Abstract
Documented male-female differences in the risk of cardiovascular and chronic kidney diseases have been largely attributed to estrogens. The cardiovascular and renal protective effects of estrogens are mediated via the activation of estrogen receptors (ERα and ERβ) and G protein-coupled estrogen receptor, and involve interactions with the renin-angiotensin-aldosterone system. Aromatase, also called estrogen synthase, is a cytochrome P-450 enzyme that plays a pivotal role in the conversion of androgens into estrogens. Estrogens are biosynthesized in gonadal and extra-gonadal sites by the action of aromatase. Evidence suggests that aromatase inhibitors, which are used to treat high estrogen–related pathologies, are associated with the development of cardiovascular events. We review the potential role of aromatization in providing cardio-renal protection and highlight several meta-analysis studies on cardiovascular events associated with aromatase inhibitors. Overall, we present the potential of aromatase enzyme as a fundamental contributor to cardio-renal protection.
Keywords: Cardiovascular, Kidney, Estrogen synthase, Aromatase inhibitors, Meta-analysis
1. Introduction
1.1. Prevalence of cardiovascular and chronic kidney diseases
Cardiovascular disease (CVD) is one of the major causes of death worldwide, and can impose a significant financial and health burden on patients in the United States and globally [1]. The Global Burden of Disease study, conducted in 195 countries, reported that the prevalence of CVD cases almost doubled from 271 million in 1990 to 523 millions in 2019, while the number of CVD deaths progressively rose from 12.1 million in 1990 to 18.6 millions in 2019 [2]. In 2020, CVD was responsible for 928,741 fatalities in the United States and approximately 19.1 million deaths worldwide [3].
Chronic kidney disease (CKD) is also one of the leading causes of death in the 21st century, with over 850 million people worldwide suffering from CKD [4,5]. The latest estimations (2017–2020) of CKD prevalence in the United States adult population revealed a 14.5% overall prevalence [6]. Globally, a total of 3.16 million deaths from kidney disease were reported in 2019 according to the Global Burden of Disease study [2]. Notably, the relationship between CVD and CKD is reciprocal. In other words, CVD can be an underlying cause of CKD and vice versa [7,8].
1.2. Age and sex factors contributing to cardiovascular health
CVD is considered as an age-related pathology in both men and women, because age is a major factor that influences the cardiovascular health [9]. CVD is a major cause of death in individuals aged 65 years or older, accounting for 40% of mortality [9]. By 2030, it is projected that over 20% of the population in the United States will be 65 years or older [9,10]. According to the American Heart Association, the incidence of CVD among adult Americans is 40% between the ages of 40 and 59, 75% between the ages of 60 and 79, and 86% in individuals over the age of 80 [11].
The American Heart Association also reports that between 2013 and 2017, 77.8% of females and 70.8% of males aged 65–74 were diagnosed with hypertension; diagnosis rates were 85.6% for women and 80.0% for males over the age of 75 years [12]. Previous studies have also revealed that women are at higher risk of stroke than men [13,14]. On the contrary, men typically develop CVD at a younger age and have a higher risk of coronary heart disease than women [15]. Several clinical studies reported that women prior to menopause are more protected from CVD, but this risk sharply increases after menopause [16,17]. This difference in the cardiovascular risk between premenopausal and postmenopausal women is attributed to estrogen and its associated receptors, which consequently contribute to the disparities in disease outcomes between men and women [18].
1.3. Age and sex factors contributing to renal health
CKD is recognized as a common clinical problem with elderly patients. Recent estimates by the Center of Disease Control and Prevention showed that CKD is more prevalent in individuals over the age of 65 (38%) compared to those between the ages of 45–64 (12%) and 18–44 (6%) [19]. CKD is slightly more common in women (14%) than men (12%) [19]. A systematic meta-analysis showed that CKD is more prevalent in females and that its prevalence increases with age [20]. The estimations in this meta-analysis were based on the use of creatinine levels to determine the estimated glomerular filtration rate, while albuminuria or proteinuria was not detected in many of the included studies [20].. This alligns with reports of increased prevelance in CKD stage 3–5 among women, but not men, between 2002 and 2007 [21]. In contrast, other studies reported higher incidence of kidney failure in men [22]. Harris and Zhang concluded that although women have a larger prevalence of CKD, the incidence of end stage renal disease is 50% higher in adult men than in women [23]. Therefore, further investigation is needed to better understand how sex affect CKD incidence, prevalence, and progression, in addition to potential sex-specific disease markers to determine whether sex hormones are related to the onset or progression of renal disease [24].
2. Estrogens
2.1. Types of estrogens and primary sites of production
Estrogens are steroidal sex hormones that include estrone (E1), estradiol (E2), estriol (E3), and estetrol (E4) [25]. The predominant circulating female hormone is E2, which is commonly referred to as “estrogen”, due to its physiological importance and prevalence during the reproductive years [16,25]. E1 is commonly detected at higher levels after menopause, while E3, and E4 are produced only during pregnancy [26].
The ovaries, specifically the granulosa cells, are the primary source of E2 in premenopausal women, acting as a circulating hormone on distal tissues [25,27]. In men, E2 is produced in minute amounts by the testes [28]. Estrogens are also produced in extra-gonadal sites such as adipose tissue, brain, skin, muscles, bones, vascular endothelium, vascular smooth muscles, intestine, liver, and adrenal glands, where they act locally in a paracrine or intracrine manner [27,29]. In a study investigating the source of elevated estrogen after menopause, an increase in the expression of aromatase (the enzyme catalyzing estrogen biosynthesis) was detected in the subcutaneous abdominal adipose tissue of ovariectomized rats [30]. This finding coincides with another study, which concluded that the conversion of androstenedione to estrogen was higher in obese women [31]. Additionally, a cross-sectional study on postmenopausal women found a link between rising body mass index and circulating estrogens (E1 and E2) [32]. However, the contribution of extra-gonadal E2 biosynthesis in different organ systems to the systemic levels of sex hormones remains debatable.
2.2. Biosynthesis of estrogens
The biosynthesis of estrogen takes place through a series of reactions catalyzed by a number of cytochrome P450 enzymes and different hydroxysteroid dehydrogenases [33]. It starts by the conversion of cholesterol to pregnenolone by cytochrome P450 cholesterol side-chain cleavage enzyme (CYP11A) [33]. Pregnenolone can either be converted to 17-hydroxypregnolone and consequently to dehydroepiandrosterone by 17α-hydroxylase (CYP17), or it can be converted to progesterone by 3β-hydroxysteroid dehydrogenase (3β-HSD) [33]. Both dehydroepiandrosterone and progesterone are then converted to androstenedione by 3β-HSD and CYP17, respectively [33]. Afterwards, the androstenedione can be either converted to testosterone by 17β-hydroxysteroid dehydrogenase (17β-HSD), or to E1 by the aromatase enzyme (CYP19A1) [33]. Then, 17β-HSD catalyzes the conversion of E1 to E2 [33]. Fig. 1 demonstrates the steps of estrogen biosynthesis.
Fig. 1.
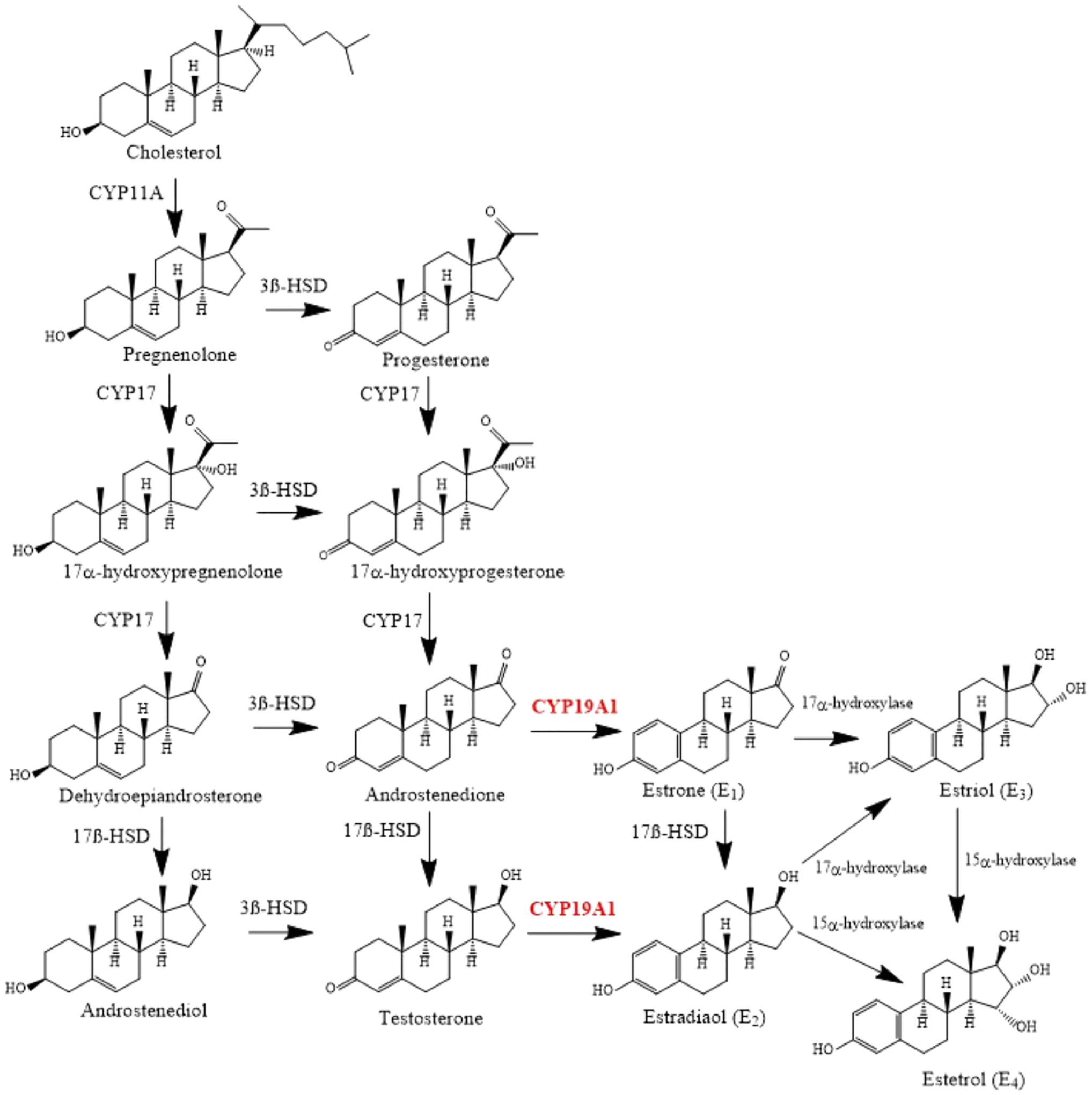
The Biosynthesis of estrogens. Created by Chemdraw Software.
2.3. Estrogen receptors
Estrogens exhibit a wide range of physiological functions on different body tissues, including the cardiovascular, reproductive, skeletal, adipose, and central nervous systems [34–36]. E2 exerts its functions through acting on the estrogen receptors (ERα and ERβ) which are encoded by the ESR1 and ESR2 genes, respectively [37]. In addition, E2 also binds to a recently discovered G protein-coupled estrogen receptor 1 (GPER1) or G protein-coupled receptor 30 (GPER30), also known as the membrane estrogen receptor [25]. Table 1 lists the gene and protein designations for estrogen receptors.
Table 1.
Protein, gene and HGNC ID designations for estrogen receptors.
| Protein | Gene | HGNC ID |
|---|---|---|
| Estrogen receptor alpha (ERα) | ESR1 | 3467 |
| Estrogen receptor beta (ERβ) | ESR2 | 3468 |
| G protein-coupled estrogen receptor 1 (GPER1) | GPER1 | 4485 |
The expression of ERs has been identified in a wide range of cells and tissues. ERα is primarily found in the mammary glands, uterus, ovary (thecal cells), bones, male reproductive organs (testes and epididymis), prostate (stroma), liver, and adipose tissue [37,38]. ERβ is present in the prostate (epithelium), bladder, ovary (granulosa cells), colon, adipose tissue, and immune system [37,38]. In addition, both ERα and ERβ are markedly expressed in the cardiovascular and central nervous systems [37,38]. Within the cardiovascular system, ERα and ERβ are expressed in endothelial cells, vascular smooth muscle cells, and a variety of cardiac tissue, including cardiomyocytes, and cardiac fibroblasts [18,28]. Stained human renal biopsies showed that ERα is mainly expressed the renal glomeruli and tubules [39], while both ERα and ERβ are expressed in the kidney proximal tubule [40]. According to several studies on rodents and humans, GPER1 is ubiquitously expressed within the reproductive system [41], cardiovascular system [42], renal system [43], brain [44], adrenal glands [45], adipocytes [46], and bones [47].
2.4. The cardio-renal protective effect of estrogen
Postmenopausal women have a higher risk of CVD than premenopausal women [48,49]. The protection against CVD prior to menopause has been largely attributed to estrogens, which have a variety of advantageous effects on arterial walls, cardiac and renal functions, as well as tissue regeneration [18]. A direct effect of estrogen involves its vascular actions regulating vascular tone, cell proliferation, and migration [50]. Estrogens also act directly on cardiomyocytes in a favourable way [50]. Besides, estrogen can exert an indirect cardioprotective effect through regulating the lipid profile and reducing the coagulating factors and reactive oxygen species [51]. For instance, a comparative study showed that postmenopausal women had considerably higher serum total cholesterol, triglyceride and low density lipoprotein cholesterol levels than premenopausal women, which may be related to reduced estrogen levels [52]. Estrogen also inhibits low-density lipoprotein transcytosis by reducing the expression of endothelial scavenger receptor class B type 1 [53].
The vascular and cardiac protective effects of estrogens are exerted via ERα, ERβ and GPER1 [16]. E2 binds to ERα and ERβ receptors, leading to activation of classical and non-classical pathways [54]. Previous studies conducted on ERα-knockout mice demonstrated the cardioprotective effect of ERα in cardiomyocytes in both males and females by improving the effectiveness of cardiac repair following a cardiac injury, such as ischemic-reperfusion injury or induced myocardial infarction (MI) [55,56]. ERα has been shown to contribute largely to the vasoprotective effects of E2 as well [18]. In male and female transgenic mice model, the overexpression of ERβ in cardiomyocytes improved the survival and the cardiac function, and decreased maladaptive remodeling following MI [57]. In a study on ERβ-deficient mice subjected to MI, there was an increase in mortality and exacerbated clinical and biochemical markers associated with heart failure (HF) [58]. In addition, ERβ activation rescues pre-existing severe HF in male mice by inducing cardiac angiogenesis, suppression of fibrosis, and restoration of hemodynamic parameters [59].
Emerging evidence points to the role of GPER1 in mediating cardiovascular protection and maintaining blood pressure [60]. Through activation of GPER1, estrogen can reduce ischemia and preserve heart function [61]. Kabir et al. found that GPER1-knockout hearts of male mice failed to demonstrate cardio-protection following ischemic-reperfusion after treatment with E2, indicating the function of GPER1 in ameliorating cardiovascular disorders [62]. GPER1 activation also reduces cardiac myocyte hypertrophy and wall thickness and enhances myocardial relaxation in hypertensive female mRen2. Lewis rats placed on a high-salt diet [63]. The activation of GPER1 with G1 (a selective GPER1 agonist) demonstrated cardio protective effects against doxorubicin-induced cardiotoxicity in male rats [64], and reversed cardiopulmonary dysfunction in ovariectomized rats [65]. Recent studies showed that the infusion of G1 into the renal medulla promotes Na+ excretion via an endothelin-1-dependent pathway in female, but not in male rats [66,67]. Additionally, GPER1 activation with G1 lowers blood pressure in ovariectomized rats [66]. These findings pave the way for additional clinical testing of novel GPER1 agonists for the treatment CVD in females following endogenous estrogen loss, perhaps removing the negative side effects of estrogen replacement therapy.
In terms of kidney function, women experience a slower decline in renal function than men, which supports the hypothesis that sex hormones play a significant role in the prevalence and severity of cardiovascular and kidney disorders [68]. E2 was found to preserve kidney function and prevent the development of glomerulosclerosis in the female rat remnant kidney model [69]. A number of studies have also shown that targeting ERs signaling pathways might have protective effects against certain renal disorders [70], including acute kidney injury [71,72] and CKD [73,74]. For instance, E2 was found to ameliorate glomerulosclerosis and tubulointerstitial fibrosis in the ageing Dahl salt-sensitive rat [73]. In addition, the activation of GPER1 via G1 demonstrated a protective effect against proteinuria and albuminuria in female Dahl salt-sensitive rats [75]. On the other hand, Mankhey et al. concluded that E2 deficiency worsens the kidney function in diabetic ovariectomized female rats, which is antagonized by E2 replacement therapy [76]. E2 deficiency increases the risk of renal pathology specially in diabetic patients through the overactivity of renin angiotensin aldosterone system (RAAS) [77].
2.5. The RAAS-estrogen interactions
The RAAS plays a central role in the regulation of the cardiovascular and renal systems. It is a major contributor to the maintenance of blood pressure and body fluid homeostasis [78]. Hyper activation of the RAAS is associated with cardiovascular disorders and their complications, such as HF, hypertension, cardiac hypertrophy, atherosclerosis, coronary heart disease, myocardial dysfunction, and renal failure [79]. The inhibition of RAAS by angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor type 1 (AT1) blockers were shown to improve cardiac and renal-related conditions [79].
The RAAS is composed of a series of enzyme–catalyzed interactions between its components, which regulate cardiovascular and renal function. The main precursor of the RAAS (angiotensinogen) is produced by the liver [80]. Angiotensinogen is cleaved by the renin secreted by the kidney to produce angiotensin I (Ang I), which is then hydrolyzed by ACE in the lungs to produce angiotensin II (Ang II) [80–82]. The latter acts on the cell-surface G protein-coupled receptor (AT1), causing an increase in blood pressure through different mechanisms, most importantly, vasoconstriction and aldosterone secretion [81,82]. Ang II also binds to cell surface type II receptors (AT2) to induce vasodilation, natriuresis, and nitric oxide generation [81,82]. Meanwhile, a biologically active heptapeptide Ang-(1−7) can be produced in response to the degradation of Ang I by endopeptidases or the degradation of Ang II by angiotensin-converting enzyme 2 (ACE2) [83]. Ang-(1−7) interact with AT2 receptors and Mas G protein-coupled receptors (MasR), which are present in the heart, vasculature, and kidneys, promoting vasodilation, nitric oxide production, and increased arterial baroreflex sensitivity [84]. Similar cardioprotective effects occur when Ang-(1−7) interacts with AT2 receptors [84]. In animal models, it has been shown that Ang-(1−7) has antihypertensive, antifibrotic, antiarrhythmogenic, and antithrombotic effects [84]. In the context of this review, we will pay more attention to the impact of estrogens on the components of the RAAS.
The angiotensinogen mRNA is expressed in several body organs including the heart, vascular system, kidneys, and adrenal glands [79]. The levels of angiotensinogen are generally higher in premenopausal women than in postmenopausal women [79]. In addition, oral estrogen replacement therapy considerably increases plasma angiotensinogen levels, which counteracts the beneficial cardioprotective effects of estrogen [79]. However, evidence indicates that plasma renin is lowered by estrogen due to the suppression of renin secretion from renal juxta-glomerular cells [79]. This may account for the cardiovascular protective effects of estrogen.
The effect of estrogen replacement therapy on Ang II levels is unclear. A study showed a protective effect against hypertension through estrogen-mediated reduction in the plasma levels of Ang II and amplification of the vasodilatory effect of Ang-(1−7) [85]. Estrogen also appeared to protect the heart against hypertrophy and fibrosis by demonstrating an inhibitory effect on Ang II-induced fibroblast-mediated remodeling and proliferation [86,87]. On the contrary, another study showed an increase in Ang II levels, which can be attributed to the suppression of RAAS by negative feedback [88]. Furthermore, a study revealed that Ang II induces albuminuria in male, but not female, rats during treatment with an ACE inhibitor [89]. Additional studies are needed to identify how estrogen dosing, route, and duration of administration and hormonal status of the recipient may impact key components in the RAAS system.
The expression of AT1 receptors in vascular smooth muscles is downregulated by estrogen, which can contribute to the association between estrogen deficiency and the incidence of CVD in post-menopausal women [90,91]. In the adrenal glands, estrogen reduces AT1 receptors and minimizes Ang II-induced aldosterone secretion [92]. It is thought that the activation of AT2 receptors will counteract the stimulation of AT1 receptors [93]. In estrogen-treated ovariectomized mice, the expression of AT2 receptors in the kidneys is increased, resulting in a reduced AT1/AT2 receptor ratio that favors vasodilation [81,94]. This may contribute to the preventive effects of estrogen on the progression of renal disease.
Estrogen was also found to regulate ACE. In ovariectomized rats, estrogen therapy reduces ACE activity in the plasma, kidneys, and aorta [85]. However, studies in ovary-intact female rats showed that during pregnancy, when estrogen level is elevated, ACE2 expression and renal and urinary Ang-(1−7) levels are increased [95,96]. Similarly, E2 pro motes the production of Ang-(1−7) in human endothelial cells via ERα, which induces E2-mediated vasodilatory effects [97]. Meanwhile, the expression of MasR varies between sexes in the renal system [98,99]. In comparison to males, the kidneys of female rats have greater levels of MasR mRNA, which can be attributed to sex hormonal factors [98,99].
Aldosterone is known to cause several types of tissue damage, including cardiac hypertrophy, cardiac fibrosis, proteinuria, vasoconstriction, and salt retention [79]. A number of studies showed that estrogen decreases Ang II-induced aldosterone secretion [100].
Thus, there are two major arms in the RAAS that has opposing actions. The first arm is the Ang II–ACE–AT1 that favors vasoconstriction and is known as the hypertensive axis, while the second arm is the Ang-(1−7)–ACE2–MasR/AT2 that favors vasodilation and is recognized as the antihypertensive axis [78,83]. Overall, estrogen promotes the production of angiotensinogen while inhibiting the production of renin and ACE. Also, the expression of AT1 is reduced by estrogen, while AT2 expression is increased. Therefore, estrogen shifts the balance of the RAAS towards the Ang-(1−7)–ACE2–MasR/AT2 receptor pathways, promoting cardiovascular protection [78]. Conversely, estrogen declination after menopause upregulates the vasoconstrictive arm of the RAAS [77]. However, the specific mechanisms by which estrogens interact with the RAAS to provide cardio-renal protection are still not fully understood and require further investigations. Fig. 2 illustrates the RAAS pathway and how estrogen modulates RAAS components favoring cardiovascular and renal protection.
Fig. 2.
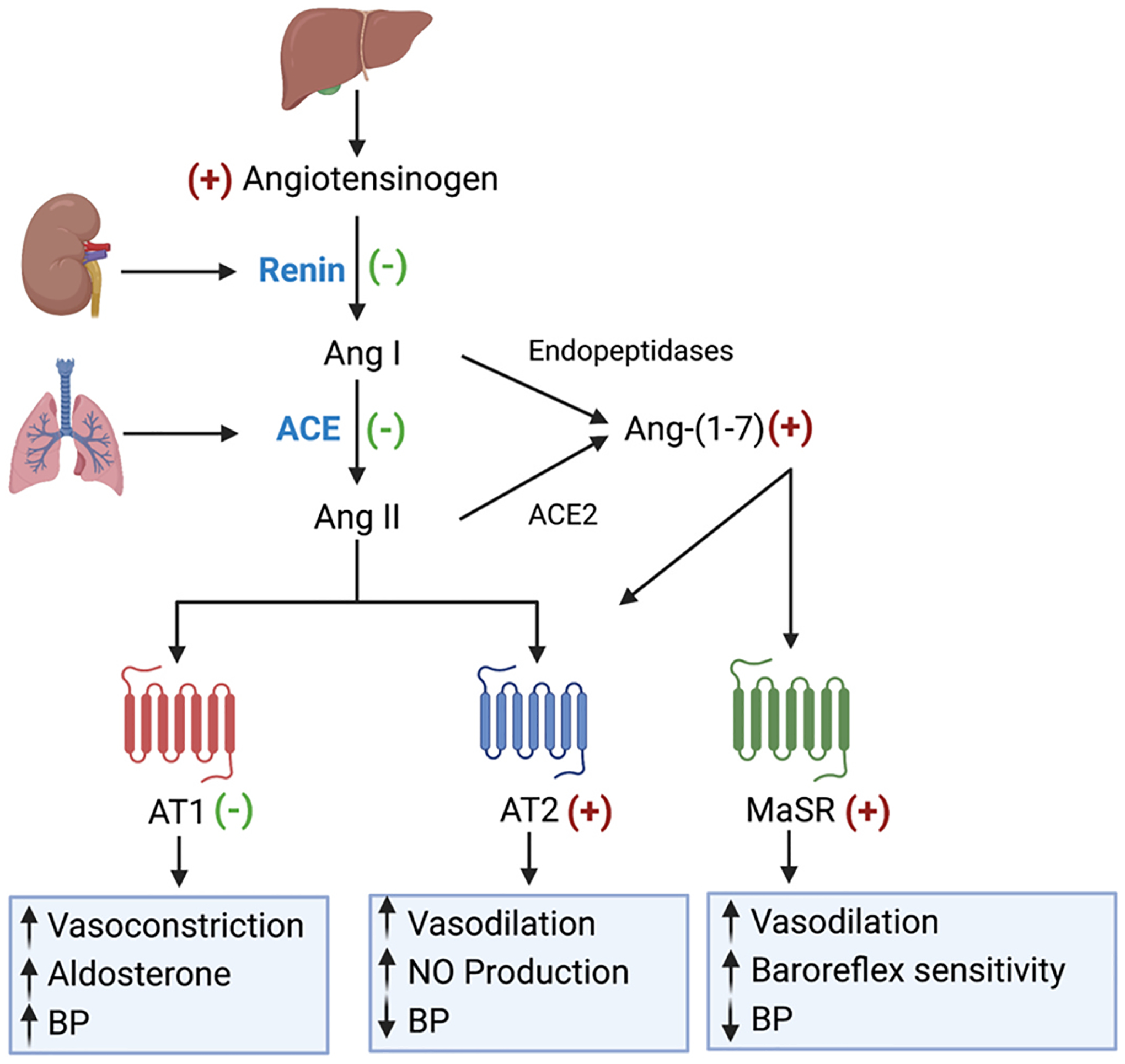
Overview of renin angiotensin aldosterone system (RAAS) pathways showing primary receptor-mediated cardiovascular effects and influence of estrogen on RAAS components. *ACE: angiotensin-converting enzyme, Ang I: angiotensin I, Ang II: angiotensin II, Ang (1 7): angiotensin 1–7, AT1: angiotensin II receptor 1, AT2: angiotensin II receptor 2, MasR: Mas receptor, (+) upregulation by estrogen, (-) downregulation by estrogen. Created in BioRender.com.
2.6. Estrogen in ageing
With ageing, fluctuations in endogenous estrogen production as well as ERs expression start to emerge [101]. The expression of ERs is influenced by ageing in a tissue- and sex-specific manner, which may provide insight on the pleiotropic effects of estrogen on the cardiovascular system [43]. Further, this age-related change in the expression of ERs can be affected by illness and hormonal exposure, which consequently affect the response to estrogen [102,103].
In the context of CVD, the fall in estrogen levels accompanied by the alterations in ER expression and/or signaling brought on by ageing, plays a role in the reduced ability of estrogen to protect the arteries, which might be associated with cardiovascular and renal disorders [18]. For instance, ERs are lower in atherosclerotic human coronary arteries compared to normal human coronary arteries, regardless of menopausal state [104]. In a comparative study using a small sample of postmortem coronary arteries, the expression of ER in vascular smooth muscle cells in postmenopausal women is lower than premenopausal women [104]. Gavin et al. reported a 30% reduction in the expression of ERα in endothelial cells during the early follicular (low estrogen) phase compared to the late follicular (high estrogen) phase of the menstrual cycle [105]. Similarly, the expression of ERα is 33% less in endothelial cells in postmenopausal women compared with the late follicular phase of the menstrual cycle in premenopausal women [105]. Animal studies also showed that endothelial ERα mRNA and protein expression decline after prolonged hypoestrogenic activity and is restored by estrogen replacement therapy [106]. Recently, Connelly et al. suggested that reduced estrogen levels cause a change in ERα:ERβ receptor ratios [103]. Meanwhile, the protein abundance of ERα and ERβ is slightly reduced with age in the aorta of female spontaneously hypertensive rats [107]. In a study conducted by Gurrala et al. to compare the transcript levels of murine ERs within the cardiovascular and renal systems across age and sex, it was revealed that the cardiac ERα mRNA transcript level is reduced in aged female mice compared to middle-aged females [43]. However, the level of cardiac ERα mRNA in male mice is not age-dependent [43]. Renal GPER1 increases with age only in female, but not male, mice; whereas cardiac GPER1 increases in both sexes with age [43]. Notably, other organ systems elicit changes in ER expression with ageing as well. For example, Arimoto et al. reported that ERα, but not ERβ, is increased with age in rat cortical astrocytes [108]. Whereas, the expression of ERα declines in the hippocampus with advancing age, causing a decrease in cognition [109]. Additional studies are required to determine the age-related changes in ER signaling and its role in the development of CVD.
3. Aromatase enzyme
The aromatase enzyme, alternatively known as estrogen synthase, is a mono-oxygenase that belongs to the cytochrome P450 family and is encoded by the CYP19A1 gene [33]. This enzyme catalyzes the demethylation of carbon 19 in androgens causing their aromatization into 18-carbon estrogens [110]. Androstenedione, testosterone, and 16-hydroxytestosterone are the physiological substrates of aromatase, which are then transformed into E1, E2, and E3, respectively [111]. Collectively, the synthesis of estrogen is catalyzed by the aromatase enzyme, which converts endogenous androgens into estrogens [111].
3.1. The mechanism of aromatization
The aromatization process advances through a number of steps elaborated in Fig. 3. First, the methyl group at C19 in androstenedione is hydroxylated to produce 19-hydroxyandrostenedione, which is then followed by a second hydroxylation reaction to produce 19-dihydroxyandrostenedione [112,113]. The latter is then dehydrated to 19-oxoandrostenedione [112,113]. Finally, the steroid ring-A is subjected to oxidative cleavage of the C10-C19 bond followed by release of formic acid, leading to the formation of estrogen [112,113].
Fig. 3.
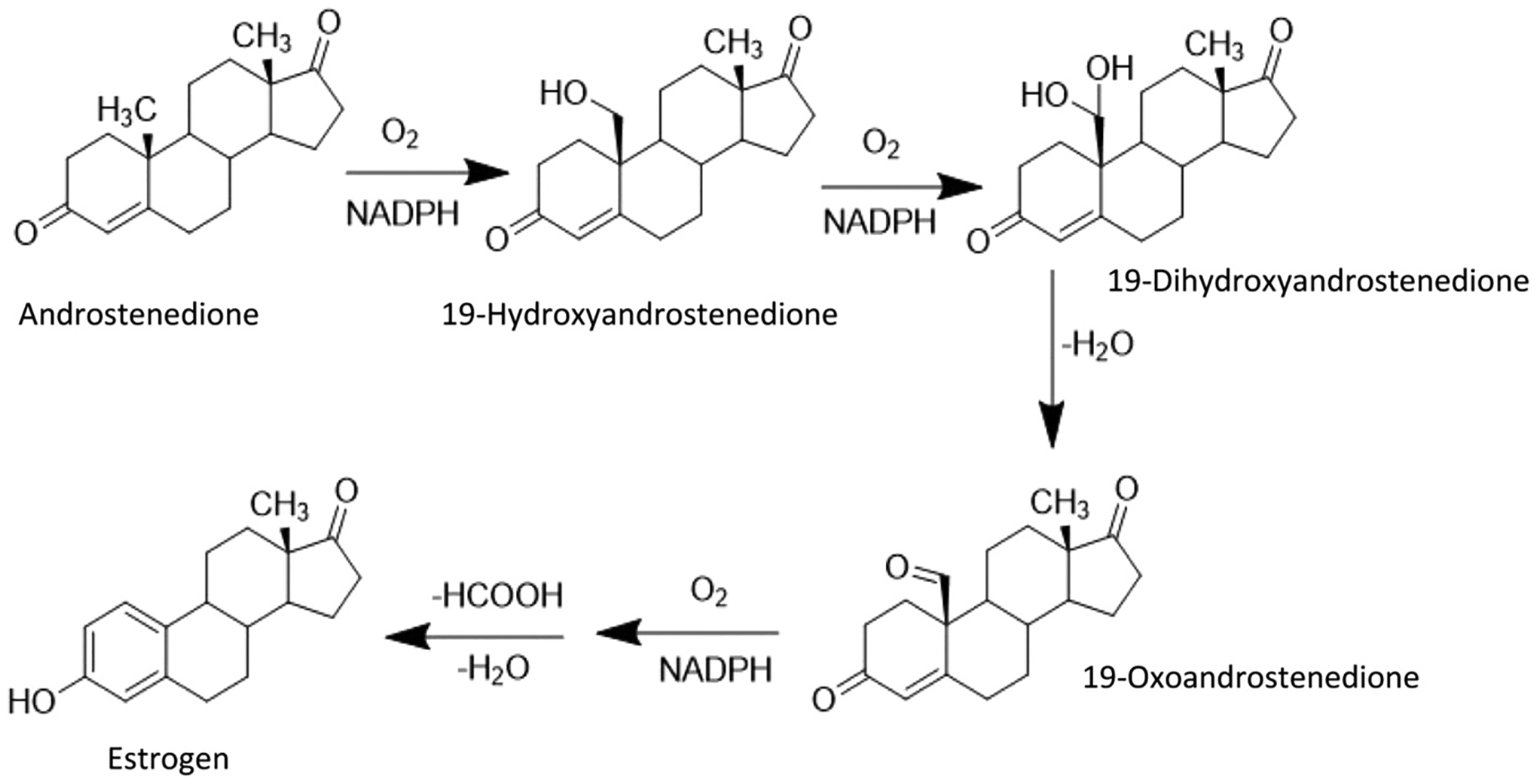
Biosynthesis of estrogen by aromatase. Created by Chemdraw Software.
3.2. Distribution of aromatase
Estrogens are produced by gonadal and extragonadal sites. Gonadally and extragonadally-driven estrogens share the same chemical structure and biological activity, but differ in their metabolic pathways of synthesis [29]. Extra-gonadal estrogens are produced when C19 precursors are supplied to any tissue that expresses aromatase [29]. Noteworthy, aromatase is primarily produced by ovarian granulosa cells in premenopausal women and adipose cells in postmenopausal women [114].
On the gonadal level, aromatase is expressed in both ovaries and testis. In the ovaries, aromatase expression is limited to differentiated preovulatory granulosa cells and luteal cells, and it is not expressed by undifferentiated granulosa cells in preantral follicles [115]. The follicle-stimulating hormone stimulates the growth and maturation of preantral follicles to the preovulatory stage, and the differentiation of granulosa cells, inducing the activation of aromatase [115]. Aromatase is downregulated after ovulation as granulosa cells develop into luteal cells [115]. Meanwhile, the detection of high amounts of estrogens in the male semen can be explained by the expression of aromatase in different testicular cells. Carreau et al. reported the presence of physiologically active aromatase in Leydig cells, Sertoli cells, spermatocytes, spermatids, and ejaculated spermatozoa in males [116].
Aromatase is also highly expressed in the placenta of both human and non-human primates [117], as well as other extra-gonadal tissues including the thalamus, hypothalamus, and hippocampus, indicating that aromatase is expressed widely in numerous regions of human brain in both men and women [118]. Aromatase activity has also been reported in stromal cells and adipocytes [119]. In bone tissue, aromatase has been identified within the human fetal osteoblastic cell line (SV-HFO) [120], and human osteoblasts [121]. The human hepatocellular carcinoma cells and HepG2 hepatoma cells showed increase in estrogen biosynthesis upon treatment with androgen precursors such as testosterone or androstenedione, indicating elevated aromatase activity [122]. Western blotting and immunohistochemistry showed that aromatase is expressed in the adrenal cortex as well as in adrenocortical tumors [123,124]. In addition, aromatase activity was demonstrated by 3[H2O] assay and gas chromatography-mass spectrometry in the parietal cells of the gastric mucosa [125]. It has also been previously reported that aromatase is expressed in epidermal keratinocytes and dermal fibroblasts [126,127]. In situ, hybridization revealed the presence of aromatase in human vascular smooth muscle cells but not in endothelial cells [128]. In men, aromatase is also expressed in the prostate [129]. Although extra-gonadal estrogen is synthesized in small amounts, its concentration is high enough to exert a biological effect locally [29]. Therefore, the disruption of aromatase homeostasis, accompanied by a disturbance in estrogen levels, will result in organ-specific effects.
3.3. Disruption of aromatase homeostasis
Both high and low levels of aromatase, and consequently high and low levels of estrogen, can cause a wide range of diseases and side effects [130]. Aromatase or estrogen excess-driven pathologies include breast, prostate, lung, gastric, and hepatic cancers, polycystic ovary syndrome, endometriosis, obesity, short stature, male hypogonadism, gynecomastia, and testicular hypertrophy [130–132]. Aromatase or estrogen deficiency-induced pathologies include cardiovascular problems [36, 133], osteoporosis [36,130], hot flushes [134], vaginal dryness and vaginal atrophy [134], skin ageing, thinning and pigmentation [135, 136], schizophrenia [130], Alzheimer’s disease [130], depression [137], insomnia [134], neuropathies [36], and elevated aldosterone levels [138,139]. Fig. 4 illustrates the sites of aromatase expression and estrogen disturbance-related effects.
Fig. 4.
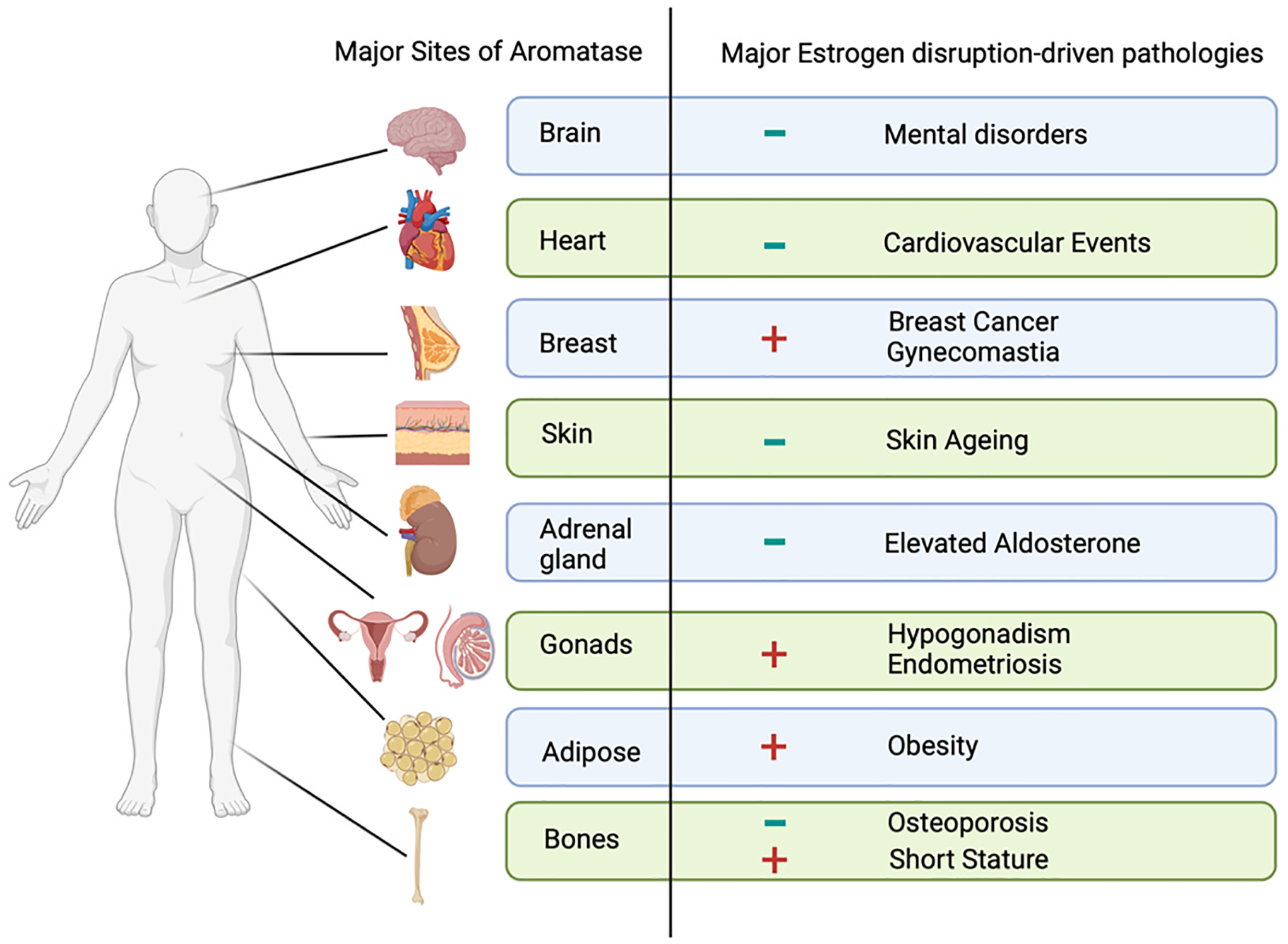
The major sites of aromatase and the associated estrogen-disruption pathologies. (-) estrogen deficiency, (+) estrogen surplus. Created in BioRender.com.
3.4. Regulation of aromatase enzyme
Aromatase enzyme is highly expressed in the ovarian granulosa cells [140]. The expression of aromatase is mainly induced by the follicle stimulating hormone which activates the transcription factor GATA4 that afterward activates other kinases, including ERK1/2, PKA, and PI3K [141]. Cyclic adenosine monophosphate (cAMP) is also involved in the transcription of aromatase [142]. In addition, it has been shown that the transcription of cardiac aromatase was stimulated by the administration of E2 therapy that can bind to ER to form a dimer which translocate into the nucleus where it can bind directly to the estrogen responsive element site on the aromatase gene (CYP19A1) [143]. E2 can also act indirectly by stimulating transcription factors [143].
4. Aromatase inhibitors (AIs)
4.1. Discovery of AIs
Historically, oophorectomy and adrenalectomy have been used to treat breast cancer [144]. The anti-epileptic medication, aminoglutethimide, was found to reduce the production of adrenal steroid hormones by blocking cytochrome P450 enzymes [145]. It was then recommended as a potential medical substitute to adrenalectomy for the treatment of breast cancer [146,147]. Later, it was discovered that the key mechanism of aminoglutethimide was the suppression of aromatase enzyme, which subsequently leads to a reduction in estrogen levels [148,149]. Aminoglutethimide was recognized as the first-generation AI [114]. In 1981, the effect of using 4-hydroxy-androstenedione (4-OH-A) against breast cancer in post-menopausal women was reported [150, 151]. By the middle of the 1980 s, 4-OH-A was named formestane and was recognized as the first selective AI against breast cancer [152]. Formestane is considered as a second-generation AI [114].
Currently-used AIs (shown in Fig. 5) are classified into irreversible steroidal inhibitors (exemestane) and reversible non-steroidal inhibitors (anastrozole and letrozole) [153]. These AIs are nominated as third-generation AIs [114]. The third-generation AIs have an advantage over the first and second generations as they are well tolerated and highly selective for the aromatase enzyme [154]. In addition, third-generation AIs outperform the first- and second-generation AIs in terms of clinical benefit and near-complete specificity in clinical application [155]. However, long-term adverse effects of these drugs, such as skeletal and cardiovascular problems, must be carefully monitored [155].
Fig. 5.
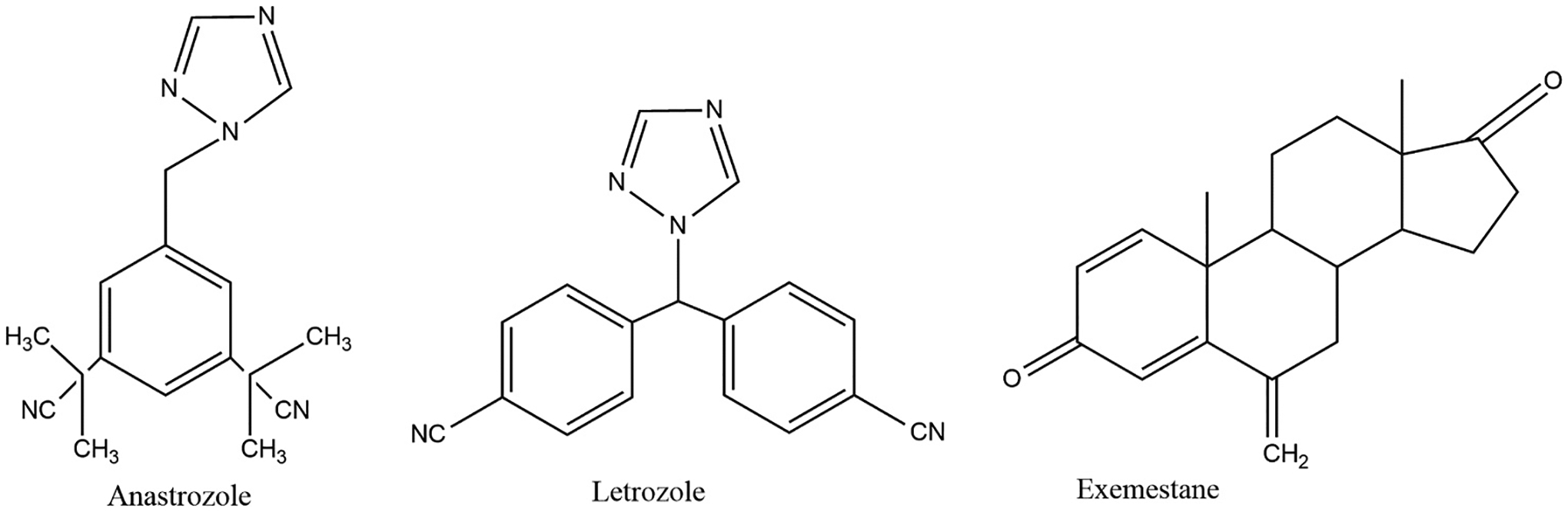
Third generation aromatase inhibitors. Created by ChemDraw Software.
4.2. Uses and side effects of AIs
AIs are currently approved by the United States Food and Drug Administration (FDA) for clinical use against ER-positive breast cancer in postmenopausal women [152,155]. However, AIs are not yet FDA-approved as risk-lowering agents in women who have not been diagnosed with breast cancer [156]. Although AIs demonstrated potential role against other estrogen-dependent conditions, their use is not yet FDA-approved. For instance, AIs showed therapeutic efficacy in improving endometriosis-associated pain [157–159], but their use for endometriosis is still considered off-label [160]. Similarly, a case of male breast cancer responding to a combination of letrozole and palbociclib was reported [161]. Yet, AIs are not FDA-approved for male breast cancer [161].
Given that AIs work by suppressing the aromatization of androgens to estrogens, causing reduction in the estrogen level in the ovaries and peripheral organs [114,162], they reduce the favorable effects of estrogen on lipid profile and bone health [158]. The most common side effect associated with AIs is the joint pain and stiffness [158], which is probably associated with the suppression of cartilage-protective effect of estrogen [163]. Other side effects of AIs include hot flashes, mood disturbances, skin ulcers, and liver function abnormality, which are normally associated with the gradual decline in ovarian estrogen production after menopause [164].
The reduction in circulating estrogens caused by AIs, and subsequently, the decrease in estrogen-mediated protective effects on the cardiovascular system, may potentially lead to an increased risk of unfavorable cardiovascular events [157,158]. Indeed, several studies found that AI users had a higher incidence of adverse CVD outcomes, but the findings are not yet universally accepted [165]. In the next sub-section, we will discuss a number of meta-analysis studies analyzing randomized controlled trials (RCTs) and cohort studies. These studies report cardiovascular events associated with AIs vs. a comparator.
4.3. Controversies regarding the cardiotoxicity of AIs
Due to the documented role of estrogen in developing breast cancer, treatment options including ER blockers such as tamoxifen (TAM) or AIs [162] have been introduced as effective therapeutic options against invasive ER-positive breast cancer in postmenopausal women [166]. The use of AIs was evaluated in three different settings: (i) monotherapy/upfront therapy (AIs instead of TAM), (ii) sequential therapy (TAM switched to AIs or vice versa), and (iii) extended therapy (AIs following five years treatment with TAM) [162]. Although TAM is the gold standard breast cancer therapy, a shift from TAM to AIs in the management of ER-positive breast cancer has occurred [166]. On the other hand, some studies reported that AIs are associated with greater incidence of cardiovascular events compared to TAM. Yet, the increased risk of cardiovascular events is still unclear whether it is due to actual cardiac toxicity of AIs or due to the potential cardioprotective effect of TAM [162].
TAM appeared to have positive cardioprotective effect throughout different mechanisms. Particularly, TAM reduces acetylcholine-induced vasoconstriction and potentiates adenosine-induced vasodilatory response, which thereby reduces blood pressure and coronary artery disease in ovariectomized spontaneously hypertensive rats, pointing to potential beneficial actions of TAM on coronary vascular health after menopause [167]. TAM has also demonstrated lipid-lowering effects, which is one of the prominent factors of protection against developing CVD. A study on postmenopausalpatients with early breast cancer showed that TAM reduces the total cholesterol and low-density lipoprotein cholesterol levels while it does not affect high-density lipoprotein cholesterol [168]. A recent study also affirmed the favorable effect of TAM on lipid profile and ameliorating dyslipidemia [169]. Of note, TAM is a selective ER modulator that elicits antagonistic activity against ERα and ERβ, however, it has been shown to elicit agonistic activity towards GPER1 [170]. Whether GPER1 agonistic activity contributes to the protective actions of TAM remains to be tested.
4.3.1. Meta-analyses showing significant increase of CVD with AIs
A number of meta-analysis studies reported an increase in the cardiovascular events associated with the use of AIs vs. a comparator. In general, the studied population in the clinical trials consisted of postmenopausal women with ER-positive breast cancer. Amir et al. conducted a meta-analysis on seven RCTs comparing AIs with TAM in postmenopausal women with early-stage breast cancer [171]. Data were extracted from two trials evaluating monotherapy with AIs vs. TAM, four trials evaluating switching from TAM to AIs vs. TAM, and one trial evaluating switching from TAM to AIs vs. AIs [171]. The findings revealed that only using AIs vs. TAM or switching from TAM to AIs vs. AIs showed a statistically significant association between AIs and CVD [171]. In addition, the pooled analysis of the data for all three treatment settings showed that the use of AIs for a longer duration was related to a significant increase in the risks of acquiring CVD compared to short duration of AIs or the use of TAM [171]. One meta-analysis study revealed that AIs used in monotherapy and sequential therapy settings are correlated with higher incidence of cardiovascular events when compared with TAM [172]. A meta-analysis conducted by Khosrow-Khavar et al. reported that the pooled analysis of eight RCTs comparing AIs (monotherapy) vs. TAM and four RCTs comparing AIs vs. TAM followed by AIs (sequential therapy), showed an elevated risk of CVD associated with the use of AIs [173]. Goldvaser et al. collated seven RCTs in order to compare AIs (extended therapy) to placebo or no treatment [174]. The pooled analysis of the included studies reported a significant increase in the likelihood of developing cardiovascular events with the extended AIs therapy [174]. In a recent meta-analysis, the findings revealed that patients receiving AIs (monotherapy or sequential therapy) vs. TAM alone or TAM followed by AIs, were at greater risk of developing cardiovascular events [175].
Recently, Yoo et. al conducted a meta-analysis of twenty five studies to investigate the CVD side effects associated with AIs and to evaluate the relation between AIs and the changes in lipid profile in adult female breast cancer patients above 19 years old [176]. By comparing the post-AIs lipid profile to the baseline group after six months of treatment, a significant reduction in high-density lipoprotein cholesterol was observed [176].
4.3.2. Meta-analyses showing non-significant increase of CVD with AIs
Despite the findings of the above studies that support the correlation between the use of AIs and cardiovascular events, some studies demonstrate statistically non-significant relationship between AIs and the occurrence of cardiovascular events. Yu et al. conducted meta-analysis study based on six RCTs, thirteen prospective cohort studies, and one retrospective cohort [177]. It was found that AIs were associated with an insignificant increase in the incidence of stroke, angina, MI, and HF in breast cancer patients compared to TAM [177]. Likewise, Sun et al. found that the pooled results of eight cohort studies revealed that there was no significant difference between AIs users and non-users in the incidence of MI, and HF [178]. Likewise, the combined analysis of four RCTs evaluating AIs (sequential therapy) vs. TAM showed a non-statistically significant correlation between AIs and cardiovascular events [171]. In addition, some studies reported that the extended therapy with AIs is not associated with cardiovascular events [172,173, 175]. Further, the incidence of MI and HF was relatively higher in the AIs group than TAM group, but this difference did not reach statistical significance [176].
This lack of consistency in the data obtained from meta-analyes on the cardiovascular toxicity of AIs can be attributed to multiple factors. There is substantial population heterogeneity since some studies included subjects from different countries. Studies also varied in the inclusion of subjects with or without history of CVD and differed in the follow-up time after treatment with AIs. Importanly, there were differences in how cardiovascular events were defined as some meta-analyses included cardiovascular-related risk factors such as hypertension and hypercholesteremia. Finally, methodological difference in the meta-analyses, such as the type of studies included whether RCTs or cohort studies, and whether AIs are compared to TAM, placebo or without a comparator, also contribute to variation in the findings.
4.4. AIs and renal toxicity
The impact of AIs on the renal system has not been sufficiently studied. There is a lack of RCTs that investigate the relationship between AIs and kidney function. However, a case of anastrozole-induced glomerulonephritis [179], and a case of letrozole-induced acute interstitial nephritis [180] were previously reported. Animal studies also showed an increase in the biomarkers of renal proximal tubular injury by chronic aromatase suppression with anastrozole in female rats [181]. The levels of urinary albumin and plasma urea were elevated in anastrozole-treated female rats fed a high salt diet [181]. Anastrozole increased the urinary excretion of the renal proximal tubule injury biomarker (kidney injury molecule-1), but the level of the glomerular injury biomarker (nephrin) was not elevated [181]. AIs can also induce renal toxicity by altering calcium reabsorption in the kidneys [182]. However, anastrozole attenuates diabetic renal disease in male rats as demonstrated by decreasing albuminuria, glomerulosclerosis, and tubulointerstitial fibrosis [183]. Because the former study was conducted on male rats, the results cannot be extrapolated to include females. In another study, letrozole administration in female rats resulted in a reduction in renal functions as well as micromorphological deteriorations [184]. This is due to the direct oxidative damage caused by letrozole and its metabolites [184]. In particular, letrozole causes a decrease in the expression of cytoprotective detoxification genes (nuclear factor erythroid 2-related factor 2, cytochrome-c, and caspase-3), an increase in hepatorenal lipid peroxides, and a decrease in glutathione and catalase enzyme [184]. Anastrazole treatment also produced similar results in female rats [185]. In light of these findings, further investigation is needed to determine if renal toxicity is a potential adverse effect of AIs.
5. Extra-gonadal aromatase and cardio-renal protection
A unique feature of extra-gonadally synthesized estrogens is being produced locally in concentrations high enough to exert local biological effects with limited systematic effects [29]. Despite the documented cardioprotective effects of estrogen, the use of estrogen-replacement therapy as a cardioprotective agent is still controversial [16]. This is due to the fact that estrogens have major off-target effects which include increased risks of breast and endometrial cancer, as well as thromboembolisms and strokes [186]. However, evidence suggests that the regulation of aromatase enzyme activity and expression protects against cardiac and vascular damage [187]. The aromatase enzyme was found to be expressed in the coronary endothelium and has an effect on the cardiac function and structural modelling as a result of the localized conversion of androgens to E2 in an acute MI male mouse model [187]. Bayard et al. also demonstrated the activity of aromatase enzyme using female rat arterial smooth muscle cells and bovine coronary endothelial cells in in-vitro models [188,189]. Another research revealed that aromatase activity is demonstrated in human arterial smooth muscle cells and therefore hypothesized that E2 produced in vascular smooth muscle cells regulates cardiac contractility and vascular tone (autocrine activity) while stimulating nitric oxide production and angiogenesis in endothelial cells (paracrine activity) [128]. In order to investigate whether upregulation of cardiac aromatase expression could improve ischemic resilience, Bell et al. conducted a study on hearts from male transgenic aromatase-overexpressing mice (AROM+), using an expression vector for human P-450 aromatase [190]. The male AROM+ mice have lower testosterone and higher E2 levels than wild-type male mice. Interestingly, ischemic contracture are attenuated in AROM+ hearts, suggesting that aromatase regulation modulates cardiac performance after ischemia [190]. Another recent study found that HF is associated with local deficiency of cardiac estrogen and downregulation of aromatase, thus suggesting that the restoring the transcript level of cardiac aromatase can protect against HF [191]. Taken together, the abovementioned information indicate a need for more investigation into the potential role of extra-gonadal aromatization and the importance of restoring cardiac aromatase and enhancing estrogen signalling in conferring cardio-renal protection.
6. Conclusion and future prospects
Aromatase is localized in gonadal and extra-gonadal sites in the human body and plays a pivotal role in estrogen biosynthesis. For instance, it is established now that the brain can synthesize estrogen, and that brain aromatase is crucial for neuroprotection [192]. In this context, we propose that estrogenesis within the cardiovascular and renal systems may function to provide cardio-renal protection as well. We argue that aromatase has a fundamental effect in cardio-renal protection through increasing the level of estrogen. In other words, aromatase can function as a component of androgen metabolism that directly supplies estrogen to cardiovascular tissues. Future studies are required to properly understand this complex relationship, and identify the role of aromatization in preserving cardiovascular and renal health.
Funding
This work was supported by the National Institutes of Health (R00 DK119413) and the American Society of Nephrology (Carl Gottschalk Research Scholar Grant).
Footnotes
CRediT authorship contribution statement
Manar Eissa: Writing – original draft preparation. Eman Gohar: Writing – review & editing.
Declaration of Competing Interest
none.
Data availability
No data was used for the research described in the article.
References
- [1].Mozaffarian D, Benjamin EJ, Go AS, Executive summary: heart disease and stroke statistics-2016 update: a report from the American Heart Association, Circulation 133 (2016) 447–454, 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- [2].Roth GA, Mensah GA, Johnson CO, et al. , Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study, J. Am. Coll. Cardiol 76 (2020) 2982–3021, 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tsao CW, Aday AW, Almarzooq ZI, et al. , Heart disease and stroke statistics - 2023 update: a report from the American Heart Association, Circulation 147 (2023) E93–E621, 10.1161/CIR.0000000000001123. [DOI] [PubMed] [Google Scholar]
- [4].Jager KJ, Kovesdy C, Langham R, et al. , A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases, Nephrol. Dial. Transpl 34 (2019) 1803–1805, 10.1093/ndt/gfz174. [DOI] [PubMed] [Google Scholar]
- [5].Kovesdy CP, Epidemiology of chronic kidney disease: an update 2022, Kidney Int. Suppl 12 (2022) 7–11, 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].United States Renal Data System. USRDS Annual Data Report: Epidemiology of kidney disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, (2022). https://adr.usrds.org/ (accessed May 4, 2023). [Google Scholar]
- [7].Ammirati AL, Kidney disease: chronic kidney disease, Rev. Assoc. Med. Bras 66 (2020) 53–59. [DOI] [PubMed] [Google Scholar]
- [8].Jankowski J, Floege J, Fliser D, et al. , Cardiovascular disease in chronic kidney disease pathophysiological insights and therapeutic options, Circulation 143 (2021) 1157–1172, 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].North BJ, Sinclair DA, The intersection between aging and cardiovascular disease, Circ. Res 110 (2012) 1097–1108, 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Colby SL, Ortman JM, Projections of the Size and Composition of the U.S. Population: 2014 to 2060, 2015.
- [11].Yazdanyar A, Newman AB, The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs, Clin. Geriatr. Med 25 (2009) 563–577, 10.1038/nature08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Benjamin EJ, Muntner P, Alonso A, et al. , Heart disease and stroke statistics-2019 update: a report from the American Heart Association, Circulation 139 (2019) e56–e528, 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- [13].Leppert MH, Ho PM, Burke J, et al. , Young women had more strokes than young men in a large, United States Claims Sample, Stroke 51 (2020) 3352–3355, 10.1161/STROKEAHA.120.030803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ekker MS, Verhoeven JI, Vaartjes I, et al. , Stroke incidence in young adults according to age, subtype, sex, and time trends, Neurology 92 (2019) e2444–e2454, 10.1212/WNL.0000000000007533. [DOI] [PubMed] [Google Scholar]
- [15].Bots SH, Peters SAE, Woodward M, Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010, BMJ Glob. Heal 2 (2017) 1–8, 10.1136/bmjgh-2017-000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Iorga A, Cunningham CM, Moazeni S, et al. , The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy, Biol. Sex. Differ 8 (2017) 33, 10.1186/s13293-017-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rodgers JL, Jones J, Bolleddu SI, et al. , Cardiovascular risks associated with gender and aging, J. Cardiovasc. Dev. Dis 6 (2019) 19, 10.3390/jcdd6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davezac M, Buscato M, Zahreddine R, et al. , Estrogen receptor and vascular aging, Front. Aging 2 (2021), 727380, 10.3389/fragi.2021.727380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Centers for Disease Control and Prevention, Chronic Kidney Disease in the United States, 2021. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, (2021). [Google Scholar]
- [20].Mills KT, Xu Y, Zhang W, et al. , A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010, Kidney Int 88 (2015) 950–957, 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Juutilainen A, Kastarinen H, Antikainen R, et al. , Trends in estimated kidney function: the FINRISK surveys, Eur. J. Epidemiol 27 (2012) 305–313, 10.1007/s10654-012-9652-3. [DOI] [PubMed] [Google Scholar]
- [22].Hecking M, Bieber BA, Ethier J, et al. , Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the dialysis outcomes and practice patterns study (DOPPS), PLoS Med 11 (2014), e1001750, 10.1371/journal.pmed.1001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harris RC, Zhang M-Z, The role of gender disparities in kidney injury, Ann. Transl. Med 8 (2020) 514, 10.21037/atm.2020.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bairey Merz CN, Dember LM, Ingelfinger JR, et al. , Sex and the kidneys: current understanding and research opportunities, Nat. Rev. Nephrol 15 (2019) 776–783, 10.1038/s41581-019-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fuentes N, Silveyra P, Estrogen receptor signaling mechanisms, Adv. Protein Chem. Struct. Biol 116 (2019) 135–170, 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Samavat H, Kurzer MS, Estrogen metabolism and breast cancer, Cancer Lett 365 (2015) 231–243, 10.1016/j.canlet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bianchi VE, Bresciani E, Meanti R, et al. , The role of androgens in women’s health and wellbeing, Pharmacol. Res 171 (2021), 105758. [DOI] [PubMed] [Google Scholar]
- [28].Mahmoodzadeh S, Dworatzek E, The role of 17β-estradiol and estrogen receptors in regulation of Ca2+ channels and mitochondrial function in Cardio myocytes, Front. Endocrinol 10 (2019), 10.3389/fendo.2019.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Barakat R, Oakley O, Kim H, et al. , Extra-gonadal sites of estrogen biosynthesis and function, BMB Rep 49 (2016) 488–496, 10.5483/BMBRep.2016.49.9.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhao H, Tian Z, Hao J, et al. , Extragonadal aromatization increases with time after ovariectomy in rats, Reprod. Biol. Endocrinol 3 (2005), 10.1186/1477-7827-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Grodin J, Sitteri PK, MacDonald P, Source of estrogen production in postmenopausal women, J. Clin. Endocrinol. Metab 36 (1973) 207. [DOI] [PubMed] [Google Scholar]
- [32].Lukanova A, Lundin E, Zeleniuch-Jacquotte A, et al. , Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women, Eur. J. Endocrinol 150 (2004) 161–171, 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- [33].Simpson ER, Mahendroo MS, Means GD, et al. , Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis, Endocr. Rev 15 (1994) 342–355, 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- [34].Gillies GE, McArthur S, Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines, Pharmacol. Rev 62 (2010) 155–198, 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee H-R, Kim T-H, Choi K-C, Functions and physiological roles of two types of estrogen receptors, ERα and ERβ, identified by estrogen receptor knockout mouse, Lab. Anim. Res 28 (2012) 71, 10.5625/lar.2012.28.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Paterni I, Granchi C, Katzenellenbogen J, et al. , Estrogen receptors alpha and beta subtype-selective ligands and clinical potential, Steroids 90 (2014) 13–29, 10.1016/j.steroids.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Morselli E, Santos RS, Criollo A, et al. , The effects of oestrogens and their receptors on cardiometabolic health, Nat. Rev. Endocrinol 13 (2017) 352–364, 10.1038/nrendo.2017.12. [DOI] [PubMed] [Google Scholar]
- [38].Dahlman-Wright K, Cavailles V, Fuqua SA, et al. , International union of pharmacology. LXIV. Estrogen receptors, Pharmacol. Rev 58 (2006) 773–781, 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- [39].Yu W, Zhao B, Zhong H, et al. , Estrogen receptor alpha expression in renal tissue and its relationship with prognosis in immunoglobulin A nephropathy, 2020. www.ijcep.com/. [PMC free article] [PubMed]
- [40].Burris D, Webster R, Sheriff S, et al. , Estrogen directly and specifically downregulates NaPi-IIa through the activation of both estrogen receptor isoforms (ER and ER) in rat kidney proximal tubule, Ren. Physiol. Am. J. Physiol. Ren. Physiol 308 (2015) 522–534, 10.1152/ajprenal.00386.2014.-We. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Otto C, Fuchs I, Kauselmann G, et al. , GPR30 does not mediate estrogenic responses in reproductive organs in mice, Biol. Reprod 80 (2009) 34–41, 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- [42].Wang H, Sun X, Chou J, et al. , Cardiomyocyte-specific deletion of the G protein-coupled estrogen receptor (GPER) leads to left ventricular dysfunction and adverse remodeling: a sex-specific gene profiling analysis, Biochim. Biophys. Acta- Mol. Basis Dis 1863 (2017) 1870–1882, 10.1016/j.bbadis.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gurrala R, Kilanowski-Doroh IM, Hutson DD, et al. , Alterations in the estrogen receptor profile of cardiovascular tissues during aging, GeroScience 43 (2021) 433–442, 10.1007/s11357-021-00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hazell GGJ, Yao ST, Roper JA, et al. , Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues, J. Endocrinol 202 (2009) 223–236, 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Trejter M, Jopek K, Celichowski P, et al. , Expression of estrogen, estrogen related and androgen receptors in adrenal cortex of intact adult male and female rats, Folia Histochem. Cytobiol 53 (2015) 133–144, 10.5603/FHC.a2015.0012. [DOI] [PubMed] [Google Scholar]
- [46].Davis KE, Carstens EJ, Irani BG, et al. , Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis, Horm. Behav 66 (2014) 196–207, 10.1016/j.yhbeh.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Heino TJ, Chagin AS, Sävendahl L, The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone, J. Endocrinol 197 (2008), 10.1677/JOE-07-0629. [DOI] [PubMed] [Google Scholar]
- [48].Arnold AP, Cassis LA, Eghbali M, et al. , Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases, Arterioscler. Thromb. Vasc. Biol 37 (2017) 746–756, 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mosca L, Barrett-Connor E, Kass Wenger N, Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes, Circulation 124 (2011) 2145–2154, 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Menazza S, Murphy E, The expanding complexity of estrogen receptor signaling in the cardiovascular system, Circ. Res 118 (2016) 994–1007, 10.1161/CIRCRESAHA.115.305376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mendelsohn ME, Protective effects of estrogen on the cardiovascular system, Am. J. Cardiol 89 (2002) 12E–18E, 10.1016/S0002-9149(02)02405-0. [DOI] [PubMed] [Google Scholar]
- [52].Reddy Kilim S, Rao Chandala S, A comparative study of lipid profile and oestradiol in pre- and post-menopausal women, J. Clin. Diagn. Res 7 (2013) 1596–1598, 10.7860/JCDR/2013/6162.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ghaffari S, Nabi FN, Sugiyama MG, et al. , Estrogen inhibits LDL (low-density lipoprotein) transcytosis by human coronary artery endothelial cells via GPER (G-protein-coupled estrogen receptor) and SR-BI (scavenger receptor class B type 1), Arterioscler. Thromb. Vasc. Biol 38 (2018) 2283–2294, 10.1161/ATVBAHA.118.310792. [DOI] [PubMed] [Google Scholar]
- [54].McDevitt MA, Glidewell-Kenney C, Jimenez MA, et al. , New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock out and knock in mice, Occup. Environ. Med 290 (2008) 24–30, 10.1016/j.mce.2008.04.003.New. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang M, Crisostomo P, Wairiuko GM, et al. , Estrogen receptor-α mediates acute myocardial protection in females, Am. J. Physiol. Hear. Circ. Physiol 290 (2006) H2204–H2209, 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- [56].Zhai P, Eurell TE, Cooke PS, et al. , Myocardial ischemia-reperfusion injury in estrogen receptor-α knockout and wild-type mice, Am. J. Physiol. Hear. Circ. Physiol 278 (2000) 1640–1647, 10.1152/ajpheart.2000.278.5.h1640. [DOI] [PubMed] [Google Scholar]
- [57].Schuster I, Mahmoodzadeh S, Dworatzek E, et al. , Cardiomyocyte-specific overexpression of oestrogen receptor β improves survival and cardiac function after myocardial infarction in female and male mice, Clin. Sci 130 (2016) 365–376, 10.1042/CS20150609. [DOI] [PubMed] [Google Scholar]
- [58].Pelzer T, Loza PAA, Hu K, et al. , Increased mortality and aggravation of heart failure in estrogen receptor-β knockout mice after myocardial infarction, Circulation 111 (2005) 1492–1498, 10.1161/01.CIR.0000159262.18512.46. [DOI] [PubMed] [Google Scholar]
- [59].Iorga A, Umar S, Ruffenach G, et al. , Estrogen rescues heart failure through estrogen receptor Beta activation, Biol. Sex. Differ 9 (2018), 10.1186/s13293-018-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gohar EY, G protein-coupled estrogen receptor 1 as a novel regulator of blood pressure, Am. J. Physiol. Ren. Physiol 319 (2020) F612–F617, 10.1152/ajprenal.00045.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Prossnitz ER, Maggiolini M, Mechanisms of estrogen signaling and gene expression via GPR30, Mol. Cell. Endocrinol 308 (2009) 32–38, 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kabir ME, Singh H, Lu R, et al. , G protein-coupled estrogen receptor 1 mediates acute estrogen-induced cardioprotection via MEK/ERK/GSK-3β Pathway after Ischemia/Reperfusion, PLoS One 10 (2015), 10.1371/journal.pone.0135988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Jessup JA, Lindsey SH, Wang H, et al. , Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2. Lewis Rats, PLoS One 5 (2010), 10.1371/journal.pone.0015433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].De Francesco EM, Rocca C, Scavello F, et al. , Protective role of GPER Agonist G-1 on cardiotoxicity induced by doxorubicin, J. Cell. Physiol 232 (2017) 1640–1649, 10.1002/jcp.25585. [DOI] [PubMed] [Google Scholar]
- [65].Alencar AKN, Montes GC, Costa DG, et al. , Cardioprotection induced by activation of GPER in ovariectomized rats with pulmonary hypertension, J. Gerontol. Ser. A Biol. Sci. Med. Sci 73 (2018) 1158–1166, 10.1093/gerona/gly068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gohar EY, Daugherty EM, Aceves JO, et al. , Evidence for G- protein–coupled estrogen receptor as a pronatriuretic factor, J. Am. Heart Assoc 9 (2020), e015110, 10.1161/JAHA.119.015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gohar EY, Pollock DM, Functional interaction of endothelin receptors in mediating natriuresis evoked by G protein–Coupled estrogen receptor 1, J. Pharmacol. Exp. Ther 376 (2021) 98–105, 10.1124/jpet.120.000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Silbiger SR, Neugarten J, The impact of gender on the progression of chronic renal disease, Am. J. Kidney Dis 25 (1995) 515–533, 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- [69].Antus B, Hamar P, Kokeny G, et al. , Estradiol is nephroprotective in the rat remnant kidney, Nephrol. Dial. Transpl 18 (2003) 54–61, 10.1093/ndt/18.1.54. [DOI] [PubMed] [Google Scholar]
- [70].Ma HY, Chen S, Du Y, Estrogen and estrogen receptors in kidney diseases, Ren. Fail 43 (2021) 619–642, 10.1080/0886022X.2021.1901739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ikeda M, Swide T, Vayl A, et al. , Estrogen administered after cardiac arrest and cardiopulmonary resuscitation ameliorates acute kidney injury in a sex- and age-specific manner, Crit. Care 19 (2015), 10.1186/s13054-015-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wu CC, Chang CY, Chang ST, et al. , 17β-estradiol accelerated renal tubule regeneration in male rats after ischemia/reperfusion-induced acute kidney injury, Shock 46 (2016) 158–163, 10.1097/SHK.0000000000000586. [DOI] [PubMed] [Google Scholar]
- [73].Maric C, Sandberg K, Hinojosa-Laborde C, Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17β-estradiol in the aging Dahl salt sensitive rat, J. Am. Soc. Nephrol 15 (2004) 1546–1556, 10.1097/01.ASN.0000128219.65330.EA. [DOI] [PubMed] [Google Scholar]
- [74].Gross ML, Adamczak M, Rabe T, et al. , Beneficial effects of estrogens on indices of renal damage in uninephrectomized SHRsp rats, J. Am. Soc. Nephrol 15 (2004) 348–358, 10.1097/01.ASN.0000105993.63023.D8. [DOI] [PubMed] [Google Scholar]
- [75].Gohar EY, Almutlaq RN, Daugherty EM, et al. , Activation of G protein-coupled estrogen receptor 1 ameliorates proximal tubular injury and 2 proteinuria in Dahl salt-sensitive female rats, Am. J. Physiol. Integr. Comp. Physiol 320 (2021) R297–R306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mankhey RW, Bhatti F, Maric C, 17β-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy, Am. J. Physiol. Ren. Physiol 288 (2005), 10.1152/ajprenal.00195.2004. [DOI] [PubMed] [Google Scholar]
- [77].O’Donnell E, Floras JS, Harvey PJ, Estrogen status and the renin angiotensin aldosterone system, Am. J. Physiol. Regul. Integr. Comp. Physiol 307 (2014) R498–R500, 10.1152/ajpregu.00182.2014. [DOI] [PubMed] [Google Scholar]
- [78].Xue B, Zhang Z, Beltz TG, et al. , Estrogen regulation of the brain renin-angiotensin system in protection against angiotensin II-induced sensitization of hypertension, Am. J. Physiol. Hear. Circ. Physiol 307 (2014) H191–H198, 10.1152/ajpheart.01012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Komukai K, Mochizuki S, Yoshimura M, Gender and the renin-angiotensinaldosterone system, Fundam. Clin. Pharmacol 24 (2010) 687–698, 10.1111/j.1472-8206.2010.00854.x. [DOI] [PubMed] [Google Scholar]
- [80].Lumbers ER, Delforce SJ, Pringle KG, et al. , The lung, the heart, the novel coronavirus, and the renin-angiotensin system; the need for clinical trials, Front. Med 7 (2020), 10.3389/fmed.2020.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Miller AJ, Arnold AC, The renin–angiotensin system in cardiovascular autonomic control: recent developments and clinical implications, Clin. Auton. Res 29 (2019) 231–243, 10.1007/s10286-018-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lemarié CA, Schiffrin EL, The angiotensin II type 2 receptor in cardiovascular disease, JRAAS J. Renin-Angiotensin-Aldosterone Syst 11 (2010) 19–31, 10.1177/1470320309347785. [DOI] [PubMed] [Google Scholar]
- [83].Medina D, Mehay D, Arnold AC, Sex differences in cardiovascular actions of the renin–angiotensin system, Clin. Auton. Res 30 (2020) 393–408, 10.1007/s10286-020-00720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Medina D, Arnold AC, Angiotensin-(1–7): translational avenues in cardiovascular control, Am. J. Hypertens. 32 (2019) 1133–1142, 10.1093/ajh/hpz146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Brosnihan KB, Li P, Ganten D, et al. , Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS, Am. J. Physiol. Regul. Integr. Comp. Physiol 273 (1997) R1908–R1915, 10.1152/ajpregu.1997.273.6.r1908. [DOI] [PubMed] [Google Scholar]
- [86].Stewart JA, Cashatt DO, Borck AC, et al. , 17β-estradiol modulation of angiotensin II-stimulated response in cardiac fibroblasts, J. Mol. Cell. Cardiol 41 (2006) 97–107, 10.1016/j.yjmcc.2006.04.019. [DOI] [PubMed] [Google Scholar]
- [87].Wu M, Han M, Li J, et al. , 17β-estradiol inhibits angiotensin II-induced cardiac myofibroblast differentiation, Eur. J. Pharmacol 616 (2009) 155–159, 10.1016/j.ejphar.2009.05.016. [DOI] [PubMed] [Google Scholar]
- [88].Xu X, Xiao JC, Luo LF, et al. , Effects of ovariectomy and 17β-estradiol treatment on the renin-angiotensin system, blood pressure, and endothelial ultrastructure, Int. J. Cardiol 130 (2008) 196–204, 10.1016/j.ijcard.2007.08.041. [DOI] [PubMed] [Google Scholar]
- [89].Sartori-Valinotti JC, Iliescu R, Yanes LL, et al. , Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked, Hypertension 51 (2008) 1170–1176, 10.1161/HYPERTENSIONAHA.107.106922. [DOI] [PubMed] [Google Scholar]
- [90].Nickenig G, Ba AT, Grohe C, et al. , Estrogen modulates AT1 receptor gene expression in vitro and in vivo, Circulation 97 (1998) 2197–2201. [DOI] [PubMed] [Google Scholar]
- [91].Nickenig G, Strehlow K, Wassmann S, et al. , Differential effects of estrogen and progesterone on AT1 receptor gene expression in vascular smooth muscle cells, Circulation 102 (2000) 1828–1833, 10.1161/01.CIR.102.15.1828. [DOI] [PubMed] [Google Scholar]
- [92].Wu Z, Maric C, Roesch DM, et al. , Estrogen regulates adrenal angiotensin AT1 receptors by modulating AT1 receptor translation, Endocrinology 144 (2003) 3251–3261, 10.1210/en.2003-0015. [DOI] [PubMed] [Google Scholar]
- [93].Horiuchi M, Akishita M, Dzau VJ, Recent progress in Angiotensin II type 2 receptor research in the cardiovascular system, Hypertension 33 (1999) 613–621. [DOI] [PubMed] [Google Scholar]
- [94].Armando I, Jezova M, Juorio AV, et al. , Estrogen upregulates renal angiotensin II AT2 receptors, Am. J. Physiol. Ren. Physiol 283 (2002) F934–F943, 10.1152/ajprenal.00145.2002. [DOI] [PubMed] [Google Scholar]
- [95].Neves LAA, Williams AF, Averill DB, et al. , Pregnancy enhances the angiotensin (Ang)-(1–7) vasodilator response in mesenteric arteries and increases the renal concentration and urinary excretion of Ang-(1–7), Endocrinology 144 (2003) 3338–3343, 10.1210/en.2003-0009. [DOI] [PubMed] [Google Scholar]
- [96].Brosnihan KB, Neves LAA, Joyner JN, et al. , Enhanced Renal Immunocytochemical Expression of ANG-(1–7) and ACE2 During Pregnancy, Hypertension 42 (2003) 749–753, 10.1161/01.HYP.0000085220.53285.11. [DOI] [PubMed] [Google Scholar]
- [97].Mompeón A, Lázaro-Franco M, Bueno-Betí C, et al. , Estradiol, acting through ERα, induces endothelial non-classic renin-angiotensin system increasing angiotensin 1–7 production, Mol. Cell. Endocrinol 422 (2016) 1–8, 10.1016/j.mce.2015.11.004. [DOI] [PubMed] [Google Scholar]
- [98].Choopani S, Nematbakhsh M, Sex difference in MasR expression and functions in the renal system, J. Renin. Angiotensin. Aldosterone. Syst 2022 (2022) 1327839, 10.1155/2022/1327839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Hilliard LM, Sampson AK, Brown RD, et al. , The “his and hers” of the renin-angiotensin system, Curr. Hypertens. Rep 15 (2013) 71–79, 10.1007/s11906-012-0319-y. [DOI] [PubMed] [Google Scholar]
- [100].Roesch DM, Tian Y, Zheng WEI, et al. , Estradiol attenuates angiotensin-induced aldosterone secretion in ovariectomized rats, Endocrinology 141 (2000) 4629–4636. [DOI] [PubMed] [Google Scholar]
- [101].Cui J, Shen Y, Li R, Estrogen synthesis and signaling pathways during ageing: from periphery to brain, Trends Mol. Med 19 (2013) 197–209, 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Novella S, Heras M, Hermenegildo C, et al. , Effects of estrogen on vascular inflammation: a matter of timing, Arterioscler. Thromb. Vasc. Biol 32 (2012) 2035–2042, 10.1161/ATVBAHA.112.250308. [DOI] [PubMed] [Google Scholar]
- [103].Connelly PJ, Casey H, Montezano AC, et al. , Sex steroids receptors, hypertension, and vascular ageing, J. Hum. Hypertens. 36 (2022) 120–125, 10.1038/s41371-021-00576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Losordo DW, Kearney M, Kim EA, et al. , Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women, Circulation 89 (1994) 1501–1510, 10.1161/01.CIR.89.4.1501. [DOI] [PubMed] [Google Scholar]
- [105].Gavin KM, Seals DR, Silver AE, et al. , Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women, J. Clin. Endocrinol. Metab 94 (2009) 3513–3520, 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Pinna C, Cignarella A, Sanvito P, et al. , Prolonged ovarian hormone deprivation impairs the protective vascular actions of estrogen receptor α agonists, Hypertension 51 (2008) 1210–1217, 10.1161/HYPERTENSIONAHA.107.106807. [DOI] [PubMed] [Google Scholar]
- [107].Wynne FL, Payne JA, Cain AE, et al. , Age-related reduction in estrogen receptor-mediated mechanisms of vascular relaxation in female spontaneously hypertensive rats, Hypertension 43 (2004) 405–412, 10.1161/01.HYP.0000111833.82664.0c. [DOI] [PubMed] [Google Scholar]
- [108].Arimoto JM, Wong A, Rozovsky I, et al. , Age increase of estrogen receptor-α (ERα) in cortical astrocytes impairs neurotrophic support in male and female rats, Endocrinology 154 (2013) 2101–2113, 10.1210/en.2012-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bean LA, Ianov L, Foster TC, Estrogen receptors the hippocampus and memory, Neuroscientist 20 (2014) 534–545, 10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Blakemore J, Naftolin F, Aromatase: contributions to physiology and disease in women and men, Physiology 31 (2016) 258–269, 10.1152/physiol.00054.2015. [DOI] [PubMed] [Google Scholar]
- [111].Di Nardo G, Gilardi G, Human aromatase: perspectives in biochemistry and biotechnology, Biotechnol. Appl. Biochem 60 (2013) 92–101, 10.1002/bab.1088. [DOI] [PubMed] [Google Scholar]
- [112].Miyairi S, Fishman J, Radiometric analysis of oxidative reactions in aromatization by placental microsomes, J. Biol. Chem 260 (1985) 320–325. [PubMed] [Google Scholar]
- [113].Akhtar M, Calder MR, Corina DL, et al. , Mechanistic studies on C-19 demethylation in oestrogen biosynthesis, Biochem. J 201 (1982) 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Słopień R, Mȩczekalski B, Aromatase inhibitors in the treatment of endometriosis, Prz. Menopauzalny. 15 (2016) 43–47, 10.5114/pm.2016.58773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Stocco Carlos, Tissue physiology and pathology of aromatase, Steroids 77 (2012) 27–35, 10.1016/j.steroids.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Carreau S, de Vienne C, Galeraud-Denis I, Aromatase and estrogens in man reproduction: a review and latest advances, Adv. Med. Sci 53 (2008) 139–144, 10.2478/v10039-008-0022-z. [DOI] [PubMed] [Google Scholar]
- [117].Kragie L, Aromatase in primate pregnancy: a review, Endocr. Res 28 (2002) 121–128, 10.1081/ERC-120015041. [DOI] [PubMed] [Google Scholar]
- [118].Sasano H, Takahashi K, Satoh F, et al. , Aromatase in the human central nervous system, Clin. Endocrinol 48 (1998) 325–329. [DOI] [PubMed] [Google Scholar]
- [119].Cleland WH, Mendelson CR, Simpson ER, Aromatase activity of membrane fractions of human adipose tissue stromal cells and adipocytes, Endocrinology 113 (1983) 2155–2160. [DOI] [PubMed] [Google Scholar]
- [120].Watanabe M, Simpson ER, Pathirage N, et al. , Aromatase expression in the human fetal osteoblastic cell line SV-HFO, J. Mol. Endocrinol 32 (2004) 533–545, 10.1677/jme.0.0320533. [DOI] [PubMed] [Google Scholar]
- [121].Enjuanes A, Garcia-Giralt N, Supervía A, Nogués X, et al. , Regulation of CYP19 gene expression in primary human osteoblasts: effects of vitamin D and other treatments, Eur. J. Endocrinol 148 (2003) 519–526, 10.1530/eje.0.1480519. [DOI] [PubMed] [Google Scholar]
- [122].Castagnetta LAM, Agostara B, Montalto G, et al. , Local estrogen formation by nontumoral, cirrhotic, and malignant human liver tissues and cells, Cancer Res 63 (2003) 5041–5045. [PubMed] [Google Scholar]
- [123].Nicol MR, Papacleovoulou G, Evans DB, et al. , Estrogen biosynthesis in human H295 adrenocortical carcinoma cells, Mol. Cell. Endocrinol 300 (2009) 115–120, 10.1016/j.mce.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Barzon L, Masi G, Pacenti M, et al. , Expression of aromatase and estrogen receptors in human adrenocortical tumors, Virchows Arch 452 (2008) 181–191, 10.1007/s00428-007-0542-0. [DOI] [PubMed] [Google Scholar]
- [125].Ueyama T, Shirasawa N, Numazawa M, et al. , Gastric parietal cells: potent endocrine role in secreting estrogen as a possible regulator of gastro-hepatic axis, Endocrinology 143 (2002) 3162–3170, 10.1210/endo.143.8.8974. [DOI] [PubMed] [Google Scholar]
- [126].Berkovitz GD, Carter KM, Brown TR, et al. , Aromatase activity in cultured human genital skin fibroblasts get, J. Clin. Endocrinol. Metab 59 (1984) 665–671, 10.1016/0303-7207(90)90012-W. [DOI] [PubMed] [Google Scholar]
- [127].Berkovitz GD, Brown TR, Fujimoto M, Aromatase activity in human skin fibroblasts grown in cell culture, Steroids 50 (1987) 281–295, 10.1016/0039-128X(83)90078-8. [DOI] [PubMed] [Google Scholar]
- [128].Harada N, Sasano H, Murakami H, et al. , Localized expression of aromatase in human vascular tissues, Circ. Res 84 (1999) 1285–1291. [DOI] [PubMed] [Google Scholar]
- [129].Takase Y, Lévesque MH, Luu-The V, et al. , Expression of enzymes involved in estrogen metabolism in human prostate, J. Histochem. Cytochem 54 (2006) 911–921, 10.1369/jhc.6A6927.2006. [DOI] [PubMed] [Google Scholar]
- [130].Patel S, Disruption of aromatase homeostasis as the cause of a multiplicity of ailments: a comprehensive review, J. Steroid Biochem. Mol. Biol 168 (2017) 19–25, 10.1016/j.jsbmb.2017.01.009. [DOI] [PubMed] [Google Scholar]
- [131].Patel S, Homaei A, Raju AB, et al. , Estrogen: the necessary evil for human health, and ways to tame it, Biomed. Pharmacother 102 (2018) 403–411, 10.1016/j.biopha.2018.03.078. [DOI] [PubMed] [Google Scholar]
- [132].Chandhoke G, Shayegan B, Hotte SJ, Exogenous estrogen therapy testicular cancer and the male to female transgender population: a case report, J. Med. Case Rep 12 (2018), 10.1186/s13256-018-1894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Sabale PM, Sabale VP, Potey LC, et al. , Aromatase and aromatase inhibitors in breast cancer treatment Prafulla, Curr. Pharm. Res 9 (2018) 2636–2655. [Google Scholar]
- [134].Santoro N, Epperson CN, Mathews SB, Menopausal symptoms and their management, Endocrinol. Metab. Clin. North Am 44 (2015) 497–515, 10.1016/j.ecl.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Thornton MJ, Estrogens and aging skin, Dermatoendocrinol 5 (2013) 264–270, 10.4161/derm.23872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Emmerson E, Hardman MJ, The role of estrogen deficiency in skin ageing and wound healing, Biogerontology 13 (2012) 3–20, 10.1007/s10522-011-9322-y. [DOI] [PubMed] [Google Scholar]
- [137].Bosworth HB, Bastian LA, Kuchibhatla MN, et al. , Depressive symptoms, menopausal status, and climacteric symptoms in women at midlife, Psychosom. Med 63 (2001) 603–608, 10.1097/00006842-200107000-00013. [DOI] [PubMed] [Google Scholar]
- [138].Macova M, Armando I, Zhou J, et al. , Estrogen reduces aldosterone, upregulates adrenal angiotensin II AT2 receptors and normalizes adrenomedullary Fra-2 in ovariectomized rats, Neuroendocrinology 88 (2009) 276–286, 10.1159/000150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Caroccia B, Seccia TM, Barton M, et al. , Estrogen signaling in the adrenal cortex: implications for blood pressure sex differences, Hypertension 68 (2016) 840–848, 10.1161/hypertensionaha.116.07660. [DOI] [PubMed] [Google Scholar]
- [140].Liu T, Huang Y, Lin H, Estrogen disorders: interpreting the abnormal regulation of aromatase in granulosa cells (Review), Int. J. Mol. Med 47 (2021), 10.3892/ijmm.2021.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Kwintkiewicz J, Cai Z, Stocco C, Follicle-stimulating hormone-induced activation of Gata4 contributes in the up-regulation of Cyp19 expression in rat granulosa cells, Mol. Endocrinol 21 (2007) 933–947, 10.1210/me.2006-0446. [DOI] [PubMed] [Google Scholar]
- [142].Overes HWTM, de Leeuw R, Kloosterboer HJ, Regulation of aromatase activity in FSH-primed rat granulosa cells in vitro by follicle-stimulating hormone and various amounts of human chorionic gonadotrophin, Hum. Reprod 7 (1992) 191–196, 10.1093/oxfordjournals.humrep.a137615. [DOI] [PubMed] [Google Scholar]
- [143].Chan HJ, Petrossian K, Chen S, Structural and functional characterization of aromatase, estrogen receptor, and their genes in endocrine-responsive and -resistant breast cancer cells, J. Steroid Biochem. Mol. Biol 161 (2016) 73–83, 10.1016/j.jsbmb.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Atkins H, A comparison of adrenalectomy and oophorectomy with hypophysectomy in the treatment of advanced cancer of the breast, J. R. Soc. Med 53 (1960) 638–641, 10.1177/003591576005300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Cash R, Brough AJ, Cohen MNP, et al. , Aminoglutethimide (Elipten-Ciba) as an inhibitor of adrenal steroidogenesis: mechanism of action and therapeutic trial, J. Clin. Endocrinol. Metab 27 (1967) 1239–1248. [DOI] [PubMed] [Google Scholar]
- [146].Griffiths CT, Hall TC, Saba Z, et al. , Preliminary trial of aminoglutethimide in breast cancer, Cancer 32 (1973) 31–37, . [DOI] [PubMed] [Google Scholar]
- [147].Santen RJ, Santner S, Davis B, et al. , Aminoglutethimide Inhibits Extraglandular Estrogen Production in Postmenopausal Women with Breast Carcinoma, 1978. [DOI] [PubMed]
- [148].Santen RJ, Worgul TJ, Samojlik E, et al. , A randomized trial comparing surgical adrenalectomy with aminoglutethimide plus hydrocortisone in women with advanced breast cancer, N. Engl. J. Med 305 (1981) 545–551. [DOI] [PubMed] [Google Scholar]
- [149].Santen RJ, Brodie H, Simpson ER, et al. , History of aromatase: Saga of an important biological mediator and therapeutic target, Endocr. Rev 30 (2009) 343–375, 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- [150].Cunningham D, Powles TJ, Dowsett M, et al. , Oral 4-hydroxyandrostenedione, a new endocrine treatment for disseminated breast cancer, Cancer Chemother. Pharmacol 20 (1987) 253–255. [DOI] [PubMed] [Google Scholar]
- [151].Coombes RC, Goss P, Dowsett M, et al. , 4-Hydroxyandrostenedione in treatment of postmenopausal patients with advanced breast cancer, Lancet 1 (1984) 1237–1239. [DOI] [PubMed] [Google Scholar]
- [152].Chumsri S, Howes T, Bao T, et al. , Aromatase, aromatase inhibitors, and breast cancer, J. Steroid Biochem. Mol. Biol 125 (2011) 13–22, 10.1016/j.jsbmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Jonat W, Hilpert F, Kaufmann M, Aromatase inhibitors: a safety comparison, Expert Opin. Drug Saf 6 (2007) 165–174, 10.1517/14740338.6.2.165. [DOI] [PubMed] [Google Scholar]
- [154].Hong S, Didwania A, Olopade O, et al. , The expanding use of third-generation aromatase inhibitors: what the general internist needs to know, J. Gen. Intern. Med 24 (2009), 10.1007/s11606-009-1037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Hong Y, Chen S, Aromatase inhibitors: Structural features and biochemical characterization, in: Ann. N. Y. Acad. Sci, Blackwell Publishing Inc, 2006, pp. 237–251, 10.1196/annals.1386.022. [DOI] [PubMed] [Google Scholar]
- [156].Wendler R, Diagnosed with breast cancer after menopause? Aromatase inhibitors can help, (2022). https://www.mdanderson.org/cancerwise/diagnosed-with-breast-cancer-after-menopause–aromatase-inhibitors-can-help.h00–159542112.html (accessed March 3, 2023).
- [157].Polyzos NP, Fatemi HM, Zavos A, et al. , Aromatase inhibitors in post-menopausal endometriosis, Reprod. Biol. Endocrinol 9 (2011), 10.1186/1477-7827-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Patwardhan S, Nawathe A, Yates D, et al. , Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis, BJOG Int. J. Obstet. Gynaecol 115 (2008) 818–822, 10.1111/j.1471-0528.2008.01740.x. [DOI] [PubMed] [Google Scholar]
- [159].Ferrero S, Gillott DJ, Venturini PL, et al. , Use of aromatase inhibitors to treat endometriosis-related pain symptoms: a systematic review, Reprod. Biol. Endocrinol 9 (2011), 10.1186/1477-7827-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Quaas AM, Weedin EA, Hansen KR, On-label and off-label drug use in the treatment of endometriosis, Fertil. Steril 103 (2015) 612–625, 10.1016/j.fertnstert.2015.01.006. [DOI] [PubMed] [Google Scholar]
- [161].Sorscher S, First A, Case of male breast cancer responding to combined aromatase inhibitor/palbociclib therapy, Int. J. Cancer Clin. Res 3 (2016) 69. [Google Scholar]
- [162].Foglietta J, Inno A, de Iuliis F, et al. , Cardiotoxicity of aromatase inhibitors in breast cancer patients, Clin. Breast Cancer 17 (2017) 11–17, 10.1016/j.clbc.2016.07.003. [DOI] [PubMed] [Google Scholar]
- [163].Nielsen RH, Christiansen C, Stolina M, et al. , Oestrogen exhibits type II collagen protective effects and attenuates collagen-induced arthritis in rats, Clin. Exp. Immunol 152 (2008) 21–27, 10.1111/j.1365-2249.2008.03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Abdulhaq H, Geyer C, Safety of adjuvant endocrine therapy in postmenopausal women with breast cancer, Am. J. Clin. Oncol. Cancer Clin. Trials 31 (2008) 595–605, 10.1097/COC.0b013e31816d9171. [DOI] [PubMed] [Google Scholar]
- [165].Matthews A, Stanway S, Farmer RE, et al. , Long term adjuvant endocrine therapy and risk of cardiovascular disease in female breast cancer survivors: systematic review, BMJ 363 (2018), 10.1136/bmj.k3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Kelly E, Lu CY, Albertini S, et al. , Longitudinal trends in utilization of endocrine therapies for breast cancer: an international comparison, J. Clin. Pharm. Ther 40 (2015) 76–82, 10.1111/jcpt.12227. [DOI] [PubMed] [Google Scholar]
- [167].Borgo MV, Lopes AB, Gouvêa SA, et al. , Effect of tamoxifen on the coronary vascular reactivity of spontaneously hypertensive female rats, Braz. J. Med. Biol. Res 44 (2011) 786–792, 10.1590/S0100-879x2011007500099. [DOI] [PubMed] [Google Scholar]
- [168].Lin C, Chen LS, Kuo SJ, et al. , Adjuvant tamoxifen influences the lipid profile in breast cancer patients, Breast Care 9 (2014) 35–39, 10.1159/000358752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Owoade AO, Adetutu A, Ogundipe OO, et al. , Effects of tamoxifen administration on lipid profile in female albino rats, Asian J. Res. Biochem (2022) 10–22, 10.9734/ajrb/2022/v10i330224. [DOI] [Google Scholar]
- [170].Jacenik D, Cygankiewicz AI, Krajewska WM, The G protein-coupled estrogen receptor as a modulator of neoplastic transformation, Mol. Cell. Endocrinol 429 (2016) 10–18, 10.1016/j.mce.2016.04.011. [DOI] [PubMed] [Google Scholar]
- [171].Amir E, Seruga B, Niraula S, et al. , Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis, J. Natl. Cancer Inst 103 (2011) 1299–1309, 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- [172].Aydiner A, Meta-analysis of breast cancer outcome and toxicity in adjuvant trials of aromatase inhibitors in postmenopausal women, Breast 22 (2013) 121–129, 10.1016/j.breast.2013.01.014. [DOI] [PubMed] [Google Scholar]
- [173].Khosrow-Khavar F, Filion KB, Al-Qurashi S, et al. , Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: a systematic review and meta-analysis of randomized controlled trials, Ann. Oncol 28 (2017) 487–496, 10.1093/annonc/mdw673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Goldvaser H, Barnes TA, Seruga B, et al. , Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis, J. Natl. Cancer Inst 110 (2018) 31–39, 10.1093/jnci/djx141. [DOI] [PubMed] [Google Scholar]
- [175].Boszkiewicz K, Piwowar A, Petryszyn P, Aromatase inhibitors and risk of metabolic and cardiovascular adverse effects in breast cancer patients—a systematic review and meta-analysis, J. Clin. Med 11 (2022), 10.3390/jcm11113133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Yoo JJ, Jung EA, Kim Z, et al. , Risk of cardiovascular events and lipid profile change in patients with breast cancer taking aromatase inhibitor: a systematic review and meta-analysis, Curr. Oncol 30 (2023) 1831–1843, 10.3390/curroncol30020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Yu Q, Xu Y, Yu E, et al. , Risk of cardiovascular disease in breast cancer patients receiving aromatase inhibitors vs. tamoxifen: a systematic review and meta-analysis, J. Clin. Pharm. Ther 47 (2022) 575–587, 10.1111/jcpt.13598. [DOI] [PubMed] [Google Scholar]
- [178].Sun J-C, Sun Z-F, He C-J, et al. , Association between aromatase inhibitors and myocardial infarction morbidity in women with breast cancer: a meta-analysis of observational studies, Cancer Control 29 (2022) 1–11, 10.1177/10732748221132512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Kalender ME, Sevinc A, Camci C, et al. , Anastrozole-associated sclerosing glomerulonephritis in a patient with breast cancer, Oncology 73 (2008) 415–418, 10.1159/000136797. [DOI] [PubMed] [Google Scholar]
- [180].Puri P, Fadia M, Puri P, et al. , Letrozole-Induced acute interstitial nephritis, J. Clin. Med. Case Rep (2020) 1–3, 10.31487/j.jcmcr.2020.01.06. [DOI] [Google Scholar]
- [181].Almutlaq RN, Newell-Fugate AE, Evans LC, et al. , Aromatase inhibition increases blood pressure and markers of renal injury in female rats, Am. J. Physiol. Ren. Physiol 323 (2022) F349–F360, 10.1152/ajprenal.00055.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Öz OK, Hajibeigi A, Howard K, et al. , Aromatase deficiency causes altered expression of molecules critical for calcium reabsorption in the kidneys of female mice. In: J. Bone Miner. Res, 2007, pp. 1893–1902, 10.1359/jbmr.070808. [DOI] [PubMed] [Google Scholar]
- [183].Manigrasso MB, Sawyer RT, Marbury DC, et al. , Inhibition of estradiol synthesis attenuates renal injury in male streptozotocin-induced diabetic rats, Am. J. Physiol. Ren. Physiol 301 (2011), 10.1152/ajprenal.00718.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Hassan N, Rashad M, Elleithy E, et al. , L-Carnitine alleviates hepatic and renal mitochondrial-dependent apoptotic progression induced by letrozole in female rats through modulation of Nrf-2, Cyt c and CASP-3 signaling, Drug Chem. Toxicol 46 (2023) 357–368, 10.1080/01480545.2022.2039180. [DOI] [PubMed] [Google Scholar]
- [185].Galal AAA, Mohamed AAR, Khater SI, et al. , Beneficial role of biochanin A on cutaneous and renal tissues of ovariectomized rats treated with anastrozole, Life Sci 201 (2018) 9–16, 10.1016/j.lfs.2018.03.037. [DOI] [PubMed] [Google Scholar]
- [186].Rossouw JE, Anderson GL, Prentice RL, et al. , Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial, J. Am. Med. Assoc 288 (2002) 321–333, 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- [187].Jazbutyte V, Stumpner J, Redel A, et al. , Aromatase inhibition attenuates desflurane-induced preconditioning against acute myocardial infarction in male mouse heart in vivo, PLoS One 7 (2012), 10.1371/journal.pone.0042032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Bayard F, Clamens S, Delsol G, et al. , Oestrogen synthesis, oestrogen metabolism and functional oestrogen receptors in bovine aortic endothelial cells., in: Ciba Found. Symp. 191 Non-Reproductive Actions Sex Steroids, Chichester, UK: John Wiley & Sons, Ltd, 1995: pp. 122–138. [DOI] [PubMed] [Google Scholar]
- [189].Bayard F, Clamens S, Meggetto F, et al. , Estrogen synthesis, estrogen metabolism, and functional estrogen receptors in rat arterial smooth muscle cells in culture, Endocrinology 136 (1995) 1523–1529, 10.1210/endo.136.4.7895662. [DOI] [PubMed] [Google Scholar]
- [190].Bell JR, Bernasochi GB, Varma U, et al. , Aromatase transgenic upregulation modulates basal cardiac performance and the response to ischemic stress in male mice, Am. J. Physiol. Hear. Circ. Physiol 306 (2014) 1265–1274, 10.1152/ajpheart.00012.2014. [DOI] [PubMed] [Google Scholar]
- [191].Iorga A, Li J, Sharma S, et al. , Rescue of pressure overload-induced heart failure by estrogen therapy, J. Am. Heart Assoc 5 (2016) 1–12, 10.1161/JAHA.115.002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Roselli CF, Brain aromatase: roles in reproduction and neuroprotection, J. Steroid Biochem. Mol. Biol 106 (2007) 143–150, 10.1016/j.jsbmb.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


