This study attempts assess to the association of β-blocker treatment withdrawal on changes in the percentage of predicted peak oxygen consumption across indexed left ventricular diastolic and systolic volumes, and left ventricular ejection fraction in patients with heart failure with preserved ejection fraction and chronotropic incompetence.
Key Points
Question
Among patients with heart failure with preserved ejection fraction (HFpEF) and chronotropic incompetence, do left ventricular dimensions and systolic function modulate the association of β-blocker treatment withdrawal with maximal functional capacity?
Findings
In this post hoc analysis of a randomized clinical trial that enrolled 52 patients with HFpEF and chronotropic incompetence taking β-blocker treatment, lower left ventricular systolic volumes identified patients in which β-blocker cessation was associated with a greater short-term improvement in maximal functional capacity.
Meaning
In this study, results showed that in patients with HFpEF and chronotropic incompetence, β-blocker cessation may be particularly beneficial in patients with smaller end-systolic left ventricular volume.
Abstract
Importance
Increasing the patient’s heart rate (HR) has emerged as a therapeutic option in patients with heart failure with preserved ejection fraction (HFpEF). However, the evidence is conflicting, and the profile of patients who benefit most from this strategy remains unclear.
Objective
To assess the association of β-blocker treatment withdrawal with changes in the percentage of predicted peak oxygen consumption (VO2) across indexed left ventricular diastolic (iLVEDV) and indexed left ventricular systolic volumes (iLVESV), and left ventricular ejection fraction (LVEF) in patients with HFpEF and chronotropic incompetence.
Design, Setting, and Participants
This post hoc analysis was conducted using data from the investigator-blinded multicenter, randomized, and crossover clinical trial, PRESERVE-HR, that took place from October 1, 2018, through December 31, 2020, to investigate the short-term effects (2 weeks) of β-blocker withdrawal on peak oxygen consumption (peak VO2). Patients with stable HFpEF (New York Heart Association functional class II to III) receiving treatment with β-blocker and chronotropic incompetence were included.
Intervention
Participants in the PRESERVE-HR trial were randomized to withdraw vs continue with β-blocker treatment. After 2 weeks, they were crossed over to receive the opposite intervention. This crossover randomized clinical trial examined the short-term effect of β-blocker withdrawal on peak VO2.
Main Outcomes and Measures
The primary outcome was to evaluate the association between β-blocker withdrawal and short-term changes in percentage of peak VO2 across iLVEDV, iLVESV, and LVEF in patients with HFpEF and chronotropic incompetence treated with β-blocker.
Results
A total of 52 patients (mean age, 73 [SD, 13] years; 60% female) were randomized. The mean resting HR, peak HR, peak VO2, and percentage of peak VO2 were 65 (SD, 9) beats per minute (bpm), 97 (SD, 15) bpm, 12.4 (SD, 2.9) mL/kg per minute, and 72.4% (SD, 17.7%), respectively. The medians (minimum-maximum) of iLVEDV, iLVESV, and LVEF were 44 mL/m2 (IQR, 19-82), 15 mL/m2 (IQR, 7-32), and 64% (IQR, 52%-78%), respectively. After stopping β-blocker treatment, the median increase in peak HR was plus 30 bpm (95% CI, 25-35; P < .001). β-Blocker cessation was differentially associated with change of percentage of peak VO2 across the continuum of iLVESV (P for interaction = .02), indicating a greater benefit in those with lower iLVESV.
Conclusions and Relevance
In this study, results showed that in patients with HFpEF and chronotropic incompetence receiving treatment with β-blocker, lower iLVESV may identify those with a greater short-term improvement in maximal functional capacity after stopping β-blocker treatment. Further studies are warranted for further investigation.
Trial Registration
ClinicalTrials.gov (NCT03871803)
Introduction
Chronotropic incompetence has emerged as a relevant pathophysiological mechanism in heart failure with preserved ejection fraction (HFpEF).1,2 Specifically, chronotropic incompetence has been associated with worse clinical outcomes and functional capacity.3,4 For this reason, increasing heart rate (HR) has emerged as a promising therapeutic option. However, recent trials have revealed conflicted findings, and the profile of patients who benefit from increases in HR remains unclear.5,6,7 We postulate that increased HR may be especially beneficial in patients with steeper left ventricular (LV) end-systolic pressure-volume relations, such as patients with small LV chambers and high LV ejection fraction (EF).8,9 In this study, we evaluated the association between β-blocker withdrawal and short-term changes in the percent of predicted peak oxygen consumption (VO2) across indexed left ventricular volumes end-diastolic (iLVEDV), indexed left ventricular volumes end-systolic (iLVESV), and left ventricular ejection fraction (LVEF) in patients with stable HFpEF and chronotropic incompetence taking treatment with β-blocker.
Methods
Study Sample and Procedures
This substudy is a post hoc analysis of a multicenter, investigator-blinded (open-label for patients), randomized, crossover study. Between October 2018 and December 2020, the study included 52 patients with HFpEF, New York Heart Association functional class (NYHA) II to III/IV, chronotropic incompetence, and previous stable treatment with β-blocker. The study design and main findings were previously published.5,10 Briefly, patients were first randomized to withdraw (arm A: n = 26) vs continue (arm B: n = 26) β-blocker treatment and afterwards were crossed over to receive the opposite intervention (15 days each period). The outcome measure was peak VO2. Maximal functional capacity was assessed through cardiopulmonary exercise testing (CPET) on a bicycle ergometer, starting at 10 W and increasing by 10 W per minute until symptom-induced cessation or a respiratory exchange ratio 1.05 or more.
Chronotropic incompetence was defined as a chronotropic index less than 0.62 during a maximal CPET (chronotropic index = [peak exercise HR − HR at rest] / [220 − age − HR at rest]). LVEF and LV volumes were assessed on the same day of CPET using a 2-dimensional transthoracic echocardiogram. LVEF was assessed by the biplane Simpson method. iLVEDV and iLVESV were calculated by dividing LV volumes by surface body area. Quality of life (QoL) was evaluated by the Minnesota Living with Heart Failure Questionnaire (MLHFQ).
Statistical Analysis
Continuous variables are expressed as mean (SD) or median (IQR) and categorical variables are presented as numbers (%). The Spearman coefficient assessed the correlations between baseline LV volumes and percentage of peak VO2. A linear mixed regression model was used to test the association between the intervention (β-blocker withdrawal vs β-blocker continuation) and the change in percentage of peak VO2 across the LV volumes and LVEF. Likewise, the associations between change of percentage of peak VO2 and changes in HR at peak exercise (change of peak HR) across the median of LV volumes and LVEF categories were assessed. Because of hierarchical levels of nesting (treatment sequence within patient identification and the latter among study centers), the models included study center and patient identification as random effects. Estimates were adjusted for the baseline value of the percentage of peak VO2 (analysis of covariance design) and sinus rhythm. A similar approach was used to analyze change of MLHFQ. Estimates are presented as least square means with 95% CIs and P values. Stata version 17.0 (Stata Corp) was used for the analyses.
Results
Baseline Characteristics
The mean age was 73 (SD, 13) years. Among the 52 participants, 60% were female, 81% were in sinus rhythm, and 67% were in stable NYHA class II. The means of peak VO2, percentage of peak VO2, rest HR, and peak HR were 12.4 (SD, 2.9) mL/kg per minute, 72.4% (SD, 17.7%), 65 (SD, 9) beats per minute (bpm), and 97 (SD, 15) bpm, respectively. The mean chronotropic incompetence was 0.41 (SD, 0.14). The medians (minimum and maximum) of baseline iLVEDV, iLVESV, LVEF, and MLHFQ were 44 mL/m2 (IQR, 19-82), 15 mL/m2 (IQR, 7-32), 64% (IQR, 52%-78%), and 25 (IQR, 13-35), respectively. Bisoprolol was the most prescribed β-blocker (88.5%), with a median dose of 2.5 mg daily (IQR, 2.5-5.0). There were no significant differences between the 2 treatment groups, including LV volumes and LVEF (Table). At baseline, iLVEDV, iLVESV, and LVEF were not correlated with percentage of peak VO2 (r = −0.16; P = .26, r = −0.20 ; P = .16, and r = 0.21; P = .13, respectively).
Table. Baseline Characteristics Across Treatment Arms .
| Variables | β-Blocker withdrawal, No. (%) | β-Blocker maintenance, No. (%) | P value |
|---|---|---|---|
| Total participants | 26 (50) | 26 (50) | NA |
| Demographic and medical history | |||
| Age, y , median (IQR) | 73.0 (68-77) | 76.5 (72-80) | .10 |
| Sex | |||
| Female | 14 (53.9) | 17 (65.4) | .57 |
| Male | 12 (46.1) | 9 (34.6) | |
| NYHA III/IV | 8 (30.8) | 10 (38.5) | .77 |
| BMI,a mean (SD) | 30.9 (5.3) | 31.3 (4.0) | .74 |
| Previous admission for AHF | 26 (100) | 26 (100) | 1.00 |
| Hypertension | 22 (84.6) | 24 (92.3) | .67 |
| Diabetes | 9 (34.6) | 12 (46.2) | .57 |
| Current smoker | 4 (15.4) | 0 (0) | .11 |
| History of IHD | 7 (26.9) | 5 (19.2) | .74 |
| History of atrial fibrillation | 11(42.3) | 9 (34.6) | .78 |
| Vital signs at rest, mean (SD) | |||
| Heart rate, bpm | 64.9 (10.8) | 64.8 (8.8) | .53 |
| Systolic blood pressure, mm Hg | 124.5 (14.2) | 122.4 (16.8) | .64 |
| Diastolic blood pressure, mm Hg | 64.2 (8.0) | 67.1 (8.7) | .23 |
| Echocardiographic parameters | |||
| iLVEDV, mL/m2, median (IQR) | 43.2 (36.8-58.0) | 46.2 (36.9-54.0) | .71 |
| iLVESV, mL/m2, median (IQR) | 15.0 (11.3-21.8) | 14.7 (11.8-17.8) | .43 |
| LVEF, %, mean (SD) | 63.8 (7.1) | 65.7 (7.0) | .34 |
| Left atrial volume index, mL/m2, mean (SD) | 42.5 (16.3) | 37.1 (10.2) | .16 |
| Left ventricular mass index, g/m2, mean (SD) | 108.1 (31.1) | 107.9 (32.7) | .98 |
| Septal E/e’ ratio,b median (IQR) | 14.3 (11.6-19.1) | 15.2 (13.4-18.5) | .49 |
| Cardiopulmonary exercise testing and quality of life variables | |||
| Peak VO2, mL/kg/min, mean (SD) | 12.2 (2.9) | 12.5 (2.9) | .69 |
| Percentage of predicted peak VO2, mean (SD) | 67.6 (17.9) | 77.2 (16.6) | .05 |
| MLHFQ, mean (SD) | 25 (13-35) | 27 (11-40) | .87 |
Abbreviations: AHF, acute heart failure; BMI, body mass index; bpm, beats per minute; IHD, ischemic heart disease; iLVEDV: indexed left ventricular end-diastolic volume; iLVESV, indexed left ventricular end-systolic volume; LVEF, left ventricular ejection fraction, MLHFQ, Minnesota Living With Heart Failure Questionnaire; NA, not applicable; NYHA, New York Heart Association functional class; VO2, oxygen consumption.
Calculated as weight in kilograms divided by height in meters squared.
Septal E/e’ ratio, ratio of mitral peak velocity of early filling [E] to early septal diastolic mitral annular velocity [e′].
HR Response to β-Blocker Withdrawal
After stopping β-blocker treatment, the change in peak HR increased by 30 bpm (95% CI, 25-35; P < .001) without significant differences across the median of iLVEDV (P for interaction = .15), iLVESV (P for interaction = .36), and LVEF less than 65%, vs 65% or more and less than 70%, vs 70% or more (P for interactions = .24 and .15).
β-Blocker Withdrawal and Changes in Peak VO2 and QoL Across LV Volumes and LVEF
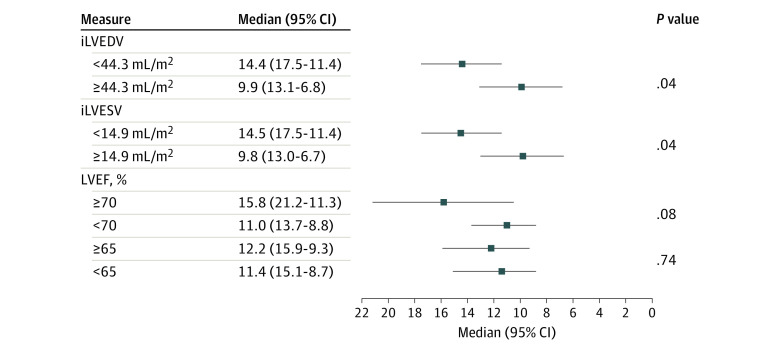
The effect of β-blocker cessation on percentage of peak VO2 across iLVEDV, iLVESV, and LVEF is presented in Figure 1. The study team found a greater increase in change in percentage of peak VO2 at smaller volumes and higher LVEF. The differential effect was significant for iLVESV (P for interaction = .02). The between-treatment comparison showed a stepwise increase in change in peak VO2 after stopping β-blocker treatment in those with lower baseline iLVESV (Figure 1B). These differential associations were not significant for iLVEDV (P for interaction = .07; Figure 1A) and LVEF (P for interaction = .06; Figure 1C), but with trends parallel to iLVESV. The study team did not find statistical evidence for heterogeneous findings across sex. However, there was a signal of a greater benefit in women with lower volumes and higher LVEF (eFigure 1 in Supplement 1). When the association of the intervention across the median LV volumes was analyzed, it was confirmed that those with LV volumes lower than the median had an association with greater improvement in change of percentage of peak VO2 (Figure 2). Regarding changes in MLHFQ, there was not a significant difference in the association of withdrawing β-blocker treatment across LV volumes and LVEF (eFigure 2 in Supplement 1).
Figure 1. Effect of β-Blocker Withdrawal on the Percentage of Predicted Peak Oxygen Consumption (VO2) Across the Continuum of Left Ventricular Volumes and Left Ventricular Ejection Fraction.
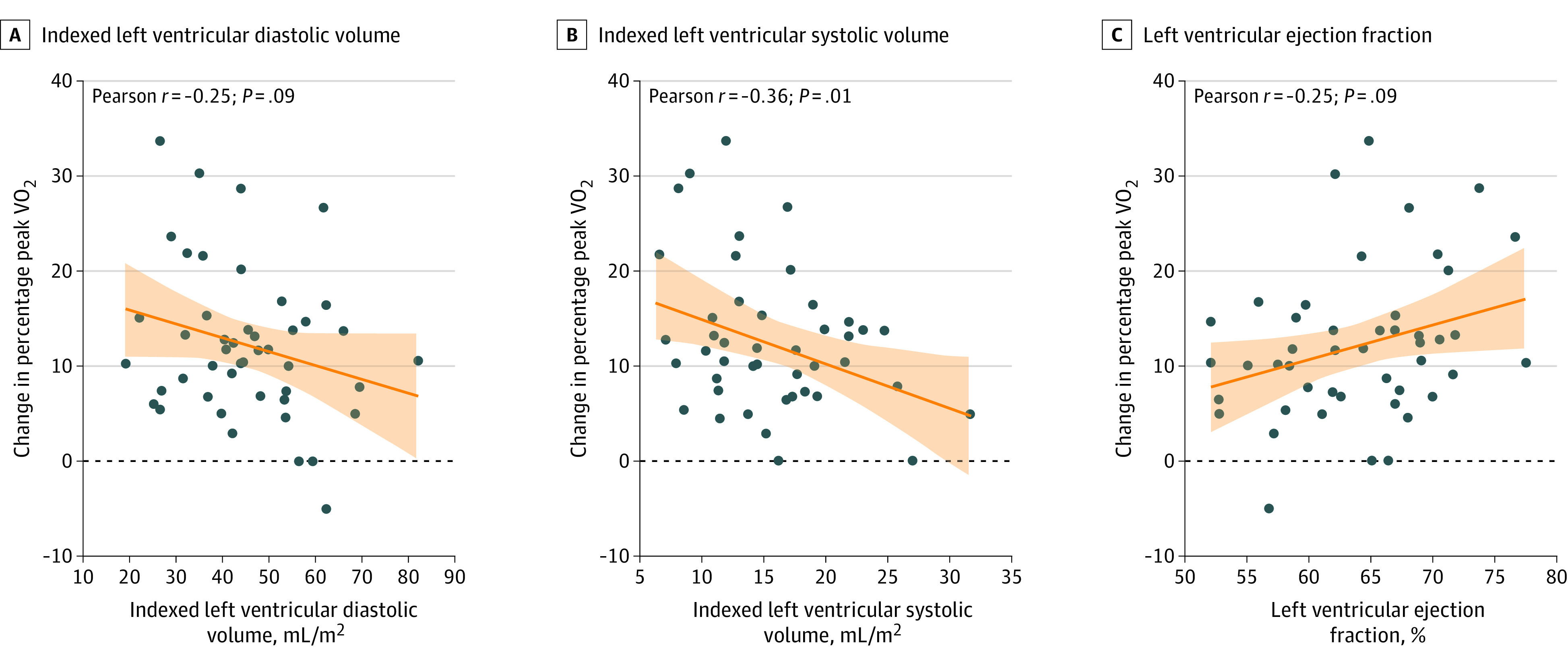
Individual patient data are shown. The orange line indicates the regression lines and the shaded area represents the 95% CIs.
Figure 2. Effect of the β-Blocker Withdrawal on Percentage of Predicted Peak Oxygen Consumption (VO2) Across the Median of Left Ventricular (LV) Volumes and Left Ventricular Ejection Fraction (LVEF).
X-axis refers to association with predicted percentage changes in peak VO2 after stopping β-blocker treatment across subgroups (LV volumes and LVEF). iLVEDV indicates indexed left ventricular diastolic volume; iLVESV; indexed left ventricular systolic volume.
Changes in Peak HR After Stopping β-Blocker and Changes in Peak VO2 Across LV Volumes and LVEF
The association between change in peak HR and the change in percentage of peak VO2 differed depending on the median of iLVESV (P for interaction = .05). In individuals with iLVESV below the median, an increase of 10 bpm in change in peak HR was associated with a greater improvement in peak VO2 (1.93%; 95% CI, 0.76-3.10; P = .001). However, this association was not found in individuals with iLVESV above or equal to the median (0.72%; 95% CI, −0.54 to 1.98; P = .27, per increase in 10 bpm). Change in peak HR was not differentially associated with change in percentage of peak VO2 across the median of iLVEDV (P for interaction = .18) or LVEF categories (less than 65% vs 65% or more; P for interaction = .69 and less than 70% vs 70% or more; P for interaction = .13). Detailed estimates are presented in the eTable in Supplement 1.
Discussion
In this substudy of the PRESERVE-HR trial, β-blocker cessation was associated with a short-term association with improvement in peak VO2 among patients with a lower iLVESV, though it is important to note that an improvement was found in the whole sample. Furthermore, in those patients with an iLVESV below the median, the increase in peak HR achieved by β-blocker cessation was associated with a greater functional improvement than those with an iLVESV above the median. While we did not find a differential association across sex, given the trend of the data there is a possibility that women may experience a greater effect.
In patients with HFpEF, β-blockers are linked to a higher risk of HF-related hospitalizations.11 Increasing HRs has emerged as a potential management strategy for HFpEF and blunted chronotropic response.8 However, the evidence is limited and seemingly inconsistent. In the PRESERVE-HR and myPACE randomized trials, strategies to increase HR revealed advantageous effects,5,6 whereas rate-adaptive pacing in the double-masked, randomized crossover RAPID-HF trial had neutral effects.7
Recent evidence has indicated that individuals with supranormal LVEF have distinct characteristics and may face higher mortality risks, even among those without cardiovascular disease, especially those with lower stroke volume.9,12,13 These patients often display smaller LV volumes, more women, and nonischemic heart disease.14 The current findings align with the notion that higher HRs might be particularly beneficial for patients with HFpEF with smaller LV volumes and hyperdynamic systolic function, which are features observed in individuals with lower iLVESV. For these patients, an increased HR could potentially enhance cardiac output and counterbalance the decrease in stroke volume during exercise.15
Limitations
There are important limitations to be addressed. First, this is an open-label, small study with short-term follow-up. Second, the current findings may not be generalizable and applied only to stable patients with HFpEF and chronotropic incompetence on treatment with β-blocker, especially those receiving bisoprolol. Third, this is a nonprespecified analysis. Fourth, the current study may not have enough power to examine QoL and other subgroups, such as sex-related differences. Fifth, echocardiographic parameters were limited and obtained in a rest supine position. Sixth, the anaerobic threshold was not registered. Lastly, although the increased HR at peak exercise mediated most of the functional capacity improvement in the PRESERVE-HF trial,5 we cannot definitively unravel the hemodynamic mechanism behind these findings. This hypothesis-generating analysis lays the groundwork for future prospective, well-powered, and controlled studies that assess the increase in HR as a therapeutic measure in HFpEF, chronotropic incompetence, and small LV dimensions.
Conclusions
In this study, in patients with HFpEF and chronotropic incompetence on treatment with β-blocker, lower iLVESV could identify patients in whom β-blocker withdrawal was associated with a greater short-term improvement in maximal functional capacity.
eTable. Between-treatment differences in percent of predicted peakVO2, per increase in 10 bpm of peak HR
eFigure 1. Effect of β-blocker treatment (β-blockers continuation vs. β-blocker withdrawal) on the percent of predicted peakVO2 across the continuum of LV volumes and LVEF
eFigure 3. Effect of β-blocker treatment (β-blockers continuation vs. β-blocker withdrawal) on the MLHFQ across the continuum of LV volumes and LVEF
eFigure 4. β-Blocker withdrawal and changes in MLHFQ along left ventricular volumes and LVEF
Data sharing statement
References
- 1.Zweerink A, van der Lingen ACJ, Handoko ML, van Rossum AC, Allaart CP. Chronotropic incompetence in chronic heart failure. Circ Heart Fail. 2018;11(8):e004969. doi: 10.1161/CIRCHEARTFAILURE.118.004969 [DOI] [PubMed] [Google Scholar]
- 2.Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. 2023;329(10):827-838. doi: 10.1001/jama.2023.2020 [DOI] [PubMed] [Google Scholar]
- 3.Palau P, Domínguez E, Seller J, et al. Chronotropic index and long-term outcomes in heart failure with preserved ejection fraction. Rev Esp Cardiol (Engl Ed). 2023;76(7):511-518. doi: 10.1016/j.recesp.2022.08.002 [DOI] [PubMed] [Google Scholar]
- 4.Domínguez E, Palau P, Núñez E, et al. Heart rate response and functional capacity in patients with chronic heart failure with preserved ejection fraction. ESC Heart Fail. 2018;5(4):579-585. doi: 10.1002/ehf2.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palau P, Seller J, Domínguez E, et al. Effect of β-blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. 2021;78(21):2042-2056. doi: 10.1016/j.jacc.2021.08.073 [DOI] [PubMed] [Google Scholar]
- 6.Infeld M, Wahlberg K, Cicero J, et al. Effect of personalized accelerated pacing on quality of life, physical activity, and atrial fibrillation in patients with preclinical and overt heart failure with preserved ejection fraction: the myPACE randomized clinical trial. JAMA Cardiol. 2023;8(3):213-221. doi: 10.1001/jamacardio.2022.5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy YNV, Koepp KE, Carter R, et al. Rate-adaptive atrial pacing for heart failure with preserved ejection fraction: the RAPID-HF randomized clinical trial. JAMA. 2023;329(10):801-809. doi: 10.1001/jama.2023.0675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer M, LeWinter MM. Heart rate and heart failure with preserved ejection fraction: time to slow β-blocker use? Circ Heart Fail. 2019;12(8):e006213. doi: 10.1161/CIRCHEARTFAILURE.119.006213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah S, Segar MW, Kondamudi N, et al. Supranormal left ventricular ejection fraction, stroke volume, and cardiovascular risk: findings from population-based cohort studies. JACC Heart Fail. 2022;10(8):583-594. doi: 10.1016/j.jchf.2022.05.007 [DOI] [PubMed] [Google Scholar]
- 10.Palau P, Seller J, Domínguez E, et al. Beta-blockers withdrawal in patients with heart failure with preserved ejection fraction and chronotropic incompetence: effect on functional capacity rationale and study design of a prospective, randomized, controlled trial (the preserve-HR trial). Clin Cardiol. 2020;43(5):423-429. doi: 10.1002/clc.23345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman DN, Plante TB, Infeld M, et al. Association of β-blocker use with heart failure hospitalizations and cardiovascular disease mortality among patients with heart failure with a preserved ejection fraction: a secondary analysis of the TOPCAT trial. JAMA Netw Open. 2019;2(12):e1916598. doi: 10.1001/jamanetworkopen.2019.16598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wehner GJ, Jing L, Haggerty CM, et al. Routinely reported ejection fraction and mortality in clinical practice: where does the nadir of risk lie? Eur Heart J. 2020;41(12):1249-1257. doi: 10.1093/eurheartj/ehz550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Essen BJ, Tromp J, Ter Maaten JM, et al. Characteristics and clinical outcomes of patients with acute heart failure with a supranormal left ventricular ejection fraction. Eur J Heart Fail. 2023;25(1):35-42. doi: 10.1002/ejhf.2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebhard C, Maredziak M, Messerli M, et al. Increased long-term mortality in women with high left ventricular ejection fraction: data from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) long-term registry. Eur Heart J Cardiovasc Imaging. 2020;21(4):363-374. doi: 10.1093/ehjci/jez321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitzman DW, Upadhya B, Pandey A. Rate-adaptive pacing for heart failure with preserved ejection fraction. JAMA. 2023;329(10):797-799. doi: 10.1001/jama.2023.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Between-treatment differences in percent of predicted peakVO2, per increase in 10 bpm of peak HR
eFigure 1. Effect of β-blocker treatment (β-blockers continuation vs. β-blocker withdrawal) on the percent of predicted peakVO2 across the continuum of LV volumes and LVEF
eFigure 3. Effect of β-blocker treatment (β-blockers continuation vs. β-blocker withdrawal) on the MLHFQ across the continuum of LV volumes and LVEF
eFigure 4. β-Blocker withdrawal and changes in MLHFQ along left ventricular volumes and LVEF
Data sharing statement