Abstract
Background/Objectives:
Pregnant women are exposed to numerous endocrine disrupting chemicals (EDCs) that can affect hormonal pathways regulating pregnancy outcomes and fetal development. Thus, we evaluated overall and fetal sex-specific associations of phthalate/replacement, paraben, and phenol biomarkers with sex-steroid and thyroid hormones.
Methods:
Illinois women (n = 302) provided plasma for progesterone, estradiol, testosterone, free T4 (FT4), total T4 (TT4), and thyroid stimulating hormone (TSH) at median 17 weeks gestation. Women also provided up-to-five first-morning urine samples monthly across pregnancy (8–40 weeks), which we pooled to measure 19 phthalate/replacement metabolites (reflecting ten parent compounds), three parabens, and six phenols. We used linear regression to evaluate overall and fetal sex-specific associations of biomarkers with hormones, as well as weighted quantile sum and Bayesian kernel machine regression (BKMR) to assess cumulative associations, non-linearities, and chemical interactions.
Results:
In women of relatively high socioeconomic status, several EDC biomarkers were associated with select hormones, without cumulative or non-linear associations with progesterone, FT4, or TT4. The biomarker mixture was negatively associated with estradiol (only at higher biomarker concentrations using BKMR), testosterone, and TSH, where each 10% mixture increase was associated with −5.65% (95% CI: −9.79, −1.28) lower testosterone and −0.09 μIU/mL (95% CI: −0.20, 0.00) lower TSH. Associations with progesterone, testosterone, and FT4 did not differ by fetal sex. However, in women carrying females, we identified an inverted u-shaped relationship of the mixture with estradiol. Additionally, in women carrying females, each 10% increase in the mixture was associated with 1.50% (95% CI: −0.15, 3.18) higher TT4, whereas in women carrying males, the mixture was associated with −1.77% (95% CI: −4.08, 0.58) lower TT4 and −0.18 μIU/mL (95% CI: −0.33, −0.03) lower TSH. We also identified select chemical interactions.
Conclusion:
Some biomarkers were associated with early-to-mid pregnancy hormones. There were some sex-specific and non-linear associations. Future studies could consider how these findings relate to pregnancy/birth outcomes.
Keywords: Endocrine disrupting chemical, Hormone, Pregnancy, Phthalate, Phenol, Paraben, Fetal sex
1. Introduction
Successful, healthy pregnancies require a multitude of coordinated physiological changes, including shifts in many hormones. Sex-steroid hormones, such as progesterone, estradiol, and testosterone, are derived from cholesterol, synthesized along the same biosynthetic pathways, and play various roles in healthy pregnancy, including maintaining pregnancy, preventing uterine contractions, and increasing uterine blood supply (Hacker et al., 2010). Thyroid hormones, such as free T4 (FT4), total T4 (TT4), and thyroid stimulating hormone (TSH), also have roles in pregnancy and fetal development, with both hypothyroidism and hyperthyroidism linked to preterm delivery, pre-eclampsia, intrauterine growth restriction, and developmental disabilities (Silva et al., 2018). There is also sex-steroid and thyroid hormone crosstalk, with thyroid hormones influencing sex-steroid hormone synthesis, transport, and elimination and sex-steroid hormones influencing thyroid hormones through feedback loops (Duarte-Guterman et al., 2014; Ren and Zhu, 2022). As normal hormonal processes need to be intricately maintained during pregnancy, any factors that perturb gestational hormones could pose health concerns and should be identified.
Pregnant women are ubiquitously exposed to non-persistent endocrine disrupting chemicals (EDCs), with virtually all women having detectable concentrations of EDC biomarkers in their urine, despite rapid metabolism and excretion from the body (CDC, 2019; Woodruff et al., 2011). EDCs are found in many consumer products (CDC, 2019; Haggerty et al., 2021). For example, di-2-ethylhexyl phthalate (DEHP) is a plasticizer used in food processing and diethyl phthalate (DEP) is a scent stabilizer used in personal care products and cosmetics ( Guo and Kannan, 2013; Hauser and Calafat, 2005; National Research Council US Committee on the Health, 2008). Parabens, such as propylparaben, are predominately used as antimicrobials in personal care products and cosmetics (Guo and Kannan, 2013; Wei et al., 2021). Phenols are a broad chemical group used for many purposes. For example, bisphenols, such bisphenol A (BPA), are used in plastics, benzophenone-3 (BP-3) is used in UV blockers, and dichlorophenols, such as 2,4-dichlorophenol (2,4-DCP), are found in pesticides (Chen et al., 2016; Chen et al., 2023; Dodson et al., 2007; Mao et al., 2022; Sun et al., 2023; Vandenberg et al., 2007). Because of potential reproductive and developmental hazards of several EDCs, such as DEHP, replacements like di-2-ethylhexyl terephthalate (DEHTP) and di(isononyl) cyclohexane-1,2-dicarboxylate (DiNCH) were introduced (Silva et al., 2013; Silva et al., 2015; Silva et al., 2017; Zota et al., 2014). Likewise, bisphenol S (BPS) and F (BPF) were introduced as replacements for BPA (Ye et al., 2015). Unfortunately, consistent with the concept of regrettable substitution, recent studies have demonstrated that some phthalate and bisphenol replacements may have similar reproductive (Lee et al., 2020; Yland et al., 2022), cardiovascular (Abrantes-Soares et al., 2022), and oncological (Edaes and de Souza, 2022) impacts as the chemicals they were meant to replace, likely due to their endocrine disrupting properties.
Certain phthalates, parabens, and phenols have been characterized as EDCs based on decades of experimental evidence (Vandenberg et al., 2012). Unsurprisingly, many epidemiological studies have investigated relationships of single non-persistent EDCs with maternal sex-steroid (Aker et al., 2019; Banker et al., 2021; Cathey et al., 2019; Johns et al., 2015; Kolatorova et al., 2018; Pacyga et al., 2021; Sathyanarayana et al., 2014; Sathyanarayana et al., 2017) and thyroid (Aker et al., 2019; Aker et al., 2016; Aung et al., 2017; Berger et al., 2018; Derakhshan et al., 2021; Derakhshan et al., 2019; Huang et al., 2022; Nakiwala et al., 2022; Romano et al., 2018; Sarzo et al., 2022; Souter et al., 2020; Yang et al., 2022) hormones. Generally, most studies investigating associations of phthalates, parabens, and phenols with sex-steroid hormones have been mixed. For example, a study from Michigan Mother-Infant Pairs (MMIP) studied six phenols and four parabens and reported negative associations of BPS with estradiol and propylparaben and methylparaben with progesterone but reported no associations with testosterone. In contrast, the PROTECT study from Puerto Rico investigated the same EDC biomarkers and only reported that triclosan was positively associated with 2nd trimester testosterone (Aker et al., 2019). Similarly, studies assessing associations of phthalates with sex-steroid hormones have reported null findings (Banker et al., 2021), select positive associations (Cathey et al., 2019; Pacyga et al., 2021), and select negative associations (Cathey et al., 2019; Johns et al., 2015; Sathyanarayana et al., 2014; Sathyanarayana et al., 2017). Studies assessing EDCs and thyroid hormones are similarly mixed, though, in general, higher exposure has been associated with disrupted T4 and TSH concentrations (Berger et al., 2018; Huang et al., 2018; Romano et al., 2018; Wang et al., 2017; Yao et al., 2016). Although pregnant women are not exposed to single chemicals, few studies have considered potential mixture effects and non-linear interactions. To the best of our knowledge, no studies have assessed associations of EDC mixtures with gestational sex-steroid hormones. In contrast, recent studies have utilized various mixtures approaches to assess cumulative associations of EDCs and thyroid hormones, but with mixed results, likely due to use of potentially problematic methods, such as including co-exposures as covariates or only assessing single classes of EDCs (Berger et al., 2018; Derakhshan et al., 2021; Huang et al., 2022; Nakiwala et al., 2022; Romano et al., 2018; Sarzo et al., 2022; Souter et al., 2020; Yang et al., 2022). While many studies reported null findings, some identified negative associations of various non-persistent EDC mixtures with maternal serum free T3 (FT3), total T3 (TT3), total T4 (TT4), thyroid peroxidase antibody (TPOAB), and T3/T4 ratio. Despite the fact that maternal hormone levels differ by fetal sex (Meulenberg and Hofman, 1991; Sitoris et al., 2022; Toriola et al., 2011), only a few studies assessed and identified differences in the relationship between single EDCs and hormones by fetal sex (Banker et al., 2021; Pacyga et al., 2021; Sathyanarayana et al., 2014). Of the studies assessing EDCs as a mixture, only one explored differences by fetal sex, reporting a negative association of a bisphenol mixture with FT3 in women carrying males and a positive association in women carrying females (Huang et al., 2022).
Because women are exposed to multiple non-persistent EDCs during pregnancy and proper hormone balance is critical for pregnancy health, our primary objective was to evaluate individual and cumulative associations of multiple classes of non-persistent EDC biomarkers with early-to-mid pregnancy sex-steroid and thyroid hormones. One difficulty in studying EDCs is the potential presence of non-monotonic dose response curves, such as low dose responses, which have been identified in in vitro and in vivo experiments for many EDCs, including phthalates and phenols (Vandenberg et al., 2012; Vandenberg et al., 2007). Therefore, to better understand how individual biomarkers within the mixture interact to affect gestational hormones, we additionally explored potential non-linear dose–response relationships and chemical-chemical interactions. As maternal sex-steroid and thyroid hormone levels may differ in women carrying females and males (Klinga et al., 1978; Sitoris et al., 2022; Toriola et al., 2011), we also evaluated whether associations differed by fetal sex.
2. Materials and methods
2.1. Illinois KIDS development study (I-KIDS) study design and population
Pregnant women were recruited into I-KIDS, a prospective pregnancy and birth cohort, from two local obstetric clinics in Champaign-Urbana, Illinois to evaluate associations of prenatal environmental chemical exposures with neurodevelopment. Recruitment and enrollment have been previously detailed (Pacyga et al., 2021; Pacyga et al., 2022; Pacyga et al., 2023). To be eligible to participate, women had to be ≤ 15 weeks pregnant at enrollment, 18–40 years old, fluent in English, in a low-risk pregnancy (determined by a medical provider), not carrying multiples, residing within 30 min of the study site, and not planning on moving before their child’s first birthday. The current study includes 302 women enrolled between 2015 and 2018, who remained in the study through the birth of their child, had urinary EDC biomarkers measured, and had available measurements of at least one sex-steroid or thyroid hormone in maternal plasma (Supp. Fig. 1). All women provided written informed consent, and the study was approved by the University of Illinois’ Institutional Review Board. The analysis of de-identified specimens at the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute human subjects’ research.
2.2. Collection of maternal sociodemographic, lifestyle, and health information
After enrollment (median 13 weeks gestation), I-KIDS staff conducted home visits to interview women about various sociodemographic and lifestyle factors. Pre-pregnancy body mass index (BMI) (kg/m2) was calculated from self-reported pre-pregnancy weight and height. To measure early pregnancy stress levels, women completed the Perceived Stress Scale (PSS), a ten-item questionnaire asking about thoughts and feelings during the last month (Cohen et al., 1983; Cohen and Williamson, 1988). At median 13 weeks gestation, women also completed a semi-quantitative food-frequency questionnaire (FFQ) adapted from the full-length Block-98 FFQ (NutritionQuest, Berkely, CA) and validated in pregnant populations (Bodnar and Siega-Riz, 2002; Boucher et al., 2006; Laraia et al., 2007). Dietary intakes representing diet patterns from the previous three months were used to calculate early pregnancy Alternative Healthy Eating Index (AHEI-2010), an 11-component diet quality index (out of 110 total points) based on foods and nutrients shown to be predictive of chronic disease risk and mortality, where a higher score indicates better overall diet quality (Chiuve et al., 2012; McCullough et al., 2002). As the AHEI-2010 considers moderate alcohol consumption beneficial but guidelines recommend avoiding alcohol in pregnancy, we removed the alcohol component to create a ten-component diet quality index (maximum: 100 points).
2.3. Assessment of urinary phthalate/replacement, paraben, triclocarban, and phenol biomarker concentrations
Because non-persistent EDCs have relatively short biological half-lives (6–24 h depending on the chemical) and high within-person variability (Shin et al., 2019a; Shin et al., 2023; Shin et al., 2019b), we measured EDC biomarkers in five across-pregnancy urine samples that were physically pooled prior to chemical biomarker measurement. At study clinic/home visits or routine prenatal care clinic visits, women provided at least three and up to five first-morning urine samples at median 13, 17, 23, 28, 34 weeks gestation. Most women contributed all five samples (97 %) and the rest provided three or four samples (Pacyga et al., 2023). Details about urine collection, processing, and storage have been previously published (Pacyga et al., 2023). Briefly, women collected urine into polypropylene urine cups and refrigerated them for up to 24 h until we aliquoted samples for long-term storage. To create the pool, we added 900 μL of urine from the first collection to a five mL cryovial tube. At each subsequent visit, we layered fresh urine onto the previous frozen sample and immediately stored the sample at −80 °C. At the end of pregnancy, we thawed and vortexed the sample to measure specific gravity.
Frozen pooled urines were shipped to the CDC’s Division of Laboratory Sciences in four batches (batch 1: enrolled December 2013 – February 2015; batch 2: enrolled February 2015 - July 2016; batch 3: enrolled July 2016 – August 2018; batch 4: enrolled September 2018 – November 2019). Only women with chemicals measured in batches 2 and 3 were included in the current study because they had all chemical data and at least some hormone data. Using previously published isotope-dilution mass spectrometry methods with rigorous quality assurance/quality control protocols and high long-term reproducibility (Calafat et al., 2006; Calafat et al., 2010; Schantz et al., 2015; Silva et al., 2013; Silva et al., 2007; Silva et al., 2019; Ye et al., 2014), CDC laboratory staff quantified 19 phthalate/replacement metabolites: monocarboxynonyl phthalate (MCNP), monocarboxyoctyl phthalate (MCOP), monooxononyl phthalate (MONP), mono-isononyl phthalate (MiNP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(3-carboxypropyl) phthalate (MCPP), monobenzyl phthalate (MBzP), mono-n-butyl phthalate (MBP), monohydroxybutyl phthalate (MHBP), mono-isobutyl phthalate (MiBP), monohydroxy-isobutyl phthalate (MHiBP), monoethyl phthalate (MEP), cyclohexane-1,2-dicarboxylic acid-mono(carboxyoctyl) ester (MCOCH), cyclohexane-1,2-dicarboxylic acid-monohydroxy isononyl ester (MHiNCH), mono(2-ethyl-5-hydroxyhexyl) terephthalate (MEHHTP), and mono(2-ethyl-5-carboxypentyl) terephthalate (MECPTP). In addition, the CDC measured concentrations of triclocarban, four parabens (butylparaben, ethylparaben, methylparaben, propylparaben), and seven phenols (bisphenol A (BPA), bisphenol F (BPF), bisphenol S (BPS), triclosan (TCS), benzophenone-3 (BP-3), 2,4-dichlorphenol (2,4-DCP), 2,5-dichlorophenol (2,5-DCP)). The limits of detection (LOD) were 0.1–1.7 ng/mL, depending on the biomarker (Table 2).
Table 2.
Distributions of pooled urinary EDC biomarkers and early-to-mid pregnancy (median 17 weeks gestation) plasma hormones (n = 302) (2015–2018).
| Biomarker | LOD (ng/mL) | % ≥ LOD | Median (25th, 75th percentile) |
|---|---|---|---|
|
| |||
| Phthalate/replacement | |||
| Mono(2-ethylhexyl) phthalate (MEHP), ng/mL | 0.8 | 76.2 | 1.31 (0.85, 2.13) |
| Mono(2-ethyl5-carboxypentyl) phthalate (MECPP), ng/mL | 0.4 | 100.0 | 8.32 (6.07, 12.70) |
| Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), ng/mL | 0.4 | 100.0 | 5.36 (3.61, 8.17) |
| Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), ng/mL | 0.2 | 100.0 | 3.99 (2.86, 6.32) |
| Mono(3-carboxypropyl) phthalate (MCPP), ng/mL | 0.4 | 96.4 | 1.28 (0.87, 1.91) |
| Monobenzyl phthalate (MBzP), ng/mL | 0.3 | 99.3 | 5.17 (2.55, 10.31) |
| Monoethyl phthalate (MEP), ng/mL | 1.2 | 100.0 | 26.72 (13.68, 47.38) |
| Monocarboxynonyl phthalate (MCNP), ng/mL | 0.2 | 100.0 | 1.83 (1.35, 2.62) |
| Mono-n-butyl phthalate (MBP), ng/mL | 0.4 | 100.0 | 12.67 (8.50, 17.17) |
| Mono-hydroxybutyl phthalate (MHBP), ng/mL | 0.4 | 90.1 | 1.20 (0.75, 1.82) |
| Mono-isobutyl phthalate (MiBP), ng/mL | 0.8 | 99.7 | 8.66 (5.75, 13.99) |
| Mono-hydroxy-isobutyl phthalate (MHiBP), ng/mL | 0.4 | 99.7 | 3.11 (2.13, 5.43) |
| Monocarboxyoctyl phthalate (MCOP), ng/mL | 0.3 | 100.0 | 7.11 (4.59, 13.65) |
| Mono-isononyl phthalate (MiNP), ng/mL | 0.9 | 31.5 | 0.61 (<LOD, 1.08) |
| Monooxononyl phthalate (MONP), ng/mL | 0.4 | 100.0 | 2.63 (1.72, 4.63) |
| Cyclohexane-1,2-dicarboxylic acid-monohydroxy isononyl ester (MHiNCH), ng/mL | 0.4 | 91.1 | 1.13 (0.69, 2.21) |
| Cyclohexane-1,2-dicarboxylic acid-mono(carboxyoctyl) ester (MCOCH), ng/mL | 0.5 | 66.9 | 0.70 (<LOD, 1.20) |
| Mono(2-ethyl-5-hydroxyhexyl terephthalate (MEHHTP), ng/mL | 0.4 | 100.0 | 8.21 (3.83, 20.26) |
| Mono(2-ethyl5-carboxypentyl terephthalate (MECPTP), ng/mL | 0.2 | 100.0 | 59.56 (25.32, 138.29) |
| Paraben | |||
| Butyl paraben, ng/mL | 0.1 | 33.4 | <LOD (<LOD, 0.15) |
| Ethyl paraben, ng/mL | 1.0 | 57.0 | 1.37 (<LOD, 6.64) |
| Methyl paraben, ng/mL | 1.0 | 100.0 | 49.01 (19.30, 138.03) |
| Propyl paraben, ng/mL | 0.1 | 99.7 | 8.75 (2.31, 28.34) |
| Phenol | |||
| Bisphenol A (BPA), ng/mL | 0.2 | 96.7 | 0.85 (0.52, 1.46) |
| Bisphenol S (BPS), ng/mL | 0.1 | 99.0 | 0.48 (0.29, 0.80) |
| Bisphenol F (BPF), ng/mL | 0.2 | 57.0 | 0.29 (<LOD, 1.05) |
| Benzophenone 3 (BP-3), ng/mL | 0.4 | 99.7 | 117.44 (37.47, 293.71) |
| Triclosan (TCS), ng/mL | 1.7 | 92.1 | 8.67 (3.51, 56.28) |
| 2,4-Dichlorophenol (2,4-DCP), ng/mL | 0.1 | 100.0 | 0.58 (0.36, 0.99) |
| 2,5-Dichlorophenol (2,5-DCP), ng/mL | 0.1 | 99.7 | 1.40 (0.84, 3.28) |
| Other | |||
| Triclocarban, ng/mL | 0.1 | 29.8 | < LOD (<LOD, 0.14) |
| RR | % ≥ lower limit of RR | Median (25th, 75th percentile) | |
| Hormone | |||
| Progesterone, ng/mL | 0.20 – 40.00 | 100.0 | 28.30 (23.60, 32.90) |
| Estradiol, pg/mL | 20.00 – 2000.00 | 100.0 | 2745.00 (1983.30, 3830.00)* |
| Testosterone, ng/dL | 20.00 – 1600.00 | 87.29 | 46.50 (34.90, 66.70) |
| Free thyroxine (FT4), ng/dL | 0.30–0.60 | 100.0 | 0.93 (0.86, 0.99) |
| Total thyroxine (TT4), μ/dL | 1.00 – 24.00 | 100.0 | 8.94 (7.97, 9.89) |
| Thyroid stimulating hormone (TSH), μU/mL | Up to 75.00 | 99.7 | 1.77 (1.16, 2.46) |
Specific gravity-adjusted EDC biomarker concentrations.
Values are outside of manufacturer’s reportable range because estradiol was diluted 10x prior to analysis and machine-read values were multiplied by 10. EDCs, endocrine disrupting chemicals; LOD, limit of detection; RR, reportable range.
2.4. Collection and quantification of plasma maternal hormone concentrations
We collected maternal plasma at median 17 weeks gestation in glass heparin-containing vacutainer tubes, centrifuged them at room temperature for 20 min, and aliquoted them for storage at −80 °C. We sent the samples to the University of Michigan Diabetes Research Center (MDRC) Clinical Core Chemistry Laboratory for quantification of progesterone, estradiol, total testosterone, FT4, TT4, and TSH using solid-phase, enzyme-labeled chemiluminescent competitive immunoassay (IMMULITE 1000, Siemens). Briefly, 10–75 μL of plasma was incubated for 30 min with a polyclonal rabbit antibody coated bead (solid-phase) and bovine calf intestine alkaline phosphate conjugated to hormone (liquid-phase). After repeated washes and centrifugation to remove unbound hormone, the chemiluminescent substrate was added to measure the signal indicating the proportion of hormone bound. Progesterone had a reportable range of 0.20 – 40.0 ng/dL, an LOD of 0.20 ng/dL, and a functional sensitivity of 0.46 ng/mL. Estradiol had reportable range of 20.0 – 2000.0 pg/mL and an analytic sensitivity of 15.0 pg/mL. Testosterone had a calibration range of 20.0 – 1600.0 ng/dL and an analytic sensitivity of 15.0 ng/dL. FT4 had a reportable range of 0.3 – 6.0 ng/dL, an analytic sensitivity of 0.13 ng/dL, an LOD of 0.28 ng/dL, and a functional sensitivity of 0.3 ng/dL. TT4 had a calibration range of 1.0 – 24.0 μg/dL and an analytic sensitivity of 0.4 μg/dL. Finally, TSH had a calibration range of up to 75.0 μIU/mL and an analytic sensitivity of 0.01 μIU/mL. Based on protocol recommendations for when estradiol concentrations are above the assay’s reportable range, estradiol samples were diluted 10x before analysis and machine-read values were multiplied by ten to obtain final concentrations. For total testosterone, we considered values below the calibration range as missing (n = 37). We used the following formula for calculating one missing TSH value: minimum detected TSH concentration /√2.
2.5. Statistical analysis
2.5.1. Derivation of analytic sample
The derivation of our analytic sample is detailed in Supplemental Fig. 1. Briefly, of 10,178 women who completed a reply card, 688 enrolled in the study and 531 remained active through the birth of their child. Of these women, 302 had chemical biomarkers > 0, at least some hormone information, and all covariate information (all women were in chemical analysis batches 2 and 3). Of our analytic sample, 295 women had complete data on progesterone and estradiol, 250 women had complete testosterone data, and 294 women had complete FT4, TT4, and TSH data (Supp. Fig. 1). We summarized sociodemographic, health, and lifestyle factors in the reference population and our analytic sample as frequency (percent) or median (25th, 75th percentile) (Table 1).
Table 1.
Characteristics of I-KIDS women in analytic sample.
| Full samplea (n = 531) | Analytic sampleb (n = 302) | Women carrying females (n = 155) | Women carrying males (n = 147) | |
|---|---|---|---|---|
|
| ||||
| Characteristic | n (%) | |||
| Race/ethnicity | ||||
| Non-Hispanic White (ref) | 424 (80.0) | 246 (81.5) | 124 (80.0) | 122 (83.0) |
| Others | 106 (20.0) | 56 (18.5) | 31 (20.0) | 25 (17.0) |
| Education | ||||
| Some college or less (ref) | 103 (19.4) | 51 (16.9) | 21 (13.5) | 30 (20.4) |
| College graduate or higher | 428 (80.6) | 251 (83.1) | 134 (86.5) | 117 (79.6) |
| Ever smoker | ||||
| No (ref) | 435 (82.2) | 250 (82.8) | 123 (79.4) | 127 (86.4) |
| Yes | 94 (17.8) | 52 (17.2) | 32 (20.6) | 20 (13.6) |
| Parity | ||||
| No children (ref) | 272 (51.3) | 163 (54.0) | 83 (53.5) | 80 (54.4) |
| 1 + children | 258 (48.7) | 139 (46.0) | 72 (46.5) | 67 (45.6) |
| Fetal sex | ||||
| Male (ref) | 271 (51.1) | 147 (48.7) | - | - |
| Female | 259 (48.9) | 155 (51.3) | - | - |
| Median (25th, 75th percentile) | ||||
| Maternal age (years) | 30.0 (27.1, 32.7) | 30.4 (27.5, 32.8) | 30.5 (27.9, 32.7) | 30.3 (27.8, 32.9) |
| Pre-pregnancy BMI (kg/m2) | 24.6 (21.9, 29.4) | 24.7 (21.9, 28.9) | 24.4 (21.6, 28.2) | 25.0 (22.5, 30.3) |
| Early pregnancy Alternative Healthy Eating Index 2010*‡ | 51.5 (43.9, 59.7) | 51.7 (44.6, 59.3) | 53.2 (45.8, 61.8) | 50.1 (43.4, 57.8) |
| Early pregnancy perceived stress | 10.9 (6.9, 16.1) | 11.1 (7.1, 16.5) | 11.5 (7.2, 16.9) | 10.5 (6.9, 16.1) |
| Gestational age at blood collection | 16.9 (16.3, 17.7) | 16.9 (16.3, 17.6) | 16.8 (16.3, 17.7) | 17.0 (16.3, 17.6) |
Percentages may not add up to 100% due to missing (n missing): perceived stress score (8 missing in reference population; 2 missing in analytic sample), gestational age (1 missing in reference population).
Alcohol intake was removed from the index (total score out of 100).
Median (25th, 75th percentile) Alternative Healthy Eating Index 2010 excludes women whose diet data have not yet been analyzed (n = 49).
Women with at least one chemical biomarker.
Women with all chemical biomarkers and at least one hormone measurement. BMI, body mass index; I-KIDS, Illinois Kids Development Study.
2.5.2. Modeling of urinary chemical concentrations
For non-zero biomarker concentrations below the LOD, we used instrument-read values to avoid bias associated with imputing concentrations < LOD (73). In our statistical analyses, we only included chemical biomarkers with concentrations > 0 in at least 60 % of women (data not shown). This resulted in butylparaben, BPF, and triclocarban being excluded from further analyses. To avoid undefined estimates for ln-transformed zero concentrations (ethylparaben n = 3; BPA n = 2; and BPS, BP-3, and 2,4-DCP n = 1), we used the formula [ln(chemical concentration + 0.0001)] in linear regression and weighted quantile sums regression (WQSR). In Bayesian kernel machine regression (BKMR) models, we used the following formula in place of zero concentrations as it improved model fit and convergence: minimum measured concentration/√2. Including BPF in WQSR models had minimal impact on estimates and weights (data not shown).
We evaluated specific gravity adjusted phthalate/replacement, paraben, and phenol biomarkers as molar sums or individual biomarkers using the previously reported formula for specific gravity adjustment (Meeker et al., 2009). For phthalates/replacements, we used the urinary metabolite concentrations to approximate pregnant women’s exposure to phthalate/replacement parent compounds. We calculated parent molar sums (nmol/mL) by summing metabolites from common precursors: MEHP, MEHHP, MEOHP, and MECPP for the sum of DEHP metabolites (∑DEHP); MCOP, MiNP, and MONP for the sum of metabolites of di-isononyl phthalate (∑DiNP); MBP and MHBP for the sum of di-n-butyl phthalate metabolites (∑DBP); MiBP and MHiBP for the sum of di-isobutyl phthalate metabolites (∑DiBP); MHiNCH and MCOCH for the sum of DiNCH metabolites (∑DiNCH); and MEHHTP and MECPTP for the sum of DEHTP metabolites (∑DEHTP). Specific formulas have been published elsewhere (Pacyga et al., 2021) and are reported in table footers. Molar concentrations were back-converted to ng/mL by multiplying ∑DEHP, ∑DiNP, ∑DBP, ∑DiBP, ∑DiNCH, and ∑DEHTP by the molecular weights of MECPP, MCOP, MBP, MiBP, MHiNCH, and MECPTP, respectively (Pacyga et al., 2022; Rodriguez-Carmona et al., 2020; Zhang et al., 2020). We estimated exposure to di-isodecyl phthalate, di-n-octyl phthalate, benzylbutyl phthalate (BBzP), and DEP using ng/mL concentrations of their urinary metabolites MCNP, MCPP, MBzP, and MEP, respectively.
2.5.3. Covariate selection
Based on previous literature and our data (Huang et al., 2022; Nakiwala et al., 2022; Romano et al., 2018; Souter et al., 2020; Yang et al., 2022), we generated a directed acyclic graph (DAG) to identify a minimum sufficient adjustment set of covariates (Supp. Fig. 2). We assessed correlations between covariates to test for potential multicollinearity; however, all covariates were only weakly or moderately correlated (r < 0.4; data not shown). Our final linear regression, WQSR, and BKMR models accounted for maternal age, race/ethnicity, educational attainment, pre-pregnancy BMI, early pregnancy diet quality (AHEI-2010), stress (PSS 10), ever smoking, parity, gestational age at hormone assessment, and fetal sex. These variables may represent latent constructs, such as reproductive health (maternal age and parity), socioeconomic status (race/ethnicity and education), and health/lifestyle (pre-pregnancy BMI, stress, ever smoking, and diet quality). Age, pre-pregnancy BMI, diet quality, stress, and gestational age were continuous variables, whereas all other variables were categorized with the reference group as indicated in Table 1.
2.5.4. Evaluating associations of non-persistent EDC biomarkers with maternal hormones
To address our main objective, we evaluated associations between EDC biomarkers and gestational hormones using unadjusted and multivariable linear regression and WQSR. When modeled individually, we ln-transformed all phthalate/replacement, paraben, and phenol biomarkers due to their right-skewed distributions. In single-pollutant and WQSR models, because of non-normal distributions, we ln-transformed progesterone, estradiol, testosterone, FT4, and TT4. TSH was normally distributed and thus not transformed.
WQSR models cumulative associations and identifies individual biomarkers responsible for most of the mixture effect, while handling moderately and highly correlated co-exposures. Our mixture included 19 non-persistent EDC biomarkers, including ten phthalate/replacement metabolites or sums, three parabens, and six phenols (as listed in Tables 3 and 4). WQSR is a supervised mixture method that creates a weighted index by transforming exposure biomarkers into quantiles (deciles in this study) and evaluates the cumulative association of the index with the outcome using multiple linear regression (Carrico et al., 2015). We generated a distribution of results using 100 iterations (repeated holdouts), each with 100 bootstrap replications. Within each iteration, data were randomly split 40/60 % into training and validation datasets, respectively (Tanner et al., 2019). To determine the relative importance (weight) of single co-exposures within the mixture, we used the standard cut-off (1/# of co-exposures; 1/19 = 0.05) to identify meaningful contributors (Carrico et al., 2015).
Table 3.
Associations between urinary EDC biomarkers and sex-steroid hormones in I-KIDS women.
| Biomarkers | Progesterone |
Estradiol |
Testosterone |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All women (n = 295) | Carrying females (n = 151) | Carrying males (n = 144) | P int | All women (n = 295) | Carrying females (n = 150) | Carrying males (n = 145) | P int | All women (n = 250) | Carrying females (n = 128) | Carrying males (n = 122) | P int | |
|
| ||||||||||||
| %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | ||||
| Phthalates & Replacements1 | ||||||||||||
| ΣDEHP | −0.03 (−0.44, 0.39) | −0.15 (−0.74, 0.45) | 0.09 (−0.48, 0.66) | 0.57 | −0.11 (−0.83, 0.61) | −0.35 (−1.38, 0.70) | 0.10 (−0.89, 1.10) | 0.54 | −0.65 (−1.49, 0.20) | −0.49 (−1.72, 0.76) | −0.79 (−1.93, 0.36) | 0.72 |
| MCPP | 0.02 (−0.36, 0.39) | 0.09 (−0.40, 0.59) | −0.09 (−0.67, 0.49) | 0.63 | −0.42 (−1.08, 0.24) | −0.49 (−1.36, 0.39) | −0.33 (−1.34, 0.69) | 0.82 | −0.64 (−1.42, 0.14) | −0.58 (−1.65, 0.51) | −0.71 (−1.84, 0.43) | 0.86 |
| MCNP | 0.05 (−0.42, 0.52) | −0.21 (−0.83, 0.41) | 0.40 (−0.31, 1.11) | 0.20 | −0.52 (−1.33, 0.29) | −0.20 (−1.28, 0.88) | −0.94 (−2.15, 0.30) | 0.38 | −0.11 (−1.12, 0.91) | 0.22 (−1.06, 1.52) | −0.65 (−2.26, 1.00) | 0.41 |
| MBzP | −0.04 (−0.31, 0.23) | −0.17 (−0.54, 0.20) | 0.10 (−0.29, 0.48) | 0.32 | −0.17 (−0.64, 0.30) | −0.38 (−1.03, 0.27) | 0.05 (−0.62, 0.72) | 0.36 | 0.01 (−0.54, 0.57) | −0.06 (−0.83, 0.73) | 0.08 (−0.70, 0.87) | 0.81 |
| MEP | 0.02 (−0.29, 0.33) | −0.24 (−0.67, 0.18) | 0.28 (−0.15, 0.71) | 0.08 | −0.35 (−0.88, 0.19) | −0.33 (−1.07, 0.42) | −0.36 (−1.10, 0.39) | 0.96 | 0.52 (−0.13, 1.18) | 0.71 (−0.16, 1.58) | 0.29 (−0.67, 1.25) | 0.51 |
| ΣDiNP | 0.08 (−0.24, 0.40) | 0.15 (−0.25, 0.56) | −0.04 (−0.57, 0.49) | 0.57 | −0.17 (−0.74, 0.39) | −0.36 (−1.07, 0.35) | 0.14 (−0.78, 1.07) | 0.40 | –0.23 (−0.91, 0.46) | −0.09 (−0.98, 0.80) | −0.41 (−1.46, 0.64) | 0.64 |
| ΣDBP | 0.03 (−0.41, 0.47) | −0.21 (−0.77, 0.34) | 0.38 (−0.28, 1.05) | 0.16 | −0.63 (−1.38, 0.13) | −0.74 (−1.70, 0.23) | −0.47 (−1.62, 0.68) | 0.72 | 0.11 (−0.78, 1.02) | 0.19 (−0.95, 1.35) | −0.01 (−1.39, 1.40) | 0.83 |
| ΣDiBP | −0.01 (−0.36, 0.34) | −0.14 (−0.58, 0.29) | 0.23 (−0.35, 0.83) | 0.31 | −0.49 (−1.08, 0.11) | −0.38 (−1.13, 0.38) | −0.67 (−1.63, 0.30) | 0.64 | 0.37 (−0.33, 1.08) | 0.32 (−0.55, 1.20) | 0.46 (−0.73, 1.67) | 0.85 |
| ΣDiNCH | 0.00 (−0.29, 0.30) | −0.19 (−0.59, 0.20) | 0.24 (−0.19, 0.68) | 0.14 | −0.07 (−0.58, 0.44) | −0.26 (−0.95, 0.44) | 0.16 (−0.59, 0.91) | 0.42 | 0.15 (−0.47, 0.77) | 0.06 (−0.81, 0.93) | 0.24 (−0.65, 1.14) | 0.77 |
| ΣDEHTP | −0.16 (−0.41, 0.09) | −0.38 (−0.73, −0.02)* | 0.04 (−0.30, 0.38) | 0.09 | −0.17 (−0.61, 0.27) | −0.06 (−0.69, 0.57) | −0.27 (−0.87, 0.33) | 0.63 | –0.14 (−0.67, 0.39) | 0.20 (−0.56, 0.97) | −0.45 (−1.16, 0.27) | 0.22 |
| Parabens | ||||||||||||
| Ethylparaben | −0.02 (−0.16, 0.12) | −0.08 (−0.28, 0.12) | 0.03 (−0.15, 0.21) | 0.44 | −0.16 (−0.40, 0.09) | −0.06 (−0.41, 0.30) | −0.24 (−0.56, 0.08) | 0.44 | –0.09 (−0.37, 0.20) | −0.19 (−0.59, 0.22) | 0.00 (−0.38, 0.38) | 0.49 |
| Methylparaben | −0.13 (−0.35, 0.08) | −0.26 (−0.56, 0.04) | 0.00 (−0.31, 0.31) | 0.23 | −0.03 (−0.41, 0.35) | 0.12 (−0.40, 0.64) | −0.19 (−0.74, 0.35) | 0.41 | –0.30 (−0.75, 0.16) | −0.23 (−0.85, 0.39) | −0.38 (−1.03, 0.28) | 0.75 |
| Propylparaben | −0.13 (−0.29, 0.02) | −0.17 (−0.40, 0.05) | −0.10 (−0.32, 0.12) | 0.64 | 0.05 (−0.23, 0.33) | 0.20 (−0.19, 0.60) | −0.11 (−0.49, 0.28) | 0.27 | –0.45 (−0.78, −0.11) | −0.49 (−0.96, −0.02)* | −0.40 (−0.87, 0.07) | 0.79 |
| Phenols | ||||||||||||
| BPA | −0.11 (−0.38, 0.15) | −0.13 (−0.43, 0.16) | −0.01 (−0.62, 0.61) | 0.72 | −0.12 (−0.58, 0.35) | 0.11 (−0.40, 0.63) | −1.11 (−2.16, −0.05)* | 0.04 | 0.24 (−0.29, 0.77) | 0.48 (−0.10, 1.06) | −0.98 (−2.26, 0.33) | 0.05 |
| BPS | −0.02 (−0.29, 0.25) | 0.11 (−0.3, 0.52) | −0.12 (−0.49, 0.25) | 0.41 | 0.07 (−0.40, 0.55) | 0.73 (0.02, 1.44)* | −0.46 (−1.09, 0.18) | 0.01 | –0.35 (−0.94, 0.25) | −0.49 (−1.39, 0.41) | −0.23 (−1.01, 0.55) | 0.67 |
| BP-3 | −0.01 (−0.18, 0.16) | −0.11 (−0.32, 0.10) | 0.17 (−0.11, 0.44) | 0.12 | −0.10 (−0.39, 0.19) | −0.08 (−0.45, 0.28) | −0.14 (−0.62, 0.35) | 0.86 | 0.13 (−0.21, 0.47) | 0.15 (−0.27, 0.58) | 0.08 (−0.49, 0.65) | 0.84 |
| TCS | 0.04 (−0.12, 0.20) | 0.07 (−0.15, 0.29) | 0.01 (−0.22, 0.23) | 0.70 | 0.03 (−0.25, 0.31) | −0.03 (−0.41, 0.36) | 0.09 (−0.31, 0.49) | 0.67 | –0.36 (−0.69, −0.02) | −0.34 (−0.80, 0.11) | −0.37 (−0.86, 0.12) | 0.93 |
| 2,4-DCP | 0.17 (−0.17, 0.50) | 0.11 (−0.37, 0.58) | 0.22 (−0.24, 0.69) | 0.73 | −0.18 (−0.76, 0.41) | −0.09 (−0.92, 0.75) | −0.26 (−1.07, 0.55) | 0.77 | –0.73 (−1.40, −0.05) | −0.32 (−1.28, 0.64) | −1.11 (−2.03, −0.17)* | 0.25 |
| 2,5-DCP | 0.08 (−0.14, 0.30) | 0.02 (−0.32, 0.37) | 0.11 (−0.16, 0.39) | 0.68 | −0.22 (−0.61, 0.16) | −0.06 (−0.66, 0.54) | −0.32 (−0.80, 0.16) | 0.50 | –0.52 (−0.97, −0.07) | −0.36 (−1.06, 0.35) | −0.62 (−1.18, −0.06)* | 0.55 |
Data are presented as the percent change (%Δ) and 95% CI in plasma hormone concentrations with every 10% increase in chemical biomarker. All models account for educational attainment, age, diet quality, pre-pregnancy body mass index, perceived stress in early pregnancy, lifetime smoking status, parity, race/ethnicity, gestational age at plasma hormone assessment, and fetal sex. Linear regression models evaluated associations of individual chemical biomarkers with plasma hormones. Bold signifies potentially meaningful findings with asterisk
denoting statistically significant findings at P < 0.05.
ΣDEHP = (MEHP/278) + (MEHHP/ 294) + (MEOHP/292) + (MECPP/308); ΣDiNP = (MiNP/292) + (MCOP/322) + (MONP/306); ΣDBP = (MBP/222) + (MHBP/238); ΣDiBP = (MiBP/222) + (MHiBP/238); ΣDiNCH = (MHiNCH/ 314) + (MCOCH/328); and ΣDEHTP = (MEHHTP/294) + (MECPTP/308).
Abbreviations: BPA, bisphenol A; BPF, bisphenol F; BPS, bisphenol S; CI, confidence interval; EDCs, endocrine disrupting chemicals; ZDEHP, sum of di-2-ethylhexyl phthalate metabolites; Pint, Pinteraction; ΣDEHTP, sum of di-2-ethylhexyl terephthalate metabolites; ΣDiNP, sum of di-isononyl phthalate metabolites; ΣDiBP, sum of di-iso-butyl phthalate metabolites; ΣDiNCH, sum of di (isononyl) cyclohexane-1,2-dicarboxylate metabolites; TCS, triclosan; 2,4-DCP, 2,4-dichlorophenol; 2,5-DCP, 2,5-dichlorophenol.
Women missing covariates in progesterone and estradiol analyses (n = 5). Diet (n = 3), stress (n = 2).
Women missing covariates in testosterone analyses (n = 4). Diet (n = 2), stress (n = 2).
Table 4.
Associations between urinary EDCs biomarkers and thyroid hormones in I-KIDS women.
| Biomarkers | Free T4 |
Total T4 |
TSH |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All women (n = 294) | Carrying females (n = 150) | Carrying males (n = 144) | P int | All women (n = 294) | Carrying females (n = 150) | Carrying males (n = 144) | P int | All women (n = 294) | Carrying females (n = 150) | Carrying males (n = 144) | P int | |
|
| ||||||||||||
| %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | %Δ (95 % CI) | μIU/mL Δ (95 % CI) | μIU/mL Δ (95 % CI) | μIU/mL Δ (95 % CI) | ||||
| Phthalates & Replacements 1 | ||||||||||||
| ΣDEHP | 0.02 (−0.16, 0.19) | −0.02 (−0.28, 0.24) | 0.05 (−0.20, 0.29) | 0.71 | 0.03 (−0.23, 0.30) | 0.28 (−0.11, 0.66) | −0.19 (−0.55, 0.18) | 0.09 | 0.01 (−0.01, 0.02) | 0.01 (−0.02, 0.04) | 0.00 (−0.02, 0.03) | 0.62 |
| MCPP | 0.05 (−0.12, 0.21) | 0.15 (−0.06, 0.37) | −0.10 (−0.35, 0.15) | 0.13 | 0.06 (−0.18, 0.31) | 0.18 (−0.14, 0.51) | −0.09 (−0.46, 0.29) | 0.28 | 0.00 (−0.02, 0.01) | 0.00 (−0.02, 0.02) | −0.01 (−0.03, 0.02) | 0.74 |
| MCNP | 0.02 (−0.18, 0.23) | 0.09 (−0.18, 0.35) | −0.06 (−0.36, 0.25) | 0.48 | 0.20 (−0.10, 0.51) | 0.20 (−0.20, 0.60) | 0.21 (−0.25, 0.67) | 0.98 | 0.00 (−0.02, 0.02) | 0.00 (−0.03, 0.03) | 0.00 (−0.03, 0.03) | 0.85 |
| MBzP | 0.02 (−0.10, 0.14) | −0.01 (−0.17, 0.16) | 0.05 (−0.12, 0.21) | 0.66 | 0.23 (0.05, 0.40) * | 0.36 (0.12, 0.60) * | 0.09 (−0.16, 0.33) | 0.12 | 0.01 (0.00, 0.02) * | 0.00 (−0.01, 0.02) | 0.02 (0.00, 0.04) * | 0.11 |
| MEP | 0.04 (−0.10, 0.17) | −0.02 (−0.20, 0.16) | 0.10 (−0.09, 0.28) | 0.37 | −0.04 (−0.24, 0.16) | −0.07 (−0.35, 0.21) | 0.00 (−0.28, 0.28) | 0.74 | 0.00 (−0.02, 0.01) | 0.00 (−0.02, 0.02) | 0.00 (−0.02, 0.01) | 0.66 |
| ΣDiNP | −0.05 (−0.19, 0.09) | 0.09 (−0.08, 0.26) | −0.28 (−0.50, −0.06)* | 0.01 | 0.01 (−0.20, 0.22) | 0.10 (−0.16, 0.37) | −0.14 (−0.48, 0.20) | 0.26 | 0.00 (−0.02, 0.01) | 0.00 (−0.01, 0.02) | −0.01 (−0.04, 0.01) | 0.22 |
| ΣDBP | 0.01 (−0.17, 0.20) | −0.01 (−0.25, 0.23) | 0.04 (−0.24, 0.33) | 0.80 | 0.00 (−0.28, 0.28) | 0.12 (−0.24, 0.48) | −0.17 (−0.60, 0.26) | 0.29 | 0.01 (−0.01, 0.03) | −0.01 (−0.03, 0.02) | 0.04 (0.01, 0.07) * | 0.01 |
| ΣDiBP | 0.03 (−0.12, 0.18) | 0.06 (−0.13, 0.25) | −0.01 (−0.26, 0.24) | 0.66 | −0.08 (−0.31, 0.15) | 0.07 (−0.21, 0.35) | −0.36 (−0.73, 0.02) | 0.08 | −0.01 (−0.02, 0.01) | −0.02 (−0.04, 0.00) * | 0.01 (−0.02, 0.04) | 0.08 |
| ΣDiNCH | 0.06 (−0.07, 0.18) | 0.08 (−0.09, 0.25) | 0.03 (−0.15, 0.22) | 0.73 | −0.05 (−0.24, 0.14) | 0.07 (−0.19, 0.32) | −0.18 (−0.46, 0.10) | 0.21 | 0.00 (−0.02, 0.01) | −0.01 (−0.02, 0.01) | 0.00 (−0.02, 0.02) | 0.68 |
| ΣDEHTP | 0.05 (−0.06, 0.16) | 0.04 (−0.12, 0.19) | 0.05 (−0.09, 0.20) | 0.89 | 0.02 (−0.14, 0.18) | 0.07 (−0.16, 0.31) | −0.03 (−0.26, 0.19) | 0.51 | 0.00 (−0.01, 0.01) | 0.00 (−0.02, 0.02) | 0.00 (−0.02, 0.01) | 0.79 |
| Parabens | ||||||||||||
| Ethylparaben | −0.01 (−0.07, 0.05) | −0.01 (−0.10, 0.08) | −0.01 (−0.09, 0.07) | 0.97 | −0.02 (−0.11, 0.07) | −0.05 (−0.18, 0.08) | 0.00 (−0.12, 0.12) | 0.53 | −0.01 (−0.01, 0.00) * | 0.00 (−0.01, 0.01) | −0.01 (−0.02, 0.00) * | 0.20 |
| Methylparaben | 0.04 (−0.05, 0.13) | 0.05 (−0.08, 0.18) | 0.03 (−0.11, 0.16) | 0.78 | −0.02 (−0.16, 0.13) | 0.11 (−0.09, 0.30) | −0.15 (−0.35, 0.05) | 0.07 | −0.01 (−0.02, 0.00) * | −0.01 (−0.02, 0.00) | −0.01 (−0.02, 0.00) | 0.96 |
| Propylparaben | 0.02 (−0.05, 0.09) | 0.05 (−0.05, 0.15) | −0.01 (−0.11, 0.08) | 0.36 | 0.02 (−0.09, 0.12) | 0.11 (−0.03, 0.26) | −0.07 (−0.22, 0.07) | 0.07 | −0.01 (−0.01, 0.00) * | −0.01 (−0.02, 0.00) | −0.01 (−0.02, 0.00) | 0.82 |
| Phenols | ||||||||||||
| BPA | 0.01 (−0.10, 0.13) | 0.06 (−0.07, 0.19) | −0.19 (−0.45, 0.08) | 0.10 | 0.07 (−0.10, 0.24) | 0.11 (−0.09, 0.30) | −0.07 (−0.47, 0.32) | 0.43 | −0.01 (−0.02, 0.01) | 0.00 (−0.02, 0.01) | −0.02 (−0.05, 0.01) | 0.31 |
| BPS | −0.03 (−0.15, 0.08) | 0.02 (−0.15, 0.20) | −0.08 (−0.24, 0.08) | 0.39 | −0.05 (−0.23, 0.12) | 0.08 (−0.18, 0.34) | −0.16 (−0.39, 0.08) | 0.19 | −0.01 (−0.02, 0.01) | −0.01 (−0.03, 0.00) | 0.00 (−0.01, 0.02) | 0.17 |
| BP-3 | −0.03 (−0.10, 0.04) | −0.02 (−0.11, 0.07) | −0.05 (−0.17, 0.07) | 0.71 | 0.02 (−0.09, 0.12) | −0.02 (−0.16, 0.11) | 0.08 (−0.10, 0.26) | 0.36 | 0.00 (−0.01, 0.01) | 0.00 (−0.01, 0.00) | 0.01 (0.00, 0.02) | 0.09 |
| TCS | −0.03 (−0.10, 0.03) | 0.00 (−0.09, 0.10) | −0.07 (−0.17, 0.02) | 0.26 | −0.04 (−0.15, 0.06) | −0.02 (−0.17, 0.12) | −0.07 (−0.21, 0.08) | 0.68 | 0.00 (−0.01, 0.01) | 0.00 (−0.01, 0.01) | 0.00 (−0.01, 0.01) | 0.86 |
| 2,4-DCP | 0.10 (−0.04, 0.24) | 0.19 (−0.01, 0.40) | 0.01 (−0.18, 0.21) | 0.22 | 0.15 (−0.07, 0.36) | 0.11 (−0.20, 0.42) | 0.18 (−0.12, 0.48) | 0.74 | 0.00 (−0.01, 0.02) | −0.01 (−0.03, 0.02) | 0.01 (−0.02, 0.03) | 0.49 |
| 2,5-DCP | 0.05 (−0.05, 0.14) | −0.02 (−0.17, 0.13) | 0.09 (−0.02, 0.21) | 0.22 | 0.17 (0.03, 0.32) * | 0.27 (0.04, 0.49) * | 0.12 (−0.06, 0.30) | 0.30 | 0.00 (−0.01, 0.01) | 0.00 (−0.02, 0.01) | −0.01 (−0.02, 0.01) | 0.54 |
Data are presented as the percent change (%Δ) and 95% CI in plasma hormone concentrations with every 10% increase in chemical biomarker. For TSH, data are presented as |iIU/mL change and 95% CI in TSH for every 10% increase in chemical biomarker. All models account for educational attainment, age, diet quality, pre-pregnancy body mass index, perceived stress in early pregnancy, lifetime smoking status, parity, race/ethnicity, gestational age at plasma hormone assessment, and fetal sex. Linear regression models evaluated associations of individual chemical biomarkers with plasma hormones. Bold signifies potentially meaningful findings with asterisk (*) denoting statistically significant findings at P < 0.05.
ΣDEHP = (MEHP/278) + (MEHHP/294) + (MEOHP/292) + (MECPP/308); ΣDiNP = (MiNP/292) + (MCOP/322) + (MONP/306); ΣDBP = (MBP/222) + (MHBP/238); ΣDiBP = (MiBP/222) + (MHiBP/238); ΣDiNCH = (MHiNCH/ 314) + (MCOCH/328); and ΣDEHTP = (MEHHTP/294) + (MECPTP/308).
Abbreviations: BPA, bisphenol A; BPF, bisphenol F; BPS, bisphenol S; CI, confidence interval; EDCs, endocrine disrupting chemicals; ΣDEHP, sum of di-2-ethylhexyl phthalate metabolites; ΣDEHTP, sum of di-2-ethylhexyl terephthalate metabolites; ΣDiNP, sum of di-isononyl phthalate metabolites; ΣDiBP, sum of di-iso-butyl phthalate metabolites; ΣDiNCH, sum of di(isononyl) cyclohexane-1,2-dicarboxylate metabolites; Pint, Pinteraction; TCS, triclosan; 2,4-DCP, 2,4-dichlorophenol; 2,5-DCP, 2,5-dichlorophenol; T4, thyroxine; TSH, thyroid stimulating hormone.
Women missing covariates in thyroid hormones analyses (n = 5). Diet (n = 3), stress (n = 2).
2.5.5. Identifying non-linear relationships and chemical-chemical interactions within the EDC biomarker mixture
BKMR uses kernel machine regression to estimate a non-parametric, high-dimensional exposure–response function to identify non-linear chemical and hormone dose–response relationships and chemical-chemical interactions within a mixture (Bobb et al., 2018; Bobb et al., 2015). After ln-transforming, centering, and scaling our co-exposures, outcomes, and continuous covariates, we fit BKMR models with 200,000 iterations and 50 knots for the same mixture of EDC biomarkers described in Section 2.5.4. To assess a cumulative non-linear mixture association, we created dose–response curves where the full mixture increases by various quantiles. We also calculated posterior inclusion probabilities (PIPs) to identify important chemical biomarkers contributing to associations between the mixture and hormones (Supp. Table 6). By interpreting univariable dose–response relationships where all other exposure biomarkers are fixed at their median, we can identify non-linear relationships, within the range of the cohort’s exposures, between single EDC biomarkers and gestational hormones. To identify interactions between biomarkers, we interpreted bivariate exposure–response plots where we visualized one biomarker’s dose–response relationship with hormones while a second chemical biomarker was held at 10th, 25th, 50th, 75th, and 90th percentiles. We only explored chemical-chemical interactions when there was evidence of potential mixture associations from either WQSR or BKMR, and we determined interactions by identifying non-parallel or non-overlapping dose–response curves.
2.5.6. Evaluating differences in associations of EDC biomarkers with maternal hormones by fetal sex
Because pregnancy hormones differ by fetal sex, our second objective was to identify fetal sex-specific associations between EDC biomarker mixtures and early-to-mid pregnancy hormones. In linear regression, WQSR, and BKMR models, we assessed fetal-sex specific associations of EDC biomarkers individually and as a mixture with maternal sex-steroid and thyroid hormones. Specifically, in linear regression models, we included a multiplicative interaction (Pinteraction) between chemical biomarkers and fetal sex. We examined general trends and reported potentially meaningful results based on the associations’ direction, strength, and precision, regardless of interaction P-value. For WQSR and BKMR, we stratified our sample by fetal sex and identified potentially meaningful results by comparing direction and strength of association.
2.5.7. Reporting of findings and interpreting meaningful associations
For single-chemical biomarker linear regression results, except TSH, our β-estimates and 95 % confidence intervals (CIs) represent the percentage change (%Δ) in gestational hormone concentration associated with a 10 % increase in chemical biomarker concentration as we performed the back-transformation [(1.10β − 1)*100] for progesterone, estradiol, testosterone, FT4, and TT4 and [βln(1.10)] for TSH. For WQSR results, except TSH, the β-estimates and 95 % CIs represent the percentage change (%Δ) in hormone concentration associated with a 10 % increase in the mixture index as our mixture exposure biomarkers were divided into deciles, all hormones were ln-transformed, and we back-transformed our mixtures results using [(eβ − 1)*100]. For TSH, the β-estimates and 95 % CIs represent the μIU/mL change in TSH for each 10 % increase in chemical biomarker concentrations or the EDC mixture. We identified potentially meaningful findings from single pollutant and WQSR models by assessing the direction, strength, and precision of the associations. To ensure we met model assumptions, we performed regression diagnostics based on residuals for single pollutant models, assessed scatterplots in WQSR, and checked for convergence with the Markov Chain Monte Carlo procedure in BKMR. We performed linear regression analyses in SAS version 9.4 (SAS Institute Inc. Cary, NC) using PROC GLM and completed WQSR and BKMR analyses in R Statistical Software using R packages “gWQS: Generalized Weighted Quantile Sum Regression” (Stefano Renzetti et al., 2023) and “bkmr: Bayesian Kernel Machine Regression” (Bobb, 2022).
3. Results
3.1. Illinois KIDS development study (I-KIDS) characteristics
Most women were non-Hispanic White (82 %), college-educated (83 %), with an annual household income >$60,000 (73 %) (Table 1), and characteristics did not differ greatly from the full I-KIDS sample of 531 women (those with at least one chemical biomarker measurement, reflecting women who stayed in the study through the birth of their infant). The median age was 30.4 years. The median pre-pregnancy BMI was 24.7 kg/m2 with 47 % of women having overweight or obesity. The median (25th, 75th percentile) diet quality (AHEI-2010) was 51.7 (44.6, 59.3) out of 100 points. Most women never smoked cigarettes (83 %). Most women reported having low early-pregnancy stress (62 %) and more than half of women were nulliparous (54 %). Fetal sex was approximately evenly distributed between females (51 %) and males (49 %).
3.2. Concentrations of maternal urinary chemical biomarkers
Most chemicals had concentrations ≥ LOD in the vast majority of women, except MiNP, MCOCH, butyl paraben, ethyl paraben, bisphenol F, and triclocarban, which were only detectable (≥ LOD) in 31.5 %, 66.9 %, 33.4 %, 57.0 %, 57.0 %, and 29.8 % of women, respectively (Table 2). Only a few chemical biomarkers were strongly correlated with each other, including ΣDiNP with MCPP (r = 0.8), 2,4-DCP with 2,5-DCP (r = 0.7), and methylparaben with propylparaben (r = 0.7; Supp. Fig. 3).
3.3. Maternal plasma hormone concentrations
All women had measureble concentrations of progesterone, estradiol, FT4, and TT4; however, 87% and 99% of women had concentrations at or above the lower limit of the reportable ranges for testosterone and TSH, respectively (Table 2). The median (25th, 75th percentile) concentration of hormones are reported in Table 2.
3.4. Linear, non-linear, and interactive relationships of EDC biomarkers with early-to-mid pregnancy sex-steroid hormones
3.4.1. Associations with progesterone
In general, despite a few single pollutant associations, in all women or women carrying females or males, there were no cumulative or non-linear associations of EDC biomarkers with progesterone or chemical-chemical interactions (Table 3; Figs. 1–2; Supp. Figs. 4, 10–12).
Fig. 1. Cumulative associations of WQSR mixture with A) progesterone, B) estradiol, C) testosterone, D) free T4, E) total T4, F) TSH.
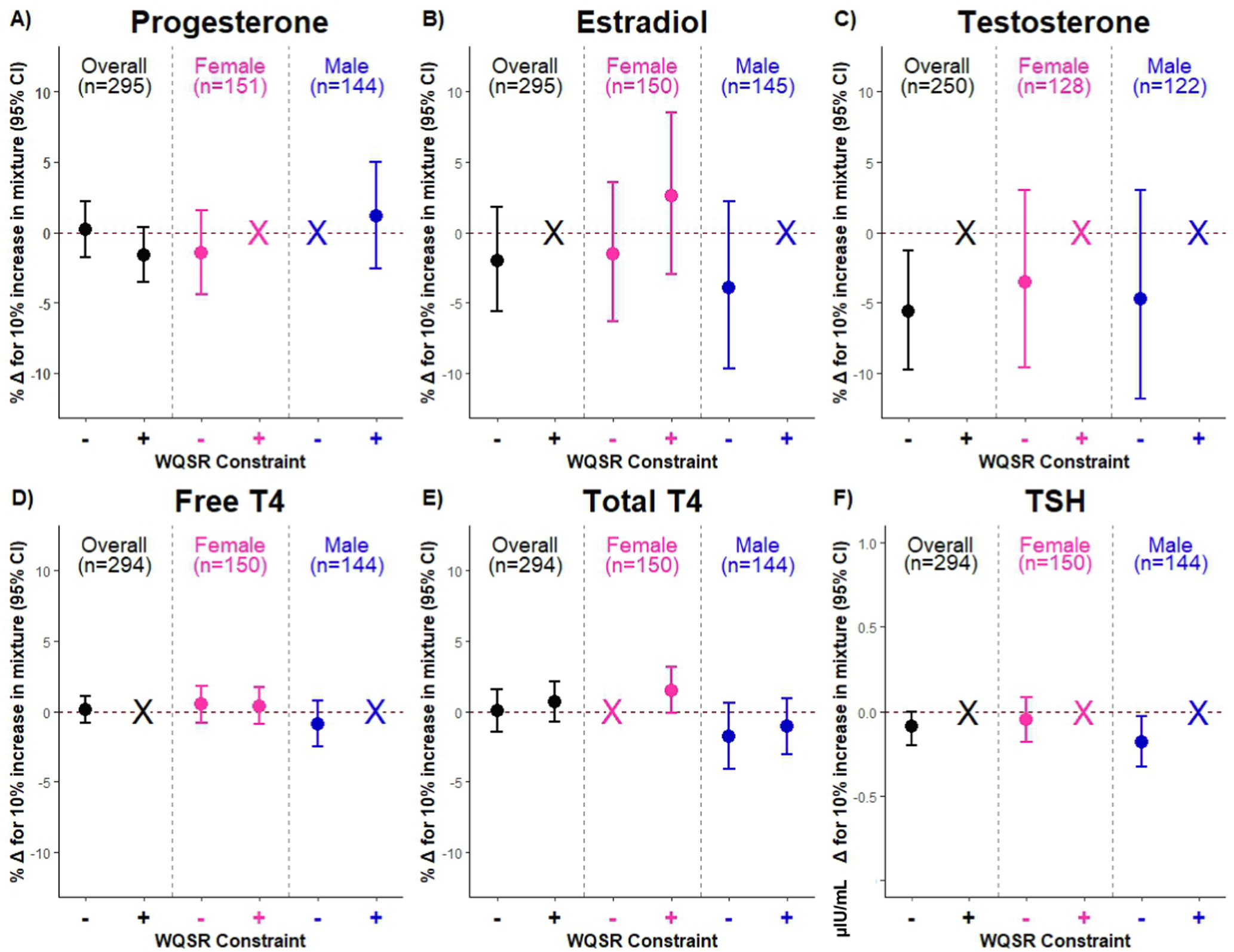
Negatively (−) and positively (+) constrained WQSR models were fit with 100 bootstraps and 100 repeated holdouts accounting for educational attainment, age, diet quality, pre-pregnancy body mass index, perceived stress in early pregnancy, lifetime smoking status, parity, race/ethnicity, gestational age at plasma hormone assessment, women carrying females, and women carrying males. Data are presented as the percent change (%Δ) and 95 % CI in plasma hormone concentrations with every 10 % increase in the WQSR mixture. For TSH, data are presented as μIU/mL change and 95 % CI in TSH for every 10 % increase in the WQSR mixture. Estimate and interval color signifies analytic sample (all women: black; women carrying females: pink; women carrying males: blue), and X indicates non-positive or non-negative β-estimates. For TSH, y-axis is on a different scale for data visualization. Abbreviations: CI, confidence interval; T4, thyroxine; TSH, thyroid stimulating hormone; WQSR, Weighted Quantile Sum Regression.
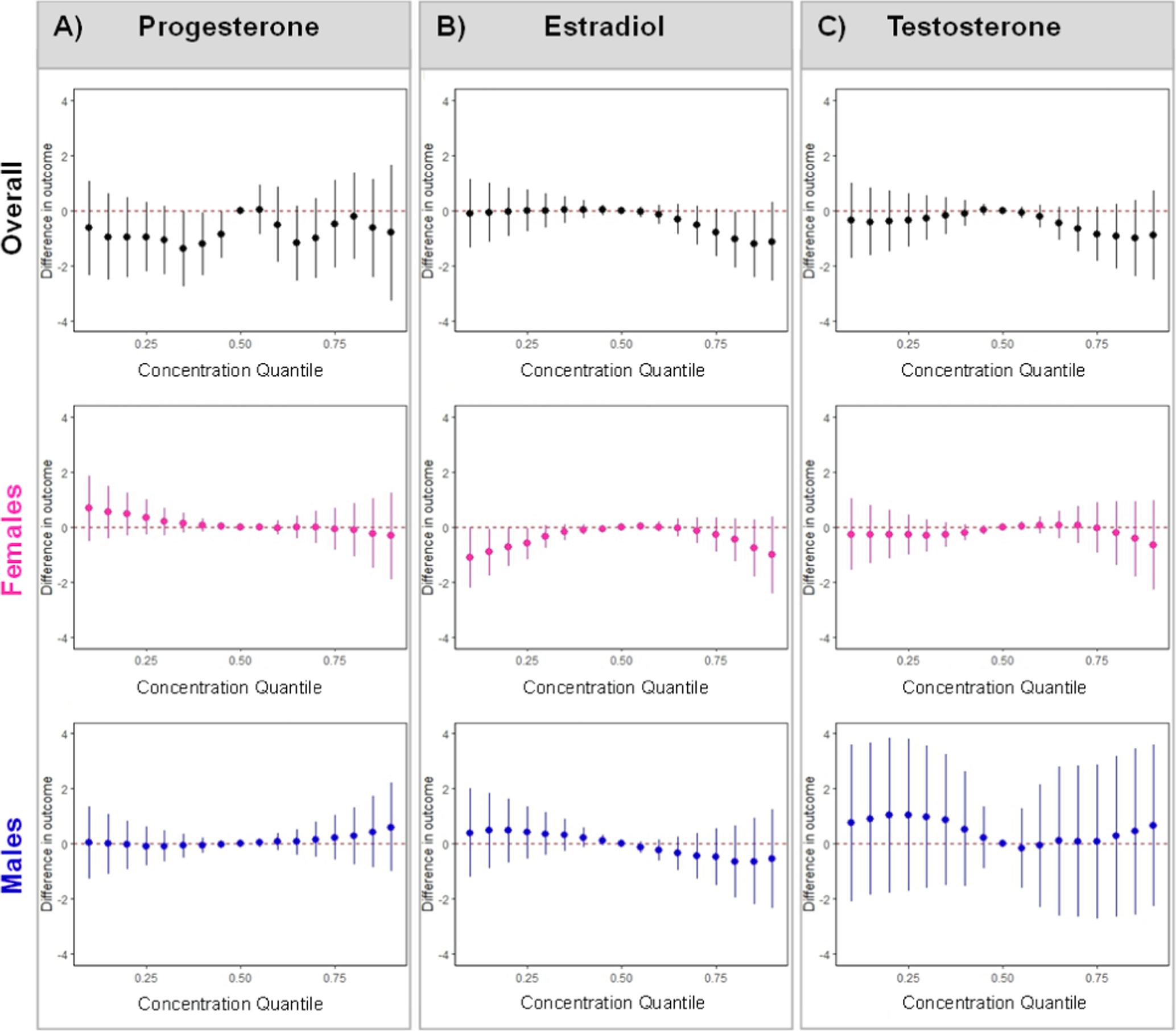
Fig. 2. Associations of BKMR mixture with A) progesterone, B) estradiol, C) testosterone in all women, women carrying females, and women carrying males.
BKMR models were fit with 200,000 iterations and 50 knots accounting for educational attainment, age, diet quality, pre-pregnancy body mass index, perceived stress in early pregnancy, lifetime smoking status, parity, race/ethnicity, gestational age at plasma hormone assessment. Data are presented as effect estimates and 95% credible intervals which are interpreted as the association between the mixture at each quantile and gestational hormone compared to when all co-exposures are fixed at the median. Estimate and interval color signifies analytic sample (all women: black; women carrying females: pink; women carrying males: blue). Abbreviations: BKMR, Bayesian Kernel Machine Regression.
3.4.2. Associations with estradiol
There was a potentially non-linear relationship between the mixture and estradiol, with negative associations at higher EDC biomarker mixture concentrations (Fig. 2). Additionally, BPA exhibited an s-shaped relationship with estradiol when all co-exposures were held at their median. This relationship was attenuated at lower concentrations of MEP and higher concentrations of BP-3, ΣDiNP, methylparaben, and propylparaben (Supp. Figs. 5, 13). When modeled individually, BPS was associated with higher estradiol in women carrying females and BPA was associated with lower estradiol in women carrying males (Table 3), but sex-specific WQSR mixture associations were only marginally meaningful (Supp. Table 2, 4). However, using BKMR, the mixture was negatively associated with estradiol at both lower and higher concentrations in women carrying females, driven by BPA (PIP: 0.95) and propylparaben (PIP: 0.75) (Fig. 2; Supp. Table 6). Importantly, in women carrying females, BPA had an inverted u-shape relationship with estradiol when all EDC biomarker concentrations were fixed at their median, which was attenuated at the highest concentrations of propylparaben (Supp. Figs. 5b, 14). In women carrying males, the relationship of BPA with estradiol was attenuated at higher concentrations of MEP and lower concentrations of MCPP (Supp. Figs. 5c, 15).
3.4.3. Associations with testosterone
Propylparaben, triclosan, 2,4-DCP, and 2,5-DCP were negatively associated with testosterone (Table 3), which, along with BPS and ΣDEHTP, drove the WQSR mixture association, such that a 10 % increase in the mixture was associated with a −5.65 % (95 % CI: −9.79, −1.28) lower testosterone (Fig. 1; Supp. Table 2, 4). Using BKMR, there were no cumulative or non-linear associations (Fig. 2); however, there was a non-linear relationship between BPS and testosterone when all co-exposures were fixed at their median (Supp. Fig. 6a). We also identified some chemical-chemical interactions, such that negative relationships of propylparaben, TCS, and 2,5-DCP with testosterone were stronger at lower concentrations of MEP, whereas associations of TCS and 2,4-DCP with testosterone were attenuated at lower concentrations of BP-3 (Supp. Fig. 16). There was no evidence of meaningful sex-specific or non-linear relationships (Table 3; Figs. 1–2; Supp. Figs. 6b,c). However, in women carrying females, propylparaben interacted with BPA, and in women carrying males, propylparaben interacted with ΣDEHTP, 2,5-DCP, ΣDiNCH, and methylparaben, whereas 2,5-DCP interacted with ΣDEHTP and methylparaben (Supp. Figs. 17–18).
3.5. Linear, non-linear, and interactive relationships of EDC biomarkers with early-to-mid pregnancy thyroid hormones
3.5.1. Associations with FT4
Overall, neither individual EDC biomarkers nor the mixture were associated with FT4 (Table 4; Figs. 1, 3; Supp. Table 2). Only in women carrying females, 2,4-DCP was associated with higher FT4, whereas only in women carrying males, ΣDiNP was associated with lower FT4 (Table 4). There was no evidence of meaningful sex-specific associations, or cumulative/non-linear associations (Figs. 1, 3; Supp. Figs. 7; 19–21).
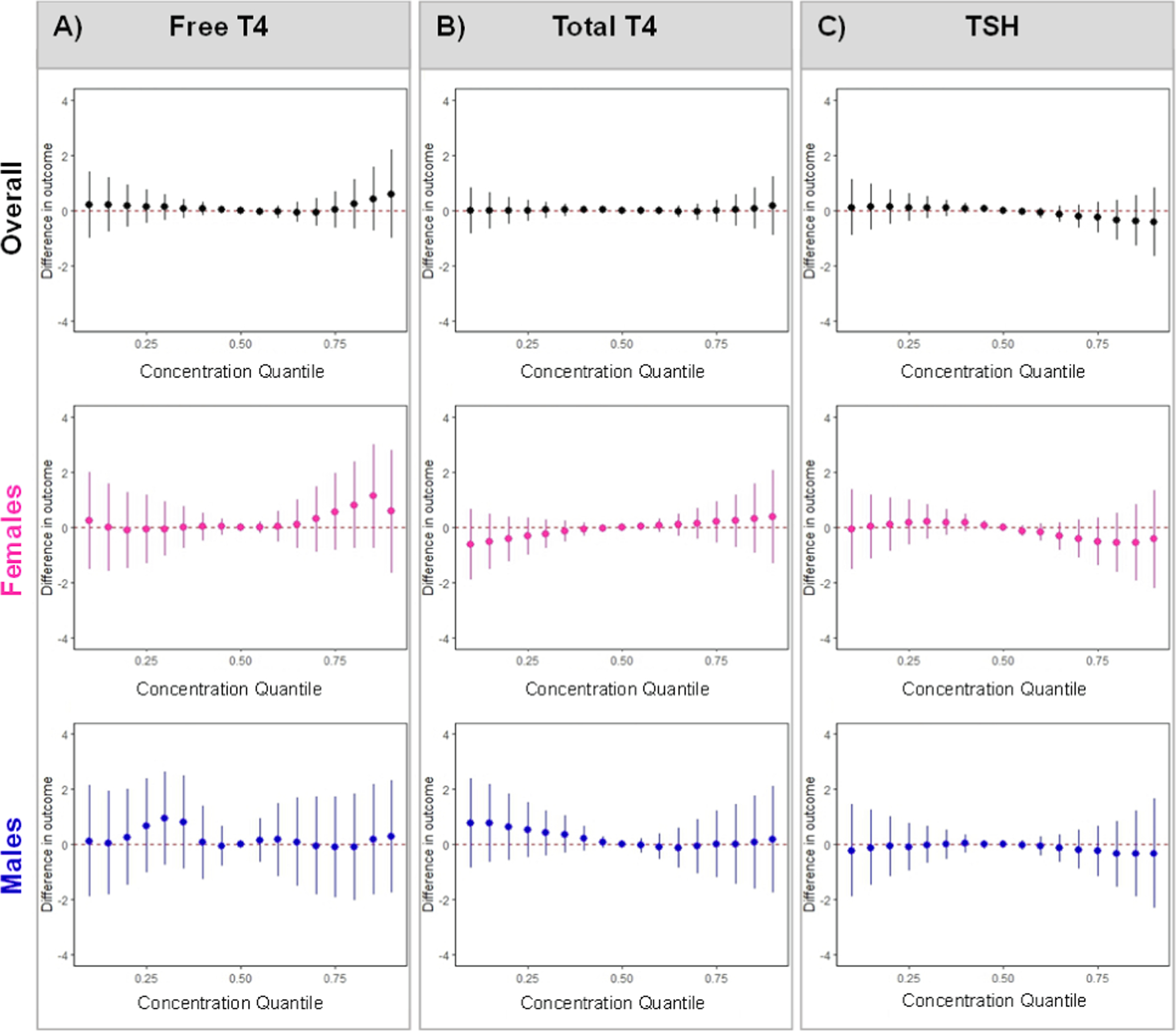
Fig. 3. Associations of BKMR mixture with A) free T4, B) total T4, C) TSH in all women, women carrying females, and women carrying males.
BKMR models were fit with 200,000 iterations and 50 knots accounting for educational attainment, age, diet quality, pre-pregnancy body mass index, perceived stress in early pregnancy, lifetime smoking status, parity, race/ethnicity, gestational age at plasma hormone assessment. Data are presented as effect estimates and 95% credible intervals which are interpreted as the association between the mixture at each quantile and gestational hormone compared to when all co-exposures are fixed at the median. Estimate and interval color signifies analytic sample (all women: black; women carrying females: pink; women carrying males: blue). Abbreviations: BKMR, Bayesian Kernel Machine Regression; T4, thyroxine; TSH, thyroid stimulating hormone.
3.5.2. Associations with TT4
Despite a few individual chemical associations, we found no evidence of cumulative or non-linear associations of the EDC biomarkers mixture with TT4 or chemical-chemical interactions (Table 4; Figs. 1, 3; Supp. Fig. 8). Only in women carrying males, higher ΣDiBP was associated with lower TT4, whereas associations of MBzP and 2,5-DCP with TT4 were stronger in women carrying females (Table 4). There was also a sex-specific association of the WQSR mixture with TT4 (Fig. 1), such that each 10 % mixture increase in women carrying females was associated with 1.50 % (95 % CI: −0.15, 3.18) higher TT4 (driven by MBzP and 2,5-DCP) and each 10 % mixture increase in women carrying males was associated with −1.77 % (95 % CI: −4.08, 0.58) lower TT4 (driven by DiBP, BPS, and ΣDiNCH) (Supp. Tables 2, 4). Using BKMR, there were no cumulative non-linear associations of EDC biomarkers with TT4 (Fig. 3). However, in women carrying males, there were non-linear relationship of BPS and TCS with TT4 when other biomarkers were held at their medians, and associations of TCS and BPS with TT4 were attenuated at higher concentrations of ΣDEHP (Supp. Figs. 8c, 24).
3.5.3. Associations with TSH
In all women, methyl-, ethyl-, and propylparaben were inversely associated with TSH, whereas MBzP was positively associated with TSH (Table 4). Parabens, along with BPA, BPS, 2,5-DCP, ΣDiNCH, ΣDiBP, and TCS, drove the WQSR mixture association, such that each 10 % mixture increase was associated with −0.09 μIU/mL (95 % CI: −0.19, 0.00) lower TSH (Fig. 1; Supp. Tables 2, 5). Using BKMR, there were no cumulative or non-linear associations of EDC biomarkers with TSH (Fig. 3); however, there was a non-linear relationship of BPA with TSH that was attenuated at higher concentrations of BP-3 (Supp. Figs. 9a, 25). In women carrying females, higher ΣDiBP was associated with lower TSH, whereas in women carrying males, higher MBzP and ΣDBP were associated with higher TSH and higher ethylparaben was associated with lower TSH (Table 4). In women carrying males, ethylparaben, along with 2,5-DCP, DiNP, MEP, BPA, MCNP, TCS, and methylparaben, drove the WQSR mixture association, such that each 10 % mixture increase was associated with −0.18 μIU/mL (95 % CI: −0.33, −0.03) lower TSH (Fig. 1; Supp. Tables 2, 5). Using BKMR, there was no evidence of sex-specific non-linear associations or chemical-chemical interactions (Fig. 3; Supp. Figs. 9b,c, 25–27).
4. Discussion
4.1. Summary of major findings
In a relatively homogenous, higher socioeconomic status sample of midwestern U.S. pregnant women, a mixture of phthalate/replacement, paraben, and phenol metabolites was associated with lower early-to-mid pregnancy testosterone in all women (driven by propylparaben and triclosan), TT4 in women carrying females (driven by MBzP, 2,5-DCP, and propylparaben), and TSH in women carrying males (driven by 2,5-DCP and propylparaben). We also identified potential non-linear associations between the EDC biomarker mixture and estradiol in all women (at high concentrations) and in women carrying females (at low and high concentrations). In general, the mixture was not associated with progesterone or FT4.
4.2. A mixture of non-persistent EDC biomarkers was not associated with early-to-mid pregnancy progesterone
Despite identifying a negative association between propylparaben and progesterone, we did not observe any meaningful mixture associations, consistent with recent studies evaluating single chemicals (Aker et al., 2019; Banker et al., 2021; Cathey et al., 2019; Johns et al., 2015; Kolatorova et al., 2018; Sathyanarayana et al., 2014; Sathyanarayana et al., 2017). Similar to our results, the Michigan Mother-InfantPairs (MMIP) study reported negative associations of methylparaben and propylparaben with first trimester progesterone (Banker et al., 2021). However, a study of Puerto Rican women (PROTECT), who had higher paraben and phthalate biomarker concentrations and lower biomarkers of phthalate replacement concentrations compared to I-KIDS, reported no associations of four parabens and seven phenols with 2nd and 3rd trimester progesterone (Aker et al., 2019) and negative associations between some phthalates biomarkers and progesterone (Cathey et al., 2019; Johns et al., 2015). Our findings, using two robust mixture methods, suggest that a mixture of non-persistent EDC biomarkers does not affect early-to-mid pregnancy progesterone; however, single chemical results suggest a relationship of parabens with progesterone that warrants further investigation, especially because methylparaben and propylparaben share common sources of exposure and thus could have cumulative effects.
4.3. An EDC biomarker mixture was associated with estradiol, with evidence of sex-specific and non-linear relationships
Our single-pollutant null findings add to already-mixed literature with regards to EDCs and estradiol. For example, PROTECT reported no associations of EDC biomarkers with estriol or estradiol (Aker et al., 2019; Cathey et al., 2019; Johns et al., 2015). However, the Infant Development and Environment Study (TIDES), comprised of women with slightly higher phthalate biomarker concentrations compared to I-KIDS, identified positive associations of phthalate biomarkers with estradiol (Sathyanarayana et al., 2017), whereas the MMIP study reported negative associations of BPS with estradiol and of methylparaben with estradiol and estrone (Banker et al., 2021). Previously, we identified many positive associations between phthalate/replacement biomarkers and urinary estrogens across pregnancy (Pacyga et al., 2021). Those prior results could differ from our current results in part due to hormone assessment timing (median 13, 28, and 34 weeks in our prior study versus 17 weeks in the current study) and hormone assessment medium (urine in the prior study versus blood in the current study). While reported sex-specific associations of EDC biomarkers with estrogens are mixed (Banker et al., 2021; Pacyga et al., 2021; Sathyanarayana et al., 2014), our results support sex-specific associations of BPS and BPA with estradiol, which should be further explored.
We identified a non-linear relationship of BPA and propylparaben with estradiol in women carrying females, which is consistent with experimental and epidemiologic studies showing various non-linear dose–response curves when evaluating EDCs and estradiol (u-shaped curves, inverted u-shape curves, s-shape curves, etc.) (reviewed by (Vandenberg et al., 2012)), likely due to dose-dependent disruption of genes, proteins, and receptors. However, as the range of urinary biomarker concentrations in I-KIDS is modest, associations at our highest concentrations may represent average concentrations in higher exposed populations, such as women in the PROTECT cohort. A recent study of pregnant Chinese women, with much higher urinary concentrations of BPA, reported non-linear negative low-dose relationships of BPA with estriol, estradiol, and estrone, with no fetal-sex differences (Li et al., 2020). However, this study also differs from ours in study design (spot urine samples for BPA and estrogen assessment) and method (single-pollutant models). Interestingly, in our study, BPA interacted with other EDC biomarkers, such as BP-3, methylparaben, and propylparaben, and the negative relationship between BPA and estradiol in women carrying females was stronger when propylparaben concentrations were relatively low. Parabens and bisphenols both disrupt estrogen (Liang et al., 2023), so it is plausible they both act at similar cellular targets. To this end, one recent experimental study reported mixtures of bisphenols and benzophenone derivatives showed synergistic or additive effects at human-relevant concentration (Kudlak et al., 2022).
4.4. Non-persistent EDC biomarkers, individually and as a mixture, were associated with lower testosterone
Several phthalates and phenols have been characterized as anti-androgenic based on animal and human epidemiologic studies (Gray et al., 2006; Parks et al., 2000); however, many studies were conducted within the context of male reproductive health. Our results suggest that select non-persistent EDC biomarkers are negatively associated with early-to-mid pregnancy testosterone, primarily driven by phenols and propylparaben, which differs somewhat from prior studies. In a previous study investigating urinary rather than plasma hormones, we identified positive associations of MBzP and MEP with urinary testosterone at 28 weeks gestation (Pacyga et al., 2021), and the Study for Future Families (SFF) reported positive associations of MEP with serum second and third trimester testosterone (Sathyanarayana et al., 2014). Despite no association between MEP and testosterone in this study, we identified that MEP attenuates some negative relationships. Associations of TCS, propylparaben, 2,4-DCP, and 2,5-DCP with lower testosterone in our study differed from one PROTECT study that reported non-significant positive associations of TCS, 2,4-DCP, and 2,5-DCP with second trimester serum testosterone (Aker et al., 2019). However, this study relied on urinary biomarker concentrations from spot urine samples collected at 16–20 weeks gestation, whereas we utilized concentrations from a pooled sample composed of up to five first-morning urine samples collected throughout gestation. Importantly, unlike prior studies that assessed single EDC biomarkers, our study used two different mixture methods that identified TCS as being a meaningful predictor of testosterone. The roles of androgens in pregnancy are not entirely clear, but testosterone at normal levels regulates key processes of pregnancy and birth, such as cervical remodeling (Makieva et al., 2014), and at higher levels is associated with pregnancy conditions like gestational diabetes, pre-eclampsia, and pre-term birth (Cathey et al., 2021; Morisset et al., 2013; Salamalekis et al., 2006). Future studies should investigate if lower testosterone levels are linked to adverse pregnancy health or birth outcomes.
4.5. Non-persistent EDC biomarkers were associated with total T4 but not with free T4
We did not identify any cumulative or non-linear associations between EDC biomarkers and FT4 overall or by fetal sex, which is consistent with prior studies (Huang et al., 2022; Nakiwala et al., 2022; Romano et al., 2018; Sarzo et al., 2022; Souter et al., 2020; Yang et al., 2022). However, our and others’ results identified relationships between EDC biomarkers and TT4. We identified positive associations of MBzP and 2,5-DCP with TT4; however, we did not identify a cumulative association of the EDC mixture with TT4. In contrast, the Health Outcomes and Measures of the Environment (HOME) study reported an inverse association of a nine-phthalate biomarkers mixture using WQSR with maternal serum TT4 (at 16 weeks gestation) that was primarily driven by MEP and MCPP, neither of which met the WQSR threshold in our study (Romano et al., 2018). One major difference between this study and ours is that HOME measured phthalate biomarkers in two spot urines at 16 and 26 weeks gestation (compared to our pooled sample of phthalates/replacements, parabens, and phenols), which could affect both temporality and precision of the exposure assessment. One recent study assessed biomarkers of phthalates, parabens, and phenols as an 11-chemical mixture using BKMR and reported a negative association with TT3/TT4 ratio (nine weeks gestation), which may indicate EDCs are associated with higher TT4 (Nakiwala et al., 2022). This study utilized a dimension reduction method by limiting chemical biomarkers included in their mixture to those exhibiting biological activity in a toxicological database, whereas we included any phthalate/replacement, paraben, and phenol biomarkers analyzed by the CDC with measurable concentrations. While no other studies identified sex-specific associations of EDC biomarkers mixtures with TT4, our results suggest a positive relationship between EDCs and TT4 in women carrying females and a negative relationship in women carrying males. Overall, our findings, and those from prior studies, suggest non-persistent EDC biomarkers may affect early-to-mid pregnancy maternal TT4 but not FT4. FT4 and TT4 are both biomarkers of maternal thyroid function, but it is unclear what implications altered TT4 would have (compared to FT4), as TT4 exhibits higher variability during early pregnancy, is poorly related to TSH levels, and is not associated with adverse pregnancy outcomes, such as pre-eclampsia, premature delivery, and abnormal birthweight (Korevaar et al., 2016).
4.6. Non-persistent EDC biomarkers were associated with lower TSH, primarily in women carrying males
We observed negative associations of parabens with TSH and identified a negative mixture association with TSH, driven by BPA, BPS, ethylparaben, and 2,5-DCP. However, no prior studies assessing non-persistent EDC biomarker mixtures with TSH have reported meaningful associations (Berger et al., 2018; Derakhshan et al., 2021; Huang et al., 2022; Nakiwala et al., 2022; Romano et al., 2018; Sarzo et al., 2022; Souter et al., 2020; Yang et al., 2022). Key differences between our study and prior studies include urine measurement timing, single spot urine versus pooled urine sampling, and the mixture composition. The only other study that modeled biomarkers of phthalates, parabens, and phenols (using an a priori-driven method for chemical inclusion described above) did not identify any associations with TSH (Nakiwala et al., 2022). While low TSH levels can occur in normal pregnancy (Laurberg et al., 2016), hyperthyroidism, characterized by high thyroid hormones and low TSH, are linked to increased risk of pre-eclampsia, miscarriage, and low birthweight (Marx et al., 2008). TSH is secreted by the pituitary gland, acts on the thyroid gland, and is regulated by TT4 and TT3. Because of complex hormonal feedback loops, EDCs could act at the pituitary or thyroid gland. The exact mechanism of action is hard to elucidate; however, animal studies have reported that bisphenols can act as thyroid hormone receptor antagonists (Kim and Park, 2019). As thyroid hormones play significant roles in pregnancy, fetal growth, and neurodevelopment (Marx et al., 2008), future studies should investigate the potential effects of other EDCs on TSH.
Unlike prior studies, we also identified a negative association of the mixture with TSH in women carrying males, which could be due to various factors. The placenta, which is responsible for thyroid hormone regulation and transport during pregnancy, is a sexed organ (XX and XY), as it develops from the zygote. Numerous studies have demonstrated that there are differences between male and female placentas in terms of gene expression, function, and morphology (Gabory et al., 2013; Graves, 2010; Meakin et al., 2021; Rich-Edwards et al., 2001). Additionally, other studies have shown that male placentas are more responsive to stressors than female placentas (Bale, 2016; Bronson and Bale, 2016; Eriksson et al., 2010). Potentially due to placental differences, the concentrations of maternal TSH have been shown to differ between women carrying male or female fetuses, which could indicate differences in thyroid homeostasis (Sitoris et al., 2022; Wang et al., 2019). Additionally, maternal sex-steroid hormones, which we have shown to also to be sexually dimorphic, play a role in regulating thyroid hormones that could explain some of the differences in TSH by fetal sex. There are likely other mechanisms that could be further investigated using experimental models.
4.7. Strengths and limitations
This current study has some limitations and many strengths. First, I-KIDS quantified EDC biomarkers in a pool of up to five first-morning urine samples collected throughout pregnancy, and some exposures occurred after our outcomes of interest. The pooled sample reduces exposure assessment error, provides a more stable estimate of gestational exposure, and can be considered a reflection of exposure during pregnancy (Shin et al., 2019a; Vernet et al., 2019). Additionally, the urinary concentrations for some biomarkers were much lower compared to other cohorts; however, this study investigated a large panel of non-persistent EDCs from multiple chemical classes and I-KIDS women have comparable EDC biomarker concentrations to reproductive-aged women in the nationally-representative National Health and Nutrition Examination Survey (Pacyga et al., 2022). Second, because we were limited to a single early second-trimester measurement of select hormones, our findings might not be generalizable to other timepoints and hormones (such as pregnancy triiodothyronine; TT3). However, we assessed six early-to-mid pregnancy plasma hormones that reflect sex-steroid and thyroid hormones known to be critical for pregnancy health and fetal development. Third, we cannot rule out unmeasured confounding, such as by poor sleep quality during pregnancy which may impact hormone levels and indirectly impact chemical exposure through alterations of behaviors and habits; however, I-KIDS collected pertinent sociodemographic, lifestyle, and health information that allowed us to account for many other important covariates, and we utilized a priori consideration and previous literature to inform decisions about covariate selection. Fourth, we may have been underpowered for analyses stratified by fetal sex, but we had adequate sample sizes overall. Fifth, the I-KIDS cohort is a relatively homogenous sample of non-Hispanic White, well-educated, married women, which limits generalizability. However, as we are investigating biological hypotheses, a homogenous sample may reduce unmeasured confounding. Lastly, BKMR results can be difficult to interpret within the context of human health (Hoskovec et al., 2021) and WQSR assumes homogeneity in direction of association (Carrico et al., 2015; Czarnota et al., 2015); however, these two robust and reliable methods allowed us to estimate cumulative effects, identify meaningful drivers of associations, and assess non-linearities and chemical-chemical interactions.
5. Conclusion
To our knowledge, this is the first study to investigate the relationship between a broad mixture of EDC urinary biomarkers and plasma pregnancy sex-steroid hormones. Additionally, we have contributed to a growing body of literature investigating EDC biomarker mixtures and pregnancy thyroid hormones. Our results suggest a mixture of non-persistent EDC biomarkers is associated with testosterone, estradiol, and TSH in this population of women. We also identified important sex-specific results, non-linear relationships, and chemical-chemical interactions. Future studies should explore similar relationships in more diverse cohorts, including those with women in high-risk pregnancies, such as pregnancies complicated by gestational diabetes, hypertension, or pre-eclampsia, to increase the understanding of associations of EDCs with pregnancy and birth outcomes. Additionally, more expansive mixtures will need to be considered that include other classes of EDCs, such as pesticides, herbicides, and per- and polyfluoroalkyl substances. Furthermore, mixture approaches could consider a priori classifying chemical biomarkers using unsupervised statistical methods, such as principal component analysis, or grouping individual biomarkers by class or proposed mechanism of action to better understand the relationship of EDCs as classes with hormonal outcomes.
Supplementary Material
Acknowledgments
Biological specimens from the Carle Foundation Hospital were used in this study. We thank contributors, patients, and their families whose help and participation made this work possible. We also would like to acknowledge BioRender, which we used in the creation of our graphical abstract.
Funding sources:
This publication was made possible by the National Institute for Environmental Health Sciences (NIH/NIEHS) grants ES024795, ES032227, ES022848, ES007255, the U.S. Environmental Protection Agency grant RD83543401, and National Institute of Health Office of the Director grant UHOD023272. Dr. Aung was supported in part by NIEHS core center grant P30ES007048. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA or NIH. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. This project was also supported by the USDA National Institute of Food and Agriculture, Michigan AgBioResearch, and by Grant Number P30DK020572 (MDRC) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations:
- 2,4-DCP
2,4-dichlorophenol
- 2,5-DCP
2,5-dichlorophenol
- AHEI-2010
Alternative Healthy Eating Index
- BP-3
benzophenone-3
- BPA
bisphenol A
- BPS
bisphenol S
- BPF
bisphenol F
- BKMR
Bayesian kernel machine regression
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- DAG
directed acylic graph
- DBP
di-n-butyl phthalate
- DEHP
di-2-ethylhexyl phthalate
- DEHTP
di-2-ethylhexyl terephthalate
- DiBP
di-isobutyl phthalate
- DiNCH
di(isononyl) cyclohexane-1,2-dicarboxylate
- DiNP
di-isononyl phthalate
- EDC
endocrine disrupting chemical
- FFQ
food frequency questionnaire
- FT4
free T4
- I-KIDS
Illinois Kids Development Study
- LOD
limit of detection
- MDRC
University of Michigan Diabetes Research Center Clinical Core Chemistry Laboratory
- PSS
perceived stress scale
- MBP
mono-n-butyl phthalate
- MBzP
monobenzyl phthalate
- MCNP
monocarboxynonyl phthalate
- MCOCH
cyclohexane-1,2-dicarboxylic acid-mono(carboxyoctyl) ester
- MCOP
monocarboxyoctyl phthalate
- MCPP
mono(3-carboxypropyl) phthalate
- MECPTP
mono(2-ethyl-5-carboxypentyl) terephthalate
- MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEHHTP
mono(2-ethyl-5-hydroxyhexyl) terephthalate
- MEHP
mono(2-ethylhexyl) phthalate
- MEOHP
mono(2-ethyl-5-oxohexyl) phthalate
- MECPP
mono(2-ethyl-5-carboxypentyl) phthalate
- MEP
monoethyl phthalate
- MHBP
monohydroxybutyl phthalate
- MHiBP
monohydroxyisobutyl phthalate
- MiBP
mono-isobutyl phthalate
- MiNP
mono-isononyl phthalate
- MHiNCH
cyclohexane-1,2-dicarboxylic acid-monohydroxy isononyl ester
- MONP
monooxononyl phthalate
- TCS
triclosan
- TPOAb
thyroid peroxidase antibody
- TSH
thyroid stimulating hormone
- TT4
total T4
- WQSR
weighted quantile sums regression
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2024.108433.
Data availability
The authors do not have permission to share data.
References
- Abrantes-Soares F, et al. , 2022. Effects of BPA substitutes on the prenatal and cardiovascular systems. Crit. Rev. Toxicol. 52, 469–498. [DOI] [PubMed] [Google Scholar]
- Aker AM, et al. , 2016. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ. Res. 151, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker AM, et al. , 2019. A repeated measures study of phenol, paraben and Triclocarban urinary biomarkers and circulating maternal hormones during gestation in the Puerto Rico PROTECT cohort. Environ. Health 18, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MT, et al. , 2017. Thyroid hormone parameters during pregnancy in relation to urinary bisphenol A concentrations: A repeated measures study. Environ. Int. 104, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, 2016. The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialogues Clin. Neurosci. 18, 459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker M, et al. , 2021. Association of maternal-neonatal steroids with early pregnancy endocrine disrupting chemicals and pregnancy outcomes. J. Clin. Endocrinol. Metab. 106, 665–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K, et al. , 2018. Associations of maternal exposure to triclosan, parabens, and other phenols with prenatal maternal and neonatal thyroid hormone levels. Environ. Res. 165, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, et al. , 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16, 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, et al. , 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 17, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, 2022. bkmr: Bayesian Kernel Machine Regression. [DOI] [PMC free article] [PubMed]
- Bodnar LM, Siega-Riz AM, 2002. A diet quality index for pregnancy detects variation in diet and differences by sociodemographic factors. Public Health Nutr. 5, 801–809. [DOI] [PubMed] [Google Scholar]
- Boucher B, et al. , 2006. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 9, 84–93. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Bale TL, 2016. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 41, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, et al. , 2010. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ. Health Perspect. 118, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, et al. , 2006. Human exposure assessment to environmental chemicals using biomonitoring. Int J Androl. 29, 166–71; discussion 181–5. [DOI] [PubMed] [Google Scholar]
- Carrico C, et al. , 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J. Agric. Biol. Environ. Stat. 20, 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey AL, et al. , 2019. Associations of phthalates and phthalate replacements with CRH and other hormones among pregnant women in Puerto Rico. J. Endocr. Soc. 3, 1127–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey AL, et al. , 2021. Gestational hormone concentrations are associated with timing of delivery in a Fetal Sex-dependent manner. Front Endocrinol (lausanne). 12, 742145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2019. Fourth national report on human exposure to environmental chemicals: updated tables, January 2019, Volume one. [Google Scholar]
- Chen D, et al. , 2016. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity – a review. Environ. Sci. Tech. 50, 5438–5453. [DOI] [PubMed] [Google Scholar]
- Chen X, et al. , 2023. Adverse effects of triclosan exposure on health and potential molecular mechanisms. Sci. Total Environ. 879, 163068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiuve SE, et al. , 2012. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 142, 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, et al. , 1983. A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson GM, 1988. Perceived Stress in a Probability Sample of the United-States. Social Psychology of Health. 31–67. [Google Scholar]
- Czarnota J, et al. , 2015. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform. 14, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshan A, et al. , 2019. Association of urinary bisphenols and triclosan with thyroid function during early pregnancy. Environ. Int. 133, 105123. [DOI] [PubMed] [Google Scholar]
- Derakhshan A, et al. , 2021. Association of urinary bisphenols during pregnancy with maternal, cord blood and childhood thyroid function. Environ. Int. 146, 106160. [DOI] [PubMed] [Google Scholar]
- Dodson RE, et al. , 2007. Measured and modeled personal exposures to and risks from volatile organic compounds. Environ. Sci. Tech. 41, 8498–8505. [DOI] [PubMed] [Google Scholar]
- Duarte-Guterman P, et al. , 2014. Mechanisms of crosstalk between endocrine systems: regulation of sex steroid hormone synthesis and action by thyroid hormones. Gen. Comp. Endocrinol. 203, 69–85. [DOI] [PubMed] [Google Scholar]
- Edaes FS, de Souza CB, 2022. BPS and BPF are as carcinogenic as BPA and are not viable alternatives for its replacement. Endocr Metab Immune Disord Drug Targets 22, 927–934. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, et al. , 2010. Boys live dangerously in the womb. Am. J. Hum. Biol. 22, 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabory A, et al. , 2013. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol. Sex Differ. 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves JA, 2010. Review: Sex chromosome evolution and the expression of sex-specific genes in the placenta. Placenta 31 (Suppl), S27–S32. [DOI] [PubMed] [Google Scholar]
- Gray LE Jr, et al. , 2006. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int. J. Androl. 29. [DOI] [PubMed] [Google Scholar]
- Guo Y, Kannan K, 2013. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ. Sci. Tech. 47, 14442–14449. [DOI] [PubMed] [Google Scholar]
- Hacker NF, et al. , 2010. Hacker and Moore’s essentials of obstetrics and gynecology. Saunders/Elsevier, Philadelphia, PA. [Google Scholar]
- Haggerty DK, et al. , 2021. REPRODUCTIVE TOXICOLOGY: Pregnancy exposure to endocrine disrupting chemicals: implications for women’s health. Reproduction 162, F169–F180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat AM, 2005. Phthalates and human health. Occup. Environ. Med. 62, 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskovec L, et al. , 2021. Model choice for estimating the association between exposure to chemical mixtures and health outcomes: a simulation study. PLoS One 16, e0249236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HB, et al. , 2018. Longitudinal assessment of prenatal phthalate exposure on serum and cord thyroid hormones homeostasis during pregnancy - Tainan birth cohort study (TBCS). Sci. Total Environ. 619–620, 1058–1065. [DOI] [PubMed] [Google Scholar]
- Huang H, et al. , 2022. Associations of bisphenol exposure with thyroid hormones in pregnant women: a prospective birth cohort study in China. Environ. Sci. Pollut. Res. Int. 29, 87170–87183. [DOI] [PubMed] [Google Scholar]
- Johns LE, et al. , 2015. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod. Biol. Endocrinol. 13, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Park YJ, 2019. Bisphenols and thyroid hormone. Endocrinol Metab (seoul). 34, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinga K, et al. , 1978. Maternal peripheral testosterone levels during the first half of pregnancy. Am. J. Obstet. Gynecol. 131, 60–62. [DOI] [PubMed] [Google Scholar]
- Kolatorova L, et al. , 2018. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ. Res. 163, 115–122. [DOI] [PubMed] [Google Scholar]
- Korevaar TI, et al. , 2016. Maternal total T4 during the first half of pregnancy: physiologic aspects and the risk of adverse outcomes in comparison with free T4. Clin. Endocrinol. 85, 757–763. [DOI] [PubMed] [Google Scholar]
- Kudlak B, et al. , 2022. Enhanced toxicity of bisphenols together with UV filters in water: identification of synergy and antagonism in three-component mixtures. Molecules 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraia BA, et al. , 2007. Pregravid body mass index is negatively associated with diet quality during pregnancy. Public Health Nutr. 10, 920–926. [DOI] [PubMed] [Google Scholar]
- Laurberg P, et al. , 2016. Dynamics and predictors of serum TSH and fT4 reference limits in early pregnancy: a study within the Danish national birth cohort. J. Clin. Endocrinol. Metab. 101, 2484–2492. [DOI] [PubMed] [Google Scholar]
- Lee G, et al. , 2020. Exposure to organophosphate esters, phthalates, and alternative plasticizers in association with uterine fibroids. Environ. Res. 189, 109874. [DOI] [PubMed] [Google Scholar]
- Li J, et al. , 2020. Trimester-specific, gender-specific, and low-dose effects associated with non-monotonic relationships of bisphenol A on estrone, 17beta-estradiol and estriol. Environ. Int. 134, 105304. [DOI] [PubMed] [Google Scholar]
- Liang J, et al. , 2023. Studying paraben-induced estrogen receptor- and steroid hormone-related endocrine disruption effects via multi-level approaches. Sci. Total Environ. 869, 161793. [DOI] [PubMed] [Google Scholar]
- Makieva S, et al. , 2014. Androgens in pregnancy: roles in parturition. Hum. Reprod. Update 20, 542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JF, et al. , 2022. Assessment of human exposure to benzophenone-type UV filters: a review. Environ. Int. 167, 107405. [DOI] [PubMed] [Google Scholar]
- Marx H, et al. , 2008. Hyperthyroidism and pregnancy. BMJ 336, 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ML, et al. , 2002. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am. J. Clin. Nutr. 76, 1261–1271. [DOI] [PubMed] [Google Scholar]
- Meakin AS, et al. , 2021. Let’s talk about placental sex, baby: understanding mechanisms that drive female- and male-specific fetal growth and developmental outcomes. Int. J. Mol. Sci. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, et al. , 2009. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ. Health Perspect. 117, 1587–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg PM, Hofman JA, 1991. Maternal testosterone and fetal sex. J. Steroid Biochem. Mol. Biol. 39, 51–54. [DOI] [PubMed] [Google Scholar]
- Morisset AS, et al. , 2013. Androgens in the maternal and fetal circulation: association with insulin resistance. J. Matern. Fetal Neonatal Med. 26, 513–519. [DOI] [PubMed] [Google Scholar]
- National Research Council (US) Committee on the Health Risks of Phthalates. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. Washington (DC): National Academies Press (US); 2008. [PubMed] [Google Scholar]
- Nakiwala D, et al. , 2022. Phenol and phthalate effects on thyroid hormone levels during pregnancy: relying on in vitro assays and adverse outcome pathways to inform an epidemiological analysis. Environ. Health Perspect. 130, 117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga DC, et al. , 2021. Maternal phthalate and phthalate alternative metabolites and urinary biomarkers of estrogens and testosterones across pregnancy. Environ. Int. 155, 106676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga DC, et al. , 2022. Identification of profiles and determinants of maternal pregnancy urinary biomarkers of phthalates and replacements in the Illinois Kids Development Study. Environ. Int. 162, 107150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga DC, et al. , 2023. Associations of individual and cumulative urinary phthalate and replacement biomarkers with gestational weight gain through late pregnancy. Sci. Total Environ. 855, 158788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks LG, et al. , 2000. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 58, 339–349. [DOI] [PubMed] [Google Scholar]
- Ren B, Zhu Y, 2022. A new perspective on thyroid hormones: crosstalk with reproductive hormones in females. Int. J. Mol. Sci. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards J, et al. , 2001. Maternal experiences of racism and violence as predictors of preterm birth: rationale and study design. Paediatr. Perinat. Epidemiol. 15 (Suppl 2), 124–135. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Carmona Y, et al. , 2020. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J. Eposure Sci. Environ. Epidemiol. 30, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano ME, et al. , 2018. Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Int. J. Hyg. Environ. Health 221, 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamalekis E, et al. , 2006. Androgen levels in the third trimester of pregnancy in patients with preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 126, 16–19. [DOI] [PubMed] [Google Scholar]
- Sarzo B, et al. , 2022. Association between phenols and thyroid hormones: The role of iodothyronine deiodinase genes. Environ. Pollut. 311, 119926. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, et al. , 2014. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction 147, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, et al. , 2017. Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. J. Clin. Endocrinol. Metab. 102, 1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz MM, et al. , 2015. Development of urine standard reference materials for metabolites of organic chemicals including polycyclic aromatic hydrocarbons, phthalates, phenols, parabens, and volatile organic compounds. Anal. Bioanal. Chem. 407, 2945–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HM, et al. , 2019a. Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ. Int. 122, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MY, et al. , 2019b. Pharmacokinetic profile of propyl paraben in humans after oral administration. Environ. Int. 130, 104917. [DOI] [PubMed] [Google Scholar]
- Shin MY, et al. , 2023. Pharmacokinetics of transdermal methyl-, ethyl-, and propylparaben in humans following single dermal administration. Chemosphere 310, 136689. [DOI] [PubMed] [Google Scholar]
- Silva MJ, et al. , 2007. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 860, 106–112. [DOI] [PubMed] [Google Scholar]
- Silva MJ, et al. , 2013. Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in U.S. adults (2000–2012). Environ. Res. 126, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, et al. , 2015. Identification of di-2-ethylhexyl terephthalate (DEHTP) metabolites using human liver microsomes for biomonitoring applications. Toxicol. In Vitro 29, 716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, et al. , 2017. Exposure to di-2-ethylhexyl terephthalate in a convenience sample of U.S. adults from 2000 to 2016. Arch. Toxicol. 91, 3287–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JF, et al. , 2018. Thyroid hormones and female reproduction. Biol. Reprod. 99, 907–921. [DOI] [PubMed] [Google Scholar]
- Silva MJ, et al. , 2019. Exposure to di-2-ethylhexyl terephthalate in the U.S. general population from the 2015–2016 National Health and Nutrition Examination Survey. Environ. Int. 123, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitoris G, et al. , 2022. Does foetal gender influence maternal thyroid parameters in pregnancy? Eur Thyroid J. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souter I, et al. , 2020. Urinary concentrations of phthalate metabolite mixtures in relation to serum biomarkers of thyroid function and autoimmunity among women from a fertility center. Environ. Health Perspect. 128, 67007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano Renzetti PC, Just Allan C, Bello Ghalib, Gennings Chris, 2023. gWQS: Generalized Weighted Quantile Sum Regression.“https://cran.r-project.org/web/packages/gWQS/gWQS.pdf”. [Google Scholar]
- Sun C, et al. , 2023. Triclosan and related compounds in the environment: Recent updates on sources, fates, distribution, analytical extraction, analysis, and removal techniques. Sci. Total Environ. 870, 161885. [DOI] [PubMed] [Google Scholar]
- Tanner EM, et al. , 2019. Repeated holdout validation for weighted quantile sum regression. MethodsX. 6, 2855–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriola AT, et al. , 2011. Determinants of maternal sex steroids during the first half of pregnancy. Obstet. Gynecol. 118, 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, et al. , 2007. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, et al. , 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 33, 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet C, et al. , 2019. An empirical validation of the within-subject biospecimens pooling approach to minimize exposure misclassification in biomarker-based studies. Epidemiology 30, 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. , 2017. Maternal urinary triclosan concentration in relation to maternal and neonatal thyroid hormone levels: a prospective Study. Environ. Health Perspect. 125, 067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. , 2019. Maternal thyroid-stimulating hormone level in the first trimester and sex ratio at birth. Endocr. Pract. 25, 315–319. [DOI] [PubMed] [Google Scholar]
- Wei F, et al. , 2021. Parabens as chemicals of emerging concern in the environment and humans: a review. Sci. Total Environ. 778, 146150. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, et al. , 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect. 119, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, et al. , 2022. Associations between phthalate exposure and thyroid function in pregnant women during the first trimester. Ecotoxicol. Environ. Saf. 242, 113884. [DOI] [PubMed] [Google Scholar]
- Yao HY, et al. , 2016. Maternal phthalate exposure during the first trimester and serum thyroid hormones in pregnant women and their newborns. Chemosphere 157, 42–48. [DOI] [PubMed] [Google Scholar]
- Ye X, et al. , 2014. Urinary concentrations of 2,4-dichlorophenol and 2,5-dichlorophenol in the U.S. population (National Health and Nutrition Examination Survey, 2003–2010): trends and predictors. Environ. Health Perspect. 122, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, et al. , 2015. Urinary concentrations of Bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ. Sci. Tech. 49, 11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yland JJ, et al. , 2022. Phthalate and DINCH urinary concentrations across pregnancy and risk of preterm birth. Environ. Pollut. 292, 118476. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. , 2020. Association of parental preconception exposure to phthalates and phthalate substitutes with preterm birth. JAMA Netw. Open 3, e202159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, et al. , 2014. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect. 122, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.


