Supplemental Digital Content is Available in the Text.
This study establishes the prevalence of co-occurring chronic pain and anxiety/depression symptoms in the US adult general population and characterizes the associated functional limitations.
Keywords: Chronic pain, Anxiety, Depression, Co-occurring, Comorbidity, Mental health screening, PHQ-8, GAD-7, Population prevalence, Mental health, Mental illness, Epidemiology, Global functioning, Functional limitations
Abstract
Co-occurrence of chronic pain and clinically significant symptoms of anxiety and/or depression is regularly noted in the literature. Yet, little is known empirically about population prevalence of co-occurring symptoms, nor whether people with co-occurring symptoms constitute a distinct subpopulation within US adults living with chronic pain or US adults living with anxiety and/or depression symptoms (A/D). To address this gap, this study analyzes data from the 2019 National Health Interview Survey, a representative annual survey of self-reported health status and treatment use in the United States (n = 31,997). Approximately 12 million US adults, or 4.9% of the adult population, have co-occurring chronic pain and A/D symptoms. Unremitted A/D symptoms co-occurred in 23.9% of US adults with chronic pain, compared with an A/D prevalence of 4.9% among those without chronic pain. Conversely, chronic pain co-occurred in the majority (55.6%) of US adults with unremitted A/D symptoms, compared with a chronic pain prevalence of 17.1% among those without A/D symptoms. The likelihood of experiencing functional limitations in daily life was highest among those experiencing co-occurring symptoms, compared with those experiencing chronic pain alone or A/D symptoms alone. Among those with co-occurring symptoms, 69.4% reported that work was limited due to a health problem, 43.7% reported difficulty doing errands alone, and 55.7% reported difficulty participating in social activities. These data point to the need for targeted investment in improving functional outcomes for the nearly 1 in 20 US adults living with co-occurring chronic pain and clinically significant A/D symptoms.
1. Introduction
Anxiety and/or depression symptoms (A/D) often co-occur among people living with chronic pain28; similarly, chronic pain often co-occurs among people experiencing A/D.36,55
Biologically, chronic pain and A/D are connected.41,55 Regions in the brain, eg, the insular cortex, prefrontal cortex, anterior cingulate, thalamus, hippocampus, and amygdala, are associated with pain processing and modulations and with A/D.47 Pain and A/D are associated with neuroinflammation,74,78 and different types of pain have been shown to increase central nervous system and serum inflammatory cytokines,9 which are associated with surgical pain, sleep disturbances, and cognitive impairment.71 Imaging studies suggest the possibility of shared neurobehavioral chronification mechanisms.7
Clinically, both chronic pain and A/D share several distinctive features. Objective measurement of symptoms is not possible—clinical assessment requires reliance on self-report.1,15,17 Reported symptom severity does not deterministically predict the degree of functional limitation.39,46 Prevalence and functional resilience are patterned by social determinants of health, including income, urbanization, age, gender, race/ethnicity, and education.2,22 Body movement and sleep are profoundly impactful for symptom reduction and functional resilience.4,13,32,60–62,70
The lived experience of both chronic pain and A/D may include invalidating interactions with family, friends, coworkers, and healthcare providers.18,23,75 Stigma is often internalized by those living with chronic pain and A/D.42,80 Experiences of social exclusion and injustice,3—including the contexts of health care and research,59—undermine functional resilience.57,79,85 In both, unhelpful cognitive patterns65 and emotional dysregulation34,37 increase the likelihood that symptoms translate to loss of function.12,35 Both chronic pain and A/D are risk factors for many of the same negative health outcomes, including new or exacerbated substance misuse, social isolation, and suicide.26,58,63,64,66,67,76
Co-occurring symptoms may create a compounding and mutually reinforcing effect.28,29,78 A/D may heighten individuals' perception of pain,11,45,72 reinforce unhelpful cognitive patterns,27 reduce treatment engagement, undermine self-efficacy, and increase the likelihood of adverse functional outcomes.14,43,44,49 It may increase the likelihood that acute pain becomes chronic.21,68 Anxiety is associated with worse postoperative recovery outcomes, including pain, prolonged hospital stays, readmission for wound complications, and mortality risk.8 Co-occurrence increases risks from prescribed substances48; eg, postsurgical pain control for patients with A/D may require higher doses of opioids,40 increasing the risk of substance misuse or disorders.33 Co-occurrence is associated with greater functional limitations compared with chronic pain alone or A/D alone.5,10,56
Although countless health conditions may frequently co-occur with chronic pain, the observable commonalities of chronic pain and A/D across biological, clinical, and policy domains present an opportunity for transformative innovations in research, prevention, and treatment.
Several studies of general or patient populations have found that chronic pain or chronic pain-associated health conditions, frequently co-occur with mental disorders, anxiety, depression, or psychological distress.6,19,20,24,30,31,77 Yet, few studies have examined the population prevalence of co-occurrence using the International Association for the Study of Pain (IASP) classification of chronic pain for International Classification of Diseases (ICD-11)73 and validated screening and diagnostic scales for A/D.38,69 To advance this work, this study examines the population prevalence and associated functional limitations of co-occurring chronic pain and A/D symptoms among US adults.
2. Methods
2.1. Data Source
The National Health Interview Survey (NHIS) is a representative, publicly available, deidentified data set suitable for prevalence calculations among the US noninstitutionalized civilian population. The NHIS is a primary data collection program of the National Center for Health Statistics (NCHS); it has surveilled the health of the US general population since 1957. Data obtained through the National Health Interview Survey are routinely used to benchmark progress on national population health objectives.52–54
Population representativeness is achieved using a complex survey design involving geographic stratification, clustering, and weighting procedures. The NHIS data are collected through interview continuously throughout the year by contracted US Census Bureau Field Representatives. The 2019 NHIS data set contained interview data from 31,997 sample adults, representing an estimated population of 244.6 million US adults (95% CI: 237.7-251.4 million). The final sample adult response rate was 59.1%.53 The NHIS uses sampling weights to adjust for the representativeness of the sample. Applying such weights ensures that final estimates are not biased in favor of those respondents with higher likelihood of selection. The value of the weight for any respondent reflects a multistep adjustment starting with a base weight. The base weight is inverse to the probability of selection.53 Weights are subsequently adjusted for nonresponse patterns. In previous years (1997-2018), the nonresponse adjustment was based purely on geography. Since 2019, the nonresponse adjustment approach uses multilevel regression models, including paradata variables that predict the likelihood of survey response and key health outcomes. The nonresponse adjustments are calibrated to US Census Bureau population projections for age, sex, race and ethnicity, educational attainment, Census division, and Metropolitan Statistical Area.53
The NHIS has been identified as the best single source for surveillance of chronic pain in the United States.25 In prior research, the NHIS survey has been used to estimate US chronic pain prevalence,22,50,81,84 examine chronic pain disparities by race/ethnicity,83 and estimate the prevalence of chronic pain treatment strategies used by the US adult general population.51,84 In 2019, the survey for the first time included validated clinical scales for anxiety and depression symptoms.82
2.2. Measures
Chronic pain was operationalized as a binary variable, based on the survey question: “In the past 3 months, how often did you have pain? Would you say never, some days, most days, or every day?” Respondents who answered “most days” or “every day” were coded as having chronic pain. Respondents coded as having chronic pain were additionally coded as either having or not having high-impact chronic pain, based on a survey item that assessed the impact of chronic pain on respondents' global functioning: “Over the past 3 months, how often did your pain limit your life or work activities? Would you say never, some days, most days, or every day?” Respondents who answered “most days” or “every day” were coded as having high-impact chronic pain. These operationalizations are consistent with the International Association for the Study of Pain's (IASP) classification of chronic pain for International Classification of Disease (ICD-11), as well as previously published literature analyzing NHIS data on chronic pain and high-impact pain.51,84 ICD-11 went into effect globally in January 2022.73
In 2019, the National Health Interview Survey-Adults incorporated 2 widely used validated clinical screening and diagnostic scales for symptoms of anxiety and depression: (1) the General Anxiety Disorder-7 (GAD-7), a validated scale used to screen for and diagnose anxiety, and (2) the Patient Health Questionnaire-8 (PHQ-8), a validated scale used to screen for and diagnose depression. These scales are widely used in primary care, emergency departments, and other settings to identify patients for whom further mental health assessment is indicated. The GAD-7 uses summative scoring to categorize patient symptoms as one of the following: none (0-4), mild (5-9), moderate (10-14), and severe (15-21). A score greater or equal to 10 on the GAD-7 is the standard cut point for probable generalized anxiety disorder.69 The PHQ-8 also uses summative scoring to categorize patient symptoms as follows: none/minimal (0-4), mild (5-9), moderate (10-14), and severe (15-24). A score of greater than or equal to 10 on the PHQ-8 is the standard cut point for probable depression.38
Survey respondents were coded as having anxiety symptoms if they scored equal to or greater than 10 on the GAD-7. Survey respondents were coded as having depression symptoms if they scored equal to or greater than 10 on the PHQ-8. Among survey respondents with co-occurring chronic pain and mental health symptoms, the majority had clinically significant symptoms of both anxiety and depression (Supplemental Table 1, available at http://links.lww.com/PAIN/B921). Survey respondents were coded as having anxiety/depression symptoms if they scored greater than or equal to 10 on the PHQ-8, the GAD-7, or both.
Functional limitations were assessed along 3 dimensions: “Because of a physical, mental, or emotional condition, do you have difficulty doing errands alone such as visiting a doctor's office or shopping?,” “Because of a physical, mental, or emotional condition, do you have difficulty participating in social activities such as visiting friends, attending clubs and meetings, or going to parties?” and “Are you limited in the kind OR amount of work you can do because of a physical, mental or emotional problem?”. The survey item regarding work limitations is binary with response categories “Yes” and “No.” The survey items related to activities of daily living and participating in social life are ordinal, with response categories “No difficulty,” “Some difficulty,” “A lot of difficulty,” and “Cannot do at all.” Ordinal variables were binarized to facilitate comparison across all 3 functional dimensions. Respondents who answered, “some difficulty,” “a lot of difficulty,” or “cannot do at all” to the ordinal survey questions were coded as having functional limitation, whereas those who answered “no difficulty” were coded as not having functional limitation. For each subpopulation, numeric responses to “Days missed at work in the past 12 months due to injury, illness, or disability” were assessed.
The NHIS 2019 adult data set includes 787 missing observations (responses of “refused,” “not ascertained,” and “don't know,” 2.5% of data); these observations were excluded from pain and A/D prevalence calculations. An additional 21, 3, and 20 observations were missing data on work, errands, and social participation, respectively; these were excluded from the corresponding analyses of functional limitations.
2.3. Analyses
Population prevalence and population mean estimates were calculated using SAS statistical software version 9.4 accounting for the stratification, clustering, and weighting procedures of the complex NHIS survey design.53 Adjusted Rao-Scott χ2 test statistics were used to compare the prevalence between the different groups. Two-sample t-tests were used to compare mean differences. R was used to generate graphics. Results are reported below.
3. Results
In 2019, 20.5% of US adults reported experiencing chronic pain and 8.8% reported A/D symptoms. The national prevalence of A/D symptoms without chronic pain was 3.9%, corresponding to an estimated 9.6 million people, and the prevalence of chronic pain without A/D symptoms was 15.6%, corresponding to an estimated 38.1 million people. The national prevalence of co-occurring chronic pain and A/D symptoms was 4.9%, corresponding to an estimated 12.0 million people living with co-occurring symptoms (Fig. 1). Table 1 presents complete prevalence statistics related to the co-occurrence of these symptoms.
Figure 1.
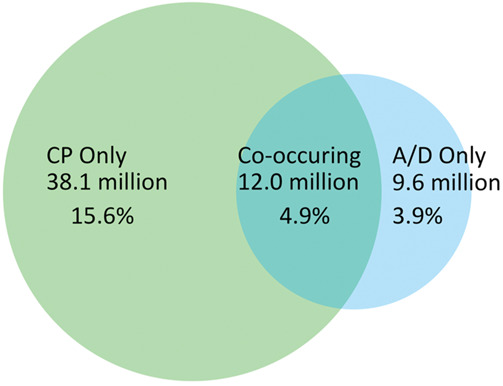
Visualizing prevalence of chronic pain alone, anxiety and/or depression symptoms alone, and co-occurrence. Data Source: National Center for Health Statistics, National Health Interview Survey, 2019. A/D, anxiety/depression; CP, chronic pain.
Table 1.
Prevalence of chronic pain, anxiety/depression symptoms, and co-occurrence in the US adult general population.
| n* | N† (millions) | 95 CI (millions) | Pop. %‡ | 95 CI | |
|---|---|---|---|---|---|
| Neither chronic pain nor A/D symptoms§ | 22,886 | 184.1 | 178.7, 189.5 | 75.5% | 74.9, 76.2 |
| Chronic pain only | 5559 | 38.1 | 36.4, 39.7 | 15.6% | 15.1, 16.1 |
| A/D symptoms only | 1166 | 9.6 | 8.9, 10.3 | 3.9% | 3.6, 4.2 |
| Co-occurring symptoms | 1599 | 12.0 | 11.1, 12.8 | 4.9% | 4.6, 5.2 |
Data Source: National Center for Health Statistics, National Health Interview Survey, 2019.
n = number of responses in survey sample.
N = national prevalence, reported in millions of US adults.
Pop. % = national prevalence as a proportion of the US adult general population.
A/D symptoms are defined as clinically significant symptoms of anxiety, depression, or both.
A/D, anxiety/depression.
Of note, US adults with co-occurring symptoms had a significantly higher prevalence of reported functional limitations compared with those with either chronic pain alone or A/D symptoms alone. Among those with co-occurring symptoms, an estimated 8.3 million (69.4%) reported that work was limited due to a health problem, an estimated 5.2 million (43.7%) reported difficulty doing errands alone, and an estimated 6.6 million (55.7%) reported difficulty participating in social activities (Fig. 2). Compared with those with chronic pain only (all P values <0.001), people with co-occurring symptoms were approximately 3.0 times more likely to report difficulty doing errands alone, 3.5 times more likely to report difficulty participating in social activities, and 1.6 times more likely to report that work is limited. Compared with those with A/D only (all P values <0.001), people with co-occurring symptoms were approximately 1.9 times more likely to report difficulty doing errands alone, 1.6 times more likely to report difficulty participating in social activities, and 1.9 times more likely to report work limitations. For additional detail, see Supplemental Table 2 (available at http://links.lww.com/PAIN/B921).
Figure 2.
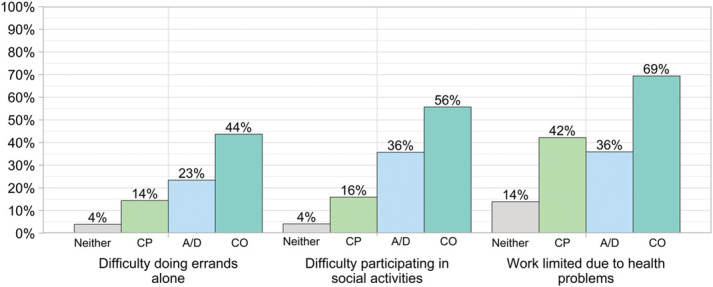
Visualizing prevalence of functional disparities among US adults who have chronic pain only, depression and/or anxiety symptoms only, and co-occurring symptoms. A/D, anxiety/depression only; CP, chronic pain only; CO, co-occurring chronic pain and anxiety/depression symptoms; Neither, neither chronic pain nor anxiety/depression symptoms are present. Data Source: National Center for Health Statistics, National Health Interview Survey, 2019.
Those with co-occurring symptoms averaged more than twice as many missed workdays (µ = 17.5 days [95% CI: 14.1, 21.0]) as those with chronic pain alone (µ = 8.5 days [95% CI: 7.5, 9.6], P < 0.001) and more than 3 times as many workdays as those with A/D symptoms alone (µ = 5.8 days [95% CI: 4.7, 7.0], P < 0.001).
Tables 2 and 3 examine the contingent prevalence of chronic pain and A/D symptoms. These tables are constructed to answer the questions “Given chronic pain, how much A/D?” (Table 2) or “Given A/D, how much chronic pain?” (Table 3).
Table 2.
Prevalence of anxiety/depression symptoms, contingent on presence/absence of chronic pain.
| Clinically significant anxiety and/or depression symptoms | |||||
|---|---|---|---|---|---|
| n* | N† (millions) | 95 CI (millions) | Pop. %‡ | 95 CI | |
| No chronic pain | 1166 | 9.6 | 8.9, 10.3 | 4.9% | 4.6, 5.3 |
| Chronic pain | 1599 | 12.0 | 11.1, 12.8 | 23.9% | 22.6, 25.2 |
| Lower-impact CP | 616 | 4.8 | 4.3, 5.3 | 15.0% | 13.7, 16.4 |
| High-impact CP | 989 | 7.2 | 6.6, 7.8 | 39.5% | 37.1, 41.9 |
Data Source: National Center for Health Statistics, National Health Interview Survey, 2019.
n = number of responses in survey sample.
N = national prevalence, reported in millions of US adults.
Pop. % = national prevalence as a proportion of the US adult general population.
CP, chronic pain.
Table 3.
Prevalence of chronic pain and high-impact chronic pain, contingent on presence/absence of anxiety/depression symptoms.
| Any chronic pain | Lower-impact CP | High-impact CP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n* | N† | 95 CI | Pop. %‡ | 95 CI | N | Pop. % | 95 CI | N | Pop. % | 95 CI | |
| No A/D symptoms§ | 5559 | 38.0 | 3.6, 4.0 | 17.1% | 16.6, 17.7 | 27.0 | 12.2% | 11.7, 12.7 | 11.0 | 5.0% | 4.7, 5.2 |
| A/D symptoms | 1599 | 12.0 | 11.1, 12.8 | 55.6% | 53.2, 57.9 | 4.8 | 22.2% | 20.3, 24.1 | 7.2 | 33.4% | 31.1, 35.6 |
| Depression only | 503 | 3.7 | 3.3, 4.1 | 55.9% | 51.8, 60.1 | 1.6 | 24.0% | 10.6, 27.6 | 2.1 | 31.9% | 28.0, 35.7 |
| Anxiety only | 255 | 1.9 | 1.6, 2.2 | 41.5% | 36.4, 46.6 | 1.1 | 23.9% | 19.3, 28.5 | 0.8 | 17.6% | 13.9, 21.3 |
| Depression and anxiety | 829 | 6.3 | 5.8, 6.8 | 61.3% | 58.1, 64.5 | 2.1 | 20.2% | 17.6, 22.8 | 4.2 | 41.1% | 37.9, 44.4 |
Data Source: National Center for Health Statistics, National Health Interview Survey, 2019.
n = number of responses in survey sample.
N = national prevalence, reported in millions of US adults.
Pop. % = national prevalence as a proportion of the US adult general population.
A/D symptoms are defined as clinically significant symptoms of anxiety, depression, or both.
A/D, anxiety/depression; CP, chronic pain.
Table 2 shows that among an estimated 50.1 million US adults with chronic pain, an estimated 12.0 million (23.9%) have co-occurring A/D symptoms. Of those with high-impact chronic pain, 7.2 million (39.5%) have co-occurring A/D symptoms. Among US adults without chronic pain, only 4.9% (9.6 million) have A/D symptoms. When comparing those with and without chronic pain, A/D symptoms are approximately 4.9 times more prevalent in US adults with chronic pain (P < 0.001).
Table 3 reverses the contingency. Among the 21.6 million US adults with A/D symptoms, an estimated 12.0 million (55.6%) have chronic pain and 7.2 million (33.4%) have high-impact chronic pain. Among the estimated 162.1 million US adults without A/D symptoms, an estimated 38.0 million (17.1%) have chronic pain and an estimated 11.0 million (5.0%) have high-impact chronic pain. When comparing those with and without A/D, chronic pain is approximately 3.3 times more prevalent in US adults with A/D (P < 0.001). Among the estimated 12.0 million respondents with symptoms of both anxiety and depression, 6.3 million (61.3%) report having chronic pain and 4.2 million (41.1%) report having high-impact chronic pain.
In exploring the disproportionate burden of pain-related functional limitation among people with A/D symptoms and co-occurring chronic pain, we see that among adults with chronic pain but without A/D symptoms, only 19.8% experience high-impact pain. Among those with chronic pain and A/D symptoms, the majority (60.1%) experience their pain as high impact.
4. Discussion
Very few studies within the past 15 years have examined the prevalence of co-occurring chronic pain and anxiety/depression symptoms among US adults. This study establishes the national prevalence of co-occurring chronic pain and unremitted A/D symptoms in the US adult general population using (1) the International Association for the Study of Pain (IASP) classification of chronic pain for International Classification of Disease (ICD-11)73 and (2) validated clinical scales for anxiety and depression based on the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria. The PHQ-8 and the GAD-7 are used as routine diagnostic and screening tools across a wide variety of clinical settings; they are also used for population-based surveillance of anxiety and depression.38,69 We found that an estimated 12.0 million people, 4.9% of the US adult population or approximately 1 in 20 US adults, experience co-occurrence of chronic pain and anxiety/depression symptoms.
Blyth et al.6 report that among adults with chronic pain in New South Wales, Australia, increasing levels of psychological distress are significantly associated with pain interference in respondent activities. In their 2005 analysis of the 2001 to 2005 National Comorbidity Survey Replication (NCS-R), Von Korff et al.77 found that 30.5% of US adults with chronic spinal pain had a comorbid mental disorder. They note that comorbidity in chronic spinal pain is significantly associated with role disability, recommending that the social impact of chronic spinal pain is best understood in the context of comorbidity. Demyttenaere et al.24 used cross-national data to confirm the association of mental disorders among persons with self-reported chronic neck or back pain.
Consistent with these earlier epidemiological studies, we found that unremitted anxiety and/or depression symptoms are 5 times more common in people with chronic pain than in those without chronic pain and that people living with chronic pain make up more than half of US adults with unremitted anxiety and/or depression symptoms. These findings highlight an underappreciated population need and potential clinical opportunity.
In addition to estimating prevalence, this study identified that those with co-occurring symptoms are at an elevated risk of functional limitations at work, activities of daily living, and social participation. The prevalence of high-impact pain and functional limitations were significantly higher among US adults with co-occurring symptoms than among those with either mental health symptoms alone or chronic pain alone. This included missing more days of work and difficulty participating in errands and social activities. In other words, among US adults with unremitted anxiety/depression symptoms, the majority also have co-occurring chronic pain, and the majority of that chronic pain is associated with limited functioning in life and work.
This study provides estimates using 2 distinct operationalizations of co-occurrence. The most applicable operationalization depends on the intended use of the estimates. The first operationalization (Table 1) categorizes the US adult population into 4 mutually exclusive and exhaustive subpopulations: (1) those with co-occurrence of chronic pain and mental health symptoms, (2) those with chronic pain alone, (3) those with anxiety/depression alone, and (4) those with neither chronic pain nor anxiety/depression symptoms. Three dimensions of global function, namely, work limitation, activities of daily living, and social participation, are explored. Mutually exclusive and exhaustive prevalence estimates may be most applicable to policymakers and researchers interested in understanding the dynamics of chronic pain and A/D symptom co-occurrence at a population level.
The second operationalization (Tables 2 and 3) examines the contingencies between chronic pain and A/D. Table 2 presents the prevalence of A/D, given chronic pain, whereas Table 3 presents the converse. Contingent prevalence estimates may be most applicable to health systems research or to those planning interventions or implementations where recruitment comes from populations of identified chronic pain patients or A/D patients, among which a smaller subset of patients are expected to have co-occurring symptoms.
In the review of the literature, commonalities in chronic pain and anxiety/depression in both preclinical and clinical research were noted. In the authors' view, co-occurrence is neither a contamination nor a complication for chronic pain or mental health research and must not be treated as such. Instead, the co-occurrence of chronic pain and A/D symptoms should be viewed as an opportune research target with potential to produce synergistic advances in the areas of chronic pain and mental illness prevention, treatment, education, and policy.
5. Limitations
The data are cross-sectional and thus cannot support inferences as to the directionality of the causal relationships between chronic pain, anxiety/depression, and functional limitation. The NHIS includes residents of households and noninstitutional group quarters such as homeless shelters and group homes. Persons with no fixed address, active-duty military personnel and civilians living on military bases, persons in long-term care institutions, persons in correctional facilities, and US nationals living abroad are not represented in this survey.53 This analysis focuses exclusively on unremitted symptoms; it does not speak to the national prevalence of symptoms that have been successfully remitted through past or ongoing treatment. Our operationalization of chronic pain is identical to the ICD-11 classification, and our operationalization of anxiety and depression symptoms uses validated screening and diagnostic scales that correspond to DSM criteria. Nonetheless, it is still possible that self-reported diagnostic data collected by the National Health Interview Survey may be less accurate than self-reported diagnostic data collected by a clinician.
6. Conclusion
Prioritizing the health and functional outcomes of people with co-occurring chronic pain and anxiety/depression symptoms would benefit all who are impacted by chronic pain or anxiety/depression. Translational and reverse translational research on co-occurrence may present an opportunity to develop interventions that—if effective to improve outcomes in the context of co-occurrence—can be fully rolled out to benefit both clinical populations.
Overall, the evidence is consistent with the narrative that the co-occurrence of chronic pain and A/D symptoms makes achieving positive health outcomes for either or both conditions more challenging. The clinical literature suggests that comprehensive mental and physical health treatment is the best treatment approach for patients with co-occurring symptoms.
Yet, little is known about A/D treatment use and the effectiveness of A/D treatment to control A/D symptoms and improve function among patients who have both chronic pain and mental health needs. Further research should clarify the existing dynamics of treatment referral, use, and effectiveness of mental health treatment for patients with chronic pain. Further research should precisely identify and address systemic disparities in treatment and associated outcomes among those living with co-occurring symptoms.
To ameliorate the profound functional impacts associated with the co-occurrence of chronic pain and mental health symptoms, research to address unmet needs and improve outcomes must be prioritized. Emphasizing function, in the case of chronic pain and anxiety/depression, may be an avenue to better meet patients where they are, vs focusing exclusively on symptom control per se.
The 2020 to 2022 global coronavirus pandemic may have durably altered the national prevalence of chronic pain and anxiety/depression since 2019.16 Numerous surveys were initiated during the pandemic to understand trends and changes in chronic pain and mental health associated with Covid-19. This study may function as a baseline index for pandemic-related comparative research in mental health, chronic pain, and co-occurrence. In 2025, the National Health Interview Survey will again use both the PHQ-8 and GAD-7, along with the chronic pain module. When the data are released, the authors will publish a comparative analysis of 2019 vs 2025, including the provision of updated national prevalence estimates on co-occurrence of chronic pain and unremitted anxiety/depression symptoms among US adults.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B921.
Acknowledgements
Funding for this study was provided by the Comprehensive Pain and Addiction Center, University of Arizona Health Sciences.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Akca YEA, Slootmaekers L, Boskovic I. Verifiability and symptom endorsement in genuine, exaggerated, and malingered pain. Psychol Inj L 2020;13:235–45. [Google Scholar]
- [2].Alegría M, NeMoyer A, Falgas I, Wang Y, Alvarez K. Social determinants of mental health: where we are and where we need to go. Curr Psychiatry Rep 2018;20:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Allen SF, Gilbody S, Atkin K, van der Feltz-Cornelis C. The associations between loneliness, social exclusion and pain in the general population: a N=502,528 cross-sectional UK Biobank study. J Psychiatr Res 2020;130:68–74. [DOI] [PubMed] [Google Scholar]
- [4].Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep 2013;36:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Backe IF, Patil GG, Nes RB, Clench-Aas J. The relationship between physical functional limitations, and psychological distress: considering a possible mediating role of pain, social support and sense of mastery. SSM Popul Health 2017;4:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. PAIN 2001;89:127–34. [DOI] [PubMed] [Google Scholar]
- [7].Brandl F, Weise B, Mulej Bratec S, Jassim N, Hoffmann Ayala D, Bertram T, Ploner M, Sorg C. Common and specific large-scale brain changes in major depressive disorder, anxiety disorders, and chronic pain: a transdiagnostic multimodal meta-analysis of structural and functional MRI studies. Neuropsychopharmacology 2022;47:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Britteon P, Cullum N, Sutton M. Association between psychological health and wound complications after surgery. Br J Surg 2017;104:769–76. [DOI] [PubMed] [Google Scholar]
- [9].Bromander S, Anckarsäter R, Kristiansson M, Blennow K, Zetterberg H, Anckarsäter H, Wass CE. Changes in serum and cerebrospinal fluid cytokines in response to non-neurological surgery: an observational study. J Neuroinflammation 2012;9:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brooks JM, Polenick CA, Bryson W, Naslund JA, Renn BN, Orzechowski NM, Almeida M, Bartels SJ. Pain intensity, depressive symptoms, and functional limitations among older adults with serious mental illness. Aging Ment Health 2019;23:470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burston JJ, Valdes AM, Woodhams SG, Mapp PI, Stocks J, Watson DJG, Gowler PRW, Xu L, Sagar DR, Fernandes G, Frowd N, Marshall L, Zhang W, Doherty M, Walsh DA, Chapman V. The impact of anxiety on chronic musculoskeletal pain and the role of astrocyte activation. PAIN 2019;160:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bushnell MC, Čeko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Butera KA, Fox EJ, George SZ. Toward a transformed understanding: from pain and movement to pain with movement. Phys Ther 2016;96:1503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cheng S-T, Leung CMC, Chan KL, Chen PP, Chow YF, Chung JWY, Law ACB, Lee JSW, Leung EMF, Tam CWC. The relationship of self-efficacy to catastrophizing and depressive symptoms in community-dwelling older adults with chronic pain: a moderated mediation model. PLoS One 2018;13:e0203964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chojnowska S, Ptaszyńska-Sarosiek I, Kępka A, Knaś M, Waszkiewicz N. Salivary biomarkers of stress, anxiety and depression. J Clin Med 2021;10:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Clauw DJ, Häuser W, Cohen SP, Fitzcharles M-A. Considering the potential for an increase in chronic pain after the COVID-19 pandemic. PAIN 2020;161:1694–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Craig KD, Badali MA. Introduction to the special series on pain deception and malingering. Clin J Pain 2004;20:377. [DOI] [PubMed] [Google Scholar]
- [18].Crumb L, Mingo TM, Crowe A. “Get over it and move on”: the impact of mental illness stigma in rural, low-income United States populations. Ment Health Prev 2019;13:143–8. [Google Scholar]
- [19].Csupak B, Sommer JL, Jacobsohn E, El-Gabalawy R. A population-based examination of the co-occurrence and functional correlates of chronic pain and generalized anxiety disorder. J Anxiety Disord 2018;56:74–80. [DOI] [PubMed] [Google Scholar]
- [20].Currie SR, Wang J. Chronic back pain and major depression in the general Canadian population. PAIN 2004;107:54–60. [DOI] [PubMed] [Google Scholar]
- [21].Currie SR, Wang J. More data on major depression as an antecedent risk factor for first onset of chronic back pain. Psychol Med 2005;35:1275–82. [DOI] [PubMed] [Google Scholar]
- [22].Dahlhamer J. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].De Ruddere L, Craig KD. Understanding stigma and chronic pain: a-state-of-the-art review. PAIN 2016;157:1607. [DOI] [PubMed] [Google Scholar]
- [24].Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, Levinson D, de Girolamo G, Nakane H, Mneimneh Z, Lara C, de Graaf R, Scott KM, Gureje O, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J, Von Korff M. Mental disorders among persons with chronic back or neck pain: results from the World Mental Health Surveys. PAIN 2007;129:332–42. [DOI] [PubMed] [Google Scholar]
- [25].Duca LM, Helmick CG, Barbour KE, Nahin RL, Von Korff M, Murphy LB, Theis K, Guglielmo D, Dahlhamer J, Porter L, Falasinnu T, Mackey S. A review of potential national chronic pain surveillance systems in the United States. J Pain 2022;23:1492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garey L, Olofsson H, Garza T, Rogers AH, Kauffman BY, Zvolensky MJ. Directional effects of anxiety and depressive disorders with substance use: a review of recent prospective research. Curr Addict Rep 2020;7:344–55. [Google Scholar]
- [27].Gatchel RJ, Neblett R, Kishino N, Ray CT. Fear-avoidance beliefs and chronic pain. J Orthop Sports Phys Ther 2016;46:38–43. [DOI] [PubMed] [Google Scholar]
- [28].Gerrits MM, van Oppen P, Leone SS, van Marwijk HW, van der Horst HE, Penninx BW. Pain, not chronic disease, is associated with the recurrence of depressive and anxiety disorders. BMC Psychiatry 2014;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gerrits MMJG, Vogelzangs N, van Oppen P, van Marwijk HWJ, van der Horst H, Penninx BWJH. Impact of pain on the course of depressive and anxiety disorders. PAIN 2012;153:429–36. [DOI] [PubMed] [Google Scholar]
- [30].Gureje O, Von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, Lepine JP, Angermeyer MC, Levinson D, de Girolamo G, Iwata N, Karam A, Guimaraes Borges LG, de Graaf R, Browne MO, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J. The relation between multiple pains and mental disorders: results from the World Mental Health Surveys. PAIN 2008;135:82–91. [DOI] [PubMed] [Google Scholar]
- [31].Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a world health organization study in primary care. JAMA 1998;280:147–51. [DOI] [PubMed] [Google Scholar]
- [32].Haack M, Simpson N, Sethna N, Kaur S, Mullington J. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology 2020;45:205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hah JM, Sharifzadeh Y, Wang BM, Gillespie MJ, Goodman SB, Mackey SC, Carroll IR. Factors associated with opioid use in a cohort of patients presenting for surgery. Pain Res Treat 2015;2015:829696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hamilton NA, Karoly P, Kitzman H. Self-Regulation and chronic pain: the role of emotion. Cogn Ther Res 2004;28:559–76. [Google Scholar]
- [35].Hampton SN, Nakonezny PA, Richard HM, Wells JE. Pain catastrophizing, anxiety, and depression in hip pathology. Bone Joint J 2019;101-B:800–7. [DOI] [PubMed] [Google Scholar]
- [36].de Heer EW, Gerrits MMJG, Beekman ATF, Dekker J, van Marwijk HWJ, de Waal MWM, Spinhoven P, Penninx BWJH, van der Feltz-Cornelis CM. The association of depression and anxiety with pain: a study from NESDA. PLoS One 2014;9:e106907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Koechlin H, Coakley R, Schechter N, Werner C, Kossowsky J. The role of emotion regulation in chronic pain: a systematic literature review. J Psychosom Res 2018;107:38–45. [DOI] [PubMed] [Google Scholar]
- [38].Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–73. [DOI] [PubMed] [Google Scholar]
- [39].Lamé IE, Peters ML, Vlaeyen JWS, Kleef Mv, Patijn J. Quality of life in chronic pain is more associated with beliefs about pain, than with pain intensity. Eur J Pain 2005;9:15–24. [DOI] [PubMed] [Google Scholar]
- [40].Larach DB, Sahara MJ, As-Sanie S, Moser SE, Urquhart AG, Lin J, Hassett AL, Wakeford JA, Clauw DJ, Waljee JF, Brummett CM. Patient factors associated with opioid consumption in the month following major surgery. Ann Surg 2021;273:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li J-X. Pain and depression comorbidity: a preclinical perspective. Behav Brain Res 2015;276:92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Livingston JD, Boyd JE. Correlates and consequences of internalized stigma for people living with mental illness: a systematic review and meta-analysis. Soc Sci Med 2010;71:2150–61. [DOI] [PubMed] [Google Scholar]
- [43].Martinez-Calderon J, Flores-Cortes M, Morales-Asencio JM, Luque-Suarez A. Pain-related fear, pain intensity and function in individuals with chronic musculoskeletal pain: a systematic review and meta-analysis. J Pain 2019;20:1394–415. [DOI] [PubMed] [Google Scholar]
- [44].Martinez-Calderon J, Jensen MP, Morales-Asencio JM, Luque-Suarez A. Pain catastrophizing and function in individuals with chronic musculoskeletal pain. Clin J Pain 2019;35:279–93. [DOI] [PubMed] [Google Scholar]
- [45].McGrath PA. Psychological aspects of pain perception. Arch Oral Biol 1994;39:S55–62. [DOI] [PubMed] [Google Scholar]
- [46].McKnight PE, Monfort SS, Kashdan TB, Blalock DV, Calton JM. Anxiety symptoms and functional impairment: a systematic review of the correlation between the two measures. Clin Psychol Rev 2016;45:115–30. [DOI] [PubMed] [Google Scholar]
- [47].Meerwijk EL, Ford JM, Weiss SJ. Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging Behav 2013;7:1–14. [DOI] [PubMed] [Google Scholar]
- [48].Michaelides A, Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med 2019;131:438–44. [DOI] [PubMed] [Google Scholar]
- [49].Miró J, Castarlenas E, de la Vega R, Galán S, Sánchez-Rodríguez E, Jensen MP, Cane D. Pain catastrophizing, activity engagement and pain willingness as predictors of the benefits of multidisciplinary cognitive behaviorally-based chronic pain treatment. J Behav Med 2018;41:827–35. [DOI] [PubMed] [Google Scholar]
- [50].Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 2015;16:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nahin RL. Use of multimodal multidisciplinary pain management in the US. JAMA Netw Open 2022;5:e2240620. [DOI] [PubMed] [Google Scholar]
- [52].National Center for Health Statistics. National health interview survey English brochure, 2019. Available at: https://www.cdc.gov/nchs/nhis/participants/survey-brochure.htm. Accessed July 7, 2023. [Google Scholar]
- [53].National Center for Health Statistics. Survey description, national health interview survey, 2019. Hyattsville, MD. 2020. Available at: https://ftp.cdc.gov/pub/health_Statistics/NCHs/Dataset_Documentation/NHIS/2019/srvydesc-508.pdf. Accessed July 7, 2023. [Google Scholar]
- [54].National Center for Health Statistics. National health interview survey, 2019. Public-use data file and documentation. Hyattsville, MD, 2020. Available at: https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm. Accessed July 7, 2023. [Google Scholar]
- [55].Nekovarova T, Yamamotova A, Vales K, Stuchlik A, Fricova J, Rokyta R. Common mechanisms of pain and depression: are antidepressants also analgesics? Front Behav Neurosci 2014;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nissen A, Hynek KA, Scales D, Hilden PK, Straiton M. Chronic pain, mental health and functional impairment in adult refugees from Syria resettled in Norway: a cross-sectional study. BMC Psychiatry 2022;22:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Onwumere J, Stubbs B, Stirling M, Shiers D, Gaughran F, Rice ASC, C de C Williams A, Scott W. Pain management in people with severe mental illness: an agenda for progress. PAIN 2022;163:1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Oquendo MA, Malone KM, Mann JJ. Suicide: risk factors and prevention in refractory major depression. Depress Anxiety 1997;5:202–11. [PubMed] [Google Scholar]
- [59].Palermo TM, Davis KD, Bouhassira D, Hurley RW, Katz JD, Keefe FJ, Schatman M, Turk DC, Yarnitsky D. Promoting inclusion, diversity, and equity in pain science. Pain Rep 2023;8:e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Palermo TM, Kiska R. Subjective sleep disturbances in adolescents with chronic pain: relationship to daily functioning and quality of life. J Pain 2005;6:201–7. [DOI] [PubMed] [Google Scholar]
- [61].Palermo TM, Law EF, Kim A, de la Vega R, Zhou C. Baseline sleep disturbances modify outcome trajectories in adolescents with chronic pain receiving internet-delivered psychological treatment. J Pain 2022;23:1245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Payne P, Crane-Godreau MA. Meditative movement for depression and anxiety. Front Psychiatry 2013;4:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Racine M. Chronic pain and suicide risk: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry 2018;87:269–80. [DOI] [PubMed] [Google Scholar]
- [64].Riquino MR, Priddy SE, Howard MO, Garland EL. Emotion dysregulation as a transdiagnostic mechanism of opioid misuse and suicidality among chronic pain patients. Borderline Personal Disord Emot Dysregul 2018;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Shigetoh H. Hypervigilance to pain affects activities of daily living: an examination using the Japanese version of the pain vigilance awareness questionnaire. J Phys Ther Sci 2017;29:2094–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Smit T, Rogers AH, Garey L, Allan NP, Viana AG, Zvolensky MJ. Anxiety sensitivity and pain intensity independently predict opioid misuse and dependence in chronic pain patients. Psychiatry Res 2020;294:113523. [DOI] [PubMed] [Google Scholar]
- [67].Smith TO, Dainty JR, Williamson E, Martin KR. Association between musculoskeletal pain with social isolation and loneliness: analysis of the English Longitudinal Study of Ageing. Br J Pain 2019;13:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Smitherman TA, Maizels M, Penzien DB. Headache chronification: screening and behavioral management of comorbid depressive and anxiety disorders. Headache J Head Face Pain 2008;48:45–50. [DOI] [PubMed] [Google Scholar]
- [69].Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. [DOI] [PubMed] [Google Scholar]
- [70].Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology 2011;114:940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology 2018;154:204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Syrjala KL, Jensen MP, Mendoza ME, Yi JC, Fisher HM, Keefe FJ. Psychological and behavioral approaches to cancer pain management. J Clin Oncol 2014;32:1703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang S-J. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). PAIN 2019;160:19–27. [DOI] [PubMed] [Google Scholar]
- [74].Vergne-Salle P, Bertin P. Chronic pain and neuroinflammation. Joint Bone Spine 2021;88:105222. [DOI] [PubMed] [Google Scholar]
- [75].Volkow ND, Gordon JA, Koob GF. Choosing appropriate language to reduce the stigma around mental illness and substance use disorders. Neuropsychopharmacology 2021;46:2230–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016;374:1253–63. [DOI] [PubMed] [Google Scholar]
- [77].Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R. Chronic spinal pain and physical–mental comorbidity in the United States: results from the national comorbidity survey replication. PAIN 2005;113:331. [DOI] [PubMed] [Google Scholar]
- [78].Walker AK, Kavelaars A, Heijnen CJ, Dantzer R. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev 2014;66:80–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Wallace B, Varcoe C, Holmes C, Moosa-Mitha M, Moor G, Hudspith M, Craig KD. Towards health equity for people experiencing chronic pain and social marginalization. Int J Equity Health 2021;20:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Waugh OC, Byrne DG, Nicholas MK. Internalized stigma in people living with chronic pain. J Pain 2014;15:550.e1–e10. [DOI] [PubMed] [Google Scholar]
- [81].Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. PAIN 2022;163:e328–32. [DOI] [PubMed] [Google Scholar]
- [82].Zablotsky B, Weeks JD, Terlizzi EP, Madans JH, Blumberg SJ. Assessing anxiety and depression: a comparison of national health interview survey measures. Hyattsville, MD: National Center for Health Statistics, 2022. [PubMed] [Google Scholar]
- [83].Zajacova A, Grol-Prokopczyk H, Fillingim R. Beyond Black vs White: racial/ethnic disparities in chronic pain including Hispanic, Asian, Native American, and multiracial US adults. PAIN 2022;163:1688–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zelaya C, Dahlhamer J, Lucas J, Connor E. Chronic pain and high-impact chronic pain among U.S. adults, 2019. Hyattsville, MD: National Center for Health Statistics, 2020. Available: https://www.cdc.gov/nchs/data/databriefs/db390-H.pdf. Accessed February 25, 2023. [Google Scholar]
- [85].Ziadni MS, Sturgeon JA, Bissell D, Guck A, Martin KJ, Scott W, Trost Z. Injustice appraisal, but not pain catastrophizing, mediates the relationship between perceived ethnic discrimination and depression and disability in low back pain. J Pain 2020;21:582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B921.


