Abstract

[18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F were designed by introducing sulfonyl 18F-fluoride onto glutamine, arginine, and tyrosine, respectively. [18F]FSY-OSO2F can be prepared directly by sulfur 18F-fluoride exchange, while [18F]Gln-OSO2F and [18F]Arg-OSO2F require a two-step labeling method. Those tracers retain their typical transport characteristics for unmodified amino acids. Both PET imaging and biodistribution confirmed that [18F]FSY-OSO2F visualized MCF-7 and 22Rv1 subcutaneous tumors with high contrast, and its tumor-to-muscle ratio was better than that of [18F]FET. However, [18F]Gln-OSO2F and [18F]Arg-OSO2F poorly image MCF-7 subcutaneous tumors, possibly due to differences in the types and amounts of transporters expressed in tumors. All three tracers can visualize the U87MG glioma. According to our biological evaluation, none of the tracers evaluated in this study exhibited obvious defluorination, and subtle structural changes led to different imaging characteristics, indicating that the application of sulfur 18F-fluoride exchange click chemistry in the design of radioactive sulfonyl fluoride amino acids is feasible and offers significant advantages.
Keywords: Tracer, Radiolabeling, Amino acid, sulfur 18F-fluoride exchange click chemistry, Positron emission tomography
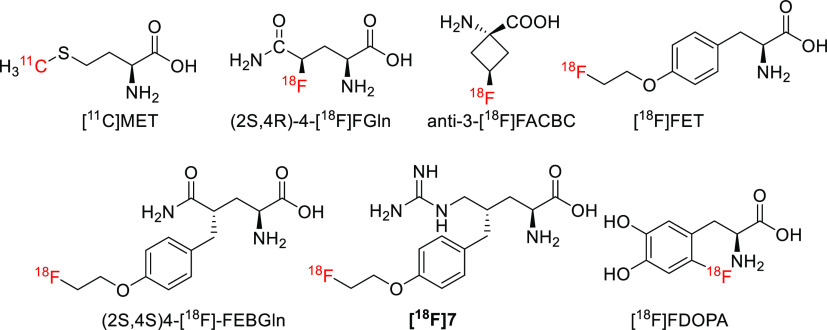
Radiopharmaceuticals are of great significance for the diagnosis, treatment, and efficacy evaluation of diseases1−3 and the invention of new drugs.4−6 Currently, [18F]FDG is the most commonly used PET tracer in clinical practice, but it has certain limitations.7−9 Further breakthroughs are still needed for the development of new tracers. Amino acids are the second largest source of energy for tumor cell growth and reproduction, after glucose. Different tumor cells consume different types of amino acids. Due to these differences, radionuclides are introduced into amino acid structures to synthesize radiotracers for the diagnosis and treatment of tumors.10,11 Unlike [18F]FDG, a commonly used clinical tracer for glucose metabolism, radiolabeled amino acids have the following advantages:10,11 (1) The diverse structure of amino acids ensures the diversity of tracers. (2) The physiological and pathological functions of each amino acid are different. (3) The type, quantity, and membrane uptake rate of amino acid transporters are closely related to the type, invasion, migration, and proliferation rate of tumor cells.11 Therefore, the development of radiolabeled amino acids that target different types of amino acid transporters can provide more detailed and reliable information for the precise diagnosis and treatment of tumors. A series of 11C- and 18F-labeled tracers have been reported, including L-type tracers, [11C]MET,12,13 (S)-[18F]FET,14−16 [18F]FDOPA,17,18 [18F]FMT,19 and (2S,4S)4-[18F]FEBGln;20 ASC-type tracers, 5-[11C]Gln,21 (2S,4R)4-[18F]FGln,22,23anti-3-[18F]FACBC,24 and (2S,4S)4-[18F]FPGln;25 A-type tracers, [18F]-cis-FPro, (S)-[18F]FAMP, and (S)-[18F]MeFAMP; XC–-type tracers, (2S,4R)-4-[18F]FGlu,26 and (2S,4S)[18F]FSPG;27 and CAT-type tracers, (2S,4S)4-[18F]FPArg28 and (S)-[18F]AFETP29 (Figure 1). After decades of development, research on radiolabeled amino acids has made important progress, and [11C]MET, (S)-[18F]FET, and [18F]FDOPA have been applied to the diagnosis of clinical brain tumors. The anti-3-[18F]FACBC (Axumin) has been approved by the U.S. FDA for the diagnosis of recurrent prostate cancer. Although significant progress has been made in research on radiolabeled amino acids, there are still some limitations in clinical applications. For example, a tracer labeled with 11C has a short half-life and a complex preparation process, which are not conducive to long-distance transportation or high-dose production. 18F-labeled tracers often have strict labeling conditions and are prone to chiral racemization during the radiolabeling process, which leads to low radiochemical yields and poor reproducibility in clinical high-dose production. Therefore, improving the radiochemical yields of [18F]-labeled tracers is the key to facilitating the clinical application of radiolabeled amino acids. Sulfur 18F-fluoride exchange click chemistry is a recently reported high-efficiency, high-yield, mild labeling method that is expected to solve the problem of radiolabeled amino acids.30 By this method, sulfonyl fluoride was introduced into the skeletons of glutamine, arginine, and tyrosine and then radiolabeled and biologically evaluated. Head-to-head comparisons were made with other previously reported tracers,31 such as (2S,4S)-4-[18F]FEBGln, [18F]7, and [18F]FET.
Figure 1.
Chemical structures of [11C]MET, (2S,4R)-4-[18F]FGln, anti-3-[18F]FCABA, [18F]FET, (2S,4S)4-[18F]FEBGln, [18F]7, and [18F]FDOPA.
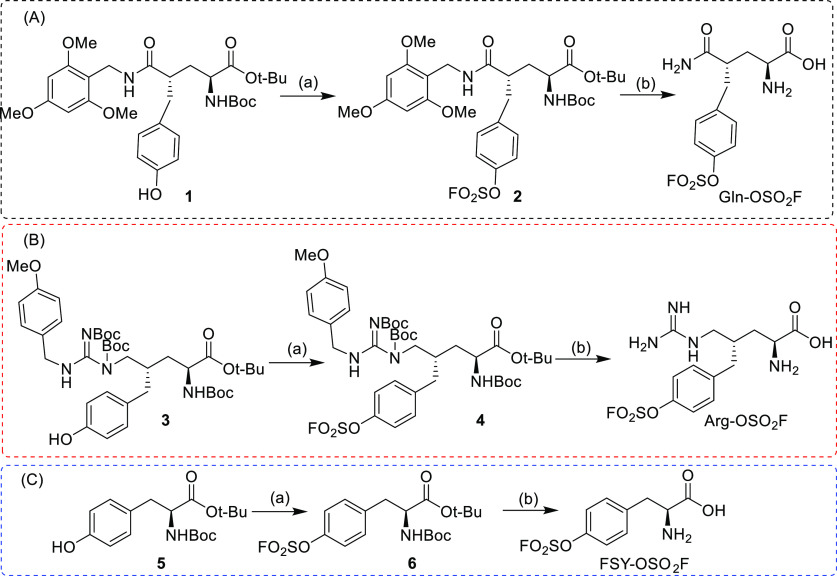
According to the reported sulfur 18F-fluoride exchange click chemistry,30 sulfonyl fluoride is usually introduced on a phenol group. Therefore, if the sulfonyl fluoride labeling method is applied in amino acid radiolabeling, then phenol functional groups need to be introduced into glutamine and arginine. A phenol group is present in the tyrosine backbone, so sulfonyl fluoride is easily introduced. Following the glutamine and arginine derivatives previously designed by our group, compounds 1 and 3 are suitable for the introduction of sulfonyl fluoride groups. Therefore, 1, 3, and 5 were first introduced with sulfonyl fluorine and then deprotected to obtain the target compounds Gln-OSO2F, Arg-OSO2F, and FSY-OSO2F, respectively (Scheme 1).
Scheme 1. Synthesis of Gln-OSO2F (A), Arg-OSO2F (B), and FSY-OSO2F (C).
Reagents and conditions: (a) 1,1′-sulfonyldiimidazole, KF, DIPEA, TFA/H2O, rt, overnight; (b) TFA, rt, 2 h.
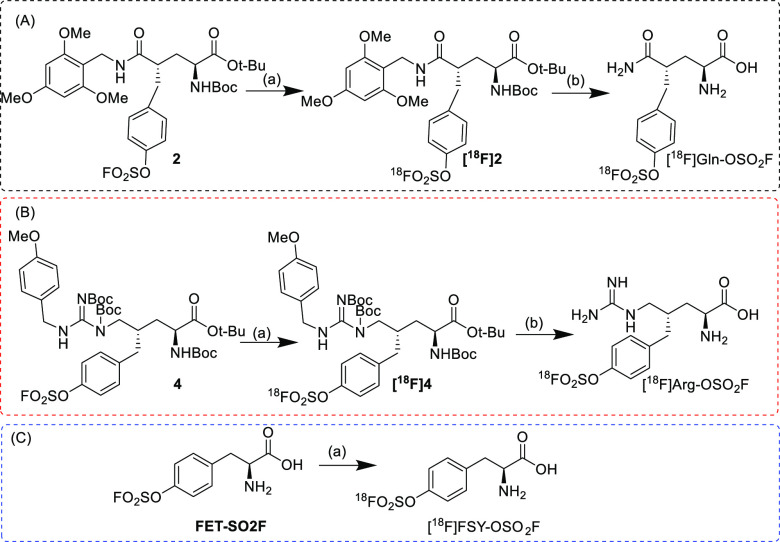
After the target compound was obtained, radiolabeling was attempted. According to the reported method,30 [18F]fluoride and sulfonyl fluoride derivatives can be used to quickly obtain radioactive compounds in ACN by 19F/18F isotope exchange. However, Gln-OSO2F and Arg-OSO2F failed to yield [18F]Gln-OSO2F and [18F]Arg-OSO2F under these conditions, while FSY-OSO2F was converted to [18F]FSY-OSO2F with a yield of 10% (Scheme 2, Scheme S1, and Figure S6). Attempts to optimize the reaction time and increase the temperature were unsuccessful in obtaining [18F]Gln-OSO2F and [18F]Arg-OSO2F directly. This difference may be due to the deterioration of the amide group of glutamine and the guanidine group of arginine during the reaction process, which resulted in the failure to obtain the target compound. The specific reasons still need further detailed research. In view of the unsuccessful direct exchange method, the exchange method was carried out for compounds 2 and 4, which have a protective group. As expected, [18F]2 and [18F]4 were successfully prepared in yields of approximately 20% (Scheme 2, Figures S1 and S2). After TFA hydrolysis, [18F]Gln-OSO2F and [18F]Arg-OSO2F were obtained (Figures S4 and S5). The two-step radiolabeling method yielded the target compound in a total yield of 10%. [18F]FSY-OSO2F can also be obtained by first exchanging [18F]fluoride in compound 6 and then hydrolyzing it with TFA (Scheme S1, Figure S3). Furthermore, in vitro plasma stability experiments showed that [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F all had high stabilities and are worthy of further study (Figure S7).
Scheme 2. Radiolabeling of [18F]Gln-OSO2F (A), [18F]Arg-OSO2F (B), and [18F]FSY-OSO2F (C).
Reagents and conditions: (a) 18F-fluoride/K222/K2CO3, ACN, rt, 1 min; (b) TFA, 50 °C ([18F]Gln-OSO2F) and 60 °C ([18F]Arg-OSO2F), 5 min.
The transport mechanisms of [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F were evaluated through in vitro uptake assays with MCF-7 cells in the absence and presence of amino acid transport inhibitors. As shown in Figure 2a–c, there was no uptake inhibition of [18F]Gln-OSO2F, [18F]Arg-OSO2F, or [18F]FSY-OSO2F in the presence of MeAIB, indicating that [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F did not use system A to enter MCF-7 cells. As a derivative of glutamine, [18F]Gln-OSO2F was inhibited by 68.4% in the presence of glutamine (Figure 2a), indicating that this compound uses a transporter similar to glutamine. Like in (2S,4S)-4-[18F]FEBGln,32 [18F]Gln-OSO2F also enters cells through the ASC and L transporters (Figure 2a). The uptake of [18F]Arg-OSO2F decreased by approximately 50% in the presence of cationic amino acid transporter substrates such as arginine and RHK (Figure 2b), indicating that the entry of [18F]Arg-OSO2F into MCF-7 cells was mediated by the cationic amino acid transporter. [18F]Arg-OSO2F can also partially enter cells through the ASC and L transporters. Like in the case of [18F]FET, in the presence of ASC, Tyr, and BCH, the uptake of [18F]FSY-OSO2F was significantly reduced (Figure 2c), indicating that the entry of [18F]FSY-OSO2F into cells is regulated mainly by L, ASC, and ASC2 transporters. The detailed mechanism by which [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F enter cells still needs further investigation.
Figure 2.

In vitro uptake of [18F]Gln-OSO2F (a), [18F]Arg-OSO2F (b), and [18F]FSY-OSO2F (c) in MCF-7 cells in the presence and absence of competitive inhibitors of amino acid transporter. MeAIB = 10 mM N-methyl α-aminoisobutyric acid (system A inhibitor); BCH = 10 mM 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid (system L inhibitor); ASC = 3.3 mM each of l-Ala, l-Ser, and l-Cys. ASC2 = 10 mM l-γ-glutamyl-p-nitroanilide; Arg = 10 mM arginine; Lys = 10 mM lysine; RKH = l-Arg, l-Lys, l-His mixture (3.3 mM of each amino acid); Tyr = 10 mM tyrosine. The PBS control condition was compared to the MeAIB BCH, ASC, ASC2, Gln, Arg, Lys, and RKH (n.s., non-significant; *p < 0.05 and **p < 0.01) conditions using two-tailed t tests. (d) In vitro cell uptakes of [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F in MCF-7 cells.
The uptake of [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F by MCF-7 cells was further studied. Compared with [18F]FSY-OSO2F, [18F]Gln-OSO2F and [18F]Arg-OSO2F had a lower uptake (Figure 2d). The uptake of [18F]FSY-OSO2F reached a peak of 18.61 ± 2.02%/100 μg protein at 30 min and then decreased to 8.48 ± 0.20%/100 μg protein at 120 min. Unlike that of [18F]FSY-OSO2F, the uptake of [18F]Gln-OSO2F and [18F]Arg-OSO2F was lower and increased slowly, and the uptake of [18F]Arg-OSO2F was slightly greater than that of [18F]Gln-OSO2F (Figure 2d).
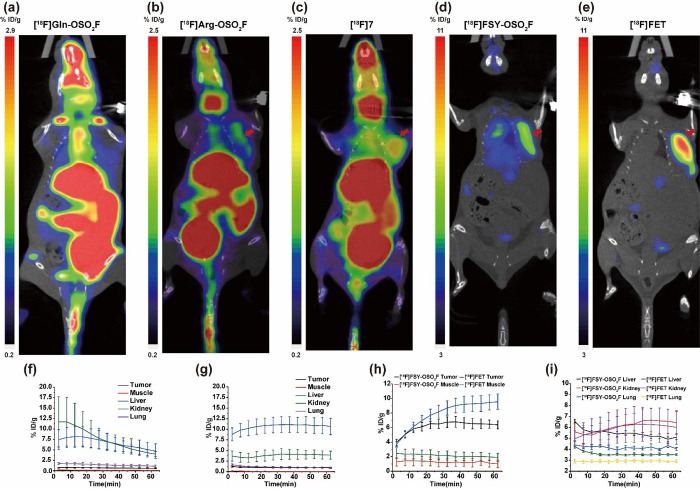
Further investigation of the tumor visualization ability of the tracer through PET imaging was conducted. In MCF-7 subcutaneous tumors, [18F]Gln-OSO2F has a greater lipid solubility and uptake in the liver than (2S,4R)-4-[18F]FGln (Figure 3a,f). MCF-7 tumors do not take up [18F]Gln-OSO2F; therefore, [18F]Gln-OSO2F cannot accurately locate MCF-7 tumors. Like [18F]Gln-OSO2F, [18F]Arg-OSO2F is metabolized by the liver and kidneys (Figure 3g), but its uptake into tumors is increased (Figure 3b). Compared with that of arginine derivative [18F]7 (Figure 3c), the uptake of [18F]Arg-OSO2F by tumors was significantly reduced (Figure 3g). Compared with [18F]Gln-OSO2F and [18F]Arg-OSO2F, [18F]FSY-OSO2F had a greater uptake in tumors (Figure 3d). After 60 min of administration, the tumor-to-muscle ratio of [18F]FSY-OSO2F was 6 (Figure 3h), while that of [18F]FET was approximately 4.5 (Figures 3e,h). According to the MCF-7 imaging results for [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F, the lipid solubility of these tracers increases with the introduction of sulfonylfluorophenol groups, resulting in increased liver uptake (Figure 3f,g,i). Consistent with the in vitro cellular uptake of MCF-7 cells, [18F]FSY-OSO2F had significantly greater tumor uptake than [18F]Gln-OSO2F and [18F]Arg-OSO2F. Luckily, [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F did not undergo significant defluorination (Figure 3a,b,d).
Figure 3.
MicroPET-CT images of [18F]Gln-OSO2F (a), [18F]Arg-OSO2F (b), [18F]7 (c), [18F]FSY-OSO2F (d), and [18F]FET (e) in MCF-7 subcutaneous tumors, where red arrow indicates tumor. Time–activity curves of [18F]Gln-OSO2F (f), [18F]Arg-OSO2F (g), and [18F]FSY-OSO2F and [18F]FET (h, i) in MCF-7 subcutaneous tumors.
Further attempts were made to expand the tumor application range of [18F]FSY-OSO2F, and static imaging of 22Rv1 subcutaneous tumors was performed at 1 h. Similar to MCF-7, it can be seen from Figure 4 that the tumor uptake of [18F]FSY-OSO2F was high (6.58 ± 0.63% ID/g), while lung, liver, and muscle uptake (0.91 ± 0.15% ID/g) was low. Compared to [18F]FSY-OSO2F, [18F]FET has higher tumor uptake (10.07 ± 1.49% ID/g) but also higher uptake in lungs, liver, and muscles (3.69 ± 0.71% ID/g). Therefore, [18F]FSY-OSO2F has a higher tumor-to-muscle ratio than [18F]FET (7.23 vs 2.72, p < 0.05). After pretreatment with FET, the tumor uptake of [18F]FSY-OSO2F was not inhibited (Figure 4), and the tumor-to-muscle ratio was increased (7.23 vs 8.0), while the background uptake of [18F]FET decreased significantly (3.69 ± 0.71% vs 2.29 ± 0.48% ID/g, p < 0.05), and the tumor-to-muscle ratio was increased (4.07 vs 2.72, p < 0.05). The increase in the target-to-non-target ratio and the uninhibited tumor uptake may be caused by the fact that the expression level of amino acid transporters in vivo is much higher than that of specific proteins such as PSMA. With the addition of inhibitors, specific proteins such as PSMA can be inhibited in scintigraphy. Under similar conditions, low expression of transporters in backgrounds such as muscle uptake can be inhibited, while in tumors high expression of transporters cannot be inhibited with small amount of inhibitors; therefore, the target-to-non-target ratio is increased after the addition of inhibitors.
Figure 4.
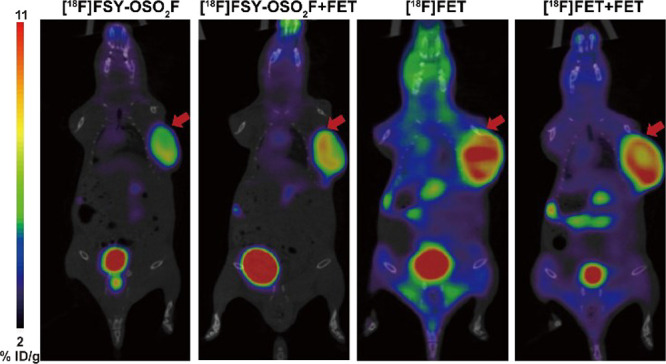
Representative microPET-CT images of 22Rv1 subcutaneous tumors with [18F]FSY-OSO2F and [18F]FET with or without FET (0.4 mg/mouse) at 60 min post injection.
The PET imaging results of [18F]FSY-OSO2F were further verified by a MCF-7 subcutaneous tumor biodistribution assay. After 60 min of administration, the tumor uptake of [18F]FSY-OSO2F was 8.31 ± 1.92% ID/g (Figure 5). Consistent with the results of PET imaging, it is metabolized mainly by the liver and kidneys (Figure 5). Its tumor-to-muscle ratio was 3.14 ± 0.91 at 60 min time points. The lung uptake of [18F]FSY-OSO2F was 4.24 ± 0.36% ID/g. The tumor-to-lung ratio was 1.95 ± 0.60, so [18F]FSY-OSO2F is expected to be useful for diagnosing lung cancer. Consistent with the results of PET imaging, [18F]FSY-OSO2F did not undergo significant defluorination.
Figure 5.

Biodistribution studies of [18F]FSY-OSO2F in mice bearing MCF-7 xenograft at 60 time points after intravenous injection (n = 4).
The greatest advantage of using radiolabeled amino acids in tumors is the ability to image gliomas.33 Therefore, [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F have also been applied to the imaging of U87MG intracranial glioma. After 60 min of administration, gliomas could be visualized by all three tracers (Figure 6), with tumor and brain uptake values of 1.93 ± 0.05/0.83 ± 0.02, 1.41 ± 0.03/0.48 ± 0.02, and 4.98 ± 0.08/3.39 ± 0.14% ID/g, and target-to-non-target ratios of 2.32, 2.93, and 1.46, respectively. In this mouse model, no significant defluorination was found for any of the three tracers. Therefore, these three tracers have the potential to be applied in the diagnosis of gliomas.
Figure 6.

MicroPET-CT images of [18F]Gln-OSO2F (a), [18F]Arg-OSO2F (b), and [18F]FSY-OSO2F (c) in U87MG orthotopic glioma at 60 min post injection, where red arrow indicates tumor.
Radiolabeled amino acids are highly useful for diagnosing tumors and central nervous system diseases. Various types of radiolabeled amino acids have been developed. All of these tracers are chiral, so their radiolabeling conditions need to be strictly controlled. The conventional high-energy-barrier, activated ester nucleophilic substitution labeling method cannot solve the fundamental problem of harsh labeling conditions for radiolabeled amino acids. Low-activation-energy [18F]fluorine exchange methods, such as [18F]fluorine–aluminum exchange,34 [18F]fluoroboron exchange,35,36 [18F]fluorophosphine exchange,37 and [18F]fluorosulfur exchange,38 have important applications in radiopharmaceutical development. The [18F]fluorine–aluminum exchange method is suitable for the design of large-molecule drugs because it requires complexing agent coordination.34 The [18F]fluoroboron exchange method has been applied to the study of radiolabeled amino acids, such as [18F]FBQ;39 its labeling yield has been greatly improved, and the labeling conditions have been simplified. However, due to in vivo defluorination, further clinical application of these tracers is limited.
Sulfur 18F-fluoride exchange click chemistry is a relatively new and efficient labeling method that has been reported in recent years. Moreover, glutamine, arginine, and tyrosine are closely related to the occurrence and progression of many tumors. Therefore, the use of sulfur 18F-fluoride exchange click chemistry to design tracers of these three amino acids can help diagnose a wide range of tumors. Accordingly, [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F were designed and evaluated. As expected, FSY-OSO2F can be smoothly converted to [18F]FSY-OSO2F at room temperature, which greatly solves the problem of difficult amino acid labeling. However, [18F]Gln-OSO2F and [18F]Arg-OSO2F cannot be prepared by direct exchange of cold compounds, possibly due to further reactions of glutamine and arginine with sulfonyl fluoride under alkaline conditions; the exact reasons still need to be further studied. In the presence of protective groups, compounds 2, 4, and 6 can be rapidly converted to [18F]2, [18F]4, and [18F]6, respectively. After further TFA hydrolysis, [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F were obtained.
Like [18F]7 and (2S,4S)4-[18F]FEBGln, [18F]Gln-OSO2F and [18F]Arg-OSO2F are prepared by a two-step labeling method. However, the intermediates of [18F]7 and (2S,4S)-4-[18F]FEBGln have low radiochemical yields (less than 10%) and require harsh reaction conditions, whereas the intermediates of [18F]Gln-OSO2F and [18F]Arg-OSO2F were obtained in 20% yield under mild conditions. Therefore, sulfur 18F-fluoride exchange click chemistry still has great advantages in amino acid labeling. Experiments on the cell transport mechanism showed that these tracers had characteristics of unmodified amino acids. PET scintigraphy revealed that the tumor did not take up [18F]Gln-OSO2F, had low uptake of [18F]Arg-OSO2F, and had high uptake of [18F]FSY-OSO2F. This approach also provides a new method for identifying breast cancer types. Compared with the tracers (2S,4S)-4-[18F]FEBGln, [18F]7, and [18F]FET, which have not been replaced by sulfonyl fluoride, lower uptake of [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F was observed in the MCF-7 tumor, which may be due to changes in the transporters used for radiolabeling amino acids. Further glioma PET imaging revealed that the target-to-non-target ratios of [18F]Gln-OSO2F and [18F]Arg-OSO2F were greater than that of [18F]FSY-OSO2F. From the perspective of uptake in different tumor models, the uptake of different types of radiolabeled amino acids varies greatly, which may be due to the different amounts and types of transporters expressed by different tumors. For example, the L-type transporter is highly expressed in tumors, but the L-type transporter can transport the tracer into and out of the tumor,10 and then the tracer is difficult to enrich in the tumor, so the tracer has a short tumor residence time. The A-type transporter can transport the tracer into the tumor in one direction,10 and then the tracer can be enriched in the tumor, so the tracer has a long tumor residence time. Therefore, the amounts and types of amino acid transporters expressed by the tumor determine the tumor imaging ability of the tracer. The study of tracers targeting transporters of different subtypes is essential for the accurate diagnosis of tumors. From a structural point of view, there are only slight differences among the three tracers, but the imaging characteristics are quite different. Therefore, in the future, when designing radiolabeled amino acids, the imaging characteristics of the tracer can be controlled by subtly adjusting functional groups.
Notably, most of the tracers designed by sulfur 18F-fluoride exchange click chemistry have been reported to be defluorinated.30,40 Our group has also designed tracers targeting PSMA, FAP (fibroblast activation protein), and SERT (serotonin transporter) based on sulfur 18F-fluoride exchange click chemistry,41 all of which have obvious defluorination. There was no obvious defluorination of the compounds reported in this paper, which may be due to the enhanced stability of the tracer during the transport process. Unlike receptor-binding tracers, which require high contrast activity, metabolically radiolabeled amino acids require relatively low specific activity. Therefore, sulfonyl 18F-fluoride amino acids deserve further research to develop novel tracers with excellent properties.
In summary, [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F were designed by using sulfur 18F-fluoride exchange click chemistry. FSY-OSO2F can be converted directly to [18F]FSY-OSO2F by a one-step labeling method, while [18F]Glu-SO2F and [18F]Arg-OSO2F need to be prepared by a two-step method. Transport mechanism experiments showed that [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F had transporter characteristics similar to those of unmodified amino acids. [18F]FSY-OSO2F and [18F]Arg-OSO2F visualized MCF-7 tumors at high and low contrast, respectively, while [18F]Gln-OSO2F did not image tumors. All three tracers can visualize gliomas. Importantly, no significant defluorination of radioactive sulfonyl fluoride amino acids was found, so the design of new radiolabeled amino acids based on sulfur 18F-fluoride exchange click chemistry is worthy of further development.
Acknowledgments
We thank Dr. Yajing Liu from the School of Pharmaceutical Science of Capital Medical University for revision of this manuscript.
Glossary
Abbreviations
- ACN
acetonitrile
- DIPEA
N,N-diisopropylethylamine
- FET
fluoroethyltyrosine
- K2.2.2
Kryptofix 222
- PET
positron emission tomography
- PSMA
prostate-specific membrane antigen
- rt
room temperature
- TFA
trifluoroacetic acid
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00557.
Procedure for the synthesis, radiosynthesis, and biodistribution of [18F]Gln-OSO2F, [18F]Arg-OSO2F, and [18F]FSY-OSO2F; NMR spectra for all compounds reported herein (PDF)
Author Contributions
‡ Yong Huang and Hualong Chen contributed equally to this paper.
This research was funded by the Beijing Natural Science Foundation (7222299) and Medical Innovation Capability Improvement Plan of Capital Medical University (12300124), National Cancer Center, National Clinical Research Center for Cancer, Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen (SZ2020MS008 and E010321007), Shenzhen High-level Hospital Construction Fund, the National Natural Science Foundation of China (82372018 and 82102115), The Shenzhen Science and Technology Program of China (JCYJ20220818101804009), and Shenzhen Clinical Research Center for Cancer (No. (2021) 287).
The authors declare no competing financial interest.
Supplementary Material
References
- Kreisl W. C.; Kim M.-J.; Coughlin J. M.; Henter I. D.; Owen D. R.; Innis R. B. PET imaging of neuroinflammation in neurological disorders. Lancet Neurology 2020, 19, 940–950. 10.1016/S1474-4422(20)30346-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodei L.; Herrmann K.; Schöder H.; Scott A. M.; Lewis J. S. Radiotheranostics in oncology: current challenges and emerging opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 534–550. 10.1038/s41571-022-00652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K.; Schwaiger M.; Lewis J. S.; Solomon S. B.; McNeil B. J.; Baumann M.; Gambhir S. S.; Hricak H.; Weissleder R. Radiotheranostics: a roadmap for future development. Lancet Oncol. 2020, 21, e146–e156. 10.1016/S1470-2045(19)30821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly D. J. Small Molecule PET Tracers in Drug Discovery. Semin. Nucl. Med. 2017, 47, 454–460. 10.1053/j.semnuclmed.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Durham T. B.; Blanco M.-J. Target Engagement in Lead Generation. Bioorg. Med. Chem. Lett. 2015, 25, 998–1008. 10.1016/j.bmcl.2014.12.076. [DOI] [PubMed] [Google Scholar]
- Piel M.; Vernaleken I.; Roesch F. Positron Emission Tomography in CNS drug discovery and drug monitoring. J. Med. Chem. 2014, 57, 9232–9258. 10.1021/jm5001858. [DOI] [PubMed] [Google Scholar]
- Pijl J. P.; Nienhuis P. H.; Kwee T. C.; Glaudemans A.; Slart R.; Gormsen L. C. Limitations and Pitfalls of FDG-PET/CT in Infection and Inflammation. Semin. Nucl. Med. 2021, 51, 633–645. 10.1053/j.semnuclmed.2021.06.008. [DOI] [PubMed] [Google Scholar]
- van Leer B.; van Rijsewijk N. D.; Nijsten M. W. N.; Slart R.; Pillay J.; Glaudemans A. Practice of (18)F-FDG-PET/CT in ICU Patients: A Systematic Review. Semin. Nucl. Med. 2023, 53, 809–819. 10.1053/j.semnuclmed.2023.05.003. [DOI] [PubMed] [Google Scholar]
- Sarikaya I.; Sarikaya A. Assessing PET Parameters in Oncologic (18)F-FDG Studies. J. Nucl. Med. Techol. 2020, 48, 278–282. 10.2967/jnmt.119.236109. [DOI] [PubMed] [Google Scholar]
- McConathy J.; Goodman M. M. Non-natural amino acids for tumor imaging using positron emission tomography and single photon emission computed tomography. Cancer Metast. Rev. 2008, 27, 555–573. 10.1007/s10555-008-9154-7. [DOI] [PubMed] [Google Scholar]
- McConathy J.; Yu W.; Jarkas N.; Seo W.; Schuster D. M.; Goodman M. M. Radiohalogenated nonnatural amino acids as PET and SPECT tumor imaging agents. Med. Res. Rev. 2012, 32, 868–905. 10.1002/med.20250. [DOI] [PubMed] [Google Scholar]
- Leskinen-Kallio S.; Nagren K.; Lehikoinen P.; Ruotsalainen U.; Joensuu H. Uptake of 11C-methionine in breast cancer studied by PET. An association with the size of S-phase fraction. Br. J. Cancer 1991, 64, 1121–1124. 10.1038/bjc.1991.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal T.; Narayanan T. K.; Jain V.; Mukherjee J.; Mantil J. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol. Imaging and Biol. 2008, 10, 1–18. 10.1007/s11307-007-0115-2. [DOI] [PubMed] [Google Scholar]
- Hutterer M.; Nowosielski M.; Putzer D.; Waitz D.; Tinkhauser G.; Kostron H.; Muigg A.; Virgolini I. J.; Staffen W.; Trinka E.; Gotwald T.; Jacobs A. H.; Stockhammer G. O-(2–18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J. Nucl. Med. 2011, 52, 856–864. 10.2967/jnumed.110.086645. [DOI] [PubMed] [Google Scholar]
- Pauleit D.; Stoffels G.; Bachofner A.; Floeth F. W.; Sabel M.; Herzog H.; Tellmann L.; Jansen P.; Reifenberger G.; Hamacher K.; Coenen H. H.; Langen K.-J. Comparison of 18F-FET and 18F-FDG PET in brain tumors. Nucl. Med. Biol. 2009, 36, 779–787. 10.1016/j.nucmedbio.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Pöpperl G.; Kreth F. W.; Mehrkens J. H.; Herms J.; Seelos K.; Koch W.; Gildehaus F. J.; Kretzschmar H. A.; Tonn J. C.; Tatsch K. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur. J. Nucl. Med. Mol. I maging 2007, 34, 1933–1942. 10.1007/s00259-007-0534-y. [DOI] [PubMed] [Google Scholar]
- Minn H.; Kauhanen S.; Seppanen M.; Nuutila P. 18F-FDOPA: a multiple-target molecule. J. Nucl. Med. 2009, 50, 1915–1918. 10.2967/jnumed.109.065664. [DOI] [PubMed] [Google Scholar]
- Koerts J.; Leenders K. L.; Koning M.; Portman A. T.; van Beilen M. Striatal dopaminergic activity (FDOPA-PET) associated with cognitive items of a depression scale (MADRS) in Parkinson’s disease. Eur. J. Neurosci. 2007, 25, 3132–3136. 10.1111/j.1460-9568.2007.05580.x. [DOI] [PubMed] [Google Scholar]
- VanBrocklin H. F.; Blagoev M.; Hoepping A.; O’Neil J. P.; Klose M.; Schubiger P. A.; Ametamey S. A new precursor for the preparation of 6-[18F]Fluoro-l-m-tyrosine ([18F]FMT): efficient synthesis and comparison of radiolabeling. Appl. Radiat. Isot. 2004, 61, 1289–1294. 10.1016/j.apradiso.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Liu Y.; Li C.; Li Z.; Chen H.; Zhang L.; Liang Y.; Wu Z. Evaluation of (2S,4S)-4-[18F]FEBGln as a Positron Emission Tomography Tracer for Tumor Imaging. Mol. Pharmaceutics 2023, 20, 5195–5205. 10.1021/acs.molpharmaceut.3c00544. [DOI] [PubMed] [Google Scholar]
- Qu W.; Oya S.; Lieberman B. P.; Ploessl K.; Wang L.; Wise D. R.; Divgi C. R.; Chodosh L. A.; Thompson C. B.; Kung H. F. Preparation and characterization of L-[5–11C]-glutamine for metabolic imaging of tumors. J. Nucl. Med. 2012, 53, 98–105. 10.2967/jnumed.111.093831. [DOI] [PubMed] [Google Scholar]
- Qu W.; Zha Z.; Ploessl K.; Lieberman B. P.; Zhu L.; Wise D. R.; Thompson C. B.; Kung H. F. Synthesis of optically pure 4-fluoro-glutamines as potential metabolic imaging agents for tumors. J. Am. Chem. Soc. 2011, 133, 1122–1133. 10.1021/ja109203d. [DOI] [PubMed] [Google Scholar]
- Ploessl K.; Wang L.; Lieberman B. P.; Qu W.; Kung H. F. Comparative evaluation of 18F-labeled glutamic acid and glutamine as tumor metabolic imaging agents. J. Nucl. Med. 2012, 53, 1616–1624. 10.2967/jnumed.111.101279. [DOI] [PubMed] [Google Scholar]
- Turkbey B.; Mena E.; Shih J.; Pinto P. A.; Merino M. J.; Lindenberg M. L.; Bernardo M.; McKinney Y. L.; Adler S.; Owenius R.; Choyke P. L.; Kurdziel K. A. Localized Prostate Cancer Detection with (18)F FACBC PET/CT: Comparison with MR Imaging and Histopathologic Analysis. Radiology 2014, 270, 849–856. 10.1148/radiol.13130240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Zha Z.; Li G.; Lieberman B. P.; Choi S. R.; Ploessl K.; Kung H. F. [(18)F](2S,4S)-4-(3-Fluoropropyl)glutamine as a tumor imaging agent. Mol. Pharmaceutics 2014, 11, 3852–3866. 10.1021/mp500236y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belokon Y. N.; Maleev V. I.; Savel’eva T. F.; Moskalenko M. A.; Pripadchev D. A.; Khrustalev V. N.; Saghiyan A. S. Asymmetric synthesis of enantiomerically and diastereoisomerically enriched 4-[F or Br]-substituted glutamic acids. Amino Acids 2010, 39, 1171–1176. 10.1007/s00726-010-0551-1. [DOI] [PubMed] [Google Scholar]
- Baek S.; Choi C. M.; Ahn S. H.; Lee J. W.; Gong G.; Ryu J. S.; Oh S. J.; Bacher-Stier C.; Fels L.; Koglin N.; Hultsch C.; Schatz C. A.; Dinkelborg L. M.; Mittra E. S.; Gambhir S. S.; Moon D. H. Exploratory clinical trial of (4S)-4-(3-[18F]fluoropropyl)-L-glutamate for imaging xC- transporter using positron emission tomography in patients with non-small cell lung or breast cancer. Clin. Cancer Res. 2012, 18, 5427–5437. 10.1158/1078-0432.CCR-12-0214. [DOI] [PubMed] [Google Scholar]
- Wu R.; Liu S.; Liu Y.; Sun Y.; Cheng X.; Huang Y.; Yang Z.; Wu Z. Synthesis and biological evaluation of [18F](2S,4S)4-(3-fluoropropyl) arginine as a tumor imaging agent. Eur. J. Med. Chem. 2019, 183, 111730. 10.1016/j.ejmech.2019.111730. [DOI] [PubMed] [Google Scholar]
- McConathy J.; Martarello L.; Malveaux E. J.; Camp V. M.; Simpson N. E.; Simpson C. P.; Bowers G. D.; Olson J. J.; Goodman M. M. Radiolabeled amino acids for tumor imaging with PET: radiosynthesis and biological evaluation of 2-amino-3-[18F]fluoro-2-methylpropanoic acid and 3-[18F]fluoro-2-methyl-2-(methylamino)propanoic acid. J. Med. Chem. 2002, 45, 2240–2249. 10.1021/jm010241x. [DOI] [PubMed] [Google Scholar]
- Zheng Q.; Xu H.; Wang H.; Du W.-G. H.; Wang N.; Xiong H.; Gu Y.; Noodleman L.; Sharpless K. B.; Yang G.; Wu P. Sulfur [18F]Fluoride Exchange Click Chemistry Enabled Ultrafast Late-Stage Radiosynthesis. J. Am. Chem. Soc. 2021, 143, 3753–3763. 10.1021/jacs.0c09306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Li C.; Li Z.; Xie Y.; Chen H.; Li S.; Liang Y.; Wu Z. Design, Synthesis, and Biological Evaluation of a Novel [18F]-Labeled Arginine Derivative for Tumor Imaging. Pharmaceuticals 2023, 16, 1477. 10.3390/ph16101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Liu S.; Wu R.; Zhang L.; Zhang Y.; Hong H.; Zhang A.; Xiao H.; Liu Y.; Wu Z.; Zhu L.; Kung H. F. Synthesis and preliminary evaluation of a novel glutamine derivative: (2S,4S)4-[18F]FEBGln. Bioorg. Med. Chem. Lett. 2019, 29, 1047–1050. 10.1016/j.bmcl.2019.03.012. [DOI] [PubMed] [Google Scholar]
- Huang C.; McConathy J. Radiolabeled amino acids for oncologic imaging. J. Nucl. Med. 2013, 54, 1007–1010. 10.2967/jnumed.112.113100. [DOI] [PubMed] [Google Scholar]
- Kumar K.; Ghosh A. 18F-AlF Labeled Peptide and Protein Conjugates as Positron Emission Tomography Imaging Pharmaceuticals. Bioconjugate Chem. 2018, 29, 953–975. 10.1021/acs.bioconjchem.7b00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Pourghiasian M.; Radtke M. A.; Lau J.; Pan J.; Dias G. M.; Yapp D.; Lin K. S.; Bénard F.; Perrin D. M. An organotrifluoroborate for broadly applicable one-step 18F-labeling. Angew. Chem., Int. Ed. 2014, 53, 11876–11880. 10.1002/anie.201406258. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Lin K.-S.; Bénard F.; Pourghiasian M.; Kiesewetter D. O.; Perrin D. M.; Chen X. One-step 18F labeling of biomolecules using organotrifluoroborates. Nat. Protoc. 2015, 10, 1423–1432. 10.1038/nprot.2015.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H.; Zhang L.; Xie F.; Zhuang R.; Jiang D.; Liu H.; Li J.; Yang H.; Zhang X.; Nie L.; Li Z. Rapid one-step 18F-radiolabeling of biomolecules in aqueous media by organophosphine fluoride acceptors. Nat. Commun. 2019, 10, 989. 10.1038/s41467-019-08953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Li J.; Li S.; Li G.; Sharpless K. B.; Wu P. SuFEx Click Chemistry Enabled Late-Stage Drug Functionalization. J. Am. Chem. Soc. 2018, 140, 2919–2925. 10.1021/jacs.7b12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Li C.; Hong H.; Liu H.; Wang C.; Xu M.; Han Y.; Liu Z. Side Chain Optimization Remarkably Enhances the in Vivo Stability of 18F-Labeled Glutamine for Tumor Imaging. Mol. Pharmaceutics 2019, 16, 5035–5041. 10.1021/acs.molpharmaceut.9b00891. [DOI] [PubMed] [Google Scholar]
- Hu X.; Lv G.; Hua D.; Zhang N.; Liu Q.; Qin S.; Zhang L.; Xi H.; Qiu L.; Lin J. Preparation and Bioevaluation of 18F-Labeled Small-Molecular Radiotracers via Sulfur(VI) Fluoride Exchange Chemistry for Imaging of Programmed Cell Death Protein Ligand 1 Expression in Tumors. Mol. Pharmaceutics 2023, 20, 4228–4235. 10.1021/acs.molpharmaceut.3c00355. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Ji X.; Sun Y.; Chen H.; Zheng W.; Yang T., Yu Z.. A sulfonyl fluoride compound and its application. CN Patent ZL202110795819.1, Oct 26, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.