Abstract
Antibiotic failure is one of the most worrisome threats to global health. Among the new therapeutic efforts that are being explored, the use of bacteriophages (viruses that kill bacteria), also known as ‘phages’, is being extensively studied as a strategy to target bacterial pathogens. However, one of the main drawbacks of phage therapy is the plethora of defence mechanisms that bacteria use to defend themselves against phages. This review aims to summarize the therapeutic approaches that are being evaluated to overcome the bacterial defence systems, including the most innovative therapeutic approaches applied: circumvention of phage receptor mutations; modification of prophages; targeting of CRISPR-Cas systems and the biofilm matrix; engineering of safer and more efficacious phages; and inhibition of the anti-persister strategies used by bacteria.
Introduction
Antimicrobial resistance causes almost 5 million deaths annually worldwide and is predicted to become the leading cause of death.1 Bacterial antibiotic resistance is driven by natural evolution, and antibiotic resistance genes are common, even in DNA isolated from ancient sediments.1–3 However, the massive use of antibacterial agents over decades, together with their release in untreated wastewater, exerts a selective pressure that has led to a global health crisis and could lead to an era without effective antibiotics.4,5
Throughout history, numerous different outbreaks of severe infectious diseases have been caused by heterogeneous pathogens (belonging to different phyla) that have acquired some resistance mechanisms. Examples of such bacterial pathogens include carbapenem-resistant Klebsiella pneumoniae,6,7 colistin-resistant plasmid-mediated Enterobacteriaceae,8–10 XDR Pseudomonas aeruginosa,11 carbapenem-resistant Acinetobacter baumannii,12 MDR Stenotrophomonas maltophilia,13–15 MDR Burkholderia cepacia13 and MRSA.16,17 The latter are some of the most common examples, but there are many more.
Although some researchers remain optimistic about a renewed antibiotic pipeline, vaccines or antibody–antibiotic conjugates,18 every time a new antibiotic is introduced in the clinical setting, bacterial resistance arises against it as the result of accelerated evolution.19
One of the most recently discovered antibiotics, teixobactin, was detected in 2015 by screening uncultured soil bacteria by using iChip technology.20
Teixobactin is a depsipeptide synthesized by the soil bacterium Eleftheria terrae and has shown extremely good efficacy and toxicity profiles in vivo against Gram-positive bacterial infections.21
To date, the resistance reported against teixobactin is slow and very costly in terms of fitness;22 however, such successes are rare. The restoration of old strategies to combat resistant pathogens (such as phage therapy), together with the use of non-antibiotic compounds [antimicrobial peptides (AMPs) and repurposed drugs], is gaining much attention.23,24
Bacteriophages
Bacteriophages, also called phages, are viruses that infect bacteria. They are the most abundant organisms on Earth, being found in all environments including the soil, ocean, sewage and mammalian gut. It is thought that there are around 10 phages per bacterium, yielding an estimated 1031 viral particles.25 Phages are generally classified according to their life cycle into lytic phages and lysogenic phages, although other types of phage infection are also possible, such as chronic infection or pseudolysogeny.26,27 Lytic phages attach to the bacterial surface, inject their DNA, use bacterial machinery to replicate and subsequently lyse the cells to release multiple viral particles; by contrast, lysogenic phages inject their DNA and integrate it into the bacterial genome, so that it is passed on to progeny, where it may act as a vector for horizontal gene transfer (HGT). However, lysogenic phages can be induced and released from cells under stress situations, provoking cell lysis.28
Bacteria–phage interactions: an arms race
Bacteria and phages are in a permanent state of co-evolution referred to as an arms race, because when one develops a mechanism to evade the other, this causes the latter to adapt and avoid this defence system (Figure 1). Thus, bacteria have evolved defence mechanisms to protect themselves from predators, while phages, in turn, have evolved counterdefence strategies to evade these systems.29–32 The main mechanisms of defence that bacteria have developed against phages are summarized in Table 1, and the phage resistance mechanisms are summarized in Table 2.
Figure 1.

Original digital illustration (made in Adobe Photoshop from a sketch) representing the bacteria–phage ‘arms race’, a co-evolutionary state in which one develops a mechanism to evade the other and adapt. Bacteria display defence mechanisms to protect themselves from phages, while these evolve counterdefence strategies to evade these systems.
Table 1.
Bacterial defence mechanisms against phages
| General mechanism | Specific mechanism | Description | Ref |
|---|---|---|---|
| Adsorption resistance | Modification of bacterial receptor | Either by (i) mutations of cell surface receptors; (ii) inhibitors that compete with the phage; or (iii) alteration of polysaccharide patterns that hide the receptor molecules. | 29 |
| Biofilms | Adsorption of the phage to its bacterial receptor becomes restricted when bacteria form biofilms. | 33 | |
| Outer-membrane vesicles (OMVs) | Fragments of the outer membrane of Gram-negative bacteria containing phage receptors may be secreted and act as a bait, leading to phage adsorption and resulting in less successful infections. | 34–36 | |
| Blocked uptake | Prophages | SIE mechanism: prevention of infection by other phages. | 37 |
| Small molecules | Anthracyclines, aminoglycosides and viperins: molecules with anti-phage properties, can be secreted by bacteria. | 38 | |
| Restriction | Restriction modification | Methyltransferase adds methyl groups to the host DNA to distinguish it from the foreign material (especially viral DNA), and the restriction endonuclease recognizes the viral DNA and cuts it. | 39 |
| CRISPR-Cas | Bacteria capture short sequences (protospacers) of invading phages and integrate them in their chromosome (CRISPR array). For subsequent phage infections, the system activates and degrades the phage DNA. | 40 | |
| Other mechanisms | Abortive infection systems and CBASS | Cells prevent the release of functional virions at the expense of their host cell survival (e.g. inducing programmed cell death). | 41,42 |
| TA | The bacterial toxin induces a global metabolic latency so that phage infection does not progress (no successful phage replication or assembly). | 43,44 | |
| QS | Phage adsorption is reduced in the presence of some auto-inducers of the QS network. | 45,46 |
Table 2.
Phage evasion mechanisms against bacterial defences
| Mechanism | Description | Target | References |
|---|---|---|---|
| Alternative adsorption to bacterial surface | Modification of receptor binding protein (RBP) by acquiring mutations that allow recognition of the mutated version of the receptor, or sometimes even a completely different receptor. | Receptor | 47 |
| Bacterial capsule degradation | Enzymes like depolymerases allow phages to degrade the extracellular polysaccharides present in the bacterial capsules. In some phages, the RBPs themselves have intrinsic depolymerase activity. | Capsule | 48,49 |
| Evasion of Abi | Mutation of specific genes in the phage. | Abi systems | 50 |
| Evasion of restriction modification | Reduction in the number or masking of restriction targets incorporating modified bases. | RM systems | 51,52 |
| Evasion of TA | Inhibition of a protease that would normally cleave the antitoxin or encoding own antitoxin protein. | TA systems | 53,54 |
| Evasion of CRISPR-Cas | (i) Single nucleotide substitution; (ii) complete deletion in the protospacer region or in the conserved motif adjacent to PAMa; (iii) anti-CRISPR systems (acr-aca). | CRISPR-Cas systems | 55 |
aPAM, protospacer adjacent motif.
The use of phages for therapeutic purposes is of renewed interest in Western countries, where it fell into disuse in the 1940s with the advent of commercial antibiotics. Due to the rising antibiotic resistance rates, the use of phages in therapy has regained interest. Currently, the following minimum criteria are required for the use of phages for therapeutic purposes: (i) the phages must be strictly lytic in nature, lacking genes for lysogeny such as integrases and recombinases; (ii) they must have clear antimicrobial activity against the target pathogen; and (iii) bacterial debris and endotoxins must be removed to below predetermined thresholds of safety.56 Although common in some eastern countries such as Georgia (Eastern Europe), phage therapy was completely abandoned with the advent of antibiotics in Western countries.57 However, phage therapy has shown promising results, good safety profiles and efficacy in some relevant clinical cases,58–61 as discussed below. Importantly, a recent retrospective and observational study focused on 100 consecutive cases of personalized phage therapy in Belgium revealed that more than 77% of difficult-to-treat infections experienced some clinical improvement, with bacterial eradication achieved in 61% of the total cases considered, and only 15% presenting adverse events (among which, 7% were considered mild to moderate).62 Similarly, in 2023, Green et al.63 summarized several cases of patients treated with personalized phage therapy and also concluded that there were no major adverse reactions and phage–antibiotic synergy (PAS) was frequently observed, consistent with the previously mentioned 2023 report by Pirnay et al.62
PAS is a well-known phenomenon consisting of an improved outcome after the combination of phage and antibiotics, relative to their separate effects.64,65
The synergism between conventional antibiotics and a pre-adapted lytic phage (K. pneumoniae phage M1) was made use of to resolve a severe fracture-related infection in a 30-year-old bomb victim in Belgium.65 Pre-adapted phages were considered those mutants with broadest infectivity after 15 rounds of the Appelman protocol, which consists of growing phages iteratively on mostly refractory bacterial isolates, until the adapted phage can lyse the phage-resistant strains.66 Although ceftazidime/avibactam reduced K. pneumoniae bacterial counts in mature biofilms, they did not completely eradicate them, and high doses of the lytic phage M1 alone failed. Nonetheless, combinations of phage M1 and moderate concentrations of ceftazidime/avibactam were significantly more effective, which also suggests a synergistic effect.65 Similarly, P. aeruginosa phage PNM and colistin (0.5 mg/L), aztreonam (8 mg/L) and gentamicin (2 mg/L) displayed strong synergistic activity against one of the P. aeruginosa isolates that caused severe post-liver transplantation sepsis in a toddler.67 A similar synergistic phenomenon was observed with another P. aeruginosa isolate, although the effect was slightly less intense with colistin. Finally, the treatments allowed a second liver transplantation and resulted in complete resolution of the infection.67 The most significant case reports using phages against antibiotic-resistant isolates are summarized in Table 3.
Table 3.
Clinical case reports focusing on phage therapy
| Date and country | Cause of infection | Patient(s) | Pathology/disease | Treatment | Administration route | Clinical outcome | Observation | Reference |
|---|---|---|---|---|---|---|---|---|
| 2011, Australia | P. aeruginosa | 67-year-old female | Urinary tract infection after intra-abdominal resections and pelvic irradiation for adenocarcinoma | 6 lytic phage cocktail | Pyophage #051007 was instilled directly into the bladder every 12 h for 10 days | Resolution of infection and microbiological improvement | No antibiotic- or phage-resistant bacteria arose | 68 |
| 2016, Bangladesh | E. coli | 225 children | Acute bacterial diarrhoea | 11 T4-like phage cocktail and Microgen ColiProteus phage cocktail | Oral, 4 days | Failure | Overgrowth of Streptococcus gallolyticus and Streptococcus salivarius in the stool | 69 |
| 2017, USA | MDR A. baumannii | 68-year-old | Diabetes, necrotizing pancreatitis (MDR A. baumannii-infected pancreatic pseudocyst) | Cocktail of 4 anti A. baumannii phages | Intracavitary and IV administration | Resolution of the patient’s infection | Increased antibiotic sensitivity when phage and antibiotics were simultaneously administered | 70 |
| 2018, USA | MDR A. baumannii | 77-year-old male | Postoperative craniectomy complication: cerebritis infection | Lytic A. baumannii phage | IV, 98 doses | The patient died | High degree of severity of underlying assault injury | 71 |
| 2018, Belgium | XDR P. aeruginosa | Male toddler | Sepsis post liver transplantation | Phage–antibiotic combination | IV therapy for 86 days | Complete resolution of all infections, second liver transplantation | Resistant phage mutants but not sufficient to re-establish proper infection | 67 |
| 2018, USA | Bacteraemia caused by P. aeruginosa | 2-year-old boy | DiGeorge syndrome, congenital heart disease | 2 lytic phage cocktail | IV every 6 h during 36 h | Death by end-stage cardiac failure | Repeated blood sterilization after each introduction of phage therapy (therapy had to be interrupted) | 72 |
| 2019, USA | MDR P. aeruginosa and MDR Burkholderia dolosa | 67-year-old male, 57-year-old female and 28-year-old female | Three lung transplant recipients, the third one with cystic fibrosis | Cocktails of P. aeruginosa phages in combination with antibiotics; 1 lytic phage against B. dolosa | Nebulized and IV, both antibiotics and phages | Resolution of patient 1 and 2 infections; patient 3 died due to liver and kidney failure | Resistance arose after suspension of phage therapy; challenge in finding lytic phages against B. dolosa | 73 |
| 2019, USA | M. abscessus | 15-year-old | Cystic fibrosis with a disseminated M. abscessus infection | Three-phage cocktail | IV phage treatment, every 12 h for at least 32 weeks | Sternal wound closure, improved liver function, substantial resolution of infected skin nodules | Genome engineering | 74 |
| 2020, USA | Mycobacterium chelonae | 56-year-old man | Refractory disseminated cutaneous M. chelonae infection | Muddy phage | IV | Skin lesions improved and no evidence of granulomas | No resistance observed | 75 |
| 2022, Belgium | PDR K. pneumoniae | 30-year-old bombing victim | Fracture-related antibiotic-resistant infection | Phage M1 and ceftazidime/avibactam, tigecycline and ciprofloxacin | Catheter in place, locally administered 3 times a day for 5 days | Skin graft vascularized, sinus tract closed and dry, no signs of infection, gain of muscle | Phage-neutralizing antibodies on Days 8–18 post-phage application (unlikely to interfere) | 65 |
| 2023, Spain |
MDR P. aeruginosa | 58-year-old man | Infection of axillo-bifemoral bypass graft | Phages PT07, 14/01, PNM and ceftazidime/avibactam | IV 7 days | Failure | Decrease in the MIC of the antimicrobials | 76 |
PDR, pandrug resistant.
The strategies of phage training (pre-adaptation) and PAS do not always produce the intended results, which is why it is necessary to look for methods that specifically circumvent bacterial defence mechanisms. This review summarizes some of the most relevant studies in this regard, in order to illuminate this increasingly vast field, while considering the defence mechanisms in relation to the latest advances in phage therapy.
Innovative strategies in phage therapy: counteracting bacterial defence mechanisms
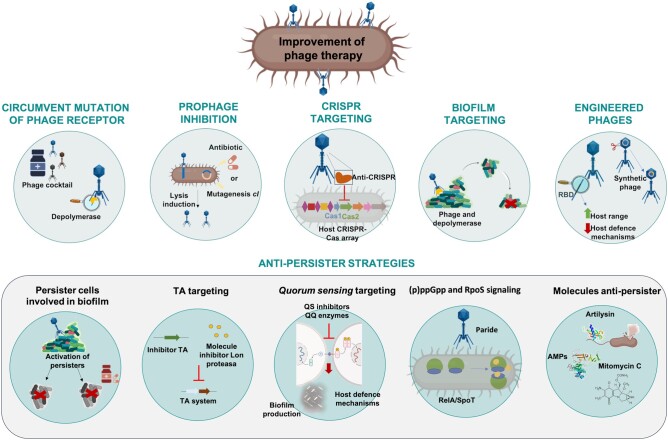
One of the greatest disadvantages of phage therapy is possibly the frequent and rapid generation of phage-resistant bacterial mutants during treatment.77 In this regard, the strategy of counteracting these phage defence systems may be a useful way of enhancing the therapeutic outcomes of phages in clinical settings (Figure 2).
Figure 2.
Strategies to improve the efficacy of phage therapy, using bacterial defence mechanisms to phage infection as a target. RBD, receptor-binding domain.
Circumventing mutation of phage receptors
Binding between a phage and its receptor is a critical point in phage infection. However, bacteria have developed defence mechanisms to block phage binding. To circumvent these mechanisms and obtain better results in phage therapy, phage cocktails can be optimized.78 For example, phage combinations that target different receptors can be prioritized, thereby reducing the likelihood of resistance emergence. For example, Yang et al.79 designed a cocktail for treating P. aeruginosa that contained two phages, both with a different receptor, one targeting the O antigen and the other targeting a truncated form of the O antigen that the pathogen produced when it became resistant to the initial O antigen-targeting phage. One of the phage mutants (PaoP5-m1) was found to be excellent for eliminating P. aeruginosa mutants with truncated O-antigen structures, as it was able to adsorb and infect all of the strains, regardless of their O-antigen structure. On the other hand, phages may encode enzymes capable of crossing barriers, such as biofilms, capsule layers or the outside of the cell, or the LPSs of the outer membrane. These proteins are usually located in tail fibres, tail spikes or baseplates. Depolymerases, for example, are a type of enzyme used by phages to access receptors hidden by polysaccharides, or in cases where the polysaccharide chain is the receptor itself, to cleave it, thus stabilizing the binding.80
Prophage modification
Although phage therapy uses lytic phages, most bacteria have prophages in their genomes, often resulting in a well-documented phage resistance mechanism known as ‘superinfection exclusion’ (SIE).37,81 In this situation, a prophage residing in a host cell prevents infection by other similar phage by blocking injection of DNA.82 However, the presence of prophages in the genome is not always related to SIE defence mechanisms.81,83,84 Many researchers have claimed that the competitive advantage of lysogens over prophage-free competitors reflects the mutualistic (rather than parasitic) relationship between prophages and bacteria.85–87
An interesting approach that paves the way for alternative research aimed at a better understanding of phage therapy is to target these prophages, more specifically using the gene cI, repressor of the lytic cycle, as a therapeutic target. Disruption of the repressor-operator cI by interaction with a small molecule leads to activation of the lytic phase in prophages, thus allowing them to infect their non-lysogenic counterparts and leading to their elimination.88
The conversion of a lysogenic phage into a lytic phage displaying activity against multiple clinical isolates of A. baumannii is also an example of the use of engineered phages for therapeutic purposes.89 In this research, the authors combined the mutant phage with subinhibitory concentrations of different antibiotics and observed a decrease in the frequency of occurrence of phage-resistant bacteria; they also found that combinations including the converted lytic phage increased the survival of infected Galleria mellonella larvae. Along the same line, a three-phage cocktail composed of engineered phages was administered IV every 12 h for at least 32 weeks to a 15-year-old cystic fibrosis patient with a disseminated Mycobacterium abscessus infection.74 Importantly, one of these phages (ZoeJ) was lysogenic and converted into a lytic phage by mutation of its repressor gene. This M. abscessus isolate was resistant to all antibiotics tested: clarithromycin, amikacin, tobramycin, ciprofloxacin, moxifloxacin, cefoxitin, co-trimoxazole, doxycycline and linezolid. The phage treatment resulted in a drastic clinical improvement at all levels: sternal wound closure; enhanced liver and lung functions; resolution of infected skin nodules; and weight increase. No adverse effects were observed after this first use of therapeutic phages to treat a human mycobacterial infection.74
Prophage induction therapy (a term coined by Lakshminarasimhan in 2022) can potentially be applied to clinical settings to target antibiotic-resistant strains of bacteria. Some researchers have investigated this phenomenon in the gut environment and concluded that medications (including non-antibiotic drugs) inhibiting bacterial growth led to an increase in phage particles due to prophage induction.90 Importantly, the authors considered this factor an important driver of phage–bacteria dynamics in the gut. One advantage of the prophage induction therapy versus the administration of exogenous lytic phages is that the viral particles released from the pathogenic bacteria will be highly localized, with lower titres than used for phage therapy, thus minimizing activation of the immune response.88
Clustered regularly interspaced short palindromic repeats (CRISPR-Cas) targeting
CRISPR-Cas systems are the only adaptive immune system observed to date in the prokaryotic world. These are composed of short repeated sequences where spacers become intercalated, conforming the CRISPR-array; this is usually followed by the encoding sequences of CRISPR-associated endonucleases (Cas). Spacer sequences are phage DNA fragments that are cut and integrated into the bacterial CRISPR array, so that bacteria can recognize future infections by phages that they have previously encountered and degrade the DNA of the phages via the Cas endonucleases.40 The main way that phages evade prokaryotic CRISPR-Cas immunity is by using anti-CRISPR proteins. These basically consist of Acr proteins (typically small proteins of 80–150 amino acids) that inhibit bacterial CRISPR-Cas activity by binding directly to the Cas protein, thereby inactivating it, so that the phages can successfully replicate in the bacterial host.91 Acr proteins and the Aca (Acr-associated) proteins work via diverse mechanisms to inhibit critical steps of CRISPR immunity, including cas gene expression,92 assembly of CRISPR ribonucleoprotein complexes,93,94 recognition of target nucleic acids,95,96 and recruitment of effector nucleases.95 Therefore, the use of phages carrying specific acr (or other CRISPR-Cas evasion mechanisms) could be a promising therapeutic approach against MDR bacteria, and further research is needed in this regard.97
Biofilm targeting
As previously mentioned, biofilm populations are particularly prone to being resistant to antimicrobials and phage attack. Under most conditions, biofilms will allow phage-susceptible bacteria to be protected from phage exposure, if they are growing alongside other cells that are phage resistant; this phenomenon has implications regarding the ecology of phage–bacteria interactions, as well as in the development of phage-based antimicrobial therapeutics.98 In turn, some phages possess genes coding for extracellular polysaccharide depolymerases that can specifically degrade the polysaccharidic components of biofilms and facilitate the access of phages to deeper layers.99
The interaction between phages and bacteria depends on the ability of the phage particles to diffuse through the biofilm, in which cells aggregate and adhere, sometimes hiding phage receptors.98,100 One interesting example of how a bacteriophage can adapt to biofilm-growing bacteria is the study conducted by Blasco et al.101 in 2022, in which phages of A. baumannii exhibited genomic rearrangement: 10 ORFs were lost and four new ORFs were produced, all of them encoding tail proteins. As a result of this recombination event, a depolymerase-expressing phenotype was visible in 81% of the strains tested. In the same study, a phage cocktail was made with this mutated and adapted phage, together with a phage known to have a depolymerase (B3), and strong activity against 24-h-old biofilms (measured by cfu—enumeration and crystal violet staining) was observed.
Tkhilaishvili et al.102 tested the anti-biofilm activity of a Staphylococcus aureus-specific phage, Sb-1, and observed that Sb-1 degraded the extracellular matrix formed after 24 h and also targeted persister cells. These researchers assessed the minimum biofilm eradicating concentration (MBEC) and performed confocal laser scanning microscopy (CLSM), which revealed that tail enzymes present in Sb-1 degraded the extracellular polysaccharide component of the matrix of S. aureus ATCC 43300. This is consistent with the findings of Son et al.,103 who reported that a depolymerase present in the lytic S. aureus SAP-2 phage was able to disrupt 48-h-old biofilms [evaluated after safranin staining and scanning electronic microscopy (SEM)]. In another study, Gutiérrez et al.104 reported that lytic phages were a promising option for fighting biofilm formation in staphylococcal infections. In fact, the results of the study confirmed that lytic phages disrupted 4-h-old biofilms, measured by quantification of cfu and crystal violet staining. With the same pathogen, Alves et al.105 combined the use of the S. aureus phage K and a newly isolated phage, DRA88, and observed a reduction in growth of a 48-h-old S. aureus biofilm, as observe by crystal violet staining. Rahman et al.106 combined the phage SAP-26 with erythromycin, vancomycin and rifampicin to treat 24-h-old S. aureus biofilms and obtained a reduction of approximately 28% in the biofilm, as measured by viable cell count, with SAP-26 alone. Erythromycin and vancomycin decreased viable cell counts by 25% and 17%, respectively. However, treatment of the biofilm with the combination of phage and rifampicin yielded a 65% reduction in growth. The combinations of phage–azithromycin and phage–vancomycin reduced the cell counts by 60% and 40%, respectively, after 24 h.107
Another study focusing on the eradication of in vitro biofilms using phages is that conducted by Akturk et al.,108 in which they generated dual-species (S. aureus and P. aeruginosa) 24-h-old biofilms in an in vitro artificial dermis model and treated it with specific phages targeting these pathogens (SAFA and EPA1, respectively) and gentamicin. The most effective treatment concerning reduction in viable cells was obtained after multiple doses of EPA1 + SAFA + gentamicin, whereas the lowest reduction was produced by application of phages only, followed by another dose of the combined phage treatment.
Many studies have revealed the potential use of phages to eradicate biofilm formation by MDR strains.107 For example, Khalifa et al.109 evaluated the lytic activity of an isolated phage from sewage water, EFDG1, against various Enterococcus faecalis and Enterococcus faecium strains, and observed effective activity against planktonic and 2-week-old biofilms, measured by crystal violet staining and CLSM. Importantly, this phage acted on various clinical isolates regardless of their antibiotic resistance profile. In addition, the EFDG1 phage efficiently prevented ex vivo E. faecalis root canal infection, which may be important to prevent persistent infections associated with root canal treatment failure.
Another example is the study conducted by Lehman et al.,110 who evaluated the effect of pretreating hydrogel-coated silicone catheters with mixtures of P. aeruginosa and Proteus mirabilis phages. The authors used a multiday continuous-flow in vitro model with artificial urine medium, in which they produced single- and dual-species biofilms during 96 h. They obtained a 2–4 log reduction in biofilm counts for both species over a period of 48 h. The results of this study suggest that pretreatment of catheters with phage cocktails can significantly reduce mixed-species biofilm formation. In a similar way, Fu et al.111 showed the potential of a phage cocktail to prevent P. aeruginosa biofilms forming on hydrogel-coated catheters in an in vitro model system. The authors observed a reduction in the biofilm viable counts, determined by cfu counts and SEM. Both studies suggest the potential of applying phage cocktails to the surfaces of indwelling medical devices to minimize biofilm formation.110,111
Furthermore, Pallavali et al.112 assessed the effect of lytic phages on 96-h-old biofilms of P. aeruginosa, K. pneumoniae, Escherichia coli and S. aureus, and observed a reduction of nearly 80% in the biomass of biofilms, quantified after cfu enumeration and crystal violet staining. Moreover, Alves et al.113 showed that a novel bacteriophage cocktail reduced and dispersed P. aeruginosa biofilms under static and flow conditions, therefore having a therapeutic ability to control P. aeruginosa infections. For the static model, after contact for 4 h with the phage suspension at an moi of 10, more than 95% of 48-h-old biofilm biomass was eliminated, as measured by crystal violet staining. On the other hand, in the 48-h-old biofilm flow model, slower activity was observed by CLSM; however, 48 h after addition of phage cocktail the biofilm had dispersed. Another example is the case of the phages ϕMR299-2 and ϕNH-4, which were able to eliminate the 24-h-old biofilm of P. aeruginosa in vitro (using cystic fibrosis lung airway cells) and in vivo (using a murine lung model).114,115 Furthermore, Lu et al.116 reported enhanced dispersion of biofilms with engineered phages, designed from the T7 E. coli phage expressing the dispersin B (DspB). The engineered enzymatic phage reduced bacterial biofilm cell counts by 99.97%.
Other studies have demonstrated the potential of a combined therapy to eradicate biofilms. For instance, the E. coli phage T4 was used in combination with the antibiotic cefotaxime to eradicate 24-h-old E. coli biofilms. The results revealed that the addition of T4 reduced the MBEC of cefotaxime against E. coli biofilms by between 2- and 8-fold, indicating that the combination of T4 and cefotaxime significantly enhanced biofilm eradication.117 In a similar way, 8-day-old K. pneumoniae biofilms were also eradicated using a combination of phage at an moi of 0.01 with amoxicillin (512 µg/mL). The results of this study showed a significant reduction in the bacterial counts in biofilms after application of combined therapy.115 In addition, a 12-h-old biofilm of K. pneumoniae was treated by a combination of phage KPO1K2 at an moi of 1 and ciprofloxacin (1 mg/mL), and no significant differences in biofilm removal (quantified by cfu counts) were obtained. However, the combined treatment significantly prevented the emergence of resistance.118 Other groups used the phage T4 of E. coli and PB-1 of P. aeruginosa in combination with tobramycin at different concentrations (2 and 0.5 µg/mL, respectively) to remove 48-h-old biofilms. The combined treatment led to a 99.99% decrease in the survival of E. coli biofilms measured by cfu/mL count relative to the use of tobramycin alone. However, the combination of tobramycin and P. aeruginosa phage was as effective as tobramycin alone, although the combination reduced the emergence of antibiotic- and phage-resistant cells.119 Finally, Zhang et al.120 reported that a mixture of RNA phages of P. aeruginosa and chloride reduced the 72-h-old biofilm growth by 94% and removed it in 88% of the cases; the biofilm growth was measured by crystal violet staining.
In general, the main two approaches implemented in regard to the administration of phages to control biofilms are IV bacteriophage therapy and the direct injection of bacteriophages to the site of biofilm on surgical intervention. The first example is documented by Doub et al.,121 but unsuccessful therapeutic outcomes were obtained since the patient continued to have culture-positive S. aureus knee infection, suggesting the inability of IV therapy to eradicate the biofilm infection. The second route of administration is documented by Tkhilaishvili et al.,122,123 who treated a chronic relapsing periprosthetic knee infection and chronic osteomyelitis of the femur, caused by an MDR P. aeruginosa, with a combined treatment between antibiotics (colistin, meropenem and ceftazidime) and phages, locally applied during surgery.
A systematic review analysing 68 articles on this topic included correlation analysis that revealed some phage parameters relevant to the treatment outcome: higher phage concentrations were strongly associated with better biofilm control; and phages with higher burst sizes and shorter latent periods were the best candidates for controlling biofilms.124
The most significant cases of biofilm removal using phages are summarized in Table 4.
Table 4.
Treatments and innovation strategies: biofilm targeting
| Authors (year) | Mechanisms | Species | References |
|---|---|---|---|
| Pearl et al. (2008) Chegini et al. (2020) |
Production of enzymes that degrade extracellular matrix and infect persister cell |
P. aeruginosa
E. coli |
125,126 |
| Eskenazi et al. (2022) | ‘Trojan horses’ strategies where phage was used to destroy biofilm and activate persister cell | K. pneumoniae | 65 |
| Dunsing et al. (2019) | Phage carried depolymerase enzyme to counteract the defence of bacteria | Pantoea stewartia | 127 |
| Blasco et al. (2022) | Phage cocktail with a host-adapted phage Ab105-2ϕΔ404ad and phage vB_AbaP_B3 show strong antibiofilm activity | A. baumannii | 101 |
| Vidakovic et al. (2018) Chaudhry et al. (2020) Darch et al. (2017) |
Phages are retained in biofilms; they confer protection barrier against other bacteria and phages |
E. coli
P. aeruginosa |
128–130 |
| Tkhilaishvili et al. (2018) | Anti-biofilm activity of phage Sb-1; Sb-1 degrades biofilm matrix and target persister cell | S. aureus | 102 |
| Son et al. (2010) | Phage SAP-2 carried depolymerase enzyme able to disrupt the biofilm | S. aureus | 103 |
| Gutiérrez et al. (2015) | Lytic phages can be efficient biofilm-disrupting agent | Staphylococcus species | 104 |
| Khalifa et al. (2015) | Anti-biofilm activity of phage EFDG1 | E. faecalis, E. faecium | 109 |
| Lehman et al. (2015) | Effect of pretreating hydrogel-coated silicone catheters with phages; these phage cocktails can significantly reduce mixed-species biofilm formation | P. aeruginosa, Proteus mirabilis | 110 |
| Alves et al. (2014) | Phage cocktail of phages K and DRA88 reduce biofilm formation | S. aureus | 105 |
| Rahman et al. (2011) | Application of phage SAP-26 in combination with different antibiotics against 24-h-old biofilm | S. aureus | 106 |
| Akturk et al. (2023) | In vitro dual-species biofilm in combination with gentamicin | P. aeruginosa, S. aureus | 131 |
| Fu et al. (2010) | Phage cocktails prevent biofilm formation on catheters in an in vitro model | P. aeruginosa | 111 |
| Pallavali et al. (2021) | Lytic phages against 96-h-old multispecies biofilms |
P. aeruginosa,
K. pneumoniae, E. coli and S. aureus |
112 |
| Alves et al. (2016) | Novel phage cocktail reduces and disperses biofilm under static and flow conditions | P. aeruginosa | 113 |
| Alemayehu et al. (2012) | Phage ϕMR299-2 and ϕNH-4 eliminate P. aeruginosa in murine lung and on cystic fibrosis lung airway cell | P. aeruginosa | 114 |
| Lu et al. (2007) | Dispersion of biofilm with ‘engineered enzymatic phages’ | E. coli | 116 |
| Ryan et al. (2012) | Combined therapy to eradicate biofilm: T4 phage with cefotaxime | E. coli | 117 |
| Manmeet Sakshi Bedi et al. (2009) | Combined therapy to eradicate biofilm: phages of K. pneumoniae with amoxicillin | K. pneumoniae | 115 |
| Verma et al. (2009) | Combined therapy to eradicate 12-h-old biofilms: phage KP01K2 with ciprofloxacin | K. pneumoniae | 118 |
| Coulter et al. (2014) | Combined therapy to eradicate 48-h-old biofilms: phage T4 of E. coli and PB-1 of P. aeruginosa with tobramycin | E. coli, P. aeruginosa | 119 |
| Zhang et al. (2013) | Mixture of RNA phages and chloride to reduce biofilm | P. aeruginosa | 120 |
| Doub et al. (2022) | Recalcitrant MRSA prosthetic knee and femoral lateral plate infection | S. aureus | 121 |
| Tkhilaishvili et al. (2019) | Direct phage injection in a periprosthetic joint infection and osteomyelitis | P. aeruginosa | 122 |
Engineered phages
Phage engineering is also a very important and powerful tool for increasing the likelihood of successful phage therapy; in particular, great effort has been made to extend the host range of phages by genetic engineering, as this is a major limitation of the application of phages in therapy. This strategy could significantly reduce the amount of work needed to search for phages to treat specific bacteria and could lead to the selection of a few well-studied phages that could become ‘scaffolds’ for the generation of customized phages. Furthermore, this would reduce the variability between treatments and enhance translation to clinical applications.132
Yehl et al.133 targeted regions in the tail fibre of coliphage T3 for mutagenesis, in order to create highly diverse phage libraries that were screened to identify phages with altered host ranges. The authors showed that these engineered phages not only had a broader host range, but they were also able to suppress bacterial resistance to phage infection. They targeted naturally occurring phage-resistant bacterial mutants, which could potentially delay or even prevent the onset of phage resistance.134 One example of this is a study in which phages containing engineered anti-CRISPR (acr) genes were explored by including type I anti-CRISPR genes (acrIF1, acrIF2 and acrIF3) in the P. aeruginosa phage DMS3/DMS3m to obtain the potential to block bacterial replication and infection.135 The results indicated that bacterial inhibition required the production of Acrs to be above a specific threshold, so that successful phage replication may be dependent on the competition between CRISPRs and Acrs. This work led to an innovative treatment in which anti-CRISPR phages could be used to treat intractable Pseudomonas disease.
Additionally, natural, engineered and chemically synthesized genomes and re-engineered functional phages that infect Gram-negative bacteria and acid-fast mycobacteria have been assembled and shown to be efficient.136
A plethora of studies have used genetically modified phages to enhance their potential for biofilm control.137 Some of these are listed below. For example, expression of enzymes such as depolymerase or DspB is used to enhance phage activity.116 Alternatively, insertion of restriction endonuclease genes or modified holin genes or deletion of export protein genes have been explored to minimize inflammatory responses and thus improve phage therapy outcomes.138–140 One example of the latter is the endosialidases of some coliphages, specialized tail spike proteins that degrade the polysialic acid (polySia) capsule of E. coli K1, not only reducing the virulence of the pathogen but, importantly, limiting the tissue damage and the inflammatory processes that occur in response to bacterial invasion.141
Simultaneously, Park et al.142 integrated a CRISPR-Cas system targeting the nuc gene (encoding a thermostable nuclease uniquely present in S. aureus, so that the microbiome will not be affected) into the temperate phage ϕSaBov. They also removed the virulence factors and infected CTH96 S. aureus strains and did not recover any viable cells after treatment with an moi of 100. The authors conducted in vivo studies with a skin infection model in C57BL/6 mice and obtained a reduction in cfu of more than two orders of magnitude after applying ϕSaBov-Cas9-nuc embedded into a hydrogel, relative to ϕSaBov-Cas9-null. To increase the host specificity, the ϕSaBov tail fibre protein was complemented with that from the phage ϕ11, which exhibited a broader spectrum, resulting in the specificity being extended to the human pathogenic clones ST1, ST5, ST8 and ST36.142 Furthermore, this same group studied the effects of ϕSaBov-Cas9-nuc on biofilms, both in vitro and in vivo, and enhanced clearance in the biofilm using 108 pfu/mL of the CRISPR-Cas-transformed phage ϕSaBov-Cas9-nuc.143
Altogether, these findings provide evidence that engineered phages may be a viable alternative approach for use in patients with difficult-to-treat bacterial infections, including those caused by MDR bacteria that do not respond to conventional antibiotic therapy, and also to treat phage-resistant bacteria that have acquired immunity via the CRISPR-Cas system. The most relevant studies concerning engineered phages are summarized in Table 5.
Table 5.
Report cases of engineered phages
| Authors (year) | Target | Methods | References |
|---|---|---|---|
| Pei and Lamas-Samanamud (2014) | T7 phage was constructed to encode a lactonase enzyme for quenching of QS | Phage-displayed vector | 144 |
| Park et al. (2017) | Temperate phage ϕSaBov of S. aureus | CRISPR-Cas9 genomic editing technique | 142 |
| Yehl et al. (2019) | A region in the tail fibre of coliphage T3 | Mutagenesis | 133 |
| Blasco et al. (2019) | Repressor gene cI of lysogenic phage of A. baumannii | Knockout by homologous recombination | 89 |
| Dedrick et al. (2019) | Repressor gene of lysogenic phages of M. abscessus | Phage recombineering of electroporated DNA (BRED) | 74 |
| Pires et al. (2021) | Reduction of genome encoding hypothetical proteins (48%) | Yeast-based phage-engineering platform | 145 |
| Qin et al. (2022) | Introducing type I anti-CRISPR genes (AcrIF1, AcrIF2 and AcrIF3) into the P. aeruginosa phage DMS3/DMS3m | Homologous recombination | 135 |
| Mitsunaka et al. (2022) | Deleting the c2 lysis-repressor gene in P22 Salmonella phage, host-range increase of T3 coliphage with T7 tail fibre, engineering of mycophage D29 to show luminescence (addition of the Nluc gene) | In vitro genome assembly and installation into appropriate host bacteria or a cell-free transcription and translation (TXTL) system | 136 |
| Nick et al. (2022) | Repressor gene 45 of lytic phage of M. abscessus | Phage recombineering of electroporated DNA (BRED) | 146 |
| Emslander et al. (2022) | E. coli, Yersinia pestis and K. pneumoniae | Cell-free method and proteomic characterization (MS) | 147 |
| Liyanagedera et al. (2022) | Incorporate a SpyTag moiety on the capsid head to enable rapid post-synthetic modification | Scaffold phage | 148 |
| Fa-arun et al. (2023) | Phage tail for the delivery of a Cas9 antimicrobial into clinically relevant human gut pathogens Shigella flexneri and E. coli O157:H7 | Cosmid system | 149 |
| Prokopczuk et al. (2023) | Repressor and the excisionase genes of Pf4 phage in P. aeruginosa PAO1 strain | Mutagenesis | 150 |
Anti-persister strategies
Persister cells are a subpopulation of bacteria in a dormant state: they neither grow nor replicate and can re-establish infections once antibiotic stress has been removed.151
Persister cells involved in biofilm
The subpopulations of resistant phenotypes within the biofilm have been referred to as persisters.152
Phages have two properties that make biofilms susceptible to their action: they produce enzymes that degrade the extracellular matrix; and they can infect persister cells, remaining dormant within them, but reactivating when cells become metabolically active;125,126 the phages themselves may be the main actors in this reactivation.153 Furthermore, ‘Trojan horse’ strategies where phages are used in a first instance to destroy biofilms and activate persister cells, rendering them more susceptible to the antibiotics subsequently introduced, can be considered an elegant pathway to synergy. This strategy takes advantage of the ability of some phages to revert resistance to antibiotics, resensitizing bacteria.65
Toxin–antitoxin (TA) targeting
TA modules are generally encoded by two adjacent genes: a stable toxin; and an unstable antitoxin, which is degraded under stress conditions by protease systems,154 leading to activation of the toxin that often results in reduced bacterial metabolism.155 One of the main functions of the TA systems is to provide bacteria with another phage resistance mechanism, using the toxin as a general metabolic switch. Therefore, these systems would be a good target for increasing the success of phage therapy. For example, genetically engineered phages that harbour genes encoding toxin inhibitors, or molecules that protect the degradation of the antitoxin by Lon proteases, could potentially be used in phage therapy.53
Quorum sensing (QS) targeting
QS is bacterial cell-to-cell communication mediated by the production and recognition of small molecules called autoinducers.156 Several studies clearly show that QS also affects the susceptibility of bacteria to phage infection and the coordination of defence strategies against phages.45,157–159 Inhibition of QS may be important to enhance phage therapy, as recently demonstrated by Høyland-Kroghsbo et al.,160 who observed overexpression of QS genes in P. aeruginosa infected by the JBD44 phage, demonstrating that QS up-regulates the phage defence systems in the bacterial hosts. The QS network modulates several phage defence mechanisms to appropriately combat infections, shaping the outcomes of phage–host interactions and representing a crucial target to improve phage therapy. In the same line, Shah et al.161 found that Pseudomonas lysogenic phage DMS3 encoded a QS anti-activator protein, Aqs1, which acted as an inhibitor of LasR, the master regulator of QS. The authors also found that Aqs1 protein silences multiple anti-phage defence mechanisms simultaneously, such as Abi systems. Therefore, infection studies with DMS3ΔAqs1 resulted in 100-fold fewer viable cells, which proved that the presence of Aqs1 inhibited Abi-mediated resistance.
Consistently, other studies have linked the inhibition of QS and improved phage infection; for instance, Mion et al.162 demonstrated the role of the disruption of QS, a strategy known as quorum quenching (QQ), to reduce bacterial virulence and increase both antibiotic and phage treatment efficiency. These researchers used the QQ enzyme SsoPox-W263I, a lactonase able to degrade acyl-homoserine lactones (AHLs), to reduce the virulence and biofilm formation by clinical strains of P. aeruginosa from diabetic foot ulcers, thereby enhancing the susceptibility of phage and antibiotic-sensitive and phage-resistant bacteria to bacteriophages and antibiotics. The study results revealed a reduction of more than 70% in biofilm formation in 6 of 10 strains. Another example is the study by Qin et al.,163 who showed the effect of a QS inhibitor, a penicillic acid, on infection of the strain PAK-AR2 and PAO1 of P. aeruginosa. The results show that supplementation with the QS inhibitor increased the productive infection of the cells by the P. aeruginosa lytic phage C11 and increased the burst size of lytic phage K5 by 45% when cells were infected at the logarithmic phase, relative to the control in the absence of penicillic acid. Finally, the study conducted by Severin et al.164 demonstrated that QS also up-regulated a different phage defence system in Vibrio cholerae, the cyclic oligonucleotide-based antiphage signalling system (CBASS).
Nevertheless, if QS regulates multiple bacterial systems, inhibition of this network could in some cases lead to failure when phages are used as therapeutic agents. For example, Xuan et al.165 reported that QS up-regulated the expression of phage receptor in P. aeruginosa PAO1 (the O antigen of the LPS), increasing phage adsorption and infection rates. Furthermore, Broniewski et al.166 showed that a QS inhibitor decreased the phage adsorption rates due to down-regulation of the type IV pilus, which acted as the phage receptor. This caused delayed lysis of bacterial cultures and favoured CRISPR immunity. Therefore, the results of this group suggested that the inhibition of QS may reduce rather than improve the therapeutic efficacy of pilus-specific phages. Similarly, Ghosh et al.167 demonstrated that a cocktail of different synthetic AHLs or AHL-producing strains led to induction of prophages in E. coli, 168 which could lead to the propagation of these and the subsequent integration in other commensal bacteria. Finally, similar situations have been observed in E. faecalis, V. cholerae and Pseudomonas spp., in which prophage induction was promoted after exposure to autoinducer 2 (AI-2), autoinducer 3,5-dimethylpyrazin-2-ol (DPO) and 2-heptyl-3-hydroxy-4-quinolone (PQS) respectively.169–174
Overall, prophage induction is a desirable feature in terms of evading phage resistance mediated by prophages (SIE) as well as provoking bacterial lysis; however, caution is required to prevent horizontal gene transfer and activation of prophage-encoded toxins, among other risks worthy of consideration.168,175
These studies confirm the current debate concerning the effects of the QS network on phage defence systems and phage therapy outcomes.
(p)ppGpp and RpoS signalling
Most phages enter a more or less stable state of hibernation in deep-dormant host cells (persister cells) and do not produce the lysis of the bacteria cells. However, Maffei et al.172 found a newly isolated P. aeruginosa phage, named Paride, that can directly replicate and induce the lysis of deep-dormant hosts. Efficient replication of Paride on growth-arrested hosts specifically requires cellular stress responses in the form of (p)ppGpp and RpoS signalling that are dispensable for infections of growing hosts. Interestingly, the authors showed that a combination of Paride and meropenem could sterilize deep-dormant cultures in vitro and greatly reduced a resilient bacterial infection of a tissue cage implant in mice.
Anti-persister molecules
Innovative treatments to tackle dormant, persister bacterial cells, against which antibiotics are not efficient, are needed. In this context, phage therapy could be of benefit. An example of the use of lytic phages against an imipenem-persister clinical isolate of K. pneumoniae has been reported by Pacios et al.173 in a study in which a lytic phage was used in combination with the repurposed anticancer drug mitomycin C (also considered a natural antibiotic) and the conventional imipenem. Both combinations resulted in the death of persister cells and decreased the emergence of in vitro resistant mutants. These results were confirmed in the in vivo G. mellonella model, in which the combination significantly reduced the mortality rate of the larvae. Briers et al.174 also described a novel artilysin (an outer membrane-penetrating, phage-derived endolysin), named Art-175, that is able to rapidly and effectively pass through the outer membrane of P. aeruginosa persister cells and exert its bactericidal activity within the cell, without the need for any metabolic activity. Endolysins are peptidoglycan-degrading enzymes synthesized by phages, used at the end of their replication cycle to hydrolyse the peptidoglycan from within and allow the release of newly formed virions.33–35,175
All of these studies provide some insights into the treatment of persister cells, prone to forming biofilms that are difficult to eradicate.
Conclusions
Because of the constant adaptation over hundreds of millions of years of co-evolution, bacteria and phages have acquired a plethora of mechanisms to defend themselves against infection and to neutralize these defence systems. This review summarizes some of the most relevant bacterial and phage defence mechanisms in order to throw some light on this increasingly vast topic, and it outlines the latest advances in phage therapy with particular focus on targeting the main bacterial defence systems (mutation of phage receptors, prophages, CRISPR-Cas, biofilm, TA systems, QS). The latter two are involved in the development of persister cells. It is therefore essential to develop more anti-persister strategies to counteract these bacterial phenotypes. In this context, phage engineering is of particular interest to improve the potential use of personalized phages in therapy.
Contributor Information
Inés Bleriot, Grupo de Microbiología Traslacional y Multidisciplinar (MicroTM)-Servicio de Microbiología Instituto de Investigación Biomédica A Coruña (INIBIC), Hospital A Coruña (CHUAC), Universidad de A Coruña (UDC), A Coruña, Spain; Study Group on Mechanisms of Action and Resistance to Antimicrobials (GEMARA) on behalf of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), Madrid, Spain.
Olga Pacios, Grupo de Microbiología Traslacional y Multidisciplinar (MicroTM)-Servicio de Microbiología Instituto de Investigación Biomédica A Coruña (INIBIC), Hospital A Coruña (CHUAC), Universidad de A Coruña (UDC), A Coruña, Spain; Study Group on Mechanisms of Action and Resistance to Antimicrobials (GEMARA) on behalf of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), Madrid, Spain.
Lucia Blasco, Grupo de Microbiología Traslacional y Multidisciplinar (MicroTM)-Servicio de Microbiología Instituto de Investigación Biomédica A Coruña (INIBIC), Hospital A Coruña (CHUAC), Universidad de A Coruña (UDC), A Coruña, Spain; Study Group on Mechanisms of Action and Resistance to Antimicrobials (GEMARA) on behalf of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), Madrid, Spain.
Laura Fernández-García, Grupo de Microbiología Traslacional y Multidisciplinar (MicroTM)-Servicio de Microbiología Instituto de Investigación Biomédica A Coruña (INIBIC), Hospital A Coruña (CHUAC), Universidad de A Coruña (UDC), A Coruña, Spain; Study Group on Mechanisms of Action and Resistance to Antimicrobials (GEMARA) on behalf of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), Madrid, Spain.
María López, Grupo de Microbiología Traslacional y Multidisciplinar (MicroTM)-Servicio de Microbiología Instituto de Investigación Biomédica A Coruña (INIBIC), Hospital A Coruña (CHUAC), Universidad de A Coruña (UDC), A Coruña, Spain; Study Group on Mechanisms of Action and Resistance to Antimicrobials (GEMARA) on behalf of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), Madrid, Spain.
Concha Ortiz-Cartagena, Grupo de Microbiología Traslacional y Multidisciplinar (MicroTM)-Servicio de Microbiología Instituto de Investigación Biomédica A Coruña (INIBIC), Hospital A Coruña (CHUAC), Universidad de A Coruña (UDC), A Coruña, Spain; Study Group on Mechanisms of Action and Resistance to Antimicrobials (GEMARA) on behalf of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), Madrid, Spain.
Antonio Barrio-Pujante, Grupo de Microbiología Traslacional y Multidisciplinar (MicroTM)-Servicio de Microbiología Instituto de Investigación Biomédica A Coruña (INIBIC), Hospital A Coruña (CHUAC), Universidad de A Coruña (UDC), A Coruña, Spain; Study Group on Mechanisms of Action and Resistance to Antimicrobials (GEMARA) on behalf of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), Madrid, Spain.
Rodolfo García-Contreras, Microbiology and Parasitology Department Faculty of Medicine, UNAM, Mexico City, Mexico.
Jean-Paul Pirnay, Laboratory for Molecular and Cellular Technology, Queen Astrid Military Hospital, Brussels, Belgium.
Thomas K Wood, Department of Chemical Engineering, Pennsylvania State University, University Park, PA, USA.
María Tomás, Grupo de Microbiología Traslacional y Multidisciplinar (MicroTM)-Servicio de Microbiología Instituto de Investigación Biomédica A Coruña (INIBIC), Hospital A Coruña (CHUAC), Universidad de A Coruña (UDC), A Coruña, Spain; Study Group on Mechanisms of Action and Resistance to Antimicrobials (GEMARA) on behalf of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), Madrid, Spain; MePRAM, Proyecto de Medicina de Precisión contra las resistencias Antimicrobianas, A Coruña, Spain.
Funding
This study was funded by projects PI19/00878 and PI22/00323 awarded to M. Tomás within the State Plan for R + D + I 2013–2016 (National Plan for Scientific Research, Technological Development and Innovation 2008–2011) and co-financed by the ISCIII—Deputy General Directorate for Evaluation and Promotion of Research—European Regional Development Fund ‘A way of Making Europe’ and Instituto de Salud Carlos III FEDER, the Study Group on Mechanisms of Action and Resistance to Antimicrobials, GEMARA (SEIMC, http://www.seimc.org). This research was also supported by CIBERINFEC (CIBER21/13/00095) and by a grant from the Instituto de Salud Carlos III (MePRAM Project, PMP22/00092), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, funded by NextGeneration European Union funds that support the actions of the Resilience and Recovery Facility. O. Pacios, L. Fernández-García and M. López were financially supported by grants IN606A-2020/035, IN606B-2021/013 and IN606C-2022/002, respectively (GAIN, Xunta de Galicia). I. Bleriot was financially supported by the pFIS programme (ISCIII, FI20/00302). T. Wood was supported by the Novo Nordic Foundation Exploratory Interdisciplinary Synergy Programme (NNF19OC0058357).
Transparency declarations
None to declare.
References
- 1. Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wright GD, Poinar H. Antibiotic resistance is ancient: implications for drug discovery. Trends Microbiol 2012; 20: 157–9. 10.1016/j.tim.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 3. D’Costa VM, King CE, Kalan L et al. Antibiotic resistance is ancient. Nature 2011; 477: 457–61. 10.1038/nature10388 [DOI] [PubMed] [Google Scholar]
- 4. Bartlett JG, Gilbert DN, Spellberg B. Seven ways to preserve the miracle of antibiotics. Clin Infect Dis 2013; 56: 1445–50. 10.1093/cid/cit070 [DOI] [PubMed] [Google Scholar]
- 5. Trastoy R, Manso T, Fernandez-Garcia L et al. Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clin Microbiol Rev 2018; 31: e00023-18. 10.1128/CMR.00023-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gu D, Dong N, Zheng Z et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis 2018; 18: 37–46. 10.1016/S1473-3099(17)30489-9 [DOI] [PubMed] [Google Scholar]
- 7. Zeng L, Yang C, Zhang J et al. An outbreak of carbapenem-resistant Klebsiella pneumoniae in an intensive care unit of a major teaching hospital in Chongqing, China. Front Cell Infect Microbiol 2021; 11: 656070. 10.3389/fcimb.2021.656070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Du H, Chen L, Tang YW et al. Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect Dis 2016; 16: 287–8. 10.1016/S1473-3099(16)00056-6 [DOI] [PubMed] [Google Scholar]
- 9. Trongjit S, Assavacheep P, Samngamnim S et al. Plasmid-mediated colistin resistance and ESBL production in Escherichia coli from clinically healthy and sick pigs. Sci Rep 2022; 12: 2466. 10.1038/s41598-022-06415-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Majewski P, Gutowska A, Smith DGE et al. Plasmid mediated mcr-1.1 colistin-resistance in clinical extraintestinal Escherichia coli strains isolated in Poland. Front Microbiol 2021; 12: 547020. 10.3389/fmicb.2021.547020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Witney AA, Gould KA, Pope CF et al. Genome sequencing and characterization of an extensively drug-resistant sequence type 111 serotype O12 hospital outbreak strain of Pseudomonas aeruginosa. Clin Microbiol Infect 2014; 20: O609–18. 10.1111/1469-0691.12528 [DOI] [PubMed] [Google Scholar]
- 12. Thoma R, Seneghini M, Seiffert SN et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob Resist Infect Control 2022; 11: 12. 10.1186/s13756-022-01052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diniz Rocha VF, Cavalcanti TP, Azevedo J et al. Outbreak of Stenotrophomonas maltophilia and Burkholderia cepacia bloodstream infections at a hemodialysis center. Am J Trop Med Hyg 2020; 104: 848–53. 10.4269/ajtmh.20-1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guyot A, Turton JF, Garner D. Outbreak of Stenotrophomonas maltophilia on an intensive care unit. J Hosp Infect 2013; 85: 303–7. 10.1016/j.jhin.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 15. Menekşe Ş, Tanrıverdi ES, Oğuş H et al. Stenotrophomonas maltophilia outbreak with a commercial blood gas injector as the culprit and interventions for source and prevention: a possible passage between patient and ECMO water heater device. Am J Infect Control 2023; 51: 533–8. 10.1016/j.ajic.2022.07.012 [DOI] [PubMed] [Google Scholar]
- 16. Möllers M, von Wahlde MK, Schuler F et al. Outbreak of MRSA in a gynecology/obstetrics department during the COVID-19 pandemic: a cautionary tale. Microorganisms 2022; 10: 689. 10.3390/microorganisms10040689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubin IM, Hansen TA, Klingenberg AM et al. A sporadic four-year hospital outbreak of a ST97-IVa MRSA with half of the patients first identified in the community. Front Microbiol 2018; 9: 1494. 10.3389/fmicb.2018.01494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hutchings MI, Truman AW, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol 2019; 51: 72–80. 10.1016/j.mib.2019.10.008 [DOI] [PubMed] [Google Scholar]
- 19. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T 2015; 40: 277–83. [PMC free article] [PubMed] [Google Scholar]
- 20. Qi YK, Tang X, Wei NN et al. Discovery, synthesis, and optimization of teixobactin, a novel antibiotic without detectable bacterial resistance. J Pept Sci 2022; 28: e3428. 10.1002/psc.3428 [DOI] [PubMed] [Google Scholar]
- 21. Gunjal VB, Thakare R, Chopra S et al. Teixobactin: a paving stone toward a new class of antibiotics? J Med Chem 2020; 63: 12171–95. 10.1021/acs.jmedchem.0c00173 [DOI] [PubMed] [Google Scholar]
- 22. Lloyd DG, Schofield BJ, Goddard MR et al. De novo resistance to Arg10-teixobactin occurs slowly and is costly. Antimicrob Agents Chemother 2020; 65: e01152-20. 10.1128/AAC.01152-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pacios O, Blasco L, Bleriot I et al. Strategies to combat multidrug-resistant and persistent infectious diseases. Antibiotics (Basel) 2020; 9: 65. 10.3390/antibiotics9020065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parmanik A, Das S, Kar B et al. Current treatment strategies against multidrug-resistant bacteria: a review. Curr Microbiol 2022; 79: 388. 10.1007/s00284-022-03061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergh O, Børsheim KY, Bratbak G et al. High abundance of viruses found in aquatic environments. Nature 1989; 340: 467–8. 10.1038/340467a0 [DOI] [PubMed] [Google Scholar]
- 26. Mäntynen S, Laanto E, Oksanen HM et al. Black box of phage-bacterium interactions: exploring alternative phage infection strategies. Open Biol 2021; 11: 210188. 10.1098/rsob.210188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howard-Varona C, Hargreaves KR, Abedon ST et al. Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J 2017; 11: 1511–20. 10.1038/ismej.2017.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Du Toit A. Viral infection: the language of phages. Nat Rev Microbiol 2017; 15: 134–5. 10.1038/nrmicro.2017.8 [DOI] [PubMed] [Google Scholar]
- 29. Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nat Rev Microbiol 2010; 8: 317–27. 10.1038/nrmicro2315 [DOI] [PubMed] [Google Scholar]
- 30. Samson JE, Magadán AH, Sabri M et al. Revenge of the phages: defeating bacterial defences. Nat Rev Microbiol 2013; 11: 675–87. 10.1038/nrmicro3096 [DOI] [PubMed] [Google Scholar]
- 31. Ambroa A, Blasco L, López M et al. Genomic analysis of molecular bacterial mechanisms of resistance to phage infection. Front Microbiol 2022; 12: 784949. 10.3389/fmicb.2021.784949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bleriot I, Blasco L, Pacios O et al. Proteomic study of the interactions between phages and the bacterial host Klebsiella pneumoniae. Microbiol Spectr 2023; 11: e0397422. 10.1128/spectrum.03974-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Visnapuu A, Van der Gucht M, Wagemans J et al. Deconstructing the phage-bacterial biofilm interaction as a basis to establish new antibiofilm strategies. Viruses 2022; 14: 1057. 10.3390/v14051057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reyes-Robles T, Dillard RS, Cairns LS et al. Vibrio cholerae outer membrane vesicles inhibit bacteriophage infection. J Bacteriol 2018; 200: e00792-17. 10.1128/JB.00792-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stephan MS, Broeker NK, Saragliadis A et al. Analysis of O-antigen-specific bacteriophage P22 inactivation by Salmonella outer membrane vesicles. Front Microbiol 2020; 11: 510638. 10.3389/fmicb.2020.510638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yasuda M, Yamamoto T, Nagakubo T et al. Phage genes induce quorum sensing signal release through membrane vesicle formation. Microbes Environ 2022; 37: ME21067. 10.1264/jsme2.ME21067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Susskind MM, Wright A, Botstein D. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. IV. Genetics and physiology of sieB exclusion. Virology 1974; 62: 367–84. 10.1016/0042-6822(74)90399-7 [DOI] [PubMed] [Google Scholar]
- 38. Hardy A, Kever L, Frunzke J. Antiphage small molecules produced by bacteria—beyond protein-mediated defenses. Trends Microbiol 2023; 31: 92–106. 10.1016/j.tim.2022.08.001 [DOI] [PubMed] [Google Scholar]
- 39. Rusinov IS, Ershova AS, Karyagina AS et al. Avoidance of recognition sites of restriction-modification systems is a widespread but not universal anti-restriction strategy of prokaryotic viruses. BMC Genomics 2018; 19: 885. 10.1186/s12864-018-5324-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barrangou R, Fremaux C, Deveau H et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007; 315: 1709–12. 10.1126/science.1138140 [DOI] [PubMed] [Google Scholar]
- 41. Aframian N, Eldar A. Abortive infection antiphage defense systems: separating mechanism and phenotype. Trends Microbiol 2023; 31: 1003–12. 10.1016/j.tim.2023.05.002 [DOI] [PubMed] [Google Scholar]
- 42. Lopatina A, Tal N, Sorek R. Abortive infection: bacterial suicide as an antiviral immune strategy. Annu Rev Virol 2020; 7: 371–84. 10.1146/annurev-virology-011620-040628 [DOI] [PubMed] [Google Scholar]
- 43. Bleriot I, Blasco L, Pacios O et al. The role of PemIK (PemK/PemI) type II TA system from Klebsiella pneumoniae clinical strains in lytic phage infection. Sci Rep 2022; 12: 4488. 10.1038/s41598-022-08111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hazan R, Engelberg-Kulka H. Escherichia coli mazEF-mediated cell death as a defense mechanism that inhibits the spread of phage P1. Mol Genet Genomics 2004; 272: 227–34. 10.1007/s00438-004-1048-y [DOI] [PubMed] [Google Scholar]
- 45. Tan D, Svenningsen SL, Middelboe M. Quorum sensing determines the choice of antiphage defense strategy in Vibrio anguillarum. mBio 2015; 6: e00627. 10.1128/mBio.00627-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoque MM, Naser IB, Bari SM et al. Quorum regulated resistance of Vibrio cholerae against environmental bacteriophages. Sci Rep 2016; 6: 37956. 10.1038/srep37956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meyer JR, Dobias DT, Weitz JS et al. Repeatability and contingency in the evolution of a key innovation in phage lambda. Science 2012; 335: 428–32. 10.1126/science.1214449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scholl D, Adhya S, Merril C. Escherichia coli K1’s capsule is a barrier to bacteriophage T7. Appl Environ Microbiol 2005; 71: 4872–4. 10.1128/AEM.71.8.4872-4874.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cornelissen A, Ceyssens PJ, Krylov VN et al. Identification of EPS-degrading activity within the tail spikes of the novel Pseudomonas putida phage AF. Virology 2012; 434: 251–6. 10.1016/j.virol.2012.09.030 [DOI] [PubMed] [Google Scholar]
- 50. Labrie SJ, Tremblay DM, Moisan M et al. Involvement of the major capsid protein and two early-expressed phage genes in the activity of the lactococcal abortive infection mechanism AbiT. Appl Environ Microbiol 2012; 78: 6890–9. 10.1128/AEM.01755-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iida S, Streiff MB, Bickle TA et al. Two DNA antirestriction systems of bacteriophage P1, darA, and darB: characterization of darA− phages. Virology 1987; 157: 156–66. 10.1016/0042-6822(87)90324-2 [DOI] [PubMed] [Google Scholar]
- 52. Murphy J, Mahony J, Ainsworth S et al. Bacteriophage orphan DNA methyltransferases: insights from their bacterial origin, function, and occurrence. Appl Environ Microbiol 2013; 79: 7547–55. 10.1128/AEM.02229-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sberro H, Leavitt A, Kiro R et al. Discovery of functional toxin/antitoxin systems in bacteria by shotgun cloning. Mol Cell 2013; 50: 136–48. 10.1016/j.molcel.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Otsuka Y, Yonesaki T. Dmd of bacteriophage T4 functions as an antitoxin against Escherichia coli LsoA and RnlA toxins. Mol Microbiol 2012; 83: 669–81. 10.1111/j.1365-2958.2012.07975.x [DOI] [PubMed] [Google Scholar]
- 55. Deveau H, Barrangou R, Garneau JE et al. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J Bacteriol 2008; 190: 1390–400. 10.1128/JB.01412-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Young R, Gill JJ. Phage therapy redux—what is to be done? Science 2015; 350: 1163–4. 10.1126/science.aad6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ferry T, Kolenda C, Briot T et al. Past and future of phage therapy and phage-derived proteins in patients with bone and joint infection. Viruses 2021; 13: 2414. 10.3390/v13122414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Uyttebroek S, Chen B, Onsea J et al. Safety and efficacy of phage therapy in difficult-to-treat infections: a systematic review. Lancet Infect Dis 2022; 22: e208–20. 10.1016/S1473-3099(21)00612-5 [DOI] [PubMed] [Google Scholar]
- 59. Tamma PD, Souli M, Billard M et al. Safety and microbiological activity of phage therapy in persons with cystic fibrosis colonized with Pseudomonas aeruginosa: study protocol for a phase 1b/2, multicenter, randomized, double-blind, placebo-controlled trial. Trials 2022; 23: 1057. 10.1186/s13063-022-07047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gómez-Ochoa SA, Pitton M, Valente LG et al. Efficacy of phage therapy in preclinical models of bacterial infection: a systematic review and meta-analysis. Lancet Microbe 2022; 3: e956–68. 10.1016/S2666-5247(22)00288-9 [DOI] [PubMed] [Google Scholar]
- 61. Steele A, Stacey HJ, de Soir S et al. The safety and efficacy of phage therapy for superficial bacterial infections: a systematic review. Antibiotics (Basel) 2020; 9: 754. 10.3390/antibiotics9110754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pirnay JP, Djebara S, Steurs G et al. Retrospective, observational analysis of the first one hundred consecutive cases of personalized bacteriophage therapy of difficult-to-treat infections facilitated by a Belgian consortium. medRxiv 2023; 10.1101/2023.08.28.23294728 [DOI]
- 63. Green SI, Clark JR, Santos HH et al. A retrospective, observational study of 12 cases of expanded access customized phage therapy: production, characteristics, and clinical outcomes. Clin Infect Dis 2023; 77: 1079–91. 10.1093/cid/ciad335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Comeau AM, Tétart F, Trojet SN et al. Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One 2007; 2: e799. 10.1371/journal.pone.0000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eskenazi A, Lood C, Wubbolts J et al. Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae. Nat Commun 2022; 13: 302. 10.1038/s41467-021-27656-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burrowes BH, Molineux IJ, Fralick JA. Directed in vitro evolution of therapeutic bacteriophages: the Appelmans protocol. Viruses 2019; 11: 241. 10.3390/v11030241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Van Nieuwenhuyse B, Van der Linden D, Chatzis O et al. Bacteriophage-antibiotic combination therapy against extensively drug-resistant Pseudomonas aeruginosa infection to allow liver transplantation in a toddler. Nat Commun 2022; 13: 5725. 10.1038/s41467-022-33294-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khawaldeh A, Morales S, Dillon B et al. Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J Med Microbiol 2011; 60: 1697–700. 10.1099/jmm.0.029744-0 [DOI] [PubMed] [Google Scholar]
- 69. Sarker SA, Sultana S, Reuteler G et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine 2016; 4: 124–37. 10.1016/j.ebiom.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schooley RT, Biswas B, Gill JJ et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 2017; 61: e00954-17. 10.1128/AAC.00954-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. LaVergne S, Hamilton T, Biswas B et al. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect Dis 2018; 5: ofy064. 10.1093/ofid/ofy064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Duplessis C, Biswas B, Hanisch B et al. Refractory Pseudomonas bacteremia in a 2-year-old sterilized by bacteriophage therapy. J Pediatric Infect Dis Soc 2018; 7: 253–6. 10.1093/jpids/pix056 [DOI] [PubMed] [Google Scholar]
- 73. Aslam S, Courtwright AM, Koval C et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am J Transplant 2019; 19: 2631–9. 10.1111/ajt.15503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dedrick RM, Guerrero-Bustamante CA, Garlena RA et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 2019; 25: 730–3. 10.1038/s41591-019-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Little JS, Dedrick RM, Freeman KG et al. Bacteriophage treatment of disseminated cutaneous Mycobacterium chelonae infection. Nat Commun 2022; 13: 2313. 10.1038/s41467-022-29689-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Blasco L, López-Hernández I, Rodríguez-Fernández M et al. Case report: analysis of phage therapy failure in a patient with a Pseudomonas aeruginosa prosthetic vascular graft infection. Front Med (Lausanne) 2023; 10: 1199657. 10.3389/fmed.2023.1199657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage 2011; 1: 111–4. 10.4161/bact.1.2.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gordillo Altamirano FL, Barr JJ. Unlocking the next generation of phage therapy: the key is in the receptors. Curr Opin Biotechnol 2021; 68: 115–23. 10.1016/j.copbio.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 79. Yang Y, Shen W, Zhong Q et al. Development of a bacteriophage cocktail to constrain the emergence of phage-resistant Pseudomonas aeruginosa. Front Microbiol 2020; 11: 327. 10.3389/fmicb.2020.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Knecht LE, Veljkovic M, Fieseler L. Diversity and function of phage encoded depolymerases. Front Microbiol 2020; 10: 2949. 10.3389/fmicb.2019.02949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bleriot I, Trastoy R, Blasco L et al. Genomic analysis of 40 prophages located in the genomes of 16 carbapenemase-producing clinical strains of Klebsiella pneumoniae. Microb Genom 2020; 6: e000369. 10.1099/mgen.0.000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Donnelly-Wu MK, Jacobs WR, Hatfull GF. Superinfection immunity of mycobacteriophage L5: applications for genetic transformation of mycobacteria. Mol Microbiol 1993; 7: 407–17. 10.1111/j.1365-2958.1993.tb01132.x [DOI] [PubMed] [Google Scholar]
- 83. Hao G, Shu R, Ding L et al. Bacteriophage SRD2021 recognizing capsular polysaccharide shows therapeutic potential in serotype K47 Klebsiella pneumoniae infections. Antibiotics (Basel) 2021; 10: 894. 10.3390/antibiotics10080894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Serian D, Churin Y, Hammerl JA et al. Characterization of temperate LPS-binding Bordetella avium phages that lack superinfection immunity. Microbiol Spectr 2023; 11: e0370222. 10.1128/spectrum.03702-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bondy-Denomy J, Davidson AR. When a virus is not a parasite: the beneficial effects of prophages on bacterial fitness. J Microbiol 2014; 52: 235–42. 10.1007/s12275-014-4083-3 [DOI] [PubMed] [Google Scholar]
- 86. Nanda AM, Thormann K, Frunzke J. Impact of spontaneous prophage induction on the fitness of bacterial populations and host-microbe interactions. J Bacteriol 2015; 197: 410–9. 10.1128/JB.02230-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fillol-Salom A, Alsaadi A, Sousa JAM et al. Bacteriophages benefit from generalized transduction. PLoS Pathog 2019; 15: e1007888. 10.1371/journal.ppat.1007888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lakshminarasimhan A. Prophage induction therapy: activation of the lytic phase in prophages for the elimination of pathogenic bacteria. Med Hypothesis 2022; 169: 110980. 10.1016/j.mehy.2022.110980 [DOI] [Google Scholar]
- 89. Blasco L, Ambroa A, Lopez M et al. Combined use of the Ab105-2phiDeltaCI lytic mutant phage and different antibiotics in clinical isolates of multi-resistant Acinetobacter baumannii. Microorganisms 2019; 7: 556. 10.3390/microorganisms7110556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sutcliffe SG, Shamash M, Hynes AP et al. Common oral medications lead to prophage induction in bacterial isolates from the human gut. Viruses 2021; 13: 455. 10.3390/v13030455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li Y, Bondy-Denomy J. Anti-CRISPRs go viral: the infection biology of CRISPR-Cas inhibitors. Cell Host Microbe 2021; 29: 704–14. 10.1016/j.chom.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Osuna BA, Karambelkar S, Mahendra C et al. Listeria phages induce Cas9 degradation to protect lysogenic genomes. Cell Host Microbe 2020; 28: 31–40.e9. 10.1016/j.chom.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mahendra C, Christie KA, Osuna BA et al. Broad-spectrum anti-CRISPR proteins facilitate horizontal gene transfer. Nat Microbiol 2020; 5: 620–9. 10.1038/s41564-020-0692-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Knott GJ, Thornton BW, Lobba MJ et al. Broad-spectrum enzymatic inhibition of CRISPR-Cas12a. Nat Struct Mol Biol 2019; 26: 315–21. 10.1038/s41594-019-0208-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bondy-Denomy J, Garcia B, Strum S et al. Multiple mechanisms for CRISPR-Cas inhibition by anti-CRISPR proteins. Nature 2015; 526: 136–9. 10.1038/nature15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Meeske AJ, Jia N, Cassel AK et al. A phage-encoded anti-CRISPR enables complete evasion of type VI-A CRISPR-Cas immunity. Science 2020; 369: 54–9. 10.1126/science.abb6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vyas P, Harish. Anti-CRISPR proteins as a therapeutic agent against drug-resistant bacteria. Microbiol Res 2022; 257: 126963. 10.1016/j.micres.2022.126963 [DOI] [PubMed] [Google Scholar]
- 98. Simmons EL, Bond MC, Koskella B et al. Biofilm structure promotes coexistence of phage-resistant and phage-susceptible bacteria. mSystems 2020; 5: e00877-19. 10.1128/mSystems.00877-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu S, Lu H, Zhang S et al. Phages against pathogenic bacterial biofilms and biofilm-based infections: a review. Pharmaceutics 2022; 14: 427. 10.3390/pharmaceutics14020427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Heilmann S, Sneppen K, Krishna S. Coexistence of phage and bacteria on the boundary of self-organized refuges. Proc Natl Acad Sci U S A 2012; 109: 12828–33. 10.1073/pnas.1200771109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Blasco L, Bleriot I, de Aledo MG et al. Development of an anti-Acinetobacter baumannii biofilm phage cocktail: genomic adaptation to the host. Antimicrob Agents Chemother 2022; 66: e0192321. 10.1128/aac.01923-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tkhilaishvili T, Lombardi L, Klatt AB et al. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int J Antimicrob Agents 2018; 52: 842–53. 10.1016/j.ijantimicag.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 103. Son JS, Lee SJ, Jun SY et al. Antibacterial and biofilm removal activity of a podoviridae Staphylococcus aureus bacteriophage SAP-2 and a derived recombinant cell-wall-degrading enzyme. Appl Microbiol Biotechnol 2010; 86: 1439–49. 10.1007/s00253-009-2386-9 [DOI] [PubMed] [Google Scholar]
- 104. Gutiérrez D, Vandenheuvel D, Martínez B et al. Two phages, phiIPLA-RODI and phiIPLA-C1C, lyse mono- and dual-species staphylococcal biofilms. Appl Environ Microbiol 2015; 81: 3336–48. 10.1128/AEM.03560-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Alves DR, Gaudion A, Bean JE et al. Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation. Appl Environ Microbiol 2014; 80: 6694–703. 10.1128/AEM.01789-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rahman M, Kim S, Kim SM et al. Characterization of induced Staphylococcus aureus bacteriophage SAP-26 and its anti-biofilm activity with rifampicin. Biofouling 2011; 27: 1087–93. 10.1080/08927014.2011.631169 [DOI] [PubMed] [Google Scholar]
- 107. Pires DP, Melo L, Vilas Boas D et al. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr Opin Microbiol 2017; 39: 48–56. 10.1016/j.mib.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 108. Akturk E, Melo LDR, Oliveira H et al. Combining phages and antibiotic to enhance antibiofilm efficacy against an in vitro dual species wound biofilm. Biofilm 2023; 6: 100147. 10.1016/j.bioflm.2023.100147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Khalifa L, Brosh Y, Gelman D et al. Targeting Enterococcus faecalis biofilms with phage therapy. Appl Environ Microbiol 2015; 81: 2696–705. 10.1128/AEM.00096-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lehman SM, Donlan RM. Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob Agents Chemother 2015; 59: 1127–37. 10.1128/AAC.03786-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fu W, Forster T, Mayer O et al. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother 2010; 54: 397–404. 10.1128/AAC.00669-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pallavali RR, Degati VL, Narala VR et al. Lytic bacteriophages against bacterial biofilms formed by multidrug-resistant Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus isolated from burn wounds. Phage (New Rochelle) 2021; 2: 120–30. 10.1089/phage.2021.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Alves DR, Perez-Esteban P, Kot W et al. A novel bacteriophage cocktail reduces and disperses Pseudomonas aeruginosa biofilms under static and flow conditions. Microb Biotechnol 2016; 9: 61–74. 10.1111/1751-7915.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Alemayehu D, Casey PG, McAuliffe O et al. Bacteriophages ϕMR299-2 and ϕNH-4 can eliminate Pseudomonas aeruginosa in the murine lung and on cystic fibrosis lung airway cells. mBio 2012; 3: e00029-12. 10.1128/mBio.00029-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sakshi Bedi M, Verma V, Chhibber S. Amoxicillin and specific bacteriophage can be used together for eradication of biofilm of Klebsiella pneumoniae B5055. World J Microbiol Technol 2009; 25: 1145–51. 10.1007/s11274-009-9991-8 [DOI] [Google Scholar]
- 116. Lu TK, Collins JJ. Dispersing biofilms with engineered enzymatic bacteriophage. Proc Natl Acad Sci U S A 2007; 104: 11197–202. 10.1073/pnas.0704624104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ryan EM, Alkawareek MY, Donnelly RF et al. Synergistic phage-antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunol Med Microbiol 2012; 65: 395–8. 10.1111/j.1574-695X.2012.00977.x [DOI] [PubMed] [Google Scholar]
- 118. Verma V, Harjai K, Chhibber S. Restricting ciprofloxacin-induced resistant variant formation in biofilm of Klebsiella pneumoniae B5055 by complementary bacteriophage treatment. J Antimicrob Chemother 2009; 64: 1212–8. 10.1093/jac/dkp360 [DOI] [PubMed] [Google Scholar]
- 119. Coulter LB, McLean RJ, Rohde RE et al. Effect of bacteriophage infection in combination with tobramycin on the emergence of resistance in Escherichia coli and Pseudomonas aeruginosa biofilms. Viruses 2014; 6: 3778–86. 10.3390/v6103778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang Y, Hu Z. Combined treatment of Pseudomonas aeruginosa biofilms with bacteriophages and chlorine. Biotechnol Bioeng 2013; 110: 286–95. 10.1002/bit.24630 [DOI] [PubMed] [Google Scholar]
- 121. Doub JB, Ng VY, Lee M et al. Salphage: salvage bacteriophage therapy for recalcitrant MRSA prosthetic joint infection. Antibiotics (Basel) 2022; 11: 616. 10.3390/antibiotics11050616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tkhilaishvili T, Winkler T, Müller M et al. Bacteriophages as adjuvant to antibiotics for the treatment of periprosthetic joint infection caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 2019; 64: e00924-19. 10.1128/AAC.00924-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tkhilaishvili T, Wang L, Perka C et al. Using bacteriophages as a Trojan horse to the killing of dual-species biofilm formed by Pseudomonas aeruginosa and methicillin resistant Staphylococcus aureus. Front Microbiol 2020; 11: 695. 10.3389/fmicb.2020.00695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Meneses L, Brandão AC, Coenye T et al. A systematic review of the use of bacteriophages for in vitro biofilm control. Eur J Clin Microbiol Infect Dis 2023; 42: 919–28. 10.1007/s10096-023-04638-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chegini Z, Khoshbayan A, Taati Moghadam M et al. Bacteriophage therapy against Pseudomonas aeruginosa biofilms: a review. Ann Clin Microbiol Antimicrob 2020; 19: 45. 10.1186/s12941-020-00389-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pearl S, Gabay C, Kishony R et al. Nongenetic individuality in the host–phage interaction. PLoS Biol 2008; 6: e120. 10.1371/journal.pbio.0060120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dunsing V, Irmscher T, Barbirz S et al. Purely polysaccharide-based biofilm matrix provides size-selective diffusion barriers for nanoparticles and bacteriophages. Biomacromolecules 2019; 20: 3842–54. 10.1021/acs.biomac.9b00938 [DOI] [PubMed] [Google Scholar]
- 128. Vidakovic L, Singh PK, Hartmann R et al. Dynamic biofilm architecture confers individual and collective mechanisms of viral protection. Nat Microbiol 2018; 3: 26–31. 10.1038/s41564-017-0050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chaudhry W, Lee E, Worthy A et al. Mucoidy, a general mechanism for maintaining lytic phage in populations of bacteria. FEMS Microbiol Ecol 2020; 96: fiaa162. 10.1093/femsec/fiaa162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Darch SE, Kragh KN, Abbott EA et al. Phage inhibit pathogen dissemination by targeting bacterial migrants in a chronic infection model. mBio 2017; 8: e00240-17. 10.1128/mBio.00240-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Akturk E, Oliveira H, Santos SB et al. Synergistic action of phage and antibiotics: parameters to enhance the killing efficacy against mono and dual-species biofilms. Antibiotics (Basel) 2019; 8: 103. 10.3390/antibiotics8030103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Harada LK, Silva EC, Campos WF et al. Biotechnological applications of bacteriophages: state of the art. Microbiol Res 2018; 212-13: 38–58. 10.1016/j.micres.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 133. Yehl K, Lemire S, Yang AC et al. Engineering phage host-range and suppressing bacterial resistance through phage tail fiber mutagenesis. Cell 2019; 179: 459–469.e9. 10.1016/j.cell.2019.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Latka A, Lemire S, Grimon D et al. Engineering the modular receptor-binding proteins of Klebsiella phages switches their capsule serotype specificity. mBio 2021; 12: e00455-21. 10.1128/mBio.00455-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Qin S, Liu Y, Chen Y et al. Engineered bacteriophages containing anti-CRISPR suppress infection of antibiotic-resistant P. aeruginosa. Microbiol Spectr 2022; 10: e0160222. 10.1128/spectrum.01602-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Mitsunaka S, Yamazaki K, Pramono AK et al. Synthetic engineering and biological containment of bacteriophages. Proc Natl Acad Sci U S A 2022; 119: e2206739119. 10.1073/pnas.2206739119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Motlagh AM, Bhattacharjee AS, Goel R. Biofilm control with natural and genetically-modified phages. World J Microbiol Biotechnol 2016; 32: 67. 10.1007/s11274-016-2009-4 [DOI] [PubMed] [Google Scholar]
- 138. Hagens S, Habel A, von Ahsen U et al. Therapy of experimental pseudomonas infections with a nonreplicating genetically modified phage. Antimicrob Agents Chemother 2004; 48: 3817–22. 10.1128/AAC.48.10.3817-3822.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Matsuda T, Freeman TA, Hilbert DW et al. Lysis-deficient bacteriophage therapy decreases endotoxin and inflammatory mediator release and improves survival in a murine peritonitis model. Surgery 2005; 137: 639–46. 10.1016/j.surg.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 140. Peng H, Chen IA. Phage engineering and the evolutionary arms race. Curr Opin Biotechnol 2021; 68: 23–9. 10.1016/j.copbio.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B. Bacteriophages and phage-derived proteins—application approaches. Curr Med Chem 2015; 22: 1757–73. 10.2174/0929867322666150209152851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Park JY, Moon BY, Park JW et al. Genetic engineering of a temperate phage-based delivery system for CRISPR/Cas9 antimicrobials against Staphylococcus aureus. Sci Rep 2017; 7:44929. 10.1038/srep44929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cobb LH, Park J, Swanson EA et al. CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS One 2019; 14: e0220421. 10.1371/journal.pone.0220421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pei R, Lamas-Samanamud GR. Inhibition of biofilm formation by T7 bacteriophages producing quorum-quenching enzymes. Appl Environ Microbiol 2014; 80: 5340–8. 10.1128/AEM.01434-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pires DP, Monteiro R, Mil-Homens D et al. Designing P. aeruginosa synthetic phages with reduced genomes. Sci Rep 2021; 11: 2164. 10.1038/s41598-021-81580-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Nick JA, Dedrick RM, Gray AL et al. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell 2022; 185: 1860–1874.e12. 10.1016/j.cell.2022.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Emslander Q, Vogele K, Braun P et al. Cell-free production of personalized therapeutic phages targeting multidrug-resistant bacteria. Cell Chem Biol 2022; 29: 1434–1445.e7. 10.1016/j.chembiol.2022.06.003 [DOI] [PubMed] [Google Scholar]
- 148. Liyanagedera SBW, Williams J, Wheatley JP et al. SpyPhage: a cell-free TXTL platform for rapid engineering of targeted phage therapies. ACS Synth Biol 2022; 11: 3330–42. 10.1021/acssynbio.2c00244 [DOI] [PubMed] [Google Scholar]
- 149. Fa-Arun J, Huan YW, Darmon E et al. Tail-engineered phage P2 enables delivery of antimicrobials into multiple gut pathogens. ACS Synth Biol 2023; 12: 596–607. 10.1021/acssynbio.2c00615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Prokopczuk FI, Im H, Campos-Gomez J et al. Engineered superinfective Pf phage prevents dissemination of Pseudomonas aeruginosa in a mouse burn model. mBio 2023; 14: e0047223. 10.1128/mbio.00472-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Balaban NQ, Helaine S, Lewis K et al. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 2019; 17: 441–8. 10.1038/s41579-019-0196-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2004; 2: 95–108. 10.1038/nrmicro821 [DOI] [PubMed] [Google Scholar]
- 153. Akanda ZZ, Taha M, Abdelbary H. Current review—the rise of bacteriophage as a unique therapeutic platform in treating peri-prosthetic joint infections. J Orthop Res 2018; 36: 1051–60. 10.1002/jor.23755 [DOI] [PubMed] [Google Scholar]
- 154. Chan WT, Espinosa M, Yeo CC. Keeping the wolves at bay: antitoxins of prokaryotic type II toxin-antitoxin systems. Front Mol Biosci 2016; 3: 9. 10.3389/fmolb.2016.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Song S, Wood TK. A primary physiological role of toxin/antitoxin systems is phage inhibition. Front Microbiol 2020; 11: 1895. 10.3389/fmicb.2020.01895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Castillo-Juárez I, Maeda T, Mandujano-Tinoco EA et al. Role of quorum sensing in bacterial infections. World J Clin Cases 2015; 3: 575–98. 10.12998/wjcc.v3.i7.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Luthe T, Kever L, Thormann K et al. Bacterial multicellular behavior in antiviral defense. Curr Opin Microbiol 2023; 74: 102314. 10.1016/j.mib.2023.102314 [DOI] [PubMed] [Google Scholar]
- 158. Høyland-Kroghsbo NM, Maerkedahl RB, Svenningsen SL. A quorum-sensing-induced bacteriophage defense mechanism. mBio 2013; 4: e00362-12. 10.1128/mBio.00362-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Saucedo-Mora MA, Castañeda-Tamez P, Cazares A et al. Selection of functional quorum sensing systems by lysogenic bacteriophages in Pseudomonas aeruginosa. Front Microbiol 2017; 8: 1669. 10.3389/fmicb.2017.01669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Høyland-Kroghsbo NM, Bassler BL. Phage infection restores PQS signaling and enhances growth of a Pseudomonas aeruginosa lasI quorum-sensing mutant. J Bacteriol 2022; 204: e0055721. 10.1128/jb.00557-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Shah M, Taylor VL, Bona D et al. A phage-encoded anti-activator inhibits quorum sensing in Pseudomonas aeruginosa. Mol Cell 2021; 81: 571–583.e6. 10.1016/j.molcel.2020.12.011 [DOI] [PubMed] [Google Scholar]
- 162. Mion S, Rémy B, Plener L et al. Quorum quenching lactonase strengthens bacteriophage and antibiotic arsenal against Pseudomonas aeruginosa clinical isolates. Front Microbiol 2019; 10: 2049. 10.3389/fmicb.2019.02049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Qin X, Sun Q, Yang B et al. Quorum sensing influences phage infection efficiency via affecting cell population and physiological state. J Basic Microbiol 2017; 57: 162–70. 10.1002/jobm.201600510 [DOI] [PubMed] [Google Scholar]
- 164. Severin GB, Ramliden MS, Ford KC et al. Activation of a Vibrio cholerae CBASS anti-phage system by quorum sensing and folate depletion. mBio 2023; 14: e0087523. 10.1128/mbio.00875-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Xuan G, Lin H, Tan L et al. Quorum sensing promotes phage infection in Pseudomonas aeruginosa PAO1. mBio 2022; 13: e0317421. 10.1128/mbio.03174-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Broniewski JM, Chisnall MAW, Høyland-Kroghsbo NM et al. The effect of quorum sensing inhibitors on the evolution of CRISPR-based phage immunity in Pseudomonas aeruginosa. ISME J 2021; 15: 2465–73. 10.1038/s41396-021-00946-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ghosh D, Roy K, Williamson KE et al. Acyl-homoserine lactones can induce virus production in lysogenic bacteria: an alternative paradigm for prophage induction. Appl Environ Microbiol 2009; 75: 7142–52. 10.1128/AEM.00950-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hu J, Ye H, Wang S et al. Prophage activation in the intestine: insights into functions and possible applications. Front Microbiol 2021; 12: 785634. 10.3389/fmicb.2021.785634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Rossmann FS, Racek T, Wobser D et al. Phage-mediated dispersal of biofilm and distribution of bacterial virulence genes is induced by quorum sensing. PLoS Pathog 2015; 11: e1004653. 10.1371/journal.ppat.1004653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Fernández-Piñar R, Cámara M, Dubern JF et al. The Pseudomonas aeruginosa quinolone quorum sensing signal alters the multicellular behaviour of Pseudomonas putida KT2440. Res Microbiol 2011; 162: 773–81. 10.1016/j.resmic.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 171. Silpe JE, Bassler BL. A host-produced quorum-sensing autoinducer controls a phage lysis-lysogeny decision. Cell 2019; 176: 268–280.e13. 10.1016/j.cell.2018.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Maffei E, Woischnig A-K, Burkolter MR et al. Phage Paride can kill dormant, antibiotic-tolerant cells of Pseudomonas aeruginosa by direct lytic replication. Nat Commun 2024; 15: 175. 10.1038/s41467-023-44157-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Pacios O, Fernández-García L, Bleriot I et al. Enhanced antibacterial activity of repurposed mitomycin C and imipenem in combination with the lytic phage vB_KpnM-VAC13 against clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother 2021; 65: e0090021. 10.1128/AAC.00900-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Briers Y, Walmagh M, Grymonprez B et al. Art-175 is a highly efficient antibacterial against multidrug-resistant strains and persisters of Pseudomonas aeruginosa. Antimicrob Agents Chemother 2014; 58: 3774–84. 10.1128/AAC.02668-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Schmelcher M, Donovan DM, Loessner MJ. Bacteriophage endolysins as novel antimicrobials. Future Microbiol 2012; 7: 1147–71. 10.2217/fmb.12.97 [DOI] [PMC free article] [PubMed] [Google Scholar]