Abstract
There is a complex interrelationship between the nervous system and the cardiovascular system. Comorbidities of cardiovascular diseases (CVD) with mental disorders, and vice versa, are prevalent. Adults with mental disorders such as anxiety and depression have a higher risk of developing CVD, and people with CVD have an increased risk of being diagnosed with mental disorders.
Oxidative stress is one of the many pathways associated with the pathophysiology of brain and cardiovascular disease. Nicotinamide adenine dinucleotide phosphate oxidase (NOX) is one of the major generators of reactive oxygen species (ROS) in mammalian cells, as it is the enzyme that specifically produces superoxide.
This review summarizes recent findings on the consequences of NOX activation in thrombosis and depression. It also discusses the therapeutic effects and pharmacological strategies of NOX inhibitors in CVD and brain disorders. A better comprehension of these processes could facilitate the development of new therapeutic approaches for the prevention and treatment of the comorbidity of thrombosis and depression.
Keywords: NOX, Platelets, Endothelium, Neurons, Microglia, Psychiatry disorders
1. Introduction
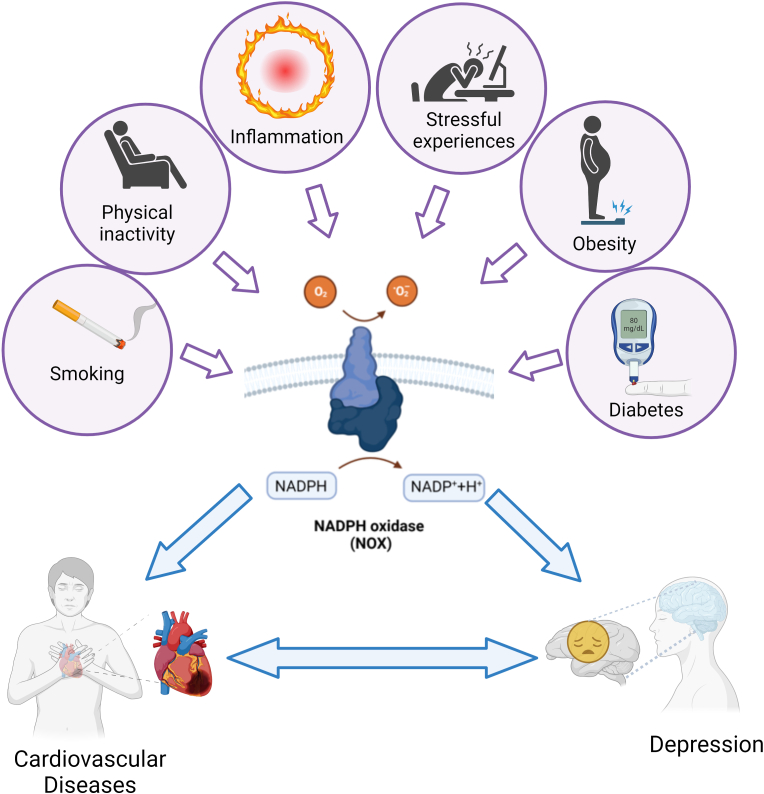
Cardiovascular diseases (CVDs) and depression are currently the most prevalent causes of disability in high-income countries. According to the WHO, CVDs are complicated illness causing considerable number of deaths worldwide (https://apps.who.int/iris/handle/10665/44701). Major depression is a prevalent mental disorder characterized by persistent poor mood, anhedonia, exhaustion, sadness, insomnia, and increased risk of suicide [1]. CVDs and depression are two seemingly unrelated conditions, but they can be linked in different ways, so it is important to consider both when treating patients. Obesity, physical inactivity, smoking, diabetes, stress and chronic inflammation are common risk factors for both CVDs and depression [2], and a possible bidirectional relationship between CVD and depression has been suggested (Fig. 1). Indeed, patients with CVD are more likely to develop depressive symptoms, and depressed people have a higher risk of developing CVD [3]. Depression is 2–3 times more prevalent in CVDs patients than in the general population. The incidence of depression in CVD patients is between 10 % and 40 %, and it increases with the severity of the heart disease [4].
Fig. 1.
Common risk factors associated with cardiovascular disease and depression
Various environmental factors such as obesity [[5], [6], [7], [8]], physical inactivity [6,9,10], smoking [[11], [12], [13]], diabetes [14,15], stress [16,17,18] and chronic inflammation [5,7], which are also common risk factors for CVDs and depression, can regulate the expression/activity of NOXs.
Depression correlated with adverse cardiovascular outcomes and mortality in CVDs patients [3,[19], [20], [21]]. The relationship between depression and both arterial and venous thrombosis has been established in numerous human researches. Depression is associated with a worse prognosis in acute coronary syndrome patients, a manifestation of CVD resulting from coronary artery thrombosis [22], and increases the relative risk of venous thrombosis [23]. Conversely, venous thromboembolism increased the risk of depression [24], suggesting a reciprocal risk model of depression and thrombosis. The strong interaction between depression and thrombosis has been also proved by the use of experimental models of depression and thrombosis. Several studies have shown that animal models such as chronic restraint stress, chronic mild stress and lipopolysaccharide injection, which are commonly used to promote depressive-like disorders in rodents [[25], [26], [27], [28]], enhanced arterial thrombosis [[16], [29], [30], [31], [32]], platelets activation [33] and promoted the development of venous thrombosis [30,34]. On the other hand, coronary artery ligation, which mimics human coronary occlusion by thrombosis, causes depression-like behavior in animals [35,36].
It is well documented that depression is associated with worsening psychosocial variables since its onset, which may increase the likelihood of unhealthy lifestyle habits such as poor diet, tobacco smoking, alcohol consumption, and physical inactivity [[37], [38], [39], [40]]. It is noteworthy that these unhealthy behaviors have traditionally been studied in the area of non-communicable diseases and the American Heart Association has included these modifiable factors in the list of “Life's Simple 7″ that have a definite impact on the development and progression of cardiovascular disease [41]. These observations clearly indicate that, in addition to the direct effects of depression on the pathophysiological mechanisms leading to CVDs, increased behavioral risk profiles in depressed patients also contribute to their higher risk of CVDs [42]. For this reason, managing unhealthy behaviors should be included in the strategy to reduce cardiovascular risk associated with depression [2].
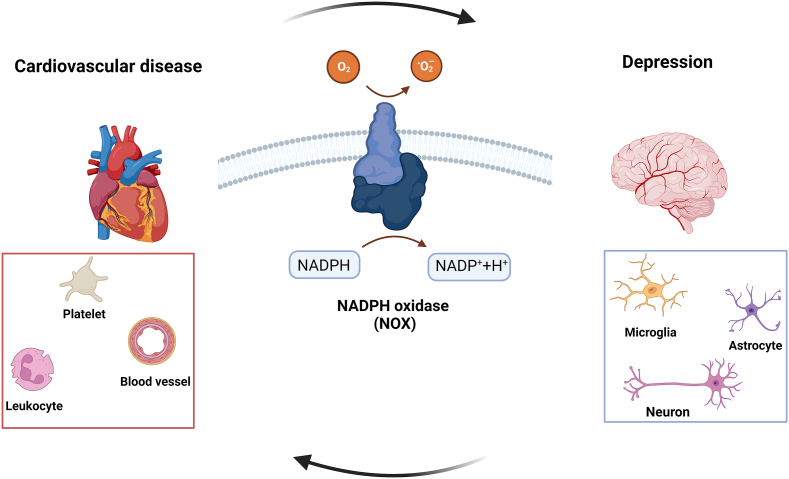
It is well known that depression can alter the body's stress response and inflammatory pathways, the activation of endothelial cells and platelets, and the increased oxidative stress production, which can enhance the risk of blood clot formation and consequently the probability of a thrombotic event [43,44]. Similarly, thrombosis and the resulting health consequences can lead to autonomic nervous system dysfunction, inflammation, endothelial dysfunction, platelet hyperactivity and generation of reactive oxygen species (ROS), that in turn can trigger or exacerbate depression [43,44]. Various mechanisms have been proposed for the link between depression and thrombosis, with oxidative stress being one of the most interesting. In all cells there is a dynamic balance between pro- and antioxidant factors. The most important oxidants are ROS, endogenous byproducts of oxidative respiration, intracellular signals, and substances used to defend against pathogens. On the other hand, some mechanisms can prevent damage caused by radical species, such as small antioxidant molecules and enzymatic systems. If antioxidants succeed in balancing the formation of ROS, homeostasis is maintained. However, if ROS formation is not adequately counteracted, a state referred to as oxidative stress occurs, leading to various cellular damages. ROS affects the primary structures of cells such as proteins, lipids and DNA that in turn can promote cell death [45]. Increase in ROS production has been associated to CVDs (hypertension, atherosclerosis, thrombosis, heart failure, and arrhythmia) and depression [[46], [47], [48], [49], [50]]. Among ROS promoting the onset of CVD and depression, superoxide () hydroxyl radicals, (OH•) hydrogen peroxide (H2O2), and peroxynitrite anion are the main culprits, and their main sources are xanthine oxidase, lipoxidases, myeloperoxidases and nicotinamide adenine dinucleotide phosphate oxidase (NOX). NOXs are exclusively accountable for ROS production and are the main cellular ROS source in mammalian [51,52]. NOXs are expressed in the cardiovascular system [53], in various cell types including platelets [54,55], endothelial cells [[56], [57], [58], [59]] and leukocytes [60] playing a role in thrombotic events, as well as in the brain cells relevant to depression including neurons, microglia, and astrocytes (Fig. 2; Table 1) [61]. It is noteworthy that the activity and expression of NOXs are regulated by obesity [[5], [6], [7], [8]], physical inactivity [6,9,10], smoking [[11], [12], [13]], diabetes [14,15], stress [16,17,18] and chronic inflammation [5,7], which are also some of the common risk factors for CVDs and depression (Fig. 1).
Fig. 2.
NOXs: a common player in cardiovascular disease and depression
NOX activity is altered in different cellular types involved in both thrombosis and depression and may be a common potential therapeutic target for the comorbidity of these diseases.
Table 1.
NOX isoforms expression in thrombosis-related cells and in brain cells.
| NOX isoforms | Product | Thrombosis-related cells | Source | Brain cells | Source |
|---|---|---|---|---|---|
| NOX1 | O2−∙ | Platelets | [48,62] | Neurons | [[63], [64], [65], [66], [67]] |
| Endothelium | [68,69] | Microglia | [70] | ||
| Leukocytes | [54,71] | Asrocytes | [72] | ||
| NOX2 | O2−∙ | Platelets | [48,62] | Neurons | [[65], [66], [67]] |
| Endothelium | [68,69] | Microglia | [70] | ||
| Leukocytes | [54,71] | Asrocytes | [67,72] | ||
| NOX3 | O2−∙ | N.A. | Neurons | [67] | |
| Oligodendrocytes | [73] | ||||
| Asrocytes | [67] | ||||
| NOX4 | H2O2 | Platelets | [48] | Neurons | [67] |
| Endothelium | [68,74] | Microglia | [67] | ||
| Astrocytes | [67,72] | ||||
| NOX5 | O2−∙ | Platelets | [48,75] | Oligodendrocytes | [73] |
| Endothelium | [68] |
Here we will examine the impact of NOX activation on thrombosis and depression. Therapeutic effects and pharmacological strategies related to NOX inhibitors in CVD and depression are also summarized to pave the way for developing new therapeutic methods that could be effective in preventing and treating the comorbidity of thrombosis and depression.
1.1. Thrombosis mechanisms
Thrombosis is a multifaceted, complex process that plays a significant role in the pathophysiology of CVD. Myocardial infarction and stroke are caused by arterial thrombosis, whereas venous thrombosis causes venous thromboembolism and pulmonary embolism. Arterial thrombosis is usually triggered by erosion and rupture of an atherosclerotic plaque, followed by platelet activation/aggregation, thrombus development and vessel occlusion [76]. In contrast, venous thrombosis is the result of a disturbance in the balance between blood flow and venous endothelial health [77].
Thrombosis is mediated by endothelial cells, platelets, leukocytes and circulating coagulation proteins. Adhesion of platelets to the exposed subendothelial matrix and/or activated endothelium leads to platelet activation/aggregation, which results in the expression of specific receptors in the cell membrane and the release of negatively charged factors important for the formation of coagulation complexes, and the release of small molecules that can alter the properties of leukocytes, endothelial cells and other vascular cells [78,79].
A disrupted endothelium expresses/releases a variety of factors that contribute to the thrombotic response, including procoagulants, cell adhesion molecules, nitric oxide and von Willebrand Factor. These factors actively contribute to the thrombotic process by influencing platelet activation/adhesion and fibrin formation [80,81]. Endothelial cell activation also leads to leukocyte adhesion and activation. In particular, neutrophil granulocytes, the most abundant leukocytes in the bloodstream, release molecules that can influence coagulation through various mechanisms, such as activation of clotting factors and platelet activation and aggregation [82]. Monocytes are an important source of intravascular expression of Tissue Factor (TF) and provide a membrane surface for the initiation of coagulation under various conditions, and initiate the expression of TF, which in turn activates the coagulation cascade [83].
NOX-dependent production of ROS is also involved in the thrombotic process, mainly by affecting platelet responsiveness [84] and its role in determining the risk of thrombosis [85,86] has been demonstrated in several studies.
1.2. Depression mechanisms
Depression is a complex mental illness involving various molecular and cellular mechanisms in the brain [87,88]. Although the precise etiology of depression is yet unknown, recent research suggests that different cell types are involved. Neurons, the primary signaling cells in the central nervous system, have a key role in depression, as alterations in neurotransmitter signaling, neuroplasticity and synaptic connectivity have been implicated in the pathophysiology [[87], [88], [89]]. Imbalances in glutamate and gamma-aminobutyric acid (GABA) transmission, as well as deficits in monoamines and neurotrophic factors, disrupt the circuit in the limbic and cortical area of the brain that regulate emotions and moods [89]. These abnormalities may be a component of the atrophy and loss of neurons seen in depressed patients and animal models of stress, along with stress-activated signaling pathways that regulate neurotrophic factors and inflammatory cytokines. Microglia and astrocytes contribute to neuroinflammation and immune responses by releasing pro-inflammatory cytokines and amplifying the inflammatory cascade [[90], [91], [92]].
Hormonal, immunological, and aging variables, as well as epigenetic, neurotrophic, and oxidative stress, have all been linked to depression. According to the neurotrophic theory of depression, synaptic and neuronal changes associated with depression are caused by a deficiency of growth factors, in particular the brain-derived neurotrophic factors (BDNF), the highest abundant neurotrophin detected in the adult brain [93]. Indeed, reduction of peripheral BDNF in depressed patient and in the brain regions of rodent model of depression have been reported [[94], [95], [96]].
Increased damages caused by oxidative stress (lipid peroxidation, DNA damage and protein oxidation) have been described in the postmortem brain tissue of depressed humans and in animal models of depression, which can promote neuronal damage and exacerbate neuronal dysfunction [[97], [98], [99]]. ROS can be generated by various brain cells (neurons, microglia and astrocytes) and alter synaptic plasticity [[100], [101], [102]]. There is increasing evidence that NOXs are expressed in all brain cells and that NOX-dependent ROS generation plays a key role in depression [17,[71], [103], [104], [105]].
2. NOX isoforms, structure and activation
NOXs are multimembered transmembrane protein complexes that transfer electrons throughout the membranes with the unique task of producing and H2O2. The NOX family comprises seven different enzymes, NOX1-3 and NOX5, which produce and NOX4 and DUOX1-2, which produce H2O2. NOX proteins share six conserved transmembrane domains, four conserved heme-binding histidine domains, the FAD-binding domain, and the NADPH-binding domain [106]. Despite the similarity of their structure, each isoform of NOX requires association with a specific subset of proteins to be activated.
The first member identified and characterized in phagocytes was NOX2, also termed as gp91phox, which is constitutively associated with the transmembrane subunit p22phox [107,108] and its activation needs the translocation of other factors present in the cytosol. Indeed, NOX2 complex also includes the cytosolic regulatory factors p47phox, p67phox, small GTPase Rac, and the activity modulator p40phox [109,110]. NOX2 activation involve the interactions of different proteins, beginning with phosphorylation of the cytosolic p47phox/p67phox complex, which in turn causes a conformational shift that facilitates the interaction with the p22phox subunit. After the complex is translocated into the membrane, p67phox bind to NOX2 and recruits the p40phox and GTPase Rac subunits, which modulate NOX2 activity [[110], [111], [112], [113]]. After the complex is assembled, it becomes active and produces by transferring electrons from cytosolic NADPH to oxygen in the extracellular or luminal region.
NOX1 interacts in the membrane with NOXO1, NOXA1 and Rac by forming a constitutive complex with p22phox. NOX3 similarly interacts with p22phox and NOXO1, whereas NOX4 is constitutively active and exclusively linked with p22phox. Activation of NOX5 and DUOX1/2 is modulated by a calcium-calmodulin-binding protein or directly by Ca2+ [110,111,[113], [114], [115]].
2.1. NOXs in leukocytes
NOX2 has been firstly identified in the membrane of phagocytes, and has an important role in the mechanisms of host defense against pathogens such as bacterial and fungal [116]. In addition to the involvement of NOX2 in the phagocytic role of leukocytes, they have also been extensively studied for their contribution to inflammation, atherosclerosis, restenosis, and hypertension [[117], [118], [119], [120], [121], [122]].
NOX1 and NOX2 are critical for the differentiation of monocyte into macrophage and the polarization M2-type cells, as found in macrophages without NOX1 and NOX2 [123]. It has also been described that the ROS produced by NOXs in macrophages contribute to fatty liver disease [124], neurotoxic processes for retinal microglia [125], and NLRP3 inflammasome activation [[126], [127], [128]].
It has been shown that NOX2 is the predominant isoform in the neutrophils of patients with chronic granulomatous disease [129] and NOX2 knock-out mice [130].
Several protein kinases, including AKT2 and protein kinase C, phosphorylate p47phox at eight to nine serine sites during neutrophil stimulation [131,75]. Phosphorylation of p47phox promotes membrane translocation and the recruitment of additional cytosolic subunits, resulting in NOX2 enzymatic activity.
In dendritic cells (DCs), the expression of NOX subunits is low compared to other immune cells such as monocytes and the levels of p47phox and NOX2, but not p40phox and p67phox, were selectively increased during maturation and activation of DCs after TLR activation [132]. Several studies have found that NOX2 plays a key function in of the phagosome regulation. Indeed, DCs have a neutral or at least slightly alkaline pH, and deletion of NOX2 leads to the formation of acidic phagosomes [62,133], which leads to increased antigen degradation and compromised cross-presentation to CD8+ T cells via MHC class I [133,134].
Recently, it was found that the levels of p22phox, but not NOX2, were raised in addition to NOX5 expression in a subset of DCs during differentiation from circulating monocytes [135].
The role of NOXs in lymphocytes is complex and based mainly on animal models and human pathological conditions associated with alterations in NOX2 function, as reported by Paige et al. [136]. NOX2 signaling is required for balanced development of CD4+ T cells [137] and T regulatory cells (Tregs) [138]. NOX2 is essential for blocking TCR signal transduction in CD8+ Tregs because it is employed by CD8+ Tregs to produce a new Treg-mediated inhibition of CD4+ T cells [139]. In B cells, NOX2 is employed to rapidly create ROS in response to intracellular bacteria [140], as well as negatively modulate ROS-driven BCR-induced proliferation [141].
2.2. NOXs in platelets
ROS have a significant impact on platelet function [142,143] and platelets themselves can generate ROS including , H2O2, and OH• [144]. Platelets may promote the release of ROS in pathological conditions, which in turn lead to changes in platelet functions [[145], [146], [147]], resulting in potential thrombotic phenotype. Although ROS may be elicited by various source, several studies have reported NOX activity in platelets (Table 1) [54,[68], [74], [148]]. In vitro studies have shown that the radical species produced by platelets act as an intracellular signaling pathway and contribute to the prothrombotic phenotype. In vitro, collagen causes the production of NOX-dependent , in platelets, which increases the released ADP and sustains thrombus growth [149]. In platelets, inhibition of NOX decreased not only the formation of ROS, but also aggregation induced by collagen/thrombin and the release of thromboxane B2 promoted by collagen [148].
Human platelets or megakaryocytic cell line (MEG-01) homogenates can produce ROS through NADH/NADPH-dependent enzymatic activity [69]. Further studies have shown that platelets and/or megakaryocytes express various subunits that form NOX, (p22phox, p67phox, p47phox [69], and small G protein Rac, but not NOX2 [149,150]). In contrast to previous work, some years later Chlopicki and colleagues demonstrated that platelets express also NOX2 at levels comparable to neutrophils [148]. Consistent with this observation, Pignatelli et al. confirmed in vivo that the production of from platelets depends on the expression of the NOX2 subunit. The absence of this subunit has a direct effect on the expression of CD40 ligand promoted by collagen, arachidonic acid and, thrombin [151]. Currently, it has been confirmed that in platelets the subunits p47phox, p67phox, p22phox, NOX2 and Rac1/2 form the NOX active enzyme [55].
Although NOX2 is the most studied and characterized isoform in platelets [148,74], human platelets also express NOX1 [74], and NOX5 [68] isoforms. Interestingly, the NOX4 mRNA but not the protein was detected in human platelets [54].
Activation of NOX2 in platelets is mediated by collagen/GPVI, sCD40L/CD40L, and oxidized lipids (ox-LDL)/CD36. In particular, the signal mediated by CD40 is mainly involved in atherosclerotic processes [152], while the signals mediated by collagen/GPVI and oxLDL/CD36 are preferentially associated with thrombotic and thromboembolic processes [63,64].
The association of the cytoplasmic tails of GPVI, a membrane glycoprotein expressed only by platelets and known as a collagen receptor [65], with the tumor necrosis factor receptor-associated factor 4 (TRAF4) and with p47phox subunit leads to ROS production via a NOX2-dependent pathway, and to GPIIb/IIIa activation, which allow the binding of fibrinogen and consequently aggregation of platelets and formation of thrombus [63].
Ox-LDL, which are not only important for atherosclerosis, are also important activators of platelets [86,[66], [67], [153], [154]]. The interaction of endogenous ox-LDLs with the CD36 receptor on platelets promotes platelet hyperactivity through the formation of ROS, which has a critical function in the prothrombotic phenotype [64]. Binding of ox-LDL to CD36 mediates the production of ROS through NOX2 activation, as demonstrated by specific CD36 and NOX2 inhibitors and by NOX2 deletion [86,155].
Finally, triple NOX knockout mice (ie. NOX1−/−/NOX2−/−/NOX4−/−) displayed impair platelet activation and reduced thrombus formation in vitro and in vivo, confirming the relevance of NOXs in the physiopathology of thrombosis [156].
2.3. NOXs in endothelium
Endothelial cells synthesize small amounts of mRNA encoding NOX subunits and have a low rate of oxidant production compared with phagocytes [157]. In addition, much of the is produced intracellularly, whereas neutrophil is mainly produced in the extracellular compartment [158]. In view of these features, the generation of oxidant by these cells is usually considered to be a 'redox-sensitive' intracellular pathway that plays a central role in physiological and pathological situations.
Different studies explored the composition of the enzyme in endothelial cells showing that they generally contain only the catalytic subunit NOX2 and the regulatory p22phox [159]. Only one study showed the presence of NOX2, p22phox, p67phox, and p47phox subunits but not of p40phox and the flavocytochrome b558 [157]. Of note, Gorlach et al. showed that the NOX2 subunit was identical in endothelial cells and phagocytes [160], whereas Bayraktutan et al. found distinct differences in the domain of NADPH-binding and in sites of glycosylation of endothelial NOX2 [159].
Endothelial cells express NOX1, NOX2, and NOX5, which regulate the superoxide generation, and NOX4, the constitutively active enzyme, that generates H2O2 (Table 1) [70].
NOX1, NOX2, and NOX5 have been found to enhance endothelial dysfunction, inflammation, and apoptosis in the arterial wall in arteries from individuals with coronary artery disease and in animals with experimentally generated hypertension, diabetes, or atherosclerosis. NOX4, on the other hand, is vasoprotective by enhancing NO bioavailability and decreasing cell death pathways [161].
In endothelium, NOX2 and NOX1 expression are increased under pathological conditions, leading to excessive formation, especially in the extracellular compartment [162]. The release of ROS contributes to the inactivation of NO, the reduction of its vasoactive function, and the generation of peroxynitrite, which is responsible for the damage of lipids, proteins and DNA, and promotes cell damage and death. This results in the loss of NO vasoprotective action, macromolecule damage, and stimulation of proinflammatory mechanisms, leading to endothelial dysfunction [163].
In particular, it has been shown that different types of stimuli (e.g. cholesterol, TNF-α, homocysteine, and endostatin) enhance the NOX1 and NOX2 expression. As a result, NOX2 tends to cluster in lipid rafts and translocate p47phox from the cytosol into the plasmatic membrane [164]. These clusters promote the formation of large amounts of and are a point of attraction for NOX2-expressing macrophages. The simultaneous presence of NOX2 in endothelial lipid rafts and in macrophages promotes the formation of ROS in the luminal space of the vessel, which explains why vascular lesions are more often associated with extracellular than with intracellular protein damage [165].
2.4. NOXs in neurons
The expression of almost all NOX subunits has been described in neuronal cells (Table 1), although the role of NOX1 and NOX2 has mainly been investigated [18,[166], [167], [168], [169]].
Levels of NOX subunits are low expressed, in physiological conditions, whereas they are upregulated in pathological conditions. High levels of p47phox and NOX2 were described in pyramidal cortical neurons and GABAergic neurons in preclinical experimental models of depression and in postmortem patients with depression, respectively [18,168].
At the neuronal subcellular level, NOX2, p47phox, and p22phox have been localized in the soma, neurites, and synapses, respectively, whereas the exact neuronal distribution of the other NOX subunits has not yet been analyzed in detail [113].
Physiological production of oxidant by NOXs have positive effects in neurogenesis, neurite growth and branching, synaptic plasticity, and synaptic weakening have been described [100,170,171]. Postsynaptic NOX2 regulates long-term depression by reducing AMPA-mediated transmission [171]. Increased generation of ROS by members of the NOX family has adverse effects on adult hippocampal neurogenesis, dendritic arborization, synaptic plasticity, glutamate receptor regulation, excitotoxicity, and neuronal survival [109,172,72].
Interestingly, in rodent models of depression, upregulation of NOX2 promoted the reduction of parvalbumin-positive GABAergic neurons in the prefrontal cortex [173], whereas overexpression of NOX1 oxidized NMDA receptor 1 and decreased expression of BDNF through epigenetic modifications [105].
2.5. NOXs in microglia
Microglia are the tissue-specific macrophages in the brain, playing a significant function in neuroinflammation in different brain diseases, as psychiatric disorders and depression [92,174]. In healthy condition, microglia exhibit high motility, monitor the brain from damaged or dying cells, for pathogens and debris maintaining local homeostasis. In addition, microglia interact with neuronal synapses, monitor synapse status, and contribute to the maintenance of neuronal circuits [175,176]. Activated microglia release pro-inflammatory cytokines, excitatory neurotransmitters, proteinases, and ROS that promote neuroinflammation and brain disease. In fact, production of ROS by microglia is considered one of the key factors in dysfunction, damage, and death of neurons [177]. Microglial activation was observed both in patients with depression and in experimental models, and an association between microglial activation and depression severity has also been described [92,178]. Moreover, depletion of microglia prevented the production of ROS and depression-like phenotypes in mice exposed to social stress [179]. Among brain cells, microglia have the greatest levels of NOX, particularly NOX2 (Table 1) [177] and inflammation-induced overexpression of NOX2 occurs first in microglia and then in neurons [180]. Furthermore, the stress-induced anxiety-like phenotype was related with a rise in NOX2 in microglial cells and was prevented in NOX2 knock-out mice [181].
NOX1 is localized in intracellular vesicles, some of which are lysosomes, whereas NOX2 is distributed in the plasma membrane but redistributed intracellularly into small vesicles after microglial activation [167,177,182]. Several studies have shown that the increase in NOX family members are associated with microglia activation in different brain regions [177,182,183]. Interestingly, recent research studies have shown that microglia release several chemicals compounds including chemokines, cytokines and growth factors that may have a critical function in controlling brain and cardiovascular activity [184]. On the other hand, myocardial infarction promotes a persistent microglia activation in specific brain areas [185,186]. These pieces of evidence suggest that microglia may have a key role in linking brain and heart disease.
2.6. NOX in astrocytes
Astrocytes make up about 30 % of the cells in the brain, interact with neurons and blood vessels, involved in the pathophysiology of depression [187]. Astrocytes are homeostatic and defensive cells that play a role in maintaining brain homeostasis by releasing transmitters such as glutamate, gamma-aminobutyric acid (GABA), and adenosine 5′-triphosphate (ATP) or trophic factors such as BDNF. Furthermore, astrocytes have a role in neuroinflammation by producing proinflammatory cytokines [188]. Astrocytes become reactive and participate in the recovery of injured brain tissue in neurodegenerative and psychiatric conditions [189]. NOX4 is the most abundant isoform in astrocytes, although NOX1 and NOX2 have also been detected (Table 1) [169,190]. Interestingly, anxiety and depression-like symptoms have been reported to be associated with astrocytosis and overexpression of NOX1 in a model of brain injury [191].
3. NOXs and thrombosis
The clinical relevance of NOX-dependent production of ROS in promoting platelet responsiveness [84] and, consequently, in determining the risk of thrombosis [85,86] has been demonstrated in several studies.
However, contradictory results on the effects of deletion of NOX1 on platelet response and the resulting thrombotic phenotype have been reported. Delaney et al. demonstrated that platelets derived from NOX1 knock-out mice (NOX1-/Y) showed decreased ROS production and impaired aggregation promoted by thrombin and thromboxane A2 analog U46619 but a normal response to collagen-related peptide (CRP). Moreover, NOX1-/Y mice did not exhibit alterations in in vivo thrombus formation [54]. In contrast, Vara et al. showed that washed platelets isolated from NOX1-/Y mice have reduced platelet aggregation induced by collagen, whereas they showed normal responses to thrombin. Furthermore, ferric chloride-induced coronary artery thrombosis was delayed in NOX1-/Y mice, whereas tail bleeding was similar to that in control mice, suggesting that NOX1 has an important role in pathological arterial thrombosis but not in hemostasis [192].
ROS derived from activation of the NOX2 isoform, appears to be a major contributor to pathological signaling, as seen in NOX2 deficient patients [193]. In particular, granulomatous disease patients with p47phox or NOX2 deficiency present deactivation of NO resulting in arterial dilatation associated with a low carotid intima-media thickness. Taking together these observations suggest that reduction of NOX2 activity prevents or retards the development and the progression of atherosclerotic plaques [129]. Similarly, hypercholesterolemic children showed reduced flow mediated-dilation and enhanced carotid intima-media thickness, related to increased NOX2 expression on platelets [194]. Of note, platelets NOX2−/− patients display defective arterial thrombosis [54,155]. Platelets obtained from individuals with hereditary NOX2 deficiency were incapable of producing , expressed low levels of CD40L and showed impaired activation [151].
These observations were also confirmed by experimental animal models lacking NOX2 [195] or specific p47phox deficiency [196], in which reduced platelet activation and venous and arterial thrombosis was found. The importance of NOX2 platelets in thrombosis was provided by the defective arterial thrombus formation observed in control thrombocytopenic mice after injection of NOX2−/− platelets [195]. Recently, it has been showed that NOX2 deletion in mice reduced platelet aggregation induced by thrombin without affecting the platelet response to collagen, and it was not sufficient to affect pathological arterial thrombosis and hemostasis [192].
Regarding the effects of altered NOX activity on endothelial cells, numerous studies have demonstrated the involvement of distinct NOX isoforms in endothelial dysfunction or activation, which consequently contributes to a thrombotic phenotype. In particular, NOX1 overexpression in vascular smooth muscle cells may lead to effects on the adjacent endothelial cell layer [197]. This may cause tetrahydrobiopterin oxidation in endothelial cells and uncoupling of endothelial nitric oxide synthase (eNOS), leading to reduction of NO bioavailability and endothelial dysfunction [197]. In addition, the superoxide anion can react rapidly with NO leading to the formation of peroxynitrite, a highly reactive and cytotoxic molecule, resulting in a loss of NO bioactivity. NO regulates vascular tone, inhibits platelet aggregation, and has antithrombotic properties. The reduction in NO bioavailability caused by increased superoxide anions contributes to the pathogenesis of thrombotic conditions associated with endothelial dysfunction, vascular inflammation and platelets activation [198].
Deletion of NOX1 attenuates hypertensive response to angiotensin-II (Ang-II) and it is protective against oxidative stress, endothelial dysfunction, aortic dissection and vascular hypertrophy [[199], [200], [201]]. In addition, NOX1 deletion is related to reduced formation of ROS, expression of chemokines, proinflammatory and profibrotic markers, and adhesion and recruitment of leukocytes into vessel wall [202].
NOX2 deletion in mice reduced endothelial dysfunction [203] and vascular hypertrophy, but had little or no effect on blood pressure [204,205] in an experimental hypertension model induced by Ang-II stimulation, suggesting that NOX2 may play a marginal role in the regulation of blood pressure. Endothelial overexpression of NOX2 in mice increased endothelial dysfunction induced by Ang-II. Similarly, targeted overexpression of NOX2 in the endothelium of ApoE−/− mice promoted vascular production, activated endothelial cells, and enhanced macrophage infiltration [206]. In addition, bone marrow transplant studies showed that deletion of endothelial NOX2 significantly reduced neutrophil-platelet interactions during vascular inflammation [207].
Regarding the possible role of NOX5 in atherothrombosis, almost all studies have been performed on cells isolated from human tissues. NOX5 has been found in the endothelium of normal arteries and increased in the neointima of diseased arteries and in the smooth muscle of advanced atherosclerotic arteries [208]. Overexpression of NOX5 generated large amounts of , which in turn can react with NO to produce peroxynitrite reducing the availability of NO. Endothelial cells attempted to compensate for the reduction in NO by boosting NO activity synthase [209].
As already mentioned, NOX4 activity promotes the generation of H2O2, which, unlike superoxide, does not react to any significant extent with NO and thus does not reduce the availability of NO [210]. Moreover, H2O2 is known to oxidize reactive cysteine residues selectively and reversibly, changing the activity of protein targets, such as ion channels, transcription factors, kinases, and phosphatases [211]. H2O2 can itself work as a relaxing factor derived from the endothelium [212]. Therefore, it's not surprising that NOX4 has protective roles in the vessel wall. NOX4 is required for endothelial progenitor cell (EPC) proliferation and migration, and it protects EPCs against cell death caused by proinflammatory cytokines. These NOX4 features may aid in the effective functioning of EPCs [213]. NOX4 has been shown to be an eliminating enzyme and its deletion promotes the accumulation of and reduction of eNOS expression [214]. Similarly, loss of NOX4 under stress conditions promoted by ischemia or Ang-II led to a reduction of expression of eNOS and production of NO, which in turn stimulates inflammation and cell death in NOX4−/− mice [214]. In contrast, selective overexpression in the epithelium of NOX4 leads to beneficial cardiovascular effects by increasing eNOS expression [215,216], exhibiting increased endothelial-dependent vasodilation and blood pressure decrease compared to control animals [217].
Regarding the involvement of NOXs of leukocytes in thrombotic mechanisms, most information focuses on neutrophils. Through the interaction of P-selectin and its ligands, the neutrophils are the first blood cells to reach the site of inflammation, adhere there and roll over the endothelium [218]. Creeping neutrophils rapidly migrate through the inflamed endothelium, worsening the progression of the disease by interacting with platelets and/or clogging the vessel lumen [219].
Infusion of platelets or neutrophils from NOX2 knock-out mice into control mice showed that NOX2 plays a critical role in regulating the interaction between neutrophils and platelets during vascular inflammation [207]. In contrast, bone marrow transplantation experiments provided evidence that NOX2 deletion did not affect the adhesion and roll over of neutrophil to endothelium [207]. Importantly, neutrophil-platelet interactions can induce the formation of neutrophil extracellular traps (NETs) [220], which have a crucial role in immunothrombosis processes [221]. Considering that the production of ROS by NOX2 are crucial for NETosis [222,223], it would be relevant to specifically investigate the function of AKT2-NOX2 pathway in NETosis.
4. NOXs and depression
Some progress in understanding the molecular relationship between oxidative stress and psychiatric disorders, including depression, has been made in experimental models, primarily through the use of stress paradigms. Indeed, chronic stress experimental models have consistently reported to promote behavior deficit, along with corticolimbic brain areas atrophy, hippocampal neurogenesis reduction, neurotransmissions alteration, enhanced neuroinflammation, and disruption of the hypothalamic-pituitary-adrenal (HPA) axis, all of which have been described in stress-related psychiatric disorders [180,[224], [225], [226]]
Seo et al. observed that chronic restraint stress promoted depression-like phenotypes paralleled by increased ROS levels and lipid peroxidation in the prefrontal cortex and hippocampus of mice. Moreover, enhanced gene and protein levels of the p47phox and p67phox subunits were detected. Remarkably, pharmacological inhibition of NOXs by the antioxidant/NOX inhibitor apocynin, molecular downregulation of p47phox in the hippocampus, or the use of heterozygous p47phox knockout mice suppressed the increase in oxidative stress and depression-like behavior induced by chronic stress, indicating the important role of NOX2 in the depression-like phenotype promoted by chronic stress in mice [227].
Similar results were also obtained using different stress paradigms, e.g. chronic mild stress, social isolation rearing and single prolonger stress. Indeed, chronic mild stress promoted the increase in microglial of NOX2 levels leading to an increase in oxidative stress in the ventral hippocampus and behavioral alterations in mice [181]. In addition, the molecular and behavioral changes induced by chronic mild stress were attenuated in NOX2 knockout mice or by the treatment with apocynin [181]. Social isolation rearing and single prolonged stress enhanced NOX2 levels in the hippocampus, nucleus accumbens and prefrontal cortex, thereby promoting a rise in oxidative stress and microglia that are associated with reduction in parvalbumin immunoreactivity and behavioral impairments [173,228]. Dysfunction of parvalbumin-positive GABAergic interneurons were correlated with various psychiatric disorders such as depression, schizophrenia, posttraumatic stress disorder and Alzheimer's disease [229]. The increased in p47phox was mainly observed in pyramidal cells in the prefrontal cortex, whereas there was no evidence of microglial activation [228]. Both loss-of-function p47phox subunit or treatment with the inhibitor apocynin prevented all these alterations [173,228]. In addition, aged mice (18 months old) displayed elevated levels of NOX2, p47phox, and ROS in the hippocampus and were more susceptible to stress-induced depression-like phenotypes [71].
In clinical studies, NOX2 has been suggested as a possible biomarker for suicidality, which is a common hallmark of several psychiatric disorders, such as depression. It's interesting to note that depressed people with a history of suicide attempts have higher levels of oxidative stress and lower overall antioxidant levels [230]. and increased NOX2 levels have been specifically detected in GABAergic neurons in the cortical region of postmortem subjects who committed asphyxial suicide [168].
It has also been suggested that depressive-like behaviors and NOX1 in the mesocortical pathway are causally linked. Compared to other NOX isoforms, NOX1 is a non-phagocytic version of the protein that is less prevalent in the nervous system. Social defeat stress or chronic treatment with corticosteroid, which is elevated in depressed patients, specifically raise NOX1 levels in the ventral tegmental area (VTA), but not in other parts of the brain [105]. The VTA has a crucial function in regulating reward processing and altered cellular functions in the VTA have been linked to depression [231]. The VTA contains dopaminergic, GABAergic, and glutamatergic neurons projecting to different brain areas including the prefrontal cortex. The increase in NOX1 levels in the VTA promoted by chronic administration of corticosterone was paralleled by the development of depressive behavior, enhanced oxidative stress, atrophy of dendritic arborization and reduction in BDNF levels in the prefrontal cortex [105]. Molecular downregulation of NOX1 in the VTA or use of a NOX1 knockout mice prevented all the molecular and behavioral alterations promoted by chronic treatment with corticosterone [105]. Consistent with these findings, another paper reported that the depressive-like phenotypes promoted by chronic unpredictable mild stress were associated with upregulation of NOX1, ROS, and apoptosis in the frontal cortex of mice [232]. Again, the downregulation of NOX1 in the frontal cortex prevented all of these molecular and behavioral changes promoted by chronic unpredictable mild stress [232].
The investigation of variations in NOX1 plasma levels in patients suffering from major depressive disorder has only been carried out in one study to date. It was discovered that people with a first episode of depression who did not smoke and were not treated with psychotropic drugs had higher serum NOX1 levels compared to healthy people. In addition, a strong and positive correlation between plasma NOX1 levels and depressive symptoms was found, suggesting that NOX1 could be a potential biomarker for depression [104].
5. NOXs and inflammation in thrombosis and depression
Thrombosis and depression are characterized not only by an increase in oxidative stress, but also by an increase in inflammatory markers [233,234]. Indeed, the NOX-dependent ROS production promotes inflammation, which maintaining oxidative stress, creating a vicious cycle that exacerbates chronic inflammation. To summarize the concept that oxidative stress and inflammation are intertwined processes that influence and reinforce each other, the term OxInflammation was coined [235].
Oxidative stress can activate numerous redox-sensitive transcription factors such as NF-κB, which in turn regulate the expression of pro-inflammatory molecules. When stimulated, inflammatory cells release chemokines and cytokines that can further promote the generation of ROS via NOX enzymes and other mechanisms, contributing to oxidative stress and leading to a positive feedback loop that can promote or exacerbate the development of different chronic diseases, including thrombosis and depression [236].
It is well known that NOXs regulate thromboinflammatory responses [237]. Specifically, NOX inhibition limits production of NETs, modulator of thromboinflammation, attenuating in vivo thrombus formation in both arterioles and venules [238].
During TNF-α-induced vascular inflammation, thrombus formation is markedly influenced by the exposure of P-selectin after agonistic stimulation and ligand binding activity of GPIbα mediated by ROS produced by platelet NOX2 [207]. In addition, pharmacological inhibition of NOX and deletion of p47phox prevented endothelial dysfunction induced by cytokine cocktail [11,239]. Interestingly, deletion of NOXO1 was recently reported to selectively reduce the plasmatic proinflammatory cytokine profile and the development of atherosclerosis in female mice [240].
Depression is associated with chronic and low-grade inflammatory conditions. Elevated levels of various cytokines, including TNFα, IL1β, IL6, IL18 have been detected in the serum of depressed patients [233,241] and in various brain regions of animal models of depression [242]. Intranasal administration of lipopolysaccharide induced a depressive-like phenotype associated with an increase in NOX2, p47phox and lipid peroxidation in the hippocampus and amygdala of mice. Treatment with apocynin prevented all of these changes induced by lipopolysaccharide administration [243]. Chronic social defeat stress in mice promoted depressive-like phenotypes associated with increased levels of NOX2, IL6 and TNFα in the striatum, prefrontal cortex and hippocampus [244]. Similarly, the depressive-like behaviors promoted by chronic unpredictable mild stress were paralleled by the increase of NOX1, TNFα, IL1β and IL6 in the prefrontal cortex of mice [232].
6. Consequence of NOX inhibitors on thrombosis and depression
Given the role of NOX in regulating various processes involved in both thrombosis and depression, the use of agents targeting this enzyme has gained interest in counteracting these pathologies and especially their coexistence.
Most of the compounds that have been tested to reduce oxidative stress-induced thrombosis and depression are inhibitors of NOX1 and NOX2 activation [104,111].
In the following section, we briefly summarize natural and non-natural compounds that have been tested in the field of thrombosis and depression (Table 2).
Table 2.
Effect of NOX inhibitors on thrombosis and depression.
| Compound | Thrombosis source | Depression source | ||
|---|---|---|---|---|
|
Apocynin |
↓ platelet aggregation | [132] |
↓ p47phox and p67phox ↓ HDACs ↑ H3Ac |
[84] |
| ↓ thrombus formation in vitro | [216,217] | |||
| ↓ adhesion of platelets | [204] | ↓ depressive-like phenotype | [16,84,85,87,208,[221], [222], [223]] | |
|
↓ arterial thrombosis in vivo ↓ NOX1 expression in megakaryocytes |
[16] | |||
|
↓ endothelial dysfunction NO signalling |
[220] |
|||
|
Celastrol |
↓ P-selectin and GPIIbIIIa on platelets | [224] | ||
| ↓ arterial thrombosis in vivo | [225] | ↓ depressive-like phenotype | [227,228] | |
|
↓ endothelial dysfunction in vivo |
[226] |
|||
|
Ebselen |
↓ platelet aggregation | [232] | ||
| ↓ arterial and venous thrombosis in vivo | [233] | ↓ depressive-like phenotype | [236] | |
|
↓ NOX2 expression in macrophages |
[234] |
|||
|
Suramin |
↓ platelet aggregation ↓ calcium mobilization |
[238] | ||
| ↓ venous thrombosis in vivo | [239] | N.A. | N.A. | |
|
↓monocyte adhesion ↓ LDL oxidation ↑ NOX4 |
[240] |
|||
|
Perhexiline |
↓ platelet aggregation |
[243] |
N.A. |
N.A. |
|
Berberine |
↓ NOX4 in endothelial cells | [245] | ↓ depressive-like phenotype | [246,247] |
| ↓ platelet aggregation | [248] | ↑ BDNF | [246,247] | |
| ↓ macrophage activation | [249] | ↑ 5HT, DA, NE | [247,250] | |
| ↓ leukocyte adhesion to endothelium | [251] |
↓ neuroinflammation ↓kynurenine metabolism ↓iNOs |
[171,252] |
|
|
↓ endothelial dysfunction |
[253] |
|||
|
2-APT/ML171 |
↓ platelet aggregation to collagen | [62,174,254] |
N.A. |
N.A. |
| ↓ platelet adhesion collagen | ||||
| ↓ thrombus formation in vitro collagen | ||||
| = platelet response to thrombin | ||||
| = platelet adhesion to fibrinogen | ||||
|
Phox-I |
↓ platelet activation in vitro and ex vivo ↓ platelet adhesion in vivo = bleeding |
[255] |
N.A. |
N.A. |
| VAS2870 and VAS2870 |
↓ platelet aggregation ↓ platelet granule release ↓ GPIIbIIIa activation = bleeding ↓ arterial thrombosis in vivo |
[256] | N.A. | N.A. |
| ↓ platelet adhesion ex vivo | [257] | |||
| ↓ vascular reactivity | [258] | |||
Apocynin is a naturally occurring methoxy-substituted catechol, extracted from the roots of Picrorhiza kurroa and was one of the first NOX inhibitors described. Apocynin prevents the shift of p47phox subunit from the cytosol to the plasma membrane as well as the overexpression of NADPH oxidase subunits [259,260].
Apocynin is a compound extensively studied as a NOX inhibitor in experimental models of cardiovascular disease and depression.
It prevents platelet aggregation triggered by various stimuli (e.g., collagen, thrombin, and ADP) [148], significantly decreases thrombin-induced GPIIbIIIa activation [261], reduces the thrombus formation on collagen under high shear stress [261,245], and prevents platelet adhesion in subject with advanced atherosclerotic plaques [223].
In endothelial cells, the activity of NOX represents an important signaling pathway, and complete inhibition of this enzyme could lead to deleterious effects in cells and tissues [248]. In this regard, apocynin could be considered sufficiently safe, since the levels of myeloperoxidase, the enzyme that converts the prodrug into its active form, are very low in blood vessels and are raised fivefold in cardiovascular disease [249]. Therefore, it is likely that apocynin has no effect on the endothelium under physiological conditions, when endothelial NOX levels are low, and only inhibits excessive NOX activity under pathological conditions. Specifically, apocynin prevents endothelial dysfunction by not only inhibiting NOX activity but also affecting NO signal [251], resulting in an antithrombotic phenotype.
We recently showed that apocynin reduces the formation of ROS and the NOX1 levels in bone marrow megakaryocytes, decreases platelet hyperactivity and plasmatic levels of malondialdehyde and prevents arterial thrombosis in stressed mice [16]. In addition, we reported that apocynin is able to reduce the increase of oxidative stress, p47phox and p67phox in the hippocampus in an experimental animal model of depression [17]. Moreover, apocynin prevents stress-induced epigenetic and behavioral changes [17]. Consistent with our data, several other studies have described the antidepressant properties of apocynin in various stress-induced experimental paradigms of depression by reducing levels of oxidative stress in the hippocampus, prefrontal cortex and striatum [103,105,227,253]. Interestingly, apocynin also reduces the depression-like phenotype in nonstress-related depression models such as the progressive multiple sclerosis and acute lung injury mouse models by reducing levels of oxidative stress in the hippocampus and amygdala [243,246]. Several studies have shown that apocynin is able to reduce the NF-κB signaling pathway, inflammasome activation, neuroinflammation and microglial activation [247,252]. In addition, apocynin protects against autophagy activation and neuronal cell death [252,250].
Celastrol is an orange triterpene obtained from Tripterygium wilfordii Hook F. (Thunder God vine) and Celastrus regelii. This compound is a NOX inhibitor that acts on the interaction between p47phox and p22phox and is a promising inhibitor of platelet activation. It inhibits the expression of P-selectin promoted by thrombin, ADP, and 12-myristate-13-acetate and GPIIbIIIa expression in platelets stimulated with ADP. In addition, celastrol moderately reduces platelet adhesion to fibrinogen and aggregation induced by ADP [262]. Interestingly, Ouyang et al. reported that this molecule reduces thrombosis in thrombogenic mice administered with high-fat diet [263]. Inhibition of NOX2 by celastrol is also able to inhibit Ang–II–mediated endothelial dysfunction [254].
Few works have investigated the potential function of celastrol in animal models of depression. In one study, celastrol administration reduced the depression-like phenotype in a mouse model of the comorbidity of obesity and depression. These behavioral changes are associated with the suppression of the overexpression of TNFα and the downregulation of BDNF [264]. In addition, celastrol showed antidepressant effects in a winter depressive-like model in medaka fish [265]. It has been also demonstrated that celastrol is able to reduced oxidative stress, production of IL1β, activation of NF-κB, glia activation and neurodegeneration [250].
Ebselen, a synthetic organoselenium molecule with anti-inflammatory, antioxidant, and cytoprotective activity, is another compound that has been studied as a NOX inhibitor [266]. Ebselen was shown to prevent the translocation of p47phox into membranes and its interaction with p22phox [255].
This molecule is able to inhibit aspirin-mediated increases in intracellular calcium in human platelets [267] and, more importantly, reduces cardiovascular risk in animal models. Specifically, ebselen attenuated platelet aggregation in a mouse model of chronic kidney disease known to be at higher risk for developing CVD [256]. In addition, ebselen delayed and/or completely prevented venous and arteriolar thrombus formation in photochemical and ferric chloride thrombosis models [257]. Interestingly, ebselen reduces aortic lesions in diabetic ApoE−/− mice by decreasing NOX2 levels in macrophages, suggesting its potential therapeutic role in atherothrombotic disease [258]. Although ebselen has been suggested as a potential treatment for depressed patients resistant to drug treatments [268], its efficacy as an antidepressant has been little studied. Ebselen rescues depressive-like phenotypes in CREB-regulated transcriptional coactivator-1 knockout mice, a gene involved in regulating brain metabolism and associated with psychiatric disorders [269]. Moreover, it has been reported that ebselen can modulate oxidative stress levels, apoptotic pathway, adult hippocampal neurogenesis, synaptic protein levels, and long-term potentiation in the brain of different animal model [[270], [271], [272], [273], [274]].
Suramin, a purinergic receptor antagonist used as an antiparasitic agent, inhibits NOX2 activity by competitive inhibition [275].
Suramin completely blocks platelet aggregation induced by platelet-activating factor, thrombin, arachidonic acid, and alkyllysophosphatic acid. Calcium mobilization mediated by thrombin was also inhibited by this compound [276]. It also reduced venous thrombus formation in mouse models [277]. Studies in leukocyte cells have also focused on suramin therapeutic effect in atherosclerosis-related events. Specifically, it inhibits oxLDL-induced adhesion of monocyte cell lines and oxLDL-associated ROS production and induction of NOX4 [278].
No specific studies have been performed to assess the potential role of suramin as an antidepressant. However, suramin improved social behavior and memory performance [279,280], two features common in depressed patients. It is noteworthy that suramin reduces the expression and release of microglial cytokines, oxidative stress and neuronal cell death in vitro [281,282] as well as the activation of microglia [283].
Perhexiline is an anti-anginal agent working as a noncompetitive inhibitor of NOX2, although the precise mechanism of action has not yet been identified [275].
Perhexiline is associated with increased responsiveness to NO. Specifically, in platelets, it enhances the effect of the NO donor sodium nitroprusside, which inhibits aggregation [284].
Only one study examined the effect of perhexiline in endothelial and leukocyte cells, showing that it can inhibit the preassembled NOX complex in neutrophil and human umbilical vein endothelial cells [73]. The relevance of these findings to specific pathologies has not yet been investigated.
No data are available on the possible effects of perhexiline on depression. Only one article describes how two patients taking fluoxetine and paroxetine, selective serotonin reuptake medications, suffered severe toxicity from perhexiline [285].
Berberine is an ammonium salt of alkaloid found in various plants such as Berberidacea, Coptis, and Hydrastis. Berberine has promising therapeutic effects on CVDs including atherosclerosis, myocardial infarction heart failure, cardiac hypertrophy, and hypertension [260,286] and could be a natural compound useful in the treatment of depression [287].
Berberine has a protective function in cardiovascular cells by inhibiting NOXs, particularly NOX4. Treatment with berberine decreased ROS plasma levels in healthy humans and inhibited NOX4 upregulation and ROS production in cultured endothelial cells [288].
In addition, berberine was reported to inhibit platelet aggregation, ROS production and NADPH oxidase activation. It can also act synergistically with the no specific inhibitor of NOX (DPI/VAS2870) and reduce agonist-induced platelet aggregation [289].
The role of berberine on leukocyte cells has been mainly associated with autoimmune, inflammatory, or cancer diseases. However, recent studies have shown that berberine is able to decrease oxLDL-induced macrophage activation [290], to reduce adhesion of leukocyte to LPS-treated endothelial cells [291], and to protect against endothelial injury and dysfunction [292]. All these data could suggest that berberine may also be beneficial in atherothrombotic disease, although none of the previous studies directly linked these effects to NOX inhibition.
The antidepressant effects of berberine have been demonstrated especially in stress-related experimental models of depression. Berberine reduces depressive-like phenotypes in stressed rodents by rising the production of dopamine, norepinephrine, serotonin, and BDNF [293,294]. In addition, berberine prevented depressive-like phenotypes by reducing oxidative stress, kynurenine metabolism, and neuroinflammation in the hippocampus of mice exposed to chronic stress [188,295]. Berberine also reduced depressive-like phenotypes in rat models of chronic pain and diabetes, and this was paralleled by the reduction of oxidative stress in several brain areas important for mood regulation [296,297]. Berberine also promotes neuroprotective effects by reducing oxidative stress, mitochondrial dysfunction and inflammatory reactions and controlling autophagy [298].
Among the synthetic compounds that may affect NOX activity, they have been studied only in CVD models, and currently no data are available on their possible effect on depression.
2-acetylphenothiazine (2-APT, also known as ML171) is a NOX inhibitor that significantly blocks the NOX1-dependent production of ROS and has only marginal effects on the other NOXs [299].
It has been consistently reported that 2-APT blocks superoxide anion production, platelet aggregation, adhesion, and thrombus formation in a collagen-dependent manner without effects on platelet response to thrombin or platelet adhesion to fibrinogen [74,192,300]. Similar results were also obtained with a 2-APT derivative, 1-(10H-phenothiazin-2-yl) vinyl tert-butyl carbonate (2APT-D6) [192].
2-APT, together with other compounds that have higher specificity for NOX1 and/or NOX2 than for NOX4 [299,301,302], represent a good therapeutic agent that can be used in endothelial vascular pathogenesis because they preserve NOX4 activity, which plays a beneficial role in endothelial function. However, very little evidence is available in vivo, so they are far from true therapeutic use.
Phox-I is a novel small molecule specifically designed to inhibit the interaction of Rac1 with p67phox [303]. Phox-I inhibits ROS production and platelet activation in vitro and ex vivo experiments, and platelet adhesion and accumulation in vivo models, without altering the hemostatic response to damage [304].
VAS2870 and its derivative VAS3947 are pan-NOX inhibitors that promote conformational changes and block formation of the NOX multicomplex without affecting xanthine oxidase activity, eNOS, and ROS scavenger [305].
VAS2870 and VAS3947 decreased collagen- and thrombin-induced platelet aggregation in mice, blocked downstream PKC signaling, decreased calcium mobilization, GPIIbIIIa activation, and platelet granule release. In vivo, administration of VAS reduced thrombus formation without impairing regular hemostasis [306]. In ex vivo studies, VAS2870 inhibited human platelet adhesion under static settings and thrombus formation under physiological flow settings promoted by Aβ1-42 treatment [307].
Specifically, VAS2870 was reported to prevent carotid artery reactivity promoted by chronic stress in rats [308].
7. Open question and conclusions
CVDs and depression are both common. Compared to the general population, CVD patients suffer more frequently from depression. In addition, depressed patients have a higher mortality rate and a higher risk of cardiovascular disease in later life compared to the general population. The outcomes of CVD patients who are also depressed are worse than those of people who are not depressed. The risk of CVDs and subsequent mortality varies according to the severity of depression [171,[309], [310], [311], [312], [313], [314], [315], [316], [317], [318], [319]]. In addition, CVDs and depression share some common risk factors, such as stressful experiences, obesity, smoking, sedentary lifestyle, and chronic inflammation. Unfortunately, the two pathologies have been studied almost exclusively, both in clinical studies and in preclinical experimental models: as consequence bidirectionality of the relationship between CVDs and depression makes this subject particularly complex. Indeed, it has been suggested that both depression is a risk factor for CVDs and that CVDs is a risk factor for depression [320] (Fig. 1, Fig. 3).
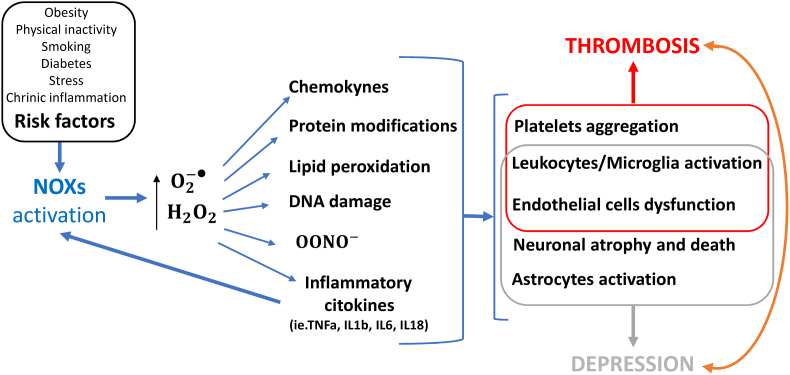
Fig. 3.
Molecular and cellular mechanisms regulated by NOXs in thrombosis and depression
Environmental factors can regulate the activity of NADPH oxidases (NOXs) and promote the production of superoxide () and hydrogen peroxide (H2O2), which promote the formation of peroxynitrite anions , the production of chemokines and cytokines, protein modifications, lipid peroxidation and DNA damage, which in turn can modulate the activity of platelets, leukocytes, microglia, endothelial cells, neurons and astrocytes, ultimately favoring the occurrence of thrombosis and depression.
To the best of our knowledge, there is no epidemiological information available regarding how the above-mentioned NOX inhibitors affect the association between depression and thrombosis. Nevertheless, some clinical trials have been conducted or are underway aimed at separately defining the effect of these compounds on cardiovascular disease and depressive disorders. For berberine and perhexiline in particular, the studies focus on CVD, while for ebselen there is only one study on depressive symptoms (Table 3). Further studies are needed to understand whether these molecules might be effective in reducing thrombosis-depression comorbidity.
Table 3.
Clinical trials on NOX inhibitor and cardiovascular or depressive disease.
| Compounds | Condition | Treatment | Recruitment status | ClinicalTrials.gov ID |
|---|---|---|---|---|
| Perhexiline | Hypertrophic Cardiomyopathy | Perhexiline vs Placebo | Unknown status | NCT04426578 |
| Perhexiline | Diabetes Cardiomyopathy | Perhexiline vs Placebo | Unknown status | NCT00628056 |
| Perhexiline | Hypertrophic Cardiomyopathy | Perhexiline vs Placebo | Withdrawn | NCT02431221 |
| Perhexiline | Diastolic Heart Failure | Perhexiline vs Placebo | Completed | NCT00839228 |
| Perhexiline | Hypertrophic Cardiomyopathy | Perhexiline vs Placebo | Completed | NCT00500552 |
| Perhexiline | Hypertrophic Cardiomyopathy | Perhexiline vs Placebo | Completed | NCT00841139 |
| Perhexiline | Hypertrophic Cardiomyopathy | Perhexiline vs Placebo | Completed | NCT02862600 |
| Perhexiline | Myocardial Reperfusion Injury | Perhexiline vs Placebo | Completed | NCT00845364 |
| Perhexiline | Myocardial Reperfusion Injury | Perhexiline vs Placebo | Unknown status | NCT00989508 |
| Berberine | Cardiovascular Risk Factor | Berberine vs Placebo | Completed | NCT03770325 |
| Berberine | Metabolic Syndrome | Berberine | Not yet recruiting | NCT05105321 |
| Berberine | Dyslipidemia, Hypertension, Prediabetes | Berberine vs Placebo | Not yet recruiting | NCT05749874 |
| Berberine | Stable coronary artery disease | Berberine, Aspirin, Clopidogrel, Statin | Unknown status | NCT04434365 |
| Berberine | Coronary artery disease | Berberine, Aspirin, Clopidogrel | Recruiting | NCT03378934 |
| Berberine | Hypertension, Endothelial dysfunction | Berberine vs Placebo | Unknown status | NCT04790942 |
| Ebselen | Depressive Disorder | Ebselen vs Placebo | Recruiting | NCT05117710 |
As for drugs currently used to treat depression and thrombosis, there is evidence of their effectiveness in inhibiting NOX activation. In particular, there is evidence that statins block platelet activity by inhibiting platelet NOX formation of ROS [155] and that aspirin reduces the production of ROS via a reduction in NOX4 expression in endothelial cells [[321], [322], [323]] and microglial cells [323].
Similarly, antidepressants such as fluoxetine and mirtazapine can reduce the activity and expression of the NOX subunits and the subsequent production of ROS in microglial cells [53,324,325].
We have provided here a comprehensive overview of how the overactivation of NOX and the consequent generation of ROS is a possible common mechanism in CVDs, especially thrombosis, and depression. Indeed, the common risk factors underlying CVDs and depression may promote NOX activation and production of ROS, contributing to both diseases (Fig. 3). Therefore, it would be important to investigate whether inhibition of NOX by pharmacological treatments could be effective in preventing and treating the comorbidity of thrombosis and depression. More importantly, addressing cardiovascular health and mental well-being together may be beneficial in the prevention and treatment of both conditions.
CRediT authorship contribution statement
Patrizia Amadio: Writing – original draft, Conceptualization. Leonardo Sandrini: Writing – original draft, Conceptualization. Marta Zarà: Writing – review & editing. Silvia S. Barbieri: Writing – review & editing, Conceptualization. Alessandro Ieraci: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was partially supported by Fondazione Cariplo, Italy (2018–0525), and Italian Ministry of Health, Rome, Italy (Ricerca Corrente RC 2022, Ricerca Finalizzata RF2019_12370907) to SSB.
Contributor Information
Silvia S. Barbieri, Email: silvia.barbieri@cardiologicomonzino.it.
Alessandro Ieraci, Email: alessandro.ieraci@uniecampus.it.
Data availability
No data was used for the research described in the article.
References
- 1.Malhi G.S., Mann J.J. Depression. Lancet. 2018;392:2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 2.Sobolewska-Nowak J., Wachowska K., Nowak A., Orzechowska A., Szulc A., Plaza O., Galecki P. Exploring the heart-mind connection: unraveling the shared pathways between depression and cardiovascular diseases. Biomedicines. 2023;11 doi: 10.3390/biomedicines11071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hare D.L., Toukhsati S.R., Johansson P., Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur. Heart J. 2014;35:1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- 4.Dhar A.K., Barton D.A. Depression and the link with cardiovascular disease. Front. Psychiatr. 2016;7:33. doi: 10.3389/fpsyt.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Favor J.D., Dubis G.S., Yan H., White J.D., Nelson M.A., Anderson E.J., Hickner R.C. Microvascular endothelial dysfunction in sedentary, obese humans is mediated by NADPH oxidase: influence of exercise training. Arterioscler. Thromb. Vasc. Biol. 2016;36:2412–2420. doi: 10.1161/ATVBAHA.116.308339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morawietz H., Brendel H., Diaba-Nuhoho P., Catar R., Perakakis N., Wolfrum C., Bornstein S.R. Cross-talk of NADPH oxidases and inflammation in obesity. Antioxidants. 2023;12 doi: 10.3390/antiox12081589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youn J.Y., Siu K.L., Lob H.E., Itani H., Harrison D.G., Cai H. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes. 2014;63:2344–2355. doi: 10.2337/db13-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasipe B., Li S., Laher I. Exercise and vascular function in sedentary lifestyles in humans. Pflügers Archiv. 2023;475:845–856. doi: 10.1007/s00424-023-02828-6. [DOI] [PubMed] [Google Scholar]
- 10.Laufs U., Wassmann S., Czech T., Munzel T., Eisenhauer M., Bohm M., Nickenig G. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005;25:809–814. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 11.Barbieri S.S., Amadio P., Gianellini S., Zacchi E., Weksler B.B., Tremoli E. Tobacco smoke regulates the expression and activity of microsomal prostaglandin E synthase-1: role of prostacyclin and NADPH-oxidase. Faseb. J. 2011;25:3731–3740. doi: 10.1096/fj.11-181776. [DOI] [PubMed] [Google Scholar]
- 12.Carnevale R., Loffredo L., Pignatelli P., Nocella C., Bartimoccia S., Di Santo S., Martino F., Catasca E., Perri L., Violi F. Dark chocolate inhibits platelet isoprostanes via NOX2 down-regulation in smokers. J. Thromb. Haemostasis. 2012;10:125–132. doi: 10.1111/j.1538-7836.2011.04558.x. [DOI] [PubMed] [Google Scholar]
- 13.Rafacho B.P., Azevedo P.S., Polegato B.F., Fernandes A.A., Bertoline M.A., Fernandes D.C., Chiuso-Minicucci F., Roscani M.G., Dos Santos P.P., Matsubara L.S., Matsubara B.B., Laurindo F.R., Paiva S.A., Zornoff L.A., Minicucci M.F. Tobacco smoke induces ventricular remodeling associated with an increase in NADPH oxidase activity. Cell. Physiol. Biochem. 2011;27:305–312. doi: 10.1159/000327957. [DOI] [PubMed] [Google Scholar]
- 14.Farhan A., Hassan G., Ali S.H.L., Yousaf Z., Shafique K., Faisal A., Younis B.B., Mirza S. Spontaneous NETosis in diabetes: a role of hyperglycemia mediated ROS and autophagy. Front. Med. 2023;10 doi: 10.3389/fmed.2023.1076690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasce A., Gariani K., Jornayvaz F.R., Szanto I. NADPH oxidases connecting fatty liver disease, insulin resistance and type 2 diabetes: current knowledge and therapeutic outlook. Antioxidants. 2022;11 doi: 10.3390/antiox11061131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandrini L., Ieraci A., Amadio P., Popoli M., Tremoli E., Barbieri S.S. Apocynin prevents abnormal megakaryopoiesis and platelet activation induced by chronic stress. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/9258937. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbieri S.S., Sandrini L., Musazzi L., Popoli M., Ieraci A. Apocynin prevents anxiety-like behavior and histone deacetylases overexpression induced by sub-chronic stress in mice. Biomolecules. 2021;11 doi: 10.3390/biom11060885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiavone S., Jaquet V., Sorce S., Dubois-Dauphin M., Hultqvist M., Backdahl L., Holmdahl R., Colaianna M., Cuomo V., Trabace L., Krause K.H. NADPH oxidase elevations in pyramidal neurons drive psychosocial stress-induced neuropathology. Transl. Psychiatry. 2012;2:e111. doi: 10.1038/tp.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cukic M., Savic D., Sidorova J. When heart beats differently in depression: review of nonlinear heart rate variability measures. JMIR Ment Health. 2023;10 doi: 10.2196/40342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiga T. Depression and cardiovascular diseases. J. Cardiol. 2022 doi: 10.1016/j.jjcc.2022.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Vaccarino V., Badimon L., Bremner J.D., Cenko E., Cubedo J., Dorobantu M., Duncker D.J., Koller A., Manfrini O., Milicic D., Padro T., Pries A.R., Quyyumi A.A., Tousoulis D., Trifunovic D., Vasiljevic Z., de Wit C., Bugiardini R., E.S.C.S.D.G. Reviewers Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. Eur. Heart J. 2020;41:1687–1696. doi: 10.1093/eurheartj/ehy913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtman J.H., Froelicher E.S., Blumenthal J.A., Carney R.M., Doering L.V., Frasure-Smith N., Freedland K.E., Jaffe A.S., Leifheit-Limson E.C., Sheps D.S., Vaccarino V., Wulsin L., American Heart Association Statistics Committee of the Council on, Prevention, C. the Council on, N. Stroke Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350–1369. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 23.Lin C.E., Chung C.H., Chen L.F., Chien W.C. Increased risk for venous thromboembolism among patients with concurrent depressive, bipolar, and schizophrenic disorders. Gen. Hosp. Psychiatr. 2019;61:34–40. doi: 10.1016/j.genhosppsych.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen H., Horvath-Puho E., Laugesen K., Braekkan S.K., Hansen J.B., Sorensen H.T. Venous thromboembolism and risk of depression: a population-based cohort study. J. Thromb. Haemostasis. 2023;21:953–962. doi: 10.1016/j.jtha.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Millett C.E., Phillips B.E., Saunders E.F.H. The sex-specific effects of LPS on depressive-like behavior and oxidative stress in the Hippocampus of the mouse. Neuroscience. 2019;399:77–88. doi: 10.1016/j.neuroscience.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Petkovic A., Chaudhury D. Encore: behavioural animal models of stress, depression and mood disorders. Front. Behav. Neurosci. 2022;16 doi: 10.3389/fnbeh.2022.931964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J., Kim Y.K. Animal models for the study of depressive disorder. CNS Neurosci. Ther. 2021;27:633–642. doi: 10.1111/cns.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin R., Zhang K., Li Y., Tang Z., Zheng R., Ma Y., Chen Z., Lei N., Xiong L., Guo P., Li G., Xie Y. Lipopolysaccharide-induced depression-like model in mice: meta-analysis and systematic evaluation. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1181973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S., Piao L., Wan Y., Huang Z., Meng X., Inoue A., Wang H., Yue X., Jin X., Shi G.P., Kuzuya M., Cheng X.W. CTSS modulates stress-related carotid artery thrombosis in a mouse FeCl(3) model. Arterioscler. Thromb. Vasc. Biol. 2023;43:e238–e253. doi: 10.1161/ATVBAHA.122.318455. [DOI] [PubMed] [Google Scholar]
- 30.Wang X. Lipopolysaccharide augments venous and arterial thrombosis in the mouse. Thromb. Res. 2008;123:355–360. doi: 10.1016/j.thromres.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Stampfli S.F., Camici G.G., Keller S., Rozenberg I., Arras M., Schuler B., Gassmann M., Garcia I., Luscher T.F., Tanner F.C. Restraint stress enhances arterial thrombosis in vivo--role of the sympathetic nervous system. Stress. 2014;17:126–132. doi: 10.3109/10253890.2013.862616. [DOI] [PubMed] [Google Scholar]
- 32.Sandrini L., Ieraci A., Amadio P., Veglia F., Popoli M., Lee F.S., Tremoli E., Barbieri S.S. Sub-chronic stress exacerbates the pro-thrombotic phenotype in BDNF(Val/Met) mice: gene-environment interaction in the modulation of arterial thrombosis. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19103235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng G., Yang X., Li Y., Wang X., Tan S., Chen F. LPS enhances platelets aggregation via TLR4, which is related to mitochondria damage caused by intracellular ROS, but not extracellular ROS. Cell. Immunol. 2018;328:86–92. doi: 10.1016/j.cellimm.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Dong T., Cheng Y.W., Yang F., Sun P.W., Zhu C.J., Zhu L., Zhang G.X. Chronic stress facilitates the development of deep venous thrombosis. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/384535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frey A., Popp S., Post A., Langer S., Lehmann M., Hofmann U., Siren A.L., Hommers L., Schmitt A., Strekalova T., Ertl G., Lesch K.P., Frantz S. Experimental heart failure causes depression-like behavior together with differential regulation of inflammatory and structural genes in the brain. Front. Behav. Neurosci. 2014;8:376. doi: 10.3389/fnbeh.2014.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun N., Mei Y., Hu Z., Xing W., Lv K., Hu N., Zhang T., Wang D. Ghrelin attenuates depressive-like behavior, heart failure, and neuroinflammation in postmyocardial infarction rat model. Eur. J. Pharmacol. 2021;901 doi: 10.1016/j.ejphar.2021.174096. [DOI] [PubMed] [Google Scholar]
- 37.Kwapong Y.A., Boakye E., Khan S.S., Honigberg M.C., Martin S.S., Oyeka C.P., Hays A.G., Natarajan P., Mamas M.A., Blumenthal R.S., Blaha M.J., Sharma G. Association of depression and poor mental health with cardiovascular disease and suboptimal cardiovascular health among young adults in the United States. J. Am. Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strine T.W., Mokdad A.H., Dube S.R., Balluz L.S., Gonzalez O., Berry J.T., Manderscheid R., Kroenke K. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen. Hosp. Psychiatr. 2008;30:127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 39.van Gool C.H., Kempen G.I., Penninx B.W., Deeg D.J., Beekman A.T., van Eijk J.T. Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: results from the Longitudinal Aging Study Amsterdam. Age Ageing. 2003;32:81–87. doi: 10.1093/ageing/32.1.81. [DOI] [PubMed] [Google Scholar]
- 40.Velten J., Lavallee K.L., Scholten S., Meyer A.H., Zhang X.C., Schneider S., Margraf J. Lifestyle choices and mental health: a representative population survey. BMC Psychol. 2014;2:58. doi: 10.1186/s40359-014-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lloyd-Jones D.M., Hong Y., Labarthe D., Mozaffarian D., Appel L.J., Van Horn L., Greenlund K., Daniels S., Nichol G., Tomaselli G.F., Arnett D.K., Fonarow G.C., Ho P.M., Lauer M.S., Masoudi F.A., Robertson R.M., Roger V., Schwamm L.H., Sorlie P., Yancy C.W., Rosamond W.D., American Heart Association Strategic Planning Task, C. Statistics Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 42.Penninx B.W. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neurosci. Biobehav. Rev. 2017;74:277–286. doi: 10.1016/j.neubiorev.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Amadio P., Zara M., Sandrini L., Ieraci A., Barbieri S.S. Depression and cardiovascular disease: the viewpoint of platelets. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21207560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huffman J.C., Celano C.M., Beach S.R., Motiwala S.R., Januzzi J.L. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013;2013 doi: 10.1155/2013/695925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Pol A., van Gilst W.H., Voors A.A., van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur. J. Heart Fail. 2019;21:425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindqvist D., Dhabhar F.S., James S.J., Hough C.M., Jain F.A., Bersani F.S., Reus V.I., Verhoeven J.E., Epel E.S., Mahan L., Rosser R., Wolkowitz O.M., Mellon S.H. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76:197–205. doi: 10.1016/j.psyneuen.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhatt S., Nagappa A.N., Patil C.R. Role of oxidative stress in depression. Drug Discov. Today. 2020;25:1270–1276. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Kattoor A.J., Pothineni N.V.K., Palagiri D., Mehta J.L. Oxidative stress in atherosclerosis. Curr. Atherosclerosis Rep. 2017;19:42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 49.Samman Tahhan A., Sandesara P.B., Hayek S.S., Alkhoder A., Chivukula K., Hammadah M., Mohamed-Kelli H., O'Neal W.T., Topel M., Ghasemzadeh N., Ko Y.A., Aida H., Gafeer M., Sperling L., Vaccarino V., Liang Y., Jones D.P., Quyyumi A.A. Association between oxidative stress and atrial fibrillation. Heart Rhythm. 2017;14:1849–1855. doi: 10.1016/j.hrthm.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsutsui H., Kinugawa S., Matsushima S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 51.Brieger K., Schiavone S., Miller F.J., Jr., Krause K.H. Reactive oxygen species: from health to disease. Swiss Med. Wkly. 2012;142 doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 52.Panth N., Paudel K.R., Parajuli K. Reactive oxygen species: a key hallmark of cardiovascular disease. Adv. Met. Med. 2016 doi: 10.1155/2016/9152732. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miao R., Wang L., Chen Z., Ge S., Li L., Zhang K., Chen Y., Guo W., Duan X., Zhu M., Zhao G., Lin F. Advances in the study of nicotinamide adenine dinucleotide phosphate oxidase in myocardial remodeling. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.1000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delaney M.K., Kim K., Estevez B., Xu Z., Stojanovic-Terpo A., Shen B., Ushio-Fukai M., Cho J., Du X. Differential roles of the NADPH-oxidase 1 and 2 in platelet activation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2016;36:846–854. doi: 10.1161/ATVBAHA.116.307308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuentes E., Gibbins J.M., Holbrook L.M., Palomo I. NADPH oxidase 2 (NOX2): a key target of oxidative stress-mediated platelet activation and thrombosis. Trends Cardiovasc. Med. 2018;28:429–434. doi: 10.1016/j.tcm.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Li J.M., Shah A.M. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J. Biol. Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 57.Meza C.A., La Favor J.D., Kim D.H., Hickner R.C. Endothelial dysfunction: is there a hyperglycemia-induced imbalance of NOX and NOS? Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petry A., Djordjevic T., Weitnauer M., Kietzmann T., Hess J., Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxidants Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 59.Van Buul J.D., Fernandez-Borja M., Anthony E.C., Hordijk P.L. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxidants Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 60.Bokoch G.M., Knaus U.G. NADPH oxidases: not just for leukocytes anymore. Trends Biochem. Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 61.Serrano F., Kolluri N.S., Wientjes F.B., Card J.P., Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- 62.Accapezzato D., Visco V., Francavilla V., Molette C., Donato T., Paroli M., Mondelli M.U., Doria M., Torrisi M.R., Barnaba V. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J. Exp. Med. 2005;202:817–828. doi: 10.1084/jem.20051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arthur J.F., Qiao J., Shen Y., Davis A.K., Dunne E., Berndt M.C., Gardiner E.E., Andrews R.K. ITAM receptor-mediated generation of reactive oxygen species in human platelets occurs via Syk-dependent and Syk-independent pathways. J. Thromb. Haemostasis. 2012;10:1133–1141. doi: 10.1111/j.1538-7836.2012.04734.x. [DOI] [PubMed] [Google Scholar]
- 64.Podrez E.A., Byzova T.V., Febbraio M., Salomon R.G., Ma Y., Valiyaveettil M., Poliakov E., Sun M., Finton P.J., Curtis B.R., Chen J., Zhang R., Silverstein R.L., Hazen S.L. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nieswandt B., Watson S.P. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 66.Colas R., Sassolas A., Guichardant M., Cugnet-Anceau C., Moret M., Moulin P., Lagarde M., Calzada C. LDL from obese patients with the metabolic syndrome show increased lipid peroxidation and activate platelets. Diabetologia. 2011;54:2931–2940. doi: 10.1007/s00125-011-2272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davi G., Guagnano M.T., Ciabattoni G., Basili S., Falco A., Marinopiccoli M., Nutini M., Sensi S., Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 68.Bartimoccia S., Carnevale R., Sanguigni V., De Falco E., Frati G., Loffredo L., Plebani A., Soresina A., Pignatelli P., Violi F. NOX 5 is expressed in platelets from patients with chronic granulomatous disease. Thromb. Haemostasis. 2016;116:198–200. doi: 10.1160/TH15-12-0999. [DOI] [PubMed] [Google Scholar]
- 69.Seno T., Inoue N., Gao D., Okuda M., Sumi Y., Matsui K., Yamada S., Hirata K.I., Kawashima S., Tawa R., Imajoh-Ohmi S., Sakurai H., Yokoyama M. Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb. Res. 2001;103:399–409. doi: 10.1016/s0049-3848(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 70.Nisimoto Y., Diebold B.A., Cosentino-Gomes D., Lambeth J.D. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J.E., Kwon H.J., Choi J., Seo J.S., Han P.L. Aging increases vulnerability to stress-induced depression via upregulation of NADPH oxidase in mice. Commun. Biol. 2020;3:292. doi: 10.1038/s42003-020-1010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plaza-Briceno W., Estay S.F., de la Fuente-Ortega E., Gutierrez C., Sanchez G., Hidalgo C., Chavez A.E., Haeger P.A. N-Methyl-d-Aspartate receptor modulation by nicotinamide adenine dinucleotide phosphate oxidase type 2 drives synaptic plasticity and spatial memory impairments in rats exposed pre- and postnatally to ethanol. Antioxidants Redox Signal. 2020;32:602–617. doi: 10.1089/ars.2019.7787. [DOI] [PubMed] [Google Scholar]
- 73.Kennedy J.A., Beck-Oldach K., McFadden-Lewis K., Murphy G.A., Wong Y.W., Zhang Y., Horowitz J.D. Effect of the anti-anginal agent, perhexiline, on neutrophil, valvular and vascular superoxide formation. Eur. J. Pharmacol. 2006;531:13–19. doi: 10.1016/j.ejphar.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 74.Vara D., Campanella M., Pula G. The novel NOX inhibitor 2-acetylphenothiazine impairs collagen-dependent thrombus formation in a GPVI-dependent manner. Br. J. Pharmacol. 2013;168:212–224. doi: 10.1111/j.1476-5381.2012.02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen J., Tang H., Hay N., Xu J., Ye R.D. Akt isoforms differentially regulate neutrophil functions. Blood. 2010;115:4237–4246. doi: 10.1182/blood-2009-11-255323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Badimon L., Padro T., Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care. 2012;1:60–74. doi: 10.1177/2048872612441582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koupenova M., Kehrel B.E., Corkrey H.A., Freedman J.E. Thrombosis and platelets: an update. Eur. Heart J. 2017;38:785–791. doi: 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nieswandt B., Pleines I., Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J. Thromb. Haemostasis. 2011;9(Suppl 1):92–104. doi: 10.1111/j.1538-7836.2011.04361.x. [DOI] [PubMed] [Google Scholar]
- 80.Wang M., Hao H., Leeper N.J., Zhu L., Early Career C. Thrombotic regulation from the endothelial cell perspectives. Arterioscler. Thromb. Vasc. Biol. 2018;38:e90–e95. doi: 10.1161/ATVBAHA.118.310367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yau J.W., Teoh H., Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015;15:130. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swystun L.L., Liaw P.C. The role of leukocytes in thrombosis. Blood. 2016;128:753–762. doi: 10.1182/blood-2016-05-718114. [DOI] [PubMed] [Google Scholar]
- 83.Owens A.P., 3rd, Mackman N. Tissue factor and thrombosis: the clot starts here. Thromb. Haemostasis. 2010;104:432–439. doi: 10.1160/TH09-11-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carnevale R., Loffredo L., Sanguigni V., Plebani A., Rossi P., Pignata C., Martire B., Finocchi A., Pietrogrande M.C., Azzari C., Soresina A.R., Martino S., Cirillo E., Martino F., Pignatelli P., Violi F. Different degrees of NADPH oxidase 2 regulation and in vivo platelet activation: lesson from chronic granulomatous disease. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dayal S., Wilson K.M., Motto D.G., Miller F.J., Jr., Chauhan A.K., Lentz S.R. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation. 2013;127:1308–1316. doi: 10.1161/CIRCULATIONAHA.112.000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magwenzi S., Woodward C., Wraith K.S., Aburima A., Raslan Z., Jones H., McNeil C., Wheatcroft S., Yuldasheva N., Febbriao M., Kearney M., Naseem K.M. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood. 2015;125:2693–2703. doi: 10.1182/blood-2014-05-574491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hassamal S. Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatr. 2023;14 doi: 10.3389/fpsyt.2023.1130989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jeon S.W., Kim Y.K. Neuron-microglia crosstalk in neuropsychiatric disorders. Adv. Exp. Med. Biol. 2023;1411:3–15. doi: 10.1007/978-981-19-7376-5_1. [DOI] [PubMed] [Google Scholar]
- 89.Duman R.S., Sanacora G., Krystal J.H. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102:75–90. doi: 10.1016/j.neuron.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neuroinflammation . 1 ed. Springer Singapore; 2023. Gut-Brain Axis and Immunity in Neuropsychiatric Disorders. [Google Scholar]
- 91.Durkee C., Kofuji P., Navarrete M., Araque A. Astrocyte and neuron cooperation in long-term depression. Trends Neurosci. 2021;44:837–848. doi: 10.1016/j.tins.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H., He Y., Sun Z., Ren S., Liu M., Wang G., Yang J. Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflammation. 2022;19:132. doi: 10.1186/s12974-022-02492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 94.Amadio P., Macchi C., Favero C., Zara M., Solazzo G., Dioni L., Sandrini L., Vigna L., Greco M.F., Buoli M., Sirtori C.R., Pesatori A.C., Ieraci A., Ruscica M., Barbieri S.S., Bollati V. Brain-derived neurotrophic factor and extracellular vesicle-derived miRNAs in an Italian cohort of individuals with obesity: a key to explain the link between depression and atherothrombosis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.906483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bjorkholm C., Monteggia L.M. Bdnf - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castren E., Monteggia L.M. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol. Psychiatr. 2021;90:128–136. doi: 10.1016/j.biopsych.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 97.Gawryluk J.W., Wang J.F., Andreazza A.C., Shao L., Young L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- 98.Salim S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Therapeut. 2017;360:201–205. doi: 10.1124/jpet.116.237503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Velzen L.S., Wijdeveld M., Black C.N., van Tol M.J., van der Wee N.J.A., Veltman D.J., Penninx B., Schmaal L. Oxidative stress and brain morphology in individuals with depression, anxiety and healthy controls. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;76:140–144. doi: 10.1016/j.pnpbp.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 100.De Pasquale R., Beckhauser T.F., Hernandes M.S., Giorgetti Britto L.R. LTP and LTD in the visual cortex require the activation of NOX2. J. Neurosci. 2014;34:12778–12787. doi: 10.1523/JNEUROSCI.1414-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Massaad C.A., Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxidants Redox Signal. 2011;14:2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tornese P., Sala N., Bonini D., Bonifacino T., La Via L., Milanese M., Treccani G., Seguini M., Ieraci A., Mingardi J., Nyengaard J.R., Calza S., Bonanno G., Wegener G., Barbon A., Popoli M., Musazzi L. Chronic mild stress induces anhedonic behavior and changes in glutamate release, BDNF trafficking and dendrite morphology only in stress vulnerable rats. The rapid restorative action of ketamine, Neurobiol Stress. 2019;10 doi: 10.1016/j.ynstr.2019.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang X., Xiaokaiti Y., Yang J., Pan J., Li Z., Luria V., Li Y., Song G., Zhu X., Zhang H.T., O'Donnell J.M., Xu Y. Inhibition of phosphodiesterase 2 reverses gp91phox oxidase-mediated depression- and anxiety-like behavior. Neuropharmacology. 2018;143:176–185. doi: 10.1016/j.neuropharm.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 104.Hursitoglu O., Kurutas E.B., Strawbridge R., Oner E., Gungor M., Tuman T.C., Uygur O.F. Serum NOX1 and Raftlin as new potential biomarkers of Major Depressive Disorder: a study in treatment-naive first episode patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2023;121 doi: 10.1016/j.pnpbp.2022.110670. [DOI] [PubMed] [Google Scholar]
- 105.Ibi M., Liu J., Arakawa N., Kitaoka S., Kawaji A., Matsuda K.I., Iwata K., Matsumoto M., Katsuyama M., Zhu K., Teramukai S., Furuyashiki T., Yabe-Nishimura C. Depressive-like behaviors are regulated by NOX1/NADPH oxidase by redox modification of NMDA receptor 1. J. Neurosci. 2017;37:4200–4212. doi: 10.1523/JNEUROSCI.2988-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheng G., Cao Z., Xu X., van Meir E.G., Lambeth J.D. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 107.Parkos C.A., Dinauer M.C., Jesaitis A.J., Orkin S.H., Curnutte J.T. Absence of both the 91kD and 22kD subunits of human neutrophil cytochrome b in two genetic forms of chronic granulomatous disease. Blood. 1989;73:1416–1420. [PubMed] [Google Scholar]
- 108.Dinauer M.C., Pierce E.A., Bruns G.A., Curnutte J.T., Orkin S.H. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J. Clin. Invest. 1990;86:1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fang J., Sheng R., Qin Z.H. NADPH oxidases in the central nervous system: regional and cellular localization and the possible link to brain diseases. Antioxidants Redox Signal. 2021;35:951–973. doi: 10.1089/ars.2021.0040. [DOI] [PubMed] [Google Scholar]
- 110.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Y., Murugesan P., Huang K., Cai H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat. Rev. Cardiol. 2020;17:170–194. doi: 10.1038/s41569-019-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Groemping Y., Lapouge K., Smerdon S.J., Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 113.Villegas L., Norremolle A., Freude K., Vilhardt F. Nicotinamide adenine dinucleotide phosphate oxidases are everywhere in brain disease, but not in huntington's disease? Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.736734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu R., Song K., Wu J.X., Geng X.P., Zheng L., Gao X., Peng H., Chen L. Structure of human phagocyte NADPH oxidase in the resting state. Elife. 2022;11 doi: 10.7554/eLife.83743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Noreng S., Ota N., Sun Y., Ho H., Johnson M., Arthur C.P., Schneider K., Lehoux I., Davies C.W., Mortara K., Wong K., Seshasayee D., Masureel M., Payandeh J., Yi T., Koerber J.T. Structure of the core human NADPH oxidase NOX2. Nat. Commun. 2022;13:6079. doi: 10.1038/s41467-022-33711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Babior B.M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 117.Brown D.I., Griendling K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015;116:531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cathcart M.K. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- 119.Helfinger V., Palfi K., Weigert A., Schroder K. The NADPH oxidase Nox4 controls macrophage polarization in an NFkappaB-dependent manner. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/3264858. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manea A., Manea S.A., Gan A.M., Constantin A., Fenyo I.M., Raicu M., Muresian H., Simionescu M. Human monocytes and macrophages express NADPH oxidase 5; a potential source of reactive oxygen species in atherosclerosis. Biochem. Biophys. Res. Commun. 2015;461:172–179. doi: 10.1016/j.bbrc.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 121.Manea S.A., Vlad M.L., Fenyo I.M., Lazar A.G., Raicu M., Muresian H., Simionescu M., Manea A. Pharmacological inhibition of histone deacetylase reduces NADPH oxidase expression, oxidative stress and the progression of atherosclerotic lesions in hypercholesterolemic apolipoprotein E-deficient mice; potential implications for human atherosclerosis. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ouerd S., Idris-Khodja N., Trindade M., Ferreira N.S., Berillo O., Coelho S.C., Neves M.F., Jandeleit-Dahm K.A., Paradis P., Schiffrin E.L. Endothelium-restricted endothelin-1 overexpression in type 1 diabetes worsens atherosclerosis and immune cell infiltration via NOX1. Cardiovasc. Res. 2021;117:1144–1153. doi: 10.1093/cvr/cvaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu Q., Choksi S., Qu J., Jang J., Choe M., Banfi B., Engelhardt J.F., Liu Z.G. NADPH oxidases are essential for macrophage differentiation. J. Biol. Chem. 2016;291:20030–20041. doi: 10.1074/jbc.M116.731216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim S.Y., Jeong J.M., Kim S.J., Seo W., Kim M.H., Choi W.M., Yoo W., Lee J.H., Shim Y.R., Yi H.S., Lee Y.S., Eun H.S., Lee B.S., Chun K., Kang S.J., Kim S.C., Gao B., Kunos G., Kim H.M., Jeong W.I. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nat. Commun. 2017;8:2247. doi: 10.1038/s41467-017-02325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wolf A., Herb M., Schramm M., Langmann T. The TSPO-NOX1 axis controls phagocyte-triggered pathological angiogenesis in the eye. Nat. Commun. 2020;11:2709. doi: 10.1038/s41467-020-16400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meissner F., Seger R.A., Moshous D., Fischer A., Reichenbach J., Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010;116:1570–1573. doi: 10.1182/blood-2010-01-264218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Latz E. NOX-free inflammasome activation. Blood. 2010;116:1393–1394. doi: 10.1182/blood-2010-06-287342. [DOI] [PubMed] [Google Scholar]
- 128.Moon J.S., Nakahira K., Chung K.P., DeNicola G.M., Koo M.J., Pabon M.A., Rooney K.T., Yoon J.H., Ryter S.W., Stout-Delgado H., Choi A.M. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat. Med. 2016;22:1002–1012. doi: 10.1038/nm.4153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Violi F., Carnevale R., Loffredo L., Pignatelli P., Gallin J.I. NADPH oxidase-2 and atherothrombosis: insight from chronic granulomatous disease. Arterioscler. Thromb. Vasc. Biol. 2017;37:218–225. doi: 10.1161/ATVBAHA.116.308351. [DOI] [PubMed] [Google Scholar]
- 130.Pollock J.D., Williams D.A., Gifford M.A., Li L.L., Du X., Fisherman J., Orkin S.H., Doerschuk C.M., Dinauer M.C. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 131.El Benna J., Faust R.P., Johnson J.L., Babior B.M. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. J. Biol. Chem. 1996;271:6374–6378. doi: 10.1074/jbc.271.11.6374. [DOI] [PubMed] [Google Scholar]
- 132.Vulcano M., Dusi S., Lissandrini D., Badolato R., Mazzi P., Riboldi E., Borroni E., Calleri A., Donini M., Plebani A., Notarangelo L., Musso T., Sozzani S. Toll receptor-mediated regulation of NADPH oxidase in human dendritic cells. J. Immunol. 2004;173:5749–5756. doi: 10.4049/jimmunol.173.9.5749. [DOI] [PubMed] [Google Scholar]
- 133.Savina A., Jancic C., Hugues S., Guermonprez P., Vargas P., Moura I.C., Lennon-Dumenil A.M., Seabra M.C., Raposo G., Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 134.Mantegazza A.R., Savina A., Vermeulen M., Perez L., Geffner J., Hermine O., Rosenzweig S.D., Faure F., Amigorena S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marzaioli V., Hurtado-Nedelec M., Pintard C., Tlili A., Marie J.C., Monteiro R.C., Gougerot-Pocidalo M.A., Dang P.M., El-Benna J. NOX5 and p22phox are 2 novel regulators of human monocytic differentiation into dendritic cells. Blood. 2017;130:1734–1745. doi: 10.1182/blood-2016-10-746347. [DOI] [PubMed] [Google Scholar]
- 136.Mortimer P.M., Mc Intyre S.A., Thomas D.C. Beyond the extra respiration of phagocytosis: NADPH oxidase 2 in adaptive immunity and inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.733918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crotzer V.L., Matute J.D., Arias A.A., Zhao H., Quilliam L.A., Dinauer M.C., Blum J.S. Cutting edge: NADPH oxidase modulates MHC class II antigen presentation by B cells. J. Immunol. 2012;189:3800–3804. doi: 10.4049/jimmunol.1103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee K., Won H.Y., Bae M.A., Hong J.H., Hwang E.S. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9548–9553. doi: 10.1073/pnas.1012645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wen Z., Shimojima Y., Shirai T., Li Y., Ju J., Yang Z., Tian L., Goronzy J.J., Weyand C.M. NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs. J. Clin. Invest. 2016;126:1953–1967. doi: 10.1172/JCI84181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kovacs I., Horvath M., Lanyi A., Petheo G.L., Geiszt M. Reactive oxygen species-mediated bacterial killing by B lymphocytes. J. Leukoc. Biol. 2015;97:1133–1137. doi: 10.1189/jlb.4AB1113-607RR. [DOI] [PubMed] [Google Scholar]
- 141.Bertolotti M., Farinelli G., Galli M., Aiuti A., Sitia R. AQP8 transports NOX2-generated H2O2 across the plasma membrane to promote signaling in B cells. J. Leukoc. Biol. 2016;100:1071–1079. doi: 10.1189/jlb.2AB0116-045R. [DOI] [PubMed] [Google Scholar]
- 142.Ambrosio G., Golino P., Pascucci I., Rosolowsky M., Campbell W.B., DeClerck F., Tritto I., Chiariello M. Modulation of platelet function by reactive oxygen metabolites. Am. J. Physiol. 1994;267:H308–H318. doi: 10.1152/ajpheart.1994.267.1.H308. [DOI] [PubMed] [Google Scholar]
- 143.Handin R.I., Karabin R., Boxer G.J. Enhancement of platelet function by superoxide anion. J. Clin. Invest. 1977;59:959–965. doi: 10.1172/JCI108718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Finazzi-Agro A., Menichelli A., Persiani M., Biancini G., Del Principe D. Hydrogen peroxide release from human blood platelets. Biochim. Biophys. Acta. 1982;718:21–25. doi: 10.1016/0304-4165(82)90004-6. [DOI] [PubMed] [Google Scholar]
- 145.Ambrosio G., Tritto I., Golino P. Reactive oxygen metabolites and arterial thrombosis. Cardiovasc. Res. 1997;34:445–452. doi: 10.1016/s0008-6363(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 146.Forde R.C., Fitzgerald D.J. Reactive oxygen species and platelet activation in reperfusion injury. Circulation. 1997;95:787–789. doi: 10.1161/01.cir.95.4.787. [DOI] [PubMed] [Google Scholar]
- 147.Leo R., Pratico D., Iuliano L., Pulcinelli F.M., Ghiselli A., Pignatelli P., Colavita A.R., FitzGerald G.A., Violi F. Platelet activation by superoxide anion and hydroxyl radicals intrinsically generated by platelets that had undergone anoxia and then reoxygenated. Circulation. 1997;95:885–891. doi: 10.1161/01.cir.95.4.885. [DOI] [PubMed] [Google Scholar]
- 148.Chlopicki S., Olszanecki R., Janiszewski M., Laurindo F.R., Panz T., Miedzobrodzki J. Functional role of NADPH oxidase in activation of platelets. Antioxidants Redox Signal. 2004;6:691–698. doi: 10.1089/1523086041361640. [DOI] [PubMed] [Google Scholar]
- 149.Krotz F., Sohn H.Y., Gloe T., Zahler S., Riexinger T., Schiele T.M., Becker B.F., Theisen K., Klauss V., Pohl U. NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood. 2002;100:917–924. doi: 10.1182/blood.v100.3.917. [DOI] [PubMed] [Google Scholar]
- 150.Soulet C., Gendreau S., Missy K., Benard V., Plantavid M., Payrastre B. Characterisation of Rac activation in thrombin- and collagen-stimulated human blood platelets. FEBS Lett. 2001;507:253–258. doi: 10.1016/s0014-5793(01)02984-2. [DOI] [PubMed] [Google Scholar]
- 151.Pignatelli P., Sanguigni V., Lenti L., Ferro D., Finocchi A., Rossi P., Violi F. gp91phox-dependent expression of platelet CD40 ligand. Circulation. 2004;110:1326–1329. doi: 10.1161/01.CIR.0000134963.77201.55. [DOI] [PubMed] [Google Scholar]
- 152.Lievens D., Zernecke A., Seijkens T., Soehnlein O., Beckers L., Munnix I.C., Wijnands E., Goossens P., van Kruchten R., Thevissen L., Boon L., Flavell R.A., Noelle R.J., Gerdes N., Biessen E.A., Daemen M.J., Heemskerk J.W., Weber C., Lutgens E. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116:4317–4327. doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Korporaal S.J., Van Eck M., Adelmeijer J., Ijsseldijk M., Out R., Lisman T., Lenting P.J., Van Berkel T.J., Akkerman J.W. Platelet activation by oxidized low density lipoprotein is mediated by CD36 and scavenger receptor-A. Arterioscler. Thromb. Vasc. Biol. 2007;27:2476–2483. doi: 10.1161/ATVBAHA.107.150698. [DOI] [PubMed] [Google Scholar]
- 154.Wraith K.S., Magwenzi S., Aburima A., Wen Y., Leake D., Naseem K.M. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase-signaling pathways. Blood. 2013;122:580–589. doi: 10.1182/blood-2013-04-491688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carnevale R., Bartimoccia S., Nocella C., Di Santo S., Loffredo L., Illuminati G., Lombardi E., Boz V., Del Ben M., De Marco L., Pignatelli P., Violi F. LDL oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis. 2014;237:108–116. doi: 10.1016/j.atherosclerosis.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 156.Vara D., Mailer R.K., Tarafdar A., Wolska N., Heestermans M., Konrath S., Spaeth M., Renne T., Schroder K., Pula G. NADPH oxidases are required for full platelet activation in vitro and thrombosis in vivo but dispensable for plasma coagulation and hemostasis. Arterioscler. Thromb. Vasc. Biol. 2021;41:683–697. doi: 10.1161/ATVBAHA.120.315565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jones S.A., O'Donnell V.B., Wood J.D., Broughton J.P., Hughes E.J., Jones O.T. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am. J. Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 158.Lassegue B., Clempus R.E. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 159.Bayraktutan U., Blayney L., Shah A.M. Molecular characterization and localization of the NAD(P)H oxidase components gp91-phox and p22-phox in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000;20:1903–1911. doi: 10.1161/01.atv.20.8.1903. [DOI] [PubMed] [Google Scholar]
- 160.Gorlach A., Brandes R.P., Nguyen K., Amidi M., Dehghani F., Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ. Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 161.Burtenshaw D., Hakimjavadi R., Redmond E.M., Cahill P.A. Nox, reactive oxygen species and regulation of vascular cell fate. Antioxidants. 2017;6 doi: 10.3390/antiox6040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Drummond G.R., Sobey C.G. Endothelial NADPH oxidases: which NOX to target in vascular disease? Trends Endocrinol. Metabol. 2014;25:452–463. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 163.Juan C.A., Perez de la Lastra J.M., Plou F.J., Perez-Lebena E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang A.Y., Yi F., Zhang G., Gulbins E., Li P.L. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/01.HYP.0000196727.53300.62. 10.1161/ [DOI] [PubMed] [Google Scholar]
- 165.Woods A.A., Linton S.M., Davies M.J. Detection of HOCl-mediated protein oxidation products in the extracellular matrix of human atherosclerotic plaques. Biochem. J. 2003;370:729–735. doi: 10.1042/BJ20021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.McCann S.K., Dusting G.J., Roulston C.L. Early increase of Nox4 NADPH oxidase and superoxide generation following endothelin-1-induced stroke in conscious rats. J. Neurosci. Res. 2008;86:2524–2534. doi: 10.1002/jnr.21700. [DOI] [PubMed] [Google Scholar]
- 167.Savchenko V.L. Regulation of NADPH oxidase gene expression with PKA and cytokine IL-4 in neurons and microglia. Neurotox. Res. 2013;23:201–213. doi: 10.1007/s12640-012-9327-6. [DOI] [PubMed] [Google Scholar]
- 168.Schiavone S., Neri M., Mhillaj E., Morgese M.G., Cantatore S., Bove M., Riezzo I., Tucci P., Pomara C., Turillazzi E., Cuomo V., Trabace L. The NADPH oxidase NOX2 as a novel biomarker for suicidality: evidence from human post mortem brain samples. Transl. Psychiatry. 2016;6:e813. doi: 10.1038/tp.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cooney S.J., Bermudez-Sabogal S.L., Byrnes K.R. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J. Neuroinflammation. 2013;10:155. doi: 10.1186/1742-2094-10-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Le Belle J.E., Orozco N.M., Paucar A.A., Saxe J.P., Mottahedeh J., Pyle A.D., Wu H., Kornblum H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yi J.H., Kim D.H., Piers T.M., Kim S.C., Whitcomb D.J., Regan P., Cho K. Postsynaptic p47phox regulates long-term depression in the hippocampus. Cell Discov. 2018;4:44. doi: 10.1038/s41421-018-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Russo R., Cattaneo F., Lippiello P., Cristiano C., Zurlo F., Castaldo M., Irace C., Borsello T., Santamaria R., Ammendola R., Calignano A., Miniaci M.C. Motor coordination and synaptic plasticity deficits are associated with increased cerebellar activity of NADPH oxidase, CAMKII, and PKC at preplaque stage in the TgCRND8 mouse model of Alzheimer's disease. Neurobiol. Aging. 2018;68:123–133. doi: 10.1016/j.neurobiolaging.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 173.Liu F.F., Yang L.D., Sun X.R., Zhang H., Pan W., Wang X.M., Yang J.J., Ji M.H., Yuan H.M. NOX2 mediated-parvalbumin interneuron loss might contribute to anxiety-like and enhanced fear learning behavior in a rat model of post-traumatic stress disorder. Mol. Neurobiol. 2016;53:6680–6689. doi: 10.1007/s12035-015-9571-x. [DOI] [PubMed] [Google Scholar]
- 174.Brisch R., Wojtylak S., Saniotis A., Steiner J., Gos T., Kumaratilake J., Henneberg M., Wolf R. The role of microglia in neuropsychiatric disorders and suicide. Eur. Arch. Psychiatr. Clin. Neurosci. 2022;272:929–945. doi: 10.1007/s00406-021-01334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wake H., Moorhouse A.J., Miyamoto A., Nabekura J. Microglia: actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013;36:209–217. doi: 10.1016/j.tins.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 176.Kettenmann H., Hanisch U.K., Noda M., Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 177.Haslund-Vinding J., McBean G., Jaquet V., Vilhardt F. NADPH oxidases in oxidant production by microglia: activating receptors, pharmacology and association with disease. Br. J. Pharmacol. 2017;174:1733–1749. doi: 10.1111/bph.13425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Setiawan E., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Rajkowska G., Suridjan I., Kennedy J.L., Rekkas P.V., Houle S., Meyer J.H. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatr. 2015;72:268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Guevara C.A., Del Valle P., Mercedes C.R. Microglia and reactive oxygen species are required for behavioral susceptibility to chronic social defeat stress. J. Neurosci. 2020;40:1370–1372. doi: 10.1523/JNEUROSCI.2175-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cardoner N., Andero R., Cano M., Marin-Blasco I., Porta-Casteras D., Serra-Blasco M., Via E., Vicent-Gil M., Portella M. Impact of stress on brain morphology: insights into structural biomarkers of stress-related disorders. Curr. Neuropharmacol. 2023 doi: 10.2174/1570159X21666230703091435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lv H., Zhu C., Wu R., Ni H., Lian J., Xu Y., Xia Y., Shi G., Li Z., Caldwell R.B., Caldwell R.W., Yao L., Chen Y. Chronic mild stress induced anxiety-like behaviors can Be attenuated by inhibition of NOX2-derived oxidative stress. J. Psychiatr. Res. 2019;114:55–66. doi: 10.1016/j.jpsychires.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 182.Cheret C., Gervais A., Lelli A., Colin C., Amar L., Ravassard P., Mallet J., Cumano A., Krause K.H., Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J. Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Simpson D.S.A., Oliver P.L. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants. 2020;9 doi: 10.3390/antiox9080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang M., Pan W., Xu Y., Zhang J., Wan J., Jiang H. Microglia-mediated neuroinflammation: a potential target for the treatment of cardiovascular diseases. J. Inflamm. Res. 2022;15:3083–3094. doi: 10.2147/JIR.S350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dworak M., Stebbing M., Kompa A.R., Rana I., Krum H., Badoer E. Sustained activation of microglia in the hypothalamic PVN following myocardial infarction. Auton. Neurosci. 2012;169:70–76. doi: 10.1016/j.autneu.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 186.Rana I., Stebbing M., Kompa A., Kelly D.J., Krum H., Badoer E. Microglia activation in the hypothalamic PVN following myocardial infarction. Brain Res. 2010;1326:96–104. doi: 10.1016/j.brainres.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 187.Dolotov O.V., Inozemtseva L.S., Myasoedov N.F., Grivennikov I.A. Stress-induced depression and alzheimer's disease: focus on astrocytes. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23094999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ge P.Y., Qu S.Y., Ni S.J., Yao Z.Y., Qi Y.Y., Zhao X., Guo R., Yang N.Y., Zhang Q.C., Zhu H.X. Berberine ameliorates depression-like behavior in CUMS mice by activating TPH1 and inhibiting Ido1-associated with tryptophan metabolism. Phytother Res. 2023;37:342–357. doi: 10.1002/ptr.7616. [DOI] [PubMed] [Google Scholar]
- 189.Liao Y., Xing Q., Li Q., Zhang J., Pan R., Yuan Z. Astrocytes in depression and Alzheimer's disease. Front. Med. 2021;15:829–841. doi: 10.1007/s11684-021-0875-0. [DOI] [PubMed] [Google Scholar]
- 190.Nayernia Z., Jaquet V., Krause K.H. New insights on NOX enzymes in the central nervous system. Antioxidants Redox Signal. 2014;20:2815–2837. doi: 10.1089/ars.2013.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ravula A.R., Rodriguez J., Younger D., Perumal V., Shao N., Rama Rao K.V., Pfister B., Chandra N. Animal model of repeated low-level blast traumatic brain injury displays acute and chronic neurobehavioral and neuropathological changes. Exp. Neurol. 2022;349 doi: 10.1016/j.expneurol.2021.113938. [DOI] [PubMed] [Google Scholar]
- 192.Vara D., Tarafdar A., Celikag M., Patinha D., Gulacsy C.E., Hounslea E., Warren Z., Ferreira B., Koeners M.P., Caggiano L., Pula G. NADPH oxidase 1 is a novel pharmacological target for the development of an antiplatelet drug without bleeding side effects. Faseb. J. 2020;34:13959–13977. doi: 10.1096/fj.202001086RRR. [DOI] [PubMed] [Google Scholar]
- 193.Violi F., Pignatelli P. Platelet NOX, a novel target for anti-thrombotic treatment. Thromb. Haemostasis. 2014;111:817–823. doi: 10.1160/TH13-10-0818. [DOI] [PubMed] [Google Scholar]
- 194.Martino F., Loffredo L., Carnevale R., Sanguigni V., Martino E., Catasca E., Zanoni C., Pignatelli P., Violi F. Oxidative stress is associated with arterial dysfunction and enhanced intima-media thickness in children with hypercholesterolemia: the potential role of nicotinamide-adenine dinucleotide phosphate oxidase. Pediatrics. 2008;122:e648–e655. doi: 10.1542/peds.2008-0735. [DOI] [PubMed] [Google Scholar]
- 195.Sonkar V.K., Kumar R., Jensen M., Wagner B.A., Sharathkumar A.A., Miller F.J., Jr., Fasano M., Lentz S.R., Buettner G.R., Dayal S. Nox2 NADPH oxidase is dispensable for platelet activation or arterial thrombosis in mice. Blood Adv. 2019;3:1272–1284. doi: 10.1182/bloodadvances.2018025569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang X., Zhang S., Ding Y., Tong H., Xu X., Wei G., Chen Y., Ju W., Fu C., Qi K., Li Z., Zeng L., Xu K., Qiao J. p47phox deficiency impairs platelet function and protects mice against arterial and venous thrombosis. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dikalova A.E., Gongora M.C., Harrison D.G., Lambeth J.D., Dikalov S., Griendling K.K. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H673–H679. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Guzik T.J., West N.E., Pillai R., Taggart D.P., Channon K.M. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002;39:1088–1094. doi: 10.1161/01.hyp.0000018041.48432.b5. [DOI] [PubMed] [Google Scholar]
- 199.Gavazzi G., Banfi B., Deffert C., Fiette L., Schappi M., Herrmann F., Krause K.H. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 200.Gavazzi G., Deffert C., Trocme C., Schappi M., Herrmann F.R., Krause K.H. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension. 2007;50:189–196. doi: 10.1161/HYPERTENSIONAHA.107.089706. [DOI] [PubMed] [Google Scholar]
- 201.Matsuno K., Yamada H., Iwata K., Jin D., Katsuyama M., Matsuki M., Takai S., Yamanishi K., Miyazaki M., Matsubara H., Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 202.Du J., Fan L.M., Mai A., Li J.M. Crucial roles of Nox2-derived oxidative stress in deteriorating the function of insulin receptors and endothelium in dietary obesity of middle-aged mice. Br. J. Pharmacol. 2013;170:1064–1077. doi: 10.1111/bph.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jung O., Schreiber J.G., Geiger H., Pedrazzini T., Busse R., Brandes R.P. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109:1795–1801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 204.Wang H.D., Xu S., Johns D.G., Du Y., Quinn M.T., Cayatte A.J., Cohen R.A. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 205.Touyz R.M., Mercure C., He Y., Javeshghani D., Yao G., Callera G.E., Yogi A., Lochard N., Reudelhuber T.L. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension. 2005;45:530–537. doi: 10.1161/01.HYP.0000158845.49943.5e. [DOI] [PubMed] [Google Scholar]
- 206.Douglas G., Bendall J.K., Crabtree M.J., Tatham A.L., Carter E.E., Hale A.B., Channon K.M. Endothelial-specific Nox2 overexpression increases vascular superoxide and macrophage recruitment in ApoE(-)/(-) mice. Cardiovasc. Res. 2012;94:20–29. doi: 10.1093/cvr/cvs026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim K., Li J., Tseng A., Andrews R.K., Cho J. NOX2 is critical for heterotypic neutrophil-platelet interactions during vascular inflammation. Blood. 2015;126:1952–1964. doi: 10.1182/blood-2014-10-605261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang Q., Malik P., Pandey D., Gupta S., Jagnandan D., Belin de Chantemele E., Banfi B., Marrero M.B., Rudic R.D., Stepp D.W., Fulton D.J. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler. Thromb. Vasc. Biol. 2008;28:1627–1633. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Guzik T.J., Chen W., Gongora M.C., Guzik B., Lob H.E., Mangalat D., Hoch N., Dikalov S., Rudzinski P., Kapelak B., Sadowski J., Harrison D.G. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J. Am. Coll. Cardiol. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dikalov S.I., Dikalova A.E., Bikineyeva A.T., Schmidt H.H., Harrison D.G., Griendling K.K. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic. Biol. Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Thomas S.R., Witting P.K., Drummond G.R. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxidants Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 212.Matoba T., Shimokawa H., Morikawa K., Kubota H., Kunihiro I., Urakami-Harasawa L., Mukai Y., Hirakawa Y., Akaike T., Takeshita A. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler. Thromb. Vasc. Biol. 2003;23:1224–1230. doi: 10.1161/01.ATV.0000078601.79536.6C. [DOI] [PubMed] [Google Scholar]
- 213.Hakami N.Y., Ranjan A.K., Hardikar A.A., Dusting G.J., Peshavariya H.M. Role of NADPH oxidase-4 in human endothelial progenitor cells. Front. Physiol. 2017;8:150. doi: 10.3389/fphys.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schroder K., Zhang M., Benkhoff S., Mieth A., Pliquett R., Kosowski J., Kruse C., Luedike P., Michaelis U.R., Weissmann N., Dimmeler S., Shah A.M., Brandes R.P. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 215.Craige S.M., Chen K., Pei Y., Li C., Huang X., Chen C., Shibata R., Sato K., Walsh K., Keaney J.F., Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Drummond G.R., Cai H., Davis M.E., Ramasamy S., Harrison D.G. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ. Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- 217.Ray R., Murdoch C.E., Wang M., Santos C.X., Zhang M., Alom-Ruiz S., Anilkumar N., Ouattara A., Cave A.C., Walker S.J., Grieve D.J., Charles R.L., Eaton P., Brewer A.C., Shah A.M. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler. Thromb. Vasc. Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 218.Phillipson M., Kubes P. The neutrophil in vascular inflammation. Nat. Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kim K.H., Barazia A., Cho J. Real-time imaging of heterotypic platelet-neutrophil interactions on the activated endothelium during vascular inflammation and thrombus Formation in live mice. J. Vis. Exp. 2013 doi: 10.3791/50329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Etulain J., Martinod K., Wong S.L., Cifuni S.M., Schattner M., Wagner D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126:242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wienkamp A.K., Erpenbeck L., Rossaint J. Platelets in the NETworks interweaving inflammation and thrombosis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.953129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hook J.S., Cao M., Potera R.M., Alsmadi N.Z., Schmidtke D.W., Moreland J.G. Nox2 regulates platelet activation and NET formation in the lung. Front. Immunol. 2019;10:1472. doi: 10.3389/fimmu.2019.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu Y., Davidson B.P., Yue Q., Belcik T., Xie A., Inaba Y., McCarty O.J., Tormoen G.W., Zhao Y., Ruggeri Z.M., Kaufmann B.A., Lindner J.R. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ Cardiovasc Imaging. 2013;6:74–82. doi: 10.1161/CIRCIMAGING.112.975193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.McEwen B.S., Bowles N.P., Gray J.D., Hill M.N., Hunter R.G., Karatsoreos I.N., Nasca C. Mechanisms of stress in the brain. Nat. Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sanacora G., Yan Z., Popoli M. The stressed synapse 2.0: pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat. Rev. Neurosci. 2022;23:86–103. doi: 10.1038/s41583-021-00540-x. [DOI] [PubMed] [Google Scholar]
- 226.Musazzi L., Mingardi J., Ieraci A., Barbon A., Popoli M. Stress, microRNAs, and stress-related psychiatric disorders: an overview. Mol. Psychiatr. 2023 doi: 10.1038/s41380-023-02139-3. [DOI] [PubMed] [Google Scholar]
- 227.Seo J.S., Park J.Y., Choi J., Kim T.K., Shin J.H., Lee J.K., Han P.L. NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J. Neurosci. 2012;32:9690–9699. doi: 10.1523/JNEUROSCI.0794-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schiavone S., Sorce S., Dubois-Dauphin M., Jaquet V., Colaianna M., Zotti M., Cuomo V., Trabace L., Krause K.H. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol. Psychiatr. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 229.Ruden J.B., Dugan L.L., Konradi C. Parvalbumin interneuron vulnerability and brain disorders. Neuropsychopharmacology. 2021;46:279–287. doi: 10.1038/s41386-020-0778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Vargas H.O., Nunes S.O., Pizzo de Castro M., Bortolasci C.C., Sabbatini Barbosa D., Kaminami Morimoto H., Venugopal K., Dodd S., Maes M., Berk M. Oxidative stress and lowered total antioxidant status are associated with a history of suicide attempts. J. Affect. Disord. 2013;150:923–930. doi: 10.1016/j.jad.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 231.Nestler E.J., Carlezon W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatr. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 232.Zou T., Zhang J., Liu Y., Zhang Y., Sugimoto K., Mei C. Antidepressant-like effect of geniposide in mice exposed to a chronic mild stress involves the microRNA-298-5p-mediated Nox1. Front. Mol. Neurosci. 2020;13:131. doi: 10.3389/fnmol.2020.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jazvinscak Jembrek M., Orsolic N., Karlovic D., Peitl V. Flavonols in action: targeting oxidative stress and neuroinflammation in major depressive disorder. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24086888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Robea M.A., Balmus I.M., Girleanu I., Huiban L., Muzica C., Ciobica A., Stanciu C., Cimpoesu C.D., Trifan A. Coagulation dysfunctions in non-alcoholic fatty liver disease-oxidative stress and inflammation relevance. Medicina (Kaunas) 2023;59 doi: 10.3390/medicina59091614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Valacchi G., Virgili F., Cervellati C., Pecorelli A. OxInflammation: from subclinical condition to pathological biomarker. Front. Physiol. 2018;9:858. doi: 10.3389/fphys.2018.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.S.K. Wood, Chapter 8 - the role of inflammation and oxidative stress in depression and cardiovascular disease, in: P.D. Chantler, K.T. Larkin (Eds.) Cardiovascular Implications of Stress and Depression, Academic Press2020, pp. 175-209.
- 237.Stark K., Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021;18:666–682. doi: 10.1038/s41569-021-00552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ansari J., Vital S.A., Yadav S., Gavins F.N.E. Regulating neutrophil PAD4/NOX-dependent cerebrovasular thromboinflammation. Int. J. Biol. Sci. 2023;19:852–864. doi: 10.7150/ijbs.77434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Barbieri S.S., Zacchi E., Amadio P., Gianellini S., Mussoni L., Weksler B.B., Tremoli E. Cytokines present in smokers' serum interact with smoke components to enhance endothelial dysfunction. Cardiovasc. Res. 2011;90:475–483. doi: 10.1093/cvr/cvr032. [DOI] [PubMed] [Google Scholar]
- 240.Buchmann G.K., Schurmann C., Warwick T., Schulz M.H., Spaeth M., Muller O.J., Schroder K., Jo H., Weissmann N., Brandes R.P. Deletion of NoxO1 limits atherosclerosis development in female mice. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Al-Hakeim H.K., Al-Rammahi D.A., Al-Dujaili A.H. IL-6, IL-18, sIL-2R, and TNFalpha proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J. Affect. Disord. 2015;182:106–114. doi: 10.1016/j.jad.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 242.Kitaoka S. Inflammation in the brain and periphery found in animal models of depression and its behavioral relevance. J. Pharmacol. Sci. 2022;148:262–266. doi: 10.1016/j.jphs.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 243.Nadeem A., Siddiqui N., Al-Harbi N.O., Attia S.M., AlSharari S.D., Ahmad S.F. Acute lung injury leads to depression-like symptoms through upregulation of neutrophilic and neuronal NADPH oxidase signaling in a murine model. Int. Immunopharm. 2017;47:218–226. doi: 10.1016/j.intimp.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 244.Ben-Azu B., Emokpae O., Ajayi A.M., Jarikre T.A., Orhode V., Aderibigbe A.O., Umukoro S., Iwalewa E.O. Repeated psychosocial stress causes glutamic acid decarboxylase isoform-67, oxidative-Nox-2 changes and neuroinflammation in mice: prevention by treatment with a neuroactive flavonoid, morin. Brain Res. 2020;1744 doi: 10.1016/j.brainres.2020.146917. [DOI] [PubMed] [Google Scholar]
- 245.Begonja A.J., Teichmann L., Geiger J., Gambaryan S., Walter U. Platelet regulation by NO/cGMP signaling and NAD(P)H oxidase-generated ROS. Blood Cells Mol. Dis. 2006;36:166–170. doi: 10.1016/j.bcmd.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 246.Peres D.S., Theisen M.C., Fialho M.F.P., Dalenogare D.P., Rodrigues P., Kudsi S.Q., Bernardes L.B., Ruviaro da Silva N.A., Luckemeyer D.D., Sampaio T.B., Pereira G.C., Mello F.K., Ferreira J., Bochi G.V., Oliveira S.M., de David Antoniazzi C.T., Trevisan G. TRPA1 involvement in depression- and anxiety-like behaviors in a progressive multiple sclerosis model in mice. Brain Res. Bull. 2021;175:1–15. doi: 10.1016/j.brainresbull.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 247.Dang D.K., Shin E.J., Nam Y., Ryoo S., Jeong J.H., Jang C.G., Nabeshima T., Hong J.S., Kim H.C. Apocynin prevents mitochondrial burdens, microglial activation, and pro-apoptosis induced by a toxic dose of methamphetamine in the striatum of mice via inhibition of p47phox activation by ERK. J. Neuroinflammation. 2016;13:12. doi: 10.1186/s12974-016-0478-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Konior A., Schramm A., Czesnikiewicz-Guzik M., Guzik T.J. NADPH oxidases in vascular pathology. Antioxidants Redox Signal. 2014;20:2794–2814. doi: 10.1089/ars.2013.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Anatoliotakis N., Deftereos S., Bouras G., Giannopoulos G., Tsounis D., Angelidis C., Kaoukis A., Stefanadis C. Myeloperoxidase: expressing inflammation and oxidative stress in cardiovascular disease. Curr. Top. Med. Chem. 2013;13:115–138. doi: 10.2174/1568026611313020004. [DOI] [PubMed] [Google Scholar]
- 250.Liu N., Lin M.M., Huang S.S., Liu Z.Q., Wu J.C., Liang Z.Q., Qin Z.H., Wang Y. NADPH and mito-apocynin treatment protects against KA-induced excitotoxic injury through autophagy pathway. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.612554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Olukman M., Orhan C.E., Celenk F.G., Ulker S. Apocynin restores endothelial dysfunction in streptozotocin diabetic rats through regulation of nitric oxide synthase and NADPH oxidase expressions. J. Diabet. Complicat. 2010;24:415–423. doi: 10.1016/j.jdiacomp.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 252.Ma M.W., Wang J., Dhandapani K.M., Wang R., Brann D.W. NADPH oxidases in traumatic brain injury - promising therapeutic targets? Redox Biol. 2018;16:285–293. doi: 10.1016/j.redox.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pereira G.C., Piton E., Dos Santos B.M., da Silva R.M., de Almeida A.S., Dalenogare D.P., Schiefelbein N.S., Fialho M.F.P., Moresco R.N., Dos Santos G.T., Marchesan S., Bochi G.V. Apocynin as an antidepressant agent: in vivo behavior and oxidative parameters modulation. Behav. Brain Res. 2020;388 doi: 10.1016/j.bbr.2020.112643. [DOI] [PubMed] [Google Scholar]
- 254.Li M., Liu X., He Y., Zheng Q., Wang M., Wu Y., Zhang Y., Wang C. Celastrol attenuates angiotensin II mediated human umbilical vein endothelial cells damage through activation of Nrf2/ERK1/2/Nox2 signal pathway. Eur. J. Pharmacol. 2017;797:124–133. doi: 10.1016/j.ejphar.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 255.Carbone F., Teixeira P.C., Braunersreuther V., Mach F., Vuilleumier N., Montecucco F. Pathophysiology and treatments of oxidative injury in ischemic stroke: focus on the phagocytic NADPH oxidase 2. Antioxidants Redox Signal. 2015;23:460–489. doi: 10.1089/ars.2013.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pang P., Abbott M., Abdi M., Fucci Q.A., Chauhan N., Mistri M., Proctor B., Chin M., Wang B., Yin W., Lu T.S., Halim A., Lim K., Handy D.E., Loscalzo J., Siedlecki A.M. Pre-clinical model of severe glutathione peroxidase-3 deficiency and chronic kidney disease results in coronary artery thrombosis and depressed left ventricular function. Nephrol. Dial. Transplant. 2018;33:923–934. doi: 10.1093/ndt/gfx304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lindenblatt N., Schareck W., Belusa L., Nickels R.M., Menger M.D., Vollmar B. Anti-oxidant ebselen delays microvascular thrombus formation in the rat cremaster muscle by inhibiting platelet P-selectin expression. Thromb. Haemostasis. 2003;90:882–892. doi: 10.1160/TH02-09-0093. [DOI] [PubMed] [Google Scholar]
- 258.Chew P., Yuen D.Y., Koh P., Stefanovic N., Febbraio M.A., Kola I., Cooper M.E., de Haan J.B. Site-specific antiatherogenic effect of the antioxidant ebselen in the diabetic apolipoprotein E-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 2009;29:823–830. doi: 10.1161/ATVBAHA.109.186619. [DOI] [PubMed] [Google Scholar]
- 259.Barbieri S.S., Cavalca V., Eligini S., Brambilla M., Caiani A., Tremoli E., Colli S. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic. Biol. Med. 2004;37:156–165. doi: 10.1016/j.freeradbiomed.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 260.Yousefian M., Shakour N., Hosseinzadeh H., Hayes A.W., Hadizadeh F., Karimi G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine. 2019;55:200–213. doi: 10.1016/j.phymed.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 261.Begonja A.J., Gambaryan S., Geiger J., Aktas B., Pozgajova M., Nieswandt B., Walter U. Platelet NAD(P)H-oxidase-generated ROS production regulates alphaIIbbeta3-integrin activation independent of the NO/cGMP pathway. Blood. 2005;106:2757–2760. doi: 10.1182/blood-2005-03-1047. [DOI] [PubMed] [Google Scholar]
- 262.Hu H., Straub A., Tian Z., Bassler N., Cheng J., Peter K. Celastrol, a triterpene extracted from Tripterygium wilfordii Hook F, inhibits platelet activation. J. Cardiovasc. Pharmacol. 2009;54:240–245. doi: 10.1097/FJC.0b013e3181b21472. [DOI] [PubMed] [Google Scholar]
- 263.Ouyang M., Qin T., Liu H., Lu J., Peng C., Guo Q. Enhanced inflammatory reaction and thrombosis in high-fat diet-fed ApoE-/- mice are attenuated by celastrol. Exp. Clin. Endocrinol. Diabetes. 2021;129:339–348. doi: 10.1055/a-1010-5543. [DOI] [PubMed] [Google Scholar]
- 264.Zhu C., Yang J., Zhu Y., Li J., Chi H., Tian C., Meng Y., Liu Y., Wang J., Lin N. Celastrol alleviates comorbid obesity and depression by directly binding amygdala HnRNPA1 in a mouse model. Clin. Transl. Med. 2021;11:e394. doi: 10.1002/ctm2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Nakayama T., Okimura K., Shen J., Guh Y.J., Tamai T.K., Shimada A., Minou S., Okushi Y., Shimmura T., Furukawa Y., Kadofusa N., Sato A., Nishimura T., Tanaka M., Nakayama K., Shiina N., Yamamoto N., Loudon A.S., Nishiwaki-Ohkawa T., Shinomiya A., Nabeshima T., Nakane Y., Yoshimura T. Seasonal changes in NRF2 antioxidant pathway regulates winter depression-like behavior. Proc. Natl. Acad. Sci. U. S. A. 2020;117:9594–9603. doi: 10.1073/pnas.2000278117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wang J., Wang P., Dong C., Zhao Y., Zhou J., Yuan C., Zou L. Mechanisms of ebselen as a therapeutic and its pharmacology applications. Future Med. Chem. 2020;12:2141–2160. doi: 10.4155/fmc-2019-0218. [DOI] [PubMed] [Google Scholar]
- 267.Brune B., Diewald B., Ullrich V. Ebselen affects calcium homeostasis in human platelets. Biochem. Pharmacol. 1991;41:1805–1811. doi: 10.1016/0006-2952(91)90118-o. [DOI] [PubMed] [Google Scholar]
- 268.Ramli F.F., Cowen P.J., Godlewska B.R. The potential use of ebselen in treatment-resistant depression. Pharmaceuticals. 2022;15 doi: 10.3390/ph15040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cherix A., Poitry-Yamate C., Lanz B., Zanoletti O., Grosse J., Sandi C., Gruetter R., Cardinaux J.R. Deletion of Crtc1 leads to hippocampal neuroenergetic impairments associated with depressive-like behavior. Mol. Psychiatr. 2022;27:4485–4501. doi: 10.1038/s41380-022-01791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.De Luca S.N., Brassington K., Chan S.M.H., Dobric A., Mou K., Seow H.J., Vlahos R. Ebselen prevents cigarette smoke-induced cognitive dysfunction in mice by preserving hippocampal synaptophysin expression. J. Neuroinflammation. 2022;19:72. doi: 10.1186/s12974-022-02432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Herrera D.G., Yague A.G., Johnsen-Soriano S., Bosch-Morell F., Collado-Morente L., Muriach M., Romero F.J., Garcia-Verdugo J.M. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc. Natl. Acad. Sci. U. S. A. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Johnsen-Soriano S., Bosch-Morell F., Miranda M., Asensio S., Barcia J.M., Roma J., Monfort P., Felipo V., Romero F.J. Ebselen prevents chronic alcohol-induced rat hippocampal stress and functional impairment. Alcohol Clin. Exp. Res. 2007;31:486–492. doi: 10.1111/j.1530-0277.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- 273.Martini F., Rosa S.G., Klann I.P., Fulco B.C.W., Carvalho F.B., Rahmeier F.L., Fernandes M.C., Nogueira C.W. A multifunctional compound ebselen reverses memory impairment, apoptosis and oxidative stress in a mouse model of sporadic Alzheimer's disease. J. Psychiatr. Res. 2019;109:107–117. doi: 10.1016/j.jpsychires.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 274.Xie Y., Tan Y., Zheng Y., Du X., Liu Q. Ebselen ameliorates beta-amyloid pathology, tau pathology, and cognitive impairment in triple-transgenic Alzheimer's disease mice. J Biol Inorg Chem. 2017;22:851–865. doi: 10.1007/s00775-017-1463-2. [DOI] [PubMed] [Google Scholar]
- 275.Gatto G.J., Jr., Ao Z., Kearse M.G., Zhou M., Morales C.R., Daniels E., Bradley B.T., Goserud M.T., Goodman K.B., Douglas S.A., Harpel M.R., Johns D.G. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J. Enzym. Inhib. Med. Chem. 2013;28:95–104. doi: 10.3109/14756366.2011.636360. [DOI] [PubMed] [Google Scholar]
- 276.Siafaka-Kapadai A., Svetlov S., Hanahan D.J., Javors M.A. Effects of suramin on human platelet aggregation and Ca2+ mobilization induced by thrombin and other agonists. Life Sci. 1998;63:1769–1777. doi: 10.1016/s0024-3205(98)00451-2. [DOI] [PubMed] [Google Scholar]
- 277.Fernandes R.S., Assafim M., Arruda E.Z., Melo P.A., Zingali R.B., Monteiro R.Q. Suramin counteracts the haemostatic disturbances produced by Bothrops jararaca snake venom. Toxicon. 2007;49:931–938. doi: 10.1016/j.toxicon.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 278.Kuang Y., Liu H., Guo S., Wang Y., Zhang H., Qiao Y. The antagonist of P2Y11 receptor NF157 ameliorates oxidized LDL-induced vascular endothelial inflammation. Artif. Cells, Nanomed. Biotechnol. 2019;47:1839–1845. doi: 10.1080/21691401.2019.1610412. [DOI] [PubMed] [Google Scholar]
- 279.Naviaux J.C., Schuchbauer M.A., Li K., Wang L., Risbrough V.B., Powell S.B., Naviaux R.K. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Transl. Psychiatry. 2014;4 doi: 10.1038/tp.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Naviaux J.C., Wang L., Li K., Bright A.T., Alaynick W.A., Williams K.R., Powell S.B., Naviaux R.K. Antipurinergic therapy corrects the autism-like features in the Fragile X (Fmr1 knockout) mouse model. Mol. Autism. 2015;6:1. doi: 10.1186/2040-2392-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Chan C.F., Lin-Shiau S.Y. Site of action of suramin and reactive blue 2 in preventing neuronal death induced by dequalinium. J. Neurosci. Res. 2000;62:692–699. doi: 10.1002/1097-4547. (20001201)62:5<692::AID-JNR8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 282.Shieh C.H., Heinrich A., Serchov T., van Calker D., Biber K. P2X7-dependent, but differentially regulated release of IL-6, CCL2, and TNF-alpha in cultured mouse microglia. Glia. 2014;62:592–607. doi: 10.1002/glia.22628. [DOI] [PubMed] [Google Scholar]
- 283.Wu Y., Willcockson H.H., Maixner W., Light A.R. Suramin inhibits spinal cord microglia activation and long-term hyperalgesia induced by formalin injection. J. Pain. 2004;5:48–55. doi: 10.1016/j.jpain.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 284.Willoughby S.R., Stewart S., Chirkov Y.Y., Kennedy J.A., Holmes A.S., Horowitz J.D. Beneficial clinical effects of perhexiline in patients with stable angina pectoris and acute coronary syndromes are associated with potentiation of platelet responsiveness to nitric oxide. Eur. Heart J. 2002;23:1946–1954. doi: 10.1053/euhj.2002.3296. [DOI] [PubMed] [Google Scholar]
- 285.Alderman C.P., Hundertmark J.D., Soetratma T.W. Interaction of serotonin re-uptake inhibitors with perhexiline. Aust. N. Z. J. Psychiatr. 1997;31:601–603. doi: 10.3109/00048679709065084. [DOI] [PubMed] [Google Scholar]
- 286.Feng X., Sureda A., Jafari S., Memariani Z., Tewari D., Annunziata G., Barrea L., Hassan S.T.S., Smejkal K., Malanik M., Sychrova A., Barreca D., Ziberna L., Mahomoodally M.F., Zengin G., Xu S., Nabavi S.M., Shen A.Z. Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. Theranostics. 2019;9:1923–1951. doi: 10.7150/thno.30787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhu W.Q., Wu H.Y., Sun Z.H., Guo Y., Ge T.T., Li B.J., Li X., Cui R.J. Current evidence and future directions of berberine intervention in depression. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.824420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Cheng F., Wang Y., Li J., Su C., Wu F., Xia W.H., Yang Z., Yu B.B., Qiu Y.X., Tao J. Berberine improves endothelial function by reducing endothelial microparticles-mediated oxidative stress in humans. Int. J. Cardiol. 2013;167:936–942. doi: 10.1016/j.ijcard.2012.03.090. [DOI] [PubMed] [Google Scholar]
- 289.Paul M., Hemshekhar M., Kemparaju K., Girish K.S. Berberine mitigates high glucose-potentiated platelet aggregation and apoptosis by modulating aldose reductase and NADPH oxidase activity. Free Radic. Biol. Med. 2019;130:196–205. doi: 10.1016/j.freeradbiomed.2018.10.453. [DOI] [PubMed] [Google Scholar]
- 290.Pei C., Zhang Y., Wang P., Zhang B., Fang L., Liu B., Meng S. Berberine alleviates oxidized low-density lipoprotein-induced macrophage activation by downregulating galectin-3 via the NF-kappaB and AMPK signaling pathways. Phytother Res. 2019;33:294–308. doi: 10.1002/ptr.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wu Y.H., Chuang S.Y., Hong W.C., Lai Y.J., Chang G.J., Pang J.H. Berberine reduces leukocyte adhesion to LPS-stimulated endothelial cells and VCAM-1 expression both in vivo and in vitro. Int. J. Immunopathol. Pharmacol. 2012;25:741–750. doi: 10.1177/039463201202500320. [DOI] [PubMed] [Google Scholar]
- 292.Wang Y., Huang Y., Lam K.S., Li Y., Wong W.T., Ye H., Lau C.W., Vanhoutte P.M., Xu A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc. Res. 2009;82:484–492. doi: 10.1093/cvr/cvp078. [DOI] [PubMed] [Google Scholar]
- 293.Zhan Y., Han J., Xia J., Wang X. Berberine suppresses mice depression behaviors and promotes hippocampal neurons growth through regulating the miR-34b-5p/miR-470-5p/BDNF Axis. Neuropsychiatric Dis. Treat. 2021;17:613–626. doi: 10.2147/NDT.S289444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Huang M., He Y., Tian L., Yu L., Cheng Q., Li Z., Gao L., Gao S., Yu C. Gut microbiota-SCFAs-brain axis associated with the antidepressant activity of berberine in CUMS rats. J. Affect. Disord. 2023;325:141–150. doi: 10.1016/j.jad.2022.12.166. [DOI] [PubMed] [Google Scholar]
- 295.Liu Y.M., Niu L., Wang L.L., Bai L., Fang X.Y., Li Y.C., Yi L.T. Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res. Bull. 2017;134:220–227. doi: 10.1016/j.brainresbull.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 296.Zhang J.H., Yang H.Z., Su H., Song J., Bai Y., Deng L., Feng C.P., Guo H.X., Wang Y., Gao X., Gu Y., Zhen Z., Lu Y. Berberine and ginsenoside Rb1 ameliorate depression-like behavior in diabetic rats. Am. J. Chin. Med. 2021;49:1195–1213. doi: 10.1142/S0192415X21500579. [DOI] [PubMed] [Google Scholar]
- 297.Arora V., Chopra K. Possible involvement of oxido-nitrosative stress induced neuro-inflammatory cascade and monoaminergic pathway: underpinning the correlation between nociceptive and depressive behaviour in a rodent model. J. Affect. Disord. 2013;151:1041–1052. doi: 10.1016/j.jad.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 298.Tian E., Sharma G., Dai C. Neuroprotective properties of berberine: molecular mechanisms and clinical implications. Antioxidants. 2023;12 doi: 10.3390/antiox12101883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Gianni D., Taulet N., Zhang H., DerMardirossian C., Kister J., Martinez L., Roush W.R., Brown S.J., Bokoch G.M., Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem. Biol. 2010;5:981–993. doi: 10.1021/cb100219n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Walsh T.G., Berndt M.C., Carrim N., Cowman J., Kenny D., Metharom P. The role of Nox1 and Nox2 in GPVI-dependent platelet activation and thrombus formation. Redox Biol. 2014;2:178–186. doi: 10.1016/j.redox.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ranayhossaini D.J., Rodriguez A.I., Sahoo S., Chen B.B., Mallampalli R.K., Kelley E.E., Csanyi G., Gladwin M.T., Romero G., Pagano P.J. Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration. J. Biol. Chem. 2013;288:36437–36450. doi: 10.1074/jbc.M113.521344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Rey F.E., Cifuentes M.E., Kiarash A., Quinn M.T., Pagano P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ. Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- 303.Bosco E.E., Kumar S., Marchioni F., Biesiada J., Kordos M., Szczur K., Meller J., Seibel W., Mizrahi A., Pick E., Filippi M.D., Zheng Y. Rational design of small molecule inhibitors targeting the Rac GTPase-p67(phox) signaling axis in inflammation. Chem. Biol. 2012;19:228–242. doi: 10.1016/j.chembiol.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Akbar H., Duan X., Piatt R., Saleem S., Davis A.K., Tandon N.N., Bergmeier W., Zheng Y. Small molecule targeting the Rac1-NOX2 interaction prevents collagen-related peptide and thrombin-induced reactive oxygen species generation and platelet activation. J. Thromb. Haemostasis. 2018;16:2083–2096. doi: 10.1111/jth.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Radermacher K.A., Wingler K., Langhauser F., Altenhofer S., Kleikers P., Hermans J.J., Hrabe de Angelis M., Kleinschnitz C., Schmidt H.H. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxidants Redox Signal. 2013;18:1418–1427. doi: 10.1089/ars.2012.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lu W.J., Li J.Y., Chen R.J., Huang L.T., Lee T.Y., Lin K.H. VAS2870 and VAS3947 attenuate platelet activation and thrombus formation via a NOX-independent pathway downstream of PKC. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-55189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Abubaker A.A., Vara D., Visconte C., Eggleston I., Torti M., Canobbio I., Pula G. Amyloid peptide beta1-42 induces integrin alphaIIbbeta3 activation, platelet adhesion, and thrombus formation in a NADPH oxidase-dependent manner. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/1050476. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Coco H., Pernomian L., Pereira P.C., Gomes M.S., Marchi K.C., Lopes A.H., Cunha T.M., Tirapelli C.R., de Oliveira A.M. Chronic restraint stress increases angiotensin II potency in the rat carotid: role of cyclooxygenases and reactive oxygen species. J. Pharm. Pharmacol. 2017;69:52–65. doi: 10.1111/jphp.12659. [DOI] [PubMed] [Google Scholar]
- 309.Bush D.E., Ziegelstein R.C., Tayback M., Richter D., Stevens S., Zahalsky H., Fauerbach J.A. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am. J. Cardiol. 2001;88:337–341. doi: 10.1016/s0002-9149(01)01675-7. [DOI] [PubMed] [Google Scholar]
- 310.Camkurt M.A., Findikli E., Izci F., Kurutas E.B., Tuman T.C. Evaluation of malondialdehyde, superoxide dismutase and catalase activity and their diagnostic value in drug naive, first episode, non-smoker major depression patients and healthy controls. Psychiatr. Res. 2016;238:81–85. doi: 10.1016/j.psychres.2016.01.075. [DOI] [PubMed] [Google Scholar]
- 311.Jiang W., Alexander J., Christopher E., Kuchibhatla M., Gaulden L.H., Cuffe M.S., Blazing M.A., Davenport C., Califf R.M., Krishnan R.R., O'Connor C.M. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch. Intern. Med. 2001;161:1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 312.Junger J., Schellberg D., Muller-Tasch T., Raupp G., Zugck C., Haunstetter A., Zipfel S., Herzog W., Haass M. Depression increasingly predicts mortality in the course of congestive heart failure. Eur. J. Heart Fail. 2005;7:261–267. doi: 10.1016/j.ejheart.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 313.Lesperance F., Frasure-Smith N., Talajic M., Bourassa M.G. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105:1049–1053. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 314.Oglodek E.A. Changes in the concentrations of inflammatory and oxidative status biomediators (MIP-1 alpha, PMN elastase, MDA, and IL-12) in depressed patients with and without posttraumatic stress disorder. Pharmacol. Rep. 2018;70:110–118. doi: 10.1016/j.pharep.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 315.Oglodek E.A., Szota A., Just M.J., Mos D., Araszkiewicz A. Comparison of chemokines (CCL-5 and SDF-1), chemokine receptors (CCR-5 and CXCR-4) and IL-6 levels in patients with different severities of depression. Pharmacol. Rep. 2014;66:920–926. doi: 10.1016/j.pharep.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 316.Rumsfeld J.S., Havranek E., Masoudi F.A., Peterson E.D., Jones P., Tooley J.F., Krumholz H.M., Spertus J.A., Cardiovascular Outcomes Research C. Depressive symptoms are the strongest predictors of short-term declines in health status in patients with heart failure. J. Am. Coll. Cardiol. 2003;42:1811–1817. doi: 10.1016/j.jacc.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 317.Sherwood A., Blumenthal J.A., Trivedi R., Johnson K.S., O'Connor C.M., Adams K.F., Jr., Dupree C.S., Waugh R.A., Bensimhon D.R., Gaulden L., Christenson R.H., Koch G.G., Hinderliter A.L. Relationship of depression to death or hospitalization in patients with heart failure. Arch. Intern. Med. 2007;167:367–373. doi: 10.1001/archinte.167.4.367. [DOI] [PubMed] [Google Scholar]
- 318.Wagner C.J., Musenbichler C., Bohm L., Farber K., Fischer A.I., von Nippold F., Winkelmann M., Richter-Schmidinger T., Muhle C., Kornhuber J., Lenz B. LDL cholesterol relates to depression, its severity, and the prospective course. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;92:405–411. doi: 10.1016/j.pnpbp.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 319.Wyatt R.J., Portnoy B., Kupfer D.J., Snyder F., Engelman K. Resting plasma catecholamine concentrations in patients with depression and anxiety. Arch. Gen. Psychiatr. 1971;24:65–70. doi: 10.1001/archpsyc.1971.01750070067009. [DOI] [PubMed] [Google Scholar]
- 320.Mosovich S.A., Boone R.T., Reichenberg A., Bansilal S., Shaffer J., Dahlman K., Harvey P.D., Farkouh M.E. New insights into the link between cardiovascular disease and depression. Int. J. Clin. Pract. 2008;62:423–432. doi: 10.1111/j.1742-1241.2007.01640.x. [DOI] [PubMed] [Google Scholar]
- 321.Cammisotto V., Carnevale R., Nocella C., Stefanini L., Bartimoccia S., Coluccia A., Silvestri R., Pignatelli P., Pastori D., Violi F. Nox2-mediated platelet activation by glycoprotein (GP) VI: effect of rivaroxaban alone and in combination with aspirin. Biochem. Pharmacol. 2019;163:111–118. doi: 10.1016/j.bcp.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 322.Tsai K.L., Huang P.H., Kao C.L., Leu H.B., Cheng Y.H., Liao Y.W., Yang Y.P., Chien Y., Wang C.Y., Hsiao C.Y., Chiou S.H., Chen J.W., Lin S.J. Aspirin attenuates vinorelbine-induced endothelial inflammation via modulating SIRT1/AMPK axis. Biochem. Pharmacol. 2014;88:189–200. doi: 10.1016/j.bcp.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 323.Chen B., Zhao J., Zhang S., Wu W., Qi R. Aspirin inhibits the production of reactive oxygen species by downregulating Nox4 and inducible nitric oxide synthase in human endothelial cells exposed to oxidized low-density lipoprotein. J. Cardiovasc. Pharmacol. 2012;59:405–412. doi: 10.1097/FJC.0b013e318248acba. [DOI] [PubMed] [Google Scholar]
- 324.Caruso G., Grasso M., Fidilio A., Torrisi S.A., Musso N., Geraci F., Tropea M.R., Privitera A., Tascedda F., Puzzo D., Salomone S., Drago F., Leggio G.M., Caraci F. Antioxidant activity of fluoxetine and vortioxetine in a non-transgenic animal model of alzheimer's disease. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.809541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chung E.S., Chung Y.C., Bok E., Baik H.H., Park E.S., Park J.Y., Yoon S.H., Jin B.K. Fluoxetine prevents LPS-induced degeneration of nigral dopaminergic neurons by inhibiting microglia-mediated oxidative stress. Brain Res. 2010;1363:143–150. doi: 10.1016/j.brainres.2010.09.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.