Highlights
Recent quantitative measurements have revealed dynamics of transcription factor DNA binding, preinitiation complex assembly, chromatin remodeling, and transcriptional bursting in living cells.
Timescale comparison between transcription initiation events (input) and transcriptional burst kinetics (output) reveals mechanisms of transcription regulation.
Transcription dynamics are shaped by the complex interplay of transient DNA binding of regulatory factors and the local environment (chromatin, clustering of regulatory factors, and enhancer–promoter interactions).
Emerging imaging technologies are beginning to facilitate the dissection of input–output relationships at single genes to understand how regulatory factors affect rate-limiting steps.
Keywords: transcriptional bursting, gene regulation, DNA binding dynamics, chromatin, clustering, enhancer
Abstract
Recent imaging studies have captured the dynamics of regulatory events of transcription inside living cells. These events include transcription factor (TF) DNA binding, chromatin remodeling and modification, enhancer–promoter (E–P) proximity, cluster formation, and preinitiation complex (PIC) assembly. Together, these molecular events culminate in stochastic bursts of RNA synthesis, but their kinetic relationship remains largely unclear. In this review, we compare the timescales of upstream regulatory steps (input) with the kinetics of transcriptional bursting (output) to generate mechanistic models of transcription dynamics in single cells. We highlight open questions and potential technical advances to guide future endeavors toward a quantitative and kinetic understanding of transcription regulation.
Recent imaging studies have captured the dynamics of regulatory events of transcription inside living cells. These events include transcription factor (TF) DNA binding, chromatin remodeling and modification, enhancer–promoter (E–P) proximity, cluster formation, and preinitiation complex (PIC) assembly. Together, these molecular events culminate in stochastic bursts of RNA synthesis, but their kinetic relationship remains largely unclear. In this review, we compare the timescales of upstream regulatory steps (input) with the kinetics of transcriptional bursting (output) to generate mechanistic models of transcription dynamics in single cells. We highlight open questions and potential technical advances to guide future endeavors toward a quantitative and kinetic understanding of transcription regulation.
The stochastic nature of gene transcription
Gene transcription is a tightly regulated process of which both input and output are dynamic. During this process, an ensemble of interactions between transcriptional proteins and DNA (input) orchestrates bursts of RNA production (output). Transcription initiation starts with sequence-specific TFs, which bind DNA motifs in gene regulatory regions positioned at the promoter or distal enhancers. TFs recruit chromatin remodelers, chromatin modifiers, and coactivators to prime the local chromatin environment for gene activation. These steps facilitate the assembly of the PIC, composed of general TFs (GTFs) and RNA polymerase II (Pol II), after which Pol II can escape the promoter and transcribe the gene into RNA. Due to random molecular collisions and the low copy number of each gene in the nucleus (i.e., two alleles for a diploid cell), each of these steps is inherently stochastic, resulting in fluctuations in polymerase loading. In addition, RNA production is often not continuous but instead shows stochastic switching between periods of high activity and periods of inactivity, called transcriptional bursting. Observations of transcriptional bursting range from the classic Miller spreads to single-molecule fluorescent microscopy approaches [1,2]. Recently, high-throughput RNA labeling and live-cell imaging of hundreds of endogenous genes revealed that in human cells, all observed genes are transcribed in bursts [3]. In addition, allele-specific single-cell RNA sequencing inferred bursting kinetics of the entire transcriptome in human and mouse cells [4]. Transcriptional bursting is thus a general phenomenon in gene transcription.
While most central players of gene transcription have been revealed, their impact on the dynamics of transcriptional bursting is still under active investigation. Studying the dynamics of the transcriptional process provides a complementary view to biochemical, structural, and genomic studies, and is essential to provide insight into the regulation of rate-limiting steps in transcription. In this review, we will discuss the most recent advances in the field of transcription dynamics, focusing on comparing the timescales of molecular regulatory events with the timescales of stochastic transcriptional ON–OFF switching. We discuss the contribution of transcriptional protein–DNA binding dynamics, the local chromatin architecture, clustering, and E–P proximity to transcriptional burst regulation (Figure 1). We also review recent observations of coupling of transcriptional bursting of multiple genes. Last, we outline potential future advances that could allow for better timescale comparison and expand our understanding of the regulatory mechanisms behind transcription dynamics.
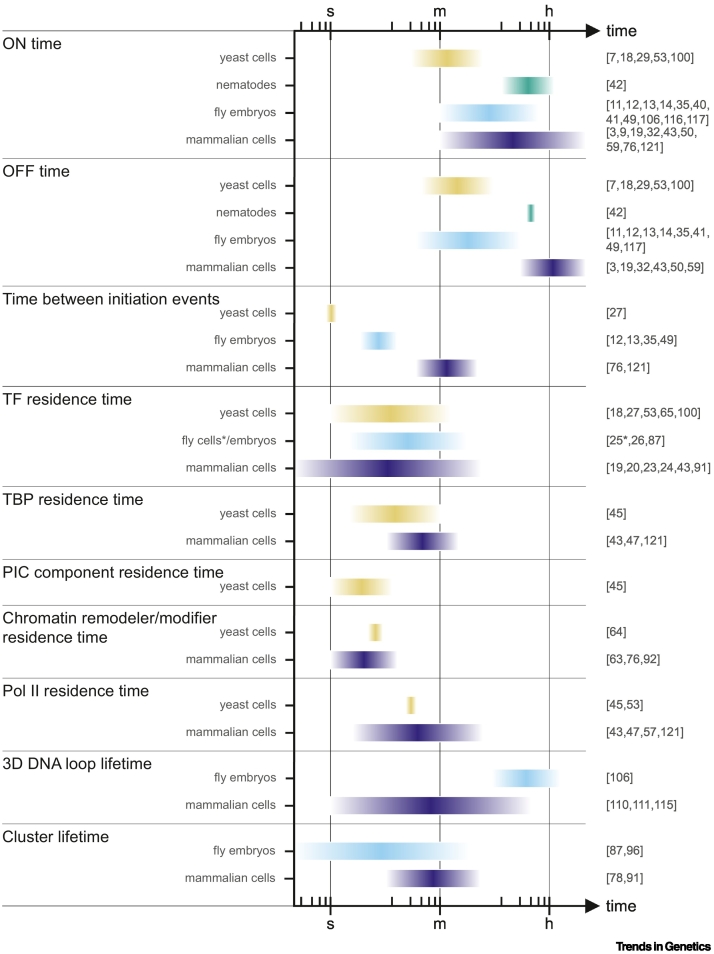
Figure 1.
The timescales of molecular events underlying transcriptional bursting.
A kinetic overview of the regulatory steps leading to transcription, based on quantitative measurements in the references of this review. Although this list is far from complete, it is evident that timescales differ between both molecular events and model organisms. Note that this variation also depends on the genomic context and experimental setup. Future developments in microscopy and kinetic modeling are required to improve the resolution and connect these timescales. An asterisk (*) indicates that these TF residence measurements were performed in fly cells, not in fly embryos. Abbreviations: h, hours; m, minutes; PIC, preinitiation complex; Pol II, RNA polymerase II; TBP, TATA-binding protein; TF, transcription factor; s, seconds. See [3,7,9,11., 12., 13., 14.,18., 19., 20.,23., 24., 25., 26., 27.,29,32,35,40., 41., 42., 43.,45,47,49,50,53,57,59,63., 64., 65.,76,78,87,91,92,96,100,106,110,111,115., 116., 117.,121].
Transcriptional bursting
To reveal the molecular basis of transcriptional regulation, a powerful approach is to visualize gene activity in real time in single living cells. Such imaging experiments have revealed that most genes are transcribed in bursts with timescales of several minutes in fly embryos, yeast, and bacteria, tens of minutes in nematodes, and tens of minutes to hours in mammalian cells [5] (Figure 1). These bursts are characterized by three parameters: the rate of switching ON, the Pol II initiation rate during the ON state, and the rate of switching OFF [6]. Gene activity can be modulated by changing any of these three rates [3,7,8], and quantification of the bursting parameters with live-cell transcription imaging provides insight into which kinetic steps are regulated by different regulatory mechanisms. As the experimental detection of the bursts has guided its descriptive terminology, we shortly discuss variations in nomenclature (Box 1) to avoid confusion while assessing the literature.
Box 1. Parameters of transcriptional bursting.
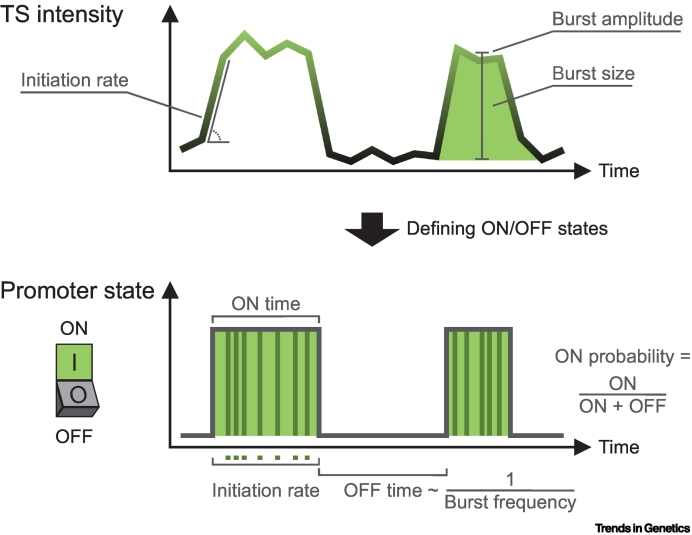
Visualizing fluctuations in RNA synthesis of specific genes over time is most commonly accomplished by labeling nascent RNA with the sensitive MS2 or PP7 labeling system [1]. Production of RNA results in the local accumulation of fluorescence at the transcription site (TS), visible as a bright dot in the nucleus, of which the intensity is proportional to the number of nascent transcripts. These fluorescence intensity time traces reflect the kinetics of transcription initiation and can thus be used to extract several kinetic parameters underlying transcriptional bursting. In the simplest two-state description of bursting, called the random telegraph model, transcriptional bursting can be described with three parameters: the rate of switching ON, the Pol II initiation rate during the ON state, and the rate of switching OFF [6]. However, other nomenclature is often found in the literature, as described in the next paragraphs (Figure I).
Using live-cell RNA imaging, the period a TS is visible is generally referred to as the burst duration, or ON time. Although this ON time is usually interpreted as the time the promoter is actively loading polymerases, it is important to realize that this measurement also includes Pol II elongation, splicing, if applicable, and/or termination and release kinetics. The time during which no TS is visible corresponds to the time between bursts, or OFF time, which is inversely related to the burst frequency. Especially in more complex eukaryotes, this OFF time is frequently composed of multiple OFF states of different duration (i.e., long and short OFF states that likely reflect different molecular compositions) [9,32,121]. Mathematically, one can then define a more complex multistate model with multiple OFF states with additional rates to describe these bursting kinetics. The ON and OFF times together determine the active fraction or ON probability [8,13]. This parameter can also be determined using snapshot approaches, such as smFISH, by determining the number of actively transcribing alleles.
A technically more challenging rate to measure is the initiation rate. One approach is to measure the slope at the beginning of bursts [35], which captures the initial Pol II loading rate but cannot detect fluctuations in initiation rate during the entire burst and is not always reliable at low transcription levels [36]. Promising deconvolution approaches now allow for extraction of the initiation times directly from the entire transcription intensity traces [49,59,122]. As an alternative indirect measure of initiation rate, a commonly used approach is to determine the amplitude or intensity of the transcriptionally active periods. Burst size is defined as the number of polymerases or number of produced RNAs in a burst and depends on both the ON time and the initiation rate. Because of the stochastic nature of transcription, all these parameters show variation over time within a specific distribution, of which the shape is informative about the number of rate-limiting steps in the transitions between states. For example, an exponential distribution is indicative of a single rate-limiting step, while a gamma or multiexponential distribution indicates multiple rate-limiting steps [1].
Figure I.
Parameters of transcriptional bursting.
Top, fluorescence intensity trace of a single transcription site (TS) over time, with initiation rate (number of Pol II/time), burst amplitude (indirect measure of initiation rate), and burst size (number of Pol II per burst). Bottom, obtained promoter states with ON time (burst duration) and OFF time (time between bursts; inversely related to burst frequency). The ON probability is the fraction of time that the promoter is in the ON state.
Alt-text: Box 1
Recent insights suggest substantial variations in the dynamic range of bursting parameters, with burst frequency exhibiting much larger differences between conditions than burst duration or amplitude [3,8,9], suggesting that burst frequency is likely the main regulatory ‘knob’ to control the total RNA output. This finding is in line with a study combining Pol II chromatin immunoprecipitation with single-cell transcription measurements, which showed that burst frequency and pause release, rather than Pol II loading, are key rate-limiting steps for transcriptional regulation [10]. To some degree, the bursting parameters may even be connected, where a certain ON probability defines the other bursting parameters [11., 12., 13.], implying that regulatory mechanisms driving the ON–OFF switching are interdependent. Although these data suggest that bursting parameters are somehow constrained, there is also ample evidence for independent control of each parameter [7,8,14]. Nevertheless, the most extreme combinations of bursting parameters (e.g., large but infrequent bursts) likely do not naturally occur inside cells. In the next section, we highlight the latest insights into how an understanding of transcriptional bursting regulation sheds light on the mechanisms that control gene expression.
The molecular drivers of transcriptional bursting
TF–DNA binding
Gene activation starts with binding of TFs to regulatory DNA sequences in enhancers or promoters of the target gene. Since the first visualization of TF binding dynamics in living cells, it has become clear that TFs bind to DNA transiently [15., 16., 17.]. These studies have sparked the hypothesis that the fluctuations in transcription are driven by fluctuations in TF binding dynamics. In the most simplistic model, binding of a TF to DNA would result in loading of several polymerases, and its release would mark the transition to an OFF period. The TF residence time would thus determine the ON time. In support of this, a correlation between residence time and ON time has been observed for the budding yeast (Saccharomyces cerevisiae) TF Gal4, where a binding site mutation at the endogenous GAL3 promoter reduces both Gal4 DNA residence times in vitro as well as GAL3 ON time in vivo [18]. Similarly, longer residence times of the hormone-activated glucocorticoid receptor correspond to an increased ON time [19]. Also, for other TFs, residence time correlates with transcription levels, suggesting a similar relationship [20., 21., 22.].
However, while the ON times of transcriptional bursts typically range from minutes to hours, the duration of TF–DNA interactions generally is in the timescale of seconds [5,15,16]. How do these relatively short-lasting TF–DNA interactions result in long periods of transcriptional activity if they are not the same duration? One possibility is that the live-cell imaging methods to measure residence time cause bleaching or tracking artifacts and thereby underestimate the true residence time. Although controls are often taken along to prevent such artifacts, these do not completely exclude the existence of a subpopulation of TF–DNA binding events that lasts much longer. Recent studies indeed suggest that a small fraction of TF residence times is much longer than previously thought [23., 24., 25., 26.], partially closing the gap between TF binding and bursting timescales. Using a novel tracking method, our lab recently revealed a previously unidentified population of Gal4 that bound to the GAL10 gene in the minute timescale [27]. However, relating these TF binding events to the transcriptional bursting kinetics of the same gene showed that, despite such long binding events being observed, for the majority of bursts (84%), the Gal4 binding events were still shorter than the GAL10 ON times. Instead, we observed that TFs frequently exchange during a burst (Figure 2). Such exchange could bridge the timescale discrepancy between TF binding and transcription, where individual TF molecules bind shortly but are replaced by others to increase the period of high TF occupancy. TF exchange also implies some form of cooperativity [5], which could arise from cooperative TF binding [28], TF clustering [29], chromatin remodeling [30], or other mechanisms. Cooperativity ensures that the probability of TF binding is higher if TFs are already bound, resulting in bimodal switching between TF high and low occupancy and consequently switching between active and inactive periods [5].
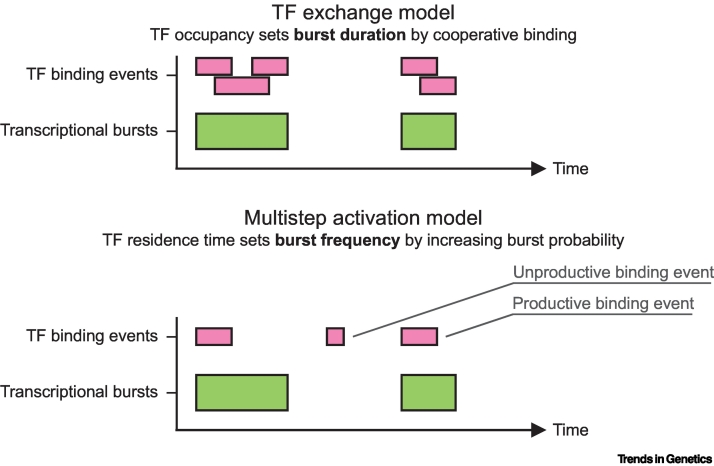
Figure 2.
Models linking transcription factor (TF) binding and transcriptional bursting.
Top, TF exchange model where TF exchange prolongs periods of high TF occupancy at the promoter, thereby increasing the burst duration. Bottom, multistep activation model where longer TF residence times initiate bursts more productively through a multistep transcriptional activation cascade, thereby increasing burst frequency.
Although TF exchange is an attractive model to explain how short binding can result in longer periods of transcription activation, not all TFs bind cooperatively, and other mechanisms likely also exist. Systematically varying TF residence times in a synthetic TF reporter setup with a single TF binding site did not affect ON time but solely affected burst frequency [21]. Based on this finding, it was proposed that TF binding can initiate a multistep gene activation pathway that enables transcription activation and keeps the burst going even after the TF has dissociated, making the TF residence time independent of the ON time (Figure 2). If longer residence times initiate bursts more productively, residence times are correlated with burst frequency [21]. It will be interesting to understand how different TFs use these different kinetic mechanisms. Methods to directly observe the functional effect of the TF binding events on transcription of a target gene will be essential to test these models further [18,19,27].
TF concentration
If TF binding dynamics affect transcriptional fluctuations, it is expected that not only the DNA residence time but also the binding probability contributes to transcriptional bursting regulation. The TF binding probability to a target site depends on the concentration of TF molecules. Surprisingly, measuring transcription of a synthetic TF reporter containing one binding site indicated that variations in TF concentration have relatively little effect on transcriptional bursting compared with variations in TF residence time [21]. Likewise, a twofold reduction in the levels of the budding yeast TF Gal4 does not linearly affect ON and OFF times [7,29], whereas reducing Gal4 residence times does linearly decrease transcription [18]. However, reducing the overall nuclear TF concentration may not reduce the local concentration at the gene to the same degree, especially if the TF is concentrated in clusters, as for Gal4 [29]. Indeed, clusters of Taf1 and Med19 buffer changes in nuclear concentration, as a twofold reduction in the global nuclear concentration does not change Klf4 burst frequency, but changes in the local Taf1 and Med19 concentration correlate linearly with burst frequency [9]. Moreover, there is abundant evidence that changes in TF concentration regulate gene expression by affecting several parameters of transcriptional bursting, as exemplified in the next paragraphs.
Since concentration directly affects the association rate of TFs, the most straightforward scenario is that increased TF association is linked to increased probability of transcription. Accordingly, activation of steroid receptors with different doses regulates the duration of the OFF state with little effect on the ON time at both a synthetic and endogenous gene [31,32]. Similarly, live-cell imaging of the tumor suppressor p53 and mRNA synthesis of its target gene p21 upon ionizing radiation showed that p53 levels modulate the frequency of p21 bursts [33], agreeing with time-resolved single-molecule RNA fluorescence in situ hybridization (smFISH) measurements [34]. At very low concentrations, the p53 levels also scale with the burst amplitude but quickly reach a maximum amplitude at higher p53 levels, suggesting that the initiation rate during a burst is rapidly saturated [33].
Similarly, during Drosophila melanogaster embryonic development, the Bicoid (Bcd) morphogen gradient tunes the initiation rate and ON probability to modulate transcription levels along the anterior–posterior axis. In addition, to obtain a sharp expression boundary of target genes, nuclei display an all-or-none response, where the fraction of active nuclei differs along the TF gradient [35]. How does such a binary response arise? Based on modeling and measuring transcription rates from various Dorsal binding site affinities, it was proposed that all-or-none responses originate from rapid nuclear cycles in combination with a kinetic barrier model, where the promoter has to undergo multiple kinetic barriers to transition from a transcriptionally OFF state to a transcriptionally ON state. Dorsal regulates the transition rates across the kinetic barriers, which thereby determines whether nuclei are able to activate within the limited time window before mitosis [36]. Moreover, cooperativity of Bcd binding acts to create a nonlinear response and to sharpen the boundary along the gradient [37,38]. In addition, Zelda and Hunchback were found to help sharpen the boundary, where Zelda lowers the Bcd concentration threshold for transcription activation, and Hunchback reduces stochastic ON–OFF switching and increases the Pol II initiation rate [39]. Besides sensing TF concentration, gene expression during Drosophila development can be induced at defined times after a previous regulatory event, as shown for Fushi tarazu (Ftz) expression [40]. Multiple mechanisms thus allow the TF gradient to facilitate precise responses during development.
In addition to regulating the frequency of transcription or the fraction of active cells, recent studies suggest that TF concentration can regulate the ON time. Both in Caenorhabditis elegans and in Drosophila, Notch signaling modulates the duration of the ON state [41,42]. Cooperative binding of the Notch intracellular domain to paired motifs is required to create longer bursts, which would be in line with the TF exchange model proposed in the previous section. Loss of cooperative interactions or reduced TF concentration may result in reduced TF exchange and thus shorter bursts. Another example of TF concentration affecting ON time rather than OFF time comes from the response to overexpression of the oncogenic TF MYC [43]. Genome-wide increased ON times are associated with changed residence times of factors associated with PIC assembly and Pol II pause release. Although the connection between PIC stability and transcription dynamics remains to be established (see later), these results suggest that MYC exerts its oncogenic function by altering the DNA-binding stability of the basal transcription machinery, resulting in prolonged transcriptional activation [43].
Pol II and PIC
Transcription initiation requires the simultaneous presence of many proteins at the promoter, including GTFs, coactivators, and subunits of Pol II. How do PIC assembly dynamics regulate transcription dynamics? Based on early in vitro biochemical experiments, it was proposed that part of the PIC stays bound to the promoter after initiation to support multiple rounds of transcription [44]. Such a ‘reinitiation complex’, stabilized by TF binding, could potentially promote bursts of several polymerases. In that case, the timescale of PIC–DNA binding should match the timescale of ON periods. Recent single-molecule tracking in budding yeast indicated that many PIC components have a residence time of around 2–10 s on DNA in living cells, suggesting that PIC assembly, RNA Pol II initiation, and promoter escape can occur within a few seconds [45]. These PIC timescales are closer to the timescales of initiation rates than ON times (Figure 1), suggesting continuous PIC assembly and disassembly for each initiation event rather than stable PIC binding throughout the entire burst.
However, the story may be different for TATA-binding protein (TBP). In the same single-molecule tracking study, TBP binding was much longer than the binding of other PIC components [45]. Although this binding measurement could be dominated by long binding at Pol I or Pol III promoters [46], another interpretation is that TBP may indeed remain bound for multiple rounds of transcription. Such long TBP binding is supported by the ability of TBP to compete with nucleosomes at TATA-containing Pol II promoters and its ability to act as a mitotic bookmarking factor [7,47]. In addition, only ∼10% of budding yeast genes are thought to show bursting transcription, and this has been linked to the presence of a TATA box [48]. In several organisms, mutation of the TATA box shortens the burst duration or the fraction of time with high initiation rates [7,49., 50., 51.], suggesting a direct link between TBP stability and ON time. Moreover, upon MYC overexpression, genome-wide ON times increase, which correlate with longer TBP residence times [43]. TBP may thus keep the promoter in the ON state to promote multiple transcription events (Figure 3).
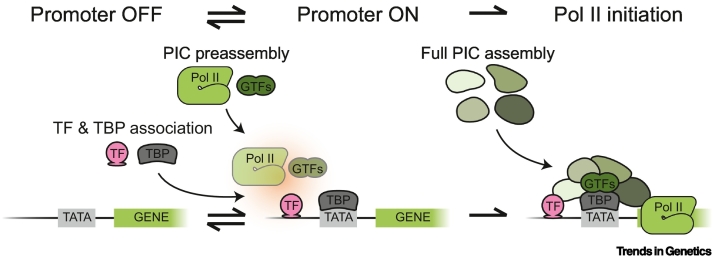
Figure 3.
Hypothesized dynamics of preinitiation complex (PIC) assembly during transcriptional bursting.
Proposed model describing the sequential assembly of multiple proteins at the promoter before transcription initiation. When the promoter progresses from the OFF to the ON state, transcription factors (TFs) and possibly TATA-binding protein (TBP) associate at the promoter. In addition, it has been suggested that several general TFs (GTFs) and RNA polymerase II (Pol II) are recruited to DNA-bound TFs to enable fast PIC assembly. During these ON periods, full PIC assembly results in Pol II initiation and RNA production.
Still, recruitment of the remaining PIC components needs to be rapid. At the GAL10 gene in budding yeast, we observed a transcription initiation rate of one Pol II per second, suggesting highly efficient and rapid Pol II loading [27]. Instead of recruiting all the PIC components separately and sequentially at the promoter, single-molecule in vitro studies in yeast extracts indicated that Pol II and several GTFs (TFIIF and TFIIE) preassemble at DNA-bound TFs, independent of binding to the promoter [52]. This prerecruitment creates an increased local concentration of PIC components that might promote rapid PIC assembly and transcriptional bursting (Figure 3). In addition, interactions between the carboxy-terminal domains of multiple Pol II molecules and interactions with Mediator may enable fast Pol II recruitment to target genes [53,54]. These mechanisms ensure that the recruitment of Pol II is not a biologically limiting factor [10,33,55].
After Pol II initiation and promoter escape, metazoan genes display Pol II promoter-proximal pausing, forming an additional layer of gene regulation [10,56]. Single-molecule footprinting and live-cell Pol II–GFP (green fluorescent protein) imaging experiments have revealed that Pol II pausing mostly represents continuous initiation and early termination of Pol II rather than stable Pol II stalling [57,58]. In agreement, recent live-cell imaging of HIV transcription and of Drosophila genes driven by an initiator-containing promoter indicated that pausing is not obligatory for each transcription round but that only a fraction of Pol II stochastically enters the paused state [49,59]. These live-cell imaging studies exemplify how residence times of PIC components, PIC assembly kinetics, and pause release regulate transcription dynamics at different steps. Future development of advanced live-cell microscopy methods to measure binding dynamics of PIC components at single genes will facilitate dissection of these steps.
Local genome architecture
Chromatin
Binding of TFs and the transcriptional machinery is affected by chromatin. Nucleosomes can form transcriptional obstacles, as they prevent PIC formation in vivo [45] and perturb TF binding in vitro by reducing the stability of TFs on DNA [60,61]. The local chromatin environment is therefore primed for transcriptional activation by chromatin remodelers, which slide and/or evict nucleosomes. While histone proteins have traditionally been viewed as relatively stable on DNA, new evidence suggests that histone exchange at promoters occurs every few minutes [62]. In this timescale, multiple nucleosome remodelers visit a single gene repeatedly, with residence times of a few seconds [63,64], suggesting that continuous nucleosome movement, assembly, and disassembly influence the ON–OFF switching of transcription. Indeed, dynamic nucleosome remodeling by the remodeler RSC is required to enable efficient TF binding for the start of each transcriptional burst [7,65]. At the TATA box of the inducible GAL10 gene in budding yeast, nucleosomes need to be removed to allow for TBP binding before the first burst only [7]. The kinetics of nucleosome movement and exchange thus influence transcription dynamics.
In addition, since nucleosome remodeling consumes ATP, it has been proposed that this energy expenditure could drive the gene regulatory system into a nonequilibrium state [66,67]. For example, instead of TF dissociation being determined by equilibrium kinetics (association/dissociation rates), TF binding at high-affinity binding sites would lead to energy-dependent chromatin remodeling that eventually leads to eviction of the TF from DNA. In agreement, remodeler-dependent TF dissociation has been observed for the mammalian glucocorticoid receptor and the yeast TFs Ace1 and Gal4 [65,68,69]. Moreover, the distribution of transcriptional ON states of the yeast PHO5 promoter was recently shown to be consistent with such a nonequilibrium model [70]. The benefit of this energy expenditure is that it enables ‘kinetic proofreading’, which provides specificity while maintaining high responsiveness from dynamic TF–DNA interactions. Nonequilibrium energy-dependent steps can also make a system more sensitive and allow for more complex signal processing [71,72].
Besides the spatial positioning by nucleosome remodelers, the local chromatin architecture can be altered by histone modifications. For example, upon binding to enhancers, TFs recruit histone acetyltransferases, such as p300. p300 acetylates H3K27, other histone sites, and nonhistone proteins, resulting in recruitment of the bromodomain-containing protein BRD4, which in turn brings P-TEFb to promoters to enable Pol II promoter escape and pause release [73., 74., 75., 76.]. In addition, p300-mediated acetylation antagonizes liquid–liquid phase separation of chromatin to increase accessibility, and clustering of p300 promotes recruitment of additional TFs [77,78], both of which may act as a positive feedback loop for transcription initiation [76]. Clusters of p300 thereby support a faster initiation rate and an increased ON time [78,79]. Conversely, for the Bmal1 circadian promoter and an HIV reporter, p300 recruitment influences mostly the OFF time [8,80]. These differences may come from the different activating mechanisms of p300, where the effects on TF recruitment, chromatin phase separation, BRD4 recruitment, and Pol II pause release depend on histone acetylation, but p300 clustering itself can also facilitate TF binding independent of acetylation [78]. Another hint that acetylation can shape transcription dynamics comes from the model organism Neurospora crassa, where recruitment of the histone deacetylase HDA3 by the White Collar Complex TF results in a long refractory period, which limits transcriptional bursting [81]. The rapid turnover of acetylation (median time of around 90 min, with a subset of sites below 30 min) suggests that dynamic cycles of acetylation and deacetylation contribute to the transcriptional bursting kinetics [75]. Nonetheless, further experiments are needed to decipher whether histone acetylation and other modifications instruct dynamic transcription patterns.
Clustering
In recent years, many components of the transcriptional machinery have been shown to form areas of high local concentration, referred to as clusters, hubs, condensates, or droplets. It was proposed that the high concentration of TFs, cofactors, and Pol II near target genes would allow bursts of transcription [82]. Although there are numerous examples that clustering facilitates transcription [83., 84., 85., 86.], clustering does not always correlate with transcription [87,88] and has even been found to inhibit transcription [22,29,89]. This discrepancy may be the result of the multitude of effects that clustering has on different processes (Figure 4). For example, multivalent homotypic and heterotypic interactions underlying clustering facilitate recruitment of TFs, cofactors, and PIC components to target genes, stabilize TF binding to DNA, enable 3D genome interactions between target genes, and increase the local concentration of the transcriptional machinery to support fast PIC assembly and Pol II loading [78,83,90., 91., 92., 93., 94.]. However, clusters in the nucleoplasm may titrate factors from target genes [22,89]. Similarly, interactions within a cluster may cause competition between productive and nonproductive interactions [29]. These findings suggest that there is an optimal degree of clustering, which may differ per system and therefore results in different effects of clustering on transcription. For example, for the budding yeast TF Gal4, self-interactions enable recruitment of Gal4 to a target gene, but clustered molecules that are not DNA bound shorten the ON time and prolong the OFF time [29]. In contrast, in Drosophila embryos, addition of polyQ tracts to TFs to increase clustering results in more frequent and higher intensity bursts [84]. These examples highlight our limited comprehension of the impact of clustering on transcription. They also indicate that clustering is not the sole driver of bursting. Nevertheless, there are emerging examples where clustering plays a role in shaping bursting patterns, some of which we will discuss below.
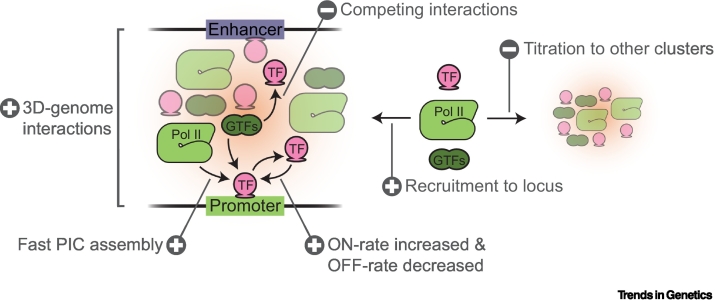
Figure 4.
The effect of clustering on transcription.
Clustering can promote transcription by facilitating 3D genome interactions, fast assembly of transcriptional proteins at the promoter, increasing transcription factor (TF) ON rates, decreasing TF OFF rates, and recruiting transcriptional proteins. However, clustering needs to be regulated since competing interactions between transcriptional proteins within clusters and their titration to other (nucleoplasmic) clusters may inhibit transcriptional activation. TFs, general TFs (GTFs), and RNA polymerase II (Pol II) are indicated at the different mechanisms.
During embryonic development of zebrafish and Drosophila, clusters form sequentially, starting with TFs, followed by cofactors and eventually resulting in initiating and elongating Pol II clusters [95,96]. Interestingly, in Drosophila, Pol II clusters are dissolved by the act of transcription, suggesting that Pol II elongation or nascent RNA disperse Pol II clusters and limit further initiation [96,97]. Novel expansion microscopy revealed a potential mechanism of this negative feedback where Pol II elongation results in release of the E–P contacts [97]. Additionally, high concentrations of RNA can dissolve clusters, resulting in a negative feedback loop that limits burst sizes [98]. Other mechanisms may potentially also contribute to this feedback. For example, one possibility is that the dissolution of the cluster in these examples is caused by DNA supercoiling generated by transcription, which can destabilize DNA-bound factors and block transcription [99,100]. In addition, such a negative feedback loop may arise if these clusters coactivate noncoding transcription at enhancers, which then dislodges DNA-bound TFs and dissolves the cluster [101]. Apart from gaining a more detailed understanding of the underlying mechanisms, future studies are needed to reveal the extent to which these self-limiting bursts are prevalent across different contexts.
Clustering may be one of the mechanisms by which enhancers communicate with promoters. An exciting single-molecule nanoscopy system has revealed that enhancers often contain nearby clusters of 5–20 transcription and regulatory molecules, such as Mediator, BRD4, and Cdk9 [76,102]. Whereas Mediator clusters remain present irrespective of transcription, clusters of BRD4 and Pol II are mostly observed during periods of active transcription [103], suggesting that their formation may be associated with the ON time. Encounters of promoters with these enhancer clusters may facilitate the start of a burst [9]. The dynamics of the clusters and the multitude of effects they have on different transcriptional processes underscore the importance of such novel live-cell imaging techniques to further elucidate the role of clustering in temporal gene regulation.
E–P proximity
In addition to clustering, other nonmutually exclusive models have been suggested to explain how enhancers communicate with promoters over large genomic distances. For instance, enhancers may locally activate TFs in a gradient around the enhancer by p300 acetylation, after which activated TFs diffuse to nearby promoters [104]. Moreover, an enhancer may be brought within proximity of its promoter by cohesin-mediated loop extrusion [105]. Although a full discussion of the different enhancer models is outside the scope of this review, the next section will discuss some recent studies of how E–P contacts regulate bursting, providing unique insight into the kinetic steps that enhancers regulate.
One of the main questions in the field has been how E–P distance affects transcriptional bursting. In Drosophila embryos, visualizing E–P distance and transcription in live cells showed that sustained E–P proximity is required for transcription activation [106]. However, similar attempts at other Drosophila genes and in mammalian cells were less successful and detected no correlation [107., 108., 109.]. This discrepancy may arise from larger time delays between E–P contact and transcriptional activation or from different chromosome dynamics, where most E–P interactions may be more transient than the stable homie interactions in Drosophila [106]. Accordingly, live-cell imaging revealed that cohesin-mediated loops in mammalian cells are dynamic, where the loop lifetime is, on average, 5–30 min in the presence of convergent CTCF sites and 5 min in the absence of CTCF sites [110,111]. Although these experiments were performed in the absence of enhancers or promoters, it is interesting to note that these timescales are comparable with or slightly shorter than reported ON times. It thus remains unclear whether an E–P contact is required throughout the burst, to only initiate it, or even whether a single contact is sufficient for burst initiation [112].
Besides direct visualization, a powerful approach has been to modulate the E–P contact probability. Forced chromatin looping, changing the genomic distance between a promoter and enhancer, degrading cohesin, or deleting enhancers mostly affects the burst frequency, indicating that enhancers mostly modulate the initiation of bursts [9,32,113., 114., 115.]. Comparing E–P distances and bursting patterns with and without cohesin degradation suggested that cohesin promotes E–P interactions within 200 nm to stimulate transcription activation [9]. Varying E–P distances genetically showed that the E–P contact probability and burst frequency followed a nonlinear relationship, suggesting a multistep process where multiple enhancer contacts may be required to initiate a burst of transcription [115]. Additionally, examples of ON time regulation by enhancers are also emerging [41], illustrating that much is still unknown. It will be exciting to dissect the exact steps that enhancers control to activate transcription. Comparing the timescales of loop extrusion at enhancers and promoters, cluster formation, E–P proximity, and transcriptional bursting will help to uncover the mechanisms that mediate E–P communication.
Gene coupling
Recent literature also reveals increasing examples of gene pairs showing coupled transcription. In budding yeast, coupled bursting is observed at neighboring GAL genes [100]. Although coupling is strongest for GAL genes in the divergent orientation, tandem genes also show some degree of coupled initiation. It is possible that Gal4 clustering results in simultaneous Gal4 binding to the promoters of these genes. Accordingly, coupling reduces upon accumulation of DNA supercoils, which destabilizes Gal4 binding [100]. In Drosophila, genes regulated by the same enhancer show coupled transcriptional bursting [116], which may arise from a shared TF cluster forming around the enhancer [84]. Additionally, coupled transcription occurs for paralogous Drosophila genes at large genomic distance [117]. These genes come in close proximity by tethering elements in the promoter, suggesting that this proximity enables the formation of a shared cluster [117,118].
In mammalian cells, analysis of DNA distances and nascent RNA of hundreds of genes showed that coupling between genes depends on physical distance rather than genomic distance [119]. Similarly, proximity mediated by cohesin also resulted in coupling between enhancer and promoter transcription [114]. The large scale of coupling between genes suggests that coupling could simply be the result of proximity and may not necessarily have a function. Nevertheless, even without a direct role for transcriptional coupling, studying the interplay between genes may serve as a valuable readout to uncover the mechanisms of transcription regulation.
Concluding remarks
As illustrated by the examples earlier, comparing the timescales of molecular events leading to transcription (such as TF binding, PIC assembly, and chromatin remodeling) with the kinetic readout of transcription (ON time, OFF time, and initiation rate) is a powerful method to dissect which regulatory processes are rate‐limiting (Figure 1). Matching timescales suggest a regulatory relationship between input and output, although correlation does not prove causation. Such a kinetic view also reveals how regulatory factors control different transcriptional steps to ensure tight control of gene expression. However, a current limitation in this timescale comparison is that most kinetic measurements of regulatory factor binding or action are global and not gene specific. Furthermore, the measurements of transcription dynamics are often limited to one or two genes, challenging straightforward interpretation and generalization. Recent developments in microscopy now allow measurements of cluster composition and TF dynamics at a single gene [18,19,27,76], which will be essential to dissect the kinetics of the regulatory steps (see Outstanding questions). Future advances to visualize multiple regulatory factors simultaneously at a single locus in vivo, similar to what is currently done in vitro [52], would create exciting opportunities in unraveling the order of events, cooperativity, and dependencies at an unprecedented level. As more and more quantitative measurements will become available and the ability to capture interdependencies between regulatory factors increases, we expect that the complexity of the system will increasingly demand mathematical and physical modeling to provide a thorough and quantitative understanding of the regulatory processes [5,115]. In addition, the presence of energy expending mechanisms in transcription demands new kinetic models to investigate the nonequilibrium nature of eukaryotic gene regulation [67,71,72,120]. Last, the ability to specifically edit mammalian genomes has substantially increased in recent years, allowing for more mechanistic studies, but it nevertheless remains essential to continue to use diverse model organisms to exploit the natural variation in nuclear environments, 3D genome architecture, and regulatory complexity to reveal the scope of possible regulatory mechanisms at different scales. Overall, novel tools and continued efforts in the field will pave the way for a quantitative and kinetic understanding of transcription regulation.
Outstanding questions.
Which kinetic mechanisms are used by cells to connect transient binding of transcriptional proteins with long transcriptional bursts?
Does a reinitiation complex exist in vivo?
How do nucleosome remodeling and modifications shape transcription dynamics?
How do enhancers communicate with promoters and how does this communication result in bursts of transcription?
How does the spatial clustering of regulatory factors contribute to different transcription regulatory steps?
How does gene coupling arise and does coupled bursting serve a function?
Alt-text: Outstanding questions
Acknowledgments
Acknowledgments
We thank Simona Antonova and Wim de Jonge for critical reading of the manuscript. This work was supported by Oncode Institute, which is partly financed by the Dutch Cancer Society, the European Research Council (ERC Starting Grant 755695 BURSTREG), the Dutch Research Council (gravitation program CancerGenomiCs.nl), and an institutional grant of the Dutch Cancer Society and of the Dutch Ministry of Health, Welfare and Sport.
Declaration of interests
The authors declare no competing interests.
References
- 1.Lenstra T.L., et al. Transcription dynamics in living cells. Annu. Rev. Biophys. 2016;45:25–47. doi: 10.1146/annurev-biophys-062215-010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tunnacliffe E., Chubb J.R. What is a transcriptional burst? Trends Genet. 2020;36:288–297. doi: 10.1016/j.tig.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Wan Y., et al. Dynamic imaging of nascent RNA reveals general principles of transcription dynamics and stochastic splice site selection. Cell. 2021;184:2878–2895. doi: 10.1016/j.cell.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsson A.J.M., et al. Genomic encoding of transcriptional burst kinetics. Nature. 2019;565:251–254. doi: 10.1038/s41586-018-0836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammers N.C., et al. A matter of time: using dynamics and theory to uncover mechanisms of transcriptional bursting. Curr. Opin. Cell Biol. 2020;67:147–157. doi: 10.1016/j.ceb.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peccoud J., Ycart B. Markovian modeling of gene-product synthesis. Theor. Popul. Biol. 1995;48:222–234. [Google Scholar]
- 7.Brouwer I., et al. Dynamic epistasis analysis reveals how chromatin remodeling regulates transcriptional bursting. Nat. Struct. Mol. Biol. 2023;30:692–702. doi: 10.1038/s41594-023-00981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mamrak N.E., et al. The kinetic landscape of human transcription factors. bioRxiv. 2022 doi: 10.1101/2022.06.01.494187. Published online June 3, 2022. [DOI] [Google Scholar]
- 9.Cheng L., et al. Mechanisms of transcription control by distal enhancers from high-resolution single-gene imaging. bioRxiv. 2023 doi: 10.1101/2023.03.19.533190. Published online March 21, 2023. [DOI] [Google Scholar]
- 10.Bartman C.R., et al. Transcriptional burst initiation and polymerase pause release are key control points of transcriptional regulation. Mol. Cell. 2019;73:519–532. doi: 10.1016/j.molcel.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berrocal A., et al. Unified bursting strategies in ectopic and endogenous even-skipped expression patterns. bioRxiv. 2023 doi: 10.1101/2023.02.09.527927. Published online February 10, 2023. [DOI] [Google Scholar]
- 12.Chen P.-T., et al. Gene activity as the predictive indicator for transcriptional bursting dynamics. arXiv. 2023 doi: 10.48550/arXiv.2304.08770. Published online April 18, 2023. [DOI] [Google Scholar]
- 13.Zoller B., et al. Diverse spatial expression patterns emerge from unified kinetics of transcriptional bursting. Cell. 2018;175:835–847. doi: 10.1016/j.cell.2018.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoshi M., et al. Dynamic modulation of enhancer responsiveness by core promoter elements in living Drosophila embryos. Nucleic Acids Res. 2022;50:92–107. doi: 10.1093/nar/gkab1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jonge W.J., et al. Following the tracks: how transcription factor binding dynamics control transcription. Biophys. J. 2022;121:1583–1592. doi: 10.1016/j.bpj.2022.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzocca M., et al. Transcription factor binding kinetics and transcriptional bursting: what do we really know? Curr. Opin. Struct. Biol. 2021;71:239–248. doi: 10.1016/j.sbi.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Wagh K., et al. Transcription factor dynamics: one molecule at a time. Annu. Rev. Cell Dev. Biol. 2023;39:277–305. doi: 10.1146/annurev-cellbio-022823-013847. [DOI] [PubMed] [Google Scholar]
- 18.Donovan B.T., et al. Live-cell imaging reveals the interplay between transcription factors, nucleosomes, and bursting. EMBO J. 2019;38 doi: 10.15252/embj.2018100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stavreva D.A., et al. Transcriptional bursting and co-bursting regulation by steroid hormone release pattern and transcription factor mobility. Mol. Cell. 2019;75:1161–1177. doi: 10.1016/j.molcel.2019.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loffreda A., et al. Live-cell p53 single-molecule binding is modulated by C-terminal acetylation and correlates with transcriptional activity. Nat. Commun. 2017;8:313. doi: 10.1038/s41467-017-00398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popp A.P., et al. Altering transcription factor binding reveals comprehensive transcriptional kinetics of a basic gene. Nucleic Acids Res. 2021;49:6249–6266. doi: 10.1093/nar/gkab443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trojanowski J., et al. Transcription activation is enhanced by multivalent interactions independent of phase separation. Mol. Cell. 2022;82:1878–1893. doi: 10.1016/j.molcel.2022.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Garcia D.A., et al. Power-law behavior of transcription factor dynamics at the single-molecule level implies a continuum affinity model. Nucleic Acids Res. 2021;49:6605–6620. doi: 10.1093/nar/gkab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hipp L., et al. Single-molecule imaging of the transcription factor SRF reveals prolonged chromatin-binding kinetics upon cell stimulation. Proc. Natl. Acad. Sci. U. S. A. 2019;116:880–889. doi: 10.1073/pnas.1812734116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang X., et al. Kinetic principles underlying pioneer function of GAGA transcription factor in live cells. Nat. Struct. Mol. Biol. 2022;29:665–676. doi: 10.1038/s41594-022-00800-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellec M., et al. The control of transcriptional memory by stable mitotic bookmarking. Nat. Commun. 2022;13:1176. doi: 10.1038/s41467-022-28855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pomp W., et al. Transcription factor exchange enables prolonged transcriptional bursts. bioRxiv. 2023 doi: 10.1101/2023.05.15.540758. Published online May 15, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang T., et al. Wild type GAL4 binds cooperatively to the GAL1-10 UASG in vitro. J. Biol. Chem. 1993;268:9629–9635. [PubMed] [Google Scholar]
- 29.Meeussen J.V.W., et al. Transcription factor clusters enable target search but do not contribute to target gene activation. Nucleic Acids Res. 2023;51:5449–5468. doi: 10.1093/nar/gkad227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sönmezer C., et al. Molecular co-occupancy identifies transcription factor binding cooperativity in vivo. Mol. Cell. 2021;81:255–267. doi: 10.1016/j.molcel.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson D.R., et al. Direct observation of frequency modulated transcription in single cells using light activation. eLife. 2013;2 doi: 10.7554/eLife.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez J., et al. Intrinsic dynamics of a human gene reveal the basis of expression heterogeneity. Cell. 2019;176:213–226. doi: 10.1016/j.cell.2018.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hafner A., et al. Quantifying the central dogma in the p53 pathway in live single cells. Cell Syst. 2020;10:495–505. doi: 10.1016/j.cels.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedrich D., et al. Stochastic transcription in the p53-mediated response to DNA damage is modulated by burst frequency. Mol. Syst. Biol. 2019;15 doi: 10.15252/msb.20199068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia H.G., et al. Quantitative imaging of transcription in living Drosophila embryos links polymerase activity to patterning. Curr. Biol. 2013;23:2140–2145. doi: 10.1016/j.cub.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alamos S., et al. Minimal synthetic enhancers reveal control of the probability of transcriptional engagement and its timing by a morphogen gradient. Cell Syst. 2023;14:220–236. doi: 10.1016/j.cels.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas T., et al. 3 minutes to precisely measure morphogen concentration. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tran H., et al. Precision in a rush: trade-offs between reproducibility and steepness of the hunchback expression pattern. PLoS Comput. Biol. 2018;14 doi: 10.1371/journal.pcbi.1006513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandes G., et al. Synthetic reconstruction of the hunchback promoter specifies the role of Bicoid, Zelda and Hunchback in the dynamics of its transcription. eLife. 2022;11 doi: 10.7554/eLife.74509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birnie A., et al. Precisely timed regulation of enhancer activity defines the binary expression pattern of Fushi tarazu in the Drosophila embryo. Curr. Biol. 2023;33:2839–2850. doi: 10.1016/j.cub.2023.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falo-Sanjuan J., et al. Enhancer priming enables fast and sustained transcriptional responses to Notch signaling. Dev. Cell. 2019;50:411–425. doi: 10.1016/j.devcel.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee C., et al. Dynamics of Notch-dependent transcriptional bursting in its native context. Dev. Cell. 2019;50:426–435. doi: 10.1016/j.devcel.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patange S., et al. MYC amplifies gene expression through global changes in transcription factor dynamics. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2021.110292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yudkovsky N., et al. A transcription reinitiation intermediate that is stabilized by activator. Nature. 2000;408:225–229. doi: 10.1038/35041603. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen V.Q., et al. Spatiotemporal coordination of transcription preinitiation complex assembly in live cells. Mol. Cell. 2021;81:3560–3575. doi: 10.1016/j.molcel.2021.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasegawa Y., Struhl K. Promoter-specific dynamics of TATA-binding protein association with the human genome. Genome Res. 2019;29:1939–1950. doi: 10.1101/gr.254466.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teves S.S., et al. A stable mode of bookmarking by TBP recruits RNA polymerase II to mitotic chromosomes. eLife. 2018;7 doi: 10.7554/eLife.35621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hornung G., et al. Noise–mean relationship in mutated promoters. Genome Res. 2012;22:2409–2417. doi: 10.1101/gr.139378.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pimmett V.L., et al. Quantitative imaging of transcription in living Drosophila embryos reveals the impact of core promoter motifs on promoter state dynamics. Nat. Commun. 2021;12:4504. doi: 10.1038/s41467-021-24461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tantale K., et al. A single-molecule view of transcription reveals convoys of RNA polymerases and multi-scale bursting. Nat. Commun. 2016;7:12248. doi: 10.1038/ncomms12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corrigan A.M., et al. A continuum model of transcriptional bursting. eLife. 2016;5 doi: 10.7554/eLife.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baek I., et al. Single-molecule studies reveal branched pathways for activator-dependent assembly of RNA polymerase II pre-initiation complexes. Mol. Cell. 2021;81:3576–3588. doi: 10.1016/j.molcel.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ling Y.H., et al. Disordered C-terminal domain drives spatiotemporal confinement of RNAPII to enhance search for chromatin targets. bioRxiv. 2023 doi: 10.1101/2023.07.31.551302. Published online November 30, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quintero-Cadena P., et al. RNA Pol II length and disorder enable cooperative scaling of transcriptional bursting. Mol. Cell. 2020;79:207–220. doi: 10.1016/j.molcel.2020.05.030. [DOI] [PubMed] [Google Scholar]
- 55.Rosen G.A., et al. Dynamics of RNA polymerase II and elongation factor Spt4/5 recruitment during activator-dependent transcription. Proc. Natl. Acad. Sci. U. S. A. 2020;117:32348–32357. doi: 10.1073/pnas.2011224117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Core L., Adelman K. Promoter-proximal pausing of RNA polymerase II: a nexus of gene regulation. Genes Dev. 2019;33:960–982. doi: 10.1101/gad.325142.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steurer B., et al. Live-cell analysis of endogenous GFP-RPB1 uncovers rapid turnover of initiating and promoter-paused RNA polymerase II. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E4368–E4376. doi: 10.1073/pnas.1717920115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krebs A.R., et al. Genome-wide single-molecule footprinting reveals high RNA polymerase II turnover at paused promoters. Mol. Cell. 2017;67:411–422. doi: 10.1016/j.molcel.2017.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tantale K., et al. Stochastic pausing at latent HIV-1 promoters generates transcriptional bursting. Nat. Commun. 2021;12:4503. doi: 10.1038/s41467-021-24462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mivelaz M., et al. Chromatin fiber invasion and nucleosome displacement by the Rap1 transcription factor. Mol. Cell. 2020;77:488–500. doi: 10.1016/j.molcel.2019.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo Y., et al. Nucleosomes accelerate transcription factor dissociation. Nucleic Acids Res. 2014;42:3017–3027. doi: 10.1093/nar/gkt1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yaakov G., et al. Measurement of histone replacement dynamics with genetically encoded exchange timers in yeast. Nat. Biotechnol. 2021;39:1434–1443. doi: 10.1038/s41587-021-00959-8. [DOI] [PubMed] [Google Scholar]
- 63.Kenworthy C.A., et al. Bromodomains regulate dynamic targeting of the PBAF chromatin-remodeling complex to chromatin hubs. Biophys. J. 2022;121:1738–1752. doi: 10.1016/j.bpj.2022.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim J.M., et al. Single-molecule imaging of chromatin remodelers reveals role of ATPase in promoting fast kinetics of target search and dissociation from chromatin. eLife. 2021;10 doi: 10.7554/eLife.69387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mehta G.D., et al. Single-molecule analysis reveals linked cycles of RSC chromatin remodeling and Ace1p transcription factor binding in yeast. Mol. Cell. 2018;72:875–887. doi: 10.1016/j.molcel.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coulon A., et al. Eukaryotic transcriptional dynamics: from single molecules to cell populations. Nat. Rev. Genet. 2013;14:572–584. doi: 10.1038/nrg3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong F., Gunawardena J. Gene regulation in and out of equilibrium. Annu. Rev. Biophys. 2020;49:199–226. doi: 10.1146/annurev-biophys-121219-081542. [DOI] [PubMed] [Google Scholar]
- 68.Fletcher T.M., et al. ATP-dependent mobilization of the glucocorticoid receptor during chromatin remodeling. Mol. Cell Biol. 2002;22:3255–3263. doi: 10.1128/MCB.22.10.3255-3263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li M., et al. Dynamic regulation of transcription factors by nucleosome remodeling. eLife. 2015;4 doi: 10.7554/eLife.06249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shelansky R., et al. A telltale sign of irreversibility in transcriptional regulation. bioRxiv. 2022 doi: 10.1101/2022.06.27.497819. Published online June 28, 2022. [DOI] [Google Scholar]
- 71.Lammers N.C., et al. Competing constraints shape the nonequilibrium limits of cellular decision-making. Proc. Natl. Acad. Sci. U. S. A. 2023;120 doi: 10.1073/pnas.2211203120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahdavi S., et al. Flexibility and sensitivity in gene regulation out of equilibrium. bioRxiv. 2023 doi: 10.1101/2023.04.11.536490. Published online April 13, 2023. [DOI] [Google Scholar]
- 73.Narita T., et al. Enhancers are activated by p300/CBP activity-dependent PIC assembly, RNAPII recruitment, and pause release. Mol. Cell. 2021;81:2166–2182. doi: 10.1016/j.molcel.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Stasevich T.J., et al. Regulation of RNA polymerase II activation by histone acetylation in single living cells. Nature. 2014;516:272–275. doi: 10.1038/nature13714. [DOI] [PubMed] [Google Scholar]
- 75.Weinert B.T., et al. Time-resolved analysis reveals rapid dynamics and broad scope of the CBP/p300 acetylome. Cell. 2018;174:231–244. doi: 10.1016/j.cell.2018.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li J., et al. Single-molecule nanoscopy elucidates RNA polymerase II transcription at single genes in live cells. Cell. 2019;178:491–506. doi: 10.1016/j.cell.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gibson B.A., et al. Organization of chromatin by intrinsic and regulated phase separation. Cell. 2019;179:470–484. doi: 10.1016/j.cell.2019.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma L., et al. Co-condensation between transcription factor and coactivator p300 modulates transcriptional bursting kinetics. Mol. Cell. 2021;81:1682–1697. doi: 10.1016/j.molcel.2021.01.031. [DOI] [PubMed] [Google Scholar]
- 79.Fraser L.C.R., et al. Reduction in gene expression noise by targeted increase in accessibility at gene loci. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2018640118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicolas D., et al. Modulation of transcriptional burst frequency by histone acetylation. Proc. Natl. Acad. Sci. U. S. A. 2018;115:7153–7158. doi: 10.1073/pnas.1722330115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oehler M., et al. Transcription activator WCC recruits deacetylase HDA3 to control transcription dynamics and bursting in Neurospora. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adh0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hnisz D., et al. A phase separation model for transcriptional control. Cell. 2017;169:13–23. doi: 10.1016/j.cell.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chowdhary S., et al. Inducible transcriptional condensates drive 3D genome reorganization in the heat shock response. Mol. Cell. 2022;82:4386–4399. doi: 10.1016/j.molcel.2022.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawasaki K., Fukaya T. Functional coordination between transcription factor clustering and gene activity. Mol. Cell. 2023;83:1605–1622. doi: 10.1016/j.molcel.2023.04.018. [DOI] [PubMed] [Google Scholar]
- 85.Schneider N., et al. Liquid-liquid phase separation of light-inducible transcription factors increases transcription activation in mammalian cells and mice. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abd3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei M.-T., et al. Nucleated transcriptional condensates amplify gene expression. Nat. Cell. Biol. 2020;22:1187–1196. doi: 10.1038/s41556-020-00578-6. [DOI] [PubMed] [Google Scholar]
- 87.Mir M., et al. Dynamic multifactor hubs interact transiently with sites of active transcription in Drosophila embryos. eLife. 2018;7 doi: 10.7554/eLife.40497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dufourt J., et al. Temporal control of gene expression by the pioneer factor Zelda through transient interactions in hubs. Nat. Commun. 2018;9:5194. doi: 10.1038/s41467-018-07613-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chong S., et al. Tuning levels of low-complexity domain interactions to modulate endogenous oncogenic transcription. Mol. Cell. 2022;82:2084–2097. doi: 10.1016/j.molcel.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y., et al. Mechanisms governing target search and binding dynamics of hypoxia-inducible factors. eLife. 2022;11 doi: 10.7554/eLife.75064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chong S., et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science. 2018;361 doi: 10.1126/science.aar2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kent S., et al. Phase-separated transcriptional condensates accelerate target-search process revealed by live-cell single-molecule imaging. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sabari B.R., et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 2018;361 doi: 10.1126/science.aar3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang W., et al. A histidine cluster determines YY1-compartmentalized coactivators and chromatin elements in phase-separated enhancer clusters. Nucleic Acids Res. 2022;50:4917–4937. doi: 10.1093/nar/gkac233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuznetsova K., et al. Nanog organizes transcription bodies. Curr. Biol. 2023;33:164–173. doi: 10.1016/j.cub.2022.11.015. [DOI] [PubMed] [Google Scholar]
- 96.Cho C.-Y., O’Farrell P.H. Stepwise modifications of transcriptional hubs link pioneer factor activity to a burst of transcription. Nat. Commun. 2023;14:4848. doi: 10.1038/s41467-023-40485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pownall M.E., et al. Chromatin expansion microscopy reveals nanoscale organization of transcription and chromatin. Science. 2023;381:92–100. doi: 10.1126/science.ade5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Henninger J.E., et al. RNA-mediated feedback control of transcriptional condensates. Cell. 2021;184:207–225. doi: 10.1016/j.cell.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Desai R.V., et al. A DNA repair pathway can regulate transcriptional noise to promote cell fate transitions. Science. 2021;373 doi: 10.1126/science.abc6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patel H.P., et al. DNA supercoiling restricts the transcriptional bursting of neighboring eukaryotic genes. Mol. Cell. 2023;83:1573–1587. doi: 10.1016/j.molcel.2023.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hamamoto K., et al. Dynamic interplay between non-coding enhancer transcription and gene activity in development. Nat. Commun. 2023;14:826. doi: 10.1038/s41467-023-36485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li J., et al. Single-gene imaging links genome topology, promoter–enhancer communication and transcription control. Nat. Struct. Mol. Biol. 2020;27:1032–1040. doi: 10.1038/s41594-020-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohishi H., et al. STREAMING-tag system reveals spatiotemporal relationships between transcriptional regulatory factors and transcriptional activity. Nat. Commun. 2022;13:7672. doi: 10.1038/s41467-022-35286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karr J.P., et al. The transcription factor activity gradient (TAG) model: contemplating a contact-independent mechanism for enhancer-promoter communication. Genes Dev. 2022;36:7–16. doi: 10.1101/gad.349160.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rinzema N.J., et al. Building regulatory landscapes reveals that an enhancer can recruit cohesin to create contact domains, engage CTCF sites and activate distant genes. Nat. Struct. Mol. Biol. 2022;29:563–574. doi: 10.1038/s41594-022-00787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen H., et al. Dynamic interplay between enhancer-promoter topology and gene activity. Nat Genet. 2018;50:1296–1303. doi: 10.1038/s41588-018-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alexander J.M., et al. Live-cell imaging reveals enhancer-dependent Sox2 transcription in the absence of enhancer proximity. eLife. 2019;8 doi: 10.7554/eLife.41769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Espinola S.M., et al. Cis-regulatory chromatin loops arise before TADs and gene activation, and are independent of cell fate during early Drosophila development. Nat. Genet. 2021;53:477–486. doi: 10.1038/s41588-021-00816-z. [DOI] [PubMed] [Google Scholar]
- 109.Platania A., et al. Competition between transcription and loop extrusion modulates promoter and enhancer dynamics. bioRxiv. 2023 doi: 10.1101/2023.04.25.538222. Published online April 26, 2023. [DOI] [Google Scholar]
- 110.Gabriele M., et al. Dynamics of CTCF- and cohesin-mediated chromatin looping revealed by live-cell imaging. Science. 2022;376:496–501. doi: 10.1126/science.abn6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mach P., et al. Cohesin and CTCF control the dynamics of chromosome folding. Nat. Genet. 2022;54:1907–1918. doi: 10.1038/s41588-022-01232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mach P., Giorgetti L. Integrative approaches to study enhancer-promoter communication. Curr. Opin. Genet. Dev. 2023;80 doi: 10.1016/j.gde.2023.102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bartman C.R., et al. Enhancer regulation of transcriptional bursting parameters revealed by forced chromatin looping. Mol. Cell. 2016;62:237–247. doi: 10.1016/j.molcel.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robles-Rebollo I., et al. Cohesin couples transcriptional bursting probabilities of inducible enhancers and promoters. Nat. Commun. 2022;13:4342. doi: 10.1038/s41467-022-31192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zuin J., et al. Nonlinear control of transcription through enhancer-promoter interactions. Nature. 2022;604:571–577. doi: 10.1038/s41586-022-04570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fukaya T., et al. Enhancer control of transcriptional bursting. Cell. 2016;166:358–368. doi: 10.1016/j.cell.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Levo M., et al. Transcriptional coupling of distant regulatory genes in living embryos. Nature. 2022;605:754–760. doi: 10.1038/s41586-022-04680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Batut P.J., et al. Genome organization controls transcriptional dynamics during development. Science. 2022;375:566–570. doi: 10.1126/science.abi7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bohrer C.H., Larson D.R. Synthetic analysis of chromatin tracing and live-cell imaging indicates pervasive spatial coupling between genes. eLife. 2023;12 doi: 10.7554/eLife.81861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zoller B., et al. Eukaryotic gene regulation at equilibrium, or non? Curr. Opin. Syst. Biol. 2022;31 doi: 10.1016/j.coisb.2022.100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Szczurek A.T., et al. Polycomb sustains promoters in a deep OFF-state by limiting PIC formation to counteract transcription. bioRxiv. 2023 doi: 10.1101/2023.06.13.544762. Published online June 14, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Douaihy M., et al. BurstDECONV: a signal deconvolution method to uncover mechanisms of transcriptional bursting in live cells. Nucleic Acids Res. 2023;51 doi: 10.1093/nar/gkad629. [DOI] [PMC free article] [PubMed] [Google Scholar]