Abstract
OBJECTIVES:
We aimed to assess the feasibility and reliability of sequential ultrasonographic and elastographic monitoring in acute liver failure (ALF).
DESIGN:
Observational study.
SETTING:
ALF is a rare, life-threatening disease that requires intensive care admission and often liver transplant, where the accurate selection of patients is crucial. Liver elastography is a noninvasive tool that can measure hepatic stiffness, but previous results have been inconclusive in ALF.
PATIENTS:
Patients admitted between October 2021 and March 2023 to the Liver Intensive Therapy Unit at King’s College Hospital with ALF were recruited, with healthy control (HC) individuals and acute-on-chronic liver failure (ACLF) used as controls.
INTERVENTION:
None.
MEASUREMENTS:
Average shear wave velocity was recorded with ElastPQ on the right and left liver lobes and the spleen. Portal vein flow, hepatic artery resistive index, and peak systolic velocity were also recorded. Physiologic and histologic data were used for comparison.
MAIN RESULTS:
Forty patients with ALF, 22 patients with ACLF, and 9 HC individuals were included in the study. At admission, liver stiffness measurement (LSM) of the right lobe was statistically different between HC individuals (5.6 ± 2 kPa), ALF (31.7 ± 17 kPa), and ACLF (76.3 ± 71 kPa) patients (ALF vs. ACLF, p = 0.0301). Spleen size and stiffness discriminated between ALF (10.4 ± 2 cm and 21.4 ± 16.6 kPa) and ACLF (14 ± 2.3 cm and 42.6 ± 26 kPa). At admission, LSM was not different between ALF patients who spontaneously survived versus patients who died or were transplanted in the following 90 days. However, the trend over the first 10 days of admission was different with a peak of LSM at day 5 in spontaneous survivors followed by reduction during the recovery phase. ALF patients with poor prognosis showed a persistently increased LSM.
CONCLUSIONS:
In ALF stiffness peaks at day 5 of admission with subsequent reduction in patients spontaneously surviving, showing significant difference according to the prognosis at day 7 of admission. LSM might be useful in distinguishing acute from acute-on-chronic liver failure together with spleen volume and stiffness.
Keywords: acute liver failure, acute-on-chronic liver failure, diagnostic ultrasound, elastography, tissue elasticity imaging
KEY POINTS
Question: Can elastography be used as diagnostic and prognostic tool in acute liver failure?
Findings: In this prospective observational cross-sectional pilot study, we showed that elastography has the potential to be used in acute liver failure and prognosticate outcome on day 7 of admission.
Meaning: Elastography could be employed in intensive care to assess patients with acute liver failure and can contribute to the decision-making process.
Acute liver failure (ALF) is a rare life-threatening disease, characterized by coagulopathy and hepatic encephalopathy occurring in people without preexisting liver conditions that usually require admission to intensive care (1). The average prevalence is approximately six people per million population per year (2) and the survival improved in the last 50 years from 17% to 62% (3). This is distinct from acute-on-chronic liver failure (ACLF) (4), occurring instead in the 35% of people with decompensated cirrhosis admitted to the hospital that develop multiple organ failure (5). The mortality rate within 28 days after admission is 32.0% in patients with two organ failures and 78.6% in those with three organ failures or more (4). Both these liver failure syndromes are characterized by intense systemic inflammation and cytokine release, with cardiovascular instability and immune dysfunction with a high incidence of death from sepsis and multiple organ failure (6, 7). The cardiovascular changes are similar to those of sepsis with a hyperdynamic circulation manifested by a high cardiac output and decreased mean arterial pressure (MAP) and systemic vascular resistance (1). Usually only if the clinical interpretation through history, examination, laboratory, and imaging modalities are inconclusive, a liver biopsy is performed to ascertain the specific etiology of the ALF and distinguish it from ACLF.
There are no pharmaceutical therapies for either immune or vascular dysfunction in ALF and, if patients do not improve with supporting measures, liver transplant (LT) is the curative option. Survival without LT has improved thanks to the selection of patients, critical care support, and liver support strategies such as plasmapheresis aimed to remove circulating cytokines and dampen the inflammatory response (8). In ACLF is still matter of debate if LT needs to be expedited and national programs are ongoing to address this issue (9).
In this setting, the accurate prognostication of patients is crucial. Current U.K. guidelines for ALF transplant listing are based on the King’s College Criteria, evaluating clinical parameters, that unfortunately have low sensitivity (10) hence worldwide efforts are needed to improve prognostic and diagnostic accuracy.
Liver elastography is a noninvasive tool that can measure stiffness which is widely used in stable chronic liver disease (11), but with inconclusive results in ALF (12–16). This is likely because in this setting the high stiffness is not related to the presence of fibrosis but to a combination of other factors, including, inflammation, necrosis, fluid status, and microthrombosis. Acute hepatitis is indeed considered a bias or a source of “false positivity” in the current guidelines (11). Liver ultrasonography, and CT, have been helpful in prognostication of ALF as reduced or collapsing liver volume, suggests poor chance of recovery (17). Spleen stiffness, on the other hand, can be of help in differentiating acute from acute-on-chronic liver disease and can predict decompensation in the chronic setting (18). Furthermore, despite being confined to an ambulatory setting since its creation, in recent years elastography gained attention from the intensive care community and in particular for its application in cardiac disease, as assessment tool of liver congestion in the setting of right heart failure (19, 20).
The aim of our pilot study was to evaluate the usefulness of a noninvasive ultrasound approach in patients with acute liver failure syndromes admitted to intensive care, to provide pilot data as a diagnostic tool that can help in both the triage of patients (distinguish ALD from ACLF) and prognostication in an LT setting. We sought to comprehensively characterize the dynamic changes in liver elastography, vascular Doppler, and systemic inflammation to provide a mechanistic explanation of the ultrasonographic findings. We hypothesized that ultrasonography could have a role in the identification of ALF, distinguishing it from ACLF at admission, and also help in the prognostication of the disease.
MATERIALS AND METHODS
Study Population
ALF patients admitted from October 2021 to March 2023 to the Liver Intensive Care of King’s College Hospital were recruited. Patients with ACLF (according to EASL_CLIF definition (4)) and healthy individuals were used as healthy control (HC) individuals. Patients presenting with pregnancy, disseminated malignancy, preexisting immunosuppressive states including drugs and HIV infection, and chronic granulomatous diseases were excluded. Patients were screened and approached for recruitment as part of the “Immunometabolism in Sepsis, Inflammation and Liver Failure Syndromes/IMET Study” (version 3.1, dated February 17, 2022, research ethics committee number 19/NW/0750, integrated research application system number 244089) study within 24 hours of admission. The study was compliant with the ethical standards of the Helsinki Declaration of 1975. Patients or family consultees in case of lack of capacity provided written informed consent. Patients with indeterminate status ALF versus ACLF were comprehensively examined by a senior hepatologist, radiologist, and pathologist and classified accordingly.
Ultrasound Protocol
Imaging was collected with a Philips Affiniti 70 (Philips, Amsterdam, The Netherlands) ultrasound machine with an elastography upgrade. Average shear wave velocity was recorded with ElastPQ (Philips, Amsterdam, The Netherlands) on the right and left liver lobes and spleen. Almost all the patients were intubated and ventilated, thus measurements were taken in supine position at the end of each expiration, to avoid movement artifacts. The right lobe was explored with an intercostal approach, the left lobe with longitudinal orientation in epigastrium, and the spleen with left intercostal approach. Liver longitudinal diameter was measured along an oblique line from the right hemidiaphragm to the inferior tip of the right lobe in the midaxillary line (Supplementary Fig. 2E, http://links.lww.com/CCX/B306). At least 10 measurements per site were recorded and the results were deemed reliable if interquartile range (IQR)/median was less than 30% (21). Portal vein flow was recorded as per standard practice with an angle between 30 and 60°, and the hepatic artery resistive index (HARI) and peak systolic velocity were also recorded. Scans were performed by a single operator (F.M.T.) daily for the first 10 days of admission or until LT, discharge, or death of the patient.
Blood Samples and Clinical Data
On days 1, 3, 7, and 10 samples of plasma and serum were collected and stored after centrifugation. Biopsy or explant histologic findings, when available, were used for comparison.
Clinical data and laboratory parameters were collected, and severity scores were calculated, including the Model for End-stage Liver Disease, Child-Pugh, and Sequential Organ Failure Assessment (SOFA) (22). When invasive monitoring with Pulse index Continuous Cardiac Output (Pulsion, Feldkirchen, Germany) was performed, the following data were measured by transpulmonary thermodilution and collected: cardiac index (CI), stroke volume variation (SVV), global end-diastolic volume index (GEDVI), extravascular lung water index (ELWI), and systemic vascular resistance index (SVRI) (23).
Cytokine Analysis
The V-PLEX Proinflammatory Panel 1 Human Kit (Meso Scale Diagnostics, Rockville, Maryland, USA) (interferon-γ, interleukin [IL]-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, tumor necrosis factor α) from Meso Scale Diagnostics, LLC was measured as per Supplementary Methods (http://links.lww.com/CCX/B306).
Enzyme-Linked Immunosorbent Assay (ELISA)
von Willebrand factor (vWF) and soluble platelet-endothelial cell adhesion molecule-1 plasma concentrations were quantified according to the manufacturer’s instructions briefly summarized in Supplementary Methods (http://links.lww.com/CCX/B306).
Histology
Liver explants/biopsies were examined by a single operator (R.M.), hematoxylin and eosin, and Sirius red staining was used. The following parameters were evaluated: Ishak confluent hepatocellular necrosis, Ishak intralobular inflammation, Ishak portal/septal inflammation, ductular reaction, ballooning, cytoplasmatic cholestasis, canalicular cholestasis, ductular cholestasis, and percentage of fat (microvacuolar and microvacuolar) (24).
Statistical Analysis
Descriptive statistics, univariate (t test and analysis of variance [ANOVA] for parametric data and Mann-Whitney and Kruskal-Wallis with Dunn’s multiple comparisons tests for not parametric data), receiver operating characteristic curve, and correlations analysis (Pearson and Spearman) were performed with GraphPad PRISM (GraphPad Software, v9, Boston, MA). Multiple linear regression has been calculated with SPSS, v28 (IBM, Chicago, IL).
RESULTS
Liver Elastography Together With Spleen Assessment is a Good Triage Tool in Acute Liver Failure Syndromes
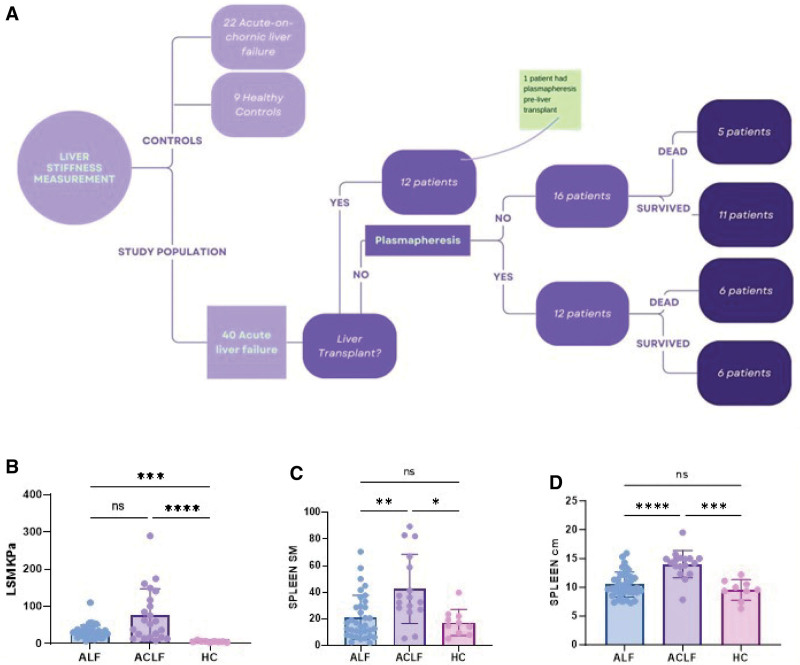
Forty-five patients with an index presentation of ALF requiring admission to liver intensive care, 17 patients with known chronic liver disease in ACLF as per CLIF definition (4), and 9 HC individuals were included in the study. After collateral history, imaging, and in some cases liver biopsy, acute patients were subsequently recharacterized as 40 ALF and 22 ACLF (Table 1). The most common etiology of ALF was paracetamol overdose, affecting 23 patients (57%). Mean age was 37.5 ± 16 and the admission median SOFA score was 14 (IQR = 6). Seventeen patients spontaneously survived, 12 patients were transplanted (30%), and 12 patients died (30%), including one after LT, within 90 days from admission. Plasmapheresis was done in 13 patients (Fig. 1A; and Supplementary Table 1, http://links.lww.com/CCX/B306). The average time from inclusion to LT was 7 ± 7 days, whereas the average time from inclusion to death was 22 ± 23 days.
TABLE 1.
Characteristics of the Population
| Acute Liver Failure, n = 40 | Acute-on-Chronic Liver Failure, n = 22 | p | |
|---|---|---|---|
| Age (yr) | 37.5 ± 16 | 44 ± 12.5 | 0.1048 |
| Female, n (%) | 20 (50%) | 8 (36%) | 0.3019 |
| Liver stiffness measurement (kPa), median (IQR) | 28.13 (19.44) | 56.6 (105.47) | 0.0301 |
| Spleen SM (kPa), median (IQR) | 15.1 (21.55) | 32.6 (36.69) | 0.0085 |
| Hepatic artery resistive index, ± sd | 0.72 ± 0.1 | 0.70 ± 0.1 | 0.4541 |
| Portal vein velocity (cm/s), median (IQR) | 16.8 (8.5) | 18.45 (19.4) | 0.8210 |
| Right lobe (cm), ± sd | 14.2 ± 3.1 | 14.5 ± 2.5 | 0.7172 |
| Spleen (cm), median (IQR) | 9.95 (2.83) | 14.3 (2.05) | < 0.0001 |
| Central venous pressure (mm Hg), ± sd | 11.85 ± 4.7 | 14.3 ± 4.8 | 0.0623 |
| Mean arterial pressure (with vasopressor support) (mm Hg), median (IQR) | 77 (11.5) | 70 (14.5) | 0.0425 |
| Heart rate (beat/min), ± sd | 90.8 ± 23.5 | 87.9 ± 13.5 | 0.6101 |
| Cardiac index, median (IQR) | 4 (2.6) | 4.8(3) | 0.2316 |
| International normalized ratio, median (IQR) | 2.7 (2.8) | 1.7 (0.45) | 0.0005 |
| Total bilirubin (mg/dL), median (IQR) | 7 (7.78) | 14 (21.145) | 0.0798 |
| Aspartate aminotransferase (U/L), median (IQR) | 3107 (7873) | 102 (80.5) | < 0.0001 |
| Lactate, median (IQR) | 2.7 (2.6) | 1.5 (0.75) | 0.0093 |
| Sequential Organ Failure Assessment score, median (IQR) | 14 (6) | 14 (5) | 0.6326 |
| Renal replacement therapy, n (%) | 36 (90%) | 13 (59%) | 0.0042 |
| Mechanical ventilation, n (%) | 33 (82.5%) | 18 (81%) | 0.9464 |
IQR = interquartile range.
Population characteristics presented as mean ± sd for parametric values, and median with IQR for nonparametric values; Student t test for parametric, Mann-Whitney for nonparametric, and chi-square for frequency have been used.
Boldface values indicate statistically significant results, where p < 0.05.
Figure 1.
Study population and main findings. A, Flowchart including the study population. B, Admission liver stiffness measurement of the right lobe (RLSM) accurately identified healthy control (HC) individuals (5.6 ± 2 kPa), acute liver failure (31.7 ± 17), and acute-on-chronic liver failure (76.3 ± 71 kPa) patients (Kruskall-Wallis). Acute liver failure (ALF) (n = 40), acute-on-chronic liver failure (ACLF) (n = 22), HC individuals (n = 9). C, Spleen size is increased in ACLF (14 ± 2.3 cm) compared with ALF (10.4 ± 2 cm) and HC individuals (9.5 ± 1.8 cm) (Kruskall-Wallis). III, Spleen stiffness is increased in ACLF (45.6 ± 26 kPa) compared with ALF (21 ± 16 kPa) and HC individuals (17.3 ± 10 kPa) (Kruskall-Wallis). D, Spleen stiffness is increased in ACLF (45.6 ± 26 kPa) compared with ALF (21 ± 16 kPa) and HC individuals (17.3 ± 10 kPa) (Kruskall-Wallis). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns = not statistically significant.
Survivors had a mean ICU stay of 15.3 ± 12.6 days and mean hospital stay of 24.1 ± 14.7 days. Four patients were repatriated to the referring hospital before being fit for discharge home. Among survivors, 9 patients were on vasopressors, 12 were mechanically ventilated, and 14 on renal replacement therapy (RRT) at admission; on day 3, 9 patients were on vasopressors, 13 mechanically ventilated and 13 on RRT; at day 7, 2 patients were on vasopressors, 7 mechanically ventilated and 7 on RRT; and at day 10, 0 patients were on pressors, 4 mechanically ventilated and 5 on RRT.
Ultrasonographic parameters are presented in Table 1, of note the average scan time was 20 minutes per day per patient.
The admission liver stiffness measurement of the right lobe liver stiffness measurement (RLSM) was significantly different between HC individuals (5.6 ± 2 kPa), ALF (31.7 ± 17), and ACLF (76.3 ± 71 kPa) patients (Kruskal-Wallis p < 0.0001) (Fig. 1B). RLSM discriminated between ALF and ACLF (Supplementary Figs. 1 and 2G, http://links.lww.com/CCX/B306) and was consistent with the histological findings. In ACLF, LSM correlated directly with liver size measured as longitudinal diameter (Supplementary Fig. 2A, http://links.lww.com/CCX/B306), and the highest values were found in alcoholic hepatitis, Wilson’s disease and in those admitted for gastrointestinal bleeding (LSM > 100 kPa).
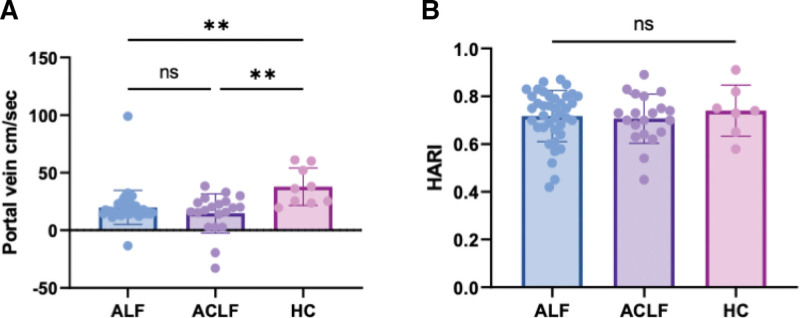
The estimation of the left lobe did not satisfy the minimum requirements of IQR/median greater than 30% in half of the patients, due to heartbeat artifacts and thus was deemed unreliable (data not shown). The admission spleen size and stiffness discriminated between ALF (10.4 ± 2 cm and 21.4 ± 16.6kPa) and ACLF (14 ± 2.3 cm and 42.6 ± 26 kPa, p < 0.0001 and p = 0.0009)(Supplementary Fig. 2G, http://links.lww.com/CCX/B306) but not between ALF and HC individuals (Fig. 1, C and D). Portal vein flow was reduced in both ALF (19.9 ± 14 cm/s) and ACLF (14.6 ± 17 cm/s) compared with HC individuals (37.9 ± 16 cm/s, Kruskal Wallis p = 0.0012), with some cases of reverse flow and no difference was seen between groups in terms of HARI (ANOVA, p = 0.7765) (Fig. 2, A and B).
Figure 2.
Vascular indices in acute liver failure. A, At admission, portal vein flow was reduced in both acute liver failure (ALF) (19.9 ± 14 cm/s) and acute-on-chronic liver failure (ACLF) (14.6 ± 17 cm/s) compared with controls (37.9 ± 16 cm/s) (Kruskall-Wallis), with some cases of reverse flow. B, No difference is seen in hepatic artery resistive index (HARI), between ALF 0.71 ± 0.1, ACLF 0.71 ± 0.1, healthy control (HC) individuals 0.74 ± 0.1 (analysis of variance). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns = not statistically significant.
Increased LSM After 1 Week Of Admission is a Poor Prognostic Feature of ALF
At admission to liver intensive care, LSM was not different between ALF patients who spontaneously survived versus those who died or were transplanted in the following 90 days (Supplementary Fig. 3A, http://links.lww.com/CCX/B306).
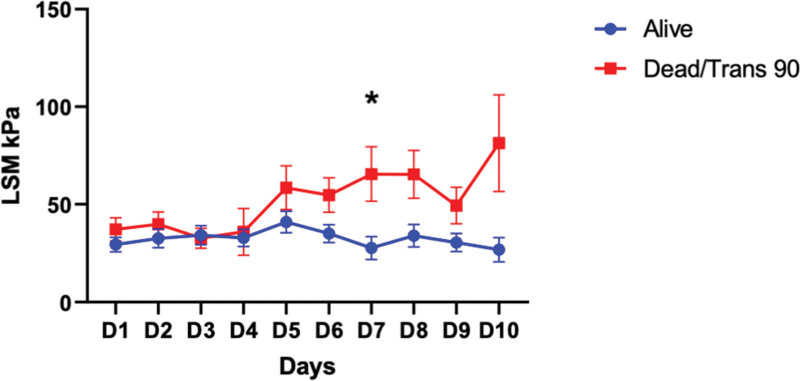
However, the trend over the first 10 days of admission was different, with a peak of LSM at day 5 in spontaneous survivors and afterward a reduction during the recovery phase. Conversely, patients deceased or requiring LT within the following 90 days showed a persistently increased LSM. This was significantly different at day 7 of admission between the two groups (Fig. 3). Furthermore, the ratio between day 7 LSM and baseline showed a trend, although not significant, of increment in ALF patients with poor prognosis (Supplementary Fig. 3A, http://links.lww.com/CCX/B306). Similar results were found if considering the disease onset rather than the admission as day 1 (Supplementary Fig. 4, http://links.lww.com/CCX/B306), after day 8 of illness patients spontaneously survived had consistently reduced values of LSM compared with the ones with poor prognosis.
Figure 3.
Trends in liver stiffness during the study period. The trend over the first 10 days of admission is different with a peak of liver stiffness measurement (LSM) at day 5 in spontaneous survivors and following reduction during the recovery phase. Conversely, patients deceased or requiring liver transplants within the following 90 days show a persistently increase LSM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
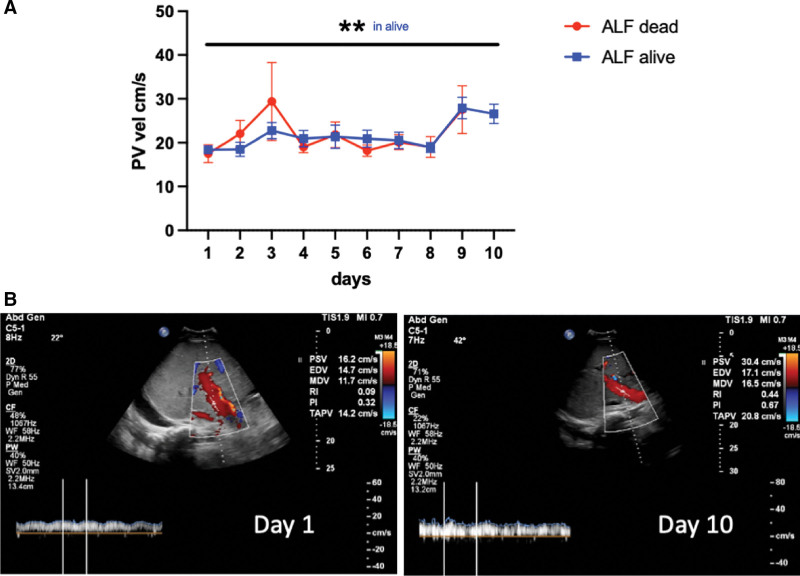
Portal vein flow was reduced in ALF and was stable during the first week with increase in velocity at days 9–10 in patients alive at 90 days postadmission and a significant difference between baseline and day 10 (Fig. 4). In patients with poor prognosis, the trend is less stable with detection also of reverse flow, and without difference between baseline and end of the follow-up.
Figure 4.
Trends in portal venous flow during the study period. A, Portal vein flow is reduced in acute liver failure (ALF), stable during the first week with increase in velocity at days 9–10 in patients alive at 90 days postadmission, with a significant difference between baseline and D10. In patients with poor prognosis, the trend is less stable with detection also of reverse flow, and without difference between baseline and end of the follow-up. B, Example of increase in portal vein velocity in acute liver failure at day 10 compared with baseline, time-averaged peak velocity (TAPV) 14.2 cm second day 1 vs. 20.8 cm/s on day 10. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
LSM Dynamic Changes During Admission Might be Related to Liver Synthetic Function and Coagulation Cascade in ALF
No correlation was found with the amount of noradrenaline used (Spearman r = 0.2846), the MAP (r = –0.109), the central venous pressure (CVP) (r = 0.1544), the heart rate (r = 0.0974), the SVRI (r = –0.3440), the SVV (r = –0.1941), the ELWI (r = 0.0455), the GEDVI (r = 0.0944). Only CI was directly correlated with LSM (Spearman r = 0.4414, p = 0.0348).
At admission, soluble platelet-endothelial cell adhesion molecule-1 (sPECAM-1) was not different between ALF, ACLF, and HC individuals, whereas vWF was increased in acute liver failure syndromes (ALF = 182.9 ± 149.6, ACLF = 215.6 ± 150.8 μg/mL) compared with HC individuals (36.76 ± 14.5) (Kruskall-Wallis p = 0.0001) (Supplementary Fig. 5A, http://links.lww.com/CCX/B306).
No correlation was found between sPECAM-1 (r = 0.565) or vWF(r = –0.335) and LSM at admission.
Proinflammatory cytokines were increased in both liver failure syndromes compared with controls (Supplementary Fig. 5C, http://links.lww.com/CCX/B306); however, no cytokines or liver function tests (including bilirubin, AST, ALP, gamma-glutamyl transferase (GGT), international normalized ratio [INR], APTR, and fibrinogen) were correlated with the LSM at baseline. Interestingly at day 7, LSM was directly correlated with INR (Spearman r = 0.6447, p = 0.0029), APTR (r = 0.8176, p < 0.0001), and fibrinogen (r = –0.5215, p = 0.022) but not with AST, bilirubin, or platelets count (Supplementary Fig. 3C, http://links.lww.com/CCX/B306). Multiple linear regression was used to test if bilirubin, fibrinogen, platelets, and LSM at day 7 significantly predicted mortality or LT within 90 days. The overall regression was statistically significant (R2 = 0.788, F(4–14) = 12.390, p < 0.001). Additionally, coefficients were further assessed to ascertain the influence of each of the factors and it was found that bilirubin (B = 0.001, t = 2.817, p = 0.014) and fibrinogen (B = –0.223, t = –3.589, p = 0.003) significantly predicted mortality.
Cytoplasmic Cholestasis was Directly Correlated With High LSM
Ten ALF explants and six ACLF explants/biopsies were analyzed. All ACLF samples presented cirrhosis, as per the definition, whereas the ALF case did not show any fibrosis. Confluent hepatocellular necrosis characterized ALF (mean score 4, vs. ACLF mean score 0.3), while intralobular inflammation was a feature present in both diseases (mean score 2 in ALF and 2.5 in ACLF). Cholestasis, in particular in the cytoplasmic compartment was present mainly in ACLF (mean score 1.6, vs. ALF mean score 0.8) and correlated with high values of LSM (Spearman r = 0.54, p = 0.034) (Supplementary Fig. 5B, http://links.lww.com/CCX/B306). Ballooning (mean score ACLF 1.3 vs. ALF 1.4) and steatosis (ALF micro 11.5 ± 12, macro 27.8 ± 28.8% vs. ACLF micro 5.8 ± 6.6%, macro 31.6 ± 28.2%) was not correlated with LSM.
DISCUSSION
The aim of this pilot study was to assess the reliability and feasibility of ultrasound and elastography monitoring in patients with acute liver failure.
The current transplant criteria identify ALF patients with poor prognosis with 58% sensitivity and 89% specificity (10) thus there is an urgent need for new bedside tools to aid in prognostication. We evaluated the prognostic role of LSM in the context of ALF given the conflicting evidence in the literature and lack of comparison with ACLF.
Upon admission to liver intensive care, usually within the first 48 hours postacute insult, there was no difference in LSM between patients who spontaneously survived and those who died or were transplanted within the following 90 days.
However, the trend during the first 10 days of admission was different between the two groups. LSM peaked at day 5 in spontaneous survivors with subsequent reduction during the recovery phase. In contrast, patients with a poor prognosis exhibited persistently increased LSM, and by day 7 of admission, the two groups displayed statistically different LSM values. We also calculated a ratio between day 7 LSM and baseline, which showed a trend, although not statistically significant, of increment in patients with a poor prognosis. Similarly, analyzing the data according to disease onset and therefore excluding the subacute live failure patients that constituted a separated group, by day 8 of illness the patients with poor prognosis showed a progressively increased LSM.
Our results also demonstrate that LSM and spleen size and stiffness have potential in the triage of patients presenting to intensive care with features of acute liver failure. Preexisting liver diseases are often unknown and a liver biopsy might not be available in a timely manner, but ACLF patients show remarkably elevated measurements of liver stiffness compared with ALF and values usually seen in stable cirrhosis.
Elastography finds, indeed, one of its major applications in chronic liver disease, gaining a role in noninvasive assessment of liver fibrosis, cirrhosis (25), and portal hypertension (11). It has been also included in the Baveno VII criteria to describe clinically significant portal hypertension (CSPH) (26). Despite being less studied increased portal pressure gradient is one of the features of ALF, associated with systemic vasodilation and a hyperkinetic circulatory state, with decreased arterial pressure and peripheral resistance, and increased cardiac output (27, 28).
In our cohort portal vein flow was reduced in ALF and remained stable during the first week, with increase in velocity at days 9–10 in patients alive at 90 days postadmission. This change showed a reversibility of portal hypertension once the liver injury improved.
We hypothesized that in part what we measure with elastography could be secondary to vascular changes, portal hypertension, and liver congestion, even if there are probably multiple factors leading to the increased stiffness in ALF. Recent literature indeed emphasizes the importance of liver stiffness in heart failure (20, 29) as a measure of liver congestion correlated with CVP. To exclude a possible confounder due to the cardiovascular status of these patients, we used invasive cardiac monitoring measurements assessing CVP together with other hemodynamic parameters. Only CI was found to be correlated with high LSM.
In cases of acute liver injury, thromboinflammation-mediated liver damage with deposition of platelet-rich thrombi within hepatic sinusoids (30) has been reported. Therefore we measured circulating vWF and PECAM-1. vWF has been shown to correlate with hepatic venous pressure gradient (31, 32) and is predictive of CSPH. This is a glycoprotein involved in arterial thrombus formation, released into the circulation by endothelial cells. It is known that vWF levels are elevated in patients with ALF (33, 34) and conversely, ADAMTS13 (also known as vWF-cleaving protease) activity is usually low. No correlation was found between vWF, sPECAM-1, and the increased LSM at admission suggesting flow in the larger vessels as opposed to microcirculatory thrombi was driving this association.
No cytokines or liver function tests (including bilirubin, AST, ALP, GGT, INR, APTR, or fibrinogen) were found to be correlated with the LSM at baseline; however, at day 7, LSM was directly correlated with INR, APTR, and fibrinogen but not with AST, bilirubin, or platelets count. This finding suggests that the persistence of increased LSM is somehow related to synthetic dysfunction rather than inflammation.
Furthermore, explants and biopsies from both ALF and ACLF were analyzed and cytoplasmic cholestasis correlated with increased LSM but not with necrosis.
In ACLF the highest values were found in alcoholic hepatitis, Wilson’s disease, and patients admitted for upper gastrointestinal bleeding (LSM > 100 kPa). LSM correlated directly with the size of the liver and this is surprising and suggests that what we were measuring was more than simple cirrhosis, in which the volume is usually reduced. What is interesting is that the very high LSM (> 50 kPa) was not explained by higher AST, bilirubin, or IL-6.
Thus, the reason behind the increased liver stiffness in acute liver failure syndromes still needs to be elucidated but inflammation alone is insufficient to explain it and cardiac output is an important determinant.
This was a pilot study to find the elements to build a feasible and useful protocol. A better practice could be a triage scan at baseline to correctly identify chronic for pure acute patients with a follow-up between days 5 and 7 of admission and a late scan at day 10 and after to help with prognostication and in the decision-making regarding the recovery. We acknowledge the presence of overlap of values between ACLF and ALF and we feel that data are not sufficient at this point to provide a clear cutoff criterion to prognosticate or discriminate. Furthermore, this study lacks external validation, but we aim to overcome all these limitations during future studies. The role of plasmapheresis in changing the inflammatory microenvironment and therefore potentially LSM was not assessed due to the small number of patients undertaking this procedure (n = 13), but will be included in future larger studies as an important covariate.
This is a real-life setting so as expected not all patients had all 10-day measurements due to clinical endpoints such as LT, discharge, or death before the end of the protocol. Furthermore, LT affects the natural history for acute liver failure, and patients who completed the 10 days of admission with conservative treatment either did not fulfill the King’s College Criteria or were excluded due to psychosocial reasons, which could represent a selection bias.
The lack of invasive hepatic pressure monitoring is another limitation in this setting, but at the same time, these patients are too unstable to go to the hemodynamic liver laboratory and are seldom clinically indicated. The reproducibility of the technique was not assessed, since all the measurements were done by a single operator and this will be addressed in future studies.
CONCLUSIONS
In ALF liver stiffness is increased, but less than in ACLF, and peaks at day 5 of admission with subsequent reduction in patients spontaneously survived. Cardiovascular changes, inflammatory markers, cytokines, vWF, and sPECAM-1 cannot yet explain the increased LSM; however at day 7 it is correlated with synthetic dysfunction (INR, APTR, and fibrinogen). Histology suggests that cytoplasmic cholestasis may contribute to higher stiffness. Further studies, in a larger cohort, are needed to better understand the diagnostic role of liver stiffness in acute liver failure and whether the addition of these values to existing prognostic criteria could improve their sensitivity and specificity.
Supplementary Material
Footnotes
This work was supported by the MRC IAA 2021 Kings College London (MR/X502923/1), King's Health Partners and King's College Hospital Charity.
The authors have no disclosures or conflicts of interest relevant to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Trovato FM, Rabinowich L, McPhail MJW: Update on the management of acute liver failure. Curr Opin Crit Care 2019; 25:157–164 [DOI] [PubMed] [Google Scholar]
- 2.Bower WA, Johns M, Margolis HS, et al. : Population-based surveillance for acute liver failure. Am J Gastroenterol 2007; 102:2459–2463 [DOI] [PubMed] [Google Scholar]
- 3.Bernal W, Hyyrylainen A, Gera A, et al. : Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol 2013; 59:74–80 [DOI] [PubMed] [Google Scholar]
- 4.Moreau R, Jalan R, Gines P, et al. ; CANONIC Study Investigators of the EASL–CLIF Consortium: Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013; 144:1426–37, 1437.e1 [DOI] [PubMed] [Google Scholar]
- 5.Mezzano G, Juanola A, Cardenas A, et al. : Global burden of disease: Acute-on-chronic liver failure, a systematic review and meta-analysis. Gut 2022; 71:148–155 [DOI] [PubMed] [Google Scholar]
- 6.Trovato FM, Zia R, Artru F, et al. : Lysophosphatidylcholines modulate immunoregulatory checkpoints in peripheral monocytes and are associated with mortality in people with acute liver failure. J Hepatol 2023; 78:558–573 [DOI] [PubMed] [Google Scholar]
- 7.Trovato FM, Zia R, Napoli S, et al. : Dysregulation of the lysophosphatidylcholine/autotaxin/lysophosphatidic acid axis in acute-on-chronic liver failure is associated with mortality and systemic inflammation by lysophosphatidic acid-dependent monocyte activation. Hepatology 2021; 74:907–925 [DOI] [PubMed] [Google Scholar]
- 8.Larsen FS, Schmidt LE, Bernsmeier C, et al. : High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol 2016; 64:69–78 [DOI] [PubMed] [Google Scholar]
- 9.Karvellas CJ, Francoz C, Weiss E: Liver transplantation in acute-on-chronic liver failure. Transplantation 2021; 105:1471–1481 [DOI] [PubMed] [Google Scholar]
- 10.McPhail MJ, Farne H, Senvar N, et al. : Ability of King’s College criteria and Model For End-Stage liver disease scores to predict mortality of patients with acute liver failure: A meta-analysis. Clin Gastroenterol Hepatol 2016; 14:516–525.e5; quiz e43 [DOI] [PubMed] [Google Scholar]
- 11.European Association for the Study of the Liver. Electronic address eee, Clinical Practice Guideline P, Chair, et al. : EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J Hepatol 2021; 75:659–689 [DOI] [PubMed] [Google Scholar]
- 12.Ferraioli G, Barr RG: Ultrasound liver elastography beyond liver fibrosis assessment. World J Gastroenterol 2020; 26:3413–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlas TF, Pfrepper C, Rosendahl J, et al. : Acoustic radiation force impulse (ARFI) elastography in acute liver failure: Necrosis mimics cirrhosis. Z Gastroenterol 2011; 49:443–448 [DOI] [PubMed] [Google Scholar]
- 14.Kuroda H, Takikawa Y, Onodera M, et al. : Serial changes of liver stiffness measured by acoustic radiation force impulse imaging in acute liver failure: A case report. J Clin Ultrasound 2012; 40:99–104 [DOI] [PubMed] [Google Scholar]
- 15.Kuroda H, Kakisaka K, Oikawa T, et al. : Liver stiffness measured by acoustic radiation force impulse elastography reflects the severity of liver damage and prognosis in patients with acute liver failure. Hepatol Res 2015; 45:571–577 [DOI] [PubMed] [Google Scholar]
- 16.Kreimeyer H, Buechter M, Best J, et al. : Performance of the liver maximum function capacity test, fibrinogen, and transient elastography in patients with acute liver injury. Dig Dis 2023; 41:259–267 [DOI] [PubMed] [Google Scholar]
- 17.Zabron A, Quaglia A, Fatourou E, et al. : Clinical and prognostic associations of liver volume determined by computed tomography in acute liver failure. Liver Int 2018; 38:1592–1601 [DOI] [PubMed] [Google Scholar]
- 18.Meister P, Dechene A, Buchter M, et al. : Spleen stiffness differentiates between acute and chronic liver damage and predicts hepatic decompensation. J Clin Gastroenterol 2019; 53:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soloveva A, Kobalava Z, Fudim M, et al. : Relationship of liver stiffness with congestion in patients presenting with acute decompensated heart failure. J Card Fail 2019; 25:176–187 [DOI] [PubMed] [Google Scholar]
- 20.Saito Y, Kato M, Nagashima K, et al. : Prognostic relevance of liver stiffness assessed by transient elastography in patients with acute decompensated heart failure. Circ J 2018; 82:1822–1829 [DOI] [PubMed] [Google Scholar]
- 21.Barr RG, Wilson SR, Rubens D, et al. : Update to the Society of Radiologists in ultrasound liver elastography consensus statement. Radiology 2020; 296:263–274 [DOI] [PubMed] [Google Scholar]
- 22.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Audimoolam VK, McPhail MJ, Willars C, et al. : Predicting fluid responsiveness in acute liver failure: A prospective study. Anesth Analg 2017; 124:480–486 [DOI] [PubMed] [Google Scholar]
- 24.Ishak K, Baptista A, Bianchi L, et al. : Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22:696–699 [DOI] [PubMed] [Google Scholar]
- 25.Trovato FM, Atzori S, Musumeci G, et al. : Liver and spleen transient elastography and acoustic radiation force impulse measurements. Performance and comparison of measurements in the same area concurrently assessed for liver fibrosis by biopsy. Adv Med Sci 2015; 60:300–306 [DOI] [PubMed] [Google Scholar]
- 26.de Franchis R, Bosch J, Garcia-Tsao G, et al. ; Baveno VII Faculty: Baveno VII—renewing consensus in portal hypertension. J Hepatol 2022; 76:959–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navasa M, Garcia-Pagan JC, Bosch J, et al. : Portal hypertension in acute liver failure. Gut 1992; 33:965–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valla D, Flejou JF, Lebrec D, et al. : Portal hypertension and ascites in acute hepatitis: Clinical, hemodynamic and histological correlations. Hepatology 1989; 10:482–487 [DOI] [PubMed] [Google Scholar]
- 29.Dhillon JK, Fong MW, Fong TL: Use of liver stiffness measurements in acute decompensated heart failure: New applications of a non-invasive technique. ESC Heart Fail 2022; 9:2800–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris SM, Chauhan A: The role of platelet mediated thromboinflammation in acute liver injury. Front Immunol 2022; 13:1037645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Mura V, Reverter JC, Flores-Arroyo A, et al. : Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut 2011; 60:1133–1138 [DOI] [PubMed] [Google Scholar]
- 32.Ferlitsch M, Reiberger T, Hoke M, et al. : von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology 2012; 56:1439–1447 [DOI] [PubMed] [Google Scholar]
- 33.Driever EG, Stravitz RT, Zhang J, et al. : VWF/ADAMTS13 imbalance, but not global coagulation or fibrinolysis, is associated with outcome and bleeding in acute liver failure. Hepatology 2021; 73:1882–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hugenholtz GC, Adelmeijer J, Meijers JC, et al. : An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: Implications for hemostasis and clinical outcome. Hepatology 2013; 58:752–761 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.