Abstract
Fundamental insight gained over the last decades led to the discovery of cytokines as pivotal drivers of inflammatory diseases such as rheumatoid arthritis, psoriasis/psoriasis arthritis, inflammatory bowel diseases, atopic dermatitis and spondylarthritis. A deeper understanding of the pro-inflammatory and anti-inflammatory effects of various cytokines has prompted new cytokine-targeting therapies, which revolutionised the treatment options in the last years for patients with inflammatory disorders. Disease-associated immune responses typically involve a complex interplay of multiple cytokines. Therefore, blockade of one single cytokine does not necessarily lead to a persistent remission in all patients with inflammatory disorders and fostered new therapeutic strategies targeting intracellular pathways shared by multiple cytokines. By inhibiting JAK-STAT signalling pathways common to families of cytokines, JAK-inhibitors (JAKinibs) have created a new paradigm for the treatment of inflammatory diseases. Multiple agents have been approved for various disorders and more are being investigated for several new indications. Second-generation selective JAKinibs have been devised with the aim to achieve an increased selectivity and a possible reduced risk of side effects. In the current review, we will summarise the current body of evidence of pan versus selective JAKinibs and the most recent insights on new side effects and indications, including COVID-19.
Keywords: Cytokines, Immune System Diseases, Inflammation
Introduction
Over the past decades, important insight were gained of the molecular components of the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway, Within this review, we will provide a brief summary of the most important findings that led to the development of inhibitors of the JAK-STAT pathway, which we will refer to as JAK-inhibitors (JAKinibs).
JAK/STAT-dependent cytokines
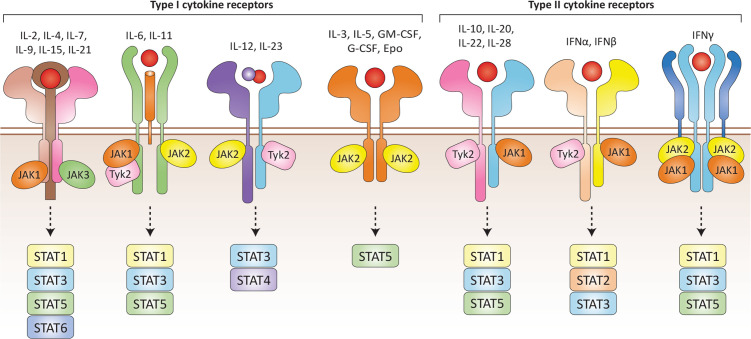
Cytokines are structurally diverse hormones that are secreted by immune and non-immune cells. They are important for the maintenance of physiological homeostasis.1 Cytokines bind receptors belonging to at least seven families, which subsequently activate multiple signalling pathways. In this review, we focus on a large cytokine family that binds type I/II cytokine receptors, all of which are in turn dependent on a small family of tyrosine kinases, JAK to function (figure 1).2 3 These cytokines can be categorised into two major classes based on cytokine folding and receptor properties (box 1).
Figure 1.
Type I/II cytokine receptors. Type I/II family cytokines signal through different heteromeric receptors which define the family (box 1). Members of the type I/II cytokine family include interleukins (ILs), interferons (IFNs), IFN-like cytokines, colony-stimulating factors, hormones and growth factors. The combinatorial complexity of cytokine receptor signalling is mediated by specific binding of JAK isoforms to intracellular domains and subsequent activation of STATs.
Box 1. Type I and type II cytokine family.
Type I cytokines
Receptors for type I cytokines harbour a conserved WSXWS motif in their extracellular domains and bind ligands sharing a four α-helical structure.277 Members of this receptor family can be further grouped based on shared receptor chains that combine with cytokine-specific chains to form the individual receptor complexes. The common γ-chain (γc, also known as interleukin (IL)-2 receptor γ subunit) cytokines include IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21. The common β-chain cytokines include IL-3, IL-5 and granulocyte macrophage colony-stimulating factor. The third major family include cytokines that bind to the glycoprotein 130 (gp30) receptor and include IL-6, IL-11, IL-27, LIF, OSM, CT-1, CNTF, CLC and IL-31. Related to the gp130 cytokines is the dimeric cytokine family, which includes IL-12, IL-23 and IL-35. Other cytokines like erythropoietin, thrombopoietin, granulocyte colony-stimulating factor and growth hormone bind to homodimeric receptors.
Type II cytokines
The type II cytokines comprise a group of >30 signalling molecules including the interferons (IFNαs, IFNβ, IFNγ, IFNk, IFNλ2 (IL-28A), IFNλ3 (IL-28B), IFNλ1 (IL-29), IFNλ4) and IL-10-related cytokines (IL-10, IL-19, IL-20, IL-22, IL-24 and IL-26). Type II cytokine receptors are related to type I receptors, but lack the characteristic WSXWS motif.
The JAK/STAT pathway
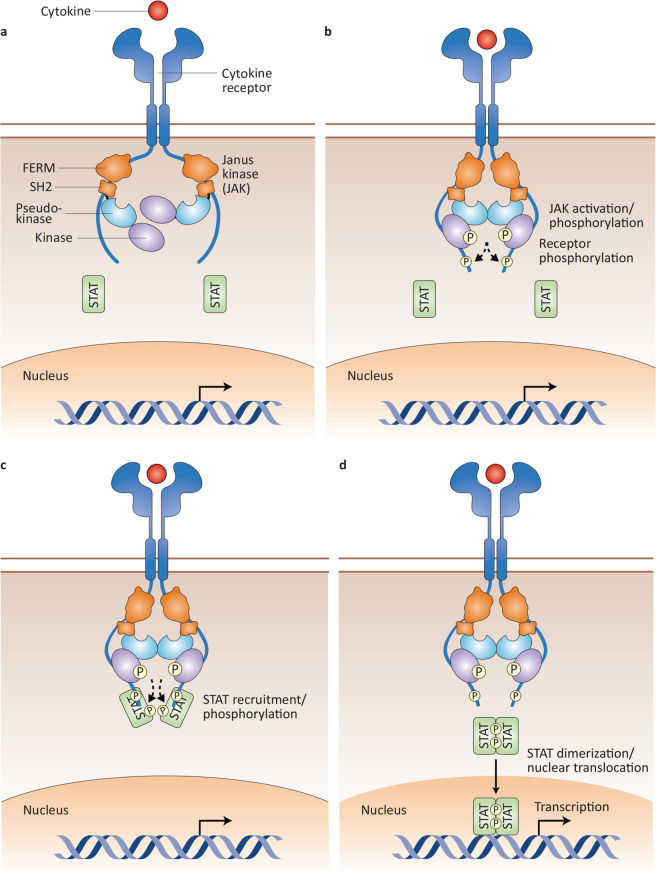
The type I/II cytokine receptors have no intrinsic catalytic activity. The receptors consist of an extracellular cytokine binding domain and a cytoplasmatic domain, which binds a combination of one to three tyrosine kinases of the JAK family. This consists of four members: JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2) (figure 1). In contrast with the other members, the expression of JAK3 is largely restricted to cells of the haematopoietic system.4 5
JAKs share conserved domain composition harbouring N-terminal FERM and SH2 domains as well as C-terminal pseudokinase and kinase domains (figure 2). JAKs are constitutively associated with the intracellular tails of receptors via the FERM and SH2 domains.6 7 JAKs are phosphotransferases, that is, they transfer phosphate from ATP to tyrosine residues. Signalling is initiated by cytokine-induced activation of receptor-associated JAKs. Recent work revealed a role for the pseudokinase domain in dimerisation and activation of the receptor complex7 8 leading to kinase domain autophosphorylation and transphosphorylation as well as phosphorylation of the receptor tails, thereby creating docking sites for latent, cytoplasmatic transcription factors termed signal transducers and activators of transcription (STATs). STATs are recruited to the receptor complex through their tyrosine-phosphate-binding SH2 domains, and become themselves phosphorylated. Thus, activated, phospho-STATs homodimerise or heterodimerise and translocate to the nucleus. Binding of dimerised STATs to DNA-regulatory elements controls transcription.9–13 STATs bind multiple sites in the genome and regulate thousands of protein-coding genes, along with long non-coding RNAs and microRNAs. Gene transcription is also regulated by modification of the chromatin structure by STATs.14 Thereby, JAK-STAT-dependent signalling is involved in many fundamental biological processes, including apoptosis, proliferation, migration, development and differentiation of a variety of cell types present in all organs of the body. Inhibition of one or more JAKs or STATs can lead to the inhibition of other family members. Not all the actions of type I/II cytokines in various tissues have been clarified and thus the molecular consequences/effects of JAK/STAT inhibition are currently not fully understood.
Figure 2.
JAK-STAT signalling pathway. (A) Individual JAKs are constitutively associated with their specific receptors through their FERM and SH2 domains. (B) On cytokine engagement, JAKs become activated and phosphorylate each other, as well as the intracellular tails of their receptors. (C) Phosphorylation of the receptor chains generates docking sites for STATs, which can bind to the cytoplasmic domain of the receptor facilitating JAK-mediated STAT phosphorylation. (D) Phosphorylated STATs dimerise, translocate to the nucleus and bind to DNA, thereby regulating gene transcription.
Cytokine-dependent activation of JAK/STAT pathways
The specificity of JAK/STAT-mediated signal transduction is determined by the cytokine receptor complex. Seven mammalian STAT family members have been identified (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6) that can be activated by a variety of different type I/II cytokine receptors and their associated JAKs.
The common γchain (γc) cytokines (interleukin (IL)-4, IL-2, IL-9, IL-7, IL-15 and IL-21), which activate receptor complexes incorporating the common-γ chain, signal through JAK1 and JAK3. JAK3 specifically binds to the common-γ chain and JAK1 is associated with cytokine specific α-chains and β-chains.15 Receptor signalling leads to the phosphorylation and nuclear translocation of STAT5A/5B by all members to a variable extent.16 IL-4 additionally activates STAT6 and IL-21 primarily activates STAT3.16 17 Signalling in response to binding of IL-6, IL-11, IL-13, oncostatin M and leukaemia inhibitory factor to the type I receptor common gp130 chain is mediated through JAK1 and JAK2, although some data point to a role for TYK2 as well18; together these signals lead to a combination of STAT3 and STAT1 activation.19 IL-12 and IL-23 activate specific receptor complexes that share the common p40 receptor chain and bind JAK2 and TYK2, which leads to the activation of STAT3 and STAT4.20–23 Receptors for IL-3, IL-5 and granulocyte macrophage colony-stimulating factor (GM-CSF), as well as erythropoietin (EPO), thrombopoietin (TPO) and granulocyte colony-stimulating factor (G-CSF) signal solely via JAK2 and lead to STAT5 phosphorylation.24
The type II receptor subfamily comprises the IL-10 and interferon (IFN) cytokine families.
The latter can be divided into three subfamilies. Type I IFNs, including the many IFNα and IFNβ require JAK1 and TYK2, which leads to activation of STAT1, STAT2 and STAT4. The type II IFN, IFNγ signals through JAK1 and JAK2 that activates STAT1 and to a lesser extent STAT3.25–27 IFNγ stimulation leads to the formation of either STAT1–STAT1 homodimers or STAT1–STAT3 heterodimers.28 The third subfamily, the type III IFNλs (IL-28A, IL-28B and IL-29) are functionally similar to the type I IFNs.
The members of the second major, group, the IL-10 family signal through JAK1 and TYK2 and activate STAT1, STAT3 and STAT5.29 This is similar to IFNγ but with STAT3 activated to a greater extent than STAT1 with the presence of STAT3 homodimers.
Genetic evidence for the significance of the Janus kinase family
Murine genetics highlight the critical role of the JAK family in mediating the actions of type I/II cytokines. This has been supported by the discovery of both loss-of-function (LOF) and gain-of-function (GOF) JAK mutations in patients (table 1).
Table 1.
JAKs and STATs with associated phenotypes
| JAK/STAT | Knockout mouse phenotype | Genetic links to human diseases |
| JAK1 | Perinatally lethal | GOF: somatic mutations are seen in ALL, AML, solid-organ malignancies |
| JAK2 | Embryonically lethal, absence of erythropoiesis | GOF: PV, PMF, ET, hypercoagulable state, haematological malignancies Polymorphisms: Behçet’s disease |
| JAK3 | Defective T and B cell maturation | LOF: T- NK- B+ severe combined immunodeficiency |
| TYK2 | Reduced response to type I interferon and IL-12, defective STAT3 activation | LOF: primary immunodeficiency characterised by dermatitis and impaired antiviral and anti-tb immunity |
| STAT1 | Impaired response to type I and II interferons, susceptibility to viral infections | LOF: primary immunodeficiency with viral susceptibility GOF: chronic mucocutaneous candidiasis, blood cytopenias |
| STAT2 | Impaired response to type I interferon and susceptibility to viral infections | LOF: increased susceptibility to viral mutations |
| STAT3 | Embryonically lethal | LOF: AD-HIES GOF: germline mutations: multisystem auto-immune diseases Somatic mutations: LGL and other T cell lymphomas Polymorphisms: Behçet’s disease |
| STAT4 | Impaired Th1 differentiation | Polymorphisms: RA, SLE, Sjögren’s syndrome LOF: mycosis |
| STAT5a/STAT5b | Neonatally lethal: few surviving animals at birth are grossly runted and die after a few weeks | Deficiency: autoimmunity, bleeding diathesis, immunodeficiency and dwarfism Somatic mutations: LGL |
| STAT6 | Impaired Th2 differentiation | Polymorphisms: asthma, atopy, increased IgE |
AD, atopic dermatitis; ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; ET, essential thrombocythemia; GOF, gain of function; HIES, hyper IgE syndrome; IL, interleukin; JAK, Janus kinase; LGL, leukaemia, large granular lymphocytic leukaemia; LOF, loss of function; PMF, primary myelofibrosis; PV, polycythemia vera; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; STAT, signal transducer and activator of transcription; Th, T helper.
JAK1-deficient mice die perinatally with impaired organogenesis and dwarfism in keeping with the many cytokines that rely on this kinase. However, isolated cells from these mice confirmed an essential role of JAK1 for signalling by all class II cytokine receptors, together with the common-γ chain and gp130 cytokine families.30 In humans JAK1 LOF mutation was shown to be associated with recurrent atypical mycobacterial infection and early onset metastatic bladder carcinoma.31 JAK1 GOF mutations were identified in one family with autosomal dominant immune dysregulatory and hypereosinophilic syndrome.32 Polymorphisms of JAK1 are associated with juvenile idiopathic arthritis (JIA).33
JAK2 has a similarly pleiotropic role including an essential role in the action of many haematopoietic growth factors. JAK2-deficient mice die in utero with bone marrow failure. There are no patients that lack JAK2 but germline JAK2 GOF and somatic mutations have been reported together with acquired JAK2 GOF mutations in patients with myeloproliferative disease.34 35 JAK2 polymorphisms are associated with Behçet’s disease.36
JAK3 deficiency causes a severe combined immunodeficiency in both mice and infants characterised by loss of T and natural killer (NK) cells. Curiously, B cell development is preserved in humans but not mice. Mice held in germ-free facilities are healthy but develop a slowly progressive inflammatory disease associated with splenomegaly as the few T cells that develop lack regulation. By contrast, human infants generally die of infection within the first year of life without medical intervention.37 38
TYK2-deficient mice are viable and have selective impairment of cytokine responses that include loss of IFN and IL-12/23 family cytokine responses with susceptibility to viral infections. TYK2 gene polymorphisms are linked to autoimmune diseases such as systemic lupus erythematosus (SLE) and Crohn’s disease (CD), ulcerative colitis (UC), psoriasis, multiple sclerosis (MS), systemic sclerosis (SS), inflammatory myopathies, primary biliary cirrhosis and type 1 diabetes.39 Variants of TYK2 have been shown to be catalytically impaired but to have residual signalling in response to IFNα/β, IL-6 and IL-10.40 Variants of TYK2 are found to be associated with protection against rheumatoid arthritis (RA), SLE, inflammatory bowel diseases (IBD) and endometriosis-related infertility.41 42 Homozygosity for the common TYK2 P1104A allele selectively disrupts the induction of IFNγ by IL-23 and is a common monogenic aetiology of tuberculosis.43 TYK2 deficiency in patients has been associated with a variety of clinical phenotypes. The first case included intracellular bacterial and viral infections and features of hyper IgE syndrome (HIES) such as atopic dermatitis (AD), high serum IgE levels and staphylococcal abscesses, although subsequent cases have demonstrated a phenotype characterised by suseptability to viral infections and heightened atopy.44–46
Mutations in STAT genes cause many immunodeficiency syndromes, and polymorphisms in these genes are associated with autoimmune diseases. Mutations in STATs can cause abnormalities in immune functions. GOF STAT1 mutations are associated with chronic mucocutaneus candidiasis, characterised by recurrent or persistent infections of skin, nails and mucosa with Candida organisms.47 Patients with inflammatory disease associated with STAT1 GOF mutations have been treated with allogeneic bone marrow transplantation with mixed success.48 49 JAKinibs have been successfully used to correct this syndrome, but it remains to be seen if they can safely be used as a long-term treatment.50 Dominant negative LOF STAT1 mutations with impaired IFN signalling have been characterised and present with susceptibility to viral infections.51 STAT2 deficiency, alongside ISG15 and ubiquitin-specific peptidase 18 (USP18) deficiencies, have been associated with severe early onset inflammation characteristic of type I interferonopathies.52
Dominant negative LOF STAT3 mutations were the first reported cause of HIES. Conversely, patients with STAT3 GOF mutations have been reported and present with an early onset inflammatory disorder characterised by joint and skin inflammation. Mutations of STAT3 have been linked to large granular lymphomas,53 Behçet’s disease,36 CD54 and psoriasis,54 whereas STAT4 polymorphisms are associated with RA and SLE.55 STAT4 deficiencies have been associated with a novel inborn error of IL-12-dependent IFNγ immunity associated with susceptibility to paracoccidioidomycosis.56
Polymorphisms in STAT6 are associated with atopy and asthma due to disturbed IL-4 signalling57 and with recurrent mycobacterial infections including disseminated BCG disease. GOF mutations is associated with primary atopic disorders.58 Autosomal recessive STAT5B mutations cause a complex syndrome characterised by dwarfism, immunodeficiency and autoimmunity and can also be associated with recurrent pneumonia and other infections.3 4 Thus, a large body of evidence points to a critical role for JAKs and STATs in the pathogenesis of rare and common disorders of human immunity.30
Negative regulators of JAK/STAT signalling
JAK/STAT signalling can be both enhanced and inhibited by many accessory proteins. There are two major families of negative regulators, the protein inhibitors of activated STAT family was the first to be discovered and are consitutively expressed and bind to activated STAT dimers within the cell nucleus. By contrast, the supressors of cytokine signalling (SOCS) family are induced by STAT signalling and translocate to the JAK bound cytokine receptor complexes to generate a negative feedback loop. There are seven SOCS family members, each of which have a different repertoire of target cytokine receptors. Activation of one STAT pathway can lead to inhibiton of a second cytokine receptor JAK/STAT pathway. A group led by Rieux-Laucat has identified five families with haplo-insufficiency of SOCS1 caused by heterozygote mutations of SOCS1. Affected members present with blood cytopenias and multiorgan autoimmune diseases that phenocopy patients with STAT1 or STAT3 GOF mutations.59 ISG15 represents an IFNα/β-induced ubiquitin-like protein and human ISG15 promotes a proviral state following IFN priming. ISG15-deficient patients do not present with any overt viral phenotype, but are highly susceptible to environmental mycobacteria and can present with autoinflammatory disease.60 61 USP18 is a key negative regulator of type I IFN signalling by blocking the access of JAK1 to the type I IFN receptor. The absence of USP18 results in unmitigated IFN-mediated inflammation and is lethal during the perinatal period.61 62
Rationale and development of JAKinibs
The inhibition of key cytokines by targeting their signal transduction pathways with small molecules was first articulated in 199563 based on genetic data. Key to the success of this approach was the realisation that it was possible to generate highly specific inhibitors of protein kinases by designing small molecules that could block the ATP docking site.
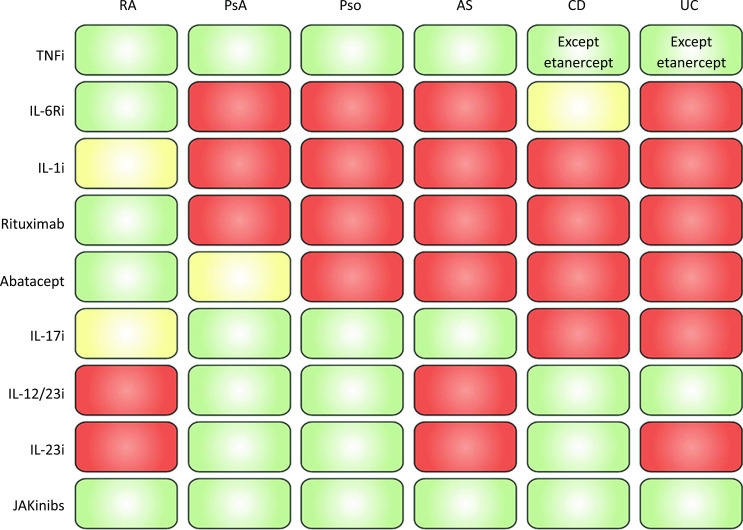
Prior to the widespread use of JAKinibs, a large number of biological disease-modifying antirheumatic drugs (bDMARDs) has been licensed in the field of rheumatology and many other areas (oncology, dermatology, gastroenterology, neurology). When focussing on rheumatic diseases, it is evident from figure 3 that most of these bDMARDs are efficacious for one or just a few diseases, while among the bDMARDs tumour necrosis factor (TNF) inhibitors are highly efficacious across all these diagnoses, but also beyond, such as IBD (figure 3) and uveitis; IL-6R inhibitors and TNF inhibitors are also approved for JIA. Despite advances in our understanding of the pathophysiology of many of these inflammatory diseases, a number of questions remain: (i) why do so many agents work selectively in one or few disorders while TNF inhibitors act so widely across diseases and (ii) why, for example in RA and psoriasis arthritis (PsA), the response rates of all these different targeted therapies are very similar. It has been hypothesised that this may be due to the pivotal role of pro-inflammatory cytokines, especially TNFα. Thus, TNFα likely represents a common shared pathway that is directly or indirectly targeted by drugs with different modes of action across different diseases.64 65 Consistent with this theory, combinations of bDMARDs do not exhibit increased efficacy,66 67 while the increase in serious infections attests to the interference with more than one immunological pathway. TNFα does not signal via JAKs, but uses the nuclear factor kappa B (NF-κB) and mitogen activated protein kinase (MAPK) pathways. Consequently, inhibitors of p38 MAPK, NF-κB and other signalling cascades, such as spleen tyrosine kinase (Syk) as used by Fc receptors or Bruton tyrosine kinase (BTK) as used by B cell receptors, have been a focus of clinical research. Interestingly, neither p38 nor Syk inhibition showed significant efficacy,68 69 while phase II data for BTK inhibition showed some efficacy,70 but the development for RA was apparently discontinued.71 Furthermore, no compound inhibition the NF-κB pathway has yet been sufficiently studied in rheumatic diseases.
Figure 3.
Efficacy of various approved agents across different therapies. Green: good efficacy; orange: low efficacy (some not approved for the respective indication); red: no efficacy (not approved for the respective indication). AS, ankylosing spondylitis; CD, Crohn’s disease; i, inhibitor(s); IL, interleukin; JAKinibs, Janus kinase inhibitors; PsA, psoriatic arthritis; PsO, psoriasis; RA, rheumatoid arthritis; TNF, tumour necrosis factor; UC, ulcerative colitis.
The first reported in vivo use of a JAKinibs was described for blocking allograft rejection.72 The first generation of JAKinibs inhibits multiple JAK family members. Subsequently, more specific inhibitors have been generated (table 2). JAKinibs have been found to have a similarly broad (and maybe even broader) breadth of efficacy in various indications as the TNFα inhibitors, even though TNFα does not signal via the JAK-STAT pathway. Thus, despite more than one decade of research into a plethora of small molecules that inhibit various signal transduction pathways, only JAKinibs have hitherto provided sufficient benefit with acceptable safety aspects to make it into clinical application for patients with rheumatic diseases. It is a riddle why inhibition of other molecules does not work to a similar extent. This may be due to redundancy of pivotal pathways so that a secondary molecular path compensates if another essential one is inhibited or due to the fact that some pathways are of such crucial importance that their inhibition is afflicted with unacceptable side effects. Thus, even though JAKinibs are essential for various organ developmental steps in utero, their inhibition in adulthood does not appear to be affected with unacceptable adverse events, while still providing sufficient anti-inflammatory efficacy.
Table 2.
In vitro selectivity of common JAKinibs for the major families of type I/II cytokines
| Type I cytokine receptor | Type II cytokine receptor | |||||||
| Receptor family | GP-130 family | IL-2R CGC family | IL-12/23 family | CβC family | IL-10 family | Type I IFNs | Type II IFNs | |
| Cytokine ligands | IL-6, 11, 27, LIF, OSM | IL-2, 4, 7, 9, 15, 21 | IL-12, 23 | IL-3, IL-5, GM-CSF | IL-10, 19, 20, 22, 26 | IFNα, β | IFNγ | |
| Asc JAKs | JAK1, JAK2, TYK2 | JAK1, JAK3 | JAK2, TYK2 | JAK2 | JAK1, JAK2, TYK2 | JAK1, TYK2 | JAK1, JAK2 | |
| Downstream STATs | STAT1, 3, 5 | STAT1, 3, 5, (6) | STAT3, 4 | STAT5 | STAT1, 3, 5 | STAT1, 2, 3 | STAT1, 3, 5 | |
| Inhibitors in increasing order of selectivity | Tofacitinib | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| Peficitinib | +++ | +++ | ++ | ++ | +++ | +++ | +++ | |
| Baricitinib | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| Upadacitinib | +++ | +++ | ++ | + | +++ | +++ | +++ | |
| Filgotinib | +++ | +++ | + | + | +++ | +++ | +++ | |
| Abrocitinib | +++ | +++ | – | – | +++ | +++ | +++ | |
The degree of inhibition is normalised against the ability of each JAKinib to inhibit JAK1 as measured by the IC50 value in nM.
+++=IC50 of the most inhibited associated JAK for a given cytokine family is lower than or equal to the IC50 for JAK1.
++=IC50 of the most inhibited associated JAK for a given cytokine family is onefold to twofold higher than the IC50 for JAK1.
+=IC50 of the most inhibited associated JAK for a given cytokine family is 2-fold to 10-fold higher than the IC50 for JAK1.
–=IC50 of the most inhibited associated JAK for a given cytokine family is >10 times higher than the IC50 for JAK1.
GM-CSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; JAK, Janus kinase; STAT, signal transducer and activator of transcription.
First-generation JAKinibs—indications and therapeutic effects
Ruxolitinib was designed as a JAK2 inhibitor after the discovery of GOF JAK2 mutations in 65%–97% of patients with the common myeloproliferative diseases, primary myelofibrosis (PMF), primary polycythaemia (polycythaemia rubra vera (PRV)) and primary or essential thrombocythemia (ET).73 It was the first Food and Drug Administration (FDA)-approved and European Medicines Agency (EMA)-approved JAKinib for the treatment of PMF. Ruxolitinib was subsequently approved for the treatment of PRV in patients with an insufficient response or intolerance to hydroxyurea. A phase II study in RA completed in 2008 (NCT00550043) remains unpublished. Ruxolitinib is effective both at reducing splenomegaly and the constitutional symptoms associated with PMF and is of benefit even in the absence of a JAK2 mutation.74 Its success is due in part to its ability to inhibit JAK1 in addition to JAK2. Conversely, this increases the incidence of viral infections in patients on ruxolitinib. Ruxolitinib is effective in treating several inflammatory conditions and was recently FDA approved for the treatment of glucocorticoid-resistant acute and chronic graft-versus-host disease (GVHD), a major complication of allogeneic bone marrow transplantation after failure of one or two lines of systemic therapy in adult and paediatric patients 12 years and older.75 In a recently published phase III open-label, randomised trial, treatment with ruxolitinib was superior to control therapies and associated with greater overall response, longer failure-free survival and reduction in symptoms among patients with glucocorticoid-refractory or glucocorticoid-dependent chronic GVHD.76 Ruxolitinib is approved by the FDA for treatment of non-segmental vitiligo and for AD.
Tofacitinib (JAK1/3 and partial JAK2 inhibitor) was the first studied and FDA-approved and EMA-approved JAKinib for the treatment of patients with RA, showing efficacy across many patient populations, including patients refractory to bDMARDs,77–79 conventional synthetic (cs)DMARDs and also patients who were methotrexate (MTX) naïve.80 Tofacitinib was originally designed as a selective inhibitor of JAK3. Pharmacological studies revealed a blockade of JAK3 and JAK1 but JAK2 and TYK2 were also affected, although to a lesser extent. Accordingly, tofacitinib has the greatest effect on IL-6, IFNγ and common γc cytokines.81 A head-to-head study comparing tofacitinib with adalimumab 40 mg every other week (in combination with background MTX) showed non-inferiority of the combination therapy of tofacitinib 5 mg two times per day (+MTX) and adalimumab 40 mg every other week (+MTX), but failed to demonstrate non-inferiority for tofacitinib 5 mg monotherapy.82 Two studies confirmed the efficacy of tofacitinib in patients with PsA with insufficient response to csDMARDs or bDMARDs and led to subsequent regulatory approval of tofacitinib for PsA.83 84 Patients with ankylosing spondylitis (AS) with insufficient response to non-steroidal anti-inflammatory drugs (NSAIDs) showed a clear dose-response relationship and significantly better outcomes compared with placebo treatment in a phase II study.85 A phase III study (NCT03502616) has confirmed the efficacy results in patients with AS.86 In patients with chronic plaque psoriasis, tofacitinib reduced skin disease significantly more compared with placebo treatment.87–90 A head-to-head trial, comparing patients treated with 10 mg of tofacitinib two times per day showed non-inferiority compared with patients treated with etanercept twice weekly.88 Tofacitinib also showed superior results compared with placebo in induction (10 mg two times per day) as well as maintenance therapy (5 mg and 10 mg two times per day) of patients with severe UC and was approved for this indication.91 No significant difference of tofacitinib compared with placebo treatment was found when treating patients with CD.92 93 Limited data are provided for efficacy of tofacinitib in patients with SLE. However, tofacitinib was found to be generally safe in subjects with SLE according to a phase I randomised controlled trial. Tofacitinib was safe in SLE meeting study’s primary end point. As secondary end points it could be shown that tofacitinib improves cardiometabolic and immunological parameters associated with the premature atherosclerosis in SLE.94 Ongoing trials currently investigate safety and efficacy in patients with SLE with skin manifestations (NCT03288324, NCT03159936). The STAT4 SLE risk allele has been associated with increased IL-12-induced IFNγ production in T cells from patients with SLE,95 suggesting beneficial effects of JAKinibs. In a randomised, double-blind, placebo-controlled clinical trial, tofacitinib has been shown to be effective and generally safe in patients with the STAT4 SLE risk allele.94
Peficitinib (pan-JAK inhibitor) was found to be modestly efficient in multicentre trials in patients with RA. However, several trials investigating Japanese patients with RA found significant improvements of disease activity and physical function with subsequent regulatory approval of peficitinib in Japan.96–101 One phase II trial showed a significant reduction of psoriatic skin disease with peficitinib compared with placebo.102 In patients with UC, a phase II trial failed to meet its primary end point, with only one dosage (150 mg) leading to significant improvements in remission induction after 8 weeks of treatment compared with placebo.103 Pefecitinib is currently not considered for approval by the FDA or EMA.
Baricitinib (LY3009104) is a dual JAK1/2 inhibitor that is functionally similar to ruxolitinib and therefore suppresses IFNγ, IL-6, IL12/23, EPO and GM-CSF signalling. Baricitinib was approved for treatment of patients with RA in the 4 mg dose by the EMA and 2 mg dose by the FDA based on various studies, showing efficacy in treatment-naïve csDMARD-experienced and bDMARD-experienced patients with active disease.104–112 In a head-to-head study, 4 mg of baricitinib (+MTX) was statistically superior to adalimumab 40 mg every other week (+MTX).113 One phase II trial showed 8 mg and 10 mg of baricitinib to be superior to placebo treatment in patients with chronic plaque psoriasis.114 In patients with moderate-to-severe AD, baricitinib significantly reduced inflammation and pruritus, as well as quality of life and skin pain and was EMA approved in December 2020.115–117 Baricitinib has not been investigated in patients with PsA, AS, UC or CD so far. In a double-blind placebo-controlled phase II trial, baricitinib at 4 mg dose, but not the 2 mg dose, significantly improved the signs and symptoms of patients with active SLE.118 However, based on results from two phase III trials to evaluate long-term safety and efficacy in patients with SLE (SLE-BRAVE I and II), baricitinib failed to provide clinical improvement in patients with active SLE receiving stable background therapy, with only baricitinib at daily dosage of 4 mg in the SLE-BRAVE I showing significant benefit compared with placebo. Other key end points were not met in either study. The use of corticosteroids was not restricted, potentially resulting in high placebo response rate.119 120 Phase II trials investigate efficacy and safety in patients with Sjögren’s syndrome (NCT05016297) and relapsing giant cell arteritis (NCT03026504). Baricitinib has recently completed phase III trials in the treatment of alopecia arreata (AA) (NCT03579749) with patients attaining a minimum of 80% of scalp recovery after 24 weeks at the 4 mg dose.121 Consequently, the drug has been approved for this indication.
Next-generation JAKinibs—indications and therapeutic effects
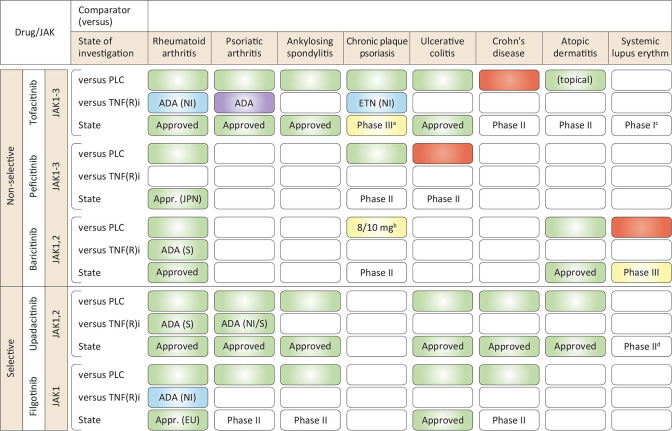
The side effects of JAKinibs are both predictable and perplexing, but to some degree can be attributed to their lack of selectivity. Tofacitinib was designed as a selective JAK3 inhibitor, yet its inhibition of JAK2 contributes to the unwanted side effects of anaemia and neutropenia. Conversely, the JAK2 inhibitor, ruxolitinib, designed to inhibit bone marrow overproduction of myeloid cells, inhibits JAK1 that will contribute to the observed increased incidence of viral infections. To address this, a second generation of inhibitors that could specifically inhibit individual JAKs were developed and investigated in a several clinical trials (figure 4). However, their success has been mixed and several agents were dropped after failing in clinical trials.
Figure 4.
Admission of approved selective versus non-selective JAKinibs. The label in the upper box indicates the comparator referenced with the colour code (green colour indicates significant differences; red colour indicates no significant difference; yellow indicates mixed results; blue colour indicates studies meeting non-inferiority; purple indicates no formal statistical comparison and numerically similar results. *No formal statistical comparison, numerically similar results. †Tofacitinib is currently not pursued for drug approval for plaque psoriasis. ‡8/10 mg reached statistical significance, no significance was observed for 2/4 mg versus placebo. §One trial, safety only (NCT02535689). ¶Phase II trial, no data/results published (NCT03978520). **No difference was observed in patients with small bowel CD,158 ADA, adalimumab; ETN, etanercept; JAK, Janus Kinase; NI, non-inferiority; PLC, placebo; S, superiority; TNF(R)i, tumour necrosis factor alpha receptor inhibitor.
Upadacitinib (ABT 494) represents a putatively selective JAK1/2 inhibitor, which has shown consistent efficacy results for RA, PsA, AS, JIA and IBD.122 123 Upadacitinib 15 mg once daily is EMA and FDA approved for treatment of RA, PsA, AS, UC and AD, with currently pending approval for CD.124–131 In patients with PsA, statistical superiority of upadacitinib 15 mg once daily (+MTX) compared with adalimumab 40 mg every other week (+MTX) in MTX non-responding patients132 was achieved. In patients with PsA with insufficient response to non-bDMARDs, upadacitinib 15 mg and 30 mg once daily were superior to placebo treatment, with upadacitinib 15 mg once daily being non-inferior to adalimumab 40 mg every other week and upadacitinib 30 mg once daily being statistically superior to adalimumab.133 Furthermore, patients with PsA with refractory disease despite previous bDMARD therapy had significant improvement of signs and symptoms as well as physical function when treated with upadacitinib, compared with placebo.134 Upadacitinib is approved for patients with AS by the EMA and FDA. In patients with AS with insufficient response to NSAIDs, upadacitinib 15 mg once daily was superior to placebo.130 131 In a phase III trial, efficacy and safety was shown in patients with active AS, refractory to biological therapy.135 Two phase II trials, investigating upadacitinib in UC demonstated that 7.5–45 mg of extended-release upadacitinib (once daily) was superior to placebo in induction of remission over 8 weeks.136 This led to a recently completed U-ACCOMPLISH phase III study using the highest 45 mg daily dose that confirmed benefit and has led to FDA approval for this JAKinib.137
By contrast, in patients with CD, higher rates of clinical and endoscopic remission were observed in patients treated with 3–24 mg of upadacitinib two times per day or 24 mg once daily, but no clear dose-response could be observed regarding endoscopic remission.138 An ongoing phase III trial currently investigates efficacy and safety of upadacitinib in patients with moderately to severely active CD who have inadequately responded to or are intolerant to biologic therapy (NCT03345836). Significant improvement of AD was observed when treating severely affected patients with AD with upadacitinib,139–141 which led to the approval for this indication by the FDA and EMA. No trial data are currently provided for the use of upadacitinib in patients with SLE. One ongoing phase II trial addresses safety and efficacy in moderately to severely active SLE (NCT03978520). Phase III trials are ongoing to address efficacy and safety in giant-cell arteritis and Takayasu arteritis (NCT03725202, NCT04161898).
Filgotinib (GLPG0634) , a designed selective JAK1 inhibitor, has demonstrated efficacy for RA and UC. Filgotinib was effective compared with placebo in the treatment of patients with bDMARD refractory RA,142 MTX-naïve patients143 and also in MTX-inadequate response (IR) patients.144–146 Furthermore, in MTX-IR patients, filgotinib 200 mg (+MTX) once daily was non-inferior to adalimumab (+MTX) based on DAS28-CRP ≤3.2. In September 2020, filgotinib received the approval for treating patients with RA and insufficient response to MTX treatment via the the EMA but remains currently unapproved by the FDA. Additionally, filgotinib 200 mg showed better efficacy compared with placebo in three separate phase II studies investigating patients with PsA, AS and CD.147–149 In a combined phase IIb/III trial, filgotinib was generally well tolerated and efficacious in inducing and maintaining clinical remission in UC.150 Safety and efficacy of filgotinib was currently investigated in phase III trials as induction and maintenance therapy for patients with moderately to severely CD (NCT02914561), but the results remain unpublished at the time of writing this manuscript. Another phase II trial in small bowel CD (DIVERGENCE-1) did not show a statistical difference when comparing filgotinib with placebo treatment.151
Abrocitinib (PF-04965842) is a selective JAK1 inhibitor, which has recently been approved by the FDA for the treatment of adults living with refractory, moderate-to-severe AD. The safety and efficacy of abrocitinib was evaluated in three phase III, randomised, placebo-controlled clinical trials: JADE MONO-1 and MONO-2 evaluated the efficacy and safety of two doses of abrocitinib monotherapy with moderate-to-severe AD.152 153 Abrocitinib showed similar responses compared with dupilumab in a head-to-head trial (JADE COMPARE) investigating adult patients with moderate-to-severe AD.154 Patients that completed 16 weeks of treatment in JADE MONO-1 and JADE MONO-2 were invited to enrol an ongoing phase III long-term extension study (JADE EXTEND—NCT034422822) including 92 weeks of treatment with abrocitinib with or without concomitant topical corticosteroids.
Decernotinib (VX-509) is a selective JAK3 inhibitor that showed some efficacy in phase II trials for the treatment of RA. However, its use is limited by multiple drug interactions, since it is metabolised by aldehyde oxidase to a metabolite that inhibits CYP3A4, which is essential for inactivation of many common drugs. The mixture of lack of efficacy, side effects and drug interactions led to an end of further development.155–157
Ritlecitinib (PF-06651600) is a selective JAK3 and TEC tyrosine kinase family inhibitor, which showed promising results in small, early studies investigating the treatment of RA and AA.158 159
Deucravacitinib (BMS-986165) is the first compound that targets the pseudokinase domain of a JAK, namely TYK2, and therefore represents a highly selective, allosteric TYK2 inhibitor that can inhibit IL-12, IL-23 and IFN signalling. Deucravacitinib was superior to placebo and apremilast treatment in a phase III trial for the treatment of patients with moderate-to-severe psoriasis160 161 and is approved by the FDA, while EMA approval is still pending. Results from a recently completed phase II trial show efficacy of deucravacitinib in PsA.162 163 In a phase II randomised, double-blind, placebo-controlled trial, safety and efficacy of deucravacitinib was shown in patients with active SLE with a higher response rate for the SLE Responder Index 4 at week 32 with an acceptable safety profile.164
Brepocitinib (PF-06700841) targets TYK2 and JAK1 selectively and was efficacious in phase II studies in patients with chronic plaque psoriasis165 and AA.159
Experimental evidence for JAKinib selectivity
Clinically approved JAKinibs have been developed with a specific target spectrum in mind,166 however selectivity for individual JAK isoforms in vivo is most likely relative and influenced by multiple variables such as dose, drug metabolism and target cell spectrum. The bulk of protein kinases have been designed as competitive ATP antagonists. The first protein kinase inhibitors were able to inhibit a limited number of kinases by virtue of a gatekeeper residue that is found within the ATP binding region only when the kinase is in the inactive state.167 This residue varies in different kinases and is both used by drug companies to generate selective inhibitors and mutated by cancer cells to escape the effect of these kinase inhibitors. All JAK family members use methionine as a gatekeeper residue posing challenges for designing highly selective JAKinibs. This can be overcome in part by the use of novel strategies such as targeting the inhibitory peudokinase domain.
Tofacitinib (CP-690,550) was originally designed as a selective JAK3 inhibitor,72 but subsequent studies employing in vitro kinase and cellular assays have determined that this compound preferentially inhibits cytokines that signal via JAK1 and/or JAK3 over JAK2.168 169 Its ability to inhibit JAK1 enables the drug to inhibit many inflammatory cytokines. Tofacitinib showed efficacy in mouse and rat models of arthritis and inhibited STAT1 and STAT3 signalling in vitro and both JAK1 and JAK3 signalling pathways in the collagen-induced arthritis model.168 170–174 This wide spectrum is likely to play a role in both the efficacy and toxicity of the drug.
Ruxolitinib and baricitinib exhibits specificity for JAK1 and JAK2 over JAK3 in kinase assays and has shown efficacy in murine arthritis models.175 176 This wide spectrum of inhibition is likely to be responsible for the unwanted immunosuppression in patients with myelofibrosis treated with ruxolitinib and the unwanted anaemia in patients with RA treated with baricitinib.
Upadacitinib and filgotinib have been described as selective inhibitors for JAK1 over other JAK isoforms. Both inhibitors showed selectivity towards JAK1 and JAK2 over JAK3 and TYK2 in pure biochemical in vitro kinase assays, but more profound selectivity for JAK1 in cellular assays.172 177 In a rat model of arthritis, a comparative analysis of upadacitinib and tofacitinib revealed that increased selectivity of upadacitinib for JAK1 resulted in a reduced effect on reticulocyte deployment and NK cell depletion relative to its efficacy.177 A direct comparison of IL-7-induced pSTAT5 and IL-6-induced pSTAT3 of patients treated with these drugs from a phase I trial also revealed a higher selectivity of upadacitinib for JAK1 vs JAK3.178 In preclinical studies, filgotinib inhibited JAK1-related pathways with higher selectivity for JAK1 over JAK2 in whole blood, peripheral blood mononuclear cells (PBMCs) and in murine arthritis models.172–174
While different degrees of JAK isoform selectivity have been described for clinically approved drugs, it remains unclear how data derived from in vitro experiments and in vivo models reflect clinical usefulness, since little difference has been noted for efficacy or safety. Only limited studies are available that actually provide comparative functional analyses on JAKinib selectivity. A recent study compared the inhibitory effects of tofacitinib, baricitinib, upadacitinib and filgotinib on cytokine-induced STAT phosphorylation patterns in whole blood cells using clinically efficacious doses. Even though minor numerical differences in cytokine receptor inhibition were observed, the overall inhibition profiles were similar across studied JAKinibs.179 An additional in vitro pharmacological analysis compared the inhibitory effect of tofacitinib, baricitinib, upadacitinib in PBMCs. Although distinct pharmacological profiles for JAKinibs have been observed in this study, no continuous inhibition of JAKinibs on individual cytokine signalling pathways could be detected.179 180
The in vivo impact of pan versus selective JAKinibs was addressed by Moodley et al who performed comparative immunological, transcriptomic and epigenetic profiling of ex vivo isolated murine cells. Selective cell type specific effects of JAKinibs could be described; however, globally there was a high overlap between compared compounds.181
Importantly, JAK selectivity as detected in vitro by using recombinant enzymes or isolated cells may not necessarily reflect the in vivo selectivity, which is likely dependent on a large inter-individual variability of pharmacokinetic and pharmacodynamic aspects, based in part on pharmacogenomic effects on drug metabolism or tissue/cell sensitivity. Since most respective receptors use JAKs as heterodimers, it is currently not possible to understand differences in selectivity if any one of the JAK1/2, JAK1/3 or JAK2/3 heterodimers are inhibited. However, since cells of the haematopoietic system use JAK2 homodimers for signal transduction, a proxy for in vivo JAK2 inhibition constitutes the occurrence of anaemia or reversal of chronic anaemia in inflammatory states. Such in vivo effects may differ from in vitro data where the complexity of an organ system or a whole organism with its genetic, epigenetic or proteomic background is missing.
In summary, current experimental data do not allow drawing a clear conclusion of the potential advantages of a higher selectivity of next-generation JAKinibs. One still needs to learn which beneficial effects and which adverse events are associated with specific JAKinib characteristics. Thus, additional comparative experimental data of pan and selective JAKinibs on ex vivo isolated cells from clinical trial participants are needed as are head-to-head comparisons of JAKinibs with presumed differences in selectivity to understand the impact on safety and also efficacy.
Topical JAKinibs
Compared with systemically acting compounds, topically applied JAKinibs potentially have certain advantages. Key is a lower risk of potential side effects due to less systemic distribution when compared with oral administration. Thus, when used topically, pan-JAKinibs could be used for conditions in which systemic long-term treatment would not be an option due to safety concerns. Target areas for potential use of topical JAKinibs are similar to indications for topical glucocorticoid treatment, being the skin, the eyes, the gastrointestinal tract and the lungs. Efficient delivery of the compound to the target tissue is an essential prerequisite of topical JAKinib treatment. Most developments therefore focus on the skin, especially because the repertoire of anti-inflammatory drug classes that are in use for topical treatment of inflammatory skin diseases is limited to glucocorticoids, calcineurin inhibitors and vitamin D analogues. Here, formulations have to assure that the compound can penetrate into the skin and reach targets cells like keratinocytes or immune cells, which in most skin diseases are mainly located within the dermis. Hyperkeratotic skin lesions with thick epidermal layers and scaling make compound penetration more difficult. Cells and cytokines in immune-mediated skin diseases are well studied,182–184 leading to many clinical trials focusing on the efficacy and safety of JAKinibs in dermatology.185 While topical glucocorticoids belong to the most potent anti-inflammatory compound class, their long-term use ultimately leads to telangiectasia, striae, easy bruising, hypertrichosis and most importantly skin atrophy with subsequent wound healing deficits. Moreover, in some types of chronic skin inflammation like psoriasis, a rebound phenomenon typically appears after termination of topical glucocorticoids. Thus, alternative immunosuppressive agents like the class of JAKinibs that do not result in skin atrophy or telangiectasia may be advantageous and could, given equal or better efficacy and a more tolerable safety profile, widely replace topical glucocorticoids.
Topical JAKinibs have been tested in the setting of a variety of inflammatory skin conditions including AA, AD, chronic hand eczema, cutaneous GVHD, discoid lupus erythematosus, hidradenitis suppurativa, necrobiosis lipoidica, psoriasis and vitiligo, as summarised in table 3. Most of the JAKinibs tested in skin diseases are applied as creams. Exceptions include tofacitinib, which is applied in an ointment and ATI-502, which has been developed as a solution.
Table 3.
Clinical developmental stages of topical JAKinibs for skin diseases
| Disease | JAKi | Target | Route | Phase of development | Trial identifier |
| Alopecia areata | Ruxolitinib | JAK1/JAK2 | Topical | Phase II | NCT02553330 |
| Tofacitinib | JAK1/JAK3 | Topical | Phase II | NCT02812342 | |
| Ifidancitinib | JAK1/JAK3 | Topical | Phase II | NCT03759340 | |
| Atopic dermatitis | Ruxolitinib | JAK1/JAK2 | Topical | Phase III | NCT03745651 |
| Topical | Phase III | NCT03745638 | |||
| Topical | Phase I (paediatric) | NCT03257644 | |||
| Topical | Phase I | NCT03920852 | |||
| Delgocitinib | Pan-JAK | Topical | Phase II | NCT03725722 | |
| Topical | Phase I | NCT03826901 | |||
| Tofacitinib | JAK1/JAK3 | Topical | Phase II | NCT02001181 | |
| Brepocitinib | JAK1/TYK2 | Topical | Phase II | NCT03903822 | |
| Ifidancitinib | JAK1/JAK3 | Topical | Phase II | NCT03585296 | |
| Chronic hand eczema | Delgocitinib | Pan-JAK | Topical | Phase III | NCT04871711 |
| Topical | Phase III | NCT05355818 | |||
| Topical | Phase II | NCT02664805 | |||
| Cutaneous GVHD | Ruxolitinib | JAK1/JAK2 | Topical | Phase II | NCT03395340 |
| Topical | Phase II | NCT03954236 | |||
| Discoid lupus erythematosus | Delgocitinib | Pan-JAK | Topical | Phase II | NCT03958955 |
| Healthy | PF-06263726 | Pan-JAK | Topical | Phase I | NCT01981681 |
| Hidradenitis suppurativa | Ruxolitinib | JAK1/JAK2 | Topical | Phase II | NCT04414514 |
| Lichen planus | Ruxolitinib | JAK1/JAK2 | Topical | Phase II | NCT03697460 |
| Necrobiosis lipoidica | Ruxolitinib | JAK1/JAK2 | Topical | Phase II | NCT04492618 |
| Psoriasis | Ruxolitinib | JAK1/JAK2 | Topical | Phase II | NCT00820950 |
| Topical | Phase II | NCT00617994 | |||
| Topical | Phase II | NCT00778700 | |||
| Tofacitinib | JAK1/JAK3 | Topical | Phase II | NCT01831466 | |
| Topical | Phase II | NCT01246583 | |||
| Topical | Phase II | NCT00678561 | |||
| Topical | Phase I | NCT02193815 | |||
| PF-06700841 | JAK1/TYK2 | Topical | Phase II | NCT03850483 | |
| Vitiligo | Ruxolitinib | JAK1/JAK2 | Topical | Phase III | NCT04057573 |
| Topical | Phase III | NCT04052425 | |||
| Topical | Phase III | NCT04530344 | |||
| Topical | Phase II | NCT02809976 | |||
| Topical | Phase II | NCT03099304 |
GVHD, graft-versus-host disease; JAK, Janus kinase; JAKinibs, JAK-inhibitors.
The number on clinical trials or case series on the use of topical JAKinibs has increased over the last few years. One case series reports on the use of either tofacitinib ointment or ruxolitinib cream in paediatric patients with AA. Regrowth of hair was reported in four out of six patients.186 Both JAKinibs, tofacitinib and ruxolitinib as topical formulations are now studied in phase II trials for AA. A further JAKinib, ATI-502 as solution is also in phase II for AA. In two phase III trials oral baricitinib was superior to placebo with respect to hair regrowth in patients with severe AA.121 Topical tofacitinib has also been tested for AD. Results from a phase II trial showed significant improvement of AD clinical scores like the eczema area and severity index (EASI), physician global assessment and body surface area. Importantly, pruritus also improved when the JAK1/JAK3 inhibitor was applied to the skin. Of note, the median plasma tofacitinib concentrations detected were very low (0.31–0.70 ng/mL).187 Likewise, ruxolitinib showed clinical improvement in AD in a phase II study.188 Topical ruxolitinib was well tolerated and no safety concerns or clinically significant application-site reactions appeared when compared with vehicle control.188 Subsequently, ruxolitinib has completed two phase III studies (TRuE-AD1 and 2) each with >500 patients. Treatment success was seen in 50% of subjects taking the 1.5% ruxolitinib cream compared with 8%–15% in vehicle controls after 8 weeks.189 Consequently, ruxolitinib cream has been approved by the FDA for this condition.
A JAKinib with a novel three-dimensional spiro motif is delgocitinib.190 This compound seems to show improved physicochemical properties for local application and showed efficacy in skin inflammation in preclinical models.191 192 Delgocitinib has gone through phase I–III studies for patients with AD demonstrating significant improvement in the EASI score193 194 and has been approved in Japan for the treatment of AD. As recently published, the modified (m)EASI-50 was achieved by 51.0% of patients compared with 11.5% that received vehicle control and mEASI-75 was observed in 26.4% of treated patients compared with 5.8 with mEASI-75 response that received vehicle control treatment at week 4. The adverse events in patients that were treated with the topical JAKinib were reported to be mild and not related to the compound.194 Long-term safety data demonstrated the absence of skin atrophy or telangiectasia, typical side effects of skin applied glucocorticosteroids.195 In vitro studies showed blockade of JAK1-3 and TYK2 and therefore delgocitinib is considered as a pan-JAK inhibitor.196
Delgocitinib has been tested in patients with chronic hand eczema. A treatment period of 8 weeks achieved treatment success in 46% of patients receiving the pan-JAKinib in an ointment compared with 15% treated with the vehicle control during a phase II trial.197 A 16-week phase IIb trial confirmed the efficacy of delgocitinib for chronic hand eczema.198 First approval for the use of this topical pan-JAKinib for chronic hand eczema is expected. As reported for its use in AD, topical delgocitinib was generally well tolerated. A Japanese phase III trial demonstrated efficacy and safety of delgocitinib 0.5% ointment two times per day in patients with moderate-to-severe AD.194 Delgocitinib ointments with 0.25% or 0.5% were tested in paediatric patients with AD. Topical delgocitinib (Corectim; 0.25% and 0.5%) is approved in Japan for the treatment of children and adults with AD. The other advanced-stage topical JAKinib developed for the treatment of AD is ruxolitinib. Data from two phase III trials demonstrated EASI-75 and EASI-90 responses in 61.8%–62.1% and 43.4%–44.3% of patients, respectively at week 8 (vehicle control at week 8 showed 14.4%–24.6% EASI-75 and 4.2%–9.5% EASI-90 responders). The FDA-approved ruxolitinib (Opzelura) for the topical treatment of patients aged 12 years and older with AD. Other skin diseases, where topical JAKinibs are under phase II clinical investigation include cutaneous GVHD, discoid lupus, hidradenitis suppurativa, lichen planus and necrobiosis lipoidica.
In psoriasis, topical JAK1/2 inhibition improved lesion thickness, erythema and scaling compared with placebo. When testing the plasma, nanomolar concentrations (0.32–2.10 nmol/L) were detected in patients who received ruxolitinib.199 Topical ruxolitinib treatment of psoriatic plaques decreased factors related to IL-17 expressing T helper cell responses, dendritic cell activation and epidermal hyperplasia.200 The use of tofacitinib ointments in psoriasis is well tolerated and has been reported to lead to an improvement by 4–8 weeks of treatment with good tolerability.201 202
Several studies exist on the use of topical JAKinibs for the treatment of vitiligo, a skin disease characterised by a cytotoxic CD8+ T cell response towards melanocytes.183 Data from a phase II trial have been reported very recently. By measuring a 25% or higher improvement from baseline in facial vitiligo area scoring index (F-VASI), a significant number of patients treated with ruxolitinib cream reached improvement at week 24 compared with vehicle control cream.203 Phase III trials on ruxolitinib cream for vitiligo (TRuE-V1 and TRuE-V2) in patients 12 years of age and older confirmed the positive effects of JAK inhibition on skin repigmentation. F-VASI-75 responses at week 24 were 29.8% and 30.9% using 1.5% ruxolitinib cream two times per day compared with the vehicle control cream two times per day with 7.4% and 11.4% of patients achieving F-VASI-75.204 Common adverse events reported included application-site acne, nasopharyngitis and pruritus. While ruxolitinib cream (Opzelura) is already approved for the treatment of vitiligo by the FDA, EMA approval is pending.
Taken together, JAKinibs have the potential to become the modern anti-inflammatory topicals. They seem to be as effective as glucocorticoids and may replace them in the long-term run in terms of tolerability. Yet, topical JAKinibs need improvements in structure and penetration to show their efficacy in the skin. In some skin diseases, hyerproliferation and/or hyperkeratosis may limit their penetration as a deep penetration to, for example, hair follicular structures may be needed. Conversely, the success of JAKinibs as skin creams may be in part due to their enhanced absorption, which raises concerns about systemic absorption and related side effects. This has led to a new generation of topical JAKinibs that have enhanced tissue retention and minimal systemic absorption. LAS194046 and AZD0449, both inhaled JAKinibs, were shown to decrease allergic lung inflammation in rats.205 206 The JAK1 inhibitor AZD0449 has completed (NCT03766399) and is recruiting (NCT04769869) for phase I trials in humans. Within a double-blind, randomised, placebo-controlled, phase I proof-of-activity study in adults with mild asthma, the JAK1 inhibitor GDC-0214, used as an inhaled formulation, caused dose-dependent reductions in exhaled nitric oxide.207
JAKinibs and COVID-19
The SARS-CoV-2 was initially described as the cause of severe acute viral pneumonia in Wuhan, China, in December 2019 leading to a global pandemic. Infection by SARS-CoV2 results in a protean disease named COVID-19 that often results in a severe acute respiratory distress syndrome which frequently requires mechanical ventilation. Despite an association with lymphopenia, patients with severe COVID-19 often present signs of an immune hyper-responsiveness which involves the activation of different immune cells, such as T helper cells, macrophages, dendritic cells and neutrophils. This hyperactivation results in abnormally high levels of pro-inflammatory cytokines and chemokines known as cytokine release syndrome (CRS; also called cytokine storm) and has been known to underlie the pathology of viral infections, which had already been observed in the pathogenesis of SARS and the Middle East respiratory syndrome. These patients present with abnormally elevated plasma levels of cytokines such as IL-1β, IL-1RA, IL-2, IL-6, IL-7, IL-10, GM-CSF, IFNγ, TNFα as well as chemokines such as IL-8, IP-10, monocyte chemoattractant protein 1, macrophage inflammatory protein (MIP)1α and MIP1β.
Besides antiviral drugs, the search for drugs to be used in patients with COVID-19 immediately focused on modulators of the hypercytokinaemia as an attractive approach to reduce COVID-19 mortality rate. In particular, IL-6 appears to be a major driver of acute inflammation and elevated levels of IL-6 in patient plasma have been correlated to respiratory failure in patients with COVID-19208 and associated with increased risk of acute respiratory distress syndrome, myocardial damage and mortality. Elevated IL-6 is also seen in patients with cancer receiving either chimeric antibody receptor T cell therapy or immune check point inhibitors. Monoclonal antibodies against IL-6, such as tocilizumab and sarilumab, which are already used in those clinical settings, have been used in patients with COVID-19 to dampen the hyperinnate immune response observed in patients with severe COVID-19 with some degree of success.209–212 Beside monoclonal antibodies specifically targeting IL-6, approved drugs inhibiting IL-6/JAK/STAT signalling may represent a valuable tool. In particular, JAKinibs, such as baricitinib, tofacitinib, ruxolitinib and fedratinib have been reported to attenuate the host inflammatory response associated with massive pro-inflammatory cytokine and chemokine release.
Cell entry, the first step of SARS-CoV-2 infection, is mediated by the ACE2 receptor on host cells in lung epithelial cells as well as in other tissues including the oral mucosa, the gastrointestinal tract, kidney, heart and blood vessels. ACE2 receptor signalling is mediated by two members of the numb-associated kinase family, the adaptor protein 2-associated kinase 1 (AAK1) and the cyclin G-associated kinase. Among the many clinically approved kinase inhibitors, baricitinib has been predicted to have the highest affinity towards these two kinases. Of note, binding of some JAKinibs including ruxolitinib, baricitinib and fedratinib to AAK1 and BMP2K (Bike) had been previously shown and could be explained by conserved binding modes between numb-associated kinases and JAKs. In vitro experiments with tofacitinib suggested that this JAKinib did not possess the same inhibitory effects towards these other kinases.
Inhibition of the JAK-mediated signalling results in an impairment of IFN-driven responses including the antiviral response. Therefore, there are concerns on the use of these drugs which have been shown to effectively inhibit the expression of IFN-regulated genes for the management of COVID-19.
Infection of rhesus macaques with SARS-CoV2 showed that baricitinib treatment was associated with reduced pneumonia, inflammatory cytokine transcripts and reduction in lymphoid and myeloid cell infiltration. There was a reduction in neutrophil extracellular traps release as well as microvascular thrombosis.213 The first sizeable clinical open-label study has reported in 113 patients who received a 2-week treatment with oral baricitinib (4 mg/day) combined with antivirals (lopinavir/ritonavir) compared with 78 patients who received the standard of care (SOC) therapy (hydroxychloroquine and lopinavir/ritonavir). Notably, the 2-week case fatality rate was significantly lower in the baricitinib-arm compared with SOC-treated patients (0% (0/113) vs 6.4% (5/78)). Moreover, intensive care unit admission was also significantly reduced (0.88% (1/113) vs 17.9% (14/78)) in patients receiving baricitinib compared with SOC patients. With the exception of anosmia, all clinical, laboratory, including CRP levels, and respiratory functions significantly improved after 1 week and SpO2 significantly improved at week 2. Moreover, only few adverse effects (transaminases increase in four patients, urinary infection in one patient and oral candidiasis in one patient) were observed.214
In a randomised controlled trial, the Adaptive COVID-19 Treatment Trial (ACTT)-2,215 the combination of remdesivir plus baricitinib (515 patients) was compared with remdesivir alone (518 patients) in moderate-to-severe COVID-19. The primary outcome was time to recovery. Patients who received both baricitinib and remdesivir recovered after a median of 7 days compared with 8 days in controls. And greater benefit was observed in patients who received supplemental oxygen or non-invasive ventilation at baseline. Interestingly, the beneficial effect was less pronounced in patients who did not require oxygen or who were intubated. A larger ACTT-4 (NCT04640168) was also performed and completed in 2021. Baricitinib in combination with remdesivir was compared with dexamethasone and remdesivir. This study showed that the two interventions were comparable effective.216 Notably, no excess of thromboembolic events emerged from the ACCT-2 study with a similar incidence of thromboembolic events in both treatment arms. Given the findings reported by the ACCT-2 study, the FDA authorised an emergency use application for baricitinib usage in combination with remdesivir for patients with severe COVID-19, requiring supplemental oxygen, invasive mechanical ventilation or extracorporeal membrane oxygenation. Treatment with baricitinib in addition to SOC was associated with reduced mortality in hospitalised adults with COVID-19.217 218 Furthermore, the double-blind, placebo-controlled study, COV-BARRIER, showed the efficacy of adding baricitinib to the SOC to treat patients hospitalised with COVID-19 (NCT04421027). Adding baricitinib (4 mg dose) to the SOC did not achieve statistical significance in the primary end point—patient progression to high flow oxygen, invasive mechanical ventilation, including ECMO, or death. Nonetheless, a significant reduction (38%) in death from any cause in all groups receiving baricitinib was observed.219 Based on the above-metioned studies, the FDA approved baricitinib, as a monotherapy, for the treatment of patients with COVID-19 including children aged over 2 years requiring supplemental oxygen and non-invasive or invasive mechanical ventilation.
Tofacitinib has also shown superiority to placebo as a treatment for hospitalised patients with COVID-19 pneumonia (NCT04469114). Patients from 15 sites were randomised to tofacitinib or placebo along with local SOC, including use of glucocorticoids, antibiotics, anticoagulants and antiviral agents. Tofacitinib treatment significantly reduced the risk of death or respiratory failure over a 28-day period.220
In a mouse model of CRS, ruxolitinib attenuated T cell activation, cytokine production and several pathological features associated with the hypercytokinemia. IFNγ deficiency significantly protected mice from lethal CRS by attenuating small bowel pathology, whereas IL-17A deficiency significantly increased mortality by augmenting small bowel pathology.221 Efficacy and safety of ruxolitinib was reported in a phase II clinical trial,222 although the primary end point was not met.
Overall, we still have an incomplete knowledge of the effects of SARS-CoV-2 infection, the role that cytokines and IFNs have in the context of the pathology and the balance between positive and negative aspects of the JAK-mediated signalling cascades. Limited and controversial data have been reported on the role of JAKinibs on incidence and severity of COVID-19 infection in patients under JAKinib treatment.223 224
Why are JAKinibs so efficacious?
Due to their central role in cytokine receptor signalling (figure 1), participating in a broad array like IL-6, IL-2, IL-12/23 and IFNs it is clear that JAKinibs impact multiple pivotal functions, including antiviral properties. Thus, in contrast to the focused activity of TNF inhibitors on a single inflammatory key factor, JAKinibs exert their efficacy not by their capacity to inhibit different cytokines at the same time, but rather by their potential to interfere with the signalling of cytokines that are differentially involved in the pathogenesis of particular diseases. Indeed, when we look at figure 1 and figure 3 in tandem, we can assume that JAKinibs have efficacy in RA due to their interference with IL-6 signalling, in PsO, PsA and IBD due to their inhibition of the IL-23 pathway and thus generation of Th17 cells. Their side-effect profile (eg, anaemia, HZ), though, may be due to the simultaneous inhibition of signalling by IFNs and growth factors. Indeed, similar to the above-mentioned combination of bDMARDs targeting different pathways, the efficacy of JAKinibs does not appear to exceed that of the most efficacious bDMARDs, but their safety profile is different and includes adverse events not commonly seen on treatment with individual bDMARDs. Indeed, at higher doses of JAKinibs, which were tested in phase III trials, such as 10 mg two times per day of tofacitinib or 30 mg once daily of upadacitinib, the benefit-risk profile was not acceptable, just as seen for DMARD combinations.
Safety of approved JAKinibs
Since the JAK-STAT pathway is used by a wide array of hormones, growth factors, colony-stimulating factors and cytokines, its function is pleiotropic. Consequently, blockade of the JAK-STAT pathway leads to a number of predictable side effects. Although evidence from clinical trials in RA,225 psoriasis226 and IBD91 support an acceptable benefit-risk profile, one must also consider off-target binding at higher doses, as well as idiosyncratic drug hypersensitivity, drug allergies and drug-drug interactions.227 Safety concerns include effects on haematopoiesis, innate and adaptive host defence as well as cell growth; overall though, large studies have demonstrated an acceptable safety profile for many (but not all) patient populations investigated228–230 (figure 5).
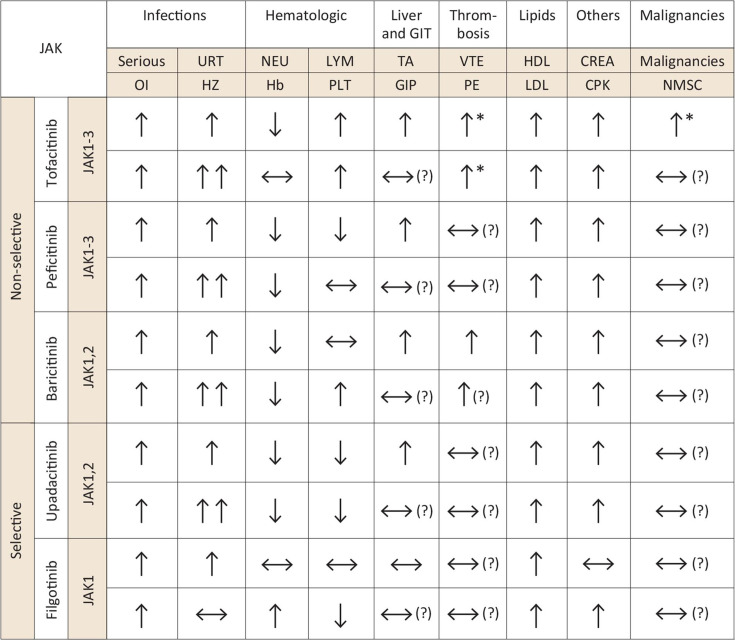
Figure 5.
Side effects of selective versus non-selective JAKinibs. *In patients with cardiovascular or VTE risk factors at baseline. Arrows indicate the respective adverse event risk compared with placebo treatment with an slightly (↑), highly (↑↑), lower (↓) or similar (↔) risk. Question marks in brackets highlight areas of uncertainty, especially for safety events that need exploration in large observational studies. JAK, Janus kinase; HZ, herpes zoster; CREA, creatinine; URT, upper respiratory tract; NEU, neutrophils; LYM, lymphocytes; Hb, haemoglobin; PLT, platelets; TA, transaminases; GIT, gastrointestinal tract; GIP, gastrointestinal perforations; VTE, venous thromboembolism; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CPK, creatine phosphokinase; NMSC, non-melanoma skin cancer; OI, opportunistic infection.
Tofacitinib, the first JAKinib licensed for indications outside of cancer treatment, was approved for RA in 2012 by the FDA but not until 2017 by EMA. The approval by the FDA was contingent on a phase IIIb/IV study to monitor all adverse effects associated with tofacitinib therapy. This was named the ORAL-SURVEILLANCE study, which has recently published its findings. The trial included 4362 patients with moderate-to-severe RA despite previous MTX treatment, who were above 50 years of age and had at least one additional cardiovascular (CV) risk factor. The participants were randomly assigned to receive tofacitinib 5 mg or 10 mg two times per day or a TNF inhibitor (either adalimumab or etanercept) and were followed for up to 6 years. The trial’s co-primary end points of non-inferiority of tofacitinib versus TNF inhibitor in major adverse cardiovascular events (MACEs) and cancer was not met. A higher risk of developing MACE was reported with an HR with any dose of tofacitinib versus TNF inhibitors of 1.33 (95% CI 0.91 to 1.94) resulting in a number needed to harm (NNH) of 412 (567 for TOFA5 two times per day and 319 for TOFA10 two times per day) and for developing cancer (excluding non-melanoma skin cancer (NMSC)), with an HR of 1.48 (95% CI 1.04 to 2.09) and an NNH of 275 (276 and 275 for for TOFA5 two times per day and TOFA10 two times per day, respectively) both crossing the predefined upper 95% CI of 1.8.231
Based on the ORAL-SURVEILLANCE study, the FDA determined in late 2021 that there is an increased risk of serious heart-related events such as heart attack or stroke, cancer, blood clots and death for patient treated with tofacitinib, assumed most JAKinibs as functionally equivalent and requested new and updated safety data for baricitinib and upadacitinib. The FDA also determined that JAKinibs should only be used after one or more TNF inhibitors have failed or are contraindicated.
In early 2022, the task force developing an update of the EULAR recommendations for the management of RA also evaluated the data of the ORAL-SURVEILLANCE trial which warranted a change compared with the 2019 version.232–234 First, the EULAR Task Force took into account that data for other JAKinibs than tofacitinib do not exist beyond registers or rather long-term extensions of trials and, therefore, one cannot exclude that a similar risk might also be observed with other JAKinibs. On the other hand, only patients with defined risk factors have been studied in ORAL-SURVEILLANCE, while registry data and LTEs of trials did not show any differences between anti-TNFs and JAKinibs in general RA populations. Based on these assessments, JAKinibs were separated from bDMARDs in the respective element and it was recommended that in patients with RA with IR to csDMARDs, JAKinibs may be used only after assessment of defined risk factors for MACEs, venous thromboembolism (VTE) and malignancy; these risk factors are then listed (see also below).
In October 2022, based on a review conducted by the Pharmacovigilance Risk Assessment Committee, EMA has concluded that the identified risks apply to all JAKinibs approved for the treatment of chronic inflammatory disorders such as RA, PsA, JIA, axial spondyloarthritis, UC, AD and AA. EMA recently recommended that in patients aged 65 years or above, those at increased risk of major CV diseases (heart attack or stroke), those who smoke and those at increased risk of cancer, JAKinibs should be used with caution and only if no suitable alternatives exist. JAKinibs should also be used with caution in patients with risk factors for VTE. Furthermore, doses should be reduced in patient groups who are at risk of VTE, cancer or major CV problems, where possible.
The profile of newer JAKinibs appear comparable with possible differences in infection rates, and haemoglobin changes. Rates in herpes zoster (HZ) infections appeared different (and without any increase compared with placebo or TNF inhibitor treatment) in randomised controlled trials investigating filgotinib, while other JAKinibs do show increased HZ rates. However, the interpretability of safety signals derived from clinical drug development programmes is limited for several reasons: (1) most trials include a selective patient population (not representative of the general population or the patient population in clinical routine), (2) even large randomised controlled trials provide relatively small patient numbers and (3) relatively short observation periods. In the light of usage of these compounds in potentially multimorbid patients with chronic IMIDs that may demand life-long therapy, the identification of rare safety signals is increasingly challenging.231 In an integrated safety analysis of the long-term extension studies in patients with RA, PsA, AD and AS treated with upadacitinib, comprising >6000 patients and 15 000 patient-years of exposure, the rates of malignancies (excluding NMSC), MACE and VTE was similar between upadacitinib and the active comparators adalimumab and MTX, respectively. Increased rates of HZ were observed in the RA and PsA population, whereas NMSC, serious infections and opportunistic infections were observed to be more frequent in upadacitinib (compared with adalimumab)-treated patients in PsA.235 Additional long-term safety studies are necessary to find definite conclusions regarding safety profiles of pan versus selective JAKinibs. This cannot be emphasised enough when discussing the safety of JAKinibs with our current experience.
Infections rates
The most common infections in clinical trials from patients with RA included nasopharyngitis, upper respiratory infections, gastroenteritis or bronchitis. Increased infection rates were observed in a systematic review investigating safety events, especially HZ, tuberculosis, cellulitis, panniculitis, septic shock and osteomyelitis.236 A higher risk for opportunistic infection, primarily owing to HZ infections were observed for patients treated with tofacitinib as compared with TNF inhibitors.231 Accordingly, most recent EULAR recommendations consider the use of HZ vaccinations for patients with rheumatic diseases.237 238 Recent reports showed that the risk of serious and fatal infections was further increased in elderly patients above 65 years of age.239 Therefore, the EMA recommended that tofacitinib should only be considered in these patients if no suitable alternative treatment is available.
The risk for developing HZ may be further influenced by concomitant use of glucocorticoids or MTX and also higher rates in certain populations, as clinical JAKinibs studies in Asian patients suggest.225 240–244 The exact mechanism remains unclear but in part may be explained by the importance of JAK-dependent cytokines in driving the development and functions of NK cells, which are important for controlling viral infections, although NK cell counts are not markedly reduced in patients treated with JAKinibs. Reduced IFNγ activity and subsequent reduced activity of neutrophils may explain an increased rate of oral candidiasis.
Nephropathy
A larger multicentre clinical trial also showed a higher incidence (14%–18%) of BK virus-associated nephropathy in renal transplant recipients treated with tofacitinib compared with ciclosporin (6%)245 246 also in combination with mycophenolate mofetil and at relatively high doses. High dose of baricitinib was also associated with BK nephropathy and BK viraemia in patients with genetic autoinflammatory disease.247 Elevations of creatinine have been observed under JAKinib treatment but have not been associated with renal failure or other clinical sequelae.248 249
Gastrointestinal perforation
Possible increased risk of gastrointestinal perforations was recognised in patients with RA treated with tofacitinib (all treated with glucocorticoids or NSAIDs).225 In August 2020, regulators in the UK have issued a warning regarding an increased risk of diverticulitis based on increased rates of diverticulitis with several patients experiencing intestinal perforations in clinical trials and postmarketing studies. Numerically higher rates of gastrointestinal perforations were observed in three upadacitinib studies compared with placebo.129 132 133
Risk of thrombotic adverse events
Epidemiological studies have shown that patients with RA are in general at risk of VTE compared with control populations.250 251 Therefore, concerns have been raised as to whether the usage of JAKinibs in RA further increases that risk. In ORAL-SURVEILLANCE patients treated with 10 mg tofacitinib two times per day showed an increased risk for developing VTE and pulmonary embolism (PE), whereas no increased risk was observed for 5 mg tofacitinib two times per day.231 History of VTE, use of oral contraceptives, GC use, increased body mass index, antidepressant use, male gender and age above 65 years were associated factors with VTE/PE, while usage of protone pump inhibitors appeared protective. A large cohort study in >50 000 patients comparing tofacitinib versus TNF inhibitors found a numerically higher, but statistically non-significant, risk of development of VTE.252
A post hoc analysis of safety data from large populations of patients with RA, PsO and PsA treated with tofacitinib assessed the risk of VTE and arterial thromboembolism (ATE), including analyses stratified by the baseline CV or VTE risk factors. Integrated safety analysis across the whole tofacitinib development programme suggested an increased risk for VTE, PE and ATE in patients with pre-existing CV and VTE risk factors.253
Curiously, the association of VTE/PE risk in patients with myeloproliferative disease receiving ruxolitinib suggests a protective effect of JAK inhibition. Myeloproliferative disease carries a significant risk of VTE/PE that is thought to be related to increased blood viscosity associated with a raised haematocrit.254 There is a mixed evidence that the presence of JAK2 mutations increases this risk254 and conversely equally mixed evidence that risk of VTE is decreased by ruxolitinib.255 This may be a reflection on its use in a different indication/patient group or due to the drug itself.
A recent multidatabase analysis comparing baricitinib with TNF inhibitors identified an increased risk for VTE (incidence rate ratio: 1.51; 95% CI 1.10 to 2.08) in baricitinib-treated patients,256one of the reasons for FDA to approve only the lower dose of baricitinib (2 mg/day) for the treatment of RA, while in most other regulatory areas the 4 mg dose is also approved. However, the results of this study were mainly driven by one of the registries and not observed by others. A randomised prospective study is currently being conducted to adequately address this question.
As yet, no clear signal for VTE/PE in upadacitinib and filgotinib trials was observed. However, package labels include warnings especially for patients with risk factors for VTE. While the JAKinibs mechanism of action leading to thromboembolism remains unclear, similar signals have been identified in multiple members of the family. If JAK selectivity plays a role remains an open debate and specifically designed safety studies comparing selective and unselective JAKinibs in a head-to-head setting are needed to evaluate risk differences of VTEs.
Haematological adverse events
Given that many haematopoietic growth factors including EPO, TPO and G-CSF signal through JAK2, changes in laboratory parameters are not unexpected. Anaemia was reported in patients treated with ruxolitinib, baricitinib, upadacitinib and peficitinib.257 While small changes in haemoglobin levels were observed in a pooled analysis of tofacitinib patients on a group level, only few patients experienced clinically meaningful haemoglobin changes.258
An inverse correlation was observed for the increase in haemoglobin and disease activity, suggesting that reduction of inflammation counterbalances the minor negative effects of tofacitinib in erythropoiesis.258 A possible reason for a smaller increase in haemoglobin with tofacitinib 10 mg two times per day is a dose-associated inhibition of JAK2, which is not observed at the lowest 5 mg two times per day dose. The greater decrease in haemoglobin levels in ruxolitinib-treated and baricitinib-treated patients as compared with tofacitinib-treated patients might in part be explained by their potent inhibition of JAK2. Reductions in haemoglobin levels seem to be dose dependent and only rarely clinically significant.259 Of note, however, it is a question of inducing anaemia and a question of reversing anaemia of chronic disease, which allows to draw conclusions on JAK2 inhibition. Anaemia is not reversed with tofacitnib, bacricitinib, peficitinib or upadacitnib, suggesting in vivo JAK2 inhibition by all these drugs. Only filogitinib improved haemoglobin levels in clinical phase II and phase III trials, and no increased incidence of anaemia was observed in patients treated with filgotinib.144 146
JAK1 and JAK3 play an essential role in lymphocyte survival and maturation and therefore all JAKinibs have been associated with lowered lymphocyte counts. Monitoring of lymphocyte counts is recommended since lymphopenia was associated with a slightly higher overall infection rate and therefore JAKinib should be interrupted when lymphocyte count is <1.0×109 cells/L. Thrombocytosis reflects disease activity in patients with RA and suppression of inflammation should reduce platelet number. While treatment with tofacitinib is associated with a decrease in platelets, an early increase in thrombocytes on baricitinib was observed, but did not appear to be associated with an increased risk for VTEs. In contrast to rheumatological patients, the use of JAKinibs in haematological patients is associated with a higher incidence of cytopenias. However, as all FDA-approved JAKinibs are competitive antagonists their effect can be overcome by pharmacological doses of cytokines. Thus, it is possible to use EPO, TPO and G-CSF to reverse the cytopenias associated with JAKinib use but maintain their effectiveness as immunosupressants in this patient group.
Malignancies
One particular concern with long-term suppression of the JAK/STAT pathway is the possible development of malignancies. Both type I and II IFNs play an important role in the process of immunoediting, which is critical for the antitumour immune response. In post-transplant patients treated with tofacitinib, the risk of lymphoproliferative malignancy was increased by JAK inhibition. However, it has to be considered that the doses of tofacitinib used in these trials were higher than the approved dose for RA and that the patients were also treated with concomitant immunosuppressants. Phase II and phase III trials for autoimmune diseases did not show an increased cancer risk associated with tofacitinib treatment.260 The risk to develop lymphoma or other malignancies based on data from the RA clinical trial data and pooled analysis of UC studies was low.243 261 However, data from ORAL-SURVEILLANCE (see above) showed an increased risk of malignancies (excluding NMSC) in tofacitinib-treated patients, receiving either the approved dose of tofacitinib 5 mg two times per day or 10 mg two times per day in comparison to TNF inhibitors (etanercept or adalimumab)-treated patients. All patients included in the study were on stable background DMARDs when entering the trial. Patients receiving tofacitinib 10 mg two times per day were switched to 5 mg two times per day as a result of a protocol modification in 2019 due to safety concerns of the 10 mg two times per day dosing. Non-inferiority was not demonstrated, as the upper limit of the HR’s 95% CI crossed the predefined margin of 1.8. HR for cancer (excluding NMSC) were 1.48 (1.04 to 2.09) in the combined tofacitinib group. HRs for tofacitinib 5 mg two times per day and 10 mg two times per day arms (vs TNF inhibitor) were 1.47 (1.00 to 2.18) and 1.48 (1.00 to 2.19), respectively. The most common malignancies observed were lung cancer and lymphoma. Also, non-adjudicated rates of melanoma skin cancer were higher in the tofacitinib arms.231 Consequently, a warning regarding the use of JAKinibs in patients with a risk of malignancy, especially smokers and previous smokers, has been raised.
Lipid profile and cardiovascular adverse events
Elevation of serum lipids have been seen in patients with RA receiving IL-6 receptor blocker tocilizumab.262 IL-6 is known to lead to insulin resistance and to support the redistribution of fatty acids from the blood to peripheral tissues which can cause low serum levels of low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglycerides. Accordingly, increase in LDL, HDL were observed in patients treated with approved JAKinibs and may be secondary to blocking of IL-6 signalling. However, long-term extension studies have not shown a higher incidence of CV events.225 Moreover, the ENTRACTE trial, which had a study design similar to ORAL-SURVEILLANCE, did not show any difference between tocilizumab and anti-TNF regarding MACEs263; this implies that the data seen in ORAL-SURVEILLANCE are unlikely to have been mediated by the inhibition of IL-6 signalling by tofacitinib. Elevation of serum lipids have been observed by 12 weeks and are generally stable thereafter with an incidence of CV events similar to placebo. LDL and HDL ratio remained stable after 24 months.225 264–268 Treatment with atorvastatin has been shown to be effective in patients with elevations in cholesterol on tofacitinib.269 Only minor, not clinically meaningful elevations of lipid levels were observed in filgotinib-treated patients in a pooled safety analysis of phase III studies.238 According to an international consensus, lipid levels should be assessed every 3 months in patients treated with JAKinibs and if increased, managed according to national guidelines.270
Regarding major adverse CV events, recent findings from the ORAL-SURVEILLANCE postmarketing trial suggested that tofacitinib was associated with higher rates of MACE than TNF inhibitors in patients with RA at high CV risk. Incidence rates of MACE, defined as CV death, non-fatal myocardial infarction or non-fatal stroke, were 0.91 for patients receiving tofacitinib 5 mg two times per day, 1.05 for those treated with tofacitinib 10 mg two times per day and 0.73 per 100 person-years for those treated with a TNF inhibitor. The estimated HR for occurrence of MACE with any dose of tofacitinib relative to the TNF-inhibitor group was 1.33 (95% CI 0.91 to 1.94).231 In an exploratory post hoc analysis of ORAL-SURVEILLANCE, it was suggested that the increased MACE risk appears to be markedly higher in patients with RA with a history of atherosclerotic CV disease (ie, coronary artery disease, cerebrovascular disease or peripheral artery disease), compared with patients without a history of atherosclerotic CV disease.271
In a large multidatabase, population-based study including 102 263 patients with RA, tofacitinib was not associated with an overall risk of composite CV outcome compared with TNF inhibitors treated in the real-world setting. However, in patients with baseline CV risk factor or history of CV disease tofacitinib is associated with an elevated risk of CV events.272 More data for baricitinib, upadacitinib or filgotinib are required to address similar safety aspects due to shared mechanisms of action with tofacitinib. A phase IV study, comparing the safety of baricitinib versus TNF inhibitors with respect to venous VTEs when given to participants with RA is currently ongoing (NCT03915964).
Potential teratogenicity and fertility
It is reasonable to assume that JAKinibs cross the placenta from the beginning of pregnancy. In animal studies, tofacitinib was teratogenic and feticidal when used at doses several times higher than those used in humans.273 No fetal deaths or congenital malformations were observed in the clinical development programmes for RA, psoriasis or IBD.274 275 However, the safety of JAKinibs during pregnancy or breast feeding has not been well established in larger cohorts and, therefore, their use in these patient populations should currently be avoided. No data are available on breast feeding with JAKinibs. However, small molecules are present in lactating rat milk, therefore breast feeding should be avoided. In preclinical animal studies, an effect of filgotinib treatment on spermatogenesis was observed, which was not seen with other JAKinibs. Studies investigating this effect (NCT03926195, NCT03201445) remain unpublished at the timepoint of writing this manuscript, however, a press release stated that no increased risk for impaired spermatogenesis was observed in two randomised controlled trials (MANTA and MANTA-RAY).276
Other laboratory variables
A randomised controlled trial assessed changes in serum creatinine and glomerular filtration rate in patients with RA. Tofacitinib-treated patients with RA showed a mild increase in creatinine levels and a decrease in glomerular filtration rate, which reversed on drug discontinuation.228 249 Slight elevations in serum creatinine rates have been observed across all JAKinib studies but were not associated with nephropathic changes or a clinical correlate leading to end-stage renal disease. Slight elevations of transaminases have been observed for all the approved JAKinibs, except for filgotinib. Abnormalities resolved after reduction or discontinuation. However, monitoring of liver function tests after initiation and during JAKinib treatment is recommended. Creatine phosphokinase elevations have been noted with all JAKinibs but have generally been asymptomatic and did not lead to rhabdomyolysis.
Conclusion and future perspectives
Inhibition of type I/II cytokine signalling through the use of JAKinibs has been a great success in the treatment of a variety of autoimmune diseases and haematological malignancies and in the increasingly recognised cytokine release syndromes driven by the use of cancer immunotherapies, the appearance of COVID-19 and other macrophage activation syndromes.
However, the use of JAKinibs has been limited by adverse events, both when applied as a monotherapy and in combination with other immuno-modulatory agents. For certain adverse events, such as MACE, malignancy and VTE, patients at risk can be identified, and for others, such as HZ, prevention by vaccination should be implemented. Regarding combination of therapy, the best regimens are yet to be fully established for some of the diseases—for RA it is usually combination with MTX.
Despite an increasing amount of work in the recent years, the importance of selectivity for an effective treatment response and also regarding reduction of adverse events still remains unclear. The introduction of increased selectivity of JAKinibs has hitherto resulted only in a limited reduction of adverse effects. Highly specific inhibitors of JAK1 are still able to block many families of cytokines and highly specific inhibitors of other JAKs have been less successful therapeutically. Nevertheless, there may be some interesting differences in the safety profile of highly selective versus less selective agents, but this still needs to be ascertained. Moreover, TYK2 inhibitors appear efficacious in certain diseases and may exhibit a different safety profile compared with JAK1, 2, 3-inhibiting agents, thus holding some promise in the future for certain disorders.
The use of topical JAKinibs either as creams in dermatology or inhalers in respiratory medicine offers an alternative strategy to overcome side effects of this group of drugs. Even when used systemically, JAKinibs exert their effects rapidly and are quickly cleared from the body. This may lead to their use as short-term agents during the early stages of transplantation or prior to the use of a more slowly acting biological therapy.
While we have made much progress and gained many insights regarding JAKs and their inhibition over the last decade, many unanswered questions remain in this rapidly progressing field and await further elucidation. These questions include, but are not limited to: which JAKs drive protection from HZ? Which JAKs are responsible for protection from clotting and MACEs? Which JAKs protect from malignancies? Which specific pathway are targeted by the same JAKinibs in different diseases? Answers to these questions will allow for a safer use of JAKinibs, and may provide clues for novel therapies against thrombosis and the development of malignancies.
Footnotes
Handling editor: David S Pisetsky
Contributors: All authors contributed to the manuscript.
Competing interests: JSS is main editor of the Annals of the Rheumatic Diseases.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Shuai K, Liu B. Regulation of JAK–STAT signalling in the immune system. Nat Rev Immunol 2003;3:900–11. 10.1038/nri1226 [DOI] [PubMed] [Google Scholar]
- 2. O’Shea JJ, Ma A, Lipsky P. Cytokines and Autoimmunity. Nat Rev Immunol 2002;2:37–45. 10.1038/nri702 [DOI] [PubMed] [Google Scholar]
- 3. Leonard WJ, O’Shea JJ. And Stats: biological implications. Annu Rev Immunol 1998;16:293–322. 10.1146/annurev.immunol.16.1.293 [DOI] [PubMed] [Google Scholar]
- 4. O’Shea JJ, Holland SM, Staudt LM. Jaks and Stats in immunity, immunodeficiency, and cancer. N Engl J Med 2013;368:161–70. 10.1056/NEJMra1202117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rane SG, Reddy EP. Jak3: a novel JAK kinase associated with terminal differentiation of hematopoietic cells. Oncogene 1994;9:2415–23. [PubMed] [Google Scholar]
- 6. Babon JJ, Liau NPD, Kershaw NJ. Jak1 takes a FERM hold of type II cytokine receptors. Structure 2016;24:840–2. 10.1016/j.str.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 7. Glassman CR, Tsutsumi N, Saxton RA, et al. Structure of a Janus kinase cytokine receptor complex reveals the basis for dimeric activation. Science 2022;376:163–9. 10.1126/science.abn8933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Philips RL, Wang Y, Cheon H, et al. The JAK-STAT pathway at 30: much learned, much more to do. Cell 2022;185:3857–76. 10.1016/j.cell.2022.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of JAK–STAT signaling in the immune system. Nat Immunol 2017;18:374–84. 10.1038/ni.3691 [DOI] [PubMed] [Google Scholar]
- 10. Levy DE, Darnell JE. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002;3:651–62. 10.1038/nrm909 [DOI] [PubMed] [Google Scholar]
- 11. McBride KM, Banninger G, McDonald C, et al. Regulated nuclear import of the Stat1 transcription factor by direct binding of Importin-alpha. EMBO J 2002;21:1754–63. 10.1093/emboj/21.7.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sekimoto T, Yoneda Y. Nuclear import and export of proteins: the molecular basis for intracellular signaling. Cytokine Growth Factor Rev 1998;9:205–11. 10.1016/s1359-6101(98)00012-4 [DOI] [PubMed] [Google Scholar]
- 13. O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention*. Annu Rev Med 2015;66:311–28. 10.1146/annurev-med-051113-024537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vahedi G, Takahashi H, Nakayamada S, et al. Stats shape the active enhancer landscape of T cell populations. Cell 2012;151:981–93. 10.1016/j.cell.2012.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leonard WJ, Lin J-X, O’Shea JJ. The Γc family of Cytokines: basic biology to therapeutic ramifications. Immunity 2019;50:832–50. 10.1016/j.immuni.2019.03.028 [DOI] [PubMed] [Google Scholar]
- 16. Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by Γc family Cytokines. Nat Rev Immunol 2009;9:480–90. 10.1038/nri2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnston JA, Kawamura M, Kirken RA, et al. Phosphorylation and activation of the Jak-3 Janus kinase in response to Interleukin-2. Nature 1994;370:151–3. 10.1038/370151a0 [DOI] [PubMed] [Google Scholar]
- 18. Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFNα signaling, although it is required for IL-12-mediated T cell function. Immunity 2000;13:561–71. 10.1016/s1074-7613(00)00055-8 [DOI] [PubMed] [Google Scholar]
- 19. Heinrich PC, Behrmann I, Müller-Newen G, et al. MüLLER-NEWEN G, et Al. Interleukin-6-type cytokine signalling through the Gp130/JAK/STAT pathway. Biochem J 1998;334 (Pt 2)(Pt 2):297–314. 10.1042/bj3340297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chua AO, Chizzonite R, Desai BB, et al. Expression cloning of a human IL-12 receptor component. A new member of the cytokine receptor Superfamily with strong Homology to Gp130. The Journal of Immunology 1994;153:128–36. 10.4049/jimmunol.153.1.128 [DOI] [PubMed] [Google Scholar]
- 21. Bacon CM, McVicar DW, Ortaldo JR, et al. Interleukin 12 (IL-12) induces tyrosine Phosphorylation of Jak2 and Tyk2: differential use of Janus family tyrosine Kinases by IL-2 and IL-12. J Exp Med 1995;181:399–404. 10.1084/jem.181.1.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghoreschi K, Laurence A, O’Shea JJ. Janus Kinases in immune cell signaling. Immunol Rev 2009;228:273–87. 10.1111/j.1600-065X.2008.00754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunter CA. New IL-12-family members: IL-23 and IL-27, Cytokines with divergent functions. Nat Rev Immunol 2005;5:521–31. 10.1038/nri1648 [DOI] [PubMed] [Google Scholar]
- 24. Arai KI, Lee F, Miyajima A, et al. Cytokines: Coordinators of immune and inflammatory responses. Annu Rev Biochem 1990;59:783–836. 10.1146/annurev.bi.59.070190.004031 [DOI] [PubMed] [Google Scholar]
- 25. Meraz MA, White JM, Sheehan KC, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK–STAT signaling pathway. Cell 1996;84:431–42. 10.1016/s0092-8674(00)81288-x [DOI] [PubMed] [Google Scholar]
- 26. Schindler C, Plumlee C. Inteferons pen the JAK–STAT pathway. Semin Cell Dev Biol 2008;19:311–8. 10.1016/j.semcdb.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schindler C, Levy DE, Decker T. JAK-STAT signaling: from Interferons to Cytokines. J Biol Chem 2007;282:20059–63. 10.1074/jbc.R700016200 [DOI] [PubMed] [Google Scholar]
- 28. Shuai K, Schindler C, Prezioso VR, et al. Activation of transcription by IFN-gamma: tyrosine Phosphorylation of a 91-kD DNA binding protein. Science 1992;258:1808–12. 10.1126/science.1281555 [DOI] [PubMed] [Google Scholar]
- 29. Fickenscher H, Hör S, Küpers H, et al. The Interleukin-10 family of Cytokines. Trends Immunol 2002;23:89–96. 10.1016/s1471-4906(01)02149-4 [DOI] [PubMed] [Google Scholar]
- 30. Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and Nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 1998;93:373–83. 10.1016/s0092-8674(00)81166-6 [DOI] [PubMed] [Google Scholar]
- 31. Eletto D, Burns SO, Angulo I, et al. Biallelic Jak1 mutations in immunodeficient patient with Mycobacterial infection. Nat Commun 2016;7:13992. 10.1038/ncomms13992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Del Bel KL, Ragotte RJ, Saferali A, et al. Jak1 gain-of-function causes an Autosomal dominant immune Dysregulatory and Hypereosinophilic syndrome. J Allergy Clin Immunol 2017;139:2016–20. 10.1016/j.jaci.2016.12.957 [DOI] [PubMed] [Google Scholar]
- 33. McIntosh LA, Marion MC, Sudman M, et al. Genome‐Wide Association Meta‐Analysis reveals novel juvenile idiopathic arthritis susceptibility Loci. Arthritis Rheumatol 2017;69:2222–32. 10.1002/art.40216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park SY, Baek S, Kim S, et al. Clinical significance of asthma clusters by longitudinal analysis in Korean asthma cohort. PLoS One 2013;8:e83540. 10.1371/journal.pone.0083540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akada H, Akada S, Hutchison RE, et al. Critical role of Jak2 in the maintenance and function of adult hematopoietic stem cells. Stem Cells 2014;32:1878–89. 10.1002/stem.1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu K, Hou S, Jiang Z, et al. Jak2 and Stat3 Polymorphisms in a Han Chinese population with Behcet’s disease. Invest Ophthalmol Vis Sci 2012;53:538–41. 10.1167/iovs.11-8440 [DOI] [PubMed] [Google Scholar]
- 37. Sic H, Speletas M, Cornacchione V, et al. An activating Janus Kinase-3 Mutation is associated with cytotoxic T lymphocyte Antigen-4-dependent immune dysregulation syndrome. Front Immunol 2017;8:1824. 10.3389/fimmu.2017.01824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lesmana H, Popescu M, Lewis S, et al. Germline gain-of-function Jak3 Mutation in familial chronic lymphoproliferative disorder of NK cells. Blood 2020;136(Supplement 1):9–10. 10.1182/blood-2020-142078 [DOI] [Google Scholar]
- 39. Tao J-H, Zou Y-F, Feng X-L, et al. Meta-analysis of Tyk2 gene Polymorphisms association with susceptibility to autoimmune and inflammatory diseases. Mol Biol Rep 2011;38:4663–72. 10.1007/s11033-010-0601-5 [DOI] [PubMed] [Google Scholar]
- 40. Li Z, Gakovic M, Ragimbeau J, et al. Two rare disease-associated Tyk2 variants are Catalytically impaired but signaling competent. J Immunol 2013;190:2335–44. 10.4049/jimmunol.1203118 [DOI] [PubMed] [Google Scholar]
- 41. Diogo D, Bastarache L, Liao KP, et al. Tyk2 protein-coding variants protect against rheumatoid arthritis and Autoimmunity, with no evidence of major pleiotropic effects on non-autoimmune complex traits. PLoS One 2015;10:e0122271. 10.1371/journal.pone.0122271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peluso C, Christofolini DM, Goldman CS, et al. Tyk2 Rs34536443 polymorphism is associated with a decreased susceptibility to Endometriosis-related infertility. Hum Immunol 2013;74:93–7. 10.1016/j.humimm.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 43. Boisson-Dupuis S, Ramirez-Alejo N, Li Z, et al. Tuberculosis and impaired IL-23–dependent IFN-Γ immunity in humans homozygous for a common Tyk2 Missense variant. Sci Immunol 2018;3:eaau8714. 10.1126/sciimmunol.aau8714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kreins AY, Ciancanelli MJ, Okada S, et al. Human Tyk2 deficiency: Mycobacterial and viral infections without hyper-IGE syndrome. J Exp Med 2015;212:1641–62. 10.1084/jem.20140280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lv G, Sun G, Wu P, et al. Novel mutations of Tyk2 leading to divergent clinical phenotypes. In Review [Preprint] 2021. 10.21203/rs.3.rs-297607/v1 [DOI] [PubMed] [Google Scholar]
- 46. Guo W, Feng X, Yang M. Mycobacterium Intracellulare infection associated with Tyk2 deficiency: A case report and review of the literature. Infect Drug Resist 2020:4347–53. 10.2147/idr.s279438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uzel G, Sampaio EP, Lawrence MG, et al. Dominant gain-of-function Stat1 mutations in Foxp3 wild-type immune dysregulation-Polyendocrinopathy-Enteropathy-X-linked-like syndrome. J Allergy Clin Immunol 2013;131:1611–23. 10.1016/j.jaci.2012.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moriya K, Suzuki T, Uchida N, et al. Ruxolitinib treatment of a patient with steroid-dependent severe Autoimmunity due to Stat1 gain-of-function Mutation. Int J Hematol 2020;112:258–62. 10.1007/s12185-020-02860-7 [DOI] [PubMed] [Google Scholar]
- 49. Bloomfield M, Kanderová V, Paračková Z, et al. Utility of Ruxolitinib in a child with chronic Mucocutaneous Candidiasis caused by a novel Stat1 gain-of-function Mutation. J Clin Immunol 2018;38:589–601. 10.1007/s10875-018-0519-6 [DOI] [PubMed] [Google Scholar]
- 50. Chaimowitz NS, Ebenezer SJ, Hanson IC, et al. Stat1 gain of function, type 1 diabetes, and reversal with JAK inhibition. N Engl J Med 2020;383:1494–6. 10.1056/NEJMc2022226 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020;370:eabd4570. 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gruber C, Martin-Fernandez M, Ailal F, et al. Homozygous Stat2 gain-of-function Mutation by loss of Usp18 activity in a patient with type I Interferonopathy. J Exp Med 2020;217:e20192319. 10.1084/jem.20192319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koskela HLM, Eldfors S, Ellonen P, et al. Somatic Stat3 mutations in large granular lymphocytic leukemia. N Engl J Med 2012;366:1905–13. 10.1056/NEJMoa1114885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ellinghaus D, Ellinghaus E, Nair RP, et al. Combined analysis of genome-wide Association studies for Crohn disease and psoriasis identifies seven shared susceptibility Loci. Am J Hum Genet 2012;90:636–47. 10.1016/j.ajhg.2012.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Remmers EF, Plenge RM, Lee AT, et al. Stat4And the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med 2007;357:977–86. 10.1056/NEJMoa073003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schimke LF, Hibbard J, Martinez-Barricarte R, et al. Paracoccidioidomycosis associated with a heterozygous Stat4 Mutation and impaired IFN-Γ immunity. J Infect Dis 2017;216:1623–34. 10.1093/infdis/jix522 [DOI] [PubMed] [Google Scholar]
- 57. Duetsch G, Illig T, Loesgen S, et al. Stat6 as an asthma candidate gene: polymorphism-screening, Association and haplotype analysis in a Caucasian Sib-pair study. Hum Mol Genet 2002;11:613–21. 10.1093/hmg/11.6.613 [DOI] [PubMed] [Google Scholar]
- 58. Takeuchi I, Yanagi K, Takada S, et al. Stat6 gain-of-function variant exacerbates multiple allergic symptoms. J Allergy Clin Immunol 2023;151:1402–9. 10.1016/j.jaci.2022.12.802 [DOI] [PubMed] [Google Scholar]
- 59. Hadjadj J, Castro CN, Tusseau M, et al. Early-onset Autoimmunity associated with Socs1 Haploinsufficiency. Nat Commun 2020;11:5341. 10.1038/s41467-020-18925-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Speer SD, Li Z, Buta S, et al. Isg15 deficiency and increased viral resistance in humans but not mice. Nat Commun 2016;7:11496. 10.1038/ncomms11496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hermann M, Bogunovic D. Isg15: in sickness and in health. Trends Immunol 2017;38:79–93. 10.1016/j.it.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 62. Alsohime F, Martin-Fernandez M, Temsah M-H, et al. JAK inhibitor therapy in a child with inherited Usp18 deficiency. N Engl J Med 2020;382:256–65. 10.1056/NEJMoa1905633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Russell SM, Tayebi N, Nakajima H, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in Lymphoid development. Science 1995;270:797–800. 10.1126/science.270.5237.797 [DOI] [PubMed] [Google Scholar]
- 64. Smolen JS, Aletaha D, Redlich K. The pathogenesis of rheumatoid arthritis: new insights from old clinical data? Nat Rev Rheumatol 2012;8:235–43. 10.1038/nrrheum.2012.23 [DOI] [PubMed] [Google Scholar]
- 65. Smolen JS, Aletaha D. Forget Personalised medicine and focus on abating disease activity. Ann Rheum Dis 2013;72:3–6. 10.1136/annrheumdis-2012-202361 [DOI] [PubMed] [Google Scholar]
- 66. Genovese MC, Cohen S, Moreland L, et al. Combination therapy with Etanercept and Anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum 2004;50:1412–9. 10.1002/art.20221 [DOI] [PubMed] [Google Scholar]
- 67. Weinblatt M, Schiff M, Goldman A, et al. Selective Costimulation modulation using Abatacept in patients with active rheumatoid arthritis while receiving Etanercept: a randomised clinical trial. Ann Rheum Dis 2007;66:228–34. 10.1136/ard.2006.055111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Genovese MC, Cohen SB, Wofsy D, et al. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a P38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. J Rheumatol 2011;38:846–54. 10.3899/jrheum.100602 [DOI] [PubMed] [Google Scholar]
- 69. Genovese MC, van der Heijde DM, Keystone EC, et al. A phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study of 2 dosing regimens of Fostamatinib in patients with rheumatoid arthritis with an inadequate response to a tumor necrosis factor-Α antagonist. J Rheumatol 2014;41:2120–8. 10.3899/jrheum.140238 [DOI] [PubMed] [Google Scholar]
- 70. Cohen S, Tuckwell K, Katsumoto TR, et al. Fenebrutinib versus placebo or Adalimumab in rheumatoid arthritis: A randomized, Double‐Blind, phase II trial. Arthritis Rheumatol 2020;72:1435–46. 10.1002/art.41275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ramadass V, Vaiyapuri T, Tergaonkar V. Small molecule NF-ΚB pathway inhibitors in clinic. Int J Mol Sci 2020;21:5164. 10.3390/ijms21145164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Changelian PS, Flanagan ME, Ball DJ, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 2003;302:875–8. 10.1126/science.1087061 [DOI] [PubMed] [Google Scholar]
- 73. Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function Mutation of Jak2 in Myeloproliferative disorders. N Engl J Med 2005;352:1779–90. 10.1056/NEJMoa051113 [DOI] [PubMed] [Google Scholar]
- 74. Verstovsek S, Mesa RA, Gotlib J, et al. The clinical benefit of Ruxolitinib across patient subgroups: analysis of a Placebo‐Controlled, phase III study in patients with myelofibrosis. Br J Haematol 2013;161:508–16. 10.1111/bjh.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jagasia M, Perales M-A, Schroeder MA, et al. Results from Reach1, a single-arm phase 2 study of Ruxolitinib in combination with corticosteroids for the treatment of steroid-refractory acute graft-vs-host disease. Blood 2018;132(Supplement 1):601. 10.1182/blood-2018-99-116342 [DOI] [Google Scholar]
- 76. Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med 2021;385:228–38. 10.1056/NEJMoa2033122 [DOI] [PubMed] [Google Scholar]
- 77. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. 10.1016/S0140-6736(12)61424-X [DOI] [PubMed] [Google Scholar]
- 78. Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 2009;60:1895–905. 10.1002/art.24567 [DOI] [PubMed] [Google Scholar]
- 79. Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. 10.1056/NEJMoa1109071 [DOI] [PubMed] [Google Scholar]
- 80. Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. 10.1056/NEJMoa1310476 [DOI] [PubMed] [Google Scholar]
- 81. Riese RJ, Krishnaswami S, Kremer J. Inhibition of JAK Kinases in patients with rheumatoid arthritis: scientific rationale and clinical outcomes. Best Pract Res Clin Rheumatol 2010;24:513–26. 10.1016/j.berh.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 82. Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and Adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL strategy): a phase 3B/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390:457–68. 10.1016/S0140-6736(17)31618-5 [DOI] [PubMed] [Google Scholar]
- 83. Mease P, Hall S, FitzGerald O, et al. Tofacitinib or Adalimumab versus placebo for Psoriatic arthritis. N Engl J Med 2017;377:1537–50. 10.1056/NEJMoa1615975 [DOI] [PubMed] [Google Scholar]
- 84. Gladman D, Rigby W, Azevedo VF, et al. Tofacitinib for Psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017;377:1525–36. 10.1056/NEJMoa1615977 [DOI] [PubMed] [Google Scholar]
- 85. van der Heijde D, Deodhar A, Wei JC, et al. Tofacitinib in patients with Ankylosing Spondylitis: a phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis 2017;76:1340–7. 10.1136/annrheumdis-2016-210322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Deodhar A, Sliwinska-Stanczyk P, Xu H, et al. Tofacitinib for the treatment of Ankylosing Spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2021;80:1004–13. 10.1136/annrheumdis-2020-219601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a phase 2B randomized Placebo‐Controlled Dose‐Ranging study. Br J Dermatol 2012;167:668–77. 10.1111/j.1365-2133.2012.11168.x [DOI] [PubMed] [Google Scholar]
- 88. Bachelez H, van de Kerkhof PCM, Strohal R, et al. Tofacitinib versus Etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015;386:552–61. 10.1016/S0140-6736(14)62113-9 [DOI] [PubMed] [Google Scholar]
- 89. Papp KA, Menter MA, Abe M, et al. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol 2015;173:949–61. 10.1111/bjd.14018 [DOI] [PubMed] [Google Scholar]
- 90. Zhang J, Tsai T-F, Lee M-G, et al. The efficacy and safety of tofacitinib in Asian patients with moderate to severe chronic plaque psoriasis: A phase 3, randomized, double-blind, placebo-controlled study. J Dermatol Sci 2017;88:36–45. 10.1016/j.jdermsci.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 91. Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:496–7. 10.1056/NEJMc1707500 [DOI] [PubMed] [Google Scholar]
- 92. Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterology Hepatology Official Clin Pract J Am Gastroenterological Assoc 2014;12:1485–93. 10.1016/j.cgh.2014.01.029 [DOI] [PubMed] [Google Scholar]
- 93. Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017;66:1049–59. 10.1136/gutjnl-2016-312735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hasni SA, Gupta S, Davis M, et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat Commun 2021;12:3391. 10.1038/s41467-021-23361-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hagberg N, Rönnblom L. Interferon-Α enhances the IL-12-induced Stat4 activation selectively in carriers of the Stat4 SLE risk allele Rs7574865[T]. Ann Rheum Dis 2019;78:429–31. 10.1136/annrheumdis-2018-213836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Takeuchi T, Tanaka Y, Iwasaki M, et al. Efficacy and safety of the oral Janus kinase inhibitor Peficitinib (Asp015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis 2016;75:1057–64. 10.1136/annrheumdis-2015-208279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Genovese MC, Greenwald M, Codding C, et al. Peficitinib, a JAK inhibitor, in combination with limited conventional synthetic disease-modifying Antirheumatic drugs in the treatment of moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol 2017;69:932–42. 10.1002/art.40054 [DOI] [PubMed] [Google Scholar]
- 98. Kivitz AJ, Gutierrez-Ureña SR, Poiley J, et al. Peficitinib, a JAK inhibitor, in the treatment of moderate-to-severe rheumatoid arthritis in patients with an inadequate response to methotrexate. Arthritis Rheumatol 2017;69:709–19. 10.1002/art.39955 [DOI] [PubMed] [Google Scholar]
- 99. Tanaka Y, Takeuchi T, Tanaka S, et al. Efficacy and safety of Peficitinib (Asp015K) in patients with rheumatoid arthritis and an inadequate response to conventional Dmards: a randomised, double-blind, placebo-controlled phase III trial (Raj3). Ann Rheum Dis 2019;78:1320–32. 10.1136/annrheumdis-2019-215163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Takeuchi T, Tanaka Y, Tanaka S, et al. Efficacy and safety of Peficitinib (Asp015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (Raj4) in Japan. Ann Rheum Dis 2019;78:1305–19. 10.1136/annrheumdis-2019-215164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Diller M, Hülser M-L, Hasseli R, et al. Ab0492 JAK-inhibition with Peficitinib and Filgotinib in fibroblast-like Synoviocytes in rheumatoid arthritis. Annual European Congress of Rheumatology, EULAR 2018, Amsterdam; June 2018:1406 10.1136/annrheumdis-2018-eular.2182 [DOI] [Google Scholar]
- 102. Papp K, Pariser D, Catlin M, et al. A phase 2A randomized, Double‐Blind, Placebo‐Controlled, sequential Dose‐Escalation study to evaluate the efficacy and safety of Asp015K, a novel Janus kinase inhibitor, in patients with Moderate‐To‐Severe psoriasis. Br J Dermatol 2015;173:767–76. 10.1111/bjd.13745 [DOI] [PubMed] [Google Scholar]
- 103. Sands BE, Sandborn WJ, Feagan BG, et al. Peficitinib, an oral Janus kinase inhibitor, in moderate-to-severe ulcerative colitis: results from a randomised, phase 2 study. J Crohns Colitis 2018;12:1158–69. 10.1093/ecco-jcc/jjy085 [DOI] [PubMed] [Google Scholar]
- 104. Keystone EC, Taylor PC, Drescher E, et al. Safety and efficacy of Baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann Rheum Dis 2015;74:333–40. 10.1136/annrheumdis-2014-206478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fleischmann R, Schiff M, van der Heijde D, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior Disease‐Modifying Antirheumatic drug treatment. Arthritis Rheumatol 2017;69:506–17. 10.1002/art.39953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Smolen JS, Kremer JM, Gaich CL, et al. Patient-reported outcomes from a randomised phase III study of Baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON). Ann Rheum Dis 2017;76:694–700. 10.1136/annrheumdis-2016-209821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dougados M, van der Heijde D, Chen Y-C, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic Dmards: results from the RA-BUILD study. Ann Rheum Dis 2017;76:88–95. 10.1136/annrheumdis-2016-210094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Keystone EC, Taylor PC, Tanaka Y, et al. Patient-reported outcomes from a phase 3 study of Baricitinib versus placebo or Adalimumab in rheumatoid arthritis: secondary analyses from the RA-BEAM study. Ann Rheum Dis 2017;76:1853–61. 10.1136/annrheumdis-2017-211259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tanaka Y, Atsumi T, Amano K, et al. Efficacy and safety of Baricitinib in Japanese patients with rheumatoid arthritis: subgroup analyses of four multinational phase 3 randomized trials. Mod Rheumatol 2018;28:583–91. 10.1080/14397595.2017.1392057 [DOI] [PubMed] [Google Scholar]
- 110. Tanaka Y, Emoto K, Cai Z, et al. Efficacy and safety of Baricitinib in Japanese patients with active rheumatoid arthritis receiving background methotrexate therapy: A 12-week, double-blind, randomized placebo-controlled study. J Rheumatol 2016;43:504–11. 10.3899/jrheum.150613 [DOI] [PubMed] [Google Scholar]
- 111. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. 10.1056/NEJMoa1507247 [DOI] [PubMed] [Google Scholar]
- 112. van der Heijde D, Durez P, Schett G, et al. Structural damage progression in patients with early rheumatoid arthritis treated with methotrexate, Baricitinib, or Baricitinib plus methotrexate based on clinical response in the phase 3 RA-BEGIN study. Clin Rheumatol 2018;37:2381–90. 10.1007/s10067-018-4221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or Adalimumab in rheumatoid arthritis. N Engl J Med 2017;376:652–62. 10.1056/NEJMoa1608345 [DOI] [PubMed] [Google Scholar]
- 114. Papp KA, Menter MA, Raman M, et al. A randomized phase 2B trial of Baricitinib, an oral Janus kinase (JAK) 1/Jak2 inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol 2016;174:1266–76. 10.1111/bjd.14403 [DOI] [PubMed] [Google Scholar]
- 115. Simpson EL, Lacour J-P, Spelman L, et al. Baricitinib in patients with Moderate‐To‐Severe Atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol 2020;183:242–55. 10.1111/bjd.18898 [DOI] [PubMed] [Google Scholar]
- 116. Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe Atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-Ad5). J Am Acad Dermatol 2021;85:62–70. 10.1016/j.jaad.2021.02.028 [DOI] [PubMed] [Google Scholar]
- 117. Reich K, Kabashima K, Peris K, et al. Efficacy and safety of Baricitinib combined with topical corticosteroids for treatment of moderate to severe Atopic dermatitis: A randomized clinical trial. JAMA Dermatol 2020;156:1333–43. 10.1001/jamadermatol.2020.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:222–31. 10.1016/S0140-6736(18)31363-1 [DOI] [PubMed] [Google Scholar]
- 119. Petri M, Bruce IN, Dörner T, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-I. Lancet 2023;401:1011–9. 10.1016/S0140-6736(22)02546-6 [DOI] [PubMed] [Google Scholar]
- 120. Petri M, Bruce IN, Dörner T, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-II. Lancet 2023;401:1011–9. 10.1016/S0140-6736(22)02546-6 [DOI] [PubMed] [Google Scholar]
- 121. King B, Ohyama M, Kwon O, et al. Two phase 3 trials of Baricitinib for Alopecia Areata. N Engl J Med 2022;386:1687–99. 10.1056/NEJMoa2110343 [Epub ahead of print Published Online First]. [DOI] [PubMed] [Google Scholar]
- 122. Bechman K, Yates M, Galloway JB. The new entries in the therapeutic Armamentarium_ the small molecule JAK inhibitors. Pharmacol Res 2019;147:104392. 10.1016/j.phrs.2019.104392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Duggan S, Keam SJ. Upadacitinib: first approval. Drugs 2019;79:1819–28. 10.1007/s40265-019-01211-z [DOI] [PubMed] [Google Scholar]
- 124. Genovese MC, Smolen JS, Weinblatt ME, et al. Efficacy and safety of ABT-494, a selective JAK-1 inhibitor, in a phase IIb study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol 2016;68:2857–66. 10.1002/art.39808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019;393:2303. 10.1016/S0140-6736(19)30419-2 [DOI] [PubMed] [Google Scholar]
- 126. Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of Upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018;391:2503–12. 10.1016/S0140-6736(18)31115-2 [DOI] [PubMed] [Google Scholar]
- 127. Kameda H, Takeuchi T, Yamaoka K, et al. Efficacy and safety of Upadacitinib in Japanese patients with rheumatoid arthritis (SELECT-SUNRISE): a placebo-controlled phase IIb/III study. Rheumatology (Oxford) 2020;59:3303–13. 10.1093/rheumatology/keaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kremer JM, Emery P, Camp HS, et al. A phase IIb study of ABT‐494, a selective JAK‐1 inhibitor, in patients with rheumatoid arthritis and an inadequate response to anti–tumor necrosis factor therapy. Arthritis Rheumatol 2016;68:2867–77. 10.1002/art.39801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of Upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet 2018;391:2513–24. 10.1016/S0140-6736(18)31116-4 [DOI] [PubMed] [Google Scholar]
- 130. van der Heijde D, Song I-H, Pangan AL, et al. Efficacy and safety of Upadacitinib in patients with active Ankylosing Spondylitis (SELECT-AXIS 1): a Multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet 2019;394:2108–17. 10.1016/S0140-6736(19)32534-6 [DOI] [PubMed] [Google Scholar]
- 131. Deodhar A, van der Heijde D, Sieper J, et al. Safety and efficacy of Upadacitinib in patients with active Ankylosing Spondylitis and an inadequate response to nonsteroidal antiinflammatory drug therapy: One‐Year results of a Double‐Blind, Placebo‐Controlled study and Open‐Label extension. Arthritis Rheumatol 2022;74:70–80. 10.1002/art.41911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Fleischmann R, Pangan AL, Song I-H, et al. Upadacitinib versus placebo or Adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019;71:1788–800. 10.1002/art.41032 [DOI] [PubMed] [Google Scholar]
- 133. McInnes IB, Kato K, Magrey M, et al. Efficacy and safety of Upadacitinib in patients with Psoriatic arthritis: 2-year results from the phase 3 SELECT-PSA 1 study. Rheumatol Ther 2023;10:275–92. 10.1007/s40744-022-00499-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis 2021;80:312–20. 10.1136/annrheumdis-2020-218870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. van der Heijde D, Baraliakos X, Sieper J, et al. Efficacy and safety of Upadacitinib for active Ankylosing Spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann Rheum Dis 2022;81:1515–23. 10.1136/ard-2022-222608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sandborn WJ, Ghosh S, Panes J, et al. Efficacy of Upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology 2020;158:2139–49. 10.1053/j.gastro.2020.02.030 [DOI] [PubMed] [Google Scholar]
- 137. Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, Multicentre, double-blind, randomised trials. Lancet 2022;399:2113–28. 10.1016/S0140-6736(22)00581-5 [DOI] [PubMed] [Google Scholar]
- 138. Sandborn WJ, Feagan BG, Loftus EV, et al. Efficacy and safety of Upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterology 2020;158:2123–38. 10.1053/j.gastro.2020.01.047 [DOI] [PubMed] [Google Scholar]
- 139. Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe Atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol 2020;145:877–84. 10.1016/j.jaci.2019.11.025 [DOI] [PubMed] [Google Scholar]
- 140. Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of Upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe Atopic dermatitis (AD up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021;397:2169–81. 10.1016/S0140-6736(21)00589-4 [DOI] [PubMed] [Google Scholar]
- 141. Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily Upadacitinib versus placebo in adolescents and adults with moderate-to-severe Atopic dermatitis (measure up 1 and measure up 2): results from two Replicate double-blind, randomised controlled phase 3 trials. Lancet 2021;397:2151–68. 10.1016/S0140-6736(21)00588-2 [DOI] [PubMed] [Google Scholar]
- 142. Genovese MC, Kalunian K, Gottenberg J-E, et al. Effect of Filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying Antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA 2019;322:315–25. 10.1001/jama.2019.9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Westhovens R, Rigby WFC, van der Heijde D, et al. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3, randomised controlled FINCH 3 trial. Ann Rheum Dis 2021;80:727–38. 10.1136/annrheumdis-2020-219213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis 2021;80:848–58. 10.1136/annrheumdis-2020-219214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tanaka Y, Matsubara T, Atsumi T, et al. Efficacy and safety of Filgotinib in combination with methotrexate in Japanese patients with active rheumatoid arthritis who have an inadequate response to methotrexate: subpopulation analyses of 24-week data of a global phase 3 study (FINCH 1. Mod Rheumatol 2022;32:263–72. 10.1093/mr/roab030 [DOI] [PubMed] [Google Scholar]
- 146. Combe BG, Tanaka Y, Buch MH, et al. Efficacy and safety of Filgotinib in patients with high risk of poor prognosis who showed inadequate response to MTX: A post hoc analysis of the FINCH 1 study. Rheumatol Ther 2023;10:71–2. 10.1007/s40744-022-00530-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mease P, Coates LC, Helliwell PS, et al. Efficacy and safety of Filgotinib, a selective Janus kinase 1 inhibitor, in patients with active Psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:2367–77. 10.1016/S0140-6736(18)32483-8 [DOI] [PubMed] [Google Scholar]
- 148. van der Heijde D, Baraliakos X, Gensler LS, et al. Efficacy and safety of Filgotinib, a selective Janus kinase 1 inhibitor, in patients with active Ankylosing Spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018;392:2378–87. 10.1016/S0140-6736(18)32463-2 [DOI] [PubMed] [Google Scholar]
- 149. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with Filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017;389:266–75. 10.1016/S0140-6736(16)32537-5 [DOI] [PubMed] [Google Scholar]
- 150. Feagan BG, Danese S, Loftus EV, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2B/3 double-blind, randomised, placebo-controlled trial. Lancet 2021;397:2372–84. 10.1016/S0140-6736(21)00666-8 [DOI] [PubMed] [Google Scholar]
- 151. D’Haens GR, Lee S, Taylor SA, et al. Filgotinib for the treatment of small bowel Crohn’s disease: the DIVERGENCE 1 trial. Gastroenterology 2023;165:289–92. 10.1053/j.gastro.2023.03.234 [Epub ahead of print Published Online First]. [DOI] [PubMed] [Google Scholar]
- 152. Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of Abrocitinib in adults and adolescents with moderate-to-severe Atopic dermatitis (JADE MONO-1): a Multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet 2020;396:255–66. 10.1016/S0140-6736(20)30732-7 [DOI] [PubMed] [Google Scholar]
- 153. Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of Abrocitinib in patients with moderate-to-severe Atopic dermatitis: A randomized clinical trial. JAMA Dermatol 2020;156:863–73. 10.1001/jamadermatol.2020.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or Dupilumab for Atopic dermatitis. N Engl J Med 2021;384:1101–12. 10.1056/NEJMoa2019380 [DOI] [PubMed] [Google Scholar]
- 155. Fleischmann RM, Damjanov NS, Kivitz AJ, et al. A randomized, Double‐Blind, Placebo‐Controlled, Twelve‐Week, Dose‐Ranging study of Decernotinib, an oral selective JAK‐3 inhibitor, as monotherapy in patients with active rheumatoid arthritis. Arthritis Rheumatol 2015;67:334–43. 10.1002/art.38949 [DOI] [PubMed] [Google Scholar]
- 156. Genovese MC, van Vollenhoven RF, Pacheco-Tena C, et al. VX-509 (Decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol 2016;68:46–55. 10.1002/art.39473 [DOI] [PubMed] [Google Scholar]
- 157. Genovese MC, Yang F, Østergaard M, et al. Efficacy of VX-509 (Decernotinib) in combination with a disease-modifying Antirheumatic drug in patients with rheumatoid arthritis: clinical and MRI findings. Ann Rheum Dis 2016;75:1979–83. 10.1136/annrheumdis-2015-208901 [DOI] [PubMed] [Google Scholar]
- 158. Robinson MF, Damjanov N, Stamenkovic B, et al. Efficacy and safety of PF‐06651600 (Ritlecitinib), a novel Jak3/TEC inhibitor, in patients with Moderate‐To‐Severe rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol 2020;72:1621–31. 10.1002/art.41316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. King B, Guttman-Yassky E, Peeva E, et al. A phase 2A randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors Ritlecitinib and Brepocitinib in Alopecia Areata: 24-week results. J Am Acad Dermatol 2021;85:379–87. 10.1016/j.jaad.2021.03.050 [DOI] [PubMed] [Google Scholar]
- 160. Krueger JG, McInnes IB, Blauvelt A. Tyrosine kinase 2 And Janus Kinase‒Signal transducer and activator of transcription signaling and inhibition in plaque psoriasis. J Am Acad Dermatol 2022;86:148–57. 10.1016/j.jaad.2021.06.869 [Epub ahead of print Published Online First]. [DOI] [PubMed] [Google Scholar]
- 161. Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and Apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol 2023;88:29–39. 10.1016/j.jaad.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 162. Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and Apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program fOr evaluation of Tyk2 inhibitor psoriasis second trial. J Am Acad Dermatol 2023;88:40–51. 10.1016/j.jaad.2022.08.061 [DOI] [PubMed] [Google Scholar]
- 163. Mease PJ, Deodhar AA, van der Heijde D, et al. Efficacy and safety of selective Tyk2 inhibitor, Deucravacitinib, in a phase II trial in Psoriatic arthritis. Ann Rheum Dis 2022;81:815–22. 10.1136/annrheumdis-2021-221664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Morand E, Pike M, Merrill JT, et al. Deucravacitinib, a tyrosine kinase 2 inhibitor, in systemic lupus erythematosus: A phase II, randomized, Double‐Blind, Placebo‐Controlled trial. Arthritis Rheumatol 2023;75:242–52. 10.1002/art.42391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Forman SB, Pariser DM, Poulin Y, et al. Tyk2/Jak1 inhibitor PF-06700841 in patients with plaque psoriasis: phase IIa, randomized, double-blind, placebo-controlled trial. J Invest Dermatol 2020;140:2359–70. 10.1016/j.jid.2020.03.962 [DOI] [PubMed] [Google Scholar]
- 166. Choy EH. Clinical significance of Janus kinase inhibitor selectivity. Rheumatology (Oxford) 2019;58:953–62. 10.1093/rheumatology/key339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Adams J, Huang P, Patrick D. A strategy for the design of Multiplex inhibitors for kinase-mediated signalling in angiogenesis. Curr Opin Chem Biol 2002;6:486–92. 10.1016/s1367-5931(02)00357-5 [DOI] [PubMed] [Google Scholar]
- 168. Meyer DM, Jesson MI, Li X, et al. Anti-inflammatory activity and neutrophil reductions mediated by the Jak1/Jak3 inhibitor, CP-690,550, in rat adjuvant-induced arthritis. J Inflamm (Lond) 2010;7:41. 10.1186/1476-9255-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 2011;186:4234–43. 10.4049/jimmunol.1003668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Lin TH, Hegen M, Quadros E, et al. Selective functional inhibition of JAK‐3 is sufficient for efficacy in Collagen‐Induced arthritis in mice. Arthritis Rheum 2010;62:2283–93. 10.1002/art.27536 [DOI] [PubMed] [Google Scholar]
- 171. Milici AJ, Kudlacz EM, Audoly L, et al. Cartilage preservation by inhibition of Janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis Res Ther 2008;10:R14. 10.1186/ar2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Van Rompaey L, Galien R, van der Aar EM, et al. Preclinical characterization of Glpg0634, a selective inhibitor of Jak1, for the treatment of inflammatory diseases. J Immunol 2013;191:3568–77. 10.4049/jimmunol.1201348 [DOI] [PubMed] [Google Scholar]
- 173. Traves PG, Murray B, Campigotto F, et al. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by Filgotinib, Upadacitinib, tofacitinib and Baricitinib. Ann Rheum Dis 2021;80:865–75. 10.1136/annrheumdis-2020-219012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Namour F, Galien R, Van Kaem T, et al. Safety, pharmacokinetics and pharmacodynamics of Glpg0974, a potent and selective Ffa2 antagonist, in healthy male subjects. Br J Clin Pharmacol 2016;82:139–48. 10.1111/bcp.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Clark JD, Flanagan ME, Telliez J-B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem 2014;57:5023–38. 10.1021/jm401490p [DOI] [PubMed] [Google Scholar]
- 176. Fridman JS, Scherle PA, Collins R, et al. Selective inhibition of Jak1 and Jak2 is efficacious in rodent models of arthritis: Preclinical characterization of Incb028050. J Immunol 2010;184:5298–307. 10.4049/jimmunol.0902819 [DOI] [PubMed] [Google Scholar]
- 177. Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the Jak1 selectivity of Upadacitinib (ABT-494). BMC Rheumatol 2018;2:23. 10.1186/s41927-018-0031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Mohamed M-EF, Beck D, Camp HS, et al. Preferential inhibition of Jak1 relative to Jak3 by Upadacitinib: exposure-response analyses of ex vivo data from 2 phase 1 clinical trials and comparison to tofacitinib. J Clin Pharmacol 2020;60:188–97. 10.1002/jcph.1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Dowty ME, Lin TH, Jesson MI, et al. Janus kinase inhibitors for the treatment of rheumatoid arthritis demonstrate similar profiles of in vitro cytokine receptor inhibition. Pharmacol Res Perspect 2019;7:e00537. 10.1002/prp2.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. McInnes IB, Byers NL, Higgs RE, et al. Comparison of Baricitinib, Upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human Leukocyte subpopulations. Arthritis Res Ther 2019;21:183. 10.1186/s13075-019-1964-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Moodley D, Yoshida H, Mostafavi S, et al. Network pharmacology of JAK inhibitors. Proc Natl Acad Sci U S A 2016;113:9852–7. 10.1073/pnas.1610253113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Alves de Medeiros AK, Speeckaert R, Desmet E, et al. Jak3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One 2016;11:e0164080. 10.1371/journal.pone.0164080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Sabat R, Wolk K, Loyal L, et al. T cell pathology in skin inflammation. Semin Immunopathol 2019;41:359–77. 10.1007/s00281-019-00742-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Welsch K, Holstein J, Laurence A, et al. Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur J Immunol 2017;47:1096–107. 10.1002/eji.201646680 [DOI] [PubMed] [Google Scholar]
- 185. Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in Dermatology. Front Immunol 2019;10:2847. 10.3389/fimmu.2019.02847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Bayart CB, DeNiro KL, Brichta L, et al. Topical Janus kinase inhibitors for the treatment of pediatric Alopecia Areata. J Am Acad Dermatol 2017;77:167–70. 10.1016/j.jaad.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 187. Bissonnette R, Papp KA, Poulin Y, et al. Topical tofacitinib for Atopic dermatitis: a phase II a randomized trial. Br J Dermatol 2016;175:902–11. 10.1111/bjd.14871 [DOI] [PubMed] [Google Scholar]
- 188. Kim BS, Howell MD, Sun K, et al. Treatment of Atopic dermatitis with Ruxolitinib cream (Jak1/Jak2 inhibitor) or triamcinolone cream. J Allergy Clin Immunol 2020;145:572–82. 10.1016/j.jaci.2019.08.042 [DOI] [PubMed] [Google Scholar]
- 189. Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of Ruxolitinib cream for the treatment of Atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol 2021;85:863–72. 10.1016/j.jaad.2021.04.085 [DOI] [PubMed] [Google Scholar]
- 190. Noji S, Hara Y, Miura T, et al. Discovery of a Janus kinase inhibitor bearing a highly three-dimensional Spiro scaffold: JTE-052 (Delgocitinib) as a new dermatological agent to treat inflammatory skin disorders. J Med Chem 2020;63:7163–85. 10.1021/acs.jmedchem.0c00450 [DOI] [PubMed] [Google Scholar]
- 191. Tanimoto A, Shinozaki Y, Yamamoto Y, et al. A novel JAK inhibitor JTE-052 reduces skin inflammation and ameliorates chronic dermatitis in rodent models: comparison with conventional therapeutic agents. Exp Dermatol 2018;27:22–9. 10.1111/exd.13370 [DOI] [PubMed] [Google Scholar]
- 192. Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by Downmodulating T cell activation and differentiation. J Dermatol Sci 2016;84:258–65. 10.1016/j.jdermsci.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 193. Nakagawa H, Nemoto O, Igarashi A, et al. Efficacy and safety of topical JTE-052, a Janus kinase inhibitor, in Japanese adult patients with moderate-to-severe Atopic dermatitis: a phase II, Multicentre, randomized, vehicle-controlled clinical study. Br J Dermatol 2018;178:424–32. 10.1111/bjd.16014 [DOI] [PubMed] [Google Scholar]
- 194. Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe Atopic dermatitis: A phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol 2020;82:823–31. 10.1016/j.jaad.2019.12.015 [DOI] [PubMed] [Google Scholar]
- 195. Nakagawa H, Nemoto O, Igarashi A, et al. Long‐Term safety and efficacy of Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with Atopic dermatitis. J Dermatol 2020;47:114–20. 10.1111/1346-8138.15173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Tanimoto A, Ogawa Y, Oki C, et al. Pharmacological properties of JTE-052: a novel potent JAK inhibitor that suppresses various inflammatory responses in vitro and in vivo. Inflamm Res 2015;64:41–51. 10.1007/s00011-014-0782-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical Delgocitinib in patients with chronic hand Eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol 2020;182:1103–10. 10.1111/bjd.18469 [DOI] [PubMed] [Google Scholar]
- 198. Worm M, Thyssen JP, Schliemann S, et al. The Pan‐JAK inhibitor Delgocitinib in a cream formulation demonstrates dose response in chronic hand Eczema in a 16‐Week randomized phase IIb trial*. Br J Dermatol 2022;187:42–51. 10.1111/bjd.21037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Punwani N, Scherle P, Flores R, et al. Preliminary clinical activity of a topical Jak1/2 inhibitor in the treatment of psoriasis. J Am Acad Dermatol 2012;67:658–64. 10.1016/j.jaad.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 200. Punwani N, Burn T, Scherle P, et al. Downmodulation of key inflammatory cell markers with a topical Janus kinase 1/2 inhibitor. Br J Dermatol 2015;173:989–97. 10.1111/bjd.13994 [DOI] [PubMed] [Google Scholar]
- 201. Papp KA, Bissonnette R, Gooderham M, et al. Treatment of plaque psoriasis with an ointment formulation of the Janus kinase inhibitor, tofacitinib: a phase 2B randomized clinical trial. BMC Dermatol 2016;16:15. 10.1186/s12895-016-0051-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Ports WC, Khan S, Lan S, et al. A randomized phase 2A efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol 2013;169:137–45. 10.1111/bjd.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of Vitiligo: a randomised, controlled, phase 2 trial. Lancet 2020;396:110–20. 10.1016/S0140-6736(20)30609-7 [DOI] [PubMed] [Google Scholar]
- 204. Rosmarin D, Passeron T, Pandya AG, et al. Two phase 3, randomized, controlled trials of Ruxolitinib cream for Vitiligo. N Engl J Med 2022;387:1445–55. 10.1056/NEJMoa2118828 [DOI] [PubMed] [Google Scholar]
- 205. Nilsson M, Rhedin M, Hendrickx R, et al. Characterization of selective and potent Jak1 inhibitors intended for the inhaled treatment of asthma. Drug Des Devel Ther 2022;16:2901–17. 10.2147/DDDT.S354291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Milara J, Ballester B, de Diego A, et al. The Pan-JAK inhibitor Las194046 reduces neutrophil activation from severe asthma and COPD patients in vitro. Sci Rep 2022;12:5132. 10.1038/s41598-022-09241-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Braithwaite IE, Cai F, Tom JA, et al. Inhaled JAK inhibitor GDC-0214 reduces exhaled nitric oxide in patients with mild asthma: A randomized, controlled, proof-of-activity trial. J Allergy Clin Immunol 2021;148:783–9. 10.1016/j.jaci.2021.02.042 [DOI] [PubMed] [Google Scholar]
- 208. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of Interleukin-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020;146:128–36. 10.1016/j.jaci.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Hermine O, Mariette X, Tharaux P-L, et al. Effect of Tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: A randomized clinical trial. Jama Intern Med 2020;181:32. 10.1001/jamainternmed.2021.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Salvarani C, Dolci G, Massari M, et al. Effect of Tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: A randomized clinical trial. JAMA Intern Med 2021;181:24–31. 10.1001/jamainternmed.2020.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of Tocilizumab in patients hospitalized with COVID-19. N Engl J Med 2020;383:2333–44. 10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Salama C, Mohan SV. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med 2021;384:1473–4. 10.1056/NEJMc2100217 [DOI] [PubMed] [Google Scholar]
- 213. Hoang TN, Pino M, Boddapati AK, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-Cov-2-infected Rhesus macaques. Cell 2021;184:460–75. 10.1016/j.cell.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Cantini F, Niccoli L, Nannini C, et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; Multicentre study. J Infect 2020;81:647–79. 10.1016/j.jinf.2020.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with COVID-19. New Engl J Medicine 2020;384:795–807. 10.1056/nejmoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Wolfe CR, Tomashek KM, Patterson TF, et al. Baricitinib versus dexamethasone for adults hospitalised with COVID-19 (ACTT-4): a randomised, double-blind, double placebo-controlled trial. Lancet Respir Med 2022;10:888–99. 10.1016/S2213-2600(22)00088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of Baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021;9:1407–18. 10.1016/S2213-2600(21)00331-3 [Epub ahead of print Published Online First]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Horby PW, Emberson JR, Group RC . Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. Medrxiv 2022;22271623. 10.1101/2022.03.02.22271623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Ely EW, Ramanan AV, Kartman CE, et al. Efficacy and safety of Baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or Extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial. Lancet Respir Med 2022;10:327–36. 10.1016/S2213-2600(22)00006-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with COVID-19 pneumonia. N Engl J Med 2021;385:406–15. 10.1056/NEJMoa2101643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kale SD, Mehrkens BN, Stegman MM, et al. ““small” intestinal Immunopathology plays a “big” role in lethal cytokine release syndrome, and its modulation by interferon-Γ, IL-17A, and a Janus kinase inhibitor”. Front Immunol 2020;11:1311. 10.3389/fimmu.2020.01311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe Coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020;146:137–46. 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Limen RY, Sedono R, Sugiarto A, et al. Janus kinase (JAK)-Inhibitors and Coronavirus disease 2019 (COVID-19) outcomes: a systematic review and meta-analysis. Expert Rev Anti Infect Ther 2022;20:425–34. 10.1080/14787210.2021.1982695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic Dmards with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician Registry. Ann Rheum Dis 2021;80:1137–46. 10.1136/annrheumdis-2021-220418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. 10.1136/annrheumdis-2016-210457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Valenzuela F, Korman NJ, Bissonnette R, et al. Tofacitinib in patients with moderate-to-severe chronic plaque psoriasis: long-term safety and efficacy in an open-label extension study. Br J Dermatol 2018;179:853–62. 10.1111/bjd.16798 [DOI] [PubMed] [Google Scholar]
- 227. Olivera P, Danese S, Peyrin-Biroulet L. Next generation of small molecules in inflammatory bowel disease. Gut 2017;66:199–209. 10.1136/gutjnl-2016-312912 [DOI] [PubMed] [Google Scholar]
- 228. Wollenhaupt J, Silverfield J, Lee EB, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. 10.3899/jrheum.130683 [DOI] [PubMed] [Google Scholar]
- 229. Taylor PC, Weinblatt ME, Burmester GR, et al. Cardiovascular safety during treatment with Baricitinib in rheumatoid arthritis. Arthritis Rheumatol 2019;71:1042–55. 10.1002/art.40841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Genovese MC, Smolen JS, Takeuchi T, et al. Safety profile of Baricitinib for the treatment of rheumatoid arthritis over a median of 3 years of treatment: an updated integrated safety analysis. The Lancet Rheumatology 2020;2:e347–57. 10.1016/S2665-9913(20)30032-1 [DOI] [PubMed] [Google Scholar]
- 231. Ytterberg SR, Bhatt DL, Connell CA. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:1768. 10.1056/NEJMc2202778 [DOI] [PubMed] [Google Scholar]
- 232. Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 2023;82:3–18. 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 233. Kerschbaumer A, Sepriano A, Bergstra SA, et al. Efficacy of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2023;82:95–106. 10.1136/ard-2022-223365 [DOI] [PubMed] [Google Scholar]
- 234. Sepriano A, Kerschbaumer A, Bergstra SA, et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2023;82:107–18. 10.1136/ard-2022-223357 [DOI] [PubMed] [Google Scholar]
- 235. Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of Upadacitinib over 15 000 patient-years across rheumatoid arthritis, Psoriatic arthritis, Ankylosing Spondylitis and Atopic dermatitis. RMD Open 2023;9:e002735. 10.1136/rmdopen-2022-002735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Strand V, Ahadieh S, French J, et al. Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying Antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Res Ther 2015;17:362. 10.1186/s13075-015-0880-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Furer V, Rondaan C, Heijstek MW, et al. Update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020;79:39–52. 10.1136/annrheumdis-2019-215882 [DOI] [PubMed] [Google Scholar]
- 238. Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis 2021;80:71–87. 10.1136/annrheumdis-2020-218398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Winthrop KL, Citera G, Gold D, et al. Age-based (&Amp;Amp;Lt;65 vs ≥65 years) incidence of infections and serious infections with tofacitinib versus biological Dmards in rheumatoid arthritis clinical trials and the US Corrona RA Registry. Ann Rheum Dis 2021;80:134–6. 10.1136/annrheumdis-2020-218992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Winthrop KL, Lebwohl M, Cohen AD, et al. Herpes Zoster in psoriasis patients treated with tofacitinib. J Am Acad Dermatol 2017;77:302–9. 10.1016/j.jaad.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 241. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes Zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2018;24:2258–65. 10.1093/ibd/izy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Burmester GR, Curtis JR, Yun H, et al. An integrated analysis of the safety of tofacitinib in Psoriatic arthritis across phase III and long-term extension studies with comparison to real-world observational data. Drug Saf 2020;43:379–92. 10.1007/s40264-020-00904-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019;17:1541–50. 10.1016/j.cgh.2018.11.035 [DOI] [PubMed] [Google Scholar]
- 244. Takeuchi T, Tanaka Y, Rokuda M, et al. A pooled safety analysis of Peficitinib (Asp015K) in Asian patients with rheumatoid arthritis treated over a median of 2 years. Mod Rheumatol 2021;31:543–55. 10.1080/14397595.2020.1836789 [DOI] [PubMed] [Google Scholar]
- 245. Vincenti F, Tedesco Silva H, Busque S, et al. Randomized phase 2B trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year: tofacitinib in kidney transplant patients. Am J Transplant 2012;12:2446–56. 10.1111/j.1600-6143.2012.04127.x [DOI] [PubMed] [Google Scholar]
- 246. Busque S, Vincenti FG, Tedesco Silva H, et al. Efficacy and safety of a tofacitinib-based immunosuppressive regimen after kidney transplantation: results from a long-term extension trial. Transplant Direct 2018;4:e380. 10.1097/TXD.0000000000000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Sanchez GAM, Reinhardt A, Ramsey S, et al. Jak1/2 inhibition with Baricitinib in the treatment of Autoinflammatory Interferonopathies. J Clin Invest 2018;128:3041–52. 10.1172/JCI98814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Isaacs JD, Zuckerman A, Krishnaswami S, et al. Changes in serum creatinine in patients with active rheumatoid arthritis treated with tofacitinib: results from clinical trials. Arthritis Res Ther 2014;16:R158. 10.1186/ar4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Kremer JM, Kivitz AJ, Simon-Campos JA, et al. Evaluation of the effect of tofacitinib on measured glomerular filtration rate in patients with active rheumatoid arthritis: results from a randomised controlled trial. Arthritis Res Ther 2015;17:95. 10.1186/s13075-015-0612-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Chung W-S, Peng C-L, Lin C-L, et al. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: a nationwide cohort study. Ann Rheum Dis 2014;73:1774–80. 10.1136/annrheumdis-2013-203380 [DOI] [PubMed] [Google Scholar]
- 251. Choi HK, Rho Y-H, Zhu Y, et al. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis 2013;72:1182–7. 10.1136/annrheumdis-2012-201669 [DOI] [PubMed] [Google Scholar]
- 252. Desai RJ, Pawar A, Weinblatt ME, et al. Comparative risk of venous thromboembolism in rheumatoid arthritis patients receiving tofacitinib versus those receiving tumor necrosis factor inhibitors: an observational cohort study. Arthritis Rheumatol 2019;71:892–900. 10.1002/art.40798 [DOI] [PubMed] [Google Scholar]
- 253. Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and Psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis 2020;79:1400–13. 10.1136/annrheumdis-2019-216761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Greenfield G, McPherson S, Mills K, et al. The Ruxolitinib effect: understanding how molecular pathogenesis and epigenetic dysregulation impact therapeutic efficacy in Myeloproliferative Neoplasms. J Transl Med 2018;16:360. 10.1186/s12967-018-1729-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Samuelson BT, Vesely SK, Chai-Adisaksopha C, et al. The impact of Ruxolitinib on thrombosis in patients with Polycythemia Vera and myelofibrosis. Blood Coagul Fibrinolysis 2016;27:648–52. 10.1097/MBC.0000000000000446 [DOI] [PubMed] [Google Scholar]
- 256. Salinas CA, Louder A, Polinski J, et al. Evaluation of VTE, MACE, and serious infections among patients with RA treated with Baricitinib compared to Tnfi: A multi-database study of patients in routine care using disease registries and claims databases. Rheumatol Ther 2023;10:201–23. 10.1007/s40744-022-00505-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Smolen JS, Genovese MC, Takeuchi T, et al. Safety profile of Baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019;46:7–18. 10.3899/jrheum.171361 [DOI] [PubMed] [Google Scholar]
- 258. Schulze-Koops H, Strand V, Nduaka C, et al. Analysis of haematological changes in tofacitinib-treated patients with rheumatoid arthritis across phase 3 and long-term extension studies. Rheumatology (Oxford) 2017;56:46–57. 10.1093/rheumatology/kew329 [DOI] [PubMed] [Google Scholar]
- 259. Kay J, Harigai M, Rancourt J, et al. Changes in selected haematological parameters associated with Jak1/Jak2 inhibition observed in patients with rheumatoid arthritis treated with Baricitinib. RMD Open 2020;6:e001370. 10.1136/rmdopen-2020-001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Kremer JM, Bingham CO, Cappelli LC, et al. Postapproval comparative safety study of tofacitinib and biological Disease‐Modifying Antirheumatic drugs: 5‐Year results from a United States–based rheumatoid arthritis Registry. ACR Open Rheumatol 2021;3:173–84. 10.1002/acr2.11232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Curtis JR, Lee EB, Kaplan IV, et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis 2016;75:831–41. 10.1136/annrheumdis-2014-205847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. McInnes IB, Thompson L, Giles JT, et al. Effect of Interleukin-6 receptor blockade on Surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis 2015;74:694–702. 10.1136/annrheumdis-2013-204345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Giles JT, Sattar N, Gabriel S, et al. Cardiovascular safety of Tocilizumab versus Etanercept in rheumatoid arthritis: A randomized controlled trial. Arthritis Rheumatol 2020;72:31–40. 10.1002/art.41095 [DOI] [PubMed] [Google Scholar]
- 264. Charles-Schoeman C, DeMasi R, Valdez H, et al. Risk factors for major adverse cardiovascular events in phase Iiiand Long‐Term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol 2019;71:1450–9. 10.1002/art.40911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Charles-Schoeman C, Gonzalez-Gay MA, Kaplan I, et al. Effects of tofacitinib and other Dmards on lipid profiles in rheumatoid arthritis: implications for the Rheumatologist. Semin Arthritis Rheum 2016;46:71–80. 10.1016/j.semarthrit.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 266. Wu JJ, Strober BE, Hansen PR, et al. Effects of tofacitinib on cardiovascular risk factors and cardiovascular outcomes based on phase III and long-term extension data in patients with plaque psoriasis. J Am Acad Dermatol 2016;75:897–905. 10.1016/j.jaad.2016.06.012 [DOI] [PubMed] [Google Scholar]
- 267. Sands BE, Taub PR, Feagan BG, et al. Op033 the effect of tofacitinib on serum lipids and cardiovascular safety in patients with ulcerative colitis: results from the tofacitinib ulcerative colitis clinical programme. Journal of Crohn’s and Colitis 2018;12(supplement_1):S023. 10.1093/ecco-jcc/jjx180.032 [DOI] [Google Scholar]
- 268. Charles-Schoeman C, Wicker P, Gonzalez-Gay MA, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum 2016;46:261–71. 10.1016/j.semarthrit.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 269. McInnes IB, Kim H-Y, Lee S-H, et al. Open-label tofacitinib and double-blind atorvastatin in rheumatoid arthritis patients: a randomised study*. Ann Rheum Dis 2014;73:124–31. 10.1136/annrheumdis-2012-202442 [DOI] [PubMed] [Google Scholar]
- 270. Kerschbaumer A, Smolen JS, Nash P, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a systematic literature research. RMD Open 2020;6:e001374. 10.1136/rmdopen-2020-001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Charles-Schoeman C, Buch MH, Dougados M, et al. Risk of major adverse cardiovascular events with tofacitinib versus tumour necrosis factor inhibitors in patients with rheumatoid arthritis with or without a history of atherosclerotic cardiovascular disease: a post hoc analysis from ORAL surveillance. Ann Rheum Dis 2023;82:119–29. 10.1136/ard-2022-222259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Khosrow-Khavar F, Kim SC, Lee H, et al. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis 2022;81:798–804. 10.1136/annrheumdis-2021-221915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Gisbert JP, Chaparro M. Safety of new Biologics (Vedolizumab and Ustekinumab) and small molecules (tofacitinib) during pregnancy: A review. Drugs 2020;80:1085–100. 10.1007/s40265-020-01346-4 [DOI] [PubMed] [Google Scholar]
- 274. Clowse MEB, Feldman SR, Isaacs JD, et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf 2016;39:755–62. 10.1007/s40264-016-0431-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Mahadevan U, Dubinsky MC, Su C, et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm Bowel Dis 2018;24:2494–500. 10.1093/ibd/izy160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Hellstrom WJG, Dolhain RJEM, Ritter TE, et al. MANTA and MANTA-ray: rationale and design of trials evaluating effects of Filgotinib on semen parameters in patients with inflammatory diseases. Adv Ther 2022;39:3403–22. 10.1007/s12325-022-02168-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Bazan JF. Structural design and molecular evolution of a cytokine receptor Superfamily. Proc Natl Acad Sci U S A 1990;87:6934–8. 10.1073/pnas.87.18.6934 [DOI] [PMC free article] [PubMed] [Google Scholar]