Abstract
PURPOSE
Adagrasib, a KRASG12C inhibitor, has demonstrated clinical activity in patients with KRASG12C-mutated non–small-cell lung cancer (NSCLC) and colorectal cancer (CRC). KRASG12C mutations occur rarely in other solid tumor types. We report evaluation of the clinical activity and safety of adagrasib in patients with other solid tumors harboring a KRASG12C mutation.
METHODS
In this phase II cohort of the KRYSTAL-1 study (ClinicalTrials.gov identifier: NCT03785249; phase Ib cohort), we evaluated adagrasib (600 mg orally twice daily) in patients with KRASG12C-mutated advanced solid tumors (excluding NSCLC and CRC). The primary end point was objective response rate. Secondary end points included duration of response, progression-free survival (PFS), overall survival, and safety.
RESULTS
As of October 1, 2022, 64 patients with KRASG12C-mutated solid tumors were enrolled and 63 patients treated (median follow-up, 16.8 months). The median number of prior lines of systemic therapy was 2. Among 57 patients with measurable disease at baseline, objective responses were observed in 20 (35.1%) patients (all partial responses), including 7/21 (33.3%) responses in pancreatic and 5/12 (41.7%) in biliary tract cancers. The median duration of response was 5.3 months (95% CI, 2.8 to 7.3) and median PFS was 7.4 months (95% CI, 5.3 to 8.6). Treatment-related adverse events (TRAEs) of any grade were observed in 96.8% of patients and grade 3-4 in 27.0%; there were no grade 5 TRAEs. TRAEs did not lead to treatment discontinuation in any patients.
CONCLUSION
Adagrasib demonstrates encouraging clinical activity and is well tolerated in this rare cohort of pretreated patients with KRASG12C-mutated solid tumors.
Adagrasib shows clinical activity in KRASG12C-mutated tumors, including #pancreascancer #cholangiocarcinoma.
INTRODUCTION
KRAS represents the most prevalent oncogenic driver in human cancer,1 with over 80% of mutations occurring at codon 12, normally occupied by a glycine residue.2,3 KRASG12C, which favors the active guanosine triphosphate (GTP)–bound form of KRAS and results in enhanced cell proliferation and survival,1,4 occurs in approximately 14% of patients with non–small-cell lung cancer (NSCLC) and 3%-4% of those with colorectal cancer (CRC).5-7 KRASG12C mutations have also been identified, although less frequently, in other solid tumors, including appendiceal (3%-4%), pancreatic (1%-3%), small bowel (1%-3%), biliary tract (1%), endometrial (1%-2%), and ovarian cancer (1%-2%).5,6,8 No approved targeted treatment options are currently available for patients with KRASG12C-driven solid tumors other than NSCLC.8,9
CONTEXT
Key Objective
This KRYSTAL-1 phase II cohort evaluated the clinical activity and safety of adagrasib, a potent covalent KRASG12C inhibitor, in patients with advanced solid tumors, excluding non–small-cell lung cancer (NSCLC) and colorectal cancer (CRC), harboring a KRASG12C mutation.
Knowledge Generated
To date, to our knowledge, this study is the largest phase II tumor-agnostic data set to evaluate KRASG12C-mutated solid tumors, excluding NSCLC and CRC. Adagrasib demonstrated an encouraging objective response rate of 35.1% in a biomarker-selected population who have declined or have no available standard-of-care treatment option. Responses were observed across a broad range of tumor types, including pancreatic ductal adenocarcinoma and biliary tract cancer; safety and tolerability profiles were manageable across tumor types. The range of responses observed suggest the potential for use of adagrasib as a tumor-agnostic agent, underscoring the importance of wider and more consistent genomic testing when assessing therapeutic approaches.
Relevance (R.G. Maki)
-
Adagrasib is a KRASG12C inhibitor that demonstrates activity in a number of cancer histologies in which the KRAS mutation is observed. This study broadens the diagnoses for which this class of agents may be useful.*
*Relevance section written by JCO Associate Editor Robert G. Maki, MD, PhD, FACP, FASCO.
After the identification of the KRAS switch II binding pocket, small molecules have been developed that can occupy this region and covalently bind to the mutant cysteine to prevent GTP binding.10-12 Adagrasib is an oral, small-molecule, covalent inhibitor that irreversibly and selectively binds KRASG12C, trapping it in its inactive guanosine diphosphate–bound state.10,11 Adagrasib was selected for favorable properties, including a long half-life of 23 hours, dose-dependent pharmacokinetics, and CNS penetration.10,13,14
Adagrasib is currently being evaluated in KRYSTAL-1, a phase I/II multiple expansion cohort trial of patients with advanced solid tumors harboring a KRASG12C mutation. Data reported previously from other cohorts of this trial demonstrated clinical activity and tolerability of adagrasib in previously treated patients with KRASG12C-mutated NSCLC and CRC.15,16 On the basis of these data, the US Food and Drug Administration (FDA) granted accelerated approval for adagrasib in previously treated KRASG12C-mutated NSCLC in December 2022.17 The FDA has also granted breakthrough therapy designation for adagrasib in combination with cetuximab, for the treatment of patients with KRASG12C-mutated CRC.18 Preliminary results have also been presented for other GI tumors, including pancreatic, biliary tract, and appendiceal cancers, as well as non-GI tumors, such as ovarian and endometrial cancers, demonstrating promising clinical activity in these tumor types.13,19,20 Herein, we report data from a phase II cohort of KRYSTAL-1 evaluating adagrasib monotherapy in patients with advanced solid tumors, excluding NSCLC and CRC, harboring a KRASG12C mutation.
METHODS
Study Oversight
This open-label, single-arm, nonrandomized study was designed by employees of Mirati Therapeutics, Inc (the sponsor) and the investigators. The data were collected by the investigators and analyzed by sponsor-employed statisticians. The study was conducted in accordance with the principles of the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice guidelines.21,22 The Protocol (online only) was approved by the relevant institutional review boards or ethics committees. All the patients provided written informed consent. Trial oversight was provided by the sponsor, the investigators, local institutional review boards, a specifically commissioned central institutional review board, and an independent data monitoring committee.
Patients
Eligible patients were age 18 years and older, with a histologically confirmed diagnosis of an unresectable or metastatic solid tumor (excluding NSCLC and CRC) with a KRASG12C mutation detected in tumor tissue and/or blood. Patients were required to have a life expectancy of ≥3 months, measurable disease per RECIST version 1.1 (v1.1), and an Eastern Cooperative Oncology Group performance status score of 0 or 1. Patients must have had no other available treatment with curative intent and have been ineligible for, declined, or had no available standard-of-care treatment option. Key exclusion criteria included previous treatment with another KRASG12C inhibitor and active CNS metastases; patients were eligible if CNS metastases were adequately treated and/or stable (no corticosteroids for ≥2 weeks before enrollment or stable/decreasing dose of ≤10 mg daily corticosteroids). Full eligibility criteria are provided in the Protocol.
Study Design and End Points
In this phase II cohort, the clinical activity of adagrasib was evaluated in patients with KRASG12C-mutated unresectable or metastatic solid tumors other than NSCLC and CRC. Patients received adagrasib 600 mg (capsule formulation) orally twice daily as monotherapy until disease progression, unacceptable adverse events, withdrawal of consent, or death. Patients experiencing clinical benefit, as judged by the investigator, could continue therapy beyond RECIST-defined disease progression. The primary end point was objective response rate (ORR) according to RECIST v1.1. Secondary end points included duration of response, progression-free survival (PFS), overall survival (OS), 1-year survival rate, safety, and tolerability.
Study Assessments
Clinical Assessments
Patients were tested for mutations in tumor tissue or circulating tumor DNA using preapproved methods for analysis (see Appendix 1 [online only] for details).
All patients underwent chest (computed tomography [CT] with contrast), abdomen, and pelvis imaging (CT with contrast or magnetic resonance imaging) at baseline, with subsequent assessment every 6 weeks throughout the first year of treatment and every 12 weeks thereafter. Disease evaluation scans continued until documentation of objective disease progression by the investigator; response assessments were evaluated by investigators and a blinded independent central review (BICR) according to RECIST v1.1. Patients with a tumor response (partial or complete response) had a confirmatory assessment at least 4 weeks after initial observation of response.
Safety
Adverse events were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). Patients were followed for adverse events for ≥28 days after the last study dose of study treatment.
Statistical Analysis
Confirmed objective responses were summarized as the frequency and percentage of complete and partial responses, with 95% CIs, on the basis of the full analysis set comprising all patients with measurable disease at baseline who received ≥1 dose of adagrasib. The ORR using currently available therapies to treat patients with advanced solid tumors harboring a KRASG12C mutation, who have previously received standard-of-care treatments, was assumed to be 10%; therefore, this rate was considered not clinically meaningful. The target ORR using adagrasib in this patient population was 30%. Median duration of response, PFS, and OS, as well as 1-year survival rate, were estimated using the Kaplan-Meier method. Median PFS was summarized on the basis of the full analysis set. OS was summarized on the basis of the enrolled population.
RESULTS
Patients
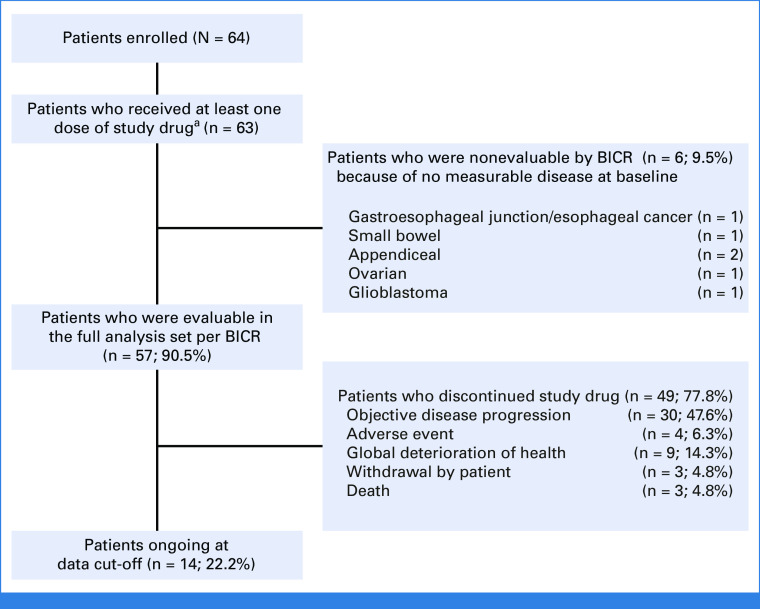
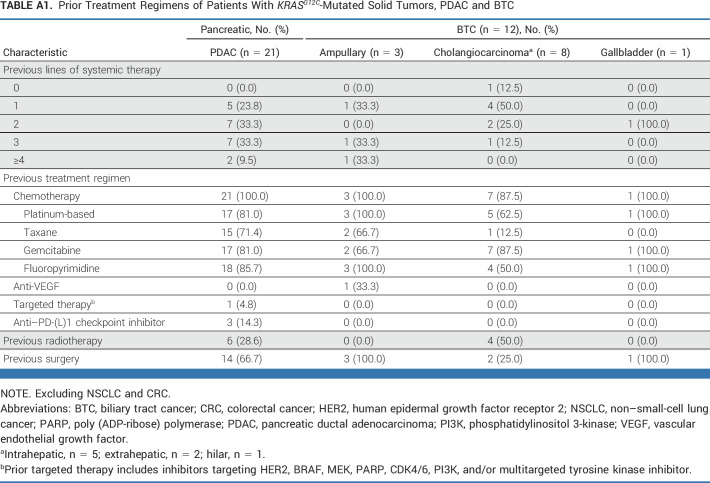
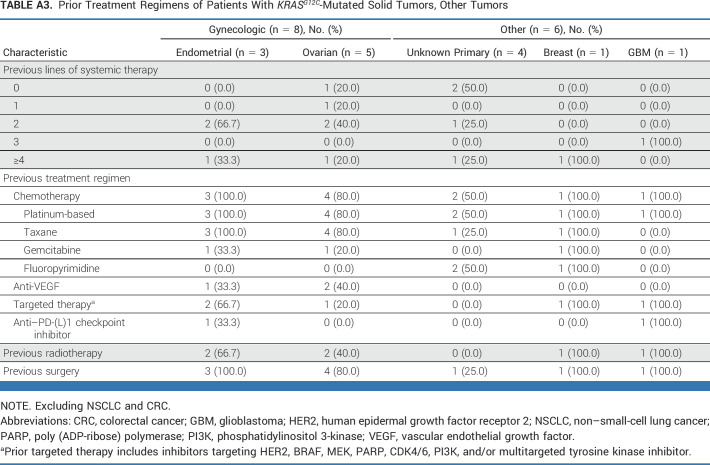
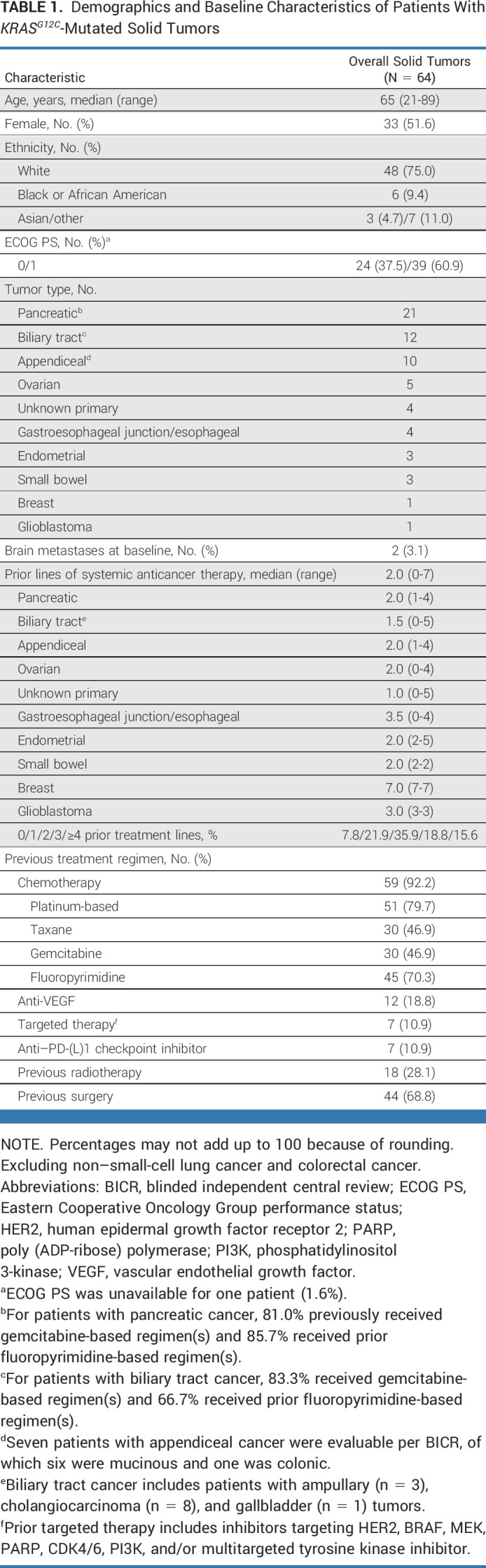
As of October 1, 2022, with an enrollment period of March 2020 to September 2022 (30 months), 64 patients with KRASG12C-mutated unresectable or metastatic solid tumors (excluding NSCLC and CRC) were enrolled. Presence of KRASG12C mutation was established using tumor tissue for 54 patients and blood circulating tumor DNA sampling for 10 patients. In total, 63 patients had received at least one dose of adagrasib at data cutoff; six patients had measurable disease at baseline according to investigator assessment, but no measurable disease according to the independent committee (Fig 1). The median follow-up was 16.8 months, and the median duration of treatment was 6.6 months. Patient demographics and baseline characteristics are shown in Table 1, with prior treatment regimens detailed in Appendix Table A1 (online only; pancreatic ductal adenocarcinoma and biliary tract cancer), Appendix Table A2 (online only; other GI tumors), and Appendix Table A3 (online only; other tumors). The median age was 65 years and the median number of prior lines of systemic therapy was 2. The cohort included patients with the following tumor types: pancreatic cancer (n = 21), biliary tract cancer (n = 12), appendiceal cancer (n = 10), ovarian cancer (n = 5), unknown primary cancer (n = 4), gastroesophageal junction/esophageal cancer (n = 4), endometrial cancer (n = 3), small bowel cancer (n = 3), breast cancer (n = 1), and glioblastoma (n = 1). As of the data cutoff, 49 patients had discontinued treatment, primarily because of disease progression (n = 30), health deterioration (n = 9), unrelated treatment emergent adverse events (n = 4), withdrawal (n = 3), and death (unrelated to treatment; n = 3), as shown in Figure 1.
FIG 1.
Patient disposition. aOne patient with appendiceal cancer was enrolled at the point of data cutoff (October 1, 2022) but had not been treated at this time. BICR, blinded independent central review.
TABLE 1.
Demographics and Baseline Characteristics of Patients With KRASG12C-Mutated Solid Tumors
Clinical Activity
Among 57 patients with measurable disease, ORR was 35.1% (20/57), all of which were partial responses as determined by BICR. Tumor responses, as determined by BICR and investigator assessment, are summarized in Table 2.
TABLE 2.
ORRs in Patients With KRASG12C-Mutated Solid Tumors (full analysis set)
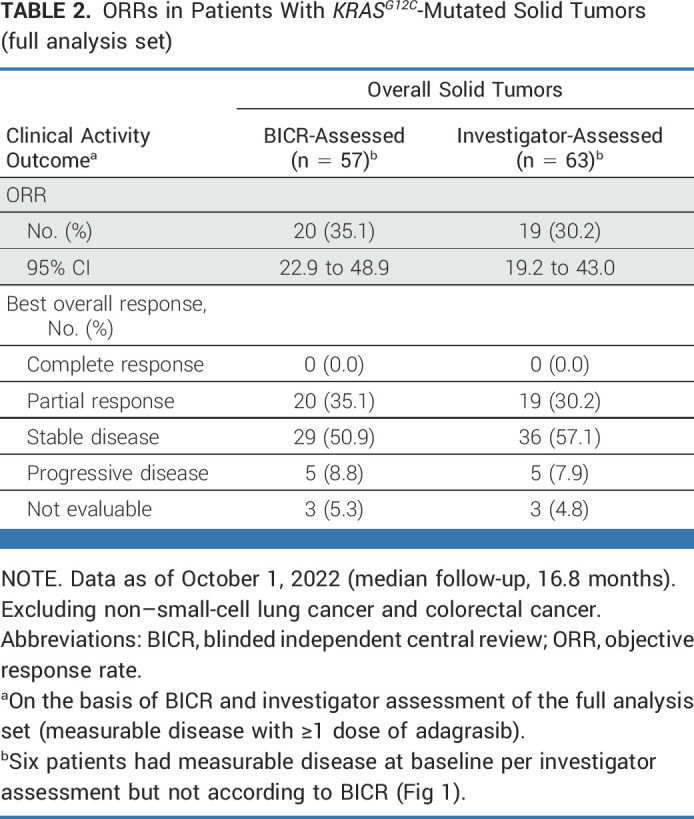
Responses by BICR were observed in nine different tumor histologies; clinical activity results according to tumor type are provided in Table 3, Figure 2A, and Appendix Figure A1 (online only). In the full analysis set, ORRs by tumor type were pancreatic (n = 7/21; 33.3%), biliary tract (n = 5/12; 41.7%), ovarian (n = 2/4; 50.0%), unknown primary (n = 1/4; 25.0%), gastroesophageal junction/esophageal (n = 1/3; 33.3%), endometrial (n = 2/3; 66.7%), small bowel (n = 1/2; 50.0%), and breast (n = 1/1; 100%). No objective responses were observed among seven patients with appendiceal cancer (including six with mucinous appendiceal adenocarcinoma); six patients (85.7%) had stable disease. Clinical activity results by tumor type according to investigator assessment are presented in Appendix Table A4 (online only); disease control rates as assessed by BICR and investigator assessment are shown in Appendix Table A5 (online only).
TABLE 3.
ORR (full analysis set), PFS (full analysis set) by Independent Central Review, and OS (enrolled population) in Patients With KRASG12C-Mutated Solid Tumors According to Tumor Type
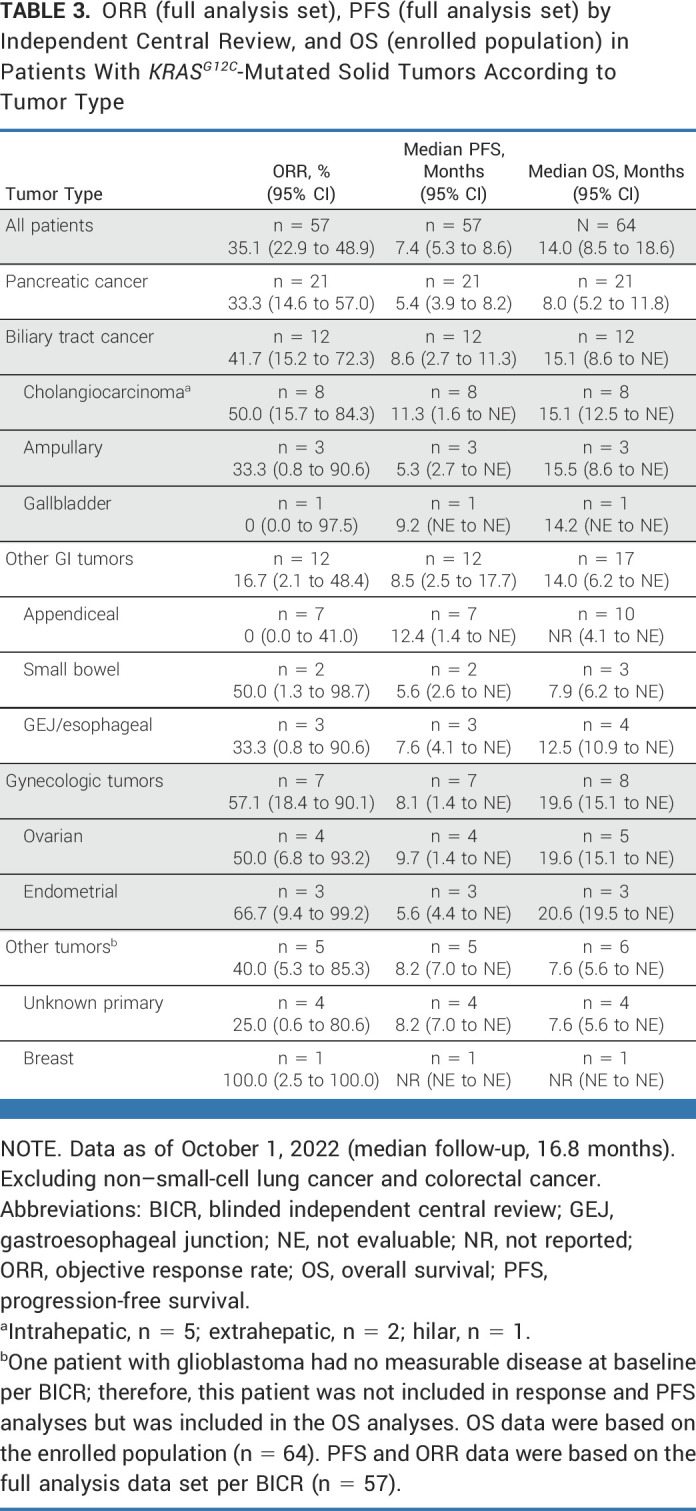
FIG 2.
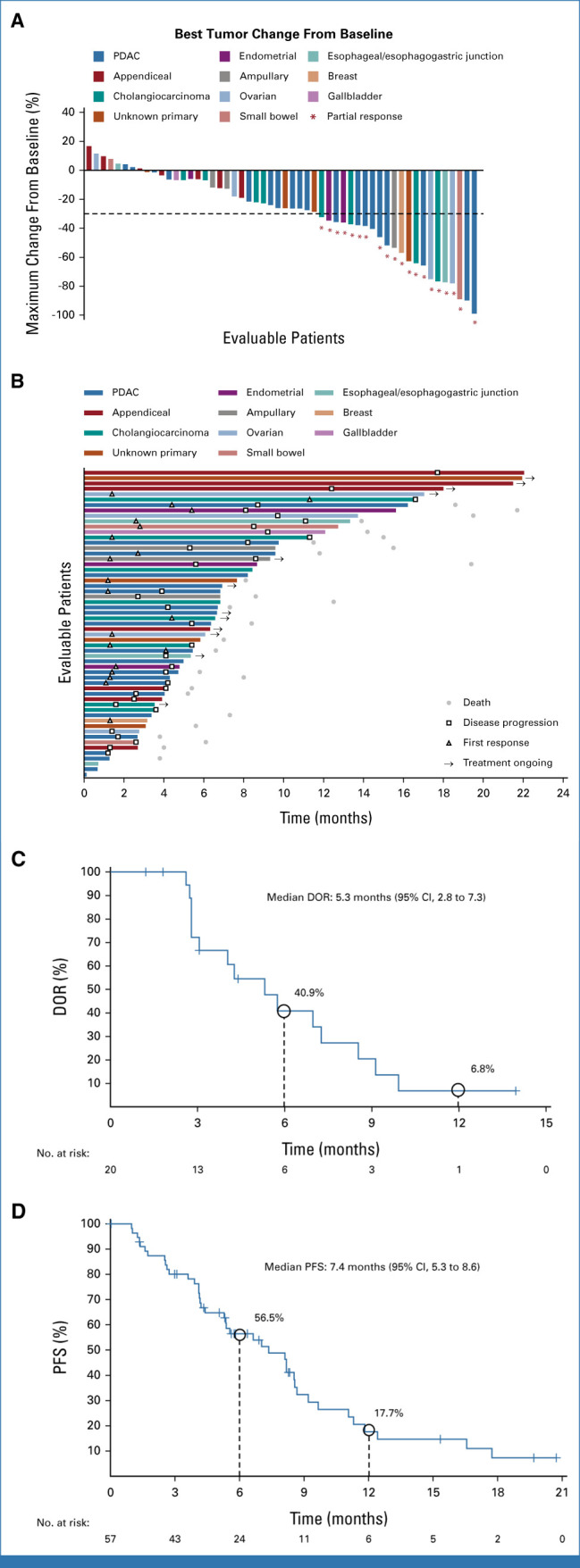
Efficacy outcomes for patients with evaluable disease by BICR. (A) Waterfall plot of best percentage tumor change from baseline (n = 54). (B) Swimmer plot showing individual duration of treatment, response, and clinical outcome at data cutoff (n = 54). (C) Kaplan-Meier graphical representation of duration of response. (D) Kaplan-Meier graphical representation of PFS. Data as of October 1, 2022 (median follow-up, 16.8 months). BICR, blinded independent central review; DOR, duration of response; PDAC, pancreatic ductal adenocarcinoma; PFS, progression-free survival.
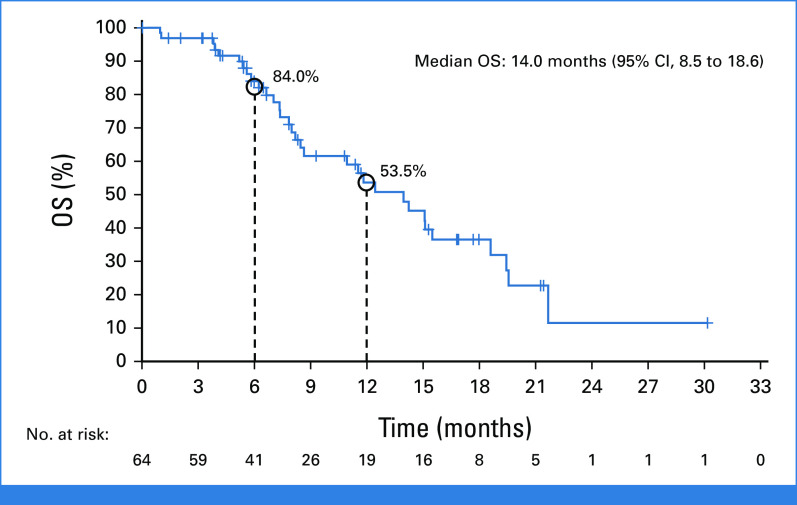
Timing and duration of responses in individual patients is shown in Figure 2B; the median time to response was 1.4 months and the median duration of response was 5.3 months (95% CI, 2.8 to 7.3) as per BICR (Fig 2C) and 7.0 months (95% CI, 3.1 to 9.9) as per investigator assessment; at the time of data cutoff, treatment was ongoing in 14 patients. Among patients with measurable disease, the median PFS was 7.4 months (95% CI, 5.3 to 8.6) as per BICR (n = 57; Fig 2D) and 6.9 months (95% CI, 5.3 to 8.3) as per investigator assessment (n = 63). Median OS was 14.0 months (95% CI, 8.5 to 18.6) and the estimated OS rate at 1 year was 53.5% (95% CI, 37.9 to 66.9; n = 64 [Appendix Fig A2, online only]). Median PFS and median OS according to tumor type are presented in Table 3. In total, 22.2% (14/63) patients from the treated population received subsequent anticancer therapy.
Safety
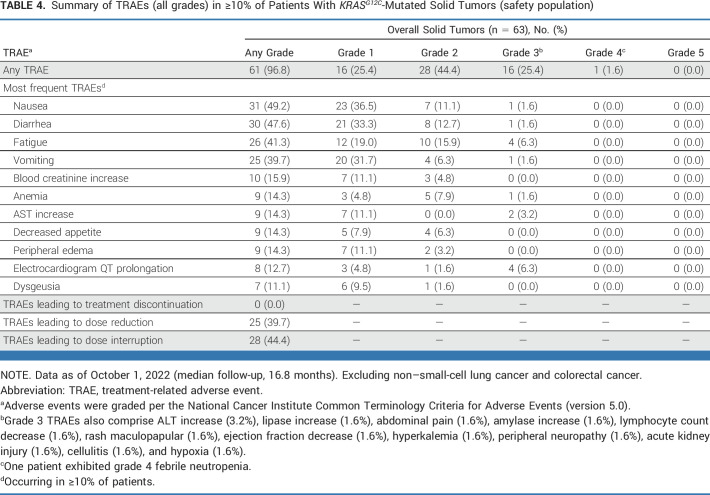
Among the 63 patients within the safety population, treatment-related adverse events (TRAEs) with adagrasib (capsule formulation) of any grade were experienced by 96.8% (Table 4), with grade 3 TRAEs reported in 25.4% of patients and grade 4 TRAEs observed in one patient (1.6%; febrile neutropenia). There were no grade 5 TRAEs. The most common any-grade TRAEs (in ≥20% of patients) were nausea (49.2%), diarrhea (47.6%), fatigue (41.3%), and vomiting (39.7%). The most common grade 3 TRAEs (in ≥5% of patients) were fatigue (6.3%) and electrocardiogram QT prolongation (6.3%). TRAEs of any grade led to adagrasib dose reduction in 25 patients (39.7%) and dose interruption in 28 patients (44.4%). Overall, no patients discontinued adagrasib because of TRAEs. Treatment-emergent adverse events are summarized in Appendix Table A6 (online only).
TABLE 4.
Summary of TRAEs (all grades) in ≥10% of Patients With KRASG12C-Mutated Solid Tumors (safety population)
DISCUSSION
Recently, KRASG12C inhibitors have demonstrated promising clinical activity in patients with KRASG12C-mutated advanced NSCLC and CRC.15,16,23,24 In December 2022, the FDA granted accelerated approval for adagrasib in previously treated KRASG12C-mutated NSCLC17 and breakthrough therapy designation for adagrasib, in combination with cetuximab, in KRASG12C-mutated CRC.18 Although biomarker-directed treatment strategies have been successful in the treatment of NSCLC, and preliminary progress has been made in CRC, targeted treatment options for other solid tumors, including pancreatic, biliary tract, and gynecologic cancers, remain extremely limited. The occurrence of the same oncogenic drivers across multiple tumor types has resulted in the treatment landscape evolving toward a tumor-agnostic approach, including recent approvals for larotrectinib and entrectinib (NTRK gene fusion–positive solid tumors), selpercatinib (RET proto oncogene-gene fusion–positive solid tumors), pembrolizumab (high microsatellite instability [MSI-H]/mismatch repair deficiency [dMMR], and tumor mutational burden-high), dostarlimab (MSI-H/dMMR), and dabrafenib plus trametinib (v-raf murine sarcoma viral oncogene homolog B1 V600E mutation–positive solid tumors).25 KRASG12C mutations occur uncommonly in solid tumors other than NSCLC and CRC, and adagrasib may represent a matched treatment option for this rare cohort of pretreated patients with KRASG12C-mutated solid tumors.
Early clinical data demonstrated encouraging clinical activity of KRASG12C inhibitors in KRASG12C-mutated solid tumors.26,27 In recent phase I trials in patients with advanced solid tumors, other than NSCLC and CRC, responses were documented in 19% of patients receiving GDC-6036 (n = 4/21) and 14% of patients receiving sotorasib (n = 4/28).26,27 Additionally, recently published data for sotorasib demonstrated an ORR of 21% in patients with KRASG12C-mutated advanced pancreatic cancer.28 In this study, adagrasib monotherapy demonstrated clinically meaningful activity and a manageable safety and tolerability profile in a variety of tumor types in a heavily pretreated patient population with tumors harboring KRASG12C mutations. Adagrasib resulted in an ORR of 35.1%, median PFS of 7.4 months, and a median OS of 14.0 months in a patient population for whom there were no available standard-of-care treatment options, and 70.3% of whom had received at least two prior therapies and 34.4% had received at least three prior therapies. Prior therapies included chemotherapy, targeted therapy, and/or checkpoint inhibitors. In addition, 78.1% (50/64) of patients within the enrolled population had GI malignancies, for whom treatment efficacy has been limited in the second-line setting and beyond.
Second-line standard chemotherapy treatment of unselected patients with metastatic pancreatic cancer has been associated with poor outcomes. Historically, a median PFS of approximately 3 months or less, a median OS of approximately 6.6 months or less, and a lack of meaningful clinical responses have been reported in phase III second-line chemotherapy studies.29,30 In this study, we observed notable clinical activity with adagrasib in pretreated patients with pancreatic cancer harboring KRASG12C mutations (>80% progressed after prior gemcitabine- and/or fluoropyrimidine-based therapy) including an ORR of 33.3% per BICR, median PFS of 5.4 months per BICR, and median OS of 8.0 months.
Initial reports of treating KRASG12C-mutated biliary tract cancers suggest limited clinical activity with sotorasib and GDC-6036, with 0/2 and 1/6 responses reported, respectively.26,27 In this study, we observed notable activity for adagrasib in the rare subgroup of 12 refractory patients with biliary tract cancers harboring KRASG12C mutations, with an ORR of 41.7%, median PFS of 8.6 months, and median OS of 15.1 months. As a point of reference, and in a biomarker-unselected patient population, the ABC-06 phase III trial investigating the efficacy of folinic acid, fluorouracil, and oxaliplatin in patients with previously treated advanced or metastatic biliary tract cancer reported an ORR of 5%, with a median PFS of 4.0 months and an OS of 6.2 months.31
Moreover, a relatively small number of patients with gynecologic cancers (n = 7) were included with a particularly encouraging observed response rate of 57%. No responses were seen in seven patients with appendiceal cancers, of which one was colonic and six were of the mucinous subtype. This lack of response is consistent with a recent report that distinct mutational subtypes of appendiceal cancers exhibit conserved clinical behavior, and RAS-mutated mucinous appendiceal cancers are relatively indolent tumors that may not be clinically aggressive.17 Colonic-type (nonmucinous) appendiceal cancers are associated with worse outcomes than mucinous appendiceal cancer and may benefit from combination therapy.32,33
Overall, the present results consistently demonstrate encouraging activity and a manageable safety and tolerability profile in patients with pretreated KRASG12C-mutated solid tumors. The most frequently occurring TRAEs are aligned with those reported previously in patients with pretreated NSCLC or heavily pretreated CRC.15,16
This study is limited by its nonrandomized, single-arm design. Additionally, KRASG12C mutations in solid tumors other than NSCLC and CRC are rare, resulting in heterogeneous populations and small patient numbers for each of the tumor types in this study.
Ongoing biomarker analyses may provide additional information on the mechanisms of primary and secondary resistance to KRASG12C inhibitors, and may be used to identify other drug targets, or combinations thereof, for specific patient populations. For example, there is a rationale for combining adagrasib with other inhibitors targeting different points of the RAS/MAPK signaling pathway, such as the combination of adagrasib and cetuximab (EGFR inhibitor) in KRASG12C-mutated CRC.16,34 On the basis of this rationale, adagrasib is being evaluated in exploratory combinations in other solid tumors, including pancreatic cancer.
Adagrasib demonstrated clinical activity across a broad range of tumor types and was well tolerated in patients with unresectable or metastatic solid tumors harboring a KRASG12C mutation. These results provide clinical evidence that KRASG12C can be therapeutically targeted across diverse tumor types, with activity observed for adagrasib in this tumor-agnostic patient population. The responses observed in tumor types such as pancreatic, where mutation analysis is not routinely conducted, underscore the importance of more consistent genomic testing. Next-generation sequencing for all tumor types is necessary to identify potential treatment targets, and testing for KRASG12C mutations across solid tumors remains critical to identify patients who may benefit from treatment with adagrasib.
ACKNOWLEDGMENT
The authors thank the patients, their families, and their caregivers, and the study investigators and their team members at each site for participation in the ongoing KRYSTAL-1 trial. A sponsor-funded medical writer (from Ashfield MedComms, an Inizio Company) provided the first draft of the manuscript and editorial assistance with later drafts of the manuscript.
APPENDIX 1. APPENDIX METHODS
Statistical Analyses
The full analysis set included all patients with measurable disease at baseline and who received ≥1 dose of adagrasib. A further six patients were included within the analysis set per investigator assessment but excluded per blinded independent central review (BICR) because of no measurable disease at baseline by BICR.
The observed responses were monitored during the trial at prespecified intervals. The futility analysis was based on the Predictive Probability Design with a maximum of approximately 60 patients. The stopping rules (rejection regions), expressed as number of responses per patients treated, were 2/26, 5/40, and 10/60. If the true objective response rate (ORR) was 10% (null hypothesis), the probability of early termination during the study would be 0.807 (under H0) and the expected sample size before termination would be 37 patients. The type 1 error would be 0.0309 and the power would be equal to 0.978. The methodology used to estimate the CI for ORRs was based on the Clopper-Pearson method; estimation of the confidence interval for median duration of response, median progression-free survival, and median overall survival was based on the Brookmeyer-Crowley method.
Tumor Mutation Screening
To establish potential eligibility for enrollment into the study, patients underwent prescreening for KRASG12C mutation in tumor tissue or circulating tumor DNA (ctDNA). Prescreening was performed historically or at the time a patient was considering study entry. The presence of KRASG12C mutation was established using sponsor preapproved methods and laboratories. Platforms used for prescreening tumor mutational analyses included polymerase chain reaction (PCR) and next-generation sequencing (NGS). The sponsor-approved laboratories were
Neogenomics Laboratories for tumor tissue, using PCR for eligibility testing and follow-up with NGS for exploratory end points
Resolution Bioscience for ctDNA in the blood, using NGS for eligibility testing
In addition, commercial and locally available tests that were preapproved by the sponsor for eligibility determination included, but were not limited to,
Neogenomics Laboratories, using PCR for tumor tissue
FoundationOne, using NGS on tumor tissue samples
PROFILE (Dana Farber Cancer Institute), using NGS on tumor tissue samples
IMPACT (Memorial Sloan Kettering Cancer Center), using NGS on tumor tissue samples
Archer VariantPlex (University of Colorado), using NGS on tumor tissue samples
Caris Life Sciences, using NGS on tumor tissue samples
StrataNGS, using NGS on tumor tissue samples
Tempus xT, using NGS on tumor tissue samples
Resolution ctDx Lung, using NGS on ctDNA
FoundationOne Liquid, using NGS on ctDNA
Guardant360CDx, using NGS on ctDNA
TABLE A1.
Prior Treatment Regimens of Patients With KRASG12C-Mutated Solid Tumors, PDAC and BTC
TABLE A2.
Prior Treatment Regimens of Patients With KRASG12C-Mutated Solid Tumors, Other GI Tumors
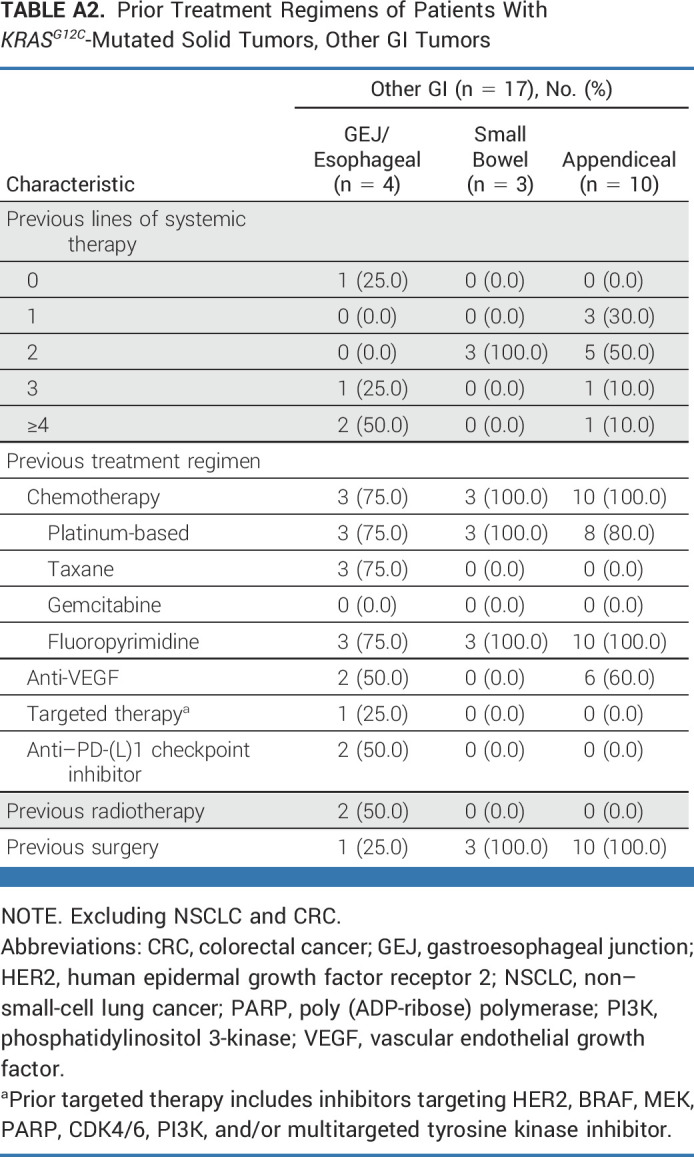
TABLE A3.
Prior Treatment Regimens of Patients With KRASG12C-Mutated Solid Tumors, Other Tumors
TABLE A4.
ORR (full analysis set), PFS (full analysis set), by Investigator Assessment, and OS (enrolled population) in Patients With KRASG12C-Mutated Solid Tumors According to Tumor Type
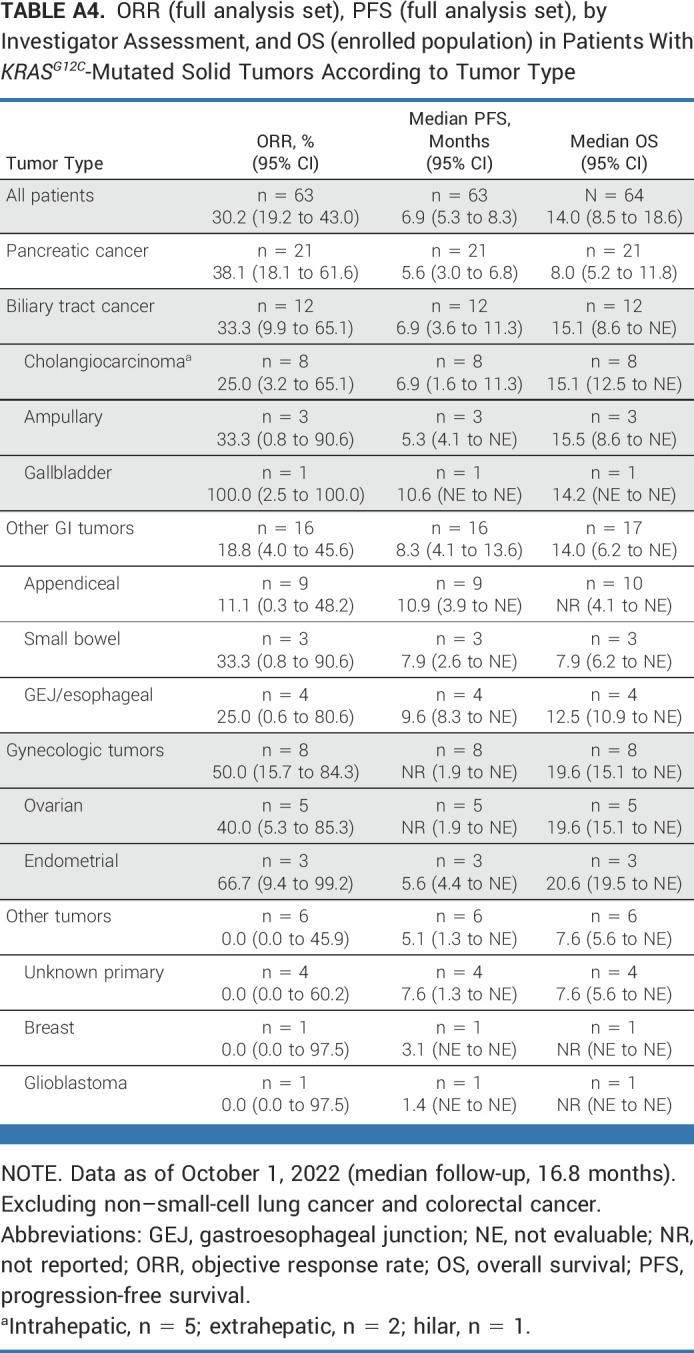
TABLE A5.
Summary of BICR and Investigator-Assessed Disease Control Rate (full analysis set), Categorized by Tumor Type
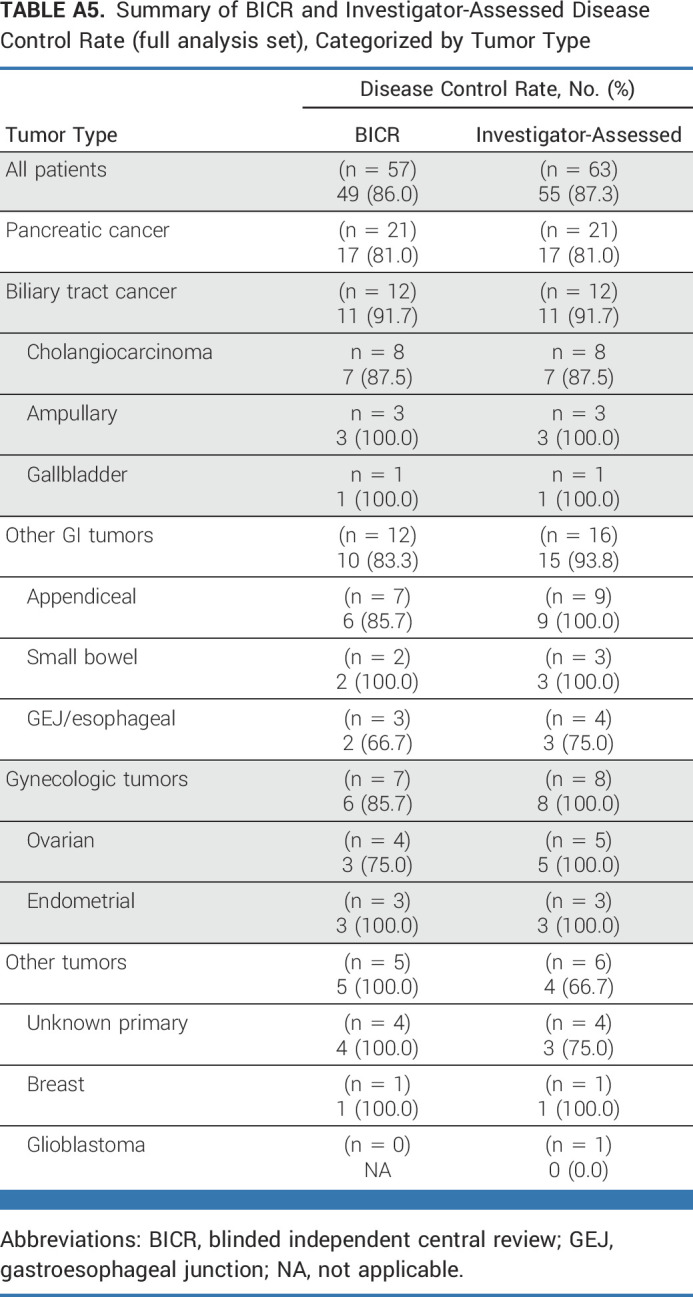
TABLE A6.
Summary of TEAEs (all grades) in ≥10% of Patients With KRASG12C-Mutated Solid Tumors (safety population)
FIG A1.
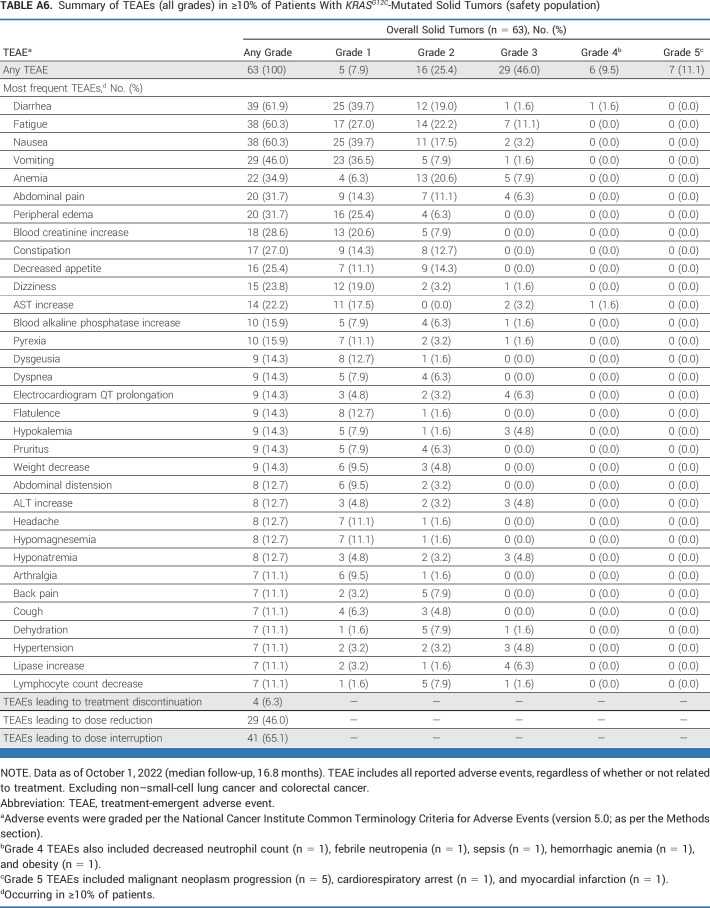
Waterfall plot of maximum percentage tumor change from baseline in patients with (A) PDAC and (B) BTC cancers. BTC, biliary tract cancer; PDAC, pancreatic ductal adenocarcinoma.
FIG A2.
OS in patients with KRASG12C-mutated solid tumorsa (N = 64). Data as of October 1, 2022 (median follow-up, 16.8 months). aExcluding non–small-cell lung cancer and colorectal cancer. OS, overall survival.
Tanios S. Bekaii-Saab
Consulting or Advisory Role: Amgen (Inst), Ipsen (Inst), Lilly (Inst), Bayer (Inst), Roche/Genentech (Inst), AbbVie, Incyte (Inst), Immuneering, Seagen (Inst), Pfizer (Inst), Boehringer Ingelheim, Janssen, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, Exact Sciences, Natera, Treos Bio, Celularity, Sobi, BeiGene, Foundation Medicine, Arcus Biosciences (Inst), Stemline Therapeutics, Kanaph Therapeutics, Deciphera, Illumina, Caladrius Biosciences, Zai Lab
Patents, Royalties, Other Intellectual Property: Patent WO/2018/183488, Patent WO/2019/055687
Other Relationship: Exelixis, Merck (Inst), AstraZeneca, Lilly, Pancreatic Cancer Action Network, FibroGen, Suzhou Kintor Pharmaceuticals, 1Globe Health Institute, Imugene, Xilis, Replimune, Sun Biopharma, UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/636276
Rona Yaeger
Honoraria: Zai Lab
Consulting or Advisory Role: Mirati Therapeutics
Research Funding: Array BioPharma (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), Mirati Therapeutics (Inst)
Alexander I. Spira
Leadership: Next Oncology (Inst)
Stock and Other Ownership Interests: Lilly
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb, Bayer
Consulting or Advisory Role: Array BioPharma (Inst), Incyte, Amgen, Novartis, AstraZeneca/MedImmune (Inst), Mirati Therapeutics, Gritstone Bio, Jazz Pharmaceuticals, Merck (Inst), Bristol Myers Squibb (Inst), Takeda, Janssen Research & Development, Mersana, Blueprint Medicines (Inst), Gritstone Bio, Daiichi Sankyo/Astra Zeneca, Regeneron, Lilly, Black Diamond Therapeutics, Sanofi, Sanofi
Research Funding: Roche (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), MedImmune (Inst), Novartis (Inst), Newlink Genetics (Inst), Incyte (Inst), AbbVie (Inst), Ignyta (Inst), LAM Therapeutics (Inst), Trovagene (Inst), Takeda (Inst), Macrogenics (Inst), CytomX Therapeutics (Inst), LAM Therapeutics, Astex Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Loxo (Inst), Arch Therapeutics (Inst), Gritstone Bio (Inst), Plexxikon (Inst), Amgen (Inst), Daiichi Sankyo (Inst), ADC Therapeutics (Inst), Janssen Oncology (Inst), Mirati Therapeutics (Inst), Rubius Therapeutics (Inst), Synthekine (Inst), Mersana (Inst), Blueprint Medicines (Inst), Regeneron, Alkermes (Inst), Revolution Medicines (Inst), Medikine (Inst), Black Diamond Therapeutics (Inst), BluPrint Oncology (Inst), Nalo Therapeutics (Inst)
Meredith S. Pelster
Honoraria: Castle Biosciences
Consulting or Advisory Role: AstraZeneca (Inst), Bayer (Inst), Novartis (Inst), Pfizer (Inst), Seagen (Inst), CytomX Therapeutics (Inst), Daiichi Sankyo (Inst), Ipsen (Inst)
Research Funding: Arcus Biosciences (Inst), Astellas Pharma (Inst), Codiak Biosciences (Inst), CytomX Therapeutics (Inst), Eisai (Inst), Gritstone Bio (Inst), HiberCell (Inst), Immune-Onc Therapeutics (Inst), OncXerna Therapeutics (Inst), Surface Oncology (Inst), SQZ Biotechnology (Inst), TransThera Sciences (Nanjing), Inc (Inst), ZielBio (Inst), BeiGene (Inst), BioNTech (Inst), Bristol Myers Squibb (Inst), Gilead Sciences (Inst), Leap Therapeutics (Inst), Panbela Therapeutics (Inst), Revolution Medicines (Inst), Translational Genomics Research Institute (Inst), 1200 Pharma (Inst)
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Navire, Pfizer, Regeneron, Medscape, Takeda
Navid Hafez
Research Funding: Genentech/Roche (Inst)
Minal Barve
Employment: Texas Oncology
Stock and Other Ownership Interests: Texas Oncology
Research Funding: Mary Crowley Research Center, Dallas Texas
Karen Velastegui
Employment: Mirati Therapeutics, Pfizer
Stock and Other Ownership Interests: Mirati Therapeutics, Arena Pharma
Xiaohong Yan
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Travel, Accommodations, Expenses: Mirati Therapeutics
Aditya Shetty
Employment: Mirati Therapeutics, Amgen
Stock and Other Ownership Interests: Mirati Therapeutics
Open Payments Link: https://openpaymentsdata.cms.gov/physician/2784722
Hirak Der-Torossian
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Travel, Accommodations, Expenses: Mirati Therapeutics
Shubham Pant
Consulting or Advisory Role: Zymeworks, Ipsen, Novartis, Janssen, Boehringer Ingelheim
Research Funding: Mirati Therapeutics (Inst), Lilly (Inst), Xencor (Inst), Novartis (Inst), Rgenix (Inst), Bristol Myers Squibb (Inst), Astellas Pharma (Inst), Purple Biotech (Inst), 4D Pharma (Inst), Boehringer Ingelheim (Inst), NGM Biopharmaceuticals (Inst), Janssen (Inst), Arcus Biosciences (Inst), Elicio Therapeutics (Inst), Bionte (Inst), Ipsen (Inst), Zymeworks (Inst), Pfizer (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at ASCO GI 2022, San Francisco, CA, January 20-23, 2022; and the 2023 ASCO Plenary Series, virtual, April 10, 2023.
SUPPORT
Supported by Mirati Therapeutics, Inc and also supported by the Cancer Center Core Grant P30 CA 008748 to Memorial Sloan Kettering. Medical writing support under the direction of the authors was provided by Flaminia Fenoaltea, MSc, and Jessica Traynor, PhD, of Ashfield MedComms, an Inizio company, and funded by Mirati Therapeutics, Inc.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
At Mirati Therapeutics, we are committed to patient care, advancing scientific understanding, and enabling the scientific community to learn from and build upon the research we have undertaken. To that end, we will honor legitimate requests for our clinical trial data from qualified researchers and investigators for conducting methodologically sound research. We will share clinical trial data, clinical study reports, study protocols, and statistical analysis plans from clinical trials for which results have been posted on clinicaltrials.gov for products and indications approved by regulators in the United States and/or European Union. Sharing is subject to protection of patient privacy and respect for the patient's informed consent. In general, data will be made available for specific requests approximately 24 months after clinical trial completion from our in-scope interventional trials. For additional information on proposals with regards to data sharing collaborations with Mirati, please e-mail us at medinfo@mirati.com.
AUTHOR CONTRIBUTIONS
Conception and design: Alexander I. Spira, Joshua K. Sabari, Hirak Der-Torossian, Shubham Pant
Administrative support: Alexander I. Spira, Karen Velastegui
Provision of study materials or patients: Tanios S. Bekaii-Saab, Rona Yaeger, Alexander I. Spira, Meredith S. Pelster, Joshua K. Sabari, Navid Hafez, Minal Barve, Shubham Pant
Collection and assembly of data: Tanios S. Bekaii-Saab, Rona Yaeger, Alexander I. Spira, Meredith S. Pelster, Joshua K. Sabari, Navid Hafez, Minal Barve, Karen Velastegui, Hirak Der-Torossian, Shubham Pant
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adagrasib in Advanced Solid Tumors Harboring a KRASG12C Mutation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tanios S. Bekaii-Saab
Consulting or Advisory Role: Amgen (Inst), Ipsen (Inst), Lilly (Inst), Bayer (Inst), Roche/Genentech (Inst), AbbVie, Incyte (Inst), Immuneering, Seagen (Inst), Pfizer (Inst), Boehringer Ingelheim, Janssen, Eisai, Daiichi Sankyo/UCB Japan, AstraZeneca, Exact Sciences, Natera, Treos Bio, Celularity, Sobi, BeiGene, Foundation Medicine, Arcus Biosciences (Inst), Stemline Therapeutics, Kanaph Therapeutics, Deciphera, Illumina, Caladrius Biosciences, Zai Lab
Patents, Royalties, Other Intellectual Property: Patent WO/2018/183488, Patent WO/2019/055687
Other Relationship: Exelixis, Merck (Inst), AstraZeneca, Lilly, Pancreatic Cancer Action Network, FibroGen, Suzhou Kintor Pharmaceuticals, 1Globe Health Institute, Imugene, Xilis, Replimune, Sun Biopharma, UpToDate
Open Payments Link: https://openpaymentsdata.cms.gov/physician/636276
Rona Yaeger
Honoraria: Zai Lab
Consulting or Advisory Role: Mirati Therapeutics
Research Funding: Array BioPharma (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), Mirati Therapeutics (Inst)
Alexander I. Spira
Leadership: Next Oncology (Inst)
Stock and Other Ownership Interests: Lilly
Honoraria: CytomX Therapeutics, AstraZeneca/MedImmune, Merck, Takeda, Amgen, Janssen Oncology, Novartis, Bristol Myers Squibb, Bayer
Consulting or Advisory Role: Array BioPharma (Inst), Incyte, Amgen, Novartis, AstraZeneca/MedImmune (Inst), Mirati Therapeutics, Gritstone Bio, Jazz Pharmaceuticals, Merck (Inst), Bristol Myers Squibb (Inst), Takeda, Janssen Research & Development, Mersana, Blueprint Medicines (Inst), Gritstone Bio, Daiichi Sankyo/Astra Zeneca, Regeneron, Lilly, Black Diamond Therapeutics, Sanofi, Sanofi
Research Funding: Roche (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), Astellas Pharma (Inst), MedImmune (Inst), Novartis (Inst), Newlink Genetics (Inst), Incyte (Inst), AbbVie (Inst), Ignyta (Inst), LAM Therapeutics (Inst), Trovagene (Inst), Takeda (Inst), Macrogenics (Inst), CytomX Therapeutics (Inst), LAM Therapeutics, Astex Pharmaceuticals (Inst), Bristol Myers Squibb (Inst), Loxo (Inst), Arch Therapeutics (Inst), Gritstone Bio (Inst), Plexxikon (Inst), Amgen (Inst), Daiichi Sankyo (Inst), ADC Therapeutics (Inst), Janssen Oncology (Inst), Mirati Therapeutics (Inst), Rubius Therapeutics (Inst), Synthekine (Inst), Mersana (Inst), Blueprint Medicines (Inst), Regeneron, Alkermes (Inst), Revolution Medicines (Inst), Medikine (Inst), Black Diamond Therapeutics (Inst), BluPrint Oncology (Inst), Nalo Therapeutics (Inst)
Meredith S. Pelster
Honoraria: Castle Biosciences
Consulting or Advisory Role: AstraZeneca (Inst), Bayer (Inst), Novartis (Inst), Pfizer (Inst), Seagen (Inst), CytomX Therapeutics (Inst), Daiichi Sankyo (Inst), Ipsen (Inst)
Research Funding: Arcus Biosciences (Inst), Astellas Pharma (Inst), Codiak Biosciences (Inst), CytomX Therapeutics (Inst), Eisai (Inst), Gritstone Bio (Inst), HiberCell (Inst), Immune-Onc Therapeutics (Inst), OncXerna Therapeutics (Inst), Surface Oncology (Inst), SQZ Biotechnology (Inst), TransThera Sciences (Nanjing), Inc (Inst), ZielBio (Inst), BeiGene (Inst), BioNTech (Inst), Bristol Myers Squibb (Inst), Gilead Sciences (Inst), Leap Therapeutics (Inst), Panbela Therapeutics (Inst), Revolution Medicines (Inst), Translational Genomics Research Institute (Inst), 1200 Pharma (Inst)
Joshua K. Sabari
Consulting or Advisory Role: AstraZeneca, Janssen Oncology, Navire, Pfizer, Regeneron, Medscape, Takeda
Navid Hafez
Research Funding: Genentech/Roche (Inst)
Minal Barve
Employment: Texas Oncology
Stock and Other Ownership Interests: Texas Oncology
Research Funding: Mary Crowley Research Center, Dallas Texas
Karen Velastegui
Employment: Mirati Therapeutics, Pfizer
Stock and Other Ownership Interests: Mirati Therapeutics, Arena Pharma
Xiaohong Yan
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Travel, Accommodations, Expenses: Mirati Therapeutics
Aditya Shetty
Employment: Mirati Therapeutics, Amgen
Stock and Other Ownership Interests: Mirati Therapeutics
Open Payments Link: https://openpaymentsdata.cms.gov/physician/2784722
Hirak Der-Torossian
Employment: Mirati Therapeutics
Stock and Other Ownership Interests: Mirati Therapeutics
Travel, Accommodations, Expenses: Mirati Therapeutics
Shubham Pant
Consulting or Advisory Role: Zymeworks, Ipsen, Novartis, Janssen, Boehringer Ingelheim
Research Funding: Mirati Therapeutics (Inst), Lilly (Inst), Xencor (Inst), Novartis (Inst), Rgenix (Inst), Bristol Myers Squibb (Inst), Astellas Pharma (Inst), Purple Biotech (Inst), 4D Pharma (Inst), Boehringer Ingelheim (Inst), NGM Biopharmaceuticals (Inst), Janssen (Inst), Arcus Biosciences (Inst), Elicio Therapeutics (Inst), Bionte (Inst), Ipsen (Inst), Zymeworks (Inst), Pfizer (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Liu P, Wang Y, Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B. 2019;9:871–879. doi: 10.1016/j.apsb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hobbs GA, Der CJ, Rossman KL. RAS isoforms and mutations in cancer at a glance. J Cell Sci. 2016;129:1287–1292. doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore AR, Rosenberg SC, McCormick F, et al. RAS-targeted therapies: Is the undruggable drugged? Nat Rev Drug Discov. 2020;19:533–552. doi: 10.1038/s41573-020-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nassar AH, Adib E, Kwiatkowski DJ. Distribution of KRAS (G12C) somatic mutations across race, sex, and cancer type. N Engl J Med. 2021;384:185–187. doi: 10.1056/NEJMc2030638. [DOI] [PubMed] [Google Scholar]
- 6. Salem ME, El-Refai SM, Sha W, et al. Landscape of KRAS(G12C), associated genomic alterations, and interrelation with immuno-oncology biomarkers in KRAS-mutated cancers. JCO Precis Oncol. 2022;6:e2100245. doi: 10.1200/PO.21.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schirripa M, Nappo F, Cremolini C, et al. KRAS G12C metastatic colorectal cancer: Specific features of a new emerging target population. Clin Colorectal Cancer. 2020;19:219–225. doi: 10.1016/j.clcc.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 8. Nollmann FI, Ruess DA. Targeting mutant KRAS in pancreatic cancer: Futile or promising? Biomedicines. 2020;8:281. doi: 10.3390/biomedicines8080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lamarca A, Edeline J, McNamara MG, et al. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat Rev. 2020;84:101936. doi: 10.1016/j.ctrv.2019.101936. [DOI] [PubMed] [Google Scholar]
- 10. Hallin J, Engstrom LD, Hargis L, et al. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christensen JG, Olson P, Briere T, et al. Targeting Kras(g12c)-mutant cancer with a mutation-specific inhibitor. J Intern Med. 2020;288:183–191. doi: 10.1111/joim.13057. [DOI] [PubMed] [Google Scholar]
- 12. Ostrem JM, Peters U, Sos ML, et al. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ou SHI, Jänne PA, Leal TA, et al. First-in-human phase I/IB dose-finding study of adagrasib (MRTX849) in patients with advanced KRASG12C solid tumors (KRYSTAL-1) J Clin Oncol. 2022;40:2530–2538. doi: 10.1200/JCO.21.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jänne P, Rybkin II, Spira A, et al. 2020. KRYSTAL-1: Updated safety and efficacy data with adagrasib (MRTX849) in NSCLC with KRASG12C mutation from a phase 1/2 study. Presented at the 32nd EORTC-NCI-AACR Symposium.
- 15. Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non–small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med. 2022;387:120–131. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 16. Yaeger R, Weiss J, Pelster MS, et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N Engl J Med. 2023;388:44–54. doi: 10.1056/NEJMoa2212419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foote MB, Walch H, Chatila W, et al. Molecular classification of appendiceal adenocarcinoma. J Clin Oncol. 2023;41:1553–1564. doi: 10.1200/JCO.22.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirati Therapeutics https://ir.mirati.com/press-releases/press-release-details/2022/Mirati-announces-Adagrasib-KRAZATI-Receives-Breakthrough-Therapy-Designation-from-FDA-for-Patients-with-Advanced-KRAS-Mutated-Colorectal-Cancer-and-NEJM-Publishes-Phase-1b2-Data-from-Adagrasib-With-or-Without-Cetuximab-in-Colorectal-Cancer/default.aspx Mirati announces adagrasib (KRAZATI™) receives breakthrough therapy designation from FDA for patients with advanced, KRAS-mutated colorectal cancer and NEJM publishes phase 1b/2 data from adagrasib with or without cetuximab in colorectal cancer.
- 19. Bekaii-Saab TS, Spira AI, Yaeger R, et al. KRYSTAL-1: Updated activity and safety of adagrasib (MRTX849) in patients (Pts) with unresectable or metastatic pancreatic cancer (PDAC) and other gastrointestinal (GI) tumors harboring a KRASG12C mutation. J Clin Oncol. 2022;40 suppl 4; abstr 519. [Google Scholar]
- 20.Johnson ML, Ou SI, Barve MA, et al. 2020. KRYSTAL-1: Activity and safety of adagrasib (MRTX849) in patients with colorectal cancer (CRC) and other solid tumors harboring a KRASG12C mutation. Presented at the the 32nd EORTC-NCI-AACR Symposium, Barcelona, Spain, October 24-25.
- 21. World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 22.International Council for Harmonisation https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2)
- 23. Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p. G12C mutation. N Engl J Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fakih MG, Kopetz S, Kuboki Y, et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK100): A prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022;23:115–124. doi: 10.1016/S1470-2045(21)00605-7. [DOI] [PubMed] [Google Scholar]
- 25. Mansinho A, Fernandes RM, Carneiro AV. Histology-agnostic drugs: A paradigm shift—A narrative review. Adv Ther. 2023;40:1379–1392. doi: 10.1007/s12325-022-02362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hong DS, Fakih MG, Strickler JH, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel M, Lee J, De Miguel M, et al. 459MO Phase Ia study to evaluate GDC-6036 monotherapy in patients with solid tumors with a KRAS G12C mutation. Ann Oncol. 2022;33:S749. [Google Scholar]
- 28. Strickler JH, Satake H, George TJ, et al. Sotorasib in KRAS p.G12C–mutated advanced pancreatic cancer. N Engl J Med. 2023;388:33–43. doi: 10.1056/NEJMoa2208470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang-Gillam A, Hubner RA, Siveke JT, et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur J Cancer. 2019;108:78–87. doi: 10.1016/j.ejca.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 30. Huffman BM, Basu Mallick A, Horick NK, et al. Effect of a MUC5AC antibody (NPC-1C) administered with second-line gemcitabine and nab-paclitaxel on the survival of patients with advanced pancreatic ductal adenocarcinoma: A randomized clinical trial. JAMA Netw Open. 2023;6:e2249720. doi: 10.1001/jamanetworkopen.2022.49720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uemura M, Qiao W, Fournier K, et al. Retrospective study of nonmucinous appendiceal adenocarcinomas: Role of systemic chemotherapy and cytoreductive surgery. BMC Cancer. 2017;17:331. doi: 10.1186/s12885-017-3327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoehn RS, Rieser CJ, Choudry MH, et al. Current management of appendiceal neoplasms. Am Soc Clin Oncol Educ Book. 2021;41:1–15. doi: 10.1200/EDBK_321009. [DOI] [PubMed] [Google Scholar]
- 34. Amodio V, Yaeger R, Arcella P, et al. EGFR blockade reverts resistance to KRAS(G12C) inhibition in colorectal cancer. Cancer Discov. 2020;10:1129–1139. doi: 10.1158/2159-8290.CD-20-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
At Mirati Therapeutics, we are committed to patient care, advancing scientific understanding, and enabling the scientific community to learn from and build upon the research we have undertaken. To that end, we will honor legitimate requests for our clinical trial data from qualified researchers and investigators for conducting methodologically sound research. We will share clinical trial data, clinical study reports, study protocols, and statistical analysis plans from clinical trials for which results have been posted on clinicaltrials.gov for products and indications approved by regulators in the United States and/or European Union. Sharing is subject to protection of patient privacy and respect for the patient's informed consent. In general, data will be made available for specific requests approximately 24 months after clinical trial completion from our in-scope interventional trials. For additional information on proposals with regards to data sharing collaborations with Mirati, please e-mail us at medinfo@mirati.com.