Summary
Moyamoya disease (MMD) is a rare cerebrovascular disorder marked by progressive stenosis of the internal carotid arteries. Assessing cerebral hemodynamics, specifically cerebrovascular reactivity (CVR), is vital for MMD management and prognosis. In this study, fMRI was performed in a prospective cohort of 47 patients with MMD and 32 healthy controls to investigate its utility in evaluating CVR and to explore the influence of cerebral posterior circulation compensation on CVR in MMD. The regions where the CVR values of participants with MMD were lower than those of healthy controls were primarily concentrated in the frontal, parietal, and temporal lobes (p < 0.05). In certain regions mainly supplied by posterior circulation, the CVR values of compensatory-normal subgroup tended to exceed those of compensatory-poor subgroup. fMRI can detect a significant decrease in CVR values in patients with MMD compared to healthy controls. Compensation for the posterior cerebral circulation may affect cerebrovascular reactivity.
Subject areas: Cardiovascular medicine, Health sciences, Internal medicine, Medical specialty, Medicine, Neurology
Graphical abstract
Highlights
-
•
Resting-state fMRI can evaluate cerebrovascular reactivity in moyamoya disease
-
•
The CVR values of MMD patients were mainly low in the frontal and temporal lobes
-
•
Posterior cerebral circulation compensation may affect CVR in MMD
Cardiovascular medicine; Health sciences; Internal medicine; Medical specialty; Medicine; Neurology
Introduction
Moyamoya disease (MMD) is a unique and rare cerebrovascular disease that leads to severe hemodynamic damage and cerebral hemorrhage; it is characterized by chronic progressive stenosis of the terminal portion of the bilateral internal carotid arteries, leading to the formation of an abnormal vascular network composed of collateral pathways.1
An essential task in diagnosing, selecting treatment strategies, guiding surgery, and predicting the prognosis of MMD is to evaluate the hemodynamic status of the brain tissue.2,3 Cerebrovascular reactivity (CVR) is the ability of the intracranial blood vessels to increase cerebral blood flow in response to vasodilatory stimulus.4 Reduced CVR is significantly associated with an increased risk of stroke recurrence in patients with symptomatic middle cerebral artery (MCA) or internal carotid artery (ICA) occlusion and is a useful marker for detecting ischemic risk in various cerebrovascular conditions.5,6 Currently, the measurement of CVR in patients with MMD mainly relies on positron emission tomography (PET) and single-photon emission computed tomography (SPECT).2,7 However, using these methods to measure CVR could be cumbersome, requiring a vasoactive challenge to patients by injecting certain drugs such as acetazolamide, inhaling CO2 gas, or performing breath holding. Although acetazolamide is generally safe, it is contraindicated in patients with sulfa allergies or sick kidney and liver diseases.8 The radiation, high cost, and availability of these examinations affect patient acceptance.
Some magnetic resonance imaging (MRI)-based imaging technologies, such as dynamic susceptibility contrast-enhanced bolus-tracking MRI and arterial spin-labeling MRI, are gradually emerging. The former still requires the injection of contrast agents, and the latter is limited by factors such as artifacts, sensitivity, and significant underestimation of cerebral blood flow, which affect the accuracy of the measurement results. However, they are much more convenient than nuclear examinations.9
Resting-state (RS) blood-oxygen-level-dependent (BOLD) functional MRI technique is a task-free, economical, and convenient method to evaluate the CVR of patients with MMD.10 However, further research is needed on the application of RS BOLD MRI in the clinical practice of MMD. The presence and appearance of steno-occlusive posterior cerebral artery lesions are not included in the diagnostic criteria of MMD.11 However, posterior circulation lesions are often involved after the progression of internal carotid artery lesions, and the clinical significance of steno-occlusive posterior circulation lesions in MMD has been demonstrated.12 Understanding the significance of posterior circulation territories is therefore of great importance when evaluating the disease progression and predicting prognosis.
This study aimed to investigate the utility of this technique in evaluating CVR in patients with MMD and to explore the influence of cerebral posterior circulation compensation on CVR using the RS BOLD MRI technique.
Results
Participants’ characteristics
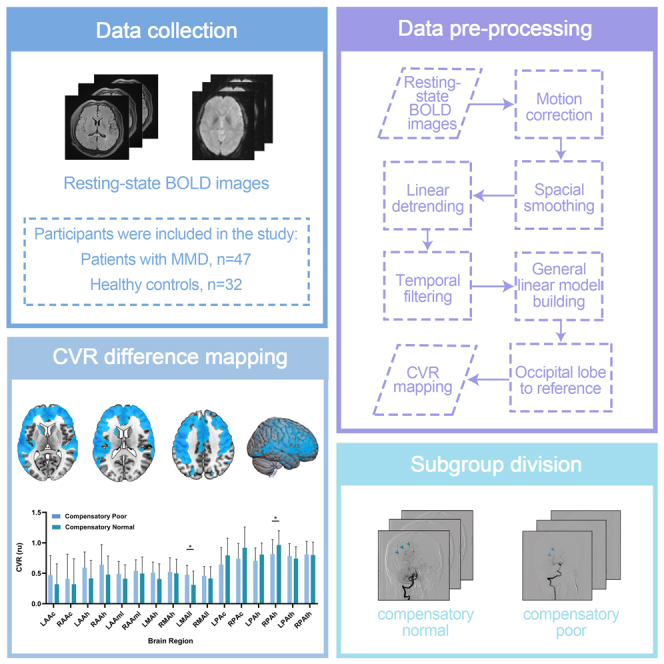
There were 32 healthy controls (mean age ±standard deviation, 35.50years ±10.25; 14 women), and 47 participants with MMD fulfilled the inclusion criteria. Among these participants with MMD, 42 showed symptoms of ischemia (mean age, 39.35years ±12.85; 18 females), 5 showed hemorrhage (mean age, 29.58years ±12.84; 1 female), and there were 5 children (mean age, 12.41years ±4.21; 2 females). The participants’ characteristics are shown in Table 1.
Table 1.
Demographic and clinical characteristics of study participants
| Variables | Compensatory-normal (n = 28) | Compensatory-poor (n = 19) | Controls (n = 32) |
|---|---|---|---|
| Sex (M:F) | 14:14 | 14:5 | 18:14 |
| Smoking history (years) | 5 (17.86%) | 8 (42.11%) | 0 |
| 19.80 ± 7.97 | 18.25 ± 9.23 | ||
| Alcohol taking (years) | 2 (7.14%) | 1 (5.26%) | 0 |
| 27.00 ± 7.00 | 10 | ||
| Age ± SD (years) | 36.93 ± 13.00 | 40.35 ± 12.89 | 35.50 ± 10.25 |
| Mean duration of symptoms ± SD (months) | 29.93 ± 43.30 | 24.72 ± 24.29 | |
| Left | |||
| 0 | 1 (3.56%) | 2 (10.53%) | |
| 1 | 2 (7.14%) | 1 (5.26%) | |
| 2 | 3 (10.71%) | 0 | |
| 3 | 13 (46.43%) | 7 (36.84%) | |
| 4 | 5 (17.86%) | 8 (42.11%) | |
| 5 | 4 (14.29%) | 1 (5.26%) | |
| 6 | 0 | 0 | |
| Right | |||
| 0 | 2 (7.14%) | 1 (5.26%) | |
| 1 | 2 (7.14%) | 2 (10.53%) | |
| 2 | 5 (17.86%) | 1 (5.26%) | |
| 3 | 6 (21.43%) | 10 (52.63%) | |
| 4 | 7 (25.00%) | 4 (21.05%) | |
| 5 | 6 (21.43%) | 1 (5.26%) | |
| 6 | 0 | 0 | |
SD, standard deviation; M, male; F, female.
Comparative results of participants with MMD and healthy controls
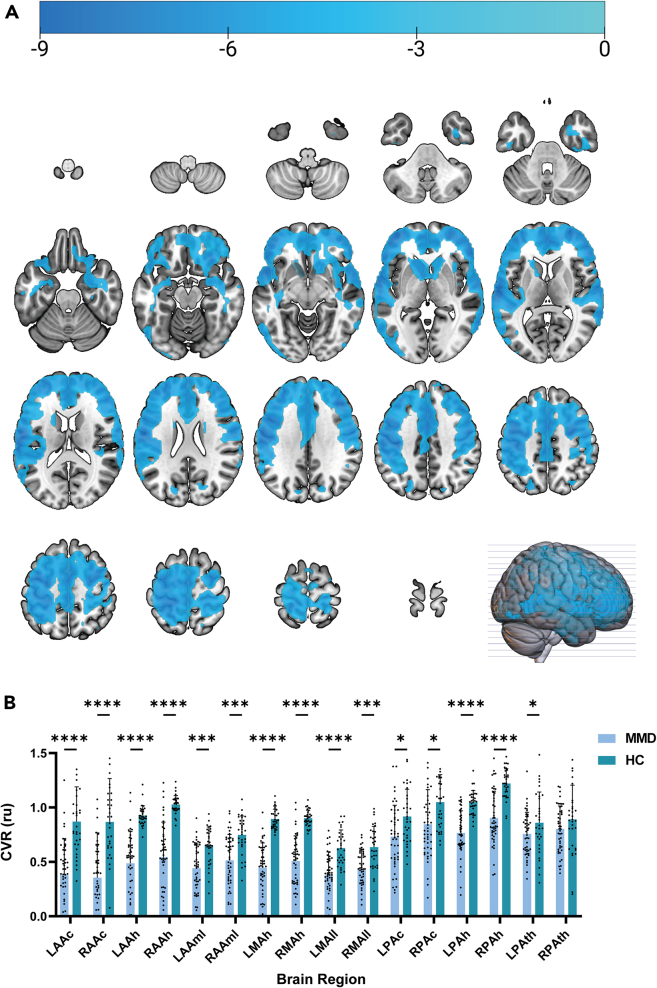
Figure 1A demonstrated that the CVR values of participants with MMD were predominantly lower in the frontal, parietal, and temporal lobes compared to healthy controls. Specifically, the CVR values were lower in participants with MMD within various territories: right anterior cerebral artery hemisphere (mean CVR value: patients group = 0.544 ± 0.315 relative units (ru) vs. control group = 1.027 ± 0.081 ru; p < 0.05 after correction), left anterior cerebral artery hemisphere (mean CVR value: patients group = 0.487 ± 0.286 ru vs. control group = 0.925 ± 0.087 ru; p < 0.05 after correction), right middle cerebral artery hemisphere (mean CVR value: patients group = 0.508 ± 0.227 ru vs. control group = 0.902 ± 0.071 ru; p < 0.05 after correction), right posterior cerebral artery hemisphere (mean CVR value: patients group = 0.903 ± 0.241 ru vs. control group = 1.220 ± 0.132 ru; p < 0.05 after correction), left posterior cerebral artery hemisphere (mean CVR value: patients group = 0.766 ± 0.199 ru vs. control group = 1.048 ± 0.104 ru; p < 0.05 after correction), left middle cerebral artery hemisphere (mean CVR value: patients group = 0.447 ± 0.222 ru vs. control group = 0.894 ± 0.088 ru; p < 0.05 after correction), right anterior cerebral artery callosal territory (mean CVR value: patients group = 0.354 ± 0.404 ru vs. control group = 0.867 ± 0.387 ru; p < 0.05 after correction), left anterior cerebral artery callosal territory (mean CVR value: patients group = 0.379 ± 0.328 ru vs. control group = 0.869 ± 0.310 ru; p < 0.05 after correction), right middle cerebral artery lateral lenticulostriate territory (mean CVR value: patients group = 0.431 ± 0.175 ru vs. control group = 0.635 ± 0.186 ru; p < 0.05 after correction), left middle cerebral artery lateral lenticulostriate territory (mean CVR value: patients group = 0.375 ± 0.212 ru vs. control group = 0.627 ± 0.160 ru; p < 0.05 after correction), right anterior cerebral artery medial lenticulostriate territory (mean CVR value: patients group = 0.514 ± 0.232 ru vs. control group = 0.748 ± 0.168 ru; p < 0.05 after correction), left anterior cerebral artery medial lenticulostriate territory (mean CVR value: patients group = 0.442 ± 0.217 ru vs. control group = 0.653 ± 0.162 ru; p < 0.05 after correction), right posterior cerebral artery callosal territory (mean CVR value: patients group = 0.846 ± 0.312 ru vs. control group = 1.050 ± 0.244 ru; p < 0.05 after correction), and left posterior cerebral artery callosal territory (mean CVR value: patients group = 0.732 ± 0.282 ru vs. control group = 0.917 ± 0.244 ru; p < 0.05 after correction) (Figure 1B).
Figure 1.
Comparison of CVR values between patients with moyamoya disease and healthy controls
(A) Mapping of T-values representing the difference in CVR between patients with moyamoya disease and healthy controls in the MNI space.
(B) Comparison histogram of CVR in different brain regions between the two cohorts. Abbreviations: LAAc (left anterior cerebral artery callosal), RAAc (right anterior cerebral artery callosal), LAAh (left anterior cerebral artery hemisphere), RAAh (right anterior cerebral artery hemisphere), LAAml (left anterior cerebral artery medial lenticulostriate), RAAml (right anterior cerebral artery medial lenticulostriate), LMAh (left middle cerebral artery hemisphere), RMAh (right middle cerebral artery hemisphere), LMAll (left middle cerebral artery lateral lenticulostriate), RMAll (right middle cerebral artery lateral lenticulostriate), LPAc (left posterior cerebral artery callosal), RPAc (right posterior cerebral artery callosal), LPAh (left posterior cerebral artery hemisphere), RPAh (right posterior cerebral artery hemisphere), LPAth (left posterior cerebral artery thalamic and midbrain perforators), and RPAth (right posterior cerebral artery thalamic and midbrain perforators). Asterisks represent statistical significance: one asterisk denotes a p value less than 0.05, three asterisks represent a p value less than 0.001, and four asterisks indicate a p value less than 0.00001. Tables S1 and S2 provide a summary of the statistical significance of all examined indices.
Considering the significant differences in age and sex between the groups, we selected them as covariates for further analyses. In these regions, the difference in CVR remained statistically significant (p < 0.05). Tables S1 and S2 provide a summary of the statistical significance of all examined indices. Our findings indicate that participants with MMD exhibit lower CVR values compared to healthy controls.
Comparative results of poor and normal compensation of posterior cerebral circulation
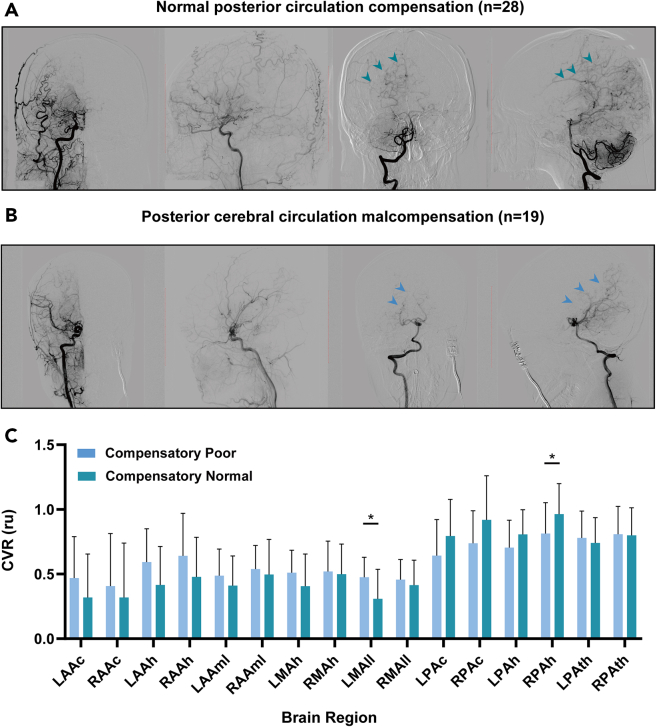
Based on imaging data, such as digital subtraction angiography (DSA) images, participants with MMD were categorized into two groups based on their compensatory state. The study included 19 participants with poor compensation of the cerebral posterior circulation and 28 participants with good compensatory state. The CVR values of the compensatory-normal group exhibited a tendency to exceed those of the compensatory-poor group in certain regions primarily supplied by the posterior circulation. This trend differed from the performance of the two groups in other areas. For instance, in the right posterior cerebral artery hemisphere territory, the mean CVR value was 0.813 ± 0.232 ru for the poor group and 0.963 ± 0.231 ru for the normal group. Similarly, in the left posterior cerebral artery hemisphere territory, the mean CVR value was 0.705 ± 0.205 ru for the poor group and 0.807 ± 0.187 ru for the normal group. Additionally, in the right posterior cerebral artery callosal territory, the mean CVR value was 0.738 ± 0.245 ru for the poor group and 0.918 ± 0.336 ru for the normal group. In the left posterior cerebral artery callosal territory, the mean CVR value was 0.642 ± 0.272 ru for the poor group and 0.794 ± 0.277 ru for the normal group (Figure 2C). The difference in CVR values between the two groups was also less pronounced in the left and right posterior cerebral artery thalamic and midbrain perforator territories compared to regions primarily supplied by the anterior or middle cerebral arteries.
Figure 2.
Conditions for the delineation of different compensatory subgroups in patients with moyamoya disease and comparison of CVR values between these two subgroups
(A) DSA screenshots illustrating the different compensation situations for the two patient groups.
(B) Comparison of CVR values between the compensatory-poor and compensatory-normal groups. Arrows indicate the DSA images of compensatory blood vessels in the posterior circulation. (C) An asterisk represents a p value before correction less than 0.05. The detailed statistical results can be found in Tables S3 and S4.
Age and sex were considered as covariates in the analysis, but the results did not reach statistical significance. The detailed statistical results can be found in Tables S3 and S4.
Discussion
The measurement of CVR requires expensive and radioactive examinations with explicit physiological maneuvers. The measurement procedure must be pre-digested; otherwise, the evaluation of vascular reactivity under a wider range of clinical and research conditions might be impractical. The RS BOLD functional MRI technique is economical, convenient, and task free and might be the best solution.
We investigated the feasibility of the RS BOLD MRI technique in evaluating CVR in patients with MMD and explored the application prospects of this technology in evaluating the impact of the compensatory state of the cerebral posterior circulation on CVR. Through the evaluation using the RS BOLD MRI technique, the lower CVR values in participants with MMD compared to healthy controls were statistically significant (p < 0.05), which was consistent with previous research.2,7,13,14 The poor compensation of the posterior circulation seemed to have a negative impact that might cause a decrease in the CVR of those regions of interest mainly supplied by the posterior circulation system, although the statistical support for this study’s results was insufficient.
Conventional CVR mapping is often performed by performing xenon-enhanced computed tomography, PET, or SPECT before and after a vasoactive challenge, such as inhalation of CO2 gas, holding breath, hyperventilation, or injecting acetazolamide, for patients with MMD.9,15 It is cumbersome and expensive to measure the CVR using these methods. In addition, acetazolamide is contraindicated in patients with sulfa allergies or kidney and liver diseases. Furthermore, patients may present with stroke-like symptoms during testing. Although these symptoms, including headache, flushing, and malaise, are often mild, transient, and rare, they still affect patients and medical staff.8,16 These stimuli limit their widespread application in patients with MMD.15,17 Unlike these conventional methods, the technique applied in this study uses natural variations in breathing patterns to measure CVR from typical RS BOLD data without requiring additional challenges and expensive equipment. Previous studies have shown that measuring CVR plays a significant role in the preoperative or postoperative evaluation and prediction of prognosis in chronic ischemic cerebrovascular diseases.10,15,17,18
Xenon-enhanced computed tomography is well established and considered the gold standard in dedicated centers for revascularization procedures to calculate the CVR because of its superior spatial resolution, accuracy, and reproducibility.19,20,21
In a prospective study on the relationship between CVR and outcomes in patients with symptomatic internal carotid or middle cerebral artery occlusion, regional cerebrovascular reactivity (rCVR) to acetazolamide was calculated at entry into the study using 133Xe SPECT and patients were prospectively followed up for 24 months. During the follow-up period, recurrent stroke occurred in 8 of 23 patients with reduced rCVR at entry and in 3 of 47 patients with normal rCVR. Cumulative recurrence-free survival rates in all patients and in each subgroup of patients with ICA or MCA occlusion and reduced CVR on entry were significantly lower than those in patients with normal rCVR (p = 0.0030, p = 0.0404, and p = 0.0310, respectively; Kaplan-Meier analysis). Among the factors considered, only a lower rCVR and resting regional cerebral blood flow were significantly associated with the risk of stroke recurrence (p = 0.0019 and p = 0.0080, respectively; Cox regression multivariate analysis). The results indicated that reduced rCVR to acetazolamide was significantly associated with an increased risk of stroke recurrence in patients with symptomatic internal carotid or middle cerebral artery occlusion.6
In a study on the preoperative and postoperative mapping of CVR in MMD using hypercapnic and hypocapnic states and BOLD MRI, three patients with angiographically confirmed MMD were evaluated before and after surgical revascularization. Presurgical CVR maps showed distinct positive and negative reactivity regions that correlated precisely with the vascular territories supplied by the severely narrowed vessels. Postsurgical reactivity maps demonstrated improvement in two patients with positive clinical outcomes and no change in the patients in whom a failed superficial temporal artery-middle cerebral artery bypass was performed. These results suggest that magnetic imaging-based CVR mapping during rapid manipulation of the end-tidal partial pressure of CO2 has considerable potential for guiding preoperative decisions and monitoring the efficacy of surgical revascularization.22
However, relatively little research has been conducted on measuring the CVR in patients with MMD using RS BOLD MRI. Previous research on this topic has been limited and focused on exploring the correlation between the measurement of CVR using the RS BOLD MRI technique and other measurement methods to determine the feasibility of evaluating CVR.10,15,17,18
In a retrospective study of the hemodynamic evaluation of patients with moyamoya angiopathy, Zerweck et al. retrospectively reviewed 27 RS MRI and 27 breath-hold MRI datasets from 26 patients with moyamoya angiopathy. The comparison of the RS maps and the breath-hold maps of 24 patients revealed a good correlation (Pearson’s r = 0.71 ± 0.13; preoperative patients: Pearson’s r = 0.71 ± 0.17; postoperative patients: Pearson’s r = 0.71 ± 0.11). The comparison of seven RS MRI datasets to the corresponding [15O] water PET datasets also revealed a high level of agreement (Pearson’s r = 0.80 ± 0.19). Therefore, RS MRI might be a promising noninvasive method, with almost no patient cooperation needed to evaluate the CVR.23
In our research, the results were also encouraging. Through the direct comparison of RS CVR between healthy controls and participants with MMD, we could clearly observe a significant decrease in CVR in the frontal, parietal, and temporal lobes of patients with MMD (p < 0.05) which suggested RS BOLD MRI has the potential to help surgeons develop appropriate diagnosis and treatment plans by evaluating patient CVR in clinical practice.
A clinical test of CVR mapping using RS BOLD MRI in healthy adults and patients with MMD suggested that CVR mapping performed using RS MRI provided a task-free method to measure the cerebrovascular reserve and depicted the treatment effect of revascularization surgery in patients with MMD, comparable to that of the reference standard of CO2 inhalation MRI. In this study, RS BOLD MRI data collected from 170 healthy controls were retrospectively evaluated to identify the optimal frequency range for temporal filtering ([0,0.1164 Hz], r = 0.74) based on spatial correlation with the reference standard CVR map obtained with CO2 inhalation. The optimized RS method was then applied to a new prospective cohort of 50 participants with MMD. RS BOLD CVR in the middle cerebral artery territory was lower in the group that did not undergo surgery (n = 30) than in the group that underwent surgery (n = 47) (mean, 0.407 ± 0.208 ru vs. 0.532 ± 0.182 ru, respectively; p = 0.006), which is corroborated with the CO2 inhalation CVR data (mean, 0.242 ± 0.273 ru vs. 0.437 ± 0.200 ru; p = 0.003). The results revealed the practicability of RS MRI in evaluating revascularization effectiveness in patients with MMD.15 In this study, the cerebellum was used to yield the reference signal time course in participants with MMD because the authors believed that posterior circulation territories are typically unaffected by MMD. However, posterior circulation is often involved in the clinical setting, and the clinical significance of steno-occlusive posterior cerebral artery lesions in MMD has been demonstrated.12
Finally, compared to this research, our study chose certain occipital areas as the reference in participants with MMD. Although some reported studies have shown that stenotic lesion in cerebral posterior circulation territory might exist in areas supplied by posterior cerebral artery in MMD, such as the temporal,12,24,25 there is almost no report about infarction of these aforementioned areas in MMD. One of the reasons may be that the occipital areas 43, 44, 47, and 48 of the Anatomical Automatic Labeling 116 Map, namely calcarine and lingual, are located at the junction of the anterior and posterior cerebral circulation and are supplied from both the temporal and occipital lobes. Therefore, the impact of insufficient cerebral perfusion caused by MMD on these areas might be relatively smaller than other cerebral posterior circulation territory. Besides, based on our single-center experience, the probability of cerebral infarction occurring in these occipital areas in MMD is relatively low. And our pre-experiment found that the specificity of the results using certain occipital areas as a reference was higher than that using the cerebellum as a reference (Figures S1 and S2). We also remain skeptical about the optimal time filtering frequency range used in this study because 0–0.1174 Hz range is much broader than the frequency band previously demonstrated to exhibit the highest correlation with end-tidal CO2 (0.02–0.04 Hz).10 In order to keep as many RS fMRI signal fluctuations as possible and minimize the interference of non-target signals as much as possible, we finally chose the optimal time filtering frequency range of 0.01–0.08 Hz.26 Our study also supplemented a direct comparison of RS CVR between the healthy controls and participants with MMD, which showed this technique could detect descending CVR in patients with MMD. Moreover, this discovery that cerebral posterior circulation compensation in MMD might have an impact on CVR in our study also showed considerable clinical significance. There was a study that showed that the occlusion of posterior cerebral artery stenosis in MMD had clinical value.12 If RS MRI could measure CVR of the area supplied by cerebral posterior circulation, it would help clinical doctors better evaluate the compensatory status of the posterior circulation and ultimately benefit patients. Understanding the significance of posterior circulation territories is of great importance when evaluating the disease progression and predicting prognosis.
In conclusion, RS BOLD functional MRI can detect a significant decrease in CVR values in patients with MMD compared to healthy controls. It has advantages of being convenient, economical, and task free and has the potential to become a routine method for measuring CVR in patients with MMD in clinical practice. Compensation of the cerebral posterior circulation might affect the local CVR measured by RS MRI in patients with MMD.
Limitations of the study
This study is objectively limited by some factors. First, the small sample size limits the scope of this study. Large-scale transnational/multi-ethnic cohorts, possibly requiring radiomic validation, are warranted to facilitate the clinical transformation of our findings. Next, the measuring results of CVR in this article were in relative rather than absolute units, which might have caused the measurement results to be mixed with redundant signals and affected the final conclusion. The determination of the CO2 concentration and a better CO2 signal separation strategy could help improve the specificity of RS BOLD MRI. Further, follow-up work and research on the correlation between clinical symptoms, CVR mapping, and cerebral posterior circulation compensation are of great significance which might help to understand the role of the cerebral posterior circulation in MMD. In addition, this study did not exclude the influence of other confounding factors, such as Suzuki staging, stenosis degree of MCA or proximal ICA, severity of anterior cerebral circulation, and so on, which might affect the assessment of the impact of posterior compensation on CVR. Finally, longitudinal studies are needed to identify the pattern of CVR measured using RS MRI throughout the course of MMD.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| Statistical Parametric Mapping (SPM12) | Wellcome Department of Imaging Neuroscience, London, UK | https://www.fil.ion.ucl.ac.uk/spm/software/spm12/ |
| MATLAB (version 2013a) | MathWorks Inc., Natick, MA, USA | https://www.mathworks.com/products/matlab.htmnlMATLAB |
| Other | ||
| a 3-T MRI machine (Verio A Tim+Dot System) | Siemens, Germany | https://www.siemens-healthineers.com/ |
| A standard 12-channel head coil (3T Head MATRIX, ATim Coil) | Siemens, Germany | https://www.siemens-healthineers.com/ |
Resource availability
Lead contact
-
•
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Rong Wang (E-mail: ronger090614@126.com, Telephone: +86 13001190333).
Materials availability
-
•
This study did not generate new unique reagents.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
There were 32 healthy controls (mean age ±standard deviation, 35.50years ±10.25; 14 women), and 47 participants with MMD fulfilled the inclusion criteria. Among these participants with MMD, 42 showed symptoms of ischemia (mean age, 39.35years ±12.85; 18 females), 5 showed hemorrhage (mean age, 29.58years ±12.84; 1 female), and there were 5 children (mean age, 12.41years ±4.21; 2 females). All participants are Han Chinese. The participants’ characteristics are shown in Table 1.
This prospective study was approved by the Research Ethics Committee of Beijing Tiantan Hospital, affiliated with Capital Medical University (KYSQ2019–058-01), and all data were obtained with written informed consent.
Method details
Participants
-
•
Two cohorts were established in this study. The experimental group comprised participants with MMD who were diagnosed according to the MRI and MRA guidelines proposed by the Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan.11 Data were collected prospectively and consecutively from the Neurosurgery Department of Beijing Tiantan Hospital which is affiliated to the Capital Medical University between June 2018 and December 2022. Moreover, the group of healthy controls included recruited volunteers who underwent extensive health screening and had no contraindications to MRI and were generally in good health with no serious or unstable medical conditions. In both cohorts, each participant underwent an RS-MRI examination for at least the corresponding time. Subsequently, based on the results of digital subtraction angiography, the participants with MMD were divided into two subgroups. Those participants with compensation of leptomeningeal from posterior to anterior, which supplied area that covered more than one-thirds of the cerebral anterior circulation distribution, were considered to belong to the normal compensated group of posterior circulation, and the poorly compensated group did not have any compensation bypass of leptomeningeal from posterior to anterior, or the compensation did not cover enough cerebral anterior circulation distribution. The angiographic outcomes were evaluated by 2 independent neuroradiologists (with at least 5 years’ experience of clinical practice) who were blinded to the clinical information. Part A and B of Figure 2 shows a comparative example of digital subtraction angiography images between these two groups.
MRI protocols
-
•
All MRI examinations were performed using a 3-T machine (Verio A Tim+Dot System; Siemens, Germany). A standard 12-channel head coil (3T Head MATRIX, ATim Coil, Siemens) was used for signal reception. Each participant laid supine with their head snugly secured using a belt and foam pads. In the RS BOLD MRI scans, the participants were asked to close their eyes, not fall asleep, and not think about anything in particular. The scanning parameters were as follows: repetition time, 2220 ms; echo time, 30.0 ms; voxel size, 3:0 × 3:0 × 3:0 mm; field-of-view, 192 mm; slice thickness, 3.0 mm; number of slices, 32; and total scanning time, 9 min and 11 s.
Quantification and statistical analysis
Data processing
-
•
For data processing, we used Statistical Parametric Mapping (SPM12, Wellcome Department of Imaging Neuroscience, London, UK; https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and in-house MATLAB (version 2013a, MathWorks Inc., Natick, MA, USA). To reduce image noise, all the resting-state images were preprocessed in seven steps: time cutting, slice-timing correction, realigning, spatial normalization, spatial smoothing, linear detrending, nuisance covariate regression, and temporal filtering. The first five volumes of individual functional images were discarded to achieve magnetization equilibrium. Subsequently. The slice-timing correction was implemented to align the resting-state images according to the middle slice. Individual images were realigned (standard of removal:3 mm or 3o) so that each part of the brain was in the same position for every volume. Next, the images were warped into the standard Montreal Neurological Institute (MNI) space by applying a transformation matrix derived by registering T1 images (co-registered with functional images) onto the MNI template using unified segmentation. Spatial smoothing with a full width at half maximum of 8 mm was applied to improve the signal-to-noise ratio and attenuate the anatomical variances caused by inaccurate intersubject registration after spatial normalization. Linear detrending was used to reduce the signal drift caused by non-neuronal activities over a large timescale, and nuisance signals were removed from each voxel’s time series to reduce the effects of non-neuronal fluctuations, including head motion profiles and cerebrospinal fluid and white matter signals. Last, the resting-state data were bandpass-filtering with a filter frequency range from 0.01 Hz to 0.08 Hz to preserve signals with physiological significance. After collecting the above data, CVR calculations were performed. CVR was calculated using the general linear model.15 The variation in BOLD was related to the reference signal time course and head motion parameters. The difference in data processing between healthy controls and patients with MMD lies in the reference object selected for the time course of the reference signal. In healthy controls, the reference area was the entire brain, whereas, in participants with MMD, the reference area was limited to the occipital areas 43, 44, 47, and 48 of the Anatomical Automatic Labeling 116 Map. Finally, the calculated CVR values were registered in the standard MNI space to obtain the CVR map, which allowed comparison between the experimental and control groups.
Statistical analysis
-
•
All the statistical analyses were performed using MATLAB scrips (version 2013a, MathWorks). Two independent sample t-tests were performed to compare the CVR maps between healthy controls and participants with MMD. Two independent sample t-tests were also performed to compare the average CVR values between the poor and good posterior circulation compensation subgroups. These tests corrected for the false discovery rate. P < 0.05 was considered statistically significant at p < 0.05.
Acknowledgments
Thanks to the health care workers in the department for their help with the project. Thanks to all participants for their support and cooperation. This research was funded by the National Natural Science Foundation of China (grant 82171887 and 82371296 to R.W., grant 8207051109 to YL.Z.). These funds covered the expenses related to data collection, testing and analysis, and interpretation of the experiment.
Author contributions
S.H. wrote the paper. H.N. provided the ideas. X.W. provided images and interpreted the data. Z.L., J.Z., X.H., Y.W., Z.Z., Y.Z., and R.W. reviewed the manuscript. All the authors have read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: January 19, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.108923.
Contributor Information
Yuanli Zhao, Email: zhaoyuanli@126.com.
Rong Wang, Email: ronger090614@126.com.
Supplemental information
References
- 1.Suzuki J., Takaku A. Cerebrovascular "moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969;20:288–299. doi: 10.1001/archneur.1969.00480090076012. [DOI] [PubMed] [Google Scholar]
- 2.Burke G.M., Burke A.M., Sherma A.K., Hurley M.C., Batjer H.H., Bendok B.R. Moyamoya disease: a summary. Neurosurg. Focus. 2009;26:E11. doi: 10.3171/2009.1.FOCUS08310. [DOI] [PubMed] [Google Scholar]
- 3.Khan N., Schuknecht B., Boltshauser E., Capone A., Buck A., Imhof H.G., Yonekawa Y. Moyamoya disease and Moyamoya syndrome: experience in Europe; choice of revascularisation procedures. Acta Neurochir. 2003;145:1061–1071. doi: 10.1007/s00701-003-0148-5. discussion 1071. [DOI] [PubMed] [Google Scholar]
- 4.Derdeyn C.P., Grubb R.L., Jr., Powers W.J. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology. 1999;53:251–259. doi: 10.1212/wnl.53.2.251. [DOI] [PubMed] [Google Scholar]
- 5.Yonas H., Smith H.A., Durham S.R., Pentheny S.L., Johnson D.W. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J. Neurosurg. 1993;79:483–489. doi: 10.3171/jns.1993.79.4.0483. [DOI] [PubMed] [Google Scholar]
- 6.Ogasawara K., Ogawa A., Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke. 2002;33:1857–1862. doi: 10.1161/01.str.0000019511.81583.a8. [DOI] [PubMed] [Google Scholar]
- 7.Hoshi H., Ohnishi T., Jinnouchi S., Futami S., Nagamachi S., Kodama T., Watanabe K., Ueda T., Wakisaka S. Cerebral blood flow study in patients with moyamoya disease evaluated by IMP SPECT. J. Nucl. Med. 1994;35:44–50. [PubMed] [Google Scholar]
- 8.Schmickl C.N., Owens R.L., Orr J.E., Edwards B.A., Malhotra A. Side effects of acetazolamide: a systematic review and meta-analysis assessing overall risk and dose dependence. BMJ Open Respir. Res. 2020;7 doi: 10.1136/bmjresp-2020-000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee M., Zaharchuk G., Guzman R., Achrol A., Bell-Stephens T., Steinberg G.K. Quantitative hemodynamic studies in moyamoya disease: a review. Neurosurg. Focus. 2009;26:E5. doi: 10.3171/2009.1.FOCUS08300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu P., Li Y., Pinho M., Park D.C., Welch B.G., Lu H. Cerebrovascular reactivity mapping without gas challenges. Neuroimage. 2017;146:320–326. doi: 10.1016/j.neuroimage.2016.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis, Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases Guidelines for Diagnosis and Treatment of Moyamoya Disease (Spontaneous Occlusion of the Circle of Willis) Neurol. Med. Chir. 2012;52:245–266. doi: 10.2176/nmc.52.245. [DOI] [PubMed] [Google Scholar]
- 12.Hishikawa T., Tokunaga K., Sugiu K., Date I. Assessment of the difference in posterior circulation involvement between pediatric and adult patients with moyamoya disease. J. Neurosurg. 2013;119:961–965. doi: 10.3171/2013.6.JNS122099. [DOI] [PubMed] [Google Scholar]
- 13.Kuwabara Y., Ichiya Y., Sasaki M., Yoshida T., Masuda K., Ikezaki K., Matsushima T., Fukui M. Cerebral hemodynamics and metabolism in moyamoya disease--a positron emission tomography study. Clin. Neurol. Neurosurg. 1997;99:S74–S78. doi: 10.1016/s0303-8467(97)00061-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuwabara Y., Ichiya Y., Otsuka M., Tahara T., Gunasekera R., Hasuo K., Masuda K., Matsushima T., Fukui M. Cerebral hemodynamic change in the child and the adult with moyamoya disease. Stroke. 1990;21:272–277. doi: 10.1161/01.str.21.2.272. [DOI] [PubMed] [Google Scholar]
- 15.Liu P., Liu G., Pinho M.C., Lin Z., Thomas B.P., Rundle M., Park D.C., Huang J., Welch B.G., Lu H. Cerebrovascular Reactivity Mapping Using Resting-State BOLD Functional MRI in Healthy Adults and Patients with Moyamoya Disease. Radiology. 2021;299:419–425. doi: 10.1148/radiol.2021203568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D.Y.T., Ishii Y., Fan A.P., Guo J., Zhao M.Y., Steinberg G.K., Zaharchuk G. Predicting PET Cerebrovascular Reserve with Deep Learning by Using Baseline MRI: A Pilot Investigation of a Drug-Free Brain Stress Test. Radiology. 2020;296:627–637. doi: 10.1148/radiol.2020192793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahanian H., Christen T., Moseley M.E., Pajewski N.M., Wright C.B., Tamura M.K., Zaharchuk G., SPRINT Study Research Group Measuring vascular reactivity with resting-state blood oxygenation level-dependent (BOLD) signal fluctuations: A potential alternative to the breath-holding challenge? J. Cereb. Blood Flow Metab. 2017;37:2526–2538. doi: 10.1177/0271678X16670921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipp I., Murphy K., Caseras X., Wise R.G. Agreement and repeatability of vascular reactivity estimates based on a breath-hold task and a resting state scan. Neuroimage. 2015;113:387–396. doi: 10.1016/j.neuroimage.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schubert G.A., Weinmann C., Seiz M., Gerigk L., Weiss C., Horn P., Thomé C. Cerebrovascular insufficiency as the criterion for revascularization procedures in selected patients: a correlation study of xenon contrast-enhanced CT and PWI. Neurosurg. Rev. 2009;32:29–36. doi: 10.1007/s10143-008-0159-z. discussion 35-26. [DOI] [PubMed] [Google Scholar]
- 20.Yonas H., Gur D., Good B.C., Latchaw R.E., Wolfson S.K., Jr., Good W.F., Maitz G.S., Colsher J.G., Barnes J.E., Colliander K.G., et al. Stable xenon CT blood flow mapping for evaluation of patients with extracranial-intracranial bypass surgery. J. Neurosurg. 1985;62:324–333. doi: 10.3171/jns.1985.62.3.0324. [DOI] [PubMed] [Google Scholar]
- 21.Webster M.W., Steed D.L., Yonas H., Latchaw R.E., Wolfson S.K., Jr., Gur D. Cerebral blood flow measured by xenon-enhanced computed tomography as a guide to management of patients with cerebrovascular disease. J. Vasc. Surg. 1986;3:298–304. [PubMed] [Google Scholar]
- 22.Mikulis D.J., Krolczyk G., Desal H., Logan W., Deveber G., Dirks P., Tymianski M., Crawley A., Vesely A., Kassner A., et al. Preoperative and postoperative mapping of cerebrovascular reactivity in moyamoya disease by using blood oxygen level—dependent magnetic resonance imaging. J. Neurosurg. 2005;103:347–355. doi: 10.3171/jns.2005.103.2.0347. [DOI] [PubMed] [Google Scholar]
- 23.Zerweck L., Roder C., Hauser T.K., Thurow J., Mengel A., Tatagiba M., Khan N., Meyer P.T., Ernemann U., Klose U. Hemodynamic evaluation of patients with Moyamoya Angiopathy: comparison of resting-state fMRI to breathe-hold fMRI and [(15)O]water PET. Neuroradiology. 2022;64:553–563. doi: 10.1007/s00234-021-02814-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto S., Kikuchi H., Karasawa J., Nagata I., Ikota T., Takeuchi S. Study of the posterior circulation in moyamoya disease. Clinical and neuroradiological evaluation. J. Neurosurg. 1984;61:1032–1037. doi: 10.3171/jns.1984.61.6.1032. [DOI] [PubMed] [Google Scholar]
- 25.Mugikura S., Takahashi S., Higano S., Shirane R., Kurihara N., Furuta S., Ezura M., Takahashi A. The relationship between cerebral infarction and angiographic characteristics in childhood moyamoya disease. AJNR. Am. J. Neuroradiol. 1999;20:336–343. [PMC free article] [PubMed] [Google Scholar]
- 26.Li M., Gao Y., Anderson A.W., Ding Z., Gore J.C. Dynamic variations of resting-state BOLD signal spectra in white matter. Neuroimage. 2022;250 doi: 10.1016/j.neuroimage.2022.118972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.