Abstract
Brain fog is one symptom that has been underexplored in traumatic brain injury (TBI). We explored the cognitive and affective correlates of brain fog in people with symptomatic mild TBI (n = 15), moderate-to-severe TBI (n = 15), and a healthy control group (n = 16). Measures across the studies assessed “brain fog” (Mental Clutter Scale), objective cognition (Useful Field of View® and Cogstate Brief Battery®), post-concussive symptoms (Post-Concussion Symptom Scale), and depressive symptoms (Profile of Moods Scale). Brain fog was higher in symptomatic mild TBI and moderate-to-severe TBI compared to healthy controls. Greater brain fog corresponded to greater depressive symptoms in symptomatic mild TBI. Greater brain fog corresponded to poorer episodic memory and working memory in moderate-to-severe TBI. Brain fog appears to reflect challenges in recovery, including depressive symptoms and worse cognitive function. Screening for brain fog might be worthwhile in people with brain injuries.
Keywords: traumatic brain injury, concussion, cognition symptoms, depression
Every year, two million people in the United States suffer external, kinetic force to the brain causing traumatic brain injury (TBI) (Frost et al., 2013), which can be either mild, moderate, or severe. As defined by the Mayo Clinic classification system, one of many taxonomies, moderate-to-severe TBIs involve injuries that result in significant loss of consciousness (>30 minutes) and posttraumatic amnesia (>24 hours), while mild TBI remain less severe or even asymptomatic (Malec, 2007). In symptomatic mild TBI and moderate-to-severe TBI considerable difficulties arise for cognition, involving trouble remembering or learning information, thinking quickly, paying attention, and everyday problem solving (Schretlen & Shapiro, 2003). Considerable research has described the wide array of performance-based cognitive difficulties experienced after a TBI. Still, we know less about the full profile of self-reported cognitive symptoms, which may provide useful information about a patient before in-depth assessment.
Self-reported cognitive symptoms can range across multiple abilities and likely depend on injury severity (Schmand et al., 1996). For mild TBI, common symptoms include trouble remembering, concentrating, and slowed thinking (de Boussard et al., 2005; Ponsford et al., 2011). While most objective cognitive problems resolve weeks to months after injury, self-reported cognitive symptoms endure up to 8 years after injury and are greater than in uninjured controls (d = .75) (Dean et al., 2012). Enduring self-reported cognitive symptoms can indicate cognitive deficits or other functional difficulties due to brain damage. In moderate-to-severe cases, self-reported cognitive symptoms typically indicate difficulties in memory and problem solving (Corrigan et al., 2004) that can last anywhere from two (d = .62) to 24 years (d = .12) post injury (Corrigan et al., 2004; Gardner et al., 2017; Hart et al., 2005).
One self-reported cognitive symptom receiving increased attention is “brain fog,” which has been revived in the clinical literature due to being a hallmark feature of SARS-Cov-2 infection (e.g., Asadi-Booya et al., 2022). Brain fog is a common colloquial term used in patient groups and clinics and has been operationalized as self-reported problems in memory, attention, and processing speed coupled with a lack of mental clarity (Katz et al., 2004; Nelson & Esty, 2015; Theoharides et al., 2015). Evidence for this self-reported cognitive symptom comes from post-concussion symptom screeners originally asking about feeling “fogginess” or “in a fog,” which showed a broad range of prevalence in the symptomatic mild TBI period (17 to 81.2%). Clinicians have labeled brain fog as a significant health challenge in patients with mild TBI and a key symptom for diagnosis by the International Conference on Concussion in Sport (McCrory et al., 2009). However, more research is needed to determine if this symptom is more severe in people with symptomatic mild TBI by inclusion of control group comparison. Also, there is a lack of understanding of how this symptom is manifested in persons with moderate-to-severe TBI, likely due to practical reasons. Studies on the effects of TBI on brain fog likely exclude moderate-to-severe cases due to substantial awareness deficits, which may render self-reported symptoms unreliable. However, self-reported symptoms may be useful during the phase where they may indicate functional issues (Nakase-Thompson et al., 2005; Stuss et al., 1999; Sherer et al., 2005). Hence, there is a need to detail the severity of brain fog during periods where it would be most advantageous to assess, i.e., symptomatic mild TBI and moderate-to-severe TBI.
Nurses have a critical role in caring for inpatients and outpatients with mild and moderate-to-severe TBIs, involving primary responsibilities for patient assessment, coordinating and communicating care, and providing care (Oyesanya, Thomas, Brown, & Turkstra, 2016). As such, nurses would benefit from application of symptom science to better understand the nature of brain fog. Symptom science is essential in nursing, involving investigation of self-reported health problems to inform early identification and treatment of health conditions. Assessing symptoms is important as they can occur across multiple conditions and often involve similar biological mechanisms, enabling the use of similar treatment approaches. Brain fog, for example, has been studied in multiple conditions also involving neuroinflammation such as chemotherapy receivers, fibromyalgia, lupus, HIV, chronic fatigue syndrome, celiac disease, postural tachycardia syndrome, and thyroid disorders (Alford et al., 2022; Mackay, 2015; Ross, Medow, Rowe, & Stewart, 2013; Samuels & Bernstein, 2022; Theoharides, Stewart, Hatziagelaki, & Kolaitis, 2015). Most recently, brain fog has been denoted as a major and long-term symptom of SARS-Cov-2-19 infection (Asadi-Pooya et al., 2022; Callan, Ladds, Husain, Pattinson, & Greenhalgh, 2022) also attributed to neuroinflammation. However, so far, studies have not been able to characterize the severity of brain fog and how it corresponds to differing degrees of neurological disorder and other major clinical symptoms.
To date, investigations of brain fog have relied on one-item assessments not capturing brain fog as a whole. Most studies of subjective cognitive function have relied on measures of self-rated memory problems or concentration problems, which do not capture difficulties in multiple cognitive abilities or a lack of mental clarity (Asadi-Pooya et al., 2022). Other studies directly ask people to indicate brain fog using only one item (Ross et al., 2013), which may not adequately capture the extent of brain fog symptoms. Furthermore, the term “brain fog” can be interpreted differently by each person, especially based on language and cultural differences, so an effort to provide a measure based on an operational definition is needed. One such measure is the Mental Clutter Scale, which assesses cognitive problems in multiple areas as well as mental clarity (Leavitt & Katz, 2011). Although validated in people with fibromyalgia, this instrument may help also differentiate severity of neurological disorders such as TBI. Such a tool would be helpful in indicating the need for more intensive clinical assessment.
To close this critical knowledge gap, our investigatory aims were two-fold: One, we sought to use a comprehensive measure of brain fog to compare symptom severities in people with symptomatic mild TBI and moderate-to-severe TBI to a healthy control group. Second, we strived to understand what major clinical factors contribute to these reports. Brain fog self-reports may help detect ongoing cognitive deficits that could potentially be ameliorated. Brain fog may also help identify people with major clinical issues to be resolved such as ongoing physical symptoms and depressive symptoms. Analyses involved integrating data from two recent studies on mild and moderate-to-severe traumatic brain injury conducted with harmonized measures. Findings provide nurses with knowledge of how brain injury corresponds to level of brain fog and how brain fog may relate to other clinical symptoms.
METHODS
Participants
The first dataset was obtained from an IRB approved study at the University of Alabama at Birmingham (Protocol Number: X160830007) seeking to identify cognitive correlates of return to driving after mild TBI. Written consent was obtained for all participants 18 years and older, and written assent was obtained for those under the age of 18 alongside consent from their legal guardians. From Fall 2016 to Summer 2018 15 individuals ages 16 to 25 within two weeks of mild TBI were recruited from local concussion clinics. We defined mild TBI using the criteria from the American Congress of Rehabilitation Medicine which requires at least one major symptom of concussion (e.g., loss of consciousness ≤ 30 minutes; loss of balance or motor coordination; disorientation or confusion; loss of memory; or dizziness; Kay et al., 1993). For this design, we selected individuals at the higher end of the mild TBI spectrum, with substantial symptoms on the Post-Concussion Symptom Scale (PCSS; individuals scoring ≥ 13) (Lovell et al., 2006). Such patients are of primary interest as they show poorer functional recovery and require continued clinical care compared to those with asymptomatic mild TBI (Collie, Makdissi, Maruff, Bennell, & McCrory, 2006). Eligibility criteria were reviewed and approved by one of our co-authors, a physician specializing in mild TBI (RDD). In addition to these participants, the first study recruited 16 healthy controls via community advertisements matching the mild TBI group on age and gender characteristics.
The second dataset was derived from a study (IRB Protocol Number: X160907003) seeking to identify the cognitive predictors of fitness to drive after moderate-to-severe TBI. All participants provided written consent with consultation of their caregivers. In the second study, we recruited 15 adult participants within 24 months of a moderate-to-severe TBI, ages 21 to 50 years, referred from the UAB Traumatic Brain Injury Model Systems. Diagnosis was based on criteria generated by the Mayo Classification System which defines a moderate-to-severe TBI as kinetic force to the brain that involves 30 minutes or greater of lost consciousness, posttraumatic amnestic greater than or equal to 24 hours, and a Glascow Coma Scale score of less than 13 (Malec et al., 2007). We additionally were able to verify moderate-to-severe TBIs using MRI and CT imaging to confirm the presence of brain injury. Our classification is generally aligned with other criteria such as from the National Institute of Neurological Disorders (NINDS) that defines a moderate-to-severe TBI as sudden damage to the brain resulting in major neuropsychological difficulties (NIH, 2018). Diagnosis of moderate-to-severe TBI was made by our co-author (TN), a clinical psychologist specialized in TBI who administered neuropsychological assessment and reviewed brain imaging results. There was no exclusion of individuals due to other underlying conditions or disorders, but people were excluded if they had had neurological conditions other than TBI that might have contributed to brain fog. All procedures were ethically approved by our Institutional Review Board. Further details about these studies have been published elsewhere (McManus, Bell, & Stavrinos, 2019; McManus, Cox, Vance, & Stavrinos, 2015; Newton et al., 2018; Stavrinos et al., 2019).
Measures
In each parent study, information was collected on demographics, brain fog, objective cognition, depressive symptoms, and post-concussive symptoms via harmonized measures described below:
Demographics.
From telephone screening, participants provided information on their age, gender, race, and ethnicity. Lastly, we collected the date of the most recent TBI.
Brain fog.
Brain fog was measured by the 16-item Mental Clutter Scale (MCS) (Leavitt & Katz, 2011). The MCS was developed to provide a detailed scale of brain fog over two dimensions: self-reported symptoms of general cognitive problems and lack of mental clarity. Questions asked participants to rate how much they have experienced different issues from 1 “Not at all” to 10 “All the time.” Example items for general cognitive symptoms included trouble with “concentration,” “memory,” or “mental speed,” whereas mental clarity included self-reported problems with “spaciness,” “fogginess,” or “information overload.” The research revealed that these two dimensions (8-items each) contained good factor stability (Leavitt & Katz, 2011). However, a one-factor score also produces strong reliability while mirroring criteria for brain fog, i.e., both subjective cognitive problems and a lack of mental clarity (Leavitt & Katz, 2011). Our study demonstrated high reliability for a total score across groups (αs ranged from .95 to .96). This total score ranges from 16 to 160, where higher values indicate greater brain fog.
Objective cognition.
Cogstate Brief Battery.®
An objective evaluation of cognitive performance was obtained by the Cogstate Brief Battery® (Collie et al., 2003). This battery is derived from the general Cogstate Battery®, which is comprehensive and tests several domains (see www.cogstate.com). However, for brevity, the current study used a brief battery, which only takes 10 to 15 minutes to complete. The Cogstate Brief Battery® consisted of four tasks:
The Detection task was a simple reaction time task in which participants pressed a “YES” key (Letter K) when they saw a card turned face-up on the screen.
The Identification task was a choice-reaction time task in which participants determined if a car was red or black and pressed the appropriate key.
The One-Back task is a working memory task like the n-back; in this task, participants selected if the card presented to them was the same as the one just before.
Lastly, in the Learning task, participants selected if a card presented was ever presented in the deck before; this required intact memory and learning ability.
Each task had a set of 1 to 3-minute practice trials to ensure comprehension of the task. To ensure optimum performance, participants wore a headset for auditory performance feedback (e.g., which makes a harsh tone for wrong answers and a light sound for correct answers). Scores were calculated using a proprietary algorithm incorporating speed, accuracy, hits, misses, and anticipations. Tests show strong construct validity with other neuropsychological measures (Maruff et al., 2009).
Useful Field of View.®
Useful Field of View (UFOV®) (Ball & Owsley, 1993) also captured objective cognition and has been used previously in persons with TBIs (Novack et al., 2006). UFOV® consisted of four tasks capturing processing speed and forms of executive function.
UFOV®1 – Stimuli Identification: Participants quickly determined if they viewed a “car” or “truck” within milliseconds of exposure. This task captured speed of processing.
UFOV®2 – Divided Attention: Participants shifted between identifying a car or truck in the center and remembering the location of a car in the periphery. The location of the car in the periphery occurred anywhere on an eight-spoke spiral around the center stimuli. As named, this task estimated divided attention but partly captured set-shifting ability.
UFOV®3 – Selective Attention I: Participants completed the same task as UFOV®2 but in the presence of distracting stimuli (47 triangles) across the screen.
UFOV®4 – Selective Attention II: The fourth subtest also tested selective attention in the presence of distractors (47 triangles) but with a new task involving the center stimuli. Participants decided if two stimuli in the center were the same (two cars or two trucks shown) or different (car and truck shown) while determining the location of the peripheral car as before. However, it involved the introduction of a novel task that increased difficulty.
Each subtest comprised visual demonstrations and a 2-minute practice to verify task comprehension. During their performance, the software provided an exposure threshold where 75% of responses were correct. These scores approximated optimal ability for each cognitive domain.
Depressed mood.
The Profile of Mood States (POMS) captured depressive symptoms for both injury and healthy control groups. The POMS was a 37-item instrument that allowed participants to denote feelings “since their injury” for the mild and moderate-to-severe TBI groups and “in the last two weeks” for the healthy control group. Participants rated how frequently they experienced various symptoms using the following Likert-type scale: “1-not at all,” “2-a little,” “3-moderately,” “4-quite a bit,” and “5- extremely.” This format provided a quickly answerable instrument with high factorial, face, and construct validity (McNair et al., 1971). To control for between- and within-group differences in negative affect, we used the depression (POMS-Dep) subscale from this instrument. This subscale includes feelings of being unhappy, sad, hopeless, discouraged, miserable, helpless, and worthless. POMS-Dep scores range from 8 (no depressive symptoms) to 40 (severe depressive symptoms) with excellent internal consistency in the current study (αs ranged from .88 to .94).
Post-Concussive Symptoms.
The Post-Concussion Symptom Scale (Lovell et al., 2006) measured injury severity. For 22 listed symptoms, participants rated their occurrence from none (0) to severe (6). Symptoms entailed cognitive (4 items), somatic (14 items), and mental/psychological difficulties (4 items). Summed responses ranged from 0 (no concussion symptoms) to 132 (high concussion symptoms). For healthy controls, scores represent general health problems. Internal consistency was high (αs ranged from .89 to .95).
Procedure.
Participants were assessed within two weeks to confirm symptomatic mild TBI and within 24 months for moderate-to-severe TBI. After verifying eligibility, we scheduled participants for an appointment in the laboratory. At this session, participants reported their level of brain fog (MCS), depressive symptoms (POMS-DEP), and post-concussive symptoms (PCSS). Afterward, participants completed the Cogstate Brief Battery® and UFOV®. We remunerated participants for their time.
Data analysis
For our first set of analyses, we calculated descriptive statistics on all variables using SPSS version 25 (see Tables 1 to 2). An ANOVA then examined the effect of group (healthy controls, symptomatic mild TBI, and moderate-to-severe TBI) on the continuous measure of brain fog (MCS total). If provided a significant omnibus test, Tukey’s post-hoc tests were then conducted to determine pairwise differences (control versus symptomatic mild TBI, control versus moderate-to-severe TBI, symptomatic mild TBI versus moderate-to-severe TBI). Because of the small sample and possible non-normality of brain fog, this was followed by a Kruskal–Wallis test, which inspected group differences based on rank. Secondly, because the moderate-to-severe TBI group was older and had greater time since injury based on the original study aims, a sensitivity analysis using a generalized linear model tested if group differences in brain fog remained after accounting for group differences in age and time since injury (value is 0 for controls). Furthermore, we examined whether group differences in brain fog could be accounted for by differences in depressive symptoms and post-concussive symptoms in sensitivity analyses. Group comparisons on demographics, objective cognition, depressive symptoms, and post-concussive symptoms are provided in Table 2.
Table 1.
Participant Demographics.
| Characteristic | Healthy Controls a | Symptomatic Mild TBI b | Moderate-to-severe TBI c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 16 | n = 15 | n = 15 | ||||||||
|
| ||||||||||
| M/% | SD/n | M/% | SD/n | M/% | SD/n |
X2/ANOVA p-value |
a vs. b | a vs. c | b vs. c | |
| Demographics | ||||||||||
| Gender | .514 | |||||||||
| Female | 62.5 | 10 | 60 | 9 | 43.8 | 7 | ||||
| Male | 37.5 | 6 | 40 | 6 | 56.3 | 9 | ||||
| Race | .441 | |||||||||
| Caucasian | 75.0 | 12 | 80.0 | 12 | 73.3 | 11 | ||||
| African American | 25.0 | 4 | 13.3 | 2 | 26.7 | 4 | ||||
| Asian | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | .513 | |||
| Bi-racial | 0.0 | 0 | 6.7 | 1 | 0.0 | 0 | ||||
| Age (years) | 17.06 | 1.57 | 16.73 | 0.80 | 33.19 | 8.74 | <.001 | .472 | <.001 | <.001 |
| Time Since Injury (mo.) | – | – | 0.40 | 0.13 | 44.26 | 36.84 | <.001 | |||
Table 2.
Differences in cognitive function, brain fog, post-concussive symptoms, and depressive symptoms across groups.
| Characteristic | Healthy Controlsa | Symptomatic Mild TBIb | Moderate-to-severe TBIc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 16 | n = 15 | n = 15 | ||||||||
|
| ||||||||||
| M | SD | M | SD | M | SD | ANOVA p-value |
a vs b | a vs c | b vs c | |
| UFOV® | ||||||||||
| UFOV®1 - Speed of Processing | 17.00 | 0.00 | 30.29 | 48.95 | 47.87 | 54.06 | .130 | |||
| UFOV®2 - Divided Attention | 17.38 | 1.50 | 52.63 | 101.99 | 119.13 | 100.74 | .005 | .202 | .004 | .073 |
| UFOV®3 - Selective Attention I | 52.00 | 24.82 | 99.33 | 114.38 | 215.00 | 141.36 | <.001 | .117 | <.001 | .012 |
| UFOV®4 - Selective Attention II | 94.63 | 58.25 | 173.42 | 141.46 | 306.20 | 123.08 | <.001 | .091 | <.001 | .007 |
| Cogstate® | ||||||||||
| Detection | 99.67 | 4.08 | 89.29 | 13.15 | 91.60 | 9.17 | .010 | .011 | .057 | .783 |
| Identification | 102.81 | 5.12 | 95.8 | 11.16 | 92.53 | 9.86 | .009 | .089 | .008 | .585 |
| Learning | 101.69 | 7.66 | 95.13 | 12.26 | 98.40 | 8.52 | .179 | |||
| One Back | 95.25 | 6.76 | 94.07 | 10.61 | 87.13 | 6.12 | .016 | .913 | .020 | .058 |
| MCS | 38.56 | 21.39 | 63.20 | 29.43 | 73.40 | 32.96 | .004 | .014 | .004 | .587 |
| Mental Clarity | 17.81 | 10.31 | 31.73 | 15.89 | 32.73 | 19.23 | .016 | .008 | .027 | .983 |
| Cognitive Problems | 20.75 | 11.42 | 31.47 | 14.44 | 40.67 | 16.13 | .001 | .031 | .001 | .185 |
| PCSS | 19.94 | 15.66 | 30.67 | 22.54 | 38.60 | 22.84 | .049 | .133 | .039 | .545 |
| POMS-Dep | 14.06 | 6.14 | 14.27 | 5.40 | 16.67 | 7.37 | .459 | |||
Notes. MCS = Mental Clutter Scale; PCSS = Post Concussive Symptom Scale; POMS-Dep = Depression subscale from Profile of Mood States; UFOV® = Useful Field of View. Boldened variables indicate significant differences.
Next, we tested group-specific relations between brain fog with objective cognition (speed of processing [UFOV®1], divided attention [UFOV®2], selective attention [UFOV®3 and UFOV®4], processing speed time [Cogstate® Detection task], processing speed accuracy [Cogstate® Identification task], working memory [Cogstate® One-Back task], and episodic memory [Cogstate® Learning task]), depressive symptoms, and postconcussive symptoms using Spearman correlations. Area under the curve analyses were conducted with non-parametric assumptions to determine how well brain fog discriminated healthy controls from people with symptomatic mild and moderate-to-severe TBI. Area under the curve analyses were also made to examine how post-concussive symptoms and depressive symptoms discriminated healthy controls from people with symptomatic mild and moderate-to-severe TBI. Associations between brain fog and objective cognition included adjustment for depressive symptoms for our second aim. For all analyses, pairwise deletion was used rather than listwise deletion to preserve sample size. We reported p-values and effect sizes for all analyses and determined significance at the .05 level.
Results
Descriptives
Demographics.
Participant demographics are shown in Table 1. For the symptomatic mild TBI group (n = 15), the average participant age was 16.73 years (SD = 0.80, range: 16 to 19). This group was predominately female (60.0%, n = 9) and Caucasian (80.0%, n = 12). The healthy control group (n = 16) appeared successfully matched to the symptomatic mild TBI group. Meanwhile, individuals with a moderate-to-severe TBI were predominately young to middle-aged adults (Mage = 33.19 years, SD = 8.74, range = 20 to 50) – significantly older than our symptomatic mild TBI and control samples (F(2,43) = 51.72, p < .001, η2 = .71). Regarding other personal characteristics, the sample consisted of a slight male majority (56.3%, n = 9) who were predominantly Caucasian (73.3%, n = 11). The moderate-to-severe TBI group were similar on sex (X2(2) = 1.33, p = .514) and race proportions (X2(2) = .20, p = .905) compared to the symptomatic mild TBI and healthy control groups. Because age was the only significant difference across groups, age was included as a covariate in later our descriptive comparisons.
Group differences in brain fog.
After conducting a one-way ANOVA, we found a significant effect of group on brain fog (F(2,43) = 6.29, p = .004; η2 = .23; 95%CI[.03 to .40]) (shown in Figure 1 and Table 2). Post-hoc tests confirmed that individuals with symptomatic mild TBI reported higher brain fog compared to healthy controls (MDiff = 24.64, p = .014; d = .96) as did individuals with moderate-to-severe TBI (MDiff = 34.84, p = .004, d = 1.25). No significant difference emerged between individuals with symptomatic mild TBI and moderate-to-severe TBI (MDiff = 10.20, p = .327). Sensitivity analyses showed that these patterns of results were similar after nonparametric testing using a nonparametric Kruskal-Wallis test as well as adjustment for age, time since injury, depressive symptoms, and post-concussive symptoms (ps < .05).
Figure 1.
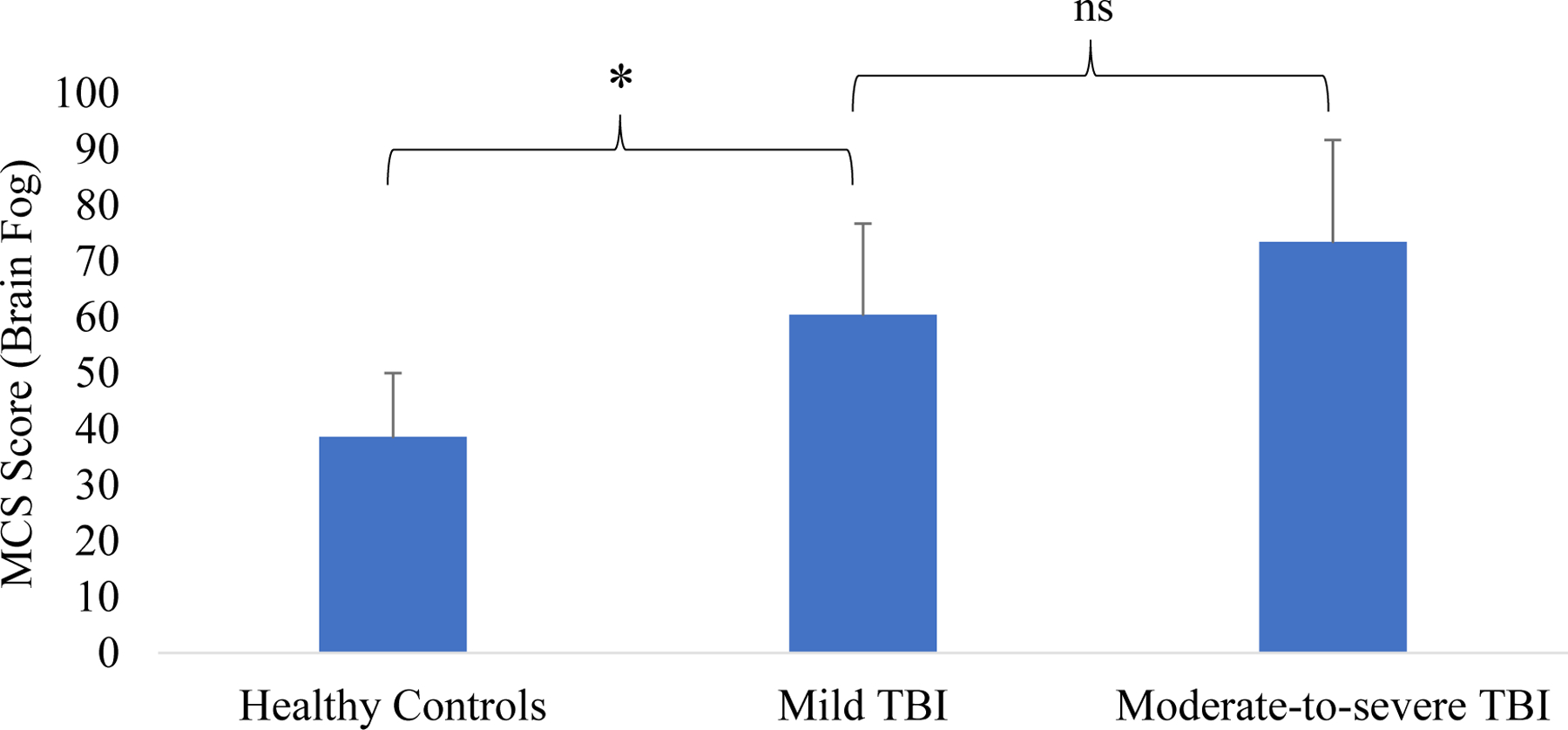
Group differences on brain fog. Notes. TBI = traumatic brain injury; asterisk represents significant differences between groups (ps < .05).
Area under the curve analyses.
Next, AUC models examined the ability of brain fog, post-concussive symptoms, and depressive symptoms, as continuous variables, to discriminate healthy controls from symptomatic mild TBI and moderate-to-severe TBI. Overall, brain fog discriminated healthy controls from individuals with symptomatic mild TBI well (AUC = .74; 95%CI: .56 to .92). Post-concussive symptoms (AUC = .64; 95%CI: .44 to .84) and depressive symptoms did poorly at discriminating healthy controls from individuals with symptomatic mild TBI (AUC = .52; 95%CI: .32 to .73). Brain fog better discriminated healthy controls from those with moderate-to-severe TBI well (AUC = .85, 95%CI: 70 to .99) as did post-concussive symptoms (AUC = .76; 95%CI: .58 to .93). Depressive symptoms did poorly at discriminating healthy controls from individuals with moderate-to-severe TBI (AUC = .62; 95%CI: .42 to .82).
Correlates of brain fog in healthy controls.
Brain fog was significantly related to depressive symptoms in healthy controls (rsp = .65, p = .006). After adjusting for depressive symptoms, brain fog did not associate with any scores on UFOV® or Cogstate® in healthy controls (|rssp| range: .02 to .36, all ps > .10), as shown in Table 3.
Table 3.
Correlations between Mental Fog and Objective Cognitive Tasks in Healthy Controls (n = 16).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. MCSa | 1 | −.03 | −.04 | −.20 | .02 | −.09 | .18 | −.03 | <.01 | .36 | .29 | .19 |
| 2. PCSS | 1 | −.08 | −.49† | .19 | −.01 | −.17 | −.06 | <.01 | .08 | .10 | .05 | |
| 3. Age | 1 | −.23 | .22 | .13 | −.17 | −.13 | <.01 | .33 | −.08 | .31 | ||
| 4. Sex | 1 | −.10 | −.10 | .07 | −.02 | <.01 | −.20 | −.25 | −.42 | |||
| 5. Detection | 1 | .67* | .33 | .53** | <.01 | −.03 | −.22 | −.29 | ||||
| 6. Identification | 1 | .44 | .27 | <.01 | −.19 | .24 | −.05 | |||||
| 7. Learning | 1 | .07 | <.01 | −.16 | −.25 | −.43 | ||||||
| 8. One Back | 1 | <.01 | −.19 | −.14 | −.35 | |||||||
| 9. UFOV®1 | 1 | <.01 | <.01 | <.01 | ||||||||
| 10. UFOV®2 | 1 | .06 | .42 | |||||||||
| 11. UFOV®3 | 1 | .59* | ||||||||||
| 12. UFOV®4 | 1 | |||||||||||
Notes. All correlations are Spearman rho. MCS = Mental Clutter Scale; PCSS = Post-Concussive Symptom Scale; POMS-Dep = Depression subscale from the Profile of Mood States; UFOV = Useful Field of View.
p < .10
p < .05
p < .01
Pre-adjusted for association with depressive symptoms using residuals from a linear regression.
Correlates of brain fog in symptomatic mild TBI.
Brain fog was significantly related to depressive symptoms in people with symptomatic mild TBI (rsp = .66, p = .008, see Figure 2). After adjusting for depressive symptoms, brain fog was related to slower processing speed on UFOV®1 (rsp = .72, p = .003) but not other scores on UFOV® or Cogstate® (|rssp| range: .06 to .27, all ps > .10) .
Figure 2.
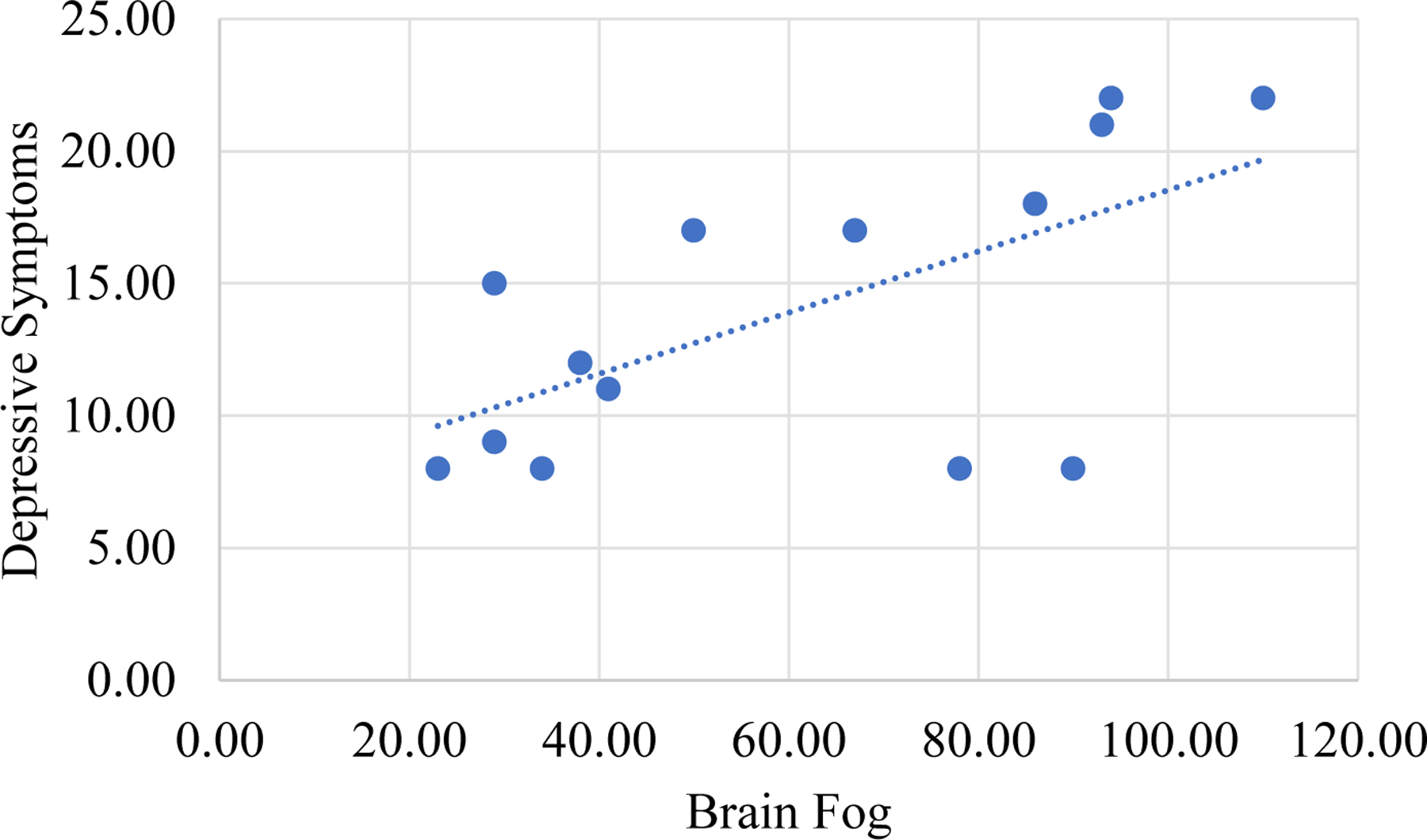
Correlation between brain fog and depressive symptoms in mild traumatic brain injury.
Correlates of brain fog in moderate-to-severe TBI.
Brain fog was unrelated to depressive symptoms in people with moderate-to-severe TBI (rsp = .26, p = .349). After adjusting for depressive symptoms, brain fog was related to objective cognition as shown in Table 5. Regarding objective cognition, greater brain fog significantly related to worse scores on the Learning (rsp = −.62, p = .023) and One Back task (rsp = −.58, p = .014; see Figure 3 and 4).
Table 5.
Correlations between Mental Fog and Objective Cognitive Tasks in Persons with Moderate-to-severe TBI (n = 15).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. MCSa | 1 | −.08 | .06 | −.20 | .25 | −.4 | −.31 | −.62* | −.58* | .12 | .03 | −.22 | −0.22 |
| 2. PCSS | 1 | −.25 | .30 | .09 | −.13 | −.31 | .20 | −.20 | .27 | .09 | .23 | −0.12 | |
| 3. Time since injury | 1 | .18 | .13 | .01 | .32 | −.03 | .10 | −.27 | −.29 | −.32 | −.24 | ||
| 4. Age | 1 | .24 | −.12 | .09 | −.02 | .40 | −.13 | −.09 | .13 | .10 | |||
| 5. Sex | 1 | .30 | −.16 | −.14 | −.16 | .15 | .17 | .06 | .17 | ||||
| 6. Detection | 1 | .46† | .36 | .08 | −.30 | −.10 | −.18 | .19 | |||||
| 7. Identification | 1 | .25 | .69** | −.28 | −.24 | −.28 | .05 | ||||||
| 8. Learning | 1 | .13 | −.58* | −.39 | −.14 | −.19 | |||||||
| 9. One Back | 1 | .13 | −.03 | .04 | .29 | ||||||||
| 10. UFOV®1 | 1 | .80*** | .66** | .62* | |||||||||
| 11. UFOV®2 | 1 | .80*** | .77*** | ||||||||||
| 12. UFOV®3 | 1 | .68** | |||||||||||
| 13. UFOV®4 | 1 | ||||||||||||
Notes. All correlations are Spearman rho. MCS = Mental Clutter Scale; PCSS = Post-Concussive Symptom Scale; POMS-Dep = Depression subscale from the Profile of Mood States; UFOV = Useful Field of View.
p < .10
p < .05
p < .01
Pre-adjusted for association with depressive symptoms using residuals from a linear regression.
Figure 3.
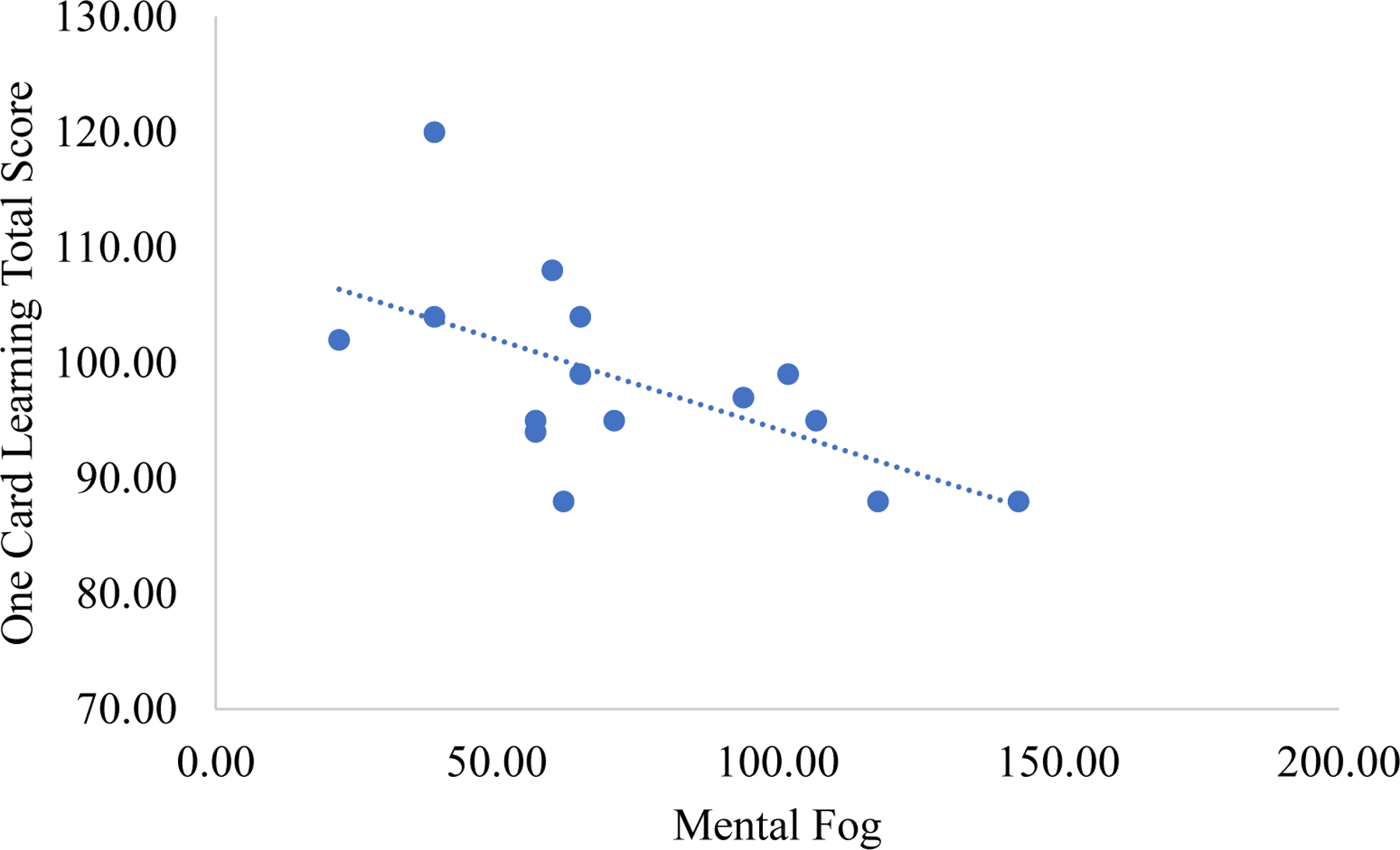
Correlation between brain fog and the Cogstate® Learning task in moderate-to-severe traumatic brain injury.
Figure 4.
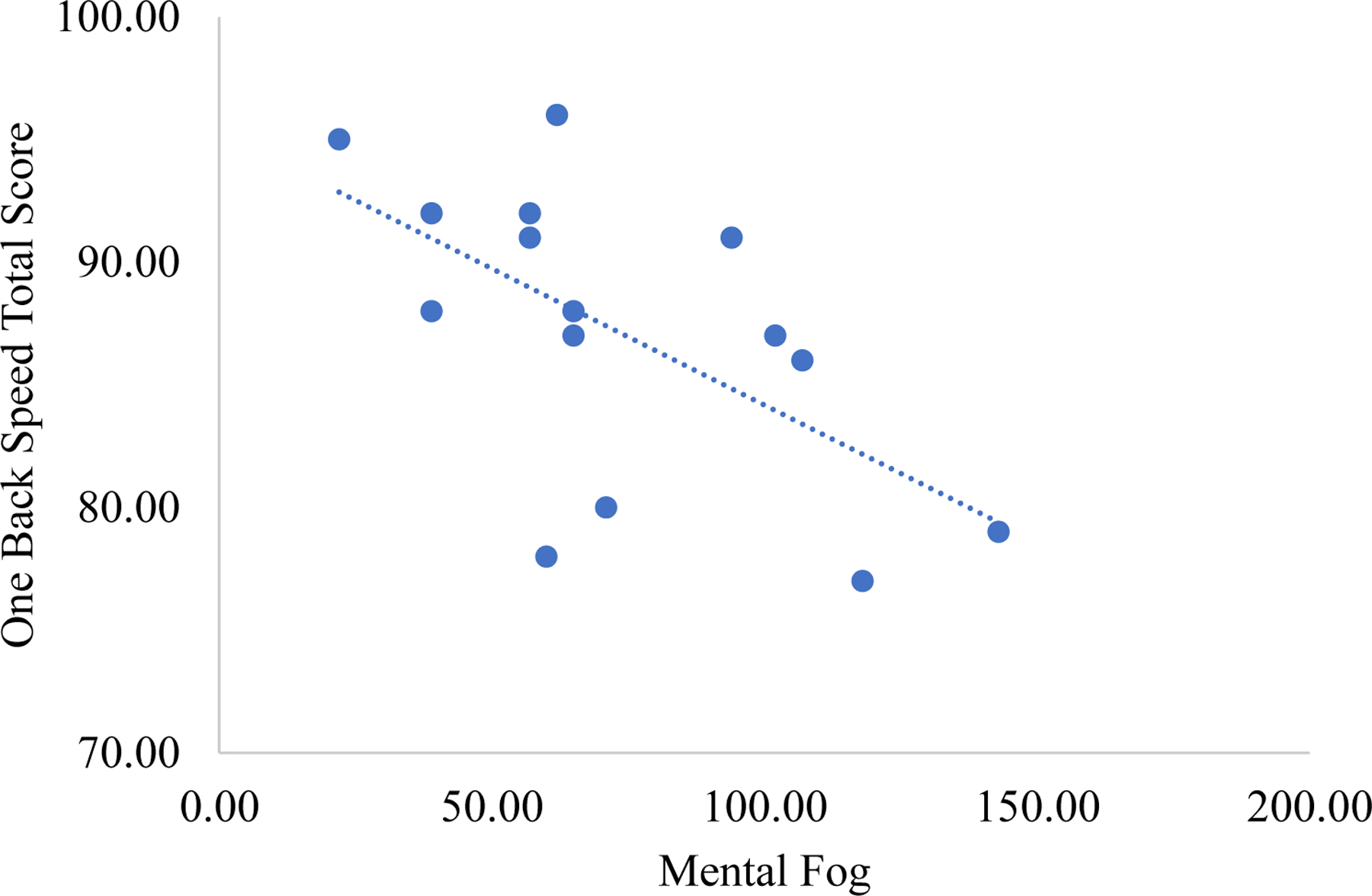
Correlation between brain fog and the Cogstate® One Back task in moderate-to-severe traumatic brain injury.
Discussion
“Brain fog” may indicate neurological disorder, making it of great clinical interest. Brain fog has been implicated in multiple conditions linked to neuroinflammation, most recently SARS-Cov-2. To date, nurses and other healthcare practitioners have few tools to measure brain fog severity, mostly relying on ratings of cognitive abilities unspecific to brain fog or one-item assessments of brain fog. This is particularly true in studies of brain injuries (Dean et al., 2012; Lovell et al., 2006). In this study, we examined the Mental Clutter Scale, a measure of brain fog validated in people with fibromyalgia (Leavitt & Katz, 2011), to capture brain fog severity. As described below, brain fog was more severe in people with mild and moderate-to-severe TBI than controls and related to other clinical symptoms differently within each group.
First, group comparisons established higher brain fog in people with symptomatic mild TBI (d = .96) and moderate-to-severe TBI (d = 1.25) than in healthy controls. Area under the curve analyses found that brain fog was able to acceptably discriminate symptomatic mild TBI (AUC = .74) and moderate-to-severe TBI from healthy controls (AUC =.85). Overall, these results are in line with the idea that brain fog captures the presence of a neurological disorder. Results are also consistent with previous work showing greater frequency of brain fog in mild TBI compared to controls, while also expanding this work to show greater severity of brain fog and to expand this finding to people with moderate-to-severe TBI (Dean et al., 2012; Lovell et al., 2006).
Second, within-group analyses revealed different clinical correlates of brain fog severity in mild and moderate-to-severe TBI. Brain fog was related to depressive symptoms and slower speed of processing in people with mild TBI. Unexpectedly, there were scant associations between brain fog and other measures of objective cognition, although this finding is consistent with the literature more broadly. Previous studies have failed to find significant associations between self-reported cognitive symptoms and objective cognitive difficulties after mild TBI (Karr et al., 2019; Spencer, Drag, Walker, & Bieliauskas, 2010; Stenberg et al., 2020), with only one other study finding an association between self-reported cognitive symptoms and slower processing speed (Schiehser et al., 2011). Furthermore, studies have found that negative affectivity, such as found in those with high depressive symptoms, contributes to the lack of correspondence between self-reported cognitive symptoms and objective cognition after mild TBI (Caplan et al., 2021). A significant correlation between brain fog and depressive symptoms in the mild TBI group may contribute to a lack of alignment between brain fog and objective cognition in people with mild TBI.
Furthermore, mechanisms underlying the association of mild TBI with depressive symptoms are likely multifactorial, involving psychological and biological components. Psychologically, depressive symptoms may arise from reduced self-efficacy resulting from being unable to keep oneself safe. Another reason could be general worries about recovery and return to normal function extending to symptom reports. Indeed, self-efficacy and general worries show strong links with self-reported cognitive symptoms in the broader literature (Aben et al., 2011; Dux et al., 2008). Biologically, depressive symptoms may correspond to inflammation related to brain damage that also underlies brain fog (Bodnar et al., 2018). In human models, blood markers of neuroinflammation (IL-6, TNFalpha, IL-10, and CRP) have been linked to depressive symptoms (Juengst, Kumar, & Wagner, 2017). In animal models, mild TBI results in neuroinflammation corresponding to anxiety-like behavior (Broussard et al., 2018). This may also explain why brain fog is related to slower processing speed, as processing speed shares negative associations with inflammation (Heringa et al., 2014). People with high brain fog severity and depressive symptoms after mild TBI may be experiencing unaddressed neuroinflammation. Further research will be needed to discover the psychobiological underpinnings of brain fog in symptomatic mild TBI.
Contrasting with the mild TBI group, reports of brain fog aligned with problems in episodic memory and working memory (as opposed to processing speed and depressive symptoms) in people with moderate-to-severe TBI. Although few studies exist in this severity group, this is consistent with one previous research showing that mental fatigue coincided with reduced working memory (Johansson et al. 2009). Thus, despite possible awareness issues, ratings of brain fog might identify persons with moderate-to-severe TBI with residual issues in episodic memory and executive function. Regarding biological mechanisms, as posited for mild TBI, brain fog may be due to persistent neuroinflammation after moderate-to-severe TBI. Recent studies have highlighted the possible role of long-term chronic neuroinflammation in explaining ongoing difficulties after moderate-to-severe TBI, including higher risk of other neurological disorders such as Parkinson’s and Alzheimer’s disease (Faden & Loane, 2015; Schimmel, Acosta, & Lozano, 2017). Brain fog, as a possible correlate of neuroinflammation, may help flag individuals having continuous difficulties in cognitive function who may also be at risk for other neurodegenerative diseases. Biomarkers of neuroinflammation may be informative in future studies.
Overall, these results have important implications for nursing, including in the area of SARS-Cov-2 infections. According to symptom science, brain fog may have similar biological mechanisms across conditions, despite different etiologies (McCall et al., 2018; Page et al., 2018; Saligan, 2019). This would explain why a measure of brain fog severity validated in people with fibromyalgia was also able to differentiate people with brain injury from controls in this study, as one common mechanism across these conditions is possible neuroinflammation (Bäckryd, Tanum, Lind, Larsson, & Gordh, 2017; Simon et al., 2017). This seems further likely as SARS-COV-2, which also involves reports of brain fog, can result in significant brain neuroinflammation (Kempuraj et al., 2020). Like investigations done in other conditions, most studies assessing brain fog in SARS-Cov-2 have relied on single item measures unable to assess severity or focus on indirect indicators of brain fog (Asadi-Pooya et al., 2022; Caspersen, Magnus, & Trogstad, 2022; Graham et al., 2021). Here we provide a measure that may be able to capture severity of brain fog which might also help differentiate those with neurological disorder after SARS-COV-2 infection. If not due to SARS-COV-2, these patients might have other contributing conditions such as brain injury which should be considered. According to the National Institutes of Health Symptom Science model, developed by the National Institute of Nursing Research, the next step for such work would be to continue phenotypic characterization of brain fog in conditions such as SARS-COV-2, followed by research on biomarker discovery and treatment application within these conditions (Cashion, Gill, Hawes, Henderson, & Saligan, 2016). The field of nursing and its focus on symptom science is well-positioned to lead such investigations.
Limitations
This study was not without limitations. First, although the symptomatic mild TBI group and healthy controls were comparable on age, the moderate-to-severe TBI cohort was much older. Therefore, descriptive comparisons involving this group should be considered with some caution. Fortunately, considerable research shows that age does not significantly affect reports of cognitive symptoms before older adulthood (Devolder & Pressley, 1991), minimizing this concern. Furthermore, differences in brain fog remained after adjusting for age differences. Second, definitions of TBI may influence results. For mild TBI, we recruited highly symptomatic mild TBI cases (> 13 on the PCSS) as these individuals are more likely to have poorer functional recovery and require continued clinical care (Collie et al., 2006). Results may not be generalizable to asymptomatic mild TBI. It is also important to note that high symptomology might influence reporting on other symptom scales like brain fog, especially if due to personality differences (Lange et al., 2010). However, adjustment for depressive symptoms lessens this concern. Furthermore, our definition of moderate-to-severe TBI was based on the Mayo Classification System, although several other definitions such as NINDS can be applied. Third, medical histories of each participant were not available for analyses of other conditions, such as mood disorders. This is particularly relevant in mild TBI where comorbid major depressive disorder and posttraumatic stress are particularly explanative of self-reported cognitive symptoms (Chamelian & Feinstein, 2006; French, Lange, & Brickell, 2014). Our analyses, however, noted no significant differences in depressive symptoms across the groups and adjusted for depressive symptoms to account for any mood differences across and within the groups. Lastly, sample sizes were modest but bolstered by the well-designed methodology to include representative clinical cases when self-reported brain fog might be most valuable. We used nonparametric statistical techniques which provide conservative testing for small samples.
Conclusions
After a TBI, individuals may experience a plethora of self-reported cognitive symptoms, some of which outlast objective cognitive impairments and may reflect residual functional concerns. Brain fog is one commonly reported symptom that has been largely overlooked despite its consideration as a common symptom of mild TBI. Here we validated the ability of a brain fog scale to differentiate people with symptomatic mild TBI from controls, providing further evidence for this measure as a potentially important diagnostic symptom. Moreover, we showed that brain fog is associated with greater depressive symptoms and slower speed of processing, outcomes that might benefit from interventions. Meanwhile, in the moderate-to-severe TBI group, brain fog appeared to represent enduring problems in episodic memory and executive function rather than depressive symptoms. In sum, the MCS appears a worthwhile tool to measure brain fog and detect the need for further clinical assessment in people with suspected head injury. Future work is needed to unravel the contributing biological mechanisms of brain fog that may be informative to other conditions, including SARS-Cov-2 infection, which is an area of heightened interest due to the pandemic.
Table 4.
Correlations between Mental Fog and Objective Cognitive Tasks in Persons with Symptomatic Mild TBI (n = 15).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. MCSa | 1 | .28 | .4 | −.03 | −.21 | −.27 | −.11 | −.06 | .16 | .72*** | .50 | .23 | .09 |
| 2. PCSS | 1 | .18 | .32 | −.24 | −.41 | .12 | −.47† | .09 | .56* | .22 | .35 | .50 | |
| 3. Time Since Injury | 1 | −.03 | −.32 | −.58* | −.65** | −.10 | −.42 | .37 | .65** | .36 | .39 | ||
| 4. Age | 1 | .04 | .18 | .35 | .06 | .25 | .02 | .03 | .43 | .12 | |||
| 5. Sex | 1 | .61* | .32 | −.25 | .49 | −.26 | −.22 | −.43 | −.22 | ||||
| 6. Detection | 1 | .69** | .18 | .55* | −.48† | −.51† | −.33 | −.77** | |||||
| 7. Identification | 1 | .09 | .65** | −.21 | −.51† | −.12 | −.42 | ||||||
| 8. Learning | 1 | .13 | −.10 | −.02 | .27 | −.19 | |||||||
| 9. One Back | 1 | −.16 | −.30 | −.49 | −.56† | ||||||||
| 10. UFOV®1 | 1 | .82*** | .45† | .48 | |||||||||
| 11. UFOV®2 | 1 | .41 | .30 | ||||||||||
| 12. UFOV®3 | 1 | .57† | |||||||||||
| 13. UFOV®4 | 1 | ||||||||||||
Notes. All correlations are Spearman rho. MCS = Mental Clutter Scale; PCSS = Post-Concussive Symptom Scale; POMS-Dep = Depression subscale from the Profile of Mood States; UFOV = Useful Field of View.
p < .10
p < .05
p < .01
Pre-adjusted for association with depressive symptoms using residuals from a linear regression.
Funding:
This work was supported by the UAB Edward R. Roybal Center for Translational Research in Aging and Mobility (5 P30 AG 022838 PI: Karlene K. Ball) and from funding provided by the University of Alabama at Birmingham (UAB) College of Arts and Sciences Interdisciplinary Team Grant Award and the UAB Department of Physical Medicine and Rehabilitation Resident Physical Collaborative Research in Functional Neurorecovery Award awarded to the last author. Work was also supported by fellowship awarded from the US Department of Transportation Federal Highway Administration (FWHA) Dwight D. Eisenhower Graduate Fellowship Program awarded to the first author. The first author was supported to conduct this work by R01AG050595 awarded to William Kremen, Carol Franz, and Michael Lyons, as well as R01AG022381.
Footnotes
Patient or Public Contribution: Patients with traumatic brain injury participated in this study.
References
- Aben L, Ponds RW, Heijenbrok-Kal MH, Visser MM, Busschbach JJ, & Ribbers GM (2011). Memory complaints in chronic stroke patients are predicted by memory self-efficacy rather than memory capacity. Cerebrovascular Diseases, 31(6), 566–572. [DOI] [PubMed] [Google Scholar]
- Alford K, Daley S, Banerjee S, Hamlyn E, Trotman D, & Vera JH (2022). “A fog that impacts everything”: a qualitative study of health-related quality of life in people living with HIV who have cognitive impairment. Quality of life Research, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi-Pooya AA, Akbari A, Emami A, Lotfi M, Rostamihosseinkhani M, Nemati H, … Farjoud-Kouhanjani M (2022). Long COVID syndrome-associated brain fog. Journal of medical virology, 94(3), 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckryd E, Tanum L, Lind A-L, Larsson A, & Gordh T (2017). Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma. Journal of pain research, 10, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, & Owsley C (1993). The Useful Field of View test: A new technique for evaluating age-related declines in visual function. Journal of the American Optometric Association, 64(1), 71–79. [PubMed] [Google Scholar]
- Bodnar CN, Morganti JM, & Bachstetter AD (2018). Depression following a traumatic brain injury: Uncovering cytokine dysregulation as a pathogenic mechanism. Neural Regeneration research, 13(10), 1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglio SP, Sosnoff JJ, & Ferrara MS (2009). The relationship of athlete-reported concussion symptoms and objective measures of neurocognitive function and postural control. Clinical Journal of Sport Medicine, 19(5), 377–382. [DOI] [PubMed] [Google Scholar]
- Broussard JI, Acion L, De Jesús-Cortés H, Yin T, Britt JK, Salas R, … Arciniegas DB (2018). Repeated mild traumatic brain injury produces neuroinflammation, anxiety-like behaviour and impaired spatial memory in mice. Brain Injury, 32(1), 113–122. [DOI] [PubMed] [Google Scholar]
- Buckley R, Saling MM, Ames D, Rowe CC, Lautenschlager NT, Macaulay SL, Martins RN, Masters CL, O’Meara T, Savage G, & others. (2013). Factors affecting subjective memory complaints in the AIBL aging study: Biomarkers, memory, affect, and age. International Psychogeriatrics, 25(8), 1307–1315. [DOI] [PubMed] [Google Scholar]
- Callan C, Ladds E, Husain L, Pattinson K, & Greenhalgh T (2022). ‘I can’t cope with multiple inputs’: a qualitative study of the lived experience of ‘brain fog’after COVID-19. BMJ open, 12(2), e056366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan B, Bogner J, Brenner L, Malec J, Hromas GA, Houck ZM, … Heaton SC (2021). Making a difference: affective distress explains discrepancy between objective and subjective cognitive functioning after mild traumatic brain injury. Journal of Head Trauma Rehabilitation, 36(3), 186–195. [DOI] [PubMed] [Google Scholar]
- Cashion AK, Gill J, Hawes R, Henderson WA, & Saligan L (2016). National Institutes of Health Symptom Science Model sheds light on patient symptoms. Nursing Outlook, 64(5), 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen IH, Magnus P, & Trogstad L (2022). Excess risk and clusters of symptoms after COVID-19 in a large Norwegian cohort. European Journal of Epidemiology, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamelian L, & Feinstein A (2006). The effect of major depression on subjective and objective cognitive deficits in mild to moderate traumatic brain injury. The Journal of Neuropsychiatry and Clinical Neurosciences, 18(1), 33–38. [DOI] [PubMed] [Google Scholar]
- Collie A, Makdissi M, Maruff P, Bennell K, & McCrory P (2006). Cognition in the days following concussion: Comparison of symptomatic versus asymptomatic athletes. Journal of Neurology, Neurosurgery & Psychiatry, 77(2), 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collie A, Maruff P, Makdissi M, McCrory P, McStephen M, & Darby D (2003). CogSport: Reliability and correlation with conventional cognitive tests used in postconcussion medical evaluations. Clinical Journal of Sport Medicine, 13(1), 28–32. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Whiteneck G, & Mellick D (2004). Perceived needs following traumatic brain injury. The Journal of Head Trauma Rehabilitation, 19(3), 205–216. [DOI] [PubMed] [Google Scholar]
- de Boussard CN, Lundin A, Karlstedt D, Edman G, Bartfai A, Borg J, & others. (2005). S100 and cognitive impairment after mild traumatic brain injury. Journal of Rehabilitation Medicine, 37(1), 53–57. [DOI] [PubMed] [Google Scholar]
- Dean PJ, O’Neill D, & Sterr A (2012). Postconcussion syndrome: Prevalence after mild traumatic brain injury in comparison with a sample without head injury. Brain Injury, 26(1), 14–26. [DOI] [PubMed] [Google Scholar]
- Devolder PA, & Pressley M (1991). Memory complaints in younger and older adults. Applied Cognitive Psychology, 5(5), 443–454. [Google Scholar]
- Dux MC, Woodard JL, Calamari JE, Messina M, Arora S, Chik H, & Pontarelli N (2008). The moderating role of negative affect on objective verbal memory performance and subjective memory complaints in healthy older adults. Journal of the International Neuropsychological Society, 14(2), 327–336. [DOI] [PubMed] [Google Scholar]
- Faden AI, & Loane DJ (2015). Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics, 12(1), 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French LM, Lange RT, & Brickell TA (2014). Subjective cognitive complaints and neuropsychological test performance following military-related traumatic brain injury. Journal of Rehabilitation Research & Development, 51(6). [DOI] [PubMed] [Google Scholar]
- Fritsch T, McClendon MJ, Wallendal MS, Hyde TF, & Larsen JD (2014). Prevalence and cognitive bases of subjective memory complaints in older adults: Evidence from a community sample. Journal of Neurodegenerative Diseases, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost RB, Farrer TJ, Primosch M, & Hedges DW (2013). Prevalence of traumatic brain injury in the general adult population: A meta-analysis. Neuroepidemiology, 40(3), 154–159. [DOI] [PubMed] [Google Scholar]
- Gardner RC, Langa KM, & Yaffe K (2017). Subjective and objective cognitive function among older adults with a history of traumatic brain injury: A population-based cohort study. PLoS Medicine, 14(3), e1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein FC, Levin HS, Goldman WP, Clark AN, & Altonen TK (2001). Cognitive and neurobehavioral functioning after mild versus moderate traumatic brain injury in older adults. Journal of the International Neuropsychological Society, 7(3), 373–383. [DOI] [PubMed] [Google Scholar]
- Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, … Ho SU (2021). Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Annals of Clinical and Translational Neurology, 8(5), 1073–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart T, Seignourel PJ, & Sherer M (2009). A longitudinal study of awareness of deficit after moderate to severe traumatic brain injury. Neuropsychological Rehabilitation, 19(2), 161–176. [DOI] [PubMed] [Google Scholar]
- Hart T, Whyte J, Kim J, & Vaccaro M (2005). Executive function and self-awareness of “real-world” behavior and attention deficits following traumatic brain injury. The Journal of Head Trauma Rehabilitation, 20(4), 333–347. [DOI] [PubMed] [Google Scholar]
- Heringa SM, van den Berg E, Reijmer YD, Nijpels G, Stehouwer CD, Schalkwijk CG, … Kappelle LJ (2014). Markers of low-grade inflammation and endothelial dysfunction are related to reduced information processing speed and executive functioning in an older population–the Hoorn study. Psychoneuroendocrinology, 40, 108–118. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Gaetz M, Lovell MR, & Collins MW (2004). Relation between subjective fogginess and neuropsychological testing following concussion. Journal of the International Neuropsychological Society, 10(6), 904–906. [DOI] [PubMed] [Google Scholar]
- Jamora CW, Young A, & Ruff RM (2012). Comparison of subjective cognitive complaints with neuropsychological tests in individuals with mild vs more severe traumatic brain injuries. Brain Injury, 26(1), 36–47. [DOI] [PubMed] [Google Scholar]
- Johansson B, Berglund P, & Rönnbäck L (2009). Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Injury, 23(13–14), 1027–1040. [DOI] [PubMed] [Google Scholar]
- Johansson B, Björk MP, & Thorvaldsson V (2020). I rate my memory quite similar at age 40 and at age 70: Findings in a Swedish longitudinal study on subjective memory over a 30-year period. GeroPsych: The Journal of Gerontopsychology and Geriatric Psychiatry, 33(4), 235–244. [Google Scholar]
- Juengst SB, Kumar RG, & Wagner AK (2017). A narrative literature review of depression following traumatic brain injury: prevalence, impact, and management challenges. Psychology research and behavior management [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamkwalala A, Hulgan T, & Newhouse P (2017). Subjective memory complaints are associated with poorer cognitive performance in adults with HIV. AIDS Care, 29(5), 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz RS, Heard AR, Mills M, & Leavitt F (2004). The prevalence and clinical impact of reported cognitive difficulties (fibrofog) in patients with rheumatic disease with and without fibromyalgia. JCR: Journal of Clinical Rheumatology, 10(2), 53–58. [DOI] [PubMed] [Google Scholar]
- Karr JE, Rau HK, Shofer JB, Hendrickson RC, Peskind ER, & Pagulayan KF (2019). Variables associated with subjective cognitive change among Iraq and Afghanistan war veterans with blast-related mild traumatic brain injury. Journal of Clinical and Experimental Neuropsychology, 41(7), 680–693. [DOI] [PubMed] [Google Scholar]
- Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K, Dahlberg C, Gerber D, Goka R, Harley P, & others. (1993). Definition of mild traumatic brain injury. Journal of Head Trauma Rehabilitation, 8(3), 86–87. [Google Scholar]
- Lange RT, Iverson GL, & Rose A (2010). Postconcussion symptom reporting and the “good-old-days” bias following mild traumatic brain injury. Archives of Clinical Neuropsychology, 25(5), 442–450. [DOI] [PubMed] [Google Scholar]
- Leavitt F, & Katz RS (2011). Development of The Mental Clutter Scale 1. Psychological Reports, 109(2), 445–452. [DOI] [PubMed] [Google Scholar]
- Lovell MR, Iverson GL, Collins MW, Podell K, Johnston KM, Pardini D, Pardini J, Norwig J, & Maroon JC (2006). Measurement of symptoms following sports-related concussion: Reliability and normative data for the Postconcussion Scale. Applied Neuropsychology, 13(3), 166–174. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Khan A, … James D (2020). COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. The Neuroscientist, 26(5–6), 402–414. [DOI] [PubMed] [Google Scholar]
- Malec JF, Brown AW, Leibson CL, Flaada JT, Mandrekar JN, Diehl NN, & Perkins PK (2007). The Mayo classification system for traumatic brain injury severity. Journal of Neurotrauma, 24(9), 1417–1424. [DOI] [PubMed] [Google Scholar]
- Mangeot S, Armstrong K, Colvin AN, Yeates KO, & Taylor HG (2002). Long-term executive function deficits in children with traumatic brain injuries: Assessment using the Behavior Rating Inventory of Executive Function (BRIEF). Child Neuropsychology, 8(4), 271–284. [DOI] [PubMed] [Google Scholar]
- Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, & Pietrzak RH (2009). Validity of the CogState Brief Battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Archives of Clinical Neuropsychology, 24(2), 165–178. [DOI] [PubMed] [Google Scholar]
- Masson F, Maurette P, Salmi L, Dartigues J, Vecsey J, Destaillats J, & Erny P (1996). Prevalence of impairments 5 years after a head injury, and their relationship with disabilities and outcome. Brain Injury, 10(7), 487–498. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewicz KM, Marshall SW, Barr W, Randolph C, Cantu RC, Onate JA, Yang J, & Kelly JP (2003). Acute effects and recovery time following concussion in collegiate football players: The NCAA Concussion Study. JAMA, 290(19), 2556–2563. [DOI] [PubMed] [Google Scholar]
- McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, & Cantu R (2009). Consensus statement on Concussion in Sport–the 3rd International Conference on Concussion in Sport held in Zurich, November 2008. South African Journal of Sports Medicine, 21(2), 434–448. [DOI] [PubMed] [Google Scholar]
- McCullagh S, & Feinstein A (2003). Outcome after mild traumatic brain injury: An examination of recruitment bias. Journal of Neurology, Neurosurgery & Psychiatry, 74(1), 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott LM, & Ebmeier KP (2009). A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders, 119(1–3), 1–8. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, & Droppleman LF (1971). POMS: profile of mood states. Educational and Industrial Testing Service: San Diego [Google Scholar]
- Mackay M (2015). Lupus brain fog: a biologic perspective on cognitive impairment, depression, and fatigue in systemic lupus erythematosus. Immunologic Research, 63(1), 26–37. [DOI] [PubMed] [Google Scholar]
- McCall MK, Stanfill AG, Skrovanek E, Pforr JR, Wesmiller SW, & Conley YP (2018). Symptom science: Omics supports common biological underpinnings across symptoms. Biological Research for Nursing, 20(2), 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus B, Bell TR, & Stavrinos D (2019). Driving simulator assessment of fitness-to-drive following traumatic brain injury [Google Scholar]
- McManus B, Cox MK, Vance DE, & Stavrinos D (2015). Predicting motor vehicle collisions in a driving simulator in young adults using the useful field of view assessment. Traffic Injury Prevention, 16(8), 818–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A, McManus B, Singichetti B, Stavrinos D, Yang JG, Kerwin T, & Bell T (2018). Driving Performance After Mild Traumatic Brain Injury in Teen Drivers. Archives of Physical Medicine and Rehabilitation, 99(11), e133. [Google Scholar]
- Nakase-Thompson R, Manning E, Sherer M, Yablon S, Gontkovsky S, & Vickery C (2005). Brief assessment of severe language impairments: Initial validation of the Mississippi Aphasia Screening Test. Brain Injury, 19(9), 685–691. [DOI] [PubMed] [Google Scholar]
- Nelson DV, & Esty ML (2015). Neurotherapy for chronic headache following traumatic brain injury. Military Medical Research, 2(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH (National Institutes of Health). Traumatic brain injury information 2018. [August 12, 2018]. https://www.ninds.nih.gov/health-information/disorders/traumatic-brain-injury
- Novack TA, Baños JH, Alderson AL, Schneider JJ, Weed W, Blankenship J, & Salisbury D (2006). UFOV performance and driving ability following traumatic brain injury. Brain Injury, 20(5), 455–461. [DOI] [PubMed] [Google Scholar]
- Oyesanya TO, Thomas MA, Brown RL, & Turkstra LS (2016). Nurses’ beliefs about caring for patients with traumatic brain injury. Western Journal of Nursing Research, 38(9), 1114–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page GG, Corwin EJ, Dorsey SG, Redeker NS, McCloskey DJ, Austin JK, … Kim MT (2018). Biomarkers as common data elements for symptom and self-management science. Journal of Nursing Scholarship, 50(3), 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Young C, Woods SP, Vigil O, Grant I, & Atkinson JH (2006). Screening for major depression in persons with HIV infection: The concurrent predictive validity of the Profile of Mood States Depression-Dejection Scale. International Journal of Methods in Psychiatric Research, 15(2), 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsford J, Cameron P, Fitzgerald M, Grant M, & Mikocka-Walus A (2011). Long-term outcomes after uncomplicated mild traumatic brain injury: A comparison with trauma controls. Journal of Neurotrauma, 28(6), 937–946. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, & Nimmo-Smith I (1996). The structure of normal human attention: The Test of Everyday Attention. Journal of the International Neuropsychological Society, 2(6), 525–534. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Medow MS, Rowe PC, & Stewart JM (2013). What is brain fog? An evaluation of the symptom in postural tachycardia syndrome. Clinical Autonomic Research, 23(6), 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saligan LN (2019). Collaborative framework to advance symptom science: an intramural perspective. Journal of Nursing Scholarship, 51(1), 17–25. [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2000). Aging and measures of processing speed. Biological Psychology, 54(1–3), 35–54. [DOI] [PubMed] [Google Scholar]
- Samuels MH, & Bernstein LJ (2022). Brain fog in hypothyroidism: What is it, how is it measured, and what can be done about it. Thyroid [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiehser DM, Delis DC, Filoteo JV, Delano-Wood L, Han SD, Jak AJ, … Bondi MW (2011). Are self-reported symptoms of executive dysfunction associated with objective executive function performance following mild to moderate traumatic brain injury? Journal of Clinical and Experimental Neuropsychology, 33(6), 704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel SJ, Acosta S, & Lozano D (2017). Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circulation, 3(3), 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmand B, Jonker C, Hooijer C, & Lindeboom J (1996). Subjective memory complaints may announce dementia. Neurology, 46(1), 121–125. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, & Shapiro AM (2003). A quantitative review of the effects of traumatic brain injury on cognitive functioning. International Review of Psychiatry, 15(4), 341–349. [DOI] [PubMed] [Google Scholar]
- Schuurmans MJ, Shortridge-Baggett LM, & Duursma SA (2003). The Delirium Observation Screening Scale: A screening instrument for delirium. Research and Theory for Nursing Practice, 17(1), 31–50. [DOI] [PubMed] [Google Scholar]
- Sherer M, Nakase-Thompson R, Yablon SA, & Gontkovsky ST (2005). Multidimensional assessment of acute confusion after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 86(5), 896–904. [DOI] [PubMed] [Google Scholar]
- Simon DW, McGeachy MJ, Bayır H, Clark RS, Loane DJ, & Kochanek PM (2017). The far-reaching scope of neuroinflammation after traumatic brain injury. Nature Reviews Neurology, 13(3), 171–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RJ, Drag LL, Walker SJ, & Bieliauskas LA (2010). Self-reported cognitive symptoms following mild traumatic brain injury are poorly associated with neuropsychological performance in OIF/OEF veterans. Journal of Rehabilitation Research & Development, 47(6). [DOI] [PubMed] [Google Scholar]
- Spencer RJ, Drag LL, Walker SJ, & Bieliauskas LA (2010). Self-reported cognitive symptoms following mild traumatic brain injury are poorly associated with neuropsychological performance in OIF/OEF veterans. Journal of Rehabilitation Research & Development, 47(6), 521–532. [DOI] [PubMed] [Google Scholar]
- Findings from the Seattle Longitudinal Study. Psychology and Aging, 33(3), 448. [DOI] [PubMed] [Google Scholar]
- Stavrinos D, Yang G, Kerwin T, McManus B, Bell TR, Newton A, & Singichetti B (2019). Driving simulator performance in the acute post-injury phase following a mild traumatic brain injury among young drivers [Google Scholar]
- Stuss DT, Toth JP, Franchi D, Alexander MP, Tipper S, & Craik FI (1999). Dissociation of attentional processes in patients with focal frontal and posterior lesions. Neuropsychologia, 37(9), 1005–1027. [DOI] [PubMed] [Google Scholar]
- Stenberg J, Karr JE, Terry DP, Håberg AK, Vik A, Skandsen T, & Iverson GL (2020). Change in self-reported cognitive symptoms after mild traumatic brain injury is associated with changes in emotional and somatic symptoms and not changes in cognitive performance. Neuropsychology, 34(5), 560. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Stewart JM, Hatziagelaki E, & Kolaitis G (2015). Brain “fog,” inflammation and obesity: key aspects of neuropsychiatric disorders improved by luteolin. Frontiers in neuroscience, 9, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer AK, & Rich JB (2002). Psychometric properties of a new metamemory questionnaire for older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57(1), P19–P27. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Curtiss G, Luis CA, & Salazar AM (2007). Long-term morbidities following self-reported mild traumatic brain injury. Journal of Clinical and Experimental Neuropsychology, 29(6), 585–598. [DOI] [PubMed] [Google Scholar]
- Vos PE, Battistin L, Birbamer G, Gerstenbrand F, Potapov A, Prevec T, Stepan CA, Traubner P, Twijnstra A, Vecsei L, & others. (2002). EFNS guideline on mild traumatic brain injury: Report of an EFNS task force. European Journal of Neurology, 9(3), 207–219. [DOI] [PubMed] [Google Scholar]
- Wallace JC (2004). Confirmatory factor analysis of the cognitive failures questionnaire: Evidence for dimensionality and construct validity. Personality and Individual Differences, 37(2), 307–324. [Google Scholar]
- Wang P-N, Wang S-J, Fuh J-L, Teng EL, Liu C-Y, Lin C-H, Shyu H-Y, Lu S-R, Chen C-C, & Liu H-C (2000). Subjective memory complaint in relation to cognitive performance and depression: A longitudinal study of a rural Chinese population. Journal of the American Geriatrics Society, 48(3), 295–299. [DOI] [PubMed] [Google Scholar]
- Whyte J, Grieb-Neff P, Gantz C, & Polansky M (2006). Measuring sustained attention after traumatic brain injury: Differences in key findings from the sustained attention to response task (SART). Neuropsychologia, 44(10), 2007–2014. [DOI] [PubMed] [Google Scholar]


