Abstract
Background:
Life’s Essential 8 (LE8) is a comprehensive construct of cardiovascular health. Yet, little is known about the LE8 score, its metabolic correlates, and their predictive implications among Black Americans and low-income individuals.
Methods:
In a nested case-control study of coronary heart disease (CHD) among 299 pairs of Black and 298 pairs of White low-income Americans from the Southern Community Cohort Study, we estimated LE8 score and applied untargeted plasma metabolomics and elastic net with leave-one-out cross-validation to identify metabolite signature (MetaSig) of LE8. Associations of LE8 score and MetaSig with incident CHD were examined using conditional logistic regression. Mediation effect of MetaSig on the LE8-CHD association was also examined. The external validity of MetaSig was evaluated in another nested CHD case-control study among 299 pairs of Chinese adults.
Results:
Higher LE8 score was associated with lower CHD risk [standardized OR=0.61 (95% CI: 0.53–0.69)]. The MetaSig, consisting of 133 metabolites, showed significant correlation with LE8 score (r=0.61) and inverse association with CHD [OR=0.57 (0.49–0.65)], robust to adjustment for LE8 score and across participants with different sociodemographic and health status (ORs: 0.42–0.69; all P<0.05). MetaSig mediated a large portion of the LE8-CHD association: 53% (32%−80%). Significant associations of MetaSig with LE8 score and CHD risk were found in validation cohort [r=0.49; OR=0.57 (0.46–0.69)].
Conclusions:
Higher LE8 score and its MetaSig were associated with lower CHD risk among low-income Black and White Americans. Metabolomics may offer an objective measure of LE8 and its metabolic phenotype relevant to CHD prevention among diverse populations.
Keywords: Life’s Essential 8, Cardiovascular Health, Coronary Heart Disease, Multi-racial Population, Metabolomics
Introduction
Coronary heart disease (CHD) is a leading cause of morbidity and mortality in the United States (US) and worldwide, with significant and persistent sociodemographic disparities1,2. To reduce the burden and life lost due to CHD and other cardiovascular diseases (CVD), the American Heart Association (AHA) has recently proposed Life’s Essential 8 (LE8) to assess and promote cardiovascular health (CVH) in individuals and populations3. LE8 includes 4 health behaviors (healthy diet, participation in physical activity, avoidance of nicotine, and healthy sleep [a new component]) and 4 health factors (weight, blood lipids, glucose, and blood pressure), and has a new scoring system with continuous scale to better reflect inter-individual differences. While a higher LE8 score has been recently associated with lower CVD incidence and mortality4–7, few studies have evaluated the LE8 score and its association with incident CHD or CVD among Black and White Americans who have low socioeconomic status (SES) and face disproportionate disease burdens. In addition, although some potential mechanisms have been identified (eg, reduced inflammation and atherosclerosis)8,9, beyond those known CVD risk pathways, mechanisms and inter-individual differences underlying the cardioprotective effects of LE8 and its included health behaviors and health factors are not fully understood.
Metabolite profiling (“metabolomics”) comprehensively measures small-molecule metabolites in biological samples and represents a powerful tool for mechanistic investigation, novel biomarker discovery, and precision medicine10,11. Metabolite profiling of blood samples may improve assessments of individuals’ alignment with LE8, particularly for behavioral factors that are prone to survey and recall biases. In addition, circulating metabolites related to LE8 may capture varied individual metabolic responses to LE8, providing novel mechanistic insights into its cardioprotective effects and informing precision medicine. While previous studies have identified metabolites related to the components of LE8, including diet12–16, physical activity17–19, tobacco exposure20–22, sleep23–26, and body mass index (BMI)27,28, to our knowledge, no study has applied untargeted plasma metabolomics to identify a comprehensive metabolite signature (MetaSig) for LE8 to enable studies with incident CHD. Given that those health behaviors and factors often correlate and interact with each other, investigating whether plasma metabolomics could provide a good objective assessment of individuals’ alignment with and metabolic responses to overall LE8 and uncovering potential pathways linking LE8 to incident CHD is highly warranted.
Here, leveraging a case-control study of CHD nested within the Southern Community Cohort Study (SCCS) involving 598 Black Americans (299 case-control pairs) and 596 White Americans (298 pairs), we assessed LE8 score and its MetaSig and examined their relations with incident CHD. The results were further replicated in another nested CHD case-control study of racially and geographically different populations: 598 Chinese adults (299 pairs) from the Shanghai Women’s Health Study and Shanghai Men’s Health Studies (SWMHS). In addition, we identified MetaSigs for the health behaviors and health factors recommended in the LE8 and evaluated their associations with incident CHD.
Methods
An overview of our study design is presented in Fig. I in the Data Supplement. Detailed methods are available in the Data Supplement. The SCCS was approved by the Institutional Review Boards of the Vanderbilt University Medical Center and Meharry Medical College; the SWMHS was approved by the Vanderbilt University Medical Center and Shanghai Cancer Institute. Informed consent was obtained from all enrolled participants. The data and code that support the findings of the present study are available upon request and approval by the Data Use Committees of the Southern Community Cohort Study (https://www.southerncommunitystudy.org/) and Shanghai Women’s Health Study & Shanghai Men’s Health Study (https://swhs-smhs.app.vumc.org/index.php).
Results
Baseline characteristics of study participants
The mean age at baseline (blood collection) was 55 years in our study participants (Table 1). The mean (SD) of LE8 score was 48.1 (12.3) in Black women, 50.0 (13.6) in Black men, 48.1 (13.4) in White women, and 48.5 (14.4) in White men. The median follow-up time for incident CHD cases was 5 (interquartile range: 3–8) years in SCCS. Incident CHD cases had significantly lower total LE8 score, health behaviors score, and health factors score than controls among subpopulations by race or sex (Fig. 1, Table 1, and Table II in the Data Supplement; all P<0.05 except for health behaviors score among male participants). The characteristics of participants in SWMHS (mean age: 61 years; mean LE8 score: 57.2 in women and 50.7 in men) are shown in Table III in the Data Supplement. There were moderate correlations between total LE8 score and individual component scores (r ranged from 0.22 with smoking to 0.47 with BMI and blood pressure scores in SCCS; Fig. II in the Data Supplement).
Table 1.
Characteristics of study participants in the Southern Community Cohort Study
| Black participants (N=598) | White participants (N=596) | |||
|---|---|---|---|---|
| CHD (N=299) | Control (N=299) | CHD (N=298) | Control (N=298) | |
| Age, years | 54.9 (8.8) | 54.7 (8.7) | 55.2 (8.7) | 55.1 (8.6) |
| Male, n (%) | 149 (49.8) | 149 (49.8) | 148 (49.7) | 148 (49.7) |
| Education, n (%) | ||||
| Less than high school | 120 (40.1) | 109 (36.5) | 108 (36.2) | 84 (28.2) |
| Completed high school | 107 (35.8) | 108 (36.1) | 118 (39.6) | 118 (39.6) |
| Vocational school or some college | 57 (19.1) | 60 (20.1) | 52 (17.4) | 54 (18.1) |
| College or graduate school | 15 (5.0) | 22 (7.4) | 20 (6.7) | 42 (14.1) |
| Income, n (%)* | ||||
| Low | 194 (64.9) | 185 (61.9) | 196 (65.8) | 167 (56.0) |
| Middle | 100 (33.4) | 104 (34.8) | 92 (30.9) | 111 (37.2) |
| High | 5 (1.7) | 10 (3.3) | 10 (3.4) | 20 (6.7) |
| Alcohol intake, n (%)† | ||||
| None | 154 (51.5) | 140 (46.8) | 175 (58.7) | 140 (47.0) |
| Moderate | 94 (31.4) | 107 (35.8) | 98 (32.9) | 129 (43.3) |
| Heavy | 51 (17.1) | 52 (17.4) | 25 (8.4) | 29 (9.7) |
| Family history of CHD, n (%) | 107 (35.8) | 89 (29.8) | 168 (56.4) | 142 (47.7) |
| History of diabetes, n (%) | 116 (38.8) | 56 (18.7) | 96 (32.2) | 40 (13.4) |
| History of dyslipidemia, n (%) | 102 (34.1) | 91 (30.4) | 155 (52.0) | 108 (36.2) |
| History of hypertension, n (%) | 210 (70.2) | 179 (59.9) | 177 (59.4) | 133 (44.6) |
| Life’s Essential 8 score | 45.9 (11.9) | 52.2 (13.2) | 45.0 (13.5) | 51.5 (13.5) |
| Life’s Essential 8 score category, n (%)‡ | ||||
| High (80–100) | 0 (0) | 7 (2.3) | 3 (1.0) | 10 (3.4) |
| Moderate (50–79) | 114 (38.1) | 174 (58.2) | 100 (33.6) | 148 (49.7) |
| Low (0–49) | 185 (61.9) | 118 (39.5) | 195 (65.4) | 140 (47.0) |
| Health behaviors score | 43.8 (18.4) | 47.4 (20.5) | 42.0 (19.2) | 46.3 (21.0) |
| Health factors score | 47.2 (20.4) | 56.7 (21.3) | 48.0 (20.7) | 56.2 (20.1) |
| Diet score | 39.8 (32.2) | 41.6 (30.6) | 36.9 (30.3) | 40.8 (32.3) |
| Physical activity score | 22.8 (39.8) | 24.2 (40.5) | 19.9 (38.8) | 21.3 (39.1) |
| Smoking score | 43.0 (42.2) | 47.8 (42.5) | 40.0 (40.3) | 46.5 (42.0) |
| Sleep score | 72.3 (29.2) | 77.1 (26.6) | 71.2 (29.7) | 78.1 (26.6) |
| Body mass index score | 50.0 (34.5) | 56.9 (35.3) | 50.4 (34.1) | 57.7 (34.2) |
| Blood lipids score | 36.8 (32.8) | 44.6 (31.8) | 25.8 (28.7) | 27.9 (27.6) |
| Blood glucose score | 54.3 (34.3) | 69.6 (30.0) | 60.9 (34.0) | 73.3 (26.7) |
| Blood pressure score | 47.7 (35.1) | 55.8 (37.3) | 54.9 (38.3) | 65.9 (38.9) |
Data were mean (standard deviation) or n (%) as indicated.
Annual household income <$15,000, $15,000 to <$25,000, and ≥$25,000 for low, middle, and high levels of income, respectively.
Alcohol intake was grouped as none, moderate (>0 to ≤2 drinks per day in men or >0 to ≤1 drink per day in women; 1 drink = 14 g ethanol), and heavy drinking (>2 drinks per day in men or >1 drink per day in women).
The cutoffs were provided by the American Heart Association3.
Figure 1.
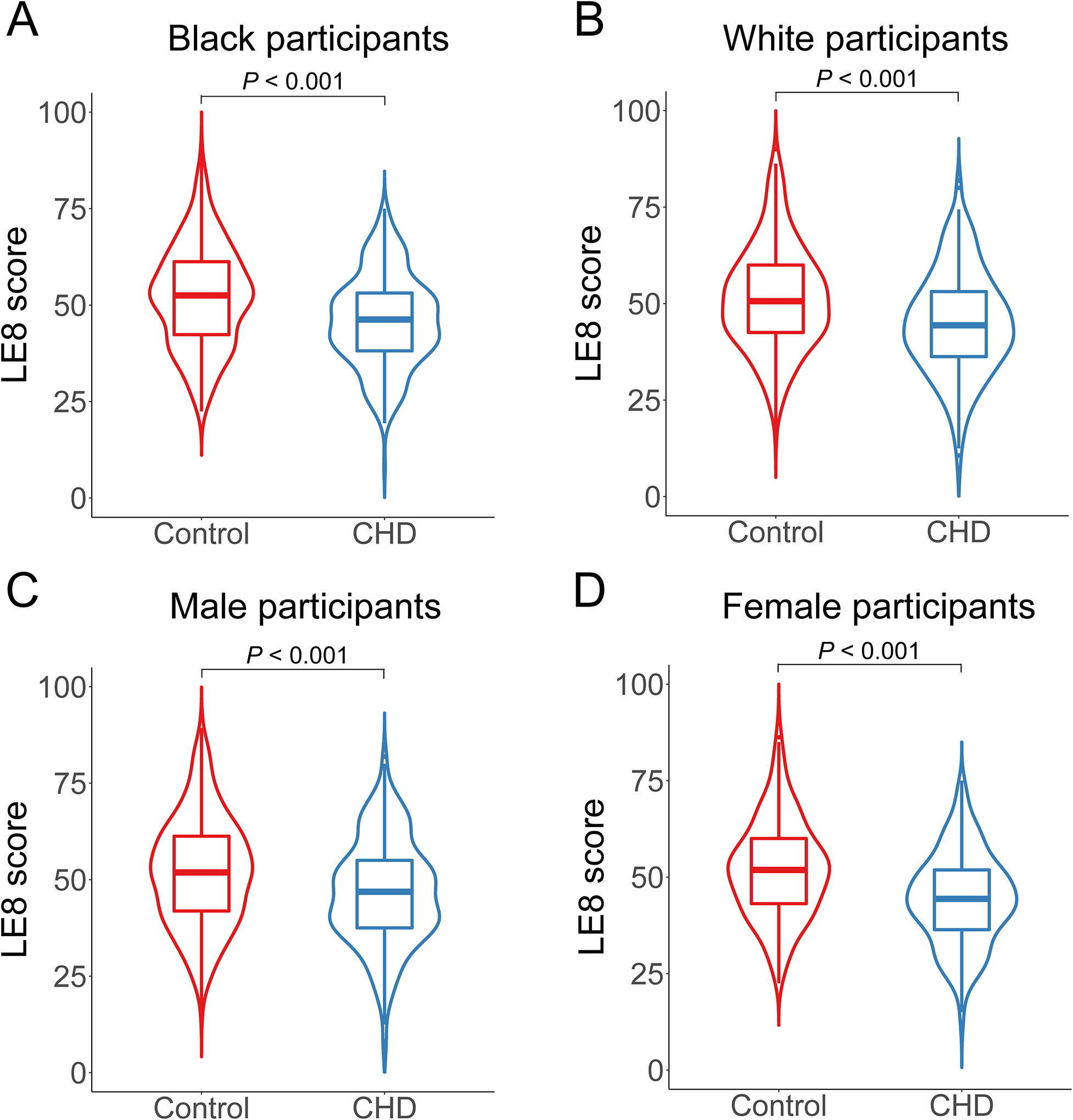
The Life’s Essential 8 score among incident coronary heart disease cases and matched controls by race and sex in the Southern Community Cohort Study. (A) The LE8 score among CHD cases and controls in Black participants. (B) The LE8 score among CHD cases and controls in White participants. (C) The LE8 score among CHD cases and controls in male participants. (D) The LE8 score among CHD cases and controls in female participants. LE8, Life’s Essential 8; CHD, coronary heart disease. P value was calculated by the Wilcoxon signed-rank test.
Metabolite signature of LE8
We identified 133 metabolites related to LE8 in elastic net regression model (top 30 are shown in Fig. 2A; the full list can be found in Table IV in the Data Supplement). We then constructed the MetaSig through a leave-one-out cross-validation approach. The MetaSig was significantly correlated with LE8 score (r = 0.61, P<0.001; Fig. 2B); meanwhile, variations in MetaSig were observed among individuals with the same LE8 score, demonstrating interindividual differences in metabolic phenotype of LE8. The MetaSig was then externally validated in SWMHS using the elastic net regression coefficients obtained from the SCCS dataset. MetaSig was also correlated with LE8 score in SWMHS (r = 0.49, P<0.001; Fig. 2C). Stratified analyses showed that correlations between LE8 score and MetaSig were consistent regardless of age, sex, race, fasting status, diabetes status, hypertension status, dyslipidemia status, and incident CHD status (r ranged from 0.54 to 0.64; Table V in the Data Supplement), suggesting the robustness of our identified LE8 metabolite signature across participants with different sociodemographic backgrounds and metabolic disease status.
Figure 2.
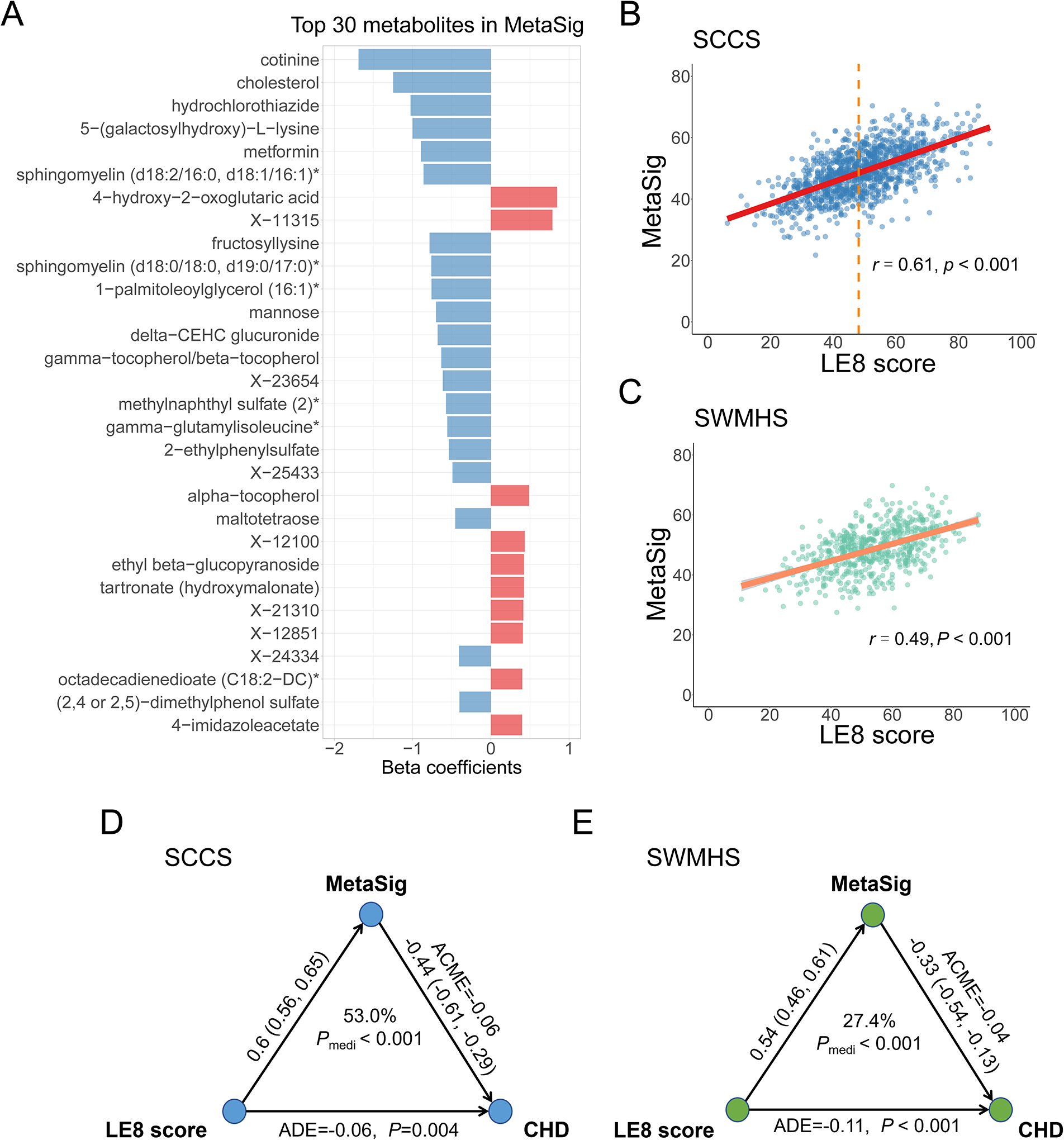
The metabolite signature of Life’s Essential 8 and its association with risk of coronary heart disease. (A) Top 30 metabolites selected by elastic net regression in SCCS. Metabolites were ranked by the absolute value of regression coefficients. (B) Spearman correlation between MetaSig and LE8 score in the SCCS. The dashed line denotes median LE8 score. (C) Spearman correlation between MetaSig and LE8 score in the SWMHS. (D) The mediation effect of MetaSig on the association between LE8 score and risk of CHD in the SCCS. (E) The mediation effect of MetaSig on the association between LE8 score and risk of CHD in the SWMHS. SCCS, Southern Community Cohort Study; SWMHS, Shanghai Women’s and Men’s Health Studies; LE8, Life’s Essential 8; MetaSig, metabolite signature; ACME, average causal mediation effects; ADE, average direct effects; CHD, coronary heart disease.
Associations with incident CHD
Higher LE8 score and its MetaSig were significantly associated with lower risk of CHD in conditional logistic regression models, adjusting for age, education, income, alcohol intake, and family history of CHD: standardized multivariable-adjusted odds ratio (OR) = 0.61 (95% CI: 0.53–0.69) for LE8 score and 0.57 (0.49–0.65) for MetaSig; both P<0.001 (Table 2). Sensitivity analysis showed that the MetaSig-CHD associations did not change after excluding any individual metabolites from the signature (Table VI in the Data Supplement). The MetaSig-CHD association did not change after excluding two drug metabolites (hydrochlorothiazide and metformin) from the MetaSig [OR (95% CI) = 0.55 (0.48–0.64); P<0.001]. Moreover, excluding 29 unknown metabolites (X-) from the MetaSig also did not change the MetaSig-CHD association [OR (95% CI) = 0.58 (0.50–0.66); P<0.001]. The scaled relative levels (Z-scores) of all 133 metabolites included in MetSig and their associations with incident CHD were shown in Table IV in the Data Supplement.
Table 2.
The associations of Life’s Essential 8 score, health behaviors score, health factors score, and their related metabolite signatures with risk of CHD*
| OR (95% CI) | P | ||
|---|---|---|---|
| SCCS | |||
| Life’s Essential 8 | LE8 score | 0.61 (0.53–0.69) | < 0.001 |
| LE8 MetaSig | 0.57 (0.49–0.65) | < 0.001 | |
| LE8 MetaSig (adjusting for LE8 score) | 0.66 (0.55–0.78) | < 0.001 | |
| Health behaviors | Health behaviors score | 0.77 (0.67–0.88) | < 0.001 |
| Health behaviors MetaSig | 0.73 (0.63–0.85) | < 0.001 | |
| Health behaviors MetaSig (adjusting for health behaviors score) | 0.79 (0.67–0.94) | 0.007 | |
| Health factors | Health factors score | 0.61 (0.53–0.7) | < 0.001 |
| Health factors MetaSig | 0.57 (0.49–0.66) | < 0.001 | |
| Health factors MetaSig (adjusting for health factors score) | 0.68 (0.55–0.84) | < 0.001 | |
| SWMHS | |||
| Life’s Essential 8 | LE8 score | 0.52 (0.42–0.65) | < 0.001 |
| LE8 MetaSig | 0.57 (0.46–0.69) | < 0.001 | |
| LE8 MetaSig (adjusting for LE8 score) | 0.69 (0.55–0.86) | < 0.001 | |
| Health behaviors | Health behaviors score | 0.73 (0.59–0.9) | 0.003 |
| Health behaviors MetaSig | 0.69 (0.55–0.87) | 0.001 | |
| Health behaviors MetaSig (adjusting for health behaviors score) | 0.77 (0.59–0.99) | 0.043 | |
| Health factors | Health factors score | 0.5 (0.4–0.62) | < 0.001 |
| Health factors MetaSig | 0.57 (0.47–0.7) | < 0.001 | |
| Health factors MetaSig (adjusting for health factors score) | 0.80 (0.62–1.04) | 0.093 | |
For associations of LE8 score and its related metabolite signature with risk of CHD, we used the conditional logistic regression, adjusted for age, education, income, alcohol intake, family history of CHD. For associations of health behaviors score and health factors score and their related metabolite signatures with risk of CHD, we used the conditional logistic regression, adjusted for age, education, income, alcohol intake, family history of CHD, and mutual adjustments of health factors score/health behaviors score. SCCS, Southern Community Cohort Study; SWMHS, Shanghai Women’s and Men’s Health Studies; CHD, coronary heart disease; LE8, Life’s Essential 8; MetaSig, metabolite signature; OR, odds ratio; CI, confidence interval.
After further adjusting for LE8 score, the MetaSig-CHD association was only slightly attenuated [OR (95% CI) = 0.66 (0.55–0.78); P<0.001; Table 2], suggesting circulating metabolites may complement LE8 assessment and contribute to CHD risk beyond LE8 score. On the other hand, the LE8-CHD association was moderately attenuated after adjusting for MetaSig [OR (95% CI) = 0.78 (0.66–0.92), P=0.003]. Mediation analysis showed that MetaSig mediated a large portion of the LE8-CHD association [53% (32%−80%); Pmediation<0.001; Fig. 2D].
Both LE8 and its MetaSig were inversely associated with CHD risk in subpopulations by race, age group, education, income, diabetes status, hypertension status, and dyslipidemia status (Pinteraction>0.05; Fig. 3), with stronger associations observed in women than in men [for LE8, OR (95% CI) = 0.53 (0.42–0.66) in women and 0.66 (0.55–0.80) in men, Pinteraction=0.017; for MetaSig, 0.48 (0.38–0.6) in women and 0.65 (0.54–0.78) in men, Pinteraction=0.016].
Figure 3.
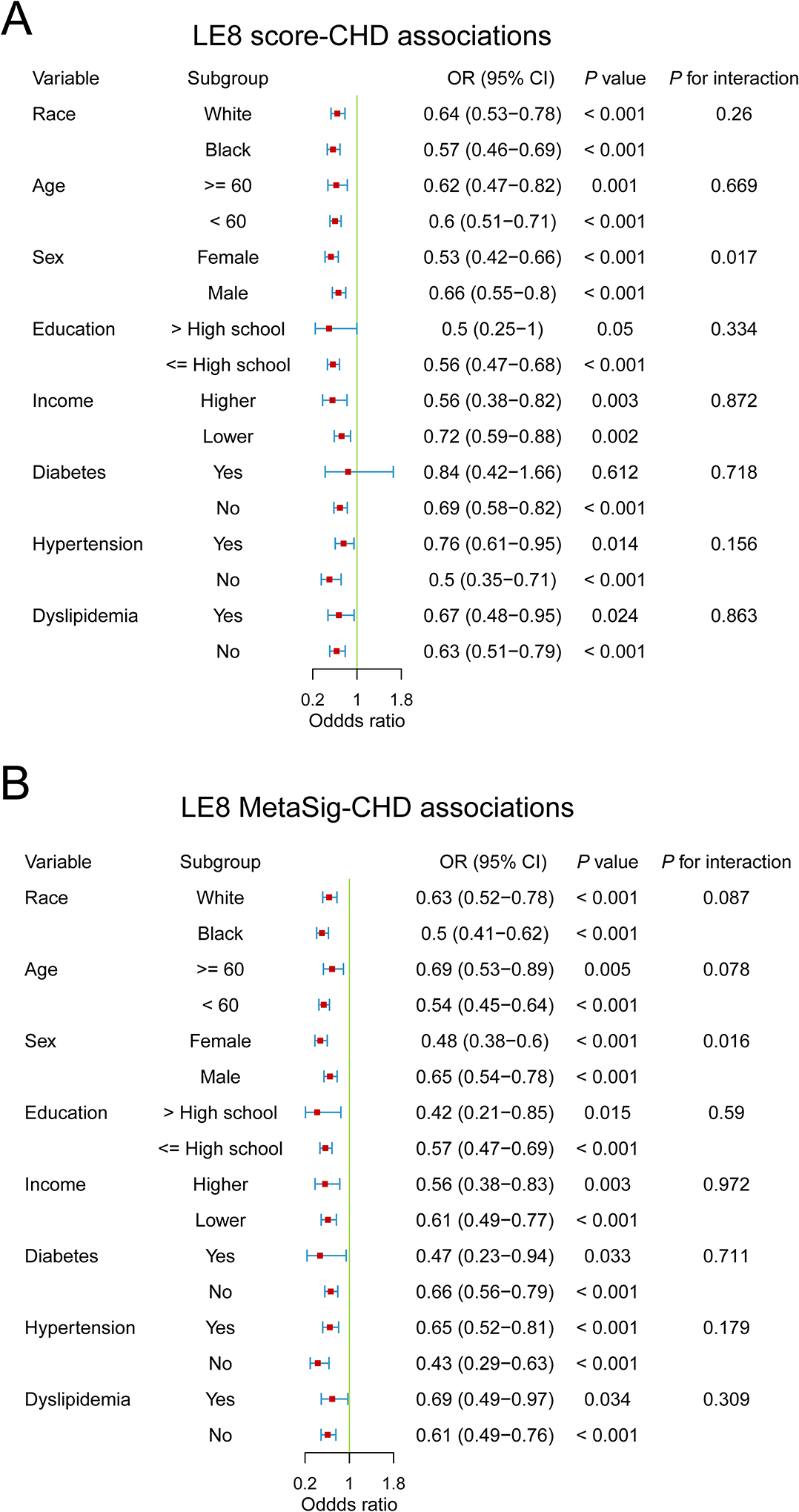
Subgroup analyses for the associations of LE8 score and its metabolite signature with risk of coronary heart disease in the Southern Community Cohort Study. (A) Subgroup analysis for the association between LE8 score and risk of CHD. (B) Subgroup analysis for the association between MetaSig and risk of CHD. Conditional logistic regression models were used, adjusted for potential confounders. For income, lower income denotes low income, and higher income denotes middle and high income. MetaSig, metabolite signature; CHD, coronary heart disease; LE8, Life’s Essential 8; OR, odds ratio; CI, confidence interval.
The association of MetaSig with incident CHD was replicated in SWMHS (Table 2), with OR (95% CI) = 0.57 (0.46–0.69) and 0.69 (0.55–0.86) after further adjusting for LE8 score (both P<0.001). The MetaSig also mediated a considerable portion of the LE8-CHD association in SWMHS [27.4% (10%−47%); Pmediation<0.001; Fig. 2E].
Metabolite signatures of health behaviors and health factors and associations with CHD
We further identified MetaSigs for health behaviors (Fig. IIIA in the Data Supplement) and health factors in SCCS (Fig. IVA in the Data Supplement; full lists of metabolites and their relative levels and associations with incident CHD are shown in Table IV in the Data Supplement), which showed significant correlations with health behaviors score (r = 0.59, P<0.001; Fig. IIIB in the Data Supplement) and with health factors score (r = 0.76, P<0.001; Fig. IVB in the Data Supplement). Significant correlations were also found among participant subgroups (Table V in the Data Supplement), suggesting the robustness of identified metabolite signatures for health behaviors and health factors. In addition, there were significant inverse associations of health behaviors score, health factors score, and their related signatures with risk of CHD (all P<0.001; Table 2). Specifically, standardized OR (95% CI) was 0.73 (0.63–0.85) for health behaviors MetaSig and 0.57 (0.49–0.66) for health factors MetaSig. Similarly, metabolites mediated large portions of the health behaviors-CHD association [43.9% (13.9%−101%), P=0.004; Fig. IIID in the Data Supplement] and health factors-CHD association [53.2% (24.8%−89%), Pmediation<0.001; Fig. IVD in the Data Supplement]. Further, all results on health behaviors MetaSig and health factors MetaSig were replicated in SWMHS (Table 2, Fig. III and Fig. IV in the Data Supplement).
Discussion
Leveraging untargeted plasma metabolites data in a nested case-control study among low-income Black and White Americans, we identified a metabolite signature that could reflect LE8 score and was associated with incident CHD, even after adjusting for LE8 and among participants with varied sociodemographic and metabolic health status, suggesting that circulating metabolite profiling may be used to help assess LE8 alignment across diverse populations and offer additional information on CVH (eg, inter-individual metabolic phenotypes related to LE8). We also identified MetaSigs for health behaviors and health factors and found consistent results showing that circulating metabolites could reflect the alignment with those recommendations and underlying metabolic phenotypes, which were further linked to incident CHD across diverse populations. All the results were further replicated in another nested case-control study of CHD among Chinese adults. Our findings demonstrate the potential utility of blood metabolomics to improve the assessment of LE8 and its underlying metabolic variations that are linked to incident CHD among sociodemographically diverse populations, towards advancing precision medicine and addressing disparities in CVH.
LE8 is the American Heart Association’s updated and enhanced guideline to measure and promote CVH for individuals and populations3. The beneficial associations of following the LE8 with lower risks of CHD, CVD, and related mortality have been demonstrated in recent studies4–7. However, multi-racial/ethnic populations with low SES remain underrepresented in research studies, even though they have persistently experienced worse CVH and CVD outcomes, as well as systemic disadvantages to improve CVH, than White and middle-class Americans2,29–31. Leveraging resources from SCCS, a large cohort of predominantly low-income Black and White Americans (in the present study: ~65% with household income <$15,000/y and ~95% with household income <$25,000/y), our study assessed CVH based on LE8 and evaluated the association of LE8 score with incident CHD. We found that a higher LE8 score (per SD increase) was associated with ~40–50% lower risk of CHD among Black Americans and individuals with low SES. While LE8 provides a comprehensive approach to quantify CVH, its assessment involves a series of procedures such as questionnaires, anthropometric and blood pressure measures, and blood draw. Particularly, health behaviors (diet, physical activity, smoking, and sleep) are usually assessed by questionnaires, which are time-consuming and prone to measurement errors and low compliance (particularly in the clinical setting). Also, the LE8 score cannot capture varied individual metabolic responses to lifestyle exposures. Hence, we incorporated untargeted plasma metabolomics data and for the first time, identified a robust metabolite signature of LE8 and then examined its association with incident CHD.
Metabolomics has been demonstrated as a powerful tool for improving exposure assessment and identifying potential novel biomarkers and mechanistic pathways in population studies, given its high-throughput characterization of thousands of metabolites in a small amount of biological samples32. Our study provides new evidence that plasma metabolite profiling may provide objective and comprehensive measures of LE8 and CVH among racially and geographically diverse populations. The identified metabolite signature may complement LE8 scores, improve the precision to stratify individuals with different future CHD risks, and potentially facilitate personalized CHD prevention strategies.
Our identified MetaSig consists of metabolites reflecting participants’ alignment with LE8 health behaviors and metabolic health status, majority of which are lipids and amino acids, and many of them have been linked to diet12–16, physical activity17–19, smoking20–22, sleep23–26, obesity27,28,33, or composite lifestyles scores34–37 in previous studies. For example, (2,4 or 2,5)-dimethylphenol sulfate, tartronate, and ethyl beta-glucopyranoside are derived from plant-based foods; cotinine is the major metabolite of nicotine from tobacco smoking; cholesterol, sphingomyelin, cortisol, and 1-palmitoleoylglycerolare are related to blood lipids; mannose, metformin, and fructosyllysine are related to prevalent diabetes and blood glucose. Particularly, drug metabolites, including hydrochlorothiazide and metformin, reflect antihypertensive and antidiabetic medications defined in LE8. Nevertheless, the associations of MetaSig with LE8 score and incident CHD were consistent among participants with or without history of hypertension or diabetes or after excluding those drug metabolites from the MetaSig. Notably, the MetaSig also contains microbial metabolites, eg, maltotetraose, anthranilate, indolebutyrate, and bile acids (taurohyocholate, glycodeoxycholate 3-sulfate, 3b-hydroxy-5-cholenoic acid, and glycohyocholate), suggesting the role of gut microbiome in host’s CVH, which cannot be captured by questionnaires or measurements of glucose, cholesterol, or blood pressure. Moreover, several metabolic pathways related to CVH and CVD development were highlighted. For example, anthranilate, indolebutyrate, picolinate, and serotonin are members of tryptophan metabolism pathway38–40; taurohyocholate, glycodeoxycholate 3-sulfate, 3b-hydroxy-5-cholenoic acid, and glycohyocholate belong to secondary bile acid metabolism pathway41,42; alpha-tocopherol, delta-tocopherol, and gamma-tocopherol/beta-tocopherol are vitamin E derivatives through tocopherol metabolism pathway43,44.
Importantly, the MetaSig was related to future CHD risk regardless of participants’ age, sex, race, SES, metabolic disease history, and even after adjustment for LE8. Further analyses indicated that circulating metabolites could play a substantial mediating role linking LE8 and reduced CHD risk. Moreover, our findings were replicated in a racially and geographically different population, suggesting external validity and potential generalizability of our findings. Taken together, our findings demonstrated that circulating metabolites could complement LE8 to improve the precision of CVH assessment and predict CHD risk among sociodemographically and geographically diverse populations.
To our knowledge, this is the first study that assessed the LE8 score, constructed its metabolite signature, and associated LE8 score and its MetaSig with incident CHD in Black and White Americans with low SES. Besides its novelty and inclusion of populations facing socioeconomic challenges and health disparities, other strengths of our study include its prospective design, comprehensive profiling of >1500 blood metabolites for a broad coverage and improved ability to construct metabolite signature for LE8, and robustness of results across populations with different sociodemographic and health status. Meanwhile, several limitations of our current study need to be acknowledged. First, as MetaSig of LE8 was identified using cross-sectional data from baseline blood samples, we cannot be certain as to the directionality of LE8-MetaSig association, and mediation analysis assumed that LE8 score preceded MetaSig. Although several population- or animal-based studies have shown the causal effects of LE8 components on blood metabolites21,26,45–48, given that blood metabolites might precede some LE8 components, the longitudinal association between LE8 adherence and circulating metabolites should be investigated. Second, given the observational nature of our study, the causality is unable to be confirmed. However, the prospective design reduces the concern of reverse causation for the LE8/MetaSig-CHD association. Third, we cannot rule out the influence of residual confounding on the LE8/MetaSig-CHD association, although we have adjusted for and stratified by major CHD risk factors. Fourth, the concentrations of fasting glucose and HbA1c and SBP and DBP were not measured in SSCS; thus, glucose score and blood pressure score were defined based on history of diabetes or hypertension, use of medications, and relative levels of glucose measured in metabolites profiling, which may influence the accuracy of LE8 score. Also, Fig. 2B–C and residual plot suggested some bias of MetaSig at the extreme levels of LE8, i.e., potential overestimation at low LE8 while underestimation at high LE8, which seems to be a common problem of metabolite/biomarker signatures. Finally, the nested case-control design may overestimate the predictive ability of the LE8 score and its MetaSig. Therefore, our results should be further validated in other prospective cohort studies.
In summary, our study assessed the LE8 score and identified MetaSig of LE8 among low-income Black and White Americans. We found that both LE8 score and its MetaSig were inversely associated with risk of CHD, consistently among participants with varied sociodemographic and metabolic health status. Our identified metabolite signature may provide an objective and comprehensive measure of LE8 and its metabolic underpinning, which may help improve the precision of CVH assessment and facilitate more effective and personalized CHD prevention strategies in diverse populations. Further examination of our identified metabolites may improve understanding of biological mechanisms as to how following LE8 benefits CHD prevention.
Supplementary Material
Acknowledgments:
The authors thank the study participants of the SCCS and the SWMHS.
Sources of Funding:
The Southern Community Cohort Study is funded by U01 CA202979, the Shanghai Women’s Health Study is funded by UM1 CA182910, and the Shanghai Men’s Health Study is funded by UM1 CA173640 from the National Cancer Institute (NCI) at the National Institutes of Health (NIH). Biospecimens of these three cohort studies are managed by the Survey and Biospecimen Shared Resource, which is supported in part by the Vanderbilt-Ingram Cancer Center (P30 CA68485). This study is supported by R01 HL149779 from the National Heart, Lung, and Blood Institute (NHLBI) at the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Nonstandard Abbreviations and Acronyms
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CMS
Centers for Medicare & Medicaid Services
- CVD
cardiovascular disease
- CVH
cardiovascular health
- DASH
Dietary Approaches to Stop Hypertension
- FFQ
food frequency questionnaire
- LE8
Life’s Essential 8
- MetaSig
metabolite signature
- MS
mass spectrometry
- NMR
nuclear magnetic resonance
- OR
odds ratio
- QC
quality control
- SCCS
Southern Community Cohort Study
- SD
standard deviation
- SES
socioeconomic status
- SWMHS
Shanghai Women’s and Men’s Health Studies
- VIF
variance inflation factor
Footnotes
Disclosures: None
Supplemental Materials:
References:
- 1.Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/s0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023. doi: 10.1161/cir.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Allen NB, Anderson CA, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G. Life’s essential 8: updating and enhancing the American Heart Association’s Construct of Cardiovascular Health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Ma H, Li X, Heianza Y, Manson JE, Franco OH, Qi L. Association of Cardiovascular Health With Life Expectancy Free of Cardiovascular Disease, Diabetes, Cancer, and Dementia in UK Adults. JAMA Intern Med. 2023. doi: 10.1001/jamainternmed.2023.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isiozor NM, Kunutsor SK, Voutilainen A, Laukkanen JA. Life’s Essential 8 and the risk of cardiovascular disease death and all-cause mortality in Finnish men. Eur J Prev Cardiol. 2023. doi: 10.1093/eurjpc/zwad040 [DOI] [PubMed] [Google Scholar]
- 6.Jin C, Li J, Liu F, Li X, Hui Y, Chen S, Li F, Wang G, Liang F, Lu X, et al. Life’s Essential 8 and 10-Year and Lifetime Risk of Atherosclerotic Cardiovascular Disease in China. Am J Prev Med. 2023. doi: 10.1016/j.amepre.2023.01.009 [DOI] [PubMed] [Google Scholar]
- 7.Yi J, Wang L, Guo X, Ren X. Association of Life’s Essential 8 with all-cause and cardiovascular mortality among US adults: A prospective cohort study from the NHANES 2005–2014. Nutr Metab Cardiovasc Dis. 2023. doi: 10.1016/j.numecd.2023.01.021 [DOI] [PubMed] [Google Scholar]
- 8.Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–1683. [DOI] [PubMed] [Google Scholar]
- 9.Gaye B, Tafflet M, Arveiler D, Montaye M, Wagner A, Ruidavets JB, Kee F, Evans A, Amouyel P, Ferrieres J. Ideal cardiovascular health and incident cardiovascular disease: heterogeneity across event subtypes and mediating effect of blood biomarkers: the prime study. Journal of the American Heart Association. 2017;6:e006389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Després JP. Predicting longevity using metabolomics: a novel tool for precision lifestyle medicine? Nat Rev Cardiol. 2020;17:67–68. doi: 10.1038/s41569-019-0310-2 [DOI] [PubMed] [Google Scholar]
- 11.Clish CB. Metabolomics: an emerging but powerful tool for precision medicine. Molecular Case Studies. 2015;1:a000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Guasch-Ferré M, Chung W, Ruiz-Canela M, Toledo E, Corella D, Bhupathiraju SN, Tobias DK, Tabung FK, Hu J, et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur Heart J. 2020;41:2645–2656. doi: 10.1093/eurheartj/ehaa209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Zhernakova DV, Kurilshikov A, Andreu-Sánchez S, Wang D, Augustijn HE, Vich Vila A, Weersma RK, Medema MH, Netea MG, et al. Influence of the microbiome, diet and genetics on inter-individual variation in the human plasma metabolome. Nat Med. 2022. doi: 10.1038/s41591-022-02014-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Baden MY, Guasch-Ferré M, Wittenbecher C, Li J, Li Y, Wan Y, Bhupathiraju SN, Tobias DK, Clish CB, et al. Plasma metabolite profiles related to plant-based diets and the risk of type 2 diabetes. Diabetologia. 2022;65:1119–1132. doi: 10.1007/s00125-022-05692-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen GC, Chai JC, Xing J, Moon JY, Shan Z, Yu B, Mossavar-Rahman Y, Sotres-Alvarez D, Li J, Mattei J, et al. Healthful eating patterns, serum metabolite profile and risk of diabetes in a population-based prospective study of US Hispanics/Latinos. Diabetologia. 2022;65:1133–1144. doi: 10.1007/s00125-022-05690-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah RV, Steffen LM, Nayor M, Reis JP, Jacobs DR, Allen NB, Lloyd-Jones D, Meyer K, Cole J, Piaggi P. Dietary metabolic signatures and cardiometabolic risk. European Heart Journal. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemppainen SM, Fernandes Silva L, Lankinen MA, Schwab U, Laakso M. Metabolite Signature of Physical Activity and the Risk of Type 2 Diabetes in 7271 Men. Metabolites. 2022;12. doi: 10.3390/metabo12010069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kujala UM, Mäkinen VP, Heinonen I, Soininen P, Kangas AJ, Leskinen TH, Rahkila P, Würtz P, Kovanen V, Cheng S, et al. Long-term leisure-time physical activity and serum metabolome. Circulation. 2013;127:340–348. doi: 10.1161/circulationaha.112.105551 [DOI] [PubMed] [Google Scholar]
- 19.Ding M, Zeleznik OA, Guasch-Ferre M, Hu J, Lasky-Su J, Lee IM, Jackson RD, Shadyab AH, LaMonte MJ, Clish C, et al. Metabolome-Wide Association Study of the Relationship Between Habitual Physical Activity and Plasma Metabolite Levels. Am J Epidemiol. 2019;188:1932–1943. doi: 10.1093/aje/kwz171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan Y, Barr DB, Ryan PB, Fedirko V, Sarnat JA, Gaskins AJ, Chang CJ, Tang Z, Marsit CJ, Corwin EJ, et al. High-resolution metabolomics of exposure to tobacco smoke during pregnancy and adverse birth outcomes in the Atlanta African American maternal-child cohort. Environ Pollut. 2022;292:118361. doi: 10.1016/j.envpol.2021.118361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu T, Holzapfel C, Dong X, Bader E, Yu Z, Prehn C, Perstorfer K, Jaremek M, Roemisch-Margl W, Rathmann W, et al. Effects of smoking and smoking cessation on human serum metabolite profile: results from the KORA cohort study. BMC Med. 2013;11:60. doi: 10.1186/1741-7015-11-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu F, Derkach A, Freedman ND, Landi MT, Albanes D, Weinstein SJ, Mondul AM, Matthews CE, Guertin KA, Xiao Q, et al. Cigarette smoking behaviour and blood metabolomics. Int J Epidemiol. 2016;45:1421–1432. doi: 10.1093/ije/dyv330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bos MM, Goulding NJ, Lee MA, Hofman A, Bot M, Pool R, Vijfhuizen LS, Zhang X, Li C, Mustafa R, et al. Investigating the relationships between unfavourable habitual sleep and metabolomic traits: evidence from multi-cohort multivariable regression and Mendelian randomization analyses. BMC Med. 2021;19:69. doi: 10.1186/s12916-021-01939-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritz J, Huang T, Depner CM, Zeleznik OA, Cespedes Feliciano EM, Li W, Stone KL, Manson JAE, Clish C, Sofer T, et al. Sleep duration, plasma metabolites, and obesity and diabetes: A metabolome-wide association study in US women. Sleep. 2022. doi: 10.1093/sleep/zsac226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topriceanu CC, Tillin T, Chaturvedi N, Joshi R, Garfield V. The association between plasma metabolites and sleep quality in the Southall and Brent Revisited (SABRE) Study: A cross-sectional analysis. J Sleep Res. 2021;30:e13245. doi: 10.1111/jsr.13245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang T, Zeleznik OA, Poole EM, Clish CB, Deik AA, Scott JM, Vetter C, Schernhammer ES, Brunner R, Hale L, et al. Habitual sleep quality, plasma metabolites and risk of coronary heart disease in post-menopausal women. Int J Epidemiol. 2019;48:1262–1274. doi: 10.1093/ije/dyy234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan XF, Chen ZZ, Wang TJ, Shu X, Cai H, Cai Q, Clish CB, Shi X, Zheng W, Gerszten RE, et al. Plasma metabolomic signatures of obesity and risk of type 2 diabetes. Obesity (Silver Spring). 2022. doi: 10.1002/oby.23549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ottosson F, Smith E, Ericson U, Brunkwall L, Orho-Melander M, Di Somma S, Antonini P, Nilsson PM, Fernandez C, Melander O. Metabolome-Defined Obesity and the Risk of Future Type 2 Diabetes and Mortality. Diabetes Care. 2022;45:1260–1267. doi: 10.2337/dc21-2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation. 2012;125:2595–2602. doi: 10.1161/circulationaha.111.070722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilkerton CS, Singh SS, Bias TK, Frisbee SJ. Changes in Cardiovascular Health in the United States, 2003–2011. J Am Heart Assoc. 2015;4:e001650. doi: 10.1161/jaha.114.001650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haynes N, Kaur A, Swain J, Joseph JJ, Brewer LC. Community-Based Participatory Research to Improve Cardiovascular Health Among US Racial and Ethnic Minority Groups. Curr Epidemiol Rep. 2022;9:212–221. doi: 10.1007/s40471-022-00298-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nature reviews Drug discovery. 2016;15:473–484. [DOI] [PubMed] [Google Scholar]
- 33.Cirulli ET, Guo L, Leon Swisher C, Shah N, Huang L, Napier LA, Kirkness EF, Spector TD, Caskey CT, Thorens B, et al. Profound Perturbation of the Metabolome in Obesity Is Associated with Health Risk. Cell Metab. 2019;29:488–500.e482. doi: 10.1016/j.cmet.2018.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delgado-Velandia M, Gonzalez-Marrachelli V, Domingo-Relloso A, Galvez-Fernandez M, Grau-Perez M, Olmedo P, Galan I, Rodriguez-Artalejo F, Amigo N, Briongos-Figuero L, et al. Healthy lifestyle, metabolomics and incident type 2 diabetes in a population-based cohort from Spain. Int J Behav Nutr Phys Act. 2022;19:8. doi: 10.1186/s12966-021-01219-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothwell JA, Murphy N, Bešević J, Kliemann N, Jenab M, Ferrari P, Achaintre D, Gicquiau A, Vozar B, Scalbert A, et al. Metabolic Signatures of Healthy Lifestyle Patterns and Colorectal Cancer Risk in a European Cohort. Clin Gastroenterol Hepatol. 2022;20:e1061–e1082. doi: 10.1016/j.cgh.2020.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Assi N, Gunter MJ, Thomas DC, Leitzmann M, Stepien M, Chajès V, Philip T, Vineis P, Bamia C, Boutron-Ruault MC, et al. Metabolic signature of healthy lifestyle and its relation with risk of hepatocellular carcinoma in a large European cohort. Am J Clin Nutr. 2018;108:117–126. doi: 10.1093/ajcn/nqy074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Si J, Li J, Yu C, Guo Y, Bian Z, Millwood I, Yang L, Walters R, Chen Y, Du H, et al. Improved lipidomic profile mediates the effects of adherence to healthy lifestyles on coronary heart disease. Elife. 2021;10. doi: 10.7554/eLife.60999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wirleitner B, Rudzite V, Neurauter G, Murr C, Kalnins U, Erglis A, Trusinskis K, Fuchs D. Immune activation and degradation of tryptophan in coronary heart disease. European journal of clinical investigation. 2003;33:550–554. [DOI] [PubMed] [Google Scholar]
- 39.Mangge H, Stelzer I, Reininghaus E, Weghuber D, Postolache T, Fuchs D. Disturbed tryptophan metabolism in cardiovascular disease. Current medicinal chemistry. 2014;21:1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G, Chen S, Zhong J, Teng K, Yin Y. Crosstalk between tryptophan metabolism and cardiovascular disease, mechanisms, and therapeutic implications. Oxidative Medicine and Cellular Longevity. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- 42.Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174–1182. doi: 10.1136/gutjnl-2020-323071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietrich M, Traber MG, Jacques PF, Cross CE, Hu Y, Block G. Does γ-tocopherol play a role in the primary prevention of heart disease and cancer? A review. Journal of the American College of Nutrition. 2006;25:292–299. [DOI] [PubMed] [Google Scholar]
- 44.Mathur P, Ding Z, Saldeen T, Mehta JL. Tocopherols in the prevention and treatment of atherosclerosis and related cardiovascular disease. Clinical cardiology. 2015;38:570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Würtz P, Wang Q, Kangas AJ, Richmond RC, Skarp J, Tiainen M, Tynkkynen T, Soininen P, Havulinna AS, Kaakinen M, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11:e1001765. doi: 10.1371/journal.pmed.1001765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lauterbach MA, Latz E, Christ A. Metabolomic Profiling Reveals Distinct and Mutual Effects of Diet and Inflammation in Shaping Systemic Metabolism in Ldlr(−/−) Mice. Metabolites. 2020;10. doi: 10.3390/metabo10090336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato S, Dyar KA, Treebak JT, Jepsen SL, Ehrlich AM, Ashcroft SP, Trost K, Kunzke T, Prade VM, Small L. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metabolism. 2022;34:329–345. e328. [DOI] [PubMed] [Google Scholar]
- 48.Cruickshank-Quinn CI, Mahaffey S, Justice MJ, Hughes G, Armstrong M, Bowler RP, Reisdorph R, Petrache I, Reisdorph N. Transient and persistent metabolomic changes in plasma following chronic cigarette smoke exposure in a mouse model. PloS one. 2014;9:e101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Signorello LB, Hargreaves MK, Blot WJ. The Southern Community Cohort Study: investigating health disparities. J Health Care Poor Underserved. 2010;21:26–37. doi: 10.1353/hpu.0.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, Li HL, Wen W, Ji BT, Li Q, et al. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–1131. doi: 10.1093/aje/kwi322 [DOI] [PubMed] [Google Scholar]
- 51.Shu XO, Li H, Yang G, Gao J, Cai H, Takata Y, Zheng W, Xiang YB. Cohort Profile: The Shanghai Men’s Health Study. Int J Epidemiol. 2015;44:810–818. doi: 10.1093/ije/dyv013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lloyd-Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, Foraker RE, Black T, Grandner MA, Allen NB, et al. Status of Cardiovascular Health in US Adults and Children Using the American Heart Association’s New “Life’s Essential 8” Metrics: Prevalence Estimates From the National Health and Nutrition Examination Survey (NHANES), 2013 Through 2018. Circulation. 2022;146:822–835. doi: 10.1161/circulationaha.122.060911 [DOI] [PubMed] [Google Scholar]
- 53.Chang RS, Xu M, Brown SH, Cohen SS, Yu D, Akwo EA, Dixon D, Lipworth L, Gupta DK. Relation of the Dietary Approaches to Stop Hypertension Dietary Pattern to Heart Failure Risk and Socioeconomic Status (from the Southern Community Cohort Study). The American journal of cardiology. 2022;169:71–77. doi: 10.1016/j.amjcard.2021.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu D, Zhang X, Xiang YB, Yang G, Li H, Gao YT, Zheng W, Shu XO. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr. 2014;100:693–700. doi: 10.3945/ajcn.113.079194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.NIH U. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, Your guide to lowering your blood pressure with DASH. DASH eating plan. DASH eating plan. 2006. [Google Scholar]
- 56.Signorello LB, Munro HM, Buchowski MS, Schlundt DG, Cohen SS, Hargreaves MK, Blot WJ. Estimating nutrient intake from a food frequency questionnaire: incorporating the elements of race and geographic region. Am J Epidemiol. 2009;170:104–111. doi: 10.1093/aje/kwp098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buchowski MS, Schlundt DG, Hargreaves MK, Hankin JH, Signorello LB, Blot WJ. Development of a culturally sensitive food frequency questionnaire for use in the Southern Community Cohort Study. Cell Mol Biol (Noisy-le-grand). 2003;49:1295–1304. [PubMed] [Google Scholar]
- 58.Shu X, Yang G, Jin F, Liu D, Kushi L, Wen W, Gao Y, Zheng W. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women’s Health Study. European journal of clinical nutrition. 2004;58:17–23. [DOI] [PubMed] [Google Scholar]
- 59.Villegas R, Yang G, Liu D, Xiang Y-B, Cai H, Zheng W, Shu XO. Validity and reproducibility of the food-frequency questionnaire used in the Shanghai men’s health study. British journal of nutrition. 2007;97:993–1000. [DOI] [PubMed] [Google Scholar]
- 60.Matthews CE, Shu X-O, Yang G, Jin F, Ainsworth BE, Liu D, Gao Y-T, Zheng W. Reproducibility and validity of the Shanghai Women’s Health Study physical activity questionnaire. American journal of epidemiology. 2003;158:1114–1122. [DOI] [PubMed] [Google Scholar]
- 61.Jurj AL, Wen W, Xiang Y-B, Matthews CE, Liu D, Zheng W, Shu X-O. Reproducibility and validity of the Shanghai Men’s Health Study physical activity questionnaire. American journal of epidemiology. 2007;165:1124–1133. [DOI] [PubMed] [Google Scholar]
- 62.Buchowski MS, Matthews CE, Cohen SS, Signorello LB, Fowke JH, Hargreaves MK, Schlundt DG, Blot WJ. Evaluation of a questionnaire to assess sedentary and active behaviors in the Southern Community Cohort Study. J Phys Act Health. 2012;9:765–775. doi: 10.1123/jpah.9.6.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–6667. doi: 10.1021/ac901536h [DOI] [PubMed] [Google Scholar]
- 64.Zou H, Hastie T. Regularization and variable selection via the elastic net. Journal of the royal statistical society: series B (statistical methodology). 2005;67:301–320. [Google Scholar]
- 65.Kuhn M Building predictive models in R using the caret package. Journal of statistical software. 2008;28:1–26.27774042 [Google Scholar]
- 66.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. Journal of statistical software. 2010;33:1. [PMC free article] [PubMed] [Google Scholar]
- 67.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. 2014.
- 68.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychological methods. 2010;15:309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


