Abstract
CD8+ T cells have been implicated as critical effector cells in protective immunity against malaria parasites developing within hepatocytes. A vaccine that protects against malaria by inducing CD8+ T cells will probably have to include multiple epitopes on the same protein or different proteins, because of parasite polymorphism and genetic restriction of T-cell responses. To determine if CD8+ T-cell responses against multiple P. falciparum proteins can be induced in primates by immunization with plasmid DNA, rhesus monkeys were immunized intramuscularly with a mixture of DNA plasmids encoding four P. falciparum proteins or with individual plasmids. All six monkeys immunized with PfCSP DNA, seven of nine immunized with PfSSP2 DNA, and five of six immunized with PfExp-1 or PfLSA-1 DNA had detectable antigen-specific cytotoxic T lymphocytes (CTL) after in vitro restimulation of peripheral blood mononuclear cells. CTL activity was genetically restricted and dependent on CD8+ T cells. By providing the first evidence for primates that immunization with a mixture of DNA plasmids induces CD8+ T-cell responses against all the components of the mixture, these studies provide the foundation for multigene immunization of humans.
A malaria vaccine will help reduce the 300 million to 500 million new Plasmodium infections and 1.5 million to 2.7 million deaths due to malaria annually (53). Many believe that the ideal vaccine may need to induce protective immunity against all stages of the parasite life cycle (7, 22). Our first step in developing such a multistage, multi-immune response vaccine is the induction of protective CD8+ T-cell responses against Plasmodium falciparum-infected hepatocytes (22). This strategy is based on the observations with mice that the sterile immunity induced by administration of radiation-attenuated sporozoites is dependent on CD8+ T cells (36, 50) directed against infected hepatocytes (20, 21, 49) and that CD8+ cytotoxic T lymphocytes (CTL) can adoptively transfer protection against challenge in the absence of other parasite-specific immune responses (20). However, T-cell responses to individual epitopes are major histocompatibility complex (MHC) restricted, and there is genetic variation within T-cell epitopes among P. falciparum isolates throughout the world (10, 12, 15). To induce this protective immune response in diverse populations and geographic regions, a vaccine may have to induce T-cell responses against multiple epitopes on multiple proteins expressed in infected hepatocytes.
In the Plasmodium yoelii rodent malaria model, DNA vaccines induce CD8+ T-cell responses and sterile protective immunity that is dependent on CD8+ T cells (11, 37). Furthermore, immunization with a mixture of DNA plasmids encoding the circumsporozoite protein (PyCSP) and hepatocyte erythrocyte protein 17 (PyHEP17) circumvents the genetic restriction of protective immunity found after immunization with each plasmid alone (11). However, immunogenicity of vaccines in nonhuman primates is generally considered to predict the immune responses in humans more accurately than does immunogenicity in mice. In developing a multiantigen, multiplasmid malaria vaccine for humans, we considered it important to know if plasmids encoding falciparum malaria genes were immunogenic in nonhuman primates and if mixing plasmids affected the response to individual component antigens. DNA plasmids encoding four different P. falciparum pre-erythrocytic (sporozoite/liver) stage proteins, PfCSP (4), PfSSP2 (33), PfExp-1 (34), and PfLSA-1 (57), have been shown individually to be immunogenic in mice (17a). We now report that these DNA plasmids induce antigen-specific, CD8+ T-cell-dependent cytolytic activity and gamma interferon (IFN-γ) in rhesus monkeys and that immunization with a mixture of plasmids did not appear to alter the CD8+ T-cell responses to any of the components of the mixture.
MATERIALS AND METHODS
P. falciparum DNA vaccines.
DNA vaccine plasmids that encoded four pre-erythrocytic proteins from the 3D7 clone of P. falciparum (47) were constructed. Details of the construction of each DNA vaccine as well as characterization of each by in vitro expression and murine immunogenicity will be published separately (17a). Briefly, vaccine plasmids were assembled by using full-length genes of PfCSP (4), PfSSP2 (33), and PfExp-1 (34) and the 3′ end of the gene of PfLSA-1 (57), encoding the C-terminal 281 amino acid residues (representing 65% of the nonrepeat region of full-length PfLSA-1). The PfExp-1 gene was cloned into plasmid VR1012 (17). This mammalian expression vector is a pUC18 derivatized plasmid that utilizes cytomegalovirus immediate-early promoter-enhancer sequences, cytomegalovirus immediate-early intron and 5′ untranslated region sequences, bovine growth hormone gene transcription termination and polyadenylation sequences, and a bacterial kanamycin resistance gene. Removing the ampicillin resistance gene from the pUC18 plasmid and substituting the kanamycin resistance gene eliminated two immunostimulatory CpG motif sequences (AACGTT) described by Sato et al. (35). No other copies of the CpG motif are present in any of these plasmid sequences. The PfCSP, PfSSP2, and PfLSA-1 genes were cloned into the plasmid VR1020 (28). This plasmid is identical to VR1012 with the exception that it additionally contains the 5′ untranslated region and leader peptide-encoding sequence (first 23 amino acid residues) of the human tissue plasminogen activator protein gene. Thus, the PfCSP, PfSSP2, and PfLSA-1 3′ genes were constructed for expression as in-frame fusions with the tissue plasminogen activator leader peptide encoded in VR1020. Plasmid DNA was prepared by a modified alkaline lysis technique and purified by cesium chloride density gradient centrifugation as previously described (17). DNA was dissolved in saline and stored at −20°C at a concentration of approximately 5 mg/ml. Endotoxin levels were 6 to 64 endotoxin units per mg of plasmid DNA for the plasmid encoding PfExp-1 and 0.5 to 6.4 endotoxin units per mg for all other plasmids in the study. The ability of each plasmid vaccine to express the encoded antigen was confirmed in vitro by utilizing antigen-specific antibodies to detect immunoreactive species of the predicted molecular weights on immunoblots of transiently transfected UM449 human melanoma cells (28). Finally, murine immunogenicity studies with each plasmid DNA showed that these vaccines induced antibody and CTL responses specific to the encoded malaria antigen (17a).
Recombinant vaccinia viruses.
Recombinant poxviruses were produced in collaboration with Virogenetics Corporation (Troy, N.Y.) (24, 42). Recombinant canary pox (ALVAC) viruses expressed PfCSP (vCP182), PfSSP2 (vCP238), and PfLSA-1 (vCP266). Recombinant vaccinia virus WR encoded PfCSP (vP1255), PfSSP2 (vP1254), and PfLSA-1 (vP1253). Recombinant COPAK virus encoded PfExp-1 (vP1385). Wild-type vaccinia virus or parental COPAK (vP993) was used as the negative control virus for labeling of target cells.
Synthetic peptides.
A synthetic peptide, MAP4 (NANP)10, consisting of a central lysine core and four branched-chain peptides, each containing 10 copies of the PfCSP B-cell epitope (26), was provided by G. P. Corradin (University of Lausanne, Lausanne, Switzerland). A 35-amino-acid peptide from PfSSP2 (amino acids 329 to 363 [SPNPEEGKGENPNGFDLPENDENPPNPPNP-PNPPN]) was provided by B. Hansen (Walter Reed Army Institute of Research, Washington, D.C.).
Immunization regimen.
Fifteen malaria-naive rhesus monkeys (Macaca mulata), age 1 to 1.5 years and weighing 1.5 to 3 kg, were randomized into five groups of three monkeys per group (Table 1). At weeks 0, 4, and 16, a 1-ml total volume of plasmid DNA in normal saline was injected intramuscularly (i.m.) with a 26 gauge-needle into four sites (triceps, tibialis anterior, deltoid, and quadriceps) as detailed in Table 1. All experiments were conducted according to the principles set forth in reference 30a.
TABLE 1.
Immunization regimens for P. falciparum plasmid DNA in rhesus monkeys
| Groupa | Plasmid(s) | i.m. injection sites | μg/muscle site (total dose)b |
|---|---|---|---|
| A | Four plasmids mixed (PfCSP, PfSSP2, PfExp-1, and PfLSA-1) | Four sites on the right sidec | 500d (2,000) |
| B | Two plasmids given separately (PfCSP and PfSSP2) | Four sites on the right side (PfCSP) and four sites on the left side (PfSSP2) | 125 (500)e |
| C | Two plasmids given separately (PfExp-1 and PfLSA-1) | Four sites on the right side (PfExp-1) and four sites on the left side (PfLSA-1) | 125 (500)e |
| D | Single plasmid (PfSSP2) | Four sites on the right side | 125 (500) |
| E | Control plasmid (VR1020) | Four sites on the right side | 500 (500) |
Each group consisted of three monkeys.
Total dose = (dose/muscle site) × four sites.
Triceps, Tibialis anterior, Deltoid, and quadriceps.
125 μg for each plasmid.
For each of the two plasmids given.
Antibody assays.
Pre- and postimmunization plasma samples were assessed simultaneously for antimalaria antibodies. Antibodies were assayed by the indirect fluorescent-antibody test (IFAT) (5) against air-dried P. falciparum (strain NF54) sporozoites, liver stage parasites (41), or blood stage parasites and by enzyme-linked immunosorbent assay (ELISA) with the synthetic peptide PfCSP MAP4 (NANP)10 or PfSSP2 35-mer. The secondary antibodies used in the ELISA were horseradish peroxidase-conjugated goat anti-human immunoglobulin G (heavy plus light chains) (Kierkegaard and Perry, Gaithersburg, Md.). For IFAT, titers are reported as the last dilution at which fluorescence was considered positive. For ELISA, titers are reported as the end point optical density at 410 nm (OD410), defined as the last dilution at which the mean OD410 for plasma from immunized monkeys was greater than the mean OD410 plus two standard deviations for preimmunization plasma.
APCs for in vitro restimulation.
Autologous B-lymphoblastoid cell lines (B-LCL) were established from purified peripheral blood mononuclear cells (PBMCs) by herpesvirus papio transformation as previously described (46). B-LCL were maintained in RPMI 1640 supplemented with 10 mM HEPES, 2 mM l-glutamine, 50 U of penicillin per ml, 50 μg of streptomycin (Life Technologies, Inc., Grand Island, N.Y.) per ml, 20 μg of gentamicin (Gibco BRL, Gaithersburg, Md.) per ml, and 12% heat-inactivated fetal bovine serum (Sigma Chemical Co., St. Louis, Mo.) (complete RPMI 1640). Antigen-presenting cells (APCs) were prepared as previously described (45) by infecting autologous B-LCL with the P. falciparum antigen-specific recombinant virus ALVAC or COPAK at 2 PFU/cell for 16 h. Ficoll-purified cells were then fixed in paraformadehyde (1.5%) for 15 min, incubated in 0.2 M glycine–phosphate-buffered saline solution for a further 15 min, and stored in 100% fetal calf serum at 4°C for up to 4 weeks.
Effector cells.
PBMCs were isolated from heparinized blood by density gradient centrifugation at 3,000 rpm for 20 min with Ficoll-Paque (Pharmacia Biotech AB, Uppsala, Sweden). Cells were harvested from the interface and washed twice at 1,200 rpm for 10 min. PBMCs were stimulated at a concentration of 107 cells/ml with fixed APCs at a ratio of 1:1 in a total volume of 4 ml in 12-well plates. Culture medium was complete RPMI 1640. Recombinant human interleukin-2 (Cetus Corp., Emeryville, Calif.) was added to each well (20 U/ml) after 24 h. Half volumes of medium supplemented with recombinant human interleukin-2 were changed every second day. At day 7, stimulated cells were subjected to Ficoll density gradient centrifugation for removal of dead cells, washed, and then restimulated with fixed APCs at a ratio of 1:1 for a further 6 days.
Target cells.
Autologous B-LCLs were incubated overnight with recombinant vaccinia virus (WR or COPAK) (2 PFU/cell) expressing the P. falciparum antigens or with wild-type vaccinia virus in the presence of 50 μCi (1 Ci = 37 GBq) of a sterile Na251CrO4 solution (Dupont New England Nuclear, Boston, Mass.). Cells were washed three times before being used as targets in the CTL assays.
51Cr release assay.
CTL activity was assessed by a 5-h chromium release assay, using 5 × 103 51Cr-labeled target cells. All assays were performed in triplicate. The results are reported as percent lysis, percent specific lysis, and mean percent specific lysis. Percent lysis was defined as [(experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm)] × 100%. Percent specific lysis was defined as (percent lysis of target cells infected with recombinant vaccinia virus expressing P. falciparum proteins) − (percent lysis of target cells infected with wild-type vaccinia virus). Mean percent specific lysis was defined as the mean of percent specific lysis values obtained at all effector/target (E/T) ratios of ≥20:1. Spontaneous release was obtained as background lysis of targets (presence of medium alone)/maximum release (presence of Triton X-100). In all cases, spontaneous release was ≤20%.
Cell depletions.
Effector cell populations were depleted of CD4+ or CD8+ T cells by using anti-CD4+- or anti-CD8+-coated Dynabeads M-450 according to the instructions of the manufacturer (Dynal, Inc., Great Neck, N.Y.). Flow cytometric analysis confirmed that cell subset depletion was >95% in all cases (data not shown).
IFN-γ reverse transcriptase PCR (RT-PCR).
IFN-γ mRNA was quantitated as previously reported (19, 43, 44). Briefly, 106 PBMCs were stimulated for 8 h with autologous B-LCL infected with recombinant viruses as described above. Total RNA was extracted by using the RNeasy kit (Qiagen Inc., Chatsworth, Calif.) and reverse transcribed by random hexamer priming. PCR was carried out with the primers specific for rhesus monkey IFN-γ (43). Various copy numbers of the nonhuman primate IFN-γ gene within plasmids were run in parallel for each PCR. Amplified products were then analyzed by gel electrophoresis and Southern blotting and hybridized to an internal IFN-γ-specific digoxigenin-labeled probe. Detection and quantification of IFN-γ mRNA levels were facilitated by densitometric analysis with the chemiluminescent Genius system (Boehringer Mannheim, Indianapolis, Ind.). The density of signal obtained with a defined copy of IFN-γ was used to derive relative copy equivalents of the appropriate IFN-γ in the samples analyzed.
RESULTS
Induction of antigen-specific antibodies.
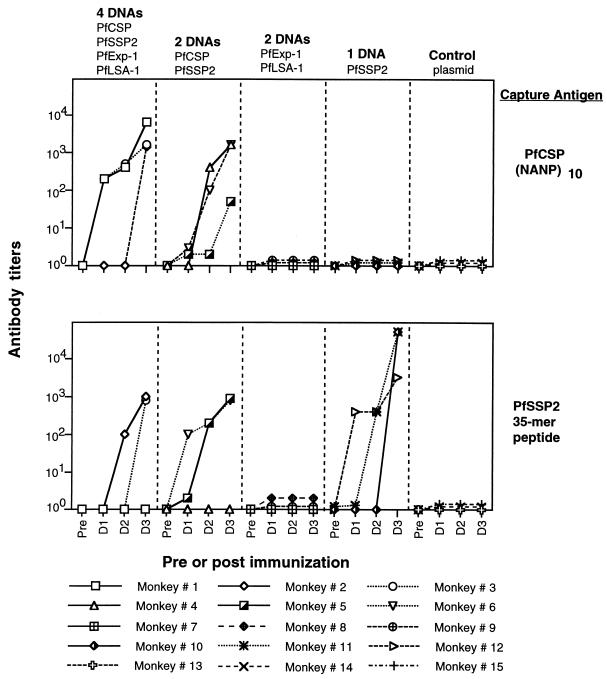
The monkeys and the immunizing plasmids are listed in Table 1. Monkeys were bled 3 weeks after each immunization, and antibodies were assessed by IFAT against air-dried sporozoites, infected hepatocytes, or erythrocytes, as well as by ELISA against synthetic peptides or recombinant proteins. No specific antibodies were detectable after the first immunization. Antibodies were detected in 8 of 12 monkeys after the second immunization and in 11 of 12 animals after the third immunization.
IFAT titers after the third immunization are reported in Table 2. We defined seroconversion as an eightfold increase above preimmune values and a final titer of ≥80. According to this criterion, 9 of the 12 immunized animals developed antibodies to whole parasites as determined by IFAT. Six of 9 monkeys recognized sporozoites, 2 of 12 monkeys recognized liver stage parasites, and 6 of 12 monkeys recognized blood stage parasites. Antibodies to sporozoites were detected in groups A, B, and D (six of nine) but not in group C or the group E control monkeys. This is consistent with the known expression of PfCSP and PfSSP2 (but not PfExp-1 or PfLSA-1) in sporozoites. IFAT titers to liver stages were observed only in group C (two of three), and the fluorescence pattern was typical of PfLSA-1. IFAT titers against infected erythrocytes were seen in groups A, B, and C (6 of 12). Five of the six responders were animals that received PfExp-1, which is a well-defined blood stage antigen. One monkey immunized with PfCSP and PfSSP2 developed antibodies to infected erythrocytes. PfSSP2/TRAP, but not PfCSP, has been reported to be expressed in infected erythrocytes (32), suggesting that this recognition is due to PfSSP2.
TABLE 2.
IFAT titers against air-dried P. falciparum sporozoites, blood stage parasites, or liver stage parasites after the third immunization
| Group (plasmid[s]) and monkey | Titera
|
|||||
|---|---|---|---|---|---|---|
| Sporozoites
|
Liver stageb
|
Blood stage
|
||||
| Prec | Postd | Pre | Post | Pre | Post | |
| A (PfCSP, PfSSP2, PfExp-1, PfLSA-1) | ||||||
| 1 | Neg | 2,560 | Neg | Neg | Neg | 20 |
| 2 | Neg | 640 | Neg | Neg | Neg | 80 |
| 3 | Neg | 320 | Neg | Neg | Neg | 160 |
| B (PfCSP, PfSSP2) | ||||||
| 4 | Neg | 640 | Neg | Neg | Neg | 80 |
| 5 | Neg | 20 | Neg | Neg | 10 | 10 |
| 6 | Neg | 20 | Neg | Neg | Neg | 40 |
| C (PfExp-1, PfLSA-1) | ||||||
| 7 | Neg | 40 | Neg | 500 | 20 | 320 |
| 8 | Neg | 20 | Neg | 500 | 20 | 160 |
| 9 | Neg | 20 | Neg | Neg | 20 | 160 |
| D (PfSSP2) | ||||||
| 10 | Neg | 320 | Neg | Neg | 20 | 20 |
| 11 | Neg | 80 | Neg | Neg | 20 | 80 |
| 12 | Neg | 40 | Neg | Neg | 20 | 40 |
| E (control) | ||||||
| 13 | Neg | 20 | Neg | Neg | 10 | 10 |
| 14 | Neg | 40 | Neg | Neg | 160 | 160 |
| 15 | Neg | 10 | Neg | Neg | 80 | 20 |
Seroconversion (boldface) has been defined as an eightfold increase above preimmune values and a final titer of ≥80. Neg, negative.
IFATs were performed at serum dilutions of 1:250 and 1:500 with P. falciparum-infected chimpanzee liver sections (41).
IFAT conducted with plasma from monkeys before immunization.
Three weeks after third immunization.
For PfCSP and PfSSP2, the results of antigen-specific ELISAs were generally consistent with the results of IFAT after the third immunization (Fig. 1). All six monkeys that received the PfCSP plasmid developed antibodies to the repeat region PfCSP peptide (the best response was in the mixture group A). Four of these six monkeys were shown to have antibodies to sporozoites by IFAT. Seven of nine monkeys that received the PfSSP2 plasmid developed antibodies to the 35-amino-acid peptide from PfSSP2 (the best response was in group D [PfSSP2 alone]). Six of these seven monkeys had antibodies to sporozoites by IFAT. Work to develop and refine ELISAs for PfExp-1 and PfLSA-1 is in progress.
FIG. 1.
Five groups of three monkeys were immunized with (i) a mixture of the four plasmids, (ii) PfCSP and PfSSP2, (iii) PfExp-1 and PfLSA-1, (iv) PfSSP2 alone, and (v) empty vector VR1020. Antibodies were assessed by ELISA with plasma collected before immunization and 3 weeks after the first (D1), second (D2) and third (D3) doses. Capture antigens were synthetic peptides PfCSP MAP4 (NANP)10 and PfSSP2 35-mer (amino acids 329 to 363). Antibody titers are reported as the end point OD410s, defined as the last dilution at which the mean OD410 for plasma from immunized monkeys was greater than the mean OD410 plus two standard deviations for preimmunization plasma.
Simultaneous induction of multiple antigen-specific CTL.
Antigen-specific CTL were assessed before immunization and after the second and third immunizations. In parallel, control CTL assays were conducted with cells from monkeys immunized with the empty control plasmid or with heterologous plasmids.
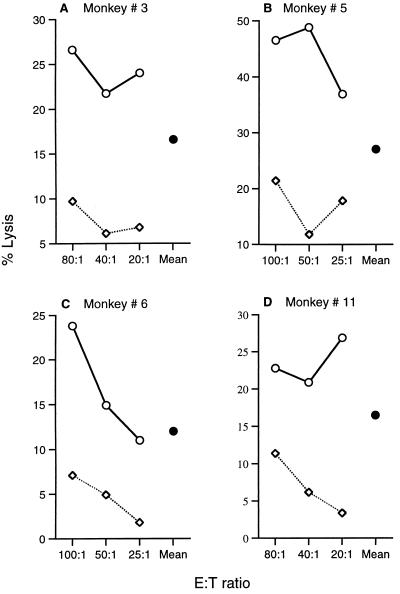
No specific CTL could be detected before immunization for any of the monkeys (data not shown). After immunization, lysis (percent lysis) which increased with higher E/T ratios was observed in a number of assays (Fig. 2C). However, in some experiments we found a paradoxical decrease in percent lysis at higher E/T ratios (Fig. 2A, B, and D). We have no definitive explanation for this phenomenon, but it may be the result of crowding at higher cell densities. We have defined the percent specific lysis for each E/T ratio as the percent lysis of target cells infected with P. falciparum recombinant vaccinia virus minus the percent lysis of targets infected with wild-type vaccinia virus (Fig. 2). To summarize the antigen-specific lytic activity of each CTL assay, we have defined the mean percent specific lysis as the average of the percent specific lyses at all E/T ratios of ≥20 (Fig. 2). The mean percent specific lysis allows us to compare the antigen-specific lytic activities in different assays despite a lack of dose response at increasing E/T ratios.
FIG. 2.
Representative CTL responses (percent lysis) at different E/T ratios. (A) PfExp-1-specific CTL activity in monkey 3 immunized with the mixture of four plasmids; (B and C) PfCSP-specific CTL activity in monkeys 5 (B) and 6 (C) immunized with PfCSP and PfSSP2; (D) PfSSP2-specific CTL activity in monkey 11 immunized with PfSSP2 alone. Adjacent to the dose-response curves, the mean percent specific lysis (•) for each assay is shown. The mean percent specific lysis for each CTL assay is the percent lysis of targets infected with recombinant P. falciparum vaccinia virus (○) − percent lysis of control targets infected with wild-type vaccinia virus (◊) averaged over the three E/T ratios.
Significance of antigen-specific CTL responses.
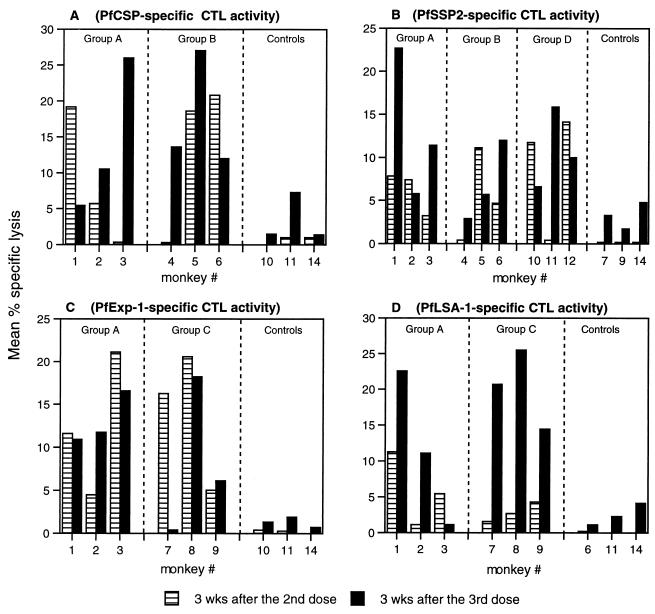
Figure 3 shows the mean percent specific lysis for each monkey after the second and third immunizations. Figure 3A shows the results for CTL activity against PfCSP in groups A and B, which received the PfCSP plasmid, and in three animals receiving control plasmid. Figure 3B, C, and D show similar data for animals immunized with plasmids PfSSP2, PfExp-1, and PfLSA-1, respectively. For each antigen tested, plasmid-immunized animals had higher mean percent specific lysis than did control-immunized animals. With positive CTL activity defined as mean percent specific lysis of >10%, no control animals were positive in 24 assays (6 assays for each panel of Fig. 3). Of the 24 assays reported for group A, 13 showed greater than 10% mean specific lysis. For group B 7 of 12 assays were positive, for group C 6 of 12 were positive, and for group D 3 of six were positive. When compared to the negative results in control animals, this degree of CTL activity was statistically significant, with P < 0.01 for each group (A, B, C, and D) (Fisher’s exact test). From this analysis, we concluded that each plasmid is immunogenic for CTL.
FIG. 3.
P. falciparum antigen-specific cytotoxic activity. Autologous B-LCL infected with recombinant viruses expressing PfCSP (A), PfSSP2 (B), PfExp-1 (C), or PfLSA-1 (D) were used as APCs. Rhesus monkeys were immunized with the mixture of four plasmids (group A), PfCSP and PfSSP2 (group B), PfExp-1 and PfLSA-1 (group C), or PfSSP2 alone (group D). Control monkeys received the empty vector VR1020 (group E) or plasmids encoding unrelated antigens. At 3 weeks (wks) after the second and third immunizations, PBMCs were stimulated with fixed APCs at a 1:1 ratio for 7 days and restimulated with fixed APCs at a 1:1 ratio for a further 6 days before use as CTL effectors. Targets were chromium-labeled autologous B-LCL infected with recombinant P. falciparum vaccinia virus or wild-type vaccinia virus. Bars represent the mean percent specific lysis for each CTL assay (percent lysis of targets infected with recombinant P. falciparum vaccinia virus − percent lysis of control targets infected with wild-type vaccinia virus averaged over all E/T ratios of ≥20:1).
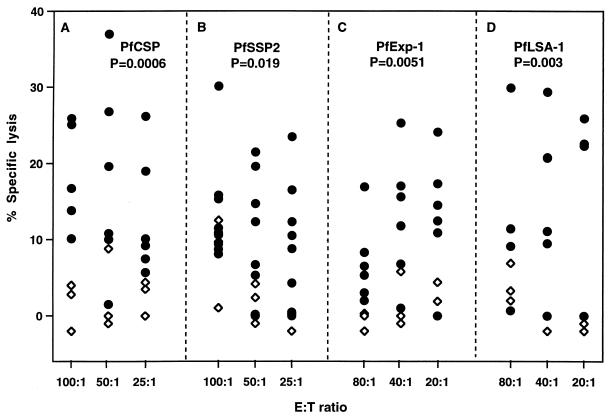
To further evaluate the DNA-induced CTL responses for each of the four antigens, irrespective of whether they were administered alone or as a mixture, a scattergram of the percent specific lysis obtained after the third immunization at each of the E/T ratios studied was compiled (Fig. 4). All data points were displayed. The peak percent specific lysis was 37.1% for PfCSP, 30.1% for PfSSP2, 25.3% for PfExp-1, and 29.9% for PfLSA-1. The Mann-Whitney U test was used to determine whether the antigen-specific lysis induced by immunization of animals with the DNA vaccines encoding P. falciparum antigens was significantly greater than that in control monkeys immunized with empty vector or unrelated plasmids. This analysis confirmed that all four P. falciparum plasmids were immunogenic in rhesus monkeys.
FIG. 4.
Scattergram of percent specific lysis for each of four plasmids 3 weeks after the third immunization. The percent specific lysis is the percent lysis of targets infected with recombinant P. falciparum vaccinia virus − percent lysis of control targets infected with wild-type vaccinia virus. •, P. falciparum plasmid-immunized monkeys; ◊, control plasmid-immunized monkeys. The Mann-Whitney U test (two tailed) was used to assess statistical significance.
CD8+ T-cell dependence and genetic restriction of the CTL response.
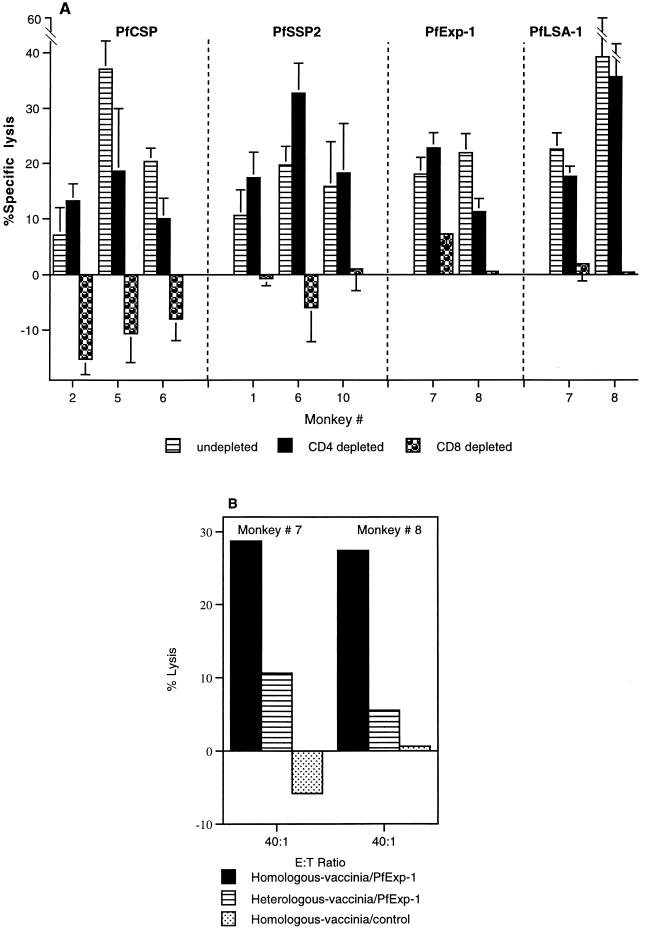
The phenotype of the induced CTL was determined by depleting effector cells in vitro of either CD8+ T cells or CD4+ T cells immediately prior to assay. In all cases, CD8+ T-cell depletion reduced or eliminated cytolytic activity (Fig. 5A), demonstrating that each of the P. falciparum DNA plasmids induced CD8+ CTL in nonhuman primates. However, in 3 of 11 cases (monkeys 5 and 6 immunized with PfCSP and PfSSP2 and monkey 8 immunized with PfExp-1 and PfLSA-1), there was approximately a 50% reduction in cytolytic activity after CD4+ T-cell depletion (Fig. 5A), suggesting a possible role for CD4+ CTL.
FIG. 5.
(A) CD8+ T-cell dependence of the P. falciparum antigen-specific cytolytic activity. CTL effectors were generated as described in the legend to Fig. 3 and were depleted of CD8+ or CD4+ T cells before use in the 51Cr release assay. Data for PfCSP and PfSSP2 show percent specific lysis (mean ± standard error of the mean) at an E/T ratio of 50:1. Data for PfExp-1 and PfLSA-1 show percent specific lysis (mean ± standard error of the mean) at an E/T ratio of 40:1. (B) MHC restriction of the P. falciparum antigen-specific cytolytic activity. Data for PfExp-1 show percent lysis at an E/T ratio of 40:1.
The monkeys used in these experiments were outbred and were not typed for MHC, so no definitive data on the MHC restriction of the CTL responses we observed are available. However, in several experiments CTL activity was observed with homologous B-LCL targets but not with heterologous targets from other animals (Fig. 5B). This would be consistent with an MHC-restricted CTL response.
Effect of mixing plasmids on induction of antigen-specific CTL responses.
To determine if mixing the plasmids had additive or inhibitory effects on the induction of antigen-specific immunity, CTL responses of monkeys immunized with the mixture of four plasmids were compared with responses of monkeys that received one or two plasmids separately (Table 3). CTL activity was scored positive if the mean percent specific lysis was greater than 10% after either the second or third immunization. This analysis revealed no significant difference between animals immunized with mixed and individual plasmids for induction of CTL.
TABLE 3.
Comparison of CTL and antibody responses of monkeys immunized with the mixture of four plasmids or with plasmids administered individually at separate sites
| Antigen | No. with positive CTL responsea/no. immunized (no. of positive assays/no. of assays)
|
No. with positive antibody response/ no. immunized
|
||
|---|---|---|---|---|
| Mixtureb | Separatec | Mixture | Separate | |
| PfCSP | 3/3 (3/6) | 3/3 (5/6) | 3/3 | 2/3 |
| PfSSP2 | 2/3 (2/6) | 5/6 (5/12) | 2/3 | 5/6 |
| PfExp-1 | 3/3 (5/6) | 2/3 (3/6) | 2/3 | 3/3 |
| PfLSA-1 | 2/3 (3/6) | 3/3 (3/6) | NDd | ND |
| Total | 10/12 (13/24) | 13/15 (16/30) | ||
CTL activity was positive if the mean percent specific lysis was >10% after the second or third immunization. Mean percent specific lysis is defined as percent lysis of antigen-labeled targets minus percent lysis of control targets, averaged for all E/T ratios of ≥20:1.
Three monkeys were immunized with the mixture of four plasmids (group A [Table 1]).
Monkeys were immunized with plasmids at separate sites (groups B, C, and D [Table 1]).
ND, not done.
Induction of IFN-γ mRNA.
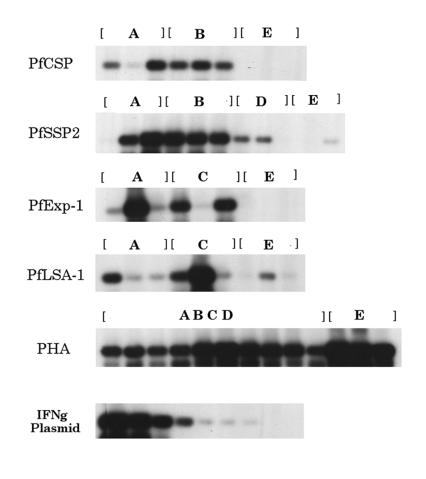
IFN-γ has been implicated in the protective immunity against liver stage parasites of malaria (11, 36, 39). Therefore, using semiquantitative RT-PCR, we measured antigen-specific production of IFN-γ mRNA in the monkeys after three immunizations with plasmid DNA. IFN-γ mRNA in PBMCs from monkeys immunized with the P. falciparum DNA vaccines was significantly elevated after 8 h of stimulation in vitro with the respective APCs (autologous B-LCL infected with P. falciparum antigen-specific recombinant virus) compared with that of monkeys immunized with the control plasmid (Fig. 6). The numbers (means ± standard errors) of IFN-γ mRNA transcripts in antigen-immunized monkeys versus those in control monkeys were 3.7 ± 1.0 versus 0 (P = 0.02; Mann-Whitney U-test) for PfCSP-immunized monkeys, 28.1 ± 14 versus 0.65 ± 0.65 (P = 0.013) for PfSSP2-immunized monkeys, 35.4 ± 28 versus 0 (P = 0.02) for PfExp-1 immunized animals, and 68.5 ± 64 versus 0.56 ± 0.65 (P = 0.05) for PfLSA-1 immunized animals. Approximately 261 ± 94 mRNA copies were detected in phytohemagglutinin-stimulated PBMCs from all monkeys regardless of the immunogen.
FIG. 6.
Semiquantitative analysis of IFN-γ mRNA. Rhesus monkeys were immunized with the mixture of four plasmids (A), PfCSP and PfSSP2 (B), PfExp-1 and PfLSA-1 (C), PfSSP2 alone (D) or empty vector VR1020 control or plasmids encoding unrelated antigens (E). PBMCs from immunized and control monkeys were stimulated in vitro for 8 h with autologous B-LCL infected with recombinant viruses encoding the respective P. falciparum antigens or with phytohemagglutinin (PHA). The IFN-γ plasmid standard represents 106, 105, 104, 103, 100, 10, 1, and 0.1 copies.
DISCUSSION
The primary objectives of this study were to determine if DNA plasmids encoding four different P. falciparum proteins (PfCSP, PfSSP2, PfExp-1, and PfLSA-1) could induce CTL in nonhuman primates and if mixing the four plasmids would alter immunogenicity. The results indicate that each of the four plasmids was immunogenic and that immunization with the plasmids as a mixture induced CD8+ T-cell responses to all components of the mixture and did not appear to decrease immunogenicity. Furthermore, of the 12 rhesus monkeys that were immunized, 8 had CTL against all of the proteins to which they were exposed, and the other 4 responded to all but one of the proteins to which they were exposed (Fig. 3).
The monkeys in these experiments were from outbred colonies and so presumably had heterogeneous genetic backgrounds. The plasmids and recombinant viruses used in this study contained DNAs from entire malaria genes or large gene fragments. Therefore, the T-cell activity against each protein may have represented responses to multiple epitopes, with different animals responding to different epitopes. It is therefore encouraging from the viewpoint of vaccine design that despite host genetic variability, 67% of the animals mounted a CD8+ T-cell response to at least one epitope on every protein to which they were exposed after plasmid injection, and CTL were detected in all of the immunized animals. This finding supports our approach of immunizing humans with a multigene DNA vaccine so as to circumvent genetic restriction of T-cell responses based on polymorphism of human HLA molecules and to eliminate parasites that may vary at CD8+ T-cell epitopes (7, 20). Because rhesus monkeys cannot be infected with P. falciparum, it cannot be determined whether such CD8+ T-cell responses would be effective in protecting against infection with P. falciparum. This can be studied only in human clinical trials.
In this study, we specifically included a group of monkeys that received all four plasmids mixed in the same syringe. In designing a DNA vaccine with multiple antigens, it is crucial to determine if mixing plasmids inhibits or enhances the immunogenicity of each component. We found that CTL responses to all antigens were generated regardless of whether they were administered mixed in the same syringe, simultaneously at separate sites, or alone. Thus, cell-mediated immunity to various DNA plasmid antigens was apparently unaffected by mixing. This finding is critically important to our long-term goal of inducing CTL against multiple parasite proteins expressed in human hepatocytes. There has been one previous study reporting immunization of monkeys with mixed plasmids encoding human immunodeficiency virus proteins, in which the same rhesus monkeys were immunized by three routes: intravenous injection, i.m. injection, and gene gun delivery (27). In that study CTL against two human immunodeficiency virus proteins were identified. However, to our knowledge this is the first report of induction of CD8+ CTL against multiple antigens after administration of a mixture of DNA plasmids by a single route.
Although CTL activity seems to be unaffected, mixing plasmids or simultaneous injection of plasmids at separate sites may have affected antibody responses. In the mixed-plasmid groups, antibodies to PfCSP were higher and antibodies to PfSSP2 were lower than in groups that received the plasmids separately. Furthermore, the highest antibody responses to PfSSP2 were observed in the group of monkeys immunized with PfSSP2 alone, as assessed by ELISA (Fig. 1). Although the small number of animals in each group precludes definitive statements, mixing or coadministering plasmids may have altered antibody responses in ways different for different antigens.
In general, cytotoxic activity was greater after the third immunization than after the second. However, there were some instances in which CTL activity could be detected after the second dose but not after the third (Fig. 3). This variability in CTL detection may be due to fluctuations in lymphocytes transiting through the peripheral blood. Similar problems with reproducibility of CTL responses from PBMCs have been observed for humans immunized with irradiated P. falciparum sporozoites, who are immune to sporozoite challenge (13, 29, 51, 52), and for individuals naturally exposed to malaria in the field, who are semi-immune (1, 8, 9, 13, 18, 23, 38). If it were possible to obtain lymphocytes from lymph nodes or spleens, sampling errors might be less and the responses might be more consistent. Nevertheless, the magnitude of the cytotoxic activity measured here was comparable to that reported for rhesus monkeys and chimpanzees with other DNA vaccines (3, 6, 14, 56), for rhesus monkeys immunized with recombinant viruses (2, 25, 40), and for rhesus monkeys immunized with other antigen delivery systems designed to induce CTL (30, 54, 55).
In mice immunized with irradiated sporozoites (36, 39) or with P. yoelii DNA plasmids (11), protective immunity is dependent on CD8+ T cells and on IFN-γ. Furthermore, we have recently shown that immunization of mice with a PySSP2 peptide induces protection which is dependent on CD4+ T cells and IFN-γ (48). In the study reported here, DNA immunization primed T cells for the antigen-specific production of IFN-γ, as measured by RT-PCR. The PBMCs were not fractionated; therefore, it is possible that the IFN-γ was produced by both CD8+ and CD4+ T cells.
Antibody responses to P. falciparum sporozoites and liver and blood stages were detected, but at relatively low levels. Because the blood-borne sporozoite enters the hepatocyte in seconds to minutes after the bite of an infected mosquito, neutralizing antibodies must already be present at a very high titer in order to protect the host against infection. The antibody titers observed in this study were modest and more variable than the T-cell responses. However, it is possible that immunizing rhesus monkeys i.m. may not be the optimal way to induce antibody responses. Work with mice (31) and Aotus monkeys (16) has shown that intradermal DNA immunization may be more effective at inducing an antibody response than i.m. injection. Similar results have been found for mice with gene gun administration of P. yoelii CSP DNA (35a). In the experiments presented here, the high dose of plasmid given i.m. may have biased the response away from antibody production. We are currently investigating ways to deliver DNA plasmid vaccines so as to induce vigorous T-cell and antibody responses in the same recipients.
While the focus of this study was on immunogenicity, the plasmid immunizations were well tolerated by the monkeys. Very high plasmid doses were administered in an attempt to increase immunogenicity. A total of 500 μg of each plasmid was given at each of three time points. Therefore, the group receiving the four-plasmid mix received a total of 6,000 μg of DNA by the end of the study. Veterinarians caring for these monkeys during the study reported that they ate and behaved normally and maintained their normal weight and growth. Complete blood count and blood chemistry results from the beginning to the end of the study were all within the normal ranges (data not shown).
These studies demonstrate for the first time that P. falciparum DNA plasmids are immunogenic in primates and that administration of a mixture of the four plasmids is as effective in inducing CD8+ CTL as is administration of each plasmid alone. Our primary strategy for eliciting sterile immunity against malaria is the induction of CD8+-T-cell responses against multiple P. falciparum proteins expressed in infected hepatocytes (20). By showing that i.m. administration of P. falciparum plasmids induces CD8+-T-cell responses in primates and that mixing of plasmids does not affect such immune responses, these studies provide the foundation for human clinical trials of a multigene plasmid DNA vaccine against malaria.
ACKNOWLEDGMENTS
We thank G. P. Corradin and F. Panea for synthesis of the MAP4 (NANP)10; B. Hansen for PfSSP2 peptide; N. Letvin for provision of protocols and assistance in establishing the CTL assays in nonhuman primates; F. Villinger for reagents, protocols, and training in measurement of cytokine mRNAs by RT-PCR; and R. Gramzinski for instruction on and assistance with immunization of nonhuman primates with DNA vaccines. We thank the Food and Drug Administration, Office of Establishment Licensing and Product Surveillence, Division of Veterinary Services, for their support. We also thank A. Figer for excellent technical assistance.
This work was supported by Naval Medical Research and Development Command Work Units 611102A.S13.00101-BFX.1431 and 612787A.870.00101.EFX.1432. This work was performed while D. L. Doolan was a National Research Council Resident Research Associate.
REFERENCES
- 1.Aidoo M, Lalvani M R C P, Allsopp C E M, Plebanski M, Meisner S J, Krausa P, Browning M, Morris-Jones S, McAdam S, Gotch F, Fidock D A, Takiguchi M, Robson K J H, Greenwood B M, Druihle P, Whittle H C, Hill A V S. Identification of conserved antigenic components for a cytotoxic T lymphocyte-inducing vaccine against malaria. Lancet. 1995;345:1003–1007. doi: 10.1016/s0140-6736(95)90754-8. [DOI] [PubMed] [Google Scholar]
- 2.Andersson S, Makitalo B, Thorstensson R, Franchini G, Tartaglia J, Limbach K, Paoletti E, Putkonen P, Biberfeld G. Immunogenicity and protective efficacy of a human immunodeficiency virus type 2 recombinant canarypox (ALVAC) vaccine candidate in cynomolgus monkeys. J Infect Dis. 1996;174:977–985. doi: 10.1093/infdis/174.5.977. [DOI] [PubMed] [Google Scholar]
- 3.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 4.Campbell J R. DNA sequence of the gene encoding a Plasmodium falciparum malaria candidate vaccine antigen. Nucleic Acids Res. 1989;17:5854. doi: 10.1093/nar/17.14.5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charoenvit Y, Leef M L, Yuan L F, Sedegah M, Beaudoin R L. Characterization of Plasmodium yoelii monoclonal antibodies directed against stage-specific sporozoite antigens. Infect Immun. 1987;55:604–608. doi: 10.1128/iai.55.3.604-608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis H L, McCluskie M J, Gerin J L, Purcell R H. DNA vaccine for hepatitis B: evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc Natl Acad Sci USA. 1996;93:7213–7218. doi: 10.1073/pnas.93.14.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doolan D L, Hoffman S L. Multi-gene vaccination against malaria: a multistage, multi-immune response approach. Parasitol Today. 1997;13:171–178. doi: 10.1016/s0169-4758(97)01040-5. [DOI] [PubMed] [Google Scholar]
- 8.Doolan D L, Houghten R A, Good M F. Location of human cytotoxic T cell epitopes within a polymorphic domain of the Plasmodium falciparum circumsporozoite protein. Int Immunol. 1991;3:511–516. doi: 10.1093/intimm/3.6.511. [DOI] [PubMed] [Google Scholar]
- 9.Doolan D L, Khamboonruang C, Beck H P, Houghten R A, Good M F. Cytotoxic T lymphocyte (CTL) low-responsiveness to the Plasmodium falciparum circumsporozoite protein in naturally-exposed endemic populations: analysis of human CTL response to most known variants. Int Immunol. 1993;5:37–46. doi: 10.1093/intimm/5.1.37. [DOI] [PubMed] [Google Scholar]
- 10.Doolan D L, Saul A J, Good M F. Geographically restricted heterogeneity of the Plasmodium falciparum circumsporozoite protein: relevance for vaccine development. Infect Immun. 1992;60:675–682. doi: 10.1128/iai.60.2.675-682.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multi-gene DNA immunization: CD8+ T cell-, interferon-γ-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doolan D L, Wizel B, Hoffman S L. Class I HLA-restricted cytotoxic T lymphocyte responses against malaria—elucidation on the basis of HLA peptide binding motif. Immunol Res. 1996;15:280–305. doi: 10.1007/BF02935313. [DOI] [PubMed] [Google Scholar]
- 13.Doolan L D, Hoffman S L, Southwood S, Wentworth P A, Sidney J, Chesnut R W, Keogh E, Appella E, Nutman T B, Gordon D M, Oloo A, Lal A A, Sette A. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and -B supertype alleles. Immunity. 1997;7:97–112. doi: 10.1016/s1074-7613(00)80513-0. [DOI] [PubMed] [Google Scholar]
- 14.Fuller D H, Murphey-Corb M, Clements J, Barnett S, Haynes J R. Induction of immunodeficiency virus-specific immune responses in rhesus monkeys following gene gun-mediated DNA vaccination. J Med Primatol. 1996;25:236–241. doi: 10.1111/j.1600-0684.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 15.Good M F. Antigenic diversity and MHC genetics in sporozoite immunity. Immunol Lett. 1994;41:95–98. doi: 10.1016/0165-2478(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 16.Gramzinski R A, Maris D C, Obaldia N, Rossan R, Sedegah M, Wang R, Hobart P, Margalith M, Hoffman S L. Optimization of antibody responses of a malaria DNA vaccine in Aotus monkeys. Vaccine Res. 1996;5:173–183. doi: 10.1016/s0264-410x(96)00270-8. [DOI] [PubMed] [Google Scholar]
- 17.Hartikka J, Sawdey M, Cornefert-Jensen F, Margalith M, Barnhart K, Nolasco M, Vahlsing L, Meek J, Marquet M, Hobart P, Norman J, Manthorpe M. An improved plasmid expression vector for direct injection into skeletal muscle. Hum Gene Ther. 1996;7:1205–1217. doi: 10.1089/hum.1996.7.10-1205. [DOI] [PubMed] [Google Scholar]
- 17a.Hedstrom, R. C., D. L. Doolan, R. Wang, A. Kumar, J. B. Sacci, Jr., M. J. Gardner, J. C. Aguiar, Y. Charoenvit, M. Sedegah, J. Tine, M. Margalith, P. Hobart, and S. L. Hoffman. In vitro expression and in vivo immunogenicity of Plasmodium falciparum prerythrocytic stage DNA vaccines. Int. J. Mol. Med., in press. [DOI] [PubMed]
- 18.Hill A V S, Elvin J, Willis A C, Aidoo M, Allsopp C E, Gotch F M, Gao X M, Takiguchi M, Greenwood B M, Townsend A R, et al. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature. 1992;360:434–439. doi: 10.1038/360434a0. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman S L, Crutcher J M, Puri S K, Ansari A A, Villinger F, Franke E D, Singh P P, Finkelman F, Gately M K, Dutta G P, Sedegah M. Sterile protection of monkeys against malaria after administration of interleukin-12. Nat Med. 1997;3:80–83. doi: 10.1038/nm0197-80. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman S L, Franke E D, Hollingdale M R, Druilhe P. Attacking the infected hepatocyte. In: Hoffman S L, editor. Malaria vaccine development: a multi-immune response approach. Washington, D.C: American Society for Microbiology; 1996. pp. 35–75. [Google Scholar]
- 21.Hoffman S L, Isenbarger D, Long G W, Sedegah M, Szarfman A, Waters L, Hollingdale M R, van der Miede P H, Finbloom D S, Ballou W R. Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science. 1989;244:1078–1081. doi: 10.1126/science.2524877. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman S L, Miller L H. Perspectives on malaria vaccine development. In: Hoffman S L, editor. Malaria vaccine development: a multi-immune response approach. Washington, D.C: American Society for Microbiology; 1996. pp. 1–13. [Google Scholar]
- 23.Lalvani A, Hurt N, Aidoo M, Kibatala P, Tanner M, Hill A V S. Cytotoxic T lymphocytes to Plasmodium falciparum epitopes in an area of intense and perennial transmission in Tanzania. Eur J Immunol. 1996;26:773–779. doi: 10.1002/eji.1830260408. [DOI] [PubMed] [Google Scholar]
- 24.Lanar D E, Tine J A, Taisne C D, Seguin M C, Cox W I, Winslow J P, Ware L A, Kauffman E B, Gordon D, Ballou W R, Paoletti E, Sadoff J C. Attenuated vaccinia virus-circumsporozoite protein recombinants confer protection against rodent malaria. Infect Immun. 1996;64:1666–1671. doi: 10.1128/iai.64.5.1666-1671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laube L S, Burrascano M, Dejesus C E, Howard B D, Johnson M A, Lee W T, Lynn A E, Peters G, Ronlov G S, Townsend K S. Cytotoxic T lymphocyte and antibody responses generated in rhesus monkeys immunized with retroviral vector-transduced fibroblasts expressing human immunodeficiency virus type-1 IIIB ENV/REV proteins. Hum Gene Ther. 1994;5:853–862. doi: 10.1089/hum.1994.5.7-853. [DOI] [PubMed] [Google Scholar]
- 26.Le T P, Preston Church L W, Corradin G, Hunter R L, Charoenvit Y, Wang R, De la Vega P, Sacci J, Ballou W R, Glenn G M, Richards R L, Alving C R, Hoffman S L. Immunogenicity of Plasmodium falciparum circumsporozoite protein multiple antigen peptide vaccine formulated with different adjuvants. Vaccine. 1998;16:305–312. doi: 10.1016/s0264-410x(97)00165-5. [DOI] [PubMed] [Google Scholar]
- 27.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, Haynes J R, Letvin N L, Wyand M, Robinson H L. Simian immunodeficiency virus DNA vaccine trial in macaques. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luke C J, Carner K, Liang X, Barbour A G. An OspA-based DNA vaccine protects mice against infection with Borrelia burgdorferi. J Infect Dis. 1997;175:91–97. doi: 10.1093/infdis/175.1.91. [DOI] [PubMed] [Google Scholar]
- 29.Malik A, Egan J E, Houghton R A, Sadoff J C, Hoffman S L. Human cytotoxic T lymphocytes against Plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci USA. 1991;88:3300–3304. doi: 10.1073/pnas.88.8.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller M D, Gould-Fogerite S, Shen L, Woods R M, Koenig S, Mannino R J, Letvin N L. Vaccination of rhesus monkeys with synthetic peptide in a fusogenic proteoliposome elicits simian immunodeficiency virus-specific CD8+ cytotoxic T lymphocytes. J Exp Med. 1992;176:1739–1744. doi: 10.1084/jem.176.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 31.Raz E, Carson D A, Parker S E, Parr T B, Abai A M, Aichinger G, Gromkowski S H, Singh M, Lew D, Yankauckas M E, Baird S M, Rhodes G H. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91:9519–9523. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robson K J, Hall J R, Jennings M W, Harris T J, Marsh K, Newbold C I, Tate V E, Weatherall D J. A highly conserved amino-acid sequence in thrombospondin, properdin and in proteins from sporozoites and blood stages of a human malaria parasite. Nature. 1988;335:79–82. doi: 10.1038/335079a0. [DOI] [PubMed] [Google Scholar]
- 33.Rogers W O, Malik A, Mellouk S, Nakamura K, Rogers M D, Szarfman A, Gordon D M, Nussler A K, Aikawa M, Hoffman S L. Characterization of Plasmodium falciparum sporozoite surface protein 2. Proc Natl Acad Sci USA. 1992;89:9176–9180. doi: 10.1073/pnas.89.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez G I, Rogers W O, Mellouk S, Hoffman S L. Plasmodium falciparum: exported protein-1, a blood stage antigen, is expressed in liver stage parasites. Exp Parasitol. 1994;79:59–62. doi: 10.1006/expr.1994.1060. [DOI] [PubMed] [Google Scholar]
- 35.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, Silverman G J, Lotz M, Carson D A, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 35a.Scheller, •. •. Submitted for publication.
- 36.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R S, Nussenzweig V. Gamma-interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 37.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedegah M, Sim B K L, Mason C, Nutman T, Malik A, Roberts C, Johnson A, Ochola J, Koech D, Were B, Hoffman S L. Naturally acquired CD8+ cytotoxic T lymphocytes against the Plasmodium falciparum circumsporozoite protein. J Immunol. 1992;149:966–971. [PubMed] [Google Scholar]
- 39.Seguin M C, Klotz F W, Schneider I, Weir J P, Goodbary M, Slayter M, Raney J J, Aniagolu J U, Green S J. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J Exp Med. 1994;180:353–358. doi: 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen L, Chen Z W, Miller M D, Stallard V, Mazzara G P, Panicali D L, Letvin N L. Recombinant virus vaccine-induced SIV-specific CD8+ cytotoxic T lymphocytes. Science. 1991;252:440–443. doi: 10.1126/science.1708168. [DOI] [PubMed] [Google Scholar]
- 41.Szarfman A, Lyon J A, Walliker D, Quakyi I, Howard R J, Sun S, Ballou W R, Esser K, London W T, Wirtz R A, Carter R. Mature liver stages of cloned Plasmodium falciparum share epitopes with proteins from sporozoites and asexual blood stages. Parasite Immunol. 1988;10:339–351. doi: 10.1111/j.1365-3024.1988.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 42.Tine J A, Lanar D E, Smith D M, Wellde B T, Schultheiss P, Ware L A, Kauffman E B, Wirtz R A, Taisne C D, Hui G S N, Chang S P, Church P, Hollingdale M R, Kaslow D C, Hoffman S, Guito K P, Ballou W R, Sadoff J C, Paoletti E. NYVAC-Pf7: a poxvirus-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. Infect Immun. 1996;64:3833–3844. doi: 10.1128/iai.64.9.3833-3844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villinger F, Hunt D, Mayne A, Vuchetich M, Findley H, Ansari A A. Qualitative and quantitative studies of cytokines synthesized and secreted by non-human primate peripheral blood mononuclear cells. Cytokine. 1993;5:469–479. doi: 10.1016/1043-4666(93)90038-7. [DOI] [PubMed] [Google Scholar]
- 44.Villinger F, Brar S S, Mayne A, Chikkala N, Ansari A A. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J Immunol. 1995;155:3946–3954. [PubMed] [Google Scholar]
- 45.Voss G, Li J, Manson K, Wyand M, Sodroski J, Letvin N L. Human immunodeficiency virus type 1 envelope glycoprotein-specific cytotoxic T lymphocytes in simian-human immunodeficiency virus-infected rhesus monkeys. Virology. 1995;208:770–775. doi: 10.1006/viro.1995.1209. [DOI] [PubMed] [Google Scholar]
- 46.Voss G, Nick S, Stahl-Hennig C, Ritter K, Hunsmann G. Generation of macaque B lymphoblastoid cell lines with simian Epstein-Barr-like viruses: transformation procedure, characterization of the cell lines and occurrence of simian foamy virus. J Virol Methods. 1992b;39:185–195. doi: 10.1016/0166-0934(92)90137-3. [DOI] [PubMed] [Google Scholar]
- 47.Walliker D, Quakyi I A, Wellems T E, McCutchan T F, Szarfman A, London W T, Corcoran L M, Burkot T R, Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- 48.Wang R, Charoenvit Y, Corradin G, de la Vega P, Franke E D, Hoffman S L. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide: induction of CD4+ T cell- and IFN-γ-dependent elimination of infected hepatocytes. J Immunol. 1996;157:4061–4067. [PubMed] [Google Scholar]
- 49.Weiss W R, Mellouk S, Houghten R A, Sedegah M, Kumar S, Good M F, Berzofsky J A, Miller L H, Hoffman S L. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. J Exp Med. 1990;171:763–773. doi: 10.1084/jem.171.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss W R, Sedegah M, Beaudoin R L, Miller L H, Good M F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci USA. 1988;85:573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wizel B, Houghten R, Church P, Tine J A, Lanar D E, Gordon D M, Ballou W R, Sette A, Hoffman S L. HLA-A2-restricted cytotoxic T lymphocyte responses to multiple Plasmodium falciparum sporozoite surface protein 2 epitopes in sporozoite-immunized volunteers. J Immunol. 1995;155:766–775. [PubMed] [Google Scholar]
- 52.Wizel B, Houghten R A, Parker K, Coligan J E, Church P, Gordon D M, Ballou W R, Hoffman S L. Irradiated sporozoite vaccine induces HLA-B8-restricted cytotoxic T lymphocyte responses against two overlapping epitopes of the Plasmodium falciparum surface sporozoite protein 2. J Exp Med. 1995;182:1435–1445. doi: 10.1084/jem.182.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization. Malaria fact sheet no. 94. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 54.Yasutomi Y, Koening S, Haun S S, Stover C K, Jackson R K, Conard P, Conley A J, Emini E A, Fuerst T R, Letvin N L. Immunization with recombinant BCG-SIV elicits SIV-specific cytotoxic T lymphocytes in rhesus monkeys. J Immunol. 1993;150:3101–3107. [PubMed] [Google Scholar]
- 55.Yasutomi Y, Palker T J, Gardner M B, Haynes B F, Letvin N L. Synthetic peptide in mineral adjuvant elicits simian immunodeficiency virus-specific CD8+ cytotoxic T lymphocytes in rhesus monkeys. J Immunol. 1993;151:5096–5105. [PubMed] [Google Scholar]
- 56.Yasutomi Y, Robinson H L, Lu S, Mustafa F, Lekutis C, Arthos J, Mullins J I, Voss G, Manson K, Wyand M, Letvin N L. Simian immunodeficiency virus-specific cytotoxic T-lymphocyte induction through DNA vaccination of rhesus monkeys. J Virol. 1996;70:678–681. doi: 10.1128/jvi.70.1.678-681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu J, Hollingdale M R. Structure of Plasmodium falciparum liver stage antigen-1. Mol Biochem Parasitol. 1991;48:223–226. doi: 10.1016/0166-6851(91)90117-o. [DOI] [PubMed] [Google Scholar]