Abstract
Cryptosporidium parvum, which causes intractable diarrhea and lethal wasting in people with AIDS, occupies an unusual intracellular but extracytoplasmic niche. No reliable therapy for cryptosporidiosis exists, though the aminoglycoside paromomycin is somewhat effective. We report that paromomycin and the related compound geneticin manifest their major in vitro anti-C. parvum activity against intracellular parasites via a mechanism that does not require drug trafficking through the host cell cytoplasm. We used both normal and transformed aminoglycoside-resistant Caco-2 or MDBK cells in these studies. Timed-exposure experiments demonstrated that these drugs inhibit intracellular but not extracellular parasites. Apical but not basolateral exposure of infected cells to these drugs led to very significant parasite inhibition, indicating an apical topological restriction of action. We estimated intracytoplasmic concentrations of paromomycin, using an intracellular bacterial killing assay, and found that C. parvum infection did not lead to increased paromomycin concentrations compared to those in uninfected cells. Global [3H]paromomycin uptake by Caco-2 cells was ∼200-fold higher than the estimated intracytoplasmic paromomycin concentration, suggestive of host cell vesicular uptake and concentration (as has been reported with other cell lines). However, preinfection exposure of Caco-2 cells to paromomycin did not result in subsequent inhibition of parasite development, indicating that if exogenous paromomycin enters the infected host cell vesicular compartment, it does not effectively communicate with the parasite. Thus, the apical membranes overlying the parasite and parasitophorous vacuole may be the unsuspected major route of entry for paromomycin and may be of importance in the design and discovery of novel drug therapies for the otherwise untreatable C. parvum.
Cryptosporidiosis in immunocompromised hosts can be a devastating and fatal disease (5). Over 100 antiparasitic and antibacterial compounds have been reported to be ineffective or marginally effective in this infection (11, 20, 27). Paromomycin, an aminoglycoside antibiotic, is one of the few agents found to have modest activity against cryptosporidiosis in people with AIDS (PWA) (37). Paromomycin inhibits both bacterial and eukaryotic ribosomal protein synthesis, though eukaryotic organisms are 10 to 15 times less sensitive than prokaryotic organisms (39). Paromomycin is poorly absorbed from the intestinal tract (4), as more than 99% of an orally administered dose of the drug is excreted fecally (22), and does not enter the cytosol of eukaryotic cells to any appreciable extent (6). These facts constitute a potential conundrum, however, for there is no published evidence supporting the notion that orally administered paromomycin inhibits or kills Cryptosporidium parvum when the parasite is freely extracellular in the lumen of the gut.
The intracellular location of C. parvum is unusual, as this eukaryote is both intracellular and extracytoplasmic (34, 35). After making contact with a host cell, the infectious sporozoites or merozoites are enveloped by host apical membranes with a rim of host cytoplasm between them. The space between the parasite and the host membranes becomes a parasitophorous vacuole. Unlike related parasites such as Toxoplasma, Plasmodium, Eimeria, and Cyclospora, which once intracellular are completely surrounded by the parasitophorous vacuole (23, 35), Cryptosporidium basal membranes fuse with host membranes. Thus, the parasitophorous vacuole extends only over the apical domain of the parasite, and the basal parasite domain is bound by a fused parasite and host membrane, termed the feeder organelle membrane. It becomes redundant and folded during parasite maturation, increasing its surface area for possible nutrient uptake or transfer. Beneath this fused membrane lie electron-dense bands, which extend disk-like beneath the parasite and lie within the host cytoplasm and have been shown by electron microscopy to intersect the host cell apical membranes. Their role is unknown, though they may serve an anchoring or sieving function.
In this study we report that both paromomycin and a related aminoglycoside antibiotic, geneticin, are able to inhibit the growth of intracellular C. parvum in a dose- and time-dependent fashion in Caco-2 cells. In contrast to paromomycin, geneticin more freely enters host cells and will kill eukaryotic cells in the absence of an inactivating enzyme or altered drug target. Thus, a number of experiments were conducted with transformed Caco-2 cells that express aminoglycoside phosphotransferase (APH), which inactivates geneticin and paromomycin (3). Using this system, we were able to demonstrate that the effects of these drugs on the parasite are not likely to involve trafficking through the host cytosol. As described below, we have also found that cells incubated with paromomycin before infection were still excellent hosts for C. parvum, indicating that other potential intracellular compartments, such as the vesicular system, do not effectively communicate with the parasite. We found that apical but not basolateral geneticin exposure affected the parasite, as did apical but not basolateral exposure to paromomycin. These results extend our prior finding that there is an apical cell defect or alteration in C. parvum-infected Caco-2 cells (21). We postulate on the basis of these data that the host-derived membranes that overlie the parasite may allow extracellular geneticin and paromomycin to enter the parasitophorous vacuole and thus gain entry into the intracellular parasite. Understanding this route of entry may be of importance in the design or discovery of effective drug therapy for cryptosporidiosis.
MATERIALS AND METHODS
Cell cultures.
Caco-2 and MDBK-F5D2 cells were grown in 75-cm2 plastic flasks (Costar, Arlington, Mass.) in an 8% CO2 atmosphere at 37°C with 20% fetal calf serum (FCS; Gibco, Grand Island, N.Y.) in Dulbecco’s modified Eagle medium (DMEM; Gibco) with 3.5 g of glucose per liter, 50 U of penicillin, and 50 μg of streptomycin per ml, as per our prior description (21). Passage was effected by washing flask monolayers with Hank’s balanced salt solution without calcium and magnesium and incubating with 0.05% trypsin and 0.53 mM EDTA in the same solution. Released cells were pelleted in complete medium and split 1:3 into new flasks. Geneticin (G418) was purchased from Sigma, and paromomycin (Parke-Davis, Morris City, N.J.) was purchased commercially.
Transformation of Caco-2 cells with the pCΔj-SV2 plasmid and construction of the APH-Caco-2 cell line.
Parental Caco-2 cells were transfected with pCΔj-SV2, a mammalian expression vector that encodes an APH activity (33), by the calcium phosphate precipitation method (24). Initial selection was performed using 800 μg of geneticin per ml, and drug-resistant colonies were isolated with glass cloning cylinders (13). A single clone was selected and expanded for all of the described experiments which utilized this transformant. We refer to this clone of cells as the APH-Caco-2 cell line because of their ability to synthesize APH.
Isolation and preparation of C. parvum.
The isolate GCH1 (Grafton Cryptosporidium human isolate 1, obtained from an AIDS patient), used in these studies, is described elsewhere (36). It was propagated in experimentally infected calves from which oocysts were purified and concentrated (10, 36). Oocysts from the same animal were used in each experiment. Feces with oocysts were pooled, homogenized, and coarsely filtered; the material then underwent two Sheather’s sugar flotations and a Percoll step gradient purification step. All further manipulations were done on ice or at 4°C with prechilled solutions to prevent premature excystation and loss of sporozoite infectivity (31).
Oocysts were suspended in ice-cold 10% commercial bleach solution in distilled water (0.545% sodium hypochloride) for 10 min, washed twice with a 50-fold excess of ice-cold distilled water, and enumerated in a hemacytometer before use. Oocyst suspensions of 107/ml were serially diluted with tissue culture media. For some experiments, control suspensions containing the same number of oocysts were subjected to two rapid cycles of freezing (15 min on dry ice) and thawing (15 min incubation in a 37°C water bath) to disrupt oocysts and inactivate sporozoites. We have verified that this process effectively disrupted oocysts (21). Excystation was monitored by parallel incubations in tissue culture media at 37°C. Four sporozoites are released from every viable oocyst (34).
Preparation of sporozoites.
Oocysts (2 × 106) were resuspended in Leibowitz L-15 medium containing 0.75% taurocholic acid and incubated at 37°C for 45 min, with occasional shaking. Sporozoites were centrifuged at 3,000 × g in a Sorvall RC3B centrifuge at 4°C for 20 min. The supernatant was aspirated, and the sporozoites were resuspended in tissue culture medium at 1 × 106 to 2 × 106/ml. For some experiments, up to 107 oocysts were allowed to excyst, and the sporozoites were purified from the mixture of oocyst remnants and sporozoites by syringe filtration (2-μm pore size).
Inoculation of monolayers with parasites.
Cells (2 × 105) were seeded atop 12-mm-diameter Costar Transwell permeable polycarbonate filters (0.4-μm pore size) after filters were wet with complete medium for ≥30 min and grown to confluence. This procedure allowed separate access to apical and basolateral reservoirs. Medium was replaced the day after seeding and two to three times per week thereafter with medium with 10% FCS unless stated otherwise (21). In other experiments, 1 × 105 to 2 × 105 cells were seeded in each 0.64-cm2 well of 8-well LabTek slides and maintained under the same conditions as monolayers on filters. Confluent monolayers were inoculated with 100 μl containing from 104 to 106 viable oocysts, 400 μl containing 106 sporozoites, 106 freeze-thawed oocysts, or tissue culture medium. Inocula were added to 500 μl of complete tissue culture medium in the apical reservoir of the Transwell chamber of a LabTek slide; basolateral reservoirs held 1.5 ml of complete medium. Infected and control monolayers were subsequently maintained in culture for 1 to 3 days.
Transmonolayer electrical resistance.
Monolayers grown in Transwell chambers were washed in fresh DMEM without FCS and immediately allowed to equilibrate for at least 20 min in their usual incubator. To measure resistance, calomel electrodes were connected to the apical and basolateral reservoirs of the Transwell chambers via 3% agar bridges (flexible plastic tubing) filled with Ringer’s solution and joined to a dual-channel voltage clamp device (University of Iowa). The spontaneous potential difference (E0) was subtracted from the potential difference seen during the passage of a 0.100-mA current (E100 − E0), and the resistance of the monolayer was calculated with Ohm’s law and expressed in ohms · centimeter2. The mean resistance of a group of blank, unseeded chambers was subtracted from the individual resistances of experimental monolayers to obtain the resistance due to the cell monolayers (21). Caco-2 cell monolayers were used in these experiments after 7 or more days of culture; APH-Caco-2 cells were used after 14 days of culture, as preliminary data had indicated that a stable transmonolayer resistance was obtained after 10 days.
Indirect immunofluorescence assay (IFA) for detection of Cryptosporidium parasites.
Parasites were detected by methods we have described previously (21). Briefly, cell monolayers atop filters or in 8-well slides were washed with plain DMEM and fixed with absolute methanol. Nonspecific binding was blocked by incubation of the monolayers with phosphate-buffered saline (PBS) containing 1% normal goat serum (NGS; Sigma) for 30 min. Blocked monolayers were then incubated with 50 μl of a 1:1,000 dilution of a polyclonal anti-Cryptosporidium rabbit antiserum in 1% NGS. After 30 min, monolayers were washed thrice in PBS and further incubated with 50 μl of a 1:1,000 dilution of fluorescein isothiocyanate-labeled goat anti-rabbit antibody (Cappel) in 1% NGS for 30 min. Monolayers on slides were mounted with coverslips with 1 mg of phenylenediamine (Sigma) per ml in 90% glycerol to retard the quenching of fluorescence (21). Monolayers on filters were dried, wet on the basolateral surface with the same mounting solution, cut out of the Transwell plastic support with a scalpel, and mounted under coverslips as described above. Monolayers were examined by standard fluorescence microscopy.
Assessment of cell viability and permeability by fluorescein diacetate (FDA) and propidium iodide (PI) vital staining.
Intact monolayers on permeable filters were stained with FDA-PI after transmonolayer electrical resistance had been measured. Two hundred microliters of 1:1 DMEM and FCS, containing 0.1 mg of FDA per ml and 0.03 mg of PI per ml, was added to the apical (or 1.5 ml was added to the basolateral) reservoir after the apical or basolateral contents were aspirated. Monolayers were exposed to the vital stains for 2 min at 37°C and then placed at 4°C. Monolayers were fixed with absolute methanol for 10 min at 4°C, mounted under coverslips as described above, and observed under fluorescent illumination at 523 nm. We have previously shown that the number of Caco-2 cells that stain intensely with PI is directly correlated with the number of infecting parasites (21).
LDH assays.
Apical and basolateral reservoirs of infected and control monolayers were individually aspirated and assayed for lactase dehydrogenase (LDH) activity at the time of resistance measurement. Aspirated media and controls were placed on ice, transported to the clinical chemistry laboratory of St. Elizabeth’s Medical Center of Boston, Boston, Mass., and assayed with a commercial clinical chemistry laboratory analyzer (Kodak). Enzyme activity was expressed in international units per liter. We have previously shown that apical reservoir LDH release after infection with the GCH1 parasite isolate is highly correlated with the number of infectious parasites and is a more sensitive marker of infection than transmonolayer resistance (TMR) changes (21).
Extracellular EIEC inhibition assay.
Logarithmic-phase enteroinvasive Escherichia coli (EIEC) EI-34 bacterial cells (the kind gift of A. Donohue-Rolfe) were diluted in Luria-Bertani broth to a density of 5 × 104 to 6 × 104 CFU/ml. A 0.9-ml portion of this culture was added to 0.1 ml of PBS containing various concentrations of paromomycin. The mixture of bacteria and paromomycin was incubated at 37°C, with constant shaking. The number of CFU at the end of 3 h was measured by the limiting dilution plating method. Bacterial growth in paromomycin was expressed as a percentage of CFU in medium without paromomycin, and a standard curve was generated by plotting the mean percentage of CFU against the concentration of paromomycin.
Intracellular EIEC inhibition assay.
The effective intracellular paromomycin concentration in Caco-2 cells was measured by a modification of the bacterial invasion assay of Donnenberg et al. (16). Logarithmic-phase EIEC cells were washed in PBS and resuspended in tissue culture medium at 6 × 107 to 8.0 × 107 CFU/ml. Confluent monolayers of Caco-2 cells grown in 8-well slides were washed once with PBS, pH 7.4, and covered with 200 μl of EIEC suspension. In order to increase the contact between the bacteria and the Caco-2 monolayer, 8-well slides were placed in a microplate carrier and centrifuged in a Beckman model TJ6 centrifuge at 25 × g for 10 min. Plates were incubated for 3 h at 37°C in 8% CO2, washed three times with PBS, and incubated for 3 h in medium containing 0, 500, 1,000, or 2,000 μg of paromomycin per ml. In samples not exposed to paromomycin, 10 μg of gentamicin per ml was added to kill extracellular EIEC. Since gentamicin at this concentration does not enter epithelial cells, only the extracellular bacteria were killed, and the intracellular bacteria were able to grow (16). The monolayers were then washed with PBS, and the Caco-2 cells were lysed with 200 μl of 1% Triton X-100 in PBS for 20 min to liberate intracellular bacteria. In the presence of PBS, this concentration of Triton X-100 does not affect bacterial viability (15–17). Surviving bacteria were counted by plating 100 μl of serially diluted Triton X-100 lysate. The intracellular bacterial growth in hosts exposed to paromomycin was expressed as a percentage of the CFU of bacteria grown in cells in the absence of paromomycin. When we used C. parvum-infected Caco-2 cells, the monolayers were infected with 5 × 105 oocysts/monolayer on LabTek 8-well slides for 3 h and washed thrice with PBS before being exposed to 200 μl of EIEC suspension.
[3H]leucine incorporation assay.
[3H]leucine incorporation by confluent Caco-2 cells grown on Transwell permeable filters was assayed as described previously (25). Briefly, medium containing the aminoglycoside (paromomycin at 2,000 μg/ml, geneticin at 1,000 μg/ml) was placed in the apical or basolateral reservoir for 24 h before the assay. The cells were then washed with PBS, and leucine-free medium was added to both of the reservoirs. The leucine-free medium contained the relevant aminoglycoside on the same (apical or basolateral) side. [3H]leucine (2 μCi or 40 to 50 Ci/mmol) was added to the apical reservoir and incubated for 1 h at 37°C in an 8% CO2 atmosphere. [3H]leucine incorporated into cellular proteins was measured by a method that involved trichloroacetic acid precipitation onto glass filters and was expressed as counts per minute. Radioactivity was measured with a Beckman LS 6000 SE scintillation counter.
Uptake of [3H]paromomycin by Caco-2 cells.
Tritiated paromomycin was synthesized by the method of Capmau et al. (9). [3H]paromomycin was separated from other reaction components on a silica gel column as described previously. We achieved yields of ∼25% in several syntheses and specific activities of ∼1.14 × 104 cpm/μg of paromomycin.
Replicate uninfected or infected Caco-2 cells grown on LabTek 8-well glass slides were incubated with [3H]paromomycin at concentrations of 0 to 2,000 μg/ml for 0, 2, 4, and 6 h. In some experiments, after being washed thrice with medium, cells were infected as described earlier. LDH release and parasite numbers, as judged by indirect fluorescent-antibody assay (IFA), were assessed at 24 and 48 h after infection. [3H]paromomycin cellular uptake was assessed by standard means, counting cellular lysates after the removal of medium and washing.
Estimation of Caco-2 cellular volume.
We crudely estimated the volume of Caco-2 host cells based on the area covered by 4 × 105 Caco-2 cells (0.64 cm2) in LabTek wells and the average range of cell heights on LabTek slides in unpublished confocal microscopy studies (21a) (2 to 4 μm, with a rough mean height of ∼3 μm). Thus, we have crudely estimated the volume of 4 × 105 Caco-2 cells grown on LabTek slides to be ∼0.192 μl. We are unaware of any independent, more rigorous published volume estimates for Caco-2 cells, despite an exhaustive search of the literature (>1,180 abstracts were reviewed in a Medline search).
Statistical analysis.
Data were analyzed with the Statistical Package for the Social Sciences (SPSS) for Windows operating in OS/2 or Windows NT. When monolayers were infected, representative groups were measured before infection and then daily during infection. The sizes of the representative groups varied between 3 and 12, depending upon the specific experimental goals. When multiple comparisons were conducted, individual groups had to be statistically significantly different with two-tailed t tests by Tukey’s Honestly Significant Difference test and by Scheffé’s test before we would accept the means of group values as being truly different. F ratios and P values were calculated for linear regressions. Linear correlations (least-squares fit) of data are generally reported with the more rigorous correlation coefficient of determination R2 rather than the correlation statistic R. Experiments were repeated at least three times.
RESULTS
Geneticin (G418) inhibits the growth of C. parvum in APH-Caco-2 cells.
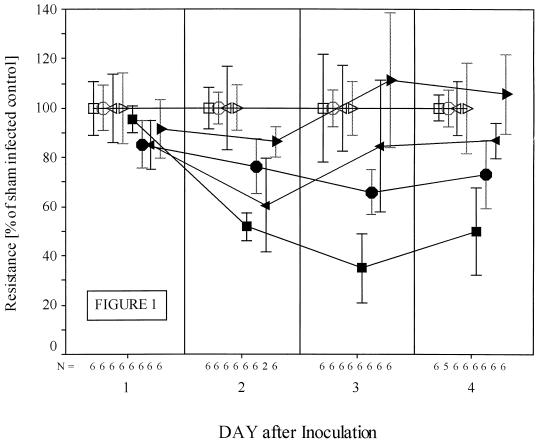
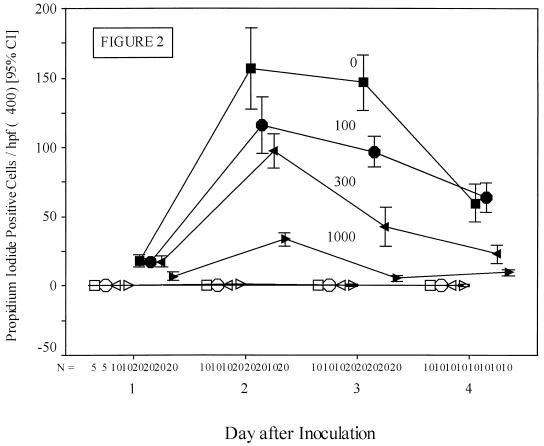
The TMR of replicate monolayers of APH-Caco-2 cells grown on Transwell filters was measured over time after inoculation with 106 oocysts in the presence and absence of G418. There was a dose-dependent protective effect of G418 against a C. parvum-induced fall in TMR, which in regression analysis was highly significant (Fig. 1). We have previously shown that infected cells become permeable to PI (21). The number of host cells that became highly stained with PI was inversely related to the concentration of G418 in the media (Fig. 2). One day after inoculation, there was a statistically significant fall in the number of PI-positive cells in the monolayers exposed to 1,000 μg of G418 per ml compared to any of the other infected groups (6.95 ± 1.30 [mean ± standard deviation] versus 17.55 ± 1.91 PI-positive cells per high-power field; 1,000 versus 300 μg of G418 per ml; n = 20 each group; P < 0.001). This protective effect of G418 was more evident on days 2, 3, and 4 after infection, with a clear incremental effect of dose over time (Fig. 2). This protective effect was again highly significant on days 2, 3, and 4 (F ratios = 80.92, 127.80, and 110.51, respectively; P < 0.0001 on each day).
FIG. 1.
Effect of geneticin on APH-Caco-2 (pCΔj-SV2 plasmid-transformed) cells. Replicate monolayers of transformed cells were grown atop Transwell permeable membranes as outlined in Materials and Methods. Geneticin was included in the medium until the day before infection to ensure high levels of expression of APH. Monolayers were infected with 106 C. parvum oocysts that were either viable (solid symbols) or freeze-thaw inactivated (open symbols). At the time of infection, medium containing 0, 100, 300, or 1,000 μg of geneticin per ml was used to sustain the cells. The mean TMR of groups of freeze-thaw-infected monolayers was normalized to 100% for each concentration of geneticin. Squares, 0; circles, 100; left-pointing triangles, 300; and right-pointing triangles, 1,000 μg of geneticin per ml. Bars represent confidence intervals in the 95th percentile. Each experimental group generally comprised six monolayers (see figure). Exposure to geneticin was very highly protective against a parasite-induced fall in TMR in regression analysis (e.g., on day 3, F = 21.36, P = 0.0001; on day 4, F = 17.80, P = 0.0004). Otherwise, infection without exposure to geneticin led to highly significant falls in TMR compared to TMR in sham-infected controls (e.g., day 3, 63.1 ± 9.8 versus 180.7 ± 15.3 ohms · cm2 [mean ± standard error of the mean]; n = 6 each group; P < 0.001).
FIG. 2.
PI-stained host cells in C. parvum-infected monolayers exposed to various concentrations of geneticin. Monolayers of APH-Caco-2 (pCΔj-SV2 plasmid-transformed) cells were infected and stained with PI as outlined in Materials and Methods. The number of PI-positive cells per ×400 visual field was quantitated and plotted over time by concentration of geneticin. Monolayers were infected with 106 C. parvum oocysts that were either viable (solid symbols) or freeze-thaw inactivated (open symbols). Squares, 0; circles, 100; left-pointing triangles, 300; and right-pointing triangles, 1,000 μg of geneticin per ml. Bars, confidence intervals (CI) in the 95th percentile. hpf, high-power field.
Apical reservoir concentrations of LDH, an intracellular enzyme that is released during cell death, were also very strongly inversely correlated with the concentration of G418 in a dose-related fashion. Two days after infection in the experiment shown in Fig. 1, the apical reservoir concentrations of LDH in monolayers exposed to 0, 100, 300, and 1,000 μg of G418 were 1,042 ± 64, 765 ± 84, 574 ± 46, and 317 ± 20 IU/liter, respectively, more than that found in sham-infected monolayers (F ratio = 47.81; P < 0.0001). On day 4 after infection, the protective effect of G418 was still highly significant (F ratio = 11.42; P = 0.0028). The number of parasites detectable per ×400 high-power visual field by IFA also decreased in the presence of G418. On monolayers infected with 106 oocysts, exposure of the cells and parasites to 1,000 μg of G418 per ml resulted in 136 ± 8, 199 ± 24, 189 ± 6, and 172 ± 15 parasites, respectively, at 24, 48, 72, and 96 h after inoculation. In contrast, in identically infected control monolayers not exposed to G418, 239 ± 23, 439 ± 25, 647 ± 65, and 213 ± 10 parasites, respectively, were seen 24, 48, 72, and 96 h after inoculation. This decreased numbers of parasites detected in the G418-exposed group were statistically significant (P = 0.001 at 24 h, P < 0.001 at 48 h, and P = 0.002 at 72 h).
Thus, by every criterion we and others have identified (TMR, apical LDH release, propidium iodide staining of host cell nuclei, and actual number of parasites) (1, 21), the aminoglycoside G418 inhibited parasite growth and its deleterious effects on host cells in a transformed aminoglycoside-resistant Caco-2 cell line.
G418 and paromomycin inhibit intracellular parasites.
APH-Caco-2 cells grown on 8-well LabTek slides were infected with 2 × 105 parasites in the presence or absence of 1,000 μg of G418 per ml. Three hours after the inoculation, the medium was replaced by fresh medium that did or did not contain the same concentration of G418. Eleven hours after inoculation, and 8 h after the initial medium change, a second medium change (with or without G418) was conducted in selected groups. These time points (3 and 11 h after inoculation) were chosen so as to isolate the first intracellular stage of the parasite, after invasion has occurred but before schizogony and merozoite release. It is known that after cell inoculation, a period of approximately 14 to 17 h is needed before the first asexual cycle of reproduction results in the release of extracellular merozoites (12). LDH release was assayed at 48 h to assess the inhibitory effects of G418 on cell death induced by C. parvum when the drug was administered in this timed fashion.
The presence of G418 during the first 3 h of infection, when the process of excystation, extracellular attachment and invasion, and initial intracellular growth are found, did not lead to any significant decrease in the release of LDH induced by the parasite (Table 1, groups 1a, 1b, 2a, and 2b). In contrast, monolayers treated with G418 throughout the experiment (0 to 48 h) or during the period from hour 3 to 48 had a marked reduction in LDH release compared to untreated controls (groups 1a, 1b, 4a, and 4b; P < 0.001 for each comparison). LDH release in monolayers exposed to G418 during the period from hours 3 to 11, when only the first intracellular stage was present, was midway between the LDH release seen in infected monolayers that were not exposed to G418 and that in infected monolayers exposed to G418 for periods from 3 to 48 h or 0 to 48 h (groups 1b, 3a, 3b, and 4a; P < 0.001 for all comparisons). We found no difference between the LDH release in monolayers exposed during the period 0 to 11 h and that in monolayers exposed during the period 3 to 11 h (groups 3b and 4b). In sum, we found that exposure of the infected monolayer during the period of the first intracellular parasite stage led to highly significant decreases in host cell LDH release and that extension of the G418 exposure period beyond 11 to 48 h led to further decreases in LDH release (Table 1). Similar results were obtained whether we grew the cells on permeable membranes or glass slides (data not shown).
TABLE 1.
Effect on C. parvum-induced release of LDH from APH-transformed cells by timed exposure to 1,000 μg of geneticin (G418) per ml
| Group no.a | Exposure conditions | LDH in IU/liter (mean ± SEM) | Significance compared to untreated infected control |
|---|---|---|---|
| 1a | Infected, no G418 treatment, medium not changed at 11 h | 808 ± 31 | |
| 1b | Infected, no G418 treatment, medium changed at 11 h | 792 ± 22 | |
| 2a | G418 during inoculation, removed at 3 h, medium not changed at 11 h (0–3 h) | 803 ± 48 | P > 0.05 (not significantly different) |
| 2b | G418 during inoculation, removed at 3 h, medium changed at 11 h (0–3 h) | 823 ± 32 | P > 0.05 (not significantly different) |
| 3a | G418 after 3 h, medium not changed at 11 h (3–48 h) | 393 ± 14 | P < 0.001 versus 1a |
| 3b | G418 after 3 h, medium changed at 11 h (3–11 h) | 548 ± 17 | P < 0.001 versus 1b, P < 0.001 versus 3a, and P<0.001 versus 4a |
| 4a | G418 at all times (0–48 h) | 451 ± 12 | P < 0.001 versus 1a |
| 4b | G418 until 11 h (washed at that time) (0–11 h) | 596 ± 15 | P < 0.001 versus 1a and P < 0.001 versus 4a |
| 5a | Uninfected control with G418 at all times (0–48 h) | 386 ± 21 | P < 0.001 versus 1a |
| 5b | Uninfected control with G418 until 11 h (0–11 h) | 386 ± 21 | P < 0.001 versus 1b |
| 6a | Uninfected control, no G418 at any time | 472 ± 18 | P < 0.001 versus 1a |
| 6b | Uninfected control, no G418 at any time, washed at 11 h | 472 ± 18 | P < 0.001 versus 1b |
n = 8 for all groups. Groups 3a, 4a, 5a, and 6a were not significantly different from one another by a Student-Newman-Keuls analysis of variance.
After performing the above-described experiments with timed exposure of infected APH-transformed cells to G418, we conducted similar timed-exposure experiments with paromomycin. It is known that a dose-response inhibitory relationship exists between paromomycin and the number of parasites detected at 48 h in the monolayer in a variety of cell lines, such as HT-29, Caco-2, and MDBK (18, 21). We found that treatment of infected cells with paromomycin during periods when only intracellular parasites are present inhibited parasite replication. Monolayers of cells were inoculated with parasites, washed at 2 h, and incubated with medium containing 2,000 μg of paromomycin per ml. Paromomycin exposure for as little as 2 h, during the period 2 to 4 h after inoculation, was sufficient to inhibit parasite replication and host cell LDH release nearly as much as 48 h of exposure (Table 2). This effect of paromomycin was found in both transformed and nontransformed Caco-2 and MDBK cells (data not shown).
TABLE 2.
Timed exposure of infected MDBK cells to 2,000 μg of paromomycin per ml
| Period of exposure (h)a | Mean no. ± SEM of parasites per ×100 field as detected by IFA at 48 h after infection |
|---|---|
| 0 (untreated infected control) | 510 ± 27 |
| 0–48 | 87 ± 9b |
| 2–4 | 136 ± 12b |
| 2–6 | 127 ± 8b |
| 2–8 | 128 ± 11b |
| 2–10 | 111 ± 12b |
| 2–48 | 110 ± 11b |
n = 16 for all groups.
Significantly different from value obtained for untreated control (P < 0.001).
Apical host cell exposure to G418 or to paromomycin was far more inhibitory to C. parvum than basolateral exposure.
APH-Caco-2 cells were grown atop permeable Transwell filters and infected with 106 oocysts after a stable TMR had been attained. Apical LDH levels were assessed during infection after apical, basolateral, apical and basolateral, or no exposure of the monolayers to 1,000 μg of G418 per ml. (In prior studies we have found that basolateral LDH levels do not become elevated during infection in this system (21), and in data not shown in this study we again found no increase in basolateral reservoir LDH levels during these studies of apical or basolateral drug exposure.) We found that apical drug exposure was far more inhibitory to the effects of C. parvum infection than basolateral exposure. For example, at 24 h, it was possible to rank order the amount of cellular LDH released by the G418 exposure group: uninfected, apical and basolateral sides, apical side, basolateral side, and no exposure (Table 3). Similar experiments were conducted with parental Caco-2 cells and 2,000 μg of paromomycin per ml, with comparable results that again demonstrated the effectiveness of apical exposure (Table 3). Thus, apical exposure to G418 (with APH-Caco-2 cells) or paromomycin (with parental Caco-2 cells), whether in conjunction with basolateral exposure or not, was highly effective at decreasing parasite-induced cell death, as measured by LDH release. In contrast, basolateral exposure was only marginally effective at preventing host cell death 24 h after infection. At 48 h after infection, when TMR changes are reliably seen after infection (21), this same pattern of apical protection was seen. Table 4 demonstrates the highly significant protective effects of apical paromomycin on TMR and host cell LDH release in Caco-2 cells at 48 h after infection.
TABLE 3.
Apical LDH release from Caco-2 or APH-Caco-2 cell monolayers 24 h after infection with 106 C. parvum oocysts and effects of apical, basolateral, apical plus basolateral, or no exposure to 2,000 μg of paromomycin per ml or 1,000 μg of geneticin per ml
| Drug exposure group | Mean ± SEM of apical LDH (IU/liter) released at 24 h after infection
|
|
|---|---|---|
| Paromomycin-exposed Caco-2 cellsa | Geneticinexposed APH-Caco-2 cellsb | |
| Control, no infection (n = 20 each)c | 470 ± 18 | 308 ± 6 |
| Infected, apical exposure alone (n = 5 each) | 651 ± 103 | 847 ± 78 |
| Infected, apical plus basolateral exposure (n = 5 each) | 844 ± 22 | 694 ± 28 |
| Infected, basolateral exposure alone (n = 5 each) | 1,739 ± 191 | 1,179 ± 23 |
| Infected, no exposure to drugs (n = 4 for geneticin, n = 5 for paromomycin) | 2,189 ± 93 | 1,795 ± 280 |
Multiple regression significance of apical exposure in preventing LDH release, P < 0.0001; for basolateral exposure, P = 0.3648 (not significantly different).
Multiple regression significance of apical exposure in preventing LDH release, P < 0.0001; for basolateral exposure, P = 0.0149.
Uninfected control monolayers also received no, apical, basolateral, or both-sided exposure to paromomycin or geneticin (n = 5 each group), but since there were no differences between these control groups, they are represented as a homogeneous group (n = 20).
TABLE 4.
Effects of apical, apical and basolateral, basal, or no exposure to 2,000 μg of paromomycin per ml on Caco-2 cell TMR and apical LDH release 48 h after inoculation with 106 C. parvum oocystse
| Type of paromomycin exposure | Uninfected control cellsc
|
Infected cellsd
|
||
|---|---|---|---|---|
| TMR in Ω·cm2 | Apical LDH release (IU/liter) | TMR in Ω·cm2 | Apical LDH release (IU/liter) | |
| None | 136 ± 6 | 322 ± 10 | 8 ± 3a | 4,434 ± 102a |
| Apical plus | 129 ± 11 | 349 ± 11 | 121 ± 11 | 1,681 ± 31a |
| Apical plus basolateral | 154 ± 17 | 428 ± 3 | 123 ± 12 | 1,467 ± 103a |
| Basolateral | 134 ± 14 | 411 ± 6 | 56 ± 8a,b | 3,280 ± 309a,b |
P ≤ 0.001 in individual t tests of uninfected control versus infected cells.
Infected cells exposed to basolateral paromomycin were significantly different from all other infected exposure groups (P ≤ 0.001 for all comparisons of TMR, and P ≤ 0.005 for all comparisons of apical LDH release.)
n = 4 for each group.
n = 6 for each group.
Results are means ± SEMs.
Paromomycin was also tested with the APH-Caco-2 cell line. The results of these experiments paralleled the results outlined above. At 48 h after infection with 106 oocysts, TMR declined by 68.8% in untreated APH-Caco-2 monolayers, 52.4% with basolateral paromomycin, 40.7% with apical and basolateral exposure, and 35.2% with apical paromomycin, compared to uninfected control monolayers also exposed to 2,000 μg of paromomycin per ml. Apical paromomycin was significantly protective against a decrease in resistance (P < 0.001), whereas basolateral exposure was not (P = 0.442). Similarly, in the same experiment, apical LDH release during infection was inhibited by apical paromomycin (1,880 ± 166 versus 4,216 ± 290 IU/liter; apical exposure versus no apical exposure, P < 0.001; n = 12 each group) but not by basolateral paromomycin (3,101 ± 402 versus 3,095 ± 468; P = 0.992 [not significant]; n = 12 each group).
Thus, apical exposure to paromomycin in either parental Caco-2 cells or APH-Caco-2 cells was far more inhibitory to parasite growth, parasite-induced falls in TMR, and eventual host cell death (as measured by LDH release) than basolateral exposure.
Sporozoite or excysting oocyst exposure to geneticin or paromomycin did not inhibit the parasite.
Oocysts were excysted for 45 min at 37°C in L-15 medium with 0, 500, or 1,000 μg of G418 per ml or with 0, 500, 1,000, or 2,000 μg of paromomycin per ml in a shaking water bath. The rate of excystation was 80%, and there were no significant differences in the rates of excystation between samples that were treated with different concentrations of the drugs (data not shown). The sporozoites were removed from drug-containing medium by centrifugation, resuspended in drug-free medium, and tested for infectivity on monolayers grown on LabTek slides. No detectable differences in the infectivity of the parasites could be found after this extracellular exposure either to G418 or to paromomycin, as measured by IFA parasite counts at 24 and 48 h postinfection. For example, in a representative experiment with paromomycin, the mean parasite counts were 991, 978, 1,081, 998, and 871 with 0, 250, 500, 1,000, and 2,000 μg of paromomycin per ml, respectively. LDH release from Caco-2 cell monolayers was also measured, and no decrease in LDH release was seen with increasing concentrations (3,113 ± 89, 3,202 ± 62, 3,151 ± 22, 3,811 ± 158, and 3,104 ± 73 IU/liter at 0, 250, 500, 1,000, and 2,000 μg/ml, respectively) of sporozoite paromomycin exposure (mean release, ∼3,276 ± 72 IU/liter at all concentrations, R = 0.0995 for LDH release by concentration [not significant]). Similar results were obtained with geneticin-exposed sporozoites (data not shown). These experiments were repeated with purified sporozoites (Materials and Methods) that had been filtered free from oocysts, and identical results were obtained for both geneticin and paromomycin (data not shown).
Paromomycin did not lead to significant inhibition of normal Caco-2 host cell protein synthesis, whereas apical G418 did.
Caco-2 cell monolayers were grown to confluence on permeable filters and exposed to either apical or basolateral paromomycin (2,000 μg/ml) or G418 (1,000 μg/ml). Leucine incorporation was measured by standard means (Materials and Methods). We found that only apical G418 led to any significant decrease (33.5%; 102,578 ± 7,183 cpm per monolayer [exposed] versus 153,575 ± 308 [control]; n = 6 in each group; P < 0.001) in leucine incorporation of host cells after 48 h of exposure to the drug. Basal exposure to G418 or apical or basal exposure to paromomycin did not lead to significant decreases in leucine incorporation (data not shown). These results for paromomycin were expected, since paromomycin does not kill Caco-2 cells, whereas geneticin kills Caco-2 cells unless they are APH transformed. We interpreted this data as suggesting that geneticin entry into normal Caco-2 cells was apically restricted.
Infection of Caco-2 cells with C. parvum did not lead to increased effective intracellular levels of paromomycin as measured by the killing of intracellular EIEC.
In order to measure the effective intracellular concentration of paromomycin, we utilized EIEC, which is sensitive to aminoglycosides such as paromomycin and gentamicin (16). In our study, more than 99% of these EIEC were inhibited by 3 h of exposure to 30 μg of paromomycin per ml with incubation at 37°C, and there was an excellent linear correlation between the concentration of paromomycin and EIEC inhibition (R2 = 0.9330; P < 0.001). One to five percent of EIEC that are added to a Caco-2 monolayer invade the cells and become intracellular by 3 h afterward (data not shown). We estimated the effective intracellular concentration achieved by externally added paromomycin by determining the percentage of inhibition of intracellular EIEC on the basis of this standard curve of extracellular EIEC inhibition by paromomycin.
The number of intracellular EIEC CFU decreased to a similar degree in uninfected and C. parvum-infected Caco-2 cells that had been incubated with 0, 500, 1,000, or 2,000 μg of paromomycin per ml. For example, the CFU counts of EIEC, or the percentage of inhibition of EIEC, after host cells were incubated in 2,000 μg of paromomycin per ml did not significantly differ between C. parvum-infected and uninfected cells (percentage of inhibition, 76.3% ± 9.7% in monolayers uninfected by C. parvum versus 86.7% ± 3.6% in C. parvum-infected monolayers; P = 0.359 [not significant]; CFU per ml, 5.4 × 104 ± 1.4 × 104 in cells not infected by C. parvum versus 7.5 × 104 ± 1.3 × 104 in C. parvum-infected cells; P = 0.313 [not significant]). Multiple regression analysis revealed that the presence or absence of C. parvum infection was not significant (P = 0.356), whereas the concentration of paromomycin was very significantly inversely correlated with the decrease in CFU (P < 0.0001 if either actual CFU or percentage of inhibition was analyzed). Overall, we found that ∼80% of intracellular EIEC were killed (in infected or uninfected cells) with an extracellular paromomycin concentration of 2,000 μg/ml. On the basis of our standard curve of EIEC killing by extracellular paromomycin, we were able to calculate that the effective intracytosolic concentration of paromomycin was ∼23 μg/ml in both infected and uninfected cells under these conditions.
Uninfected and infected Caco-2 cells reversibly take up significant amounts of [3H]paromomycin.
C. parvum-infected cells (5 × 105 oocysts per LabTek well) and uninfected Caco-2 cells were exposed to 2,000 μg of [3H]paromomycin per ml for 2 or 4 h, 4 h after infection. After 2 h of exposure (hours 4 to 6 of infection), cells were chilled, followed by medium removal and cell lysis. The lysate radioactivity was then counted as described in Materials and Methods, and the total cellular uptake of [3H]paromomycin was expressed as the estimated total uptake per milliliter of cell volume with the estimated cellular volume of Caco-2 cells (Materials and Methods; 0.192 μl for the 4 × 105 cells on a LabTek slide). The mean total uptake of [3H]paromomycin was 5,168 ± 441 μg per ml of cell volume (n = 4) in uninfected cells and 5,938 ± 595 μg per ml of cell volume (n = 4) in infected cells (not significant). After 4 h of exposure (hours 4 to 8 of infection), [3H]paromomycin uptake was 5,548 ± 161 and 4,762 ± 406 μg per ml of cell volume in uninfected and infected cells, respectively (n = 4 for each [not significant]). In order to quantify the retention of paromomycin in cells over time, we then exposed Caco-2 cells to [3H]paromomycin for 6 h, removed the [3H]paromomycin-containing medium and replaced it with paromomycin-free medium, and quantified the amount remaining in the cells 2 and 4 h later. Two hours after the medium was removed, only a mean of 33.9% of the [3H]paromomycin measured when the medium was removed remained in the cells, and 4 h after the medium was removed, only a mean of 28.0% remained (n = 4 for each). Thus, exposed cells were found to take up significant amounts of paromomycin, and there was no significant difference between the rates of uptake for infected and uninfected cells 6 and 8 h after infection. In addition, this uptake had a reversal component, as a mean of less than 30% of the [3H]paromomycin initially associated with the cell remained 4 h later.
Preinfection exposure of Caco-2 cells to paromomycin did not inhibit subsequent infection.
In order to assess the possibility that a noncytosolic compartment, such as the host vesicular system, might be involved in the delivery of paromomycin to the intracellular parasite, we exposed Caco-2 cells grown on LabTek glass slides to 0, 2,000, 5,000, or 10,000 μg of paromomycin per ml for 0, 2, 4, or 6 h. After this period, the paromomycin-containing medium was removed and cells were infected with C. parvum. The infection was allowed to proceed under standard conditions, and the level of infection was quantified by LDH assays and IFA. In repeated experiments, there was no significant decrease in the level of infection as measured by these parameters in the cells that had been preincubated with paromomycin compared to the level of infection in control, unexposed monolayers (data not shown).
DISCUSSION
By every criterion we and others have identified (1, 21), geneticin inhibits C. parvum in cells that express a geneticin-inactivating enzyme, APH. This finding contrasts sharply with results for the related coccidian parasite Toxoplasma gondii (14), which is able to survive in APH-transformed cells. Intracellular Toxoplasma parasites are completely surrounded by the host cell cytoplasm, and thus host cell cytoplasmic APH can protect Toxoplasma from exposure to exogenously added geneticin. We believe the abilities of geneticin and paromomycin to inhibit C. parvum are related to the unique intracellular location of this intracellular but extracytoplasmic parasite.
It is indeed the intracellular parasite that is relevant to the action of these drugs. We found by timed-exposure experiments that geneticin and paromomycin inhibit intracellular (but not extracellular) stages. Exposure of infected monolayers for only 2 h to 2,000 μg of paromomycin per ml was nearly as inhibitory as 2 days of exposure. Invasion of host cells is believed to be rapid after excystation or merogony, and parasites are unlikely to remain extracellular for a prolonged period either in vitro or in vivo (11, 27, 34). Via electron microscopy, we have documented that C. parvum excysts, attaches, invades, and develops into early intracellular trophozoite stages in Caco-2 cells within 30 min of inoculation under these conditions (20a). Based on these data, we cannot give any credence to the theory that paromomycin or geneticin significantly inhibits extracellular C. parvum.
We found that the apical route of drug exposure was far more inhibitory to intracellular C. parvum than the basolateral route, implying a topological restriction to the entry or action of these drugs. The apical surface of the infected host cell has two domains: the dome-like host membranes overlying the parasite and the (presumably) uninvolved apical surface of the cell. The latter, but not the former, leads directly to the host cell cytoplasm, which interfaces with the parasite feeder organelle membrane. On the basis of our results, significant paromomycin trafficking through the normal or infected host cell cytoplasm is unlikely, and in APH-transformed cell lines this possibility is essentially eliminated for both paromomycin and geneticin. In some experiments (see Table 4), we found that basolateral exposure to these agents had a small (but statistically detectable) inhibitory effect on the parasite. These living monolayers are not absolute barriers to paracellular drug leakage from one side to the other, and this phenomenon explains the small basolateral effect. Prior studies have documented the transmonolayer transfer of extracellular molecules, even in uninfected monolayers (21, 29, 30). It is likely that a fraction of the drugs added to the basolateral side would leak to the apical side, and vice versa, over time. However, even assuming the extreme case that the added drugs completely equilibrated over time across the monolayer, this potential equilibration could not have produced these results. Given the volumes of the apical (0.5 ml) and the basolateral (1.5 ml) reservoirs, with initial apical exposure to 2,000 μg of paromomycin per ml, the final expected equilibrated concentration (apical plus basolateral) would have been 500 μg of paromomycin per ml; with basolateral exposure, the final expected equilibrated concentration would have been 1,500 μg of paromomycin per ml. In that case, basolateral exposure should have been more inhibitory to the parasite than apical exposure, and it was not. Given our results, we believe the most rational conclusion to be that the small but real basolateral inhibition we sometimes saw may have been due to drug leakage from the basolateral to the apical side.
We found no decrease in leucine incorporation after host cell exposure to paromomycin. These results reinforce prior data suggesting that eukaryotic cells are not substantively affected by short-term paromomycin exposure, though long-term exposure may shorten the cell life span (6, 7, 39). We confirmed that geneticin inhibits Caco-2 cells in two ways. First, Caco-2 cells die in the presence of geneticin, and indeed this was the selective principle that was used for development of the APH-Caco-2 cell line. Second, we measured decreased leucine incorporation in cells exposed to apical geneticin, consistent with an inhibition of protein synthesis. We have also shown that the intracytosolic concentrations of paromomycin in infected and uninfected cells are similar as measured by the EIEC assay. These experiments indicated that paromomycin achieves intracytosolic concentrations that are only ∼1% of the external milieu in either uninfected or infected cells during the studied time periods. This concentration was sufficient to kill ∼80% of the intracellular EIEC while not affecting the eukaryotic host cell. Thus, potent intracellular inhibition of the eukaryote C. parvum was not accompanied by increased host cytoplasmic concentrations of paromomycin or by measurable inhibitory effects on the eukaryotic host cell.
We must therefore reconcile the intracellular inhibition of this eukaryotic parasite by drugs that do not inhibit the eukaryotic host. Further, we must reconcile this inhibition with intracytosolic drug levels that are not increased during infection and that must approach zero in transformed APH-Caco-2 cells. Based on the topology of the parasite, there are only two major logical options to explain this process. Either the drugs enter the host cell via a noncytoplasmic route, e.g., the vesicular system, and traffic via the vesicular system to the parasite, they enter the parasite via the membranes covering the parasite on the apical surface of the infected cell, or both.
While we have evidence consistent with the vesicular uptake of paromomycin, we also have evidence that this does not affect the parasite. We found that Caco-2 cells are capable of taking up substantial amounts of [3H]paromomycin (∼4 to 6 mg per ml of cell volume), while effective intracellular concentrations were low (∼23 μg per ml). Our results also show that this uptake is, at least in large part, reversible. The most rational explanation for this is uptake into a separate compartment from the cytosol via the vesicular system.
No independent data regarding the uptake of paromomycin into Caco-2 cells are available. Buchanan et al. documented that both geneticin and paromomycin accumulate within the vesicular compartment of MRC-5 and fetal lung fibroblasts (6, 7). These researchers estimated cellular uptake of dansylated paromomycin in MRC-5 cells as ∼3 mg per g of protein when the external concentration of dansylated paromomycin was ∼0.32 mM—one-tenth of the concentration we used (2,000 μg/ml = 3.2 mM paromomycin)—and dansylation decreases uptake by about 1 order of magnitude (7). Further, they have shown that ∼80% of cellular paromomycin was released after 24 h in paromomycin-free medium (6), consistent with our kinetic result that ∼70% of cellular paromomycin was released within 4 h. Thus, the magnitude of our uptake results is reasonably similar to theirs. Paromomycin accumulates in lysosomal myeloid bodies within skin fibroblasts, reflecting a steady state between aminoglycoside endocytosis and exocytosis (28). Thus, our results are consistent with those of previous studies and with vesicular uptake.
However, we do not believe that paromomycin within the vesicular host cell compartment affects the parasite, since preloading Caco-2 cells with paromomycin-containing medium (up to 10,000 μg/ml) did not inhibit parasite growth. Our kinetic data indicated that ∼30% of paromomycin is retained after 4 h of incubation in paromomycin-free medium. As exposure to paromomycin 2 to 4 h after infection is highly inhibitory, we attempted to replicate the intracellular vesicular conditions found with constant exposure to 2,000 μg of paromomycin per ml by preloading cells with 10,000 μg of paromomycin per ml. In none of these studies did we see inhibition of intracellular C. parvum. Since concentrations as low as 500 μg of exogenous paromomycin per ml will reliably inhibit C. parvum, we believe it unlikely that any significant amount of paromomycin in the host cell vesicular system finds its way to the parasite.
We fully recognize that our crude estimate of Caco-2 cell volume is inexact and may be over- or underestimated and thus under- or overestimate the true uptake of [3H]paromomycin as adjusted by cell volume. For example, shrinkage during fixation could lead to underestimation of cell height. However, the magnitude of this potential error does not change our finding that cellular paromomycin uptake is far higher (2 orders of magnitude) than would be expected from our estimate of the intracytosolic concentration obtained by our EIEC studies. Furthermore, our conclusion that paromomycin entering the host cell vesicular system does not affect intracellular parasites will be unaltered.
These points lead us to conclude that on a structural basis, the remnant host cell membranes and the parasitophorous vacuole, overlying the parasite, are the major routes of entry for these drugs to the parasite. These routes of entry (i) are consistent with the differences in inhibition by apical or basolateral exposure, (ii) do not require trafficking of paromomycin through the host cytoplasm, (iii) account for the inhibition of intracellular parasites in APH-expressing Caco-2 cells, (iv) do not involve the host cell vesicular system, and (v) resolve the clinical conundrum posed by paromomycin’s efficacy in killing an intracellular parasite while being insignificantly absorbed. This conclusion was unexpected for paromomycin, which unlike geneticin is unable to apically penetrate normal Caco-2 cells.
This route of drug entry may also explain how paromomycin inhibits one eukaryotic organism (the parasite) while not inhibiting another, the host. Eukaryotes are 10 to 15 times less sensitive to paromomycin than are prokaryotes (39). Prokaryotes have an A1408 to A1493 16S rRNA base pair that is essential for paromomycin binding and ribosomal inhibition. In eukaryotes, G1408 replaces A1408, with concomitant weak binding and resistance to paromomycin (19). Both Cryptosporidium muris (8) and C. parvum (28a) (GenBank accession no. L16997) have the eukaryotic G1408 to A1493 base pair which predicts paromomycin resistance. The susceptibilities of the host and the parasite to paromomycin might reasonably be expected to be roughly equal. Therefore, one could postulate that the parasite cytoplasmic paromomycin concentration is higher than that in the host cell cytoplasm during drug exposure, possibly because the host and parasite membranes overlying the intracellular parasite are more permeable to paromomycin than normal host cell membranes, allowing greater entry.
Relatively little is known about the specific nature of these overlying apical membranes. As judged by electron microscopy, host cell cytoplasm becomes excluded from between the two sets of host membranes that overlie the parasite and form the apical portion of the parasitophorous vacuole (35). A recent study has suggested that parasite antigens can be found within the host-derived membranes (26). Drug entry could be fostered by alterations in the set of overlying host membranes and by as-yet-uncharacterized changes in the intracellular parasite membranes. Our data do not allow us to comment further on this possibility or others, such as an unknown novel mechanism of action in Cryptosporidium organisms.
Our findings with paromomycin may be contrasted with those for drugs such as sulphasalazine and trimethoprim and the macrolides. Paromomycin does not efficiently enter host cell cytoplasms (∼1% entry) but kills intracellular C. parvum, whereas these other drugs readily enter host cells but are far less efficacious (2, 20). The latter group is far more active against other intracellular coccidian parasites (Plasmodium, Toxoplasma, Eimeria, Cyclospora) than C. parvum (35). Structurally, these other coccidians are completely surrounded by a permeable parasitophorous vacuole that is open to nutrients and low-molecular-weight compounds (23, 32). In contrast, C. parvum’s major contact with the host cell cytoplasm is the feeder organelle membrane. We postulate that the feeder organelle does not allow significant trafficking of these other agents from the host cell into the parasite, which may be of significance in drug therapy (35).
These results may shed light on paromomycin’s excellent efficacy in vitro and in animal models but modest efficacy in PWA. The concentration of paromomycin that we used in this in vitro study was 2,000 μg/ml (2 g/liter). A typical daily dose of paromomycin in adult PWA is 2 g, which is enough to make 1 liter of intestinal juices at this concentration. PWA and cryptosporidiosis may excrete 10 to 24 liters of diarrheal fluid in 24 h, suggesting that paromomycin may become too diluted in vivo to be effective. We have successfully treated C. parvum-infected gnotobiotic piglets with doses ∼35 times higher than those used in humans, without obvious harm (36), though others have noted hearing problems in some PWA treated with total daily doses of paromomycin above 2 g (38). Thus, the doses of paromomycin used in PWA may be too low to be effective. Other alternate explanations include parasite resistance to paromomycin, but our data do not allow us to speculate on this possibility.
In sum, we present evidence that suggests that the intracellular C. parvum parasite is the target of extracellular paromomycin and geneticin. These drugs’ route of entry into the parasite is likely to be the modified host-derived apical membranes and the parasitophorous vacuole overlying the intracellular parasite. This information may be of use in the design of novel anticryptosporidial agents, perhaps based on paromomycin, that take advantage of the parasite’s unique intracellular location.
ACKNOWLEDGMENTS
We gratefully acknowledge the expert technical assistance of Sheryl Dooley and Heidi Scaltreto.
We acknowledge support from the National Institute of Allergy and Infectious Diseases (award U01 AI33384-05).
REFERENCES
- 1.Adams R O B, Guerrant R L, Zu S, Fang G, Roche J K. Cryptosporidium parvum infection of intestinal epithelium: morphological and functional studies in an in vitro model. J Infect Dis. 1994;169:170–177. doi: 10.1093/infdis/169.1.170. [DOI] [PubMed] [Google Scholar]
- 2.Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991;175:880–885. doi: 10.1016/0006-291x(91)91647-u. [DOI] [PubMed] [Google Scholar]
- 3.Ballistini C, Franceschi G, Zarini F, Cassinelli G, Arcamone F, Sanfilippo A. Semisynthetic aminoglycoside antibiotics. IV. 3′4′-Dideoxyparomomycin and analogues. J Antibiot. 1982;35:98–101. doi: 10.7164/antibiotics.35.98. [DOI] [PubMed] [Google Scholar]
- 4.Bissuel F, Cotte F, Michele de Montclos M, Rabodonirina M, Trepo C. Absence of systemic absorption of oral paromomycin during long-term, high-dose treatment for cryptosporidiosis in AIDS. J Infect Dis. 1994;170:749–750. doi: 10.1093/infdis/170.3.749. [DOI] [PubMed] [Google Scholar]
- 5.Blanshard C, Jackson A M, Shanson D C, Francis N, Gazzard B G. Cryptosporidiosis in HIV-seropositive patients. Q J Med. 1992;85:813–823. [PubMed] [Google Scholar]
- 6.Buchanan J H, Rattan S I S, Stevens A, Holliday R. Intracellular accumulation of a fluorescent derivative of paromomycin in human fibroblasts. J Cell Biochem. 1982;20:71–80. doi: 10.1002/jcb.240200108. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan J H, Stevens A, Sidhu J. Aminoglycoside antibiotic treatment of human fibroblasts: intracellular accumulation, molecular changes and the loss of ribosomal accuracy. Eur J Cell Biol. 1987;43:141–147. [PubMed] [Google Scholar]
- 8.Cai J, Collins M D, McDonald V, Mitchell G H. PCR cloning and nucleotide sequence determination of the 18S rRNA genes and internal transcribed spacer 1 of the protozoan parasites Cryptosporidium parvum and Cryptosporidium muris. Biochim Biophys Acta. 1992;1131:317–320. doi: 10.1016/0167-4781(92)90032-u. [DOI] [PubMed] [Google Scholar]
- 9.Capmau M-L, Moreau B, Le Goffic F. A one pot tritiation of aminoglycoside antibiotics. J Antibiot. 1983;36:735–737. doi: 10.7164/antibiotics.36.735. [DOI] [PubMed] [Google Scholar]
- 10.Current W L. Techniques and laboratory maintenance of Cryptosporidium. In: Dubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals. Boca Raton, Fla: CRC Press; 1990. pp. 41–44. [Google Scholar]
- 11.Current W L, Garcia L S. Cryptosporidiosis. Clin Microbiol Rev. 1991;4:325–358. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Current W L, Reese N C. A comparison of endogenous development of three isolates of Cryptosporidium in suckling mice. J Protozool. 1986;33:98–108. doi: 10.1111/j.1550-7408.1986.tb05567.x. [DOI] [PubMed] [Google Scholar]
- 13.Davies J, Jimenez A. A new selective agent for eukaryotic cloning vectors. Am J Trop Med Hyg. 1980;29:1089–1092. doi: 10.4269/ajtmh.1980.29.1089. [DOI] [PubMed] [Google Scholar]
- 14.Donald R G, Roos D S. Stable molecular transformation of Toxoplasma gondii: a selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc Natl Acad Sci USA. 1993;90:11703–11707. doi: 10.1073/pnas.90.24.11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnenberg M S, Donohue-Rolfe A, Keusch G T. Epithelial cell invasion: an overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor J. Infect Dis. 1989;160:452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- 16.Donnenberg M S, Donohue-Rolfe A, Keusch G T. A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol Lett. 1990;69:83–86. doi: 10.1016/0378-1097(90)90417-o. [DOI] [PubMed] [Google Scholar]
- 17.Falkow, S., P. Small, R. Isberg, S. F. Hayes, and D. A. Corwin. 1987. A molecular strategy for the study of bacterial invasion. Rev. Infect. Dis. 9(Suppl.):S450–S455. [DOI] [PubMed]
- 18.Flanigan T P, Aji T, Marshall R, Soave R, Aikawa M, Kaetzel C. Asexual development of Cryptosporidium parvum within a differentiated human enterocyte cell line. Infect Immun. 1991;59:234–239. doi: 10.1128/iai.59.1.234-239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fourmy D, Recht M I, Blanchard S C, Puglisi J D. Structure of the A site of Escherichia coli 16S ribosomal RNA complexed with an aminoglycoside antibiotic. Science. 1996;274:1367–1371. doi: 10.1126/science.274.5291.1367. [DOI] [PubMed] [Google Scholar]
- 20.Griffiths J K. Human cryptosporidiosis: epidemiology, transmission, clinical disease, treatment, and diagnosis. Adv Parasitol. 1998;40:37–85. doi: 10.1016/s0065-308x(08)60117-7. [DOI] [PubMed] [Google Scholar]
- 20a.Griffiths, J. K., R. Moore, and S. Tzipori. Unpublished data.
- 21.Griffiths J K, Moore R, Dooley S, Keusch G T, Tzipori S. Cryptosporidium parvum infection of Caco-2 cell monolayers induces an apical monolayer defect, selectively increases transmonolayer permeability, and causes epithelial cell death. Infect Immun. 1994;62:4506–4514. doi: 10.1128/iai.62.10.4506-4514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Griffiths, J. K., and S. Tzipori. Unpublished data.
- 22.Iwaki S, Honke K, Nishida N, Taniguchi N. The absorption, excretion and influence on bowel flora of oral paromomycin sulfate. Jpn J Antibiot. 1981;34:1078–1081. [PubMed] [Google Scholar]
- 23.Joiner K A. Vacuolar membranes surrounding intracellular pathogens: where do they come from and what do they do? Infect Agents Dis. 1993;2:215–219. [PubMed] [Google Scholar]
- 24.Jordan M, Schallhorn A, Wurm F M. Transfecting mammalian cells: optimization of critical parameters affecting calcium phosphate precipitate formation. Nucleic Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keusch G T, Donohue-Rolfe A, Jacewicz M Z, Kane A V. Shiga toxin: production and purification. Methods Enzymol. 1988;165:152–161. doi: 10.1016/s0076-6879(88)65025-7. [DOI] [PubMed] [Google Scholar]
- 26.McDonald V, McCrossan M V, Petry F. Localization of parasite antigen in Cryptosporidium parvum-infected epithelial cells using monoclonal antibodies. Parasitology. 1995;110:259–268. doi: 10.1017/s0031182000080847. [DOI] [PubMed] [Google Scholar]
- 27.O’Donoghue P J. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 28.Oshima M, Hashiguchi M, Nakasuji M, Shindo N, Shibata S. Biochemical mechanisms of aminoglycoside cell toxicity. II. Accumulation of phospholipids during myeloid body formation and histological studies on myeloid bodies using twelve aminoglycoside antibiotics. J Biochem. 1989;106:794–797. doi: 10.1093/oxfordjournals.jbchem.a122932. [DOI] [PubMed] [Google Scholar]
- 28a.Pieniazek, N. J., et al. Unpublished data.
- 29.Pinto M, Robine-Leon S, Appay M-D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assman P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 30.Ranaldi G, Islam K, Sambuy Y. Epithelial cells in culture as a model for the intestinal transport of antimicrobial agents. Antimicrob Agents Chemother. 1992;36:1374–1381. doi: 10.1128/aac.36.7.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson L J, Campbell A T, Smith H V. In vitro excystation of Cryptosporidium parvum. Parasitology. 1993;106:13–19. doi: 10.1017/s003118200007476x. [DOI] [PubMed] [Google Scholar]
- 32.Schwab J C, Beckers C J M, Joiner K A. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci USA. 1994;91:509–513. doi: 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsujimoto Y. Overexpression of the human BCL-2 gene product results in growth enhancement of Epstein-Barr virus-immobilized B-cells. Proc Natl Acad Sci USA. 1989;86:1958–1962. doi: 10.1073/pnas.86.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzipori S. Cryptosporidiosis in perspective. Adv Parasitol. 1988;27:63–129. doi: 10.1016/S0065-308X(08)60353-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzipori S, Griffiths J K. Natural history and biology of Cryptosporidium parvum. Adv Parasitol. 1998;40:5–36. doi: 10.1016/s0065-308x(08)60116-5. [DOI] [PubMed] [Google Scholar]
- 36.Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin Diagn Lab Immunol. 1994;1:450–463. doi: 10.1128/cdli.1.4.450-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White A C, Jr, Chappell C L, Hayat C S, Kimball K T, Flanigan T P, Goodgame R W. Paromomycin for cryptosporidiosis and AIDS: a prospective, double-blind, trial. J Infect Dis. 1994;170:419–424. doi: 10.1093/infdis/170.2.419. [DOI] [PubMed] [Google Scholar]
- 38.White A C, Jr, Goodgame R W, Chappell C L. Reply. J Infect Dis. 1995;171:1071. . (Letter.) [Google Scholar]
- 39.Wilhelm J M, Pettitt S E, Jessop J J. Aminoglycoside antibiotics and eukaryotic protein synthesis: stimulation of errors in the translation of natural messengers in extracts of cultured human cells. Biochemistry. 1978;17:1143–1153. doi: 10.1021/bi00600a002. [DOI] [PubMed] [Google Scholar]