Summary
Large seasonal outbreaks of bronchiolitis put pressure on healthcare systems and particularly on intensive care units (ICUs). ICU admission is necessary to provide respiratory support to the severest cases, otherwise bronchiolitis can result in substantial mortality. ICU resources are often insufficient and there is scant evidence to guide the ICU clinical management. Most available studies do not cover the ICU-admitted cases and do not consider the associated public health issues. We review this topic through a multidisciplinary approach from both the clinical and public health perspectives, with an analysis based on pathophysiology and cost-effectiveness. We suggest ways to optimise respiratory care, minimise ICU stay, “protect” ICU beds and, whenever possible, make them available for other critically ill children. We also provide guidance on how to prepare ICUs to work under stressful conditions due to outbreaks and to reduce the risk of nosocomial cross-contamination, particularly in ICUs caring for high-risk children.
Funding
None.
Keywords: RSV, NICU, PICU, Respiratory failure, Outbreak, Infant
Search strategy and selection criteria.
References for this review were identified through searches of PubMed with the following words and/or MeSH terms: “bronchiolitis”, “intensive care unit (ICU)”, “critical care”, “respiratory support”, “respiratory syncytial virus (RSV)”, and “infant”. No year or language limitations were applied. Articles were also identified through searches of the authors’ own files, references cited in the retrieved articles and contacting colleagues cited in the acknowledgements. The final reference list was generated on the basis of originality and relevance to the broad scope of this review.
Introduction
Bronchiolitis is the commonest respiratory infection in early infancy. It results in considerable mortality in the absence of respiratory critical care,1 whereas, when this is provided, bronchiolitis is associated with a significant burden of care, as well as long and expensive hospital stay translating into relevant public health and societal consequences.2 Respiratory critical care is provided in intensive care units (ICUs), which therefore play a pivotal role in the current management of bronchiolitis. In recent years, seasonal outbreaks of respiratory syncytial virus (RSV), the main aetiologic agent of bronchiolitis, have been particularly severe and have stressed the healthcare systems in several high-income countries. In Europe and North America, neonatal and paediatric ICU beds have proven insufficient, and infants have had to be transferred hundreds of kilometres away from home to receive appropriate care. This has received wide media coverage and reached the headlines (“RSV hammers the hospitals”).3 On a smaller scale, this situation was similar to that observed in adult critical care during the recent pandemics,2 and is complicated, at least in Europe, by funding that seems relatively insufficient compared to the current needs.
Additional factors complicate the situation. Depending on local policies, patients with bronchiolitis are admitted to paediatric, neonatal or mixed ICUs, which may have different clinical backgrounds and levels of preparedness to work during outbreaks and prevent nosocomial cross-contamination. In fact, there are substantial variations in ICU management of bronchiolitis.4 Most available literature focuses on patients in emergency departments and general paediatric wards, without covering the severest cases who reach the ICU. Only one clinical practice guideline is dedicated to these patients, but given the paucity of specific ICU studies, it has many uncertainties and does not consider the public health issues (e.g. shortage of ICU beds, the associated costs and the risk of nosocomial outbreaks).5 Thus, guidance is needed to manage children with bronchiolitis needing critical care and, to the best of our knowledge, is lacking in the literature. We aim to fill this gap: here we review the topic from both clinical and public health perspectives and suggest how to optimise respiratory care, minimise ICU stay and the risk of nosocomial outbreaks, “protect” ICU beds and, whenever possible, make them available for other critically ill patients. We based our suggestions on the analysis of the available literature for these purposes, focusing on the duration of ICU stay and availability of beds as main indicators.
Epidemiological context
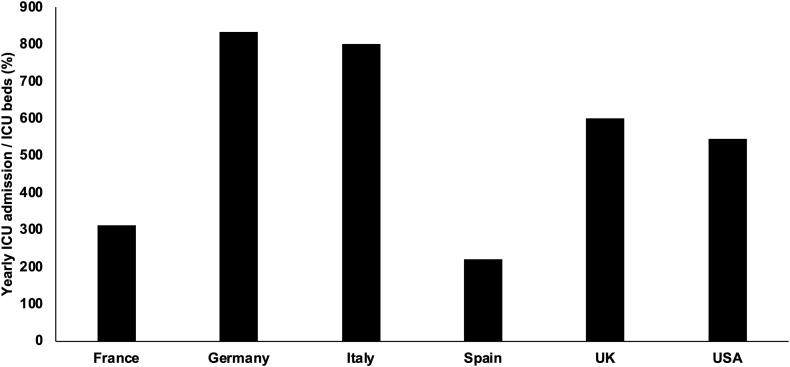
Bronchiolitis is an acute, infectious, usually viral and very contagious (i.e. basic reproduction number between 6 and 8)6 disease associated with inflammatory reaction of the small airways and, in the severest cases, extending to the lung parenchyma. It is usually a seasonal disease with annual or biannual epidemics from October to April in the northern hemisphere, and this seasonality is linked to indoor crowding and the opening of kindergartens, although is less evident in tropical zones.6 Environmental factors, such as pollution, parental smoke and impairment of ciliary function and innate immune defences due to low temperatures can also influence disease severity.6 Finally, younger age, male sex, prematurity, exclusive bottle feeding, failure to thrive, low socio-economic status, major comorbidities and immunodeficiencies are known to be risk factors for more severe disease.7 Bronchiolitis especially affects infants and toddlers under two years of age, with a peak between two and six months.8 Infants often need hospitalisation and approximately 5% of them require ICU admission for respiratory monitoring and support.9 A relevant proportion (≈10%) of ICU patients develops acute respiratory distress syndrome (ARDS) and survivors have negative outcomes such as long ICU stay, invasive respiratory support, oxygen supplementation and high associated costs.10 Given the number of infants with bronchiolitis needing critical care each year, several countries may suffer from relative ICU bed shortage. The duration of ICU stay can be variable depending on local factors, but considering a median of one week,7 and the concentration of cases during the winter season, the figures clearly show that the cases outweigh the available ICU beds (Fig. 1). The situation may be worse in some regions where there is a structural insufficiency of ICU resources, irrespective of the bronchiolitis outbreak.11 Conversely, in low-middle income countries, where intensive care is unavailable, bronchiolitis caused by RSV accounts for more than 120,000 deaths/year.8
Fig. 1.
Estimation of burden of care induced by bronchiolitis on ICUs. These should be considered illustrative estimations of the situation encountered in several high-income countries. Numbers represent the ratio between the annual cases of bronchiolitis needing ICU admission and the available beds in ICUs (paediatric, neonatal or mixed). Numbers have been obtained using local registries or personal communications from local colleagues (see acknowledgements). They represent estimations, as during the outbreaks the ICU beds might be temporarily increased by reducing elective surgical activities or changing the criteria for ICU admission of other patients, or admitting cases to neonatal ICUs that usually were not accepting infants with bronchiolitis. Abbreviation: ICU: intensive care unit.
Pathophysiology and biology
Bronchiolitis can be caused by several viruses, although RSV is the commonest and is associated with the most severe cases.7 Viruses invade airway cells causing a local inflammatory response, ciliary destruction and increased mucus secretion, although these features seem variable depending on the virus involved. Parietal inflammation, mucus and cellular debris reduce the cross-sectional area available for airflow and, in the worst-case scenario, may produce bronchiolar plugs and atelectasis. These processes lead to gas trapping with a typically obstructive respiratory failure.12
When the infection is severe enough, and the inflammatory reaction spreads from the airways to the lung parenchyma, the aforementioned phenomena may be associated with various degrees of alveolar injury. In these cases, from a pathological perspective, bronchiolitis co-exists with pneumonitis and respiratory failure has a restrictive or mixed mechanics.12 These cases present with significant alveolar inflammation and necrosis, as well as surfactant dysfunction13 and patients may qualify for neonatal (NARDS) or paediatric acute respiratory distress syndrome (PARDS).14,15 Thus, the first pathophysiological (obstructive) pattern may progress to the second (restrictive/mixed), and bronchiolitis can act as a trigger for ARDS.16,17 Most viruses increase the expression of inflammatory cytokines and reduce that of anti-inflammatory mediators. This may translate in surfactant injury which contribute to the restrictive pattern in more severe cases. Unsurprisingly, RSV-triggered ARDS presents with a high level of secretory phospholipase A2 and surfactant damage.18, 19, 20
How to prepare the ICU for the seasonal outbreak?
The seasonality observed in temperate zones offers the opportunity to be prepared for the next epidemics, but the literature lacks suggestions to prepare paediatric or neonatal ICU for an outbreak of bronchiolitis. The common ground is that ICU teams taking care of patients with bronchiolitis should have a solid pathophysiological background and significant expertise in: 1) respiratory critical care, and 2) infection control. A formal management protocol including these issues should be distributed to the whole team, with adequate simulations and refresher courses.
Additionally, the following steps should be considered to help ICU clinicians coping with the outbreak. They should be prepared to work dynamically during the stressful conditions created by an epidemic. Moreover, as patients with bronchiolitis frequently have obstructive or mixed respiratory failure, their lung mechanics is quite different from that of the most common types of neonatal respiratory disorders. Thus, if these patients are admitted to a neonatal ICU, additional training may be needed. A correct patient flow should be planned in collaboration with paediatric emergency and general paediatrics wards. The flow should be based on the local clinicians’ expertise and protocols specifically designed to recognise the severest cases using objective criteria for ICU admission and discharge, while providing serial monitoring for the other cases. These steps are crucial to avoid wasting resources, protect ICU beds from incorrect use and make them available for other children.21
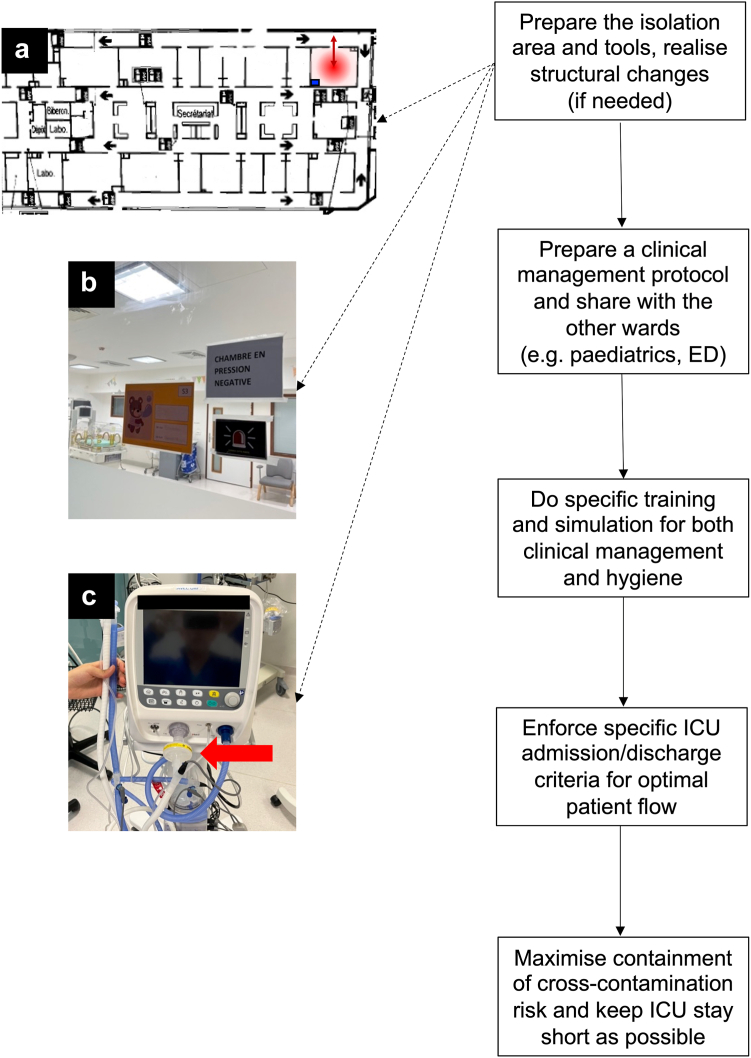
Then, it is pivotal to reduce the odds of cross-contamination using adequate hygiene measures,22 since the median transmission risk (i.e. attack rate) of nosocomial RSV outbreaks is 28.5%,23 and its mortality in paediatric/neonatal ICUs ranges between 8% and 12%.24,25 The backbone of containment is represented by patient and staff cohorting accompanied by the use of personal protective equipment.26 Thus, isolation should be enforced with extensive use of alcoholic gels or foams, as well as masks, glasses and gowns to avoid the contamination of personnel and further spread. As contagion can happen through patients’ coughing and/or aerosol transmission, dedicated isolation rooms, ideally equipped with negative pressure ventilation should be used. This is particularly important if the ICU admits infants with high-risk comorbidities (e.g. prematurity, bronchopulmonary dysplasia (BPD), congenital heart defects, neuromuscular diseases, genetic syndromes, malignancies and congenital or acquired immunodeficiencies). In some buildings, negative pressure areas can be created with small technical interventions, even if the room was not originally conceived for this; ventilators used in these areas should have high-efficiency filters inserted in the expiratory limb of their circuit. Devices used in these rooms should not be shared with patients out of the isolation area. These rooms should also allow the entrance of at least one parent, who must be adequately informed about the isolation procedures. While we recognize that not all hospital or countries have access to ideal settings and means, it seems important, considering the available literature, to highlight the best way to reduce nosocomial spread. In our experience, the strict application of these principles has avoided any nosocomial cross-contamination during the last seasonal outbreaks and a recent review confirmed the efficacy of this strategy.23 Palivizumab has been used to control already established nosocomial outbreaks and to protect the frailer patients, and therefore might be considered upon multidisciplinary discussions.24 Fig. 2 summarizes the essential points in preparing an ICU to manage the seasonal outbreak.
Fig. 2.
Flow chart of critical steps to prepare an ICU for the management of a bronchiolitis outbreak. The isolation area and instruments to be used in it should be prepared beforehand: images A and B show an isolation room (red circle) which is located at the end of an ICU and equipped with negative pressure ventilation, filter and personal protective equipment (blue square), as well as single door through which patients are admitted and discharged (red dual arrow) without entering other zones of the ICU (pictures from the “A. Béclère” Hospital, APHP-Paris Saclay University); image C shows a ventilator equipped with a HEPA filter on the expiratory limb (red arrow). A protocol for clinical management should then be prepared and shared between the wards concerned. Specific training and simulation should be performed, and this is particularly important for those neonatal ICUs that are versed only in managing neonates with different types of respiratory failure. Specific criteria for ICU admission/discharge and continuous communication between wards should be enforced to guarantee an optimised patient flow. Finally, containment to reduce the risk of cross-contamination should be enforced and ICU stay should be kept as short as possible. Abbreviations. ED: emergency department; HEPA: high-efficiency particulate absorption; ICU: intensive care unit.
ICU admission and discharge criteria
Criteria for ICU admission and discharge should be formally circulated within the hospital and approved by all concerned medical teams. They may vary from one centre to another, based on local epidemiology, medical geography and clinical experience. Nonetheless, it seems reasonable to admit (and discharge) patients to (and from) the ICU when any of the following conditions are present or absent, respectively:
-
1.
Hypoxemia defined with one of the following criterion: A) peripheral haemoglobin saturation (pulse oximetry, SpO2) <90% or partial pressure of O2 < 60 mmHg, despite low-flow (<2 L/min) oxygen supplementation or inspired oxygen fraction (FiO2) >0.30; or B) supplemental oxygen to maintain SpO2 >88% and oxygenation index ((OI) = mean airway pressure (Paw) x FiO2 x 100/PaO2) <4 or oxygen saturation index ((OSI)=Paw x FiO2 x 100/SpO2) <5 (during non-invasive respiratory support, OI and OSI should be considered as estimations since Paw may be subjected to relevant leaks,27 these estimations are based on Paw values shown by the ventilator while patients are positioned and, if certain interfaces are used, their mouth is gently closed to reduce leaks: this is internationally accepted in neonatal ICUs, thus may be potentially applied to several bronchiolitis patients14; other non-Paw based metrics might also be used), or C) need for respiratory support (i.e. continuous positive airway pressure (CPAP), non-invasive (NIV) or invasive ventilation) beside the oxygen supplementation.
-
2.
Clinically relevant dyspnoea based on signs of increased work of breathing.
-
3.
Apnoea, periodic or superficial breathing.
-
4.
Hypercarbia (i.e. partial pressure of CO2 > 65 mmHg or CO2 ≥ 60 with pH ≤ 7.25 measured on arterialised capillary samples).
-
5.
Haemodynamic compromise.
-
6.
Neurological compromise (i.e. reduced alertness, hypotonia or seizures).
-
7.
Severe dehydration.
Young (<6 months) age and history of prematurity might be considered as additional criteria.
The first criterion is essentially represented by the oxygenation thresholds needed to describe a patient as “at risk of PARDS” according to the current international definition.10,15 While it has not been clearly established whether these high-risk patients have more unfavourable outcomes, bronchiolitis can surely worsen into ARDS,12 particularly in younger, frailer or RSV-positive infants.10 OI can be calculated in a non-invasive fashion using PaO2 measured by transcutaneous devices calibrated according to current guidelines28 or using blood gas values obtained from arterialised capillary samples.17 OSI should be calculated considering the SpO2 measured with devices using motion artifact-removing algorithms.29 This criterion implies that anybody needing non-invasive (CPAP or any form of NIV) or invasive respiratory support would receive this in an ICU. In some settings, however, CPAP is provided out of ICUs and helps sparing ICU admission,30 so these criteria should be interpreted considering the local policies. The picture might be complicated by the use of heated humidified high-flow nasal cannulas (HHHFNC). As HHHFNC provide inconstant pressure, they could be associated with increased work of breathing (WOB) compared to CPAP.31 Despite HHHFNC can rescue some infants failing low flow oxygen therapy,32 they have a relevant failure rate, are less efficacious than CPAP, irrespective of the flow rate,33,34 and may increase ICU admission and length of stay.35 In fact, reducing HHHFNC use as a quality improvement project decreased ICU stay in some hospitals36 and they should be reserved for particular cases, ideally admitted to hospitals where a dedicated ICU is available in the event of failure or complications.37
The second criterion (i.e. the presence of severe dyspnoea) can be evaluated with several clinical scoring systems (online Appendix). More complex tools including oxygenation metrics and other parameters are also available, but scores are often poorly validated, and there is no evidence to prefer one score over the others to predict any outcome.38 Nonetheless, it is important to implement the use of a dyspnoea score and share it between ICU and other wards to facilitate communication and serial objective evaluations.
ICU clinical management
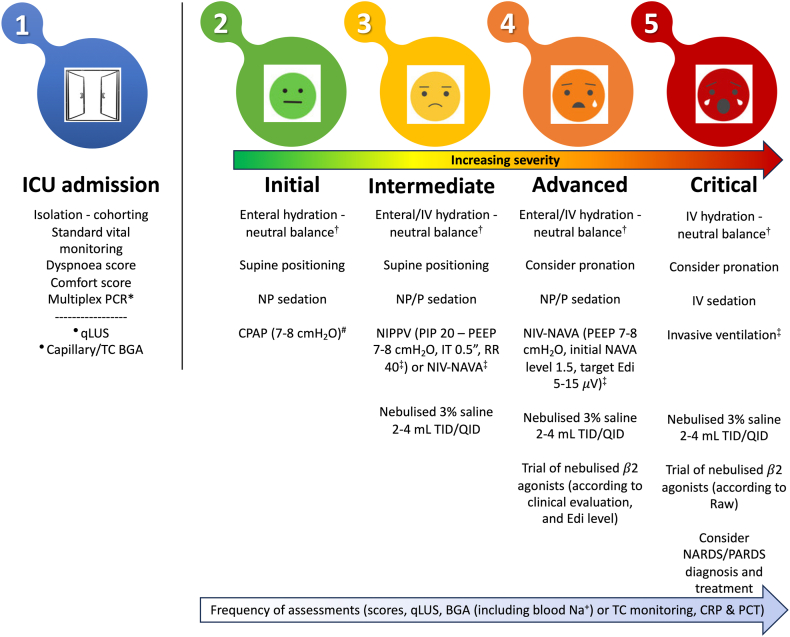
The ICU management of critically ill infants with bronchiolitis should follow the best evidence available,5 but also consider that: 1) most of this evidence has been produced in out-of-ICU settings, and 2) some interventions, despite lacking robust evidence for the improvement of relevant clinical outcomes, are safe and have the pathophysiological potential to shorten ICU stay. Thus, they may have a public health value and should be considered once the severest cases are admitted to an ICU. A suggested protocol for ICU management is illustrated in Fig. 3.
Fig. 3.
Suggested protocol for the clinical management of infants with severe bronchiolitis needing ICU admission. Isolation and cohorting should be enforced during the whole ICU stay. ∗Multiplex PCR might be performed upon ICU admission (if not performed earlier). Other tests may be performed upon ICU admission or later depending on clinical severity or if secondary infections are suspected. Standard vital monitoring (i.e. peripheral haemoglobin saturation, heart and respiratory rate) as well as calculation of a dyspnoea and a comfort score should also be performed upon admission and serially during the ICU stay. •Quantitative lung ultrasound and capillary or transcutaneous blood gas analysis should be done upon ICU admission based on clinical evaluation. #CPAP generator (continuous or variable flow or other) and interface (nasal masks/prongs, facial mask or helmet) should be chosen depending on team expertise and patient comfort. †Enteral and intravenous hydration should be given using nasogastric tubes and isotonic solutions, respectively. ‡Ventilatory parameters should be adjusted depending on chest expansion, blood gases, synchrony and comfort. Colours depict increasing clinical severity. Non-pharmacological sedation is performed with parental presence, installation, feeding, pacifiers with sucrose solutions; pharmacological sedation in non-invasively supported infants can be provided with mild sedative drugs (hydroxyzine, midazolam, chloral hydrate or dexmetomidine). The (light blue) arrow illustrates the frequency of tests and monitoring to be repeated during the ICU stay depending on clinical severity; capillary blood gas analysis allows measurement of blood sodium and indicates whether its supplementation is needed. HHHFNC are not included in the protocol as there is no evidence for their use in ICU, they can fail significantly more often compared to CPAP and prolong ICU stay.5 More details in the text. Abbreviations: BGA: blood gas analysis; CPAP: continuous positive airway pressure; CRP: C-reactive protein; Edi: electronic diaphragmatic activity; HEPA: high-efficiency particulate absorbing; HHHFNC: heated humidified high flow nasal cannula; ICU: intensive care unit; IV: intravenous; NARDS: neonatal acute respiratory distress syndrome; NIPPV: non-invasive positive pressure ventilation; NIV-NAVA: non-invasive ventilation with neurally assisted ventilation adjust; NP: non-pharmacological; P: pharmacological; PARDS: paediatric acute respiratory distress syndrome; PCR: polymerase chain reaction; PCT: procalcitonin; qLUS: quantitative lung ultrasound; QID: quater in die (i.e. every 6 h); TC: transcutaneous; TID: tris in die (i.e. every 8 h).
General management
According to available guidelines, ICU-admitted infants should routinely receive5: 1) standard vital monitoring, 2) enteral nutrition orally or through a gastric tube, depending on general clinical condition and feeding tolerance, 3) intravenous hydration with isotonic (balanced or not) solution when enteral nutrition is not possible or in complicated cases (with sodium supplementation being reserved for infants who develop hyponatraemia), 4) accurate control of fluid balance to strictly avoid overload as this is associated with longer ventilation and ICU stay, 4) non-pharmacological sedation (e.g. parental presence, installation, feeding, pacifiers with sucrose solutions). Mildly sedative drugs (e.g. either hydroxyzine, chloral hydrate, midazolam or dexmedetomidine) might be used when these fail and there is still evidence of relevant discomfort due to the non-invasive respiratory support or factors unrelated to bronchiolitis. These drugs have no strong evidence, and, thus, it is impossible to provide a generalised advice, but expert opinion suggest their use when non-pharmacologic measures fail.5 Scoring systems for the evaluation of comfort and sedation might be helpful to inform this choice. Deeper sedation should obviously be used in case of invasive ventilation.
Laboratory tests and imaging
The diagnosis of bronchiolitis is essentially based on epidemiology, clinical appearance, and medical history, so laboratory tests and imaging have a limited role, and this makes things easier during large outbreaks with increasing patient influx. Nonetheless, the identification of the responsible infectious agent might be useful in managing the isolation and immunisation schedule: in fact, if the patient is RSV-positive and is receiving monthly palivizumab, this can be stopped.39 Several respiratory viruses can now be detected on a single sample by multiplex real-time polymerase chain reaction. Nasopharyngeal lavage samples are preferred to nasal swabs as they are easier to collect and provide higher recovery rates and accuracy.40
Routine chest-X rays should not be performed, as they do not provide any clinically meaningful information, while exposing infants to radiation; chest-X rays can, however, be useful when complications (e.g. pneumothorax or other air leaks, pneumonia or secondary infections) are suspected.39 Nonetheless, point-of-care lung ultrasound is more accurate than conventional radiology in detecting loss of lung aeration and pneumothorax, and this has been specifically confirmed in patients with complicated bronchiolitis.41 Imaging is also needed to diagnose PARDS or NARDS,14,15 and ultrasound findings have been used to this end.42,43 Moreover, a quantitative lung ultrasound score can be used to guide the respiratory interventions44 or predict outcomes.45 Last but not least, point-of-care lung ultrasound can be performed by ICU physicians using hand-held devices or wireless probes dedicated to isolated patients and this may reduce the risk of cross-contamination.46
Respiratory support
Since there is no causal therapy for bronchiolitis, respiratory support is the mainstay of clinical management to buy time and keep patients alive, while the immune system overcomes the infection. The first level of respiratory support is simple oxygen supplementation: this is often started before ICU admission and most guidelines recommend it when SpO2 is ≤ 90–92%.6 Starting oxygen supplementation at higher SpO2 thresholds is not associated with better outcomes.47 As hypoxaemia worsens or relevant dyspnoea appears, respiratory support needs to be escalated.
The application of 7–8 cm H2O of CPAP is the second step and is usually enough to reduce WOB, increase expiratory time and improve dyspnoea,48 so it is suggested as initial respiratory support in the ICU.5 CPAP requires skills, equipments and patient monitoring. Patients with any risk factor such as prematurity, congenital heart defects or neuromuscular disorders are more likely to fail CPAP, which can still succeed in approximately 90% of cases.49 CPAP is associated with a reduced duration of respiratory support and ICU stay49, 50, 51 and may carry financial and public health benefits, since ICU admission is the highest driver of increased hospital charges.52 There are no data to prefer one particular system to generate the pressure. CPAP can be delivered with helmets, binasal prongs and facial or nasal masks. Interfaces smaller than total face mask might be associated with more leaks and failure,53 while helmets have fewer pressure leaks and provide better comfort.54 Team experience and patient comfort should ultimately inform the choice. Conversely, the current knowledge does not justify the use of HHHFNC instead of CPAP, and, in fact, the available evidence-based guidelines state that there is insufficient evidence to use them in ICU.5 When needed, superficial nasal suction should be serially performed to guarantee optimal CPAP transmission, but deep suctioning should be avoided.6
When CPAP is not sufficient to relieve dyspnoea and/or hypoxaemia, the next step is usually represented by NIV with two pressure levels,5 targeting normocarbia and a relatively low oxygen need (FiO2 ≤0.40). Transcutaneous devices may be useful for close monitoring and reduction of blood gas analyses.28 NIV should be synchronised whenever feasible, although this is not possible with several neonatal ventilators. When ventilation is not efficacious because of dyssynchrony or patient discomfort, NIV with neurally adjusted ventilatory assist (NAVA) may be an useful option as it increases comfort and ventilatory efficiency.55 NAVA should target a peak electrical diaphragmatic activity between 5 and 15 μV,56 and, in our experience, has helped to spare intubation in critically ill infants.
Invasive ventilation should be the last resort, when NIV is unable to guarantee normal gas exchange or complications (e.g. air leaks, pneumonitis, ARDS) arise. Non-invasive respiratory support is more likely to fail in patients qualifying for ARDS diagnosis when another organ failure is evident.57 There are no specific data to suggest one particular ventilatory mode in patients who need intubation, and general guidelines for paediatric mechanical ventilation should be followed.58
Ancillary therapies
Some ancillary ICU interventions for patients with bronchiolitis have an interesting pathophysiological background but lack strong evidence in favour or against their use:
-
-
Nebulised β2-agonist and epinephrine. These drugs do not change clinical outcomes, in populations affected by bronchiolitis as a whole, because bronchiolitic wheeze is mainly caused by mucous obstruction and oedema, rather than bronchospasm.59 Thus, their use is not indicated outside of the ICU.6 However, it is now well recognised that multiple phenotypes exist with heterogeneous clinical presentation and pathobiology, which impact on airway reactivity and responsiveness to these drugs.60 Therefore, we need to acknowledge this complexity and seek a more phenotype-specific strategy. A relevant airway muscular constriction seems more frequent: a) when rhinoviruses are the aetiologic agents, particularly outside the classic outbreak season, b) in infants aged more than 6 months and in those with asthma or an atopic family history or a tendency to exaggerated Th2 response, c) in patients clinically presenting with wheezing and dyspnoea rather than hypoxaemia as the predominant sign. In these patients, these drugs can facilitate ventilation during an acute phase of their disease. In our experience, it may be useful to perform a short trial with an objective, before-and-after efficacy evaluation, in critical situations, for instance when intubation is pending.5 This attempt should be based on dyspnoea scores and electrical diaphragmatic activity, or airway resistance measurements in non-invasively or invasively ventilated infants, respectively. Prompt recognition of side effects is possible in ICUs and makes these drugs relatively safe.
-
-
Nebulised hypertonic saline. This may decrease epithelial oedema, improve mucociliary clearance and reduce airway plugging, causing bronchiolar obstruction: one ICU study is available and found a decreased duration of ventilation in treated infants.5 It is unclear what concentration should be used, although 3% seems the commonest solution.5 Hypertonic saline has a well-established safety and might be considered in severe cases with large amounts of secretions.
-
-
Pronation. This is an effective and inexpensive intervention, strongly recommended for ARDS in adults.61 Few paediatric data are available, but pronation seems to improve oxygenation in paediatric lung injury too.62 A randomised cross-over trial demonstrated decreased WOB and improved ventilatory efficiency in non-invasively ventilated infants with bronchiolitis.63 Pronation also safely improves gas exchange in neonates with NARDS and, interestingly, in infants with evolving BPD, whose lung mechanics may be similar to that of bronchiolitis.64 Pronation is easier to apply in infants compared to adults and its risk/benefit ratio suggests that it should be tried in the severest cases.
-
-
Surfactant. Surfactant might shorten ventilation and ICU stay according to a Cochrane meta-analysis of trials recruiting patients severe enough to be intubated.65 This seems supported by data demonstrating surfactant injury in these cases.20 Nonetheless, this is likely to apply only when bronchiolitis evolved into ARDS.66 Moreover, there are uncertainties about the dose, timing and technique of administration which warrant further research.66
Finally, the rheological properties of Heliox might help to ameliorate WOB, but its cost and lack of effect in terms of duration of ventilation and ICU stay5 contraindicate its use. Other interventions (e.g. anti-leukotrienes, deoxyribonuclease, methylxanthines, nitric oxide, magnesium sulphate, physiotherapy, ribavirin and other antivirals, corticosteroids and other anti-inflammatory drugs) do not have a clear pathobiological background, have not been studied in ICU, lack possible short-term benefits, or may have significant side effects and shall not be used.
Cost-effectiveness
These suggestions are based on current knowledge of the pathophysiology and biology of bronchiolitis, as well as of safety, since the proposed approach consists of ICU interventions well known for their safety and suitability. However, most ICU therapies are also likely to result in potential public health benefits. In fact, the need for ICU admission (which usually equals the need for respiratory support) is the main determinant of hospital charges,52 and the number of patients admitted to the ICU (and particularly those receiving non-invasive respiratory support) has significantly increased in recent years.67 Therefore, if an intervention can shorten respiratory support, it may entail a reduced use of ICU resources. This can: 1) increase ICU bed availability for patients needing them for bronchiolitis or other reasons, 2) reduce inter-hospital transfers with further consequences for the cost of care, 3) reduce the number of lost workdays and other negative effects on families.
Available data show that the ICU management of bronchiolitis, despite being expensive in absolute terms, consists of interventions that are likely to be cost-effective.50,51,68, 69, 70, 71, 72, 73, 74 Data are summarised in Table 1: this is not a formal cost-effectiveness analysis, since it is difficult to precisely assess some of these effects (e.g. the cost of inter-hospital transfer or the effects on families, which may vary according to several factors) and studies have different designs and outcomes. Also, cost-effectiveness was not always proven specifically for the ICU, but rather for the hospital care in general; however, when the cost-effectiveness of a given intervention was proven in terms of reduced hospital admission or length of stay, the cost of ICU care was also considered and had a relevant weight in the outcome.52
Table 1.
Cost-effectiveness of interventions to treat or prevent bronchiolitis that may be used in the ICU.
| Intervention/[reference] | Country | Outcome | Cost-effectivenessa proven in: |
|
|---|---|---|---|---|
| Hospital setting | ICU setting | |||
| Treatment of bronchiolitis | ||||
| CPAP/49, 50, 51,74 | Netherlands, France, Colombia | ICU length of stay and duration of respiratory support, QALY | no | yes |
| Selective use of nebulised β2-agonists/70 | Colombia | Hospital and ICU admission and length of stay | yes | yes |
| Hypertonic saline/71, 72, 73 | Many countriesb | Hospital admission or length of stay, QALY | yes | no |
| Pronation | n.a. | n.a. | n.a. | n.a. |
| Surfactant | n.a. | n.a. | n.a. | n.a. |
| Prevention of nosocomial outbreaks | ||||
| Containment/68 | USA | Incidence density | yes | yes |
| Palivizumab/69 | UAE | Duration of O2 therapy | yes | no |
Abbreviations: CPAP: continuous positive airway pressure; n.a. not available; ICU: intensive care unit; QALY: quality-adjusted life year; UAE: United Arab Emirates; UK: United Kingdom; USA: United States of America.
Cost-effectiveness was proven as dominant scenario versus comparators (i.e. the intervention results in better health effects and cost savings) in the hospital or specifically in the ICU setting; when the cost-effectiveness was proven in the hospital setting (e.g.: reduced hospital admission or length of stay), the cost of ICU admission was also considered and had a relevant weight in the outcome.52 More details in the text.
Data extracted from systematic reviews and/or meta-analyses.
It is generally clear that the proposed interventions may carry relevant benefits in terms of public health. In fact, CPAP may shorten respiratory support and ICU stay, while other therapies (i.e. nebulised β2-agonists and hypertonic saline) may have an indirect but positive cost-effectiveness evaluation. Pronation is a totally inexpensive intervention and surfactant is a high-priced drug, but it would be used in just a few very critically ill infants needing invasive ventilation and is not likely to significantly impact the general ICU budget.75 Finally, containment measures are cost-effective in decreasing the spread of nosocomial RSV outbreaks within the ICU.
Outstanding questions
We have comprehensively reviewed the ICU management of severe bronchiolitis in an era of relative shortage of ICU resources and lack of ICU-specific evidence-based data. With a multidisciplinary approach, we have highlighted the important points in preparing the ICU and its personnel for seasonal outbreaks. We provide suggestions for a clinical protocol that optimises ICU care, avoids nosocomial cross-contamination, “protects” ICU beds and makes them available for other patients, whenever possible. Dedicated studies are needed to clarify the still outstanding questions about several uncertainties and to further refine ICU respiratory care and pharmacological therapy of patients.
Contributors
DDL conceived the manuscript, wrote the first draft and collected the literature information; DDL also has direct access to and verified all the information present in the manuscript. LP helped in the draft preparation and collected the literature information; she also prepared part of the iconography. EB, LV, MDN, ML, MP, WR and GC contributed to literature analysis, collection of information and preparation of the iconography. MRG supervised the whole work, performed the literature analysis of cost-effectiveness and contributed to literature analysis. All authors critically reviewed the manuscript, contributed important intellectual content, approved the final version of the manuscript and accept responsibility for its submission.
Data sharing statement
Not applicable.
Declaration of interests
DDL has received lecture fees or research and educational support or from Chiesi Farmaceutici, Getinge, Vyaire, Radiometer, Medtronic, AstraZeneca, Boehringer Ingelheim, Airway Therapeutics, Natus, Masimo and BD. He has equity options from Ophirex ltd; he also participated to a data safety monitoring board for EXO biologics. All these were unrelated to this work and the field of bronchiolitis in general; finally, he is the Immediate Past President of the European Society for Paediatric and Neonatal Intensive Care (ESPNIC). MDN participated to the medical advisory board of Eurosets, unrelated to this work and the field of bronchiolitis in general; he is also the Secretary of ESPNIC. EB has received consultancy and lecture fees and has participated in advisory boards for AstraZeneca and Sanofi, all outside of the present work. MRG received a lecture fee from Sanofi, unrelated to this work and the field of bronchiolitis in general. The other authors have no interest to declare. This work did not receive any funding.
Acknowledgements
The authors are grateful to Dr. Thomas Conlon (Philadelphia, PA-USA), Prof. Akash Deep (London, UK), Dr. Simon Nadel (London, UK) and Dr. Karl Schettler (Munich, Germany) who contributed to the estimation of ICU burden of care by providing data on yearly ICU admission and beds in their respective countries. The views expressed in this article are the personal views of Prof. Daniele De Luca and cannot be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its committees or working parties. No medical writer and no artificial intelligence-based tools were involved in it. This work did not receive any funding.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102450.
Appendix A. Supplementary data
References
- 1.Cohen C., Zar H.J. Deaths from RSV in young infants—the hidden community burden. Lancet Glob Health. 2022;10:e169–e170. doi: 10.1016/S2214-109X(21)00558-1. [DOI] [PubMed] [Google Scholar]
- 2.De Luca D., Sanchez-Luna M., Schettler M., Bont L., Baraldi E. Universal infant immunisation against respiratory syncytial virus and European inequalities: the pandemics lesson has not been learned. Lancet Reg Health Eur. 2023;34 doi: 10.1016/j.lanepe.2023.100753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fairbank R. RSV wave hammers hospitals — but vaccines and treatments are coming. Nature. 2022 doi: 10.1038/d41586-022-04434-5. [DOI] [PubMed] [Google Scholar]
- 4.Pierce H., Mansbach J., Fisher E., et al. Variability of intensive care management for children with bronchiolitis. Hosp Pediatr. 2015;5:174–184. doi: 10.1542/hpeds.2014-0125. [DOI] [PubMed] [Google Scholar]
- 5.Milési C., Baudin F., Durand P., et al. Clinical practice guidelines: management of severe bronchiolitis in infants under 12 months old admitted to a pediatric critical care unit. Intensive Care Med. 2023;49:5–25. doi: 10.1007/s00134-022-06918-4. [DOI] [PubMed] [Google Scholar]
- 6.Dalziel S.R., Haskell L., O'Brien S., et al. Bronchiolitis. Lancet. 2022;400:392–406. doi: 10.1016/S0140-6736(22)01016-9. [DOI] [PubMed] [Google Scholar]
- 7.Ghazaly M., Nadel S. Characteristics of children admitted to intensive care with acute bronchiolitis. Eur J Pediatr. 2018;177:913–920. doi: 10.1007/s00431-018-3138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazur N.I., Terstappen J., Baral R., et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23:e2–e21. doi: 10.1016/S1473-3099(22)00291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildenbeest J.G., Billard M.-N., Zuurbier R.P., et al. The burden of respiratory syncytial virus in healthy term-born infants in Europe: a prospective birth cohort study. Lancet Respir Med. 2023;11:341–353. doi: 10.1016/S2213-2600(22)00414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slain K.N., Rotta A.T., Martinez-Schlurmann N., Stormorken A.G., Shein S.L. Outcomes of children with critical bronchiolitis meeting at risk for pediatric acute respiratory distress syndrome criteria. Pediatr Crit Care Med. 2019;20:e70–e76. doi: 10.1097/PCC.0000000000001812. [DOI] [PubMed] [Google Scholar]
- 11.Minardi C., Conti G., Moscatelli A., Tesoro S., Bussolin L. Shortage of paediatric intensive care unit beds in Italy. Lancet. 2023;402:1525. doi: 10.1016/S0140-6736(23)01791-9. [DOI] [PubMed] [Google Scholar]
- 12.Newth C.J., Hammer J. Pulmonary function in ventilated infants with bronchiolitis. Pediatr Pulmonol. 1998;26:438–441. doi: 10.1002/(sici)1099-0496(199812)26:6<438::aid-ppul10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Grunwell J.R., Dahmer M.K., Sapru A., Quasney M.W., Flori H. On behalf of the second pediatric acute lung injury consensus conference (PALICC-2) for the pediatric acute lung injury and sepsis investigators (PALISI) network. Pathobiology, severity, and risk stratification of pediatric acute respiratory distress syndrome: from the second pediatric acute lung injury consensus conference. Pediat Crit Care Med. 2023;24:S12–S27. doi: 10.1097/PCC.0000000000003156. [DOI] [PubMed] [Google Scholar]
- 14.De Luca D., van Kaam A.H., Tingay D.G., et al. The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir Med. 2017;5:657–666. doi: 10.1016/S2213-2600(17)30214-X. [DOI] [PubMed] [Google Scholar]
- 15.Khemani R.G., Smith L.S., Zimmerman J.J., Erickson S. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology. Pediatr Crit Care Med. 2015;16:S23–S40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 16.Khemani R.G., Smith L., Lopez-Fernandez Y.M., et al. Pediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE) investigators; pediatric acute lung injury and sepsis investigators (PALISI) network. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med. 2019;7:115–128. doi: 10.1016/S2213-2600(18)30344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Luca D., Tingay D., van Kaam A., et al. Epidemiology of neonatal ARDS: prospective, multicenter, international cohort study. Pediatr Crit Care Med. 2022;23:524–534. doi: 10.1097/PCC.0000000000002961. [DOI] [PubMed] [Google Scholar]
- 18.De Luca D., Minucci A., Cogo P., et al. Secretory phospholipase A2 pathway during pediatric acute respiratory distress syndrome: a preliminary study. Pediatr Crit Care Med. 2011;12:e20–e24. doi: 10.1097/PCC.0b013e3181dbe95e. [DOI] [PubMed] [Google Scholar]
- 19.De Luca D., Lopez-Rodriguez E., Minucci A., et al. Clinical and biological role of secretory phospholipase A2 in acute respiratory distress syndrome infants. Crit Care. 2013;17(4):R163. doi: 10.1186/cc12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dargaville P.A., South M., McDougall P.N. Surfactant abnormalities in infants with severe viral bronchiolitis. Arch Dis Child. 1996;75:133–136. doi: 10.1136/adc.75.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Luca D. Managing neonates with respiratory failure due to SARS-CoV-2. Lancet Child Adolesc Health. 2020;4:e8. doi: 10.1016/S2352-4642(20)30073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manzoni P., De Luca D., Stronati M., et al. Prevention of nosocomial infections in neonatal intensive care units. Am J Perinatol. 2013;30:81–88. doi: 10.1055/s-0032-1333131. [DOI] [PubMed] [Google Scholar]
- 23.French C.E., McKenzie B.C., Coope C., et al. Risk of nosocomial respiratory syncytial virus infection and effectiveness of control measures to prevent transmission events: a systematic review. Influenza Resp Viruses. 2016;10:268–290. doi: 10.1111/irv.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosalli R., Alqarni S.A., Khayyat W.W., et al. Respiratory syncytial virus nosocomial outbreak in neonatal intensive care: a review of the incidence, management, and outcomes. Am J Infect Control. 2022;50:801–808. doi: 10.1016/j.ajic.2021.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Thorburn K., Kerr S., Taylor N., Van Saene H.K.F. RSV outbreak in a paediatric intensive care unit. J Hosp Infect. 2004;57:194–201. doi: 10.1016/j.jhin.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Groothuis J., Bauman J., Malinoski F., Eggleston M. Strategies for prevention of RSV nosocomial infection. J Perinatol. 2008;28:319–323. doi: 10.1038/jp.2008.37. [DOI] [PubMed] [Google Scholar]
- 27.Centorrino R., Dell'Orto V., Gitto E., Conti G., De Luca D. Mechanics of nasal mask-delivered HFOV in neonates: a physiologic study. Pediatr Pulmonol. 2019;54:1304–1310. doi: 10.1002/ppul.24358. [DOI] [PubMed] [Google Scholar]
- 28.Restrepo R.D., Hirst K.R., Wittnebel L., Wettstein R. AARC clinical practice guideline: transcutaneous monitoring of carbon dioxide and oxygen: 2012. Respir Care. 2012;57:1955–1962. doi: 10.4187/respcare.02011. [DOI] [PubMed] [Google Scholar]
- 29.Salyer J.W. Neonatal and pediatric pulse oximetry. Respir Care. 2003;48:386–396. [PubMed] [Google Scholar]
- 30.Agüera M., Melé-Casas M., Molina M.M., et al. Safety and effectiveness of bubble continuous positive airway pressure as respiratory support for bronchiolitis in a pediatric ward. Eur J Pediatr. 2022;181:4039–4047. doi: 10.1007/s00431-022-04616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasef N., El-Gouhary E., Schurr P., et al. High-flow nasal cannulae are associated with increased diaphragm activation compared with nasal continuous positive airway pressure in preterm infants. Acta Paediatr. 2015;104:e337–e343. doi: 10.1111/apa.12998. [DOI] [PubMed] [Google Scholar]
- 32.Luo J., Duke T., Chisti M.J., Kepreotes E., Kalinowski V., Li J. Efficacy of high-flow nasal cannula vs standard oxygen therapy or nasal continuous positive airway pressure in children with respiratory distress: a meta-analysis. J Pediatr. 2019;215:199–208.e8. doi: 10.1016/j.jpeds.2019.07.059. [DOI] [PubMed] [Google Scholar]
- 33.Milési C., Essouri S., Pouyau R., et al. Groupe Francophone de Réanimation et d'Urgences Pédiatriques (GFRUP). High flow nasal cannula (HFNC) versus nasal continuous positive airway pressure (nCPAP) for the initial respiratory management of acute viral bronchiolitis in young infants: a multicenter randomized controlled trial (TRAMONTANE study) Intensive Care Med. 2017;43:209–216. doi: 10.1007/s00134-016-4617-8. [DOI] [PubMed] [Google Scholar]
- 34.Milési C., Pierre A.F., Deho A., et al. GFRUP Respiratory Study Group A multicenter randomized controlled trial of a 3-L/kg/min versus 2-L/kg/min high-flow nasal cannula flow rate in young infants with severe viral bronchiolitis (TRAMONTANE 2) Intensive Care Med. 2018;44:1870–1878. doi: 10.1007/s00134-018-5343-1. [DOI] [PubMed] [Google Scholar]
- 35.Linssen R.S., Van Woensel J.B.M., Bont L., et al. Are changes in practice a cause of the rising burden of bronchiolitis for paediatric intensive care units? Lancet Respir Med. 2021;9:1094–1096. doi: 10.1016/S2213-2600(21)00367-2. [DOI] [PubMed] [Google Scholar]
- 36.Treasure J.D., Lipshaw M.J., Dean P., et al. Quality improvement to reduce high-flow nasal cannula overuse in children with bronchiolitis. Pediatrics. 2023;152 doi: 10.1542/peds.2022-058758. [DOI] [PubMed] [Google Scholar]
- 37.Kugelman A. High-flow nasal cannula therapy: can it be recommended as initial or rescue care for infants with moderate bronchiolitis in the paediatric ward? Eur Respir J. 2020;56 doi: 10.1183/13993003.01020-2020. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh Z., Potter E., Li Y., et al. Validity of clinical severity scores for respiratory syncytial virus: a systematic review. J Infect Dis. 2023:jiad436. doi: 10.1093/infdis/jiad436. [DOI] [PubMed] [Google Scholar]
- 39.Ralston S.L., Lieberthal A.S., Meissner H.C., et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 40.Macfarlane P. RSV testing in bronchiolitis: which nasal sampling method is best? Arch Dis Child. 2005;90:634–635. doi: 10.1136/adc.2004.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caiulo V.A., Gargani L., Caiulo S., et al. Lung ultrasound in bronchiolitis: comparison with chest X-ray. Eur J Pediatr. 2011;170:1427–1433. doi: 10.1007/s00431-011-1461-2. [DOI] [PubMed] [Google Scholar]
- 42.Potter S.K., Griksaitis M.J. The role of point-of-care ultrasound in pediatric acute respiratory distress syndrome: emerging evidence for its use. Ann Transl Med. 2019;7:507. doi: 10.21037/atm.2019.07.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Luca D., van Kaam A.H., Tingay D.G., et al. Lung ultrasound and neonatal ARDS: is Montreux closer to Berlin than to Kigali? – Authors' reply. Lancet Respir Med. 2017;5:e32. doi: 10.1016/S2213-2600(17)30380-6. [DOI] [PubMed] [Google Scholar]
- 44.Mongodi S., De Luca D., Colombo A., et al. Quantitative lung ultrasound: technical aspects and clinical applications. Anesthesiology. 2021;134:949–965. doi: 10.1097/ALN.0000000000003757. [DOI] [PubMed] [Google Scholar]
- 45.Bobillo-Perez S., Sorribes C., Gebellí P., et al. Lung ultrasound to predict pediatric intensive care admission in infants with bronchiolitis (LUSBRO study) Eur J Pediatr. 2021;180:2065–2072. doi: 10.1007/s00431-021-03978-4. [DOI] [PubMed] [Google Scholar]
- 46.Buonsenso D., Pata D., Chiaretti A. COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med. 2020;8:e27. doi: 10.1016/S2213-2600(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunningham S., Rodriguez A., Adams T., et al. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet. 2015;386:1041–1048. doi: 10.1016/S0140-6736(15)00163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Essouri S., Durand P., Chevret L., et al. Optimal level of nasal continuous positive airway pressure in severe viral bronchiolitis. Intensive Care Med. 2011;37:2002–2007. doi: 10.1007/s00134-011-2372-4. [DOI] [PubMed] [Google Scholar]
- 49.Ganu S., Gautam A., Wilkins B., Egan J. Increase in use of non-invasive ventilation for infants with severe bronchiolitis is associated with decline in intubation rates over a decade. Intensive Care Med. 2012;38:1177–1183. doi: 10.1007/s00134-012-2566-4. [DOI] [PubMed] [Google Scholar]
- 50.Borckink I., Essouri S., Laurent M., et al. Infants with severe respiratory syncytial virus needed less ventilator time with nasal continuous airways pressure then invasive mechanical ventilation. Acta Paediatr. 2014;103:81–85. doi: 10.1111/apa.12428. [DOI] [PubMed] [Google Scholar]
- 51.Essouri S., Laurent M., Chevret L., et al. Improved clinical and economic outcomes in severe bronchiolitis with pre-emptive nCPAP ventilatory strategy. Intensive Care Med. 2014;40:84–91. doi: 10.1007/s00134-013-3129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slain K.N., Malay S., Shein S.L. Hospital charges associated with critical bronchiolitis from 2009 to 2019. Pediatr Crit Care Med. 2022;23:171–180. doi: 10.1097/PCC.0000000000002878. [DOI] [PubMed] [Google Scholar]
- 53.Toni F., Cambra Lasaosa F.J., Conti G., et al. Comparison in the management of respiratory failure due to bronchiolitis in a pediatric ICU between 2010 and 2016. Respir Care. 2019;64:1270–1278. doi: 10.4187/respcare.06608. [DOI] [PubMed] [Google Scholar]
- 54.Chidini G., Piastra M., Marchesi T., et al. Continuous positive airway pressure with helmet versus mask in infants with bronchiolitis: an RCT. Pediatrics. 2015;135:e868–e875. doi: 10.1542/peds.2014-1142. [DOI] [PubMed] [Google Scholar]
- 55.Chidini G., De Luca D., Conti G., Pelosi P., Nava S., Calderini E. Early noninvasive neurally adjusted ventilatory assist versus noninvasive flow-triggered pressure support ventilation in pediatric acute respiratory failure: a physiologic randomized controlled trial. Pediatr Crit Care Med. 2016;17:e487–e495. doi: 10.1097/PCC.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 56.Kallio M., Peltoniemi O., Anttila E., Jounio U., Pokka T., Kontiokari T. Electrical activity of the diaphragm during neurally adjusted ventilatory assist in pediatric patients: Edi during NAVA in Children. Pediatr Pulmonol. 2015;50:925–931. doi: 10.1002/ppul.23084. [DOI] [PubMed] [Google Scholar]
- 57.Piastra M., De Luca D., Marzano L., et al. The number of failing organs predicts non-invasive ventilation failure in children with ALI/ARDS. Intensive Care Med. 2011;37:1510–1516. doi: 10.1007/s00134-011-2308-z. [DOI] [PubMed] [Google Scholar]
- 58.Kneyber M.C.J., De Luca D., Calderini E., et al. Respiratory failure section of the European society for paediatric and neonatal intensive care. Recommendations for mechanical ventilation of critically ill children from the paediatric mechanical ventilation consensus conference (PEMVECC) Intensive Care Med. 2017;43:1764–1780. doi: 10.1007/s00134-017-4920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yusuf F., Prayle A.P., Yanney M.P. β 2 -agonists do not work in children under 2 years of age: myth or maxim? Breathe. 2019;15:273–276. doi: 10.1183/20734735.0255-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nino G., Rodríguez-Martínez C.E., Castro-Rodriguez J.A. The use of β 2 -adrenoreceptor agonists in viral bronchiolitis: scientific rationale beyond evidence-based guidelines. ERJ Open Res. 2020;6 doi: 10.1183/23120541.00135-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grasselli G., Calfee C.S., Camporota L., et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49:727–759. doi: 10.1007/s00134-023-07050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhandari A.P., Nnate D.A., Vasanthan L., Konstantinidis M., Thompson J. Positioning for acute respiratory distress in hospitalised infants and children. Cochrane Database Syst Rev. 2022;6:CD003645. doi: 10.1002/14651858.CD003645.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baudin F., Emeriaud G., Essouri S., et al. Physiological effect of prone position in children with severe bronchiolitis: a randomized cross-over study (BRONCHIO-DV) J Pediatr. 2019;205:112–119.e4. doi: 10.1016/j.jpeds.2018.09.066. [DOI] [PubMed] [Google Scholar]
- 64.Loi B., Regiroli G., Foligno S., et al. Respiratory and haemodynamic effects of 6h-pronation in neonates recovering from respiratory distress syndrome, or affected by acute respiratory distress syndrome or evolving bronchopulmonary dysplasia: a prospective, physiological, crossover, controlled cohort study. eClinicalMedicine. 2022;55 doi: 10.1016/j.eclinm.2022.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jat K.R., Chawla D. Surfactant therapy for bronchiolitis in critically ill infants. Cochrane Database Syst Rev. 2015:CD009194. doi: 10.1002/14651858.CD009194.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Luca D., Cogo P., Kneyber M.C., et al. Surfactant therapies for pediatric and neonatal ARDS: ESPNIC expert consensus opinion for future research steps. Crit Care. 2021;25:75. doi: 10.1186/s13054-021-03489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willer R.J., Coon E.R., Harrison W.N., Ralston S.L. Trends in hospital costs and levels of services provided for children with bronchiolitis treated in children's hospitals. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.29920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Macartney K.K., Gorelick M.H., Manning M.L., Hodinka R.L., Bell L.M. Nosocomial respiratory syncytial virus infections: the cost-effectiveness and cost-benefit of infection control. Pediatrics. 2000;106:520–526. doi: 10.1542/peds.106.3.520. [DOI] [PubMed] [Google Scholar]
- 69.Saadah L.M., Chedid F.D., Sohail M.R., Nazzal Y.M., Al Kaabi M.R., Rahmani A.Y. Palivizumab prophylaxis during nosocomial outbreaks of respiratory syncytial virus in a neonatal intensive care unit: predicting effectiveness with an artificial neural network model. Pharmacotherapy. 2014;34:251–259. doi: 10.1002/phar.1333. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Martinez C.E., Nino G., Castro-Rodriguez J.A., Perez G.F., Sossa-Briceño M.P., Buendia J.A. Cost-effectiveness analysis of phenotypic-guided versus guidelines-guided bronchodilator therapy in viral bronchiolitis. Pediatr Pulmonol. 2021;56:187–195. doi: 10.1002/ppul.25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buendía J.A., Acuña-Cordero R. The cost-effectiveness of hypertonic saline inhalations for infant bronchiolitis. BMC Health Serv Res. 2020;20:1001. doi: 10.1186/s12913-020-05814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heikkilä P., Mecklin M., Korppi M. The cost-effectiveness of hypertonic saline inhalations for infant bronchiolitis: a decision analysis. World J Pediatr. 2018;14:26–34. doi: 10.1007/s12519-017-0112-8. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y.-J., Lee W.-L., Wang C.-M., Chou H.-H. Nebulized hypertonic saline treatment reduces both rate and duration of hospitalization for acute bronchiolitis in infants: an updated meta-analysis. Pediatr Neonatol. 2014;55:431–438. doi: 10.1016/j.pedneo.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 74.Buendía J.A., Feliciano-Alfonso J.E., Florez I.D. Systematic review and cost-utility of high flow nasal cannula versus continuous positive airway pressure in children with acute severe or moderate bronchiolitis in Colombia. Pediatr Pulmonol. 2022;57:3111–3118. doi: 10.1002/ppul.26142. [DOI] [PubMed] [Google Scholar]
- 75.Buendía J.A., Patiño D.G. Budget impact analysis of surfactant therapy for bronchiolitis in critically ill infants: the Colombian National Health System perspective. BMC Health Serv Res. 2021;21:334. doi: 10.1186/s12913-021-06347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.