ABSTRACT
Aminoglycosides are important treatment options for serious lung infections, but modeling analyses to quantify their human lung epithelial lining fluid (ELF) penetration are lacking. We estimated the extent and rate of penetration for five aminoglycosides via population pharmacokinetics from eight published studies. The area under the curve in ELF vs plasma ranged from 50% to 100% and equilibration half-lives from 0.61 to 5.80 h, indicating extensive system hysteresis. Aminoglycoside ELF peak concentrations were blunted, but overall exposures were moderately high.
KEYWORDS: lung epithelial lining fluid (ELF), population pharmacokinetics, amikacin, arbekacin, gentamicin, netilmicin, tobramycin, plazomicin, model-based meta-analysis, S-ADAPT
INTRODUCTION
Aminoglycosides are an important part of our armamentarium to treat serious lung infections caused by multidrug-resistant Gram-negative pathogens, such as Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae, and often used in combination with β-lactam antibiotics (1–12). Aminoglycosides are polar polycations (Table S1) (13), have small volumes of distribution (11, 14, 15), low (<20%) protein binding (16–19), and diminished activity in acidic pH (20, 21). A few studies report lung epithelial lining fluid (ELF) concentrations to range from 10% to 30% of those in plasma or serum, though these values only considered one or two time points during the first 2 h after the start of a short-term infusion (22–25). In contrast, ELF-to-plasma concentration ratios increased over time and reached 100% in all studies that determined ELF concentrations over up to 6 to 24 h post dose (Fig. S1) (26–31).
Time-course modeling can estimate the rate and extent of penetration, as employed to assess penetration of other antibiotic classes into ELF (32–37), bone (38–41), and cerebrospinal fluid (42, 43). Physiologically based modeling has been applied to predict the pulmonary pharmacokinetics (PK) of fluoroquinolones (44). Population PK modeling is highly beneficial to handle data sets with sparse sampling (e.g., only one ELF concentration per patient) and has been employed to model ELF concentrations of aminoglycosides in mice (45–47). However, we are not aware of published PK modeling analyses of the rate and extent of ELF penetration for aminoglycosides in humans. Instead, all but one prior study reported ELF-to-plasma concentration ratios at single or several time points (22–30) without even applying a non-compartmental analysis (NCA) (48). One study used bronchoscopic micro-sampling and NCA to calculate the area under the curve (AUC) in ELF and plasma and reported a ratio of 67.6% for arbekacin (31). Thus, despite extensive clinical use of aminoglycosides to combat serious lung infections for decades, their extent and rate of human ELF penetration have never been characterized via time-course modeling. Consequently, the impact of system hysteresis with ELF concentrations lagging behind those in plasma has never been quantitatively examined (22). Failure to consider the time course of exposures may lead to underestimating drug exposures in ELF in patients.
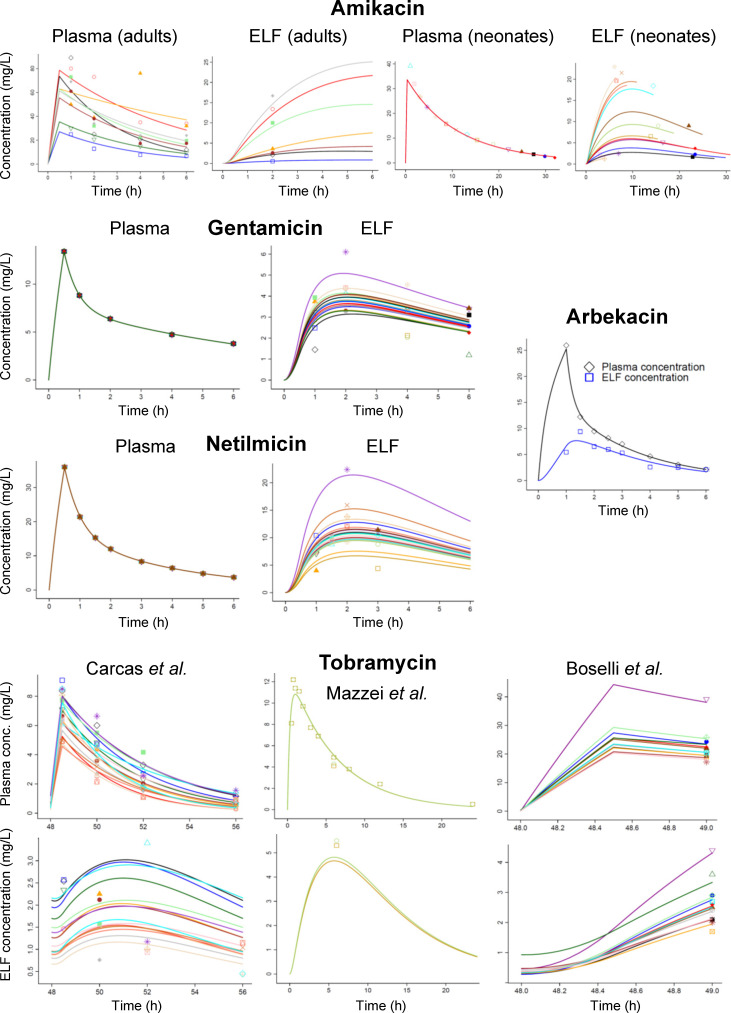
We performed model-based meta-analyses by simultaneously modeling all data from each aminoglycoside based on eight published PK studies assessing the ELF penetration in humans after intravenous or intramuscular dosing (23, 24, 26–31). We excluded studies with inhaled dosing (14, 49), one study on plazomicin reported as a poster (25, 50), and one study (51) where tobramycin concentrations in bronchoalveolar lavage fluid were expressed as a function of creatinine. Plasma (or serum) and ELF concentration values were used as reported (23, 24, 26, 28) or digitized (27, 29–31) (see Table S2 for details). For each drug, all plasma (or serum) and ELF concentrations were analyzed by population PK modeling in the S-ADAPT (version 1.57; importance sampling algorithm) and SADAPT-TRAN software packages (52–54) using previously described approaches (41, 55–59). The systemic PK of aminoglycosides was described by linear one or two compartment models (Fig. S2), plus an additional ELF compartment with a small, non-influential volume of distribution (fixed to 0.1 L) (39, 58). We estimated the ratio (FELF) for the AUC in ELF vs plasma or serum and the ELF-to-plasma equilibration half-life (t1/2,eq). The FELF characterizes the overall extent and t1/2,eq the rate of penetration.
Population PK estimated the mean ELF-to-plasma AUC ratios (FELF) between 0.502 and 1.00 for all aminoglycosides with good precision (relative standard errors, RSE ≤12%, except for 31% for amikacin, Table 1). The between-patient variability of FELF was large for amikacin (84.4% coefficient of variation) and smaller for the other aminoglycosides (≤27.6%; Fig. 1; Fig. S3). The individual subject estimates for the ELF-to-plasma AUC ratio ranged from 0.138 to 1.60 for amikacin and from 0.468 to 1.94 for gentamicin, netilmicin, and tobramycin (Table 1).
TABLE 1.
Population PK parameter estimates for one- or two-compartment models describing plasma and lung ELF concentrations of five aminoglycosides after intravenous (or intramuscular) dosinga
| Drug | Population | AUCELF/AUCPlasma | t1/2,eq (h) | CL (L/h) | CLd (L/h) | V1 (L) | V2 (L) | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean (RSEc) | BSVb (RSEc) | Median [range] | Mean | Mean | Mean | Mean | Mean | ||
| Amikacin | ICU patients (with VAP) | 0.502 (31%) | 0.844 (39%) | 0.461 [0.138–1.60] | 5.80 (58%) | 4.88 | 24.6 | ||
| Amikacin | Ventilated neonates | 0.138 | 1.61 | ||||||
| Arbekacin | Healthy volunteers | 0.535 (12%) | 0.10 (fixed) | –f | 0.613 (24%) | 3.56 | 6.77 | 3.00 | 4.77 |
| Gentamicin | VABP critically ill patients | 0.600 (7%) | 0.165 (160%) | 0.600 [0.507–0.782] | 0.857 (18%) | 3.63 | 14.9 | 13.5 | 13.9 |
| Netilmicin | ICU patients (ventilated, with pneumonia)e | 1.00 (11%) | 0.276 (67%) | 0.989 [0.627–1.94] | 1.68 (13%) | 5.61 | 6.90 | 9.36 | 7.87 |
| Tobramycind | VABP critically Ill patients and healthy volunteers | 0.642 (9%) | 0.248 (84%) | 0.635 [0.468–0.901] | 3.38 (17%) | 5.60 | 20.1 | ||
The volume of distribution of the ELF compartment was set to a small, non-influential value (0.1 L). The estimated ELF-to-plasma equilibration half-life characterized the extent of system hysteresis. AUCELF, area under the ELF concentration time curve from time zero to infinity (for a single aminoglycoside dose); AUCPlasma, area under the plasma concentration time curve from time zero to infinity (for a single aminoglycoside dose); ICU, intensive care unit; t1/2,eq, equilibration half-life between the ELF and the plasma compartment. This half-life characterizes the extent of system hysteresis and represents the slower rise of ELF concentrations compared to the rapid rise of the plasma or serum concentrations. If an aminoglycoside was dosed as a continuous infusion, the t1/2,eq would be the half-life of approaching a constant steady-state concentration in ELF; VAP / VABP, ventilator-associated (bacterial) pneumonia.
BSV, between subject variability reported as coefficient of variation.
Relative standard errors.
The estimated absorption half-life (t1/2,abs) after intramuscular dosing of tobramycin was 13.4 min.
Patients were intubated and ventilated for a variety of reasons and received antibiotics due to the development of pneumonia.
Not applicable.
Fig 1.
Fitted (lines) and observed (markers) plasma (or serum) and ELF concentrations based on published data of five aminoglycosides from eight human PK studies (23, 24, 26–31). The lines represent different subjects if individual subject data were reported (see Table S2 for details on data sets).
The estimated t1/2,eq differed between aminoglycosides with a range of 0.857 to 5.80 h in patients and 0.613 h in healthy volunteers (Table 1). It is unknown, whether these differences arose from the aminoglycoside structures (Table S1) (13), clinical factors (e.g., type of infection and inflammation) (60, 61), or both. The longest half-life was observed in neonates receiving amikacin (Fig. 1; Fig. S1). Neonates might have had an altered expression of pulmonary transporters compared to adults (62–65). Future research on such transporters and their impact on lung penetration is warranted (66–71). Owing to the sparse nature of the data sets, the differences in t1/2,eq between aminoglycosides should be interpreted cautiously.
The system hysteresis (Fig. S1) yielded blunted peak concentrations (Cmax) in ELF, which were on average 2.3- to 4-fold lower than those in plasma. Despite this, the AUC in ELF was 50% to 100% of the plasma AUC. Plazomicin modeling results are shown in Fig. S4 (50). Thus, estimating the system hysteresis via time-course modeling is important when determining the ELF penetration of aminoglycosides. To support future studies, we provided Monte Carlo simulation code to simulate ELF and plasma concentrations, as well as D-optimal sampling designs [in the PopED Lite software (72)] for amikacin, gentamicin, and tobramycin in the supplementary materials.
In mice, PK analyses estimated average ELF-to-plasma AUC ratios of 0.60 to 0.88 for amikacin, tobramycin, plazomicin, and apramycin (4, 45–47, 73), consistent with our results (Table 1). However, the ELF-to-plasma equilibration half-lives were substantially faster in mice [3 to 5 min for tobramycin and plazomicin (45, 46), and 22 to 36 min for amikacin (73)] compared to those in patients (0.857 to 5.80 h; Table 1).
This study represents the first time-course modeling to characterize the rate and extent of ELF penetration for five aminoglycosides in humans based on eight published studies. Our model-based meta-analyses revealed the average AUC in ELF to be 50% to 100% compared to those in plasma for humans. The individual subject ELF penetration ratios displayed considerable variability. Due to extensive system hysteresis, the Cmax in ELF were blunted and lower than those in plasma. With both Cmax and AUC being correlated to bacterial killing and clinical efficacy of aminoglycosides (2–11), future studies are warranted to assess whether or not blunted Cmax in ELF are clinically important. Moreover, future research is warranted to assess the impact of pH and different oxygen tensions on aminoglycoside efficacy for lung infections (74, 75). This study supports translational research to simulate the time course of ELF concentrations in in vitro infection models (76) and future clinical ELF penetration studies in animals and humans.
ACKNOWLEDGMENTS
This study was supported by awards R01AI136803 and R01AI130185 (to J.B.B.), and R01AI173064 (to Z.B., Y.L., and J.B.B.) from the National Institute of Allergy and Infectious Diseases. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the Department of Veterans Affairs.
Contributor Information
Jürgen B. Bulitta, Email: jbulitta@cop.ufl.edu.
James E. Leggett, Providence Portland Medical Center, Portland, Oregon, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.01393-23.
Supplementary figures, tables. and simulation model code.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Milatovic D, Braveny I. 1987. Development of resistance during antibiotic therapy. Eur J Clin Microbiol 6:234–244. doi: 10.1007/BF02017607 [DOI] [PubMed] [Google Scholar]
- 2. Jiao Y, Moya B, Chen MJ, Zavascki AP, Tsai H, Tao X, Sutaria DS, Louie A, Boyce JD, Deveson Lucas D, Kim TH, Tsuji BT, Bonomo RA, Drusano GL, Bulitta JB. 2019. Comparable efficacy and better safety of double β-lactam combination therapy versus β-lactam plus aminoglycoside in Gram-negative bacteria in randomized, controlled trials. Antimicrob Agents Chemother 63:e00425-19. doi: 10.1128/AAC.00425-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bland CM, Pai MP, Lodise TP. 2018. Reappraisal of contemporary pharmacokinetic and pharmacodynamic principles for informing aminoglycoside dosing. Pharmacotherapy 38:1229–1238. doi: 10.1002/phar.2193 [DOI] [PubMed] [Google Scholar]
- 4. Lepak AJ, Trang M, Hammel JP, Sader HS, Bhavnani SM, VanScoy BD, Pogue JM, Ambrose PG, Andes DR, United States Committee on Antimicrobial Susceptibility Testing . 2023. Development of modernized Acinetobacter baumannii susceptibility test interpretive criteria for recommended antimicrobial agents using pharmacometric approaches. Antimicrob Agents Chemother 67:e0145222. doi: 10.1128/aac.01452-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zavascki AP, Klee BO, Bulitta JB. 2017. Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: the pitfalls of aminoglycoside susceptibility. Expert Rev Anti Infect Ther 15:519–526. doi: 10.1080/14787210.2017.1316193 [DOI] [PubMed] [Google Scholar]
- 6. Pai MP, Rodvold KA. 2014. Aminoglycoside dosing in patients by kidney function and area under the curve: the Sawchuk-Zaske dosing method revisited in the era of obesity. Diagn Microbiol Infect Dis 78:178–187. doi: 10.1016/j.diagmicrobio.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 7. Craig WA. 2011. Optimizing aminoglycoside use. Crit Care Clin 27:107–121. doi: 10.1016/j.ccc.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 8. Turnidge J. 2003. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am 17:503–528, doi: 10.1016/s0891-5520(03)00057-6 [DOI] [PubMed] [Google Scholar]
- 9. Barclay ML, Begg EJ, Hickling KG. 1994. What is the evidence for once-daily aminoglycoside therapy? Clin Pharmacokinet 27:32–48. doi: 10.2165/00003088-199427010-00004 [DOI] [PubMed] [Google Scholar]
- 10. Tulkens PM. 1990. Efficacy and safety of aminoglycosides once-a-day: experimental and clinical data. Scand J Infect Dis Suppl 74:249–257. [PubMed] [Google Scholar]
- 11. Pechere JC, Dugal R. 1979. Clinical pharmacokinetics of aminoglycoside antibiotics. Clin Pharmacokinet 4:170–199. doi: 10.2165/00003088-197904030-00002 [DOI] [PubMed] [Google Scholar]
- 12. Serio AW, Keepers T, Andrews L, Krause KM, Bush K. 2018. Aminoglycoside revival: review of a historically important class of antimicrobials undergoing rejuvenation. EcoSal Plus 8. doi: 10.1128/ecosalplus.ESP-0002-2018 [DOI] [PubMed] [Google Scholar]
- 13. O’Shea R, Moser HE. 2008. Physicochemical properties of antibacterial compounds: implications for drug discovery. J Med Chem 51:2871–2878. doi: 10.1021/jm700967e [DOI] [PubMed] [Google Scholar]
- 14. Touw DJ. 1998. Clinical pharmacokinetics of antimicrobial drugs in cystic fibrosis. Pharm World Sci 20:149–160. doi: 10.1023/a:1008634911114 [DOI] [PubMed] [Google Scholar]
- 15. Tod M, Padoin C, Petitjean O. 2000. Clinical pharmacokinetics and pharmacodynamics of isepamicin. Clin Pharmacokinet 38:205–223. doi: 10.2165/00003088-200038030-00002 [DOI] [PubMed] [Google Scholar]
- 16. Contrepois A, Brion N, Garaud JJ, Faurisson F, Delatour F, Levy JC, Deybach JC, Carbon C. 1985. Renal disposition of gentamicin, dibekacin, tobramycin, netilmicin, and amikacin in humans. Antimicrob Agents Chemother 27:520–524. doi: 10.1128/AAC.27.4.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gordon RC, Regamey C, Kirby WM. 1972. Serum protein binding of the aminoglycoside antibiotics. Antimicrob Agents Chemother 2:214–216. doi: 10.1128/AAC.2.3.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Segal JL, Brunnemann SR, Eltorai IM. 1990. Pharmacokinetics of amikacin in serum and in tissue contiguous with pressure sores in humans with spinal cord injury. Antimicrob Agents Chemother 34:1422–1428. doi: 10.1128/AAC.34.7.1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brunnemann SR, Segal JL. 1991. Amikacin serum protein binding in spinal cord injury. Life Sci 49:PL1–PL5. doi: 10.1016/0024-3205(91)90030-f [DOI] [PubMed] [Google Scholar]
- 20. Bodem CR, Lampton LM, Miller DP, Tarka EF, Everett ED. 1983. Endobronchial pH. relevance of aminoglycoside activity in Gram-negative bacillary pneumonia. Am Rev Respir Dis 127:39–41. doi: 10.1164/arrd.1983.127.1.39 [DOI] [PubMed] [Google Scholar]
- 21. Blaser J, Lüthy R. 1988. Comparative study on antagonistic effects of low pH and cation supplementation on in-vitro activity of quinolones and aminoglycosides against Pseudomonas aeruginosa. J Antimicrob Chemother 22:15–22. doi: 10.1093/jac/22.1.15 [DOI] [PubMed] [Google Scholar]
- 22. Rodvold KA, George JM, Yoo L. 2011. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clinical Pharmacokinetics 50:637–664. doi: 10.2165/11594090-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 23. Boselli E, Breilh D, Djabarouti S, Guillaume C, Rimmelé T, Gordien J-B, Xuereb F, Saux M-C, Allaouchiche B. 2007. Reliability of mini-bronchoalveolar lavage for the measurement of epithelial lining fluid concentrations of tobramycin in critically ill patients. Intensive Care Med 33:1519–1523. doi: 10.1007/s00134-007-0688-x [DOI] [PubMed] [Google Scholar]
- 24. Najmeddin F, Shahrami B, Azadbakht S, Dianatkhah M, Rouini MR, Najafi A, Ahmadi A, Sharifnia H, Mojtahedzadeh M. 2020. Evaluation of epithelial lining fluid concentration of amikacin in critically ill patients with ventilator-associated pneumonia. J Intensive Care Med 35:400–404. doi: 10.1177/0885066618754784 [DOI] [PubMed] [Google Scholar]
- 25. Drwiega EN, Rodvold KA. 2022. Penetration of antibacterial agents into pulmonary epithelial lining fluid: an update. Clin Pharmacokinet 61:17–46. doi: 10.1007/s40262-021-01061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazzei T, Novelli A, De Lalla F, Mini E, Periti P. 1995. Tissue penetration and pulmonary disposition of tobramycin. J Chemother 7:363–370. doi: 10.1179/joc.1995.7.4.363 [DOI] [PubMed] [Google Scholar]
- 27. Panidis D, Markantonis SL, Boutzouka E, Karatzas S, Baltopoulos G. 2005. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest 128:545–552. doi: 10.1378/chest.128.2.545 [DOI] [PubMed] [Google Scholar]
- 28. Carcas AJ, García-Satué JL, Zapater P, Frías-Iniesta J. 1999. Tobramycin penetration into epithelial lining fluid of patients with pneumonia. Clin Pharmacol Ther 65:245–250. doi: 10.1016/S0009-9236(99)70103-7 [DOI] [PubMed] [Google Scholar]
- 29. Valcke YJ, Vogelaers DP, Colardyn FA, Pauwels RA. 1992. Penetration of netilmicin in the lower respiratory tract after once-daily dosing. Chest 101:1028–1032. doi: 10.1378/chest.101.4.1028 [DOI] [PubMed] [Google Scholar]
- 30. Tayman C, El-Attug MN, Adams E, Van Schepdael A, Debeer A, Allegaert K, Smits A. 2011. Quantification of amikacin in bronchial epithelial lining fluid in neonates. Antimicrob Agents Chemother 55:3990–3993. doi: 10.1128/AAC.00277-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Funatsu Y, Hasegawa N, Fujiwara H, Namkoong H, Asami T, Tasaka S, Kimizuka Y, Kamata H, Ishii M, Iketani O, Ogata H, Iwata S, Betsuyaku T. 2014. Pharmacokinetics of arbekacin in bronchial epithelial lining fluid of healthy volunteers. J Infect Chemother 20:607–611. doi: 10.1016/j.jiac.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 32. Lodise TP, Sorgel F, Melnick D, Mason B, Kinzig M, Drusano GL. 2011. Penetration of meropenem into epithelial lining fluid of patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 55:1606–1610. doi: 10.1128/AAC.01330-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lodise TP, Kinzig-Schippers M, Drusano GL, Loos U, Vogel F, Bulitta J, Hinder M, Sörgel F. 2008. Use of population pharmacokinetic modeling and Monte Carlo simulation to describe the pharmacodynamic profile of cefditoren in plasma and epithelial lining fluid. Antimicrob Agents Chemother 52:1945–1951. doi: 10.1128/AAC.00736-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Layios N, Visée C, Mistretta V, Denooz R, Maes N, Descy J, Frippiat F, Marchand S, Grégoire N. 2022. Modelled target attainment after temocillin treatment in severe pneumonia: systemic and epithelial lining fluid pharmacokinetics of continuous versus intermittent infusions. Antimicrob Agents Chemother 66:e0205221. doi: 10.1128/AAC.02052-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Das S, Fitzgerald R, Ullah A, Bula M, Collins AM, Mitsi E, Reine J, Hill H, Rylance J, Ferreira DM, Tripp K, Bertasini A, Franzoni S, Massimiliano M, Lahlou O, Motta P, Barth P, Velicitat P, Knechtle P, Hope W. 2020. Intrapulmonary pharmacokinetics of cefepime and enmetazobactam in healthy volunteers: towards new treatments for nosocomial pneumonia. Antimicrob Agents Chemother 65:e01468-20. doi: 10.1128/AAC.01468-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Felton TW, Ogungbenro K, Boselli E, Hope WW, Rodvold KA. 2018. Comparison of piperacillin exposure in the lungs of critically ill patients and healthy volunteers. J Antimicrob Chemother 73:1340–1347. doi: 10.1093/jac/dkx541 [DOI] [PubMed] [Google Scholar]
- 37. van Hasselt JGC, Rizk ML, Lala M, Chavez-Eng C, Visser SAG, Kerbusch T, Danhof M, Rao G, van der Graaf PH. 2016. Pooled population pharmacokinetic model of imipenem in plasma and the lung epithelial lining fluid. Br J Clin Pharmacol 81:1113–1123. doi: 10.1111/bcp.12901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Landersdorfer CB, Kinzig M, Höhl R, Kempf P, Nation RL, Sörgel F. 2020. Physiologically based population pharmacokinetic modeling approach for ciprofloxacin in bone of patients undergoing orthopedic surgery. ACS Pharmacol Transl Sci 3:444–454. doi: 10.1021/acsptsci.0c00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Landersdorfer CB, Kinzig M, Bulitta JB, Hennig FF, Holzgrabe U, Sörgel F, Gusinde J. 2009. Bone penetration of amoxicillin and clavulanic acid evaluated by population pharmacokinetics and Monte Carlo simulation. Antimicrob Agents Chemother 53:2569–2578. doi: 10.1128/AAC.01119-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sörgel F. 2009. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet 48:89–124. doi: 10.2165/00003088-200948020-00002 [DOI] [PubMed] [Google Scholar]
- 41. Landersdorfer CB, Kinzig M, Hennig FF, Bulitta JB, Holzgrabe U, Drusano GL, Sörgel F, Gusinde J. 2009. Penetration of moxifloxacin into bone evaluated by Monte Carlo simulation. Antimicrob Agents Chemother 53:2074–2081. doi: 10.1128/AAC.01056-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Panjasawatwong N, Wattanakul T, Hoglund RM, Bang ND, Pouplin T, Nosoongnoen W, Ngo VN, Day JN, Tarning J. 2020. Population pharmacokinetic properties of antituberculosis drugs in Vietnamese children with tuberculous meningitis. Antimicrob Agents Chemother 65:e00487-20. doi: 10.1128/AAC.00487-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kennedy JL, Bulitta JB, Chatham-Stephens K, Person MK, Cook R, Mongkolrattanothai T, Shin E, Yu P, Negron ME, Bower WA, Hendricks K. 2022. Postexposure prophylaxis and treatment of Bacillus anthracis infections: a systematic review and meta-analyses of animal models, 1947-2019. Clin Infect Dis 75:S379–S391. doi: 10.1093/cid/ciac591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aulin LBS, Tandar ST, van Zijp T, van Ballegooie E, van der Graaf PH, Saleh MAA, Välitalo P, van Hasselt JGC. 2022. Physiologically based modelling framework for prediction of pulmonary pharmacokinetics of antimicrobial target site concentrations. Clin Pharmacokinet 61:1735–1748. doi: 10.1007/s40262-022-01186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Louie A, Liu W, Fikes S, Brown D, Drusano GL. 2013. Impact of meropenem in combination with tobramycin in a murine model of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother 57:2788–2792. doi: 10.1128/AAC.02624-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drusano GL, Liu W, Fikes S, Cirz R, Robbins N, Kurhanewicz S, Rodriquez J, Brown D, Baluya D, Louie A. 2014. Interaction of drug- and granulocyte-mediated killing of Pseudomonas aeruginosa in a murine pneumonia model. J Infect Dis 210:1319–1324. doi: 10.1093/infdis/jiu237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Becker K, Aranzana-Climent V, Cao S, Nilsson A, Shariatgorji R, Haldimann K, Platzack B, Hughes D, Andrén PE, Böttger EC, Friberg LE, Hobbie SN, ENABLE consortium . 2021. Efficacy of EBL-1003 (apramycin) against Acinetobacter baumannii lung infections in mice. Clin Microbiol Infect 27:1315–1321. doi: 10.1016/j.cmi.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 48. Bulitta JB, Holford NHG. 2008. An introductory guide to non-compartmental analysis, p 1–28. In D’Agostino RB, Sullivan L, Massaro J (ed), Wiley encyclopedia of clinical trials. John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- 49. Akkerman-Nijland AM, Akkerman OW, Grasmeijer F, Hagedoorn P, Frijlink HW, Rottier BL, Koppelman GH, Touw DJ. 2021. The pharmacokinetics of antibiotics in cystic fibrosis. Expert Opin Drug Metab Toxicol 17:53–68. doi: 10.1080/17425255.2021.1836157 [DOI] [PubMed] [Google Scholar]
- 50. Cass R, Kostrub CF, Gotfried M, Rodvold K, Tack KJ, Bruss J. 2013. A double-blind, randomized, placebo-controlled study to assess the safety, tolerability, plasma pharmacokinetics and lung penetration of intravenous plazomicin in healthy subjects [poster 1637]. Abstracts from the European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany. [Google Scholar]
- 51. Braude AC, Hornstein A, Klein M, Vas S, Rebuck AS. 1983. Pulmonary disposition of tobramycin. Am Rev Respir Dis 127:563–565. doi: 10.1164/arrd.1983.127.5.563 [DOI] [PubMed] [Google Scholar]
- 52. Bulitta JB, Bingölbali A, Shin BS, Landersdorfer CB. 2011. Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J 13:201–211. doi: 10.1208/s12248-011-9257-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bulitta JB, Landersdorfer CB. 2011. Performance and robustness of the Monte Carlo importance sampling algorithm using parallelized S-ADAPT for basic and complex mechanistic models. AAPS J 13:212–226. doi: 10.1208/s12248-011-9258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bauer RJ, Guzy S, Ng C. 2007. A survey of population analysis methods and software for complex pharmacokinetic and pharmacodynamic models with examples. AAPS J 9:E60–E83. doi: 10.1208/aapsj0901007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bulitta JB, Duffull SB, Kinzig-Schippers M, Holzgrabe U, Stephan U, Drusano GL, Sörgel F. 2007. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob Agents Chemother 51:2497–2507. doi: 10.1128/AAC.01477-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bulitta JB, Okusanya OO, Forrest A, Bhavnani SM, Clark K, Still JG, Fernandes P, Ambrose PG. 2013. Population pharmacokinetics of fusidic acid: rationale for front-loaded dosing regimens due to autoinhibition of clearance. Antimicrob Agents Chemother 57:498–507. doi: 10.1128/AAC.01354-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sörgel F. 2009. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet 48:89–124. doi: 10.2165/00003088-200948020-00002 [DOI] [PubMed] [Google Scholar]
- 58. Jiao Y, Yan J, Vicchiarelli M, Sutaria DS, Lu P, Reyna Z, Spellberg B, Bonomo RA, Drusano GL, Louie A, Luna BM, Bulitta JB. 2023. Individual components of polymyxin B modeled via population pharmacokinetics to design humanized dosage regimens for a bloodstream and lung infection model in immune-competent mice. Antimicrob Agents Chemother 67:e0019723. doi: 10.1128/aac.00197-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wittau M, Paschke S, Kurlbaum M, Scheele J, Ly NS, Hemper E, Kornmann M, Henne-Bruns D, Bulitta JB. 2017. Population pharmacokinetics and target attainment of ertapenem in plasma and tissue assessed via microdialysis in morbidly obese patients after laparoscopic visceral surgery. Antimicrob Agents Chemother 61:e00952-16. doi: 10.1128/AAC.00952-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peteranderl C, Sznajder JI, Herold S, Lecuona E. 2017. Inflammatory responses regulating alveolar ion transport during pulmonary infections. Front Immunol 8:446. doi: 10.3389/fimmu.2017.00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chai AB, Ammit AJ, Gelissen IC. 2017. Examining the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation. Respir Res 18:41. doi: 10.1186/s12931-017-0526-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Arya V, Demarco VG, Issar M, Hochhaus G. 2006. Contrary to adult, neonatal rats show pronounced brain uptake of corticosteroids. Drug Metab Dispos 34:939–942. doi: 10.1124/dmd.105.007419 [DOI] [PubMed] [Google Scholar]
- 63. Brouwer KLR, Aleksunes LM, Brandys B, Giacoia GP, Knipp G, Lukacova V, Meibohm B, Nigam SK, Rieder M, de Wildt SN, Pediatric Transporter Working Group . 2015. Human Ontogeny of drug transporters: review and recommendations of the pediatric transporter working group. Clin Pharmacol Ther 98:266–287. doi: 10.1002/cpt.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smits A, Annaert P, Van Cruchten S, Allegaert K. 2020. A physiology-based pharmacokinetic framework to support drug development and dose precision during therapeutic hypothermia in neonates. Front Pharmacol 11:587. doi: 10.3389/fphar.2020.00587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Allegaert K, Simons SHP, Tibboel D, Krekels EH, Knibbe CA, van den Anker JN. 2017. Non-maturational covariates for dynamic systems pharmacology models in neonates, infants, and children: filling the gaps beyond developmental pharmacology. Eur J Pharm Sci 109S:S27–S31. doi: 10.1016/j.ejps.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 66. Brillault J, De Castro WV, Harnois T, Kitzis A, Olivier J-C, Couet W. 2009. P-glycoprotein-mediated transport of moxifloxacin in a Calu-3 lung epithelial cell model. Antimicrob Agents Chemother 53:1457–1462. doi: 10.1128/AAC.01253-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Aoki M, Iguchi M, Hayashi H, Shibasaki S, Kurosawa T, Hayashi M. 2009. Active uptake of ulifloxacin from plasma to lung that controls its concentration in epithelial lining fluid. Biol Pharm Bull 32:1095–1100. doi: 10.1248/bpb.32.1095 [DOI] [PubMed] [Google Scholar]
- 68. Deguchi Y, Sun J, Tauchi Y, Sakai S, Morimoto K. 2003. Distribution characteristics of grepafloxacin, a fluoroquinolone antibiotic, in lung epithelial lining fluid and alveolar macrophage. Drug Metab Pharmacokinet 18:319–326. doi: 10.2133/dmpk.18.319 [DOI] [PubMed] [Google Scholar]
- 69. Kim YH, Kim K-J, D’Argenio DZ, Crandall ED. 2021. Characteristics of passive solute transport across primary rat alveolar epithelial cell monolayers. Membranes 11:331. doi: 10.3390/membranes11050331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rotoli BM, Barilli A, Visigalli R, Ferrari F, Frati C, Lagrasta CA, Lascia MD, Riccardi B, Puccini P, Dall’Asta V. 2020. Characterization of ABC transporters in EpiAirway, a cellular model of normal human bronchial epithelium. Int J Mol Sci 21:3190. doi: 10.3390/ijms21093190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sun J, Deguchi Y, Tauchi Y, He Z, Cheng G, Morimoto K. 2006. Distribution characteristics of orally administered olamufloxacin, a newly synthesized fluoroquinolone antibacterial, in lung epithelial lining fluid and alveolar macrophage in rats. Eur J Pharm Biopharm 64:238–245. doi: 10.1016/j.ejpb.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 72. Aoki Y, Sundqvist M, Hooker AC, Gennemark P. 2016. PopED lite: an optimal design software for preclinical pharmacokinetic and pharmacodynamic studies. Comput Methods Programs Biomed 127:126–143. doi: 10.1016/j.cmpb.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 73. Luna BM, Bulitta JB.. Development and pharmacokinetic challenges of a murine model for Acinetobacter baumannii infection. Advancing animal models for antibacterial drug development workshop. FDA Center for Drug Evaluation and Research, Silver Spring, MD, March 5, 2020. [Google Scholar]
- 74. Shohet I, Yellin A, Meyerovitch J, Rubinstein E. 1987. Pharmacokinetics and therapeutic efficacy of gentamicin in an experimental pleural empyema rabbit model. Antimicrob Agents Chemother 31:982–985. doi: 10.1128/AAC.31.7.982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. König C, Simmen HP, Blaser J. 1993. Effect of pathological changes of pH, pO2 and pCO2 on the activity of antimicrobial agents in vitro. Eur J Clin Microbiol Infect Dis 12:519–526. doi: 10.1007/BF01970957 [DOI] [PubMed] [Google Scholar]
- 76. Bulitta JB, Hope WW, Eakin AE, Guina T, Tam VH, Louie A, Drusano GL, Hoover JL. 2019. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 63:e02307-18. doi: 10.1128/AAC.02307-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures, tables. and simulation model code.