Abstract
Objectives:
Resection of the primary (RP) in metastatic neuroendocrine tumor (NET) is controversial. The aim is to evaluate survival outcomes for RP in metastatic NET patients.
Methods:
Data were obtained from United States (US) hospitals at the National Cancer Database between 2004 and 2014. Chi-square, analysis of variance tests, univariate and multivariate cox proportional hazards models were evaluated. Kaplan-Meier curves and log-rank tests conducted to compare the survival difference of patient characteristics.
Results:
A total of 2361 patients identified. The mean age was 62.1 years (standard deviation, 13), male to female ratio 1:1; 33% were small intestine, 26.3% pancreas, and 24.4% lung; 69.6% were well differentiated grade and 42.5% underwent RP. The 5-year overall survival (OS) was significantly improved for patients who underwent RP in small intestine (5-year OS, 63.9% vs 44.2%), lung (5-year OS, 65.4% vs 20.2%), and pancreas tumors (5-year OS, 75.6% vs 30.6%). On multivariate analysis, RP (hazard ratio, 0.46; 95% confidence interval, 0.29–0.73; P < 0.001), female, year of diagnosis 2010–2014, margin, Charlson-Deyo score <2, and age <51 years, were associated with better OS.
Conclusions:
Resection of the primary in metastatic well/intermediate-differentiated NET is associated with improved OS compared to no RP.
Keywords: resection, metastatic, well/intermediate-differentiated, neuroendocrine tumor
Introduction
The annual incidence of neuroendocrine tumor (NET) is increasing, due to a true increase in incidence, increased use of improved diagnostic tools, or a combination.1,2 The clinical course of NET is highly variable and is manly determined by the pathologic grade and clinical stage. NET vary from well-differentiated, slow growing tumors to aggressive, highly proliferative poorly differentiated tumors.3 Within the well/moderately differentiated group of tumors, the functional NET pose a therapeutic challenge compared to the non-functional NET because of the impact of hormonal production on organ function and quality of life.4,5 The majority of NET are diagnosed at advanced stages with around 60–80% presenting with distant metastasis at diagnosis.6 The 5-year overall survival of patients with NET ranges from 35 to 82% in well/moderately differentiated NET.7,8
There are no prospective studies to show survival benefit of the resection of the primary tumor in patients with metastatic NET. Many retrospective studies advocate the resection of the primary pancreatic and small bowel tumors in the setting of metastatic disease.9–13 The most recent European Neuroendocrine Tumor Society (ENETS) and North American Neuroendocrine Tumor Society (NANETS) guidelines have adopted removing the primary tumor in patients with G1-G2 NET carrying distant metastases only if limited complication risks and intent-to-cure in offering treatments are provided.14,15 The utility of primary tumor resection is even more questionable for functional NET because of the minimal benefit in the palliative setting of symptom control11,16–18 and for pancreatic NET, considering the risk of postoperative complications.19, 20 The management of lung NET is similar to that of gastroenteropancreatic NET taking into consideration pathological features (mitotic count, Ki-67), somatostatin receptor expression, growth rate and disease extent21.
Stage IV well/intermediate differentiated NET treated with surgical resection of the primary site is frequently associated with improved overall survival (OS) compared to non-surgical therapy in small retrospective analyses. The aim of this study is to evaluate the impact of surgical resection of the primary tumor in patients with unresected distant metastases from NET as well as identify variables associated with prolonged survival in this patient population using the National Cancer Database (NCDB).
MATERIALS AND METHODS
Data was obtained from the NCDB between the years 2004 and 2014. With more than 1500 Commission-on-Cancer-accredited cancer programs participating, the database contains clinical and demographic information on the majority of US cancer patients. Selection criteria for the study included well/intermediate differentiated stage IV NET. Exclusion criteria were patients with missing follow up data and patients who received surgery for metastatic sites. The primary outcome was overall survival in stage IV NET patients who received surgical resection of the primary site. Patient-specific covariates included age at diagnosis, sex, race, insurance status, year of diagnosis, primary site, histology, treatment received (including surgical resection of primary site, chemotherapy and radiation), surgical margins, and Charlson-Deyo score. Ethical approval was not required for the study since patient information in the database is completely de-identified and the database is legally accessible to the public.
Statistical Analysis
The clinical and demographic characteristics of the patients were summarized using descriptive statistics as appropriate for variable type and distribution. All clinically meaningful variables were included and subsequently eliminated based on the level of significance. Chi-square and ANOVA tests were done to identify factors associated with surgical modality. Univariate and multivariate analyses were conducted to identify factors associated with patient outcome. To assess the association between patient characteristics and survival, Cox proportional hazards models were fitted with a backward elimination method (removal criteria P = 0.05). Likelihood ratio test (LRT) was used to compare the model with the covariate being assessed; both added with the model and with the assessed covariate dropped. An alpha level of 0.05 was used, and any covariate with LRT P value ˃0.05 was removed from the final multivariate model. We used backward elimination to automate the LRTs, and determine the final model with the covariates presented. In addition, sensitivity analysis was added to force the covariates with concerns back to the multivariate model to ascertain significant association with overall survival (OS). Kaplan-Meier curves were generated for overall survival. All analyses were done using SAS 9.4 (SAS Institute, Inc., Cary, NC) with a significant level of 0.05.
RESULTS
Patient Demographics and Tumor Characteristics
A total of 2361 patients with advanced stage well to intermediate-differentiated NET older than 18 years were identified (Table 1). The mean age at diagnosis was 62.1 years (standard deviation [SD], 13), with an equal male to female ratio [1:1] (Table 2). About 83% (n = 1958) were White and the majority of NET primaries were in the small intestine (n =780, 33.0%), pancreas (n = 620, 26.3%), lung (n = 576, 24.4%), and colon/rectum (n =278, 11.8%). Majority of the tumors were well differentiated tumors (n = 1643, 69.6%) followed by moderately differentiated (n = 718, 30.4%). Histology codes included neuroendocrine carcinoma (n = 1690, 71.6%), carcinoid tumor (n = 595, 25.2%), and atypical carcinoid tumor (n = 76, 3.2%). The most common metastatic site was the liver (n = 1179, 49.9%), followed by lung (n = 176, 7.5%), bone (n = 152, 6.4%), and brain (n = 44, 1.9%). Most patients had a Charlson-Deyo score of 0 (n = 1784, 75.6%), 18.0% (n = 425) had a score of 1, and 6.4% (n = 152) had a score of 2. A higher number of patients were diagnosed between 2010 and 2014 compared to 2004–2009 (% of patients,70.6% vs 29.4%). About 44.5% (n = 1051) of the patients were treated at community practices, while 40.1% (n = 946) were treated at academic or research cancer centers.
TABLE 1.
Selection/Exclusion Criteria
| Selection and Exclusion Criteria | Sample Size | Excluded |
|---|---|---|
| NCDB NET cancer cases | 130,234 | — |
| Include stage IV patients | 24,028 | 106,206 |
| Well/intermediate differentiated | 5376 | 18,652 |
| Exclude in situ | 5376 | 0 |
| Exclude patients with unknown surgical status for primary site | 5358 | 18 |
| Exclude patients who did not have pathologic confirmation | 5341 | 17 |
| Exclude CLASS OF CASE = 0* | 4831 | 510 |
| Include SEQUENCE_NUMBER in (0 1)† | 4027 | 804 |
| Exclude patients without follow up data | 3342 | 685 |
| Exclude patients who received surgery for the metastatic sites | 2361 | 981 |
Classifies cases recorded in the database.
Indicates the sequence of malignant and non-malignant neoplasms over the lifetime of the patient.
TABLE 2.
Descriptive Statistics for all Variables of Interest
| N = 2361, n (%) | |
|---|---|
| Age at Diagnosis | |
| 18–34 | 60 (2.5) |
| 35–50 | 375 (15.9) |
| 51+ | 1926 (81.6) |
| Sex | |
| Male | 1181 (50.0) |
| Female | 1180 (50.0) |
| Race | |
| White | 1958 (82.9) |
| Black | 316 (13.4) |
| Other/unknown | 87 (3.7) |
| Primary payor | |
| Not insured/unknown | 121 (5.1) |
| Private | 1103 (46.7) |
| Medicaid | 142 (6.0) |
| Medicare/other government | 995 (42.1) |
| Year of diagnosis | |
| 2004–2009 | 694 (29.4) |
| 2010–2014 | 1667 (70.6) |
| Histology | |
| Carcinoid tumor, NOS | 595 (25.2) |
| Neuroendocrine carcinoma, NOS | 1690 (71.6) |
| Atypical carcinoid tumor | 76 (3.2) |
| Primary Site | |
| Small bowel (ileum, duodenum, jejunum) | 780 (33.0) |
| Pancreas | 620 (26.3) |
| Gastric and stomach | 81 (3.4) |
| Liver | 14 (0.6) |
| Lung | 576 (24.4) |
| Kidney | 9 (0.4) |
| Prostate | 3 (0.1) |
| Rectum and colon | 278 (11.8) |
| Charlson-Deyo score | |
| 0 | 1784 (75.6) |
| 1 | 425 (18.0) |
| 2+ | 152 (6.4) |
Treatment
Surgery
Patients who underwent surgery at the primary site constituted 42.5% (n =1003), while 57.5% (n = 1358) did not have surgery (Table 3). About 67.3% (n = 675) of the patients who underwent resection had negative margins (P < 0.001) (Table 4). Surgery for primary site occurred more often in patients with private insurance (% of patients, 53.6%), year of diagnosis 2010–2014 (78.0%), small bowel primary site (% of patients, 58.2%), and 0–3 positive regional nodes (% of patients, 47.1%) compared to uninsured/Medicaid/Medicare (4.3%/5.3%/36.8%), year of diagnosis 2004–2009 (% of patients, 22.0%), pancreas/lung/colon and rectum primary site (11.1%/10.3%/17.5%), 4–86 positive regional nodes (% of patients, 34.3%) respectively (P < 0.001) (Table 4).
TABLE 3.
Treatment Received by Study Participants
| N = 2361, n (%) | |
|---|---|
| Resection | |
| No surgery for primary site | 1358 (57.5) |
| Local tumor destruction | 56 (2.4) |
| Partial resection | 681 (28.8) |
| Total resection | 231 (9.8) |
| Total or partial unknown | 35 (1.5) |
| Regional nodes examined | |
| 0 | 1415 (59.9) |
| 1–90 | 842 (35.7) |
| Not available | 104 (4.4) |
| Regional nodes positive | |
| 0–3 | 510 (21.6) |
| 4–86 | 348 (14.7) |
| Not available | 1503 (63.7) |
| Surgical margins | |
| Yes | 276 (11.7) |
| No | 675 (28.6) |
| Not available | 1410 (59.7) |
| Radiation therapy at any CoC facility | |
| No | 2084 (88.3) |
| Yes | 256 (10.8) |
| Not available | 21 (0.9) |
| Chemotherapy at any CoC facility | |
| No | 1518 (64.3) |
| Chemotherapy administered, type and number of agents not documented | 50 (2.1) |
| Single-agent chemotherapy | 272 (11.5) |
| Multi-agent chemotherapy | 431 (18.3) |
| Not available | 90 (3.8) |
CoC indicates Commission on cancer coding
TABLE 4.
Univariate Association With Surgical Modality
| Resection | |||
|---|---|---|---|
| Covariates | Yes, n = 1003, n (%) | No, n = 1358, n (%) | P |
| Primary payor | <0.001 | ||
| Not insured/unknown | 43 (4.29) | 78 (5.74) | |
| Private | 538 (53.64) | 565 (41.61) | |
| Medicaid | 53 (5.28) | 89 (6.55) | |
| Medicare/other government | 369 (36.79) | 626 (46.1) | |
| Year of diagnosis | <0.001 | ||
| 2004–2009 | 221 (22.03) | 473 (34.83) | |
| 2010–2014 | 782 (77.97) | 885 (65.17) | |
| Histology | <0.001 | ||
| Carcinoid tumor, NOS | 350 (34.9) | 245 (18.04) | |
| Neuroendocrine carcinoma, NOS | 634 (63.21) | 1056 (77.76) | |
| Atypical carcinoid tumor | 19 (1.89) | 57 (4.2) | |
| Primary site | <0.001 | ||
| Small bowel (ileum, duodenum, jejunum) | 584 (58.23) | 196 (14.43) | |
| Pancreas | 111 (11.07) | 509 (37.48) | |
| Gastric and stomach | 26 (2.59) | 55 (4.05) | |
| Liver | 0 (0) | 14 (1.03) | |
| Lung | 103 (10.27) | 473 (34.83) | |
| Kidney | 4 (0.4) | 5 (0.37) | |
| Prostate | 0 (0) | 3 (0.22) | |
| Rectum and colon | 175 (17.45) | 103 (7.58) | |
| Regional nodes examined | <0.001 | ||
| 0 | 166 (16.55) | 1249 (91.97) | |
| 1–90 | 810 (80.76) | 32 (2.36) | |
| Not available | 27 (2.69) | 77 (5.67) | |
| Regional nodes positive | <0.001 | ||
| 0–3 | 472 (47.06) | 38 (2.8) | |
| 4–86 | 344 (34.3) | 4 (0.29) | |
| Not available | 187 (18.64) | 1316 (96.91) | |
| Surgical margins | <0.001 | ||
| Yes | 276 (27.52) | 0 (0) | |
| No | 675 (67.3) | 0 (0) | |
| Not available | 52 (5.18) | 1358 (100) | |
| Radiation therapy at any CoC facility | <0.001 | ||
| No | 938 (93.52) | 1146 (84.39) | |
| Yes | 51 (5.08) | 205 (15.1) | |
| Not available | 14 (1.4) | 7 (0.52) | |
| Chemotherapy at any CoC facility | <0.001 | ||
| No | 766 (76.37) | 752 (55.38) | |
| Chemotherapy administered, type and number of agents not documented | 14 (1.4) | 36 (2.65) | |
| Single-agent chemotherapy | 95 (9.47) | 177 (13.03) | |
| Multi-agent chemotherapy | 77 (7.68) | 354 (26.07) | |
| Not available | 51 (5.08) | 39 (2.87) | |
| Charlson-Deyo score | 0.070 | ||
| 0 | 776 (77.37) | 1008 (74.23) | |
| 1 | 175 (17.45) | 250 (18.41) | |
| 2+ | 52 (5.18) | 100 (7.36) | |
P < 0.05 is the significant cutoff difference shown in bold font.
Chemotherapy and Radiation Therapy
Chemotherapy was given in 31.9% of patients (11.5% single agent, 18.3% multi-agent) and 10.8% received radiation (Table 3). In patients who underwent surgery for primary site, 18.6% received chemotherapy, compared to non-surgical candidates that received chemotherapy more often (41.8%) (Table 4). In patients who underwent surgery for primary site, 5.1% received radiation, compared to 15.1% in the non-surgery group (P < 0.001).
Overall Survival
On univariate and multivariate analyses resection of the primary (hazard ratio [HR], 0.46; 95% confidence interval [CI], 0.29–0.73; P < 0.001) was associated with improved overall survival (OS) compared to no surgery. Other covariates associated with improved survival included female sex (HR, 0.88; 95% CI, 0.78–0.99; P = 0.030), year of diagnosis 2010–2014 (HR, 0.82; 95% CI, 0.73–0.94; P = 0.003), neuroendocrine tumor histology (HR, 0.67; 95% CI, 0.57–0.78; P < 0.001), negative surgical margin (HR, 0.73; 95% CI, 0.57–0.94; P = 0.014), Charlson-Deyo score <2, and age <51 years at diagnosis (HR, 0.62; 95% CI, 0.51–0.75; P < 0.001) compared to male sex, year of diagnosis 2004–2009, other histologies, positive surgical margin, Charlson-Deyo score = 2 (HR, 1.78; 95% CI, 1.44–2.21; P < 0.001), and age >52 years at diagnosis respectively (Table 5). Chemotherapy and radiation were not associated with improved OS (Table 5). Five-year OS for resection of the primary site (5-year OS, 60.6%) was higher than for no surgical treatment (5-year OS, 28.1%) (Fig. 1). A similar pattern is seen when stratified for comorbidity score, age, and histology. Resection of the primary in stage IV well/intermediate-differentiated NET is associated with improved 5-year OS compared to patients with no surgery in small intestine (5-year OS, 63.9% vs 44.2%) (Fig. 2), lung (5-year OS, 65.4% vs 20.2%) (Fig. 3), pancreas tumors (5-year OS, 75.6% vs 30.6%) (Fig. 4), and rectum/colon tumors (5-year OS, 39.6% vs 19.7%) (Fig. 5).
TABLE 5.
Multivariable Survival Analysis of OS
| Overall Survival, mo* | |||
|---|---|---|---|
|
| |||
| Covariates | Hazard Ratio (95% CI) | HR P |
Type3 P† |
| Resection | <0.001 | ||
| Yes | 0.46 (0.29–0.73) | <0.0001 | |
| No | REF | — | |
| Age at diagnosis | <0.001 | ||
| 18–34 | 0.55 (0.34–0.90) | 0.018 | |
| 35–50 | 0.62 (0.51–0.76) | <0.001 | |
| 51+ | REF | — | |
| Sex | 0.030 | ||
| Female | 0.88 (0.78–0.99) | 0.030 | |
| Male | REF | — | |
| Year of diagnosis | 0.003 | ||
| 2010–2014 | 0.82 (0.73–0.94) | 0.003 | |
| 2004–2009 | REF | — | |
| Histology | <0.001 | ||
| Carcinoid tumor, NOS | 0.67 (0.57–0.78) | <0.001 | |
| Atypical carcinoid tumor | 1.16 (0.86–1.57) | 0.331 | |
| Neuroendocrine carcinoma, NOS | REF | — | |
| Primary Site | <0.001 | ||
| Rectum and colon | 1.81 (1.47–2.24) | <0.001 | |
| Prostate | 2.15 (0.67–6.87) | 0.195 | |
| Kidney | 2.06 (0.84–5.05) | 0.115 | |
| Lung | 1.50 (1.24–1.81) | <0.001 | |
| Liver | 1.09 (0.60–1.98) | 0.777 | |
| Gastric and stomach | 1.86 (1.34–2.57) | <0.001 | |
| Pancreas | 1.17 (0.97–1.41) | 0.106 | |
| Small bowel (ileum, duodenum, jejunum) | REF | — | |
| Surgical margins | 0.048 | ||
| Not available | 0.79 (0.48–1.31) | 0.366 | |
| No | 0.73 (0.57–0.94) | 0.014 | |
| Yes | REF | — | |
| Radiation therapy at any CoC facility | 0.001 | ||
| Not available | 0.37 (0.11–1.17) | 0.090 | |
| Yes | 1.33 (1.12–1.58) | 0.001 | |
| No | REF | — | |
| Chemotherapy at any CoC Facility | <0.001 | ||
| Not Available | 0.83 (0.56–1.22) | 0.340 | |
| Multi–agent chemotherapy | 1.34 (1.15–1.56) | <0.001 | |
| Single–agent chemotherapy | 1.03 (0.85–1.25) | 0.741 | |
| Chemotherapy administered, type and number of agents not documented | 1.39 (0.99–1.96) | 0.057 | |
| No | REF | — | |
| Charlson–Deyo Score | <0.001 | ||
| 2+ | 1.78 (1.44–2.21) | <0.001 | |
| 1 | 1.03 (0.88–1.20) | 0.692 | |
| 0 | REF | — | |
Bold values are statistically significant.
Number of observations in the original data set = 2361. Number of observations used = 2361.
Backward selection with an alpha level of removal of .20 was used. The following variables were removed from the model: Regional Nodes Examined, Regional Nodes Positive, Pathologic Stage Group, Race, Spanish Hispanic Origin, and Urban/Rural 2013.
REF indicates reference
FIGURE 1.
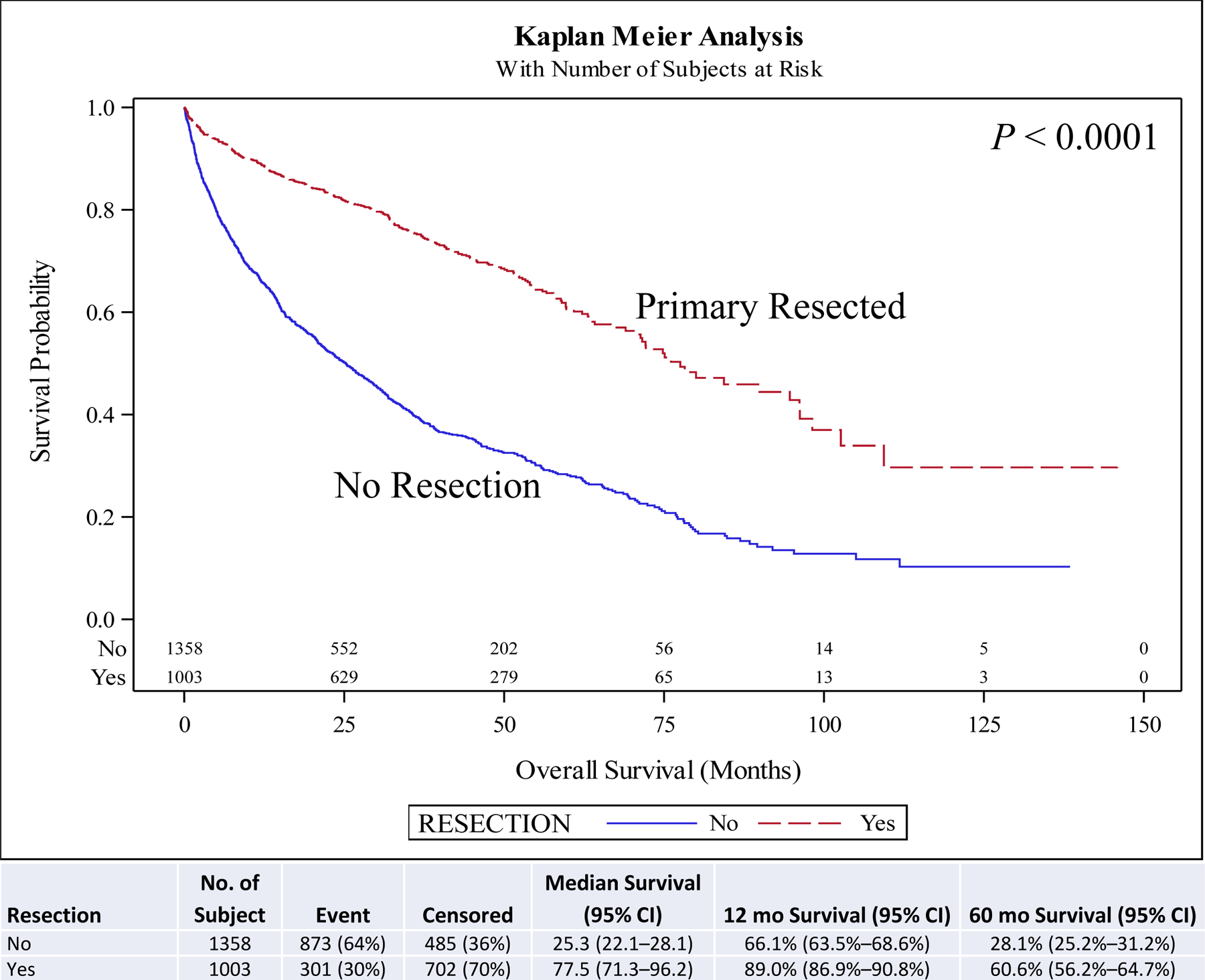
Kaplan-Meier plot for resection in all patients.
FIGURE 2.
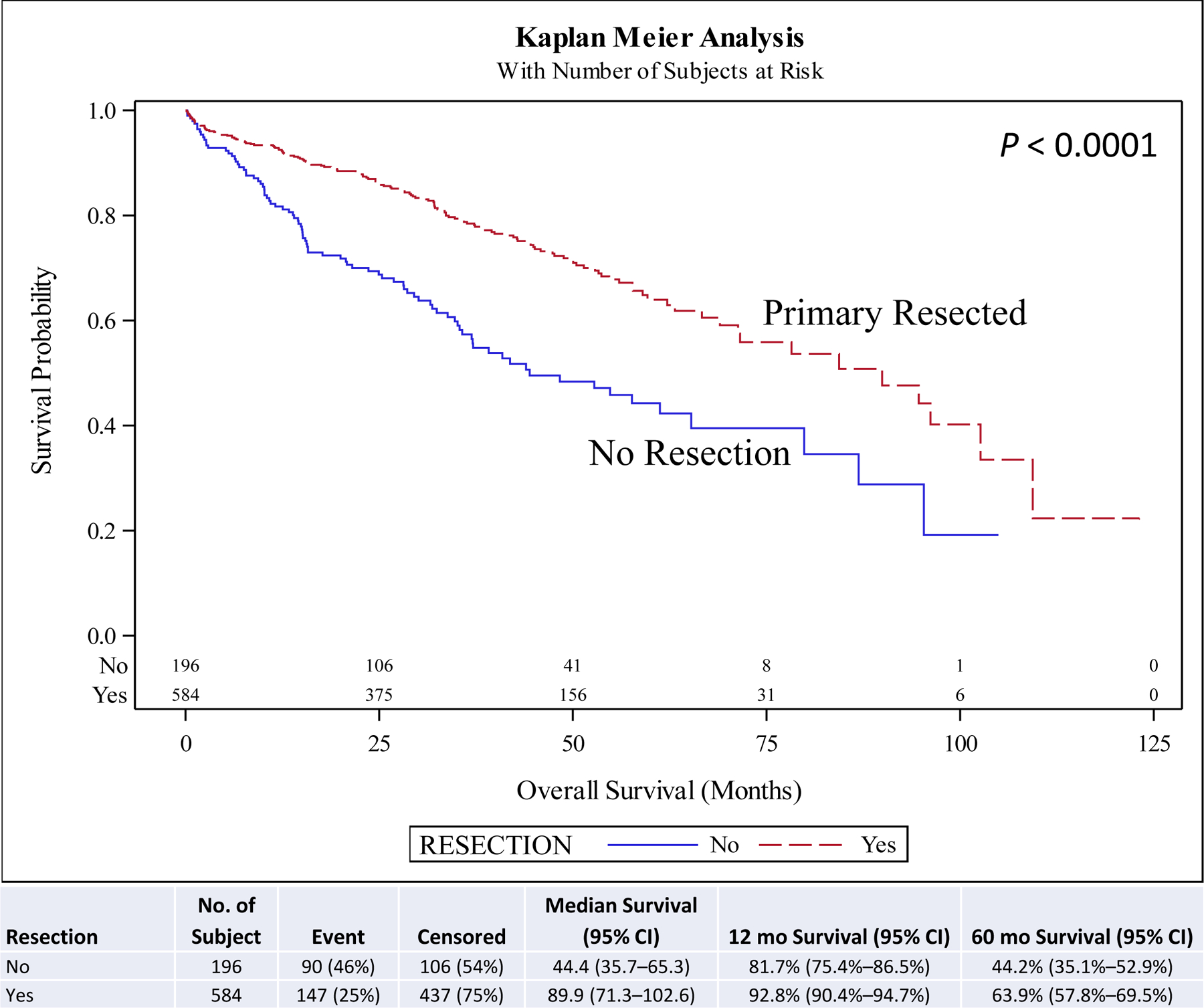
Kaplan-Meier plot for resection stratified by primary site (small bowel).
FIGURE 3.
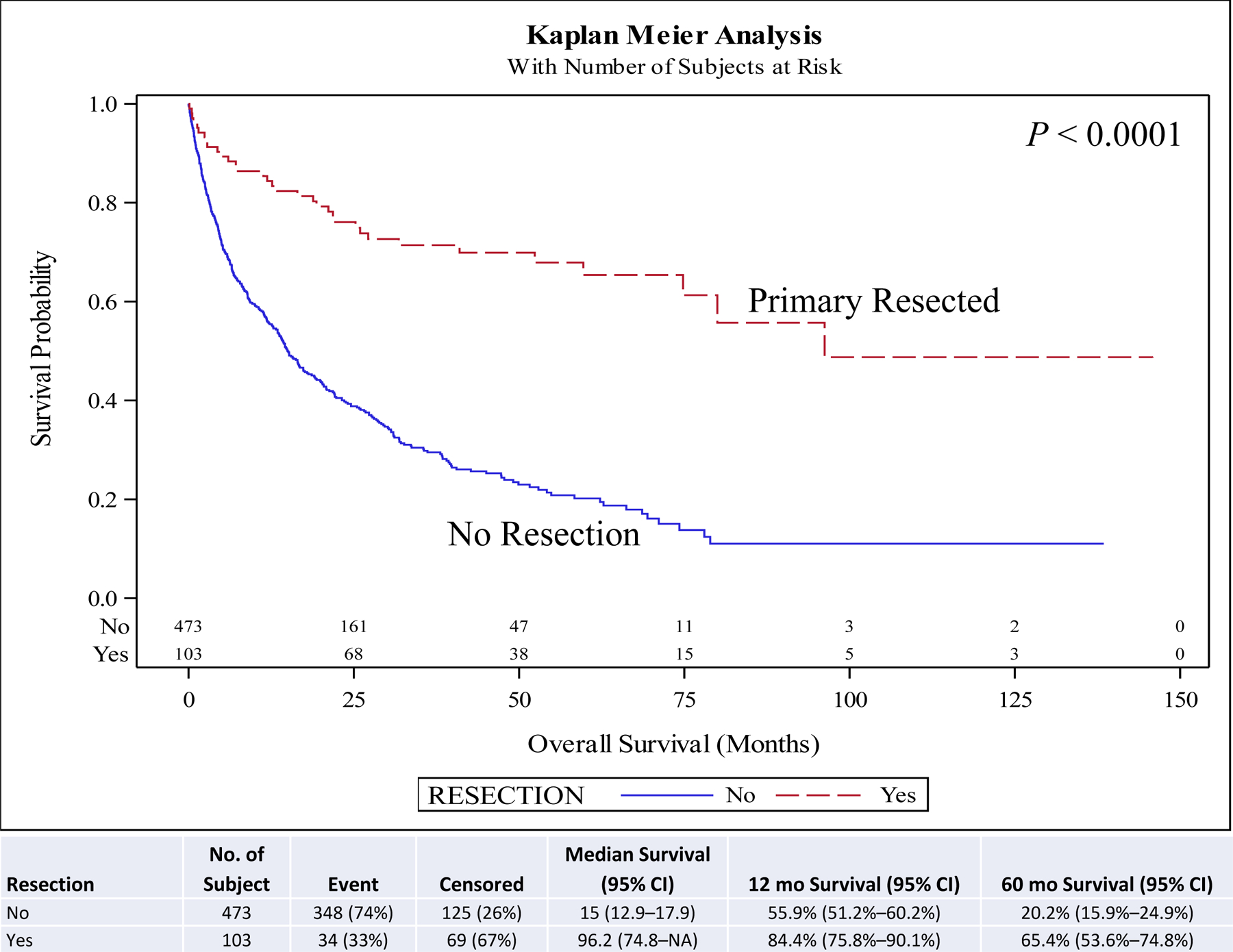
Kaplan-Meier plot for resection stratified by primary site (lung). NA, not applicable or not reached at the time of the analysis.
FIGURE 4.
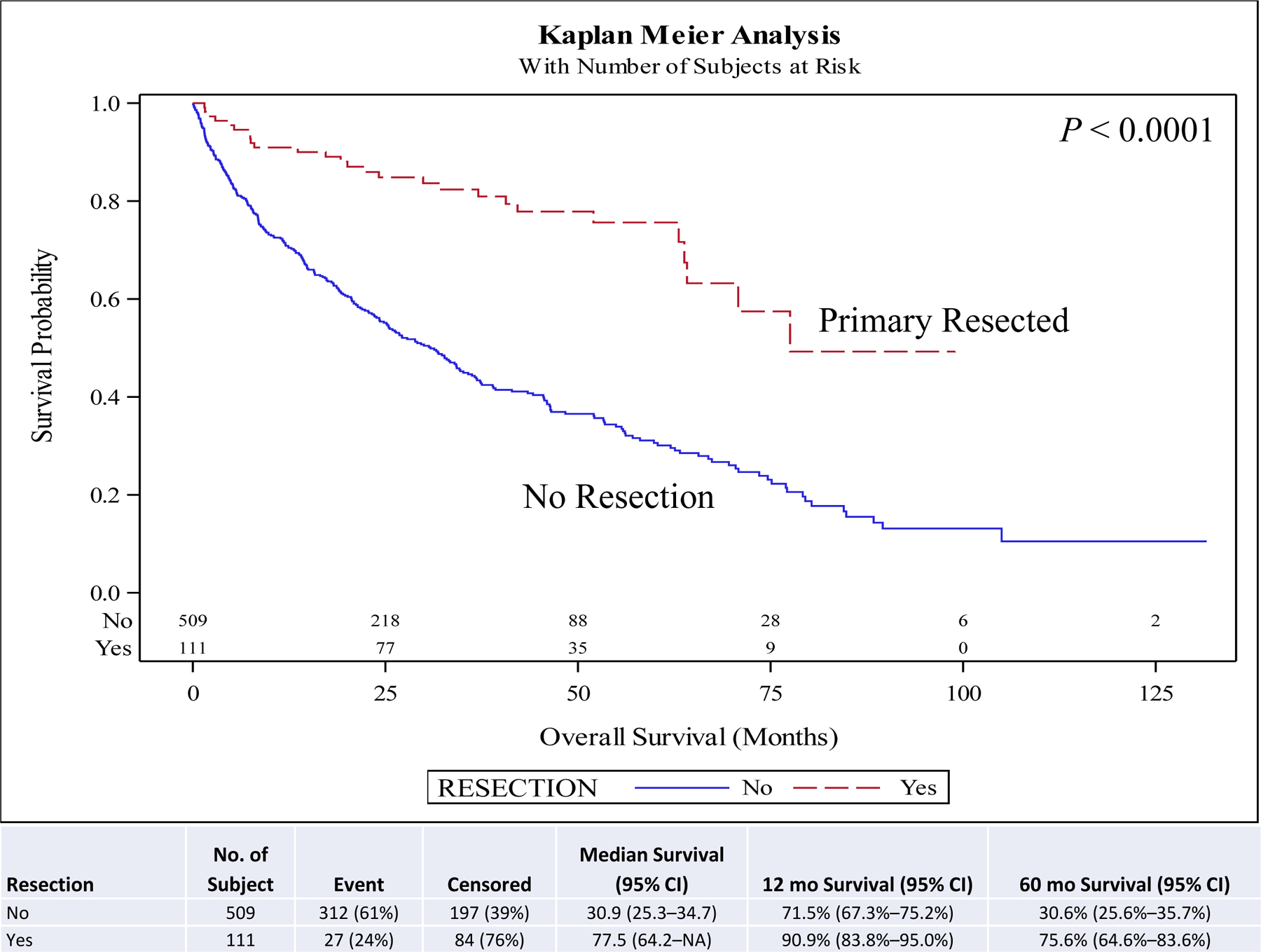
Kaplan-Meier plot for resection stratified by primary site (pancreas). NA, not applicable or not reached at the time of the analysis.
FIGURE 5.
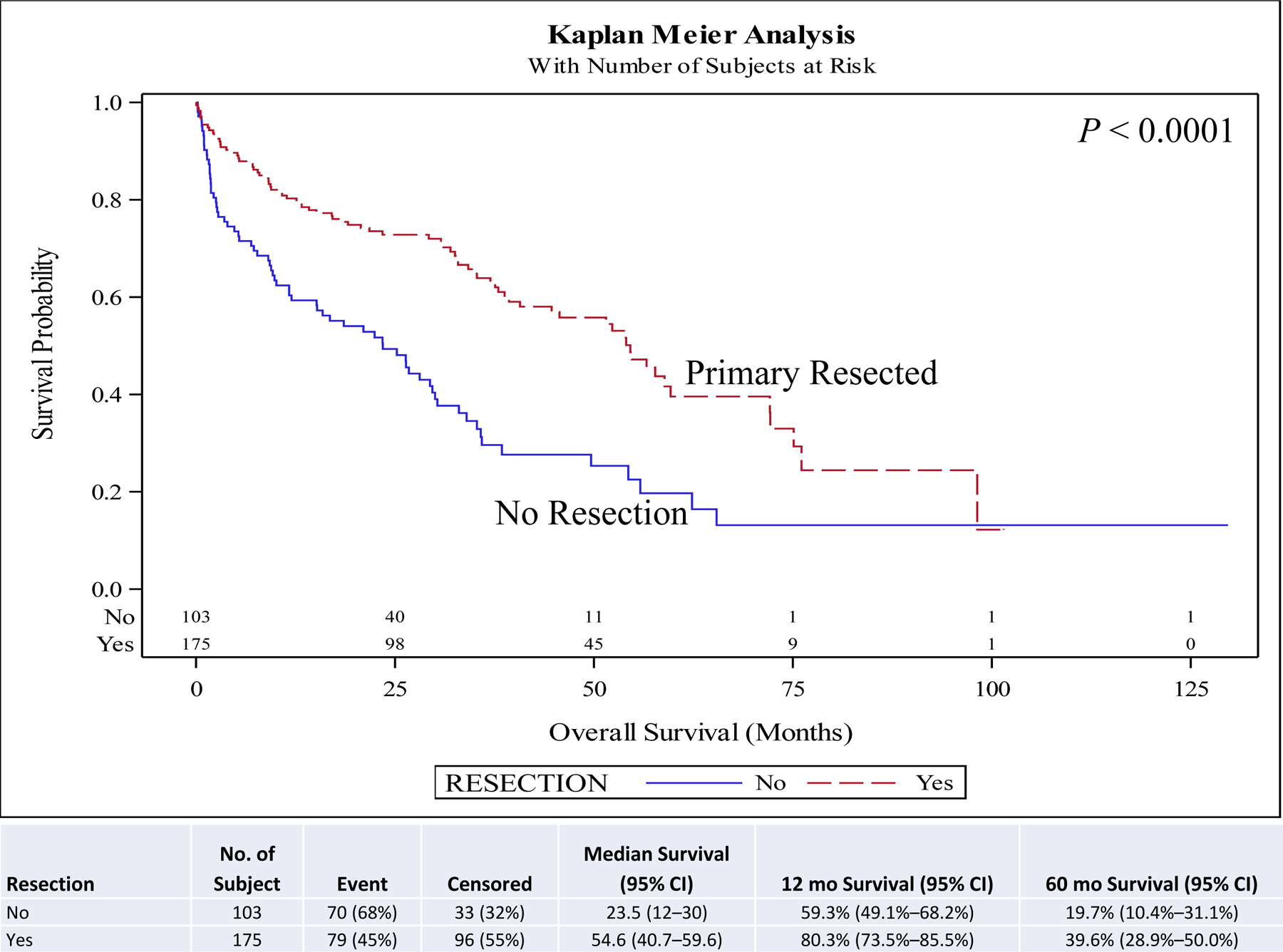
Kaplan-Meier plot for resection stratified by primary site (rectum/colon).
Subtype Analysis
For the pancreas subset, 17.9% (n =111/620) underwent resection. On univariate (HR, 0.29; 0.19–0.43; P <0.001) and multivariate (HR 0.33; 95% CI, 0.22–0.49; P < 0.001) analyses, resection of the primary was associated with improved OS compared to no surgery (Supplemental Table 1). Five-year OS for resection of the primary site (% of patients, 75.6%) was higher than for no surgical treatment (% of patients, 30.6%) (P < 0.001) (Fig. 4).
For the small bowel subset, 74.9% (n = 584/780) underwent resection. On univariate analysis, resection of the primary was associated with improved OS compared to no surgery (HR, 0.47; 95% CI, 0.36–0.61; P < 0.001). On multivariate analysis, resection of the primary leaned towards improved OS compared to no surgery, but did not reach statistical significance (HR, 0.76; 95% CI, 0.50–1.16; P = 0.210) (Supplemental Table 1). Five-year OS for resection of the primary site (% of patients, 63.9%) was higher than for no surgical treatment (% of patients, 44.2%) (P < 0.001) (Fig. 2).
For the remaining subset in the total cohort, 32.0% (n = 308/961) underwent resection. On univariate (HR, 0.39; 95% CI, 0.32–0.48; P < 0.001) and multivariate (HR, 0.44; 95% CI, 0.32–0.61; P < 0.001) analyses, resection of the primary was associated with improved OS compared to no surgery (Supplemental Table 1). Five-year OS for resection of the primary site (49.3%) was higher than for no surgical treatment (21.7%) (P < 0.001) (Supplemental Fig. 1).
DISCUSSION
Surgical resection of the primary tumor in patients with metastatic well/intermediate differentiated NET is a controversial practice.22 The majority of patients with NET have diffuse metastatic disease at presentation.23 In these patients, curative metastatectomy is challenging. There remains uncertainty whether removal of the primary tumor leads to a survival benefit.10,11,14,21,24–30 This study suggests that primary tumor resection is associated with prolonged survival for patients with well or intermediate differentiated metastatic NET of the small bowel, pancreas, lung, colon and rectum, even without metastatectomy. Similarly, a study from the United States reported a progression-free survival of 56 months after primary tumor resection in patients with non-resectable liver metastases from NET compared with 25 months observed when the primary tumor was not resected, with median survivals of 159 vs 47 months, respectively.18 In the UK and Ireland Neuroendocrine Tumor Society (UKINETS) study, resection of the primary tumor was one of the independent predictors of prolonged survival in midgut tumors with liver metastases.17 A positive impact on prognosis after resection of the primary tumor has also been reported for pancreatic NET.10,31 In addition, a study conducted using the California Cancer Registry between the years 2005 and 2011 found that primary tumor resection in gastrointestinal NET is associated with better OS, with or without liver treatment, irrespective of grade.32
Interestingly, in a study conducted by Citterio et al, the gain in survival obtained by surgical resection of the primary tumor was significant and independent from primary tumor site in the selected unfavorable population of metastatic NET.22 The observed median survival of the patients treated with somatostatin analogue and other medical therapies was 37 months,8,33 whereas patients in whom medical treatment was complemented with resection of the primary NET tumor, a median survival of 138 months was observed,22 Similarly, our study reached the conclusion that resection of the primary in stage IV well/intermediate-differentiated NET is associated with improved 5-year OS compared to patients with no surgery in small intestine, lung, and pancreas tumors.
The 2016 ENETS consensus guidelines, in the setting of unresectable metastatic disease recommend palliative resection of primary jejunal and ileal tumors, but did not comment on the role of palliative primary tumor resection in pancreatic NET.14,21,26 These recommendations are based on early data suggesting the potential for improved survival following resection of intestinal primaries, with the intention of avoiding intestinal obstruction and ischemic complications.23 The NANETS recommendation for localized pancreatic NET patients who have functional disease is to undergo surgery irrespective of size34 but no consensus guideline reached in 2020 NANETS update regarding the resection of the non-pancreatic primary in the metastatic disease setting34 except for locally symptomatic patients. In some reports, resection of the primary NET has a trend towards improved survival for patients who received peptide receptor radionuclide therapy (PRRT) after the resection35–37 with higher stabilization and objective responses after PRRT leading to better survival outcomes.35,37 This deserves future retrospective and prospective studies.
Earlier single-center studies, a series of reports using the Surveillance, Epidemiology, and End Results (SEER) database, a review from the California Cancer registry, and an NCDB study for gastroenteropancreatic NET previously established similar findings in more limited patient populations.18,27,32,36,38–40 To date, this is the largest study examining resection of the primary tumor in stage IV well/intermediate differentiated NET with different anatomical primaries (small bowel, pancreas, lung, colon, rectum) published in the literature and the first report to demonstrate in such selected subgroups of NET a clear positive impact of primary tumor resection on survival.
There are no randomized controlled trials evaluating the outcomes of palliative primary NET resection in stage IV disease.23 The majority of included studies were retrospective cohort series, which may have therefore been subject to publication bias.23 In addition, several studies made no attempt to control for confounding variables, leading to a likely bias towards patient who underwent resection.23 The limitations of this study are related to the retrospective database analysis design. Even though fairly complete and recognized to capture the largest number of cancer patients in the US, disease-specific mortality, recurrence indices, response to treatment and prior history of malignancies are not captured by the NCDB.41 Information on the specific agents of chemotherapy are not available, however, octreotide analogs are part of the systemic therapy defined by the NCDB as chemotherapy. Octreotide was not amongst the chemotherapy agents that changed their category to immunotherapy in 2013 (six drugs previously classified as chemotherapy are now classified as biological response modifier therapy (BRM)/ Immunotherapy: Alemtuzumab/Campath, Bevacizumab/Avastin, Rituximab, Trastuzumab/Herceptin, Pertuzumab/Perjeta, and Cetuximab/Erbitux), so if octreotide was used as first line treatment among the year range which our cohort was diagnosed, then it should be considered in the chemotherapy category. The chemotherapy category group reported here is likely related to the 5FU based treatments like 5FU and streptozocin combinations that were common before year 2010. The chemotherapy category excludes oral therapies which are standard of care now. The oral therapies include tyrosine kinase inhibitors, like sunitinib, and mTOR inhibitors, like everolimus and temsirolimus. In addition, capecitabine and temozolomide combination treatment is not captured by this database. The radiation data included in this analysis is likely to be palliative radiation for symptom management. Additionally, indications for surgery is not available. Patients had surgery for symptomatic or asymptomatic disease. Also, burden of disease, peritoneal, liver and bone disease which usually determine prognosis, is not available. Furthermore, the selection criteria of the patients undergoing surgery is not defined. Missing data on immunohistochemistry, Ki-67 index, mitotic rate, grade and differentiation affect survival and limit the conclusion. Subsequent therapies and exposure to PRRT affect survival and these data are missing. Another limitation of the NCDB is that we do not know if the primary tumor was ‘symptomatic’. Furthermore, the obvious contribution of good patient selection, performance status, and the ability to do the resection with minimal morbidity/mortality to the improved outcomes observed with resection of the primary cannot be overstated. This analysis included different NET primaries with separate sub-group analyses performed. Furthermore, only four distant sites of metastases were assessed and the information on involvement of other organs was unavailable. We excluded patients that received metastatectomies, therefore we were unable to study the benefit of distant site resection in addition to primary site resection. Despite these limitations, our findings have important implications.
The relatively indolent behavior of well/intermediate differentiated NET promotes a strategy of aggressive surgical intervention, even in the setting of metastatic disease. This is particularly true in symptomatic patients with good functional status.42–44 The findings of the present analysis endorse a role for primary tumor resection in small intestinal, pancreatic, lung, and colorectal tumors, provided surgery can be performed with low morbidity and mortality. Further work is necessary to evaluate any additional benefit of debulking surgery, simultaneous metastatic hepatic and/or peritoneal debulking, cytoreductive surgery with hyperthermic intra-peritoneal chemotherapy and non-surgical targeted liver therapies in extensive hepatic involvement.23
Supplementary Material
Funding Sources
There was no specific funding for this study and there are no competing financial disclosures.
Footnotes
Statements
The manuscript’s abstract was published in “Abstracts” in Journal of Clinical Oncology: J Clin Oncol 37, 2019 (suppl; abstr e15693) DOI: 10.1200/JCO.2019.37.15_suppl.e15693. Research reported in this publication was supported in part by the Winship Research Informatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The data used in the study are derived from a de-identified NCDB file. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Statement of Ethics
Ethical approval was not required for the study since patient information in the database is completely de-identified and the database is legally accessible to the public.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Xavier S, Rosa B, Cotter J. Small bowel neuroendocrine tumors: From pathophysiology to clinical approach. World J Gastrointest Pathophysiol 2016;7:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrera-Martinez AD, Hofland LJ, Galvez Moreno MA, et al. Neuroendocrine neoplasms: current and potential diagnostic, predictive and prognostic markers. Endocr Relat Cancer 2019;26:R157–r179. [DOI] [PubMed] [Google Scholar]
- 4.Pearman TP, Beaumont JL, Cella D, et al. Health-related quality of life in patients with neuroendocrine tumors: an investigation of treatment type, disease status, and symptom burden. Support Care Cancer 2016;24:3695–3703. [DOI] [PubMed] [Google Scholar]
- 5.Zerbi A, Falconi M, Rindi G, et al. Clinicopathological features of pancreatic endocrine tumors: a prospective multicenter study in Italy of 297 sporadic cases. Am J Gastroenterol 2010;105:1421–1429. [DOI] [PubMed] [Google Scholar]
- 6.Modlin IM, Gustafsson BI, Moss SF, et al. Chromogranin A--biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol 2010;17:2427–2443. [DOI] [PubMed] [Google Scholar]
- 7.Pape UF, Bohmig M, Berndt U, et al. Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a german referral center. Ann N Y Acad Sci 2004;1014:222–233. [DOI] [PubMed] [Google Scholar]
- 8.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 9.Schindl M, Kaczirek K, Passler C, et al. Treatment of small intestinal neuroendocrine tumors: is an extended multimodal approach justified? World J Surg 2002;26:976–984. [DOI] [PubMed] [Google Scholar]
- 10.Capurso G, Bettini R, Rinzivillo M, et al. Role of resection of the primary pancreatic neuroendocrine tumour only in patients with unresectable metastatic liver disease: a systematic review. Neuroendocrinology 2011;93:223–229. [DOI] [PubMed] [Google Scholar]
- 11.Capurso G, Rinzivillo M, Bettini R, et al. Systematic review of resection of primary midgut carcinoid tumour in patients with unresectable liver metastases. Br J Surg 2012;99:1480–1486. [DOI] [PubMed] [Google Scholar]
- 12.Bettini R, Mantovani W, Boninsegna L, et al. Primary tumour resection in metastatic nonfunctioning pancreatic endocrine carcinomas. Dig Liver Dis 2009;41:49–55. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson B, Kloppel G, Krenning E, et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumors--well-differentiated jejunal-ileal tumor/carcinoma. Neuroendocrinology 2008;87:8–19. [DOI] [PubMed] [Google Scholar]
- 14.Niederle B, Pape UF, Costa F, et al. ENETS Consensus Guidelines update for neuroendocrine neoplasms of the jejunum and ileum. Neuroendocrinology 2016;103:125–138. [DOI] [PubMed] [Google Scholar]
- 15.Boudreaux JP, Klimstra DS, Hassan MM, et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the jejunum, ileum, appendix, and cecum. Pancreas 2010;39:753–766. [DOI] [PubMed] [Google Scholar]
- 16.Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology 2009;89:471–476. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer 2009;16:885–894. [DOI] [PubMed] [Google Scholar]
- 18.Givi B, Pommier SJ, Thompson AK, et al. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery 2006;140:891–897; discussion 897–898. [DOI] [PubMed] [Google Scholar]
- 19.Birnbaum DJ, Turrini O, Vigano L, et al. Surgical management of advanced pancreatic neuroendocrine tumors: short-term and long-term results from an international multi-institutional study. Ann Surg Oncol 2015;22:1000–1007. [DOI] [PubMed] [Google Scholar]
- 20.Gaujoux S, Gonen M, Tang L, et al. Synchronous resection of primary and liver metastases for neuroendocrine tumors. Ann Surg Oncol 2012;19:4270–4277. [DOI] [PubMed] [Google Scholar]
- 21.Pavel M, O’Toole D, Costa F, et al. ENETS Consensus Guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 2016;103:172–185. [DOI] [PubMed] [Google Scholar]
- 22.Citterio D, Pusceddu S, Facciorusso A, et al. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur J Surg Oncol 2017;43:380–387. [DOI] [PubMed] [Google Scholar]
- 23.Almond LM, Hodson J, Ford SJ, et al. Role of palliative resection of the primary tumour in advanced pancreatic and small intestinal neuroendocrine tumours: A systematic review and meta-analysis. Eur J Surg Oncol 2017;43:1808–1815. [DOI] [PubMed] [Google Scholar]
- 24.Berardi R, Rinaldi S, Torniai M, et al. Gastrointestinal neuroendocrine tumors: Searching the optimal treatment strategy--A literature review. Crit Rev Oncol Hematol 2016;98:264–274. [DOI] [PubMed] [Google Scholar]
- 25.Oberg K, Knigge U, Kwekkeboom D, et al. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii124–130. [DOI] [PubMed] [Google Scholar]
- 26.Falconi M, Eriksson B, Kaltsas G, et al. ENETS Consensus Guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016;103:153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keutgen XM, Nilubol N, Glanville J, et al. Resection of primary tumor site is associated with prolonged survival in metastatic nonfunctioning pancreatic neuroendocrine tumors. Surgery 2016;159:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12:1083–1092. [DOI] [PubMed] [Google Scholar]
- 29.Singh S, Asa SL, Dey C, et al. Diagnosis and management of gastrointestinal neuroendocrine tumors: An evidence-based Canadian consensus. Cancer Treat Rev 2016;47:32–45. [DOI] [PubMed] [Google Scholar]
- 30.Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 2013;42:557–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertani E, Fazio N, Botteri E, et al. Resection of the primary pancreatic neuroendocrine tumor in patients with unresectable liver metastases: possible indications for a multimodal approach. Surgery 2014;155:607–614. [DOI] [PubMed] [Google Scholar]
- 32.Lewis A, Raoof M, Ituarte PHG, et al. Resection of the primary gastrointestinal neuroendocrine tumor improves survival with or without liver treatment. Ann Surg 2018. [DOI] [PubMed]
- 33.Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1–18, vii. [DOI] [PubMed] [Google Scholar]
- 34.Howe JR, Merchant NB, Conrad C, et al. The North American Neuroendocrine Tumor Society consensus paper on the surgical management of pancreatic neuroendocrine tumors. Pancreas 2020;49:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertani E, Fazio N, Radice D, et al. Resection of the primary tumor followed by peptide receptor radionuclide therapy as upfront strategy for the treatment of G1-G2 pancreatic neuroendocrine tumors with unresectable liver metastases. Ann Surg Oncol 2016;23:981–989. [DOI] [PubMed] [Google Scholar]
- 36.Bertani E, Fazio N, Radice D, et al. Assessing the role of primary tumour resection in patients with synchronous unresectable liver metastases from pancreatic neuroendocrine tumour of the body and tail. A propensity score survival evaluation. Eur J Surg Oncol 2017;43:372–379. [DOI] [PubMed] [Google Scholar]
- 37.Kaemmerer D, Twrznik M, Kulkarni HR, et al. Prior Resection of the Primary Tumor Prolongs Survival After Peptide Receptor Radionuclide Therapy of Advanced Neuroendocrine Neoplasms. Ann Surg 2021;274:e45–e53. [DOI] [PubMed] [Google Scholar]
- 38.Tierney JF, Chivukula SV, Wang X, et al. Resection of primary tumor may prolong survival in metastatic gastroenteropancreatic neuroendocrine tumors. Surgery 2019;165:644–651. [DOI] [PubMed] [Google Scholar]
- 39.Hill JS, McPhee JT, McDade TP, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer 2009;115:741–751. [DOI] [PubMed] [Google Scholar]
- 40.Tao L, Xiu D, Sadula A, et al. Surgical resection of primary tumor improves survival of pancreatic neuroendocrine tumor with liver metastases. Oncotarget 2017;8:79785–79792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DY, Teng A, Pedersen RC, et al. Racial and Socioeconomic Treatment Disparities in Adolescents and Young Adults with Stage II-III Rectal Cancer. Ann Surg Oncol 2017;24:311–318. [DOI] [PubMed] [Google Scholar]
- 42.Bacchetti S, Pasqual EM, Bertozzi S, et al. Curative versus palliative surgical resection of liver metastases in patients with neuroendocrine tumors: a meta-analysis of observational studies. Gland Surg 2014;3:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schurr PG, Strate T, Rese K, et al. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: an institutional experience. Ann Surg 2007;245:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fendrich V, Langer P, Celik I, et al. An aggressive surgical approach leads to long-term survival in patients with pancreatic endocrine tumors. Ann Surg 2006;244:845–851; discussion 852–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


