Abstract
Purpose:
Hematopoietic cell transplantation (HCT) has curative potential for myeloid malignancies, though many patients cannot tolerate myeloablative conditioning with high-dose chemotherapy alone or with total-body irradiation (TBI). Here we report long-term outcomes from a phase 1/2 study using iodine-131 (131I)-anti-CD45 antibody BC8 combined with nonmyeloablative conditioning prior to HLA-haploidentical HCT in adults with high-risk relapsed/ refractory acute myeloid or lymphoid leukemia (AML and ALL), or myelodysplastic syndrome (MDS) [ClinicalTrials.gov, NCT00589316].
Experimental Design:
Patients received a tracer diagnostic dose before a therapeutic infusion of 131I-anti-CD45 to deliver escalating doses (12 to 26 Gy) to the dose-limiting organ. Patients subsequently received fludarabine, cyclophosphamide (CY), and 2 Gy TBI conditioning before haploidentical marrow HCT. GVHD prophylaxis was post-transplant CY plus tacrolimus and mycophenolate mofetil.
Results:
Twenty-five patients (20 with AML, 4 ALL and 1 high-risk MDS) were treated; 8 had ≥ 5% blasts by morphology (range 9–20 %), and 7 had previously failed HCT. All 25 patients achieved a morphologic remission 28 days after HCT, with only two patients showing minimal residual disease (0.002 −1.8%) by flow cytometry. Median time to engraftment was 15 days for neutrophils and 23 days for platelets. Point estimates for overall survival and progression-free survival were 40% and 32% at 1 year, and 24% and 24% at 2 years, respectively. Point estimates of relapse and non-relapse mortality at 1 year were 56% and 12%, respectively.
Conclusion:
131l-anti-CD45 radioimmunotherapy prior to haploidentical HCT is feasible and can be curative in some patients, including those with disease, without additional toxicity.
INTRODUCTION
High-risk hematologic malignancies are difficult to cure with systemic chemotherapy alone, and thus allogeneic hematopoietic cell transplantation (HCT) is thought to offer the best chance for a cure in many cases. Unfortunately, the curative potential of allogenic HCT is not available for all patients with blood cancers due to the lack of fully HLA-matched donors. This is especially challenging for patients of ethnic minority groups, where some populations have a <20% chance of finding a suitably matched donor through the National Marrow Donor Program,(1) underscoring the need for alternative donors like umbilical cord blood or HLA-haploidentical donors. Early reports of post-transplant cyclophosphamide (PTCy) for GVHD prevention in combination with reduced-intensity chemotherapy to overcome earlier challenges (2–6) had less than 20% treatment-related mortality and improved long-term survival and progression free survival but was associated with a 1-year incidence of relapse of 51%.(7) Developing a safe and effective approach to HCT using HLA-haploidentical donors with improved relapse rates remains an important goal since the graft-versus-leukemia (GVL) effect is expected to be augmented in this setting.(2,8)
Efforts to decrease post-HCT relapse have focused largely on intensification of cytoreductive therapy, either by increasing the total-body irradiation (TBI) dose or chemotherapy. Controlled randomized studies have shown that relapse rates could be reduced by increasing the TBI dose.(9,10) However, higher TBI dose had a higher non-relapse mortality (NRM), leading to no overall survival (OS) improvement.(11) We have used monoclonal antibodies (Ab) specific for hematologic targets in radioimmunotherapy (RIT) as a means to deliver higher radiation doses during preparative regimens for HCT. Most recently CD45 has been targeted as a cell-surface antigen expressed on most hematologic tissues at a high copy number but not expressed on non-hematologic tissues.(12,13) In particular, anti-CD45 Ab coupled to iodine-131 (131I) has delivered 2-to-3-fold higher average radiation doses to spleen and bone marrow (BM) in combination with a high-dose chemotherapy and TBI conditioning,(14–16) and in reduced-intensity conditioning HCT regimen in older relapsed/refractory AML patients.(17) A recent HCT trial using 131I-anti-CD45 RIT prior to matched-related or unrelated-donor allogeneic HCT with reduced-intensity conditioning in younger patients reported a 73% estimated survival at 1 year where many patients had significant disease burden.(18) These studies have shown minimal additional toxicities introduced by RIT to conditioning platforms. Thus, 131I-anti-CD45 targeted RIT could be safely introduced into various conditioning platforms for patients with acute leukemias or high-risk malignancies.
We therefore hypothesize that incorporating 131I-anti-CD45 RIT before allogeneic HCT using haploidentical donors should also produce clinical benefit in high-risk patients. Herein we report on dosing 131I-anti-CD45 RIT prior to nonmyeloablative conditioning chemotherapy using haploidentical donors with PTCy for patients with high-risk leukemia or myelodysplastic syndromes. While many of these patients had significant disease burden, making them ineligible for standard HCT options, some patients were able to derive clinical benefit with this treatment, including long-term survival.
MATERIALS, METHODS, PATIENTS & TREATMENT
Patient and donor selection
Patients older than 18 years of age, with high-risk acute leukemia (i.e., beyond first remission, primary refractory), or evolved from myelodysplastic syndromes (MDS) or myeloproliferative neoplasms (MPN), or high-risk MDS were eligible for this study, although other ongoing RIT trials at the time limited enrollment to one patient to approximately every other month for this trial. Those not in remission needed to have CD45-expressing leukemic blasts, although patients in remission could have previously documented CD45-negative leukemia. All patients needed to have a related haploidentical donor (identical for one HLA haplotype and mismatched at the HLA-A, -B, or -DRB1 locus of the unshared haplotype. Additional eligibility criteria included adequate organ function, circulating blast count of < 10,000/mm3 (hydroxyurea was allowed for blast control) and Karnofsky score ≥ 70 or ECOG ≤ 2. Exclusionary criteria included the presence of circulating antibody against mouse immunoglobulin (HAMA),(17) left ventricular ejection fraction <35%, or corrected DLCO <35% and/or on continuous supplemental oxygen, prior radiation to maximally tolerated levels to any critical normal organ, uncontrolled active infections, donor-specific antibodies (DSA), symptomatic coronary artery disease or on cardiac medications for anti-arrythmia or ionotropic effect, HIV seropositivity, or refractory CNS involvement of disease. For cross-matching, potential patients were evaluated for DSA with panel reactive antibody (PRA) testing. Further evaluation for donor-directed reactivity was assessed with B and T Cell Flow cytometric crossmatch testing and T-Cell AHG CDC Crossmatch testing. A positive crossmatch is defined as presence of a known donor directive antibody as defined by the PRA studies and positivity of one or both of the confirmatory flow or CDC crossmatch tests.(19,20) Patients or their legal guardians gave written informed consent, and the study was conducted in accordance with the Declaration of Helsinki per treatment protocol approved by the institutional review board of the Fred Hutch [ClinicalTrials.gov, NCT00589316].
Production of radiolabeled antibody, biodistribution, dosimetry and treatment schema
Radiolabeled BC8 Ab (murine IgG1 anti-human CD45) was produced, tested, and radiolabeled with iodine-131 (131I; Perkin Elmer, Waltham MA) as previously described.(15,17,18) Two days prior to infusion of biodistribution dose, Lugol’s solution (iodine/potassium iodide solution) was administered orally and continued for three weeks after the therapeutic infusion to block thyroid uptake of free 131I. A tracer dose (5 mCi or 185 MBq 131I-BC8) was administered at approximately day −23 to determine biodistribution and biokinetic behavior for dosimetry. Infusions of radioimmunoconjugate required premedicating with acetaminophen 650mg po, diphenhydramine 25–50 IV or ranitidine 50mg IV over 20–30 minutes for those intolerant of diphenhydramine, ondansetron 8mg IV, hydrocortisone 100mg IV every 2 hours until completion of infusion, and IVFs until completion of infusion. The radiation-absorbed doses (Gy) to target and non-target organs per mCi of 131I was estimated from the biodistribution of 131I-BC8, via methods recommended by the Society of Nuclear Medicine and Molecular Imaging’s special committee on Medical Internal Radiation Dose, as previously described.(14,21,22) Therapy doses of 131I-BC8 were prepared with the amount of 131I calculated to deliver the target dose to the normal limiting organ (usually the liver) estimated to receive the highest radiation dose, as calculated from dosimetry data.(14) Therapy doses of radiolabeled 131I-BC8 were infused approximately day −14 with similar pre-medications and supportive care as dosimetry doses, after which patients were in radiation isolation lead-lined rooms until radiation exposure was ≤ 7 milliroentgen/hour at 1 meter. HCT conditioning consisted of fludarabine (FLU) 30mg/m2/day intravenously (IV) days −6 to −2, cyclophosphamide (CY) 14.5 mg/kg/day with mesna (dosed at 100% CY dose) on days −6 to −5, followed by total body irradiation (2 Gy from a linear accelerator) on day −1, and subsequent infusion of marrow stem-cell graft on day 0.
Immunosuppression and GVHD prophylaxis consisted of tacrolimus 1mg/IV and MMF 15mg/kg po tid, started on day +4. PTCy was dosed (50mg/kg) on day +3 with mesna support, as per the original PTCy platform.(23) A second PTCy dose on day +4 as later reported(7) was not pursued in this trial given the fear that a second PTCy dose could blunt the graft-versus-leukemia tumor effect in this high-risk population, and potentially contribute to toxicity. GCSF (5 μg/kg/day) IV or subcutaneously (SC) was started on day +4 and continued until ANC>500/mm3 for 3 days. MMF was discontinued on day +35 or earlier at the discretion of the treating provider in patients with no evidence of GVHD, and tacrolimus taper was initiated on day 84, with the goal to stop on day 180. Treatment Schema is summarized in Supplementary Figure S1. Acute GVHD was graded according to the relatively contemporary Keystone criteria.(24) On day +28, patients had standard response assessments; disease status and chimerism were again assessed on day +84, and if patients had no GVHD then tacrolimus taper was started with goal to discontinue by day +180.
Dose-escalation plan and statistical analysis
This phase I trial aimed to estimate the maximum tolerated dose (MTD) of radiation delivered via 131I-BC8 Ab when combined with pre- and post-transplant CY, FLU, 2 Gy TBI, and immunosuppression with MMF and tacrolimus when used with haploidentical donors for patients with advanced AML, ALL, or high-risk MDS. Adverse events were collected and graded according to NCI’s Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3), from first exposure of radiolabeled BC8 through day +100 after HCT, or prior to day +100 if patients were discharged before. The MTD was defined as the dose associated with a true dose-limiting toxicity (DLT) rate of 25%, with DLT defined as a grade III/IV (Bearman scale) toxicity observed within 30 days following transplant. For dose adjustments a 2-stage approach was followed,(25) with single patients entered on the first stage with dose escalation by 2 Gy increments (in the radiation dose delivered to liver) until a patient experienced a DLT. Dose escalations occurred only if the patient at the prior dose had been monitored for at least 30 days after HCT, otherwise enrolled patients would receive the same dose. Once a DLT was observed in stage 1, stage II was initiated at the next lower dose level, and patients were treated in cohorts of four given the target DLT of 25%. If no DLTs were observed in a cohort, the next cohort was treated at the next higher dose level; if 1 DLT was observed in a cohort of 4, the next cohort was treated at the same dose level; if 2 DLTs were observed within a cohort, the next cohort was treated as the next lower dose level. Overall and disease-free survival (OS, DFS) were estimated according to Kaplan-Meier method, and relapse and non-relapse mortality rates were estimated using cumulative incidence estimates. NRM was deemed a competing risk for relapse, and relapse was considered a competing risk for NRM.
Data availability
The data generated in this study are not publicly available due to some information that could compromise patient privacy or consent but are available upon reasonable request from the corresponding author.
RESULTS
Patient characteristics
Twenty-six patients with high-risk leukemias or MDS gave written informed consent. One patient did not receive therapy dose because preparation failed to meet release criteria for treatment. Of the 25 patients treated (Table 1), the majority (n=16) were male, with median age 52 years (range 25–69). Five patients were Asian, 4 Black or African American, 1 Hispanic, and the rest were white, following national statistics where the majority of allogeneic HCT patients are non-Hispanic whites (Supplementary Table S1: Study representativeness). Patients were enrolled from March 2008 through May 2016 with last follow up December 2022. Twenty patients had AML, 12 with primary AML and the other 8 with secondary AML (3 from prior MDS, 4 from prior MPN, and one with prior history of acute promyelocytic anemia). An additional 4 patients had ALL; one patient had prior CML before diagnosis with Ph+ ALL, two had T-cell ALL, and the other patient had B-cell ALL in CR2. The high-risk MDS-RAEB-2 patient (by contemporary IPSS with 2 cytopenias, and other karyotype abnormalities) had 14.5% blasts by morphology in the bone marrow.
Table 1:
Patient Characteristics
| Category | number | |||
|---|---|---|---|---|
|
| ||||
| Age, median [years (range)] | 52 (25–69) | |||
| Biological Sex | Male | 16 | ||
| Female | 9 | |||
| Diagnosis at HCT | ||||
| AML | 20 | |||
| 2` secondary AML | 8 | |||
| 1` primary AML | 12 | |||
| MDS | refractory | 1 | ||
| ALL | 4 | |||
| T-cell ALL | 2 | |||
| CML transformed to Ph+ ALL | 1 | |||
| B-cell ALL | 1 | |||
| Prior Allo-HCT | 7 | |||
| Risk Group - by ELN | 2017 Criteria | |||
| 2` AML | Favorable | 0 | ||
| Intermediate | 4 | |||
| High | 4 | |||
| 1` AML | Favorable | 1 | ||
| Intermediate | 9 | |||
| High | 2 | |||
| MDS | High | 1 | ||
| ALL | High | 4 | ||
These patients had higher risk disease; 19 of the 20 AML patients had intermediate- or high-risk disease by ELN 2017 criteria. Patients were heavily pretreated, with a median number of 4 prior cycles (range 2–10). In addition, many patients had significant disease burden, with 8 patients having >5% blasts by morphology in the marrow, and 11 of the remaining 17 that had flow cytometry evaluations had minimal residual disease by flow cytometry (range 0.035–19.1%; Table 2). This was a 2nd HCT for one of the T-cell ALL patients, 4 of the primary AML patients, and 2 of the secondary AML patients.
Table 2:
Patient Outcomes
| Pt | Age / Sex | Disease Status at Treatment | High-risk feature | # of prior treatments | Dose Level to Liver | Actual dose to Liver | PreHct BM Blast % n morph (Flow) | PreHCT MRD Status | postHCT BM Blast % morph (Flow)Δ | Relapse (Y/N) (days post HCT) | Death (Y/N) (days post HCT) | Cause of Death | Outcomes (days post HCT) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 30/M | AML-refractory relapse | FAV (inv 16) | 7 | 12 Gy | 12 Gy | 1.5 (1.5) | positive | 0 | Y (70) | Y (159) | relapse | relapsed d70, died d159 |
| 2 | 56/M | ALL | Ph+ transformed CML | 3 | 14 Gy | 14 Gy | 0 | negative | 0 | N | N | alive, disease free 5076 days | |
| 3 | 37/F | ALL- in CR2 | 6 | 16 Gy | 16 Gy | 0.03 | positive | 0 | Y (69) | Y (140) | relapse | relapse d69, died d140 | |
| 4 | 54/F | AML-prior MDS, refractory | ADV (complex karyotype) | 2 | 16 Gy | 16 Gy | 1.5 (1.5) | positive | 0 | N | Y (73) | GNR sepsis | NED |
| 5 | 35/M | AML-primary | INT (trisomy 8) | 5 | 16 Gy | 16 Gy | 0 | negative | 0 | Y (86) | Y (211) | relapse | relapsed d86, died d211 |
| 6 | 64/M | MDS-RAEB2 | HIGH | 4 | 16 Gy | 16 Gy | 14.5 (10.7) | refractory | 0 | Y (3004) | Y (4117) | cardiac arrest | relapse d3004, died d4117 without disease |
| 7 | 42/M | AML- CML blast crisis, 2nd relapse | Ph+, del Y, Blast Crisis | 9 | 16 Gy | 16 Gy | 9 (Non-diagnostic) | refractory | 0 | Y (298) | Y (709) | relapse | relapsed d298, died d709 |
| 8 | 52/F | AML-primary refractory | INT (trisomy 8) | 2 | 18 Gy | 18 Gy | 9 (11) | refractory | 0 | Y (3886) | Y (4376) | sepsis | relapse d3886, 2nd HCT (UCB), died d4376 |
| 9 | 56/F | *AML in CR2 | INT (NK) | 4 | 20 Gy | 20 Gy | 0 | negative | 0 | N | N | alive, disease-free 4067 days | |
| 10 | 37/M | *AML-primary | INT (t(9;11) | 7 | 22 Gy | 22 Gy | 2 (3.6) | positive | 0 | Y (62) | Y (75) | relapse | relapsed d62, died d75 |
| 11 | 68/M | AML-primary refractory | INT | 4 | 22 Gy | 21.7 Gy | 19 (16.7) | refractory | 0 | N | Y (516) | infection, NED | |
| 12 | 45/M | *T-ALL Primary | HIGH | 10 | 24 Gy | 22.9 Gy | 0 | negative | 0 | Y (64) | Y | relapse | DLC d90, lost to follow up |
| 13 | 55/F | AML-prior CMML | HIGH (2`, monosomy 7) | 4 | 24 Gy | 23.5 Gy | 6 (3.5) | refractory | 0 | Y (176) | Y (215) | relapse | relapsed d176, died d215 |
| 14 | 25/F | *AML Primary | INT | 4 | 26 Gy | 25.9 Gy | 0 | negative | 0 | N | Y (128) | pulmonary hemorrhage | died d128 without disease |
| 15 | 53/F | *AML- prior MF | HIGH (complex karyotype) | 3 | 26 Gy | 26.6 Gy | 0 (0.35) | positive | 0 | Y (69) | Y (81) | relapse | relapsed d69, died d81 |
| 16 | 52/M | AML - primary | HIGH (complex karyotype) | 2 | 24 Gy | 23 Gy | 2 (0.035%) | positive | 0 | Y (204) | Y (237) | relapse | relapsed d204, died d237 |
| 17 | 60/M | *AML - prior MDS, refractory | INT (NK) | 2 | 24 Gy | 24.7 Gy | 20 (14.5) | refractory | 0 | N | Y (148) | MOF, sepsis | died d148 without disease |
| 18 | 69/M | AML - primary | HIGH (complex karyotype) | 5 | 24 Gy | 23.7 Gy | 2 (5.4) | positive | 0 | Y (158) | Y (200) | relapse | relapsed d158, died d200 |
| 19 | 61/M | AML - prior PV, refractory | INT | 4 | 24 Gy | 23.5 Gy | x (9.5) | positive | 4 (1.8) | Y (27) | Y (40) | relapse | relapsed d27, died d40 |
| 20 | 55/M | ALL-Tcell | HIGH (hypodiploidy) | 4 | 26 Gy | 25.4 Gy | 0 (0.05) | positive | 0 | Y (34) | Y (85) | relapse | relapsed d34, died d85 |
| 21 | 68/F | AML - prior APL refractory | INT (NK) | 4 | 26 Gy | 25.9 Gy | 11.3 (49.2) | refractory | 0 | N | Y (1802) | bronchitis, PNA | died d1802 without disease |
| 22 | 39/M | AML - refractory | INT (NK) | 5 | 26 Gy | 26.1 Gy | 19 (20.2) | refractory | 0 | N | N | alive, disease-free 2550 days | |
| 23 | 66/F | AML - primary CR2 | HIGH (complex karyotype) | 3 | 26 Gy | 25.5 Gy | 2 (19.1) | positive | 0 (0.002) | Y (35) | Y (501) | relapse | relapsed d35, died d501 |
| 24 | 65/M | AML - prior MDS Refractory | INT | 5 | 26Gy | 25.8 Gy | 3 (1.3) | positive | 0 | Y (84) | Y (241) | relapse | relapsed d84, died d241 |
| 25 | 53/M | *AML - primary | INT (NK) | 3 | 26 | 26 Gy | 0.5 (0.4) | positive | 0 | N | Y (605) | RSV PNA | died d605 without disease |
2nd transplant
MRD by flow=zero unless otherwise noted in ( )
All patients had related haploidentical donors (children, siblings including half-siblings, mother, and fathers), mismatched at the HLA-A, -B, or -DRB1 locus of the unshared haplotype (Supplementary Table S2: Recipient – Donor characteristics).
Tracer diagnostic, biodistribution studies, and therapy infusion
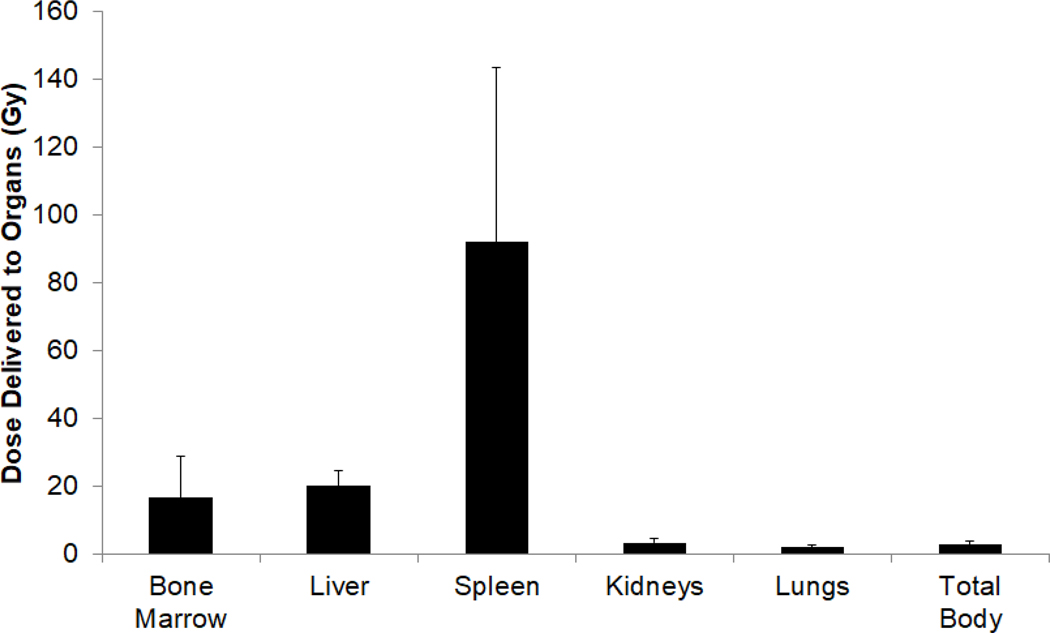
To tailor therapy and deliver radiation to the dose-limiting normal organ (liver) at target level, patients first received a tracer infusion for dosimetry using 7.3–11.5 mCi, median 8.7 mCi, (270.1 – 425.5 MBq; median 321.9 MBq) 131I on 0.5mg of anti-CD45 BC8 Ab per kg of adjusted body weight. After infusion, patients underwent gamma-camera imaging to assess relative biodistribution and clearance of radioimmunoconjugate at target CD45+ and normal, non-target organs. Based on initial dosimetry studies, the 131I amount required to deliver a target radiation dose per assigned dose level for therapy infusions was calculated after gamma-camera acquisitions and analysis by medical internal radiation dose (MIRD) approaches.(22) Dosimetry studies for all 25 patients showed that the mean radiation absorbed dose was 15.6 ± 11.6 (Gy ± S.D.) to the bone marrow, 91.4 ± 45.7 Gy to the spleen, and 21.2 ± 4.6 Gy to the liver. Non-targeted, normal organs, such as lungs and kidneys, received 2.0 ± 0.7 Gy and 3.3 ± 1.2 Gy, respectively (Figure 1). For therapeutic infusions, patients received an average of 615.6 mCi (22,773.5 MBq) 131I, with a range 290 mCi (10,730 MBq) to 1,032 mCi (38,184 MBq); median 644.7 mCi (23,853.9 MBq); using an average of 34.0 mg of BC8 (range 24.2 to 42.33mg; median 33.7 mg). After therapeutic infusion of radioimmunoconjugate, patients were hospitalized in radiation isolation for a median of 6 days (range 4–8 days) although only one patient each was in radiation isolation for 4 or 8 days each.
Figure 1: Estimated radiation absorbed dose delivered by 131I to key organs.
The total radiation absorbed dose through complete decay for 131I administered in all patients (Gy ± SEM).
In first stage of the study, patient number 14 at dose level 13, who received 26 Gy to the liver, experienced severe respiratory distress from pulmonary hemorrhaging, qualifying as a DLT, and stage II was started. Subsequent patients were enrolled in cohorts of 4, starting at the next-lower dose level of 24 Gy. However, dose levels beyond 26 Gy delivered to the liver were not pursued before funding for the study was completed. No additional adverse events occurred in second stage and the MTD was therefore considered not to be reached.
HCT and engraftment
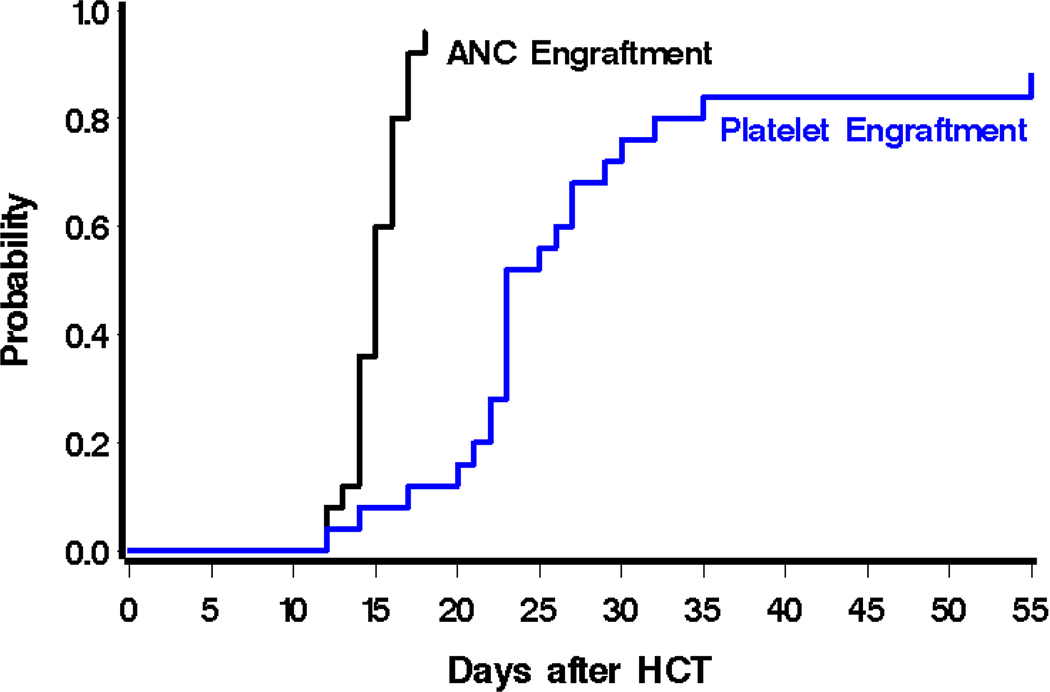
Patients were placed in isolation after therapeutic radioimmunotherapy infusion for an average of 6 days. On day −12, reduced-intensity conditioning was started with FLU, CY and 2 Gy TBI before infusion of graft. The average mononuclear cell (MNC) dose was 1.9×108 nucleated cells/kg (range 0.85 to 5.3 cells/kg). Despite additional targeted radiation via radioimmunotherapy, no delays in engraftment were noted. The median time to neutrophil engraftment, defined as > 500/mm3 for 2 consecutive days, among all 25 patients was 15 days (range 12 days to not reached), although one patient died on day 81 from multi-organ failure after relapse without full neutrophil recovery (Figure 2). Median time to platelet engraftment, defined as platelets >20,000/mm3 for 7 consecutive days without any platelet transfusions, was 23 days. Three patients died or were discharged before platelet engraftment. One of these three was the only case of primary graft failure, defined as failure to achieve ANC > 500 μL in those surviving at least 28 days; though this patient (patient 15) subsequently relapsed on day 69 with circulating blasts in peripheral blood. Another patient (patient 19) showed persistent disease on day 27 bone marrow response assessment and was discharged to home with hospice and died on day 40 after HCT. The third patient (patient 14) experienced diffuse alveolar hemorrhage and thus required platelet transfusions to maintain platelet threshold >80k, and died 138 days after HCT without evidence of disease.
Figure 2: Engraftment kinetics.
Probability of neutrophil engraftment (absolute neutrophil count (ANC) > 500/mm3 for 2 consecutive days), and platelet engraftment (platelets >20,000/mm3 for 7 consecutive days without any platelet transfusions).
Chimerism studies were performed on peripheral blood (PB) day 28 and day 84 after HCT, and on day 56 if chimerism at day 28 was not 100%. Twenty patients had PB evaluated for chimerism on day 28, and 19 of the 20 patients evaluated had 100% donor-derived CD3 chimerism (the other patient had 97% CD3 chimerism). Of those not assessed on day 28, 3 patients had 100% CD3+ donor on day 56, and the fourth patient showed 100% CD3 chimerism at day 84. Similarly, all 20 patients had CD33 chimerism of 100% at day 28. Chimerism remained high when it was last assessed before discharge on day 84, although only 17 patients had day 84 PB chimerism assessments. CD3 chimerism on day 84 was 100% in these 17 patients, with CD33 chimerism of 100% in 16 of these patients, as one patient who relapsed on day 69 had CD33 chimerism of 6%.
Toxicity
Radioimmunoconjugate infusions were well tolerated; all 25 patients completed infusion of diagnostic tracer and therapy doses. Infusion related-reactions were infrequent, and reversible with supportive medications. Infusion reactions were limited to Grade 2 or less, and included rashes, fever >100.4`F with otherwise stable vital signs, and dyspnea without hypoxia. More importantly, symptoms from infusion-related reactions resolved by the end of infusion and patients were able to proceed to gamma-camera imaging after dosimetry infusions, or to radiation isolation after therapeutic infusions.
While mild infusion-related reactions could be attributed to radioimmunoconjugate, other observed toxicities were as expected from conditioning chemotherapy (Figure 3). There was one case of a grade 5 adverse event (AE); a fatal arrythmia occurred 73 days after HCT in the context of GNR sepsis with multi-organ failure but no evidence of disease (NED). Other non-hematologic grade 4 adverse events included two cardiovascular cases (one case of SVT arrhythmia and one with constitutional symptoms fatigue); two cases of infection or febrile neutropenia; and 3 pulmonary events (two cases of hypoxia, one case of diffuse alveolar hemorrhage). Otherwise all patients experienced up to grade 4 cytopenia that were supported with blood product transfusions and prophylactic antibiotics per standard of care. Toxicity rates are similar to those undergoing ablative transplantation without RIT. Although radiotoxicity to organs like liver and kidneys is of theoretical concern, no CTCAE v3 grade 3 or higher liver or renal dysfunction was observed and attributed to radioimmunotherapy.
Figure 3: Grade ≥3 (per NCI’s CTCAE v3) non-hematologic adverse events in all 25 patients through day 100.
Adverse events were collected and graded according to NCI’s Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3), from first exposure of radiolabeled BC8 through day +100 after HCT, or prior to day +100 if patients were discharged before.
GVHD
Overall acute GVHD grade ≥ II was observed in 17 of 25 (68%) patients. However, there were only 3 cases of grade III aGVHD, with 2 of these 3 patients having both grade 3 skin and gut GVHD. There were no grade IV overall aGVHD cases observed.
Disease response and long-term outcomes.
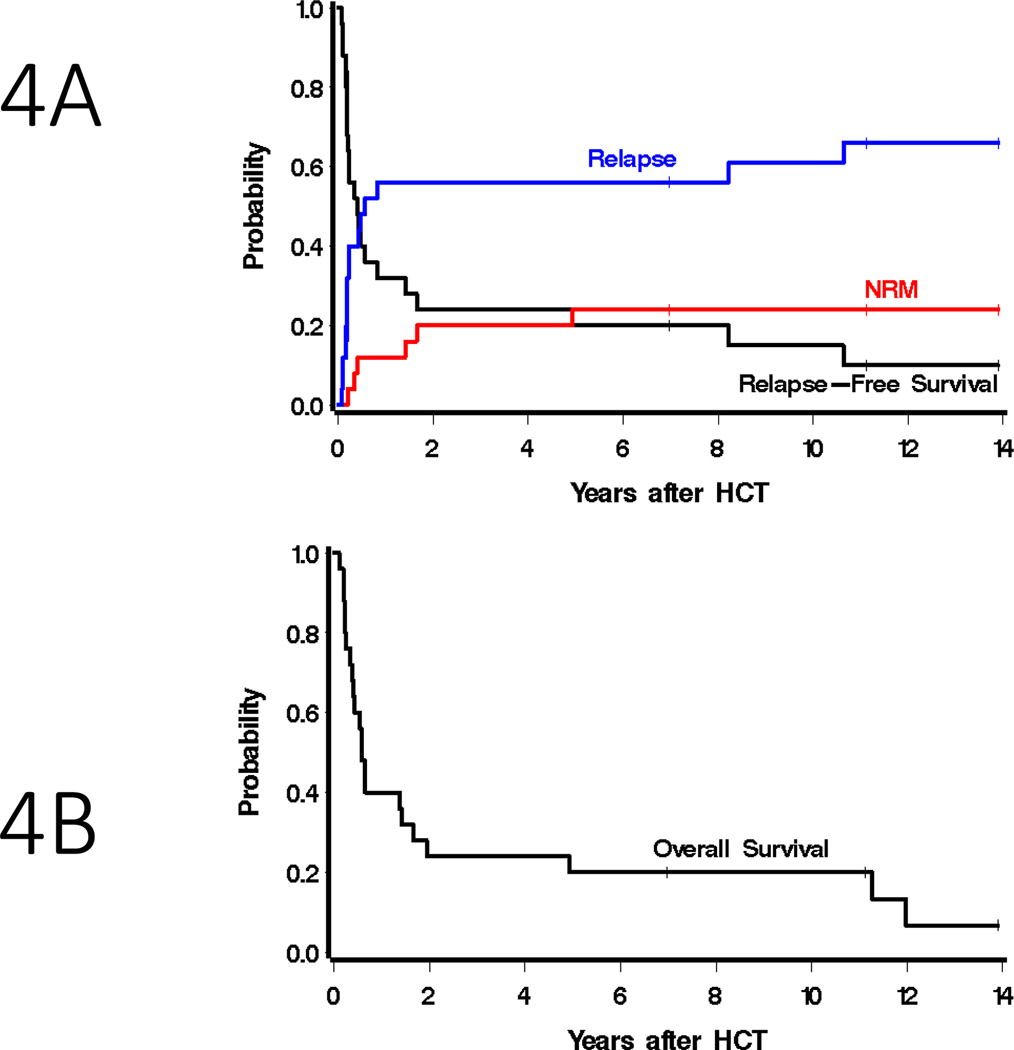
Patients were assessed for treatment response at day +28 or upon engraftment (range day 27–37). All patients survived to BM response assessment, and morphologic complete remission was documented in all treated patients (Table 2). Because allogeneic transplant patients are on medications that may suppress blood counts (e.g., MMF, ganciclovir, etc), the majority of patients achieved a morphologic remission with platelets <100 at this early day +28 time point, though 4 patients (patients 9, 11, 12, 18) did achieve a morphologic remission (CR) with complete recovery (ANC>1000 /μL and platelets > 100k/μL). The majority of patients were independent of platelet transfusions by day 28 even though only ten patients had achieved a hematologic CR (CRh, with ANC > 500/μL and platelets > 50,000/μL). In addition, 2 patients in morphologic remission after HCT had minimal residual disease (MRD) by flow cytometry (0.002% and 1.8% abnormal blasts). These 2 patients had MRD before HCT and they relapsed 501 and 40 days after HCT, respectively. Of the 8 patients who had active marrow disease with >5% blasts prior to RIT, all 8 achieved a morphologic remission without MRD by flow cytometry at day +28, and one of these patients (refractory AML with 19% blasts in the marrow) remains alive 7 years after HCT. The other 2 long term survivors had an MRD negative remission. Relapse was the major reason for treatment failure, as 16 patients would eventually relapse. The median time to relapse was 204 days (the time at which the probability of relapse crosses 50%), with relapses occurring between 27 days and 10.6 years. The one- and two-year point estimates of relapse were each 56% (95% CI, 34–73%). The point estimate of progression-free survival (PFS) at 1 and 2 years were 32% (95% CI, 15–50%) and 24% (95% CI, 10–42%), respectively (Figure 4A). However, the tolerability of radioimmunoconjugates translated into a favorable non-relapse mortality rate (NRM), estimated to be 12% (95% CI, 3–28%) at 1 year and 20% (95% CI, 7–38%) at 2 years. NRM deaths were attributed to infectious etiologies (bacterial and viral pneumonias, and sepsis). Point estimates of overall survival were 40% (95% CI, 21–58%) and 24% (95% CI, 10–42%) at 1 and 2 years, respectively (Figure 4B). Three patients remain alive 7.0, 11.1 and 13.9 years after HCT, and the 5-year survival estimate was 20% (95% CI, 7–37%; (Table 2). While numbers are small, Supplementary Figure S2 summarizes survival for patients transplanted in CR with MRD, patients transplanted in CR without MRD, and patients transplanted with frank disease. Of the 8 patients transplanted with frank disease (bone marrow blasts ≥ 5%) only 4 relapsed (range day 176 – 2886 after HCT), including 2 who relapsed >3000 days after HCT. The other 4 patients who did not relapse includes 1 long term survivor, but the other 3 died from infectious complications (days 148, 516 and 1802 after HCT). Of the 12 patients in CR with MRD+ at the time of HCT, the majority (10 patients) relapsed early (range 27–176 days after HCT).
Figure 4: Transplant outcomes.
Estimated probability of A) relapse-free survival, non-relapse mortality, and relapse and B) overall survival among all patients who received 131I-anti-CD45 RIT before allogeneic HCT using haploidentical donors.
DISCUSSION
Although AML patients with refractory disease are rarely offered HCT,(26) here we show that patients with high-risk acute leukemias or high-grade MDS were safely transplanted with RIC augmented with targeted radiation delivery to CD45+ sites of disease. This approach provided an estimated nearly 5x more dose delivered to bone marrow (15.5 Gy) compared to non-target organs like kidneys (3.3 Gy), without any significant increase in toxicity. The only grade 5 AE was a fatal arrythmia in the context of sepsis over 2 months after HCT. Grade 4 AEs were more likely attributed to conditioning chemotherapy rather than radiolabeled Ab. There was only one observed DLT (at the dose of 26 Gy to the liver), in the first stage of the trial. No additional DLT’s were observed in stage II of the study and doses beyond 26 Gy were not pursued because funding for the trial was nearing completion. However, another trial had estimated an MTD of 24 Gy to the liver when 131I-anti-CD45 RIT was combined with FLU and low-dose TBI in elderly patients pursuing HCT.(17) A different contemporary trial included younger patients with high-grade MDS and acute leukemias suggested that doses >28Gy could be tolerated when 131I-anti-CD45 RIT was combined with FLU/TBI.(18)
All treated patients herein achieved a morphologic CR 28 days after HCT, the majority MRD negative by flow cytometry. Although the majority of these morphologic remissions were MRD negative, this MRD negativity was poorly predictive of overall outcome in our study, not predictive of better outcomes as has been associated with other studies.(27,28) One explanation may be that MRD negativity was outweighed by morphologic remissions with incomplete platelet recovery which has been associated with poorer outcomes though usually in the post-induction phase.(29,30) To highlight the complexity of this nuance, 2 of the 3 long-term survivors in this study actually achieved a morphologic remission without complete platelet recovery, suggesting other contributions to survival benefit beyond count recovery. Although subgroups analysis can be misleading when inadequately powered, Supplementary Figure S2 shows survival outcomes by disease status at the time of HCT. All groups (those in CR with MRD, CR without MRD, and frank disease) reflect the impact of relapse after HCT. In this subgroup analysis, most patients in CR with MRD+ did indeed relapse (27–176 days) after HCT as MRD+ positivity at the time of HCT would predict.(28) A phase 3 registry trial comparing 131I-anti-CD45 RIT prior to HCT to standard-of-care chemotherapy options in elderly patients (NCT02665065) was just completed.(31,32) Results presented as a late-breaking abstract at TCT 2023 showed that nearly 75% of evaluable patients who received a RIT-augmented HCT achieved a CR/CRp.(31,32) Our study differed from the SIERRA trial in that SIERRA patients were limited to refractory AML only, not other high risk leukemias or MDS, and patients were 55 years or older, unlike the more diverse patients. With these limited numbers and more variable patients than the SIERRA, it is difficult to decipher who will or will not attain maximum benefit from radioactive monoclonal antibody infusion. Thus RIT-augmented HCT should be considered in high-risk leukemia patients as this approach compares well with other RIC approaches that report a NRM of 20%, although this rate is quoted for patients without disease.(33,34)
While patients with relapsed or refractory AML are difficult to treat, previous studies have suggested that allogeneic HCT could yield better results than chemotherapy alone.(35–37) One German study enrolled over 100 patients with relapsed/refractory AML, reinduced with FLAMSA (days −12 to −9) before directly proceeding to RIC HCT. Over 90% of patients achieved a CR and the 1-year estimate of OS was 54%.(38) They too documented about a third of high-risk patients relapsing within a year. Although trial comparisons are fraught with limitations, 131I-anti-CD45 RIT before FLU/TBI achieved comparable outcomes with all patients achieving a morphologic CR (100%) and similar one-year estimate of OS of 40%.
Another study that transplanted high-risk AML patients shortly after induction or re-induction chemotherapy reported a CR rate of 86% on nearly 100 patients enrolled.(39) They later reported on a multi-center trial for relapsed / refractory AML patients who received HCT after conditioning with clofarabine and melphalan.(40) In this study 60% of patients achieved a CR after HCT, with a 2-year survival probability of 52%. While our post-HCT CR rate was slightly numerically higher, relapse rates in that study differed from relapse rates observed after RIT; their highest risk patients had 17% relapse by day 100 in the original study, and 26% probability of relapse in the multi-center trial. As in our study, pre-existing bone marrow blasts did not preclude favorable long-term outcomes.
These studies investigated HCT as salvage therapy with stem cells from related or URD, but many patients, especially ethnic minority patients may not have an HLA-matched donor, for which alternative donors with UCB or haploidentical donors have been investigated. While 10 of our patients were minority patients, the majority of enrolled patients were white, supporting the generalizability of HCT using haploidentical donors. Earlier CIMBTR data supported similar outcomes after haploidentical PTCy compared to HLA-mismatched unrelated donors (MURD) for acute leukemia, irrespective of myeloablative (MA) or reduced intensity conditioning (RIC) approaches.(41) with comparable survival and NRM rates, but higher relapse rates with RIC with haploidentical HCT. However, this report included trials with different GVHD prophylaxis regimen and variable conditioning chemotherapies. A recent CIBMTR report evaluated RIC and MA approaches, all using PTCy in combination with CNI / mycophenolate mofetil for GVHD prophylaxis. In this report, haploidentical HCT using RIC regimens was associated with higher graft failure, acute grade GVHD and NRM which translated into poorer survival outcomes with a 2-year NRM, DFS and OS of 16%, 41% and 54%, respectively, compared to 8%, 55% and 67% respectively for MURDs.(42) As in our study, relapse was the main reason for transplant failure in CIBMTR’s report with a relapse rate of 42% at 2 years for patients receiving haploidentical HCT with PTCy. While patients in the CIBMTR’s report were mostly lower to intermediate risk AML patients, many of these were more likely to be in remission before proceeding to HCT, unlike the high-risk patients in our report.
Given the negative impact relapse has on HCT outcomes, we again aim to improve the cytoreductive potential of anti-CD45 RIT by labeling anti-CD45 Ab (BC8) with astatine-211 (211At), an alpha-emitter with higher decay energy (6.8 MeV; averages of two alpha decays), compared to 131I (0.66 MeV). Although higher radiation payload could cause higher toxicity, alpha decay has a shorter effective path length, depositing its decay energy over the distance of a few cell diameters (55–70 μm), limiting the potential for off-target toxicity.(43) Preclinical studies using 211At to treat multiple myeloma, non-Hodgkin lymphoma and acute myeloid leukemia by targeting CD38, CD20 and CD45 respectively in preclinical disease models have yielded long-term survivors.(43–45) Consequently our center is now enrolling patients with high-risk acute leukemias or MDS to be treated with 211At-anti-CD45 RIT before RIC conditioning with FLU/TBI (NCT03128034), as is a parallel trial with haploidentical donors (NCT03670966).
This study supports the strategy of augmenting conditioning regiment with targeted-radiation delivery before allogeneic HCT. This approach is safe without additional significant toxicity and many patients can achieve remission. Though these high-risk patients with significant disease burden experienced a relatively high rate of relapse, ongoing efforts to increase radiation payload and delivery strategies may further improve this approach to make targeted-radiation delivery before HCT a promising treatment for patients.
Supplementary Material
Statement of Translational Relevance.
Patients with refractory acute leukemias are rarely offered allogeneic hematopoietic cell transplant (HCT). In addition, not all patients have a fully matched donor, especially ethnic and minority patients, but nearly all patients should be able to identify a partially matched, haploidentical donor. Here we report the first study combining targeted radiation delivery to hematopoietic tissues before allogeneic HCT using haploidentical donors. Radiolabeled anti-CD45 antibodies delivered individualized radiation doses [per dose-escalation dose level] to the dose-limiting organ (liver) before receiving fludarabine and low-dose total-body irradiation and haploidentical donor hematopoietic stem cells. This innovative combination was well tolerated in patients with acute leukemias and high-risk myelodysplastic syndrome without increased toxicity. More importantly, this novel combination yielded complete remissions and long-term survivors.
ACKNOWLEDGEMENTS
The study was supported by NCI P01CA044991 (OWP), Be The Match Foundation (JJO). Dr. Oliver W. Press was a critical co-author and contributor to the conceptualization, securing funding, and supporter of this and other radioimmunotherapy studies before he passed away. The authors thank the patients, their families and caregivers, providers, and staff who contributed to this study. We appreciate the processing of samples by Marg Nartea, Aimee Kenoyer, and Shani Frayo and the regulatory support provided by Monina Almeda.
AKG receives Research Funding from: Merck, I-Mab bio, IgM Bio, Takeda, Gilead, Astra-Zeneca, Agios, Janssen, BMS, SeaGen, Teva, Genmab; and Consultancy/Honoraria from: Incyte, Kite, Morphosys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, Beigene, Cellectar, Janssen, SeaGen, Epizyme, I-Mab bio, Gilead, Genentech, Lilly, Caribou, Fresenius-Kabi; and has Equity Ownership in: Compliment Corporation. JJO receives research funding from: Actinium Pharmaceuticals, LLC.
Footnotes
AUTHORSHIP
The current affiliation for JMP is Loxo Oncology, Stamford, CT.
Conflict of interest disclosures:
Otherwise authors have no conflicts of interest related to this work.
REFERENCES
- 1.Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. New England Journal of Medicine 2014;371(4):339–48 doi 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty PG, Clift RA, Mickelson EM, Nisperos BB, Flournoy N, Martin PJ, et al. Marrow Transplantation from Related Donors Other than HLA-Identical Siblings. New England Journal of Medicine 1985;313(13):765–71 doi 10.1056/nejm198509263131301. [DOI] [PubMed] [Google Scholar]
- 3.Powles RL, Kay HEM, Clink HM, Barrett A, Depledge MH, Sloane J, et al. Mismatched Family Donors for Bone-Marrow Transplantation as Treatment for Acute-Leukemia. Lancet 1983;1(8325):612–5. [DOI] [PubMed] [Google Scholar]
- 4.Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner CD, et al. Effect of HLA Compatibility on Engraftment of Bone-Marrow Transplants in Patients with Leukemia or Lymphoma. New England Journal of Medicine 1989;320(4):197–204 doi 10.1056/nejm198901263200401. [DOI] [PubMed] [Google Scholar]
- 5.Ash RC, Horowitz MM, Gale RP, Vanbekkum DW, Casper JT, Gordonsmith EC, et al. Bone-Marrow Transplantation from Related Donors other than HLA-Identical Siblings- Effect of T-cell Depletion. Bone Marrow Transplantation 1991;7(6):443–52. [PubMed] [Google Scholar]
- 6.Mehta J, Singhal S, Gee AP, Chiang KY, Godder K, van Rhee F, et al. Bone marrow transplantation from partially HLA-mismatched family donors for acute leukemia: single-center experience of 201 patients. Bone Marrow Transplantation 2004;33(4):389–96 doi 10.1038/sj.bmt.1704391. [DOI] [PubMed] [Google Scholar]
- 7.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008;14(6):641–50 doi 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, et al. Effect of HLA Incompatibility on Graft-versus-Host Disease, Relapse, and Survival After Marrow Transplantation for Patients with Leukemia or Lymphoma. Hum Immunol 1990;29(2):79–91 doi 10.1016/0198-8859(90)90071-v. [DOI] [PubMed] [Google Scholar]
- 9.Clift RA, Buckner CD, Appelbaum FR, Bearman SI, Petersen FB, Fisher LD, et al. Allogeneic Marrow Transplantation in Patients with Acute Myeloid-Leukemia in 1st Remission- a Randomized Trial of 2 Irradiation Regimens. Blood. Volume 761990. p 1867–71. [PubMed] [Google Scholar]
- 10.Clift RA, Buckner CD, Appelbaum FR, Bryant E, Bearman SI, Petersen FB, et al. Allogeneic Marrow Transplantation in Patients with Chronic Myeloid-Leukemia in the Chronic Phase- a Randomized Trial of 2 Irradiation Regimens. Blood 1991;77(8):1660–5. [PubMed] [Google Scholar]
- 11.Clift RA, Buckner CD, Appelbaum FR, Sullivan KM, Storb R, Thomas ED. Long-term follow-up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood 1998;92(4):1455–6. [PubMed] [Google Scholar]
- 12.Andres TL, Kadin ME. Immunological Markers in the Differential-Diagnosis of Small Round Cell Tumors from Lymphocytic Lymphoma and Leukemia. American Journal of Clinical Pathology 1983;79(5):546–52. [DOI] [PubMed] [Google Scholar]
- 13.Omary MB, Trowbridge IS, Battifora HA. Human Homolog of Murine T200 Glycoprotein. Journal of Experimental Medicine 1980;152(4):842–52 doi 10.1084/jem.152.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagel JM, Appelbaum FR, Eary JF, Rajendran J, Fisher DR, Gooley T, et al. I-131-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood 2006;107(5):2184–91 doi 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews DC, Appelbaum FR, Eary JF, Fisher DR, Durack LD, Hui TE, et al. Phase I study of I-131-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood 1999;94(4):1237–47. [PubMed] [Google Scholar]
- 16.Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, et al. Targeted α particle immunotherapy for myeloid leukemia. Blood 2002;100(4):1233–9. [PubMed] [Google Scholar]
- 17.Pagel JM, Gooley TA, Rajendran J, Fisher DR, Wilson WA, Sandmaier BM, et al. Allogeneic hematopoietic cell transplantation after conditioning with I-131-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 2009;114(27):5444–53 doi 10.1182/blood-2009-03-213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mawad R, Gooley TA, Rajendran JG, Fisher DR, Gopal AK, Shields AT, et al. Radiolabeled Anti-CD45 Antibody with Reduced-Intensity Conditioning and Allogeneic Transplantation for Younger Patients with Advanced Acute Myeloid Leukemia or Myelodysplastic Syndrome. Biol Blood Marrow Transplant 2014;20(9):1363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood 2010;115(13):2704–8 doi 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciurea SO, Thall PF, Wang X, Wang SA, Hu Y, Cano P, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood 2011;118(22):5957–64 doi 10.1182/blood-2011-06-362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel JA, Pawlyk DA, Lee RE, Sasso NL, Horowitz JA, Sharkey RM, et al. Tumor, red marrow, and organ dosimetry for 131I-labeled anti-carcinoembryonic antigen monoclonal antibody. Cancer Res 1990;50(3 Suppl):1039s–42s. [PubMed] [Google Scholar]
- 22.Fisher DR, Badger CC, Breitz H, Eary JF, Durham JS, Hui TE, et al. Internal Radiation-Dosimetry for Clinical-Testing of Radiolabeled Monoclonal-Antibodies. Antibody Immunoconjugates and Radiopharmaceuticals 1991;4(4):655–64. [Google Scholar]
- 23.O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2002;8(7):377–86 doi 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15(6):825–8. [PubMed] [Google Scholar]
- 25.Storer BE. Small-sample confidence sets for the MTD in a phase I clinical trial. Biometrics 1993;49(4):1117–25. [PubMed] [Google Scholar]
- 26.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129(4):424–47 doi 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short NJ, Zhou S, Fu C, Berry DA, Walter RB, Freeman SD, et al. Association of Measurable Residual Disease With Survival Outcomes in Patients With Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. JAMA Oncology 2020;6(12):1890–9 doi 10.1001/jamaoncol.2020.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter RB, Ofran Y, Wierzbowska A, Ravandi F, Hourigan CS, Ngai LL, et al. Measurable residual disease as a biomarker in acute myeloid leukemia: theoretical and practical considerations. Leukemia 2021;35(6):1529–38 doi 10.1038/s41375-021-01230-4. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Xie H, Wood BL, Walter RB, Pagel JM, Becker PS, et al. Relation of Clinical Response and Minimal Residual Disease and Their Prognostic Impact on Outcome in Acute Myeloid Leukemia. Journal of Clinical Oncology 2015;33(11):1258–64 doi 10.1200/jco.2014.58.3518. [DOI] [PubMed] [Google Scholar]
- 30.Walter RB, Kantarjian HM, Huang X, Pierce SA, Sun Z, Gundacker HM, et al. Effect of Complete Remission and Responses Less Than Complete Remission on Survival in Acute Myeloid Leukemia: A Combined Eastern Cooperative Oncology Group, Southwest Oncology Group, and M. D. Anderson Cancer Center Study. Journal of Clinical Oncology 2010;28(10):1766–71 doi 10.1200/jco.2009.25.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gyurkocza B, Nath R, Seropian S, Choe H, Litzow MR, Koshy NV, et al. Clinical Experience in the Randomized Phase 3 Sierra Trial: Anti-CD45 Iodine (131I) Apamistamab [Iomab-B] Conditioning Enables Hematopoietic Cell Transplantation with Successful Engraftment and Acceptable Safety in Patients with Active, Relapsed/Refractory AML Not Responding to Targeted Therapies. Blood 2021;138(Supplement 1):1791- doi 10.1182/blood-2021-148497. [DOI] [Google Scholar]
- 32.Gyurkocza B, Seropian S, Choe H, Litzow MR, Abboud C, Koshy NV, et al. LBA3 Efficacy and Safety Results of the Sierra Trial: A Multicenter, Pivotal Phase 3 Study of Iomab-B Prior to Allogeneic Hematopoietic Cell Transplantation Versus Conventional Care in Older Patients with Active, Relapsed or Refractory Acute Myeloid Leukemia (R/R AML) Tandem Meetings | Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR. Orlando, Florida 2023. [Google Scholar]
- 33.Herr AL, Labopin M, Blaise D, Milpied N, Potter M, Michallet M, et al. HLA-identical sibling allogeneic peripheral blood stem cell transplantation with reduced intensity conditioning compared to autologous peripheral blood stem cell transplantation for elderly patients with de novo acute myeloid leukemia. Leukemia 2007;21(1):129–35 doi 10.1038/sj.leu.2404461. [DOI] [PubMed] [Google Scholar]
- 34.Storb R. Can reduced-intensity allogeneic transplantation cure older adults with AML? Best Practice & Research Clinical Haematology 2007;20(1):85–90. [DOI] [PubMed] [Google Scholar]
- 35.Breems DA, Putten WLJV, Huijgens PC, Ossenkoppele GJ, Verhoef GEG, Verdonck, et al. Prognostic Index for Adult Patients With Acute Myeloid Leukemia in First Relapse. Journal of Clinical Oncology 2005;23(9):1969–78 doi 10.1200/jco.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 36.Jabbour E, Daver N, Champlin R, Mathisen M, Oran B, Ciurea S, et al. Allogeneic stem cell transplantation as initial salvage for patients with acute myeloid leukemia refractory to high-dose cytarabine-based induction chemotherapy. American Journal of Hematology 2014;89(4):395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armistead PM, de Lima M, Pierce S, Qiao W, Wang X, Thall PF, et al. Quantifying the Survival Benefit for Allogeneic Hematopoietic Stem Cell Transplantation in Relapsed Acute Myelogenous Leukemia. Biol Blood Marrow Transplant 2009;15(11):1431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006;108(3):1092–9 doi 10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]
- 39.Stölzel F, Platzbecker U, Mohr B, Röllig C, Middeke JM, Thiede C, et al. Early intervention with allogeneic hematopoietic cell transplantation during chemotherapy-induced aplasia in patients with high-risk acute myeloid leukemia. Leukemia 2013;27(10):2068–72 doi 10.1038/leu.2013.142. [DOI] [PubMed] [Google Scholar]
- 40.Middeke JM, Herbst R, Parmentier S, Bug G, Hänel M, Stuhler G, et al. Clofarabine salvage therapy before allogeneic hematopoietic stem cell transplantation in patients with relapsed or refractory AML: results of the BRIDGE trial. Leukemia 2016;30(2):261–7 doi 10.1038/leu.2015.226. [DOI] [PubMed] [Google Scholar]
- 41.Ciurea SO, Zhang M-J, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015;126(8):1033–40 doi 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gooptu M, Romee R, St. Martin A, Arora M, Al Malki M, Antin JH, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood 2021;138(3):273–82 doi 10.1182/blood.2021011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orozco JJ, Bäck T, Kenoyer A, Balkin ER, Hamlin DK, Wilbur DS, et al. Anti-CD45 radioimmunotherapy using 211At with bone marrow transplantation prolongs survival in a disseminated murine leukemia model. Blood 2013;121(18):3759–67 doi 10.1182/blood-2012-11-467035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green DJ, Shadman M, Jones JC, Frayo SL, Kenoyer AL, Hylarides MD, et al. Astatine-211 conjugated to an anti-CD20 monoclonal antibody eradicates disseminated B-cell lymphoma in a mouse model. Blood 2015;125(13):2111–9 doi 10.1182/blood-2014-11-612770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Steen S, Comstock ML, Orozco JJ, Hamlin DK, Wilbur DS, Jones JC, et al. The α-emitter astatine-211 targeted to CD38 can eradicate multiple myeloma in a disseminated disease model. Blood 2019;134(15):1247–56 doi 10.1182/blood.2019001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are not publicly available due to some information that could compromise patient privacy or consent but are available upon reasonable request from the corresponding author.