Abstract
INTRODUCTION
Accumulating evidence indicates disproportionate tau burden and tau‐related clinical progression in females. However, sex differences in plasma phosphorylated tau (p‐tau)217 prediction of subclinical cognitive and brain changes are unknown.
METHODS
We measured baseline plasma p‐tau217, glial fibrillary acidic protein (GFAP), and neurofilament light (NfL) in 163 participants (85 cognitively unimpaired [CU], 78 mild cognitive impairment [MCI]). In CU, linear mixed effects models examined sex differences in plasma biomarker prediction of longitudinal domain‐specific cognitive decline and brain atrophy. Cognitive models were repeated in MCI.
RESULTS
In CU females, baseline plasma p‐tau217 predicted verbal memory and medial temporal lobe trajectories such that trajectories significantly declined once p‐tau217 concentrations surpassed 0.053 pg/ml, a threshold that corresponded to early levels of cortical amyloid aggregation in secondary amyloid positron emission tomography analyses. CU males exhibited similar rates of cognitive decline and brain atrophy, but these trajectories were not dependent on plasma p‐tau217. Plasma GFAP and NfL exhibited similar female‐specific prediction of medial temporal lobe atrophy in CU. Plasma p‐tau217 exhibited comparable prediction of cognitive decline across sex in MCI.
DISCUSSION
Plasma p‐tau217 may capture earlier Alzheimer's disease (AD)‐related cognitive and brain atrophy hallmarks in females compared to males, possibly reflective of increased susceptibility to AD pathophysiology.
Keywords: Alzheimer's disease, amyloid positron emission tomography, cognition, cognitively unimpaired, glial fibrillary acidic protein, medial temporal lobe, neurofilament light, plasma biomarkers, phosphorylated tau217, sex differences, verbal memory
1. BACKGROUND
Blood‐based biomarkers of Alzheimer's disease (AD) pathology are non‐invasive and cost‐effective tools for disease detection and monitoring. 1 Plasma markers of phosphorylated tau (p‐tau) strongly capture amyloid beta (Aβ)–dependent phosphorylation of tau fragments and discriminate clinical AD from controls and non‐AD dementias. 2 , 3 , 4 , 5 , 6 Recent studies demonstrate that plasma p‐tau217 and p‐tau231 track with early Aβ and tau accumulation, 3 , 7 , 8 , 9 with p‐tau217 also showing strong prediction of preclinical cognitive progression in Aβ‐positive cognitively unimpaired (CU) individuals. 10 , 11 , 12 Plasma biomarkers hold promise as clinically scalable tools in early disease, when disease‐modifying therapies may be most effective. However, person‐specific factors are likely to influence their ability to forecast AD phenotypic progression.
There is strong evidence for biological sex and gender (herein referred to as sex [i.e., female, male]) differences in AD pathophysiology, including protein aggregation and clinical expression. 13 Positron emission tomography (PET) and post mortem studies show higher levels of tau deposition in females than males, even in CU individuals. 14 , 15 , 16 Female‐related elevations in tau persist when accounting for Aβ levels, which in contrast to tau do not strongly differ by sex. 16 Moreover, Aβ and tau pathology exhibit tighter relationships with hippocampal atrophy and cognitive decline in females compared to males. 17 , 18 , 19 Despite these sex differences, most plasma AD biomarker studies control for sex without directly modeling how plasma biomarker prediction of AD phenotypic progression varies by sex. Sex‐dependent relationships between plasma AD biomarkers and disease progression outcomes may not occur uniformly across brain region or cognitive ability, 20 particularly in early pathological states in which patterns of brain atrophy and cognitive change are relatively focal.
We examined sex differences in baseline plasma p‐tau217 prediction of domain‐specific cognitive decline and regional brain atrophy in CU older adults. Sex‐specific prognostic effects of plasma p‐tau217 were compared to complementary plasma biomarkers of astroglial and axonal pathophysiology, glial fibrillary acidic protein (GFAP) and neurofilament light (NfL). Sex‐specific cognitive models were repeated in a longitudinal cohort of patients with mild cognitive impairment (MCI) to determine specificity to clinical stage. To enhance the interpretation and clinical utility of results, models probed plasma p‐tau217 concentration thresholds at which sex‐specific cognitive and brain atrophy trajectories significantly declined. Secondary Aβ PET models anchored plasma p‐tau217 concentrations to early and established levels of cortical Aβ pathology.
2. METHODS
2.1. Participants
Participants included 163 adults who underwent a baseline neurological examination, neuropsychological testing, blood draw, and study partner interview (Clinical Dementia Rating [CDR]) as part of longitudinal studies at the University of California San Francisco (UCSF) Memory and Aging Center (MAC). At baseline screening, participants were classified as CU (CDR = 0) or MCI (CDR = 0.5) per consensus case conference with board‐certified neurologists and neuropsychologists. 21 Participants were drawn from two MAC cohorts: (1) 85 community‐dwelling, CU adults (44 males, 41 females) enrolled in the Brain Aging Network for Cognitive Health (BRANCH) and (2) 78 MCI patients (37 males, 41 females) enrolled in the UCSF Alzheimer's Disease Research Center (ADRC). CU participants were recruited into BRANCH via flyers, newspaper advertisements, and community outreach events. MCI participants were recruited into the ADRC through referrals from outside clinic providers. BRANCH and ADRC enrollment criteria excluded individuals with severe systemic medical illnesses that could confound measurement of plasma biomarker concentrations (e.g., severe chronic kidney or liver disease). 22 , 23 Both studies also excluded severe psychiatric illness, non‐neurodegenerative neurological conditions that could impact cognition, and recent substance use disorders (previous 20 years). Inclusion in the current study was contingent on availability of baseline plasma biomarker and cognitive data. Study procedures were approved by the UCSF Committee on Human Research and all participants provided written informed consent.
2.1.1. Plasma biomarkers
Non‐fasting, morning blood samples were obtained by venipuncture and processed for plasma. Plasma p‐tau217 concentrations were measured using a novel single‐molecule array (Simoa) assay developed by Janssen Research and Development. 4 , 24 , 25 The functional plasma p‐tau217 assay range, accounting for sample dilution, was: lower limit of detection (LLOD) = 0.0011 pg/mL, lower limit of quantification (LLOQ) = 0.0187 g/mL, upper limit of quantification (ULOQ) = 10 pg/mL. All samples were > LLOD. GFAP and NfL concentrations were measured using the Neurology 2‐Plex B kit (Quanterix Simoa). Samples were measured in duplicate and all participants eligible for the present analysis had samples with coefficients of variance (CV) < 20%. Mean ± standard deviation CV% for study samples was 4.4% ± 3.4% (p‐tau217), 4.6% ± 3.7% (GFAP) and 4.8% ± 7.7% (NfL).
2.2. Cognitive assessment
Cognitive testing protocols 26 were harmonized across the CU and MCI cohorts, with the exception of verbal episodic memory testing. Raw test scores were converted to domain‐specific z scores based on the score distribution of the larger BRANCH cohort (all CDR = 0; n > 650 per test). 27 Cognition in each cohort was operationalized with composite z scores in four separate domains. (1) Verbal episodic memory in the CU cohort was quantified via a composite of primary metrics from the California Verbal Learning Test, second edition (CVLT‐II: total immediate recall, total long [20 minute] delay free recall, and recognition discriminability [d’]), whereas the MCI cohort completed the CVLT‐short form (CVLT‐SF: total immediate recall, total long [10 minute] delay free recall, recognition discriminability [d’]); (2) visual episodic memory was quantified via delayed (10 minute) free recall of a complex figure (modified Benson figure); (3) executive functions were quantified via a composite of digit span backward, modified Trail Making Test, Stroop Inhibition, lexical fluency (number of D‐words/60″), and design fluency (DKEFS Condition 1); and (4) language was quantified via a composite of the animal fluency task (number of animals/60″) and the 15‐item Boston Naming Test. The total number of longitudinal data observations per cognitive composite z score ranged from 288 to 328 in CU (average follow‐up = 5.6 years), and 203 to 223 in MCI (average follow‐up = 3.4 years).
2.3. Neuroimaging
A subset of the CU cohort underwent longitudinal structural magnetic resonance imaging (MRI; n = 68, 201 observations, average follow‐up = 4.8 years) at the UCSF Neuroscience Imaging Center using a Siemens Trio Tim or Prisma Fit 3T scanner. Magnetization prepared rapid gradient‐echo (MPRAGE) sequences were used to obtain whole brain T1‐weighted images sagittally using the following parameters: repetition time (TR) = 2300 ms, inversion time (TI) = 900 ms, echo time (TE) = 2.98 ms, flip angle = 9°, field‐of‐view (FOV) = 240×256 mm with 1×1 mm in‐plane resolution and 1 mm slice thickness. Parameters for both Trio and Prisma scanners had nearly identical parameters but slightly different echo times (Trio: 2.98 ms; Prisma: 2.9 ms). As previously described, 28 tissue segmentation was performed using unified segmentation in SPM12 29 and brain volumes of interest were quantified by translating a standard parcellation atlas 30 into International Consortium of Brain Mapping space and summing the gray matter within each region of interest (ROI). To focus on an early AD‐sensitive brain region, medial temporal lobe volumes were computed by summing bilateral hippocampal, entorhinal, and parahippocampal Desikan regions. 30 Comparison composite ROIs were calculated for frontal (caudal and rostral middle frontal, superior frontal, lateral and medial orbitofrontal, frontal pole, paracentral, precentral pars opercularis, pars orbitalis, pars triangularis), parietal (inferior, superior, postcentral, precuneus, supramarginal), and occipital (lingual, pericalcarine, cuneus, lateral occipital) lobes.
RESEARCH IN CONTEXT
Systematic review: The authors used PubMed to identify previous studies examining sex differences in Alzheimer's disease (AD) pathophysiology and AD biomarker prediction of cognition and brain atrophy. Sex differences in plasma phosphorylated tau (p‐tau)217 prediction of longitudinal cognitive decline and brain atrophy in cognitively unimpaired (CU) adults has not been previously studied.
Interpretation: Elevations in plasma p‐tau217 predicted verbal memory decline and medial temporal lobe atrophy in CU females, but not CU males. In patients with mild cognitive impairment, plasma p‐tau217 was a more consistent predictor of cognitive trajectories in males and females.
Future directions: These findings suggest plasma p‐tau217 may capture earlier AD‐related clinical hallmarks in females compared to males, which recapitulates prior observations that females show increased susceptibility to AD pathophysiology. Future studies with additional biomarkers that capture complementary biological pathways (e.g., sex hormones, synapses) are needed to understand the mechanisms driving sex differences in early AD‐related clinical progression.
A combined subset of the CU (n = 53) and MCI (n = 35) cohorts underwent Aβ PET imaging with either 18F‐florbetapir (n = 70 [51 CU, 19 MCI]) or 11C‐Pittsburgh compound B (PiB; n = 18 [2 CU, 16 MCI]). For both 18F‐florbetapir and PiB tracers, we measured four 5‐minute frames acquired 50–70 minutes post‐injection interval. Standardized uptake value ratio (SUVR) was calculated using mean activity in the whole cerebellum (18F‐florbetapir) or cerebellar gray matter (PiB) as the reference region. Frames were co‐registered to the corresponding MPRAGE images and global amyloid burden was estimated using a composite of frontal, cingulate, temporal, and parietal areas. 31 SUVRs were then converted to the Centiloid (CL) scale to provide a continuous measure of global cortical Aβ deposition that was harmonized across the two tracers. 32 CL values of 0 represent mean uptake in young healthy controls free of Aβ pathology, and values of 12 and 30 represent early and established levels of Aβ aggregation, respectively. 7 , 33
2.4. Statistical analysis
Cohort‐stratified sex differences in demographic, clinical, and baseline analytic variables were examined using analysis of variance (ANOVA) and chi‐square statistics with two‐tailed tests, as appropriate. A series of linear mixed‐effects models with person‐specific random slopes and intercepts examined subclinical cognitive and brain volumetric changes in the CU cohort. Time was entered as a fixed and random effect. Covariates included baseline age, apolipoprotein E (APOE) ε4 status, education (cognitive), and scanner type (structural MRI). Body mass index (BMI) was also entered as a covariate to account for its potential influence on plasma biomarker measurement. 22 , 34 We first entered a sex by time (years since baseline blood draw) interaction term for each cognitive z score and brain volume composite ROI to ascertain whether average trajectories varied by sex. Next, we added a three‐way interaction term (p‐tau217 × sex × time) to determine sex differences in plasma p‐tau217 prediction of trajectories. Standardized coefficients with bootstrapped 95% confidence intervals are reported for p‐tau217 predictive effects (p‐tau217 × time) in males and females. For significant sex‐specific p‐tau217 × time terms, we identified specific thresholds of plasma p‐tau217 concentrations at which cognitive and brain volume trajectories significantly declined using region of significance analyses. 35 , 36 Region of significance analyses use iterative simple‐slope testing across the entire range of a moderator (i.e., p‐tau217), with adjustment for multiple comparisons using the false discovery rate, to place a “floodlight” on specific segments of the moderator at which the primary predictor (i.e., time) is estimated to have a statistically significant effect on the outcome. To determine molecular specificity, plasma p‐tau217 relationships were compared to plasma GFAP and NfL. Additionally, to determine specificity of results to clinical stage, parallel p‐tau217 cognitive models were repeated in the MCI cohort. Last, the subset of CU and MCI cohort participants with Aβ PET imaging were combined for secondary analysis that modeled Aβ PET CLs as a function of plasma p‐tau217 (linear and quadratic), sex, and their interaction. Model estimates identified concentrations of plasma p‐tau217 that corresponded to early and established Aβ pathology thresholds (12 and 30 CLs, respectively). 33
3. RESULTS
3.1. Participant characteristics
Table 1 presents demographic and clinical characteristics by sex in the CU and MCI cohorts. The CU cohort was on average 75.8 years old (range: 58–99) with 17.6 years of education and 22% APOE ε4. The MCI cohort was on average 69.3 years old (range: 48–86) with average age of symptom onset at 64.2 years old (range: 43–84; 55% age of onset < 65 years old), 16.5 years of education, and 40% APOE ε4. Males and females did not statistically differ on most demographic or clinical characteristics, including medical comorbidities, in either cohort.
TABLE 1.
Participant characteristics by sex and diagnosis.
| Cognitively unimpaired | Mild cognitive impairment | ||||||
|---|---|---|---|---|---|---|---|
|
Males (n = 44) |
Females (n = 41) |
Sex P |
Males (n = 37) |
Females (n = 41) |
Sex p |
Dx a P |
|
| Age (years), mean (SD) b | 76.6 (7.6) | 74.8 (6.9) | 0.263 | 70.7 (9.5) | 68.2 (8.3) | 0.237 | <0.001 |
| Years of education, mean (SD) | 17.7 (1.9) | 17.5 (1.8) | 0.639 | 16.5 (2.5) | 16.5 (2.5) | 0.909 | 0.001 |
| APOE ε4 carrier, n (%) | 12 (27.3%) | 7 (17.1%) | 0.257 | 13 (35.1%) | 18 (43.9%) | 0.429 | 0.016 |
| Race/ethnicity (non‐Hispanic White), n (%) | 41 (93.2%) | 36 (87.8%) | 0.395 | 34 (94.4%) | 37 (90.2%) | 0.799 | 0.923 |
| BMI (kg/m2), mean (SD) | 25.3 (3.7) | 24.0 (4.2) | 0.113 | 26.5 (3.2) | 24.1 (4.40) | 0.009 | 0.433 |
| Atrial fibrillation, n (%) c | 7 (17%) | 1 (3%) | 0.057 | 2 (7%) | 1 (3%) | 0.591 | 0.346 |
| Cancer, n (%) c | 12 (29%) | 5 (13%) | 0.102 | 6 (20%) | 8 (23%) | 0.780 | 0.966 |
| Congestive heart failure, n (%) c | 3 (7%) | 1 (3%) | 0.616 | 0 (0%) | 1 (3%) | 1 | 0.380 |
| Type II diabetes, n (%) c | 3 (7%) | 2 (5%) | 1 | 1 (3%) | 0 (0%) | 0.462 | 0.224 |
| Dyslipidemia, n (%) c | 19 (46%) | 19 (49%) | 0.832 | 16 (53%) | 15 (43%) | 0.399 | 0.982 |
| Hypertension, n (%) c | 19 (46%) | 16 (41%) | 0.632 | 15 (50%) | 12 (34%) | 0.200 | 0.789 |
| Myocardial infarction, n (%) c | 3 (7%) | 1 (3%) | 0.616 | 1 (3%) | 0 (0%) | 0.462 | 0.380 |
| P‐tau217 (pg/ml), mean (SD) | 0.08 (0.04) | 0.07 (0.04) | 0.240 | 0.14 (0.1) | 0.17 (0.14) | 0.351 | <0.001 |
| GFAP (pg/ml), mean (SD) | 137.7 (54.6) | 182.09 (76.9) | 0.005 | 173.1 (75.0) | 208.4 (111.8) | 0.112 | 0.017 |
| NfL (pg/ml), mean (SD) | 19.8 (9.6) | 18.7 (10.2) | 0.600 | 19.1 (8.1) | 18.9 (8.4) | 0.943 | 0.401 |
| MMSE, mean (SD) | 29.1 (1.1) | 29.4 (0.7) | 0.138 | 26.0 (3.6) | 26.7 (3.1) | 0.415 | <0.001 |
| Visual memory z, mean (SD) d | −0.03 (1.06) | 0.07 (0.91) | 0.676 | −1.01 (1.5) | −1.46 (1.4) | 0.190 | <0.001 |
| Verbal memory z (CVLT‐II), mean (SD) d | −0.03 (0.84) | 0.31 (0.85) | 0.105 | — | — | — | — |
| Verbal memory z (CVLT‐SF), mean (SD) d | — | — | — | −2.28 (1.70) | −2.35 (1.75) | 0.879 | — |
| Executive function z, mean (SD) d | 0.06 (0.66) | −0.04 (0.74) | 0.546 | −0.97 (1.00) | −0.83 (1.20) | 0.589 | <0.001 |
| Language z, mean (SD) d | 0.01 (0.63) | 0.16 (0.84) | 0.038 | −1.02 (1.35) | −1.11 (1.69) | 0.810 | <0.001 |
| Medial temporal/TIV, mean (SD) e | 2.0 (0.2) | 2.0 (0.2) | 0.531 | — | — | — | — |
| Frontal/TIV, mean (SD) e | 13.3 (1.5) | 14.1 (1.4) | 0.038 | — | — | — | — |
| Parietal/TIV, mean (SD) e | 8.1 (1.0) | 8.6 (1.0) | 0.038 | — | — | — | — |
| Occipital/TIV, mean (SD) e | 4.9 (0.6) | 5.2 (0.6) | 0.059 | — | — | — | — |
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; CVLT‐II, California Verbal Learning Test Second Edition; CVLT‐SF, California Verbal Learning Test Short Form; Dx, diagnosis; GFAP, glial fibrillary acidic protein; MMSE, Mini‐Mental State Examination; NfL, neurofilament light; SD, standard deviation; TIV, total intracranial volume.
Diagnosis P‐values reflect differences between cognitively unimpaired (n = 85) and mild cognitive impairment (n = 78).
Age at baseline blood draw and plasma biomarker measurement.
n = 145.
Cognitive z scores based on mean (SD) of cognitively unimpaired adults from the larger Brain Aging Network for Cognitive Health cohort.
Composite volumetric region/TIV ratios are multiplied by 1000 for scaling purposes.
3.2. Sex differences in plasma biomarker concentrations
Sex differences in baseline plasma biomarker concentrations and pairwise correlations are displayed in Figure 1. CU females exhibited elevated baseline plasma GFAP compared to CU males (d = 0.64, P = 0.005), whereas baseline plasma p‐tau217 (d = −0.22, P = 0.240) and NfL (d = −0.12, P = 0.600) did not significantly differ by sex. Plasma biomarkers did not significantly differ by sex in MCI, although MCI females also exhibited non‐significant elevations in GFAP compared to MCI males (d = 0.36, P = 0.112). Pairwise correlations between biomarkers did not reach statistical significance among CU males, whereas CU females exhibited significant relationships between p‐tau217 and NfL (r = 0.34, P = 0.031) as well as GFAP and NfL (r = 0.44, P = 0.004). In both MCI males and females, each pairwise biomarker correlation was statistically significant and larger in magnitude relative to their CU counterparts.
FIGURE 1.
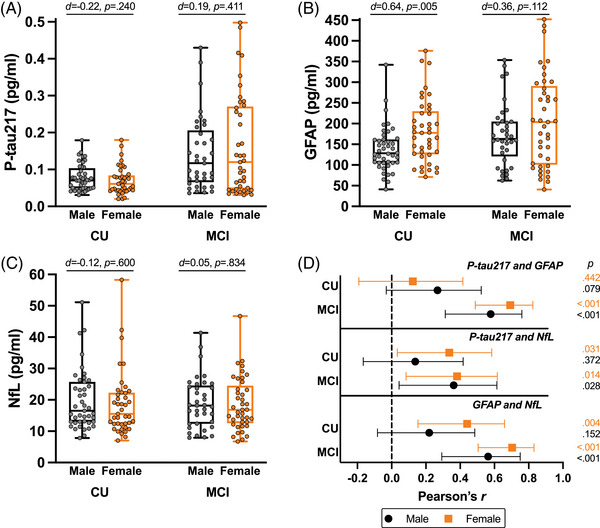
Plasma biomarker concentrations and intercorrelations by sex and clinical status. Box plots represent median, interquartile range, minimum, and maximum (A–C). Sex differences by clinical status are annotated with Cohen d estimates of effect size and P‐values. Pairwise Pearson r correlations with 95% confidence interval are plotted by sex and clinical status (D). CU, cognitively unimpaired; GFAP, glial fibrillary acidic protein; MCI, mild cognitive impairment; NfL, neurofilament light; p‐tau217; phosphorylated tau 217.
3.3. Sex differences in plasma p‐tau217 prediction of subclinical cognitive decline
In the CU cohort, time was significantly related to worsening verbal memory (β = −0.20; P < 0.001), language (β = −0.20; P < 0.001), and executive function (β = −0.10; P = 0.002), but not visual memory (β = −0.05; P = 0.260). Sex did not moderate the effect of time on any cognitive outcome (Ps > 0.625), indicating that average cognitive slopes did not differ between CU males and females (Figure S1 in supporting information). However, sex significantly moderated the effect of baseline plasma p‐tau217 on verbal memory (sex × p‐tau217 × time: β = −0.55, P = 0.004) and language trajectories (sex × p‐tau217 × time: β = −0.48, P = 0.029) such that higher plasma p‐tau217 levels related to steeper cognitive decline in CU females but not males (Figure 2A,B and Table S1 in supporting information). Region of significance analyses estimated statistically significant declines in verbal memory and language in females when baseline plasma p‐tau217 was greater than 0.053 pg/ml (Figure 2C).
FIGURE 2.
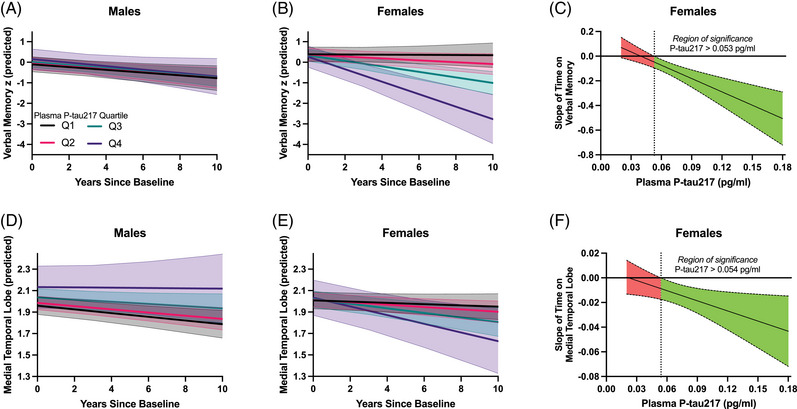
Plasma p‐tau217 prediction of verbal memory and medial temporal lobe trajectories in CU males (A, D) and females (B, E). For visualization purposes, stratified lines represent the estimated slope of time at the median of each baseline plasma p‐tau217 quartile (pg/ml; Q1 median [range]: 0.041 [0.020 to 0.046]; Q2 median [range]: 0.051 [> 0.046 to 0.068]; Q3 median [range]: 0.078 [> 0.068 to 0.092]; Q4 median [range]: 0.120 [> 0.092 to 0.200]). The effect size (unstandardized) of time on verbal memory z scores (C) and medial temporal lobe volumes (F) is plotted across baseline plasma p‐tau217 in CU females. When the X axis zero‐line is not included in the false discovery rate–adjusted confidence band, the effect of time is statistically significant at that concentration of baseline plasma p‐tau217. The region of significance occurs to the right of the dotted line, indicating that increasing time is significantly associated with worse trajectories once baseline plasma p‐tau217 concentrations surpass 0.053/0.054 pg/ml. CU, cognitively unimpaired; p‐tau217; phosphorylated tau 217.
3.4. Sex differences in plasma p‐tau217 prediction of subclinical brain atrophy
In the CU cohort, time was significantly associated with atrophy in each volumetric outcome (medial temporal: β = −0.19, P < 0.001; frontal: β = −0.22; P < 0.001; parietal: β = −0.19; P < 0.001; occipital: β = −0.20; P < 0.001). Sex did not moderate the effect of time on any volumetric outcome (P > 0.573), indicating that average atrophy slopes did not differ between males and females (Figure S2 in supporting information). Sex significantly moderated the effect of baseline plasma p‐tau217 on medial temporal lobe atrophy (sex × p‐tau217 × time: β = −0.33, P = 0.015) such that higher plasma p‐tau217 levels related to steeper medial temporal lobe atrophy in CU females but not males (Figure 2D,E and Table S2 in supporting information). Sex did not significantly moderate plasma p‐tau217 associations with any other ROI (Ps > 0.186). Region of significance analyses estimated statistically significant declines in medial temporal lobe volume when baseline plasma p‐tau217 was greater than 0.054 pg/ml (Figure 2F).
3.5. Plasma GFAP and NfL comparison models
Figure 3 displays standardized coefficients with 95% confidence intervals comparing sex‐specific plasma p‐tau217 relationships to cognitive and brain volume trajectories in CU (Figure 3A,B) to the predictive effects of plasma GFAP and NfL. Sex did not significantly moderate the relationship between GFAP or NfL with any cognitive trajectory (Table S1). However, lower order model estimates indicated that only females exhibited significant associations between higher GFAP and visual memory decline (females: β = −0.17; P = 0.001l; males: β = −0.08; P = 0.165; Figure 3C), and higher NfL and verbal memory decline (females: β = −0.18; P = 0.007; males: β = −0.10; P = 0.312; Figure 3E). These associations were smaller in magnitude than the p‐tau217 relationship with female verbal memory decline (β = −0.32, P < 0.001), which remained significant after post hoc adjustment for GFAP and NfL (β = −0.31, P < 0.001).
FIGURE 3.
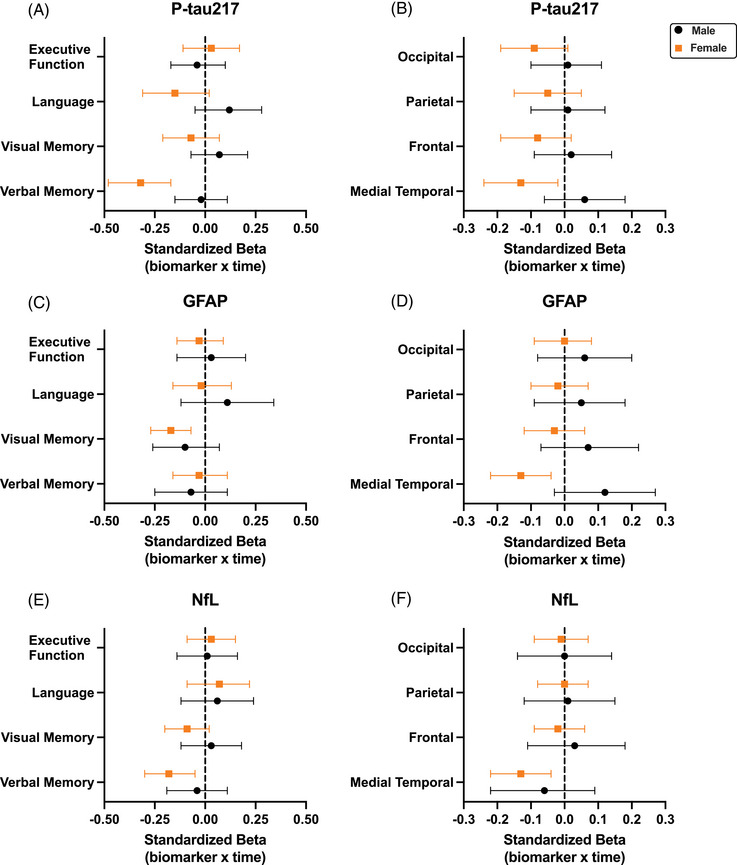
Comparison of sex‐specific plasma biomarker prediction of cognitive and brain volumetric trajectories in cognitively unimpaired adults. Values represent standardized coefficients with bootstrapped 95% confidence intervals for the interaction of baseline plasma biomarker levels and time on cognitive (A, C, E) and brain volumetric (B, D, F) outcomes in cognitively unimpaired adults, stratified by sex. Full model estimates and P‐values are presented in Tables S1 and S2 in supporting information. GFAP, glial fibrillary acidic protein; NfL, neurofilament light; p‐tau217; phosphorylated tau 217.
In imaging models, sex significantly moderated the effect of plasma GFAP on medial temporal lobe atrophy such that higher GFAP levels also related to steeper medial temporal lobe atrophy in females but not males (Figure 3D and Table S2). Sex did not interact with NfL to predict brain volumetric trajectories (Ps > 0.480). However, lower order model estimates also indicated that only females exhibited significant associations between higher NfL and steeper medial temporal lobe atrophy (Figure 3F and Table S2). Notably, all three biomarkers exhibited the same magnitude of association with female medial temporal lobe trajectories (p‐tau217: β = −0.13, P = 0.028; GFAP: β = −0.13, P = 0.010; NfL: β = −0.13, P = 0.010).
3.6. Cognitive models in MCI
In the MCI cohort, time was significantly related to worsening cognition in every domain (verbal memory: β = −0.22, P < 0.001; visual memory: β = −0.20, P < 0.001; language: β = −0.27, P < 0.001; executive function: β = −0.24, P < 0.001). Sex did not moderate the effect of time on any cognitive outcome (Ps > 0.267), indicating that average cognitive slopes also did not differ between males and females with MCI. Sex also did not significantly moderate the relationship between any baseline plasma biomarker on cognitive trajectories in MCI (Ps > 0.162). Figure 4 displays standardized coefficients with 95% confidence intervals for sex‐specific plasma p‐tau217, GFAP, and NfL prediction of cognitive trajectories in MCI. Higher baseline plasma p‐tau217 related to steeper cognitive decline across domains in MCI, with significant associations observed in both males (verbal memory, visual memory, language, executive function) and females (language, executive function). Compared to plasma p‐tau217, GFAP and NfL showed weaker and fewer statistically significant associations with cognitive trajectories in MCI, regardless of sex (Figure 4 and Table S1). Baseline age did not predict cognitive trajectories in any of the models (Ps > 0.162) and biomarker predictions of cognitive trajectories were not altered in a sensitivity analysis that removed baseline age as a covariate, suggesting that differences in age of symptom onset (highly collinear with baseline age) had little influence on MCI cohort results.
FIGURE 4.
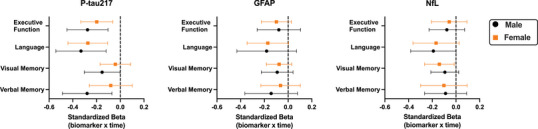
Comparison of sex‐specific plasma biomarker prediction of cognitive trajectories in mild cognitive impairment. Values represent standardized coefficients with bootstrapped 95 % confidence intervals for the interaction of baseline plasma biomarker levels and time on cognitive outcomes in mild cognitive impairment, stratified by sex. Full model estimates and P‐values are presented in Table S1 in supporting information. GFAP, glial fibrillary acidic protein; NfL, neurofilament light; p‐tau217; phosphorylated tau 217.
3.7. Plasma p‐tau217 relationship with Aβ PET by sex
Plasma p‐tau217 concentrations were plotted against Aβ PET CLs in the combined CU and MCI cohort of participants with available Aβ PET data (n = 88). Visual inspection suggested a quadratic association between plasma p‐tau217 and Aβ PET CLs (Figure 5), which was confirmed in linear regression analysis (quadratic p‐tau217: β = −0.72, P < 0.001). This relationship was not moderated by sex (P = 0.590) and remained after adjustment for age, APOE ε4, BMI, and clinical status. Based on model estimates, an early Aβ pathology threshold (12 CLs) was reached at plasma p‐tau217 concentration of 0.06 pg/ml, and an established Aβ pathology threshold (30 CLs) was reached at a plasma p‐tau217 concentration of 0.09 pg/ml.
FIGURE 5.
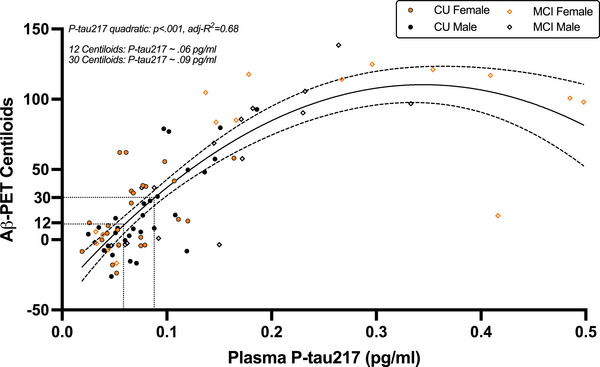
Quadratic relationship between plasma p‐tau217 and cortical Aβ burden. Plasma p‐tau217 exhibits a non‐linear relationship with Aβ PET Centiloids such that initial increases in plasma p‐tau217 correspond with steep increases in cortical Aβ burden that plateau well after established Aβ pathology levels. A single curve was plotted across sex given that the quadratic relationship between plasma p‐tau217 and Aβ PET was not moderated by sex. Thresholds for early (12 Centiloids) and established (30 Centiloids) Aβ pathology were estimated to occur at plasma p‐tau217 concentrations of 0.06 pg/ml and 0.09 pg/ml, respectively. Aβ, amyloid beta; CU, cognitively unimpaired; MCI, mild cognitive impairment; PET, positron emission tomography; p‐tau217; phosphorylated tau 217.
4. DISCUSSION
The present study examined sex‐dependent associations of plasma p‐tau217 with cognitive and brain atrophy trajectories. Among CU, baseline plasma p‐tau217 interacted with sex to predict verbal memory and language trajectories, with the strongest relationship occurring between higher plasma p‐tau217 and female‐specific verbal memory decline. CU females showed statistically significant declines in verbal memory and language z scores once baseline plasma p‐tau217 concentrations reached 0.053 pg/ml. Secondary Aβ PET analyses approximated that this threshold may reflect early levels of cortical Aβ aggregation (12 CLs). In contrast, plasma p‐tau217 only emerged as a statistically significant prognosticator of cognitive decline in both males and females at the MCI stage. Converging with plasma p‐tau217, both GFAP and NFL showed significant female‐specific associations with medial temporal lobe atrophy over time in CU, but did not evidence sex‐specific effects on cognitive trajectories. Overall, these findings lend further support for plasma p‐tau217 as an early disease stage biomarker 3 , 7 , 10 and underscore sex as an important source of divergence in its predictive utility.
Our results recapitulate prior cerebrospinal fluid (CSF) and PET findings of increased female susceptibility to AD‐related clinical change and are the first, to our knowledge, to report this sex difference with plasma p‐tau217. Females exhibit steeper hippocampal atrophy and cognitive decline in relation to lower CSF Aβ42, higher CSF total tau, and higher CSF total tau/Aβ42, 18 , 37 particularly at the MCI stage. Tau PET studies also report stronger relationships between tau deposition and cognitive outcomes in females, 17 , 38 including steeper tau‐related cognitive decline in CU individuals. 17 Recent data show stronger associations of plasma p‐tau181 with AD PET biomarkers (Aβ PET, entorhinal cortex tau PET, fluorodeoxyglucose PET) and global cognitive decline in females. 39 However, these sex differences were driven by patients with MCI and were absent among CU. In comparison, the prognostic effects of baseline plasma p‐tau217 on CU female trajectories is consistent with evidence that plasma p‐tau217 increases at earlier levels of Aβ burden than plasma p‐tau181, GFAP, and NfL, 7 and may therefore capture female vulnerability to AD‐related cognitive changes (e.g., verbal memory, language) at an earlier disease stage. Although plasma p‐tau217 was not predictive of cognitive decline in CU males, a strong and consistent plasma p‐tau217 signal was detected across domains in MCI males, thereby reinforcing plasma p‐tau217 as a useful marker for disease prognosis in males prior to dementia.
The specificity of plasma p‐tau217 to decline in medial temporal lobe volume and function (i.e., verbal memory) in CU females highlights the importance of isolating cognitive domains and brain regions most vulnerable to early AD pathology. Many studies have modeled longitudinal cognitive change with global screeners (e.g., Mini‐Mental State Examination) or global composites (e.g., modified Preclinical Alzheimer Cognitive Composite), as these metrics are relatively harmonizable across cohorts. While these global cognitive outcomes also exhibit decline in relation to plasma AD biomarker elevations, 10 , 39 the strength of biomarker associations with cognitive trajectories may be amplified when examining domain‐specific outcomes, with additional consideration for person‐specific factors that modulate risk (e.g., sex). 19
Females on average outperform males on verbal memory and language tests. 40 , 41 , 42 Notably, female verbal memory advantages persist in cross‐sectional studies of individuals with AD pathology and/or mild–moderate symptomatology. 43 , 44 , 45 Although between‐sex differences in verbal memory suggest a female verbal memory reserve, 46 longitudinal designs that model intra‐individual change indicate similar or accelerated rates of decline in females along the AD continuum. 47 , 48 Our data align with these cross‐sectional and longitudinal observations, as CU females exhibited slight verbal memory and language advantages at baseline but had similar cognitive slopes to CU males. Thus, the stronger relationship between plasma p‐tau217 and verbal memory decline in females is unlikely to represent an artifact of intercept differences or a “regression to the mean” phenomenon, but rather true biological differences.
Females consistently exhibit higher temporal tau PET signal than males for fixed levels of Aβ pathology, 16 , 17 although the exact mechanisms driving female‐related tau deposition and clinico‐radiographic sequelae are unclear. We did not observe sex differences in average plasma p‐tau217 concentrations, consistent with most prior studies, 6 , 49 but not all. 22 Plasma p‐tau217 is more tightly linked to cortical amyloid than tau in CU individuals, 50 which may explain why sex differences in tau PET deposition are not recapitulated with plasma p‐tau isoforms. A recent post mortem study highlighted microglial activation as a female‐specific mediator of Aβ‐related tau deposition. 51 Similarly, CSF microglial markers (YKL‐40, sTREM2) show stronger associations with medial temporal lobe integrity in CU females compared to males, consistent with our GFAP findings. 52 Interestingly, we also observed higher plasma GFAP in CU females than CU males, which has been previously reported. 53 Future work incorporating additional neural markers, including microglial and synaptic, 28 , 40 , 54 would help elucidate the degree to which sex‐related effects of plasma p‐tau217 reflect sex differences in neuroimmune pathways.
Our results and their interpretation should be considered in the context of our study sample. The CU cohort consisted of community‐dwelling adults without cognitive complaints or specific enrichment for familial/genetic risk of AD. APOE ε4 positivity rates in the CU cohort, particularly females (17%), were lower than would be expected for a similarly aged cohort of CU Aβ‐positive individuals (≈30%–40%). 55 Prior studies have mostly focused on plasma p‐tau217 in the context of Aβ‐positive individuals, who represent primary candidates for clinical trials. Our main plasma biomarker models were agnostic to Aβ PET status, which reflects an ecologically valid context as most clinical settings with potential access to low‐cost, blood‐based biomarker assays will not have similar access to Aβ PET. Our ability to detect a robust cognitive and neural signal of plasma p‐tau217 in females without deliberate risk stratification (e.g., APOE ε4, Aβ PET) highlights the standalone potential for plasma p‐tau217 as a predictor of cognitive and brain volumetric progression in CU females, even at “subthreshold” concentrations that align with early (vs. established) levels of cortical Aβ accumulation.
Our study is among the first to examine sex differences in plasma p‐tau217 prediction of subclinical cognitive and brain trajectories; however, we acknowledge several limitations. Sex assigned at birth is an imperfect proxy for sex‐related neurobiological variability and our data did not directly capture neurohormonal, sex chromosomal, or sociocultural factors that may mediate AD‐related sex differences. 14 , 56 , 57 , 58 Our study also lacked CSF AD biomarkers and tau PET data, which would improve biological phenotyping of study cohorts and help address questions regarding the potential role of female‐associated tau deposition as a mediator of plasma p‐tau217 relationships with medial temporal lobe structure and verbal memory. Our results converge with prior work showing disproportionate female vulnerability to AD‐related outcomes; however, our study lacked a CU replication cohort with harmonizable biomarker and neuropsychological data. Future replication of study methodology across other CU cohorts, particularly those with greater demographic diversity and MCI comparison cohorts drawn from the same recruitment source (e.g., community based), would bolster the validity and generalizability of study results. Similarly, the addition of an MCI cohort with MRI data as well as a longitudinal AD dementia cohort would enhance future studies that model sex‐dependent effects of plasma p‐tau217 across the entire AD clinical continuum.
This longitudinal study observed sex‐specific associations between baseline plasma p‐tau217 and cognitive and brain trajectories. Females were disproportionately vulnerable to the adverse effects of elevated plasma p‐tau217 on verbal memory decline and medial temporal lobe atrophy at the CU stage, whereas males exhibited p‐tau217–related cognitive decline at the MCI stage. Overall, our findings suggest plasma p‐tau217 may capture earlier AD‐related clinical hallmarks in females compared to males.
CONFLICT OF INTEREST STATEMENT
GTB and HCK are employees and stockholders of Janssen R&D (Johnson & Johnson). The remaining authors have nothing to disclose. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All study procedures were approved by the UCSF Committee on Human Research and all participants provided written informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors acknowledge the invaluable contributions of study participants as well as the assistance of support staffs at the UCSF Memory and Aging Center. This study was supported by NIH‐NIA grants R01AG032289 (PI: JHK), R01AG048234 (PI: JHK), UCSF ADRC P30AG062422 (PI: GDR), UCSF PPG P01AG019724 (PI: BLM), R01AG072475 (PI: KBC), UF1NS100608 (PI: JHK), U19AG063911 (PI: ALB), R01AG038791 (PI: ALB), K23AG073514 (PI: LV), R01AG045611 (PI: GDR), R01AG068325 (PI: DBD), R01AG079176 (PI: DBD). Our work was also supported by NIH‐NINDS grant U54NS092089 (PI: ALB), Larry L. Hillblom Foundation grant 2018‐A‐006‐NET (PI: JHK), and Alzheimer's Association grants AARF‐23‐1145318 (PI: RS) and AARF‐22‐974065 (PI: EWP).
Saloner R, VandeVrede L, Asken BM, et al. Plasma phosphorylated tau‐217 exhibits sex‐specific prognostication of cognitive decline and brain atrophy in cognitively unimpaired adults. Alzheimer's Dement. 2024;20:376–387. 10.1002/alz.13454
REFERENCES
- 1. Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's association appropriate use recommendations for blood biomarkers in Alzheimer's disease. Alzheimer's & Dementia. 2022;18:2669‐2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of Plasma Phospho‐tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324:772‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gonzalez‐Ortiz F, Kac PR, Brum WS, Zetterberg H, Blennow K, Karikari TK. Plasma phospho‐tau in Alzheimer's disease: towards diagnostic and therapeutic trial applications. Mol Neurodegener. 2023;18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doré V, Doecke JD, Saad ZS, et al. Plasma p217+tau versus NAV4694 amyloid and MK6240 tau PET across the Alzheimer's continuum. Alzheimers Dement (Amst). 2022;14:e12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asken BM, Tanner JA, VandeVrede L, et al. Plasma P‐tau181 and P‐tau217 in patients with traumatic encephalopathy syndrome with and without evidence of alzheimer disease pathology. Neurology. 2022;99:e594‐e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer's disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021;20:739‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milà‐Alomà M, Ashton NJ, Shekari M, et al. Plasma P‐tau231 and P‐tau217 as state markers of amyloid‐β pathology in preclinical Alzheimer's disease. Nat Med. 2022;28:1797‐1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mattsson‐Carlgren N, Janelidze S, Palmqvist S, et al. Longitudinal plasma P‐tau217 is increased in early stages of Alzheimer's disease. Brain. 2020;143:3234‐3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho‐Tau217 levels with tau positron emission tomography in early alzheimer disease. JAMA Neurol. 2021;78:149‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mattsson‐Carlgren N, Salvadó G, Ashton NJ, et al. Prediction of longitudinal cognitive decline in preclinical Alzheimer disease using plasma biomarkers. JAMA Neurol. 2023;80:360‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jonaitis EM, Janelidze S, Cody KA, et al. Plasma phosphorylated tau 217 in preclinical Alzheimer's disease. Brain Commun. 2023;5:fcad057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashton NJ, Janelidze S, Mattsson‐Carlgren N, et al. Differential roles of Aβ42/40, P‐tau231 and P‐tau217 for Alzheimer's trial selection and disease monitoring. Nat Med. 2022;28:2555‐2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mielke MM. Consideration of sex differences in the measurement and interpretation of Alzheimer disease‐related biofluid‐based biomarkers. J Appl Lab Med. 2020;5:158‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buckley RF, O'Donnell A, McGrath ER, et al. Menopause status moderates sex differences in tau burden: a framingham PET study. Ann Neurol. 2022;92:11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wisch JK, Meeker KL, Gordon BA, et al. Sex‐related differences in tau positron emission tomography (PET) and the effects of hormone therapy (HT). Alzheimer Dis Assoc Disord. 2021;35:164‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76:542‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buckley RF, Scott MR, Jacobs HIL, et al. Sex mediates relationships between regional tau pathology and cognitive decline. Ann Neurol. 2020;88:921‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koran MEI, Wagener M, Hohman TJ. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2017;11:205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindbergh CA, Casaletto KB, Staffaroni AM, et al. Sex‐related differences in the relationship between β‐amyloid and cognitive trajectories in older adults. Neuropsychology. 2020;34:835‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levine DA, Gross AL, Briceño EM, et al. Sex differences in cognitive decline among US adults. JAMA Netw Open. 2021;4:e210169‐e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mielke MM, Dage JL, Frank RD, et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med. 2022;28:1398‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berry K, Asken BM, Grab JD, et al. Hepatic and renal function impact concentrations of plasma biomarkers of neuropathology. Alzheimers Dement (Amst). 2022;14:e12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Groot C, Cicognola C, Bali D, et al. Diagnostic and prognostic performance to detect Alzheimer's disease and clinical progression of a novel assay for plasma P‐tau217. Alzheimer's Research & Therapy. 2022;14:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Triana‐Baltzer G, Moughadam S, Slemmon R, et al. Development and validation of a high‐sensitivity assay for measuring p217+tau in plasma. Alzheimers Dement (Amst). 2021;13:e12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16. [DOI] [PubMed] [Google Scholar]
- 27. Asken BM, VandeVrede L, Rojas JC, et al. Lower white matter volume and worse executive functioning reflected in higher levels of plasma GFAP among older adults with and without cognitive impairment. J Int Neuropsychol Soc. 2022;28:588‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saloner R, Fonseca C, Paolillo EW, et al. Combined effects of synaptic and axonal integrity on longitudinal gray matter atrophy in cognitively unimpaired adults. Neurology. 2022. doi: 10.1212/WNL.0000000000201165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839‐851. [DOI] [PubMed] [Google Scholar]
- 30. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968‐980. [DOI] [PubMed] [Google Scholar]
- 31. Rabinovici GD, Furst AJ, Alkalay A, et al. Increased metabolic vulnerability in early‐onset Alzheimer's disease is not related to amyloid burden. Brain. 2010;133:512‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klunk WE, Koeppe RA, Price JC, et al. The centiloid project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11:1‐15. e1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salvadó G, Molinuevo JL, Brugulat‐Serrat A, et al. Centiloid cut‐off values for optimal agreement between PET and CSF core AD biomarkers. Alzheimer's Research & Therapy. 2019;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Syrjanen JA, Campbell MR, Algeciras‐Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimer's & Dementia. 2022;18:1128‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson PO, Neyman J. Tests of certain linear hypotheses and their application to some educational problems. Statistical research memoirs. 1936;1:57‐93. https://psycnet.apa.org/record/1936-05538-001 [Google Scholar]
- 36. Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of educational and behavioral statistics. 2006;31:437‐448. [Google Scholar]
- 37. Sohn D, Shpanskaya K, Lucas JE, et al. Sex differences in cognitive decline in subjects with high likelihood of mild cognitive impairment due to Alzheimer's disease. Sci Rep. 2018;8:7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Banks SJ, Andrews MJ, Digma L, et al. Sex differences in Alzheimer's disease: do differences in tau explain the verbal memory gap? Neurobiol Aging. 2021;107:70‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsiknia AA, Edland SD, Sundermann EE, et al. Sex differences in plasma P‐tau181 associations with Alzheimer's disease biomarkers, cognitive decline, and clinical progression. Mol Psychiatry. 2022;27:4314‐4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saloner R, Paolillo EW, Wojta KJ, et al. Sex‐specific effects of SNAP‐25 genotype on verbal memory and Alzheimer's disease biomarkers in clinically normal older adults. Alzheimer's & Dementia. 2023;n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sundermann EE, Maki P, Biegon A, et al. Sex‐specific norms for verbal memory tests may improve diagnostic accuracy of amnestic MCI. Neurology. 2019;93:e1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pauls F, Petermann F, Lepach AC. Gender differences in episodic memory and visual working memory including the effects of age. Memory. 2013;21:857‐874. [DOI] [PubMed] [Google Scholar]
- 43. Caldwell JZK, Cummings JL, Banks SJ, Palmqvist S, Hansson O. Cognitively normal women with Alzheimer's disease proteinopathy show relative preservation of memory but not of hippocampal volume. Alzheimer's Research & Therapy. 2019;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Digma LA, Madsen JR, Rissman RA, Jacobs DM, Brewer JB, Banks SJ. Women can bear a bigger burden: ante‐ and post‐mortem evidence for reserve in the face of tau. Brain Commun. 2020;2:fcaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Edwards L, La Joie R, Iaccarino L, et al. Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer's continuum: greater tau‐PET retention in females. Neurobiol Aging. 2021;105:86‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sundermann EE, Maki PM, Rubin LH, et al. Female advantage in verbal memory: evidence of sex‐specific cognitive reserve. Neurology. 2016;87:1916‐1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bocancea DI, Svenningsson AL, van Loenhoud AC, et al. Determinants of cognitive and brain resilience to tau pathology: a longitudinal analysis. Brain. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimer's & Dementia: Translational Research & Clinical Interventions. 2015;1:103‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brickman AM, Manly JJ, Honig LS, et al. Plasma P‐tau181, P‐tau217, and other blood‐based Alzheimer's disease biomarkers in a multi‐ethnic, community study. Alzheimers Dement. 2021;17:1353‐1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Therriault J, Vermeiren M, Servaes S, et al. Association of phosphorylated tau biomarkers with amyloid positron emission tomography vs tau positron emission tomography. JAMA Neurol. 2023;80:188‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Casaletto KB, Nichols E, Aslanyan V, et al. Sex‐specific effects of microglial activation on Alzheimer's disease proteinopathy in older adults. Brain. 2022;145:3536‐3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Falcon C, Grau‐Rivera O, Suárez‐Calvet M, et al. Sex differences of longitudinal brain changes in cognitively unimpaired adults. J Alzheimers Dis. 2020;76:1413‐1422. [DOI] [PubMed] [Google Scholar]
- 53. Benedet AL, Milà‐Alomà M, Vrillon A, et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 2021;78:1471‐1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Milà‐Alomà M, Brinkmalm A, Ashton NJ, et al. CSF synaptic biomarkers in the preclinical stage of Alzheimer disease and their association with MRI and PET: a cross‐sectional study. Neurology. 2021. 10.1212/WNL.0000000000012853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jansen WJ, Janssen O, Tijms BM, et al. Prevalence estimates of amyloid abnormality across the Alzheimer disease clinical spectrum. JAMA Neurol. 2022;79:228‐243. [DOI] [PubMed] [Google Scholar]
- 56. Davis EJ, Broestl L, Abdulai‐Saiku S, et al. A second X chromosome contributes to resilience in a mouse model of Alzheimer's disease. Sci Transl Med. 2020:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sundermann EE, Panizzon MS, Chen X, Andrews M, Galasko D, Banks SJ. Sex differences in Alzheimer's‐related tau biomarkers and a mediating effect of testosterone. Biol Sex Differ. 2020;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Davis EJ, Solsberg CW, White CC, et al. Sex‐specific association of the X chromosome with cognitive change and tau pathology in aging and Alzheimer disease. JAMA Neurol. 2021;78:1249‐1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


