Abstract
Practical relevance:
Increases in cat ownership worldwide mean more cats are requiring veterinary care. Illness, trauma and surgery can result in acute pain, and effective management of pain is required for optimal feline welfare (ie, physical health and mental wellbeing). Validated pain assessment tools are available and pain management plans for the individual patient should incorporate pharmacological and non-pharmacological therapy. Preventive and multimodal analgesia, including local anaesthesia, are important principles of pain management, and the choice of analgesic drugs should take into account the type, severity and duration of pain, presence of comorbidities and avoidance of adverse effects. Nursing care, environmental modifications and cat friendly handling are likewise pivotal to the pain management plan, as is a team approach, involving the cat carer.
Clinical challenges:
Pain has traditionally been under-recognised in cats. Pain assessment tools are not widely implemented, and signs of pain in this species may be subtle. The unique challenges of feline metabolism and comorbidities may lead to undertreatment of pain and the development of peripheral and central sensitisation. Lack of availability or experience with various analgesic drugs may compromise effective pain management.
Evidence base:
These Guidelines have been created by a panel of experts and the International Society of Feline Medicine (ISFM) based on the available literature and the authors’ experience. They are aimed at general practitioners to assist in the assessment, prevention and management of acute pain in feline patients, and to provide a practical guide to selection and dosing of effective analgesic agents.
Keywords: Acute, analgesia, anti-inflammatory drugs, behaviour, dental, facial expressions, pain assessment, opioid, ovariohysterectomy
Introduction
The feline population has grown significantly in recent years, with a current estimate of around 70 million pet cats in the USA and Canada.1,2 These individuals will require veterinary care and the majority undergo at least one surgery (ie, elective neutering) during their lifetime. Others will have medical conditions or trauma requiring hospitalisation, diagnostic examinations, intravenous treatments and palliative care. Effective acute pain management is required in most, if not all, of the above scenarios and must be an integral part of feline practice. Pain has a negative impact on feline welfare. It is a basic requirement that each feline practitioner performs pain assessment during every physical examination, in addition to recording the temperature, pulse, respiration (TPR) and conducting a nutritional assessment. This requires proficient recognition and management of pain in cats, aspects of which are species-specific.

Our knowledge of feline pain management has progressed over recent years. Review articles have been published on different aspects of pain management in cats.3–6 Moreover, there are now three scales with reported validity for acute feline pain assessment that take into consideration facial expressions and the cat’s unique behaviour.7–10 All three provide clinical guidance on the need for analgesia when a cut-off value is reached. However, rescue interventions may be administered even when the cut-off is not reached if the veterinarian feels that the cat could be in pain. The advent of valid pain assessment systems has improved our ability to recognise and assess pain in the clinical setting. It has also led to more robust research and clinical studies, and provided more objective outcome measures for approval of feline-specific drugs.
We no longer rely on extrapolation of scientific information from dogs for analgesic techniques and dosage regimens in cats. The published literature contains several feline-specific pharmacokinetic-pharmacodynamic studies involving analgesics. Opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) with market authorisation specifically for cats are now available to the feline practitioner, and cat owners have come to expect safe and effective pain relief for their pets.11,12 Historically, however, feline pain management was neglected. 13 Thankfully this attitude has changed and advancements continue to be made, 14 but there are still areas for improvement. For example, pain assessment is not commonly performed in the clinical setting and validated pain assessment tools are not widely implemented in small animal practice. 15 Many veterinarians do not administer or prescribe analgesics to cats after discharge and following routine procedures (eg, neutering). 16
Here, the International Society of Feline Medicine (ISFM) provides its first guidelines on acute pain management using current evidence-based information and expert consensus. The Guidelines identify areas where critical research is needed and emphasise pain management as a key component of feline welfare. Throughout, the utility of a multi-modal and preventive analgesic approach is highlighted, involving non-pharmacological and pharmacological therapy, cat friendly handling techniques, and respect and empathy for feline patients.
Assessment and recognition of pain in cats
Principles of pain assessment
Cats are unique and so is their behaviour, especially when fear, anxiety and stress are present in the hospital setting. The assessment and treatment of pain is only one part of the pain management ‘puzzle’; every manipulation and physical examination should be performed using cat friendly handling techniques (Figure 1). 17 Pain management is not only about giving an analgesic drug: the emotional needs of the hospitalised cat must be considered (Figure 2), and the individual itself should always be treated with respect and empathy.
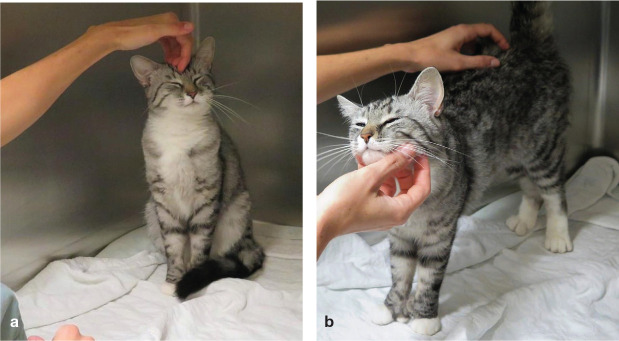
Figure 1.

Feline friendly handling techniques should be used for every interaction with cats. This will ensure optimal healthcare by reducing the cat’s stress, improving personnel safety and providing a positive outcome for the patient, client and veterinary team. After observation of the cat’s normal behaviour, the patient is calmly approached and stroked, and the painful area is gently palpated. Image courtesy of Paulo Steagall
Figure 2.

The hospital environment has an influence on the patient’s pain experience. A cat ward should be a quiet and comfortable area, considering the specific needs of this species. A hospital cage equipped with a cardboard box provides an elevated area where the cat can perch (‘a safe place’). Simple enrichment measures, such as this, can improve the hospital experience and reduce stress. Image courtesy of Paulo Steagall
Pain assessment is the first step towards appropriate treatment. Failure to recognise pain is likely the primary cause of inadequate treatment (oligoanalgesia; ie, undertreatment of acute pain). 16 Recognition of pain requires understanding of this species’ reactions within a hospital setting. Signs of pain may be subtle and misinterpreted; for example, when a cat simply reduces movement and ‘freezes’ due to fear. However, the veterinary care team and owners, too, can be trained to improve their pain assessment skills and several websites are available for this purpose.18–20 Used in isolation, physiological variables are not reliable in acute pain assessment, since they can be affected by disease, fear and stress.21–23 However, appetite may be a ‘pain indicator’, as painful individuals generally do not eat. 24
Overall, acute pain assessment is subjective, although subjectivity and bias can be reduced with specific guidelines and assessment tools (see later). In cats, and other animals, all assessments depend on the observations of a veterinary professional or carer and hence are termed ‘observer-related outcomes’. Assessment includes, but is not limited to, observation of posture, general behaviour, comfort with interactions, activity, attitude, body position and facial expressions. In conjunction with this, a dynamic and interactive approach is recommended, usually involving greeting the cat while performing gentle palpation of the wound and/or suspected painful area. This is an important step, otherwise evoked pain may be overlooked; it should be possible to palpate around surgical wounds without a strong reaction from the cat if pain is well controlled (Figure 3).
Figure 3.
Clinical pain assessment incorporates 1) observations of posture, general behaviour, comfort, activity, attitude, body position and facial expressions, and 2) a dynamic and interactive approach involving greeting the animal and performing gentle palpation of the wound/painful area. The response elicited during touch and palpation may help with clinical decisions and with determining the sensory-discriminatory domain of pain (intensity, location and duration). (a) A healthy cat is examined prior to surgery. After observing the cat’s normal behaviour, the cage is opened, and the patient is gently approached and stroked. (b) The cat responds to the interaction with the observer and shows a friendly demeanour. This should be maintained after surgery if the cat is not painful. Images reproduced from Steagall and Monteiro (2019) 5
Pain-related behaviours have been described in detail in cats (Table 1), particularly in those undergoing abdominal surgery and dental extractions (Figure 4).25,26 The development of new behaviours or loss of previous behaviours beyond the immediate postoperative period may be indicative of pain. For example, a friendly, pain-free cat normally interacts with members of the veterinary care team, tolerates gentle abdominal palpation and displays normal behaviours such as stretching, playing and yawning. The cat may sleep during hospitalisation in a ‘bagel’, ‘croissant’ or ‘pretzel’ curled-up position (Figure 5). These individuals usually have an interest in their surroundings and respond to stroking. Eating and grooming are normal behaviours that should be present in the postoperative period in comfortable cats. When a cat is painful, these behaviours may quickly disappear. It is important to note that the entire veterinary care team should be trained, and actively involved, in feline pain assessment.
Table 1.
Behaviours suggestive of acute pain in cats, depending on the source of pain
| Category of behaviour | Description of behaviour |
|---|---|
| Abdominal pain and various painful conditions | |
| Posture and activity | ✜ Crouched/hunched posture
✜ Laying in dorsolateral recumbency with pelvic limbs extended or contracted ✜ Laying down being quiet ✜ Abdominal muscle (flank) contraction ✜ Vocalisation (growling, groaning, howling, hissing) ✜ Depression/dull mentation ✜ Restlessness |
| Interaction with the observer and the environment | ✜ Lack of interest in the surroundings
✜ Withdrawing/hiding ✜ Facing the back of the cage ✜ Reluctance to move after stimulation ✜ Unwilling to play or interact with the observer ✜ Attempting to hide/escape from the observer ✜ Irritation ✜ Aggression/self-defensiveness |
| Other behaviours | ✜ Excessive attention to the wound (licking, biting)
✜ Reaction to palpation of the wound ✜ Decreased grooming and/or appetite |
| Facial expressions | ✜ Ears pulled apart and rotated outwards
✜ Squinted eyes ✜ Tense muzzle (elliptical shape) ✜ Straight whiskers (pointing forwards) ✜ Lowered head position (in relation to shoulder line) |
| Postoperative pain (eg, following dental extractions) | |
| General behaviours | ✜ Decreased activity
✜ Reluctance to move ✜ Decreased playfulness ✜ Difficulty prehending dry food ✜ Head shaking |
Adapted from Steagall (2020) 27
Figure 4.
(a) A cat with abdominal pain following ovariohysterectomy. The cat is depressed, immobile and silent. The patient has squinted eyes and is in a hunched-up position with head down. (b) A cat with abdominal pain secondary to constipation. The eyes are partially closed (‘feigned sleep’), the ears are pulled apart (ie, distance between the tips of the ears is increased when compared with a non-painful cat), and the muzzle is retracted backwards (whiskers are straight, not loose and curved). (c) Periodontal disease, such as gingivitis and periodontitis, causes pain, inflammation, dysphagia, chronic haemorrhage and weight loss. Painful cats with severe oral disease (eg, requiring multiple dental extractions) have decreased food intake, increased pain scores and present distinct behaviours such as reduced attention to surroundings and spending more time lying down, compared with pain-free cats with minimal periodontal disease. Following dental extractions, this cat scores 9/10 using the Feline Grimace Scale and requires the administration of opioids for pain relief. (d) A cat with abdominal pain can contract or extend its pelvic limbs and/or contracts its abdominal muscles (flank) spontaneously. These cats may be in dorsolateral recumbency with facial expressions of pain. (e) A tense and depressed cat in severe pain after sternotomy. This patient is sitting up due to intense discomfort, reluctant to move and not attentive to the surroundings. The cat received a score of 8/10 using the Feline Grimace Scale, indicating the need for analgesia. The cat remained uncomfortable and bothered by all bandages, catheters and nursing needs, despite infusions of opioids, ketamine and lidocaine, and the administration of NSAIDs and intercostal nerve blocks. This demonstrates that pain can be challenging to treat in some cases. The cat finally improved and analgesic infusions were tapered down before patient discharge. Images (a), (c), (d) and (e) courtesy of Paulo Steagall. Image (b) reproduced from Steagall and Monteiro (2019) 5
Figure 5.

The curled-up position of this pain-free cat is a normal posture to conserve body heat and is not usually observed in painful cats. Image reproduced from Steagall and Monteiro (2019) 5
Pain-related behaviours in cats
Pain assessment is best performed on a regular basis. However, the cat should not be disturbed while it is eating, grooming, playing and/or sleeping. The frequency of assessment should be determined based on the pain severity noted on the first assessment after surgery or trauma, the cat’s response to analgesic administration or non-pharmacological intervention and clinical judgement. Cats may be evaluated as soon as 30–45 mins after the end of surgery and then every hour in the initial postoperative period, depending on the analgesic drugs and techniques used. Assessment should be continued to ensure comfort and until the patient is discharged. 26 After discharge, assessment can continue at home with cat carers trained to look for signs of pain.
A detailed review of feline acute pain assessment is available elsewhere. 5

Pain assessment tools
The use of pain assessment tools allows a consistent, practical and more objective approach to pain evaluation. In a busy clinical setting when several team members participate in pain assessment, use of a structured tool reduces inter-observer variability.
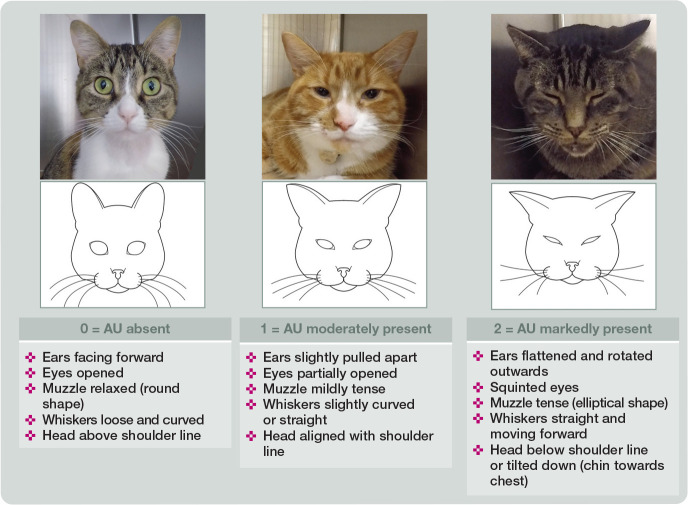
There are now three user-friendly pain assessment tools with reported validity in cats (Table 2): the Glasgow composite measure pain scale-feline, 7 the UNESP-Botucatu multidimensional feline pain assessment scale short form (Table 3) and the Feline Grimace Scale.7–9,24,28–32 The challenges facing us are how to disseminate this information, how best to incorporate these tools/systems into practice and how to train the veterinary clinic team. Facial expressions are incorporated into all three of these acute pain assessment tools; the Feline Grimace Scale (felinegrimacescale.com) is based entirely on facial ‘action units’ (Figure 6).8,29,30,33 These scales provide a cut-off/intervention score that suggests administration of analgesia is required (Table 2). Guidance to assist the veterinary nurse or technician in advocating for their patient, and the clinician with decision-making, is a clear benefit of using pain assessment tools. If the cat receives rescue intervention(s), pain assessment is repeated after 30–45 mins to ensure that treatment has been effective.
Table 2.
Overview of feline acute pain assessment tools
| UNESP-Botucatu multidimensional feline pain assessment scale short form (UFEPS-SF) | Glasgow composite measure pain scale-feline (Glasgow CMPS-Feline) | Feline Grimace Scale | |
|---|---|---|---|
| Components | ✜ Posture
✜ Comfort ✜ Activity ✜ Attitude ✜ Reaction to touching and palpation |
✜ Vocalisation
✜ Posture and activity ✜ Attention to wound ✜ Ear position ✜ Shape of muzzle ✜ Response to interaction with the observer ✜ Palpation of painful area ✜ Qualitative behaviour |
‘Action units’:
✜ Ear position ✜ Orbital tightening ✜ Muzzle tension ✜ Whisker position ✜ Head position |
| How to use the tool | There are four questions (0-3). The sum of the scores for each question is the final score for that cat (total of 12 points). Evaluation starts with observation of the undisturbed cat. Then the cat is approached for evaluation of the remaining items | Each item is scored (scores vary from item to item). The sum of the scores for all eight items is the final score for that cat. Evaluation starts with observation of the undisturbed cat, including its facial expressions. Then the cat is approached for evaluation of the remaining items | Each action unit is rated from 0 to 2, where 0 = action unit is absent; 1 = moderate appearance of the action unit, or uncertainty over its presence or absence; and 2 = obvious appearance of the action unit.
Non-visible action units are not scored. The final score is the sum of scores divided by the number of scored action units |
| Validation of tool | Comprehensive validity, reliability, sensitivity and sensibility in cats with postoperative pain following ovariohysterectomy | Evidence of construct validity and responsiveness in cats with a variety of painful conditions | Shown to be valid and reliable in cats with varied painful conditions in both real-time and image assessment, and in cats before and after dental extractions. The tool is not affected by the presence of a caregiver nor by the administration of acepromazine-buprenorphine. Inter-observer reliability has been reported for cat owners, veterinary nurses, and students and veterinarians |
| Intervention score * | ≥4/12 | ≥5/20 | ≥0.4/1.0 |
| Comments | Can be used for both medical and surgical pain with reported validity. The short-form instrument overcomes the limitations of the previous long form | Easy and quick to use | Reported high discriminative ability, good overall inter-observer reliability, excellent intra-rater reliability and excellent internal consistency. Easy and quick to use |
| Source | Video-based training on feline acute pain and use of the scale is available in the Animal Pain website | newmetrica.com/acute-pain-measurement/download-pain-questionnaire-for-cats | felinegrimacescale.com |
Adapted from Steagall (2020) 27
‘Cut-off’ value at which point administration of analgesia is required; note a veterinarian may administer rescue intervention when the cut-off is not reached if it is considered that the cat may be in pain
UNESP = Universidade Estadual Paulista; UFEPS = UNESP Feline Pain Scale
Table 3.
UNESP-Botucatu multidimensional feline pain assessment scale short form (UFEPS-SF)
| Item | Description | Score |
|---|---|---|
| Evaluate the cat’s posture in the cage for 2 mins | ||
| 1 | ✜ Natural but tense, does not move or moves little or is reluctant to move | 1 |
| ✜ Hunched position and/or dorsolateral recumbency | 2 | |
| ✜ Frequently changes position or restless | 3 | |
| ✜ The cat contracts or extends its pelvic limbs and/or contracts its abdominal muscles (flank)* | □ | |
| 2 | ✜ The cat’s eyes are partially closed (do not consider this item, if present, until 1 h after the end of anaesthesia)* | □ |
| ✜ The cat licks and/or bites the surgical wound* | □ | |
| ✜ The cat moves its tail strongly* | □ | |
| ✜ All of the above behaviours are absent | 0 | |
| ✜ Presence of one of the above behaviours | 1 | |
| ✜ Presence of two of the above behaviours | 2 | |
| ✜ Presence of three or all of the above behaviours | 3 | |
| Evaluation of comfort, activity and attitude after the cage is open and how attentive the cat is to the observer and/or surroundings | ||
| 3 | ✜ Comfortable and attentive | 0 |
| ✜ Quiet and slightly attentive | 1 | |
| ✜ Quiet and not attentive. The cat may face the back of the cage | 2 | |
| ✜ Uncomfortable, restless and slightly attentive or not attentive. The cat may face the back of the cage | 3 | |
| Evaluation of the cat’s reaction when touching, followed by pressuring around the painful site | ||
| 4 | ✜ Does not react | 0 |
| ✜ Does not react when the painful site is touched, but does react when it is gently pressed | 1 | |
| ✜ Reacts when the painful site is touched and when pressed | 2 | |
| ✜ Does not allow touch or palpation | 3 | |
From Belli et al (2021) 9 *
Tick where applicable
UNESP = Universidade Estadual Paulista; UFEPS = UNESP FEline Pain Scale
Figure 6.
Illustration and description of the Feline Grimace Scale. This pain assessment tool is composed of five action units (AU) (ear position, orbital tightening, muzzle tension, whisker position and head position). Each one is scored from 0 to 2: 0 = action unit is absent; 1 = moderate appearance of the action unit, or uncertainty over its presence or absence; and 2 = obvious appearance of the action unit. Adapted from Evangelista et al (2020) 29
Limitations and factors affecting pain assessment/recognition
The advent of novel pain assessment systems has improved our ability to recognise and treat pain in cats. However, these instruments have not fully eliminated potential confounding factors and bias. Temperament (fearful, shy, etc), the administration of some drugs (eg, sedatives or tranquillisers) and illness can still pose challenges during pain assessment.34–37 Fear and anxiety may bias pain assessment due to changes in posture, comfort and facial expressions. 38 For example, pain assessment can be a challenge in unsocialised cats in trap–neuter–return programmes (Figure 7). The administration of ketamine-based protocols produces a confounding effect early in the recovery period and may falsely increase pain scores, which could lead to unnecessary administration of rescue analgesia. 39 Residual anaesthetic and drug effects may also bias pain assessment. Pain may be difficult to differentiate from drug-induced dysphoria (see box).
Figure 7.
(a,b) Means of pain assessment can be limited in trap-neuter-return programmes or when working with other unsocialised cats. However, cats may still be evaluated using the Feline Grimace Scale, with observation of posture, comfort and facial expressions. Treatment of pain should be performed using a multimodal approach, based on the observer’s interpretation of how painful the cat’s condition could be, since wound palpation, for example, may not be possible. As with all patients, these individuals should be handled with empathy and care. Images courtesy of Sheilah Robertson
Pain scores may be influenced by the gender, training, cultural background and language of the observer, demonstrating that pain assessment is not an exact science and differences may be expected.34–36 It has been found that females and those with more training will provide higher pain scores than males and those with minimal training.13,34,36 Feline pain assessment should be integrated into the veterinary curriculum in conjunction with cat friendly handling techniques.
Cat carers should be considered part of the ‘team’ when it comes to pain management. Client education regarding pain behaviours (what to expect and how to proceed) is important and could lead to early intervention by prompting the client to seek veterinary attention. 5 For example, cat owners can identify specific behavioural alterations up to 3 days after castration or ovariohysterectomy. 42 A decrease in overall activity level and playfulness, an increase in the amount of time spent sleeping, and altered locomotion were reported by these owners. 42 ISFM has produced a Cat Carer Guide to acute pain in cats (see page 30). Finally, the Feline Grimace Scale can be used reliably by cat owners, veterinary nurses and students, providing the first feline acute pain assessment tool that may be used in the home environment 33 and by non-veterinarians, thereby paving the way towards better feline welfare.
Treatment of pain – principles and considerations
Failure to recognise and adequately treat acute pain is known as oligoanalgesia. The lack of analgesic administration to a painful cat may lead to the development of peripheral and central sensitisation,16,43 which in turn can reduce the nociceptive threshold to non-noxious and noxious stimuli (Table 4). 44 In humans, inadequate treatment of pain following surgical procedures including, but not limited to, amputation, thoracotomy, mastectomy 45 and caesarean section 46 can result in a transition to chronic or maladaptive pain states (ie, persistent postoperative pain), leading to disability and a negative impact on quality of life. A similar scenario may occur in cats; 43 for example, after onychectomy (Figure 8).
Table 4.
Glossary of terms used in the neurophysiology of pain
| Term | Explanation |
|---|---|
| Peripheral sensitisation | Reduced pain thresholds that occur following tissue damage, which are caused by an increase in the concentration of chemical mediators in the damaged tissue. There are many mediators of peripheral sensitisation and as a group they are called eicosanoids |
| Central sensitisation | Reduced pain thresholds that occur following tissue damage, which are caused by altered sensitivity of the nociceptive pathways because of repeated stimulation. The main sites of central sensitisation are the dorsal horn of the spinal cord and the higher centres of the brain, especially the cerebral cortex |
| Hyperalgesia | Increased pain due to stimulation of damaged tissue by a stimulus that would normally be perceived as painful. The stimulation is felt as more painful than it would be if the tissues were not damaged. Hyperalgesia is a clinical sign of peripheral and central sensitisation |
| Allodynia | Pain due to stimulation of damaged tissue by a stimulus that would not normally be perceived as painful. The stimulation is felt as painful because the tissues are damaged. Allodynia is a clinical sign of peripheral and central sensitisation |
| Persistent postoperative pain | Pain perceived following surgery that lasts longer than it usually would. Persistent postoperative pain is a common consequence of poor or absent pain relief at the time of surgery |
Adapted from Steagall et al (2021) 44
Figure 8.

Persistent postoperative pain in a 7-year-old female cat after onychectomy. The cat persistently keeps the left paw lifted while sitting, to avoid bearing weight on it, and also resents being touched on that paw. Image reproduced from Monteiro BP and Steagall PV. Chronic pain in cats: recent advances in clinical assessment. Journal of Feline Medicine and Surgery 2019; 21: 601–614
Understanding the principles of acute pain management aids in the development of an effective analgesic strategy and can mitigate or prevent oligoanalgesia and adverse outcomes in cats, a species in which analgesic administration has historically been neglected.
Feline drug metabolism and elimination: impact on analgesia
Drug metabolism and elimination are different in cats compared with other species. This has a significant influence on drug choice, dose and dosing intervals of analgesics. Drugs that are eliminated by metabolic conjugation (ie, glucuronidation, sulfation and/or glycosylation) are usually excreted more slowly in cats than in humans or dogs. 47 Examples include acetylsalicylic acid, acetaminophen (paracetamol), carprofen, ketoprofen and morphine. Deficient glucuronidation has significant effects on phenolic compounds such as acetaminophen, resulting in life-threatening methaemoglobinaemia due to oxidative injury to erythrocytes, anaemia and Heinz body formation.47,48 The clearance of carprofen is significantly slower in cats than in dogs (ie, the drug has a much longer elimination half-life in cats). 49 The deficient UDP-glucuronosyltransferase (UGT) pathways in cats may also be responsible for reduced morphine glucuronidation in cats. Cats eliminate morphine at a similar rate to dogs; however, they produce lower concentrations of morphine-3-glucuronide, and do not produce morphine-6-glucuronide, an active metabolite responsible for analgesia in humans. 50
Conversely, drugs that primarily undergo oxidation are often eliminated more rapidly in cats compared with dogs. Examples include meloxicam, piroxicam, buprenorphine and meperidine (pethidine). The major route of elimination of meloxicam in cats is via the gut (faeces), which is similar to dogs; the main pathway of meloxicam biotransformation is oxidation.51,52
Drugs that are excreted unchanged into urine and/or bile such as gabapentin have similar elimination half-lives in dogs and cats. 47
Feline comorbidities
The presence of comorbidities must be considered in the treatment of pain. As many as 40% of cats over 10 years of age have chronic kidney disease (CKD). 53 Drugs or metabolites that require healthy kidney function for excretion may accumulate in cats with CKD. Therefore, doses and dosing intervals may require adjustment and this will be based on clinical experience, the patient’s response and pain assessment. The effects of drugs may not be predictable because, in addition to decreased renal elimination in the face of CKD, azotaemia increases blood–brain barrier permeability, which can result in a more enhanced drug effect. A classic example is the metabolism of ketamine by the liver into the active metabolite norketamine, which is excreted by the kidneys in its active form along with ketamine itself. Ketamine and nor-ketamine accumulation may occur, with potential prolonged anaesthetic effects in cats with urethral obstruction or kidney dysfunction. Metabolic acidosis in cats with CKD can alter the biodisposition of ketamine, resulting in accumulation. Outcomes may depend on dose since accumulation does not appear to be a problem with the administration of subanaesthetic (anti-hyperalgesic) doses of ketamine given by infusion (10 µg/kg/min) as a component of a postoperative analgesic regimen after feline subcutaneous urethral bypass when renal function is improving. 27
Overall, best practice with respect to anaesthesia and analgesia is particularly important in cats with comorbidities. The administration of fluid therapy will correct dehydration and electrolyte and acid-base abnormalities while maintaining adequate renal blood flow during anaesthesia and supporting drug excretion. In some cases, the administration of a minimum effective dose of an NSAID can be considered for normovolaemic cats with stable renal disease (International Renal Interest Society [IRIS] stage I or II) undergoing surgery with a strong inflammatory component (eg, multiple dental extractions, fracture repair, large excisions) once blood pressure is controlled and there is no risk of intraoperative haemorrhage. Ideally, the cat should be eating and drinking after anaesthetic recovery before the administration of an NSAID. 54
Building an analgesic plan
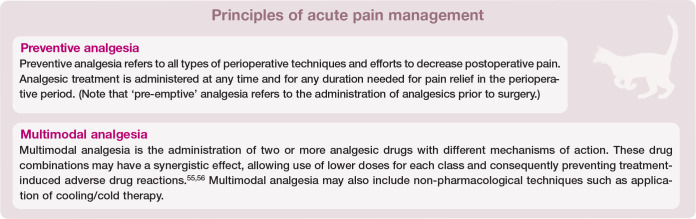
Building an effective analgesic plan relies on two fundamental principles: preventive analgesia and multimodal analgesia (see box above).
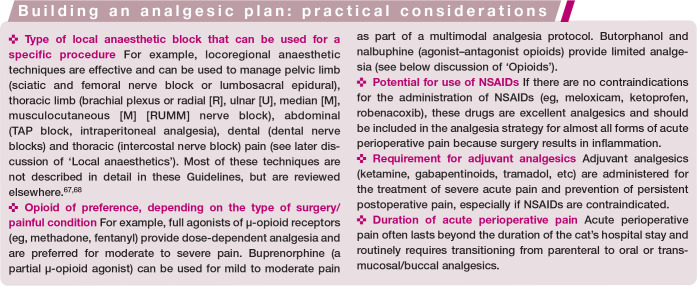
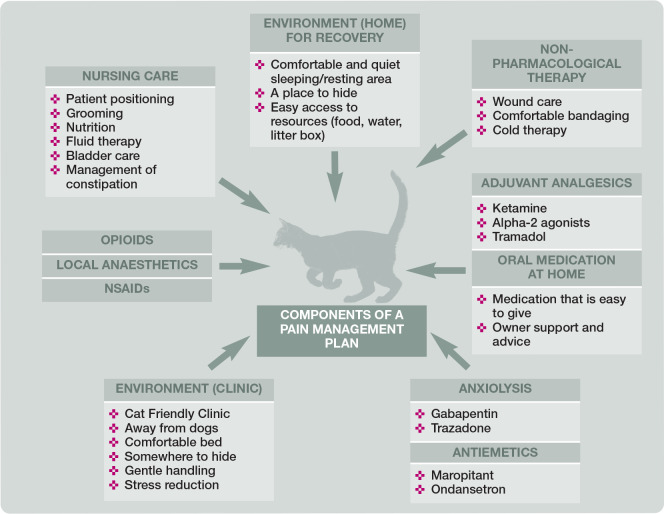
The first step of acute pain treatment involves the administration of opioids, local anaesthetics and NSAIDs. The second step of treatment involves the administration of adjuvant analgesics. 57 Further therapy should focus on analgesia and comfort, while minimising adverse drug reactions and preventing deleterious endocrine and physiological responses. Additional steps of equal importance include anxiolysis and muscle relaxation, antiemetics and non-pharmacological therapy, which should be incorporated when needed (Figure 9). Maropitant and ondansetron reduce perioperative vomiting in cats,58,59 and gabapentin or trazodone are often administered to reduce anxiety; for example, during hospitalisation.60,61 Non-pharmacological therapies should be incorporated when possible and include appropriate bandaging (wound care), cold therapy 62 (Figure 10), nursing (ie, patient positioning, fluid therapy, nutrition, cleaning and grooming), environment (ie, dry, calm, quiet and comfortable) (see Figure 2) and an area for cats that is separated from dogs (eg, as included in the requirements for ISFM Cat Friendly Clinic accreditation and the AAFP Cat Friendly Practice Program).17,63
Figure 9.
Successful management of acute pain does not just include use of analgesic agents – a combination of components is fundamental to a successful pain management plan
Figure 10.
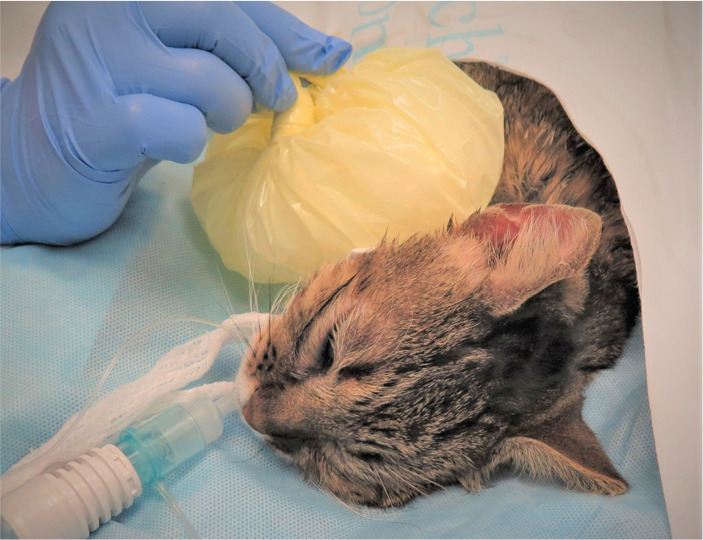
A cat undergoing ice therapy after total ear canal ablation. Cold therapy is an example of a non-pharmacological tool that provides effective analgesia, and is readily available. Image courtesy of Paulo Steagall
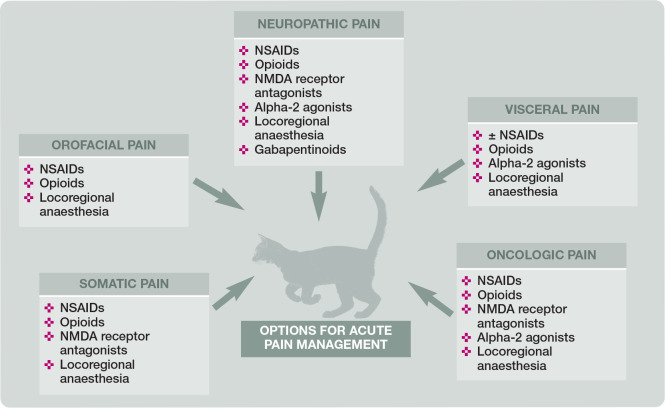
Treatment will be determined by the type, location, severity and expected duration of stimuli, and whether the cat is an inpatient or outpatient. The analgesic plan will be based on these key concepts, which can be referred to using the acronym ‘TELLS’ (Table 5 and Figure 11). For example, acute visceral pain associated with elective procedures (eg, feline neutering) may be treated effectively with NSAIDs, opioids, alpha-2 agonists64,65 and local anaesthetic techniques (eg, intratesticular, incisional, intraperitoneal or transversus abdominis plane [TAP] block). Acute somatic or neuropathic pain associated with orthopaedic or vertebral surgery/spinal cord injury may require the addition of N-methyl D-aspartate (NMDA) receptor antagonists (eg, ketamine, amantadine) and gabapentinoids.
Table 5.
Key concepts (TELLS) used for pain management,* with specific examples
| Examples | Comments | |
|---|---|---|
| Type of noxious stimuli | Visceral, neuropathic, somatic, orofacial, oncologic | Certain analgesic drugs may be more effective for certain types of pain. For example, analgesic drugs that help treat peripheral or central sensitisation (eg, ketamine or methadone) may be an appropriate choice in cases of neuropathic injury |
| Expected duration of noxious stimuli | Transient stimuli of <2 h (placement of a urinary catheter or endoscopy) vs prolonged and sustained (24-72 h) stimuli, following surgery | This should affect frequency and need for pain assessment. Analgesic drug may be determined based on duration of action. Hospitalisation and analgesic infusions may be required |
| Location of noxious stimuli | Thoracic limb, pelvic limb, orofacial, abdominal, intrathoracic | Useful when considering locoregional techniques |
| Location of patient during treatment | Outpatient or inpatient | This may influence the route and frequency of analgesic administration (dosage regimens). Also the frequency of pain assessment |
| Severity of noxious stimuli | Mild, moderate or severe | May affect the dose and choice of analgesic drug class |
These concepts help determine the dose, frequency, type and route of analgesic drug(s) to be provided
Figure 11.
Suggestions for acute pain management in patients with specific types of pain. NSAID = non-steroidal anti-inflammatory drug; NMDA = N-methyl D-aspartate
The expected duration and severity of pain also determines which analgesic drugs are best suited for each individual patient. In cats with prolonged moderate to severe pain, pain management can be performed with intermittent doses of buprenorphine 66 or methadone. Alternatives include constant rate infusions (CRIs) of opioids and/or ketamine. Specific classes of analgesics are discussed below.
The inpatient or outpatient nature of the treatment may affect the frequency and route of administration. Hospitals without after-hours or overnight staffing that either discharge patients or leave them unattended overnight may rely on long-lasting oral, sustained-release or transdermal analgesic drugs. However, some patients may require intense postoperative monitoring and analgesia, and should be transferred, if possible, to clinics that can provide such care (eg, CRIs of analgesics, fluid therapy, wound care and monitoring).
Further practical considerations when building an analgesic plan in cats are outlined in the box above.
Treatment of pain – analgesic drugs
Opioids
Opioids are commonly used analgesics for acute pain in cats.14,69 By binding to opioid receptors located within the peripheral and central nervous system they decrease the release of excitatory neurotransmitters, hyperpolarise the neuronal post-synaptic cell membrane,70–73 inhibit Ca2+ influx in afferent sensory neurons, and activate the descending inhibitory pathways. The net result is an antinociceptive effect, thus producing analgesia.
The popularity of opioids is attributable to a remarkable safety profile, potential reversibility and analgesic efficacy. Opioids can be given by a variety of routes and can reduce the minimum alveolar concentration (MAC) of volatile anaesthetics, potentially improving cardiopulmonary function during general anaesthesia. 74 However, their ability to reduce MAC in cats when administered parenterally75,76 or epidurally is lower than in dogs. For example, high doses of remifentanil reduce isoflurane requirements by a maximum of 25% in cats,77,78 compared with 79% in dogs. 79 In cats, additional anaesthetic sparing can be achieved with the inclusion of ketamine infusions. 80
At effective opioid doses, mydriasis, purring, kneading and euphoria will occur (see box on page 14).
Table 6 presents suggested dosage regimens for commonly used opioid analgesics, with comments on their clinical relevance, advantages and disadvantages. Choosing an opioid analgesic is dependent on the patient’s signalment, temperament, comorbidities, degree of pain intensity and required sedation level, and is also determined by the planned procedure, the pharmacology of the opioid itself (onset, magnitude and duration of action) and what the clinician has access to in their location. Kittens and young cats may require more frequent opioid dosing. One study showed that age can influence thermal antinociception produced by opioids in cats. 88 Specifically, hydromorphone provided a shorter duration and smaller magnitude of antinociception in cats of 6 months of age than in the same individuals at 9 and 12 months of age. 88
Table 6.
Commonly used opioid analgesics in cats, with suggested doses and routes of administration* (continued on page 16)
| Degree of acute pain management (examples) | Drug | Dose (mg/kg or µg/kg where indicated) and potential routes of administration | Duration of analgesia | Comments |
|---|---|---|---|---|
| Pure µ-opioid receptor agonist | ||||
| Moderate to severe pain (orthopaedic surgery, abdominal exploratory, cystotomy, ovariohysterectomy, neuropathic pain, thoracotomy, large or multiple mass removal[s], pancreatitis, and those listed below for other classes of opioid) | Morphine | ✜ 0.1-0.2 IV, IM or epidural | ✜ Up to 6 h for IV
or IM ✜ Up to 12-24 h for epidural |
Caution with histamine release when administered IV. Administer slowly IV. Vomiting and nausea commonly occur, particularly with IM or SC route. Urinary retention may be observed following epidural administration |
| Hydromorphone | ✜ 0.025-0.1 IV or IM | ✜ Up to 6 h for IV
or IM |
Vomiting and nausea commonly occur, particularly with IM or SC route. Opioid-induced hyperthermia occurs more frequently with the use of high doses of hydromorphone, either alone or in combination with other anaesthetic drugs. Increases in temperature are generally mild to moderate and are self-limiting (<4 h) | |
| Meperidine (pethidine) | ✜ 5-10 IM | ✜ 1-2 h | Should not be administered IV due to histamine release with potential skin reactions and hypotension. Duration of analgesia is short-lived and dose-dependent. Licensed for IM injection in European countries, including the UK | |
| Methadone | ✜ 0.2-0.6 IV, IM or
OTM |
✜ Up to 6 h | Has NMDA receptor antagonistic effect, which may be helpful to treat peripheral and central sensitisation. Minimal vomiting and nausea when compared with hydromorphone and morphine administration. Higher doses are used for OTM administration, whereas lower doses are given IV. Licensed for use in cats in the UK, Canada, Italy and some other European countries | |
| Fentanyl | ✜ 5 µg/kg bolus followed by infusion from 3-20 µg/kg/h IV
✜ 25 µg/h transdermal patch |
✜ Provides analgesia for the duration of the infusion only
✜ Up to 72 h |
Low doses are used for analgesia, whereas high doses may also provide some (15-20%) anaesthetic-sparing effect with potential simultaneous sympathetic activation.
Plasma concentrations are highly variable with use of transdermal patches (human formulations) of fentanyl in cats. Analgesia is variable and may be suboptimal. Apply fentanyl patch 18-24 h prior to noxious stimuli |
|
| Remifentanil | ✜ 6-40 µg/kg/h IV | ✜ Provides analgesia for the duration of the infusion only | Low doses are used for analgesia, whereas high doses may also provide some (15-20%) anaesthetic-sparing effect with potential simultaneous sympathetic activation. Loading doses of remifentanil are not required if started 15 mins prior to surgical stimulation. May contribute to opioid-induced hyperalgesia; however, this has not been confirmed in cats. Does not require hepatic metabolism; undergoes degradation via non-specific plasma and tissue cholinesterase | |
| Mild to moderate pain (castration,
ovariohysterectomy, small mass removal, osteoarthritis, endoscopy, urinary catheterisation, and those listed below for agonist-antagonist opioids) |
B uprenorphine – standard formulation
(0.3 mg/ml) |
✜ 0.02-0.04 IV, IM
or OTM |
✜ Up to 8 h | SC administration using the standard formulation is not recommended. There is no histamine release, vomiting or nausea after the administration of buprenorphine. Possible OTM administration for ‘hands-off’ analgesia, especially as part of multimodal analgesia. Both formulations when combined with an NSAID provide adequate analgesia for dental and neutering procedures. Not sufficient for acute pain relief following major orthopaedic procedures, especially when used as a sole analgesic. Licensed for use in cats in the UK, Canada, Australia and other countries. The high-concentration formulation is FDA-approved in the USA |
|
Buprenorphine – high
concentration (1.8 mg/ml) |
✜ 0.24 SC | ✜ Up to 24 h | ||
| Mixed µ-opioid receptor antagonist/K-agonist | ||||
| Mild pain or to help facilitate diagnostic procedures and sedation (phlebotomy, cystocentesis, physical examination, fine-needle aspiration) | Butorphanol | ✜ 0.2-0.4 IV or IM | ✜ 1-2 h | Provides appropriate analgesia when used in a multimodal protocol for orchiectomy. Does not provide adequate analgesia when used as a sole analgesic for routine surgical procedures. Higher doses do not provide additional analgesic benefit. Large inter-patient variability in analgesia has been reported. May be a better alternative than naloxone when reversing (0.02-0.05 mg/kg IV) pure opioid receptor agonists due to its ability to preserve some antinociception |
| Nalbuphine | ✜ 0.15-0.3 IV or IM | ✜ Not yet determined | May be a substitute for when butorphanol is unavailable. Minimal research has been performed to evaluate effectiveness for acute pain management. Dose to provide analgesia is yet to be determined | |
| µ-opioid receptor antagonist (reversal agent) | ||||
| Naloxone | ✜ 0.01-0.04 IV diluted to 5 ml and given slowly to effect | NA | Fast administration of 0.04 mg/kg IV can produce depressed mentation and dullness (B Simon, personal observations). Administer slowly in small increments every 60 s and to effect. Analgesia will be reversed and other analgesic techniques and drugs must be considered if pain is an issue | |
Doses, drugs and routes of administration presented in this table are suggestions and not exhaustive. Individual variability should be appreciated and opioids are better administered as part of multimodal analgesia. The analgesic effects may be variable depending on the type of pain, age, breed, surgical procedure, anaesthetic duration and route of administration. Clinical judgement is important. Veterinarians should consult drug labels if a veterinary product is available
NA = not applicable; IV = intravenous; IM = intramuscular; SC = subcutaneous; OTM = oral transmucosal; NSAID = non-steroidal anti-inflammatory drug; NMDA = N-methyl D-aspartate; FDA = Food and Drug Administration
Routes of administration
Traditionally, opioids are administered by the intramuscular (IM) or intravenous (IV) route. The onset, magnitude and duration of analgesia are influenced by the route of administration, which should be chosen on an individual basis. 89 The IV route provides reliable analgesia and a rapid onset of action. The subcutaneous (SC) route of administration provides less pain on injection than the IM route; 90 however, depending on the drug, dosage and formulation, large variations in the magnitude and duration of antinociception may be observed.82–84 For example, analgesia may be suboptimal after the SC administration of recommended doses of buprenorphine or hydromorphone in cats. 84 In contrast, a high-concentration formulation of buprenorphine (1.8 mg/ml; Simbadol, Zoetis) is approved by the US Food and Drug Administration and provides up to 24 h of mild to moderate pain relief following SC administration (Table 6). 85 This formulation is also effective when given to cats by the IV or oral transmucosal (OTM) route (off-label administration), both providing up to 8 h of analgesia. 85 The OTM route is a non-invasive alternative to parenteral administration. It bypasses the first-pass effect in the liver, leading to higher bioavailability when compared with oral administration. Variations in analgesia may still occur via the OTM route, and thus the IM or IV route is preferred when possible for acute postoperative pain management. 82
In cats, plasma drug concentrations following application of a transdermal fentanyl patch (a human product) are highly variable and may fail to produce analgesia.91,92 A trans-dermal matrix patch of buprenorphine failed to produce thermal antinociception, with high plasma concentration variability of the drug, in a preliminary study of pharmacokinetics in cats. 93
Epidural administration of opioids alone (eg, morphine or buprenorphine 94 ) provides effective control of visceral 95 and somatic 96 pain, with few adverse effects97–99 and without changes in proprioception. The bladder should be gently expressed before anaesthetic recovery to avoid the rare but reported side effect of urinary retention after the administration of epidural morphine.96,100 Regardless of which analgesic drugs are administered, an empty bladder may improve comfort during the early recovery period.
Adverse drug reactions
Some opioids may produce adverse effects, which are drug-, dose- and route-dependent (Table 6). Nausea and vomiting (Figure 12), gastro esophageal reflux, hyperthermia, dysphoria, mydriasis (Figure 12), sedation, vagally induced bradycardia, and respiratory depression with mild hypercapnia may be observed following the administration of opioids. Antiemetics (maropitant, ondansetron), prokinetics (metoclopramide), and acupuncture 101 can minimise or treat some of the adverse gastrointestinal (GI) effects associated with the administration of opioids.102–105 These GI adverse effects can be avoided by choosing methadone, fentanyl or buprenorphine. The administration of epidural opioids provides effective long-term analgesia with few side effects.98,99 Naloxone or butorphanol may be administered to effect when antagonising opioid-induced adverse effects. Analgesia may also be decreased or antagonised in this scenario and other analgesic techniques should be in place or considered.
Figure 12.
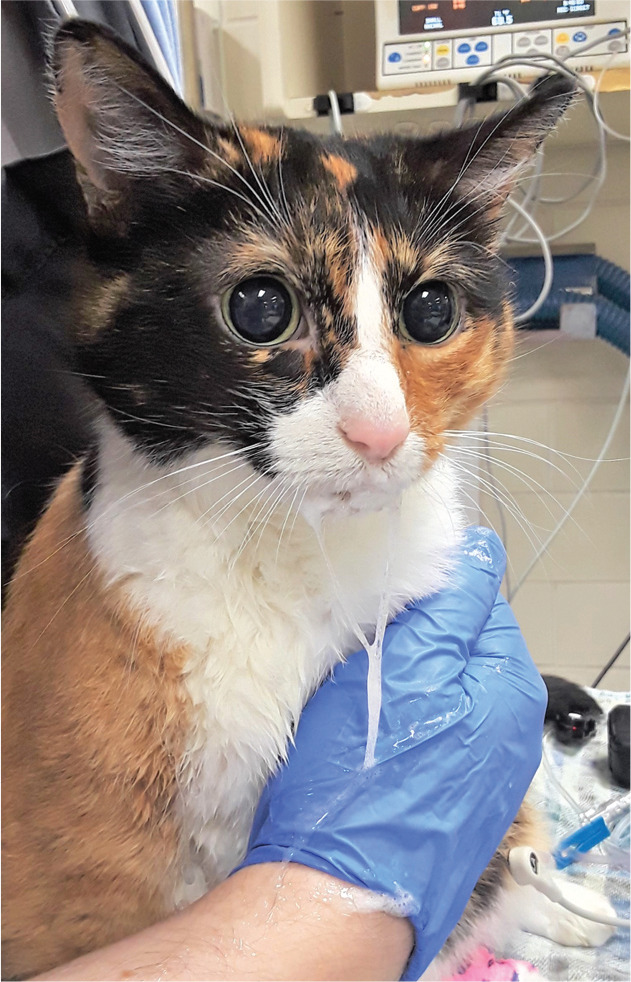
Mydriasis and hypersalivation in a cat following the intravenous administration of hydromorphone. Image courtesy of Bradley Simon
Non-steroidal anti-inflammatory drugs
NSAIDs are used extensively in cats for their analgesic, anti-inflammatory and antipyretic effects, and are crucial in the control of postoperative pain. After any contraindication for their use has been ruled out (GI disease, NSAID intolerance, unmanaged renal disease, hepatic disease, coagulopathies, hypovol-aemia and hypotension, concurrent NSAID or corticosteroid administration), NSAIDs are highly effective for the treatment of acute pain in cats as part of a multimodal approach.106–114 Recommended NSAID regimens for management of acute pain in cats are summarised in Table 7.
Table 7.
Common NSAIDs used in cats for management of acute pain*
| COX selectivity † | Formulation | Dose, route of administration, frequency | Dosing information | Indications and comments | Reference(s) |
|---|---|---|---|---|---|
| Meloxicam | |||||
| COX-2 preferential | Injection; 5 mg/ml | 0.3 mg/kg, SC, once | Administer once only | Postoperative analgesia following OVH and minor soft tissue surgery. This is the approved dose in the USA. However, the Guidelines panel members recommend using the lower doses, as listed below | 115 |
| Injection; 2 mg/ml | 0.2 mg/kg, SC, once | Can be followed by 0.05 mg/kg PO q24h for 4 days | Mild to moderate postsurgical pain | 115 | |
| Oral
suspension; 0.5 mg/ml |
0.1 mg/kg, PO, day 1; then 0.05 mg/kg, PO, q24h | Can be given indefinitely | Inflammation and pain in chronic musculoskeletal conditions.
These are approved doses in the EU. A minimum effective dose should be used for long-term treatment |
107, 115 | |
| Robenacoxib | |||||
| COX-2 selective | Injection; 20 mg/ml | 2 mg/kg, SC, q24h | Up to 3 days | Pain and inflammation associated with soft tissue surgery | 109, 116 |
| Tablets; 6 mg | 1 mg/kg, PO, q24h | Up to 6 days | Pain and inflammation associated with musculoskeletal disorders | 112, 115 | |
| Ketoprofen | |||||
| None | Injection; 10 mg/ml | 2 mg/kg, SC, q24h | Up to 3 days | Relief of acute pain and inflammation associated with musculoskeletal and other painful disorders | 115 |
| Tablets; 5 mg | 1 mg/kg, PO, q24h | Up to 5 days; can use injection instead on day 1 | As for ketoprofen injection | ||
Veterinarians should consult drug labels in their respective countries before administering NSAIDs
COX-2 preferential = greater suppression of COX-2 than COX-1; COX-2 selective = virtually no COX-1 suppression
COX = cyclooxygenase; OVH = ovariohysterectomy; SC = subcutaneous; PO = oral
Adverse effects (GI irritation, protein-losing enteropathy and nephrotoxicity) are reported when recommended doses or dosing intervals are not adhered to, or if NSAIDs are administered in combination with corticosteroids. Anorexia, inappetence, diarrhoea, vomiting and depression are usually the first signs of toxicity, and treatment should be stopped immediately if any of these occur. However, omitting NSAIDs because of fear of adverse effects is not recommended and may seriously compromise pain management.
Dosing should be based on lean body weight and cats should be normovolaemic before drug administration. Some postoperative patients may benefit from prolonged NSAID administration and this should be considered on a case-by-case basis. 115
Meloxicam and robenacoxib have good palatability, which facilitates treatment compliance,113,117 and once a day dosing also increases the likelihood of the cat receiving the prescribed medication. Injectable formulations are used in the perioperative period and can be administered IV (off-label use) or SC, depending on the drug used and country. Oral administration usually follows in the postoperative period and home environment. Many studies have shown that different perioperative NSAIDs usually perform similarly in terms of analgesic efficacy and duration of effect (ie, non-inferiority or comparison trials).110,111,113
The timing of NSAID administration is a controversial subject. NSAIDs can generally be administered preoperatively for routine neutering of healthy cats, especially if blood pressure is monitored and cats are normo-volaemic. For longer procedures, fluid therapy may be indicated, blood pressure should be monitored, and the risks and benefits related to the timing of NSAID administration need to be taken into consideration. 107 The administration of NSAIDs in patients with comorbidities such as CKD was discussed in the earlier section ‘Feline drug metabolism and elimination: impact on analgesia’.
Local anaesthetics
Local anaesthesia provides excellent analgesia by preventing transduction and transmission of nociceptive signals from the periphery to spinal and supraspinal centres. 118 Compared with other antinociceptive drugs, local anaesthetics are unique in providing complete analgesia. Locoregional anaesthesia is an important part of multimodal analgesia and should be incorporated into a pain management plan whenever possible. Anaesthetic maintenance and recovery are usually smooth when these techniques are integrated into the plan. Other advantages of locoregional anaesthesia include low cost, accessibility in all countries, muscle relaxation, decreases in injectable and volatile anaesthetic requirements, and, in turn, improved cardiopulmonary function.119–122 Additionally, the effects of long-acting local anaesthetics extend into the postoperative period, and reduce the requirements for other drugs.121,123 Recently, a liposomal bupivacaine formulation was approved for use in cats undergoing onychectomy in the USA and may provide postoperative analgesia for up to 3 days. 124 This formulation has been used off-label for incisional anaesthesia (see later). 124
Commonly used local anaesthetics are presented in Table 8.118,125 Local anaesthetic toxicity can result in severe neurological signs, cardio-respiratory collapse and death. Therefore, calculation of doses, which must always be accurately drawn up into syringes, is particularly important in cats due to their small size, especially with bupivacaine due to its cardiotoxicity. Accidental intravascular administration can be avoided by applying negative pressure to the syringe plunger before injection118,125 and repositioning the needle if blood is obtained. The most serious adverse effect reported for local anaesthetics is cardiac arrest and intravenous administration of lipid emulsion may reverse bupivacaine-induced toxicity. 127
Table 8.
Commonly used local anaesthetics for locoregional anaesthesia in cats
| Local anaesthetic | Recommended maximum dose | Onset (mins) | Duration (h)* |
|---|---|---|---|
| Lidocaine | 5 mg/kg | 1–2 | 1–2 |
| Ropivacaine | 3 mg/kg | 10–15 | 3–5 |
| Bupivacaine | 2 mg/kg | 10–15 | 4–6 |
| Liposomal bupivacaine | 5.3 mg/kg | Not determined in cats.
Several minutes in people |
24–72 |
| Local anaesthetic cream (eutectic mixture of lidocaine 2.5% and prilocaine 2.5%) | 1 ml or 1 g applied to intact skin | 30–60
(20 mins were enough to abolish response in dexmedetomidine-opioid-sedated cats 126 ) |
Not determined in cats. Several hours in people |
The information in this table represents a consensus of opinion of the Guidelines panel members
The duration of a block may vary depending on the block, technique, site of injection, anaesthetic concentration and volume, and in terms of sensory versus motor block
Lumbosacral epidural administration of opioids combined with local anaesthetics is sometimes used for the treatment of feline perioperative pain, particularly during orthopaedic procedures or hindlimb amputation. In cats, the spinal cord and dural sac terminate at the first (S1) and third (S3) sacral vertebra, respectively, while the end of the conus medullaris is located within the sacral canal (Figure 13). 128 These anatomical features make the risk of intrathecal drug administration more likely compared with dogs. 128
Figure 13.
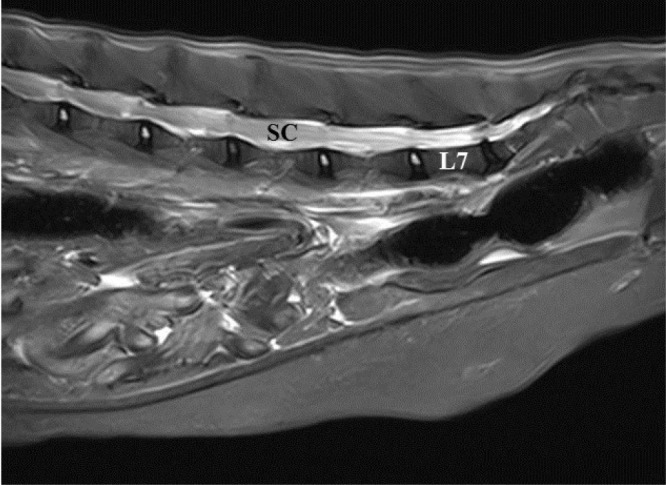
Magnetic resonance image of a feline lumbar and sacral spinal column. Note the spinal cord (SC) extending beyond the 7th lumbar (L7) vertebra. Image courtesy of Wade Friedeck, Texas A&M University College of Veterinary Medicine & Biomedical Sciences
Sacrococcygeal epidural anaesthesia has been used as an alternative to lumbosacral epidural injections for cats with urethral obstruction (Figure 14).129,130 A sacrococcygeal technique using bupivacaine or bupivacaine/morphine reduced propofol requirements and extended the time to rescue analgesia in these cats. 129 Anatomical and ultrasonographic studies have shown that the depth of needle insertion for sacrococcygeal epidural anaesthesia is approximately 0.6 cm in cats, whereas the distance between skin and the ligamentum flavum is 1 cm at the lumbosacral space. 131 The epidural space is small in cats but the technique can provide excellent perioperative analgesia and be performed safely. Appropriate anatomical landmarks should be considered and an aseptic technique used.
Figure 14.
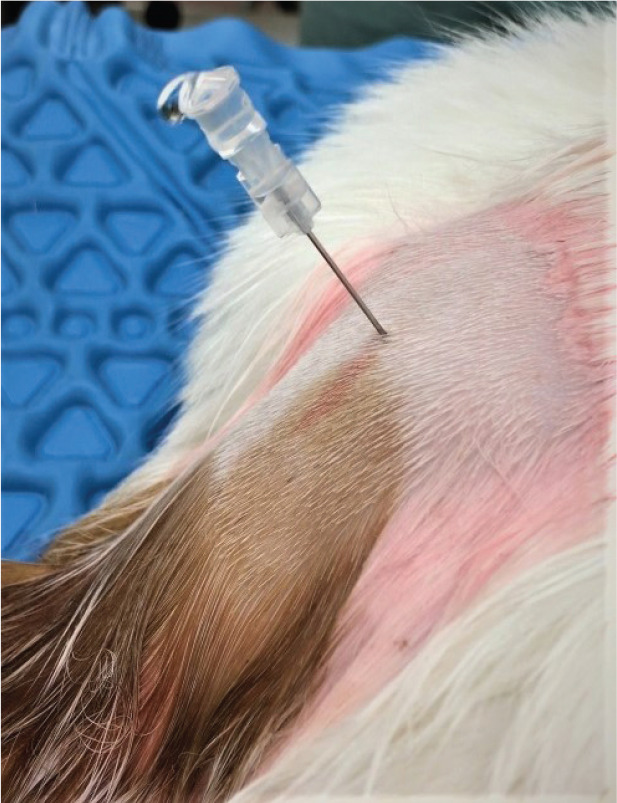
Sacrococcygeal epidural administration of local anaesthetics can be used in cats with urethral obstruction as an adequate means of pain management. Leakage of spinal fluid is less likely at this location but can occur, as shown in this image. Image courtesy of Paulo Steagall
Selected local and regional anaesthetic techniques
Several local anaesthetic techniques have been reported in cats, including sophisticated ultrasound-guided techniques, allowing a better understanding of anatomical landmarks, and improved safety and efficacy. These Guidelines provide an overview of easy-to-use, selected techniques with immediate application in feline practice (Table 9).
Table 9.
Summary of common local anaesthetic techniques used in cats
| Technique | Indications | Needle | Volume of local anaesthetic* | Landmarks | Comments |
|---|---|---|---|---|---|
|
Topical anaesthesia (lidocaine–prilocaine cream)
(Figure 15) |
Pain and stress associated with intravenous puncture or catheterisation | Not applicable (cream applied to skin) | 1 ml | Over the vein | Apply cream to clipped skin and cover with an occlusive bandage. Wait for onset – 20–30 mins in non-sedated cats |
|
Incisional (infiltration) analgesia
(Figure 16) |
OVH, castration and any other surgical procedure | 22-27 G, 1–1.5 inch | 1-2 ml or 0.4 ml/kg have been suggested, but generally this will depend on the length of the incision and on local anaesthetic concentration | Incision area | Can be performed prior to incision, but also after surgery for postoperative analgesia. It is recommended that the local anaesthetic is diluted if long incision/large volume is required |
|
Intraperitoneal analgesia
(Figure 17) |
OVH and other abdominal surgeries | 22 G, 1.16 inch catheter (without stylet) | 2 mg/kg 0.5% bupivacaine (0.4 ml/kg) diluted 1:1 with saline 0.9% (ie, 0.8 ml/kg) | Ovary pedicles and caudal uterine body, or ‘splash’ over the area of interest | Caution should be taken not to exceed the maximum recommended dose for cats (Table 8) |
|
Intratesticular block
(Figure 18) |
Castration | 25 G, 5/8 inch | 0.1-0.25 ml per testis | Into the centre of each testis | The testes become firm. Combination with incisional infiltration is recommended |
|
Dental blocks:
Infraorbital (Figures 19 [needle A] and 20) Inferior alveolar (mandibular) (Figures 19 [needle B] and 21) |
Dental cleaning, extractions, sutures, fracture repair, oral mass removal, maxillectomy and mandibulectomy | 25 G, 5/8 inch
or 27 G, 0.5 inch |
0.1–0.3 ml at each nerve (usually 0.2 ml per site) |
Infraorbital
Above premolar 3, into the infraorbital canal (4–5 mm). The infraorbital canal is short in cats and the needle should be introduced with caution to avoid globe penetration Inferior alveolar (mandibular) The lower angle of the jaw, against the medial side of the mandible in the direction of the mandibular foramen |
Infraorbital
Blocks innervation to the skin, hard and soft tissues of the rostral maxillae, including the teeth and dorsal nasal cavity Inferior alveolar (mandibular) Blocks innervations of the mandible including teeth, lower lip, part of the tongue, and hard and soft tissues |
The information in this table represents a consensus of opinion of the Guidelines panel members
Refer to Table 8 for the maximum recommended dose of the particular drug being used, to avoid toxicity OVH = ovariohysterectomy
Topical local anaesthesia
The administration of topical anaesthesia is simple and effective. In one study of cats undergoing radical mastectomy, topical application of 2 mg/kg bupivacaine in 30 ml of saline 0.9% via impregnated gauze decreased pain scores at 4 h postoperatively. 133 Although placing an intravenous catheter may seem a minor procedure, it should be remembered that each painful procedure a cat undergoes can have an additive effect and, therefore, every opportunity should be taken to decrease pain and stress. Topical local anaesthetic cream (lidocaine-prilocaine) (Table 9) reduces pain and stress during intravenous puncture or catheteristion (Figure 15), and is not systemically absorbed, thus methaemoglobinaemia or other adverse effects are avoided.126,134–136 In addition, the success rate for catheterisation is improved. 128
Figure 15.
A eutectic mixture of local anaesthetics (lidocaine and prilocaine cream) is used to desensitise the skin before venous catheterisation. The area is covered with an occlusive bandage for 20-30 mins before venepuncture. Adverse reactions have not been reported in cats. Image courtesy of Paulo Steagall

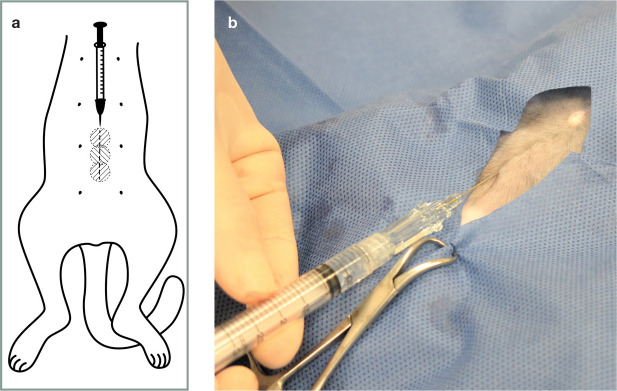
Incisional and intraperitoneal analgesia
The World Small Animal Veterinary Association (WSAVA) Global Pain Council recently published an evidence-based review on incisional (Figure 16) and intraperitoneal (Figure 17) techniques. 137 Readers are referred to that resource for a detailed discussion of indications and recommendations for use, as well as limitations. These techniques should be incorporated into multimodal pain management.132,138–143
Figure 16.
(a,b) Incisional anaesthesia is performed before or at the end of surgery by deposition of local anaesthetic subcutaneously at the incision line. Image (a) courtesy of Yael-Shilo Benjamini; image (b) courtesy of Paulo Steagall
Figure 17.
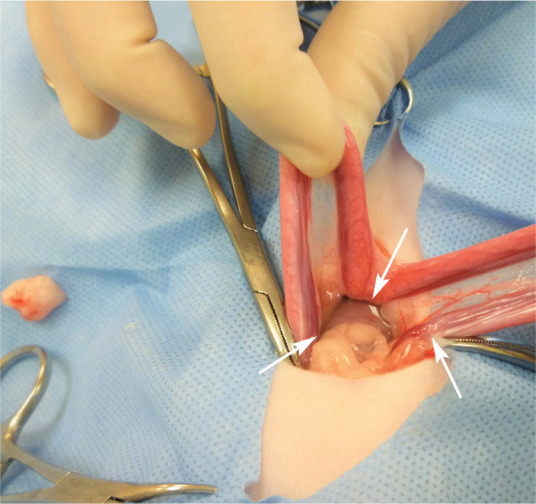
Intraperitoneal administration of local anaesthetic is performed before ovariectomy/ovariohysterectomy and other abdominal procedures. Local anaesthetics with a longer duration of action (ie, bupivacaine) are used, and the solution is divided into three parts, which are ‘splashed’ over the right and left ovarian pedicles and at the caudal aspect of the uterine body (arrows) using a 3 ml syringe. The solution is quickly absorbed by the peritoneum. A technique involving injecting bupivacaine (2 mg/kg) at the ovarian suspensory ligaments and vessels, uterine body and incisional subcutaneous tissues has also been described; 132 it resulted in lower postoperative pain scores compared with a control group and was most beneficial in larger cats (>2.7 kg). Image courtesy of Paulo Steagall
Intratesticular block
Intratesticular anaesthesia is a simple technique that may decrease sympathetic and haemodynamic responses during feline castration (Figure 18).120,144,145 It can be combined with incisional anaesthesia for complete antinociception (Figure 18b [needle B]). This technique should be performed under general anaesthesia or deep sedation, as injection and distension of the testicle can be painful.
Figure 18.
Intratesticular block. The needle is inserted into the centre of the testis and 0.1-0.25 ml of local anaesthetic is injected per testicle (a; b [needle A]). During injection the testis will become firm. The intratesticular injection can be combined with subcutaneous incisional infiltration, which is applied bilaterally at the scrotum (b [needle B]). The higher volume is used for larger testes while the lower volume is administered in kittens (c). Images (a) and (c) courtesy of Paulo Steagall; image (b) courtesy ofYael-Shilo Benjamini
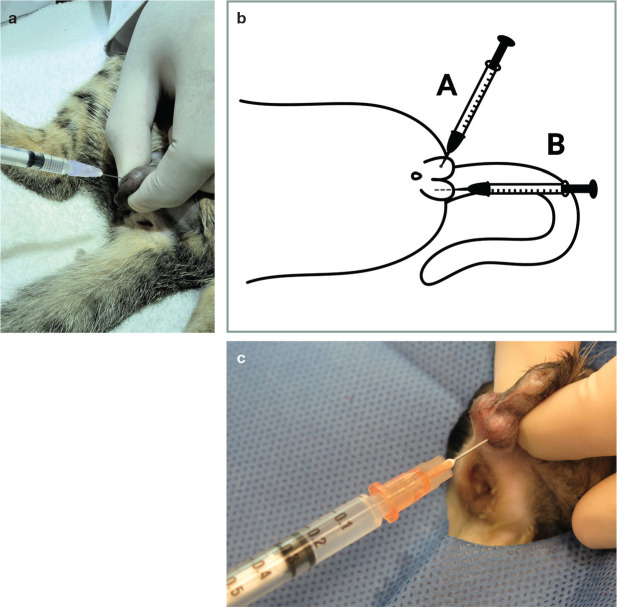
Dental blocks
There are many blocks that can be used for a variety of dental and oral procedures including extractions, oral mass removal, biopsies, maxillectomy and mandibulectomy. Injections are performed using 25–27 G needles and volumes of lidocaine of 0.1–0.3 ml at each nerve;25,125,146 however, the total dose guidelines (mg/kg, Table 8) must be adhered to. The final injectate volume will depend on the number of dental blocks to be performed. Care must be taken to carefully track the amount of drug administered, especially when injecting at multiple sites.
The infraorbital nerve block is shown in Figure 19 (needle A) and Figure 20. The maxillary nerve courses through the inferior periorbita and enters the infraorbital canal, where it becomes the infraorbital nerve.147,148 The needle should be inserted with caution into the infraorbital canal, no further than 4–5 mm, to avoid the risk of penetration of the eyeball, especially in brachycephalic cats. 149 The efficacy of this nerve block for caudal teeth (ie, feline premolar 3 and molar 1) anaesthesia has not been established in cats. 147 A maxillary nerve block has been performed as an alternative for anaesthesia of the caudal teeth, but there are reports suggesting an increased risk of penetrating the globe and, therefore, it is no longer recommended.150,151
Figure 19.
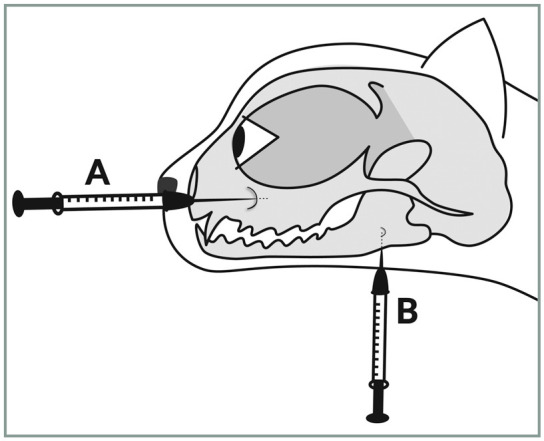
Locations for the infraorbital nerve block (needle A) and inferior alveolar (mandibular) nerve block (needle B). The techniques are illustrated photographically in Figures 20 and 21, respectively. Image courtesy ofYael-Shilo Benjamini
Figure 20.
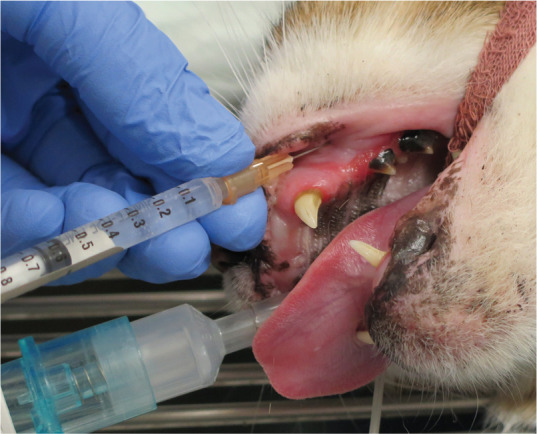
Infraorbital nerve block. The needle is inserted from a cranial to caudal direction at the level of premolar 3, approximately 4–5 mm into the infraorbital canal. Image courtesy of Paulo Steagall
The inferior alveolar (mandibular) nerve (Figure 19 [needle B] and Figure 21) enters the mandibular canal via the mandibular foramen on the medial surface of the mandibular ramus.121,148 Cardiovascular collapse was reported in a cat with a mandibular mass following inferior alveolar (mandibular) anaesthesia using bupivacaine. 152 Injection in close proximity to the tumour may have resulted in rapid bupivacaine absorption and toxic peak plasma concentrations. 152
Figure 21.
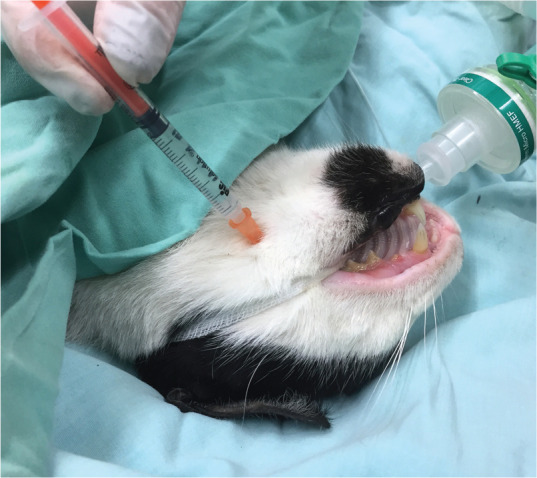
Inferior alveolar (mandibular) nerve block using an extraoral approach. The needle is inserted, perpendicularly to the skin surface, at the lower angle of the jaw, and advanced approximately 5 mm against the medial side of the mandible in the direction of the mandibular foramen. Image courtesy of Yael-Shilo Benjamini
Adjuvant analgesic drugs
✜ Alpha-2 agonists are useful in the perioperative period for premedication/sedation/ chemical restraint 153 as they also provide muscle relaxation and some analgesia. Sedation/anxiolysis may reduce the overall ‘pain experience’. Drugs with higher specificity for the alpha-2 adrenergic receptors (medetomidine and dexmedetomidine) are preferred over xylazine. Alpha-2 agonists should not be used as ‘stand-alone’ analgesics, but rather incorporated into multimodal protocols. However, their use should be reserved for cats with stable cardiovascular function. Antagonists of alpha-2 adrenergic receptors (eg, atipamezole) should always be available for drug reversal and may be required during prolonged recoveries or when cardiovascular collapse is observed.
✜ Metamizole (dipyrone) is a phenolic compound and atypical NSAID with COX-1 and some COX-2 anti-inflammatory activity. 154 The drug has potent analgesic effects that may be due to central inhibition of the COX-3 enzyme. 155 Activation of the opioid and cannabinoid systems is also a possible mechanism of action for metamizole. 156 This is a popular analgesic drug, particularly in South America, where it has been used for perioperative pain management in dogs and cats undergoing ovariohysterectomy.154,157,158 In cats, the pharmacokinetics of the two major metabolites of metamizole were described after IV, IM and PO administration (25 mg/kg). Salivation and vomiting were observed in four and two (out of six) cats after IV and IM administration, respectively. 159 However, a further study did not report these adverse effects after IV metamizole (25 mg/kg q8, 12 or 24 h). Indeed, the administration of metamizole did not produce any clinically relevant effects on Heinz body formation, biochemical profile or oxidation markers in cats undergoing ovariohysterectomy, although neither did it have an additional analgesic effect in this study in cats receiving injectable tramadol. 158 In another investigation, metamizole induced sialorrhoea when administered orally (drops) in the home environment to cats following ovariohysterectomy; rescue analgesia was required for some cats receiving either 12.5 mg/kg q12h or 25 mg/kg q24h, showing that unimodal therapy (similar to meloxicam) may not induce adequate analgesia. 154
✜ Gabapentin binds to voltage-gated calcium channels, but other mechanisms of action have been identified including activation of the descending noradrenergic inhibitory system. 160 The drug has high bioavailability in cats (90–95%) following oral administration of 10 mg/kg; peak plasma concentrations occur at between 45 mins and 2 h, and elimination half-lives were reported to be between 3 and 4 h.161,162 Repeated dosing did not affect terminal half-life and other pharmacokinetic parameters. 161
Transdermal administration of drugs is an attractive alternative to oral dosing in cats. The choice of base used to mix drugs with is important since it must be able to permeate feline skin. One study showed no significant transdermal absorption of gabapentin when compounded with ethoxydiglycol solvent and a transdermal base (Lipoderm; Professional Compounding Centers of America). 161 However, another study showed that gabapentin in the proprietary base Lipoderm did result in measurable plasma concentrations. 163 The authors of the latter study caution that their investigation involved a small number of cats and, because grooming was allowed, uptake may have occurred transmucosally. More studies using different transdermal creams, concentrations, doses and dosing intervals are needed.
There is limited information on the use of gabapentin for acute pain management.35,55,164 In one study, gabapentin (50 mg, PO, administered 12 h and 1 h before surgery) in combination with buprenorphine produced similar postoperative analgesia when compared with meloxicam and buprenorphine in cats undergoing ovariohysterectomy. 35 Further studies are thus still required to investigate the analgesic properties of gabapentinoids in cats. Gabapentin (50 or 100 mg, PO) is important in attenuating stress and fear responses in client-owned and cage-trap community cats undergoing transportation and veterinary consultation, and remains a valuable component of a holistic approach to feline pain management.60,165
✜Ketamine produces anti-hyperalgesic effects at subanaesthetic doses by non-competitive and non-specific antagonism of NMDA receptors.57,166 These receptors are important in excitatory and sustained neuro-transmission in the dorsal horn of the spinal cord. NMDA receptors are involved in the development and maintenance of central sensitisation (Table 4) and cumulative depolarisation. Therefore, ketamine is used to prevent and treat hyperalgesia and allodynia in cats with major injury and in those undergoing surgery for its anaesthetic- and opioid-sparing effects.55,80 Cats that undergo long-term therapy (days) must be hospitalised during treatment and pain should be monitored appropriately. Suggested dosing is as follows: an intravenous loading dose of 0.15–0.3 mg/kg followed by an infusion of 2–10 µg/kg/min.
✜Tramadol is a synthetic weak opioid agonist and a serotonin and noradrenaline reuptake inhibitor (dual action) analgesic drug. The injectable formulation is commonly used in some countries, especially in South America, whereas in North America, Oceania and most countries in Europe the drug is only available in an oral formulation. However, its low palatability (bitter taste) has precluded it from becoming a popular perioperative analgesic in cats. Cats may salivate profusely after drug administration and treatment compliance can be poor. Moreover, there is significant inter-patient variability in analgesic efficacy and adverse effects of tramadol in cats. Injectable formulations of tramadol (2-4 mg/kg IM) are often combined with acepromazine or dexmedetomidine for premedication.167,168
Based on the pharmacokinetics of tramadol in cats, a dosage of 4 mg/kg q6h PO has been recommended as part of a multimodal analgesic plan. 169 However, tramadol has failed to produce clinical analgesia in some studies, and pain assessment should always be performed. 167 The risk of serotonin toxicity is a concern when tramadol is administered with serotonin inhibitors (eg, fluoxetine and trazodone), monoamine oxidase inhibitors (eg, selegiline) and tricyclic antidepressants (eg, clomipramine). Clinical signs of serotonin syndrome include increased neuromuscular activity, tachycardia, fever, tachypnoea and agitation. 170
Analgesic shortages and unavailability: opioid-free or opioid-sparing techniques
Opioids are included in the WSAVA list of essential medicines for cats and dogs, and considered ‘core medicines’. 171 Nevertheless, veterinarians commonly face opioid shortages or do not always have access to this class of drug due to strict regulatory and bureaucratic constraints, and/or where medicines labelled for human use are rarely allowed to be administered to veterinary patients. These constraints affect the ability of veterinarians to treat feline pain, and this could represent a major issue for feline welfare if surgical and medical procedures are performed with limited or no perioperative analgesia.
‘Opioid-free anaesthesia’ (OFA) is a practice that excludes perioperative administration of opioids and uses non-opioid, multimodal analgesic techniques. ‘Opioid-sparing anaesthesia’ is a less restrictive technique where small amounts of opioids are used (ie, single and/or low doses of opioids perioperatively). Both techniques are now being studied in human medicine to avoid or reduce opioid-induced adverse effects, including respiratory depression and death.172–174 Although adverse effects are different between humans and cats, the study of feline OFA is integral to overcoming opioid shortages in the future and reducing opioid-induced adverse effects (vomiting, dysphoria, hyperthermia, etc). There is also an urgent need to provide humane solutions for pain management in sterilisation programmes, which have often been performed without the use of analgesics.
The veterinary literature on the subject is scant, particularly in cats. In one study, an injectable anaesthetic protocol using ketamine-midazolam and dexmedetomidine, intraperitoneal bupivacaine and postoperative administration of meloxicam did not provide optimal postoperative analgesia in more than half of the studied population. 175 Side-by-side comparisons were not performed between an opioid-based analgesic group and an OFA group, 175 and the question of ‘can we attenuate or ameliorate acute pain without opioids?’ remains. Certainly, opioid analgesics with market authorisation for cats are critical to avoid opioid drug shortages and improve feline welfare. This issue is best addressed by involving multiple stakeholders including, but not limited to, governmental bodies, industry, regulatory agencies and veterinary professionals.

Knowledge gaps and challenges
There is still a need for better understanding of feline behaviour related to pain as well as techniques for long-term pain management in the acute setting so that cats can be discharged to their owner with analgesics that continue to work in the home environment and are easy for owners to administer. Opioid-free anaesthetic techniques and the role of gabapentinoids are important to investigate. Little is known about paediatric analgesic needs, warranting further studies in this age group. There is also a need for safety and efficacy studies of in vivo loco-regional anaesthetic techniques to bridge the gap between in vitro studies and clinical practice. A major gap remains to be filled in the veterinary curriculum regarding feline analgesia and there must be a concerted effort to implement the use of pain assessment tools in feline practice.
Summary Points
✜ The 2022 ISFM Consensus Guidelines on the Management of Acute Pain in Cats provide up-to-date information on the unique features of feline anatomy, physiology and pharmacology in relation to analgesia, as well as on pain assessment tools and novel analgesic drugs and techniques.
✜ Principles of pain management include preventive analgesia and multimodal analgesia, incorporating pharmacological and non-pharmacological options. The use of cat friendly handling techniques, anxiolysis and nursing care are central to appropriate pain management.
✜ In addition to local anaesthetics, NSAIDs and opioids, adjuvant analgesics should be considered depending on the individual and its needs.
✜ The future is bright for feline pain management with the potential automation of acute pain assessment tools that could. I allow cat owners to detect pain in the home environment, the licensing of new analgesic drugs for perioperative pain f control and studies on novel analgesic drugs and techniques.
✜ Gaps in knowledge and challenges remain, but the good news is that these can be addressed with research support, educational opportunities and an enthusiastic group of feline advocates.
Supplemental Material
Supplemental material, sj-pdf-1-jfm-10.1177_1098612X211066268 for 2022 ISFM Consensus Guidelines on the Management of Acute Pain in Cats by Paulo V Steagall, Sheilah Robertson, Bradley Simon, Leon N Warne, Yael Shilo-Benjamini and Samantha Taylor in Journal of Feline Medicine and Surgery
Supplemental material, sj-pdf-2-jfm-10.1177_1098612X211066268 for 2022 ISFM Consensus Guidelines on the Management of Acute Pain in Cats by Paulo V Steagall, Sheilah Robertson, Bradley Simon, Leon N Warne, Yael Shilo-Benjamini and Samantha Taylor in Journal of Feline Medicine and Surgery
Supplemental material, sj-pdf-3-jfm-10.1177_1098612X211066268 for 2022 ISFM Consensus Guidelines on the Management of Acute Pain in Cats by Paulo V Steagall, Sheilah Robertson, Bradley Simon, Leon N Warne, Yael Shilo-Benjamini and Samantha Taylor in Journal of Feline Medicine and Surgery
Acknowledgments
ISFM is grateful to Dr Sabrine Marangoni for technical help with formatting the Guidelines, and to Nirit Roth-Benyamini for help in creating Figures 16a, 18b and 19.
Appendix: Cat Carer Guide
The Cat Carer Guide may be downloaded from icatcare.org/advice/cat-carer-guides, and is also available as supplementary material at jfms.com.
Footnotes
Supplementary material: The following files are available online:
✜ Troubleshooting dysphoria algorithm
✜ Cat Carer Guide: ‘Recognising and managing acute pain in cats: information for owners/caregivers’ (see Appendix, page 30).
Paulo V Steagall has provided consultancy services to, and received honoraria from, Boehringer Ingelheim, Dechra, Elanco, Nexyon, Vetoquinol and Zoetis. Sheilah Robertson has provided consultancy services to, and received honoraria from, Elanco and Zoetis. The other members of the Panel have no conflicts of interest to declare.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This work did not involve the use of animals and, therefore, ethical approval was not specifically required for publication in JFMS.
Informed consent: This work did not involve the use of animals (including cadavers) and, therefore, informed consent was not required. For any animals or people individually identifiable within this publication, informed consent (verbal or written) for their use in the publication was obtained from the people involved.
Contributor Information
Paulo V Steagall, Department of Clinical Sciences, Faculty of Veterinary Medicine, Universite de Montréal, Saint-Hyacinthe, Canada; and Department of Veterinary Clinical Sciences and Centre for Companion Animal Health, Jockey Club College of Veterinary Medicine and Life Sciences, City University of Hong Kong, Hong Kong, China.
Sheilah Robertson, Lap of Love Veterinary Hospice, Lutz, Florida, USA.
Bradley Simon, Department of Small Animal Clinical Sciences, Texas A&M College of Veterinary Medicine and Biomedical Sciences, College Station, Texas, USA.
Leon N Warne, Veterinary Anaesthesia & Pain Management Australia, Perth, Western Australia; and Veterinary Cannabis Medicines Australia, Perth, Western Australia, Australia.
Yael Shilo-Benjamini, Koret School of Veterinary Medicine, The Robert H Smith Faculty of Agriculture, Food and Environment, The Hebrew University of Jerusalem, Rehovot, Israel.
Samantha Taylor, International Society of Feline Medicine, Tisbury, UK.
References
- 1. American Veterinary Medical Association. U.S. pet ownership statistics. https://www.avma.org/resources-tools/reports-statistics/us-pet-ownership-statistics (2020, accessed 20 November 2021). [Google Scholar]
- 2. Canadian Veterinary Medical Association. Veterinary demographics (2020). https://www.canadianveterinarians.net/about/statistics (2021, accessed 15 July 2021). [Google Scholar]
- 3. Steagall PV, Monteiro BP, Taylor PM. A review of the studies using buprenorphine in cats. J Vet Intern Med 2014; 28: 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bortolami E, Love EJ. Practical use of opioids in cats: a state-of-the-art, evidence-based review. J Feline Med Surg 2015; 17: 283–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steagall PV, Monteiro BP. Acute pain in cats: recent advances in clinical assessment. J Feline Med Surg 2019; 21: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robertson S. How do we know they hurt? Assessing acute pain in cats. In Pract 2018; 40: 440–448. [DOI] [PubMed] [Google Scholar]
- 7. Reid J, Scott EM, Calvo G, et al. Definitive Glasgow acute pain scale for cats: validation and intervention level. Vet Rec 2017; 180: 449. DOI: 10.1136/vr.104208. [DOI] [PubMed] [Google Scholar]
- 8. Evangelista MC, Watanabe R, Leung VSY, et al. Facial expressions of pain in cats: the development and validation of a Feline Grimace Scale. Sci Rep 2019; 9: 1–11. DOI: 10.1038/s41598-019-55693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belli M, de Oliveira AR, de Lima MT, et al. Clinical validation of the short and long UNESP-Botucatu scales for feline pain assessment. PeerJ 2021; 12; 9. DOI: 10.7717/peerj.11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holden E, Calvo G, Collins M, et al. Evaluation of facial expression in acute pain in cats. J Small Anim Pract 2014; 55: 615–621. [DOI] [PubMed] [Google Scholar]
- 11. Steagall PV, Monteiro BP, Ruel HLM, et al. Perceptions and opinions of Canadian pet owners about anaesthesia, pain and surgery in small animals. J Small Anim Pract 2017; 58: 380–388. [DOI] [PubMed] [Google Scholar]
- 12. Simon BT, Scallan EM, Von Pfeil DJF, et al. Perceptions and opinions of pet owners in the United States about surgery, pain management, and anesthesia in dogs and cats. Vet Surg 2018; 47: 277–284. [DOI] [PubMed] [Google Scholar]
- 13. Rae L, MacNab N, Bidner S, et al. Attitudes and practices of veterinarians in Australia to acute pain management in cats. J Feline Med Surg. Epub ahead of print 20 September 2021. DOI: 10.1177/1098612X211043086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hunt JR, Knowles TG, Lascelles BDX, et al. Prescription of perioperative analgesics by UK small animal veterinary surgeons in 2013. Vet Rec 2015; 176: 493. DOI: 10.1136/vr.102834. [DOI] [PubMed] [Google Scholar]
- 15. Coleman DL, Slingsby LS. Attitudes of veterinary nurses to the assessment of pain and the use of pain scales. Vet Rec 2007; 160: 541–544. [DOI] [PubMed] [Google Scholar]
- 16. Simon BT, Scallan EM, Carroll G, et al. The lack of analgesic use (oligoanalgesia) in small animal practice. J Small Anim Pract 2017; 58: 543–554. [DOI] [PubMed] [Google Scholar]
- 17. Rodan I, Sundahl E, Carney H, et al. AAFP and ISFM feline-friendly handling guidelines. J Feline Med Surg 2011; 13: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feline Grimace Scale. Pain assessment in cats. https://www.felinegrimacescale.com (2020, accessed 18 November 2021). [Google Scholar]
- 19. Animal Pain. http://www.animalpain.com.br/en-us/ (2012, accessed 19 July 2019). [Google Scholar]
- 20. New Metrica. Acute pain measurement. https://www.newmet-rica.com/acute-pain-measurement/ (2019, accessed 19 July 2021). [Google Scholar]
- 21. Cambridge AJ, Tobias KM, Newberry RC, et al. Subjective and objective measurements of postoperative pain in cats. J Am Vet Med Assoc 2000; 217: 685–690. [DOI] [PubMed] [Google Scholar]
- 22. Smith JD, Allen SW, Quandt JE, et al. Indicators of postoperative pain in cats and correlation with clinical criteria. Am J Vet Res 1996; 57: 1674–1678. [PubMed] [Google Scholar]
- 23. Smith JD, Allen SW, Quandt JE. Changes in cortisol concentration in response to stress and postoperative pain in client-owned cats and correlation with objective clinical variables. Am J Vet Res 1999; 60: 432–436. [PubMed] [Google Scholar]
- 24. Brondani JT, Mama KR, Luna SPL, et al. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet Res 2013; 9: 143. DOI: 10.1186/1746-6148-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watanabe R, Frank D, Steagall PV. Pain behaviors before and after treatment of oral disease in cats using video assessment: a prospective, blinded, randomized clinical trial. BMC Vet Res 2020; 16: 1–11. DOI: 10.1186/s12917-020-02302-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waran N, Best L, Williams V. A preliminary study of behaviour-based indicators of pain in cats. Anim Welf 2007; 16: 105–108. [Google Scholar]
- 27. Steagall PV. Analgesia: what makes cats different/challenging and what is critical for cats? Vet Clin North Am Small Anim Pract 2020; 50: 749–767. [DOI] [PubMed] [Google Scholar]
- 28. Calvo G, Holden E, Reid J, et al. Development of a behaviour-based measurement tool with defined intervention level for assessing acute pain in cats. J Small Anim Pract 2014; 55: 622–629. [DOI] [PubMed] [Google Scholar]
- 29. Evangelista MC, Benito J, Monteiro BP, et al. Clinical applicability of the Feline Grimace Scale: real-time versus image scoring and the influence of sedation and surgery. PeerJ 2020; 8. DOI: 10.7717/peerj.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watanabe R, Doodnaught GM, Evangelista MC, et al. Inter-rater reliability of the Feline Grimace Scale in cats undergoing dental extractions. Front Vet Sci 2020; 7: 4–9. DOI: 10.3389/fvets.2020.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steagall PV, Monteiro BP, Lavoie A, et al. Validation of the French version of a multidimensional composite scale for the assessment of postoperative pain in cats [article in French]. Can Vet J 2017; 58: 56–64.28042156 [Google Scholar]
- 32. Della Rocca G, Catanzaro A, Conti MB, et al. Validation of the Italian version of the UNESP-Botucatu Multidimensional Composite Pain Scale for the assessment of postoperative pain in cats. Vet Ital 2018; 54: 49–61. [DOI] [PubMed] [Google Scholar]
- 33. Evangelista MC, Steagall PV. Agreement and reliability of the Feline Grimace Scale among cat owners, veterinarians, veterinary students and nurses. Sci Reports 2021; 11: 5262. DOI: 10.1038/s41598-021-84696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benito J, Monteiro BP, Beauchamp G, et al. Evaluation of inter-observer agreement for postoperative pain and sedation assessment in cats. J Am Vet Med Assoc 2017; 251: 544–551. [DOI] [PubMed] [Google Scholar]
- 35. Steagall PV, Benito J, Monteiro BP, et al. Analgesic effects of gabapentin and buprenorphine in cats undergoing ovariohysterectomy using two pain-scoring systems: a randomized clinical trial. J Feline Med Surg 2018; 20: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doodnaught GM, Benito J, Monteiro BP, et al. Agreement among undergraduate and graduate veterinary students and veterinary anesthesiologists on pain assessment in cats and dogs: a preliminary study. Can Vet J 2017; 58: 805–808. [PMC free article] [PubMed] [Google Scholar]
- 37. Benito J, Steagall PV. Postoperative analgesia between non-pregnant healthy cats versus pregnant or cats with upper respiratory tract disease [abstract]. Vet Anaesth Analg 2017; 44: 195. [Google Scholar]
- 38. Buisman M, Hasiuk MMM, Gunn M, et al. The influence of demeanor on scores from two validated feline pain assessment scales during the perioperative period. Vet Anaesth Analg 2017; 44: 646–655. [DOI] [PubMed] [Google Scholar]
- 39. Buisman M, Wagner MC, Hasiuk MM, et al. Effects of ketamine and alfaxalone on application of a feline pain assessment scale. J Feline Med Surg 2016; 18: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simon BT, Scallan EM, Baetge CL, et al. The antinociceptive effects of intravenous administration of three doses of butor-phanol tartrate or naloxone hydrochloride following hydromor-phone hydrochloride to healthy conscious cats. Vet Anaesth Analg 2019; 46: 538–547. [DOI] [PubMed] [Google Scholar]
- 41. Robertson S, Gogolski SM, Pascoe P, et al. AAFP feline anesthesia guidelines. J Feline Med Surg 2018; 20: 602–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Väisänen MA-M, Tuomikoski SK, Vainio OM. Behavioral alterations and severity of pain in cats recovering at home following elective ovariohysterectomy or castration. J Am Vet Med Assoc 2007; 231: 236–242. [DOI] [PubMed] [Google Scholar]
- 43. Adrian D, Papich M, Baynes R, et al. Chronic maladaptive pain in cats: a review of current and future drug treatment options. Vet J 2017; 230: 52–61. [DOI] [PubMed] [Google Scholar]
- 44. Steagall PV, Bustamante H, Johnson CB, et al. Pain management in farm animals: focus on cattle, sheep and pigs. Animals 2021; 11: 1483. DOI: 10.3390/ani11061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Humble SR, Dalton AJ, Li L. A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy or mastectomy. Eur J Pain 2015; 19: 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weibel S, Neubert K, Jelting Y, et al. Incidence and severity of chronic pain after caesarean section. Eur J Anaesthesiol 2016; 33: 853–865. [DOI] [PubMed] [Google Scholar]
- 47. Court MH. Feline drug metabolism and disposition: pharma-cokinetic evidence for species differences and molecular mechanisms. Vet Clin North Am Small Anim Pract 2013; 43: 1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Court MH, Greenblatt DJ. Molecular genetic basis for deficient acetaminophen glucuronidation by cats: UGT1A6 is a pseudogene, and evidence for reduced diversity of expressed hepatic UGT1A isoforms. Pharmacogenetics 2000; 10: 355–369. [DOI] [PubMed] [Google Scholar]
- 49. Taylor P, Delatour P, Landont F, et al. Pharmacodynamics and enantioselective pharmacokinetics of carprofen in the cat. Res Vet Sci 1996; 60: 144–151. [DOI] [PubMed] [Google Scholar]
- 50. Taylor PM, Robertson SA, Dixon MJ, et al. Morphine, pethidine and buprenorphine disposition in the cat. J Vet Pharmacol 2002; 24: 391–398. [DOI] [PubMed] [Google Scholar]
- 51. Grude P, Guittard J, Garcia C, et al. Excretion mass balance evaluation, metabolite profile analysis and metabolite identification in plasma and excreta after oral administration of [14C]-meloxicam to the male cat: preliminary study. J Vet Pharmacol 2010; 33: 396–407. [DOI] [PubMed] [Google Scholar]
- 52. Lehr T, Narbe R, Jöns O, et al. Population pharmacokinetic modelling and simulation of single and multiple dose administration of meloxicam in cats. J Vet Pharmacol Ther 2009; 33: 277–286. [DOI] [PubMed] [Google Scholar]
- 53. Sparkes AH, Caney S, Chalhoub S, et al. ISFM consensus guidelines on the diagnosis and management of feline chronic kidney disease. J Feline Med Surg 2016; 18: 219–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Monteiro B, Steagall PV, Lascelles BDX, et al. Long-term use of non-steroidal anti-inflammatory drugs in cats with chronic kidney disease: from controversy to optimism. J Small Anim Pract 2019; 60: 459–462. [DOI] [PubMed] [Google Scholar]
- 55. Steagall PV, Monteiro BP. Multimodal analgesia for perioperative pain in three cats. J Feline Med Surg 2013; 15: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goich M, Bascuñán A, Faúndez P, et al. Multimodal analgesia for treatment of allodynia and hyperalgesia after major trauma in a cat. JFMS Open Rep 2019; 5. DOI: 10.1177/2055116919855809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ruel HLM, Steagall PV. Adjuvant analgesics in acute pain management. Vet Clin North Am Small Anim Pract 2019; 49: 1127–1241. [DOI] [PubMed] [Google Scholar]
- 58. Hickman MA, Cox SR, Mahabir S, et al. Safety, pharmacokinetics and use of the novel NK-1 receptor antagonist maropitant (CereniaTM) for the prevention of emesis and motion sickness in cats. J Vet Pharmacol Ther 2008; 31: 220–229. [DOI] [PubMed] [Google Scholar]
- 59. Santos LCP, Ludders JW, Erb HN, et al. A randomized, blinded, controlled trial of the antiemetic effect of ondansetron on dexmedetomidine-induced emesis in cats. Vet Anaesth Analg 2011; 38: 320–327. [DOI] [PubMed] [Google Scholar]
- 60. Van Haaften KA, Forsythe LRE, Stelow EA, et al. Effects of a single preappointment dose of gabapentin on signs of stress in cats during transportation and veterinary examination. J Am Vet Med Assoc 2017; 251: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 61. Stevens BJ, Frantz EM, Orlando JM, et al. Efficacy of a single dose of trazodone hydrochloride given to cats prior to veterinary visits to reduce signs of transport- and examination-related anxiety. J Am Vet Med Assoc 2016; 249: 202–207. [DOI] [PubMed] [Google Scholar]
- 62. Wright B, Kronen PW, Lascelles D, et al. Ice therapy: cool, current and complicated. J Small Anim Pract 2020; 61: 267–271. [DOI] [PubMed] [Google Scholar]
- 63. Carney HC, Little S, Brownlee-Tomasso D, et al. AAFP and ISFM feline-friendly nursing care guidelines. J Feline Med Surg 2012; 14: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Slingsby LS, Bortolami E, Murrell JC. Methadone in combination with medetomidine as premedication prior to ovariohysterectomy and castration in the cat. J Feline Med Surg 2015; 17: 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Camargo JB, Steagall PV, Minto BW, et al. Post-operative analgesic effects of butorphanol or firocoxib administered to dogs undergoing elective ovariohysterectomy. Vet Anaesth Analg 2011; 38: 252–259. [DOI] [PubMed] [Google Scholar]
- 66. Taylor PM, Luangdilok C, Sear JW. Pharmacokinetic and pharmacodynamic evaluation of high doses of buprenorphine delivered via high-concentration formulations in cats. J Feline Med Surg 2016; 18: 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Portela DA, Verdier N, Otero PE. Regional anesthetic techniques for the limb and thorax in small animals: a review of the literature and technique description. Vet J 2018; 241: 8–19. DOI: 10.1016/j.tvjl.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 68. Portela DA, Verdier N, Otero PE. Regional anesthetic techniques for the pelvic limb and abdominal wall in small animals: a review of the literature and technique description. Vet J 2018; 238: 27–40. [DOI] [PubMed] [Google Scholar]
- 69. Bradbrook CA, Clark L. State of the art analgesia – recent developments in pharmacological approaches to acute pain management in dogs and cats. Part 1. Vet J 2018; 238: 76–82. [DOI] [PubMed] [Google Scholar]
- 70. Go VL, Yaksh TL. Release of substance P from the cat spinal cord. J Physiol 1987; 391: 141–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Torrecilla M, Marker CL, Cintora SC, et al. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci 2002; 22: 4328–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mansour A, Fox CA, Thompson RC, et al. µ-opioid receptor mRNA expression in the rat CNS: comparison to µ-receptor binding. Brain Res 1994; 643: 245–265. [DOI] [PubMed] [Google Scholar]
- 73. Taddese A, Nah S-Y, McCleskey EW. Selective opioid inhibition of small nociceptive neurons. Science 1995; 270: 1366–1369. [DOI] [PubMed] [Google Scholar]
- 74. Pascoe PJ, Ilkiw JE, Fisher LD. Cardiovascular effects of equipotent isoflurane and alfentanil/isoflurane minimum alveolar concentration multiple in cats. Am J Vet Res 1997; 58: 1267–1273. [PubMed] [Google Scholar]
- 75. Ilkiw JE, Pascoe PJ, Tripp LD. Effects of morphine, butor-phanol, buprenorphine, and U50488H on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 2002; 63: 1198–1202. [DOI] [PubMed] [Google Scholar]
- 76. Steffey EP, Eisele JH, Baggot JD, et al. Influence of inhaled anesthetics on the pharmacokinetics and pharmacodynamics of morphine. Anesth Analg 1993; 77: 346–351. [DOI] [PubMed] [Google Scholar]
- 77. Brosnan RJ, Pypendop BH, Siao KT, et al. Effects of remifentanil on measures of anesthetic immobility and analgesia in cats. Am J Vet Res 2009; 70: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 78. Ferreira TH, Aguiar AJA, Valverde A, et al. Effect of remifentanil hydrochloride administered via constant rate infusion on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res 2009; 70: 581–588. [DOI] [PubMed] [Google Scholar]
- 79. Monteiro ER, Teixeira-Neto FJ, Campagnol D, et al. Effects of remifentanil on the minimum alveolar concentration of isoflurane in dogs. Am J Vet Res 2010; 71: 150–156. [DOI] [PubMed] [Google Scholar]
- 80. Steagall PV, Aucoin M, Monteiro BP, et al. Clinical effects of a constant rate infusion of remifentanil, alone or in combination with ketamine, in cats anesthetized with isoflurane. J Am Vet Med Assoc 2015; 246: 976–981. [DOI] [PubMed] [Google Scholar]
- 81. Gaumann DM, Yaksh TL, Tyce GM, et al. Sympathetic stimulating effects of sufentanil in the cat are mediated centrally. Neurosci Lett 1988; 91: 30–35. [DOI] [PubMed] [Google Scholar]
- 82. Giordano T, Steagall PV, Ferreira TH, et al. Postoperative analgesic effects of intravenous, intramuscular, subcutaneous or oral transmucosal buprenorphine administered to cats undergoing ovariohysterectomy. Vet Anaesth Analg 2010; 37: 357–366. [DOI] [PubMed] [Google Scholar]
- 83. Steagall PV, Pelligand L, Giordano T, et al. Pharmacokinetic and pharmacodynamic modelling of intravenous, intramuscular and subcutaneous buprenorphine in conscious cats. Vet Anaesth Analg 2013; 40: 83–95. [DOI] [PubMed] [Google Scholar]
- 84. Robertson SA, Wegner K, Lascelles BDX. Antinociceptive and side-effects of hydromorphone after subcutaneous administration in cats. J Feline Med Surg 2009; 11: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Doodnaught GM, Monteiro BP, Benito J, et al. Pharmacokinetic and pharmacodynamic modelling after subcutaneous, intravenous and buccal administration of a high-concentration formulation of buprenorphine in conscious cats. PLoS One 2017; 12: e0176443. DOI: 10.1371/journal.pone.0176443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wegner K, Robertson SA, Kollias-Baker C, et al. Pharmacokinetic and pharmacodynamic evaluation of intravenous hydromor-phone in cats. J Vet Pharmacol Ther 2004; 27: 329–336. [DOI] [PubMed] [Google Scholar]
- 87. Wegner K, Robertson SA. Dose-related thermal antinociceptive effects of intravenous hydromorphone in cats. Vet Anaesth Analg 2007; 34: 132–138. [DOI] [PubMed] [Google Scholar]
- 88. Simon BT, Scallan EM, Monteiro BP, et al. The effects of aging on hydromorphone-induced thermal antinociception in healthy female cats. Pain 2019; 4: e722. DOI: 10.1097/PR9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Simon BT, Steagall PV. The present and future of opioid analgesics in small animal practice. J Vet Pharmacol Ther 2017; 40: 315–326. [DOI] [PubMed] [Google Scholar]
- 90. Gurney M, Cripps P, Mosing M. Subcutaneous pre-anaesthetic medication with acepromazine-buprenorphine is effective as and less painful than the intramuscular route. J Small Anim Pract 2009; 50: 474–477. [DOI] [PubMed] [Google Scholar]
- 91. Hofmeister EH, Egger CM. Transdermal fentanyl patches in small animals. J Am Anim Hosp Assoc 2004; 40: 468–478. [DOI] [PubMed] [Google Scholar]
- 92. Davidson CD, Pettifer GR, Henry JD. Plasma fentanyl concentrations and analgesic effects during full or partial exposure to transdermal fentanyl patches in cats. J Am Vet Med Assoc 2004; 224: 700–705. [DOI] [PubMed] [Google Scholar]
- 93. Murrell JC, Robertson SA, Taylor PM, et al. Use of a transdermal matrix patch of buprenorphine in cats: preliminary pharma-cokinetic and pharmacodynamic data. Vet Rec 2007; 160: 578–583. [DOI] [PubMed] [Google Scholar]
- 94. Duke-Novakovski T, Clark CR, Ambros B, et al. Plasma concentrations of buprenorphine after epidural administration in conscious cats. Res Vet Sci 2011; 90: 480–483. [DOI] [PubMed] [Google Scholar]
- 95. DeRossi R, Hermeto LC, Jardim PHA, et al. Postoperative pain control in cats: clinical trials with pre-emptive lidocaine epidural co-administered with morphine or methadone. J Feline Med Surg 2016; 18: 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Troncy E, Junot S, Keroack S, et al. Results of preemptive epidural administration of morphine with or without bupivacaine in dogs and cats undergoing surgery: 265 cases (1997-1999). J Am Vet Med Assoc 2002; 221: 666–672. [DOI] [PubMed] [Google Scholar]
- 97. Ambros B, Steagall PV, Mantovani F, et al. Antinociceptive effects of epidural administration of hydromorphone in conscious cats. Am J Vet Res 2009; 70: 1187–1192. [DOI] [PubMed] [Google Scholar]
- 98. Steagall PV, Milette V, Mantovani FB, et al. Antinociceptive effects of epidural buprenorphine or medetomidine, or the combination, in conscious cats. J Vet Pharmacol Ther 2009; 32: 477–484. [DOI] [PubMed] [Google Scholar]
- 99. Pypendop BH, Siao KT, Pascoe PJ, et al. Effects of epidurally administered morphine or buprenorphine on the thermal threshold in cats. Am J Vet Res 2008; 69: 983–987. [DOI] [PubMed] [Google Scholar]
- 100. Herperger LJ. Postoperative urinary retention in a dog following morphine with bupivacaine epidural analgesia. Can Vet J 1998; 39: 650–652. [PMC free article] [PubMed] [Google Scholar]
- 101. Scallan EM, Simon BT. The effects of acupuncture point Pericardium 6 on hydromorphone-induced nausea and vomiting in healthy dogs. Vet Anaesth Analg 2016; 43: 495–501. [DOI] [PubMed] [Google Scholar]
- 102. Martin-Flores M, Mastrocco A, Lorenzutti AM, et al. Maropitant administered orally 2-2.5 h prior to morphine and dexmedeto-midine reduces the incidence of emesis in cats. J Feline Med Surg 2017; 19: 876–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Martin-Flores M, Sakai DM, Learn MM, et al. Effects of maropitant in cats receiving dexmedetomidine and morphine. J Am Vet Med Assoc 2016; 248: 1257–1261. [DOI] [PubMed] [Google Scholar]
- 104. Martin-Flores M, Sakai DM, Mastrocco A, et al. Evaluation of oral maropitant as an antiemetic in cats receiving morphine and dexmedetomidine. J Feline Med Surg 2016; 18: 921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kenward H, Elliott J, Lee T, et al. Anti-nausea effects and pharmacokinetics of ondansetron, maropitant and metoclopramide in a low-dose cisplatin model of nausea and vomiting in the dog: a blinded crossover study. BMC Vet Res 2017; 13: 244. DOI: 10.1186/s12917-017-1156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Robertson SA. Managing pain in feline patients. Vet Clin North Am Small Anim Pract 2008; 38: 1267–1290. [DOI] [PubMed] [Google Scholar]
- 107. Ingwersen W, Fox R, Cunningham G, et al. Efficacy and safety of 3 versus 5 days of meloxicam as an analgesic for feline onychectomy and sterilization. Can Vet J 2012; 53: 257–264. [PMC free article] [PubMed] [Google Scholar]
- 108. Speranza C, Schmid V, Giraudel JM, et al. Robenacoxib versus meloxicam for the control of perioperative pain and inflammation associated with orthopaedic surgery in cats: a randomized clinical trial. BMC Vet Res 2015; 11: 79. DOI: 10.1186/s12917-015-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. King S, Roberts ES, King JN. Evaluation of injectable robenacoxib for the treatment of post-operative pain in cats: results of a randomized, masked, placebo-controlled clinical trial. BMC Vet Res 2016; 12: 215. DOI: 10.1186/s12917-016-0827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sattasathuchana P, Phuwapattanachart P, Thengchaisri N. Comparison of post-operative analgesic efficacy of tolfenamic acid and robenacoxib in ovariohysterectomized cats. J Vet Med Sci 2018; 80: 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Slingsby LS, Waterman-Pearson AE. Postoperative analgesia in the cat after ovariohysterectomy by use of carprofen, ketoprofen, meloxicam or tolfenamic acid. J Small Anim Pract 2000; 41: 447–450. [DOI] [PubMed] [Google Scholar]
- 112. Sano T, King JN, Seewald W, et al. Comparison of oral robenacoxib and ketoprofen for the treatment of acute pain and inflammation associated with musculoskeletal disorders in cats: a randomised clinical trial. Vet J 2012; 193: 397–403. [DOI] [PubMed] [Google Scholar]
- 113. Morton CM, Grant D, Johnston L, et al. Clinical evaluation of meloxicam versus ketoprofen in cats suffering from painful acute locomotor disorders. J Feline Med Surg 2011; 13: 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kongara K, Chambers P. Robenacoxib in the treatment of pain in cats and dogs: safety, efficacy, and place in therapy. Vet Med (Auckl) 2018; 9: 53–61. DOI: 10.2147/vmrr.s170893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sparkes AH, Heiene R, Lascelles BDX, et al. ISFM and AAFP consensus guidelines: long-term use of NSAIDs in cats. J Feline Med Surg 2010; 12: 521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Monteiro B, Steagall PV. Antiinflammatory drugs. Vet Clin North Am Small Anim Pract 2019; 49: 993–1011. [DOI] [PubMed] [Google Scholar]
- 117. Giraudel JM, Gruet P, Alexander DG, et al. Evaluation of orally administered robenacoxib versus ketoprofen for treatment of acute pain and inflammation associated with musculoskeletal disorders in cats. Am J Vet Res 2010; 71: 710–719. [DOI] [PubMed] [Google Scholar]
- 118. Grubb T, Lobprise H. Local and regional anaesthesia in dogs and cats: overview of concepts and drugs (part 1). Vet Med Sci 2020; 6: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vicente D, Bergström A. Evaluation of intraoperative analgesia provided by incisional lidocaine and bupivacaine in cats undergoing ovariohysterectomy. J Feline Med Surg 2018; 20: 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Moldal ER, Eriksen T, Kirpensteijn J, et al. Intratesticular and subcutaneous lidocaine alters the intraoperative haemodynamic responses and heart rate variability in male cats undergoing castration. Vet Anaesth Analg 2013; 40: 63–73. [DOI] [PubMed] [Google Scholar]
- 121. Aguiar J, Chebroux A, Martinez-Taboada F, et al. Analgesic effects of maxillary and inferior alveolar nerve blocks in cats undergoing dental extractions. J Feline Med Surg 2015; 17: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zilberstein LF, Moens YP, Leterrier E. The effect of local anaesthesia on anaesthetic requirements for feline ovariectomy. Vet J 2008; 178: 214–218. [DOI] [PubMed] [Google Scholar]
- 123. Robertson SA, Taylor PM. Pain management in cats – past, present and future. Part 2. Treatment of pain – clinical pharmacology. J Feline Med Surg 2004; 6: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gordon-Evans WJ, Suh HY, Guedes AG. Controlled, non-inferiority trial of bupivacaine liposome injectable suspension. J Feline Med Surg 2020; 22: 916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Niemiec B, Gawor J, Nemec A, et al. World Small Animal Veterinary Association global dental guidelines. J Small Anim Pract 2020; 61. DOI: 10.1111/jsap.13132. [DOI] [PubMed] [Google Scholar]
- 126. Oliveira RL, Soares JH, Moreira CM, et al. The effects of lidocaine-prilocaine cream on responses to intravenous catheter placement in cats sedated with dexmedetomidine and either methadone or nalbuphine. Vet Anaesth Analg 2019; 46: 492–495. [DOI] [PubMed] [Google Scholar]
- 127. O’Brien TQ, Clark-Price SC, Evans EE, et al. Infusion of a lipid emulsion to treat lidocaine intoxication in a cat. J Am Vet Med Assoc 2010; 237: 1455–1458. [DOI] [PubMed] [Google Scholar]
- 128. Maierl J, Liebich H-G. Investigations on the postnatal development of the macroscopic proportions and the topographic anatomy of the feline spinal cord. Anat Histol Embryol 1998; 27: 375–379. [DOI] [PubMed] [Google Scholar]
- 129. Pratt CL, Balakrishnan A, McGowan E, et al. A prospective randomized, double-blinded clinical study evaluating the efficacy and safety of bupivacaine versus morphine-bupivacaine in caudal epidurals in cats with urethral obstruction. J Vet Emerg Crit Care 2020; 30: 170–178. [DOI] [PubMed] [Google Scholar]
- 130. O’Hearn AK, Wright BD. Coccygeal epidural with local anesthetic for catheterization and pain management in the treatment of feline urethral obstruction. J Vet Emerg Crit Care 2011; 21: 50–52. [DOI] [PubMed] [Google Scholar]
- 131. Credie L, Luna S. The use of ultrasound to evaluate sacrococcygeal epidural injections in cats. Can Vet J 2018; 59: 143–146. [PMC free article] [PubMed] [Google Scholar]
- 132. Fudge JM, Page B, Mackrell A, et al. Evaluation of targeted bupivacaine for reducing acute postoperative pain in cats undergoing routine ovariohysterectomy. J Feline Med Surg 2020; 22: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yilmaz ÖT, Toydemir TSF, Kirşan I, et al. Effects of surgical wound infiltration with bupivacaine on postoperative analgesia in cats undergoing bilateral mastectomy. J Vet Med Sci 2014; 76: 1595–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Crisi PE, De Santis F, Giordano MV, et al. Evaluation of eutectic lidocaine/prilocaine cream for jugular blood sampling in cats. J Feline Med Surg 2021; 23: 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wagner KA, Gibbon KJ, Strom TL, et al. Adverse effects of EMLA (lidocaine/prilocaine) cream and efficacy for the placement of jugular catheters in hospitalized cats. J Feline Med Surg 2006; 8: 141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gibbon KJ, Cyborski JM, Guzinski MV, et al. Evaluation of adverse effects of EMLA (lidocaine/prilocaine) cream for the placement of jugular catheters in healthy cats. J Vet Pharmacol Ther 2003; 26: 439–441. [DOI] [PubMed] [Google Scholar]
- 137. Steagall PV, Benito J, Monteiro B, et al. Intraperitoneal and incisional analgesia in small animals: simple, cost-effective techniques. J Small Anim Pract 2020; 61: 19–23. [DOI] [PubMed] [Google Scholar]
- 138. Benito J, Monteiro B, Lavoie AM, et al. Analgesic efficacy of intraperitoneal administration of bupivacaine in cats. J Feline Med Surg 2016; 18: 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Benito J, Evangelista MC, Doodnaught GM, et al. Analgesic efficacy of bupivacaine or bupivacaine-dexmedetomidine after intraperitoneal administration in cats: a randomized, blinded, clinical trial. Front Vet Sci 2019; 6: 307. DOI: 10.3389/fvets.2019.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Benito J, Monteiro BP, Beaudry F, et al. Pharmacokinetics of bupivacaine after intraperitoneal administration to cats undergoing ovariohysterectomy. Am J Vet Res 2016; 77: 641–645. [DOI] [PubMed] [Google Scholar]
- 141. Benito J, Monteiro B, Beaudry F, et al. Efficacy and pharmaco-kinetics of bupivacaine with epinephrine or dexmedetomidine after intraperitoneal administration in cats undergoing ovariohysterectomy. Can J Vet Res 2018; 82: 124–130. [PMC free article] [PubMed] [Google Scholar]
- 142. De OL, Carapeba G, Nicácio IPGA, Stelle ABF, et al. Comparison of perioperative analgesia using the infiltration of the surgical site with ropivacaine alone and in combination with meloxicam in cats undergoing ovariohysterectomy. BMC Vet Res 2020; 16: 88. DOI: 10.1186/s12917-020-02303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nicácio IP, Stelle ABF, Bruno TS, et al. Comparison of intraperitoneal ropivacaine and ropivacaine-dexmedetomidine for postoperative analgesia in cats undergoing ovariohysterectomy. Vet Anaesth Analg 2020; 47: 396–404. [DOI] [PubMed] [Google Scholar]
- 144. Soltaninejad H, Vesal N. Plasma concentrations of lidocaine following laryngeal administration or laryngeal and intratesticular administration in cats. Am J Vet Res 2018; 79: 614–620. [DOI] [PubMed] [Google Scholar]
- 145. Fernandez-Parra R, Zilberstein L, Fontaine C, et al. Comparison of intratesticular lidocaine, sacrococcygeal epidural lidocaine and intravenous methadone in cats undergoing castration: a prospective, randomized, investigator-blind clinical trial. Vet Anaesth Analg 2017; 44: 356–363. [DOI] [PubMed] [Google Scholar]
- 146. Watanabe R, Doodnaught G, Proulx C, et al. A multidisciplinary study of pain in cats undergoing dental extractions: a prospective, blinded, clinical trial. PLoS One 2019; 14: e0213195. DOI: 10.1371/journal.pone.0213195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rochette J. Regional anesthesia and analgesia for oral and dental procedures. Vet Clin North Am Small Anim Pract 2005; 35: 1041–1058. [DOI] [PubMed] [Google Scholar]
- 148. Gross ME, Pope ER, Jarboe JM, et al. Regional anesthesia of the infraorbital and inferior alveolar nerves during noninvasive tooth pulp stimulation in halothane-anesthetized cats. Am J Vet Res 2000; 61: 1245–1247. [DOI] [PubMed] [Google Scholar]
- 149. Grubb T, Lobprise H. Local and regional anaesthesia in dogs and cats: descriptions of specific local and regional techniques (part 2). Vet Med Sci 2020; 6: 218–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Perry R, Moore D, Scurrell E. Globe penetration in a cat following maxillary nerve block for dental surgery. J Feline Med Surg 2015; 17: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Volk H, Bayley K, Fiani N, et al. Ophthalmic complications following ocular penetration during routine dentistry in 13 cats. N Z Vet J 2019; 67: 46–51. [DOI] [PubMed] [Google Scholar]
- 152. Aprea F, Vettorato E, Corletto F. Severe cardiovascular depression in a cat following a mandibular nerve block with bupivacaine. Vet Anaesth Analg 2011; 38: 614–618. [DOI] [PubMed] [Google Scholar]
- 153. Simon BT, Steagall PV. Feline procedural sedation and analgesia: when, why and how. J Feline Med Surg 2020; 22: 1029–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Pereira MA, Campos KD, Gonçalves LA, et al. Cyclooxygenases 1 and 2 inhibition and analgesic efficacy of dipyrone at different doses or meloxicam in cats after ovariohysterectomy. Vet Anaesth Analg 2021; 48: 7–16. [DOI] [PubMed] [Google Scholar]
- 155. Chandrasekharan NV, Dai H, Roos KLT, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/ antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci 2002; 99: 13926139–31. DOI: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Jasiecka A, Maślanka T, Jaroszewski JJ. Pharmacological characteristics of metamizole. Pol J Vet Sci 2014; 17: 207–214. [DOI] [PubMed] [Google Scholar]
- 157. Zanuzzo FS, Teixeira-Neto FJ, Teixeira LR, et al. Analgesic and antihyperalgesic effects of dipyrone, meloxicam or a dipyrone-meloxicam combination in bitches undergoing ovariohysterectomy. Vet J 2015; 205: 33–37. [DOI] [PubMed] [Google Scholar]
- 158. Teixeira LG, Martins LR, Schimites PI, et al. Evaluation of postoperative pain and toxicological aspects of the use of dipyrone and tramadol in cats. J Feline Med Surg 2020; 22: 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lebkowska-Wieruszewska B, Kim TW, Chea B, et al. Pharmacokinetic profiles of the two major active metabolites of metamizole (dipyrone) in cats following three different routes of administration. J Vet Pharmacol Ther 2018; 41: 334–339. [DOI] [PubMed] [Google Scholar]
- 160. Cheng J-K, Chiou L-C. Mechanisms of the antinociceptive action of gabapentin. J Pharmacol Sci 2006; 100: 471–486. [DOI] [PubMed] [Google Scholar]
- 161. Adrian D, Papich MG, Baynes R, et al. The pharmacokinetics of gabapentin in cats. J Vet Intern Med 2018; 32: 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Siao KT, Pypendop BH, Ilkiw JE. Pharmacokinetics of gabapentin in cats. Am J Vet Res 2010; 71: 817–821. [DOI] [PubMed] [Google Scholar]
- 163. Slovak JE, Costa AP. A pilot study of transdermal gabapentin in cats. J Vet Intern Med 2021; 35: 1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Vettorato E, Corletto F. Gabapentin as part of multi-modal analgesia in two cats suffering multiple injuries. Vet Anaesth Analg 2011; 38: 518–520. [DOI] [PubMed] [Google Scholar]
- 165. Pankratz KE, Ferris KK, Griffith EH, et al. Use of single-dose oral gabapentin to attenuate fear responses in cage-trap confined community cats: a double-blind, placebo-controlled field trial. J Feline Med Surg 2018; 20: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Pozzi A, Muir WW, Traverso F. Prevention of central sensitization and pain by N-methyl-D-aspartate receptor antagonists. J Am Vet Med Assoc 2006; 228: 53–60. [DOI] [PubMed] [Google Scholar]
- 167. Brondani JT, Luna LSP, Beier SL, et al. Analgesic efficacy of perioperative use of vedaprofen, tramadol or their combination in cats undergoing ovariohysterectomy. J Feline Med Surg 2009; 11: 420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Steagall PV, Taylor PM, Brondani JT, et al. Antinociceptive effects of tramadol and acepromazine in cats. J Feline Med Surg 2008; 10: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Pypendop BH, Ilkiw JE. Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats. J Vet Pharmacol Ther 2008; 31: 52–59. [DOI] [PubMed] [Google Scholar]
- 170. Indrawirawan Y, McAlees T. Tramadol toxicity in a cat: case report and literature review of serotonin syndrome. J Feline Med Surg 2014; 16: 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Steagall PV, Pelligand L, Page SW, et al. ; WSAVA Therapeutic Guidelines Group (TGG). The World Small Animal Veterinary Association (WSAVA): list of essencial medicines for cats and dogs. J Small Anim Pract 2020; 16; 61. DOI: 10.1111/jsap.13135. [DOI] [PubMed] [Google Scholar]
- 172. Beloeil H. Opioid-free anesthesia. Best Pract Res Clin Anaesthesiol 2019; 33: 353–360. [DOI] [PubMed] [Google Scholar]
- 173. Chia PA, Cannesson M, Bui CCM. Opioid free anesthesia: feasible? Curr Opin Anaesthesiol 2020; 33: 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Forget P. Opioid-free anaesthesia. Why and how? A contextual analysis. Anaesth Crit Care Pain Med 2019; 38: 169–172. [DOI] [PubMed] [Google Scholar]
- 175. Diep TN, Monteiro BP, Evangelista MC, et al. Anesthetic and analgesic effects of an opioid-free, injectable protocol in cats undergoing ovariohysterectomy: a prospective, blinded, randomized clinical trial. Can Vet J 2020; 61: 621–628. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jfm-10.1177_1098612X211066268 for 2022 ISFM Consensus Guidelines on the Management of Acute Pain in Cats by Paulo V Steagall, Sheilah Robertson, Bradley Simon, Leon N Warne, Yael Shilo-Benjamini and Samantha Taylor in Journal of Feline Medicine and Surgery
Supplemental material, sj-pdf-2-jfm-10.1177_1098612X211066268 for 2022 ISFM Consensus Guidelines on the Management of Acute Pain in Cats by Paulo V Steagall, Sheilah Robertson, Bradley Simon, Leon N Warne, Yael Shilo-Benjamini and Samantha Taylor in Journal of Feline Medicine and Surgery
Supplemental material, sj-pdf-3-jfm-10.1177_1098612X211066268 for 2022 ISFM Consensus Guidelines on the Management of Acute Pain in Cats by Paulo V Steagall, Sheilah Robertson, Bradley Simon, Leon N Warne, Yael Shilo-Benjamini and Samantha Taylor in Journal of Feline Medicine and Surgery