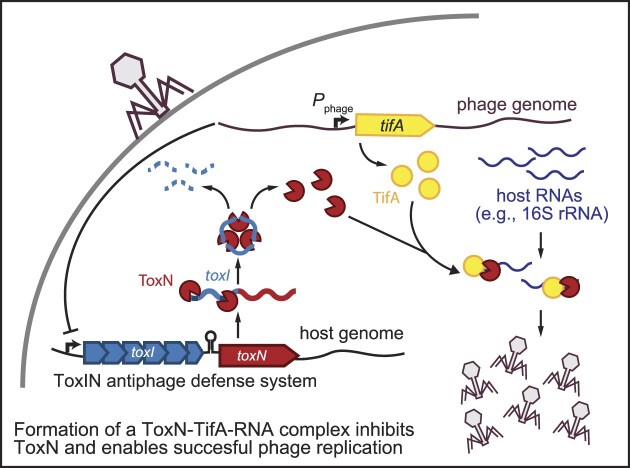
Abstract
Bacteria harbor diverse mechanisms to defend themselves against their viral predators, bacteriophages. In response, phages can evolve counter-defense systems, most of which are poorly understood. In T4-like phages, the gene tifA prevents bacterial defense by the type III toxin–antitoxin (TA) system toxIN, but the mechanism by which TifA inhibits ToxIN remains unclear. Here, we show that TifA directly binds both the endoribonuclease ToxN and RNA, leading to the formation of a high molecular weight ribonucleoprotein complex in which ToxN is inhibited. The RNA binding activity of TifA is necessary for its interaction with and inhibition of ToxN. Thus, we propose that TifA inhibits ToxN during phage infection by trapping ToxN on cellular RNA, particularly the abundant 16S rRNA, thereby preventing cleavage of phage transcripts. Taken together, our results reveal a novel mechanism underlying inhibition of a phage-defensive RNase toxin by a small, phage-encoded protein.
Graphical Abstract
Graphical Abstract.
Introduction
Predation by phages, the viruses that infect bacteria, represents a constant and potent threat to bacterial life. The need to survive this conflict has driven the evolution of numerous highly sophisticated antiphage elements in bacteria, including restriction-modification and CRISPR-Cas systems (1–3). These mechanisms, in turn, have led to the emergence of counter-defense systems in phages, highlighting the intense, ongoing evolutionary arms race between phages and their bacterial hosts (4,5). In addition to illuminating the fundamental mechanisms underlying these host-pathogen interactions, understanding this arms race is crucial for the development of phage therapy to combat the rise of antibiotic-resistant bacteria.
One prevalent class of bacterial defense genes is toxin–antitoxin (TA) systems, widespread genetic elements comprising a growth-inhibitory toxin that is neutralized by its cognate antitoxin (6–16). During phage infection, the toxin is liberated (‘activated’) from the antitoxin and goes on to block phage replication (9). Most toxins are growth inhibitory rather than bactericidal, but many TA systems have been proposed to act via an abortive infection mechanism. In such a mechanism, the infected cell does not survive phage infection (17,18). However, by blocking phage propagation, the TA system prevents spread of the phage from infected cells to the rest of a bacterial population (11,13,14,19).
TA systems are grouped into ‘types’ based on the molecular mechanism underlying antitoxin inhibition of the cognate toxin (6,8). The type III TA systems, first discovered in the plant pathogen Pectobacterium atrosepticum, typically involve an endoribonuclease (RNase) toxin and an antitoxin RNA expressed as an array of short tandem repeats (ToxN and toxI, respectively, in P. atrosepticum) (11,20–22). The toxin cleaves these repetitive antitoxin RNAs into shorter repeat monomers (typically 36–50 nucleotides in length); the interactions between the toxin and antitoxin neutralize the toxin by sequestering it from other putative substrate RNAs in the cell (20,23,24). Following infection, phage-induced shutoff of host transcription leads to rapid depletion of the cellular pool of unstable antitoxin RNAs, thereby liberating the toxin (19). Activated toxin then cleaves phage mRNAs to block production of new phage particles.
How do phages overcome or escape from the defense mediated by type III TA systems? In P. atrosepticum, the phage ΦTE can overcome toxIN by amplifying a locus expressing a noncoding RNA that resembles toxI or by acquiring toxI from the toxIN system directly (25). Further, the P. atrosepticum phage ΦM1 can acquire single-base changes in a gene of unknown function called M1-23, which interacts with the host nucleotide excision repair protein UvrA and allows ΦM1 to evade toxIN and another type III TA system called tenpIN (26). A T4-like phage that infects Serratia overcomes toxIN through mutations in the phage gene asiA, which encodes a factor that helps reprogram the host RNA polymerase to transcribe phage genes (27). The asiA mutation likely prevents full transcriptional shutoff of toxIN, which is required for ToxN activation.
We recently described a toxIN homolog from an environmental isolate of Escherichia coli that provides E. coli MG1655 with robust defense against several phages, including T4, T5, and T7 (19). We then evolved T4 to escape toxIN-mediated defense. Sequencing demonstrated that phages overcame toxIN via segmental amplification, and thus increased expression, of the T4 early gene tifA (previously 61.4), which encodes a protein inhibitor of ToxN (28). TifA is a small (85 amino acid) protein that is conserved across T-even phages. Although ToxN co-precipitates with TifA during T4 infection, the mechanistic basis of TifA inhibition of ToxN remains unclear (28) (Figure 1A).
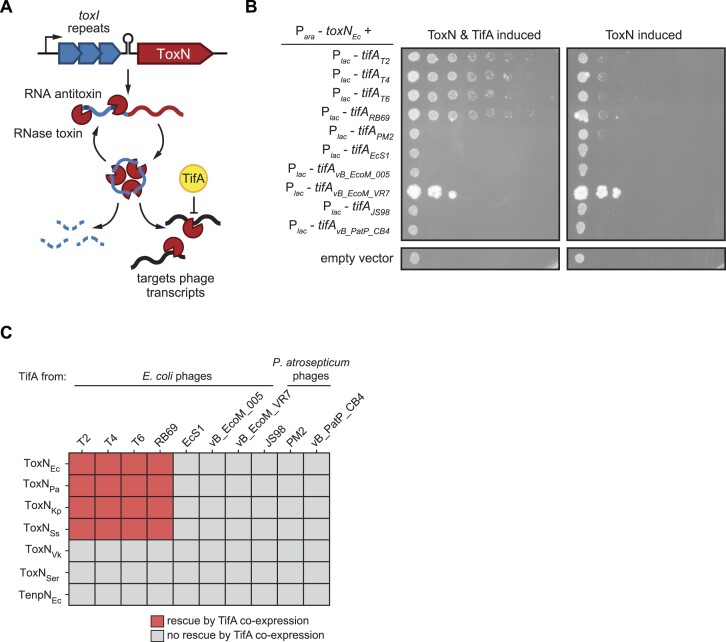
Figure 1.
TifA is a small phage-encoded inhibitor of ToxN. (A) Model for ToxIN complex formation, activation, and inhibition by TifA during phage infection. (B) Serial dilutions of E. coli cells expressing toxNEc and several tifA homologs. Plasmids harboring toxN and tifA under arabinose- and IPTG-inducible promoters, respectively, were transformed into E. coli MG1655, and expression was induced as indicated with the addition of arabinose and IPTG. (C) Heatmap showing the ability of various TifA homologs to rescue overproduction of various ToxN homologs; for plate images, see Supplementary Figure S2A-F. Bacterial species from which ToxN and TenpN homologs were cloned: Escherichia coli (Ec); Pectobacterium atrosepticum (Pa); Klebsiella pneumoniae (Kp); Shigella sonnei (Ss); Vibrio kanaloae (Vk); Serratia sp. SRS-8-S-2018 (Ser). Also see Supplementary Figures S1 and S2.
Here, we show that TifA proteins from different T-even phages are specific inhibitors of several ToxN homologs, indicating that TifA proteins recognize certain features of ToxN and are not nonspecific inhibitors of RNase activity. Using structure predictions coupled to a genetic screen, we identify the regions of TifA that likely bind ToxN, including one extended loop that may partially occlude the ToxN active site. Notably, we also show that TifA is a nucleic acid-binding protein and that TifA together with ToxN forms a high molecular weight RNA-protein complex. RNA-sequencing indicates that 16S rRNA is highly enriched in the complexes formed. We propose that TifA inhibits ToxN during phage infection by tethering it to 16S rRNAs, and possibly other cellular RNAs, thereby rendering it inert. Taken together, our results uncover a novel mechanism underlying inhibition of an RNase toxin by a phage-encoded antitoxin and highlight the role that type III TA systems play in the phage-bacterial arms race.
Materials and methods
Reagents, software and databases
All commercial instruments, reagents and software used in this study are listed in Supplementary Table S1.
Biological resources
All strains, plasmids, and primers used in this study are listed in Supplementary Tables S3, S4, and S5, respectively. Phage Thu1/R2 was isolated during the 2021 Fall LS50 course (Harvard) by freshman students under the instruction of Sriram Srikant. It was isolated from Charles River water on E. coli MG1655, and genome sequenced to taxonomically classify it in the Tevenvirinae.
Strains and growth conditions
For all cell spotting experiments, E. coli MG1655 strains were grown in M9 medium (10x stock made with 64 g/l Na2HPO4-7H2O, 15 g/l KH2PO4, 2.5 g/l NaCl, 5.0 g/l NH4Cl) supplemented with 0.4% glycerol, 0.1% casamino acids, 2 mM MgSO4, and 0.1 mM CaCl2 (M9-glycerol). When applicable, 0.4% glucose was supplemented to prevent leaky toxin expression from the arabinose-inducible PBAD promoter (M9-glucose). For plasmid construction, E. coli TOP10 or DH5α cells were cultured in Luria broth (LB) medium, supplemented with 0.4% glucose when applicable. For phage infection experiments, E. coli MG1655 strains were grown in LB medium. For protein overexpression, E. coli T7 express cells (NEB) were grown in LB medium. Antibiotics were used at the following concentrations (liquid; plates): carbenicillin (50 μg/ml; 100 μg/ml), chloramphenicol (20 μg/ml; 30 μg/ml).
Plasmid construction
pBAD33-toxN homologs: ToxN homolog sequences screened in Figure 1 were ordered in G-blocks from IDT, which also contained flanking SalI and SacI sites (Supplementary Table S6). The G-blocks were then cloned into the SalI and SacI sites of the arabinose-inducible pBAD33 vector (pBAD33-toxN). To construct pBAD33-toxNEc-CBD (chitin binding domain), toxN fused to the Mxe GyrA intein and the chitin binding domain was PCR-amplified from pTXB1-toxN with primers CKG-661/662, which also introduced complementarity to the pBAD33 backbone. pBAD33 was linearized with SalI-HF and SacI-HF, and the PCR products were ligated using Gibson assembly.
pEXT20-tifA homologs: TifA homolog sequences screened in Figure 1 were ordered in G-blocks from IDT (Supplementary Table S6) or amplified from phage genomic DNA and cloned into pKVS45 using Gibson assembly. The TifA homologs were then PCR-amplified from pKVS45 using primers listed in Supplementary Table S5 and ligated into SacI/SalI-linearized pEXT20 vector using Gibson assembly. To test for expression of TifA homologs in E. coli, pEXT20-tifAvB_PatP_CB4-FLAG and pEXT20-tifAvB_EcoM_005-FLAG were constructed using around-the-horn PCR of pEXT20-tifAvB_PatP_CB4 and pEXT20-tifAvB_EcoM_005 using primer pairs CKG-666/672 and CKG-666/667, respectively.
pBR322-toxIN and pBR322-toxI-toxN-His6 mutants: pBR322-toxI-toxN(Y115H), pBR322-toxI-toxN(L118S), pBR322-toxI-toxN(K111N), and pBR322-toxI-toxN(K111R) were constructed using around-the-horn PCR of pBR322-toxIN using primer pairs CKG-499/500, CKG-501/502, CKG-545/546 and CKG-547/546, respectively. pBR322-toxI-toxN(K55A)-His6 was constructed using around-the-horn PCR of pBR322-toxI-toxN(K55A) with the primer pair CKG-303/304.
pEXT20-tifAT4 and pEXT20-tifARB69 mutants: pEXT20-tifAT4(F41S) and pEXT20-tifAT4(F41H) were constructed using around-the-horn PCR of pEXT20-tifAT4 with primer pairs CKG-597/598 and CKG-599/598, respectively. pEXT20-tifARB69(H41F) and pEXT20-tifARB69(H41S) were constructed using around-the-horn PCR of pEXT20-tifARB69 with primer pairs CKG-600/601 and CKG-602/601, respectively. pEXT20-tifARB69(P53A T54A) and pEXT20-tifARB69(R52A K55A R56A R59A) were constructed by Gibson assembly of G-blocks (Supplementary Table S6) into pEXT20 vector linearized via digestion with SacI/SalI. pEXT20-tifARB69(K55A R56A R59A) was then constructed from pEXT20-tifARB69(R52A K55A R56A R59A) via around-the-horn PCR with primers CKG-625/626. pEXT20-tifARB69-FLAG was constructed via around-the-horn PCR of pEXT20-tifARB69 with primers CKG-665/441. To construct pEXT20-tifARB69-MBP-His6, MBP-His6 was PCR-amplified from pET-tifARB69-MBP-His6 with primers CKG-663/664. pEXT20-tifARB69 was linearized via PCR with CKG-624/665. The PCR products were then ligated together using Gibson assembly.
pKVS45-tifAT4-FLAG mutants: pKVS45-tifAT4(F41S)-FLAG, pKVS45-tifAT4(F41H)-FLAG, and pKVS45-tifAT4(P53A T54A)-FLAG were constructed via around-the-horn PCR of pKVS45-tifAT4-FLAG using primer pairs CKG-597/598, CKG-599/598, and CKG-603/604, respectively. pKVS45-tifAT4(R52A K55A R56A R59A) was constructed by around-the-horn PCR of pKVS45-tifAT4 using primers CKG-523/524. A FLAG tag was then added to pKVS45-tifAT4(R52A K55A R56A R59A) with around-the-horn PCR using primers CKG-411/404. pKVS45-tifAT4(K55A R56A R59A)-FLAG was then constructed from pKVS45-tifAT4(R52A K55A R56A R59A)-FLAG via around-the-horn PCR with CKG-627/628.
pTXB1-toxN: The toxN gene was PCR-amplified from pBAD33-toxN using primers CKG-413/415, which also introduced an extra arginine residue to the C-terminus of ToxN (to increase intein self-cleavage efficiency) and NdeI and SapI restriction sites. The PCR product was then cloned into the NdeI and SapI sites of the protein overexpression vector pTXB1 (NEB), upstream of a C-terminal intein and chitin-binding domain.
pACYC-toxI: toxI was PCR-amplified, along with its native promoter and transcription terminator, from pBR322-toxIN using CKG-416/139. pACYCDuet-1 (Novagen) was linearized via PCR with CKG-147/134 to remove the first RBS and the second T7 promoter and RBS from the vector. toxI was then ligated into linearized pACYC with Gibson assembly.
pACYC-tifARB69 and pACYC-tifARB69-FLAG: tifARB69 was PCR-amplified from pKVS45-tifARB69 with using CKG-450/451. pACYCDuet-1 was linearized via PCR with CKG-423/424 to remove the second T7 promoter and RBS from the vector. tifARB69 was then ligated into linearized pACYC with Gibson assembly. pACYC-tifARB69-FLAG was then constructed from around-the-horn PCR of pACYC-tifARB69 with CKG-441/424.
pACYCDuet-1-toxI-tifARB69, pACYCDuet-1-toxI-tifARB69-FLAG and pACYCDuet-1-toxI-tifARB69(K55A R56A R59A): toxI was PCR-amplified, along with its native promoter and transcription terminator, from pACYC-toxI using CKG-416/460. pACYCDuet-1 was linearized via PCR with CKG-147/444, and toxI was ligated downstream of the first T7 promoter into linearized pACYCDuet-1 with Gibson assembly to yield pACYCDuet-1-toxI. tifARB69 was PCR-amplified from pKVS45-tifARB69 with CKG-458/459, which also introduced flanking AvrII and NdeI restriction sites. The PCR product was then cloned into the AvrII and NdeI sites of pACYCDuet-1-toxI downstream of the second T7 and RBS promoter to yield pACYCDuet-1-toxI-tifARB69. pACYCDuet-1-toxI-tifARB69-FLAG was then constructed from around-the-horn PCR of pACYC-tifARB69 with CKG-441/575. pACYCDuet-1-toxI-tifARB69 (K55A R56A R59A) was constructed via around-the-horn PCR of pACYC-tifARB69 with CKG-656/657.
pET-tifARB69-MBP-His6: tifARB69 was introduced into pET-MBP-His6 (Addgene Plasmid #37237) using ligation-independent cloning. tifARB69 was PCR-amplified from pACYC-tifARB69 with PCR primers CKG-465/466, gel-purified, and digested with T4 DNA polymerase in the presence of dGTP to create sticky ends with homology to pET-MBP-His6. pET-MBP-His6 was linearized via restriction digestion with HpaI and then and digested with T4 DNA polymerase in the presence of dCTP to create sticky ends. The insert and vector were then incubated together at room temperature for 15 min. 1 μl 25 mM EDTA was then added, and the ligation was incubated for an additional 30 min at room temperature to yield pET-tifARB69-MBP-His6. pET-tifARB69(P53A T54A)-MBP-His6 and pET-tifARB69(K55A R56A R59A)-MBP-His6 were assembled analogously; tifARB69(P53A T54A) was PCR-amplified from pEXT20-tifARB69(P53A T54A) with primers CKG-465/466, and tifARB69(K55A R56A R59A) was PCR-amplified from pEXT20-tifARB69(K55A R56A R59A) with primers CKG-465/466.
ToxN, toxI and TifA overproduction on solid media
ToxN and TifA overproduction spotting assays were conducted as described previously (19,28). Briefly, single colonies of each strain were grown to saturation overnight at 37°C in M9-glucose. 1 ml of each culture was then harvested by centrifugation, washed twice in 1× PBS, and resuspended in 500 μl 1× PBS. Resuspended cells were serially diluted 10-fold in 1× PBS and spotted on M9L plates (M9-glycerol supplemented with 5% (v/v) LB) supplemented with 0.2% arabinose (toxin-inducing), 0.2% arabinose and 100 μM IPTG (toxin- and TifA/toxI-inducing), and 0.4% glucose (toxin-suppressing). Plates were then incubated at 37°C for 24 h (M9L-glucose) or 48 h (M9L-arabinose and M9L-arabinose-IPTG) before imaging. Images shown in figures are representative of at least three independent biological replicates.
Western blotting to measure expression levels of tifA homologs
To verify expression of selected tifA homologs, pEXT20-tifAvB_PatP_CB4-FLAG and pEXT20-tifAvB_EcoM_005-FLAG were transformed into E. coli MG1655. Overnight cultures containing each plasmid were back-diluted 1:100 and grown to mid-log phase in a shaking water bath at 37°C in M9-glycerol supplemented with 100 μM IPTG to induce tifA expression. Cells were harvested ∼6.5 h after dilution via centrifugation, and protein expression was analyzed with western blotting using an anti-FLAG antibody (Cell Signaling Technologies) as described previously (28). RpoA was used as a loading control by probing membranes with an anti-E. coli RpoA antibody (BioLegend).
tab screen
The toxN gene was mutagenized using error-prone PCR following the protocol described in (29). Briefly, toxN was PCR-amplified with primers CKG-106/12 from pBR322-toxIN in 1x Taq reaction buffer using Taq polymerase in the presence of 0.125 mM and 0.0625 mM MnCl2. The PCR products were then gel-purified and re-introduced into pBR322-toxI backbone (linearized with CKG-497/498) using Gibson assembly. Prior to Gibson assembly, the mutagenized toxN PCR products were also TOPO cloned and Sanger sequenced to ensure that there were 0–1 mutations per gene present.
2 μl of each Gibson-assembled pBR322-toxIN plasmid was then transformed into E. coli TOP10, grown overnight at 37°C in 5 ml LB + carb, and miniprepped. Miniprepped libraries, alongside wild-type pBR322-toxIN, were then transformed using electroporation into MG1655 pKVS45-tifAT4 and MG1655 pKVS45-tifARB69 and grown overnight at 37°C in 5 ml LB + carb + chlor.
The tab screen was then conducted as described in (16). Briefly, overnight cultures were back-diluted to OD600 = 1.0 in LB + carb. 100 μl of each culture was mixed separately with 100 μl of a range of T4 dilutions (5.5 × 106 to 5.5 × 1010 pfu/ml) and 20 ml of molten LB + 0.5% agar and spread to cover the bottom of an empty 15 cm Petri dish. Plates were incubated overnight at 37°C to allow microcolonies to form. Colonies from plates containing the phage dilution with the largest difference in colony-forming efficiency between the control and library samples were screened by colony PCR with CKG-105/115, to amplify and Sanger sequence the toxIN locus. Mutations in toxN were identified by Sanger sequencing and re-introduced into fresh wild-type pBR322-toxIN by around-the-horn PCR (see above) to validate and further characterize.
Phage spotting experiments
Phage spotting assays were conducted as described previously in (28). Briefly, bacterial strains of interest were mixed with LB + 0.5% agar (supplemented with 100 ng/ml aTc when necessary to induce tifA expression) and spread onto an LB + 1.2% agar + antibiotic plate. Phage stocks were serially diluted 10-fold in 1x FM buffer (20 mM Tris–HCl pH 7.4, 100 mM NaCl, 10 mM MgSO4) or LB and spotted on the bacterial lawn. Plates were then incubated overnight at 37°C before imaging. Images shown in figures are representative of at least three independent biological replicates.
Co-immunoprecipitation assays to study ToxN-TifA interactions
Co-immunoprecipitation assays were performed as described in (28). Briefly, cultures of E. coli MG1655 containing pBR322-toxIN-His6 (wild-type or mutant) and pKVS45-tifAT4-FLAG (wild-type or mutant) were grown at 37°C in LB medium supplemented with 15 μg/ml L-tryptophan and 1 μg/ml thiamine to OD600= 0.4 and then infected with T4 at MOI = 5. Cells were treated with aTc at a final concentration of 100 ng/ml to induce production of TifA 30 min prior to T4 infection. After 15 min of T4 infection, 40 ml of cells from each culture were collected and pelleted by centrifugation (5 min at 4000 g, 4°C). After removing the supernatant, pellets were flash-frozen and stored at -80°C.
To perform anti-FLAG co-IP experiments, cell pellets were resuspended in 500 μl lysis buffer (25 mM Tris–HCl pH 8, 150 mM NaCl, 1 mM EDTA pH 8, 1% Triton X-100, cOmplete protease inhibitor cocktail (Roche), 1 μl/ml ReadyLyse (Novagen), 1 μl/ml DNase I (TakaraBio), 5% glycerol) and lysed by two freeze-thaw cycles followed by incubation on an end-to-end rotor for 1 h at 30°C. Lysates were clarified by centrifugation (10 min at 14000 g, 4°C), added to anti-FLAG-M2 magnetic beads (Sigma), and incubated with the beads for 1 h at 4°C while rotating on an end-to-end rotor. The beads were washed three times with wash buffer (25 mM Tris–HCl pH 8, 150 mM NaCl, 1 mM EDTA pH 8, cOmplete protease inhibitor cocktail (Roche), 5% glycerol), and the protein was eluted from the beads by adding 1x Laemmli buffer to the beads, vortexing, and boiling at 95°C. Samples were then analyzed by western blot as described previously (28). Blots shown are representative of at least two independent biological replicates.
Cell growth assays in liquid culture
Plate reader growth assays were used to compare the growth rates of E. coli MG1655 cells transformed with pBR322-toxIN, pBR322-toxI-toxN(K111N), pBR322-toxI-toxN(K111R), pBR322-toxI-toxN(Y115H), and pBR322-toxI-toxN(L118S). Overnight cultures were back-diluted 1:100 in 4 ml LB and grown to mid-log phase at 37°C while rotating in a roller drum. Cultures were then back-diluted to OD600 = 0.001 in 1.5 ml LB, and growth was measured in 24-well plates at 15 min intervals with orbital shaking for 6 h at 37°C on a plate reader (Biotek). Data reported are the mean of two technical replicates each for four independent growth curve experiments. Growth rates of each strain were calculated using the Curveball package (30) with growth curves fit to the Richards (generalized logistic) model.
Protein purification
ToxN-TifARB69, ToxIN, and ToxN-toxI-TifARB69: Complexes containing ToxN were purified using a modified version of the IMPACT kit protocol (NEB). 2–4 L of T7 Express (NEB) cells overexpressing ToxN with a C-terminal intein fused to chitin-binding domain (CBD) (pTXB1-toxN), along with toxI (pACYC-toxI), untagged TifARB69 (pACYC-tifARB69), FLAG-tagged TifARB69 (pACYC-tifARB69-FLAG), untagged TifARB69 and toxI (pACYCDuet-1-toxI-tifARB69), untagged TifARB69(K55A R56A R59A) and toxI (pACYCDuet-1-toxI-tifARB69(K55A R56A R59A)), or FLAG-tagged TifARB69 and toxI (pACYCDuet-1-toxI-tifARB69-FLAG) were grown in LB at 37°C to OD600 ∼ 0.8. Protein overproduction was then induced with the addition of 0.4 mM IPTG, and cultures were grown for an additional ∼18 h at 16°C. Cells were then harvested by centrifugation, resuspended in lysis/column buffer (20 mM Tris–HCl pH 8.5, 500 mM NaCl, 1 mM EDTA, 0.05% Tween-20, SIGMAFAST Protease Inhibitor Tablet (Sigma), 5% glycerol), and lysed using a Microfluidizer (three passes, 18 000 psi). The cell lysate was clarified by centrifugation at 15 000 rpm for 30 min and passed over chitin resin (NEB) pre-equilibrated with lysis/column buffer at 4°C. The resin was then washed with 25 column volumes of lysis/column buffer. To induce self-cleavage of the C-terminal intein-CBD tag, the resin was quickly washed with 3 column volumes of lysis/column buffer supplemented with 50 mM DTT (column cleavage buffer), capped, and incubated overnight at 4°C. The next day, 5 column volume of lysis/column buffer was added to each column to elute untagged ToxN and its binding partners. Fractions containing protein were pooled, concentrated through an Amicon 3K Centrifugal Filter Unit, and then passed over a Superose 6 Increase 10/300 GL column (GE Healthcare) equilibrated in column buffer (20 mM Tris–HCl pH 8.5, 50 mM NaCl, 1 mM EDTA, 5% glycerol). Fractions containing ToxN and/or TifA were identified by SDS-PAGE/Coomassie staining, concentrated, flash-frozen, and stored at −80°C. Fractions from the ToxN-TifARB69-FLAG and ToxN-toxI-TifARB69-FLAG purifications were analyzed by western blot as described previously (19,28), using mouse anti-FLAG antibodies (Sigma). The SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher) was used to develop blots, and blots were imaged with the ChemiDoc MP Imaging System (Bio-Rad). The rough molecular weight of the ToxN-TifA-RNA peak on each column was estimated based on published standards (GE Healthcare). Each purification was performed and analyzed by FPLC at least three independent times.
TifA-MBP-His6: 1–4 L of T7 Express (NEB) cells overproducing TifA-MBP-His6 (pET-tifARB69-MBP-His6) were grown in LB at 37°C to OD600 ∼0.8. Protein overproduction was then induced with the addition of 0.4 mM IPTG, and cultures were grown for an additional ∼18 h at 16°C. Cells were then harvested by centrifugation, resuspended in lysis buffer (50 mM Tris–HCl pH 7.5, 500 mM NaCl, 0.05% Tween-20, 1 mM TCEP, 0.18 mg/ml PMSF, SIGMAFAST Protease Inhibitor Tablet (Sigma), 5% glycerol) and lysed using a Microfluidizer (three passes, 18000 psi). The cell lysate was clarified by centrifugation at 15 000 rpm for 30 min and passed over Ni-NTA agarose resin (QIAGEN) pre-equilibrated with buffer A (50 mM Tris–HCl pH 7.5, 500 mM NaCl, 1 mM TCEP, 0.18 mg/ml PMSF, SIGMAFAST Protease Inhibitor Tablet (Sigma), 5% glycerol, 10 mM imidazole). The resin was then washed with 25 column volumes of buffer A. The protein was eluted from the resin with 5 column volumes of buffer B (50 mM Tris–HCl pH 7.5, 500 mM NaCl, 1 mM TCEP, 0.18 mg/ml PMSF, SIGMAFAST Protease Inhibitor Tablet (Sigma), 5% glycerol, 300 mM imidazole). Fractions containing protein were pooled and then concentrated and buffer-exchanged into buffer B without imidazole or protease inhibitor using an Amicon 10K Centrifugal Filter Unit. Purified protein was then passed over a Superose 6 Increase 10/300 GL column (GE Healthcare) equilibrated in column buffer (20 mM Tris–HCl pH 8.5, 50 mM NaCl, 1 mM EDTA, 5% glycerol). Fractions containing TifA-MBP-His6 were identified by SDS-PAGE/Coomassie staining, concentrated, flash-frozen, and stored at −80°C. TifA(P53A T54A)-MBP-His6 and TifA(K55A R56A R59A)- MBP-His6 were purified analogously. Each purification was performed and analyzed by FPLC at least two independent times.
TifA-nucleic acid in vitro binding assay using fluorescence polarization
TifA binding activity to RNA, ssDNA, and dsDNA was tested using a fluorescence polarization-based method. In vitro synthesized TifARB69 was purchased from AAPPTEC and solubilized in MilliQ H2O prior to use in in vitro binding experiments. 5′ 6-FAM (fluorescein)-labeled nucleic acid molecules derived from the region surrounding the ToxN cut site in the E. coli transcript artJ (RNA oligos CKG 658–660 and ssDNA oligos CKG-674–675) and from the 16S rRNA (RNA oligos CKG-676–678) were purchased from IDT and solubilized in 10 mM Tris–HCl, pH = 7.5, 0.1 mM EDTA. To construct dsDNA fragments derived from artJ, CKG-674 and CKG-675 were annealed to complementary unlabeled 30 and 45 nt oligos, respectively, purchased from IDT. Binding reactions were assembled in a final volume of 20 μl in 1× binding buffer (150 mM NaCl, 50 mM Tris–HCl, pH 7.5, 1 mM DTT, 5% glycerol, 0.1 mg/ml ultrapure BSA (Ambion)) with varying concentrations of TifARB69 and 5 nM RNA or DNA. Reactions were incubated for 30 min at 37°C in a black flat bottom 384-well plate (Corning 3573). Fluorescence polarization was measured with a Tecan Infinite 200 Pro F Plex plate reader with 485 nm/20 nm bandwidth excitation filters and 530 nm/25 nm bandwidth emission filters fitted with polarizers. Data were fit in GraphPad Prism using the following equation: FPobs = [Protein]*FPmax + KD*FPmin / [Protein] + KD. Data shown are the result of three independent biological replicates.
RNA-seq following overexpression of ToxN-toxI-TifARB69 and ToxN-toxI-TifARB69(K55A R56A R59A)
Whole-cell lysate RNA was isolated from T7 Express cells overproducing ToxN-toxI-TifARB69-FLAG (which behaved analogously to cells overexpressing ToxN-toxI-TifARB69, see Figure 6A versus Supplementary Figure S7B) or ToxN-toxI-TifARB69(K55A R56A R59A) following lysis with a Microfluidizer via subsequent rounds of extraction with acid-buffered phenol, acid-buffered phenol chloroform, and chloroform, followed by isopropanol precipitation. RNA was isolated from purified proteins following elution from chitin resin via subsequent rounds of extraction with acid-buffered phenol chloroform and chloroform, followed by isopropanol precipitation. Each purification was performed in biological duplicate. 200 ng of each RNA sample was then used to make RNA-seq libraries using the NEBNext® Ultra™ II RNA Library Prep Kit for Illumina® following the manufacturers’ protocol for use with purified mRNA or rRNA-depleted RNA. Paired-end sequencing of the libraries was performed on an Illumina NextSeq 5000 machine at the MIT BioMicroCenter. FASTQ files were then mapped to the E. coli BL21(DE3) genome (NZ_CP053602.1) and plasmid maps for pTXB1-toxN and pACYC Duet-1-toxI-tifARB69 as previously described (19,31).
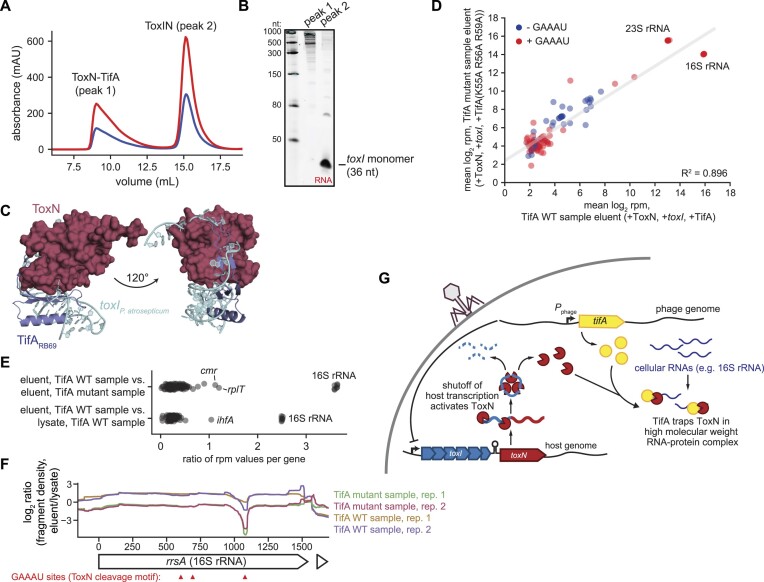
Figure 6.
ToxN co-purifies with the 16S rRNA in the presence of TifA. (A) Size-exclusion chromatogram of ToxN co-purified with toxI and TifA run a Superose 6 Increase 10/300 GL column. (B) 6% urea–PAGE gel analyzing the RNA content of the two major peaks in (A). (C) AlphaFold model for the ToxN-TifARB69 complex, with P. atrosepticum toxI (PDB: 2xdd) modelled into the complex. (D) Scatter plot comparing the mean summed RNA-seq counts (log2 RPM) for individual genes in the TifA WT sample eluent (+ToxN, +toxI, +TifARB69) and the TifA mutant sample eluent (+ToxN, +toxI, +TifARB69 (K55A R56A R59A)) (two biological replicates each). Genes with RPM values greater than or equal to 3 in all sequenced libraries (lysates and eluents) are shown. The gray line indicates the linear regression fit for the data. (E) Scatter plots showing the ratio of mean RPM values for individual transcripts in the samples indicated. (F) Plot of log2 (ratio, fragment density in eluent versus lysate) across the 16S rRNA locus rrsA for both biological replicates of the TifA WT and TifA mutant samples. Sites containing the ToxN cleavage motif (GAAAU) are indicated with red arrows. (G) Proposed model for TifA inhibition of ToxN. Following phage infection, shutoff of host transcription, including of the toxIN locus, leads to degradation of toxI and release of ToxN. TifA then interacts with activated ToxN to prevent degradation of cellular transcripts, likely by recruiting and/or sequestering ToxN to a subset of cellular RNAs, in particular the 16S rRNA, such that ToxN can no longer cleave phage transcripts. Also see Supplementary Figure S7.
For analysis of reads mapping to each gene, one count was added to the middle of each read. All reads mapping to a given genomic region were then summed and normalized by reads per million (RPM). This quantity was then used for downstream analyses. For analysis of fragment density across 16S rRNA loci, one count was added to every position in each read. Counts at each position were then divided by a sample size factor as described in (31).
Results
TifA is a small phage protein that inhibits ToxN and its homologs
Producing TifAT4 from an inducible promoter rescues cell growth when E. coli ToxN is produced from a separate inducible promoter, suggesting that TifAT4 is a bona fide antitoxin for ToxN (Figure 1B) (28). TifA homologs from the closely-related T4-like coliphages T2, T6 and RB69 are also capable of rescuing the growth defects resulting from E. coli ToxN, hereafter referred to as ToxNEc when necessary for clarity (Figure 1B) (28). Although TifAT2 and TifAT6 are closely related to TifAT4 (>96% identity), TifARB69 is more distantly related (56% identity).
To determine whether inhibition extended beyond these proteins, we constructed a panel of six additional TifA homologs from E. coli and Pectobacterium atrosepticum phages spanning seven phylogenetic clusters, as well as six additional type III toxins—five ToxN homologs and one TenpN homolog—from different environmental isolates of γ-proteobacteria species for which phages contain tifA homologs (Supplementary Figure S1A-S1B, Supplementary Table S2). We cloned each tifA and toxN homolog into vectors that allow for separate and inducible expression and co-transformed these plasmids into E. coli MG1655. We then conducted a pairwise neutralization screen on plates that induced production of both ToxN and TifA (Figure 1C, Supplementary Figure S2A-F). We judged rescue of toxicity by comparing the growth of each strain producing a given toxin +/– TifA induction.
We found that the TifA homologs from T2, T4, T6 and RB69 displayed relatively broad inhibitory activity. In addition to neutralizing ToxNEc, these TifA homologs could neutralize three other closely-related ToxNs (≥ 81% sequence similarity to ToxNEc) from P. atrosepticum, Klebsiella pneumoniae, and Shigella sonnei, but not the more distantly related ToxN homologs from Vibrio kanaloae and Serratia sp. SRS-8-S-2018 (< 33% sequence similarity to ToxNEc) or E. coli TenpN. The TifA homologs from other E. coli and P. atrosepticum phages did not neutralize any of the ToxN homologs or TenpN. To verify that a lack of rescue was not caused by low expression of TifA homologs, we used immunoblotting to confirm for two homologs that they were produced at high levels (Supplementary Figure S2G). We conclude that TifA homologs display specificity in their inhibition of ToxN proteins, suggesting that TifA is not a nonspecific inhibitor of toxin RNase activity and likely interacts with sequence or structural features of ToxN.
AlphaFold prediction of the ToxN–TifA interface
TifAT4 is sufficient, in the absence of any other phage protein, to neutralize ToxN, and prior work showed that ToxN co-precipitates with TifAT4 during T4 infection, suggesting that the two proteins interact (28). To investigate the molecular features of their interaction, we first used AlphaFold to predict the structures of TifAT4 and TifARB69 (Figure 2A, B, Supplementary Figure S3A-S3B) (32). Each protein was predicted to adopt an L-shaped structure with an N-terminal three-stranded β-sheet packed against a C-terminal α-helix predicted with high confidence. Protruding from this base is an ∼20 amino acid-long loop region predicted with lower confidence.
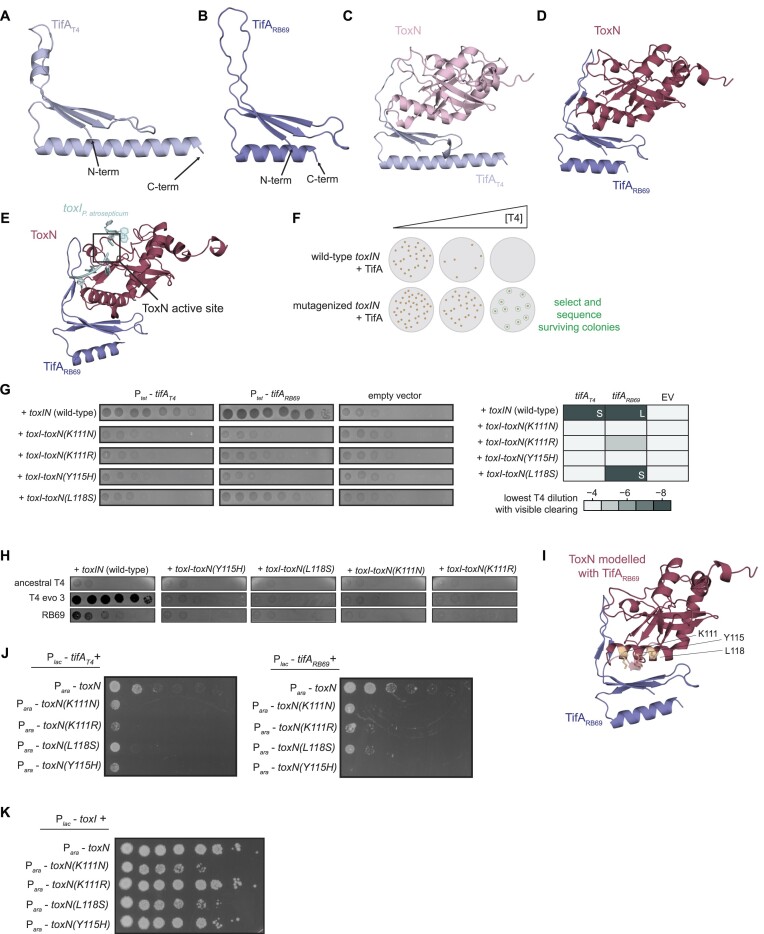
Figure 2.
Prediction and validation of the ToxN-TifA interface. (A, B) AlphaFold model for TifAT4 (A) and TifARB69 (B). (C, D) AlphaFold models for the ToxN-TifAT4 (C) and ToxN-TifARB69 (D) complexes. (E) AlphaFold model for ToxN-TifARB69 with toxIPa and the predicted ToxN active site indicated. The position for toxIPa relative to ToxN was visualized by overlaying the E. coli ToxN predicted structure and the ToxINPa crystal structure (PDB: 2xdd) and is shown to indicate the ToxN active site. (F) Schematic of tab screen used to isolate TifA-resistant ToxN mutants. (G) (Left) Spotting assays for T4 on lawns of +toxIN (either wild-type or mutant toxN) cells with TifAT4, TifARB69, or neither expressed from an anhydrous tetracycline (tet)-inducible promoter during phage infection. (Right) Heatmap quantifying spotting assays, with color indicating the lowest T4 dilution in which the cells displayed visible clearing (either plaques or lower cell density). L and S refer to large and small plaques, respectively, for samples on which T4 formed visible plaques. (H) Spotting assays for wild-type (ancestral) T4, evolved T4 with increased expression of dmd-tifA (T4 evo 3), and RB69 on lawns of +toxIN (with wild-type or mutant toxN) cells. (I) AlphaFold model for the ToxN-TifARB69 complex, with residues K111, Y115 and L118 on ToxN highlighted. (J) Serial dilutions of E. coli cells ectopically expressing toxN (wild-type or mutant) and tifAT4 or tifARB69. (K) Serial dilutions of E. coli cells ectopically expressing toxN (wild-type or mutant) and toxI. Also see Supplementary Figures S3 and S4.
We then used AlphaFold to model ToxN in complex with TifAT4 or TifARB69 (Figure 2C, D). The AlphaFold models for ToxN in both complexes closely resembled the existing crystal structure (20) of P. atrosepticum ToxN (81% sequence similarity to ToxNEc) solved in complex with its cognate toxI (RMSD of 1.87 and 0.91 Å, respectively) (Supplementary Figure S3C, D). Although AlphaFold yielded medium- to high-confidence predictions for ToxN and each of the two TifA homologs individually, the predictions for the interactions between the two proteins were less certain (Supplementary Figure S3F-I). Nevertheless, for both TifAT4 and TifARB69, the highest-ranking models predicted that the interior of the L-shaped TifA interacted with ToxN, with several residues from the N-terminal β-sheet and the predicted 20 amino acid loop of TifA oriented toward the kinked α-helix and a loop region on the surface of ToxN (Figure 2C, D, Supplementary Figure S3E). We did not observe any large rearrangements of the predicted E. coli ToxN structure relative to the crystal structure of P. atrosepticum ToxN, indicating that TifA likely does not alter the conformation of ToxN (Supplementary Figure S3C, D). However, part of the region on ToxN predicted to be bound by TifA overlaps a region of ToxN involved in RNA substrate binding, and the tip of the 20 amino-acid loop in TifA appears to partially occlude the ToxN active site (Figure 2E), suggesting that TifA may directly interfere with ToxN-mediated cleavage of RNA targets.
ToxN can escape TifA via mutations in the toxI binding pocket
To corroborate the AlphaFold predictions for the ToxN-TifA interface, we conducted a screen for mutants of ToxN that could escape TifA. Using error-prone PCR, we mutagenized toxN in the context of plasmid-borne toxIN under the control of its native promoter. We then transformed this library into E. coli MG1655 containing plasmid-borne TifAT4 or TifARB69 under the control of a tetracycline-inducible promoter (Ptet-tifA). We previously showed that high levels of TifAT4 or TifARB69 are sufficient to prevent toxIN-mediated defense against T4 infection, leading to propagation of T4. Thus, we reasoned that mutations in toxN that disrupt the ToxN-TifA interface without abolishing ToxN activity would restore the ability of ToxN to block T4 infection.
Because ToxIN is an abortive infection (Abi) system, it is impossible to directly select for cells with functional ToxIN systems following phage infection. Instead, we used a selection strategy called the tab (T4 abortive) method, which allows for isolation of microcolonies with functional Abi systems following phage infection (Figures 2F, S4A) (16,33). We challenged our library of toxIN mutants, in the context of TifAT4 or TifARB69 overproduction, with increasing concentrations of T4, mixed the cells and phage with soft agar, and allowed infection to proceed overnight. Because of limited phage diffusion through the agar, cells containing functional ToxIN systems could form microcolonies on plates containing low and intermediate concentrations of T4. We identified concentrations of T4 in which our toxIN mutant library, but not cells containing wild-type toxIN, formed microcolonies and Sanger sequenced approximately 40 surviving colonies for each TifA homolog, which resulted in 12 unique mutations. We re-cloned each mutation into pBR322-toxIN and transformed each plasmid into E. coli MG1655 containing plasmid-borne Ptet-tifAT4 or Ptet-tifARB69. We infected these strains with T4 to verify that each mutant toxN could indeed overcome TifA, using inhibition of T4 plaquing as a readout. Of the twelve original mutations, four were verified to confer defense against T4 infection in the presence of overproduced TifA (Figure 2G). There were three substitutions in ToxN that rendered cells partially or completely resistant to both TifAT4 and TifARB69 (K111N, K111R, and Y115H) and one substitution that rendered cells resistant to only TifAT4 (L118S).
We then tested the ability of each toxN mutant to avoid phage-encoded TifA (as opposed to TifA produced from an inducible promoter as in Figure 2G). We transformed pBR322-toxIN plasmids containing each mutation into E. coli MG1655 and then, for each strain, compared the plaquing efficiency of several phages (Figure 2H). Both RB69 and an evolved T4 clone (T4 evo 3 (28)) containing an amplification of tifA could form plaques on wild-type +toxIN cells, indicating that TifA was inhibiting ToxN. However, like wild-type T4, both RB69 and T4 evo 3 were restricted in the presence of the four ToxN mutants. Thus, the substitutions K111N, K111R, Y115H, and L118S in ToxN allow it to evade inhibition by TifA during phage infection.
The three residues we identified in the screen (K111, Y115, and L118) are localized on the surface of the kinked α-helix in ToxN within the ToxN-TifA interface predicted by AlphaFold (Figure 2I), suggesting that these residues may help mediate the interaction between ToxN and TifA. To further test this hypothesis, we overexpressed each ToxN mutant in uninfected cells ectopically producing TifAT4 or TifARB69. All four substitutions prevented TifA rescue of ToxN-mediated toxicity, supporting the notion that residues K111, Y115, and L118 are important for the antitoxin activity of TifA (Figure 2J).
Y115 and L118 were previously identified as residues mediating ToxN-toxI interactions in a closely-related ToxIN system from P. atrosepticum, and these residues map to the interface formed between toxI and ToxN in the crystal structure of P. atrosepticum ToxIN (20). To test whether the mutations found to disrupt ToxN-TifA interaction also weaken the ToxN-toxI interaction, we co-produced each ToxN mutant with toxI under separate, inducible promoters and monitored cell growth on medium that promotes expression of both toxN and toxI (Figure 2K). Although ToxN(K111R) and ToxN(Y115H) were as robustly inhibited by toxI as wild-type ToxN, ToxN(K111N) and ToxN(L118S) displayed significantly less sensitivity to toxI, resulting in a lower plating efficiency and smaller colonies. Thus, although substitutions in the toxI binding pocket allow ToxN to overcome TifA, they may also weaken the ability of toxI to sequester ToxN in uninfected cells. Despite this change in sensitivity to toxI, we did not observe obvious growth defects from toxIN constructs containing mutations in those positions when toxIN was expressed as an operon (Supplementary Figure S4B). However, toxI transcript levels greatly exceed those of toxN in the native locus (11) and thus may be sufficient to sequester mutant ToxN proteins during normal cell growth.
Naturally-occurring substitutions in TifA enhance inhibition of ToxN
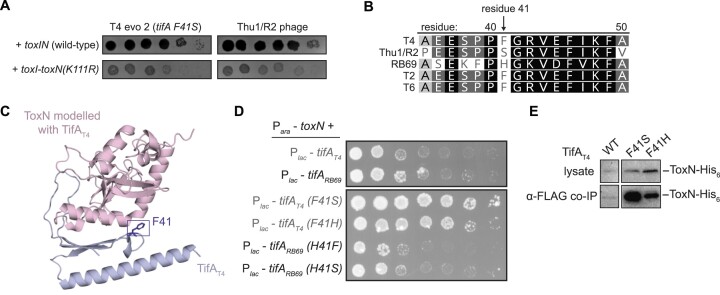
At late rounds in our original evolution of T4 to overcome toxIN, we had noticed the emergence of a mutation producing the substitution F41S in TifA in one of our evolved populations (evo 2) that had an amplification of the tifA-dmd locus. These evolved phages were slightly better at infecting +toxI-toxN(K111R) cells than other lines of evolved phages (Figures 2H, 3A) suggesting that the F41S substitution strengthens the interaction between ToxN and TifA and restores neutralization of ToxN(K111R). Notably, a serine occurs naturally at position 41 in the TifA homolog from the T4-like coliphage Thu1/R2, which has a single copy of tifA but can replicate on both +toxIN and +toxIN(K111R) cells (Figure 3A-B). For TifARB69, which is a more robust inhibitor of ToxN (Figures 1B, 3D), residue 41 is a histidine (Figure 3B). In both ToxN-TifA AlphaFold structures, residue 41 is on a loop in the predicted ToxN-TifA binding interface (Figure 3C). Thus, we hypothesized that substituting F41 with either serine or histidine improves the inhibition of ToxN by TifAT4.
Figure 3.
Mutations in TifA that strengthen its interaction with ToxN. (A) Spotting assays for evolved T4 phage with increased expression of dmd-tifA(F41S) (T4 evo 2) and Thu1/R2 phage on lawns of +toxIN and +toxI-toxN(K111R) cells. (B) Alignment of residues 35–50 of TifA homologs from several T4-like phages, with residue 41 indicated. Degree of sequence similarity is highlighted in gray/black. (C) AlphaFold model for the ToxN-TifAT4 complex, with residue F41 on TifA highlighted. (D) Serial dilutions of E. coli cells ectopically expressing toxN and wild-type or mutant tifAT4 or tifARB69. (E) Western blot of ToxN-His6 in cell lysate and following co-immunoprecipitation with TifAT4-FLAG (wild-type or mutant) during T4 infection.
To test this hypothesis, we examined whether TifAT4 (F41S or F41H) and TifARB69 (H41S or H41F) could neutralize the toxicity of ToxN in uninfected cells (Figure 3D). Both substitutions in TifAT4 substantially increased its ability to inhibit ToxN relative to wild-type TifAT4. Conversely, for TifARB69, H41F and H41S both appeared to modestly decrease inhibition of ToxN, as manifested by smaller colonies at the lowest plating dilution with visible colonies. We also performed an anti-FLAG IP on cells producing FLAG-tagged TifAT4 and ToxN-His6 during T4 infection (Figure 3E). Immunoblotting for ToxN-His6 revealed much stronger signal for the samples in which TifA harbored either the F41S or F41H substitution compared to wild-type TifA. Taken together, these observations indicate that position 41 of TifA, which is predicted to be positioned at the ToxN-TifA interface and varies in TifA homolog sequences, mediates inhibition of ToxN.
ToxN and TifA form an RNA–protein complex
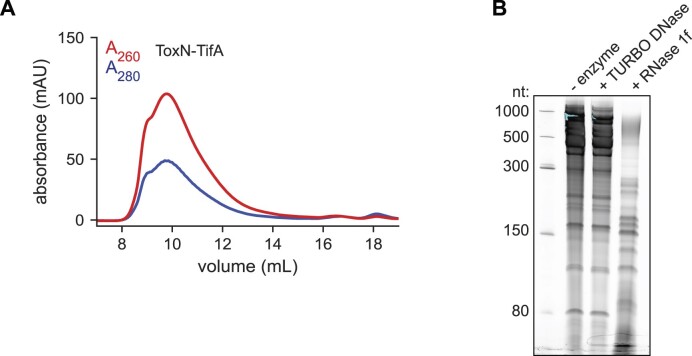
Given that TifA is sufficient to neutralize ToxN and that the proteins interact in co-IP experiments, we sought to overexpress and purify a ToxN-TifA complex to further characterize the interactions between the two proteins. We focused on the ToxN-TifARB69 complex as TifARB69 is a stronger inhibitor of ToxN than TifAT4 (Figure 2G, H). We tagged ToxN with a chitin-binding domain and, after confirming that this fusion protein was functional and rescued by TifARB69 (Supplementary Figure S5A), co-expressed it with untagged TifARB69 in E. coli BL21. After column purification on chitin agarose resin, we used size exclusion chromatography with a Superose 6 column to isolate the complex. We assessed nucleic acid and protein levels in our eluent by monitoring absorbance at 260 and 280 nm, respectively (Figure 4A). To our surprise, a major peak appeared in the void volume of the column. By repeating the ToxN-TifA purification in a strain producing FLAG-tagged TifA, which is functional (Supplementary Figure S5A), we confirmed the presence of TifA in the high molecular weight peak using western blotting (Supplementary Figure S5B). The major peak's ratio of absorbance at 260 nm to absorbance at 280 nm (A260/A280) was close to 2, suggesting that it contained both protein and nucleic acid. We extracted nucleic acid from the eluent of the chitin agarose purification, treated it with DNase or RNase, ran it on a denaturing urea-PAGE gel, and stained the gel with SYBR Gold to visualize both DNA and RNA (Figure 4B). The co-localizing nucleic acid was mostly high molecular weight material (>300 nucleotides) and was resistant to DNase treatment and sensitive to RNase treatment, suggesting that it was RNA (Figure 4B).
Figure 4.
ToxN and TifA form an RNA-protein complex.(A) Size-exclusion chromatogram of purified ToxN-TifARB69 run on a Superose 6 Increase 10/300 GL column. (B) Denaturing 6% urea-PAGE gel resolving nucleic acid that copurifies with ToxN-TifARB69 on chitin resin; nucleic acid was treated with Turbo DNase, RNase 1f, or neither before running on the gel. Also see Supplementary Figure S5.
TifA is an RNA-binding protein
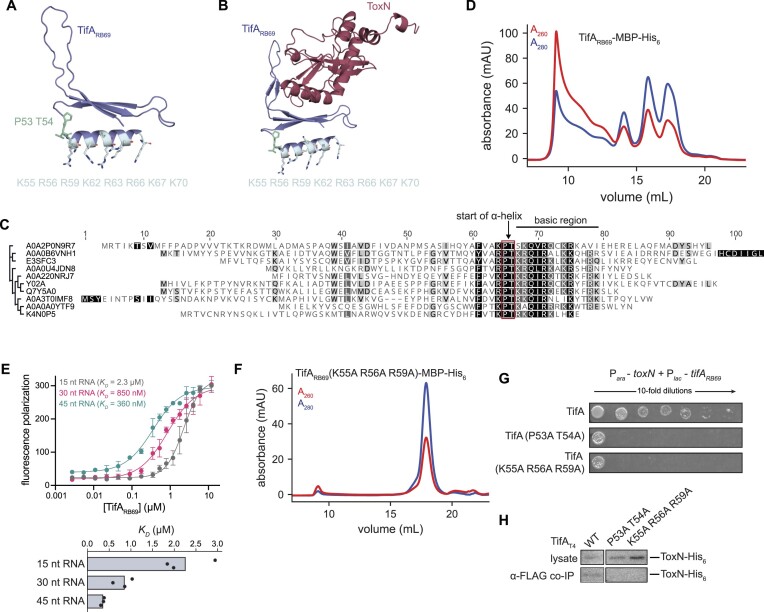
We suspected that the TifA in ToxN–TifA complexes may directly bind RNA as the AlphaFold models of both TifAT4 and TifARB69 predicted a C-terminal α-helix with a high density of solvent-exposed, basic residues, reminiscent of a nucleic acid binding motif (Figure 5A, B). The basic C-terminal region of TifA, as well as the Pro-Thr amino acids capping the α-helix in both TifAT4 and TifARB69, are conserved in TifA homologs, further suggesting that this basic α-helix plays a crucial role in TifA function (Figures 5C, S6A). In contrast, the sequence of the loop region containing residue 41 in TifAT4 and TifARB69 was conserved within closely-related sequences but not in wider taxonomic alignments (Supplementary Figure S6A). The sequence of this region, as opposed to the basic α-helix, may have evolved to interact with particular groups of ToxN homologs. To characterize TifA’s potential RNA binding activity, we overexpressed and purified TifARB69-MBP-His6 on nickel resin and then ran purified protein on a size-exclusion column (Figure 5D). We confirmed that TifARB69-MBP-His6 was a functional inhibitor of ToxN using cell growth assays (Supplementary Figure S6C). TifA-MBP-His6 eluted in several peaks, including one peak in the void volume of the column that was accompanied by an A260/A280 ratio of ∼2.0, as with the ToxN-TifA complex. Indeed, TifARB69-MBP-His6 co-purified with RNA of a range of molecular weights (Supplementary Figure S6B), suggesting that TifA interacts with RNA in the absence of ToxN.
Figure 5.
TifA is an RNA-binding protein. (A) AlphaFold model for TifARB69 with residues capping the predicted C-terminal α-helix (P53 T54) and basic residues on the surface of the predicted α-helix indicated. (B) AlphaFold model for the ToxN-TifARB69 complex with residues capping the predicted C-terminal α-helix (P53 T54) and basic residues on the surface of the predicted α-helix indicated. (C) Sequence alignment for 10 TifA homologs from different phylogenetic clusters indicating the highly-conserved PT motif, immediately followed by a cluster of basic residues. UniProtKB accession numbers for the TifA homologs in the alignment are, from top to bottom, as follows: A0A2P0N9R7; A0A0B6VNH1; E3SFC3; A0A0U4JDN8; A0A220NRJ7; Y02A; Q7Y5A0; A0A3T0IMF8; A0A0A0YTF9; K4N0P5. (D) Size-exclusion chromatogram of purified TifARB69-MBP-His6 run on a Superose 6 Increase 10/300 GL column. (E) Fluorescence polarization assay of increasing concentrations of synthesized TifARB69 incubated with several fluorescently-labeled RNA species (lengths indicated). RNA sequences were derived from the region of the E. coli transcript artJ that is cleaved by ToxN; see Supplementary Table S5 for sequences. (F) Size-exclusion chromatogram of purified TifARB69 (K55A R56A R59A)-MBP-His6 run on a Superose 6 Increase 10/300 GL column. (G) Serial dilutions of E. coli cells ectopically expressing toxN and wild-type or RNA-binding deficient tifARB69. (H) Western blot of ToxN-His6 in cell lysate and following co-immunoprecipitation with TifAT4-FLAG (wild-type or RNA-binding deficient) in T4-infected cells producing TifAT4 and ToxN. Note that the TifAT4 wild-type control is same as in Figure 3E because both experiments were run on the same blot. Also see Supplementary Figure S6.
To confirm this finding, we tested the ability of TifA protein to bind nucleic acid in vitro. We incubated increasing concentrations of directly synthesized, untagged TifARB69 with fluorescently-labeled RNAs of different lengths (15, 30 and 45 nucleotides) derived from the E. coli gene artJ and measured binding via fluorescence polarization (Figure 5E). TifARB69 interacted with all three fragments, with measured KD values of 2.3 μM, 850 nM and 360 nM for the 15, 30 and 45 nucleotide RNAs, respectively. TifARB69 also interacted with several RNA fragments derived from the 16S rRNA (Supplementary Figure S6D). We also found that TifA bound short ssDNA and dsDNA fragments, with a similar affinity (Supplementary Figure S6D), but because the nucleic acid that co-precipitated with TifA was RNase sensitive and not DNase sensitive (Supplementary Figure S6B), we conclude that TifA primarily interacts with RNA in vivo.
To determine whether TifA’s predicted C-terminal, basic α-helix facilitates RNA binding, we overexpressed and purified a variant of TifARB69-MBP-His6 in which we substituted the Pro-Thr residues capping the α-helix (P53A T54A) and a variant in which we substituted three basic residues in the α-helical region (K55A R56A R59A) (Figure 5F, Supplementary Figure S6E). Unlike wild-type TifARB69, these variants each ran as a single, well-defined peak on a size-exclusion column. The major peak for both variants had an A260/A280 ratio close to 0.5, suggesting that they no longer co-purify with RNA.
Finally, we asked whether the RNA-binding ability of TifA was necessary for neutralization of ToxN. Unlike wild-type TifARB69, neither TifARB69(P53A T54A) nor TifARB69(K55A R56A R59A) could rescue the cell growth defects of overproducing ToxN from a separate, inducible promoter (Figure 5G). Further, neither TifAT4(P53A T54A)-FLAG nor TifAT4(K55A R56A R59A)-FLAG co-immunoprecipitated with ToxN-His6 during T4 infection (Figure 5H), suggesting that these variants of TifA are no longer functional inhibitors and that RNA binding by TifA is necessary for ToxN-TifA interactions. Taken all together, our results indicate that TifA uses its C-terminal, basic α-helix to bind RNA and neutralize ToxN.
ToxN co-purifies with 16S rRNA in the presence of TifA
During normal cell growth, ToxN is sequestered via interactions with its cognate RNA antitoxin, toxI. Based on our result that ToxN-TifARB69 formed a ribonucleoprotein complex, we wondered whether TifA might form a ternary complex with ToxN and processed, 36-nucleotide toxI during T4 infection to stabilize the heterohexameric ToxIN complex. To test this idea, we overexpressed chitin binding domain (CBD)-tagged ToxN with untagged TifARB69 and toxI and then purified ToxN and its binding partners by running the cell lysate through chitin agarose resin. We analyzed the purified proteins by size exclusion chromatography, again assessing nucleic acid and protein levels in our eluent (Figure 6A). We observed two major peaks – one in the void volume (peak 1) and one centered at ∼15.5 ml (peak 2)—both of which had an A260/A280 ratio close to 2. We analyzed the contents of each peak on an RNA gel. Peak 1, which resembled the copurification of ToxN and TifA alone, contained many high-molecular weight RNAs. In peak 2, which ran in the same location as purified ToxIN complex (Supplementary Figure S7A), the predominant RNA species was the same size as processed toxI (36 nucleotides) (Figure 6B). To test if TifA was associated with one of these peaks, we repeated the CBD-tagged ToxN purification from cells expressing toxI and FLAG-tagged TifARB69 and then monitored the location of TifARB69-FLAG via western blotting (Supplementary Figure S7B). TifARB69-FLAG signal co-localized with the first, but not the second peak, indicating that TifA does not interact with heterohexameric ToxIN complexes. Taken together, these results indicate that toxI monomer and TifA interactions with ToxN are likely mutually exclusive, consistent with the AlphaFold prediction that TifA and toxI contact overlapping regions of ToxN (Figures 2D, 6C). Thus, TifA likely associates with ToxN after ToxN has dissociated from processed toxI but leads to formation of an RNA-protein complex that renders ToxN inert.
Next, we used RNA sequencing (RNA-seq) to identify the RNA species associated with CBD-tagged ToxN purified from cells expressing tagged ToxN, toxI, and TifA. We sought RNAs that were enriched following pull-down of ToxN co-expressed with toxI and TifARB69 (hereafter called the TifA WT sample) relative to the cell lysate from that purification and relative to a purification of ToxN co-expressed with toxI and TifARB69(K55A R56A R59A) (hereafter called the TifA mutant sample) that includes the TifA variant unable to bind RNA. We performed and sequenced two biological replicates of each pulldown, and sequencing reads were highly reproducible between biological replicates (Supplementary Figure S7C).
There was no evident enrichment of ToxN substrates in the TifA WT sample, indicating that ToxN cleavage of target transcripts does not guide the specificity of the ToxN-TifA-RNA complex (Figure 6D). In fact, the only RNA highly enriched in the TifA WT complex relative to either the TifA WT sample lysate or the TifA mutant sample eluent was the 16S rRNA (Figure 6D, E, Supplementary Figure S7D, E), which is transcribed from seven loci (rrsA-G) in the E. coli genome and comprises the RNA portion of the small ribosomal subunit. Because the sequences for the 16S rRNA loci are highly similar (>99% sequence similarity in E. coli BL21), RNA-seq reads for 16S rRNA were randomly distributed among the seven loci during our mapping process. Thus, we were unable to determine if one particular 16S rRNA locus was more highly enriched than others in our pulldown.
The fragment density counts for both the TifA WT sample and the TifA mutant sample (normalized to counts from their respective lysate samples) were roughly uniform across the 16S rRNA loci (example of rrsA shown in Figure 6F). However, in both samples, we noticed a depletion in fragment density near an instance of the ToxN cleavage motif (GAAAU) toward the 3′ end of the 16S rRNA, suggesting that ToxN might cleave co-purifying 16S rRNA. Notably, this cleavage valley was deeper in the TifA mutant samples compared to the TifA WT samples, providing additional evidence that TifA is a direct inhibitor of ToxN. To test whether the catalytic activity of ToxN is necessary for its interaction with TifA, we assessed TifAT4 binding to a nuclease-inactive version of ToxN (ToxN(K55A)) during T4 infection. ToxN(K55A) co-immunoprecipitated with TifAT4 with the same efficiency as wild-type ToxN (Supplementary Figure S7F), demonstrating that RNA cleavage by ToxN is not necessary for its sequestration by TifA. In sum, these findings suggest that TifA stabilizes an interaction of ToxN with 16S rRNA, with TifA binding RNA and recruiting ToxN or with TifA tethering ToxN to RNA molecules during ToxN’s target search process (Figure 6G). These stabilizing interactions likely trap ToxN in an inactive state, thereby preventing it from acting as an effective, multiple turnover RNase.
Discussion
Bacteria and phages are locked in an ongoing coevolutionary arms race, with each rapidly evolving mechanisms to subvert or overcome the other (2–4,34). Toxin-antitoxin (TA) systems are widespread antiphage defense elements in bacteria in which activation of the toxin during infection prevents phage replication and propagation through the rest of the bacterial population (6,9). Phages have been shown to overcome TA systems by several means, including by producing bona fide antitoxins from their own genomes and by preventing toxin activation (13,14,25,28,35). We recently discovered a small protein in T4 and T4-like phages, called TifA, that interferes with ToxN-mediated antiphage defense (28). The cognate antitoxin for ToxN is a short RNA molecule, suggesting that TifA must inhibit ToxN differently than toxI.
Here, we found that TifA is a small RNA-binding protein that forms an RNA-protein complex with ToxN when they are co-produced. Our observation that TifA proteins display specificity for certain ToxN homologs suggests that TifA does not function by simply coating RNA to prevent any RNase degradation; instead, its inhibition is likely mediated by specific protein-protein interactions with ToxN. Our AlphaFold predictions for the ToxN-TifA structure, and subsequent characterization of TifA-resistant ToxN mutants and naturally-occurring TifA variants, corroborate this hypothesis. We identified several residues localized in the predicted ToxN-TifA interface that strengthen or weaken TifA inhibition of ToxN, providing evidence for specific molecular interactions between ToxN and TifA. However, AlphaFold predicted several orientations for ToxN and TifA relative to one another, making the identification of precise protein-protein interaction points difficult. One possibility for why AlphaFold did not predict a single, high-confidence structure is that the complex requires the presence of RNA, which was not included in the modeling. Interestingly, two of the mutations that we found in ToxN that allow it to overcome TifA also weaken its interaction with its cognate antitoxin, toxI. Thus, toxIN may face a tradeoff in acquiring mutations that prevent inhibition by TifA, as doing so might also risk liberation of ToxN from toxI in the absence of phage infection.
Based on our results, we propose the following model for TifA inhibition of ToxN, using T4 infection as an example (Figure 6G). Following phage infection, the shutoff of host transcription, including the toxIN locus, leads to degradation of toxI precursors and release of ToxN from processed, monomeric toxI (19). tifA is an early gene in T4, so it is rapidly expressed once the T4 genome is injected into the infected cell. TifA then interacts with activated ToxN to prevent degradation of cellular transcripts, likely by trapping ToxN on 16S rRNA, and possibly other abundant cellular RNAs. In a previous study, we used RNA-seq to identify ToxN targets in uninfected E. coli cells (19). In this context, the 16S rRNA was not cleaved by ToxN, suggesting that TifA may determine the binding specificity of the ToxN-TifA complex. In sum, we propose that TifA effectively prevents ToxN from operating as an efficient, multiple-turnover RNase.
TifA as a phage-encoded antitoxin
In our model for TifA activity, it does not act like a type II protein antitoxin that binds to and neutralizes a cognate toxin in the absence of other cofactors (8). Instead, inhibition involves both a protein (TifA) and RNA. Interactions between toxins, their substrates, and inhibitory factors have been observed for other toxin–antitoxin and antiviral defense systems. For example, in the plasmid maintenance system CcdAB, the toxin CcdB binds to and inhibits DNA gyrase. Its cognate antitoxin, CcdA, can bind to the CcdB-gyrase complex to release gyrase-bound CcdB (36). Similarly, the anti-CRISPR protein AcrIIC1 from a Neisseria meningitidis mobile genetic element inhibits DNA-bound N. meningitidis Cas9 by preventing the conformational changes in the Cas9 active site required for DNA cleavage (37,38). Strikingly, it was recently discovered that the interaction between APOBEC3, a host-encoded antiviral protein, and HIV-1 Vif, an APOBEC3 antagonist, requires RNA as a ‘molecular glue’ to correctly position residues in Vif that inhibit APOBEC3. Analogous to our observation that residues in ToxN that mediate its interactions with TifA are also necessary for complete inhibition by toxI, the APOBEC3–Vif–RNA interface includes residues in APOBEC3 that are required for its restriction of viral replication in the absence of Vif (39). In the case of ToxN-TifA, it remains to be determined exactly how ToxN RNase activity is inhibited in the presence of TifA.
Concluding remarks
The diversity of systems underlying antiphage defense and phage counter-defense is readily apparent; however, the molecular underpinnings of most of these systems remain poorly characterized. Having previously discovered TifA, an anti-toxIN element in T4-like phages, we investigated the mechanism underlying inhibition of an antiphage TA system by a phage-encoded antitoxin. Our results suggest that the RNA-binding protein TifA inhibits ToxN by sequestering it onto abundant RNAs in the cell, most notably the 16S rRNA, thereby rendering it inert. Thus, our results reveal a novel mechanism underlying phage inhibition of an RNase toxin and emphasize the ongoing arms race between T4-like phages and toxIN systems.
Supplementary Material
Acknowledgements
We thank R.S. Isaac, D. Saxton and P. Culviner for comments on the manuscript; B. Wang for assistance with protein purification and in vitro biochemical experiment; R.S. Isaac for assistance with fluorescence polarization assays; and all members of the Laub lab for helpful discussions.
Author contributions: C.K.G., G.I.C.T., S.S., K.C., C.R.D. and D.A.G. generated reagents and performed experiments. C.K.G. and S.S. performed bioinformatic analyses. C.K.G. performed RNA-seq analysis. C.K.G., G.I.C.T., S.S. and C.R.D. designed experiments and analyzed data. C.K.G. and M.T.L. prepared figures and wrote the manuscript with input from co-authors.
Contributor Information
Chantal K Guegler, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Department of Genetics, Harvard Medical School, Boston, MA 02115, USA.
Gabriella I C Teodoro, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Sriram Srikant, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Keerthana Chetlapalli, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Christopher R Doering, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Dia A Ghose, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Michael T Laub, Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA; Howard Hughes Medical Institute, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.
Data availability
RNA sequencing data generated in this study is available in the Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/ under accession code GSE234211. Funding for open access charge: HHMI funds.
Supplementary data
Supplementary Data are available at NAR Online.
Funding
NSF predoctoral graduate fellowship (to C.K.G.); Howard Hughes Medical Institute Gilliam Fellowship (to C.R.D.); C.K.G. is a Merck Fellow of the Jane Coffin Childs Fund for Medical Research; S.S. is a Howard Hughes Medical Institute Awardee of the Life Sciences Research Foundation; M.T.L. is an Investigator of the Howard Hughes Medical Institute. Funding for open access charge: HHMI funds.
Conflict of interest statement. None declared.
References
- 1. Labrie S.J., Samson J.E., Moineau S.. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010; 8:317–327. [DOI] [PubMed] [Google Scholar]
- 2. Tal N., Sorek R.. SnapShot: bacterial immunity. Cell. 2022; 185:578–578. [DOI] [PubMed] [Google Scholar]
- 3. Bernheim A., Sorek R.. The pan-immune system of bacteria: antiviral defence as a community resource. Nat. Rev. Microbiol. 2020; 18:113–119. [DOI] [PubMed] [Google Scholar]
- 4. Hampton H.G., Watson B.N.J., Fineran P.C.. The arms race between bacteria and their phage foes. Nature. 2020; 577:327–336. [DOI] [PubMed] [Google Scholar]
- 5. Samson J.E., Magadán A.H., Sabri M., Moineau S.. Revenge of the phages: defeating bacterial defences. Nat. Rev. Microbiol. 2013; 11:675–687. [DOI] [PubMed] [Google Scholar]
- 6. Harms A., Brodersen D.E., Mitarai N., Gerdes K.. Toxins, targets, and triggers: an overview of toxin–antitoxin biology. Mol. Cell. 2018; 70:768–784. [DOI] [PubMed] [Google Scholar]
- 7. Song S., Wood T.K.. A primary physiological role of toxin/antitoxin systems is phage inhibition. Front Microbiol. 2020; 11:1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamaguchi Y., Park J.-H., Inouye M.. Toxin-antitoxin systems in bacteria and archaea. Genetics. 2011; 45:61–79. [DOI] [PubMed] [Google Scholar]
- 9. LeRoux M., Laub M.T.. Toxin-antitoxin systems as phage defense elements. Annu. Rev. Microbiol. 2022; 76:21–43. [DOI] [PubMed] [Google Scholar]
- 10. Dy R.L., Przybilski R., Semeijn K., Salmond G.P.C., Fineran P.C.. A widespread bacteriophage abortive infection system functions through a type IV toxin–antitoxin mechanism. Nucleic Acids Res. 2014; 42:4590–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fineran P.C., Blower T.R., Foulds I.J., Humphreys D.P., Lilley K.S., Salmond G.P.C.. The phage abortive infection system, ToxIN, functions as a protein–RNA toxin–antitoxin pair. Proc Natl. Acad. Sci. U.S.A. 2009; 106:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koga M., Otsuka Y., Lemire S., Yonesaki T.. Escherichia coli rnlA and rnlB compose a novel toxin–antitoxin system. 2011; 187:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang T., Tamman H., Wallant K.C.’t., Kurata T., LeRoux M., Srikant S., Brodiazhenko T., Cepauskas A., Talavera A., Martens C.et al.. Direct activation of a bacterial innate immune system by a viral capsid protein. Nature. 2022; 612:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. LeRoux M., Srikant S., Teodoro G.I.C., Zhang T., Littlehale M.L., Doron S., Badiee M., Leung A.K.L., Sorek R., Laub M.T.. The DarTG toxin–antitoxin system provides phage defence by ADP-ribosylating viral DNA. Nat Microbiol. 2022; 7:1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pecota D.C., Wood T.K.. Exclusion of T4 phage by the hok/sok killer locus from plasmid R1. J. Bacteriol. 1996; 178:2044–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vassallo C.N., Doering C.R., Littlehale M.L., Teodoro G.I.C., Laub M.T.. A functional selection reveals previously undetected anti-phage defence systems in the E. coli pangenome. Nat Microbiol. 2022; 7:1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelly A., Arrowsmith T.J., Went S.C., Blower T.R.. Toxin–antitoxin systems as mediators of phage defence and the implications for abortive infection. Curr. Opin. Microbiol. 2023; 73:102293. [DOI] [PubMed] [Google Scholar]
- 18. Song S., Wood T.K.. Post-segregational killing and phage inhibition are not mediated by cell death through toxin/antitoxin systems. Front. Microbiol. 2018; 9:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guegler C.K., Laub M.T.. Shutoff of host transcription triggers a toxin–antitoxin system to cleave phage RNA and abort infection. Mol. Cell. 2021; 81:2361–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blower T.R., Pei X.Y., Short F.L., Fineran P.C., Humphreys D.P., Luisi B.F., Salmond G.P.C.. A processed noncoding RNA regulates an altruistic bacterial antiviral system. Nat. Struct. Mol. Biol. 2011; 18:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blower T.R., Fineran P.C., Johnson M.J., Toth I.K., Humphreys D.P., Salmond G.P.C.. Mutagenesis and functional characterization of the RNA and protein components of the toxIN abortive infection and toxin–antitoxin locus of Erwinia▿. J. Bacteriol. 2009; 191:6029–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emond E., Dion E., Walker S.A., Vedamuthu E.R., Kondo J.K., Moineau S.. AbiQ, an abortive infection mechanism from Lactococcus lactis. Appl. Environ. Microb. 1998; 64:4748–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blower T.R., Short F.L., Rao F., Mizuguchi K., Pei X.Y., Fineran P.C., Luisi B.F., Salmond G.P.C.. Identification and classification of bacterial type III toxin–antitoxin systems encoded in chromosomal and plasmid genomes. Nucleic Acids Res. 2012; 40:6158–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goeders N., Chai R., Chen B., Day A., Salmond G.P.C.. Structure, evolution, and functions of bacterial type III toxin–antitoxin systems. Toxins. 2016; 8:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blower T.R., Evans T.J., Przybilski R., Fineran P.C., Salmond G.P.C.. Viral evasion of a bacterial suicide system by RNA–Based molecular mimicry enables infectious altruism. PLos Genet. 2012; 8:e1003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blower T.R., Chai R., Przybilski R., Chindhy S., Fang X., Kidman S.E., Tan H., Luisi B.F., Fineran P.C., Salmond G.P.C.. Evolution of pectobacterium bacteriophage ΦM1 to escape two bifunctional type III toxin–antitoxin and abortive infection systems through mutations in a single viral gene. Appl. Environ. Microb. 2017; 83:e03229-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen B., Akusobi C., Fang X., Salmond G.P.C.. Environmental T4-Family bacteriophages evolve to escape abortive infection via multiple routes in a bacterial host employing “altruistic suicide” through type III toxin–antitoxin systems. Front. Microbiol. 2017; 8:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Srikant S., Guegler C.K., Laub M.T.. The evolution of a counter-defense mechanism in a virus constrains its host range. Elife. 2022; 11:e79549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Srikant S., Gaudet R., Murray A.W.. Selecting for altered substrate specificity reveals the evolutionary flexibility of ATP-binding cassette transporters. Curr. Biol. 2020; 30:1689–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ram Y., Dellus-Gur E., Bibi M., Karkare K., Obolski U., Feldman M.W., Cooper T.F., Berman J., Hadany L.. Predicting microbial growth in a mixed culture from growth curve data. Proc National Acad Sci. 2019; 116:14698–14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Culviner P.H., Guegler C.K., Laub M.T.. A simple, cost-effective, and robust method for rRNA depletion in RNA-sequencing studies. Mbio. 2020; 11:e00010-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A.et al.. Highly accurate protein structure prediction with AlphaFold. Nature. 2021; 596:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takahashi H., Coppo A., Manzi A., Martire G., Pulitzer J.F.. Design of a system of conditional lethal mutations (tab/k/com) affecting protein-protein interactions in bacteriophage T4-infected Escherichia coli. J. Mol. Biol. 1975; 96:563–578. [DOI] [PubMed] [Google Scholar]
- 34. Stern A., Sorek R.. The phage-host arms race: shaping the evolution of microbes. Bioessays. 2011; 33:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Otsuka Y., Yonesaki T.. Dmd of bacteriophage T4 functions as an antitoxin against Escherichia coli LsoA and RnlA toxins. Mol. Microbiol. 2012; 83:669–681. [DOI] [PubMed] [Google Scholar]
- 36. Jonge N.D., Garcia-Pino A., Buts L., Haesaerts S., Charlier D., Zangger K., Wyns L., Greve H.D., Loris R.. Rejuvenation of CcdB-poisoned gyrase by an intrinsically disordered protein domain. Mol. Cell. 2009; 35:154–163. [DOI] [PubMed] [Google Scholar]
- 37. Harrington L.B., Doxzen K.W., Ma E., Liu J.-J., Knott G.J., Edraki A., Garcia B., Amrani N., Chen J.S., Cofsky J.C.et al.. A broad-spectrum inhibitor of CRISPR-Cas9. Cell. 2017; 170:1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pawluk A., Amrani N., Zhang Y., Garcia B., Hidalgo-Reyes Y., Lee J., Edraki A., Shah M., Sontheimer E.J., Maxwell K.L.et al.. Naturally occurring off-switches for CRISPR-Cas9. Cell. 2016; 167:1829–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Y.-L., Langley C.A., Azumaya C.M., Echeverria I., Chesarino N.M., Emerman M., Cheng Y., Gross J.D.. The structural basis for HIV-1 Vif antagonism of human APOBEC3G. Nature. 2023; 615:728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data generated in this study is available in the Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/ under accession code GSE234211. Funding for open access charge: HHMI funds.