Abstract
Objective
The use of minimally invasive endoluminal treatment for urethral strictures has been a subject for debate for several decades. The aim of this study was to review and discuss the safety, efficacy and factors influencing the clinical application of balloon dilation for the treatment of male urethral strictures.
Design
Systematic review and meta-analysis.
Data sources
Embase, Medline, Web of Science, Cochrane Library and Scopus were searched for publications published before 17 July 2022.
Study selection
Two independent researchers screened and assessed the results, and all clinical studies on balloon dilation for the treatment of urethral strictures in men were included.
Data extraction and synthesis
The success rate, rate of adverse events, International Prostate Symptom Scores, maximum uroflow (Qmax) and postvoid residual urine volume were the main outcomes. Stata V.14.0 was used for statistical analysis.
Results
Fifteen studies with 715 patients were ultimately included in this systematic review. The pooled results of eight studies showed that the reported success rate of simple balloon dilation for male urethral strictures was 67.07% (95% confidence interval [CI]: 55.92% to 77.36%). The maximum urinary flow rate at 3 months (risk ratio [RR]= 2.6510, 95% CI: 1.0681 to 4.2338, p<0.01) and the maximum urinary flow rate at 1 year (RR= 1.6637, 95% CI: 1.1837 to 2.1437, p<0.05) were significantly different after dilation. There is insufficient evidence to suggest that balloon dilation is superior to optical internal urethrotomy or direct visual internal urethrotomy (DVIU) (RR= 1.4754, 95% CI: 0.7306 to 2.9793, p=0.278).
Conclusion
Balloon dilation may be an intermediate step before urethroplasty and is a promising alternative therapy to simple dilation and DVIU. The balloon is a promising drug delivery tool, and paclitaxel drug-coated balloon dilation is effective in reducing retreatment rates in patients with recurrent anterior urethral strictures. The aetiology, location, length, previous treatment of urethral stricture may be associated with the efficacy of balloon dilation.
PROSPERO registration number
CRD42022334403.
Keywords: UROLOGY, Adult urology, Kidney & urinary tract disorders
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study systematically reviewed the principle, safety and efficacy of balloon dilatation and described intermittent urethral balloon self-dilation.
We provide a comprehensive analysis of factors such as aetiology, stricture location, stricture length, and prior management intervention and discuss the clinical directions for balloon dilation.
The quality of the included studies was relatively low, and there was a considerable risk of bias.
Most of the included studies were retrospective observational studies that lacked valid controls, and the results need to be interpreted with caution.
Introduction
Urethral stricture is a relatively common disease in men and is described as any abnormal narrowing of the anterior or posterior urethra. In some susceptible populations, the incidence of male urethral stricture disease is as high as 0.6%, with more than 5000 individuals hospitalised per year.1 The most common symptoms in patients are weakened urine flow and even urinary retention, which seriously affects the quality of life.2 The aetiology of urethral stricture is complex, is complex and includes trauma, infection, iatrogenic, lichen sclerosus, idiopathic, etc. Iatrogenic urethral injury is the most common type of urethral stricture in resource-rich countries, whereas urethral injuries caused by infection and trauma are more common in developing countries.3 4 With continuous developments in medical technology, the rapid increase in the incidence of iatrogenic urethral stricture warrants further investigation. Catheterisation, transurethral manipulation, prostate surgery, radiotherapy and chemotherapy can cause irreversible stricture of the urethra.5–8
Although urethroplasty has been recognised as a curative treatment for urethral strictures, dilation and direct visual internal urethrotomy (DVIU) are still widely used and effective for single bulbar urethral strictures <2 cm, for which the success rate is 35%–70%.3 9 There is currently a lack of evidence evaluating whether dilation or DVIU is more effective than the other methods, so both have the same therapeutic indications.10
Balloon dilation is a special type of dilation that has a long history of treating urethral strictures in men. Russinovich et al were first to report the outcomes of balloon dilation performed in seven males with urethral stricture in 1980; this type of dilation was painless compared with traditional dilation methods and was not prone to cause mucosal or periurethral injury.11 Subsequently, Pinot et al dilated the urethra of 25 patients using an inflatable balloon catheter, which included atraumatic catheterisation through a vascular catheter under urethroscopy, followed by inflation of the balloon catheter into a flexible guidewire.12 Dilation was controlled under the guidance of voiding urethrography and was much less uncomfortable than conventional urethral dilation; only 3 of 25 patients needed to undergo a repeat procedure. Immediately, Glesy et al designed a new coaxial balloon dilator for the treatment of urethral stricture and noted that the balloon dilator can expand slowly and gradually, which is better than traditional rapid and sudden expansion.13 Several studies have shown that balloon dilation results in minimal trauma and immediate symptom relief, with less patient discomfort and a lower complication rate.14–19 Since there is some radiation exposure with angiography, B-ultrasound has been used to facilitate control of balloon dilation, and good clinical results have been initially achieved.20 Further research revealed that balloon dilation under the guidance of cystoscopy gently, safely and effectively dilates the urethra.21
Although balloon dilation is a well-tolerated minimally invasive endoluminal surgical procedure widely used in practice, its clinical significance has not been systematically and comprehensively reviewed. Our objective was to assess the efficacy, safety and factors influencing the clinical application of balloon dilation.
Materials and methods
Search strategy
Reporting in this study was in accordance with the guidelines of the PRISMA statement22 (online supplemental table 1), and the specific protocol was registered on PROSPERO with the registration number CRD42022334403. Using Medical Subject Headings and free text terms, we searched for relevant articles published prior to 17 July 2022, in the following databases: Medline, Embase, Cochrane Library, Web of Science and Scopus. The search strategy is shown in online supplemental file.
bmjopen-2023-071923supp001.pdf (99.1KB, pdf)
bmjopen-2023-071923supp002.pdf (68.3KB, pdf)
Eligibility criteria
Two researchers (XL and CX) screened and assessed the search results independently. The inclusion criteria were as follows: (1) studies with male patients diagnosed with urethral strictures; (2) studies in which balloon dilation was applied as the main intervention, not including patient self-dilation; (3) clinical studies, retrospective or prospective and (4) studies reporting the success and adverse event rates.
Conference abstracts were eligible for inclusion if they reported sufficient outcome data. If several articles were all related to the same study, the most recent publication with the most complete data was included in the systematic review. A consensus was finally reached through consultation and discussion in the event of any disagreements or differences between the two researchers.
Quality assessment
The quality of the included studies was independently assessed by two researchers (XL and CX). All observational studies were assessed using the Newcastle-Ottawa Scale in terms of population selection, comparability and outcome evaluation.23 Randomised controlled trials (RCTs) were assessed using the Jadad Quality Scale, and articles with a score >3 were considered high-quality research.24 For single-arm clinical trials, the first eight items of the Methodological Index for Non-randomised Studies scale were used for assessment.25 The ROBINS-I tool was used to further assess the risk of bias in non-RCTs.26
Data extraction
We extracted data on the success rate, adverse event rate, International Prostate Symptom Score (IPSS), maximum uroflow (Qmax, mL/s) and postvoid residual urine volume (PVR). When disagreements arose, a third reviewer participated in the discussions and mediated to reach a consensus.
Statistical analysis
Stata V.14.0 (StataCorp) was used for statistical analysis, and the success and adverse event rates were reported as proportions. The I2 index was used to test for between-study heterogeneity. An I2>50% was considered to indicate significant heterogeneity, and the random effects model was used for pooled analysis; otherwise, less heterogeneity was considered, and the fixed effects model was used. By excluding each study one by one, we performed a sensitivity analysis of the balloon dilation success rate to assess the stability and reliability of the pooled results. Subgroup analyses were performed according to the results of the meta-regression models.
Patient and public involvement
None.
Results
Study selection
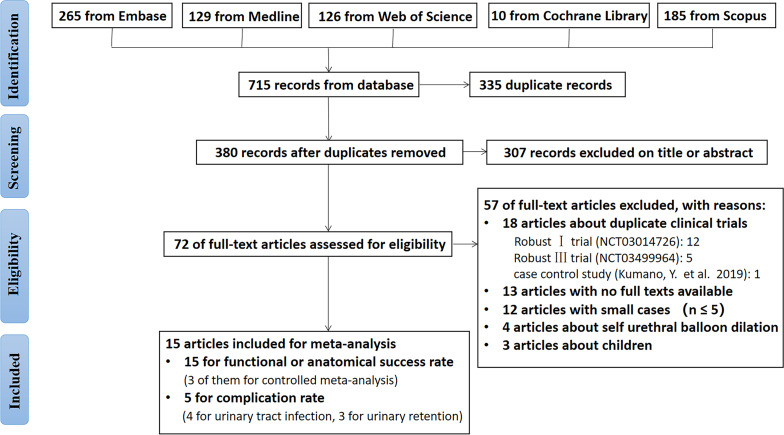
The flow chart of the study retrieval process is shown in figure 1. Fifteen studies were included in the systematic review, involving a total of 842 patients. Tables 1 and 2 present the main characteristics of the included studies. Among these, there were 1 RCT,27 2 single-arm clinical trials,28 29 2 case‒control studies30 31 and 10 retrospective case studies.32–41
Figure 1.
Flow diagram of study selection.
Table 1.
Clinical characteristics and efficiency of balloon dilation (I)
| Study | Evaluable Patients (n) | Age (average) | Aetiology | Location of the strictures | Length of stricture | Predilated state |
| Virasoro et al28 | 43 | 50.7 (22.0–81.0) | / | Anterior urethra | ≤2 cm | 1–4 prior endoscopic treatments (none within 3 months of enrolment) |
| Elliott et al27 | 60 (79):15 (48)* | 60.6±16.0 : 58.7±15.5 | Iatrogenic (21/78, 26.9%); idiopathic (42/78, 53.8%); inflammatory (1/78, 1.3%); traumatic (14/78, 17.9%); pelvic radiation (9/79, 11.4%) | Anterior urethra | ≤3 cm | ≥2 prior endoscopic treatments |
| Beeder et al32 | 91 | 61 | / | Anterior urethra (n=75, 82%); posterior urethra (n=16, 18%) | / | Most (75/91, 82%) had prior treatment for USD (endoscopic 50/91 (55%), 51/91 (56%) urethroplasty) |
| Alibekov et al33 | 7 | 52 (47–65) | Idiopathic (4/7, 57.1%); inflammatory (1/7, 14.3%); traumatic (2/7, 28.6%) | Anterior urethra | ≤1 cm | All patients had 1 urethral stone. The sizes of the stone ranged from 4 to 9 mm (median—6 mm) |
| Yi et al34 | 80 | / | / | Anterior urethra (n=59, 74%); posterior urethra (n=21, 26%) | ≤1.5 cm | Over 75% of patients had some form of prior stricture treatment, including dilation (34/80, 42.5%), DVIU (19/80, 23.8%) or urethroplasty (48/80, 60%) |
| Kumano et al30 | 13:9 | 71:63 | Iatrogenic (10/13, 76.9%); idiopathic (3/13, 23.1%) | Anterior urethra (n=9, 41%); posterior urethra (n=13, 59%) | / | / |
| Zhou et al35 | 45 | 46.6 (22–76) | Iatrogenic (19/45, 42.2%); inflammatory (5/45, 11.1%); traumatic (18/45, 40%); pelvic radiation (3/45, 6.7%) | Anterior urethra (n=36, 80%); posterior urethra (n=9, 20%) | ≤2 cm | 5 patients had a prior suprapubic cystostomy |
| Yu et al31 | 31:25 | 49 (32–67) : 44 (24–71) | Iatrogenic (7/31, 22.6%); idiopathic (1/31, 3.2%); inflammatory (2/31, 6.5%); traumatic (21/31, 67.7%); | Anterior urethra (n=45, 80%); posterior urethra (n=11, 20%) | ≤1 cm (n=48, 86%); >1 cm (n=8, 14%) | None received prior endovascular therapy |
| Chhabra et al36 | 134 (144)* | 52 (18–85) | Iatrogenic (59/144, 41.0%); idiopathic (84/144, 58.3%); pelvic radiation (1/144, 0.7%) | Anterior urethra (n=110, 76%); posterior urethra (n=8, 6%); both (n=26, 18%) | ≤1.5 cm (n=130, 90%) ; >1 cm (n=14, 10%) |
/ |
| Ishii et al37 | 10 | 70 (61–75) | Iatrogenic | Posterior urethra | / | All patients had cystourethral anastomotic stricture after radical prostatectomy |
| Mao et al38 | 37 (39)* | 55 (24–84) | / | Anterior urethra (n=17, 44%); posterior urethra (n=20, 51%); both (n=2, 5%) | ≤2 cm | / |
| Vyas et al39 | 120 | 49.86 (30-85) | / | Anterior urethra (n=114, 95%); posterior urethra (n=6, 5%) | ≤1.5 cm | / |
| Alguersuari et al40 | 65 | 63.17±16.9 | / | Anterior urethra (26.2%); posterior urethra (73.8%) | ≤2 cm (86.2%) ; >2 cm (13.8%) | / |
| MacDiarmid et al41 | 51 | / | Iatrogenic (27/51, 52.9%); idiopathic (11/51, 21.6%); inflammatory (10/51, 19.6%); traumatic (3/51, 5.9%) | Anterior urethra (n=49, 96%); posterior urethra (n=2, 4%) | / | / |
| Mohammed and Wirima29 | 6 (7)* | 35 (16–67) | Iatrogenic (1/6, 16.7%); idiopathic (2/6, 33.3%); inflammatory (2/6, 33.3%); traumatic (1/6, 16.7%) | Anterior urethra (n=4, 57%); posterior urethra (n=3, 43%) | / | / |
*A number of people who were initially assessed at baseline in the study are in parentheses, and a number of people who could be effectively assessed at the end of the follow-up are outside the brackets.
DVIU, direct visual internal urethrotomy; USD, urethral stricture disease.
Table 2.
Clinical characteristics and efficiency of balloon dilation (II)
| Study | Balloon types | Control | Definition of success rate | Reported success rate (%) | Follow-up |
| Virasoro et al28 | Optilume drug-coated balloon (DCB) | / | Functional success was defined as ≥50% reduction in International Prostate Symptom Score without need for retreatment. | 67 | 3 years |
| Elliott et al27 | Optilume DCB | dilation/DVIU | Anatomical success: the proportion of participants in whom the surgeons could atraumatically pass a 16-French flexible cystoscope or a 14-French catheter through the treated area at 6 months | 74.6:26.8 | 1 year |
| Beeder et al32 | 8 cm, 24-French UroMax Ultra balloon dilator | / | Proportion of patients who reported no recurrence of lower urinary tract symptoms or did not need further stricture treatment | 50 | 12 months (3–40) |
| Alibekov et al33 | / | / | Proportion of patients without recurrence of urethral stricture 18 months of dilation | 85.7 | 14 months (3–24) |
| Yi et al34 | 8 cm, 24-French UroMax Ultra balloon dilator | / | Proportion of patients with no postoperative recurrence of urethral stricture or who did not need further stricture treatment | 66.3 | 8.4 months (IQR, 3.9–22) |
| Kumano et al30 | Balloon dilation catheter (X-FORCE; BARD Medical, Murray Hill, New Jersey, USA) | OIU | Proportion of patients with no recurrence of stricture during the follow-up period | 84:22 | / |
| Zhou et al35 | Balloon catheter (X-Force, C.R. Bard, USA) | / | Proportion of patients who did not need further stricture treatment during the follow-up period | 86.7 | 6–24 months |
| Yu et al31 | 6 cm, 7-French balloon catheter (X-Force, C.R. Bard, USA) | DVIU | Proportion of patients with no postoperative recurrent urethral stricture or who did not need further stricture treatment | 35.5 | 14.75 months (5–36) |
| Chhabra et al36 | 8 cm, 24-French urethral Balloon catheter set (Cook Urological, Spencer, Indiana, USA) | / | Proportion of patients who did not need further stricture treatment during the follow-up period | 84.4 | 24 months (3–52) |
| Ishii et al37 | 6 cm, 6-French Balloon catheter, the X Force | / | Proportion of patients with no recurrence of strictureduring the follow-up period | 80 | 24 months (7–67) |
| Mao et al38 | 24-French Nephrostomy balloon dilation catheter, the X Force | / | Proportion of patients who did not need further stricture treatment during the follow-up period | 64.9 | / |
| Vyas et al39 | 8 cm, 24-French urethral Balloon catheter set (Cook Urological, Spencer) | / | Proportion of patients who did not need further stricture treatment during the follow-up period | 68 | 6 months (2–60) |
| Alguersuari et al40 | Fluoroscopic-guided balloon dilation | / | Proportion of patients who did not need further stricture treatment during the follow-up period | 69 | / |
| MacDiarmid et al41 | The UrethraMax (4, 6 or 8 cm; 24-French) or a coude tip balloon dilation catheter | / | Proportion of patients who did not need further stricture treatment during the follow-up period | 55 | 9 months (1–16) |
| Mohammed and Wirima29 | Olbert balloon catheter | / | Proportion of patients who did not need further stricture treatment during the follow-up period | 66.7 | 12 months (6–26) |
DVIU, direct vision internal urethrotomy; OIU, optical internal urethrotomy.
Quality analysis and risk of bias
We evaluated the quality of the 15 studies included in the systematic review, and the results are presented in online supplemental table 2. Most of the current studies in this article are retrospective, with inadequate study designs and a lack of valid controls.
bmjopen-2023-071923supp003.pdf (124KB, pdf)
We further conducted a bias analysis of 14 non-RCTs using the ROBINS-I tool, and the evaluation criteria and results are shown in online supplemental table 3. Since the operation is often influenced by the subjective preferences of the surgeons and most of the included studies are retrospective case studies, unavoidable selection bias is one of the most prominent issues. Selection bias is exacerbated in some small-sample studies of patients with specific comorbid conditions, such as coexisting urinary calculi. Some confounding factors such as age, body mass index, aetiology of the stricture, location of the stricture, length of the stricture, prior management and other factors, such as patient baseline physical condition, were present in most studies. Some of these confounding factors were not appropriately controlled for in the multivariable adjusted analysis. Some outcome measures of balloon dilation are subjective, and researchers may also exaggerate the efficacy of the procedure to publicise its advantages. Moreover, a funnel plot of eight studies included in the evaluation of the conventional balloon dilation success rate was generated, and there was no evidence of publication bias (Egger’s test: t=−2.42, p=0.052>0.05) (online supplemental figure 1). In addition, due to the small sample sizes of some of the included studies, there are some limitations in reflecting the overall clinical situation.
bmjopen-2023-071923supp004.pdf (101.8KB, pdf)
bmjopen-2023-071923supp005.pdf (376.2KB, pdf)
The principle of balloon dilation
The principle of balloon dilation is to apply radial force along the balloon span at the stricture site. The principle of traditional optical internal urethrotomy (OIU) is to achieve epithelial regeneration by incising scar tissue. Compared with the parallel force applied by simple dilation, balloon dilation applies less shear force and causes less trauma, which can reduce the risk of cavernous fibrosis development and cause less discomfort.31 42 43 Balloon dilation can also cause the fibrous scar in the stricture to more evenly fracture, resulting in 360° annular expansion, thereby increasing the inner diameter of the stenotic segment; during the balloon dilation process, the urethral pressure gradually increases, and the balloon is slowly and gently expanded to minimise damage to blood vessels and urethral tissue.13 Balloon dilation tends to achieve extrusion moulding in a single pass, and the high pressure of the balloon is effective in compressing the bleeding point. In addition, the smooth surface of balloon can prevent normal urethral mucosal damage.
Safety assessment and incidence of adverse events
Urinary tract infection, urinary retention, postoperative haematuria and dysuria are the main complications of balloon dilation. Therefore, strict aseptic and standardised operations are needed during surgery to prevent and avoid the occurrence of adverse events as much as possible.
We performed a pooled analysis of reported adverse event rates for urinary tract infection and urinary retention. The pooled incidence of infection in patients after balloon dilation was 3.27% (95% CI: 1.2% to 8.86%; heterogeneity: I2=46.2589%, p=0.1338) (online supplemental figure 2A). However, the pooled incidence of urinary retention was 8.31% (95% CI: 1.84% to 18.39%; heterogeneity: I2=84.6223%, p<0.05) (online supplemental figure 2B). Urinary tract infection is the most common complication within 30 days of balloon dilation, and some patients require antibiotic treatment.32 Some patients also have transient haematuria after surgery, but no further treatment, such as blood transfusion, is needed.31 32 Furthermore, Yu et al ’s study also revealed that the incidence of major postoperative complications, such as urethral bleeding and urinary tract infection, in the balloon dilation group was lower than that in the DVIU group (urethral bleeding: 2/31 vs 8/25, p=0.017; UTI: 1/31 vs 6/25 p=0.037).31
bmjopen-2023-071923supp006.pdf (317.2KB, pdf)
Clinical efficacy of balloon dilation for male urethral strictures
Conventional balloon dilation success rate
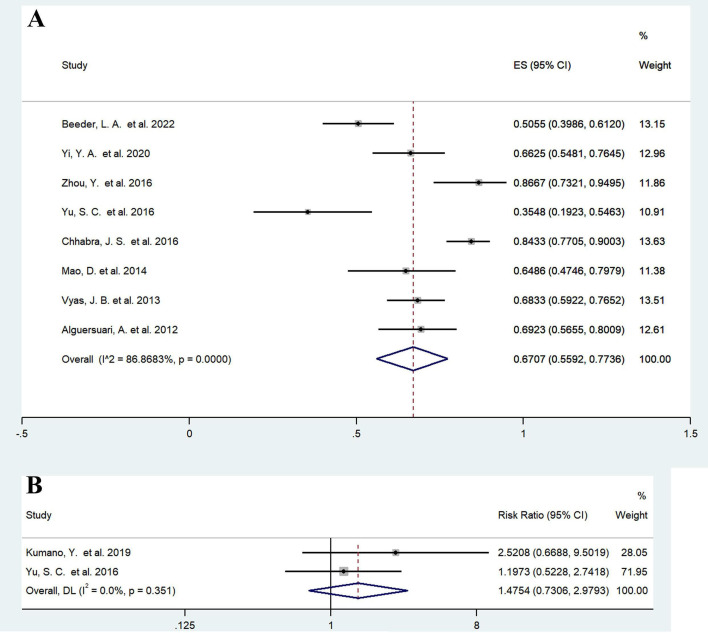
For studies with conventional balloon dilation, we defined the success of balloon dilation as no recurrence or no further stricture treatment during the follow-up period, excluding studies with a sample size of less than 30 on account of the potentially greater selection bias and merging data from eight studies published in 2012–2022.31 32 34–36 38–40 Reported success rates varied from 35.5% to 86.7%. The pooled balloon dilation success rate was 67.07% (95% CI: 55.92% to 77.36%; heterogeneity: I2=86.8683%, p<0.05) (figure 2A). Six of these studies reported follow-up, with a median pooled follow-up time of 13.50 months (95% CI: 12.86% to 14.14%; heterogeneity: I2=99.2%, p<0.05). This result needs to be interpreted with caution and most likely overestimates the efficacy of balloon dilation. Clinical data obtained during long-term follow-ups are lacking, and the real-world balloon dilation success rate should decline progressively with longer follow-ups. Moreover, the assessment of the success rate of balloon dilation involves significant subjective factors that may exaggerate efficacy.
Figure 2.
Forest plots showing the efficacy of balloon dilation. (A) Success rate of conventional balloon dilation; (B) balloon dilation (drug-coated balloons excluded) compared with simple dilation, DVIU and OIU. DVIU, direct visual internal urethrotomy; OIU, optical internal urethrotomy. Weights are from random effects model. DL, DerSimonian-Laird method.
We performed a sensitivity analysis by excluding studies one by one. The recalculated results are shown in online supplemental table 4 and online supplemental figure 3. Compared with the pooled results of all the studies, the maximum deviation rate was 5.3%, indicating that the final pooled result was relatively stable. We performed a meta-regression analysis and found that factors such as the location of the stricture (t=5.25, p<0.05), length of the stricture (t=7.97, p<0.05) and age (t=7.97, p<0.05) may be associated with high heterogeneity, and subgroup analyses of these factors were performed as described in the Clinical preference and efficacy influencing factors of balloon dilation section.
bmjopen-2023-071923supp007.pdf (62.4KB, pdf)
bmjopen-2023-071923supp008.pdf (418.9KB, pdf)
Drug-coated balloon dilation success rate
Balloons coated with drugs such as paclitaxel have achieved promising clinical results in recent years. Two studies on paclitaxel-coated balloons for recurrent urethral strictures revealed the considerable effect of these devices on recurrent urethral strictures, with a relatively objective functional success rate (67%) and an anatomical success rate (74.6%).27 28 The functional success rate was defined as the percentage of subjects with ≥50% improvement in IPSS scores who did not require retreatment. The anatomical success rate was defined as the proportion of participants for whom a 16 Fr flexible cystoscope or a 14 Fr catheter could atraumatically pass through the treated area at 6 months postoperatively. Both drug balloon studies were performed in patients with recurrent anterior urethral strictures who had received at least one prior endoscopic treatment. The patients had urethral strictures ≤12 F, all less than 3 cm in length. The IPSS scores were greater than 11, and all the patients had urinary flow rates of at least 15 mL/s or less. These studies excluded patients with prior urethroplasty, lichen sclerosus, neurogenic bladder, bladder neck contracture, artificial urinary sphincter or other confounding aetiologies.
Assessment of patient’s clinical symptoms
The changes in the urinary flow rate, PVR and IPSS are summarised in table 3. Compared with that preoperatively, the postoperative maximum urinary flow rate was greatly improved at 3 months (risk ratio [RR]=2.6510, 95% CI: 1.0681 to 4.2338; z=3.282, p<0.01; I2=96.5%, p<0.05), and the significant difference remained at 1-year postoperatively (RR=1.6637, 95% CI: 1.1837 to 2.1437; z=6.794, p<0.01; I2=78.8%, p<0.05). The patient’s IPSS scores and PVR also decreased accordingly.
Table 3.
Changes in the urinary flow rate, PVR and IPSS after balloon dilation
| Study | Location of the strictures | Length of strictures |
| Virasoro et al28 | Anterior urethra | ≤ 2 cm |
| Elliott et al27 | Anterior urethra | ≤ 3 cm |
| Zhou et al35 | Anterior urethra (n=36, 80%); posterior urethra (n=9, 20%) | ≤ 2 cm |
| Chhabra et al36 | Anterior urethra (n=110, 76%); posterior urethra (n=8, 6%); both (n=26, 18%) | ≤ 1.5 cm (n=130, 90%); |
| > 1 cm (n=14, 10%) | ||
| Vyas et al39 | Anterior urethra (n=114, 95%); posterior urethra (n=6, 5%) | ≤ 1.5 cm |
| MacDiarmid et al41 | Anterior urethra (n=49, 96%); posterior urethra (n=2, 4%) | / |
| IPSS | |||||
| Before surgery | 3 months | 6 months | 1 year | 2 years | 3 years |
| 25.2±4.5 (n=53) | 6.1±7.6 (n=51) | 4.6±5.2 (n=45) | 4.5±3.9 (n=40) | 6.9±7.7 (n=38) | 5.5±6.9 (n=33) |
| 22.0±6.8 (n=79) | 7.4±5.8 (n=74) | 8.3±6.2 (n=71) | 9.0±7.1 (n=67) | / | / |
| / | / | / | / | / | / |
| / | / | 12.7 (n=112) | 12.6 (n=112) | / | / |
| 21.6 (n=120) | 11.4 (n=120) | 12.6 (n=120) | / | / | / |
| / | / | / | / | / | / |
| Qmax (mL/s) | |||||
| Before surgery | 3 months | 6 months | 1 year | 2 years | 3 years |
| 5.0±2.6 (n=46) | 22.2±12.5 (n=51) | 19.8±10.8 (n=45) | 20.1±10.0 (n=39) | 17.5±10.4 (n=38) | 15.1±8.3 (n=33) |
| 7.6±3.4 (n=78) | 18.6±10.9 (n=71) | 16.6±8.9 (n=69) | 15.5±9.0 (n=65) | / | / |
| 5.6±1.4 (n=45) | 19.8±3.9 (n=45) | / | / | / | / |
| 5.2±2.7 (n=144) | / | 15.4±7.2 (n=112) | 12.6±5.7 (n=112) | / | / |
| 5.7 (n=120) | 14.3 (n=120) | 12.7 (n=120) | / | / | / |
| 10.4 (n=48) | 15.3 (n=43) | 17.7 (n=27) | 15.2 (n=5) | / | / |
| PVR (mL) | |||||
| Before surgery | 3 months | 6 months | 1 year | 2 years | 3 years |
| 141.4±105.1 (n=43) | 141.4±105.1 (n=51) | 30.0±42.8 (n=45) | 24.6±32.1 (n=39) | 45.5±49.5 (n=38) | 50.2±62.5 (n=33) |
| 109.8±116.9 (n=77) | 103.4±134.4 (n=70) | 73.1±117.7 (n=67) | 94.6±121.8 (n=66) | / | / |
| / | / | / | / | / | / |
| / | / | / | / | / | / |
| 90.2 (n=120) | 34.2 (n=120) | 20.2 (n=120) | / | / | / |
| / | / | / | / | / | / |
IPSS, International Prostate Symptom Scores; PVR, postvoid residual urine volume; Qmax, maximum uroflow.
Patients’ subjective perception of improvement in voiding symptoms is a crucial indicator of the true efficacy of urethral stricture treatment, and the results are summarised in table 4. The ROBUST III study28 revealed that patients’ International Prostate Symptom Score-Quality of Life scores increased significantly by 30 days after balloon dilation, indicating outstanding short-term efficacy. Moreover, 3-year follow-up results from the ROBUST I trial study27 indicated significant improvements in both QoL scores and Patient-Reported Outcome Measure for Urethral Stricture Surgery scores for patients who underwent balloon dilation compared with baseline status (p<0.0001). With the extension of follow-up time, the quality of life of the patients remained good, reflecting the long-term effectiveness of balloon dilation.
Table 4.
Changes in the USS-PROM score, IPSS-QOL and IIEF score after balloon dilation
| Study: Virasoro et al28 | ||||||
| Scoring items | Before surgery | 3 months | 6 months | 1 year | 2 years | 3 years |
| USS-PROM | 15.9±4.7 (n=53) | 3.2±5.5 (n=51) | 1.9±2.9 (n=45) | 1.4±1.8 (n=40) | 3.6±5.8 (n=38) | 2.0±3.5 (n=33) |
| IPSS QoL | 4.9±0.9 (n=53) | 0.8±1.3 (n=51) | 0.7±0.9 (n=45) | 0.7±0.9 (n=40) | 0.9±1.5 (n=38) | 0.7±1.2 (n=33) |
| IIEF-OS | 6.5±2.6 (n=53) | 7.9±2.5 (n=51) | 7.9±2.5 (n=45) | 8.1±2.5 (n=40) | 7.6±2.5 (n=38) | 8.2±2.2 (n=33) |
| IIEF-EF | 16.0±12.2 (n=53) | 20.7±12.0 (n=51) | 21.0±11.8 (n=45) | 22.1±10.9 (n=40) | 21.1±11.9 (n=38) | 22.5±11.2 (n=33) |
| Study: Elliott et al27 | ||||||
| Scoring items | Before surgery | 30 days | 3 months | 6 months | 1 year | / |
| IPSS QoL | 4.5±1.3 (n=79) | 1.7±1.4 (n=78) | 1.6±1.4 (n=74) | 1.7±1.3 (n=71) | 1.9±1.5 (n=67) | / |
| IIEF | 5.8±2.9 (n=72) | 5.9±2.8 (n=75) | 6.6±2.7 (n=71) | 6.5±2.8 (n=68) | 6.9±3.0 (n=59) | / |
IIEF, International Index of Erectile Function; IIEF, International Index of Erectile Function-overall satisfaction domain; IIEF, International Index of Erectile Function-erectile function domain; IPSS QoL, International Prostate Symptom Score-Quality of Life; USS-PROM, Patient-Reported Outcome Measure for Urethral Stricture Surgery.
Comparison of balloon dilation with other endoluminal treatments
We conducted an analysis of two studies comparing DVIU and OIU and found no significant difference in efficacy between conventional balloon dilation and internal urethrotomy (RR=1.4754, 95% CI: 0.7306 to 2.9793; z=1.085, p=0.278; heterogeneity: I2=0%, p=0.351) (figure 2B). Even though fewer comparative studies are currently available, the balloon dilation may have potentially favourable long-term results by virtue of its smaller shear force and uniform 360° circumferential dilation. Yu et al reported that the estimated stricture-free survival rate at 12 months was 77.42% after balloon dilation and 48.00% after DVIU; moreover, a significantly higher stricture-free survival rate was observed in the balloon dilation group (p=0.02<0.05, hazard ratio [HR]=0.35, 95% CI: 0.14 to 0.87).31 In Kumano et al’s study, the balloon dilation group had significantly longer stricture-free times than the OIU group (p<0.01), with median (mean) stricture-free times of 1675 (1673) and 244 (599) days, respectively.30 Currently, there are no studies comparing the clinical outcomes of simple dilation versus balloon dilation. Due to the paucity of current studies, no adequate evidence exists to suggest that balloon dilation is superior to other conventional endoluminal therapies.
Clinical preference and efficacy influencing factors of balloon dilation
Aetiology
We pooled eight studies of simple balloon dilation that addressed specific aetiologies29–31 33 35–37 41 involving a total of 307 patients. Iatrogenic urethral strictures (43.32%, 133/307) and idiopathic urethral strictures (34.20%, 105/307) accounted for the vast majority of cases. Stricture caused by trauma or inflammation accounted for 14.66% (45/307) and 6.51% (20/307), respectively. Four patients also suffered from radiation. Although this is only a one-sided epitome, it follows that iatrogenic injury may become the main aetiology of urethral stricture in males in the future.
Due to the lack of meticulous subgroup analysis in the included studies, it was difficult for us to directly compare the differences in efficacy among strictures caused by different aetiologies. The influence of aetiology on the efficacy of balloon dilation depends primarily on the type of stenotic pathology it creates and the specific stenotic segment length and location. The essence of balloon dilation is the efficient expansion of the targeted site, taking care to avoid causing additional fibrosis of scar tissue in the narrow segment. If additional fibrosis occurs, strictures are highly likely to recur. Therefore, balloon dilation may not be suitable for strictures with a high degree of fibrosis. Lichen sclerosus is a specific cause of urethral stricture. The pathological features of lichen sclerosus include hyperkeratosis or epithelial atrophy, basal cell vacuolar degeneration, lichenoid lymphocytic infiltration and upper epithelial sclerosis.44 This epithelial stromal lesion characterised by squamous atrophy or hyperplasia is distinct from the fibrotic pathological characterisation of most urethral strictures. A recent review pooling expert opinions in urology stated that dilation is unlikely to be a successful long-term solution for lichenoid sclerosing urethral stricture, potentially triggering adverse outcomes in the long term.45 Balloon dilation is essentially a physical treatment method that cannot pathologically or fundamentally improve the condition of patients with specific urethral strictures, and its clinical indications need to be strictly controlled.
Location of the urethral stricture
We combined 11 studies that identified the location of the stricture29 32–41; 74.28% (488/657) were anterior urethral stricture, 21.77% (143/657) were posterior urethral stricture and 3.95% (26/657) were both. Most patients who undergo balloon dilation have an anterior urethral stricture.
Most of the current studies have not further categorised comparisons of balloon dilation based on differences in stricture location, and the data of patients with stricture at different sites were analysed together. A subgroup analysis of eight conventional balloon dilation studies that involved the combination of success rates31 32 34–36 38–40 was performed according to the percentage of anterior urethral strictures, and the results are shown in online supplemental figure 4. The combined results of studies with mostly anterior urethral strictures (70%–90%) reported a success rate of 66.45% (95% CI: 47.58% to 83.01%) for balloon dilation.
bmjopen-2023-071923supp009.pdf (541.8KB, pdf)
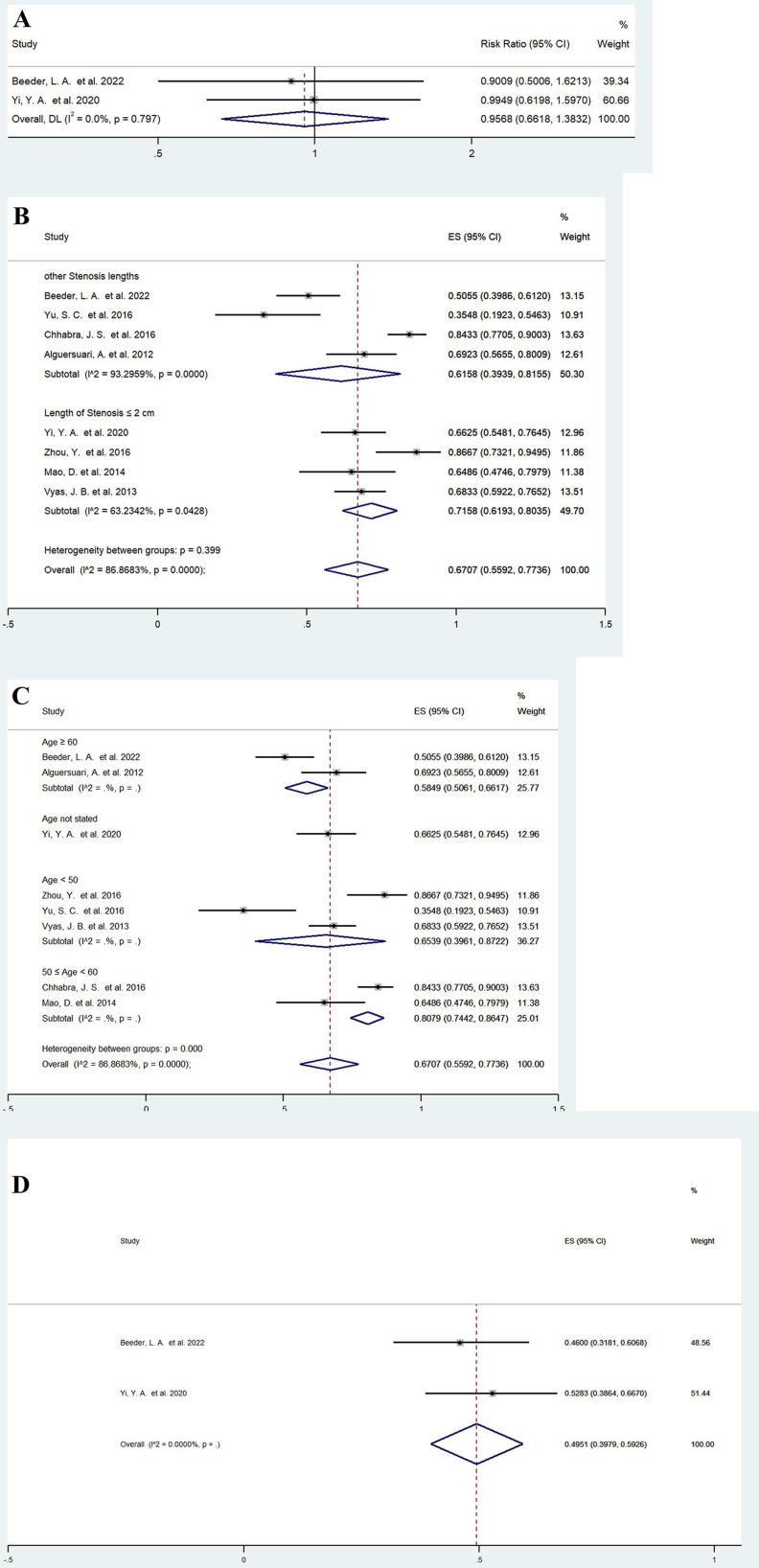
Moreover, we combined data from two studies32 34 that included a subgroup analysis of stricture location and did not find any significant difference in the efficacy of balloon dilation between anterior and posterior urethral strictures (RR=0.9568, 95% CI: 0.6618 to 1.3832, p=0.814) (figure 3A).
Figure 3.
Forest plots showing the possible influencing factors of balloon dilation. (A) location of the urethral stricture; (B) length of urethral stricture; (C) age; (D) prior endoscopic management. Weights are from random effects model. DL, DerSimonian-Laird method.
Length of urethral stricture
We previously performed a subgroup analysis of the pooled conventional balloon dilation success rate31 32 34–36 38–40 according to the length of the urethral stricture, and the results are shown in figure 3B. For shorter strictures (≤2 cm), the success rate of balloon dilation reached 71.58% (95% CI: 61.93% to 80.35%), and heterogeneity was also reduced (I2=63.2342%, p<0.05) (figure 3B). In a study of patients with anterior urethral strictures less than 1 cm in length, the success rate was as high as 85.7%.33 The reduction in heterogeneity of the pooled results suggested that the stenotic segment length is a prognostic factor, and balloon dilation for short-segment urethral strictures may have a higher success rate.
Age
We further stratified the previous eight studies31 32 34–36 38–40 according to age group, and the results are shown in figure 3C. In the 50–60 years age group, the success rate of balloon dilation was 80.79% (95% CI: 74.42% to 86.47%). However, for patients older than 60 years, the success rate decreased to 58.49% (95% CI: 50.61% to 66.17%). Interestingly, the combined success rate was 65.39% (95% CI: 39.61% to 87.22%) in relatively young patients, probably because some of the reported younger patients had more severe strictures. The aetiology of strictures in elderly patients is often iatrogenic, whereas in younger patients, more complex urethral strictures can be caused by relatively specific factors such as trauma and lichenoid sclerosis gonorrhoea. Even though the success rate is unclear, we can see a decreasing trend in the efficacy of balloon dilation in elderly patients.
Prior intervention management
A separate analysis of patients who had received prior endoscopic management (catheter/balloon dilation, DVIU) was performed in two studies,32 34 and we found that balloon dilation had a pooled success rate of 49.51% (95% CI: 39.79% to 59.26%) (figure 3D). In patients who previously underwent surgical intervention, the efficacy of balloon dilation may be lower. Based on the limited data available in these two studies,32 34 we compared the success rates of conventional balloon dilation in patients who did and did not undergo previous urethroplasty and found no significant difference (RR=1.1682, 95% CI: 0.6160 to 2.2153, p=0.634) (online supplemental figure 5A). The prevailing clinical view is that repeated endoluminal intervention may render further endoluminal treatment less effective, but this needs to be confirmed by clinical studies with larger sample sizes.
bmjopen-2023-071923supp010.pdf (532.6KB, pdf)
Other patient status
We performed a more nuanced subgroup analysis of the two studies32 34 that provided some patient baseline details. There was no statistically significant difference in balloon dilation efficacy between patients with and without a smoking history (RR=1.1052, 95% CI: 0.8083 to 1.5112, p=0.531) (online supplemental figure 5B). Chronic diseases such as coronary artery disease (RR=1.0714, 95% CI: 0.7618 to 1.5069, p=0.692), diabetes mellitus (RR=0.9144, 95% CI: 0.6118 to 1.3666, p=0.662), hypertension (RR=0.8377, 95% CI: 0.6121 to 1.1464, p=0.269) and chronic obstructive pulmonary disease (RR=1.3515, 95% CI: 0.7495 to 2.4374, p=0.317) did not significantly affect the efficacy of balloon dilation (online supplemental figure 5C–F). Our preliminary analysis suggested that patient status, such as poor lifestyle habits and chronic diseases, may not significantly impact the efficacy of balloon dilation.
Intermittent urethral balloon self-dilation
Patient self-balloon dilation is a specific form of balloon dilation, and we also briefly review its clinical evaluation. Urethral dilation is easy to perform and can be performed by the patient at home, thereby avoiding the need for repeated hospitalisations and frequent general anaesthesia.46 A study by Levine and Engebrecht47 suggested that adjuvant balloon self-dilation at home may be a potential option for patients at high risk of recurrence. In this study of 25 eligible patients, most patients noted that balloon dilation improved voiding and maintained or improved the peak urinary flow rate at an average of 18.7 months after the initial procedure. Nonetheless, 6 patients (19%) complained of balloon placement discomfort, 3 (10%) noted minor bleeding during dilation and 4 (13%) developed urinary tract infections during the follow-up period. Hennessey et al ’s initial experience with self-expanding balloon dilation in the outpatient setting was encouraging, with all 11 patients reporting that they were very satisfied or satisfied with their overall outcomes and quality of life.48 A recent study reported in 2021 stated that self-urethral balloon dilation offers patients with complex strictures, especially those with a history of radiation, an opportunity to avoid surgical intervention.49
However, the imprecision of patient self-balloon dilation may cause complications and even aggravate injury. As early as the last century, scholars have shown that short-term postoperative self-dilation techniques do not appear to prevent stricture recurrence in patients treated with endourethral incisions.50 A meta-analysis of patient self-dilation also indicated that the quality of evidence for this approach to reduce the risk of recurrent urethral strictures is very low.51 Although self-dilation is very convenient and avoids surgical complications, it is not suitable for all patients, and not all patients can master the skills and techniques of self-dilation. Self-dilation needs to be further weighed against surgery, and well-designed RCTs are needed to determine whether this benefit of convenience is sufficient to make this intervention worthwhile.
Discussion
With the gradual increase in the incidence of iatrogenic urethral strictures, surgeons should choose the appropriate treatment method according to the aetiology of the urethral stricture, the location and length of the stricture and the degree of urethral fibrosis. Even though there is no clear evidence that the clinical efficacy of balloon dilation is significantly better than that of other endoluminal treatments, such as simple dilation and DVIU, balloon dilation still has high clinical plasticity.
Both balloon dilation and simple dilation are essentially dilatation, causing tearing of scar tissue and scar remodelling at the site of the stricture. Balloon dilation involves the application of a 360° circumferential radial force at the stricture site, providing a more uniform force than simple dilation. Moreover, for harder scars that cannot be torn by simple dilation, the pressure of the balloon can be gradually increased to achieve dilatation, which has broader clinical indications.
Urethrotomy requires a radial incision at the site of the stricture. The main disadvantage of internal urethrotomy is the inability to accurately estimate the depth of scar tissue during the procedure, resulting in imprecise scar tissue incisions. There may also be damage to the corpus cavernosum below the urethra, and vascular disruption in the corpus cavernosum and localised extravasation of urine through mucosal fissures may exacerbate corpus cavernosum fibrosis, eventually leading to stricture recurrence.31 52 Some scholars believe that balloon dilation tends to be performed in fewer fibrotic cases without urethral cavernous fibrosis, suggesting that balloon dilation will not invade the deep urethral membrane; therefore, even if the dilation time is longer, the restenosis rate of balloon dilation is lower than that of OIU.30 Thus, DVIU is commonly used for posterior urethral strictures and is avoided in the penile urethra to prevent leakage of the cavernous penile veins to circumvent the risk of causing impotence. Balloon dilation has no definitive stricture site limitations and can be effective in the dilatation of hard-textured scars that cannot be incised by DVIU. Yu et al reported that the operation time of balloon dilation was much shorter than that of DVIU (13.19±2.68 min vs 18.44±3.29 min, p<0.01),31 highlighting the operational simplicity of balloon dilation. Compared with urethrotomy, balloon dilation has a lower cost and can improve the efficiency of hospital bed turnover.53
To reduce the high recurrence rate after endoluminal treatment, intraurethral lesion injections of drugs such as steroids and mitomycin C are commonly used, and balloons are considered promising forms of drug delivery.54 The advent and use of drug-coated balloons (DCBs) can reduce inflammation and relapse rates by releasing drugs such as immunosuppressants during expansion. Barbalias et al conducted animal experiments using paclitaxel-coated balloons and reported that paclitaxel could pass through the urothelial barrier and immediately distribute to the urothelium, submucosa and smooth muscle layers of the normal rabbit urethra after dilation.55 The drug can penetrate the epithelium and act on deep urethral tissue, effectively reducing inflammation and inhibiting urethral fibrosis. In the recent ROBUST I study,28 an optilume DCB was shown to maintain symptom relief for 3 years after treatment in a highly susceptible population with recurrent urethral strictures. Foorty-three patients in this trial had a functional success rate of 67%, a retreatment-free rate of 77% and an improvement in the mean IPSS from 25.2 at baseline to 5.5 at 3 years (p<0.0001). The 1-year results from another RCT (the ROBUST III study)27 showed that patients dilated with an optilume DCB had a significantly higher anatomical success rate at 6 months than those in the DVIU group (75% vs 27%, p<0.001). Both the symptoms and urinary flow rates improved significantly in both groups, but these effects were significantly more pronounced in the Optilume DCB group. The US Food and Drug Administration has approved the use of the Optilume DCB for the treatment of male urethral strictures.56 Nevertheless, in the ROBUST III study,27 the incidences of serious adverse events in the control group (DVIU/simple dilation) and DCB group were 16.7% and 10.1%, respectively. The types and incidences of adverse events in the two groups were closely matched, but the incidences of postoperative haematuria and dysuria were higher in the DCB group than in the control group (11.4% and 2.1%, respectively). In addition, rhenium-188 mercaptoacetyltriglycine-filled balloon dilation is expected to delay stricture recurrence in patients with urethral strictures. A clinical report of five patients revealed that the mean treatment interval was prolonged from 2.2 months to 10.7 months after rhenium-188 mercaptoacetyltriglycine-filled balloon dilation.57 Further consideration needs to be given to factors such as the local drug concentration achievable in dilation and the reliability of the therapeutic dose. The design of new balloons, such as cutting balloons, and the exploration of new expansion techniques may be research directions in the future.58 59 The new type of balloon should meet the biomechanical requirements to better fit the narrow urethra.
The timing of balloon dilation is closely related to the location, length and scar thickness of the stricture, and appropriate case selection is critical. Balloon dilation may be an intermediate step before urethroplasty and is a promising alternative therapy to simple dilation and urethrotomy. Like simple dilation and DVIU, balloon dilation is indicated for patients with short-segment urethral strictures. Although balloon dilatation is currently not definitively superior to simple dilation or DVIU due to the lack of long-term follow-up studies, balloon dilation has the following advantages: (1) In principle, the balloon expands with less shear force, presenting a gradual uniform 360° circular dilation to minimise the non-therapeutic urethral injuries; (2) In the penile urethra where DVIU is not recommended, simple dilation and balloon dilation can be used; (3) As long as the guidewire can be passed, simple dilation and balloon dilation can be attempted in stenotic segments in which the endoscope of the DVIU cannot pass; (4) The balloon, with its high pressure, can dilate some urethras with harder scars that are difficult to dilate with simple dilation and DVIU; (5) The balloon can be used as a promising drug delivery tool and has achieved favourable clinical results. For some patients with long complex urethral strictures, balloon dilation may even be used as an initial therapy. In patients with recurrent strictures, urethrotomy or urethral dilation followed by urethroplasty is the most cost-effective strategy.60 The use of endoscopic urethroplasty combined with balloon dilation for traumatic destruction of the prostatic membranous urethra has been previously reported.61 Balloon dilation can also be used in conjunction with repeat simple dilation, endourethrotomy and urethroplasty. If urethroplasty is not feasible, patients can undergo intermittent self-dilation to stabilise the results after endoluminal therapy. Intermittent urethral balloon self-dilation may be an option, but its safety is difficult to ensure due to the lack of direct visualisation control and difficulty in achieving the appropriate therapeutic pressure of the balloon. There is no standardised schedule for self-dilation, and the exact dilation schedule depends on the condition and the treatment recommended by the doctor. Patients are usually advised to start with more frequent dilation, even daily and then gradually increase the interval. Intermittent self-dilation can continue for a fixed period of time or indefinitely. Nevertheless, intermittent self-dilation tends to stabilise the stricture and prolong recurrence rather than keep the patient stricture free.3 The emergence of a new, safer, DCB suitable for at-home use may prolong the patient self-dilation interval and bring new hope for future treatments.
We recognise the limitations of our research. There is a considerable risk of bias in this meta-analysis, most of which stems from the retrospective design of the studies and the lack of valid controls. Evidence from retrospective observational studies needs to be interpreted with caution because of the susceptibility to selection bias, recall bias and exaggerated efficacy of balloon dilation. The assessment of the efficacy of balloon dilation is often subjective, and it is difficult to use a clear objective measure. Patients have different perceptions of their voiding status, and patients’ subjective feelings can influence their choice of therapeutic intervention. The efficacy of balloon dilation is also affected by confounding factors such as aetiology, stricture location, stricture length, prior management intervention, comorbidities and socioeconomic status. The long-term outcomes of balloon dilation need to be further investigated. RCTs with larger sample sizes and more comparable control groups are needed to further prove the efficacy and safety of balloon dilation in the future.
Conclusion
Balloon dilation may be an intermediate step before urethroplasty and a promising alternative to simple dilation and DVIU. The balloon is a promising drug delivery tool, and paclitaxel DCB dilation is effective in reducing retreatment rates in patients with recurrent anterior urethral strictures. Due to the low quality of the evidence, we have little confidence in our estimates of effects. Evidence for other comparisons and outcomes is also limited. The stricture aetiology, stricture location, stricture length and previous treatment may be associated with the efficacy of balloon dilation. However, additional high-quality studies are needed for further investigation.
Supplementary Material
Footnotes
Contributors: Conceptualisation was created by XL and CX. Investigation was performed by XL, XJ and CX. Analysis and interpretation of data were produced by XL, XJ and CX. XL and CX wrote the manuscript. XL and XJ conducted the statistical analysis. Critical revision of the manuscript for important intellectual content was produced by XL, CX, ZZ, TC, ZG and JL. Supervision was performed by JL.
XL and JL act as guarantors for this study.
Funding: This work was financially supported by the National Natural Science Foundation of China (Grant No. 8207032402).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. J Urol 2007;177:1667–74. 10.1016/j.juro.2007.01.041 [DOI] [PubMed] [Google Scholar]
- 2.Verla W, Oosterlinck W, Spinoit AF, et al. A comprehensive review emphasizing anatomy, etiology, diagnosis, and treatment of male urethral stricture disease. Biomed Res Int 2019;2019:9046430. 10.1155/2019/9046430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumen N, Campos-Juanatey F, Greenwell T, et al. European Association of Urology guidelines on urethral stricture disease (part 1): management of male urethral stricture disease. Eur Urol 2021;80:190–200. 10.1016/j.eururo.2021.05.022 [DOI] [PubMed] [Google Scholar]
- 4.Stein DM, Thum DJ, Barbagli G, et al. A geographic analysis of male urethral stricture aetiology and location. BJU Int 2013;112:830–4. 10.1111/j.1464-410X.2012.11600.x [DOI] [PubMed] [Google Scholar]
- 5.Cornu J-N, Ahyai S, Bachmann A, et al. A systematic review and meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic obstruction: an update. Eur Urol 2015;67:1066–96. 10.1016/j.eururo.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 6.Hollingsworth JM, Rogers MAM, Krein SL, et al. Determining the noninfectious complications of indwelling urethral catheters: a systematic review and meta-analysis. Ann Intern Med 2013;159:401–10. 10.7326/0003-4819-159-6-201309170-00006 [DOI] [PubMed] [Google Scholar]
- 7.Lo T, Vanni A, Cronin P. 1046 poster Urethral stricture as a complication of high dose rate brachytherapy for prostate canceR. Radiotherapy and Oncology 2011;99:S390. 10.1016/S0167-8140(11)71168-9 [DOI] [Google Scholar]
- 8.Vanni A, Cronin P, Hamawy K, et al. Urethral stricture as a complication of high-dose-rate brachytherapy for clinically localized prostate cancer. Brachytherapy 2010;9:S93. 10.1016/j.brachy.2010.02.171 [DOI] [Google Scholar]
- 9.Wessells H, Angermeier KW, Elliott S, et al. Male urethral stricture: American Urological Association guideline. J Urol 2017;197:182–90. 10.1016/j.juro.2016.07.087 [DOI] [PubMed] [Google Scholar]
- 10.Wong SSW, Aboumarzouk OM, Narahari R, et al. Simple urethral dilatation, endoscopic urethrotomy, and urethroplasty for urethral stricture disease in adult men. Cochrane Database Syst Rev 2012;12:CD006934. 10.1002/14651858.CD006934.pub3 [DOI] [PubMed] [Google Scholar]
- 11.Russinovich NAE, Lloyd LK, Griggs WP, et al. Balloon dilatation of urethral strictures. Urol Radiol 1981;2:33–7. 10.1007/BF02926693 [DOI] [PubMed] [Google Scholar]
- 12.Pinot JJ, Hermanowicz M, Bonnet JL, et al. Dilatation of the urethra by inflatable balloon catheter. Presse Med 1983;12:163–4. [PubMed] [Google Scholar]
- 13.Glesy JD, Finn JC, Hermann GD, et al. Coaxial balloon dilation and calibration of urethral strictures. The American Journal of Surgery 1984;147:611–4. 10.1016/0002-9610(84)90124-7 [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama T, Go H, Takashima A, et al. Balloon dilatation for entire urethral stricture. Urol Int 1991;46:232–4. 10.1159/000282141 [DOI] [PubMed] [Google Scholar]
- 15.Hübler J, Solt J. Balloon catheter for the dilatation of the urethra. Orv Hetil 1991;132:925–7. [PubMed] [Google Scholar]
- 16.Rosa Arias J, Valdivia Uría JG, López López JA, et al. Pneumatic dilatation of urethral stenosis. Arch Esp Urol 1989;42:147–51. [PubMed] [Google Scholar]
- 17.D’Agostino S, Campobasso P, Musi L, et al. Treatment of urethral stenoses in children using a balloon catheter. Pediatr Med Chir 1989;11:155–9. [PubMed] [Google Scholar]
- 18.Perini L, Cavallo A, Perin B, et al. Dilatation of benign urethral stenosis using a balloon catheter. Radiol Med 1988;76:8–10. [PubMed] [Google Scholar]
- 19.Acunas B, Acunas G, Gokmen E, et al. Balloon dilatation of iatrogenic urethral strictures. Eur J Radiol 1988;8:214–6. [PubMed] [Google Scholar]
- 20.Xie T, Huang X, Xu Q, et al. Balloon dilation by B ultrasound monitoring for treatment of urethral stricture: 5 case reports. Beijing Da Xue Xue Bao Yi Xue Ban 2014;46:657–8. [PubMed] [Google Scholar]
- 21.Gelman J, Liss MA, Cinman NM. Direct vision balloon dilation for the management of urethral strictures. J Endourol 2011;25:1249–51; 10.1089/end.2011.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 25.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712–6. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elliott SP, Coutinho K, Robertson KJ. One-year results for the ROBUST III randomized controlled trial evaluating the optilume® drug-coated balloon for anterior urethral strictures. Reply. J Urol 2022;207:942. 10.1097/JU.0000000000002427 [DOI] [PubMed] [Google Scholar]
- 28.Virasoro R, DeLong JM, Estrella RE, et al. A drug-coated balloon treatment for urethral stricture disease: three-year results from the ROBUST I Study. Res Rep Urol 2022;14:177–83. 10.2147/RRU.S359872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammed SH, Wirima J. Balloon catheter dilatation of urethral strictures. AJR Am J Roentgenol 1988;150:327–30. 10.2214/ajr.150.2.327 [DOI] [PubMed] [Google Scholar]
- 30.Kumano Y, Kawahara T, Mochizuki T, et al. Management of urethral stricture: High-pressure balloon dilation versus optical internal urethrotomy. Low Urin Tract Symptoms 2019;11:34–7. 10.1111/luts.12208 [DOI] [PubMed] [Google Scholar]
- 31.Yu S-C, Wu H-Y, Wang W, et al. High-pressure balloon dilation for male anterior urethral stricture: single-center experience. J Zhejiang Univ Sci B 2016;17:722–7. 10.1631/jzus.B1600096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beeder LA, Cook GS, Nealon SW, et al. Long-term experience with balloon dilation for short bulbar and membranous urethral strictures: establishing a baseline in the active drug treatment era. J Clin Med 2022;11:3095. 10.3390/jcm11113095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alibekov MM, Katibov MI, Skorovarov AS, et al. The one-stage balloon dilatation with stone extraction for a combination of short urethral stricture and urethral stone in men. Vestn Urol 2021;9:16–24. 10.21886/2308-6424-2021-9-2-16-24 [DOI] [Google Scholar]
- 34.Yi YA, Rozanski AT, Shakir NA, et al. Balloon dilation performs poorly as a salvage management strategy for recurrent bulbar urethral strictures following failed urethroplasty. Transl Androl Urol 2020;9:3–9. 10.21037/tau.2019.08.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Li G, Yan J, et al. Combination of the ureteral dilation catheter and balloon catheter under the ureteroscope in the treatment of male urethral stricture. Zhonghua Nan Ke Xue 2016;22:42–5. [PubMed] [Google Scholar]
- 36.Chhabra JS, Balaji SS, Singh A, et al. Urethral balloon dilatation: factors affecting outcomes. Urol Int 2016;96:427–31. 10.1159/000443704 [DOI] [PubMed] [Google Scholar]
- 37.Ishii G, Naruoka T, Kasai K, et al. High pressure balloon dilation for vesicourethral anastomotic strictures after radical prostatectomy. BMC Urol 2015;15:62. 10.1186/s12894-015-0059-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao D, Yeqi N, Lu Y, et al. Urethral dilatation with nephrostomy balloon dilation catheter for treatment of male patients with urethrostenosis. Chinese Journal of Andrology 2014;28:25–9. [Google Scholar]
- 39.Vyas JB, Ganpule AP, Muthu V, et al. Balloon dilatation for male urethral strictures “revisited.” Urol Ann 2013;5:245–8. 10.4103/0974-7796.120296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alguersuari A, Palaña P, Criado E. Fluoroscopic-guided balloon dilation for urethral strictures: a feasible and effective approach for an old, unsolved problem. Cardiovasc Intervent Radiol 2012;35:S279. [Google Scholar]
- 41.MacDiarmid SA, Harrigan CT, Cottone JL, et al. Assessment of a new transurethral balloon dilation catheter in the treatment of urethral stricture disease. Urology 2000;55:408–13. 10.1016/s0090-4295(99)00541-5 [DOI] [PubMed] [Google Scholar]
- 42.Parente A, Angulo JM, Romero RM, et al. Management of ureteropelvic junction obstruction with high-pressure balloon dilatation: long-term outcome in 50 children under 18 months of age. Urology 2013;82:1138–43. 10.1016/j.urology.2013.04.072 [DOI] [PubMed] [Google Scholar]
- 43.Kumar P, Nargund VH. Management of post-radical prostatectomy anastomotic stricture by endoscopic transurethral balloon dilatation. Scand J Urol Nephrol 2007;41:314–5. 10.1080/00365590601017030 [DOI] [PubMed] [Google Scholar]
- 44.Liu JS, Walker K, Stein D, et al. Lichen sclerosus and isolated bulbar urethral stricture disease. J Urol 2014;192:775–9. 10.1016/j.juro.2014.03.090 [DOI] [PubMed] [Google Scholar]
- 45.Chung ASJ, Suarez OA. Current treatment of lichen sclerosus and stricture. World J Urol 2020;38:3061–7. 10.1007/s00345-019-03030-z [DOI] [PubMed] [Google Scholar]
- 46.Searles JM, MacKinnon AE. Home-dilatation of the urethral meatus in boys. BJU Int 2004;93:596–7. 10.1111/j.1464-410x.2003.04680.x [DOI] [PubMed] [Google Scholar]
- 47.Levine LA, Engebrecht BP. Adjuvant home urethral balloon dilation for the recalcitrant urethral stricture. J Urol 1997;158:818–21. 10.1097/00005392-199709000-00034 [DOI] [PubMed] [Google Scholar]
- 48.Hennessey DB, Thomas AZ, Forde JC, et al. Management of recalcitrant urethral strictures with self-dilatation balloon catheter. Ir J Med Sci 2013;182:227–30. 10.1007/s11845-012-0866-x [DOI] [PubMed] [Google Scholar]
- 49.Monn MF, Virasoro R, DeLong J, et al. MP56-05 natural history of patients using self urethral balloon dilation to manage urethral stricture. Journal of Urology 2021;206:e970–1. 10.1097/JU.0000000000002086.05 [DOI] [Google Scholar]
- 50.Matanhelia SS, Salaman R, John A, et al. A prospective randomized study of self-dilatation in the management of urethral strictures. J R Coll Surg Edinb 1995;40:295–7. [PubMed] [Google Scholar]
- 51.Ivaz SL, Veeratterapillay R, Jackson MJ, et al. Intermittent self-dilatation for urethral stricture disease in males: a systematic review and meta-analysis. Neurourol Urodyn 2016;35:759–63. 10.1002/nau.22803 [DOI] [PubMed] [Google Scholar]
- 52.Isen K, Nalçacıoğlu V. Direct vision internal urethrotomy by using endoscopic scissors. Int Urol Nephrol 2015;47:905–8. 10.1007/s11255-015-0960-x [DOI] [PubMed] [Google Scholar]
- 53.Sur H, Dowson C, Jabbar T, et al. A cost based rationale for redefining the management of urethral strictures. J Endourol 2012;26:A391–2. [Google Scholar]
- 54.Pang KH, Chapple CR, Chatters R, et al. A systematic review and meta-analysis of adjuncts to minimally invasive treatment of urethral stricture in men. Eur Urol 2021;80:467–79. 10.1016/j.eururo.2021.06.022 [DOI] [PubMed] [Google Scholar]
- 55.Barbalias D, Lappas G, Ravazoula P, et al. Evaluation of the distribution of paclitaxel after application of a paclitaxel-coated balloon in the rabbit urethra. J Endourol 2018;32:381–6. 10.1089/end.2017.0935 [DOI] [PubMed] [Google Scholar]
- 56.FDA approves the Optilume® urethral drug coated balloon for the treatment of urethral strictures. BJU Int 2022;129:305. 10.1111/bju.15716 [DOI] [PubMed] [Google Scholar]
- 57.Shin JH, Song H-Y, Moon DH, et al. Rhenium-188 mercaptoacetyltriglycine-filled balloon dilation in the treatment of recurrent urethral strictures: initial experience with five patients. J Vasc Interv Radiol 2006;17:1471–7. 10.1097/01.RVI.0000235738.28095.04 [DOI] [PubMed] [Google Scholar]
- 58.Hosseini J, Fallah-Karkan M, Rahavian A, et al. Feasibility, complication and long-term follow-up of the newly nelaton based urethral dilation method, retrospective study. Am J Clin Exp Urol 2019;7:378–83. [PMC free article] [PubMed] [Google Scholar]
- 59.Yildirim E, Cicek T, Istanbulluoglu O, et al. Use of cutting balloon in the treatment of urethral stricture: a novel technique. Cardiovasc Intervent Radiol 2009;32:525–8. 10.1007/s00270-009-9555-1 [DOI] [PubMed] [Google Scholar]
- 60.Greenwell TJ, Castle C, Andrich DE, et al. Repeat urethrotomy and dilation for the treatment of urethral stricture are neither clinically effective nor cost-effective. J Urol 2004;172:275–7. 10.1097/01.ju.0000132156.76403.8f [DOI] [PubMed] [Google Scholar]
- 61.Stillwell TJ, Patterson DE, LeROY AJ. Endoscopic urethroplasty with balloon dilatation for traumatic disruption of the prostatomembranous urethra. Journal of Endourology 1988;2:257–61. 10.1089/end.1988.2.257 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-071923supp001.pdf (99.1KB, pdf)
bmjopen-2023-071923supp002.pdf (68.3KB, pdf)
bmjopen-2023-071923supp003.pdf (124KB, pdf)
bmjopen-2023-071923supp004.pdf (101.8KB, pdf)
bmjopen-2023-071923supp005.pdf (376.2KB, pdf)
bmjopen-2023-071923supp006.pdf (317.2KB, pdf)
bmjopen-2023-071923supp007.pdf (62.4KB, pdf)
bmjopen-2023-071923supp008.pdf (418.9KB, pdf)
bmjopen-2023-071923supp009.pdf (541.8KB, pdf)
bmjopen-2023-071923supp010.pdf (532.6KB, pdf)
Data Availability Statement
Data are available on reasonable request. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.