Abstract
Introduction
Antibrush border antibody disease (ABBA) is an autoimmune tubulointerstitial kidney disease that primarily affects older individuals and results in progressive kidney failure. It is rare with only 20 reported cases. Here, we describe a case series to further define the clinicopathologic spectrum and natural history, and to inform management.
Methods
We identified 67 patients with ABBA who underwent kidney biopsy, including 65 native and 2 transplants. Demographics, clinical findings, and laboratory data were obtained. Histopathologic data included light microscopy, immunofluorescence, electron microscopy and immunostaining for LRP2, CUBN, and AMN. Follow-up data, including treatment(s), laboratory values, and outcomes, were available from 51 patients.
Results
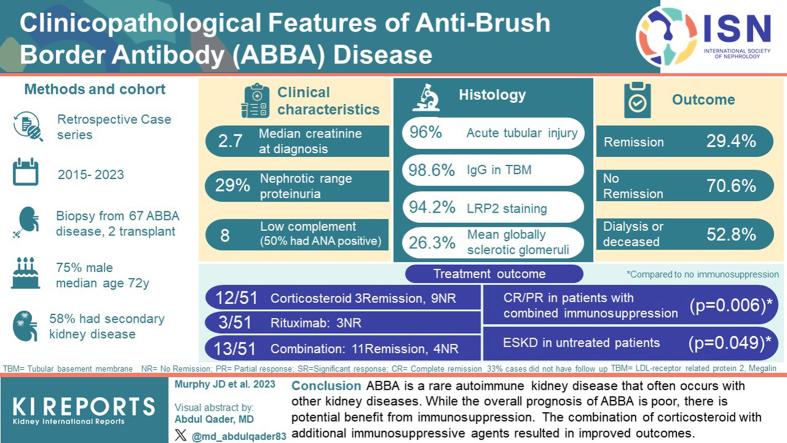
Patients with ABBA were predominantly male with a median age of 72 years. Median serum creatinine was 2.7 mg/dl, proteinuria was 2.8 g/day, and hematuria was present in two-thirds of the patients. Tubular injury with LRP2-positive tubular basement membrane (TBM) deposits were seen in 94.2% of patients. Thirty-eight patients (56.7%) had a second kidney disease, commonly glomerular diseases with high-grade proteinuria. These diseases included podocytopathies, membranous nephropathy (MN), IgA nephropathy, diabetic glomerulopathy, lupus nephritis (LN), crescentic glomerulonephritis (GN), tubulointerstitial nephritis, and involvement by lymphoma. The majority of patients were treated with immunosuppression. Of those patients with follow-up, 29.4% achieved remission, 70.6% had no response, and 52.8% required dialysis or were deceased. Untreated patients were at the highest risk.
Conclusion
ABBA is a rare autoimmune kidney disease that often occurs with other kidney diseases. Although the overall prognosis of ABBA is poor, there is potential benefit from immunosuppression.
Keywords: ABBA, AMN, antibrush border antibody disease, CUBN, LRP2, kidney biopsy
Graphical abstract
See Commentary on Page 197
ABBA is a rare autoimmune tubulointerstitial kidney disease incited by autoantibodies directed against the proximal tubular brush borders that results in a progressive decline in kidney function. ABBA was first described in case reports as an interstitial nephritis and autologous immune complex GN that resulted from development of autoantibodies against proximal tubular protein antigens.1, 2, 3 The histopathologic features are often subtle; with this constellation of findings were defined as a distinct disease entity less than a decade ago.4 A diagnosis of ABBA requires a kidney biopsy that demonstrates the presence of tubular injury with deposition of IgG along TBMs, proximal tubular brush borders, Bowman's capsule, and/or glomerular basement membranes.4,5 The autoantigen for most patients with this disease was identified through immunoprecipitation of patient serum with tubular extract, which revealed reactivity against low density lipoprotein-receptor related protein 2 (LRP2; also known as megalin). LRP2 is identified within TBM deposits and serves as a useful confirmatory biomarker of disease in clinical practice.4
Clinical features of ABBA include a male predominance, older age at onset, and presentation of progressive chronic kidney disease with subnephrotic range proteinuria.4 In the largest series of 10 patients with ABBA, half progressed to end-stage disease, indicating poor kidney survival. Following this series, multiple case reports demonstrated overlap syndromes with a second kidney disease identified concurrently with ABBA, potentially extending the morphologic spectrum of this disease. These concurrent kidney diseases included minimal change disease (n = 1),6 LN (n = 2),7 plasma cell-rich interstitial nephritis (n = 1),8 and low-grade B-cell lymphoma (n = 3).9,10
To date, only 20 ABBA cases have been reported, and therefore, we have a limited understanding of the disease spectrum. We now describe a series of 67 ABBA patients to further define the clinicopathologic spectrum and natural history of the disease, and to inform clinical management of this rare entity.
Methods
Case Selection
A retrospective clinicopathologic analysis of all patients who had a kidney biopsy diagnosis of ABBA between 2015 and 2023 was performed. The study was approved by Solutions Institutional Review Board. Sixty-seven cases were identified, including 57 new cases along with extended follow-up data from 10 previously reported cases.4,8 Seven cases were received in consultation with outside institutions, including 2 from Oregon Health Science University, 1 from the University of Pennsylvania, 1 from Centre Hospitalier de l'Universite de Montreal Pathologie in Canada, 1 from University of California-San Francisco and 2 from Ameripath Laboratories.
Inclusion criteria included the following: (i) acute tubular injury (ATI); (ii) the presence of IgG staining along TBMs and/or proximal tubular brush borders, and at least 1 of the following: (a) positive staining for LRP2 within TBM deposits, (b) positive serologic testing for antibrush border antibodies by indirect immunofluorescence assay (IFA).
Kidney Biopsy Processing
All samples were processed for light microscopy, immunofluorescence, and electron microscopy via standard techniques.11 Serial 3-μm tissue sections were stained with hematoxylin and eosin, periodic acid-Schiff, Masson's trichrome, Jones methenamine silver, and Silver methenamine Masson's trichrome stains. Frozen sections were stained with fluorescein-tagged polyclonal rabbit antihuman antibodies to IgG, IgA, IgM, C3, C1q, albumin, fibrinogen, and kappa and lambda light chains and evaluated with a semiquantitative 0 to 3+ scale.
For cases with sufficient tissue, IgG subclass staining was performed. IgG subclass antibodies included IgG1 (Sigma Aldrich # F0767, St. Louis. MO), IgG2 (Sigma Aldrich #F4516), IgG3 (Sigma Aldrich #F4641), and IgG4 (Sigma Aldrich #F9890). LRP2 staining was performed on formalin-fixed, paraffin-embedded tissue in all cases with mouse monoclonal antihuman LRP2 (1:1000; EMD Millipore, Billerica, MA) followed by a polyclonal (Rhodamine Red-X) goat antimouse IgG secondary antibody (Jackson ImmunoResearch, cat # 111-297-003). CUBN and AMN staining was performed by paraffin immunofluorescence in all cases with tissue remaining for evaluation (n = 59) with rabbit polyclonal anticubilin antibody (1:200, Thermo Fisher #PA5-83684, Waltham, MA) or rabbit polyclonal antiamnionless antibody (1:200, Sigma Aldrich #000817) followed by a polyclonal (Rhodamine Red) goat antimouse IgG secondary antibody (Jackson ImmunoResearch, cat # 111-297-003).
Additional antibodies were used in the characterization of selected cases. For cases with interstitial nephritis or plasma cell-rich interstitial infiltrates, IgG4 immunohistochemistry was performed. A cutoff of >10 IgG4+ plasma cells per high power field was used to evaluate whether IgG4+ cells were increased.12,13 Special stains for mycobacteria (acid fast bacilli) and fungal organisms (Grocott's methenamine silver) were performed when granulomatous inflammation was present to help exclude an infectious etiology. Cases with concurrent MN were stained with antibodies for phospholipase A2 receptor (rabbit polyclonal, Sigma-Aldrich, cat # HPA012657, 1:50), thrombospondin type 1 domain-containing 7A (rabbit polyclonal, Atlas antibodies, cat # AMAB91234, 1:50), exostosin 1 (rabbit polyclonal, Invitrogen, cat # PA5-60699, 1:50) and neural cell adhesion molecule 1 (mouse monoclonal, Invitrogen, cat # PA5-27958, 1:50). FITC-conjugated goat anti-mouse IgG (Jackson Immunoresearch 111-095-003, 1:100 dilution) or Rhodamine Red X Affinipure goat antirabbit IgG (Jackson Immunoresearch, cat # 111-295-144, 1:100 dilution) were used as secondary antibodies for mouse or rabbit primary antibodies, respectively.
Histopathologic Evaluation
Histopathologic parameters, including light microscopy, immunofluorescence, and electron microscopy were scored on all biopsies. Light microscopic evaluation included glomerular number, percentage of globally sclerotic glomeruli, presence or absence of segmental sclerosis, and presence or absence of spikes and/or holes along the capillary loops of glomeruli. Tubulointerstitial findings recorded included the presence or absence of acute tubular injury (ATI), interstitial edema, interstitial inflammation, tubulitis, and interstitial fibrosis and tubular atrophy (IF/TA). IF/TA was graded as mild, moderate, or severe using the thresholds of involvement by 10% to 25% of the cortical parenchyma as mild, 25% to 50% as moderate, and >50% tubulointerstitial scarring as severe. The presence of interstitial inflammation in areas of cortical parenchyma outside of regions of IF/TA was considered significant only if involving ≥25% of nonfibrotic cortical parenchyma. Tubulitis was considered significant if multifocal and present in nonatrophic tubules. In cases with both significant interstitial inflammation and tubulitis, a second diagnosis of acute interstitial nephritis was made. When significant interstitial inflammation and tubulitis involved ≥25% of both intact cortex and areas of IF/TA, a second diagnosis of chronic active interstitial nephritis was made. Inflammation within intact cortical parenchyma was required, as interstitial inflammation restricted to areas of IF/TA can be nonspecific.
Immunofluorescence findings included the presence or absence staining for immunoglobulins and complement components (IgA, IgG, IgM, C3, and C1q) along the glomerular capillary loops, Bowman's capsule, interstitium, TBMs, and proximal tubular brush borders. Each of these immune reactants, as well as IgG subclasses (IgG1, IgG2, IgG3, and IgG4) were evaluated on a 0 to 3+ scale of fluorescence intensity. LRP2, CUBN, and AMN staining was assessed for presence or absence within TBM deposits. Parameters evaluated by electron microscopy included the presence or absence of subepithelial, subendothelial, or mesangial immune deposits within glomeruli, as well as along TBMs.
IFA for Antibrush Border Antibodies
Serologic testing for ABBA was also performed on all patients where sera were available for evaluation (n = 30) including 10 with follow-up sera. The IFA examines for seroreactivity against proximal tubular brush borders in a semiquantitative manner. It is not antigen specific, and therefore, does not distinguish between LRP2, CUBN, and AMN as protein targets. For serologic testing, 4 μm frozen sections of normal human kidney were washed with Flex PBS buffer (Dako Corp., Carpinteria, CA) and patient serum was applied to tissue sections at 1:10 dilution in PBS-Tween-20 solution for 30 minutes at room temperature. Slides were reacted with fluorescein-conjugated polyclonal anti-human IgG (Kent Laboratories Inc., Bellingham, WA) as a secondary antibody at 1:200 dilution and incubated for 30 minutes, rinsed, and cover slipped with aqueous embedding medium. Slides were then evaluated by immunofluorescence microscopy for the presence or absence of staining along the proximal tubular brush borders. Cases with positive staining at the initial dilution (1:10) were further diluted (1:100, 1:500, and 1:1000) to determine a serologic titer. IgG subtype staining was performed in parallel, using the antibodies described above in place of fluorescein-conjugated polyclonal anti-human IgG.
Clinical Data
Medical information, including demographics and clinical and laboratory findings provided at the time of biopsy were obtained. Demographic data included age, race, and sex. Laboratory values included serum creatinine, quantitative proteinuria (urine protein-to-creatinine ratio or 24-hour urine collection), serum albumin level, serum complements (C3 and C4), serum protein electrophoresis results, urinalysis findings, and serologic studies. Serologic studies included antinuclear antibodies (ANA), antidouble stranded DNA antibodies, antineutrophil cytoplasmic antibodies (ANCA), as well as antibodies against HIV and viral hepatitis. Clinical data obtained included the presence or absence of hypertension, diabetes mellitus, obesity, cancer, and monoclonal gammopathy.
Acute kidney injury was defined as having an abrupt increase in serum creatinine (>50% from baseline when known), requirement for renal replacement therapy, or documentation of acute kidney injury within the patient's medical record. Chronic kidney disease was defined as having a glomerular filtration rate of ≤60 ml/min without concurrent acute kidney injury, renal dysfunction of more than 3 months, or having chronic kidney disease listed within the patient's medical record. Hematuria was identified by either having positive blood on dipstick or presence of red blood cells by urine microscopy (≥ 5 RBC/hpf). Hypoalbuminemia was defined as serum albumin <3.5 g/dl. Nephrotic syndrome was defined as urinary protein of greater than ≥3.5 g/24-hours, by urine protein-to-creatinine ratio as well as hypoalbuminemia.
Clinical follow-up data, including treatment and outcomes were also obtained. Regarding outcome analysis, patients were stratified based on the following parameters: (i) complete response or remission (CR): serum creatinine ≤1.2 mg/dl, (ii) significant response: ≥50% reduction in serum creatinine, (iii) partial response (PR): ≤50% reduction in creatinine, and (iv) NR, for which patients did not meet the above criteria. These included outcomes of no improvement, progressive disease, end-stage kidney disease (ESKD), or death.
Statistical Analysis
Continuous variables were assessed with mean ± SD for parametric continuous variables and sample medians for nonparametric variables. Categorical variables were compared using Fisher's exact test.
Results
Clinical and Laboratory Data
ABBA is exceedingly rare. Of 98,785 kidney biopsies evaluated over a 6-year period, 50 patients with ABBA were identified in our laboratory, comprising 0.05% of total cases. Seventeen additional cases were studied (including 10 previous cases from Arkana4 and 7 from outside institutions), with a final cohort of 67 patients and 73 total biopsies. Sixty-five patients had ABBA in the native kidney and 2 within allografts.
Patients with ABBA were predominately males (74.6%) with a median age of 72 years. The majority of patients were White (83%). Medical comorbidities were common, with 37.3% having diabetes, 56.7% with hypertension, 16.4% being obese (body mass index ≥30 kg/m2), and 31.3% having cancer (Table 1). Of the patients, 41.3% had monoclonal gammopathy, of which 7 had small B cell lymphoma, 3 had plasma cell dyscrasia, and 9 were of undetermined significance. Solid organ malignancies were present in 11, including prostate cancer (n = 4), breast cancer (n = 2), melanoma (n = 2, both remote), kidney cancer (n = 1), meningioma (n = 1), and thymoma (n = 1). No patients were treated with immunotherapy (i.e., checkpoint inhibitors). Autoimmune diseases were observed in 10 patients, including 5 with hypothyroidism, 2 with polymyalgia rheumatica, 1 with immune thrombocytopenic purpura, 1 with myasthenia gravis, and 1 with systemic lupus erythematosus, likely representing polyautoimmunity.14, 15, 16 Four patients required dialysis at presentation.
Table 1.
Demographic and clinical laboratory data at the time of biopsy
| Demographic data | |
| Male/female | 50 M/17 F |
| Age (yr), median (range) | 72 years (range, 24–91 yrs) |
| ≤60, n (%) | 8/67 (11.9%) |
| 61–70, n (%) | 18/67 (26.9%) |
| 71–80, n (%) | 29/67 (43.3%) |
| 81–90, n (%) | 12/67 (17.9%) |
| Race (%, of those with known race) | |
| White | 83.3% |
| Black | 4.2% |
| Hispanic | 8.4% |
| Asian | 4.2% |
| Comorbidities | |
| Hypertension, n (%) | 38 (56.7%) |
| Diabetes, n (%) | 25 (37.3%) |
| Obesity, (BMI >30 kg/m2), n (%) | 11 (16.4%) |
| Prior/concurrent malignancy, n (%) | 21 (31.3%) |
| Laboratory data at time of biopsy | |
| Creatinine (mg/dl), median (range) | 2.7 (0.91–14.7) |
| Proteinuria (g/day), median (range) | 2.8 (0.34–10.7) |
| Serum albumin (g/dl), mean (SD) | 3.3 ± 0.87 |
| Hypoalbuminemia | 17/37 (45.9%) |
| Hematuria, n (%) | 30/45 (66.7%) |
| Nephrotic syndrome, n (%) | 12/54 (22.2%) |
| Monoclonal paraprotein, n (%) | 19/46 (41.3%) |
| Positive ANA, n (%) | 21/42 (50%) |
| Positive dsDNA, n (%) | 7/29 (24.1%) |
| Positive ANCA, n (%) | 6/36 (16.7%) |
| Positive ABBA, n (%)a | 26/30 (86.7%) |
| Hypocomplementemia, n (%) | 8/36 (22.2%) |
| Low C3 only, n (%) | 4/8 (50%) |
| Low C4 only, n (%) | 0 |
| Low C3 + C4, n (%) | 4/8 (50%) |
ABBA, anti-brush border antibodies; ANA, antinuclear antibodies; ANCA, anti-neutrophil cytoplasmic antibodies; BMI, body mass index; dsDNA, double stranded DNA antibodies; n, number of patients; yr, year.
ABBA were detected by indirect immunofluorescence on normal kidney tissue sections.
The majority of patients had acute and/or chronic kidney failure at the time of diagnosis with a mean estimated glomerular filtration rate of 26.6 ± 17.4 ml/min and median creatinine of 2.7 mg/dl. Ninety-five percent of patients had proteinuria >0.5 g/day, nephrotic range in 29.3%, and 22.2% had nephrotic syndrome. Hematuria was seen in most patients (Table 1). Eight patients had hypocomplementemia. Three patients with hypocomplementemia had a concurrent proliferative GN (1 LN, 1 crescentic IgA nephropathy, and 1 proliferative and crescentic GN). Evaluation for Fanconi syndrome, including urine pH, electrolytes, and amino acids was not performed for the majority of patients; however, those tested lacked abnormalities of proximal tubular dysfunction, such as normoglycemic glycosuria, hypophosphatemia, hyponatremia, hypokalemia, hypouricemia, or metabolic acidosis.
Serologic studies revealed 50.0% of patients had antinuclear antibodies. Six patients were ANCA-positive (3 PR3+, 0 MPO+, 1 atypical ANCA that was both PR3- and MPO-negative, and 2 were positive by IFA; MPO/PR3 ELISA was not performed). One patient with ANCA-positivity had crescentic GN on biopsy. In all patients tested, 2 patients were hepatitis B-positive (1 HBsAg-positive, other status unknown), 1 was hepatitis C positive, and there were no HIV-positive patients. Serum for ABBA testing was available for 30 patients, with 26 demonstrating seroreactivity against the proximal tubular brush borders in an IFA (Table 1).
Histopathologic Features
Light microscopic evaluation revealed that 95.9% of patients demonstrated ATI, with the majority having interstitial edema. There was disproportionate interstitial fibrosis (moderate to severe in 65.2%) to the degree of glomerulosclerosis (mean 26.3% ± 19.0%, Table 2). ATI was characterized by a spectrum of epithelial thinning and simplification, loss of proximal tubular brush borders, apical cytoplasmic blebbing, and reactive appearing nuclei (Figure 1 and Supplementary Figure S1). TBM deposits were not readily visible by light microscopy in the majority of cases, although when present were characterized by the presence of periodic acid-Schiff-positivity or fushinophilia along the TBMs on Trichrome stain. Interstitial inflammation in intact cortical parenchyma was seen in 61.6%, and 26.0% had tubulitis. One biopsy showed granulomatous inflammation. Considering that a previous case study reported increased IgG4 cells in LRP2 nephropathy,8 all cases with interstitial inflammation that were plasma cell-rich were stained for IgG4 (n = 12). Four cases showed increased IgG4 plasma cells. These patients did not have systemic evidence of IgG4-related disease (although serum IgG4 levels were not available in all patients). The majority of biopsies showed mildly thickened glomerular basement membranes with segmental holes along the glomerular capillary loops on silver stains (Table 2 and Figure 1). Proliferative changes (such as endocapillary hypercellularity, fibrinoid necrosis, or crescents) were not present in the majority of cases.
Table 2.
Overview of histopathologic findings (N = 73 biopsies from 67 patients)
| Pathologic finding | Value |
|---|---|
| Light microscopy | |
| No. of glomeruli (mean ± SD) | 17.8 ± 14.2 |
| Percent globally sclerotic glomeruli (mean ± SD) | 26.3 ± 19.0 |
| Cases with segmental GBM holes via Jones silver stain, n (%) | 41 (56.2%) |
| Acute tubular injury, n (%) | 70 (95.9%) |
| Interstitial edema, n (%) | 49 (67.1%) |
| Tubulitis, n (%) | 19 (26.0%) |
| Interstitial inflammation (N = 70): none/mild/moderate/severe | none 25/70 (35.7%), mild 24/70 (34.3%), moderate 15/70 (21.4%), severe 6/70 (8.6%) |
| Increased Interstitial IgG4 cells, n (%) >10 IgG4+ plasma cells/high power field |
4 (5.4%) |
| Tubular atrophy: none/mild/moderate/severe | none 4/72 (5.6%), mild 21/72 (29.2%), moderate 33/72 (45.8%), severe 13/72 (18.1%) |
| Interstitial fibrosis: none/mild/moderate/severe | none 6/72 (8.3%), mild 19/72 (26.4%), moderate 32/72 (44.4%), severe 15/72 (20.8%) |
| Immunofluorescence | |
| IgG along proximal tubular brush borders, n (%) | 56/73 (76.7%) |
| IgG granular staining along TBMs, n (%) | 72/73 (98.6%) |
| C3 granular staining along TBMs, n (%) | 47/73 (64.3%) |
| IgG in interstitium, n (%) | 1/73 (1.4%) |
| IgG granular staining along capillary loops, n (%)a | 57/72 (79.2%) |
| Segmental (<50%) | 53/72 (73.6%) |
| Global (≥50%) | 4/72 (5.6%) |
| C3 granular staining along capillary loops, n (%)a | 34/72 (47.2%) |
| IgG staining along Bowman's capsule, n (%)a | 52/72 (72.2%) |
| Segmental (<50%) | 27/49 (55.1%) |
| Global (≥50%) | 22/49 (44.9%) |
| LRP2 immunofluorescence TBM positivity, n (%)a | 65/69 (94.2%) |
| IgG1 staining; dominance (strongest intensity) | 44/49 (89.8%); 20/49 (40.8%) |
| IgG2 staining; dominance | 7/49 (14.3%); 0/49 (0%) |
| IgG3 staining; dominance | 1/49 (2.0%); 0/49 (0%) |
| IgG4 staining; dominance | 42/49 (85.7%); 12/49 (24.5%) |
| IgG1 and IgG4 co-dominance | 17/49 (34.7%) |
| Electron microscopy (N = 63) | |
| Tubular basement membrane deposits, n (%)b | 55 (87.3%) |
| Subepithelial electron-dense deposits, n (%)b | 44 (69.8%) |
| Subendothelial electron-dense deposits, n (%)b | 2 (3.2%) |
| Mesangial deposits, n (%)b | 19 (30.2%) |
GBM, glomerular basement membrane; SD, standard deviation; TBMs, tubular basement membranes.
One case did not have glomeruli available by immunofluorescence.
Electron microscopy was not performed in 10 of the cases.
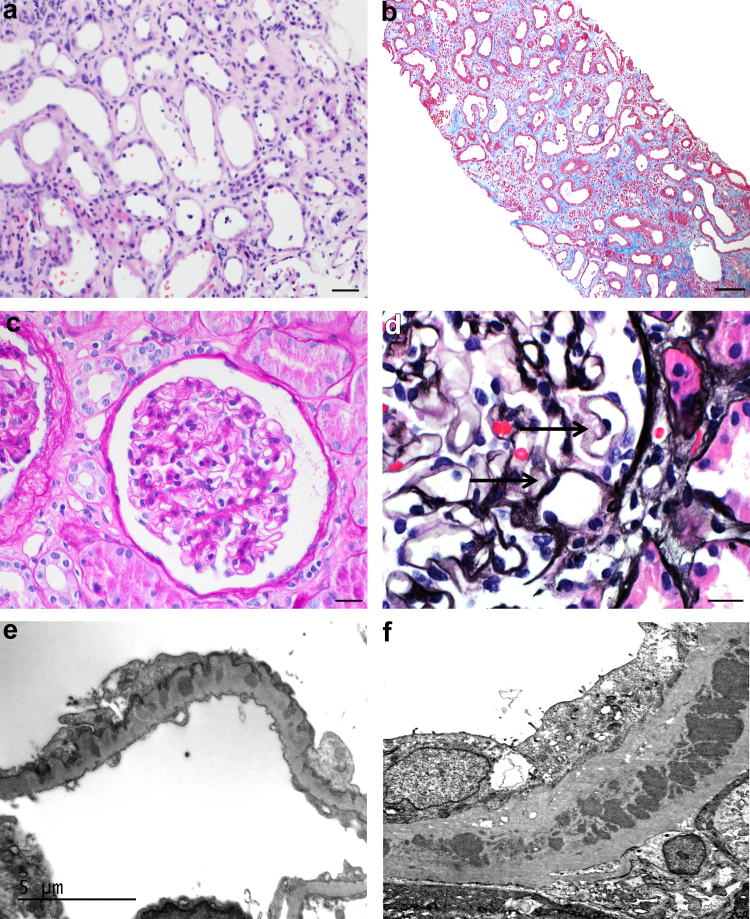
Figure 1.
Microscopic features of typical ABBA. (a) Acute tubular injury with epithelial simplification, tubular dilatation, and interstitial edema (hematoxylin and eosin stain, original magnification 400x, scale bar = 20 μm). (b) Acute on chronic tubular injury with increased spacing between injured tubules due to interstitial fibrosis (Masson-Trichrome-stain, original magnification 100x, scale bar = 100 μm). (c) Unremarkable glomerulus (Periodic acid-Schiff reaction, original magnification 400x, scale bar = 20 μm). (d) Focal segmental vacuoles along the glomerular capillary loops consistent with intramembranous immune deposits (arrows, Jones methenamine Silver stain, original magnification 600x, scale bar = 20 μm). (e) Electron photomicrograph showing subepithelial and intramembranous electron-dense immune-type deposits with podocyte foot process effacement (unstained section original magnification 12000x, scale bar = 5 μm). (f) Electron photomicrograph displaying large TBM deposits (unstained section original magnification 12000x).
Immunofluorescence studies demonstrated IgG staining along the proximal tubular brush borders in the majority of cases (Figure 2). Of the biopsies without brush border staining, most demonstrated TBM deposits in addition to glomerular staining along Bowman's capsule and/or the glomerular capillary loops. In some biopsies, the ATI was severe with loss of proximal tubular brush borders and tubular epithelial cell sloughing. IgG staining may be absent along brush borders in these cases, which is likely “false negative” because the brush borders were lost or severely attenuated in the setting of severe ATI (Supplementary Figure S2). Conversely, a lack of TBM deposits was rarely observed in the setting of mild ATI. In this instance, an IFA for ABBA within sera was used for disease confirmation (Supplementary Figure S3).
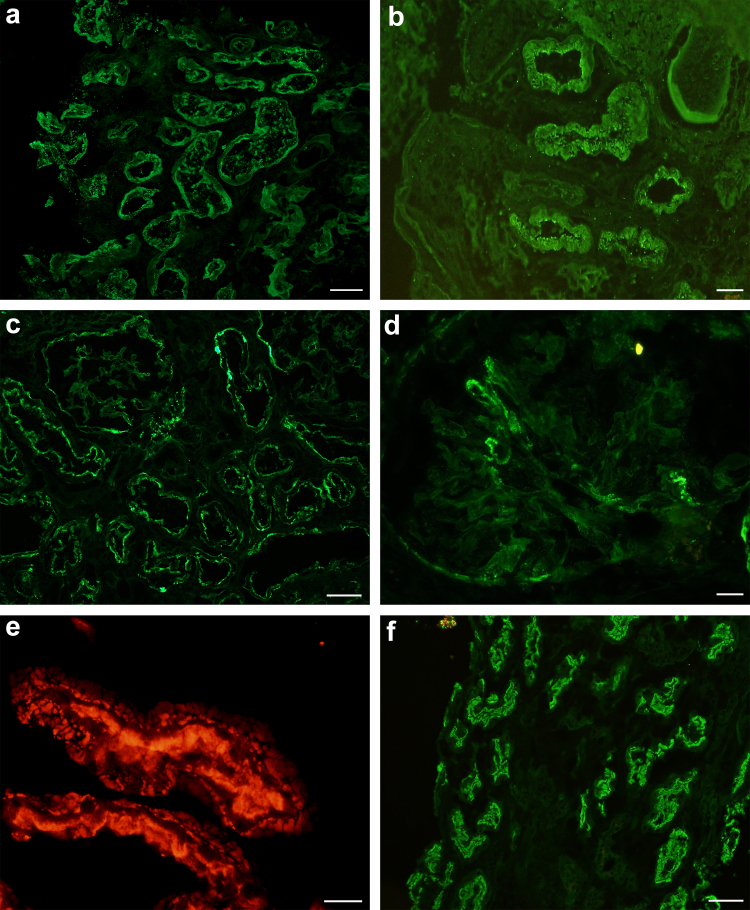
Figure 2.
Classic immunofluorescence features of ABBA. (a) IgG staining along TBMs and proximal tubular brush borders (fluorescein-conjugated anti-human IgG, original magnification 200x, scale bar = 50 μm). (b) IgG staining highlighting proximal tubular brush border and tubular epithelial cell cytoplasm (fluorescein-conjugated anti-human IgG, original magnification 400x, scale bar = 20 μm). (c) Granular IgG staining along TBMs and Bowman's capsule of a glomerulus (fluorescein-conjugated anti-human IgG, original magnification 200x, scale bar = 50 μm. (d) Segmental IgG staining along glomerular capillary loops and Bowman's capsule (fluorescein-conjugated anti-human IgG, original magnification 400x, scale bar = 20 μm). (e) LRP2 staining highlighting TBM deposits in addition to proximal tubular brush border staining (paraffin immunofluorescence staining for LRP2, original magnification 600x, scale bar = 20 μm). (f) Indirect immunofluorescence of ABBA sera demonstrates seroreactivity against the proximal tubular brush borders of normal human kidney (fluorescence conjugated anti-human IgG, original magnification 200x, scale bar = 50 μm).
There was granular TBM staining for IgG in all but 1 case (98.6%), with concomitant staining of C3 in 64.3%. C3 staining was seen along TBMs in patients with normal serum complement levels as well as those with hypocomplementemia (5/8 patients). One case demonstrated granular TBM staining with IgA, 2 with IgM, and 1 had C1q staining along TBMs. Only 1 patient had interstitial IgG staining and no IgG staining was observed within vessels. The immune deposits were polyclonal in all but 1 case, which demonstrated IgG1 lambda restriction along the proximal tubular brush borders and within TBM deposits. This case had concurrent IgG staining along Bowman's capsule, segmental IgG staining along the glomerular capillary loops, and LRP2-positive immune deposits as well as positive serology for ABBA, which are characteristic of other cases of ABBA disease. However, mono-isotypic staining is unusual and it is unclear whether this represents a monoclonal gammopathy of renal significance.
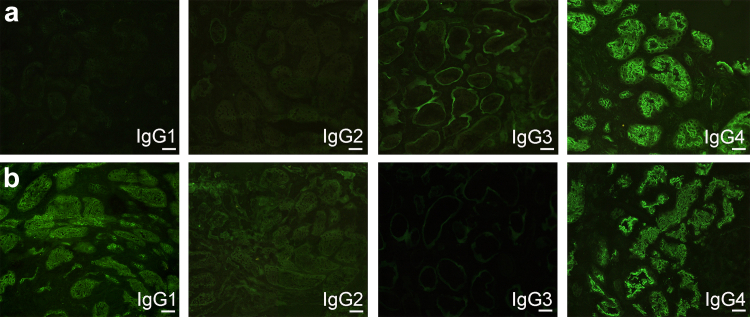
IgG subclass staining was performed in 49 cases. IgG1 staining of TBM deposits was present in 89.8%, IgG2 in 14.3%, IgG3 in 2.0%, and IgG4 in 85.7%. IgG1-dominant staining was more common, followed by IgG1/IgG4 codominance (Table 2 and Figure 3). Sixty-five cases (94.2%) demonstrated staining of LRP2 within TBM deposits (Figure 2). One case demonstrated staining for LRP2, CUBN, and AMN within TBM deposits (Supplementary Figure S6). This case lacked IgG staining along proximal tubular brush borders and had confluent TBM deposits visible by light microscopy, similar features as described in a reported case of ABBA due to antibodies against CUBN and AMN.17 There were no other cases with CUBN or AMN in TBM deposits (n = 58). The finding of positive LRP2 staining in the TBMs was confirmed to be specific for ABBA. The LRP2 stain was negative in 58 renal biopsies with TBM deposits that were not ABBA, including 21 cases of LN with TBM deposits, 4 cases of chronic tubulointerstitial nephritis associated with graft-versus-host disease, 16 cases of ATI with TBM deposits, and 17 cases of IgG4-related kidney disease. Specificity was also established in a prior study of an additional 50 control cases, including 40 with TBM deposits.4
Figure 3.
IgG subclass staining in selected cases of ABBA. (a) IgG4 dominant staining along proximal tubular brush borders (fluorescein-conjugated anti-human IgG1/2/3/4 subclass antibodies, original magnification 400x, scale bar = 20 μm). (b) IgG1 and IgG4 co-dominant staining along proximal tubular brush borders (fluorescein-conjugated anti-human IgG1/2/3/4 subclass antibodies, original magnification 400x, scale bar = 20 μm).
Ig staining within glomeruli was present in nearly all cases. Segmental glomerular capillary loop staining was seen with IgG and C3 in 79.2% and 47.2% of cases, respectively (Figure 2). Granular staining with IgG was seen along Bowman's capsule in 72.2% cases, which was global in 55.1% of cases and segmental in 44.9% of cases. There was no predilection for the tubular pole (Table 2). There was mesangial staining for IgA in 9.6%, IgM in 9.6%, and C1q in 7.7% biopsies. Four cases had global IgG capillary loop deposits, with a second diagnosis of MN. In the vast majority of cases, there was no staining for LRP2 within the glomerular immune deposits, which was only observed in a single case (Supplementary Figure S5).
Tissue was available for electron microscopy in 63 cases. Ultrastructural analysis showed granular electron dense deposits within the TBMs and subepithelial glomerular capillary loop deposits in the vast majority of cases (Table 2). Approximately one-third of cases had mesangial immune deposits and subendothelial deposits were rare (Table 2). Electron dense deposits were not seen along the proximal tubular brush borders.
“Secondary” Diagnoses in Patients With ABBA
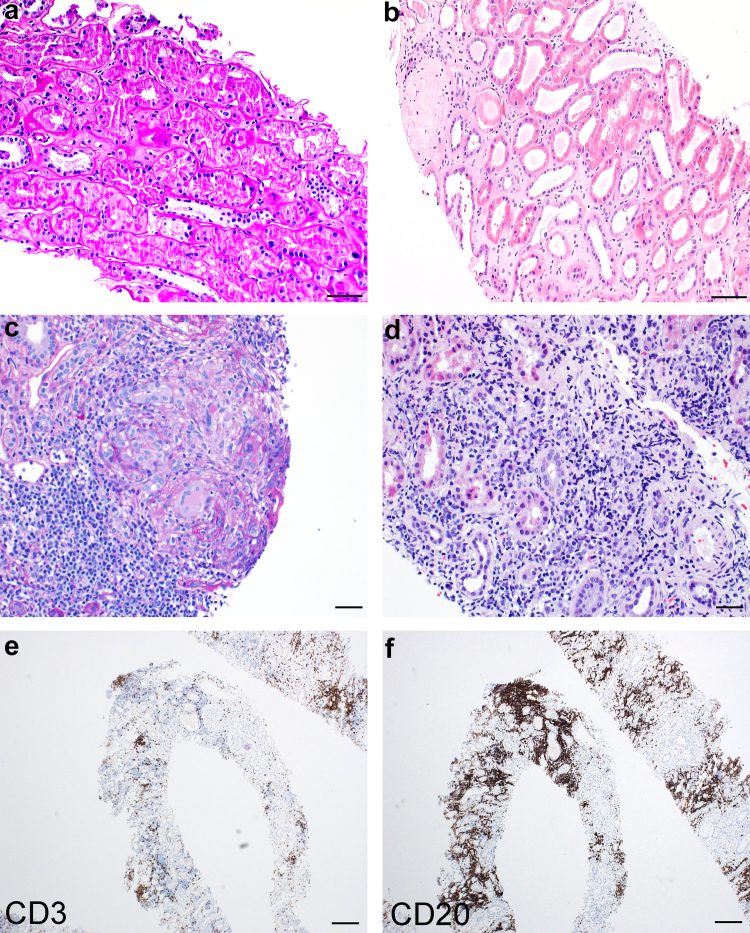
Concurrent histopathologic diagnoses were made in 38 (56.7%) cases, including both glomerular and tubulointerstitial diseases. Coincident tubulointerstitial diseases were seen in 7 patients and included acute interstitial nephritis (n = 2), nonnecrotizing granulomatous interstitial nephritis (n = 1), myoglobin cast nephropathy (n = 1), and involvement by small B-cell lymphoma (n = 3) (Figure 4). Serum complement levels were available for 2 of the 3 patients with interstitial nephritis and were normal, ruling out idiopathic hypocomplementemic interstitial nephritis. Two patients with renal involvement by lymphoma had chronic lymphocytic leukemia and 1 had lymphoplasmacytic lymphoma. Vascular diseases were seen in 7 patients, with arterionephrosclerosis (n = 6) and antibody-mediated rejection (n = 1).
Figure 4.
Histopathologic spectrum of tubulointerstitial findings in ABBA. (a) Mild acute tubular injury, characterized by apical cytoplasmic blebbing and attenuation of brush borders of the proximal tubules (PAS stain, original magnification 200x, scale bar = 50 μm). (b) Acute on chronic tubular injury with interstitial fibrosis and edema separating tubular profiles, epithelial simplification, and dilation (hematoxylin and eosin stain, original magnification 200 x, scale bar = 50 μm). (c) Granulomatous interstitial nephritis, characterized by lymphocytic interstitial inflammation, epitheliod histiocytes, and a giant cell reaction (PAS stain, original magnification 400x, scale bar = 20 μm). (d) Dense monomorphic lymphoid infiltrate within interstitium (hematoxylin and eosin stain, original magnification 400x, scale bar = 20 μm). (e) CD3 immunohistochemistry of the case shown in (d) demonstrating CD3+ T cells make up the minority of the interstitial lymphoid infiltrate (CD3 immunoperoxidase staining, original magnification 40x, scale bar = 200 μm). (f) CD20 immunohistochemistry of the case shown in (d) and (e) showing a predominance of CD20+ B cells within the lymphoid infiltrate, consistent with involvement by a low-grade B cell lymphoma in a patient with known lymphoplasmacytic lymphoma (CD20 immunoperoxidase staining, original magnification 40x, scale bar = 200 μm). ABBA, antibrush border antibody disease; PAS, periodic acid-Schiff; TBM, tubular basement membrane.
Glomerular diseases were seen in 24 patients with ABBA (Supplementary Figures S3–S6). Two patients had 2 diagnoses. Diagnoses included membranous glomerulopathy (n = 4), minimal change disease (n = 5), primary focal segmental glomerulosclerosis (tip variant, n = 1), diabetic glomerulopathy (n = 7), IgA nephropathy (n = 3), proliferative LN (n = 1), pauci-immune crescentic GN (n = 1), proliferative immune complex-mediated GN (without a history of lupus, n = 3) and amyloid light chain amyloidosis (n = 1) (Supplementary Figure S6). Membranous glomerulopathy cases were phospholipase A2 receptor, thrombospondin type 1 domain-containing 7A, and exostosin 1-negative. One patient with concurrent MN was hepatitis B positive (HBsAg-positive) and it cannot be ruled out that the patient's disease was secondary to hepatitis B virus infection. The other 3 patients with MN did not have a known inciting trigger (i.e., lupus, malignancy, infection, or medication), although 1 was antinuclear antibodies-positive. All patients with podocytopathy had nephrotic range proteinuria. Although 19 patients had a monoclonal paraprotein identified on SPEP, UPEP, or immunofixation, only 1 patient had definite evidence of a monoclonal gammopathy of renal significance (amyloid light chain amyloidosis). This rate of monoclonal gammopathy is much higher than that of the age-adjusted population, which is 3% to 5% in adults over 50 years.18 Of patients with crescentic GN, 1 had a positive p-ANCA serology.
Clinical Treatment and Outcomes
Follow-up data were available for 51 patients (76.1%). The median follow-up interval was 199 days (range = 24–1014 days for patients alive at follow-up). Patients received various treatments because there is currently no standard of care. The majority of patients received at least 1 type of immunosuppressive therapy (58.8%). Of those on immunosuppression, 12 received corticosteroids only, 3 received rituximab only, and 15 received a combination of steroids along with 1 or 2 other agents, including mycophenolate mofetil, tacrolimus, or rituximab. Four patients were treated with other modalities with 2 patients treated with a sodium-glucose cotransporter-2 inhibitor. Four patients were treated for underlying hematologic malignancy with 1 or more of the following: bortezomib, daratumumab, vincristine, cyclophosphamide, and/or doxorubicin with or without concurrent immunosuppression. Seventeen patients were not treated (33.3%). Plasmapheresis was performed in 2 patients, both of whom were transplant recipients.
CR was achieved for 5 patients (9.8%), whereas 3 patients (5.9%) had a significant response with ≥50% decrease in serum creatinine from the time of presentation. Seven patients (13.7%) had a PR with ≤50% reduction in serum creatinine. The majority (n = 36, 70.6%) had no NR or disease progression. Nineteen patients with NR had kidney failure or were deceased, with 11 developing ESKD and 8 deceased at follow-up. Of the deceased patients, 4 opted for only palliative care and died of kidney failure, 1 died from multiple myeloma and 2 died of sepsis (1 from disseminated Varicella zoster and 1 from methicillin-resistant Staphylococcus aureus bacteremia); the cause of death of the remaining patient was unknown although likely not ESKD because there was no dialysis requirement prior to death. Four patients underwent kidney transplantation, 2 of which had disease recurrence in the allograft.
Fifteen patients were treated with a single agent (9.8%). Of the 12 patients treated with corticosteroids alone, 2 had CR (16.7%), 1 had PR (8.3%), 6 had NR (75.0%), and 5 of the 9 NR patients developed ESKD requiring dialysis. Three patients discontinued steroid therapy due to complications. In the 3 patients who received rituximab only, all had NR. Of those who had received combination therapy (29.5%), 3 patients had CR (23.1%), 4 had significant response (30.8%), 2 PR (15.4%), and 4 had NR (30.8%). Patients who received combination therapy were significantly more likely to achieve complete or partial remission (P = 0.006). Of untreated patients (n = 14), and 12 had NR. Untreated patients were significantly more likely to develop ESKD (P = 0.049, Table 3). Patients with a secondary concurrent kidney disease were not at increased risk of developing ESKD. A summary of outcome data is provided in Table 3 and clinical details of patients achieving remission and not achieving remission are included in Supplementary Tables S1–S3, respectively.
Table 3.
Clinical outcomes by treatment type (N = 51 patients)
| Treatment | n | CR | SR | PR | NR | ESKD or death | aP |
|---|---|---|---|---|---|---|---|
| No immunosuppression | 17/51 (33.3%) | 0/17 (0%) | 0/17 (0%) | 1/17 (5.9%) | 16/17 (94.1%) | 8/16 with NR | N/A |
| Corticosteroids | 12/51 (23.5%) | 2/12 (16.7%) | 0/12 (0%) | 1/12 (8.3%) | 9/12 (75.0%) | 5/9 with NR | 0.28 |
| Rituximab | 3/51 (5.9%) | 0/3 (0%) | 0/3 (0%) | 0/3 (0%) | 3/3 (100%) | 0/3 with NR | 1.00 |
| Combination | 15/51 (29.4%) | 3/15 (20%) | 3/15 (20%) | 5/15 (33.3%) | 4/15 (26.7%) | 4/4 with NR | 0.0004 untx vs. treated |
| Other | 4/51 (7.8%) | 0/4 (0%) | 0/4 (0%) | 0/4 (0%) | 4/4 (100%) | 2/4 with NR | 1.00 |
| Total | 51/51 (100%) | 5/51 (9.8%) | 3/51 (5.9%) | 7/51 (13.7%) | 36/51 (70.6%) | 19/36 with NR (52.8%) | 0.01 untx vs. treated |
CR, complete response/remission; ESKD, end-stage kidney disease; n, number of patients; NR, no response; PR, partial response; SR, significant response; untx, untreated.
CR: Return of normal kidney function (Cr ≤1.2).
SR: Decrease in creatinine of ≥50%.
PR: Decrease in serum creatinine of ≤50%.
NR: Failure to meet above parameters, ESKD, or death.
P-values represent contingency analysis using Fisher's exact test, comparing remission to no response.
P-values represent Fisher's exact tests with comparison to the no immunosuppression group.
Serologic Outcomes
Regarding ABBA serologic testing, 30 patients had an IFA for ABBA performed at the time of biopsy, of which 21 had available follow-up data. In addition, 10 had subsequent serum testing at follow-up (range = 95-1307 days from the time of first serum collection). Although serologic testing was not available for all patients, there were no significant differences between patients who underwent serologic testing compared to those who did not (Supplementary Table S4).
IgG subclasses of ABBA were determined in 21 patients. Fourteen patients had antibodies of IgG1 subclass, 4 IgG2, and 18 IgG4. IgG4 antibodies were predominant in the majority of patients (11/21), followed by IgG1 + IgG4 co-dominance (6/21), and IgG1 dominance (4/11). There were no IgG3 antibrush border antibodies detected. ABBA testing of patient sera demonstrated IgG4 predominance, whereas deposits along TBMs were most often of IgG1 subclass or IgG1/IgG4 co-dominance. Fourteen patients had both serum and residual tissue for IgG subclass analysis. The majority of patients demonstrated IgG4-predominance in sera (n = 8). In comparison, IgG subclasses in tissue showed primarily IgG1 and IgG4 co-dominance (n = 11). There were no patients with IgG1 ABBA that did not show IgG1 staining in the corresponding biopsy tissue (Supplementary Table S5). It is possible that IgG1 to IgG4 isotype switching may occur later in disease , as antibodies in circulation are produced later than those already deposited within the kidney. It has been shown in MN that IgG1 to IgG4 isotype switching occurs later in the disease, for which a similar phenomenon may occur.19
Positive titers were present in 27 patients (1:10 to 1:1000) and absent in three. Six patients had a titer of 1:10, 12 with 1:100, 3 with 1:500, 6 with 1:1000 titer, and 3 had no ABBA detected. All 3 seronegative patients demonstrated TBM IgG deposits with LRP2 staining. Eight of the 10 patients with ABBA testing at follow-up had a decreased antibody titer or were negative for ABBA. Of those with reduced or absent antibodies, 3 achieved CR, 1 had a significant response, 1 had a PR, and 3 showed NR. For the 2 patients without a drop in antibody titer, both showed NR.
Discussion
We present the largest cohort of 67 patients and 73 biopsies with ABBA to expand our understanding of the disease. Prior to this study, only 20 ABBA cases were described in the literature.20 The majority of previously described patients were elderly males who presented with a decline in kidney function and subnephrotic range proteinuria, a common demographic and clinical presentation in our series. Although the disease frequency of ABBA is rare (making up only 0.05% of total biopsies), underrecognition is likely, given the insidious onset of disease in an elderly population. Older patients often have medical comorbidities, and in this setting, a slow decline in kidney function with only subnephrotic range proteinuria might be assumed to be due to diabetic nephropathy or hypertensive nephrosclerosis with these patients being less likely to undergo biopsy.
Many patients in our series had an atypical presentation with nephritic and/or nephrotic syndrome due to concurrent glomerular or tubulointerstitial diseases. Prior to this study, a total of 7 patients with ABBA with concurrent kidney diseases were reported, including minimal change disease,6 LN,7 IgG4-related kidney disease,8 and lymphoma.9,10 A second kidney disease diagnosis was identified in the majority of patients (56.7%), of which included MN, IgA nephropathy, LN, minimal change disease, primary focal segmental glomerulosclerosis, crescentic GNs, nodular diabetic glomerulosclerosis, amyloid light chain amyloidosis, acute interstitial nephritis, granulomatous interstitial nephritis, renal involvement by lymphoma or amyloidosis, arterionephrosclerosis, or antibody-mediated rejection. The histopathologic spectrum of ABBA has broadened with improved recognition, facilitated by the use of LRP2 immunostaining for disease confirmation in clinical practice. Given this broad disease spectrum, ABBA may be overlooked due to overlap with tubulointerstitial features of the “secondary” kidney diseases, such as TBM deposits being common in other autoimmune diseases, such as lupus (Supplementary Figure S6). It is important to have a high index of suspicion for ABBA in biopsies with ATI and TBM deposits, despite presence of a concurrent disease.
Interestingly, both hematologic and solid organ malignancies were common among ABBA patients. Nineteen patients had monoclonal gammopathy, of which 7 had small B cell lymphoma, 3 had plasma cell dyscrasia, and 9 were of undetermined significance. This rate of monoclonal gammopathy (41.3% with available data) is nearly 8-fold higher than in the age-adjusted population.18 It is uncertain if ABBA could be triggered secondary to malignancy or if it may represent a paraneoplastic disease manifestation. Of note, in 2 patients with concurrent lymphoma, 1 went into remission and 1 had stable kidney function following successful treatment of the underlying malignancy.
There are challenges to the diagnosis of ABBA due to differential diagnostic considerations with some overlapping features of the disease. IgG staining along proximal tubular brush borders could be missed because it is often limited to the better-preserved tubules that do not show loss or attenuation of the brush borders, which could be focal. In some cases of ABBA, TBM deposits can be dense and nearly confluent which may appear linear, possibly mimicking anti-TBM disease. Electron microscopy is useful in this setting, because electron dense deposits are detected along TBMs in ABBA but not anti-TBM disease. Extraglomerular staining along Bowman's capsule and along glomerular basement membranes is also not observed in anti-TBM disease. Cases of ABBA disease with concurrent plasma cell-rich interstitial inflammation can mimic IgG4-related kidney disease, which demonstrates IgG deposition within glomeruli in addition to TBM. Other autoimmune tubulointerstitial kidney diseases with morphologic overlap include idiopathic hypocomplementemic interstitial nephritis and extraglomerular staining in the setting of lupus and rarely in Sjogren's syndrome (Supplementary Figure S7). Utilization of LRP2 as a biomarker of disease and/or evaluation for serum antibodies can confirm diagnosis.
Although megalin is the most common antigen in ABBA, CUBN, and AMN were also identified as targets, with identification of circulating antibodies and detection of CUBN and AMN in TBM deposits reported in a recent case, further supporting immunogenicity of the protein complex.17 In this series, we observed a single case with AMN and CUBN within immune deposits in addition to LRP2, suggestive of epitope spreading to include all components of the megalin-cubilin-amnionless complex. Tubular components are known to be antigenic from animal models, because rats immunized with tubular extracts form autoantibodies directed against megalin and develop an immune complex-mediated kidney disease that mimics MN in humans.21
Immunosuppression may be beneficial in the treatment of ABBA, despite the overall poor prognosis in our cohort. Of 51 patients with available data, 36 (70.6%) did not achieve remission with 52.8% developing ESKD or death at follow-up. This was particularly pronounced among patients who did not receive any form of immunosuppression with 94.1% of those demonstrating disease progression. Steroid therapy provided a modest benefit, although statistically insignificant. Combination therapy with corticosteroids in addition to other immunosuppressive agents had superior outcomes, with 73.3% patients achieving partial remission or CR. ABBA patients previously reported in the literature also demonstrated a potential benefit of immunosuppression. Of 17 patients with available data (combined from multiple studies),20 11 (64.7%) developed ESKD or death, 4 (23.5%) had chronic kidney disease not requiring dialysis, and only 2 (11.8%) achieved CR. Both of those patients who achieved remission received combination immunosuppression. Thus albeit limited, our cohort and prior data suggest that patients with ABBA may benefit from immunosuppressive therapy.
Patients with concurrent glomerular diseases had a trend toward better outcomes, for reasons of which are unclear. These patients were not more likely to receive immunosuppressive therapy; however, there may be earlier recognition of the disease from a concurrent disease process with an acute presentation (such as nephrotic syndrome). It is likely that treatment of a concurrent glomerular disease improves overall outcomes. It is possible, and likely, that high-grade proteinuria from a disease such as a podocytopathy, MN, LN, among others incites ABBA through high-grade proteinuria overwhelming the capacity for proximal tubular epithelial cell reabsorption of protein, inducing injury and sensitization events. Therefore, correction of an inciting disease process could abrogate ABBA. Earlier studies have shown poor outcomes in patients with ABBA4; therefore, without treatment, it is possible that these patients may be at higher risk of disease progression and that recognition of a concurrent disease is important.
Limitations of our study are primarily related to the retrospective design. Follow-up intervals were variable and 32.9% of cases did not have follow-up. Clinical laboratory data were incomplete and blood samples were not available from all patients for evaluation of ABBA. Of those tested for ABBA, only a subset had follow-up sera available for comparison of antibody titers. There were no standardized doses or durations of immunosuppressive therapies, and we cannot exclude selection bias in those treated with more aggressive combined immunosuppression because these patients may have had a higher likelihood of recovery. No standard-of-care treatment exists for disease management due to its low frequency and lack of clinical trials; and although imperfect, observational data can help inform disease management.
Conclusions
In summary, ABBA is a very rare autoimmune kidney disease that often occurs with other kidney diseases. These most frequently include glomerular diseases with high-grade proteinuria. Although the overall prognosis of ABBA is poor; there is potential benefit from immunosuppression. The combination of corticosteroids with additional immunosuppressive agents resulted in improved outcomes compared to those untreated. Prospective data collection from a larger cohort and through multi-institutional collaboration may further elucidate optimal management. Nonetheless, this study represents the largest to date, and expands on the histopathologic spectrum and natural history of this rare disease.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank Sudhir Joshi, Lilli Walker, and Caitlyn Gilmer for technical and administrative assistance.
Data Availability
No mineable datasets were generated in this work for entry into a repository.
Footnotes
Figure S1. Spectrum of tubular injury in ABBA.
Figure S2. Kidney biopsy showing a case of ABBA with tubular basement membrane deposits, but a lack of proximal tubular brush border staining.
Figure S3. Kidney biopsy showing a case of ABBA with IgG staining along the proximal tubular brush borders without corresponding tubular basement membrane deposits.
Figure S4. Patient biopsy with LRP2, CUBN, and AMN within TBM deposits, suggestive of epitope spreading in the patient's ABBA disease.
Figure S5. LRP2 positivity in glomerular capillary loop deposits seen in a single case of ABBA.
Figure S6. Histopathologic spectrum of concurrent glomerular diseases in ABBA.
Figure S7. Diagnostic algorithm for tubulointerstitial kidney diseases with tubular basement membrane IgG deposits.
Table S1. Patient characteristics with post-transplant recurrence.
Table S2. Patient characteristics with CR, SR, and PR (n = 15 patients).
Table S3. Patient characteristics with no response (n = 36 patients).
Table S4. Comparison of ABBA patients who had serologic testing compared to patients without serologic disease confirmation. Two cases (1 in each group) was a limited cortical sample and could not be analyzed for all histologic parameters. ABBA, antibrush border antibody disease.
Table S5. Comparison of IgG subclass staining within serum and kidney biopsy tissue.
Supplementary Material
Figure S1. Spectrum of tubular injury in ABBA.
Figure S2. Kidney biopsy showing a case of ABBA with tubular basement membrane deposits, but a lack of proximal tubular brush border staining.
Figure S3. Kidney biopsy showing a case of ABBA with IgG staining along the proximal tubular brush borders without corresponding tubular basement membrane deposits.
Figure S4. Patient biopsy with LRP2, CUBN, and AMN within TBM deposits, suggestive of epitope spreading in the patient's ABBA disease.
Figure S5. LRP2 positivity in glomerular capillary loop deposits seen in a single case of ABBA.
Figure S6. Histopathologic spectrum of concurrent glomerular diseases in ABBA.
Figure S7. Diagnostic algorithm for tubulointerstitial kidney diseases with tubular basement membrane IgG deposits.
Table S1. Patient characteristics with post-transplant recurrence.
Table S2. Patient characteristics with CR, SR, and PR (n = 15 patients).
Table S3. Patient characteristics with no response (n = 36 patients).
Table S4. Comparison of ABBA patients who had serologic testing compared to patients without serologic disease confirmation.
Table S5. Comparison of IgG subclass staining within serum and kidney biopsy tissue.
References
- 1.Shwayder M., Ozawa T., Boedecker E., Guggenheim S., McIntosh R.M. Nephrotic syndrome associated with Fanconi syndrome. Immunopathogenic studies of tubulointerstitial nephritis with autologous immune-complex glomerulonephritis. Ann Intern Med. 1976;84:433–437. doi: 10.7326/0003-4819-84-4-433. [DOI] [PubMed] [Google Scholar]
- 2.Douglas M.F., Rabideau D.P., Schwartz M.M., Lewis E.J. Evidence of autologous immune-complex nephritis. N Engl J Med. 1981;305:1326–1329. doi: 10.1056/NEJM198111263052206. [DOI] [PubMed] [Google Scholar]
- 3.Morrison E.B., Kozlowski E.J., McPhaul J.J., Jr. Primary tubulointerstitial nephritis caused by antibodies to proximal tubular antigens. Am J Clin Pathol. 1981;75:602–609. doi: 10.1093/ajcp/75.4.602. [DOI] [PubMed] [Google Scholar]
- 4.Larsen C.P., Trivin-Avillach C., Coles P., et al. LDL receptor-related protein 2 (megalin) as a target antigen in human kidney anti-brush border antibody disease. J Am Soc Nephrol. 2017;29:644–653. doi: 10.1681/ASN.2017060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosales I.A., Collins A.B., do Carmo P.A., Tolkoff-Rubin N., Smith R.N., Colvin R.B. Immune complex tubulointerstitial nephritis due to autoantibodies to the proximal tubule brush border. J Am Soc Nephrol. 2016;27:380–384. doi: 10.1681/ASN.2015030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caliskan Y., Caza T., Mosman A., et al. A case of immune complex mediated tubulointerstitial disease and nephrotic syndrome: anti LRP-2 Nephropathy with diffuse podocyte effacement. J Nephrol. 2021;34:915–919. doi: 10.1007/s40620-020-00762-9. [DOI] [PubMed] [Google Scholar]
- 7.Dvanajscak Z., Murphy J.D., Larsen C.P., Padala S.A. Anti-brush border antibody disease (anti-LRP2 nephropathy) associated with lupus nephritis. Kidney Int Rep. 2020;5:1590–1594. doi: 10.1016/j.ekir.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinesh K.P., Raniele D., Michels K., et al. Anti-LRP2 nephropathy with abundant IgG4-positive plasma cells: A case report. Am J Kidney Dis. 2019;74:132–137. doi: 10.1053/j.ajkd.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Gamayo A., Hecox D., Dicker L., et al. Anti-LRP2 nephropathy with concurrent kidney infiltration by lymphoma. Clin Kidney J. 2020;13:468–472. doi: 10.1093/ckj/sfz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng L., Ruiz-Cordero R., Caza T., Walavalkar V. Anti-LDL receptor-related protein 2 nephropathy with synchronous primary kidney extranodal marginal zone lymphoma. Glomerular Dis. 2021;1:302–308. doi: 10.1159/000518852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker P.D., Cavallo T., Bonsib S.M. Ad Hoc Committee on Renal Biopsy Guidelines of the Renal Pathology Society. Practice guidelines for the renal biopsy. Mod Pathol. 2004;17:1555–1563. doi: 10.1038/modpathol.3800239. [DOI] [PubMed] [Google Scholar]
- 12.Raissian Y., Nasr S.H., Larsen C.P., et al. Diagnosis of IgG4-related tubulointerstitial nephritis. J Am Soc Nephrol. 2011;22:1343–1352. doi: 10.1681/ASN.2011010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshpande V., Zen Y., Chan J.K., et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 14.Rojas-Villarraga A., Amaya-Amaya J., Rodriguez-Rodriguez A., Mantilla R.D., Anaya J.M. Introducing polyautoimmunity: secondary autoimmune diseases no longer exist. Autoimmune Dis. 2012;2012 doi: 10.1155/2012/254319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cojocaru M., Cojocaru I.M., Silosi I. Multiple autoimmune syndrome. Maedica (Bucur) 2010;5:132–134. [PMC free article] [PubMed] [Google Scholar]
- 16.Ordoñez-Cañizares M.C., Mena-Vázquez N., Redondo-Rodriguez R., et al. Frequency of polyautoimmunity in patients with rheumatoid arthritis and systemic lupus erythematosus. J Clin Rheumatol. 2022;28:e38–e43. doi: 10.1097/RHU.0000000000001574. [DOI] [PubMed] [Google Scholar]
- 17.Morelle J., Caza T., Debiec H., et al. Cubilin and amnionless protein are novel target antigens in anti-brush border antibody disease. Kidney Int. 2022;101:1063–1068. doi: 10.1016/j.kint.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Kyle R.A., Therneau T.M., Rajkumar S.V., et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 19.Huang C.C., Lehman A., Albawardi A., et al. IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Mod Pathol. 2013;26:799–805. doi: 10.1038/modpathol.2012.237. [DOI] [PubMed] [Google Scholar]
- 20.Arcoverde Fechine Brito L.P., Guedes F.L., Cavalcante Vale P.H., et al. Antibrush border antibody disease: A case report and literature review. Kidney Med. 2021;3:848–855. doi: 10.1016/j.xkme.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salant D.J., Quigg R.J., Cybulsky A.V. Heymann nephritis: mechanisms of renal injury. Kidney Int. 1989;35:976–984. doi: 10.1038/ki.1989.81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No mineable datasets were generated in this work for entry into a repository.