Summary
Background
Much remains unknown regarding the associations of adversities in childhood and adulthood with incident cardiovascular diseases (CVD). We aimed to examine the independent and cumulative relations of adversities in childhood and adulthood with incident CVD and whether these associations can be mitigated by adopting a healthy lifestyle later in life.
Methods
We included 136,073 men and women [38–72 years at baseline] free of diagnosed CVD at baseline who responded to surveys on adversities in childhood and adulthood in the United Kingdom Biobank prospective cohort. They were recruited between 2006 and 2010 and were followed-up until 28 January 2021. Adversities included physical abuse, emotional abuse, sexual abuse, emotional neglect, and physical neglect. Participants were categorised into four groups according to the exposure periods, which were no adversity, childhood adversity only, adulthood adversity only, and cumulative adversity (both childhood and adulthood). The primary outcomes included incident fatal and non-fatal CVD events. The modifiable lifestyle factors were smoking, physical activity, diet, sleeping, social or leisure activities, and friend or family visits.
Findings
We identified 16,415 (10.71/1000 person-year) incident CVD during a median follow-up of 11.8 years. Compared with participants with no adversity, CVD incidence increased by 11% in those with childhood adversity only (adjusted hazard ratio [HR]: 1.11 [95% CI 1.06–1.17], p < 0.001), 4% in those with adulthood adversity only (1.04 [1.00–1.09], p = 0.05), and 21% in those with cumulative adversity (1.21 [1.16–1.26], p < 0.001). Analysis of interactions showed that adulthood adversity amplified the childhood adversity-CVD association (p for interaction = 0.03). Compared with the participants with one or fewer ideal lifestyle factors, those with more than four ideal factors had a 25%–36% lower risk of CVD across the three adversity groups.
Interpretation
Our findings suggested that childhood adversities were associated with an increased risk of CVD which can be magnified by adulthood adversities and substantially mitigated by adopting a healthy lifestyle later in life.
Funding
The National Natural Science Foundation of China and Guangzhou Foundation for Basic and Applied Basic Research.
Keywords: Cardiovascular diseases, Adverse experience, Childhood, Adulthood, Lifestyle
Research in context.
Evidence before this study
We systematically searched PubMed for articles published in English with terms including “adverse experiences”, “adversity”, “trauma”, “maltreatment”, “interpersonal trauma”, “interpersonal violence”, “intimate partner violence”, “cardiovascular disease” and “cardiovascular health” from inception up until 12 January 2024. Some studies provided evidence that childhood adversity was associated with ischaemic heart disease, cerebrovascular disease, obesity, hypertension, and diabetes, with very limited studies focusing on adulthood adversity. Some knowledge gaps still exist, such as the associations between adversity and a broad spectrum of CVD events, the interplay between childhood adversity and adulthood adversity on CVD risk, and the modification effects of adopting a healthy lifestyle on the associations of adversity with CVD.
Added value of this study
With the large well-characterised UKB prospective cohort, we assessed the independent and cumulative associations of adversities in childhood and adulthood with the incident CVD including 13 cardiovascular conditions, providing a full view of the association between adversity and CVD. We found that adversities either in childhood or in adulthood were associated with an increased risk of CVD. Despite a weaker association of adulthood adversity with CVD risk, adulthood adversities magnified the association between childhood adversity and CVD risk. Furthermore, adopting a healthier lifestyle was associated with a lower risk of CVD regardless of the experience of adversities in childhood or adulthood.
Implications of all the available evidence
With an unexpectedly high proportion of people affected, adversities in either childhood or adulthood should be taken into consideration by policymakers of cardiometabolic health prevention and control. Prevention and management of adversities both early and later in life can benefit the cardiometabolic health. Considering that the significant interaction between adulthood adversity and childhood adversity, efforts to avoid adulthood adversity are still critical, whether or not childhood adversity has already occurred. Lastly, lifestyle modifications can be considered a lifetime strategy to improve cardiometabolic health regardless of adversity exposure.
Introduction
Cardiovascular diseases (CVD) remain the leading cause of mortality and a major contributor to disability globally.1 Childhood is a critical stage of development characterised by heightened sensitivity,2 wherein exposure to adversities like physical, sexual, or emotional abuse, and physical neglect, could trigger an excessive activation of the hypothalamic-pituitary-adrenal axis, parasympathetic and sympathetic nervous system, and inflammatory and immune response to the stress.3, 4, 5 As a result, persistent detrimental effects on cardiometabolic health may ensue.6, 7, 8, 9, 10 Considering the surprisingly high prevalence of adverse childhood experiences (ACEs), for example, with over 60% of US adults reporting at least one ACE and approximately 25% reporting three or more ACEs,11 there is a substantial public health significance in exploring the associations between ACE and CVD.
Despite the detrimental association between ACEs and the risk of CVD in previous studies,12,13 important knowledge gaps regarding the associations of adversities with cardiometabolic health warrant further investigation. First, previous studies mainly focused on the outcomes of ischaemic heart disease or cardiometabolic risk factors like obesity, hypertension, and diabetes.14, 15, 16, 17 Few studies have examined a broad spectrum of cardiovascular conditions in a large prospective cohort study, making it hard to assess the strength of the associations of childhood adversities with each type of CVD. Second, although adversities happen not only in childhood but also in adulthood,18 very few studies have investigated the relation of adversities in adulthood with the risk of CVD, leaving unexplored the independent and cumulative effects of adversities in childhood and adulthood. Third, traditional modifiable lifestyle factors, such as diet, smoking, physical activity, and sleeping, have been shown to be of benefit for cardiometabolic health.19 Psychosocial support was also considered an effective approach to alleviate the harms of childhood adversities to cardiometabolism.20, 21, 22 However, it remains unclear whether and to what extent the detrimental associations between adversities and the risk of CVD could be mitigated by adopting a healthy lifestyle.
Using the data from the large United Kingdom Biobank (UKB) prospective cohort study, we aimed to examine the independent and cumulative relations of adversities in childhood and adulthood with the incidence of total CVD and 13 cardiovascular conditions and whether these detrimental associations can be modified by adopting a healthy lifestyle.
Methods
Study design and population
The UKB is a population-based prospective cohort study, which recruited more than half a million participants aged 40–69 years in 22 assessment centres across England, Scotland, and Wales from 2006–2010.23 During 2016–2017, 157,199 participants with a working email address completed an online mental health survey including questionnaires on adverse experiences in childhood and adulthood. We further excluded participants with missing data on adversities or with diagnosed CVD at baseline. Finally, a total of 136,073 participants were included in the analysis (Supplementary Figure S1).
Ethics
All participants provided informed consent to the study. This study was performed under ethical approval obtained by UKB from the National Health Service National Research Ethics Service (21/NW/0157).
Adversities in childhood and adulthood
In the UKB, childhood adversities for each participant were assessed using a validated questionnaire that inquired about experiences of physical abuse, emotional abuse, sexual abuse, emotional neglect, or physical neglect when they were growing up.14, 15, 16,24,25 The adversities in adulthood referred to adverse experiences since the age of 16 and were evaluated using a questionnaire with the same structure.
In the questionnaires, each adversity was assessed using a 5-point scale from “never true” to “very often true” (score coded from 0 to 4). According to the definition developed in previous study,26 the presence of each type of adversity was defined according to the following criteria: score ≤2 for emotional neglect, ≥1 for physical abuse, ≥1 for emotional abuse, ≥1 for sexual abuse, and ≤3 for physical neglect. Participants who preferred not to answer the questions were coded as data missing and were excluded from the analyses.
Participants reporting at least one of the five types of adversities were categorised as having experienced adverse events during childhood or adulthood. Alternatively, those who did not report any such adversities were classified as having no adverse experiences during that period. Subsequently, participants were exclusively categorised into four groups based on their experiences of adversities during childhood and adulthood. These groups included those with no adversities (no experience of adversities in either childhood or adulthood), those with childhood adversities only (experienced childhood adversities but no adulthood adversities), those with adulthood adversities only (experienced adulthood adversities but no childhood adversities), and those with cumulative adversities (experienced adversities in both childhood and adulthood). Detailed algorithm on the coding strategy is provided in Supplementary Table S1.
Ascertainment of incident CVD events
The outcomes of interest in this study were the incidence of total CVD and 13 cardiovascular conditions including CVD death, atherosclerotic diseases (ischaemic heart disease, ischaemic stroke, and peripheral arterial disease), infection-related diseases (rheumatic heart disease, myocarditis and pericarditis, and endocarditis) and others (heart failure, haemorrhage stroke, venous thromboembolism, non-rheumatic valvular disease, dysrhythmias, and aortic aneurysm). The incident cases were identified based on the linked hospital admissions and mortality data according to the International Classification of Diseases 9 and 10 code (Supplementary Table S2). The follow-up time for each participant was defined as the duration between entry into the cohort (2006–2010) and the date of occurrence of CVD or censoring (death, loss to follow-up, or the end of follow-up [January 28, 2021]), whichever occurred first.
Covariates
Characteristics collected at baseline for each participant were included in the analysis as covariates, including age, sex, race/ethnicity (white or others), Townsend deprivation index (TDI) (≤median or above), and family history of heart disease and stroke (yes or no). The TDI is a composite measure derived from indicators such as unemployment, non-car ownership, non-home ownership, and household overcrowding to assess socioeconomic deprivation on a neighbourhood level. The percentages of missing data of covariates were generally less than 1%. We performed a random forest imputation for the missingness.
Modifiable lifestyle factors
We included four behavioural factors including smoking, physical activity, diet, and sleeping and two social support-related factors including social or leisure activities and friend or family visits (Supplementary Table S3). Participants completed the questionnaires via a touchscreen interview. The ideal smoking status was never smoking. The ideal physical activity was defined as at least 150 min/week of moderate-intensity or 75 min/week of vigorous-intensity physical activity. An ideal diet was defined as intake of at least 5 healthy foods, such as more fruits, vegetables, whole grains, fish/shellfish, dairy, vegetable oils, and less refined grains, processed meats, unprocessed meats, and sugar-sweetened beverages.27, 28, 29 The ideal sleeping duration was defined as 7–9 h per day.30 The ideal social activity was defined as participating in any type of social or leisure activities, such as sports club or gym, pub or social club, religious group, adult education class, and other group activity. The ideal relationship was defined as more than one friend or family visit per month.
Statistical analysis
We employed Cox proportional hazard models to estimate the associations of adversities with incident CVD events, with adjustment for age, sex, race/ethnicity, TDI, and family history of heart disease and stroke. The proportional hazard assumption was tested with the Schoenfield residual test, and no deviation from the assumption was found. In the analysis, we treated the independent variable of adversities in 3 different ways. First, adversity exposure was entered into the model as four groups (no, childhood only, adulthood only, and cumulative adversities) with the no adversities group serving as the reference. Second, we conducted analyses for each of the 5 types of adversities with further mutual adjustment for the other types of adversities. Third, we calculated continuous scores for childhood adversities, adulthood adversities, and cumulative adversities for each participant and then examined the shape of the relationship of adversities with CVD risk. In this analysis, we included a restricted cubic spline term for adversity score with three knots at 10th, 50th, and 90th centiles in the Cox model. The nonlinearity p-value was calculated with a likelihood ratio test. We also conducted stratification analyses by sex (men vs. women) and TDI (≤median vs. above).
We conducted a series of sensitivity analyses to evaluate the robustness of the above adversity-CVD associations. First, we further adjusted for smoking status, physical activity, BMI, survey centre, and the usage of antihypertensive, anti-diabetes, or cholesterol-lowering medication, aspirin, or steroids in the model. Second, given that the mental health survey was conducted about 6 years after the participant enrolment, we excluded incident CVD cases identified between enrolment and the mental health survey to avoid potential reverse causation. Third, we employed the Fine-Gray proportional hazards model to account for competing risks of deaths.
We quantified the multiplicative interactions between adversities in childhood and adulthood by including the product term of the scores of childhood adversities and adulthood adversities in the model. We also used an interaction surface plot to visualize the synergetic effect of adversities in childhood and adulthood.31
We further conducted mediation analyses to examine whether the association of adversities in childhood and adulthood with risk of CVD was mediated by depression, BMI, inflammatory biomarker score, or biological age. The inflammatory biomarker score is a standardised z score calculated based on the levels of C-reactive protein and white blood cell count.32 The biological aging was assessed using the PhenoAge algorithm, which integrates chronological age and nine biomarkers of albumin, alkaline phosphatase, creatinine, C-reactive protein, glucose, mean cell volume, red cell distribution width, white blood cell count, and lymphocyte proportion.33 We constructed a logistic model to regress the outcome (CVD) on the exposure (adversities in childhood and adulthood) and the mediator of interest and a linear/logistic model to regress the mediator on the exposure, with adjustment for potential confounders. We then integrated these two regressions to obtain the estimates for direct and indirect effects and proportion of mediating effect using the “Mediator” R package.
Lastly, we examined if the adversity-CVD associations could be modified by lifestyle factors, such as smoking, sleep, diet, physical activity, social activity, and friend or family visits. Following the criteria mentioned above, we counted the number of ideal lifestyle factors for each participant, categorised as ≤1 (reference), 2, 3, and ≥4 ideal factors. We evaluated the associations of the number of ideal lifestyle factors with CVD risk, using ≤1 ideal factor as the reference in each adversity group. The interaction between lifestyle and adversities was quantified by including their product term in the model. Analyses were conducted using software R (version 4.2.1), with 95% confidence intervals (CI) and two-sided p values calculated for statistical inference.
Role of the funding source
The funders were not involved in study design, data collection, data analysis, data interpretation, and writing of the report. XZ, AF, and JL had full access to all the data in the study. All authors accepted responsibility for the decision to submit for publication.
Results
Baseline characteristics and prevalence of adversities
A total of 136,073 participants free of diagnosed CVD at baseline were included in this study, of whom the mean age was 55.5 years, 42.7% were men, and 97% were white. According to adversity exposure, the participants were exclusively classified into four groups including no adversities (n = 42,508, 31.2%), childhood adversities only (n = 21,120, 15.5%), adulthood adversities only (n = 31,001, 22.8%), and cumulative adversities (n = 41,444, 30.5%). Among the participants with childhood adversities only, physical abuse was the most common type of adversity (42.3%) followed by emotional neglect (39.5%). While among those with adulthood adversities only, emotional neglect (57.5%) and emotional abuse (41.1%) constituted the majority of adversities (Table 1).
Table 1.
Baseline characteristics according to adversity exposure among 136,073 participants in the UKB.
| Total | No adversities | Childhood adversities only | Adulthood adversities only | Cumulative adversitiesa | |
|---|---|---|---|---|---|
| No. | 136,073 | 42,508 | 21,120 | 31,001 | 41,444 |
| Follow-up year, Mean ± SD, y | 11.8 ± 1.0 | 11.9 ± 1.0 | 11.8 ± 1.0 | 11.8 ± 1.0 | 11.8 ± 1.0 |
| Age, Mean ± SD, y | 55.5 ± 7.7 | 56.1 ± 7.6 | 55.7 ± 7.7 | 55.2 ± 7.8 | 55.1 ± 7.8 |
| Sex, No. (%) | |||||
| Women | 78,010 (57.3) | 22,750 (53.5) | 10,473 (49.6) | 19,121 (61.7) | 25,666 (61.9) |
| Men | 58,063 (42.7) | 19,758 (46.5) | 10,647 (50.4) | 11,880 (38.3) | 15,778 (38.1) |
| Race/ethnicity, No. (%)b | |||||
| White | 132,099 (97.1) | 41,960 (98.7) | 20,575 (97.4) | 30,177 (97.3) | 39,387 (95.0) |
| Black | 1012 (0.7) | 91 (0.2) | 116 (0.5) | 203 (0.7) | 602 (1.5) |
| Asian | 1110 (0.8) | 181 (0.4) | 145 (0.7) | 269 (0.9) | 515 (1.2) |
| Other | 1852 (1.4) | 276 (0.7) | 284 (1.3) | 352 (1.1) | 940 (2.3) |
| Townsend deprivation index, Mean ± SD | −1.7 ± 2.8 | −2.2 ± 2.5 | −2.0 ± 2.7 | −1.6 ± 2.9 | −1.2 ± 3.0 |
| Family history of heart disease or stroke, No. (%) | 75,786 (55.7) | 23,996 (56.5) | 11,896 (56.3) | 17,109 (55.2) | 22,785 (55.0) |
| Smoking status, No. (%) | |||||
| Never | 79,239 (58.4) | 26,921 (63.4) | 12,143 (57.6) | 18,420 (59.5) | 21,755 (52.6) |
| Previous | 46,694 (34.4) | 13,338 (31.4) | 7561 (35.9) | 10,165 (32.9) | 15,630 (37.8) |
| Current | 9848 (7.3) | 2176 (5.1) | 1371 (6.5) | 2347 (7.6) | 3954 (9.6) |
| Sleeping duration, hours, No. (%) | |||||
| <7 | 29,130 (21.4) | 7579 (17.8) | 4339 (20.5) | 6683 (21.6) | 10,529 (25.4) |
| 7–9 | 98,888 (72.7) | 32,605 (76.7) | 15,496 (73.4) | 22,519 (72.6) | 28,268 (68.2) |
| >9 | 8055 (5.9) | 2324 (5.5) | 1285 (6.1) | 1799 (5.8) | 2647 (6.4) |
| Regular physical activityc, No. (%) | 108,249 (79.6) | 34,451 (81.0) | 16,954 (80.3) | 24,302 (78.4) | 32,542 (78.5) |
| Healthy dietd, No. (%) | 25,446 (18.7) | 7903 (18.6) | 3894 (18.4) | 5776 (18.6) | 7873 (19.0) |
| Social or leisure activities, No. (%) | |||||
| Sports club or gym | 47,451 (34.9) | 15,820 (37.3) | 7686 (36.5) | 10,564 (34.1) | 13,381 (32.4) |
| Pub or social club | 19,801 (14.6) | 5809 (13.7) | 3061 (14.5) | 4618 (14.9) | 6313 (15.3) |
| Religious group | 12,611 (9.3) | 4311 (10.2) | 2019 (9.6) | 2802 (9.1) | 3479 (8.4) |
| Adult education class | 4716 (3.5) | 1349 (3.2) | 719 (3.4) | 1083 (3.5) | 1565 (3.8) |
| Other group activity | 13,109 (9.7) | 4109 (9.7) | 1910 (9.1) | 3027 (9.8) | 4063 (9.8) |
| None of the above | 38,148 (28.1) | 11,056 (26.0) | 5689 (27.0) | 8853 (28.6) | 12,550 (30.3) |
| Frequency of friend or family visits, No. (%) | |||||
| Never or almost never | 1631 (1.2) | 249 (0.6) | 193 (0.9) | 394 (1.3) | 795 (1.9) |
| Once every few months | 9519 (7.1) | 2418 (5.7) | 1436 (6.8) | 2211 (7.2) | 3454 (8.4) |
| About once a month | 20,953 (15.5) | 6337 (15.0) | 3357 (16.0) | 4645 (15.1) | 6614 (16.1) |
| About once a week | 50,531 (37.5) | 15,908 (37.7) | 7992 (38.1) | 11,352 (37.0) | 15,279 (37.3) |
| 2–4 times a week | 39,714 (29.4) | 13,107 (31.1) | 6260 (29.9) | 9180 (29.9) | 11,167 (27.2) |
| Almost daily | 12,513 (9.3) | 4159 (9.9) | 1727 (8.2) | 2928 (9.5) | 3699 (9.0) |
| Types of adversity, No. (%) | |||||
| Emotional neglect | 57,872 (42.5) | 0 (0.0) | 8348 (39.5) | 17,821 (57.5) | 31,703 (76.5) |
| Physical abuse | 37,060 (27.2) | 0 (0.0) | 8926 (42.3) | 6331 (20.4) | 21,803 (52.6) |
| Emotional abuse | 44,309 (32.6) | 0 (0.0) | 5799 (27.5) | 12,730 (41.1) | 25,780 (62.2) |
| Sexual abuse | 17,933 (13.2) | 0 (0.0) | 3896 (18.4) | 2502 (8.1) | 11,535 (27.8) |
| Physical neglect | 32,117 (23.6) | 0 (0.0) | 5185 (24.6) | 5752 (18.6) | 21,180 (51.1) |
| Total score of adversity, median (IQR) | 3.0 (1.0, 5.0) | 0.0 (0.0, 1.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 7.0 (5.0, 10.0) |
| Depression, No. (%) | 44,569 (32.8) | 9009 (21.2) | 6324 (29.9) | 10,477 (33.8) | 18,759 (45.3) |
| BMI, Mean ± SD, kg/m2 | 26.7 ± 4.5 | 26.4 ± 4.2 | 26.8 ± 4.4 | 26.5 ± 4.6 | 27.0 ± 4.8 |
| Inflammatory scoree, Mean ± SD | −0.1 ± 1.1 | −0.2 ± 1.1 | −0.1 ± 1.1 | −0.1 ± 1.1 | 0.0 ± 1.2 |
| Biological agef, Mean ± SD, y | 48.9 ± 9.3 | 49.2 ± 9.1 | 49.0 ± 9.3 | 48.6 ± 9.3 | 48.7 ± 9.3 |
| Diabetes, No. (%) | 4346 (3.2) | 1193 (2.8) | 671 (3.2) | 1002 (3.2) | 1480 (3.6) |
Continuous variables are described as mean ± standard deviation (SD) for those with normal distribution and median (interquartile range) for those with non-normal distribution. Categorical data are described as n (%).
Cumulative adversities denote adversities in both childhood and adulthood.
Race/ethnicity: White includes individuals with British, Irish and any other white background; Black includes individuals with Caribbean, African and any other Black background; Asian includes individuals with Indian, Pakistani, Bangladeshi and any other Asian background.
Regular physical activity refers to ≥150 min/week of moderate activity or ≥75 min/week of vigorous activity.
Healthy diet refers to the intake of at least 5 healthy foods, such as more fruits, vegetables, whole grains, (shell)fish, dairy, vegetable oils, and less refined grains, processed meats, unprocessed meats, and sugar-sweetened beverages.
The inflammatory biomarker score is a standardised z score calculated based on the levels of C-reactive protein and white blood cell count.
The biological aging was assessed using the PhenoAge algorithm, which integrates chronological age and nine biomarkers of albumin, alkaline phosphatase, creatinine, C-reactive protein, glucose, mean cell volume, red cell distribution width, white blood cell count, and lymphocyte proportion.
Association of adversities with incident total CVD
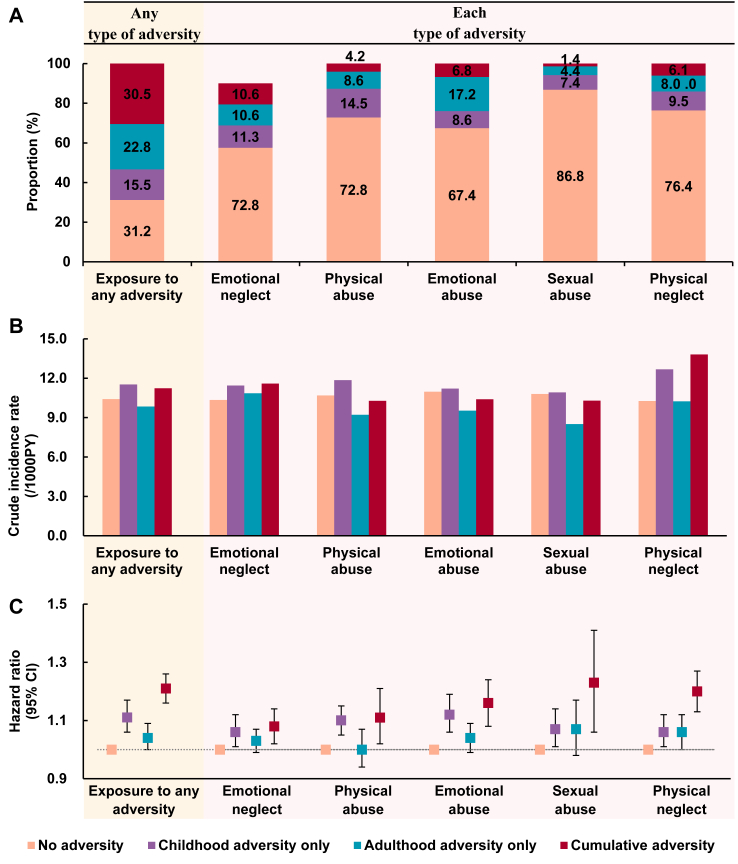
During a median follow-up of 11.8 years, we identified 16,415 (10.71/1000 person-years) incident CVD events in total. Compared with the participants with no adversities, the risk of total CVD increased 11% in those with childhood adversities only (adjusted hazard ratio [HR]: 1.11 [95% CI 1.06–1.17], p < 0.001), 4% in those with adulthood adversities only (1.04 [1.00–1.09], p = 0.049), whereas, 21% in those with cumulative adversities (1.21 [1.16–1.26], p < 0.001) (Fig. 1, Supplementary Table S4). The findings were robust when we additionally adjusted for smoking status, physical activity, BMI, survey centre, or the use of medication related to cardiometabolism in the model, considered competing risk in analysis, or excluded the incident cases occurring between the enrolment (2006–2010) and mental health survey (2016–2017) from the analysis (Supplementary Table S5).
Fig. 1.
Associations between adversities and risk of total CVD among 136,073 participants in the UKB. (A) Proportions of participants in each adversity group. (B) Crude incidence rate (per 1000 person-years) of total CVD in each adversity group. (C) Adjusted hazard ratios and their 95% CI of total cardiovascular diseases (CVD), which were derived from Cox proportional regression model with adjustment for age, sex, race/ethnicity, Townsend deprivation index, family history of heart disease, and stroke, and other types of adversity. Cumulative adversities denote adversities in both childhood and adulthood.
Our mediation analysis further indicated that, among the association between childhood adversity and risk of CVD, 25.2% was mediated by depression, 17.4% by BMI, 4.9% by biological age and 3.7% by inflammatory score. For the association between adulthood adversity and risk of CVD, 14.0% was mediated by depression, 24.3% by BMI, 25.2% by biological age and 24.2% by inflammatory score. Regarding the association between cumulative adversity and risk of CVD, 37.9% was mediated by depression, 16.8% by BMI, 10.9% by biological age and 8.5% by inflammatory score (Supplementary Table S6).
We further examined the associations of each type of adversities with the incident CVD. Compared to counterparts with no adversities, participants with childhood adversities only showed that the experience of emotional abuse was associated with the highest increased risk of total CVD (HR: 1.12 [1.06–1.19]), followed by physical abuse (HR: 1.10 [1.05–1.15]), sexual abuse (HR: 1.07 [1.01–1.14]), physical neglect (HR: 1.06 [1.01–1.12]), and emotional neglect (HR: 1.06 [1.01–1.12]). Despite the weak association between adulthood adversities and CVD risk, the associations of experiencing cumulative adversities with CVD risk increased dramatically. Sexual abuse had the strongest association with CVD risk (HR: 1.24 [1.07–1.42]), followed by physical neglect (HR: 1.20 [1.13–1.27]), emotional abuse (HR: 1.16 [1.08–1.24]), physical abuse (HR: 1.11 [1.02–1.21]), and emotional neglect (HR: 1.08 [1.02–1.14]) (Fig. 1).
Stratification analysis by sex showed stronger associations between adversities and risk of total CVD in women than in men (Supplementary Table S7). Stratification analysis by TDI revealed a significant interaction between physical abuse and TDI, indicating that physical abuse was associated with an increased risk of total CVD in the population with high TDI but not among those with low TDI (Supplementary Table S8). Taken together, women with high TDI faced a higher risk of CVD than their counterparts if they had experienced adversities in childhood and adulthood (Supplementary Table S9).
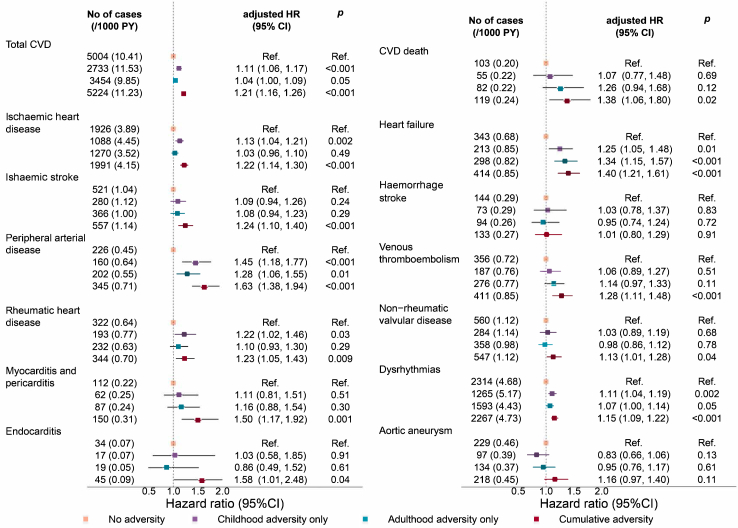
Associations of adversities with 13 cardiovascular conditions
Among the 13 studied cardiovascular conditions, childhood adversities only was associated with increased risk of 5 cardiovascular conditions including peripheral arterial disease, heart failure, rheumatic heart disease, ischaemic heart disease, and dysrhythmias (HR ranged from 1.11 for dysrhythmia to 1.45 for peripheral arterial disease); adulthood adversities only was associated with increased risk of 3 cardiovascular conditions including heart failure, peripheral arterial disease, and dysrhythmias (HR ranged from 1.07 for dysrhythmias to 1.34 for heart failure); in contrast, cumulative adversities was associated with increased risk of almost all cardiovascular conditions except for haemorrhage stroke (HR ranged from 1.13 for non-rheumatic valvular disorders to 1.63 for peripheral arterial disease) (Fig. 2).
Fig. 2.
Associations between adversities and specific cardiovascular conditions among 136,073 participants in the UKB. Hazard Ratios and 95% Cis were derived from Cox proportional regression models with adjustment for age, sex, race/ethnicity, Townsend deprivation index, and family history of heart diseases, and stroke. Cumulative adversities denote adversities in both childhood and adulthood. Abbreviations: CI, confidence interval; HR, hazard ratio; PY, person-years.
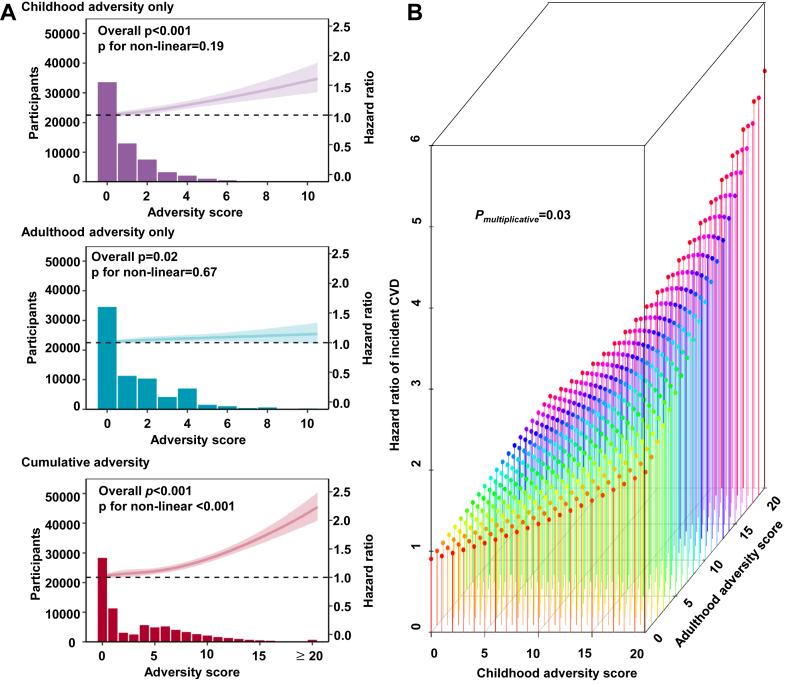
Interplay between childhood and adulthood adversities on incident CVD
The higher score of childhood adversities was associated with higher risk of total CVD in a linear manner (overall p < 0.001, p for nonlinear = 0.19). The score of adulthood adversities was also associated with increased risk of total CVD in a linear manner (overall p = 0.02, p for nonlinear = 0.67), although the strength of the association was weaker than the above observed in the analysis for childhood adversities. In contrast, the shape of the relationship between score of cumulative adversities and CVD risk became inverted “L” (overall p < 0.001, p for nonlinear <0.001). When the score of cumulative adversities was greater than 10, the strength of the association between each score increments and CVD risk became stronger (Fig. 3A). Despite the weak association of adulthood adversities with CVD risk, interaction analysis showed that adulthood adversities could amplify the childhood adversity-CVD association (p for interaction = 0.03). Such interaction effect was visualized using an interaction surface plot (Fig. 3B).
Fig. 3.
Interactions between childhood and adulthood adversities on the risk of CVD among 136,073 participants in the UKB. (A) Cox proportional regression models with restricted cubic spline terms for adversity score were used to examine the associations between the scores and risk of CVD, with adjustment for age, sex, race/ethnicity, Townsend deprivation index, and family history of heart diseases and stroke. (B) Interaction surface plot on the linear predictor of the hazard function. The z-axis shows the predictor of the hazard function for varying scores of childhood adversities and adulthood adversities, illustrating their interacting effect on the risk of CVD. Cumulative adversities denote adversities in both childhood and adulthood.
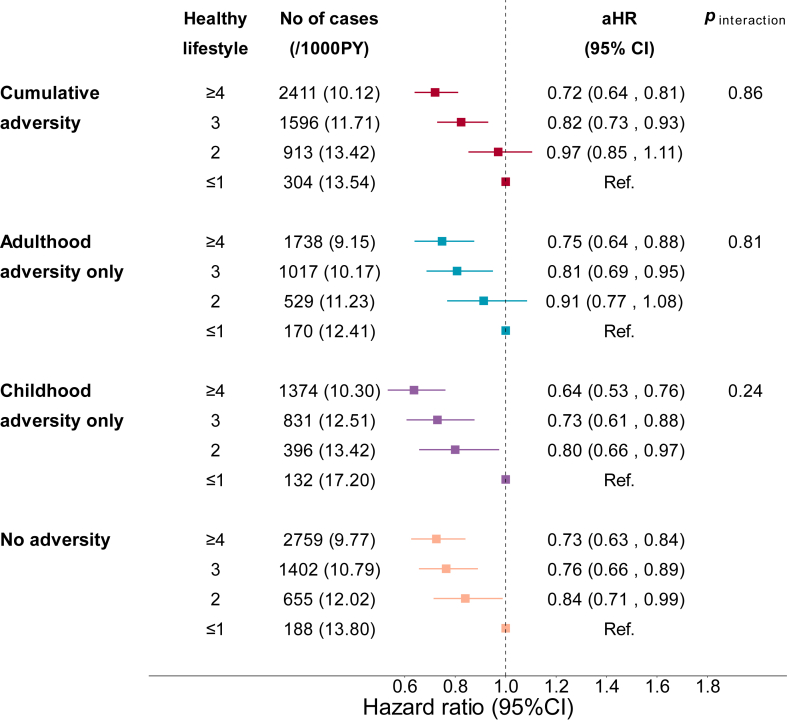
Interplay between adversities and lifestyle on the incident CVD
We analysed the associations between lifestyle factors and CVD risk according to adversity exposure. Compared with the participants with one or fewer ideal lifestyle factor, those with more than four ideal lifestyle factors had 27% lower risk of total CVD in the no adversities group (HR: 0.73 [0.63–0.84]), 36% lower risk in the childhood adversities only group (HR: 0.64 [0.53–0.76]), 25% lower risk in the adulthood adversities only group (HR: 0.75 [0.64–0.88]), and 28% lower risk in the cumulative adversities group (HR: 0.72 [0.64–0.81]). We did not find significant interaction between adversity exposure and lifestyle on CVD risk (Fig. 4, Supplementary Figure S2). We also conducted stratification analysis by sex and TDI and found that the beneficial associations between adopting healthy lifestyle behaviours and decreased risk of CVD in each adversity group were not affected by sex or TDI (Supplementary Figures S3 and S4).
Fig. 4.
Risk of CVD according to adversities and number of ideal lifestyle factors among 136,073 participants in the UKB. Ideal lifestyle factors were defined as follows: no smoking, regular physical activity (≥150 min/week of moderate activity or ≥75 min/week of vigorous activity), a healthy diet (including at least 5 healthy foods such as fruits, vegetables, whole grains, (shell)fish, dairy, vegetable oils, and fewer refined grains, processed meats, unprocessed meats, and sugar-sweetened beverages), adequate sleep (7–9 h per day), engagement in social or leisure activities (like sports clubs or gyms, pubs or social clubs, religious groups, adult education classes, and other group activities), and participating in friend or family visits at least once a week. The Cox proportional hazard model was used for the analysis, with adjustment of age, sex, race/ethnicity, Townsend deprivation index, and family history of heart diseases and stroke. p values for interaction were calculated by including the product term of adversity and lifestyle in model. Cumulative adversities denote adversities in both childhood and adulthood.
We analysed interactions between adversities and each lifestyle factor on the risk of CVD. Among the six examined lifestyle factors, smoking exhibited the strongest association with the risk of CVD, particularly for the outcome of peripheral arterial disease. Compared with smokers, those who never smoked had a 51%–63% lower risk of peripheral arterial disease across the four groups of no adversity, childhood adversity only, adulthood adversity only, and cumulative adversity (Supplementary Table S10).
Discussion
In this large prospective cohort study, we investigated the associations between adversities in childhood and adulthood and incident CVD and assessed whether such detrimental associations can be mitigated by adopting healthy lifestyle factors. Our findings indicate that experiencing adversities either in childhood or adulthood was associated with increased risk of CVD among 136,073 participants in the UKB. Despite the weaker association of adulthood adversities with CVD risk in comparison with the childhood adversities, adulthood adversities magnified the association between childhood adversities and CVD risk. Adopting healthy lifestyle was associated with decreased CVD risk regardless of whether adversities occurred in childhood or adulthood.
Previous studies have predominantly focused on examining the associations between adversities experienced during childhood and the development of ischaemic heart disease. A recent study in the UKB reported significant association between childhood maltreatment (physical, sexual or emotional abuse, emotional or physical neglect) and CVD risk, with stronger association in women than in men.14 A Danish cohort study examined the association between adversities experienced between ages 0 and 15 and the subsequent risk of developing CVD from ages 16–38. The study encompassed three dimensions of childhood adversities, namely material deprivation, family-related loss or threat of loss, and family dynamics. The findings revealed that individuals exposed to childhood adversities faced a greater risk of developing CVD in early adulthood compared to those with limited adversity experiences.17 Consistent with these findings, our study not only demonstrated that childhood adversities were associated with the increased risk of total CVD, but also revealed the strongest association with peripheral arterial disease followed by heart failure, rheumatic heart disease, ischaemic heart disease, and dysrhythmias.
Evidence regarding adulthood adversities and CVD risk remains very limited and mainly showed the significant association between intimate partner violence and cardiometabolic health among women.34, 35, 36, 37 The associations between other types of adversity and CVD risk in men and women have not been well studied. Our findings are in support of the positive association between adulthood adversities and CVD risk especially among women. Therefore, controlling adversities both early and later in life should be incorporated in the prevention and control of CVD.
To our knowledge, the interactions between childhood adversities and adulthood adversities have not been well studied in previous studies, although some noted the possibly cumulative health effects of adversities occurring at different ages.38 One novel and interesting finding of our study is that, although the adulthood adversity-CVD association was weaker than the childhood adversity-CVD association, adulthood adversity magnified the strength of association between childhood adversities and CVD risk. We found three lines of evidence for the existence of interaction between childhood and adulthood adversities in our study. First, the risk of CVD in cumulative adversities group was larger than the risks in the childhood adversity-only group and the adulthood adversities group and even the sum of the risks in these two groups. Second, among the 13 cardiovascular conditions, childhood adversities were associated with 5 cardiovascular conditions, adulthood adversities with 3, and cumulative adversities with all except for haemorrhage stroke. Third, despite the linear relationship between childhood and adulthood adversity only and CVD risk, a non-linear inverted “L” shape was found for the association between cumulative adversities and CVD risk, indicating a significant interaction effect between childhood and adulthood adversities. Considering that the significant interaction between adulthood adversities and childhood adversities, efforts to prevent adulthood adversities are of critical importance, regardless of the adversity status in childhood.
Modifiable lifestyle factors, such as healthy diet, no smoking, regular physical activity, adequate sleeping, participation in social activities, and frequent friend or family visits, have been shown to be of benefit for cardiometabolic health and key intervention targets for reducing CVD risk.19, 20, 21, 22 Recent studies demonstrated that adopting healthy lifestyle could prevent most cases of CVD even among the individuals with lower birth weight39 and is related to increased life expectancy.40,41 However, no previous studies examined if the combination of these lifestyle factors can mitigate the detrimental associations between adversities and CVD risk. Our study showed that the more favourable lifestyle factors adopted, the lower the risk of CVD, regardless of the experience of childhood or adulthood adversities. Adopting more than 4 ideal factors was associated with at least 25% lower risk of CVD among the examined population. We highlight that lifestyle modifications can be considered a lifetime strategy to improve cardiometabolic health regardless of adversity exposure in early life. In addition, the reduction of influence of adversities on risk of CVD requires interventions address individual-level lifestyle factors, social environments, and the health care systems. Therefore, future studies focusing on multi-level interventions are warranted.
The mechanisms underlying the association between childhood adversities and risk of CVD may involve the overactivation of the hypothalamic-pituitary-adrenal axis, modulation of the parasympathetic and sympathetic nervous systems, and inflammatory and immune responses to stress.12 Two prior cohort studies conducted mediation analyses and revealed that behavioural and psychological factors (such as smoking, physical activity, sleep, and depression), physical health indicators (BMI and cholesterol levels), and inflammatory factors (C-reactive protein) mediated the link between childhood adversity and the risk of CVD, with depression exhibiting the highest mediation effect.15,16 In our study, we found that depression, BMI, biological age, and inflammatory score mediated not only the association between childhood adversity and the risk of CVD, but also the association between adulthood adversity and the risk of CVD. Consistent with the previous studies, depression was the strongest mediator linking childhood adversity and CVD. However, the mediation effect of BMI, inflammation, and biological age was stronger than depression in the association between adulthood adversity and CVD risk. Our findings indicated that adopting healthy lifestyle mitigated the associations between adversities and CVD. The underlying mechanism may be that engaging in healthy behaviours, such as non-smoking, maintaining a healthy diet, regular physical activity, and ensuring adequate sleep, improved inflammatory and immune responses to adversities.42 Additionally, social support factors, such as family or friends visiting and engaging in social or leisure activities, improved psychological health and subsequently buffered the harmful effects of adversity on CVD.43
Our findings in the current study benefit from several strengths in the study design. First, we simultaneously investigated the associations of adversities in childhood and adulthood with the risk of a broad spectrum of cardiovascular conditions in a well-characterized large prospective cohort, making our findings unique for appreciating the full scope of adversity-CVD associations. Second, we took the interplay between adversities in childhood and adulthood on CVD risk into consideration and found that the associations between childhood adversities and CVD risk can be augmented by adulthood adversities, despite a weak association of the latter with CVD risk. Third, we investigated how adversities in childhood and adulthood and modifiable lifestyle factors are jointly related to CVD risk.
Some limitations should be kept in mind when interpreting the findings. First, the experience of adversities in childhood and adulthood was collected using self-report questionnaire during 2016–2017 when the participants were in their 60s, leading to inevitable recall bias. Second, the adversities assessed in the UKB were restricted in scope, and this study did not encompass other forms of adversities, such as financial stress, the loss of a spouse, or experiences of racism. In addition, psychological care, psychiatric care, and some social factors like social cohesion were not assessed in the UKB and not be accounted for in our analysis. Third, this is an observational study in nature and some residual confounding cannot be excluded, despite adjustments for potential confounders in the models. Fourth, the participants were recruited at ages 40–69, and some early cardiovascular events or deaths prior to recruitment may not have been captured, which may lead to some bias from survival and competing risks in our study. Fifth, only participants with working email received the adversity questionnaire in the UKB, potentially introducing selection bias. As shown in Supplementary Table S11, the included participants were younger, had more ideal lifestyle factors, and were in better health than those excluded. Therefore, the associations between adversities and CVD risk may be underestimated in our study. Sixth, more than 97% populations included in this study were White and caution is advised when generalizing the findings to other populations, especially individuals from minoritized communities who may experience a multitude of adverse social determinants of health. Lastly, the small number of incident cases for some specific cardiovascular conditions might render some p values not significant, which should be considered when interpreting related findings.
In this large prospective cohort study, we found significant associations of adversities in childhood and adulthood with increased risk of CVD. Although adulthood adversities alone had weak associations with CVD risk, it magnified the association between childhood adversities and CVD risk. The increased risk of CVD associated with adversities could be substantially mitigated by adopting healthy lifestyle behaviours. Addressing adverse experience in both childhood and adulthood should be considered by policy makers for CVD prevention and control.
Contributors
JL and XZ conceived the study. XZ conducted the analyses. AF and JL verified the underlying data and conducted a technical review. XZ, JL, SL, and JZ wrote the first draft of manuscript. XZ, AF, and JL had full access to all the data in the study. All authors contributed to the interpretation of the results and revision of the manuscript and accepted responsibility to submit for publication. JL and SL are equally corresponding authors and attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data sharing statement
All data used in analysis are available from UKB upon request (https://www.ukbiobank.ac.uk).
Declaration of interests
SL reports grants outside of this work from National Institute of Health and RO1. SL also reports royalties/licenses fees from UpToDate.Com, as well as consulting fees from American Society for Nutrition, Fred Hutchinson Cancer Research Centre, Guangdong General/Provincial Hospital, Novo Nordisk, Partners Healthcare System, Inc., Research Foundation of State University of New York, Twinhealth.com, and Universidade Federal de São Paulo. Additionally, SL reports support for attending meetings or travel from Chinese Nutrition Society, European Diabetes and Nutrition Study Group, American Society of Nutrition. SL participates on a data safety monitoring board for Novo Nordisk. JEM reports grant from National Institute of Health. All other authors declare no competing interests.
Acknowledgements
This research was conducted using the UK Biobank Resource under Application Number 98973. The authors would like to express their sincere gratitude to all the participants of UK Biobank and all the research assistants involved in building the UK Biobank study. JL was supported by The National Natural Science Foundation of China (82073528, 81673156, and 81302417). XZ was supported by Guangzhou Foundation for Basic and Applied Basic Research (2023A04J0539).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102458.
Contributor Information
Simin Liu, Email: simin_liu@brown.edu.
Jie Li, Email: jie_li@brown.edu.
Appendix A. Supplementary data
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tohi M., Bay J.L., Tu'akoi S., Vickers M.H. The developmental origins of health and disease: adolescence as a critical lifecourse period to break the transgenerational cycle of NCDs-a narrative review. Int J Environ Res Public Health. 2022;19(10):6024. doi: 10.3390/ijerph19106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dempster K.S., O'Leary D.D., MacNeil A.J., Hodges G.J., Wade T.J. Linking the hemodynamic consequences of adverse childhood experiences to an altered HPA axis and acute stress response. Brain Behav Immun. 2021;93:254–263. doi: 10.1016/j.bbi.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Berens A.E., Jensen S.K.G., Nelson C.A., 3rd Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med. 2017;15(1):135. doi: 10.1186/s12916-017-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine G.N., Cohen B.E., Commodore-Mensah Y., et al. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American Heart Association. Circulation. 2021;143(10):e763–e783. doi: 10.1161/CIR.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 6.Jakubowski K.P., Cundiff J.M., Matthews K.A. Cumulative childhood adversity and adult cardiometabolic disease: a meta-analysis. Health Psychol. 2018;37(8):701–715. doi: 10.1037/hea0000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deschenes S.S., Kivimaki M., Schmitz N. Adverse childhood experiences and the risk of coronary heart disease in adulthood: examining potential psychological, biological, and behavioral mediators in the whitehall II cohort study. J Am Heart Assoc. 2021;10(10) doi: 10.1161/JAHA.120.019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loucks E.B., Almeida N.D., Taylor S.E., Matthews K.A. Childhood family psychosocial environment and coronary heart disease risk. Psychosom Med. 2011;73(7):563–571. doi: 10.1097/PSY.0b013e318228c820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu A., McLaughlin K.A., Misra S., Koenen K.C. Childhood maltreatment and health impact: the examples of cardiovascular disease and type 2 diabetes mellitus in adults. Clin Psychol. 2017;24(2):125–139. doi: 10.1111/cpsp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong M., Giles W.H., Felitti V.J., et al. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110(13):1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 11.Merrick M.T., Ford D.C., Ports K.A., Guinn A.S. Prevalence of adverse childhood experiences from the 2011-2014 behavioral risk factor surveillance system in 23 states. JAMA Pediatr. 2018;172(11):1038–1044. doi: 10.1001/jamapediatrics.2018.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godoy L.C., Frankfurter C., Cooper M., Lay C., Maunder R., Farkouh M.E. Association of adverse childhood experiences with cardiovascular disease later in life: a review. JAMA Cardiol. 2021;6(2):228–235. doi: 10.1001/jamacardio.2020.6050. [DOI] [PubMed] [Google Scholar]
- 13.Hughes K., Bellis M.A., Hardcastle K.A., et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2(8):e356–e366. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- 14.Soares A.L.G., Hammerton G., Howe L.D., Rich-Edwards J., Halligan S., Fraser A. Sex differences in the association between childhood maltreatment and cardiovascular disease in the UK Biobank. Heart. 2020;106(17):1310–1316. doi: 10.1136/heartjnl-2019-316320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho F.K., Celis-Morales C., Gray S.R., et al. Child maltreatment and cardiovascular disease: quantifying mediation pathways using UK Biobank. BMC Med. 2020;18(1):143. doi: 10.1186/s12916-020-01603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soares A.G., Howe L.D., Heron J., et al. How does childhood maltreatment influence cardiovascular disease? A sequential causal mediation analysis. Int J Epidemiol. 2021;51(2):555–566. doi: 10.1093/ije/dyab085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bengtsson J., Elsenburg L.K., Andersen G.S., Larsen M.L., Rieckmann A., Rod N.H. Childhood adversity and cardiovascular disease in early adulthood: a Danish cohort study. Eur Heart J. 2023;44(7):586–593. doi: 10.1093/eurheartj/ehac607. [DOI] [PubMed] [Google Scholar]
- 18.Thurston R.C., Chang Y., Matthews K.A., et al. Interpersonal trauma and risk of incident cardiovascular disease events among women. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.024724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y.B., Chen C., Pan X.F., et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ. 2021;373 doi: 10.1136/bmj.n604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lester S., Khatwa M., Sutcliffe K. Service needs of young people affected by adverse childhood experiences (ACEs): a systematic review of UK qualitative evidence. Child Youth Serv Rev. 2020;118 doi: 10.1016/j.childyouth.2020.105429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saul H., Gursul D. What support do young people affected by adverse childhood experiences need? BMJ. 2021;375 doi: 10.1136/bmj.n2608. [DOI] [PubMed] [Google Scholar]
- 22.Lorenc T., Lester S., Sutcliffe K., Stansfield C., Thomas J. Interventions to support people exposed to adverse childhood experiences: systematic review of systematic reviews. BMC Public Health. 2020;20(1):657. doi: 10.1186/s12889-020-08789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sudlow C., Gallacher J., Allen N., et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanlon P., McCallum M., Jani B.D., McQueenie R., Lee D., Mair F.S. Association between childhood maltreatment and the prevalence and complexity of multimorbidity: a cross-sectional analysis of 157,357 UK Biobank participants. J Comorb. 2020;10 doi: 10.1177/2235042X10944344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grabe H.J., Schulz A., Schmidt C.O., et al. [A brief instrument for the assessment of childhood abuse and neglect: the childhood trauma screener (CTS)] Psychiatr Prax. 2012;39(3):109–115. doi: 10.1055/s-0031-1298984. [DOI] [PubMed] [Google Scholar]
- 26.Davis K.A.S., Coleman J.R.I., Adams M., et al. Mental health in UK Biobank - development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open. 2020;6(2) doi: 10.1192/bjo.2019.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity. Circulation. 2016;133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han H., Cao Y., Feng C., et al. Association of a healthy lifestyle with all-cause and cause-specific mortality among individuals with type 2 diabetes: a prospective study in UK Biobank. Diabetes Care. 2022;45(2):319–329. doi: 10.2337/dc21-1512. [DOI] [PubMed] [Google Scholar]
- 29.Said M.A., Verweij N., van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank study. JAMA Cardiol. 2018;3(8):693–702. doi: 10.1001/jamacardio.2018.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd-Jones D.M., Allen N.B., Anderson C.A.M., et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146(5):e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamina C., Sturm G., Kollerits B., Kronenberg F. Visualizing interaction effects: a proposal for presentation and interpretation. J Clin Epidemiol. 2012;65(8):855–862. doi: 10.1016/j.jclinepi.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Mekli K., Lophatananon A., Maharani A., Nazroo J.Y., Muir K.R. Association between an inflammatory biomarker score and future dementia diagnosis in the population-based UK Biobank cohort of 500,000 people. PLoS One. 2023;18(7) doi: 10.1371/journal.pone.0288045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao X., Geng T., Jiang M., et al. Accelerated biological aging and risk of depression and anxiety: evidence from 424,299 UK Biobank participants. Nat Commun. 2023;14(1):2277. doi: 10.1038/s41467-023-38013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breiding M.J., Black M.C., Ryan G.W. Chronic disease and health risk behaviors associated with intimate partner violence-18 U.S. states/territories, 2005. Ann Epidemiol. 2008;18(7):538–544. doi: 10.1016/j.annepidem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Vives-Cases C., Ruiz-Cantero M.T., Escribà-Agüir V., Miralles J.J. The effect of intimate partner violence and other forms of violence against women on health. J Public Health. 2011;33(1):15–21. doi: 10.1093/pubmed/fdq101. [DOI] [PubMed] [Google Scholar]
- 36.Lown E.A., Vega W.A. Intimate partner violence and health: self-assessed health, chronic health, and somatic symptoms among Mexican American women. Psychosom Med. 2001;63(3):352–360. doi: 10.1097/00006842-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Stene L.E., Jacobsen G.W., Dyb G., Tverdal A., Schei B. Intimate partner violence and cardiovascular risk in women: a population-based cohort study. J Womens Health. 2013;22(3):250–258. doi: 10.1089/jwh.2012.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levinsky M., Schiff M. Lifetime cumulative adversity and physical health deterioration in old age: evidence from a fourteen-year longitudinal study. Soc Sci Med. 2021;289 doi: 10.1016/j.socscimed.2021.114407. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y.X., Li Y., Rich-Edwards J.W., et al. Associations of birth weight and later life lifestyle factors with risk of cardiovascular disease in the USA: a prospective cohort study. EClinicalMedicine. 2022;51 doi: 10.1016/j.eclinm.2022.101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma H., Wang X., Xue Q., et al. Cardiovascular health and life expectancy among adults in the United States. Circulation. 2023;147(15):1137–1146. doi: 10.1161/CIRCULATIONAHA.122.062457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X., Ma H., Li X., et al. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in UK adults. JAMA Intern Med. 2023;183(4):340–349. doi: 10.1001/jamainternmed.2023.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorburn A.N., Macia L., Mackay C.R. Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity. 2014;40(6):833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Gluckman P.D., Buklijas T., Hanson M.A. In: The epigenome and developmental origins of health and disease. Rosenfeld C.S., editor. Academic Press; Boston: 2016. The developmental origins of health and disease (DOHaD) concept: past, present, and future; pp. 1–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.