Abstract
The cytoadhesion of Plasmodium falciparum laboratory strains and clones to Saimiri brain microvascular endothelial cells (SBEC 17), with chondroitin-4-sulfate (CSA) as the only adhesion receptor, was tested. Only one strain had significant cytoadhesion. However, CSA-specific infected erythrocytes (IRBCs) were detected in all strains after selection of a CSA-specific subpopulation by culturing the few adherent IRBCs. This demonstrates the lack of sensitivity of cytoadhesion microassays for detecting small quantities of CSA-specific IRBCs in cultures or field isolates. Cytoadhesion to CSA is maximal at 24 h of the cycle and decreases with the onset of schizogony, reaching a minimum just before reinvasion. This fluctuation must be taken into account in comparisons of the cytoadhesion of different strains or isolates. The minimum size of CSA for active inhibition was 4 kDa, and a mass of 9 kDa was required for inhibition similar to that obtained with the 50-kDa CSA. In contrast to cytoadhesion to CSA, which is pH independent or maximal at physiological pH (depending on the target endothelial cells), adhesion to CD36 and intercellular adhesion molecule 1 was pH dependent, requiring acidic conditions to be maximal in all cases. Cytoadhesion to CSA may trigger the occlusion of microvessels and cause the acidosis necessary for the other receptors to be fully efficient. If this key role in the mechanisms of sequestration were to be confirmed in vivo, prevalence studies of the CSA cytoadhesion phenotype would have to be reevaluated, because simple cytoadhesion assays do not detect CSA-specific parasites present in very low numbers, and these parasites might then be undetected in the peripheral blood but present in organs in which sequestration occurs, such as the placenta (M. Fried and P. E. Duffy, Science 272:1502–1504, 1996).
Plasmodium falciparum is responsible for almost all deaths from malaria. One possible reason for this virulence is the ability of P. falciparum-infected erythrocytes (IRBCs) to cytoadhere to the endothelial cells lining the microvessels, a phenomenon called sequestration (1, 9, 21). Various endothelial receptors involved in cytoadhesion have been identified (for a review see reference 23). Chondroitin-4-sulfate (CSA), a glycosaminoglycan, has recently been shown to mediate cytoadhesion to Saimiri brain microvascular endothelial cells (SBECs), human lung endothelial cells (28), and CHO and C32 cells (30). The involvement of this receptor in humans was confirmed by a study of IRBC sequestration on placentas (8), in which the IRBCs obtained from human placentas adhered exclusively to purified CSA. The cytoadhesion of these IRBCs to sections of fresh-frozen human placenta was inhibited by purified CSA. The authors detected no IRBCs that bound to CSA in the blood of nonpregnant donors. They therefore suggested that this parasite subpopulation preferentially sequesters and multiplies in the placenta. CSA was also found to be involved in cytoadhesion to the placenta by Gysin et al. in the primate model Saimiri sciureus (13).
However, the involvement of CSA is not limited to cytoadhesion in the placenta. Specific desequestration of IRBCs has been achieved by the injection of CSA into P. falciparum-infected Saimiri monkeys (27). For these nonpregnant monkeys it was not possible to determine the organ(s) from which the IRBCs were desequestered, but CSA is expressed throughout the microvasculature, in association with various proteoglycans, including thrombomodulin (13) and, in the placenta, beta-glycan and thrombomodulin (26).
CSA is the only receptor for which it has been possible to reverse cytoadhesion in vivo. A more detailed characterization of cytoadhesion to CSA is therefore of particular interest for the development of new antipathological therapeutic strategies.
MATERIALS AND METHODS
Parasites.
Parasites of the Palo Alto (FUP)1 (PA) (11, 12), IPL/BRE1 (BRE) (28), and FCR3 strains and of the D6 (Sierra Leone) and 3D7 (32) clones were studied. The IRBCs were cultured in RPMI 1640 containing bicarbonate, glutamine, 0.2% glucose, 50 μM hypoxanthine, 10-μg/ml gentamicin, and 10% human AB+ serum, containing O+ erythrocytes, at 37°C in a humidified atmosphere consisting of 5% O2, 5% CO2, and 90% N2.
Selection by panning.
We characterized cytoadhesion to CSA and compared it to that to CD36 and intercellular adhesion molecule 1 (ICAM-1) by panning the three strains and the two clones as previously described (25). Briefly, we selected subcultures cytoadhering specifically to CSA by incubating at 37°C gelatin-enriched suspensions of IRBCs from the various strains and clones on confluent monolayers of SBEC 17, which expresses only CSA (10). The cells were incubated for 2 h in cytoadhesion medium composed of RPMI 1640 with the pH adjusted to 6.8. They were then washed extensively with cytoadhesion medium to remove noncytoadherent IRBCs. Cytoadherent IRBCs, if present, were cultured as described above, and two additional selections by panning were performed on these subpopulations.
We also selected subpopulations of cells of the three strains used that cytoadhered to CD36 or ICAM-1. Selection for CD36 cytoadhesion was carried out as described above on a confluent monolayer of SBEC C2, which expresses CD36 and CSA, after treatment of the endothelial cells with 1-U/ml chondroitinase ABC for 1 h at 37°C. Chondroitinase ABC treatment resulted in the complete digestion of CSA at the surface of the SBECs. The only receptor remaining was CD36. For testing of ICAM-1 cytoadhesion, SBEC 3A (expressing ICAM-1 and CSA) was used, after treatment with chondroitinase ABC, as described above.
Cytoadhesion and cytoadhesion inhibition assays.
Mature-stage IRBCs from preparations enriched by gelatin sedimentation (17) were suspended at a concentration of 5 × 106 IRBCs/ml in cytoadhesion medium at pH 6.8 unless otherwise indicated. Cytoadhesion microassays were then performed on 12-well immunofluorescence assay slides (Institut Pasteur, Paris, France) on which SBECs had been cultured to confluence. Forty microliters of the IRBC suspension was added to each well, and the IRBCs were allowed to cytoadhere for 2 h at 37°C. Unattached IRBCs were removed by extensive washing, and the cells were fixed for 1 h with 2.5% glutaraldehyde (G5882; Sigma).
For cytoadhesion inhibition assays, the IRBCs were incubated with SBECs in the presence of 0.1 mg of soluble CSA (27042; Fluka, Saint Quentin Fallavier, France) per ml or with SBECs previously incubated for 1 h at 37°C in the presence of 1-U/ml chondroitinase ABC, 25-μg/ml 84H10 anti-ICAM-1 monoclonal antibody (MAb) (Immunotech, Marseille, France), or 5-μg/ml FA6-152 anti-CD36 MAb (gift from L. Edelman).
Adherent IRBCs were counted under a light microscope in four randomly selected fields (each with a 0.2827-mm2 area) at a ×300 magnification (Nikon TMS) distributed over the surface of each sample. The assays were performed in duplicate or triplicate, and the results were expressed as the number of bound IRBCs per square millimeter of target cell monolayer.
Synchronization of the IRBC cultures.
We synchronized the CSA-specific PA-infected RBC subpopulation by selecting ring-stage parasites with multiple 5% sorbitol treatments (18) until the parasites reinvaded the erythrocytes within 4 h.
Preparation of CSA molecules of different sizes.
A 10-mg/ml solution of commercially available CSA (50 kDa) in 0.15 M NaCl was digested by incubation with 0.5-U/ml chondroitinase ABC for 30 min at 20°C. The sample was boiled for 10 min to stop the reaction. Control CSA was prepared in the same way but without the addition of chondroitinase ABC. The variously sized molecules present in the digested sample were separated by exclusion chromatography on a polyacrylamide size exclusion gel, Biogel P30 (catalog no. 150-4154; Bio-Rad, Ivry sur Seine, France), and their sizes were determined by comparison with the elution profiles of standards (gift from H. Lortat-Jacob). The elution medium was 1 M NaCl. The collected fractions were dialyzed against water and lyophilized. Cytoadhesion inhibition activity was tested with dilutions of the fractions in 0.15 M NaCl at a concentration of 4 μM. The fractions were mixed with equal volumes of suspensions of 107 IRBCs/ml of cytoadhesion medium to give final concentrations of 2 μM CSA and 5 × 106 IRBCs/ml. Control CSA was treated in the same way, with the final concentration of 2 μM corresponding to 0.1 mg/ml. The molecules that we purified and tested in inhibition assays were 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 7, and 9 kDa in size.
Statistical analysis.
Results of IRBC adhesion, cytoadhesion, and cytoadhesion inhibition assays are expressed as means ± standard errors. The Mann-Whitney test was used to evaluate the statistical significance of differences for data from cytoadhesion inhibition assays and to compare cytoadhesion levels.
RESULTS
Selection by panning on SBEC 17.
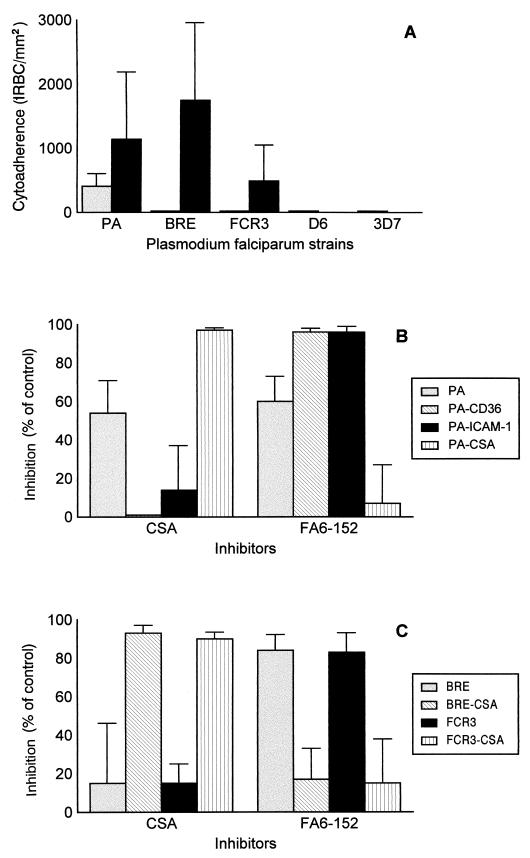
The PA strain showed significant cytoadhesion to SBEC 17 (408 ± 199 IRBCs/mm2), whereas the other two strains and the two clones cytoadhered at levels of fewer than 20 IRBCs/mm2 of cells (Fig. 1A). The few cytoadherent IRBCs of the three strains and two clones were cultured, and subpopulations that cytoadhered specifically to SBEC 17 were obtained only from the PA, BRE, and FCR3 strains. After three rounds of panning on SBEC 17, the levels of cytoadhesion to SBEC 17 of the CSA-selected subpopulations were 1,143 ± 1,044 IRBCs/mm2 for the PA subpopulation (PACSA), 1,750 ± 1,905 IRBCs/mm2 for BRECSA, and 489 ± 565 IRBCs/mm2 for FCR3CSA (Fig. 1A).
FIG. 1.
IRBC cytoadhesion to CSA, CD36, and ICAM-1. (A) The numbers of cytoadherent IRBCs per mm2 of SBEC 17 monolayer for the initial populations (left columns) of five strains were compared with those for the corresponding CSA-selected subpopulations (right columns). Selection was carried out by three successive pannings on SBEC 17, expressing only CSA cytoadhesion receptors, for PA, BRE, and FCR3 strains, or by one panning for clones D6 and 3D7. The CSA-dependent cytoadhesion phenotype was detected in the selected cultures of strains PA, BRE, and FCR3, even if it was not detectable in the corresponding initial population. The last two diagrams show the inhibition by soluble CSA (0.1 mg/ml) and anti-CD36 MAb FA6-152 (5 μg/ml) of the cytoadhesion to SBEC 1D for initial and CD36-, ICAM-1-, and CSA-selected PA-infected RBC populations (B) and for initial and CSA-selected BRE- and FCR3-infected RBC populations (C). Inhibition is expressed as a percentage relative to the corresponding control value obtained in the absence of inhibitor.
Characterization of IRBC cytoadhesion to the various endothelial receptors.
We characterized the cytoadhesion phenotypes of all the selected IRBC populations by performing cytoadhesion inhibition microassays on the various SBECs. With SBEC 1D, which expresses CSA, CD36, and ICAM-1, 54% of the PA-infected RBC cytoadhesion was inhibited by soluble CSA and 60% was inhibited by MAb FA6-152 (Fig. 1B). The cytoadhesion of the PACSA-infected RBCs to the SBEC 1D was totally inhibited by soluble CSA but was unaffected by MAb FA6-152. For the populations selected on SBEC C2 (PACD36) and SBEC 3A (PAICAM-1), there were total inhibition with MAb FA6-152 and no inhibition with soluble CSA (Fig. 1B). This result was consistent with the fact that PACD36- and PAICAM-1-infected RBCs did not cytoadhere to SBEC 17.
PACD36-infected RBCs cytoadhered to SBEC 3A (data not shown), and this cytoadhesion was insensitive to CSA and chondroitinase ABC. PAICAM-1-infected RBCs cytoadhered to SBEC C2 (not shown), and cytoadhesion was partially inhibited by FA6-152 (37% ± 8%) but not by soluble CSA. MAb 84H10, which recognizes the ICAM-1 expressed on SBEC 1D and SBEC 3A and inhibits adhesion of IRBCs to ICAM-1 (20), did not inhibit the cytoadhesion of any of the PA-selected populations to the SBECs studied (data not shown).
The results obtained with the BRE- and FCR3-selected IRBCs were similar to those obtained with the PA-selected IRBC subpopulations (Fig. 1C).
Cytoadhesion fluctuations during the IRBC cycle.
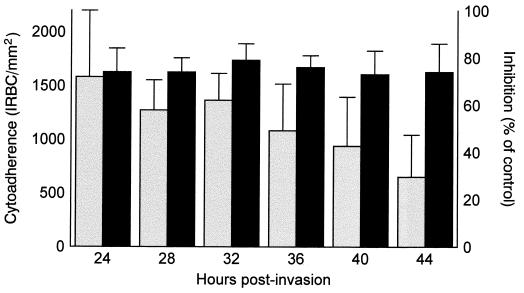
We synchronized PACSA-infected RBCs until the parasites reinvaded within 4 h. From 24 to 40 h of the cycle we performed CSA inhibition assays on SBEC 1D every 4 h. Cytoadhesion was maximal at 24 h of the cycle (Fig. 2), corresponding to the young trophozoite stage. It decreased significantly with the onset of schizogony. At 44 h of the cycle, just before the start of reinvasion, cytoadhesion was 2.4 times lower than the maximum level recorded. The differences between cytoadhesion at 24 h of the cycle and at other times were not significant until 36 h. The differences were very significant at 40 h (P = 0.0067) and extremely significant at 44 h (P = 0.0004). Cytoadhesion inhibition by CSA remained at the same level throughout the cycle, with no significant variation (Fig. 2).
FIG. 2.
Cytoadhesion changes during the parasite cycle. Time-dependent changes in cytoadhesion level (number of adherent IRBCs per square millimeter of SBEC 1D) for highly synchronized CSA-selected PA-infected RBCs (left columns) were assessed, together with cytoadhesion inhibition by CSA relative to the control cytoadhesion (right columns). The experiment began when the IRBCs reached the trophozoite stage and ended just before reinvasion.
pH-dependent cytoadhesion.
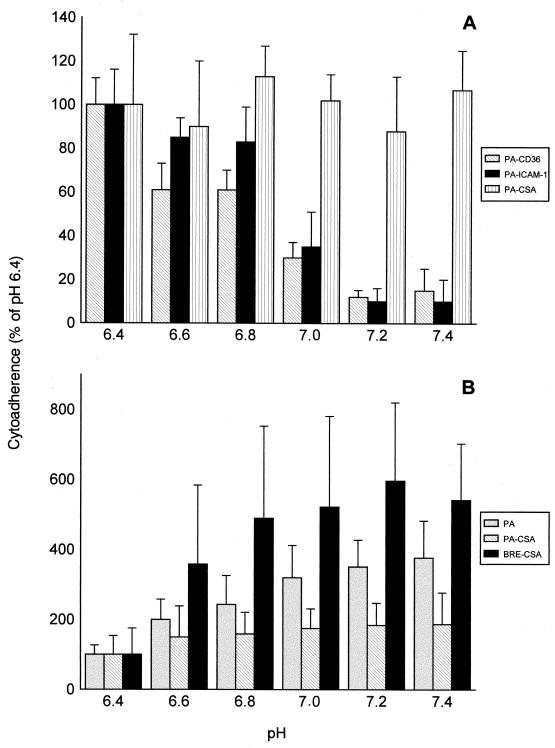
Assays were performed with IRBCs suspended in cytoadhesion medium adjusted to the desired pH with HCl or NaOH. For PA- and BRE-infected RBCs panned on cellular CD36 and ICAM-1, we observed a decrease in cytoadhesion to the SBEC 1D ranging from 70 to 90% if the pH of the cytoadhesion medium was increased from 6.4 to 7.4 (Fig. 3A). The decreases in cytoadhesion were only about 60% with SBEC C2 and 50% with SBEC 3A. With SBEC 1D, SBEC C2, and SBEC 3A, the cytoadhesion of PACSA- and BRECSA-infected RBCs was constant over the entire pH range (Fig. 3A). Finally, for the IRBCs adhering to SBEC 17 (i.e., PA-, PACSA-, and BRECSA-infected RBCs), adhesion levels were two to six times higher at pH 7.4 than at pH 6.4 (Fig. 3B).
FIG. 3.
pH-dependent changes in cytoadhesion. The cytoadhesion of various PA-infected RBC populations (infected with PACD36, PAICAM-1, and PACSA) on SBEC 1D (A) and of IRBC populations able to cytoadhere to SBEC 17 (PA, PACSA, and BRECSA) (B) was determined as a function of the pH of the medium, for pH values ranging from 6.4 to 7.4. Cytoadhesion is expressed as the percentage of adherent IRBCs at pH 6.4.
Size-dependent CSA activity.
The cytoadhesion inhibition activities for the various sizes of CSA molecules tested are presented in Table 1, together with the significance levels of the inhibitions relative to control cytoadhesion levels obtained in the absence of CSA or relative to the maximum inhibition obtained with the 50-kDa CSA. Molecules smaller than 4 kDa had no significant inhibitory activity. Inhibition increased with the size of the CSA polymer. The inhibition achieved with all molecules at least 9 kDa in size was statistically equivalent to the inhibition achieved with 50-kDa CSA. Similar results were obtained with PACSA and BRECSA.
TABLE 1.
Cytoadherence inhibitions obtained with the purified oligosaccharides of CSA
| Oligosaccharide size (kDa) | % Inhibition efficiencya |
P value vsb:
|
|
|---|---|---|---|
| Inhibition by full-length 50-kDa CSA | Cytoadherence in the absence of CSA (control) | ||
| 1 | 0 | 0.0002 (ES) | 0.065 (NQS) |
| 1.5 | 4 ± 6 | 0.0002 (ES) | 0.5054 (NS) |
| 2 | 7 ± 9 | 0.0002 (ES) | >0.9999 (NS) |
| 2.5 | 1 ± 2 | 0.0002 (ES) | 0.4418 (NS) |
| 3 | 24 ± 21 | 0.0002 (ES) | 0.083 (NQS) |
| 3.5 | 26 ± 28 | 0.0002 (ES) | 0.1949 (NS) |
| 4 | 50 ± 21 | 0.0006 (ES) | 0.0011 (VS) |
| 5 | 59 ± 11 | 0.0002 (ES) | 0.0003 (ES) |
| 6 | 73 ± 9 | 0.0011 (VS) | 0.0002 (ES) |
| 7 | 85 ± 9 | 0.0281 (S) | 0.0002 (ES) |
| 9 | 99 ± 13 | 0.6454 (NS) | 0.0002 (ES) |
| 24 | 100 | >0.9999 (NS) | 0.0002 (ES) |
Mean ± standard deviation for cytoadherence inhibition expressed as a percentage of that by 50-kDa CSA.
ES, extremely significant; VS, very significant; S, significant; NQS, not quite significant; NS, not significant.
DISCUSSION
The sequestration of IRBCs in the microvasculature is thought to play a key role in the pathogenesis of severe malaria caused by P. falciparum. If all the IRBC field isolates sequester away from the peripheral circulation when mature, only a small proportion cause life-threatening complications like cerebral malaria. The cytoadhesion of IRBCs to the endothelial cells lining the small vessels and the formation of rosettes (7, 31) lead to sequestration. Cytoadhesion involves at least seven types of endothelial receptors. Almost all isolates bind in vitro to CD36 and thrombospondin (2, 5, 22, 29), whereas only a few isolates adhere to ICAM-1, vascular endothelial cell adhesion molecule, E-selectin, platelet/endothelial cell adhesion molecule 1/CD31, and CSA (4, 5, 22, 25, 28, 30). Many studies have found no correlation between the cytoadhesion phenotypes of field isolates and the severity of the disease (5, 14, 15, 19, 24, 30). However, Ho et al. found that cytoadhesion to C32 cells was associated with the severity of the clinical case (16), and Newbold et al. found a correlation between adhesion to ICAM-1 and clinical illness in nonanemic patients and a particularly strong correlation in cerebral malaria patients (22). However, C32 cells have at least two types of cytoadhesion receptors (CD36 and CSA), making interpretation of the results difficult.
Fried and Duffy studied cytoadhesion in human placentas (8). IRBCs from all the placentas studied cytoadhered to CSA, whereas no cytoadhesion to CSA was detected with IRBCs from the peripheral blood of some of the pregnant women. This demonstrates that a cytoadhesion phenotype present in an organ may not be detectable in the peripheral circulation by adhesion assays. This could have been due to the detection limit of the adhesion assay or to the CSA-specific phenotype being limited to the placenta. The restriction of the CSA-specific phenotype to the placenta is ruled out by the desequestration of IRBCs obtained when P. falciparum-infected nonpregnant S. sciureus monkeys (27) are injected with CSA. The specificity of this desequestration demonstrates that IRBCs are able to cytoadhere to CSA in the microvasculature of nonpregnant animals of this primate species, as well as in the placenta. However, the low sensitivity of the adhesion assays is demonstrated by the results presented here. By measuring the cytoadhesion to SBEC 17 we were able to show that there were no IRBCs able to cytoadhere to CSA in the BRE1 and FCR3 strains. However, we did detect a small number of cells of these strains with the CSA phenotype by panning on SBEC 17. In light of the results obtained by Fried and Duffy with placentas (8), this detection is of importance. A few IRBCs in the peripheral blood may indicate the presence of a particular phenotype in the microvasculature of different organs, with pathological consequences, as has been noted for maternal malaria.
We characterized the cytoadhesion to CSA and compared it with that to CD36 and ICAM-1 by selecting IRBC subpopulations that specifically cytoadhered to these receptors. IRBC selection was carried out by panning on endothelial cells treated enzymatically such that only one receptor was expressed at their surface. We used this approach, rather than panning on purified receptors, to preserve the three-dimensional conformations of the molecules. We checked the specificity of the pannings by cytoadhesion inhibition assays on SBEC 1D. We showed for three P. falciparum strains that CSA cytoadhesion was not associated with other cytoadhesion phenotypes on the same IRBC (8, 30). However, CD36-specific IRBCs cytoadhered to ICAM-1-producing cells (SBEC 3A4) and ICAM-1-specific IRBCs cytoadhered to CD36-producing cells, as previously described by Baruch et al. (3).
We studied changes in the extent of cytoadhesion during maturation of the IRBC subpopulations panned on SBEC 17. The significant decrease in cytoadhesion between 24 h of the cycle and the beginning of the reinvasion must be taken into account when the levels of cytoadhesion of different strains or the levels of cytoadhesion of a strain at different times in the cycle are being compared. The percent cytoadhesion inhibition by soluble CSA remained constant over the same period, showing that the proportion of IRBCs cytoadhering to CSA remained constant throughout the mature stages. Therefore, although cytoadhesion inhibition by soluble CSA is a characteristic which can be compared for different IRBC cultures, the results must be interpreted with caution in relation to the cytoadhesion level. Indeed, with the exception of highly synchronized IRBCs, comparisons of the cytoadhesion levels of two IRBC strains, populations, or cultures should take into account the stage of the parasites, with the help of standard curves of the cytoadhesion level as a function of the maturation stage for each culture. The same considerations should be applied to field studies of receptor prevalence.
The 2.4-fold-lower level of cytoadhesion may be due to modification of the plasticity of the IRBC membrane or of the three-dimensional structure or to a rapid turnover of the parasitic ligand during maturation. In any case, although 58% of the IRBCs able to cytoadhere at 24 h of the cycle lose this capacity during schizogony, this does not necessarily mean that there is desequestration of 58% of the adherent IRBCs in vivo. We observed cytoadhesion but not dissociation of the cytoadhesion, and it is not clear whether this phenomenon is limited to in vitro adhesion.
Crandall et al. (6) reported higher levels of cytoadhesion under slightly acidic conditions. We also found that cytoadhesion to CD36 and ICAM-1 was affected by pH, in the range between pH 6.4 and 7.4. This effect depended on the endothelial cells on which cytoadhesion assays were carried out, but the level of cytoadhesion was consistently lower at physiological pH (pH 7.2 to 7.4) by 50 to 90%. For CSA-selected IRBCs, depending on the endothelial cells used, the cytoadhesion level was either constant or higher at physiological pH (on SBEC 17). If this result is not restricted to in vitro cytoadhesion it may have two major consequences. Firstly, at equivalent development stages, similar cytoadhesion levels can be obtained with two to ten times fewer IRBCs specifically binding to CSA than with IRBCs binding to CD36 or ICAM-1. The difference in the number of IRBCs required may be two to six times greater if the endothelial cells on which the cytoadhesion assay is carried out have characteristics similar to those of SBEC 17. Secondly, at pH 7.2 to 7.4 cytoadhesion to CSA is more efficient than that to CD36 or ICAM-1. Therefore, CSA-specific IRBCs may cause the occlusion of microvessels, which then leads, by a local acidosis, to the optimal conditions for cytoadhesion to CD36 and ICAM-1. This, together with the very wide distribution of CSA-expressing proteoglycans, could make cytoadhesion to CSA particularly important in the development of diseases. This aspect is reinforced by pH regulation of the various compartments in which sequestration occurs, particularly in the brain.
The differences in cytoadhesion pH sensitivity observed for individual parasite strains, depending on the target endothelial cells used in the assay, demonstrate differences in the interactions between endothelial receptors and parasitic ligands. These differences probably reflect differences in the environment of CD36, ICAM-1, and CSA at the surface of the four types of SBECs studied, inducing changes in the conformation of the molecules. These endothelial cells are the target for in vivo sequestration in S. sciureus, and they therefore exhibit aspects of the cytoadhesion observed in this model. However, caution should be used in interpretation of the cytoadhesion characteristics observed with cells which are not targets of sequestration in vivo, all the more so if adhesion is assayed on purified immobilized receptors, the environment of which is totally artificial.
In maternal malaria, and in other clinical complications in which sequestration is thought to be involved, the inhibition or reversal of cytoadhesion to CSA may be of particular value. Desequestration of IRBCs cytoadhering to CSA can be achieved by intramuscular injection of CSA in Saimiri monkeys (27). Defining the minimal active length of CSA may be of interest for limiting possible size-related reactions. All purified CSA polysaccharides smaller than 4 kDa were devoid of significant inhibitory activity. The 9-kDa polymer was the smallest molecule for which the cytoadhesion inhibition was similar to that with the 50-kDa CSA (P > 0.6). The mass of a CSA monomer [(β1-4)GlcUA(β1-3)GalNAc4SO4] is 460 Da, so significant inhibition is observed only with polysaccharides that are at least 8- to 9-mers, and maximum activity is obtained only with molecules that are at least 19-mers. If CSA is regarded as a linear chain, the defined size of the active polymer seems too large to correspond to the length of the parasitic ligand active site. Therefore, a precise three-dimensional CSA structure is presumably necessary for CSA to interact with the corresponding ligand, and this structure requires a minimum length of molecule. The CSA chain may also need to be long enough to interact with several ligands at the same time.
CSA has been shown to be a cytoadhesion receptor in vivo (8, 27). Investigations of the importance of this receptor in field isolates from Papua New Guinea (30) and Thailand (5) revealed a low prevalence of the CSA cytoadhesion phenotype. These prevalence studies, carried out with cells or purified receptors in adhesion microassays, must now be reinterpreted in light of the fact that selection by panning is necessary to detect small proportions of CSA-binding IRBCs in a strain or an isolate, particularly given the low sensitivity of the assay and changes in cytoadhesion levels during the life cycle of the parasite.
ACKNOWLEDGMENTS
We thank L. Edelman for the gift of anti-CD36 MAb FA6-152 and H. Lortat-Jacob for CSA size standards and for technical assistance in the purification of the CSA polysaccharides.
This work was supported by GDR-1077 and French Army grants (contract DSP/STTC-97/070).
REFERENCES
- 1.Aikawa M. Human cerebral malaria. Am J Trop Med Hyg. 1988;39:3–10. doi: 10.4269/ajtmh.1988.39.3. [DOI] [PubMed] [Google Scholar]
- 2.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M, Ingravallo P. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Investig. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baruch D I, Gormley J A, Ma C, Howard R J, Pasloske B L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature (London) 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 5.Chaiyaroj S C, Angkasekwinai P, Buranakiti A, Looareesuwan S, Rogerson S J, Brown G V. Cytoadherence characteristics of Plasmodium falciparum isolates from Thailand: evidence for chondroitin sulfate A as a cytoadherence receptor. Am J Trop Med Hyg. 1996;55:76–80. doi: 10.4269/ajtmh.1996.55.76. [DOI] [PubMed] [Google Scholar]
- 6.Crandall I, Smith H, Sherman I W. Plasmodium falciparum: the effect of pH and Ca2+ concentration on the in vitro cytoadherence of infected erythrocytes to amelanotic melanoma cells. Exp Parasitol. 1991;73:362–368. doi: 10.1016/0014-4894(91)90108-9. [DOI] [PubMed] [Google Scholar]
- 7.David P H, Handunnetti S M, Leech J H, Gamage P, Mendis K N. Rosetting: a new cytoadherence property of malaria-infected erythrocytes. Am J Trop Med Hyg. 1988;38:289–297. doi: 10.4269/ajtmh.1988.38.289. [DOI] [PubMed] [Google Scholar]
- 8.Fried M, Duffy P E. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 9.Fujioka H, Aikawa M. The molecular basis of pathogenesis of cerebral malaria. Microb Pathog. 1996;20:63–72. doi: 10.1006/mpat.1996.0006. [DOI] [PubMed] [Google Scholar]
- 10.Gay F, Robert C, Pouvelle B, Peyrol S, Scherf A, Gysin J. Isolation and characterization of brain microvascular endothelial cells from Saimiri monkeys. An in vitro model for sequestration of Plasmodium falciparum-infected erythrocytes. J Immunol. 1995;184:15–28. doi: 10.1016/0022-1759(95)00070-q. [DOI] [PubMed] [Google Scholar]
- 11.Gysin J, Fandeur T. Saimiri sciureus (karyotype 14-7), an alternative experimental model of Plasmodium falciparum infection. Am J Trop Med Hyg. 1983;32:461–467. doi: 10.4269/ajtmh.1983.32.461. [DOI] [PubMed] [Google Scholar]
- 12.Gysin J, Hommel M, Pereira da Silva L. Experimental infection of the squirrel monkey Saimiri sciureus with Plasmodium falciparum. J Parasitol. 1980;66:1003–1009. [PubMed] [Google Scholar]
- 13.Gysin J, Pouvelle B, Le Tonqueze M, Edelman L, Boffa M C. Chondroitin sulfate of thrombomodulin is an adhesion receptor for Plasmodium falciparum-infected erythrocytes. Mol Biochem Parasitol. 1997;88:267–271. doi: 10.1016/s0166-6851(97)00082-0. [DOI] [PubMed] [Google Scholar]
- 14.Hasler T, Albrecht G R, van Schravendijk M R, Aguiar J C, Morehead K E, Pasloske B L, Ma C, Barnwell J W, Greenwood B, Howard R J. An improved microassay for Plasmodium falciparum cytoadherence using stable transformants of Chinese hamster ovary cells expressing CD36 or intercellular adhesion molecule-1. Am J Trop Med Hyg. 1993;48:332–347. doi: 10.4269/ajtmh.1993.48.332. [DOI] [PubMed] [Google Scholar]
- 15.Hasler T, Handunnetti S M, Aguiar J C, van Schravendijk S M, Greenwood B M, Lallinger G, Cegielski P, Howard R J. In vitro rosetting, cytoadherence and microagglutination properties of Plasmodium falciparum-infected erythrocytes from Gambian and Tanzanian patients. Blood. 1990;76:1845–1852. [PubMed] [Google Scholar]
- 16.Ho M, Singh B, Looareesuwan S, Davis T M E, Bunnag D, White N J. Clinical correlates of in vitro Plasmodium falciparum cytoadherence. Infect Immun. 1991;59:873–878. doi: 10.1128/iai.59.3.873-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen J B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978;27:1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- 18.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 19.Marsh K, Marsh V M, Brown J, Whittle H C, Greenwood B M. Plasmodium falciparum: the behaviour of clinical isolates in an in vitro model of infected red blood cell sequestration. Exp Parasitol. 1988;65:202–208. doi: 10.1016/0014-4894(88)90123-3. [DOI] [PubMed] [Google Scholar]
- 20.Maubert B, Guilbert L J, Deloron P. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infect Immun. 1997;65:1251–1257. doi: 10.1128/iai.65.4.1251-1257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendis K, Carter R. Clinical disease and pathogenesis in malaria. Parasitol Today. 1995;11:PTI 1–PTI 15. doi: 10.1016/0169-4758(95)80143-x. [DOI] [PubMed] [Google Scholar]
- 22.Newbold C, Warn P, Black G, Berendt A, Craig A, Snow B, Msobo M, Peshu N, Marsh K. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am J Trop Med Hyg. 1997;57:389–398. doi: 10.4269/ajtmh.1997.57.389. [DOI] [PubMed] [Google Scholar]
- 23.Ockenhouse C F. Cell adhesion molecules in host-parasite interactions. In: Wegner C D, editor. Adhesion molecules. London, United Kingdom: Academic Press Ltd.; 1994. pp. 277–291. [Google Scholar]
- 24.Ockenhouse C F, Ho M, Tandon N N, van Seventer G A, Shaw S, White N J, Jamieson G A, Chulay J D, Webster H K. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J Infect Dis. 1991;164:163–169. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- 25.Ockenhouse C F, Tegoshi T, Maeno Y, Benjamin C, Ko M, Aikawa M, Lobb R R. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for ELAM-1 and VCAM-1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pouvelle B, Fusaï T, Gysin J. Plasmodium falciparum et chondroïtine-4-sulfate: le nouveau couple clé de la séquestration? Med Trop. 1998;58:187–198. [PubMed] [Google Scholar]
- 27.Pouvelle B, Meyer P, Robert C, Bardel L, Gysin J. Chondroitin-4-sulfate impairs in vitro and in vivo cytoadherence of Plasmodium falciparum infected erythrocytes. Mol Med. 1997;3:508–518. [PMC free article] [PubMed] [Google Scholar]
- 28.Robert C, Pouvelle B, Meyer P, Muanza K, Fujioka H, Aikawa M, Scherf A, Gysin J. Chondroitin-4-sulphate (proteoglycan), a receptor for Plasmodium falciparum-infected erythrocyte adherence on brain microvascular endothelial cells. Res Immunol. 1995;146:383–393. doi: 10.1016/0923-2494(96)81042-x. [DOI] [PubMed] [Google Scholar]
- 29.Roberts D D, Sherwood J A, Spitalnik S L, Panton L J, Howard R, Dixit V M, Frazier W A, Miller L H, Ginsburg V. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature (London) 1985;318:64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- 30.Rogerson S J, Chaiyaroj S C, Ng K, Reeder J C, Brown G. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahlgren M, Fernandez V, Scholander C, Carlson J. Rosetting. Parasitol Today. 1994;10:73–79. doi: 10.1016/0169-4758(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 32.Walliker D, Quakyi I A, Wellems T E, McCutchan T F, Szarfman A, London W T, Corcoran L M, Burkot T R, Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]