Abstract
Over the past 2 decades, population simulation modeling has evolved as an effective public health tool for surveillance of cancer trends and estimation of the impact of screening and treatment strategies on incidence and mortality, including documentation of persistent cancer inequities. The goal of this research was to provide a framework to support the next generation of cancer population simulation models to identify leverage points in the cancer control continuum to accelerate achievement of equity in cancer care for minoritized populations. In our framework, systemic racism is conceptualized as the root cause of inequity and an upstream influence acting on subsequent downstream events, which ultimately exert physiological effects on cancer incidence and mortality and competing comorbidities. To date, most simulation models investigating racial inequity have used individual-level race variables. Individual-level race is a proxy for exposure to systemic racism, not a biological construct. However, single-level race variables are suboptimal proxies for the multilevel systems, policies, and practices that perpetuate inequity. We recommend that future models designed to capture relationships between systemic racism and cancer outcomes replace or extend single-level race variables with multilevel measures that capture structural, interpersonal, and internalized racism. Models should investigate actionable levers, such as changes in health care, education, and economic structures and policies to increase equity and reductions in health-care–based interpersonal racism. This integrated approach could support novel research approaches, make explicit the effects of different structures and policies, highlight data gaps in interactions between model components mirroring how factors act in the real world, inform how we collect data to model cancer equity, and generate results that could inform policy.
At present, minoritized populations experience substantially poorer cancer outcomes than the overall population, reflecting the impact of systemic racism on cancer control (1-12). Systemic racism stems from policies and institutions that bolster the US racial hierarchy (13). The term systemic racism broadly captures the multiple levels (14,15) of racism including structural, interpersonal, and internalized racism, which are deeply embedded in systems, laws, written or unwritten policies, and entrenched practices and beliefs that produce, condone, and perpetuate widespread unfair treatment of minoritized populations, with adverse health consequences (16).
There is an extensive body of literature describing the impact of systemic racism on cancer incidence, progression, and death (14,17–25). For example, despite lower incidence than other groups, stage-for-stage, self-identified African American or Black women have persistently higher breast cancer mortality than all other racial and ethnic groups (1-3). Additionally, policies like redlining and segregation have been linked to lower breast cancer survival (26,27). Targeted smoking policies can increase rates of lung cancer in minoritized groups (24), and lack of access to screening can increase rates of avoidable colorectal cancer mortality (25).
Policies and practices like discrimination in housing, education, politics, and employment lead to increased exposure to carcinogens that increase risk of certain cancers while decreasing access to and funding for cancer prevention, screening, and treatment. Each of the multiple levels of systemic racism can also increase the incidence of comorbidities like cardiovascular disease, hypertension, and diabetes, each of which can increase risk of treatment toxicity, decrease the efficacy of cancer treatment, and increase overall mortality. Despite this evidence, there has been limited focus thus far on incorporating the direct effects of systemic racism on cancer outcomes in simulation modeling research.
The National Cancer Institute–funded Cancer Intervention and Surveillance Modeling Network (CISNET) is a consortium of researchers who collaborate to use independent models to describe trends in cancer rates, including inequities over time. The modeling teams depict underlying relationships in the cancer control process and estimate how intervention at any point in the process, such as screen detection at an early stage, affects probability of cancer death (28,29).
Historically, CISNET models have approached equity analyses by stratifying overall population input parameters like screening rates by racial group (ie, Black vs White), where self-reported race was considered as a sociopolitical and not a biological factor (30,31). However, this approach does not fully explain the observed cancer mortality disparities in the United States. For example, a previous breast cancer modeling study showed that only 44%-62% of mortality disparities could be explained by race-specific input parameters, including screening use and treatment efficacy (30,32). In analyses of screening guidelines, most past CISNET analyses have focused on the overall population ignoring potential differences in the cancer control process that impact effectiveness of screening (or treatment) by race (33,34). Additionally, one CISNET study illustrated that screening strategies that started at earlier ages for Black vs White women would be needed to achieve equity in breast cancer mortality, given higher age-specific incidence rates at young ages and decreased treatment effectiveness due to delays, dose reductions, and incomplete cycles (31,35–37). These data could support implementation of race-based guidelines as one approach to increase equity. However, studies that investigate race-based guidelines are at risk for creating or perpetuating inequity if they fail to incorporate frameworks that conceptualize race as a proxy for systemic racism (38-40). Race does not have meaningful biological underpinnings because the origins of segmenting populations into groups based on geographic ancestry and skin color rest on racism (eg, policies resulting in racial segregation) (41). Thus, future progress in using models to guide efforts to improve equity will require replacing race-specific inputs with factors that better capture the multilevel nature of systemic racism, with a specific focus on actionable, policy-relevant variables.
In this paper, we provide a health equity framework that can serve as a road map to guide future modeling to support equitable cancer care. This road map is intended to guide the development of future models that consider systemic racism and its downstream impact on cancer control processes, underscore different assumptions about relationships when data are uncertain, and identify critical new data needed to better depict structural racism. The ultimate goal of this framework is to facilitate the identification of strategic intervention leverage points in the cancer control continuum to redress effects of systemic racism and use the results to inform discussions about policy, community-, and individual-level interventions to accelerate achievement of equity in cancer care (42,43).
Systemic racism framework
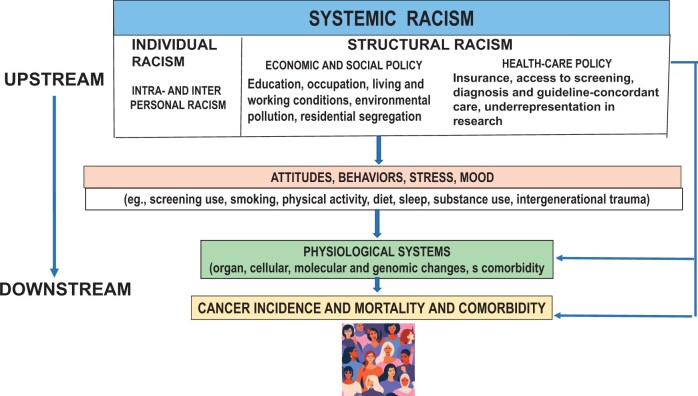
Our framework encompasses Bailey’s definition with a focus on examples specifically related to cancer outcomes. For instance, structural racism can be reflected in health-care organization and policies that affect access to care, including screening, prompt diagnosis and treatment initiation, and receipt of guideline-concordant therapy and survivorship care (17). Individualized racism refers to the interpersonal and internalized manifestations of racial discrimination (14). Structural racism includes the compounding impacts of the cultural norms, policies, laws, and practices that produce and maintain racial inequity (14,44). As defined by Bailey and colleagues (44), structural racism refers to the totality of ways in which societies foster racial discrimination through mutually reinforcing systems of housing, education, employment, earnings, benefits, credit, media, health care, and criminal justice. A focus on structural racism offers a concrete, feasible, and promising approach toward advancing health equity and improving population health (44). These definitions are similar to those in past models (18,19,23,45–47), although there has been wide variation in specific definitions and measures of these constructs in studies of cancer mortality (48). In our framework, systemic racism is considered an upstream influence acting on subsequent downstream events, including behavioral responses and their physiological effects on cancer incidence and mortality and competing comorbidities (Figure 1). As described by Lett and colleagues (14), the framework includes 2 domains of racism (structural and individualized).
Figure 1.
Conceptual framework of systemic racism.
Upstream systemic racism
Structural racism and cancer
We emphasize 2 components of structural racism with the greatest salience for simulating cancer control processes: inequitable economic and social policy and practice and health-care policy. Equitable economic and social and health-care policies therefore represent tractable levers to improve cancer equity.
Social and economic policy and practice. Many social and economic policies and practices, such as housing policy like redlining and lending bias, adversely affect impact health (49) and cancer outcomes (26,50). For instance, redlining, which maintains segregation, can facilitate disparate heat, carcinogen, and toxin exposure and is associated with cancer mortality (26,50,51). Redlining also affects the quality and number of resources available for education (48). Education, in turn, affects subsequent occupation and economic position, including adequate housing without the stresses of crowding, availability of employer-sponsored health insurance, paid health-care leave, and access to community resources that promote health (eg, access to green space, gyms, grocery stores). Education also influences health literacy, awareness of the need for healthy behaviors (and risks for disease), and knowledge and use of services like cancer screening and effects of health-care spending (52,53).
Disparate carcinogen exposure also can occur without residential segregation through policies that permit use of culturally based racist practices, like using hip-hop imagery in tobacco ads, and geographically based practices, like disproportionate placement of cigarette billboards in predominantly Black communities (24). Given the salience of policies like redlining and practices like race- and culturally based tobacco advertising, tractable solutions include laws that change lending practices, improve the safety of work environments, reduce air pollution and heat, and prohibit racist carcinogen advertisements (eg, tobacco, alcohol) (54-57). A detailed list of additional policies are detailed in a review by Egede and colleagues (58) on the consequences of redlining. To begin to capture these forces in model analyses, area data on redlining and area- and individual-level data on education are fairly readily available, which could be incorporated into equity-centered cancer simulation models (59). However, racism and education interact, such that similar levels of education do not always result in similar social and economic returns for minoritized populations (60). Given this, race-education interaction terms may be useful to include in simulation models as proxies for racism until improved measures of education-related discrimination are reported.
Finally, structural resilience or social cohesion and resources in communities can offset some effects of systemic racism and serve as other targets to improve equity (61). One example of this approach is the training of community health workers as a community resource to overcome systemic racism–related barriers to care and increase cancer awareness and screening (62-64).
Health-care policy. Although health-care policy is a subset of economic and social policies, we portray it separately because it is especially salient in modeling cancer equity. Insurance for health-care services, goods, and pharmaceuticals has been consistently linked to downstream health behaviors and cancer care (65). As noted previously, health-care organization and policies affect screening, prompt diagnosis and treatment initiation, and receipt of guideline-concordant therapy and survivorship care. Additionally, inequitable health policy leads to racial disparities in the prevalence and adequate control of comorbidities like cardiovascular disease, diabetes, and obesity, which increase cancer risk, decrease therapeutic efficacy, and lead to higher treatment toxicity. For example, obesity increases the risk of multiple cancers like breast and colorectal cancer and is associated with cancer recurrence, development of second primary cancers, and cancer mortality (66-69). Having obesity can also impair access to surgery because of elevated operative risks or preclude optimal imaging or radiotherapy because of weight limits. Similarly, renal disease or cardiac disease, which are far more common in Black patients, can preclude receipt of specific chemotherapeutic regimens. Health-care policy therefore represents a lever for increasing equity. For example, changes in federal policies on drug pricing, Medicare and Medicaid coverage of clinical trial participation, or coverage of new screening technology or therapeutic approaches are all variables that could be built into cancer simulation models to test and identify how policies that set prices or guarantee coverage could increase equity or prevent the emergence of inequities (70). This is critical for cancers with relatively new or evolving future care options, where inequities arising from uneven access haven’t yet emerged. At the state level, participation in Medicaid expansion such as occurred under the Affordable Care Act or limiting Medicaid payment for cancer surgery to high-volume hospitals has resulted in statistically significant decreases in cancer mortality (71-74). However, regionalization of care can also have unintended consequences, as minoritized populations might be less likely to access high-volume hospitals because of transportation issues or insurance barriers (eg, hospitals refusing to accept Medicaid). Hospital-level segregation often results in inequitable facility-level practices (ie, separate is rarely equal), leading to delays in follow-up of abnormal screening results and initiation of treatment (75). Additionally, access variables will not alone capture variability in realized access within the cancer care system related to the effects of racism in the quality of treatment offered or ongoing distrust resulting from systemic racism (76-78). At a local hospital system level, practice guidelines, audits, and electronic medical record triggers that prompt providers to prescribe tobacco cessation aids or offer human papillomavirus vaccines or specific cancer screening tests also provide additional levers to target structural and interpersonal racism.
Finally, because the exclusion of certain racial and ethnic groups from cancer research is another form of structural racism, equity will depend on meaningful increases in research representation of minoritized populations and initiation of trials that specifically seek to address racial equity. Lack of sufficient research representation precludes conclusions about efficacy and effectiveness of new cancer care approaches in all populations. In turn, lack of evidence is often cited as a barrier to action to address equity. Remedying this will likely require designing trials that are broadly accessible in terms of numbers of visits, time off work, social support required, and insurance coverage (79). Location of trials within community settings or minority-serving institutions where priority populations obtain care is critical. Additionally, enrollment can be facilitated by omitting unnecessary exclusions for concurrent diseases, addressing interpersonal racism at the point of care for trial enrollment, and training a greater number of minority investigators (who can help facilitate equitable enrollment) (80,81).
Individual-level racism
Racism is deeply embedded in modern society, so we conceptualize individualized racism as the dominant form of racism from which ongoing interpersonal and internalized racism originate (18). Intrapersonal (ie, internalized acceptance of negative messages about their own abilities and worth) and interpersonal racism (ie, prejudice about the abilities, motives, and intentions of others based on their race) can also affect downstream behaviors and attitudes of health-care professions and individuals at risk for cancer, ultimately impairing physiological homeostasis (18).
Interpersonal racism. Racism manifests at interpersonal levels to affect downstream events affecting health, including cancer mortality (14,82,83). Interpersonal racism at the point of care results in decreased receipt of preventive care, such as human papillomavirus vaccinations, care for depression, smoking cessation, and screening referrals, all of which are levers to improve equity and could be captured in modeling (29,84). Interpersonal racism also plays a major role in medical treatment receipt and clinical trial enrollment (85,86). Interpersonal racism (eg, bias in provider communication practices, discrimination practices) on the part of health-care providers affects the care offered and contributes to medical distrust (87). Data from group-based ratings of discrimination and patient-provider communication (eg, Consumer Assessment of Healthcare Surveys) might also be useful to explore to capture interpersonal racism from health-care professionals. Finally, interpersonal racism outside of health care can be relevant for cancer outcomes, as discrimination can be impactful coming from anyone who serves as a gatekeeper for optimal cancer prevention, screening, or treatment receipt including employers declining time off request for cancer screening, salesclerks targeting minoritized patients for tobacco sales, or landlords failing to investigate requests for toxic concerns).
Intrapersonal and internalized racism. Structural and interpersonal racism can ultimately result in internalized racism, impacting downstream attitudes and behaviors related to cancer control. Internalized racism can contribute to fatalism, decreasing motivation to stop smoking, maintaining a healthy diet, or adhering to guidelines for physical activity or treatment regimens (76,88,89).
Although individual-level racism is not particularly tractable in simulation models because of the lack of capture of these variables in large data sets, it may be useful to include variables from national survey data with broad representation, like smoking cessation or screening rates by educational level, in simulation models to capture these downstream effects of racism on attitudes and health behaviors.
Downstream effects of systemic racism
Physiological systems
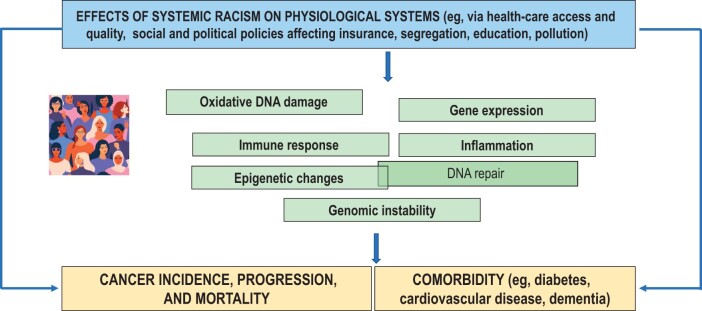
Continuing downstream, systemic racism can affect physiological homeostasis and disease development directly or indirectly via effects on behaviors and attitudes (17,44). Exposures to environmental toxins in a living or work situation can be a direct result of social and economic policies. Disruptions in sleep, poor physical activity, or smoking behaviors as a result of residential segregation, violence, or noise pollution can lead to disruptions of and damage to physiological homeostasis. These physiological changes can affect cellular-level integrity via factors like inflammation and DNA damage, which in turn can lead to comorbid diseases and cancer development or progression (Figure 2) (46,90–92). High community rates of HIV in segregated neighborhoods are leading to earlier age of onset of non-AIDS–defining cancers like lung cancer than seen in people without HIV infection as a consequence of disordered inflammation and immune responses; at the same time, screening guidelines fail to consider how to achieve equity for people living with HIV, a group overrepresented by minoritized persons (93-96).
Figure 2.
Impact of systemic racism on physiological systems.
The chronic stress of systemic racism can also have direct effects on physiological processes and homeostasis. For example, physiological adaptations of biological systems to chronic stress, known as allostatic load, have been found to consistently affect systemic inflammation, epigenetic changes, gene expression, and genetic instability (97-108). Epigenetic changes can also perpetuate intergenerational trauma and chronic stress and its physiological effects (109). Together these changes give rise to cancer development, progression, and mortality and death from comorbid disease (41,46,103,105,107,110–113). Downstream interventions to protect physiological systems like stress management techniques or coaching for sleep hygiene may be useful for specific individuals (114-117) but do not address systemic inequities at the root of most differences we see in physiological systems and tumor biology that manifest at the population level. Overall, investment in the development of systems biology models that span physiological systems through population levels will be challenging but useful to expand the ability to capture the downstream effects of systemic racism on cancer incidence and mortality (48,118).
One major challenge is ascertaining whether racial trends in cancer outcomes are partially influenced by genetic-based ancestry patterns vs being driven by common, present-day effects of racism. For example, numerous studies have demonstrated that the incidence of triple-negative breast cancer is increased in Black American and West African women. Because most Black American women are of predominantly West African ancestry due to the transatlantic slave trade, some researchers have hypothesized that the increased incidence in both groups is related to a genetic predisposition (118). However, there are potential limits to this idea and difficulty with accurately measuring the effects of racism. First, screening rates are low in most places in West Africa in part because of the legacy of colonialism. In this instance, detection biases may occur such that ductal carcinoma in situ and slower-growing invasive cancers can go undetected, leading to a seeming increase in the incidence of the more rapid-growing triple-negative cancers. Within the United States, there is emerging evidence that social stress in the form of poverty and other stressors is associated with increased triple-negative incidence, even among White women. Given this, some of the increased triple-negative incidence in Black American and West African women could be due to ongoing racism and related stressors and not necessarily genetics. Further complicating the issue is the effects of racism manifest differently in the United States and West Africa, as each context may have certain protective factors. Determining whether genetic-based ancestry play any role in racial trends in cancer incidence and survival therefore requires a more extensive understanding of genetics and carcinogenesis specifically, as well as more detailed measurement and investigation of the role of racism and other societal stressors on cancer outcomes (48).
Cancer incidence and mortality
The most downstream impact of systemic racism on disruption of physiological homoeostasis is increased risk of certain cancers (eg, cervical cancer, lung cancer), more advanced stage at diagnosis, and ultimately, inequities in life years that could be saved by effective cancer control (1-11). In the next section we summarize how simulation models have captured the pervasive influence of systemic racism and suggest approaches for future model research specifically designed to evaluate the effects of interventions targeting levers to change components of systemic racism and improve equity.
Application of equity framework for future modeling
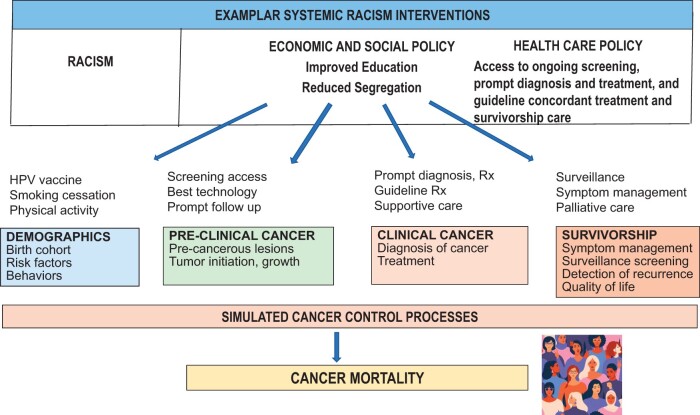
The papers in this issue used racial group stratification of input parameters from multiple national data sources to evaluate the relative contributions of prevention, preclinical and clinical detection, treatment, and survivorship to mortality differences between the overall population and self-identified Black persons. Using this approach, the models stated their intention to indirectly capture some of the upstream effects of systemic racism on structural factors that are known to impact use of screening and prompt therapy (eg, insurance status, having employer-based insurance) and were explicit that they were using race as a sociopolitical and not a biological construct. In future models designed to capture relationships between systemic racism and cancer outcomes, race could be extended or replaced by additional variables with more direct links to specific manifestations of individualized and structural racism and actionable levers, such as insurance policy, education quality, and economic opportunities and income (Figure 3). If/when race is included in models, principles such as those outlined by the US Centers for Disease Control and Prevention and other working groups (eg, Curricular Praxis Workgroup of Radical Public Health) should be employed to capture links between data levels and information about interactions across variables and levels (119).
Figure 3.
Framework for incorporating systemic racism into simulation models. Rx = prescription.
One example of this approach is the use of educational level as a proxy for economic opportunity in recent lung cancer simulation modeling. In that analysis, Cao and colleagues (120) modeled smoking behavior as a risk for lung cancer based on national data on education level, where smoking rates were modeled as decreasing more over time among birth cohorts with higher vs lower educational levels. However, this relationship varied by ethnic group, with Hispanic groups with low education levels having the lowest smoking rates, underscoring the complexity of this work and the need to consider education, race and racism interactions explicitly in future work.
Insurance is another potential lever to increase equity because it has effects on health behaviors, screening use, and access to therapy and survivorship care (121). For example, National Health Interview Survey data from different time periods could be used to construct probability of obtaining a first screening exam or later regular screening based on age, education, and insurance coverage. This approach is planned for simulation modeling of multiple myeloma and endometrial cancer, 2 of the new CISNET incubator sites (122), and the lung cancer modeling team (59).
Policies that provide universal insurance coverage or include mandates for evidence-based coverage of prevention and early detection could increase screening for multiple cancer sites and access to prescriptions for nicotine replacement and counterbalance racism effects on lower access, provider bias in prescribing or self-efficacy for quitting.
Income level has also been used as an indirect measure of the downstream effects of systemic racism on structures related to economic opportunity. This approach has recently been used in lung cancer modeling that considers the impact of income on smoking patterns (123). Although smoking prevalence has been decreasing over time, the relative differences between low- and high-income groups have increased substantially, with persons from high-income groups having statistically significant lower smoking rates with lower lung cancer mortality rates than those from low-income groups (123).
Other approaches to capturing systemic racism would be to use the data linking the downstream effects of racism on chronic stress on epigenetic age or effects on DNA damage and damage on cancer incidence or mortality. This is theoretically possible, however, there are limited national datasets with individual data and biospecimens that could be used to construct model input parameters to capture these indirect effects of racism on cancer outcomes. One example of data with the potential for use in constructing model inputs is from the long-term follow-up of women enrolled in the Women’s Health Study, which includes detailed data and specimen collection many years before and after cancer onset (124).
Each of the above approaches to modeling systemic racism relies on individual-level data. However, most work in this area to date has relied on associations of area-level policy to group outcomes. This approach can be useful for exploring relationships between community factors and cancer outcomes and identifying characteristics of areas in need of intervention (125). However, area-level data limit inference about causality and can limit the ability to develop model input parameters conditional on individual attributes needed for the microsimulations.
Although there are some useful national datasets with individual data, purposeful investment in collecting longitudinal individual- and systems-level data will be necessary to better define the temporal relationships between systemic racism and time course of initiation of cancer progression, especially because there is wide variability in these processes across cancer types.
Finally, there are other policy levers to reduce systemic racism that may not have existing data linking the intervention to some aspect of proximate or distal components of the cancer control process captured in population simulation models. In these cases, modeling could be useful to determine thresholds of association with cancer risk or access needed to change mortality via approaches like payments for value-based systems, increases in minimum wage, investment in green space for physical activity, or provision of incentives for grocery store placement in food deserts (58).
The proposed model integrating systemic racism in population simulation models is intended to change the culture of modeling cancer disparities and provide a feasible roadmap to addressing the multilevel nature of systemic racism. This integrated approach can support novel research approaches, make explicit the effects of different structures and policies, highlight data gaps in relationships between model components, inform how we collect data to model cancer outcomes, and generate results that could inform policy decisions (23).
Acknowledgments
This work was supported by National Cancer Institute grant CA253911 (JM, CC, VLS). This research was also supported in part by National Cancer Institute grant R35CA197289 and R35CA283926 to JM, National Cancer Institute grant P30CA051008 to CD, National Cancer Institute grant P30CA051008 for support of a pilot award to JJ, National Cancer Institute grant K99 CA241397 to JJ, and the Institute on Aging at the National Institutes of Health grant R21AG075008 to JM.
Role of the funder: The views, statements, and opinions in this publication are solely the responsibility of the authors and do not necessarily represent the views of the National Institutes of Health or the Department of Veterans Affairs. The study sponsors did not have any role in the design of the study, the collection, analysis, and interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Contributor Information
Christina Chapman, Department of Radiation Oncology, Baylor College of Medicine, and the Center for Innovations in Quality, Effectiveness, and Safety in the Department of Medicine, Baylor College of Medicine and the Houston VA, Houston, TX, USA.
Jinani Jayasekera, Health Equity and Decision Sciences Research Laboratory, National Institute on Minority Health and Health Disparities, Intramural Research Program, National Institutes of Health, Bethesda, MD, USA.
Chiranjeev Dash, Office of Minority Health and Health Disparities Research and Cancer Prevention and Control Program, Georgetown Lombardi Comprehensive Cancer Center, Washington, DC, USA.
Vanessa Sheppard, Department of Health Behavior and Policy and Massey Cancer Center, Virginia Commonwealth University, Richmond, VA, USA.
Jeanne Mandelblatt, Departments of Oncology and Medicine, Georgetown University Medical Center, Cancer Prevention and Control Program at Georgetown Lombardi Comprehensive Cancer Center and the Georgetown Lombardi Institute for Cancer and Aging Research, Washington, DC, USA.
Data Availability
No new data were generated or analyzed in support of this research.
Author contributions
Christina Chapman, MD (Conceptualization; Data curation; Methodology; Writing—original draft; Writing—review & editing), Jinani Jayasekera, PhD (Conceptualization; Methodology; Writing—original draft; Writing—review & editing), Chirandeev Dash, MBBS, PhD (Conceptualization; Writing—original draft; Writing—review & editing), Vanessa Sheppard, PhD (Conceptualization; Methodology; Writing—original draft; Writing—review & editing), and Jeanne S. Mandelblatt, MD (Conceptualization; Formal analysis; Funding acquisition; Methodology; Project administration; Visualization; Writing—original draft; Writing—review & editing)
Funding
No funding was used for this study.
Monograph sponsorship
This article appears as part of the monograph “Reducing Disparities to Achieve Cancer Health Equity: Using Simulation Modeling to Inform Policy and Practice Change,” sponsored by the National Cancer Institute, National Institutes of Health ([Comparative Modeling of Precision Breast Cancer Control Across the Translational Continuum; 3 U01 CA253911-03S2]).
References
- 1. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL.. Cancer statistics for African Americans, 2019. CA A Cancer J Clin. 2019;69(3):211-233. doi: 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 3. Lawrence WR, McGee-Avila JK, Vo JB, et al. Trends in cancer mortality among Black individuals in the US from 1999 to 2019. JAMA Oncol. 2022;8(8):1184-1189. doi: 10.1001/jamaoncol.2022.1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Association for Cancer Rearch. AACR cancer disparities progress report. https://cancerprogressreport.aacr.org/disparities/. Accessed September 1, 2022.
- 5. Howlader N NA, Krapcho M, Miller D, et al. (eds). SEER Cancer Statistics Review, 1975-2017. Bethesda, MD: National Institute of Health; 2019. [Google Scholar]
- 6. Hoskins KF, Calip GS, Huang HC, Ibraheem A, Danciu OC, Rauscher GH.. Association of social determinants and tumor biology with racial disparity in survival from early-stage, hormone-dependent breast cancer. JAMA Oncol. 2023;9(4):536. doi: 10.1001/jamaoncol.2022.7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL.. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15(4):248-254. doi: 10.1038/nrc3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams DR, Mohammed SA, Shields AE.. Understanding and effectively addressing breast cancer in African American women: unpacking the social context. Cancer. 2016;122(14):2138-2149. doi: 10.1002/cncr.29935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadigh G, Gray RJ, Sparano JA, et al. Assessment of racial disparity in survival outcomes for early hormone receptor-positive breast cancer after adjusting for insurance status and neighborhood deprivation: a post hoc analysis of a randomized clinical trial. JAMA Oncol. 2022;8(4):579-586. doi: 10.1001/jamaoncol.2021.7656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vince RA Jr, Jiang R, Bank M, et al. Evaluation of social determinants of health and prostate cancer outcomes among black and white patients: a systematic review and meta-analysis. JAMA Netw Open. 2023;6(1):e2250416. doi: 10.1001/jamanetworkopen.2022.50416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doubeni CA, Selby K, Levin TR.. Disparities in preventable mortality from colorectal cancer: are they the result of structural racism? Gastroenterology. 2021;160(4):1022-1025. doi: 10.1053/j.gastro.2020.12.071 [DOI] [PubMed] [Google Scholar]
- 12. Newman LA. Race and ethnicity as a sociopolitical construct that is biologically relevant in breast cancer. JAMA Surg. 2023;158(6):592. doi: 10.1001/jamasurg.2023.0313 [DOI] [PubMed] [Google Scholar]
- 13. Rothstein R. The Color of Law: A Forgotten History of How Our Government Segregated America. New York, NY: Liveright Publishing; 2017. [Google Scholar]
- 14. Lett E, Asabor E, Beltrán S, Cannon AM, Arah OA.. Conceptualizing, contextualizing, and operationalizing race in quantitative health sciences research. Ann Fam Med. 2022;20(2):157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones CP. Levels of racism: a theoretic framework and a gardener’s tale. Am J Public Health. 2000;90(8):1212-1215. doi: 10.2105/ajph.90.8.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Braveman PA, Arkin E, Proctor D, Kauh T, Holm N.. Systemic and structural racism: definitions, examples, health damages, and approaches to dismantling: study examines definitions, examples, health damages, and dismantling systemic and structural racism. Health Aff (Millwood). 2022;41(2):171-178. [DOI] [PubMed] [Google Scholar]
- 17. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT.. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453-1463. doi: 10.1016/s0140-6736(17)30569-x [DOI] [PubMed] [Google Scholar]
- 18. Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896(1):173-188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 19. Williams DR, Lawrence JA, Davis BA.. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40:105-125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams DR, Neighbors HW, Jackson JS.. Racial/ethnic discrimination and health: findings from community studies. Am J Public Health. 2008;98(suppl 9):S29-S37. doi: 10.2105/ajph.98.supplement_1.s29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams DR, Yu Y, Jackson JS, Anderson NB.. Racial differences in physical and mental health: socioeconomic status, stress, and discrimination. J Health Psychol. 1997;2(3):335-351. [DOI] [PubMed] [Google Scholar]
- 22. Williams RM, Li T, Luta G, et al. Lung cancer screening use and implications of varying eligibility criteria by race and ethnicity: 2019 Behavioral Risk Factor Surveillance System data. Cancer. 2022;128(9):1812-1819. doi: 10.1002/cncr.34098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diez Roux AV. Conceptual approaches to the study of health disparities. Annu Rev Public Health. 2012;33:41-58. doi: 10.1146/annurev-publhealth-031811-124534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. James L. Landmark settlement of “kool mixx” tobacco lawsuits. https://ag.ny.gov/press-release/2004/landmark-settlement-kool-mixx-tobacco-lawsuits. Published 2004. Accessed June 1, 2023.
- 25. Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, Van Ballegooijen M, Zauber AG, Jemal A.. contribution of screening and survival differences to racial disparities in colorectal cancer rates contribution of screening and survival to CRC disparities. Cancer Epidemiol Biomarkers Prev. 2012;21(5):728-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beyer KMM, Zhou Y, Laud PW, et al. Mortgage lending bias and breast cancer survival among older women in the United States. J Clin Oncol. 2021;39(25):2749-2757. doi: 10.1200/j.clin.oncol.21.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seewaldt VL, Winn RA.. Residential racial and economic segregation and cancer mortality in the US—speaking out on inequality and injustice. JAMA Oncol. 2023;9(1):126-127. doi: 10.1001/jamaoncol.2022.5272 [DOI] [PubMed] [Google Scholar]
- 28. Malinowski C, Lei X, Zhao H, Giordano SH, Chavez-MacGregor M.. Association of Medicaid expansion with mortality disparity by race and ethnicity among patients with de novo stage IV breast cancer. JAMA Oncol. 2022;8(6):863-870. doi: 10.1001/jamaoncol.2022.0159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doubeni CA, Corley DA, Zhao W, Lau Y, Jensen CD, Levin TR.. Association between improved colorectal screening and racial disparities. N Engl J Med. 2022;386(8):796-798. doi: 10.1056/NEJMc2112409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Ravesteyn NT, Schechter CB, Near AM, et al. Race-specific impact of natural history, mammography screening, and adjuvant treatment on breast cancer mortality rates in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(1):112-122. NOT IN FILE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174(12):1637-1646. doi: 10.7326/m20-6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang Y, Schechter CB, van Ravesteyn NT, et al. Collaborative modeling of the impact of obesity on race-specific breast cancer incidence and mortality. Breast Cancer Res Treat. 2012;136(3):823-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mandelblatt JS, Cronin KA, Bailey S, et al. ; for the Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different U.S. Breast Cancer Screening Strategies. Ann Intern Med. 2016;164(4):215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25(18):2522-2527. doi: 10.1200/jco.2006.10.2749 [DOI] [PubMed] [Google Scholar]
- 36. Wu AH, Kurian AW, Kwan ML, et al. Diabetes and other comorbidities in breast cancer survival by race/ethnicity: the California Breast Cancer Survivorship Consortium (CBCSC). Cancer Epidemiol Biomarkers Prev. 2015;24(2):361-368. doi: 10.1158/1055-9965.Epi-14-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurian AW, Lichtensztajn DY, Keegan TH, et al. Patterns and predictors of breast cancer chemotherapy use in Kaiser Permanente Northern California, 2004-2007. Breast Cancer Res Treat. 2013;137(1):247-260. doi: 10.1007/s10549-012-2329-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wright JL, Davis WS, Joseph MM, et al. ; for the AAP Board Committee on Equity. Eliminating race-based medicine. Pediatrics. 2022;150(1):e2022057998. doi: 10.1542/peds.2022-057998 [DOI] [PubMed] [Google Scholar]
- 39. Cerdeña JP, Plaisime MV, Tsai J.. From race-based to race-conscious medicine: how anti-racist uprisings call us to act. Lancet. 2020;396(10257):1125-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yearby R. Race based medicine, colorblind disease: how racism in medicine harms us all. Am J Bioeth. 2021;21(2):19-27. [DOI] [PubMed] [Google Scholar]
- 41. Carlos RC, Obeng-Gyasi S, Cole SW, et al. Linking structural racism and discrimination and breast cancer outcomes: a social genomics approach. J Clin Oncol. 2022;40(13):1407-1413. doi: 10.1200/jco.21.02004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carethers JM, Doubeni CA.. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158(2):354-367. doi: 10.1053/j.gastro.2019.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Doubeni CA, Corley DA, Zauber AG.. Colorectal cancer health disparities and the role of US law and health policy. Gastroenterology. 2016;150(5):1052-1055. doi: 10.1053/j.gastro.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bailey ZD, Feldman JM, Bassett MT.. How structural racism works — racist policies as a root cause of U.S. racial health inequities. N Engl J Med. 2021;384(8):768-773. doi: 10.1056/NEJMms2025396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alvidrez J, Castille D, Laude-Sharp M, Rosario A, Tabor D.. The National Institute on Minority Health and Health Disparities research framework. Am J Public Health. 2019;109(suppl 1):S16-S20. doi: 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noren Hooten N, Pacheco NL, Smith JT, Evans MK.. The accelerated aging phenotype: the role of race and social determinants of health on aging. Ageing Res Rev. 2022;73:101536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Glass TA, McAtee MJ.. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. 2006;62(7):1650-1671. doi: 10.1016/j.socscimed.2005.08.044 [DOI] [PubMed] [Google Scholar]
- 48. Jayasekera J, El kefi S, Fernandez JR, et al. Opportunities, challenges, and future directions for simulation modeling the effects of structural racism on cancer mortality in the U.S.: a scoping review. J Natl Cancer Inst Monogr. 2023;2023(62):231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Swope CB, Hernández D, Cushing LJ.. The relationship of historical redlining with present-day neighborhood environmental and health outcomes: a scoping review and conceptual model. J Urban Health. 2022;99(6):959-983. doi: 10.1007/s11524-022-00665-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol Biomarkers Prev. 2021;30(1):53-60. doi: 10.1158/1055-9965.Epi-20-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hoffman JS, Shandas V, Pendleton N.. The effects of historical housing policies on resident exposure to intra-urban heat: a study of 108 US urban areas. Climate. 2020;8(1):12. [Google Scholar]
- 52. Woudstra AJ, Smets EMA, Verdam MGE, Fransen MP.. The role of health literacy in explaining the relation between educational level and decision making about colorectal cancer screening. Int J Environ Res Public Health. 2019;16(23):4644. doi: 10.3390/ijerph16234644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. LaVeist TA, Pérez-Stable EJ, Richard P, et al. The economic burden of racial, ethnic, and educational health inequities in the US. JAMA. 2023;329(19):1682-1692. doi: 10.1001/jama.2023.5965 [DOI] [PubMed] [Google Scholar]
- 54. Gomez SL, Shariff-Marco S, DeRouen M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: current research, methodological considerations, and future directions. Cancer. 2015;121(14):2314-2330. doi: 10.1002/cncr.29345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wray AJD, Minaker LM.. Is cancer prevention influenced by the built environment? A multidisciplinary scoping review. Cancer. 2019;125(19):3299-3311. doi: 10.1002/cncr.32376. [DOI] [PubMed] [Google Scholar]
- 56. Alvarez CH. Structural racism as an environmental justice issue: a multilevel analysis of the state racism index and environmental health risk from air toxics. J Racial Ethn Health Disparities. 2023;10(1):244-258. doi: 10.1007/s40615-021-01215-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roberts JD, Dickinson KL, Hendricks MD, Jennings V.. “I Can’t Breathe”: examining the legacy of American racism on determinants of health and the ongoing pursuit of environmental justice. Curr Environ Health Rep. 2022;9(2):211-227. doi: 10.1007/s40572-022-00343-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Egede LE, Walker RJ, Campbell JA, Linde S, Hawks LC, Burgess KM.. Modern day consequences of historic redlining: finding a path forward. J Gen Intern Med. 2023;38(6):1534-1537. doi: 10.1007/s11606-023-08051-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Levy DT, Tam J, Jeon J, Holford TR, Fleischer NL, Meza R.. Summary and concluding remarks: patterns of birth cohort‒specific smoking histories. Am J Prev Med. 2023;64(4 suppl 1):S72-S79. doi: 10.1016/j.amepre.2022.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bertrand MM. Are Emily and Greg more employable than Lakisha and Jamal? A field experiment on labor market discrimination. National Bureau of Economic Research working paper 9873. July 2003. http://www.nber.org/papers/w9873. Accessed May 1, 2023.
- 61. Szanton SL, LaFave SE, Thorpe RJ Jr. Structural racial discrimination and structural resilience: measurement precedes change. J Gerontol A Biol Sci Med Sci. 2022;77(2):402-404. doi: 10.1093/gerona/glab344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roland KB, Milliken EL, Rohan EA, et al. Use of community health workers and patient navigators to improve cancer outcomes among patients served by federally qualified health centers: a systematic literature review. Health Equity. 2017;1(1):61-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wells KJ, Luque JS, Miladinovic B, et al. Do community health worker interventions improve rates of screening mammography in the United States? A systematic review. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1580-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Umaretiya PJ, Vinci RJ, Bona K.. A structural racism framework to guide health equity interventions in pediatric oncology. Pediatrics. 2022;149(5):e2021054634. [DOI] [PubMed] [Google Scholar]
- 65. Yabroff KR, Reeder-Hayes K, Zhao J, et al. Health insurance coverage disruptions and cancer care and outcomes: systematic review of published research. J Natl Cancer Inst. 2020;112(7):671-687. doi: 10.1093/jnci/djaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K; for the International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer–viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794-798. doi: 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1244-1259. doi: 10.1158/1055-9965.Epi-12-0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sung H, Hyun N, Leach CR, Yabroff KR, Jemal A.. Association of first primary cancer with risk of subsequent primary cancer among survivors of adult-onset cancers in the United States. JAMA. 2020;324(24):2521-2535. doi: 10.1001/jama.2020.23130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Petrelli F, Cortellini A, Indini A, et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e213520. doi: 10.1001/jamanetworkopen.2021.3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lowry KP, Trentham-Dietz A, Schechter CB, et al. Long-term outcomes and cost-effectiveness of breast cancer screening with digital breast tomosynthesis in the United States. J Natl Cancer Inst. 2020;112(6):582-589. doi: 10.1093/jnci/djz184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barnes JM, Johnson KJ, Adjei Boakye E, et al. Early Medicaid expansion and cancer mortality. J Natl Cancer Inst. 2021;113(12):1714-1722. doi: 10.1093/jnci/djab135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lam MB, Phelan J, Orav EJ, Jha AK, Keating NL.. Medicaid expansion and mortality among patients with breast, lung, and colorectal cancer. JAMA Netw Open. 2020;3(11):e2024366. doi: 10.1001/jamanetworkopen.2020.24366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee G, Dee EC, Orav EJ, et al. Association of Medicaid expansion and insurance status, cancer stage, treatment and mortality among patients with cervical cancer. Cancer Rep (Hoboken). 2021;4(6):e1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nattinger AB, Bickell NA, Schymura MJ, et al. Centralization of initial care and improved survival of poor patients with breast cancer. J Clin Oncol. 2023;41(11):2067-2075. doi: 10.1200/j.clin.oncol.22.02012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lawson MB, Bissell MCS, Miglioretti DL, et al. Multilevel factors associated with time to biopsy after abnormal screening mammography results by race and ethnicity. JAMA Oncol. 2022;8(8):1115-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sheppard VB, Wang J, Yi B, et al. ; for the Latin American Cancer Research Coalition. Are health-care relationships important for mammography adherence in Latinas? Latin American Cancer Research Coalition. J Gen Intern Med. 2008;23(12):2024-2030. doi: 10.1007/s11606-008-0815-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sheppard VB, Williams KP, Wang J, Shavers V, Mandelblatt JS.. An examination of factors associated with healthcare discrimination in Latina immigrants: the role of healthcare relationships and language. J Natl Med Assoc. 2014;106(1):15-22. doi: 10.1016/s0027-9684(15)30066-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sutton AL, Hagiwara N, Perera RA, Sheppard VB.. Assessing perceived discrimination as reported by Black and White women diagnosed with breast cancer. J Racial Ethn Health Disparities. 2021;8(3):589-595. doi: 10.1007/s40615-020-00817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Niranjan SJ, Wenzel JA, Martin MY, et al. Perceived institutional barriers among clinical and research professionals: minority participation in oncology clinical trials. JCO Oncol Pract. 2021;17(5):e666-e675. doi: 10.1200/op.20.00970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. National Academies of Sciences Engineering and Medicine. Improving the Evidence Base for Treatment Decision Making for Older Adults with Cancer: Proceedings of a Workshop—in Brief. New York, NY: National Academies Press; 2021. [Google Scholar]
- 81. Bertagnolli MM, Singh H.. Treatment of older adults with cancer - addressing gaps in evidence. N Engl J Med. 2021;385(12):1062-1065. doi: 10.1056/NEJMp2106089 [DOI] [PubMed] [Google Scholar]
- 82. Paradies Y, Ben J, Denson N, et al. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015;10(9):e0138511. doi: 10.1371/journal.pone.0138511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Williams DR, Mohammed SA.. Racism and health I: pathways and scientific evidence. Am Behav Sci. 2013;57(8):1152-1173. doi: 10.1177/0002764213487340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hooks-Anderson DR, Salas J, Secrest S, Skiöld-Hanlin S, Scherrer JF.. Association between race and receipt of counselling or medication for smoking cessation in primary care. Fam Pract. 2018;35(2):160-165. doi: 10.1093/fampra/cmx099 [DOI] [PubMed] [Google Scholar]
- 85. Schulman KA, Berlin JA, Harless W, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med. 1999;340(8):618-626. doi: 10.1056/NEJM199902253400806 [DOI] [PubMed] [Google Scholar]
- 86. Shavers VL, Brown ML.. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334-357. doi: 10.1093/jnci/94.5.334 [DOI] [PubMed] [Google Scholar]
- 87. Bazargan M, Cobb S, Assari S.. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19(1):4-15. doi: 10.1370/afm.2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. O’Malley AS, Sheppard VB, Schwartz M, Mandelblatt J.. The role of trust in use of preventive services among low-income African-American women. Prev Med. 2004;38(6):777-785. [DOI] [PubMed] [Google Scholar]
- 89. Sheppard VB, Faul LA, Luta G, et al. Frailty and adherence to adjuvant hormonal therapy in older women with breast cancer: CALGB protocol 369901. J Clin Oncol. 2014;32(22):2318-2327. doi: 10.1200/jco.2013.51.7367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Carroll J, Bower JE, Ganz PA.. Cancer-related accelerated ageing and biobehavioural modifiers: a framework for research and clinical care. Nat Rev Clin Oncol. 2022;19(3):173-187. doi: 10.1038/s41571-021-00580-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Carroll JE, Cole SW, Seeman TE, et al. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav Immun. 2016;51:223-229. doi: 10.1016/j.bbi.2015.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Slopen N, Lewis TT, Williams DR.. Discrimination and sleep: a systematic review. Sleep Med. 2016;18:88-95. doi: 10.1016/j.sleep.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Frega S, Ferro A, Bonanno L, Guarneri V, Conte P, Pasello G.. Lung cancer (LC) in HIV positive patients: pathogenic features and implications for treatment. Int J Mol Sci. 2020;21(5):1601. doi: 10.3390/ijms21051601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hessol NA, Martínez-Maza O, Levine AM, et al. Lung cancer incidence and survival among HIV-infected and uninfected women and men. AIDS. 2015;29(10):1183-1193. doi: 10.1097/qad.0000000000000690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kirk GD, Merlo C, O’ Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45(1):103-110. doi: 10.1086/518606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sellers SA, Edmonds A, Ramirez C, et al. Optimal lung cancer screening criteria among persons living with HIV. J Acquir Immune Defic Syndr. 2022;90(2):184-192. doi: 10.1097/qai.0000000000002930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Clark R, Anderson NB, Clark VR, Williams DR.. Racism as a stressor for African Americans. A biopsychosocial model. Am Psychol. 1999;54(10):805-816. doi: 10.1037//0003-066x.54.10.805 [DOI] [PubMed] [Google Scholar]
- 98. Eckerling A, Ricon-Becker I, Sorski L, Sandbank E, Ben-Eliyahu S.. Stress and cancer: mechanisms, significance and future directions. Nat Rev Cancer. 2021;21(12):767-785. doi: 10.1038/s41568-021-00395-5 [DOI] [PubMed] [Google Scholar]
- 99. Forrester S, Jacobs D, Zmora R, Schreiner P, Roger V, Kiefe CI.. Racial differences in weathering and its associations with psychosocial stress: the CARDIA study. SSM - Population Health. 2019;7:100319. doi: 10.1016/j.ssmph.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD.. Do US Black women experience stress-related accelerated biological aging?: a novel theory and first population-based test of Black-White differences in telomere length. Hum Nat. 2010;21(1):19-38. doi: 10.1007/s12110-010-9078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lim S, Nzegwu D, Wright ML.. The impact of psychosocial stress from life trauma and racial discrimination on epigenetic aging-a systematic review. Biol Res Nurs. 2022;24(2):202-215. doi: 10.1177/10998004211060561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shonkoff JP, Garner AS, Siegel BS, et al. ; for the Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232-e246. doi: 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- 103. Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939-944. doi: 10.1038/nm1447 [DOI] [PubMed] [Google Scholar]
- 104. Thaker PH, Lutgendorf SK, Sood AK.. The neuroendocrine impact of chronic stress on cancer. Cell Cycle. 2007;6(4):430-433. doi: 10.4161/cc.6.4.3829 [DOI] [PubMed] [Google Scholar]
- 105. Van Dyke ME, Baumhofer NK, Slopen N, et al. Pervasive discrimination and allostatic load in African American and White adults. Psychosom Med. 2020;82(3):316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS.. Price of adaptation–allostatic load and its health consequences. Arch Intern Med. 1997;157(19):2259-2268. [PubMed] [Google Scholar]
- 107. Rentscher KE, Carroll JE, Repetti RL, Cole SW, Reynolds BM, Robles TF.. Chronic stress exposure and daily stress appraisals relate to biological aging marker p16(INK4a). Psychoneuroendocrinology. 2019;102:139-148. doi: 10.1016/j.psyneuen.2018.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Polsky LR, Rentscher KE, Carroll JE.. Stress-induced biological aging: a review and guide for research priorities. Brain Behav Immun. 2022;104:97-109. doi: 10.1016/j.bbi.2022.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Scorza P, Duarte CS, Hipwell AE, et al. ; for the Program Collaborators for Environmental influences on Child Health Outcomes. Research review: intergenerational transmission of disadvantage: epigenetics and parents’ childhoods as the first exposure. J Child Psychol Psychiatry. 2019;60(2):119-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Beydoun MA, Beydoun HA, Noren Hooten N, et al. Epigenetic clocks and their association with trajectories in perceived discrimination and depressive symptoms among US middle-aged and older adults. Aging (Albany NY). 2022;14(13):5311-5344. doi: 10.18632/aging.204150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042-7052. doi: 10.1158/0008-5472.Can-10-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Thames AD, Irwin MR, Breen EC, Cole SW.. Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology. 2019;106:277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Linnenbringer E, Gehlert S, Geronimus AT.. Black-White disparities in breast cancer subtype: the intersection of socially patterned stress and genetic expression. AIMS Public Health. 2017;4(5):526-556. doi: 10.3934/publichealth.2017.5.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Antoni MH, Pereira DB, Marion I, et al. Stress management effects on perceived stress and cervical neoplasia in low-income HIV-infected women. J Psychosom Res. 2008;65(4):389-401. doi: 10.1016/j.jpsychores.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chang A, Sloan EK, Antoni MH, Knight JM, Telles R, Lutgendorf SK.. Biobehavioral pathways and cancer progression: insights for improving well-being and cancer outcomes. Integr Cancer Ther. 2022;21:15347354221096081. doi: 10.1177/15347354221096081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Steel JL, Antoni M, Pathak R, et al. Adverse childhood experiences (ACEs), cell-mediated immunity, and survival in the context of cancer. Brain Behav Immun. 2020;88:566-572. doi: 10.1016/j.bbi.2020.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Taub CJ, Lippman ME, Hudson BI, et al. The effects of a randomized trial of brief forms of stress management on RAGE-associated S100A8/A9 in patients with breast cancer undergoing primary treatment. Cancer. 2019;125(10):1717-1725. doi: 10.1002/cncr.31965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Davis MB, Newman LA.. Oncologic anthropology: an interdisciplinary approach to understanding the association between genetically defined African ancestry and susceptibility for triple negative breast cancer. Curr Breast Cancer Rep. 2021;13(4):247-258. doi: 10.1007/s12609-021-00426-y [DOI] [Google Scholar]
- 119. Collaboratory for Health Justice. Best Practices for Using Race in Public Health Research.2021. https://publichealth.uic.edu/community-engagement/collaboratory-for-health-justice/best-practices-race-public-health-research/. Accessed May 2023.
- 120. Cao P, Jeon J, Tam J, et al. Smoking disparities by level of educational attainment and birth cohort in the U.S. Am J Prev Med. 2023;64(4 suppl 1):S22-S31. doi: 10.1016/j.amepre.2022.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sheni R, Qin J, Viswanathan S, Castellucci E, Kalnicki S, Mehta V.. Predictive factors for cancer treatment delay in a racially diverse and socioeconomically disadvantaged urban population. JCO Oncology Practice. 2023;19(6):e904-e915. doi: 10.1200/OP.22.00779. [DOI] [PubMed] [Google Scholar]
- 122. Trikalinos. Approaches to Developing de Novo Cancer Population Models to Examine Racial Disparities in Bladder, Gastric and Endometrial Cancer and Multiple Myeloma Mortality: The CISNET Incubator Program.2023. [DOI] [PMC free article] [PubMed]
- 123. Jeon J, Cao P, Fleischer NL, et al. Birth cohort‒specific smoking patterns by family income in the U.S. Am J Prev Med. 2023;64(4 suppl 1):s32-s41. doi: 10.1016/j.amepre.2022.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Paskett ED, Caan BJ, Johnson L, et al. The Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) Study: description and baseline characteristics of participants Women’s Health Initiative LILAC study. Cancer Epidemiol Biomarkers Prev. 2018;27(2):125-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chantarat T, Van Riper DC, Hardeman RR.. The intricacy of structural racism measurement: a pilot development of a latent-class multidimensional measure. EClinicalMedicine. 2021;40:101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.