK12 in Arabidopsis upon low oxygen (hypoxia) stress (Fan et al., 2023). CPKs play important roles in plant development and stress response by sensing the calcium signals induced by developmental and environmental stimuli and phosphorylating different substrate proteins (Yip Delormel and Boudsocq, 2019). CPK12 is activated during hypoxia stress and translocated from the cytoplasm to the nucleus, where it interacts and stabilizes the core regulator of hypoxia sensing group VII ethylene-responsive transcription factors (ERF-VII). PA binds to CPK12, and the application of PA promotes the nuclear translocation of CPK12, whereas the inhibition of PLD-mediated PA production abolishes it upon hypoxia stress (Fan et al., 2023).
4.3. PA molecular species display binding selectivity for protein interactions
PA can exist as various molecular species due to the numbers of carbons and double bonds of two fatty acyl chains. PA-binding proteins exhibit varied binding preferences to different PA molecular species (Zhang et al., 2004; Guo et al., 2011; Kim et al., 2013; Yao et al., 2013). For example, ABSCISIC ACID INSENSITIVE 1 (ABI1), a protein phosphatase 2C that negatively regulates plant response to the stress hormone ABA, displayed binding to di18:1-PA but not to di16:0-PA (Fig. 6; Zhang et al., 2004). This binding property is in stark contrast with LHY that binds 16:0-containing PA species (e.g. di16:0 and 16:0/18:1-PA) but not di18:1-PA (Kim et al., 2019; Fig. 6). Another PA-binding transcription factor AHL4 binds PA containing unsaturated fatty acids (e.g. 16:0/18:1 and di18:1-PA), but not PA containing two saturated fatty acids (e.g. di16:0 and di18:0-PA; Cai et al., 2020). By comparison, sphingosine kinases bind to 16:0/18:1-PA and di18:1-PA equally well, but not di18:0-PA or di18:2-PA (Guo et al., 2011; Fig. 6), whereas GAPCs displayed no specific preference for PA species tested (Kim et al., 2013). The binding specificity has been verified using liposomal binding and in some cases with SPR (Guo et al., 2011; Kim et al., 2013). In addition, LHY immunoprecipitated from plants has 16C-containg PA associated (Kim et al., 2019).
Lipidomic analyses revealed selective changes of PA species abundance as affected by genetic alterations of lipid metabolic enzymes and stress conditions. For example, among 23 PA species measured, the level of 34:3-PA was decreased by disruption of pPLAIIIβ whereas the levels of 34:3-PA and 7 major PA species, such as 34:1, 34:2, 36:2, 36:3, 36:4, 36:5 and 36:6-PA, were increased by overexpression of pPLAIIIβ (Li et al, 2011). A recent study reported that the nuclear levels of 34:2-, 34:3-, and 36:6-PA were decreased in DGK5-KO, while 34:1-, 34:2-, 36:2-, 36:3-, and 36:4-PA -were increased in DGK5-OE, compared to WT, with the levels of other PA species comparable to those of WT (Li et al., 2024). NaCl stress decreased the nuclear levels of 34:3-, 36:2-, 36:4-, and 36:5-PA in DGK5-KO and increased those of 34:2-, 34:3-, and 36:4-PA in DGK5-OE, with the nuclear levels of other PA species in the DGK5-altered plants being comparable to those in WT(Li et al., 2024). Heat stress increased the levels of 34:2, 34:3, 36:3 and 36:4-PA in nuclei while increasing levels of the most species of total cellular PA (Kim et al., 2022). The contents of PA species vary depending on photoperiod and circadian conditions as well. 34:2, 34:3, 34:6, 36:4, 36:5 and 36:6-PA highly accumulated at dawn when compared to dusk, and 34:4 and 36:6-PA levels oscillated diurnally (Maatta et al., 2012; Kim et al., 2019).
Such specific interactions and their biological significances are supported by structural analysis. For example, the hydrophobic residues at the C-terminus of the Arabidopsis actin capping protein (AtCP) are inserted into the lipid bilayer containing PA, and this interaction is likely to be regulated by the length of the PA acyl chains (Huang et al., 2006; Pleskot et al., 2012). Changes in PA levels in specific membranes can directly affect the PA-targeted proteins, their localization, activity, and biological functions. These results suggest that the acyl chain composition of PA is an important determinant of PA interactions with specific target proteins.
5. PA effects on nuclear and physiological processes
The involvement of PA in nuclear functions is supported currently by two categories of evidence. One is the interaction of PA with proteins that function in nuclei, such as transcription factors. The other is the impact of the genes and enzymes that affect nuclear PA production and cellular processes. Manipulations of the nuclear PA-producing reactions shed light on the role of nuclear PA in specific cellular and physiological processes.
5.1. Lipid modulation of circadian clock function
The finding that PA binding to the core regulators of the circadian clock LHY/CCA1 provided valuable insights into the interconnection between lipid metabolism and molecular clock functioning (Fig. 7). LHY and CCA1, together with TIMING OF CAB EXPRESSION1 (TOC1), constitute a central loop in the molecular clock of Arabidopsis. LHY and CCA1 accumulate in the morning and suppress TOC1 expression by binding to its promoter while TOC1 in turn, represses LHY/CCA1 expression in the evening (Alabadí et al., 2001; Gendron et al., 2012; Huang et al., 2012). Increasing levels of 16C-PA species that bind to LHY impeded the LHY binding to TOC1pro, interfering with LHY’s association with target gene promoters (Kim et al., 2019). The effect of the PA-LHY/CCA1 interaction on the circadian clock is supported by the finding that perturbations of PA metabolism alter clock function (Fig. 7A, B). The pah1pah2 that had elevated levels of PA exhibited longer periods in TOC1 expression and leaf movements than those of WT (Fig. 7B). Conversely, when the PA production by PLD or DGK was suppressed, the oscillation of leaf movement was approximately one hour shorter than solvent controls (Kim et al., 2019). The opposing changes in circadian period length from the increased and decreased PA levels suggest that altered PA metabolism affects the PA-LHY/CCA1 interaction and impacts clock function (Kim et al., 2019).
Fig. 7. Interconnection between the circadian clock and lipid metabolism,

A. Reciprocal regulation between LHY/CCA1 and PA, B. Altered PA levels affect clock outputs. Increased PA by loss of PAH1 and PAH2 (pah1pah2) lengthens the oscillation of vertical leaf movement. Plants were entrained to 12-h light/12-h dark cycle for 5 days and leaf movement was monitored under constant light. Values are means ± S.D. (n=12) normalized to initial leaf position. Period length is in parenthesis. PAH-com, pah1pah2 complemented with PAH1. C. PA-LHY/CCA1 bind the evening element (EE) and/or CBS of KASIII and promotes its expression that mediates the first condensation reaction of fatty acid biosynthesis. PA inhibits LHY/CCA1 binding to KASIII promoter, suppressing LHY/CCA1’s promotion.
Studies in animal systems also indicate an interplay between lipids and the circadian clock. Clock misalignments, such as shift work, chronic jet lag, and sleep deprivation, and clock gene mutations are associated with various lipid metabolic diseases, such as obesity and nonalcoholic fatty liver disease (Shi et al., 2013; Panda, 2016; Chaix et al., 2019). The core clock regulators BMAL1 and CLOCK play an important role in the modulation of fat storage, utilization, and adipocyte differentiation (Friedrichs et al., 2018). C16:0, a common fatty acid in the human diet, alters the expression of clock-regulated genes and disrupts circadian rhythms (Guo et al., 2018), and high-fat diets impaired BMAL1 recruitment to target chromatin sites and rhythmic expression of clock genes (Vollmers et al., 2009; Eckel-Mahan et al., 2013; Wehrens et al., 2017; Sato et al., 2018; Budai et al., 2019). The effect of a high-fat diet on circadian gene expression is mediated in part through PPARγ effect on BMAL/CLOCK (Eckel-Mahan et al., 2013). PPARs bind to CRY1/2 (Kriebs et al., 2017), and PPARα binds to PER2 and modulates the circadian expression of BMAL1 (Canaple et al., 2006; Schmutz et al., 2010), which is also regulated by PPARγ (Wang et al., 2008). PPARs’ role in circadian regulation is through interaction with lipids because fatty acids, eicosanoids, and PA are ligands for PPAR activity. PA binds to PPARα and reduces its ability to bind the promoter of epidermal growth factor receptor (EGFR), repressing the EGFR expression in cancer cells (Mahankali et al., 2015). In addition, the level of an 18:0-containing PA species oscillates in the nucleus in mouse liver cells (Aviram et al., 2016). Suppression of PA production inhibited the rhythmic expression of BMAL1 in human cells and the effect was associated with its attenuation of the mTOR pathway (Walton et al., 2018). These results indicate that PA may directly and/or indirectly regulate clock functions in mammals.
5.2. Transcriptional regulation of lipid synthesis and seed oil production
Genetic alterations of the clock transcriptional regulators LHY/CCA1 affect lipid metabolism and accumulation (Hsiao et al., 2014; Kim et al., 2019). The levels of specific species, including 16C-containing PA, display cycling with a period of ~24-hour in WT, but not in lhy cca1 (Kim et al., 2019). Similar lipid oscillation was observed previously, but whether it was due to diel cycles in the environment or under circadian regulation was unclear (Maatta et al., 2012). The analysis with lhy cca1 mutants suggests that the lipid level changes observed in WT are influenced by the circadian clock, rather than just as a response to a light-dark cycle. Furthermore, the storage TAG content in seeds was decreased in lhy cca1 but increased in LHY-OE plants, compared to WT (Kim et al., 2019, 2023). Similarly, CCA1-OE plants also displayed an increase in seed oil content (Hsiao et al., 2014). The opposite effects on oil content in lhy cca1 and their OE plants are consistent with their effect on circadian periods because lhy cca1 plants are short-period (i.e. ~19 hours) whereas LHY-/CCA1-OE plants are long-period to arrhythmic, as the level of overexpression increases (Farinas and Mas, 2011; Rawat et al., 2011).
As evidence for how the perturbation of the clock and changed circadian periods lead to the opposite effects on lipid accumulation, our recent study shows that LHY/CCA1 regulate the initial condensation step of fatty acid biosynthesis in Arabidopsis (Kim et al., 2023). Increased LHY expression enhanced FA synthesis, and the expression of KASIII that encoded β-ketoacyl-ACP synthase III was oppositely changed in developing seeds of LHY/CCA1-OEs and lhycca1. Chromatin immunoprecipitation, electrophoretic mobility shift, and transactivation assays indicated that LHY directly bound and activated the promoter of KASIII (Fig. 7C). PA, a metabolic precursor of TAG, inhibited LHY binding to KASIII promoter elements, suggesting that PA acts as a negative effector modulating the LHY/CCA1 promotion of storage lipid production (Fig. 7C; Kim et al., 2023).
5.3. Transcriptional regulation of lipid degradation and seed germination
Transcriptional control plays important roles in metabolic regulation because it can affect the expression of a network of multiple genes. In lipid biosynthesis, transcription factors, such as WRINKLED 1 (WRI1), LEAFY COTYLEDONs (LECs), and FUSCA 3 (FUS3), regulate the expression of multiple genes contributing to lipid synthesis and TAG accumulation (Focks and Benning, 1998; Luerssen et al., 1998; Santos Mendoza et al., 2005; Santos‐Mendoza et al., 2008; Shen et al., 2010; Elahi et al., 2015). However, little was known about the transcriptional regulation of lipid degradation in plants.
The characterization of the PA-AHL4 interaction led to the finding that AHL4 suppresses the expression of genes for TAG lipases and for enzymes in fatty acid β-oxidation during seedling establishment and growth (Cai et al., 2020). AHL4 bound to the promoter regions of the genes encoding the TAG lipases SDP1 and DALL5 and acyl-thioesterase KAT5 involved in fatty acid β-oxidation (Cai et al., 2020). Those genes contained AHL4-binding cis-elements and the AHL4 interaction with the promoter region was suppressed by PA species that bound to AHL4. The expression levels of AHL4-targeted genes, SDP1, DALL5, and KAT5, were decreased in pldα1pldδ that had a lower PA content but increased in pah1pah2 mutants with a higher PA level (Cai et al., 2020). The rate of seed TAG degradation during and after germination was lower in the seeds and seedlings of AHL4 OEs, but higher in those of AHL4 KOs. These results indicate that an increase in PA releases the suppression of AHL4 on its target genes, and, in turn, increases TAG hydrolysis and fatty acid oxidation to provide the energy and substrates for seedlings establishment and development.
5.4. PA in hormone signaling and production
PLD, NPC, and PA have been shown to mediate ABA signaling and auxin distribution (Zhang et al., 2004; Mishra et al., 2006; Li and Xue, 2007; Peters et al., 2010; Wang et al., 2019). The protein phosphatase 2C ABI1 negatively regulates ABA response, which requires its catalytic activity and nuclear localization (Moes et al., 2008; Ma et al., 2009). PA binds ABI1 and inhibits phosphatase activity of ABI1, which in turn promotes ABA response. The pldα displayed decreased ABA-induced PA production and impaired ABA-mediated stress response but had increased nuclear accumulation of ABI1 (Zhang et al., 2004; Mishra et al., 2006). PA application increased the membrane association of the PA-binding protein PP2AA1, which enhances protein phosphatase 2A (PP2A) activity (Gao et al., 2013). In addition, PA binds PINOID kinase (PID), increases the plasma membrane association of PID, and promotes PID-dependent phosphorylation of PIN, which regulates the efflux and redistribution of auxin (Wang et al., 2019). These results indicate that PA is involved in ABA response and auxin distribution by modulating the protein activity and/or subcellular distribution in and outside nuclei.
Recent results indicate that PA action in nuclei is involved in hormone signaling and metabolism (Fig. 8). PA interacts with the GA receptor GID1 in rice and affects the translocation of GID1 into nuclei (Cao et al., 2021). The GA-induced nuclear localization of GID1 and degradation of the DELLA protein SLENDER RICE1 (SLR1) are impaired in pldα6 (Cao et al., 2021). Another study suggested that the inhibition of PA production by 1-butanol abolished the salicylic acid (SA)-induced nuclear localization of the SA receptors non-expresser of pathogenesis-related protein (NPR1) (Janda et al., 2015). SA plays a critical role in plant defense response, and the nuclear localization of NPR1 is required to activate the expression of pathogenesis-related (PR) genes (Kinkema et al., 2000; Yan and Dong, 2014).
Fig. 8. Effects of PA on nuclear processes in hormone signaling and production.

Refer the text for the effect on specific processes. For brevity, effect on specific pathways is shown, but crosstalk among pathways exists. In addition, PA from PLDα1 and PLDδ both are involved, but in different steps of ABA signaling. Please refer to text for abbreviations and details.
In addition, DGK5 and its product PA bind the ABA synthesizing enzyme ABA2 and reduce the enzymatic activity of ABA2 (Fig. 8; Li et al., 2023a). ABA content was increased in DGK5-KO plants but decreased in DGK5-OE plants. DGK5-KO plants were more resistant to water and salt stress, but DGK5-OE plants were more sensitive to those stressors. In addition, both DGK5 and ABA2 were localized in and outside nuclei, and the in vivo interaction between DGK5 and ABA2 mainly occurred in nuclei. Moreover, ABA2 was accumulated less in nuclei in DGK5-KOs plants (Li et al., 2023a). These results indicate that DGK5 and PA regulate ABA production by regulating the enzymatic activity and/or subcellular distribution of ABA2.
5.5. PA in hypoxia responses
PA levels have been reported to increase in response to hypoxia in Arabidopsis and wheat (Xie et al., 2015; Xu et al., 2020). Both pldα and pldδ displayed increased sensitivity to hypoxia (Zhou et al., 2022). Hypoxia stress, which is usually caused by root waterlogging and submergence in plants, has a negative effect on plant growth and production (León et al., 2021; Xie et al., 2021). The submergence of plants in water causes the gaseous hormone ethylene to entrap in the submerged tissues, and ethylene has been found to play an important role in hypoxia acclimation and metabolic adjustment in response to flooding-induced hypoxia stress (Hartman et al., 2021). In the absence of ethylene, the Raf-like protein kinase CONSTITUTIVE TRIPLE RESPONSE1 (CTR1) phosphorylates the C-terminal domain of ETHYLENE-INSENSITIVE2 (EIN2), preventing the nuclear localization of EIN2, whereas, in presence of ethylene, CTR1 perceives the signal from the ethylene receptor complex and becomes inactive (Fig. 8). The unphosphorylated C-terminal domain of EIN2 is then cleaved and translocated from the ER membrane into nuclei, where it regulates its downstream transcription factors (Ju et al., 2012). A previous study showed that PA bound CTR1, inhibited its kinase activity, and impaired the interaction between CTR1 and the ethylene receptor ETR1 (Testerink et al., 2007). Additionally, exogenous PA promotes the nuclear translocation of EIN2 (Xie et al., 2015). A recent study shows that PA binds CPK12 and promotes its nuclear translocation (Fan et al., 2023; Fig. 8). The cytoplasm to nuclear translocation of CPK12 under hypoxia stabilizes the core regulator of hypoxia signaling and response (Fan et al., 2023).
In addition, PA also promotes the translocation of an ethylene-responsive transcription factor RAP2.12 from the PM into the nuclei (Zhou et al., 2022). RAP2.12 is involved in the regulation of plant metabolism under hypoxia stress, and low oxygen induces the translocation of RAP2.12 into the nucleus, where it induces the expression of hypoxia-responsive genes (Hinz et al., 2010; Kosmacz et al., 2015; Paul et al., 2016). The binding of PA to mitogen-activated protein kinase 6 (MPK6) stimulates its kinase activity and increases the MPK6-mediated phosphorylation of RAP2.12, which activates the transcriptional activity of RAP2.12 (Yu et al., 2010; Zhou et al., 2022). These results indicate that PA is involved in plant hypoxia response via directly or indirectly modulating the nuclear accumulation of proteins that regulate hypoxia sensing and response (Fig. 8).
5.6. GAPC nuclear moonlighting in plant stress responses
GAPC is a cytosolic metabolic enzyme involved in glycolysis and has also moonlighting functions in plant responses to different stress conditions. One mode of GAPC’s non-metabolic actions is via its intracellular translocation from the cytoplasm to the nucleus under stress, including cadmium, hydrogen sulfide, bacterial flagellin, and heat (Vescovi et al., 2013; Henry et al., 2015; Aroca et al., 2017; Kim et al., 2020). Nuclear GAPC has been reported to mediate stress responses as a transcriptional regulator (Zhang et al., 2017a; Kim et al., 2020). In response to heat, for example, some of GAPC molecules were translocated into nuclei, where it bound and activated a transcription factor known to regulate the expression of heat-inducible genes, enhancing the thermotolerance of Arabidopsis (Fig. 2; Kim et al., 2020).
As a mechanism of how heat induced GAPC nuclear translocation, a recent study shows that PLDδ and its lipid product PA mediates the nuclear translocation of GAPC in Arabidopsis under heat (Fig. 2; Kim et al., 2023). Previously, heat stress was shown to induce a rapid PA increase, which resulted mainly from PLD activation even though specific PLD(s) for the process remained unknown (Mishkind et al., 2009). In addition, both PLDδ and PA were found to interact with GAPC in Arabidopsis (Guo et al., 2012; Kim et al., 2013). PLDδ is associated with the plasma membrane, and its intracellular distribution is not affected by heat stress (Wang and Wang, 2001; Zhang et al., 2017b). Thus, PLDδ may not directly co-move with GAPC for the nuclear translocation of GAPC under heat stress. Instead, the PLDδ-mediated PA production is required for the nuclear translocation of GAPC, and colocalization and interaction of PA-GAPC in the nucleus during heat stress are inhibited by the membrane trafficking inhibitor BFA (Kim et al., 2022). These results indicate that during heat stress, PA produced by PLDδ mediates the translocation of GAPC into the nucleus, where GAPC promotes the expression of heat-inducible genes and regulates the thermotolerance of Arabidopsis (Fig. 2).
5.7. PA effects on root architecture
The development of proper root architecture is crucial to the water and nutrient uptake of plants and communication with other plants and the environment (Motte et al., 2019; Maurel et al., 2020). The analysis of DGKs in rice reveals that DGK1, which is predicted to be associated with the nucleus, and its associated lipid mediators, DAG and PA, play an important role in root architecture in rice (Yuan et al., 2019). KO plants of OsDGK1 had more lateral roots and smaller seminal root radius than those of WT whereas overexpression of OsDGK1 resulted in fewer lateral roots with larger radius. Exogenous DAG and PA had the opposite effect on lateral root number and seminal root radius, and the addition of PA, which is the product of DGK-mediated phosphorylation of DAG, restored the root phenotype of OsDGK1 KO to that of WT (Yuan et al., 2019). DAG in animal cells is a potent cellular messenger (Dong et al., 2012; Eichmann and Lass, 2015), but the signaling functions of DAG remain elusive in plants. The loss of NPC5, which hydrolyzes phospholipids to produce DAG decreased the DAG content in roots and caused fewer lateral roots under mild salt stress (Peters et al., 2014). The application of exogenous DAG restored the lateral root number of NPC5-KO to that of WT, but the addition of PA failed to rescue the later root phenotype of NPC5-KO. In contrast to the inhibitory effect of PA on lateral root development, PA promotes the elongation of primary roots. Inhibition of DAG conversion to PA by a DGK inhibitor caused shorter primary roots (Gómez-Merino et al., 2005). These results indicate that PA and DAG have opposite effects on lateral root development.
Several PLDs, which hydrolyze phospholipids to produce PA, are involved in primary root elongation. The pldα1pldδ mutants, which had lower PA contents, had shorter primary roots under salt stress, and exogenous PA restored the primary root length of pldα1pldδ to that of WT under salt stress (Wang et al., 2019). The loss of PLDζ in Arabidopsis decreased the primary root length under phosphate deficiency (Li et al., 2006; Su et al., 2018). Moreover, the overexpression of PLDɛ, which increased PA contents, increased primary root length in Arabidopsis, canola, and soybean (Hong et al., 2009; Lu et al., 2016; Yao et al., 2022). These results together show that PA and DAG play important roles in the development of root architecture; PA enhances primary root elongation but inhibits lateral root development whereas DAG promotes lateral root development. However, further studies are needed to establish the direct connection between nuclear PA and its effects on root architecture.
6. PA in nuclear membrane remodeling and homeostasis
In addition to their signaling roles, PA and DAG are metabolic precursors for membrane synthesis, and changes in their cellular levels affect nuclear membrane remodeling, homeostasis, and functions. Defects in PAH that convert PA to DAG lead to an abnormal nuclear envelope (NE) in yeast and metazoan. The loss of PAH homolog Smp2 in yeast caused enlarged nuclei with long nuclear membranes (Santos-Rosa et al., 2005). Down-regulation of PAH homolog Lipin-1 by RNAi in Caenorhabditis elegans impaired the breakdown of NE during mitosis and resulted in binucleated cells (Gorjánácz and Mattaj, 2009). The catalytic activity of animal PAH homolog lipin was also found to be involved in increased nuclear eccentricity (Peterson et al., 2011). One possibility is that the PA/DAG ratio serves as a signal for feedback regulatory pathways that control overall lipid homeostasis. In budding yeast, PA accumulates in nuclear envelope herniations that form from the hyperactivation of the ESCRT-III (endosomal sorting complex required for transport III) nuclear envelope remodeling machinery (Thaller et al., 2021). The ESCRT machinery plays key roles in membrane remodeling and protecting the nuclear envelope integrity (Vietri et al., 2020). PA binds to the NE-specific ESCRT, Chm7, and an increase in cellular PA levels leads to the translocation of Chm7 from the cytosol to the NE/ER membrane (Thaller et al., 2021). The data suggest that the local accumulation of PA on NE recruits Chm7 to the fusion sites of the inner and outer nuclear membranes and contributes to the NE sealing during nuclear pore complex mis-assembly (Thaller et al., 2021).
In Arabidopsis, pah1pah2 that lost both PA phosphohydrolases exhibited overexpansion of the ER membrane although the volume of the nucleus was not greatly enlarged in the cells of pah1pah2 leaves (Eastmond et al., 2010). Compared to WT, pah1pah2 had a higher level of nuclear PA, and the major nuclear PA species (34:2, 34:3, 34:4, 36:2, 36:3, 36:4, 36:5, and 36:6) were all increased (Cai et al., 2020). Conversely, the PLD mutants pldα1pldδ had a lower level of nuclear PA, with the levels of major nuclear PA species being decreased compared to those in WT. The nuclear PA changes in response to stress, such as high temperature and salinity. Under heat stress, nuclear PA levels increased in WT, but not in pldδ, suggesting that PA produced by PLDδ in the plasma membrane possibly moves to the nucleus (Kim et al., 2022). Under salt stress, DGK5-KOs had a 40% lower nuclear PA level but a 17% higher nuclear DAG level than WT, whereas DGK5-OEs displayed a 45% higher nuclear PA level but a 16% lower nuclear DAG level than WT. Thus, the nuclear PA/DAG ratio was lower in DGK5-KOs, but higher in DGK5-OEs (Li et al., 2024). These results indicate that DGK5 regulates the nuclear PA/DAG homeostasis in Arabidopsis. Mechanisms that control nuclear membrane remodeling are essential to maintain the integrity and function of the nucleus, but they remain to be fully elucidated.
7. Future perspectives
PA has emerged as an important class of cellular mediators, and perturbations of its metabolism and signaling function affect various cellular and physiological processes. The mode of PA’s action has been studied primarily outside the nuclei, but the recent findings of PA signaling and function in nuclei, as described here, open a new direction to investigate and understand the regulatory function of PA. The nuclear function of PA may underlie a basis for its role in regulating gene expression, cell proliferation, and stress responses. However, the precise mechanisms of PA actions in nuclei require further elucidation. One open question is whether nuclear PA changes are associated with the nuclear envelope, nucleoplasm reticulum, and/or nucleoplasm. Developing effective nuclear PA probes enabling nuclear PA detection and quantification in vivo will help address this question. Another question is how the PA binding to a nuclear protein affects the protein functions, such as its structure, membrane association, catalytic activity, and/or interaction with other proteins or other nuclear components, such as nucleic acids and chromatin. In addition, how does PA move into and out of nuclei, such as via membrane contact site, lipid movements in the continuous membrane between ER and nuclear envelope, or vesicular trafficking? How does PA affect protein trafficking into nuclei, such as via vesicular trafficking, nuclear pore complex, and/or interaction with other proteins? Increasing results indicate the importance of the acyl chain composition of PA in PA-protein interactions, which could underlie a basis for the specificity and diverse functions of PA. However, except for binding specificity for proteins, little is known about the effect of the acyl groups on the biological functions of PA and biochemical reactions and specific conditions by which the specific PA acyl species are produced. Furthermore, besides being the substrate and product of PA metabolism (Fig. 1), DAG is a mediator in plants, and the role of PA/DAG homeostasis in nuclei and plant growth, development, and stress responses requires more attention.
Moreover, how PA, despite having the simplest structure, has such diverse functions has been a long-standing question in the field. The characterization of PA-metabolizing enzymes, such as PLD, DGK, and NPC families, has begun to shed light on the issue. Many of the individual members of a given family, such as PLD, display distinguishable regulatory properties, subcellular associations, stimulus-induced temporal and spatial expression, and/or substrate preferences (Table 1). It is conceivable that those characteristics collectively lead to cellular regulation of PA changes in terms of specific temporal and spatial patterns and acyl composition, as well as in response to specific stimuli. The PA signature is decoded in the cell, at least in part, by its interaction with different effector proteins, such as transcription factors and those involved in hormone signaling, transport, and production (Fig. 8). The production and distribution of the effector proteins themselves are tightly regulated. Those properties in combination could underly a basis for the diverse cellular effects of PA. The discovery of PA signaling in nuclei and further elucidation of the process will advance the understanding of the mechanism by which PA mediates cellular functions, which may unveil new regulatory mechanisms for gene expression, lipid metabolism, and stress responses.
Acknowledgments
The research in X.W. lab was supported by grants from the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM141374, the National Science Foundation under Grants No.2222157 and 2302424, and the USDA National institute of Foods and Agriculture 2020-67013-30908/project accession number 1022148.
Abbreviation:
- PA
phosphatidic acid
- DAG
diacylglycerol
- PLA
phospholipase A
- PLD
phospholipase D
- DGK
DAG kinase
- PLC
phospholipase C
- NPC
nonspecific phospholipase C
- PI-PLC
phosphatidylinositol-specific PLC
- TAG
triacylglycerol
- PC
phosphatidylcholine
- MGDG
monogalactosyldiacylglycerol
- PG
phosphatidyl glycerol
- ABA
abscisic acid
- GA
gibberellic acid
- MAPK
mitogen-activated protein kinase
- PAP
phosphatidic acid phosphatase
- PKC
protein kinase C
- LPP
lipid phosphate phosphatase
- IAA
indole acetic acid
- BL
brassinolide
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- IP3
inositol 1,4,5-trisphosphate
- PI(4)P
phosphatidylinositol-4-phosphate
- PI
phosphatidylinositol
- ABI1
ABA insensitive 1
- ABA2
ABA insensitive 2
- GAPC
cytosolic glyceraldehyde-3-phosphate dehydrogenase
- NADPH
nicotinamide adenine dinucleotide phosphate
- SPHK
sphingosine kinase
- WER
werewolf
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Alabadí D, Oyama T, Yanovsky MJ, Harmon FG, Más P, Kay SA (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science. 293, 880–883. [DOI] [PubMed] [Google Scholar]
- 2.Ali U, Lu S, Fadlalla T, Iqbal S, Yue H, Yang B, Hong Y, Wang X, Guo L (2022) The functions of phospholipases and their hydrolysis products in plant growth, development and stress responses. Prog Lipid Res. 86, 101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arii J (2021) Host and viral factors involved in nuclear egress of herpes simplex virus 1. Viruses. 13, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aroca A, Schneider M, Scheibe R, Gotor C, Romero LC (2017) Hydrogen Sulfide Regulates the Cytosolic/Nuclear Partitioning of Glyceraldehyde-3-Phosphate Dehydrogenase by Enhancing its Nuclear Localization. Plant Cell Physiol. 58, 983–992. [DOI] [PubMed] [Google Scholar]
- 5.Aviram R, Manella G, Kopelman N, Neufeld-Cohen A, Zwighaft Z, Elimelech M, Adamovich Y, Golik M, Wang C, Han X, Asher G (2016) Lipidomics Analyses Reveal Temporal and Spatial Lipid Organization and Uncover Daily Oscillations in Intracellular Organelles. Mol Cell. 62, 636–648. [DOI] [PubMed] [Google Scholar]
- 6.Barbaglia AM, Tamot B, Greve V, Hoffmann-Benning S. (2016) Phloem Proteomics Reveals New Lipid-Binding Proteins with a Putative Role in Lipid-Mediated Signaling. Front Plant Sci 7,563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barger SR, Penfield L, Bahmanyar S (2022) Coupling lipid synthesis with nuclear envelope remodeling. Trends Biochem Sci. 47, 52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhosle VK, Rivera JC, Chemtob S (2019). New insights into mechanisms of nuclear translocation of G-protein coupled receptors. Small GTPases 10, 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohdanowicz M, Schlam D, Hermansson M, Rizzuti D, Fairn GD, Ueyama T, Somerharju P, Du G, Grinstein S (2013) Phosphatidic acid is required for the constitutive ruffling and macropinocytosis of phagocytes. Mol Biol Cell. 24, 1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boldyrev IA, Zhai X, Momsen MM, Brockman HL, Brown RE, Molotkovsky JG (2007) New BODIPY lipid probes for fluorescence studies of membranes. J Lipid Res. 48, 1518–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown HA, Thomas PG, Lindsley CW (2017) Targeting phospholipase D in cancer, infection and neurodegenerative disorders. Nat. Rev. Drug Discov 16, 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budai Z, Balogh L, Sarang Z (2019) Short-term high-fat meal intake alters the expression of circadian clock-, inflammation-, and oxidative stress-related genes in human skeletal muscle. Int J Food Sci Nutr. 70, 749–758. [DOI] [PubMed] [Google Scholar]
- 13.Bullen HE, Soldati-Favre D (2016) A central role for phosphatidic acid as a lipid mediator of regulated exocytosis in apicomplexa. FEBS Lett. 590, 2469–81. [DOI] [PubMed] [Google Scholar]
- 14.Burns LT, Wente SR (2012) Trafficking to uncharted territory of the nuclear envelope. Curr. Opin. Cell Biol 24, 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai G, Kim SC, Li J, Zhou Y, Wang X (2020) Transcriptional Regulation of Lipid Catabolism during Seedling Establishment. Mol Plant. 13, 984–1000. [DOI] [PubMed] [Google Scholar]
- 16.Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, Michalik L, Delaunay F, Wahli W, Laudet V (2006) Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol Endocrinol. 20, 1715–1727. [DOI] [PubMed] [Google Scholar]
- 17.Cao H, Gong R, Yuan S, Su Y, Lv W, Zhou Y, Zhang Q, Deng X, Tong P, Liang S, Wang X, Hong Y (2021) Phospholipase Dα6 and phosphatidic acid regulate gibberellin signaling in rice. EMBO Rep. 22, e51871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carman GM, Han GS (2019) Fat-regulating phosphatidic acid phosphatase: a review of its roles and regulation in lipid homeostasis. J Lipid Res. 60, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaix A, Lin T, Le HD, Chang MW, Panda S (2019) Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 29, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Greer MS, Weselake RJ (2013) Plant phospholipase A: advances in molecular biology, biochemistry, and cellular function. Biomol Concepts. 4, 527–532. [DOI] [PubMed] [Google Scholar]
- 21.Corbeil D, Santos MF, Karbanová J, Kurth T, Rappa G, Lorico A (2020) Uptake and Fate of Extracellular Membrane Vesicles: Nucleoplasmic Reticulum-Associated Late Endosomes as a New Gate to Intercellular Communication. Cells. 9, 1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cocco L, Gilmour RS, Ognibene A, Letcher AJ, Manzoli FA, Irvine RF (1987) Synthesis of polyphosphoinositides in nuclei of Friend cells. Evidence for polyphosphoinositide metabolism inside the nucleus which changes with cell differentiation. Biochem J. 248, 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contreras FX, Sánchez-Magraner L, Alonso A, Goñi FM (2010) Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett. 584, 1779–1786. [DOI] [PubMed] [Google Scholar]
- 24.Corrotte M, Chasserot-Golaz S, Huang P, Du G, Ktistakis NT, et al. (2006) Dynamics and function of phospholipase D and phosphatidic acid during phagocytosis. Traffic 7, 365–377. [DOI] [PubMed] [Google Scholar]
- 25.Dong W, Lv H, Xia G, Wang M (2012) Does diacylglycerol serve as a signaling molecule in plants? Plant Signal Behav. 7, 472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du G, Frohman MA (2009) A lipid-signaled myosin phosphatase surge disperses cortical contractile force early in cell spreading. Mol Biol Cell 20, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eastmond PJ, Quettier A, Kroon JTM, Craddock C, Adams N, Slabas AR (2010) Phosphatidic acid phosphohydrolase 1 and 2 regulate phospholipid synthesis at the endoplasmic reticulum in Arabidopsis. Plant Cell. 22, 2796–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckel-Mahan KL, Patel VR, de Mateo S, Orozco-Solis R, Ceglia NJ, Sahar S, Dilag-Penilla SA, Dyar KA, Baldi P, Sassone-Corsi P (2013) Reprogramming of the circadian clock by nutritional challenge. Cell. 155, 1464–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eichmann TO, Lass A (2015) DAG tales: the multiple faces of diacylglycerol--stereochemistry, metabolism, and signaling. Cell Mol Life Sci. 72, 3931–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elahi N, Duncan RW, Stasolla C (2015) Decreased seed oil production in FUSCA3 Brassica napus mutant plants. Plant Physiol Biochem. 96, 222–230. [DOI] [PubMed] [Google Scholar]
- 31.Fan B, Liao K, Wang LN, Shi LL, Zhang Y, Xu LJ, Zhou Y, Li JF, Chen YQ, Chen QF, Xiao S (2023) Calcium-dependent activation of CPK12 facilitates its cytoplasm-to-nucleus translocation to potentiate plant hypoxia sensing by phosphorylating ERF-VII transcription factors. Mol Plant. 16, 979–998. [DOI] [PubMed] [Google Scholar]
- 32.Fan L, Zheng S, Cui D, Wang X (1999) Subcellular distribution and tissue expression of phospholipase Dalpha, Dbeta, and Dgamma in Arabidopsis. Plant Physiol. 19, 1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farinas B, Mas P (2011) Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. Plant J. 66, 318–329. [DOI] [PubMed] [Google Scholar]
- 34.Ferraz-Nogueira JP, Díez-Guerra FJ, Llopis J (2014) Visualization of phosphatidic acid fluctuations in the plasma membrane of living cells. PLoS One. 9, e102526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Focks N, Benning C (1998) wrinkled1: A novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 118, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedrichs M, Kolbe I, Seemann J, Tsang AH, Cherradi L, Klein J, Oster H (2018) Circadian clock rhythms in different adipose tissue model systems. Chronobiol Int. 35, 1543–1552. [DOI] [PubMed] [Google Scholar]
- 37.Froidure S, Canonne J, Daniel X, Jauneau A, Brière C, Roby D, Rivas S (2010) AtsPLA2-alpha nuclear relocalization by the Arabidopsis transcription factor AtMYB30 leads to repression of the plant defense response Proc Natl Acad Sci U S A. 2010;107, 15281–15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao HB, Chu YJ, Xue HW (2013) Phosphatidic acid (PA) binds PP2AA1 to regulate PP2A activity and PIN1 polar localization. Mol Plant. 6, 1692–1702. [DOI] [PubMed] [Google Scholar]
- 39.Gao K, Liu YL, Li B, Zhou RG, Sun DY, Zheng SZ (2014) Arabidopsis thaliana phosphoinositide-specific phospholipase C isoform 3 (AtPLC3) and AtPLC9 have an additive effect on thermotolerance. Plant Cell Physiol. 55, 1873–1883. [DOI] [PubMed] [Google Scholar]
- 40.Gendron JM, Pruneda-Paz JL, Doherty CJ, Gross AM, Kang SE, Kay SA (2012) Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc Natl Acad Sci U S A. 109, 3167–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gómez-Merino FC, Arana-Ceballos FA, Trejo-Téllez LI, Skirycz A, Brearley CA, Dörmann P, Mueller-Roeber B (2005) Arabidopsis AtDGK7, the smallest member of plant diacylglycerol kinases (DGKs), displays unique biochemical features and saturates at low substrate concentration: the DGK inhibitor R59022 differentially affects AtDGK2 and AtDGK7 activity in vitro and alters plant growth and development. J Biol Chem. 280, 34888–34899. [DOI] [PubMed] [Google Scholar]
- 42.Gorjánácz M, Mattaj IW (2009) Lipin is required for efficient breakdown of the nuclear envelope in Caenorhabditis elegans. J Cell Sci. 122, 1963–1969. [DOI] [PubMed] [Google Scholar]
- 43.Guo L, Mishra G, Taylor K, Wang X (2011) Phosphatidic acid binds and stimulates Arabidopsis sphingosine kinases. J. Biol. Chem 286, 13336–13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo L, Devaiah SP, Narasimhan R, Pan X, Zhang Y, Zhang W, Wang X (2012) Cytosolic glyceraldehyde-3-phosphate dehydrogenases interact with phospholipase Dδ to transduce hydrogen peroxide signals in the Arabidopsis response to stress. Plant Cell. 24, 2200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo R, Zhao B, Wang Y, Wu D, Wang Y, Yu Y, Yan Y, Zhang W, Liu Z, Liu X (2018) Cichoric Acid Prevents Free-Fatty-Acid-Induced Lipid Metabolism Disorders via Regulating Bmal1 in HepG2 Cells. J Agric Food Chem. 66, 9667–9678. [DOI] [PubMed] [Google Scholar]
- 46.Harlos K, Eibl H (1981) Hexagonal phases in phospholipids with saturated chains: phosphatidylethanolamines and phosphatidic acids. Biochemistry. 20, 2888–92. [DOI] [PubMed] [Google Scholar]
- 47.Hartman S, Sasidharan R, Voesenek LACJ (2021) The role of ethylene in metabolic acclimations to low oxygen. New Phytol. 229, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henkels KM, Miller TE, Ganesan R, Wilkins BA, Fite K, Gomez-Cambronero J (2016) A Phosphatidic acid (PA) conveyor system of continuous intracellular transport from cell membrane to nucleus maintains EGF receptor homeostasis. Oncotarget. 7, 47002–47017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henry E, Fung N, Liu J, Drakakaki G, Coaker G (2015) Beyond glycolysis: GAPDHs are multi-functional enzymes involved in regulation of ROS, autophagy, and plant immune responses. PLoS Genet. 11, e1005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinz M, Wilson IW, Yang J, Buerstenbinder K, Llewellyn D, Dennis ES, Sauter M, Dolferus R (2010) Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiol. 153, 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Homan R, Pownall HJ (1988) Transbilayer diffusion of phospholipids: dependence on headgroup structure and acyl chain length. Biochim Biophys Acta. 938, 155–66. [DOI] [PubMed] [Google Scholar]
- 52.Hong Y, Devaiah SP, Bahn SC, Thamasandra BN, Li M, Welti R, Wang X (2009) Phospholipase Dε and phosphatidic acid enhance Arabidopsis nitrogen signaling and growth. Plant J. 58, 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong Y, Zhang W, Wang X (2010) Phospholipase D and phosphatidic acid signalling in plant response to drought and salinity. Plant Cell Environ. 33, 627–635. [DOI] [PubMed] [Google Scholar]
- 54.Hong Y, Zhao J, Guo L, Kim S, Deng X, Wang G, Li M, Wang X (2016) Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res 62, 55–74. [DOI] [PubMed] [Google Scholar]
- 55.Hsiao AS, Haslam RP, Michaelson LV, Liao P, Napier JA, Chye ML (2014). Gene expression in plant lipid metabolism in Arabidopsis seedlings. PLoS One. 9, e107372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang S, Gao L, Blanchoin L, Staiger CJ (2006) Heterodimeric capping protein from Arabidopsis is regulated by phosphatidic acid. Mol Biol Cell 17, 1946–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang W, Pérez-García P, Pokhilko A, Millar AJ, Antoshechkin I, Riechmann JL, Mas P (2012) Mapping the core of the Arabidopsis circadian clock defines the network structure of the oscillator. Science. 336, 75–79. [DOI] [PubMed] [Google Scholar]
- 58.Janda M, Šašek V, Chmelařová H, Andrejch J, Nováková M, Hajšlová J, Burketová L, Valentová O (2015) Phospholipase D affects translocation of NPR1 to the nucleus in Arabidopsis thaliana. Front Plant Sci. 6, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jang JH, Lee CS, Hwang D, Ryu SH (2012) Understanding of the roles of phospholipase D and phosphatidic acid through their binding partners. Prog Lipid Res. 51, 71–81. [DOI] [PubMed] [Google Scholar]
- 60.Ju C, Yoon GM, Shemansky JM, Lin DY, Ying ZI, Chang J, Garrett WM, Kessenbrock M, Groth G, Tucker ML, Cooper B, Kieber JJ, Chang C (2012) CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci U S A. 109, 19486–19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalachova T, Škrabálková E, Pateyron S, Soubigou-Taconnat L, Djafi N, Collin S, Sekereš J, Burketová L, Potocký M, Pejchar P, Ruelland E (2022) DIACYLGLYCEROL KINASE 5 participates in flagellin-induced signaling in Arabidopsis. Plant Physiol. 190, 1978–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kay JG, Koivusalo M, Ma X, Wohland T, Grinstein S (2012) Phosphatidylserine dynamics in cellular membranes. Mol Biol Cell. 23, 2198–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SC, Edgeworth KN, Nusinow NA, Wang X (2023) Circadian clock factors regulate the first condensation reaction of fatty acid synthesis in Arabidopsis. Cell Reports (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim SC, Guo L, Wang X (2013) Phosphatidic acid binds to cytosolic glyceraldehyde-3-phosphase dehydrogenase and promotes its cleavage in Arabidopsis. J Biol Chem. 288, 11834–11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim SC, Guo L, Wang X (2020) Nuclear moonlighting of cytosolic glyceraldehyde-3-phosphate dehydrogenase regulates Arabidopsis response to heat stress. Nat Commun. 11, 3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim SC, Nusinow DA, Sorkin ML, Pruneda-Paz J, Wang X (2019) Interaction and Regulation Between Lipid Mediator Phosphatidic Acid and Circadian Clock Regulators. Plant Cell. 31, 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim SC, Yao S, Zhang Q, Wang X (2022) Phospholipase Dδ and phosphatidic acid mediate heat-induced nuclear localization of glyceraldehyde-3-phosphate dehydrogenase in Arabidopsis. Plant J. 112, 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SC, Wang X (2020) Phosphatidic acid: an emerging versatile class of cellular mediators. Essays Biochem. 64, 533–546. [DOI] [PubMed] [Google Scholar]
- 69.Kinkema M, Fan W, Dong X (2000) Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell. 12, 2339–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klymchenko AS, Kreder R (2014) Fluorescent probes for lipid rafts: from model membranes to living cells. Chem Biol. 21, 97–113. [DOI] [PubMed] [Google Scholar]
- 71.Kolesnikov Y, Kretynin S, Bukhonska Y, Pokotylo I, Ruelland E, Martinec J, Kravets V (2022) Phosphatidic Acid in Plant Hormonal Signaling: From Target Proteins to Membrane Conformations. Int J Mol Sci. 23, 3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kooijman EE, Burger KN (2009) Biophysics and function of phosphatidic acid: a molecular perspective. Biochim Biophys Acta. 1791, 881–888. [DOI] [PubMed] [Google Scholar]
- 73.Kooijman EE, Tieleman DP, Testerink C, Munnik T, Rijkers DT, Burger KN, de Kruijff B (2007) An electrostatic/hydrogen bond switch as the basis for the specific interaction of phosphatidic acid with proteins. J Biol Chem. 282, 11355–11364. [DOI] [PubMed] [Google Scholar]
- 74.Kosmacz M, Parlanti S, Schwarzländer M, Kragler F, Licausi F, Van Dongen JT (2015) The stability and nuclear localization of the transcription factor RAP2.12 are dynamically regulated by oxygen concentration. Plant Cell Environ. 38, 1094–1103. [DOI] [PubMed] [Google Scholar]
- 75.Kriebs A, Jordan SD, Soto E, Henriksson E, Sandate CR, Vaughan ME, Chan AB, Duglan D, Papp SJ, Huber AL, Afetian ME, Yu RT, Zhao X, Downes M, Evans RM, Lamia KA (2017) Circadian repressors CRY1 and CRY2 broadly interact with nuclear receptors and modulate transcriptional activity. Proc Natl Acad Sci U S A. 114, 8776–8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwiatek JM, Gutierrez B, Izgu EC, Han GS, Carman GM (2022) Phosphatidic Acid Mediates the Nem1-Spo7/Pah1 Phosphatase Cascade in Yeast Lipid Synthesis. J Lipid Res. 63, 100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.León J, Castillo MC, Gayubas B (2021) The hypoxia-reoxygenation stress in plants. J Exp Bot. 72, 5841–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li G, Xue HW (2007) Arabidopsis PLDzeta2 regulates vesicle trafficking and is required for auxin response. Plant Cell. 19, 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, Yao S, Kim SC, Wang X (2024) Lipid Phosphorylation Interacts with Abscisic Acid Production to Mediate Plant Stress Responses. Molecular Plant (acceptance pending revision) [Google Scholar]
- 80.Li M, Bahn SC, Guo L, Musgrave W, Berg H, Welti R, Wang X (2011) Patatin-related phospholipase pPLAIIIβ-induced changes in lipid metabolism alter cellulose content and cell elongation in Arabidopsis. The Plant Cell. 23, 1107–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li M, Qin C, Welti R, Wang X (2006a) Double knockouts of phospholipases Dzeta1 and Dzeta2 in Arabidopsis affect root elongation during phosphate-limited growth but do not affect root hair patterning. Plant Physiol. 140, 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li M, Welti R, Wang X. (2006b) Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of phospholipases Dζ1 and Dζ2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorusstarved plants. Plant Physiol. 142, 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li T, Xiao X, Liu Q, Li W, Li L, Zhang W, Munnik T, Wang X, Zhang Q (2023b) Dynamic responses of PA to environmental stimuli imaged by a genetically encoded mobilizable fluorescent sensor. Plant Commun. 4, 100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li W, Li M, Zhang W, Welti R, Wang X (2004) The plasma membrane-bound phospholipase Dδ enhances freezing tolerance in Arabidopsis. Nature Biotech. 22, 427–433. [DOI] [PubMed] [Google Scholar]
- 85.Li W, Song T, Wallrad L, Kudla J, Wang X, Zhang W (2019) Tissue-specific accumulation of pH-sensing phosphatidic acid determines plant stress tolerance. Nat Plants. 5, 1012–1021. [DOI] [PubMed] [Google Scholar]
- 86.Lu S, Yao S, Wang G, Guo L, Zhou Y, Hong Y, Wang X (2016) Phospholipase Dε enhances Braasca napus growth and seed production in response to nitrogen availability. Plant Biotechnol J. 14, 926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luerssen H, Kirik V, Herrmann P, Miséra S (1998) FUSCA3 encodes a protein with a conserved VP1/AB13-like B3 domain which is of functional importance for the regulation of seed maturation in Arabidopsis thaliana. Plant J. 15, 755–764. [DOI] [PubMed] [Google Scholar]
- 88.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 324, 1064–1068. [DOI] [PubMed] [Google Scholar]
- 89.Maatta S, Scheu B, Roth MR, Tamura P, Li M, Williams TD, Wang X, Welti R (2012) Levels of Arabidopsis thaliana leaf phosphatidic acids, phosphatidylserine, and most trienoate-containing polar lipid molecular species increase during the dark period of the diurnal cycle. Front Plant Sci. 3, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahankali M, Farkaly T, Bedi S, Hostetler HA and Gomez-Cambronero J (2015) Phosphatidic acid (PA) can displace PPARalpha/LXRalpha binding to the EGFR promoter causing its transrepression in luminal cancer cells. Sci Reports. 5, 15379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maurel C, Nacry P (2020) Root architecture and hydraulics converge for acclimation to changing water availability. Nat Plants. 6, 744–749. [DOI] [PubMed] [Google Scholar]
- 92.McLoughlin F, Arisz SA, Dekker HL, Kramer G, de Koster CG, Haring MA, Munnik T, Testerink C. (2013) Identification of novel candidate phosphatidic acid-binding proteins involved in the salt-stress response of Arabidopsis thaliana roots. Biochem. J 450, 573–581. [DOI] [PubMed] [Google Scholar]
- 93.Mansfeld J, Ulbrich-Hofmann R (2007) Secretory phospholipase A2-alpha from Arabidopsis thaliana: functional parameters and substrate preference. Chem Phys Lipids. 150, 156–166. [DOI] [PubMed] [Google Scholar]
- 94.Mishkind M, Vermeer JE, Darwish E, Munnik T (2009) Heat stress activates phospholipase D and triggers PIP accumulation at the plasma membrane and nucleus. Plant J. 60, 10–21. [DOI] [PubMed] [Google Scholar]
- 95.Mishra G, Zhang W, Deng F, Zhao J, Wang X (2006) A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science. 312, 264–266. [DOI] [PubMed] [Google Scholar]
- 96.Moes D, Himmelbach A, Korte A, Haberer G, Grill E (2008) Nuclear localization of the mutant protein phosphatase abi1 is required for insensitivity towards ABA responses in Arabidopsis. Plant J. 54, 806–819. [DOI] [PubMed] [Google Scholar]
- 97.Motte H, Vanneste S, Beeckman T (2019) Molecular and Environmental Regulation of Root Development. Annu Rev Plant Biol. 70, 465–488. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura Y, Koizumi R, Shui G, Shimojima M, Wenk MR, Ito T, Ohta H (2009) Arabidopsis lipins mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation. Proc Natl Acad Sci U S A. 106, 20978–20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakamura Y, Ngo AH (2020) Non-specific phospholipase C (NPC): an emerging class of phospholipase C in plant growth and development J Plant Res. 133, 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakanishi H, de los Santos P, Neiman AM (2004) Positive and negative regulation of a SNARE protein by control of intracellular localization. Mol Biol Cell. 15, 1802–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nelson RK, Frohman MA (2015) Physiological and pathophysiological roles for phospholipase D. J Lipid Res. 56, 2229–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen VC, Nakamura Y (2023) Distinctly localized lipid phosphate phosphatases mediate endoplasmic reticulum glycerolipid metabolism in Arabidopsis. Plant Cell. 35, 1548–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nishioka T, Frohman MA, Matsuda M, Kiyokawa E (2010) Heterogeneity of phosphatidic acid levels and distribution at the plasma membrane in living cells as visualized by a Föster resonance energy transfer (FRET) biosensor. J Biol Chem. 285, 35979–35987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pagliuso A, Valente C, Giordano LL, Filograna A, Li G, Circolo D, Turacchio G, Marzullo VM, Mandrich L, Zhukovsky MA, Formiggini F, Polishchuk RS, Corda D, Luini A (2016) Golgi membrane fission requires the CtBP1-S/BARS-induced activation of lysophosphatidic acid acyltransferase δ. Nat Commun. 7, 12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Panda S (2016) Circadian physiology of metabolism. Science. 354, 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paul MV, Iyer S, Amerhauser C, Lehmann M, van Dongen JT, Geigenberger P (2016) Oxygen Sensing via the Ethylene Response Transcription Factor RAP2.12 Affects Plant Metabolism and Performance under Both Normoxia and Hypoxia. Plant Physiol. 172, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng X, Frohman MA (2012) Mammalian phospholipase D physiological and pathological roles. Acta Physiol (Oxf). 204, 219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peters C, Kim S, Devaiah S, Wang X (2014) Non-specific phospholipase C5 and diacylglycerol promote lateral root development under mild salt stress in Arabidopsis. Plant Cell Environ. 37, 2002–2013. [DOI] [PubMed] [Google Scholar]
- 109.Peters C, Li M, Narasimhan R, Roth M, Welti R, Wang X (2010) Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis. Plant Cell. 22, 2642–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM (2011) mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 146, 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pleskot R, Pejchar P, Žárský V, Staiger CJ, Potocký M (2012) Structural insights into the inhibition of actin-capping protein by interactions with phosphatidic acid and phosphatidylinositol (4,5)-bisphosphate. PLoS Comput Biol. 8, e1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Potocký M, Pleskot R, Pejchar P, Vitale N, Kost B, Žárský V (2014). Live-cell imaging of phosphatidic acid dynamics in pollen tubes visualized by Spo20p-derived biosensor. New Phytol. 203, 483–494. [DOI] [PubMed] [Google Scholar]
- 113.Rawat R, Takahashi N, Hsu PY, Jones MA, Schwartz J, Salemi MR, Phinney BS, Harmer SL (2011) REVEILLE8 and PSEUDO-REPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock. PLoS Genet. 7, e1001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ren H, Federico H, Huang H, Sunkara M, Drennan T, Frohman MA, Smyth SSS, Morris AJ (2010) A Phosphatidic acid binding/nuclear localization motif determines lipin1 function in lipid metabolism and adipogenesis. Mol Biol Cell. 21, 3171–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ren H, Gao K, Liu Y, Sun D, Zheng S (2017) The role of AtPLC3 and AtPLC9 in thermotolerance in Arabidopsis. Plant Signal Behav. 12, e1162368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Santos-Mendoza M, Dubreucq B, Baud S, Parcy F, Caboche M, Lepiniec L (2008) Deciphering gene regulatory networks that control seed development and maturation in Arabidopsis. Plant J. 54, 608–620. [DOI] [PubMed] [Google Scholar]
- 117.Santos Mendoza M, Dubreucq B, Miquel M, Caboche M, Lepiniec L (2005) LEAFY COTYLEDON 2 activation is sufficient to trigger the accumulation of oil and seed specific mRNAs in Arabidopsis leaves. FEBS Lett. 579, 4666–4670. [DOI] [PubMed] [Google Scholar]
- 118.Santos-Rosa H, Leung J, Grimsey N, Peak-Chew S, Siniossoglou S (2005) The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 24, 1931–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sato F, Kohsaka A, Bhawal UK, Muragaki Y (2018) Potential Roles of Dec and Bmal1 Genes in Interconnecting Circadian Clock and Energy Metabolism. Int J Mol Sci. 19, 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U (2010) The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev. 24, 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schneider M, Knuesting J, Birkholz O, Heinisch JJ, Scheibe R (2018) Cytosolic GAPDH as a redox-dependent regulator of energy metabolism. BMC Plant Biol. 18, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen B, Allen WB, Zheng P, Li C, Glassman K, Ranch J, Nubel D, Tarczynski MC (2010) Expression of ZmLEC1 and ZmWRI1 increases seed oil production in maize. Plant Physiol. 153, 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH (2013) Circadian disruption leads to insulin resistance and obesity. Curr Biol. 23, 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shin JJ, Loewen CJ (2011) Putting the pH into phosphatidic acid signaling. BMC Biol. 9, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Siniossoglou S (2013) Phospholipid metabolism and nuclear function: roles of the lipin family of phosphatidic acid phosphatases. Biochim. Biophys. Acta 1831, 575–81. [DOI] [PubMed] [Google Scholar]
- 126.Smith CD, Wells WW (1983) Phosphorylation of rat liver nuclear envelopes. II. Characterization of in vitro lipid phosphorylation. J Biol Chem. 258, 9368–73. [PubMed] [Google Scholar]
- 127.Starr ML, Hurst LR, Fratti RA (2016) Phosphatidic Acid Sequesters Sec18p from cis-SNARE Complexes to Inhibit Priming. Traffic. 17, 1091–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Su Y, Li M, Guo L, Wang X (2018) Different effects of phospholipase Dζ2 and non-specific phospholipase C4 on lipid remodeling and root hair growth in Arabidopsis response to phosphate deficiency. Plant J. 94, 315–326. [DOI] [PubMed] [Google Scholar]
- 129.Tanguy E, Wang Q, Moine H, Vitale N (2019) Phosphatidic Acid: From Pleiotropic Functions to Neuronal Pathology. Front Cell Neurosci. 13, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tei R, Baskin JM (2022) Click chemistry and optogenetic approaches to visualize and manipulate phosphatidic acid signaling. J Biol Chem. 298, 101810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Testerink C, Larsen PB, van der Does D, van Himbergen JA, Munnik T (2007) Phosphatidic acid binds to and inhibits the activity of Arabidopsis CTR1. J Exp Bot. 58, 3905–3914. [DOI] [PubMed] [Google Scholar]
- 132.Testerink C, Munnik T (2011) Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. J Exp Bot. 62(7):2349–2361. [DOI] [PubMed] [Google Scholar]
- 133.Thaller DJ, Tong D, Marklew CJ, Ader NR, Mannino PJ, Borah S, King MC, Ciani B, Lusk CP (2021) Direct binding of ESCRT protein Chm7 to phosphatidic acid-rich membranes at nuclear envelope herniations. J Cell Biol. 220, e202004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Valente C, Luini A, Corda D (2013) Components of the CtBP1/BARS-dependent fission machinery. Histochem Cell Biol. 140, 407–421. [DOI] [PubMed] [Google Scholar]
- 135.Vescovi M, Zaffagnini M, Festa M, Trost P, Lo Schiavo F, Costa A (2013) Nuclear accumulation of cytosolic glyceraldehyde-3-phosphate dehydrogenase in cadmium-stressed Arabidopsis roots. Plant Physiol. 162, 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vietri M, Radulovic M, Stenmark H (2020) The many functions of ESCRTs. Nat Rev Mol Cell Biol. 21, 25–42. [DOI] [PubMed] [Google Scholar]
- 137.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S (2009) Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 106, 21453–21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vu HS, Shiva S, Roth MR, Tamura P, Zheng L, Li M, Sarowar S, Honey S, McEllhiney D, Hinkes P, Seib L, Williams TD, Gadbury G, Wang X, Shah J, Welti R (2014) Lipid changes after leaf wounding in Arabidopsis thaliana: Expanded lipidomic data form the basis for lipid co-occurrence analysis. Plant J 80, 728–743. [DOI] [PubMed] [Google Scholar]
- 139.Vu HS, Tamura P, Galeva NA, Chaturvedi R, Roth MR, Williams TD, Wang X, Shah J, Welti R (2012) Direct infusion mass spectrometry of oxylipin-containing Arabidopsis membrane lipids reveals varied patterns in different stress responses. Plant Physiol 158, 324–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Walton ZE, Patel CH, Brooks RC, Yu Y, Ibrahim-Hashim A, Riddle M, Porcu A, Jiang T, Ecker BL, Tameire F, Koumenis C, Weeraratna AT, Welsh DK, Gillies R, Alwine JC, Zhang L, Powell JD, Dang CV (2018) Acid Suspends the Circadian Clock in Hypoxia through Inhibition of mTOR. Cell. 174, 72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang C, Wang X (2001) A novel phospholipase D of Arabidopsis that is activated by oleic acid and associated with the plasma membrane. Plant Physiol. 127, 1102–12. [PMC free article] [PubMed] [Google Scholar]
- 142.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, Yang T (2008) Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 8, 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang P, Shen L, Guo J, Jing W, Qu Y, Li W, Bi R, Xuan W, Zhang Q, Zhang W (2019) Phosphatidic Acid Directly Regulates PINOID-Dependent Phosphorylation and Activation of the PIN-FORMED2 Auxin Efflux Transporter in Response to Salt Stress. Plant Cell. 31, 250–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang X, Devaiah SD, Zhang W, Welti R (2006) Signaling functions of phosphatidic acid. Prog Lipid Res 45, 250–278. [DOI] [PubMed] [Google Scholar]
- 145.Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD (2017) Meal Timing Regulates the Human Circadian System. Curr Biol. 27, 1768–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Welti R, Li l, Li M, Sang Y, Biesiada H, Zhou H-E, Rajashekar CB, Williams TD, Wang X (2002) Profiling membrane lipids in plant stress responses: role of phospholipase Da in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem 277, 31994–32002. [DOI] [PubMed] [Google Scholar]
- 147.Xie LJ, Chen QF, Chen MX, Yu LJ, Huang L, Chen L, Wang FZ, Xia FN, Zhu TR, Wu JX, Yin J, Liao B, Shi J, Zhang JH, Aharoni A, Yao N, Shu W, Xiao S (2015) Unsaturation of very-long-chain ceramides protects plant from hypoxia-induced damages by modulating ethylene signaling in Arabidopsis. PLoS Genet. 11, e1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xie LJ, Zhou Y, Chen QF, Xiao S (2021) New insights into the role of lipids in plant hypoxia responses. Prog Lipid Res. 81, 101072. [DOI] [PubMed] [Google Scholar]
- 149.Xu L, Pan R, Zhang W (2020) Membrane lipids are involved in plant response to oxygen deprivation. Plant Signal Behav.15, 1771938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yao H, Wang G, Guo L, Wang X (2013) Phosphatidic acid interacts with a MYB transcription factor and regulates its nuclear localization and function in Arabidopsis. Plant Cell. 25, 5030–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yao S, Wang G, Wang X (2022) Effects of Phospholipase Dε Overexpression on Soybean Response to Nitrogen and Nodulation. Front Plant Sci. 13, 852923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yao S, Wang X (2023) Monitoring lipid-protein interactions in planta using Förster resonance energy transfer. Methods Enzymol. 683, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yip Delormel T, Boudsocq M (2019) Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Phytol. 224, 585–604. [DOI] [PubMed] [Google Scholar]
- 154.Young BP, Shin JJ, Orij R, Chao JT, Li SC, Guan XL, Khong A, Jan E, Wenk MR, Prinz WA, Smits GJ, Loewen CJ (2010) Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Science. 329, 1085–1088. [DOI] [PubMed] [Google Scholar]
- 155.Yu L, Nie J, Cao C, Jin Y, Yan M, Wang F, Liu J, Xiao Y, Liang Y, Zhang W (2010) Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol. 188, 762–773. [DOI] [PubMed] [Google Scholar]
- 156.Yuan S, Kim SC, Deng X, Hong Y, Wang X (2019) Diacylglycerol kinase and associated lipid mediators modulate rice root architecture. New Phytol. 223, 261–276. [DOI] [PubMed] [Google Scholar]
- 157.Zhang H, Zhao Y, Zhou DX (2017a) Rice NAD+-dependent histone deacetylase OsSRT1 represses glycolysis and regulates the moonlighting function of GAPDH as a transcriptional activator of glycolytic genes. Nucleic Acids Res. 45, 12241–12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang P, Reue K (2017) Lipin proteins and glycerolipid metabolism: Roles at the ER membrane and beyond. Biochim Biophys Acta Biomembr. 1859, 1583–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang Q, Song P, Qu Y, Wang P, Jia Q, Guo L, Zhang C, Mao T, Yuan M, Wang X, Zhang W (2017b) Phospholipase Dδ negatively regulates plant thermotolerance by destabilizing cortical microtubules in Arabidopsis. Plant Cell Environ. 40, 2220–2235. [DOI] [PubMed] [Google Scholar]
- 160.Zhang Q, van Wijk R, Shahbaz M, Roels W, Schooten BV, Vermeer JEM, Zarza X, Guardia A, Scuffi D, García-Mata C, Laha D, Williams P, Willems LAJ, Ligterink W, Hoffmann-Benning S, Gillaspy G, Schaaf G, Haring MA, Laxalt AM, Munnik T (2018) Arabidopsis Phospholipase C3 is Involved in Lateral Root Initiation and ABA Responses in Seed Germination and Stomatal Closure. Plant Cell Physiol. 59, 469–486. [DOI] [PubMed] [Google Scholar]
- 161.Zhang W, Qin C, Zhao J, Wang X (2004) Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc. Natl. Acad. Sci. USA 101, 9508–9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X (2009) Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell. 21, 2357–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao J Devaiah SP, Wang C, Welti R, Wang X (2013) Phospholipase Dβ1 modulates defense responses to bacterial and fungal pathogens in Arabidopsis. New Phytol. 199, 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao J, Wang C, Bedair M, Welti R, Sumner LW, Baxter B, Wang X (2011) Suppression of phospholipase Dγs confers increased aluminum resistance in Arabidopsis thaliana. PLoS One. 6, e28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou Y, Zhou DM, Yu WW, Shi LL, Zhang Y, Lai YX, Huang LP, Qi H, Chen QF, Yao N, Li JF, Xie LJ, Xiao S (2022) Phosphatidic acid modulates MPK3- and MPK6-mediated hypoxia signaling in Arabidopsis. Plant Cell. 34, 889–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhukovsky MA, Filograna A, Luini A, Corda D, Valente C (2019) Phosphatidic acid in membrane rearrangements. FEBS Lett. 593: 2428–2451. [DOI] [PubMed] [Google Scholar]


