Abstract
Background
Remimazolam, a new benzodiazepine, is known for its quick onset of effects and recovery time. Recently, it has been licensed for general anesthesia and sedation in Korea and its use is increasing in other countries. However, less is known about its effect on postoperative recovery. We used a patient-reported outcome questionnaire to examine the effect of remimazolam on postoperative recovery.
Methods
Patients who underwent hysteroscopy on day surgery basis were administered an induction dose of remimazolam 6 mg/kg/h followed by a maintenance dose of 1–2 mg/kg/h. After surgery, the translated Korean version of 15-item Quality of Recovery scale (QoR-15K) including post-discharge nausea and vomiting (PDNV) and/or pain, was surveyed 24 h after surgery to evaluate patient recovery.
Results
Total of 38 patients were enrolled in this prospective, observational study. All patients successfully completed QoR-15K. Only one patient scored low for moderate pain and PDNV. On average, patients scored 9 and above for all QoR-15K items except for moderate pain (8.66 ± 1.68). When QoR-15K items were grouped into dimensions, all dimensions scored an average of 9 or higher on a 10-point scale. In addition, 19 out of 38 patients gave score range of 148 to 150 out of possible 150.
Conclusion
Psychometric evaluation based on postoperative QoR-15K among patients receiving remimazolam shows satisfactory patient recovery profiles without significant pain or PDNV. Considering its effectiveness and safety, remimazolam could be one of useful agents for general anesthesia of day surgery in terms of postoperative recovery.
Keywords: Ambulatory surgical procedure; Anesthesia, general; Anesthesia recovery period; Patient outcome assessment; Remifentanil; Remimazolam
INTRODUCTION
Remimazolam is a novel ultra-short acting benzodiazepine with rapid onset of effects, short maintenance and faster recovery time [1]. Remimazolam’s pharmacological action is similar to midazolam, but there is a difference in its metabolic pathway. While midazolam is metabolized via cytochrome P450, remimazolam is metabolized by tissue esterases [2]. Compared to the metabolite of midazolam, CNS7054, which is the metabolite of remimazolam, shows 50 times less potent sedative effect and this difference is thought to contribute to remimazolam’s rapid onset and systemic clearance [3]. In fact, the mean terminal elimination half-life of remimazolam is 0.75 h compared to 4.3 h of midazolam [4]. One major disadvantage of midazolam is its greater cumulative effects due to long-acting metabolite that causes slow recovery of neuropsychiatric function compared to propofol [5,6]. However, the context-sensitive half time of remimazolam remains constant even after a long-term continuous infusion, and thus the likelihood of delayed recovery after general anesthesia is low [1]. In addition, compared to other intravenous anesthetics, especially propofol, remimazolam-induced sedation can be reversed by flumazenil and this availability of an antagonist is highly advantageous in clinical practice.
Due to its recent development, few studies have investigated the effect of remimazolam on postoperative recovery, primarily focusing on objective parameters such as physiologic endpoints, recovery time, and possible adverse events [2,7-10]. Although these parameters are crucial and require evaluation, they overlook the quality of recovery (QoR) from the patient’s perspective. To date, there has been no study investigating remimazolam’s impact on patients’ QoR.
Various measurement tools have been developed for the psychometric assessment of quality of recovery, including the 24-h functional ability questionnaire, postoperative quality of recovery score, and the Korean version of QoR-15 (QoR-15K) questionnaire [11-14]. Among these assessments, the QoR-15K questionnaire covers a wide range of components, including physical comfort, pain, psychological and emotional state, and cognition, as well as patient’s satisfaction [12]. In a previous randomized controlled study that investigated patient anesthesia satisfaction as a secondary endpoint, patients receiving remimazolam anesthesia reported high satisfaction levels, non-inferior to those of propofol [15]. While we hypothesize that patients will show high satisfaction scores in the recovery assessment, similar to the previous study [15], this is the first study to evaluate additional QoR parameters such as physical independence, pain, and psychological support, for which the associated scores are currently unknown.
Therefore, the aim of this study was to comprehensively evaluate various QoR of patients who received remimazolam general anesthesia, specifically remimazolam-remifentanil total intravenous anesthesia (TIVA) for hysteroscopy performed as day surgery. We used the translated Korean version of the 15-item QoR-15K questionnaire, which has been previously validated in the Korean surgical population, to assess QoR [12,14].
MATERIALS AND METHODS
Study design
This was a prospective, observational study, assessing the QoR and safety of remimazolam-remifentanil TIVA in patients undergoing day surgery. This study was approved by the institutional review board of the Seoul National University Bundang Hospital (Chairperson Hak Chul Jang, IRB no. B-2109-708-309), and registered at ClinicalTrials.gov (Trial no. NCT05320016). All participants provided written informed consent before study entry and the study was conducted in accordance with the Declaration of Helsinki. In addition, all methods were conducted following the Strengthening the Reporting of Observational Studies in Epidemiology guideline [16].
Study participants
Patients over 19 years of age with a physical status I or II of the American Society of Anesthesiology, who were scheduled for elective hysteroscopy as day surgery under the general anesthesia from November 2021 to December 2021, were included in this study. The exclusion criteria were, (1) history of liver dysfunction, renal insufficiency, cranial nervous system disorders, and glaucoma; (2) a body mass index over 35 kg/m2; (3) diagnosed with sleep apnea, severe or acute respiratory failure; (4) history of alcohol or drug dependence; (5) lactose intolerance; (6) dextran 40 hypersensitivity; (7) shock or coma; (8) allergy or contraindications to both benzodiazepines and opioids. Based on the study flow diagram (Fig. 1), 38 patients were included in the final analysis.
Fig. 1.
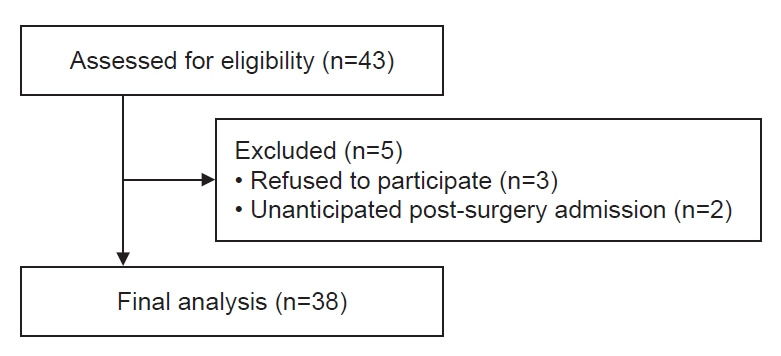
Study flow diagram.
Anesthesia
Premedication with intravenous midazolam (0.02 mg/kg) was administered before entering the operating room. Routine monitoring, including noninvasive blood pressure measurements, electrocardiography, and pulse oximetry were conducted. In addition, bispectral index (BIS complete 2-channel monitor, Covidien) was applied on the forehead to monitor the depth of anesthesia.
General anesthesia was induced with remimazolam (Byfavo Inj., Hana Pharm Co., Ltd., 6 mg/kg/h) and remifentanil (Ultiva Inj., GlaxoSmithKline Manufacturing S.p.A., 3.0 ng/ml of effect site concentration on the target-controlled infusion mode, the Minto model). If all of the following conditions were satisfied, LMA (SupremeTM, Teleflex) was inserted: (1) BIS value < 60; (2) Observer’s assessment of alertness/sedation (OAA/S) score = 0; (3) Remifentanil Ce = Cp = 3 ng/ml; (4) loss of spontaneous breathing.
For the appropriate depth of general anesthesia (BIS value between 40 and 60), continuous infusion of remimazolam was carried out by controlling the infusion rate within the range of 1–2 mg/kg/h. Remifentanil was controlled within the range of 2 ng/ml to 6 ng/ml according to the depth of anesthesia. At the end of the surgery, remimazolam and remifentanil were discontinued. If all of the following conditions were satisfied, LMA was removed, (1) BIS value > 80; (2) OAA/S score > 3; (3) Remifentanil Ce < 1 ng/ml; (4) Spontaneous breathing. If the patient’s recovery was delayed 15 min after discontinuation of remimazolam, 0.2 mg of flumazenil was administered.
Recovery
In the post-anesthesia care unit (PACU) and day surgery center after the operation, the degree of consciousness, the level of postoperative pain, and the incidence of nausea and/or vomiting are investigated. Modified OAA/S scores were assessed as soon as patients arrived at the PACU and every 10 min thereafter. If the modified OAA/S score was < 2 in the PACU, 0.1 mg of flumazenil was administered. The total amount of flumazenil administered in the PACU did not exceed 0.5 mg. Twenty-four h after surgery, patients were rated on a scale of 1 to 10 using the translated Korean version of the15-item QoR-15K questionnaire (Supplementary Fig. 1) [14]. QoR-15K dimensions and corresponding QoR-15K items can be found in Supplementary Table 1.
Sample size calculation
The following formula was used for sample size calculation because we aimed to compare the means of two related QoR questionnaire items and dimensions within the same group of patients using a paired t-test:
Where n is the required sample size, σ is the estimated standard deviation, Zα is the Z-score corresponding to chosen significance level (α), Z1-β is the Z-score fo chosen power level (1-β), and E is the effect size. The estimated standard deviation was 2 for QoR-15K items and dimension scores, as reported in previous studies [14,17]. The Z-score for a two-tailed test at a 5% significance level (α=0.05) is approximately 1.96, and the Z-score for a power of 0.90 is approximately 1.28. The sample size was calculated to detect a difference of 2 points in the QoR measurements on a 10-point scale. Substituting these values into the equation above yields a minimum sample size of 22 patients. Assuming a sample size with a dropout rate of 20% [14], the adjusted required sample size was 28 patients, which was below the number analyzed in this study.
Statistical analysis
The normal distribution of continuous variable was evaluated using the Shapiro-Wilk test. Normally distributed continuous variable was presented as mean (standard deviations) and if the distribution was not normal, median (1Q, 3Q) was presented. In case of the QoR-15 questionnaire, mean and standard deviation of each item was calculated. The student t-test and one-way ANOVA were used to compare mean scores of QoR-15 dimensions. Inter-item and –dimension correlations were measured using the Spearman correlation coefficient (ρ). Reliability was measured for the consistency of QoR-15K and it was assessed by internal consistency, split-half reliability and test-retest reliability. Internal consistency was measured using Cronbach α and test-retest reliability was measured using the intra-class correlation coefficient (ICC). All statistical analyses were performed via SPSS software, version 25.0 (IBM Co.). Values were considered statistically significant when P < 0.05.
RESULTS
Clinical and demographic characteristics of the total 38 patients are presented in Table 1. The mean age of patients was 48.4 ± 10.2 years with median anesthesia duration of 40.0 (range, 40.0, 56.3) min and median PACU length of stay of 29.5 (range, 22.8, 34.3) min. Two patients (5.3%) received flumazenil in the operating room because it required 15 min to meet our recovery criteria. One (2.6%) patient required flumazenil in the PACU because the modified OAA/S score decreased to 1. All patients successfully answered all items of QoR-15K questionnaires.
Table 1.
Demographic Characteristics and Clinical Data
| Variable | Value (n=38) |
|---|---|
| Age (yr) | 48.4 ± 10.2 |
| BMI (kg/m2) | 22.6 (20.7, 25.1) |
| ASA (I/II) | 20 (52.6)/18 (47.4) |
| Duration of anesthesia (min) | 40.0 (40.0-56.3) |
| Flumazenil in the operating room/in the PACU | 2 (5.3)/1 (2.6) |
| PACU length of stay (min) | 29.5 (22.8, 34.3) |
| Total remimazolam (mg) | 71.5 (60.0, 91.5) |
| Total remifentanil (μg) | 209.0 (170.8, 269.0) |
Values are presented as mean ± SD, median (1Q, 3Q), or number (%). BMI: body mass index, ASA: American Society of Anesthesiologists, PACU: post-anesthesia care unit.
Mean scores of QoR-15K questionnaire items are summarized in Table 2 and the number of score ranges per QoR-15 item are shown in Fig. 2. The mean score of QoR-15K items was highest for able to communicate with family or friends (10 ± 0) followed by able to look after personal hygiene unaided (9.97 ± 0.16) and severe pain (9.95 ± 0.23). In contrast, the mean score of QoR-15K items was lowest for moderate pain (8.66 ± 1.68) followed by feeling rested (9.24 ± 1.17) and having a feeling of general well-being (9.29 ± 1.06) (Table 2). This is because while no patient gave a score of 1–7 for severe pain, seven out of 38 (18.4%) patients claimed to experience moderate pain within a score range of 1–7 (Fig. 2).
Table 2.
The QoR-15K Scores
| QoR-15K items | Number of patients (n) |
10-point score | ||
|---|---|---|---|---|
| Score (1–3) | Score (4–7) | Score (8–10) | ||
| Able to breathe easy | 0 | 1 | 37 | 9.82 ± 0.61 |
| Been able to enjoy food | 0 | 1 | 37 | 9.76 ± 0.88 |
| Feeling rested | 0 | 3 | 35 | 9.24 ± 1.17 |
| Have had a good sleep | 0 | 1 | 37 | 9.71 ± 0.69 |
| Able to look after personal hygiene unaided | 0 | 0 | 38 | 9.97 ± 0.16 |
| Able to communicate with family or friends | 0 | 0 | 38 | 10 ± 0 |
| Getting support from hospital doctor and nurse | 0 | 0 | 38 | 9.92 ± 0.36 |
| Able to return to work or usual home activities | 0 | 3 | 35 | 9.53 ± 1.13 |
| Feeling comfortable and in control | 0 | 0 | 38 | 9.84 ± 0.55 |
| Having a feeling of general well-being | 0 | 3 | 35 | 9.29 ± 1.06 |
| Moderate pain | 1 | 6 | 31 | 8.66 ± 1.68 |
| Severe pain | 0 | 0 | 38 | 9.95 ± 0.23 |
| Nausea or vomiting | 1 | 2 | 35 | 9.47 ± 1.52 |
| Feeling worried or anxious | 0 | 0 | 38 | 9.84 ± 0.55 |
| Feeling sad or depressed | 0 | 0 | 38 | 9.89 ± 0.45 |
Values are presented as number only or mean ± SD. QoR-15K: Korean version of 15-item Quality of Recovery.
Fig. 2.
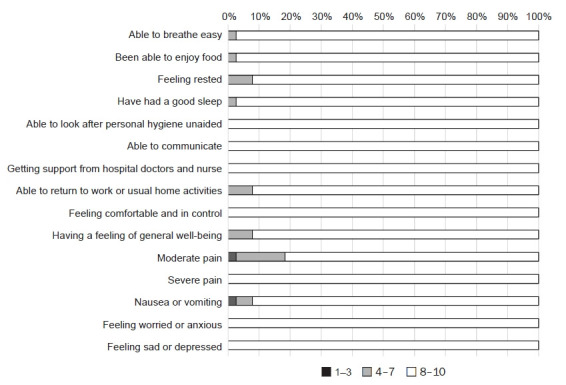
Percentage of patients who scored in range of 1–3, 4–7, and 8–10 for each item listed in the translated Korean version of the 15-item Quality of Recovery questionnaire.
For further analysis, the QoR-15K items were grouped into dimensions (i.e. pain, physical comfort, physical independence, psychological support and emotional state), the QoR-15K dimension scores were averaged to a 10-point scale. When comparing the 10-point scale score of pain to that of physical comfort (9.3 vs. 9.6), no statistically significant difference was again noted (P = 0.227, Fig. 3). With regards to physical comfort, one patient and two patients claimed to have experienced nausea or vomiting with a score of 1–3 and 4–7, respectively. No patient experienced difficulty with the ability to breathe easy, enjoy food, feel rested and have a good sleep in regards to physical comfort. Similarly no discernable difficulty (score of 1–3) was reported for QoR-15K items pertaining to physical independence and psychological support.
Fig. 3.
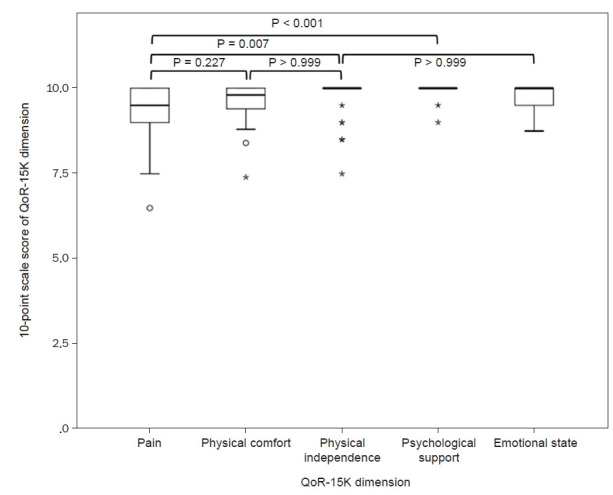
Boxplots of 10-point scale score of the 15-item Quality of Recovery (QoR-15K) dimension for each QoR-15K dimension.
The inter-item and inter-dimension correlation matrices of QoR-15K are shown in Table 3 and Table 4, respectively. Inter-item Cronbach α and split-half reliability were 0.737 and 0.858 for 24-h QoR-15K, respectively. The test-retest ICC was 0.678 (95% confidence interval [CI], 0.506–0.810). Inter-dimension Cronbach α and split-half reliability were 0.722 and 0.769, respectively.
Table 3.
Inter-item Correlation for QoR-15K Scores Taken 24 h after Surgery
| QoR-15K item | Total QoR-15K Score | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Able to breathe easy | 0.354† | - | ||||||||||||||
| Been able to enjoy food | 0.169 | 0.403† | - | |||||||||||||
| Feeling rested | 0.676† | –0.005 | –0.145 | - | ||||||||||||
| Have had a good sleep | 0.302 | 0.079 | 0.099 | 0.301 | - | |||||||||||
| Able to look after personal toilet and hygiene unaided | 0.258 | –0.056 | –0.056 | 0.222 | 0.377† | - | ||||||||||
| Able to communicate with family or friends | * | * | * | * | * | * | - | |||||||||
| Getting support from hospital doctors and nurse | 0.264 | 0.302 | –0.081 | 0.074 | –0.111 | –0.039 | * | - | ||||||||
| Able to return to work or usual home activities | 0.483† | 0.288 | 0.269 | 0.108 | –0.046 | 0.322 | * | 0.182 | - | |||||||
| Feeling comfortable and in control | 0.370† | 0.226 | –0.100 | 0.188 | 0.132 | 0.562† | * | 0.805 | 0.336† | - | ||||||
| Having a feeling of general well-being | 0.534† | 0.351 | –0.090 | 0.255 | –0.039 | –0.128 | * | 0.067 | 0.152 | –0.015 | - | |||||
| Moderate pain | 0.599† | –0.015 | –0.010 | 0.414† | 0.388† | 0.127 | * | 0.174 | 0.022 | 0.216 | 0.314 | - | ||||
| Severe pain | 0.218 | –0.081 | –0.081 | 0.067 | –0.111 | –0.039 | * | 0.486† | 0.175 | 0.368† | 0.000 | 0.250 | - | |||
| Nausea or vomiting | 0.520† | 0.286 | 0.024 | 0.326† | –0.056 | 0.299 | * | –0.111 | 0.275 | 0.086 | 0.361† | –0.008 | 0.159 | - | ||
| Feeling worried or anxious | 0.397† | –0.100 | –0.100 | 0.436† | 0.132 | 0.562† | * | 0.379† | 0.336† | 0.638† | –0.076 | 0.216 | 0.368† | 0.086 | - | |
| Feeling sad or depressed | 0.234 | 0.282 | 0.282 | 0.294 | –0.111 | –0.039 | * | –0.056 | 0.143 | –0.069 | 0.257 | 0.023 | –0.056 | 0.159 | 0.368† | - |
QoR-15K: Korean version of 15-item Quality of Recovery.
Following QoR-15K item had zero variance because all patients gave a score of 10/10 and thus, the inter-item correlation could not be calculated.
P < 0.05 was considered statistically significant.
Table 4.
Inter-dimension Correlation for QoR-15K Scores Taken 24 h after Surgery
| QoR-15K dimension | Total QoR-15 score | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Pain | 0.601* | - | ||||
| Physical comfort | 0.827* | 0.304 | - | |||
| Physical independence | 0.489* | 0.025 | 0.281 | - | ||
| Psychological support | 0.264 | 0.191 | 0.112 | 0.174 | - | |
| Emotional state | 0.709* | 0.406* | 0.461* | 0.389* | 0.367* | - |
QoR-15K: Korean version of 15-item Quality of Recovery.
P < 0.05 was considered statistically significant.
No significant correlation was found between total QoR-15K score and age (ρ = 0.094; 95% CI, –0.209 to 0.381; P = 0.573), PACU length of stay (ρ = –0.185; 95% CI, –0.439 to 0.137; P = 0.265) and duration of anesthesia (ρ = –0.223; 95% CI, –0.527 to 0.128; P = 0.178), thus excluding possible confounding effects.
Box-and-whisker plot and histogram of total QoR-15K score is shown in Fig. 4. Since patients scored on average 9 or above for each item, the total QoR-15K score did not show normal distribution. The skewness and kurtosis values of total QoR-15K score were –0.725 and –0.658, respectively. Out of the total 38 patients, 19 (50.0%) patients gave score range of 148 to 150 out of possible 150 for the total QoR-15K score.
Fig. 4.
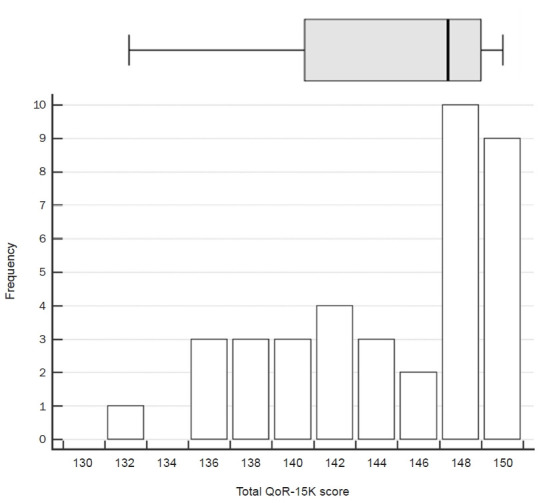
Box-and-whisker plot and histogram of the 15-item Quality of Recovery (QoR-15K) total score. Right and left whiskers denote maximum and minimum values excluding outliers, respectively.
DISCUSSION
This is the first study to evaluate the QoR in patients receiving remimazolam-remifentanil TIVA. On average, the patients scored 9 and above for all QoR-15K items except for moderate pain, which averaged at 8.7. When categorizing and averaging the QoR-15 item scores into dimensions, all QoR-15-dimension scores exceeded 9 points. These results were obtained without the influence of confounding factors such as patient age, PACU length of stay, and anesthesia duration. Internal consistency, as measured by Cronbach’s α and split-half reliability, remained above recommended levels (0.70–0.90) [18]. In addition, throughout this study, no patient experienced severe pain, and only one patient reported a discernible post-discharge nausea and vomiting (PDNV).
While previous studies have investigated the effects of remimazolam on objective postoperative recovery parameters, such as physiologic endpoints, recovery time, and possible adverse events [2,7-10], there has been a gap in research regarding the QoR as perceived by patients who received remimazolam anesthesia. This study represents the first attempt to evaluate QoR as a primary endpoint in patients who underwent remimazolam-remifentanil TIVA. Furthermore, this study employed a widely used and validated psychometric assessment tool, the QoR-15, to thoroughly assess various aspects of recovery, including pain, physical comfort, physical independence, psychological support, and emotional state. These findings underscore the clinical significance of the study’s results.
In a prior study conducted by Shi et al. [15], cirrhotic patients undergoing endoscopic variceal ligation under general anesthesia were randomly assigned to either the remimazolam or propofol group. As a secondary endpoint, patient anesthesia satisfaction was assessed using a 10-point visual analog scale (VAS) [15]. While this scoring method was not as comprehensive as the QoR-15K questionnaire employed in this study, patients in the remimazolam group reported high satisfaction with their anesthesia experience, non-inferior to the propofol group [15]. Similar to Shi et al.’s study [15], we observed that the level of physical comfort, which encompasses factors such as the ability to breathe, have a good sleep, enjoy food, feel rested, and experience PDNV, demonstrated the strongest correlation with the total QoR-15K score. Particularly, the item ‘feel rested’ exhibited the highest correlation among all the QoR-15K items. These findings suggest that a significant portion of anesthesia satisfaction can be attributed to the quality of physical comfort experienced during remimazolam anesthesia.
In regards to postoperative nausea and vomiting (PONV), a prior study has reported that propofol possesses a direct antiemetic effect and can reduce the incidence of PONV [19]. Conversely, remimazolam lacks antiemetic properites, raising the possibility of a higher incidence of PONV with its use. However, a previous investigation comparing the frequency of PONV in craniotomy patients under either remimazolam or propofol found no significant difference in the incidence of PONV [9]. Furthermore, a study conducted by Zhang et al. [20], which involved patients undergoing hysteroscopy under general anesthesia using remimazolam versus propofol, revealed that none of the patients in the remimazolam group experienced PONV, in contrast to 24% in the propofol group. Although the reason for remimazolam’s potential to result in fewer instances of PONV remains unclear, our results appear to align with those of previous studies. In our study, only one patient experienced severe PDNV with a score of 2, while two patients reported moderate PDNV with scores of 6 and 7.
This study has several limitations. First, it is an observational study of remimazolam, rather than a randomized controlled trial that includes a control group administered with different anesthetic agents, such as propofol or inhalation anesthetics. This observational study also cannot establish a direct cause-and-effect relationship and may have introduced bias. However, the encouraging results suggest the need for further investigation through randomized controlled trials comparing remimazolam to other anesthetic agents. Furthermore, the results of this study allow us to make approximate conclusions about the impact of remimazolam on QoR by comparing them to a previous study that assessed QoR profiles of propofol and desflurane [21]. Lee et al. [21] conducted a study comparing patient recovery using the QoR-40 questionnaire in individuals who received propofol TIVA and desflurane anesthesia. On postoperative day 1, patients in the propofol TIVA and desflurane groups rated the QoR-40 dimensions as follows: physiological support 9.4 and 8.9, emotional status 8.9 and 8.4, physical comfort 8.7 and 7.8, physical independence 8.4 and 7.2, and pain 8 and 7.7 out of 10, respectively [21]. In comparison to these findings, our study recorded scores for each dimension as follows: physiological support 9.96, emotional status 9.7, physical comfort 9.6, physical independence 9.75, and pain 9.3 out of 10. Notably, these scores exceeded those of the propofol TIVA group. While the previous study focused on thyroid surgery, while ours was on hysteroscopic surgery, which may differ in invasiveness, these results still offer valuable insights into QoR after remimazolam general anesthesia and can provide a foundation for future controlled comparative studies. Second, our results were obtained from a single tertiary university hospital and study population was limited to patients receiving gynecologic day surgery, specifically hysteroscopic surgery. Some other effects of remimazolam including antiemetic effects as well as some aspects of QoR-15K scores may need to be verified through further studies involving various surgeries. Third, we used translated QoR-15K, which has been validated in a previous study [14] but there are other tools to evaluate patient recovery [11-14], some of which are more comprehensive such as QoR-40 [13]. Additionally, differences in questionnaires may have affected the measurement of recovery outcomes. Fourth, the QoR-15K questionnaire was only surveyed 24 h after surgery without further serial assessment. However, all of the patients had short postoperative hospital stay because of day surgery. Since patient’s mental and physical status can undergo rapid changes during acute postoperative phase, further investigation may be needed to evaluate changes in QoR-15K score for longer hospital stays after major surgeries.
In conclusion, patients receiving remimazolam-remifentanil TIVA showed satisfactory recovery, as indicated by 24-h postoperative QoR-15K scores, with no severe pain or PDNV. The results of this observational study suggest that remimazolam may be a suitable option for general anesthesia in day surgery, particularly in terms of patients’ QoR. However, further comparisons with other commonly used anesthetic agents, such as propofol, are warranted to better understand its comparative effectiveness.
Footnotes
FUNDING
None.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Writing - original draft: Insun Park, J Kim. Writing - review & editing: SH Chung, Hyo-Seok Na, Seung Hyun Chung. Conceptualization: Insun Park, Hyo-Seok Na, Seung Hyun Chung. Data curation: Insun Park, Junkyu Kim. Formal analysis: Insun Park, Seung Hyun Chung. Methodology: Seung Hyun Chung. Project administration: Seung Hyun Chung. Funding acquisition: Seung Hyun Chung. Investigation: Seung Hyun Chung. Resources: Seung Hyun Chung. Supervision: Seung Hyun Chung. Validation: Seung Hyun Chung.
SUPPLEMENTARY MATERIALS
Supplementary data is available at https://doi.org/10.17085/apm.23102.
QoR-15K dimensions and correspoding QoR-15K items
The translated Korean version of the 15-item quality of recovery score (QoR-15K) questionnaire.
REFERENCES
- 1.Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115:274–83. doi: 10.1213/ANE.0b013e31823f0c28. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto Y. Efficacy and safety profile of remimazolam for sedation in adults undergoing short surgical procedures. Ther Clin Risk Manag. 2022;18:95–100. doi: 10.2147/TCRM.S304556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, et al. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60–6. doi: 10.1097/01.anes.0000267503.85085.c0. [DOI] [PubMed] [Google Scholar]
- 4.Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. Modern anesthetics. 2008:335–60. doi: 10.1007/978-3-540-74806-9_16. [DOI] [PubMed] [Google Scholar]
- 5.Sipe BW, Rex DK, Latinovich D, Overley C, Kinser K, Bratcher L, et al. Propofol versus midazolam/meperidine for outpatient colonoscopy: administration by nurses supervised by endoscopists. Gastrointest Endosc. 2002;55:815–25. doi: 10.1067/mge.2002.124636. [DOI] [PubMed] [Google Scholar]
- 6.Ulmer BJ, Hansen JJ, Overley CA, Symms MR, Chadalawada V, Liangpunsakul S, et al. Propofol versus midazolam/fentanyl for outpatient colonoscopy: administration by nurses supervised by endoscopists. Clin Gastroenterol Hepatol. 2003;1:425–32. doi: 10.1016/s1542-3565(03)00226-x. [DOI] [PubMed] [Google Scholar]
- 7.Tang F, Yi JM, Gong HY, Lu ZY, Chen J, Fang B, et al. Remimazolam benzenesulfonate anesthesia effectiveness in cardiac surgery patients under general anesthesia. World J Clin Cases. 2021;9:10595–603. doi: 10.12998/wjcc.v9.i34.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dou D, Feng Y, Jiang L. Efficiency and safety of remimazolam and midazolam in digestive endoscopic sedation: Systematic review and meta-analysis. Dig Endosc. 2022;34:653. doi: 10.1111/den.14219. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Nishiwaki K. Comparison of remimazolam and propofol in anesthetic management for awake craniotomy: a retrospective study. J Anesth. 2022;36:152–5. doi: 10.1007/s00540-021-03021-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Wang J, Ran R, Peng Y, Xiao Y. Efficacy and safety of remimazolam tosylate in hysteroscopy: A randomized, single-blind, parallel controlled trial. J Clin Pharm Ther. 2022;47:55–60. doi: 10.1111/jcpt.13525. [DOI] [PubMed] [Google Scholar]
- 11.Bowyer A, Jakobsson J, Ljungqvist O, Royse C. A review of the scope and measurement of postoperative quality of recovery. Anaesthesia. 2014;69:1266–78. doi: 10.1111/anae.12730. [DOI] [PubMed] [Google Scholar]
- 12.Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118:1332–40. doi: 10.1097/ALN.0b013e318289b84b. [DOI] [PubMed] [Google Scholar]
- 13.Myles PS, Weitkamp B, Jones K, Melick J, Hensen S. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84:11–5. doi: 10.1093/oxfordjournals.bja.a013366. [DOI] [PubMed] [Google Scholar]
- 14.Yoon S, Joo H, Oh YM, Lee J, Bahk JH, Lee HJ. Validation and clinical utility of the Korean version of the Quality of Recovery-15 with enhanced recovery after surgery: a prospective observational cohort study. Br J Anaesth. 2020;125:614–21. doi: 10.1016/j.bja.2020.06.040. [DOI] [PubMed] [Google Scholar]
- 15.Shi F, Chen Y, Li H, Zhang Y, Zhao T. Efficacy and Safety of remimazolam yosilate versus propofol for general anesthesia in cirrhotic patients undergoing eEndoscopic variceal ligation. Int J Gen Med. 2022;15:583–91. doi: 10.2147/IJGM.S345390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9. [Google Scholar]
- 17.De Vlieger JCN, Luiting WH, Lockyer J, Meyer P, Fleer J, Sanderman R, et al. Validation of the Dutch translation of the quality of recovery-15 scale. BMC Anesthesiol. 2022;22:243. doi: 10.1186/s12871-022-01784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDowell I. The theoretical and technical foundations of health measurement. Oxford: Oxford University Press; 1996. Measuring Health; A Guide to Rating Scales and Questionnaires; pp. 10–46. [Google Scholar]
- 19.Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol. 2009;605:1–8. doi: 10.1016/j.ejphar.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-centre randomized controlled trial. BMC Anesthesiol. 2021;21:156. doi: 10.1186/s12871-021-01373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WK, Kim MS, Kang SW, Kim S, Lee JR. Type of anaesthesia and patient quality of recovery: a randomized trial comparing propofol-remifentanil total i.v. anaesthesia with desflurane anaesthesia. Br J Anaesth. 2015;114:663–8. doi: 10.1093/bja/aeu405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
QoR-15K dimensions and correspoding QoR-15K items
The translated Korean version of the 15-item quality of recovery score (QoR-15K) questionnaire.


