Abstract
Background
Patients who report penicillin allergies may receive alternative antibiotics. Such substitution contributes to antimicrobial resistance, lower treatment efficacy, increased frequency of adverse events, and increased costs. Approximately 90% of individuals who report a penicillin allergy can tolerate a penicillin.
Objective
To identify the barriers to and facilitators of removal by health care workers of inaccurate antimicrobial allergies from patient records, known as delabelling.
Data Sources
The MEDLINE database was searched from inception to December 29, 2020.
Study Selection and Data Extraction
Qualitative studies evaluating health care professionals’ perceptions of barriers to and/or facilitators of the act of delabelling a patient’s antimicrobial allergies were included in the meta-synthesis.
Data Synthesis
The Theoretical Domains Framework was used to code and group individual utterances from the included studies, which were mapped to the Behaviour Change Wheel and corresponding intervention function and policy categories.
Results
Four studies met the inclusion criteria. Eight themes were identified as representing barriers to delabelling: delabelling skills, patient education skills, knowledge, electronic health records (EHRs), communication frameworks, time, fear about allergic reactions, and professional roles. Behaviour change interventions that may overcome these barriers include education, training, algorithms and toolkits, changes to EHRs, use of dedicated personnel, policies, incentivization of correct labelling, and an audit system.
Conclusions
Eight themes were identified as barriers to delabelling of antimicrobial allergies. Future behaviour change interventions to address these barriers were proposed. Confidence in the findings of this study was judged to be moderate, according to the GRADE CERQual approach.
Keywords: antibiotic, penicillin, allergy, delabelling, electronic health records, implementation science
RÉSUMÉ
Contexte
Les patients qui signalent des allergies à la pénicilline peuvent recevoir d’autres antibiotiques. Une telle substitution contribue à la résistance aux antimicrobiens, à une moindre efficacité du traitement, à une fréquence accrue des événements indésirables et à une augmentation des coûts. Environ 90 % des personnes qui déclarent une allergie à la pénicilline peuvent la tolérer.
Objectif
Identifier les obstacles à l’élimination par les travailleurs de la santé des allergies antimicrobiennes inexactes des dossiers des patients, ce que l’on appelle « le désétiquetage », et les facteurs qui le favorisent.
Sources des données
La base de données MEDLINE a été consultée depuis sa création jusqu’au 29 décembre 2020.
Sélection de l’étude et extraction des données
Des études qualitatives évaluant les perceptions des professionnels de la santé quant aux obstacles à l’acte de désétiquetage des allergies aux antimicrobiens d’un patient et les facilitateurs de celui-ci ont été incluses dans la métasynthèse.
Synthèse des données
Le cadre théorique des domaines a été utilisé pour coder et regrouper les énoncés individuels, qui ont ensuite été associés à la roue du changement de comportement ainsi qu’aux catégories de fonctions et de politiques d’intervention correspondantes.
Résultats
Quatre études répondaient aux critères d’inclusion. Huit thèmes ont été identifiés comme représentant des obstacles au désétiquetage: les compétences en la matière, les compétences en matière d’éducation des patients, les connaissances, les dossiers de santé électroniques (DSE), les cadres de communication, le temps, la peur des réactions allergiques et les rôles professionnels. Les interventions visant le changement de comportement qui peuvent surmonter ces obstacles comprennent l’éducation, la formation, les algorithmes et les boîtes à outils de désétiquetage, la modification des DSE, le recours à du personnel dédié, des politiques, l’incitation à un étiquetage correct et un système d’audit.
Conclusions
Huit thèmes ont été identifiés comme étant des obstacles au désétiquetage des allergies aux antimicrobiens. De futures interventions ciblant le changement de comportement pour les surmonter ont été proposées. La confiance dans les résultats de cette étude a été jugée modérée, selon l’approche GRADE CERQual.
Mots-clés: antibiotique, pénicilline, allergie, désétiquetage, dossiers de santé électroniques, science de la mise en œuvre
INTRODUCTION
Antibiotic use is common in Canada, with a daily consumption rate of 16.3 antimicrobial doses per 1000 people.1 Approximately 25% of hospitalized patients report an antimicrobial allergy, and 10% of these reported allergies are to penicillin.2–6 However, upon further testing, approximately 90% of individuals who self-report a penicillin allergy are able to tolerate this antibiotic.3,6–9 Inaccurate documentation of penicillin allergies in medical records may result in patients receiving broad-spectrum and/or non–β-lactam antibiotics, which may lead to decreased effectiveness, increased rates of adverse events, emergence of multidrug-resistant microorganisms, and increased costs.2 In one Canadian study, the use of alternative antibiotics in penicillin-allergic patients incurred an additional cost averaging $326.50 per patient.10
There are many reasons for inaccurate documentation of medication allergies.4,5,11 However, even true allergic reactions may wane over time, and the risk of repeat immunoglobulin E–mediated hypersensitivity reactions to structurally related antimicrobials diminishes by up to 80% over 10 years.12,13
Delabelling is the identification and removal of inaccurate and spurious antimicrobial allergy labels from patients’ medical records.7,14 To optimize antimicrobial prescribing and improve antimicrobial stewardship, patients reporting an antimicrobial allergy should be asked to provide a comprehensive allergy history and should undergo oral challenge and/or skin testing, as indicated.4,7,11 Patients with negative test results can safely have the allergy label removed from their record and can be advised that their risk of allergy is the same as that of the general population.4,7
Multiple organizations have recommended that health care professionals verify patients’ self-reported antimicrobial allergies and perform delabelling when indicated.4 The Canadian Society of Allergy and Clinical Immunology, in its contribution to the Choosing Wisely Canada campaign, recommends against using non–β-lactam antibiotics in patients with a history of penicillin allergy without an appropriate allergy evaluation.15 The Infectious Diseases Society of America has recommended that antimicrobial stewardship programs conduct allergy assessments and, if indicated, penicillin skin testing in patients with a history of penicillin allergy.16 Despite these recommendations, clinicians rarely perform antimicrobial allergy delabelling activities.4,11
Delabelling antimicrobial allergies requires a change in the behaviour of health care professionals. The first step to enabling health care professionals to delabel spurious allergies is identifying barriers to and facilitators of this activity.17,18 Behaviour change interventions (BCIs), defined as coordinated sets of activities intended to change specified behaviour patterns, can then be designed and implemented to enhance facilitators and overcome barriers.18,19
Several small qualitative studies describing barriers to and facilitators of delabelling antimicrobial allergies have been published.3,4 A meta-synthesis of these studies would create a broader, more germane description of the factors that influence health care workers in delabelling antimicrobial allergies. Therefore, the primary aim of this study was to describe barriers to and facilitators of delabelling inaccurate antimicrobial allergies in all health care settings through use of a validated framework and model of behaviour change. The secondary aim was to link the barriers to potential interventions and/or policies that would allow them to be overcome and that could be used to inform the design of future BCIs.
METHODS
The study protocol was published in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42021225260). The reporting of study results adhered to the ENTREQ statement, “Enhancing transparency in reporting the synthesis of qualitative research”.20
Inclusion Criteria
Qualitative studies evaluating health care professionals’ perceptions of barriers to and/or facilitators of the act of delabelling patients’ antimicrobial allergies were included in the meta-synthesis. Published English-language, full-text articles or reports based on any qualitative method of obtaining data were included. Studies using mixed methods were included if the qualitative data were analyzed independently of the quantitative data. Studies were not excluded because of methodological limitations; however, any such limitations were considered in assessing confidence in the review findings. Participants in the included studies were health care professionals who had the opportunity to perform allergy assessments in all health care settings in high-income countries (as defined by the World Bank Index of High-Income Countries21).
Search Strategy
The STARLITE mnemonic (outlined in the Cochrane Handbook for Systematic Reviews of Interventions) was used to formulate the literature search strategy.22,23 Information specialists from libraries at the University of British Columbia Biomedical Branch (Vancouver, British Columbia) and the Kelowna General Hospital (Kelowna, British Columbia) reviewed and refined the search strategy (Supplement 1, Part 1, available from https://www.cjhp-online.ca/index.php/cjhp/article/view/3490/). The MEDLINE database was searched from inception to December 29, 2020.
Study Selection and Data Extraction
Two of the authors (J.M., V.C.) independently reviewed titles and abstracts of all citations to assess eligibility. The full text of each potentially relevant study was retrieved and independently reviewed by these authors for inclusion. Disagreements were resolved by consensus, and the reasons for exclusion were documented. Covidence software (https://www.covidence.org/) was used for study screening, selection, and generation of the trial flow diagram.
The same 2 authors (J.M., V.C.) independently performed data extraction using a modified data collection form based on that of Heslehurst and others.24 Disagreements were resolved by discussion. The following data were extracted: title, lead author contact details, study context, study design, study participants, data analysis, key findings, and conclusions.
Quality Assessment
The methodological limitations of each study were independently assessed by 2 authors (J.M., V.C.) using the Critical Appraisal Skills Programme (CASP) tool.25,26 This tool uses a checklist of 10 questions, with a total of 29 signalling questions to help users interpret the checklist items.26 Studies were not excluded because of low quality.25 Disagreements in CASP tool scores were resolved by discussion.
Data Analysis and Synthesis
Data analysis was performed in duplicate by 2 authors (J.M., V.C.). Portable Document Files (PDFs) of included studies were imported into NVivo Pro 12 software (QSR International). The full text of each study was reviewed. Utterances from each study that were regarded as either barriers to or facilitators of antimicrobial allergy delabelling were identified. A coding guide, developed a priori, was used to categorize utterances into domains of the Theoretical Domains Framework (TDF) (Supplement 1, Part 2).24,25
The TDF was originally developed to provide a comprehensive, theory-informed approach to identifying the determinants of a behaviour.18,27 It has been widely used to identify influences on behaviours.18 The 14 domains of the TDF may be grouped according to the COM-B model, which posits that capability, opportunity, and motivation interact to produce behaviour.19 The Behaviour Change Wheel can then be used to map the COM-B categories to any of 9 intervention functions, which may be mapped to any of 7 policy categories (Supplement 1, Part 3).18,19 The Behaviour Change Wheel characterizes all possible intervention types and matches these features to the behavioural target, the target population, and the context in which the intervention will be delivered.18,19
To illustrate themes and ensure coding consistency across studies and between authors, participants’ quotes were documented. Paragraphs and sentences could be divided into sections and coded to separate TDF domains; however, each such section could be assigned to only 1 TDF domain. Disagreements were discussed, with resolution by a third author (S.G.) if required.
Themes were generated through consideration of the overall coding frequency of barriers and facilitators within each TDF domain, as well as consideration of the concepts providing the richest data. The consensus of 2 authors (J.M., V.C.) was required for all themes. Themes from the meta-synthesis were compared with themes from the primary studies to ensure internal validity.
Themes were grouped into the capability, opportunity, and motivation categories of the COM-B model using an established mapping process.18,19 The Behaviour Change Wheel was used to select the most appropriate BCIs to address barriers to and facilitators of antimicrobial allergy delabelling. This mapping was performed by a single author (J.M.).
Assessment of Confidence in Evidence from Reviews of Qualitative Research (GRADE-CERQual)
One of the authors (J.M.) used the GRADE-CERQual approach (https://www.cerqual.org/) to assess confidence in each theme of the meta-synthesis. The GRADE-CERQual approach is a tool to evaluate confidence in the findings of a qualitative evidence synthesis using the following 4 components: methodological limitations of included studies, coherence of each review finding, adequacy of the data contributing to a review finding, and relevance of the included studies to the review question.25,28–34
After each of the 4 components was assessed, a judgment was made regarding overall confidence in the evidence supporting the review finding. Confidence could be classified as high, moderate, low, or very low. As per the CERQual method, initial confidence in findings was assumed to be high, and confidence was downgraded if there were important concerns regarding any of the CERQual components.
RESULTS
Study Selection and Study Characteristics
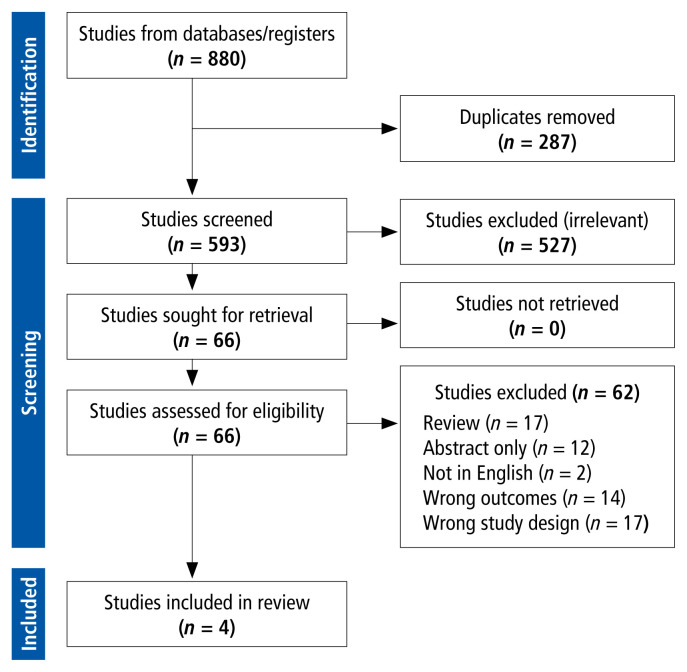
A total of 593 unique studies were identified, of which 4 met the inclusion criteria and were included in the qualitative synthesis (see Figure 1, Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] flow diagram, and Supplement 1, Part 4). Two of the studies were conducted in the United Kingdom and one each in the Netherlands and the United States.4,35–37 Physicians and pharmacists were the health care professionals most commonly interviewed; one study included a medical microbiologist, and another study interviewed only nurses and infection preventionists.35,37 The study settings were varied, with clinicians providing inpatient care (2 studies) or community care (2 studies). All of the studies collected data using either focus groups or semistructured interviews.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Quality Assessment
According to the CASP tool for assessing quality,26 the individual studies met between 2 and 8 of the 10 possible criteria (Supplement 1, Part 5). The methodological strengths and limitations in each quality domain were summarized for each study (Supplement 1, Part 5).
TDF Coding and Frequency Analysis
Overall, 200 utterances were coded, of which 118 represented barriers and 82 represented facilitators. Barriers to and facilitators of delabelling were identified in 12 and 11 TDF domains, respectively, with some overlap; the only TDF domain not represented in the analysis was “intentions” (Supplement 1, Part 6). The most frequently cited and salient domains were environmental context and resources, skills, social or professional role and identity, knowledge, and beliefs about consequences. The TDF codes were then subclassified into barriers or facilitators (Supplement 1, Parts 7 and 8). When study participants discussed strategies to overcome barriers, these utterances were coded as facilitators.
COM-B Themes
Eight themes describing barriers to antimicrobial delabelling emerged from the data, and these themes were grouped as follows: delabelling skills, patient education skills, and knowledge were categorized as capability-related themes; electronic health records (EHRs), communication frameworks, and time as opportunity-related themes; and fears about allergic reactions and professional roles as motivation-related themes.
Capability-Related Themes
Delabelling Skills
Physicians and pharmacists stated that they lacked the ability to distinguish allergies from adverse drug reactions and concurrent viral illnesses; they also noted that guidance to distinguish allergic reactions from adverse reactions was inadequate. Health care professionals requested additional education, such as a toolkit or a training module for delabelling antimicrobial allergies, with ongoing support.
Patient Education Skills
Some participants mentioned the importance of skill in educating patients about the risks of spurious allergy labels to ensure patients’ acceptance of delabelling and to prevent relabelling of allergies.
Knowledge
Study participants reported a lack of knowledge of key aspects of antimicrobial allergy delabelling. Inadequate knowledge was noted regarding the magnitude of the problem of inaccurate allergy labels and the consequences of inaccurate labels; more specifically, knowledge about distinguishing hypersensitivity reactions from adverse drug reactions was lacking. Participants suggested that education and clear definitions would aid in the delabelling process.
Opportunity-Related Themes
Electronic Health Records
Participants believed that limitations of current documentation tools and systems precluded clear documentation of medication allergies and intolerances. To overcome barriers to delabelling, health care professionals recommended modifying current EHR systems to differentiate among allergic reactions, adverse drug reactions, and patient preferences.
Communication Frameworks
Most EHRs used by participants were reported to be “stand-alone”; as such, when one health care provider delabelled an allergy, their EHR did not communicate the change to the EHRs of other health care providers. Participants expressed concern that if communication between health care professionals was poor, the onus could fall on the patient to inform other health care providers that their allergy had been delabelled. To mitigate this problem, participants suggested electronic communication among general practices, pharmacies, and hospitals, with such communication to include detailed descriptions of the type of reaction.
Time
Participants acknowledged that clarifying and delabelling spurious allergy labels in EHRs would be time-consuming, given the magnitude of contamination of current allergy records with inappropriate and incomplete documentation; furthermore, participants were aware that nurses already felt overwhelmed by administrative duties. To overcome the barrier of time constraints, participants recommended assigning dedicated personnel to the task of delabelling.
Motivation-Related Themes
Fears about Allergic Reactions
Family physicians, pharmacists, and nurses reported the fear of adverse reactions as a barrier to delabelling. Even though some health care professionals doubted the validity of patients’ reported penicillin allergies, they were unwilling to delabel the allergies, as they were concerned that by doing so, they might contribute to future potential allergic reactions.
Professional Roles
Many participants felt that physicians, pharmacists, and nurse practitioners should perform delabelling, as these professionals are able to accurately evaluate allergy symptoms. Some nurses thought that allergy assessment and/or delabelling was outside their scope of practice. However, nurses did agree that they could perform an allergy assessment and deliver the information to a physician, pharmacist, or nurse practitioner who would then delabel the allergy. Participants suggested that to overcome these barriers, health care professionals should collaborate in assessing allergy labels.
Logic Model
A logic model was created to connect barriers to delabelling antimicrobial allergies with BCIs that would address these barriers (Supplement 1, Part 9). The barriers were mapped to intervention functions (activities to promote uptake and optimal use of effective clinical services) using the Behaviour Change Wheel.18,19 The following intervention functions would increase the likelihood of successful behaviour change: education, persuasion, incentivization, coercion, training, restrictions, environmental restructuring, modelling, and enablement (Table 1). Intervention functions were then mapped to policy categories that could be used to deliver the intervention functions. The following policy categories were identified: communication/marketing, guidelines, fiscal action, legislation, regulation, environmental/social planning, and service provision (Table 2).
TABLE 1.
Intervention Functions to Support Antimicrobial Delabelling
| Intervention Function | Examples |
|---|---|
| Education: increased knowledge or understanding | Implement educational programs when rolling out delabelling toolkits for clinicians and patients |
| Persuasion: using communication to induce positive or negative feelings or stimulate action | Employ delabelling stewards on each nursing ward to motivate staff |
| Incentivization: creating expectation of reward | Offer a monthly prize for the hospital ward with the lowest number of patients with unconfirmed/unverified allergies |
| Coercion: creating expectation of punishment or cost | Use audit systems to give feedback to providers regarding number of patients with unconfirmed/unverified allergies |
| Training: imparting skills | Develop training modules and toolkits |
| Restrictions: using rules to reduce the opportunity to engage in the target behaviour (or to increase the target behaviour by reducing the opportunity to engage in competing behaviour) | Create allergy assessment policies within health authorities |
| Environmental restructuring: changing the physical or social context | Modify EHRs to enable distinct documentation of allergies vs adverse drug reactions vs patients’ medication preferences |
| Modelling: providing an example for people to aspire to or imitate | Employ delabelling stewards with additional training in delabelling to demonstrate procedures to staff |
| Enablement: increasing means/reducing barriers to increase capability or opportunity | Create a toolkit for clinicians to develop the skills, knowledge, and confidence to delabel allergies Provide dedicated personnel or protected time for delabelling services |
EHR = electronic health record.
TABLE 2.
Policy Categories to Support Antimicrobial Delabelling
| Policy Category | Examples |
|---|---|
| Communication/marketing: using print, electronic, telephonic, or broadcast media | Initiate an allergy delabelling campaign to educate the public through brochures in hospitals, physician offices, and pharmacies, and through commercials on television and radio |
| Guidelines: creating documents that recommend or mandate practice, including all changes to service provision | Develop clinical practice guidelines for delabelling antimicrobial allergies by professional associations (e.g., Infectious Diseases Society of America) |
| Fiscal action: using the tax system to reduce or increase the financial cost | Not applicable |
| Legislation: making or changing laws | Not applicable |
| Regulation: establishing rules or principles of behaviour or practice | Establish regulations endorsed by the licensing bodies and/or professional associations of physicians, pharmacists, and nurses |
| Environmental/social planning: designing and/or controlling the physical or social environment | Modify EHRs to enable distinct documentation of allergies vs adverse drug reactions vs patients’ medication preferences Improve electronic communication of allergies between outpatient and inpatient medical facilities (e.g., physician clinics, pharmacies, and hospitals) |
| Service provision: delivering a service | Provide support from content experts to health care teams in performing delabelling activities |
EHR = electronic health record.
Assessment of Confidence in Evidence from Reviews of Qualitative Research
Using the GRADE-CERQual approach, we judged overall confidence in the findings of the meta-synthesis as moderate. Details describing the CERQual findings are presented in Supplement 1, Parts 10 and 11.
DISCUSSION
To our knowledge, this qualitative meta-synthesis, based on a validated framework and model of behaviour change, is the first to identify barriers to and facilitators of delabelling of antimicrobial allergies by health care workers. Themes relating to health care workers’ capabilities, opportunities, and motivations were identified as barriers to antimicrobial allergy delabelling.
Two studies identified in our search provided the richest data for the meta-synthesis.36,37 Multiple studies were used to inform all themes except that of time constraints, which was derived from a single study.37 Consequently, we reported lower confidence in the finding of time constraints as a barrier to delabelling.
Themes relating to capability involved lack of delabelling skills, poor patient education skills, and lack of knowledge. Themes relating to opportunity included the inability of EHRs to allow accurate documentation of antimicrobial allergies and intolerances and the lack of means to communicate such information between different electronic systems. Some participants cited lack of time as a barrier to delabelling and recommended assigning dedicated personnel (physicians, pharmacists, or nurse practitioners) to perform this task. One theme relating to motivation pertained to clinicians’ fears about subsequent allergic reactions following delabelling of inaccurate antimicrobial allergies, despite understanding that the likelihood of a true allergic reaction was low. A systematic review found that, in the United States, malpractice lawsuits claiming medical negligence when patients with penicillin allergies received β-lactam antibiotics were uncommon, and defendants were rarely found liable.38,39 In particular, the authors of the systematic review found no cases in which patients experienced an allergic reaction following delabelling. Health care professionals’ liability can be mitigated by documenting patient consent when allergies are delabelled. A second theme relating to motivation was that of scope of practice. There was uncertainty among disciplines (e.g., nurse, pharmacist, physician) and within disciplines (e.g., family physician or allergist) as to whose role and responsibility encompassed antimicrobial allergy delabelling, documentation of findings in the medical record, and communication of findings to other health care professionals.
The barriers identified in this meta-synthesis are consistent with those identified in previous literature. In a rapid review, Wanat and others11 sought to explore patient and/or clinician views and experiences of penicillin allergy testing services and the influences on antibiotic prescribing behaviour in the context of penicillin allergy. Barriers to referral for allergy testing identified by these authors included patients declining allergy testing, lack of time and/or forgetting to discuss allergy testing during consultations, health care professionals anticipating that patients would not want to risk having a reaction during allergy testing, and physicians’ lack of awareness of patients’ antimicrobial allergies. These barriers are similar to the barriers identified in our meta-synthesis (lack of patient education skills, lack of time, fear of allergic reaction, lack of knowledge, and lack of delabelling skills).
An additional qualitative study has been published since completion of our meta-synthesis.40 The authors of that study identified barriers to delabelling similar to those found in our study: insufficient knowledge, lack of priority, limitations of registration in EHRs, fear of medical liability, and patients interpreting adverse effects as allergies.40 These authors also identified the following themes: individual characteristics of care providers; patient factors; professional interactions; incentives and resources; capacity for organizational change; and social, political, and legal factors. Some of their themes were similar to ours: “individual characteristics of care providers” was similar to our “delabelling skills”, “patient education skills”, and “knowledge” themes; “professional interactions” was similar to our “professional roles” and “communication frameworks” themes; and “legal factors” was similar to our “fears about allergic reaction” theme. However, some of their themes differed from ours: for example, “barriers due to patient centred factors” had no counterpart in our study, and Sijbom and others40 identified no patient-specific themes.
Strengths and Limitations
To our knowledge, this is the first qualitative meta-synthesis to describe barriers to and facilitators of antimicrobial allergy delabelling. The included studies involved a variety of health care professionals and thus provided a multidisciplinary perspective on antimicrobial allergy delabelling. One limitation was the small number of studies included in the meta-synthesis. Only one study was performed in North America, which may limit external validity. However, the quality of the studies included was adequate to achieve moderate confidence in the findings of this meta-synthesis.
CONCLUSION
Key barriers to and facilitators of antimicrobial allergy delabelling derived from multiple qualitative studies were identified and coded to themes of the TDF. Themes identified as barriers to antimicrobial allergy delabelling generated from the data pertained to delabelling skills, patient education skills, knowledge, EHRs, communication frameworks, time, fears about allergic reaction, and professional roles. In addition, BCIs with the potential to overcome barriers to antimicrobial allergy delabelling were identified. Next steps will include implementing BCIs, with future research evaluating the effect of these interventions on behaviour.
Supplementary Information
Acknowledgement
The authors would like to thank information specialists Ursula Ellis (Biomedical Branch Library, The University of British Columbia) and Michelle Main (Henderson Library, Kelowna General Hospital) for their assistance with the literature search.
Footnotes
Competing interests: None declared.
Funding: None received.
References
- 1.Canadian antimicrobial resistance surveillance system report. Public Health Agency of Canada; 2021. [cited 2023 Jun 30]. Available from: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/drugs-health-products/canadian-antimicrobial-resistance-surveillance-system-report-2021/canadian-antimicrobial-resistance-surveillance-system-report-2021.pdf. [Google Scholar]
- 2. Wu JHC, Langford BJ, Schwartz KL, Zvonar R, Raybardhan S, Leung V, et al. Potential negative effects of antimicrobial allergy labelling on patient care: a systematic review. Can J Hosp Pharm. 2018;71(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- 3. Savic L, Gurr L, Kaura V, Toolan J, Sandoe JAT, Hopkins PM, et al. Penicillin allergy de-labelling ahead of elective surgery: feasibility and barriers. Br J Anaesth. 2018;123(1):e110–e116. doi: 10.1016/j.bja.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 4. Wanat M, Anthierens S, Butler CC, Savic L, Savic S, Pavitt SH, et al. Patient and primary care physician perceptions of penicillin allergy testing and subsequent use of penicillin-containing antibiotics: a qualitative study. J Allergy Clin Immunol Pract. 2019;7(6):1888–93.e1. doi: 10.1016/j.jaip.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 5. Patrick DM, Al Mamun A, Smith N, Rempel E, Calissi P, Blondel-Hill E. Beta-lactam allergy: benefits of de-labeling can be achieved safely. B C Med J. 2019;61(9):350–1,361. [Google Scholar]
- 6. Macy E, Contreras R. Adverse reactions associated with oral and parenteral use of cephalosporins: a retrospective population-based analysis. J Allergy Clin Immunol. 2015;135(3):745–52.e5. doi: 10.1016/j.jaci.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 7. Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(12):188–99. doi: 10.1001/jama.2018.19283. [DOI] [PubMed] [Google Scholar]
- 8. Harandian F, Pham D, Ben-Shoshan M. Positive penicillin allergy testing results: a systematic review and meta-analysis of papers published from 2010 through 2015. Postgrad Med. 2016;128(6):557–62. doi: 10.1080/00325481.2016.1191319. [DOI] [PubMed] [Google Scholar]
- 9. Sacco KA, Bates A, Brigham TJ, Imam JS, Burton MC. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy. 2017;72(9):1288–96. doi: 10.1111/all.13168. [DOI] [PubMed] [Google Scholar]
- 10. Picard M, Bégin P, Bouchard H, Cloutier J, Lacombe-Barrios J, Paradis J, et al. Treatment of patients with a history of penicillin allergy in a large tertiary-care academic hospital. J Allergy Clin Immunol Pract. 2013;1(3):252–7. doi: 10.1016/j.jaip.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 11. Wanat M, Anthierens S, Butler CC, Wright JM, Dracup N, Pavitt SH, et al. Patient and prescriber views on penicillin allergy testing and subsequent antibiotic use: a rapid review. Antibiotics (Basel) 2018;7(3):71. doi: 10.3390/antibiotics7030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanca M, Torres MF, García JJ, Romano A, Mayorga C, de Ramon E, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5 Pt 1):918–24. doi: 10.1016/S0091-6749(99)70439-2. [DOI] [PubMed] [Google Scholar]
- 13. Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68(3):171–80. doi: 10.1016/0091-6749(81)90180-9. [DOI] [PubMed] [Google Scholar]
- 14. Jani YH, Williams I, Krishna MT. Sustaining and spreading penicillin allergy delabelling: a narrative review of the challenges for service delivery and patient safety. Br J Clin Pharmacol. 2020;86(3):548–59. doi: 10.1111/bcp.14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canadian Society of Allergy, Clinical Immunology. Seven tests and treatments to question. Choosing Wisely Canada; 2021. Aug, [cited 2023 Dec 23]. Available from: https://choosingwiselycanada.org/recommendation/allergy-clinical-immunology/ [Google Scholar]
- 16. Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77. doi: 10.1093/cid/ciw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atkins L, Frances J, Islam R, O’Connor D, Patey A, Ivers N, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. 2017;12:77. doi: 10.1186/s13012-017-0605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Westland H, Bos-Touwen ID, Trappenburg JCA, Schröder CD, de Wit NJ, Schuurmans MJ. Unravelling effectiveness of a nurse-led behaviour change intervention to enhance physical activity in patients at risk for cardiovascular disease in primary care: study protocol for a cluster randomised controlled trial. Trials. 2017;18(1):79. doi: 10.1186/s13063-017-1823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tong A, Flemming K, McInnes E, Oliver S, Craig J. Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol. 2012;12:181. doi: 10.1186/1471-2288-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Classifying countries by income. The World Bank; 2019. Sep 9, [cited 2023 Jun 21]. Available from: https://datatopics.worldbank.org/world-development-indicators/stories/the-classification-of-countries-by-income.html. [Google Scholar]
- 22.Noyes J, Booth A, Cargo M, Flemming K, Harden A, Harris J, et al. Chapter 21: Qualitative evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions. Version 6.1. Cochrane; 2020. [updated 2020 Sep; cited 2020 Sep 25]. Available from: www.training.cochrane.org/handbook. [Google Scholar]
- 23. Booth A. Brimful of STARLITE: towards standards for reporting literature searches. J Med Libr Assoc. 2006;94(4):421–9,e205. [PMC free article] [PubMed] [Google Scholar]
- 24. Heslehurst N, Newham J, Maniatopoulos G, Fleetwod C, Robalino S, Rankin J. Implementation of pregnancy weight management and obesity guidelines: a meta-synthesis of healthcare professionals’ barriers and facilitators using the Theoretical Domains Framework. Obes Res. 2014;15(6):462–86. doi: 10.1111/obr.12160. [DOI] [PubMed] [Google Scholar]
- 25. Noyes J, Booth A, Flemming K, Garside R, Harden A, Lewin S, et al. Cochrane Qualitative and Implementation Methods Group guidance series—paper 3: methods for assessing methodological limitations, data extraction and synthesis, and confidence in synthesized qualitative findings. J Clin Epidemiol. 2018;97:49–58. doi: 10.1016/j.jclinepi.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 26.CASP checklists [website] Critical Appraisal Skills Programme; 2018. CASP qualitative studies checklist. [cited 2020 Sep 14]. Available from: https://casp-uk.net/casp-tools-checklists/ [Google Scholar]
- 27. Michie S, Johnston M, Francis J, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol. 2008;57(4):660–80. doi: 10.1111/j.1464-0597.2008.00341.x. [DOI] [Google Scholar]
- 28. Lewin S, Booth A, Glenton C, Munthe-Kaas H, Rashidian A, Wainwright M, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings: introduction to the series. Implement Sci. 2018;13(Suppl 1):2. doi: 10.1186/s13012-017-0688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewin S, Bohren M, Rashidian A, Munthe-Kaas H, Glenton C, Colvin CJ, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings—paper 2: how to make an overall CERQual assessment of confidence and create a summary of qualitative findings table. Implement Sci. 2018;13(Suppl 1):10. doi: 10.1186/s13012-017-0689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Munthe-Kaas H, Bohren MA, Glenton C, Lewin S, Noyes J, Tunçalp Ö, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings—paper 3: how to assess methodological limitations. Implement Sci. 2018;13(Suppl 1):9. doi: 10.1186/s13012-017-0690-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colvin CJ, Garside R, Wainwright M, Munthe-Kaas H, Glenton C, Bohren MA, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings—paper 4: how to assess coherence. Implement Sci. 2018;13(Suppl 1):13. doi: 10.1186/s13012-017-0691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glenton C, Carlsen B, Lewin S, Munthe-Kaas H, Colvin CJ, Tunçalp O, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings—paper 5: how to assess adequacy of data. Implement Sci. 2018;13(Suppl 1):14. doi: 10.1186/s13012-017-0692-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noyes J, Booth A, Lewin S, Carlsen B, Glenton C, Colvin CJ, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings—paper 6: how to assess relevance of the data. Implement Sci. 2018;13(Suppl 1):4. doi: 10.1186/s13012-017-0693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Booth A, Lewin S, Glenton C, Munthe-Kaas H, Toews I, Noyes J, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings—paper 7: understanding the potential impacts of dissemination bias. Implement Sci. 2018;13(Suppl 1):12. doi: 10.1186/s13012-017-0694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carter EJ, Greendyke WG, Furuya EY, Srinivasan A, Shelley AN, Bothra A, et al. Exploring the nurses’ role in antibiotic stewardship: a multisite qualitative study of nurses and infection preventionists. Am J Infect Control. 2018;46(5):492–7. doi: 10.1016/j.ajic.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Clercq K, Cals JWL, de Bont EGPM. Inappropriate antibiotic allergy documentation in health records: a qualitative study on family physicians’ and pharmacists’ experiences. Ann Fam Med. 2020;18(4):326–33. doi: 10.1370/afm.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Powell N, Wilcock M, Roberts N, Sandoe J, Tonkin-Crine S. Focus group study exploring the issues and the solutions to incorrect penicillin allergy-labelled patients: an antibiotic stewardship patient safety initiative. Eur J Hosp Pharm. 2019;28(2):71–5. doi: 10.1136/ejhpharm-2019-001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeffres MN, Hall-Lipsy EA, King ST, Cleary JD. Systematic review of professional liability when prescribing β-lactams for patients with known penicillin allergy. Ann Allergy Asthma Immunol. 2018;121(5):530–6. doi: 10.1016/j.anai.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 39. Solensky R. Penicillin allergy and the law [editorial] Ann Allergy Asthma Immunol. 2018;121(5):517–8. doi: 10.1016/j.anai.2018.09.451. [DOI] [PubMed] [Google Scholar]
- 40. Sijbom M, Braun KK, Büchner FL, van Bodegom-Vos L, Hendriks BJC, de Boer MGF, et al. Cues to improve antibiotic-allergy registration: a mixed-method study. PloS One. 2022;17(4):e0266473. doi: 10.1371/journal.pone.0266473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.